Abstract
Hydroxylated polybromodiphenyl ethers (OH-PBDEs) are emerging aquatic pollutants, but their origins in the environment are not fully understood. There is evidence that OH-PBDEs are formed from bromophenols, but the underlying transformation processes remain unknown. Here we investigate if the photoformation of OH-PBDEs from 2,6-dibromophenol in aqueous solution involves 2,6-bromophenoxyl radicals. After UV irradiation of an aqueous 2,6-dibromophenol solution, HPLC-LTQ-Orbitrap MS and GC/MS analysis revealed the formation of a OH-PBDE and a dihydroxylated polybrominated biphenyl (di-OH-PBB). Both dimeric photoproducts were tentatively identified as 4′-OH-BDE73 and 4,4′-di-OH-PBB80. In addition, three debromination products (4-OH-BDE34, 4′-OH-BDE27, and 4,4′-di-OH-PBBs) were observed. Electron paramagnetic resonance spectroscopy revealed the presence of a 2,6-dibromophenoxyl radical with a six-line spectrum (aH (2 meta) = 3.45 G, aH (1 para) = 1.04 G, g = 2.0046) during irradiation of a 2,6-dibromophenol solution in water. The 2,6-dibromophenoxyl radical had a relatively long half-life (122 ± 5 μs) according to laser flash photolysis experiments. The para-para C-C and O-para-C couplings of these 2,6-dibromophenoxyl radicals are consistent with the observed formation of both dimeric OH-PBDE and di-OH-PBB photoproducts. These findings show that bromophenoxyl radical-mediated phototransformation of bromophenols is a source of OH-PBDEs and di-OH-PBBs in aqueous environments that requires further attention.
Keywords: hydroxylated polybromodiphenyl ether, bromophenols, photochemical process, brominated phenoxyl radicals, electron paramagnetic resonance (EPR)
INTRODUCTION
Hydroxylated polybrominated diphenyl ethers (OH-PBDEs) are a class of emerging environmental pollutants. There is increasing evidence that OH-PBDEs are ubiquitous in biotic and abiotic environments.1,2 These pollutants have been observed in abiotic compartments such as rain, snow and wastewater influent and effluent.2–5 Recent studies report that the sum of OH-PBDEs (ΣOH-PBDEs) are 15–170 pg m−2 d−1 in rain and 3.5–190 pg m−2 d−1 in snow2. Levels of ΣOH-PBDEs are in the pg L−1 range in wastewater/sewage treatment plant effluent.5 OH-PBDEs have been detected in various aquatic organisms including algae, sponges, mussels, fish and marine mammals.1, 6–8 Levels of OH-PBDEs in fish from the Detroit River can be as high as 198 pg (g wet weight)−1.9 OH-PBDEs have also been detected in humans, including women and their fetuses,10,11 thus raising human health concerns. OH-PBDEs display toxic effects in organisms, including disruption of thyroid function and sensitization/uncoupling or ryanodine receptors.10,12 They can undergo further environmental transformation and biotransformation to toxic compounds.11,13 For example, OH-PBDEs can photochemically generate highly toxic polybrominate dibenzo-p-dioxins (PBDDs) via a ring closure reaction.14 Moreover, OH-PBDEs directly photolyze into hydroquinone or di-OH-PBDEs, highly reactive transformation products that react with DNA and cause chromosomal alternations.13
The sources and origins of OH-PBDEs in the environment are not fully understood. Polybrominated diphenyl ethers (PBDEs) have been used as flame retardants in a variety of consumer and industrial polymer products since the 1970s and are considered to be an important precursor of OH-PBDEs in the environment. Three pathways have been proposed for the transformation of PBDEs to OH-PBDEs: (1) cytochrome P450 enzyme-mediated biotransformation in organism;15 (2) hydroxylation via a reaction with atmospheric hydroxyl radical;16 and (3) oxidation during wastewater treatment processes.2 OH-PBDEs are also natural products formed by cyanobacteria that have been found to be the producer of OH-PBDEs, and evidence for their natural production in marine ecosystems is provided by radiocarbon data.17,18 These findings indicate that OH-PBDEs have different sources and origins in natural environments.
