Abstract
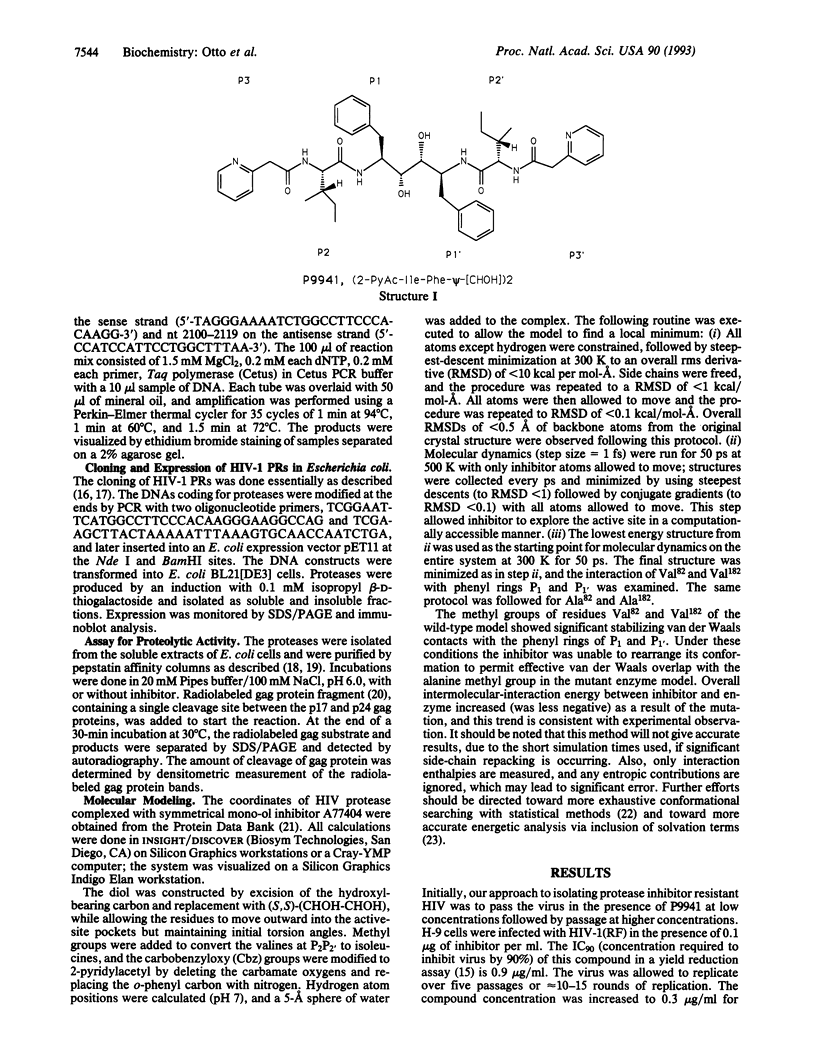
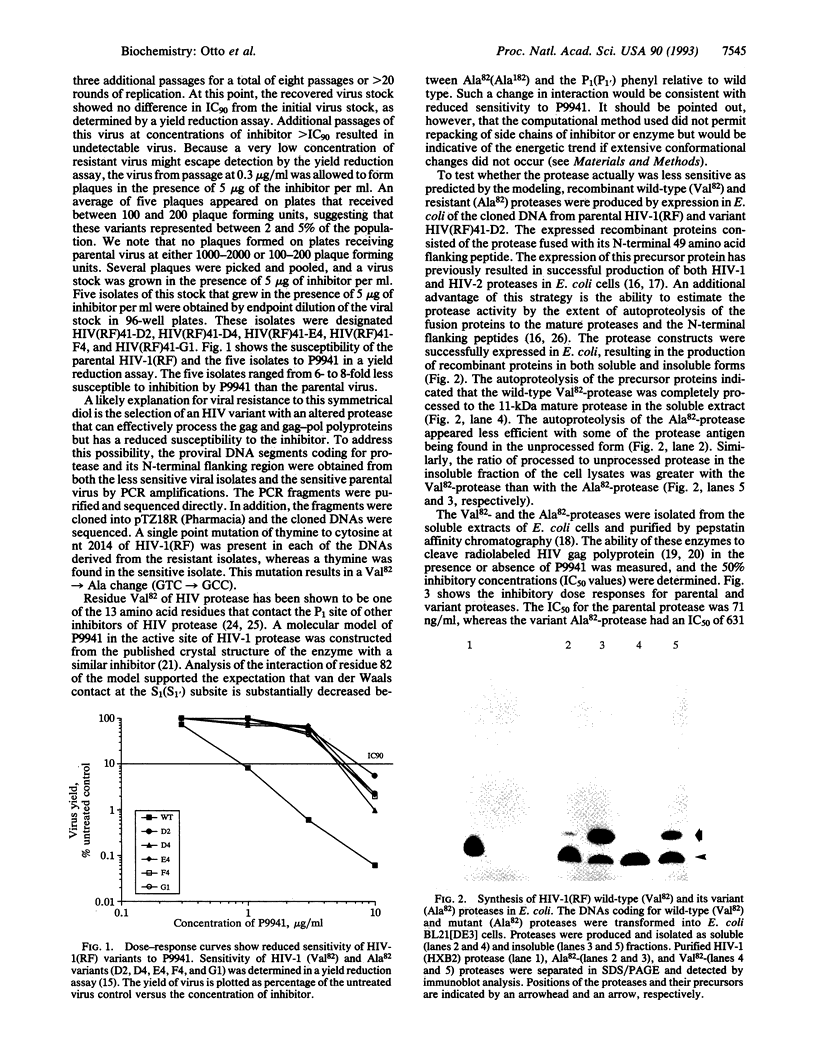
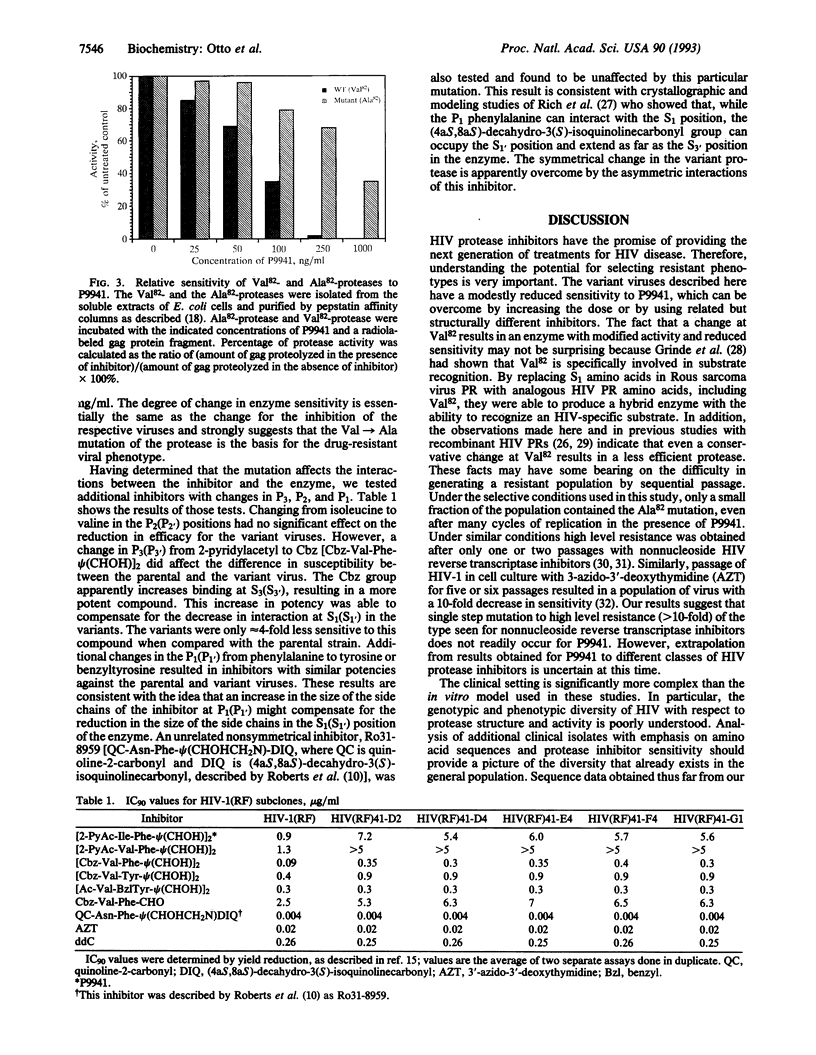
Protease inhibitors are another class of compounds for treatment of human immunodeficiency virus (HIV)-caused disease. The emergence of resistance to the current anti-HIV drugs makes the determination of potential resistance to protease inhibitors imperative. Here we describe the isolation of an HIV type 1 (HIV-1) resistant to an HIV-protease inhibitor. Serial passage of HIV-1 (strain RF) in the presence of the inhibitor, [2-pyridylacetylisoleucylphenylalanyl-psi (CHOH)]2 (P9941), failed to yield a stock of virus with a resistance phenotype. However, variants of the virus with 6- to 8-fold reduced sensitivity to P9941 were selected by using a combination of plaque assay and endpoint titration. Genetic analysis and computer modeling of the variant proteases revealed a single change in the codon for amino acid 82 (Val-->Ala), which resulted in a protease with lower affinity and reduced sensitivity to this inhibitor and certain, but not all, related inhibitors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng Y. S., McGowan M. H., Kettner C. A., Schloss J. V., Erickson-Viitanen S., Yin F. H. High-level synthesis of recombinant HIV-1 protease and the recovery of active enzyme from inclusion bodies. Gene. 1990 Mar 15;87(2):243–248. doi: 10.1016/0378-1119(90)90308-e. [DOI] [PubMed] [Google Scholar]
- Cheng Y. S., Patterson C. E., Rucker R. G., Otto M. J., Rizzo C. J., Korant B. D. Molecular characterization of HIV-2 (ROD) protease following PCR cloning from virus infected H9 cells. Adv Exp Med Biol. 1992;312:83–88. doi: 10.1007/978-1-4615-3462-4_7. [DOI] [PubMed] [Google Scholar]