Bromophenols (BPs) are widely used in various industrial products such as dyes, pulp, paints, polymer intermediates, flame retardants, herbicides and wood preservatives.19,20 In 2001, the global production of 2,4,6-tribromophenol (2,4,6-TBP) alone reached 9500 tons.21 BPs have been found in a variety of environmental matrices19,20,22–25 because of their release from industrial processes/products and their natural biosynthesis by aquatic organisms.20 BP levels in aquatic environment are typically in the ng/L to μg/L range.19,26,27 In a previous study, we proposed that the photochemical formation of OH-PBDEs from BPs is a currently unexplored source of OH-PBDEs in natural water. Specifically, we demonstrated based on mass spectroscopic evidence that 2′-OH-BDE68 was photogenerated in aqueous solution by photodimerization of 2,4-dibromophenol (2,4-DBP);28 however, the mechanism of this phototransformation process remains unknown. Two recent computational studies show that BPs can generate bromophenoxyl radicals under certain conditions via cleavage of the O-H bond or by attack of oxidizing free radicals, such as H•, HO•, O(3P) and Cl•.29,30 Direct experimental evidence for the formation of bromophenoxyl radicals during UV irradiation of aqueous solutions of BPs is still missing.
Here we investigate the hypothesis that irradiation of BPs in water results in the formation of bromophenoxyl radicals, which are key intermediates in the photoformation of dimeric products, such as OH-PBDEs. We employed electron paramagnetic resonance spectroscopy (EPR) and laser flash photolysis (LFP) techniques to study the mechanism for the photoformation of OH-PBDE from BPs. We selected 2,6-dibromophenol (2,6-DBP), one of the most widely detected BPs in aquatic environments,26,31 as a model compound and systematically investigated the formation and structure of bromophenoxyl radicals during irradiation and propose a phototransformation pathway for BPs. These findings advance our fundamental understanding of sources and origins of OH-PBDEs and other BP photoproducts in abiotic environment, and the ecological risk of BP contamination in sunlit water bodies.
EXPERIMENTAL
Chemicals
2,6-DBP (>99% purity) was purchased from Acros Organics (New Jersey, USA). 3-Carboxy-PROXYL (3-CxP; CAS# 2154-68-9), 3′-hydroxy-2,4,4′-tribromodiphenyl ether (3′-OH-BDE28), 3-hydroxy-2,2′,4,4′-tetrabromodiphenyl ether (3-OH-BDE47), 4′-hydroxy-2,2′,4,5′-tetrabromodiphenyl ether (4′-OH-BDE49), 6-hydroxy-2,2′,3,4,4′-pentabromodiphenyl ether (6-OH-BDE85) and 2-hydroxy-5-chlorobipheny were obtained from AccuStandard Inc. (New Haven, CT, USA). 5,5-Dimethylpyrroline-N-oxide (DMPO) was purchased from Dojindo (Gaitherburg, MD, USA). Diazomethane dissolved in diethyl ether was supplied by Dalian Kaifei Chemical Reagent Co (Dalian, China). All organic solvents, such as dichloromethane (DCM), n-hexane, methyl tert-butyl ether (MTBE) and methanol, used for the extraction and derivatization procedures were HPLC grade. All aqueous solutions were prepared using ultrapure water (18 MΩ) obtained from an OKP Ultrapure Water System (Shanghai Lakecore Instrument Co.; Shanghai, China).
Steady-state irradiation
A merry-go-round reactor (Xujiang Technology Co., Nanjing, China) equipped with a 350-W Xenon lamp was used for steady-state irradiation experiments. The lamp with a 290 nm cutoff filter was housed inside an immersion well where tap water (approximately 15 °C) was circulated to keep the reaction temperature inside the reactor constant. Solutions of 2,6-DBP (80 μmol/L) were placed into a quartz tube and analyzed at various intervals after irradiation. This concentration was selected based on our earlier study and facilitates the characterization of unknown photoproducts.28 The light intensity during the photo-polymerization studies with the 290 nm filter was 110 mW cm−2 as determined using an IL1400 BL radiometer with an UVGAL.NIT detector (International Light Inc.; Peabody, MA, USA).
Product analysis
Samples were acidified with HCl to pH ~2, and then extracted three times with 10 mL DCM. The combined organic phases were condensed to 1 mL under a gentle stream of nitrogen and divided into two aliquots. One aliquot (0.5 mL) was blown to dryness, re-dissolved in 100 μL acetonitrile for analysis by HPLC-LTQ Orbitrap XL (Thermo Scientific Co., MC, USA). The solvent of the other aliquot (0.5 mL) was exchanged to n-hexane (0.5 mL) and derivatized by addition of diazomethane. After storing for approximately 12 h at 4 °C, the derivatized samples were evaporated to dryness under a gentle stream of nitrogen and reconstituted in 100 μL of hexane for analysis on an Agilent 6890GC/5975MS (Agilent Co., CA, USA). 2,6-DBP was quantified using an external standard calibration curve by HPLC-LTQ Orbitrap XL. Because authentic standards of the photoproducts were not available, their apparent concentrations were determined based on external standard calibration curves of 3-OH-BDE47. This approach is reasonably accurate for the quantification of unknown OH-PBDEs because we and other have shown that the response factors for OH-PBDEs are very similar.10,32 For example, response factors of 3′-OH-BDE28, 3-OH-BDE47, 4′-OH-BDE49, and 6-OH-BDE85 vary by less than 8%. The detailed analytical conditions of GC-MS and HPLC-MS are shown in the Supporting Information. The apparent product yields were calculated using33
| (1) |
where [product]apparent is the concentration of a particular photoproduct and [2,6-DBP]0 is the initial reaction concentration of 2,6-DBP.
Electron Paramagnetic Resonance (EPR) Measurements
A Bruker EMX EPR spectrometer (Karlsruhe, Germany) equipped with a 4103TM cavity was used for the EPR studies. The samples were degased with argon to remove the majority of the oxygen and then transferred to a flat quartz cell. The flat cell was placed into the TM cavity of the EPR spectrometer, and the sample was irradiated with a 150 W Photomax xenon arc lamp (Oriel; Stratford, CT, USA) at a distance of approximately 50 cm. The power of the lamp was set at 30 W. A 280 nm (WG 280) cutoff filter (cut off the shorter wavelengths; specification 50% transmittance) from Schott (Duryea, PA, USA) was inserted into the lamp to simulate sunlight irradiation. Typical EPR instrument parameters were: microwave frequency, 9.8 GHz (X-band); microwave power, from 0.1 to 20 mW; modulation amplitude, ≤ 3.0 G; time constant, 164 ms; scan rate, 25 G/20.9 s to 120 G/42 s; receiver gain, 5.02 ×104. To study the formation of carbon-center radicals the spin trap DMPO was added to obtain a final concentration of 50 mM (from a 1 M aqueous stock). Measurement of absolute concentrations of radicals or spin adducts was accomplished by double integration of the spectra and comparison to a reference with a known concentration of 3-carboxy-PROXYL using the same instrument settings and experimental setup.34 Simulation of EPR data was performed by using WinSim software (NIEHS) and Bruker WinEPR (Karlsruhe, Germany). The Spin Trap Database (NIEHS) was referred to in order to interpret and simulate the EPR spectra.35,36
Laser Flash Photolysis (LFP) Experiments
A LP920 LFP spectrometer (Edinburgh Instruments Ltd., England) equipped with a frequency tripled Q-switched Nd: YAG laser (Brilliant) that provided a 266 nm pulse was used for the LFP experiments. The samples were purged with nitrogen gas for 15 min, and then transferred into a 1 cm quartz cuvette, and sealed from the atmosphere. The samples were irradiated by a laser (pulse width of 4 ns, average laser pulse power of 10 mJ), and a xenon lamp (450 W) was used as the probe light to monitor the transient formation and subsequent decay. The temporal profiles were recorded using a monochromator (TMS300) equipped with a photomultiplier (Hamamatsu R928) and a digital oscilloscope (Tektronix, TDS3012C). Transient absorption spectra were measured by an intensified charged-coupled device (ICCD) with a gate time of 1.5 μs and a 0.2 μs time delay (Andor, DH 720).