- Cheng Y. S., Yin F. H., Foundling S., Blomstrom D., Kettner C. A. Stability and activity of human immunodeficiency virus protease: comparison of the natural dimer with a homologous, single-chain tethered dimer. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9660–9664. doi: 10.1073/pnas.87.24.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke P. L., Kohl N. E., Hanobik M. G., Leu C. T., Vacca J. P., Guare J. P., Heimbach J. C., Dixon R. A. Interaction of mutant forms of the HIV-1 protease with substrate and inhibitors. Adv Exp Med Biol. 1991;306:483–487. doi: 10.1007/978-1-4684-6012-4_61. [DOI] [PubMed] [Google Scholar]
- Darke P. L., Nutt R. F., Brady S. F., Garsky V. M., Ciccarone T. M., Leu C. T., Lumma P. K., Freidinger R. M., Veber D. F., Sigal I. S. HIV-1 protease specificity of peptide cleavage is sufficient for processing of gag and pol polyproteins. Biochem Biophys Res Commun. 1988 Oct 14;156(1):297–303. doi: 10.1016/s0006-291x(88)80839-8. [DOI] [PubMed] [Google Scholar]
- Dreyer G. B., Metcalf B. W., Tomaszek T. A., Jr, Carr T. J., Chandler A. C., 3rd, Hyland L., Fakhoury S. A., Magaard V. W., Moore M. L., Strickler J. E. Inhibition of human immunodeficiency virus 1 protease in vitro: rational design of substrate analogue inhibitors. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9752–9756. doi: 10.1073/pnas.86.24.9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson-Viitanen S., Manfredi J., Viitanen P., Tribe D. E., Tritch R., Hutchison C. A., 3rd, Loeb D. D., Swanstrom R. Cleavage of HIV-1 gag polyprotein synthesized in vitro: sequential cleavage by the viral protease. AIDS Res Hum Retroviruses. 1989 Dec;5(6):577–591. doi: 10.1089/aid.1989.5.577. [DOI] [PubMed] [Google Scholar]
- Erickson J., Neidhart D. J., VanDrie J., Kempf D. J., Wang X. C., Norbeck D. W., Plattner J. J., Rittenhouse J. W., Turon M., Wideburg N. Design, activity, and 2.8 A crystal structure of a C2 symmetric inhibitor complexed to HIV-1 protease. Science. 1990 Aug 3;249(4968):527–533. doi: 10.1126/science.2200122. [DOI] [PubMed] [Google Scholar]
- Fontenot G., Johnston K., Cohen J. C., Gallaher W. R., Robinson J., Luftig R. B. PCR amplification of HIV-1 proteinase sequences directly from lab isolates allows determination of five conserved domains. Virology. 1992 Sep;190(1):1–10. doi: 10.1016/0042-6822(92)91186-x. [DOI] [PubMed] [Google Scholar]