Quality Assurance and Quality Control
All experiments were repeated three times and data are presented as the mean ± one standard deviation. Blank samples representing experimental, extraction and analytical procedures were analyzed in parallel to ensure that no background contamination was present. No 2,6-DBP photoproducts were detected in procedural blank samples. Spike and recovery studies were performed to assess the extraction efficiency of the photoproducts. Briefly, aqueous solutions of 2,6-DBP (100 mL, n = 3) were spiked with 5 ng of 2-hydroxy-5-chlorobiphenyl, which is structurally similar to OH-PBDEs, and subjected to the extraction procedure. Recoveries for 2-hydroxy-5-chlorobiphenyl ranged from 72 to 99%, with a relative standard deviation < 7%. Instrumental precision was determined by triplicate analysis of each sample. The limits of detection (LODs) of the OH-PBDE and di-OH-PBB photoproducts were determined based on water blanks (100 mL) and defined as the concentration that results in a signal-to-noise ratio of 3. The LODs were 2–9 pg/mL for OH-PBDEs and 4–12 pg/mL for di-OH-PBBs.
RESULTS AND DISCUSSION
Identification of photochemical products
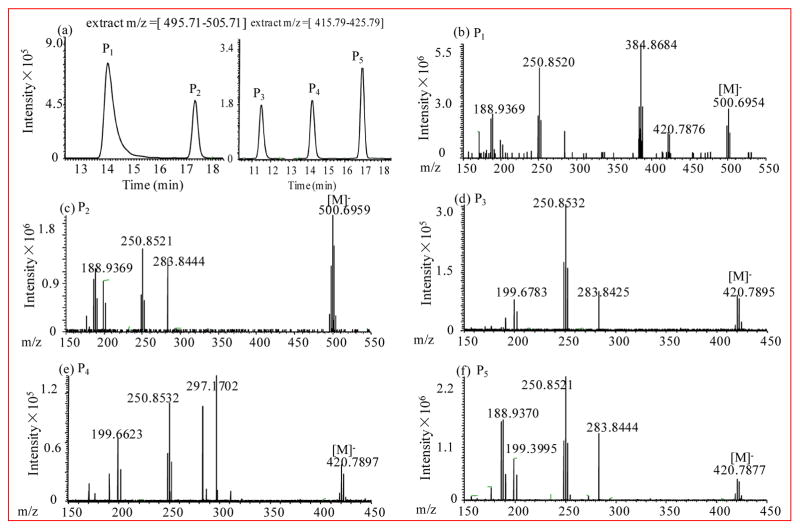
Samples of 2,6-DBP in water were analyzed by HPLC-LTQ-Orbitrap MS and GC-MS after a 1 h irradiation period to identify photoproducts. The HPLC-LTQ-Orbitrap chromatogram showed two peaks (P1, P2) with molecular ion clusters at m/z 496.69, 498.69, 500.69, 502.69 and 504.69 (abundance ratio: 1:4:6:4:1) at retention times of 14.12 min and 17.43 min, respectively (Figure 1b, c). Because the two bromine isotopes have an atomic mass of 78.9 and 80.9, and a natural abundance of 50.5% and 49.5%, respectively, these two peaks correspond to tetrabrominated photoproducts with a molecular formula C12Br4H6O2. This observation suggests that dimeric products of 2,6-DBP are generated during UV irradiation. In addition, three peaks (P3, P4, P5) with m/z 418.79, 420.79, 422.79, and 424.79 (abundance ratio: 1:3:3:1) (Figure 1d, e, f) at retention times of 11.50 min, 14.26 min and 16.95 min were observed. Based on the isotope pattern, these photoproducts are tribrominated products with a molecular formula C12Br3H7O2.
Figure 1.
Irradiation (1 h) of an aqueous solution of 2,6-dibromophenol (80 μmol/L) results in the formation of dimeric products. Representative (a) HPLC-LTQ-Orbitrap spectra of two tetrabrominated compounds which extract m/z = 495.71–505.71 and three tribrominated compounds which extract m/z = 415.79–425.79. (b–f) HPLC-LTQ-Orbitrap MS spectrum of each peak.
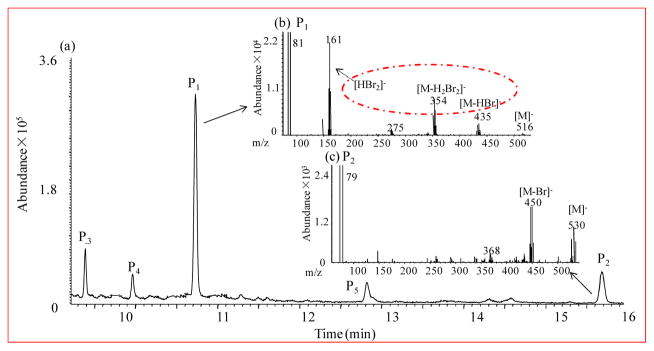
Earlier studies have shown that irradiation of phenol results in the formation of both hydroxydiphenyl ether and dihydroxybiphenyl.37 To identify the number of OH groups in the dimeric 2,6-DBP photoproducts based on the mass difference of mono- vs. dimethoxylated compounds, a second aliquot of each sample was derivatized with diazomethane and the resulting methoxylated derivatives were analyzed by GC-MS. Consistent with the HPLC-LTQ-Orbitrap analysis, the GC-MS mass spectra also showed two tetrabrominated compounds, but with different m/z ion clusters (Figure 2). The tetrabrominated photoproduct at 10.85 min had a cluster of ions centered at m/z 516 (abundance ratio: 1:4:6:4:1), which corresponds to a monomethoxylated photoproduct (P1). The second photoproduct at 15.69 min displayed an ion cluster centered at m/z 530 (abundance ratio: 1:4:6:4:1), which corresponds to a dihydroxylated compound. Similar to the photoproducts of phenol, this product likely is a dihydroxylated polybrominated biphenyl (di-OH-PBB) (P2). To the best of our knowledge, this is the first report of the photoformation of di-OH-PBBs from bromophenols in aqueous solution. In addition, fragment ion clusters characteristic of MeO-PBDEs, including (M-HBr)−, (M-H2Br2)− and (HBr2)−,38 were observed in the mass spectrum of the monohydroxylated compound (Figure 2b). Therefore, this photoproduct was tentatively identified as a OH-PBDE, which is consistent with our earlier finding that UV irradiation of 2,4-DBP results in the formation of OH-PBDEs.28 The mass spectrum of the dihydroxylated photoproduct did not show these ion clusters characteristic of (di-)methoxylated PBDEs (Figure 2c),39 which indicates that both dimeric products have different cleavage pathways under the same MS condition.
Figure 2.
(a) GC spectra of 2,6-DBP (aqueous solution, 80 μmol/L) after irradiation time of 1 h; and (b,c) mass spectra of the two tetrabrominated compounds at retention time of 10.85 min and 15.69 min, respectively.
In addition to the two tetrabrominated dimeric products, GC-MS analysis also revealed to formation of three tribrominated compounds (Figure S1). The two tribrominated photoproducts (P3, P4) at 9.39 min and 9.97 min had a cluster of ions centered at m/z 434 (abundance ratio:1:3:3:1), which correspond to a monohydroxylated photoproduct (Figure S1b, c). The third tribrominated photoproduct (P5) at 12.83 min displayed an ion cluster centered at m/z 452 (abundance ratio: 1:3:3:1), which corresponds to a dihydroxylated compound (Figure S1d). The main product produced during the irradiation of 2,6-DBP was a debrominated hydroxylated compound, monobromo-dihydroxybenzene (P6), with molecular ion clusters at m/z 186.94 and 188.94 (abundance ratio: 1:1) (Figure S2). Unlike our previous phototransformation study with 2,4-DBP,28 no dibromo-dihydroxybenzene product was detected in this study. Additional photoproducts of 2,6-DBP were formed during UV irradiation; however, these products were not further investigated due to their low concentration or low extraction efficiency.
The time-dependent formation of the six photoproducts, expressed as the apparent product yield calculated with Eq. 1, is shown in Figure 3. The apparent total yields of these products were 31 ± 8% at pH = 7.4 after a 2 h irradiation period. The apparent yields of two tetrabrominated dimeric products (P1, P2) initially increased and then decreased with irradiation time, and reached 0.68 ± 0.20 % in 2 h, which provides further evidence that they were formed during the irradiation of 2,6-DBP. The tribrominated product had slightly lower yields (0.51 ± 0.16 %, 2 h) than the tetrabrominated product. A lag time was observed for the formation of the tribrominated photoproducts (P3, P4, P5), which suggest that these compounds are debromination products derived from the tetrabrominated compounds (Figure 3, Figure S3). Monobromo-dihydroxybenzene, was a main product and its apparent yield reached 29.5 ± 7.5% after 2 h.
Figure 3.