- Grinde B., Cameron C. E., Leis J., Weber I. T., Wlodawer A., Burstein H., Skalka A. M. Analysis of substrate interactions of the Rous sarcoma virus wild type and mutant proteases and human immunodeficiency virus-1 protease using a set of systematically altered peptide substrates. J Biol Chem. 1992 May 15;267(14):9491–9498. [PubMed] [Google Scholar]
- Grobelny D., Wondrak E. M., Galardy R. E., Oroszlan S. Selective phosphinate transition-state analogue inhibitors of the protease of human immunodeficiency virus. Biochem Biophys Res Commun. 1990 Jun 29;169(3):1111–1116. doi: 10.1016/0006-291x(90)92010-w. [DOI] [PubMed] [Google Scholar]
- Gustchina A., Weber I. T. Comparative analysis of the sequences and structures of HIV-1 and HIV-2 proteases. Proteins. 1991;10(4):325–339. doi: 10.1002/prot.340100406. [DOI] [PubMed] [Google Scholar]
- Kempf D. J., Marsh K. C., Paul D. A., Knigge M. F., Norbeck D. W., Kohlbrenner W. E., Codacovi L., Vasavanonda S., Bryant P., Wang X. C. Antiviral and pharmacokinetic properties of C2 symmetric inhibitors of the human immunodeficiency virus type 1 protease. Antimicrob Agents Chemother. 1991 Nov;35(11):2209–2214. doi: 10.1128/aac.35.11.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf D. J., Norbeck D. W., Codacovi L., Wang X. C., Kohlbrenner W. E., Wideburg N. E., Paul D. A., Knigge M. F., Vasavanonda S., Craig-Kennard A. Structure-based, C2 symmetric inhibitors of HIV protease. J Med Chem. 1990 Oct;33(10):2687–2689. doi: 10.1021/jm00172a002. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Emini E. A., Schleif W. A., Davis L. J., Heimbach J. C., Dixon R. A., Scolnick E. M., Sigal I. S. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Coates K. E., Kemp S. D. Zidovudine-resistant human immunodeficiency virus selected by passage in cell culture. J Virol. 1991 Oct;65(10):5232–5236. doi: 10.1128/jvi.65.10.5232-5236.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Levitt M. Accurate prediction of the stability and activity effects of site-directed mutagenesis on a protein core. Nature. 1991 Aug 1;352(6334):448–451. doi: 10.1038/352448a0. [DOI] [PubMed] [Google Scholar]