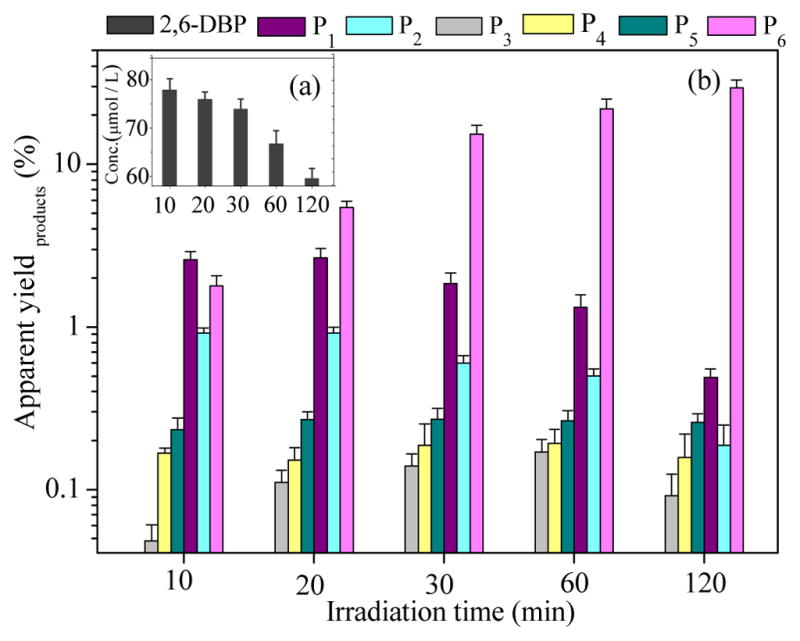
Formation of photoproducts from direct phototransformation of 2,6-DBP (aqueous solution, 80 μmol/L) with different time. Irradiated samples were analyzed by a HPLC-LTQ-Orbitrap and products were quantified with the internal standard method. P1, P2 are tetrabrominated products, P3-P5 are tribrominated product, P6 is monobromo-dihydroxybenzene. The symbols and error bars represent the mean and SD of three replicates. The calculated different product yields with reaction time are listed in Table S1.
Formation of bromophenoxyl radicals by irradiation of 2,6-DBP
To determine whether the formation of dimeric photoproducts from BPs involves a radical mechanism, an aqueous solution of 2,6-DBP (pH = 7.4) was subjected to room temperature EPR analysis before and during UV irradiation. No EPR spectrum corresponding to a 2,6-dibromophenoxyl radical was observed without UV irradiation (Figure 4a), whereas a six-line EPR spectrum was observed immediately upon irradiation of the sample (Figure 4b). The observed six-line spectrum had hyperfine splitting constants of aH (2 meta) = 3.45 G and aH (1 para) = 1.04 G, and center g-value of 2.0046. By comparison to the literature and theoretical calculations,40,41 this radical was identified as a 2,6-dibromophenoxyl radical. The arguments that support this assignment are as follows:
Figure 4.
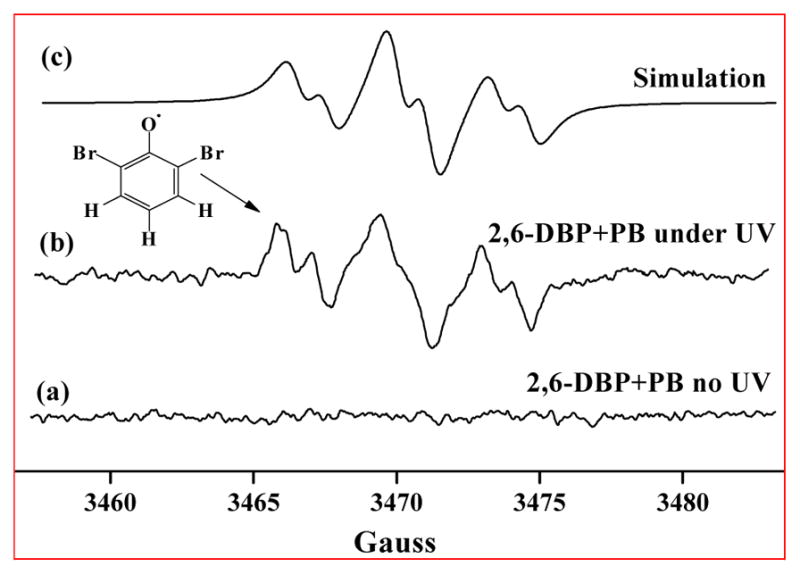
EPR spectra of 2,6-dibromophenoxyl radical generated during UV irradiation of 2,6-DBP (10 mmol/L). Spectra were obtained using a modulation amplitude of 1 G, time constant of 164 ms, receiver gain of 5.02 × 104, microwave power of 13 dB, and a scan time of 7 min over 50 G (αH (2 meta) = 3.45 G, αH (1 para) = 1.05 G, g = 2.0046). PB, phosphate buffer (pH 7.4).
The g-values of the EPR spectra are indicative of the types of radicals present and measure how the magnetic environment of the unpaired electrons differs from that of a free, gas-phase electron (g = 2.0023). In principle, the g-values of EPR spectra can be used to determine whether a radical is carbon-centered or oxygen-centered. As a general guide, the g-value increases the closer an unpaired electron is to an oxygen, with the general rank order gcarbon-centered radicals < 2.0030,42 gcarbon-centered radicals near an oxygen = 2.0030–2.0040,43 and goxygen-centered radicals > 2.0040.44 In this study, the g-value was 2.0046, which suggests that an oxygen-centered radical is formed during UV irradiation of an aqueous solution of 2,6-DBP.
The spectrum exhibits 1:2:1 pattern with a small hyperfine splitting on each line, indicating that all three hydrogens at positions 3, 4, and 5 of 2,6-DBP are present. This hyperfine splitting pattern in therefore consistent with an oxygen-centered 2,6-dibromophenoxyl radical.
The observed spectrum was reproduced using WinSim software (NIEHS) and the above reported experimental spectral parameters for a 2,6-dibromophenoxyl radical (Figure 4c). Satisfactory agreement was achieved with a correlation coefficient greater than 0.98.
The effect of increasing microwave power on the saturation behavior of a sample can give information regarding the relaxation properties of the sample, which can help to identify unknown signals. The EPR spectrum observed in this study was saturated at high microwave power (Figure S4), which is consistent with the nonlinear behavior of more readily saturated phenoxyl-type radicals such as tyrosyl radical.45
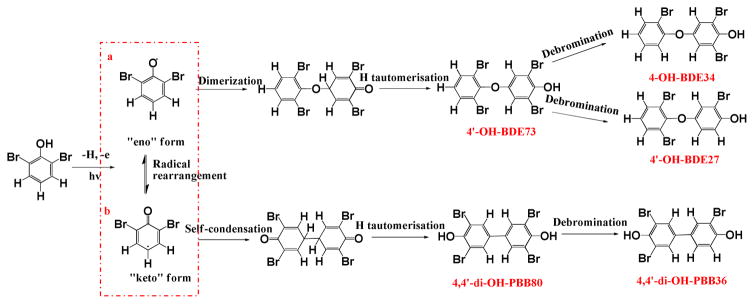
Characterization of carbon-centered radicals generated by irradiation of 2,6-DBP
Previous studies show that phenoxyl radicals exist in equilibrium of the oxygen-centered radical (“eno” form) and carbon-centered radical (“keto” form)46 (Scheme 1). The EPR spectrum shown in Figure 5 was observed when an aqueous solution containing 2,6-DBP (10 mmol/L) and the spin trap DMPO (50 mmol/L) was irradiated at 280 nm in the absence of dioxygen. Computer simulation of this EPR spectrum indicates that the spin adduct corresponds to a carbon-centered radical with hyperfine splittings aN = 16.1 G, αH = 23.6 G, NoH = 0.68, where NoH is the ratio of the nitrogen splitting constant to the hydrogen splitting constant (NoH = aN/aH).47 The similarity of this adduct and the previously reported 2-chlorophenyl-DMPO adduct (aN = 15.8 G, αH = 23.8 G, NoH = 0.65)48 suggested that it was formed by reaction of the spin trap with an aryl radical generated during the photochemical reaction of 2,6-DBP. EPR spin trapping experiments with DMPO demonstrated that high levels of keto radical were produced during the irradiation of 2,6-DBP (Figure 5). Irradiation of solvent alone produced background EPR signals that are approximately 50-times weaker (Figure 5a, b), indicating that 2,6-DBP was directly involved in radical formation.