- Loeb D. D., Swanstrom R., Everitt L., Manchester M., Stamper S. E., Hutchison C. A., 3rd Complete mutagenesis of the HIV-1 protease. Nature. 1989 Aug 3;340(6232):397–400. doi: 10.1038/340397a0. [DOI] [PubMed] [Google Scholar]
- McQuade T. J., Tomasselli A. G., Liu L., Karacostas V., Moss B., Sawyer T. K., Heinrikson R. L., Tarpley W. G. A synthetic HIV-1 protease inhibitor with antiviral activity arrests HIV-like particle maturation. Science. 1990 Jan 26;247(4941):454–456. doi: 10.1126/science.2405486. [DOI] [PubMed] [Google Scholar]
- Mellors J. W., Dutschman G. E., Im G. J., Tramontano E., Winkler S. R., Cheng Y. C. In vitro selection and molecular characterization of human immunodeficiency virus-1 resistant to non-nucleoside inhibitors of reverse transcriptase. Mol Pharmacol. 1992 Mar;41(3):446–451. [PubMed] [Google Scholar]
- Moore M. L., Bryan W. M., Fakhoury S. A., Magaard V. W., Huffman W. F., Dayton B. D., Meek T. D., Hyland L., Dreyer G. B., Metcalf B. W. Peptide substrates and inhibitors of the HIV-1 protease. Biochem Biophys Res Commun. 1989 Mar 15;159(2):420–425. doi: 10.1016/0006-291x(89)90008-9. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Schleif W. A., Boots E. J., O'Brien J. A., Quintero J. C., Hoffman J. M., Emini E. A., Goldman M. E. Viral resistance to human immunodeficiency virus type 1-specific pyridinone reverse transcriptase inhibitors. J Virol. 1991 Sep;65(9):4887–4892. doi: 10.1128/jvi.65.9.4887-4892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich D. H., Sun C. Q., Vara Prasad J. V., Pathiasseril A., Toth M. V., Marshall G. R., Clare M., Mueller R. A., Houseman K. Effect of hydroxyl group configuration in hydroxyethylamine dipeptide isosteres on HIV protease inhibition. Evidence for multiple binding modes. J Med Chem. 1991 Mar;34(3):1222–1225. doi: 10.1021/jm00107a049. [DOI] [PubMed] [Google Scholar]
- Rittenhouse J., Turon M. C., Helfrich R. J., Albrecht K. S., Weigl D., Simmer R. L., Mordini F., Erickson J., Kohlbrenner W. E. Affinity purification of HIV-1 and HIV-2 proteases from recombinant E. coli strains using pepstatin-agarose. Biochem Biophys Res Commun. 1990 Aug 31;171(1):60–66. doi: 10.1016/0006-291x(90)91356-w. [DOI] [PubMed] [Google Scholar]
- Roberts N. A., Martin J. A., Kinchington D., Broadhurst A. V., Craig J. C., Duncan I. B., Galpin S. A., Handa B. K., Kay J., Kröhn A. Rational design of peptide-based HIV proteinase inhibitors. Science. 1990 Apr 20;248(4953):358–361. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- Royer W. E., Jr, Love W. E., Fenderson F. F. Cooperative dimeric and tetrameric clam haemoglobins are novel assemblages of myoglobin folds. Nature. 1985 Jul 18;316(6025):277–280. doi: 10.1038/316277a0. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Schneider J., Kent S. B. Enzymatic activity of a synthetic 99 residue protein corresponding to the putative HIV-1 protease. Cell. 1988 Jul 29;54(3):363–368. doi: 10.1016/0092-8674(88)90199-7. [DOI] [PubMed] [Google Scholar]
- Tomasselli A. G., Hui J. O., Sawyer T. K., Staples D. J., Bannow C., Reardon I. M., Howe W. J., DeCamp D. L., Craik C. S., Heinrikson R. L. Specificity and inhibition of proteases from human immunodeficiency viruses 1 and 2. J Biol Chem. 1990 Aug 25;265(24):14675–14683. [PubMed] [Google Scholar]
- Vacca J. P., Guare J. P., deSolms S. J., Sanders W. M., Giuliani E. A., Young S. D., Darke P. L., Zugay J., Sigal I. S., Schleif W. A. L-687,908, a potent hydroxyethylene-containing HIV protease inhibitor. J Med Chem. 1991 Mar;34(3):1225–1228. doi: 10.1021/jm00107a050. [DOI] [PubMed] [Google Scholar]
- Wilson C., Mace J. E., Agard D. A. Computational method for the design of enzymes with altered substrate specificity. J Mol Biol. 1991 Jul 20;220(2):495–506. doi: 10.1016/0022-2836(91)90026-3. [DOI] [PubMed] [Google Scholar]