Scheme 1.
Photoformation pathways of dimeric products from 2,6-DBP under UV irradiation. Representative path (a) shows the formation of OH-PBDEs from eno and keto form radicals with dimerization, H tautomerisation and debromination; and path (b) shows the formation of di-OH-PBBs from keto form radical with self-condensation, H tautomerisation and debromination after irradiation.
Figure 5.
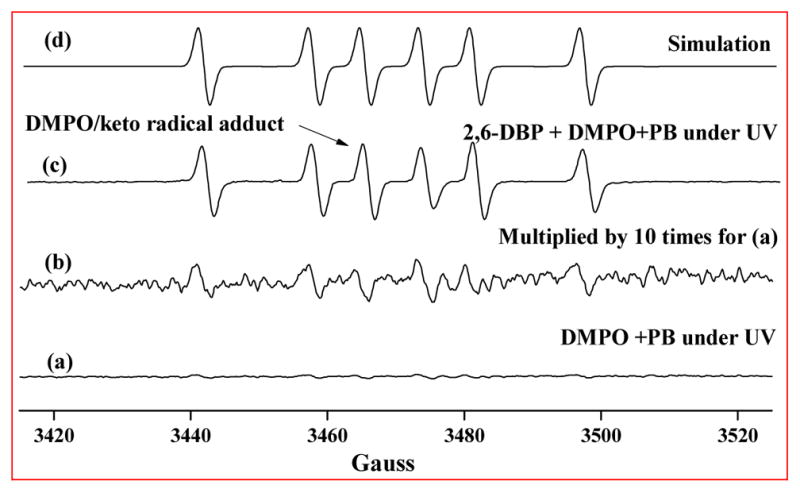
EPR spectra from a solution containing 2,6-DBP (10 mmol/L) and DMPO (50 mmol/L) after 5.6 min of 280 nm excitation in the presence of air. Modulation amplitude 1.0 G, gain 5.02 × 104, power 20 mW, 7 min scan with 164 ms time constant (αN = 16.07 G, αH = 23.58 G).
The formation of 2,6-dibromophenoxyl radicals during the direct irradiation of 2,6-DBP provides compelling evidence that the photoformation of dimeric products from 2,6-DBP and other bromophenols28 involves the formation of bromophenoxyl radicals as an initial step. Previous studies have demonstrated that the photochemical formation of phenoxyl radicals from a neutral molecule or the phenolate anion have different pathways.37 Since 2,6-DBP has a pKa of 7.52,49 57% and 43% of 2,6-DBP in the reaction solution (pH = 7.4) exist in the phenol and phenolate forms, respectively. The effect of pH on the formation of 2,6-bromophenoxyl radicals was investigated to determine which form contributes to the formation of bromophenoxyl radicals. As the pH was increased from 6.5 to 10.3, the UV absorbance increased as well as the generation of 2,6-bromophenoxyl radicals (Figure S5), which indicates that an increase in phenolate content is favorable for the formation of 2,6-bromophenoxyl radicals. Therefore, 2,6-bromophenoxyl radicals are mainly formed by direct photodissociation of the 2,6-dibromophenolate ion in the current reaction system. In brief, the 2,6-dibromophenolate ion in aqueous solution takes up photoenergy during irradiation. The excited 2,6-dibromophenolate then rapidly looses an electron to generate 2,6-dibromophenoxyl radicals.
Based on the resonance structure of 2,6-dibromophenoxyl radicals (Scheme 1), we expected that 2,6-dibromophenoxyl radicals would have a relative long half-life. Therefore, an additional LFP experiment was performed to study the stability of 2,6-dibromophenoxyl radicals in aqueous solution. Briefly, a solution of 2,6-DBP was irradiated with a 266 nm laser pulse (λmax, 2,6-DBP = 285 nm, Figure S6) in the presence of nitrogen. The transient absorption spectra showed two peaks centered near 400 nm that are located in the absorption region of phenoxyl radicals (Figure S7a).50 The molecular decay kinetics at λ=385 nm absorption showed that the 2,6-dibromophenoxyl radical decay in water solution followed first-order process in the absence of a quencher. Its decay rate constant was 8.2 (± 0.6) × 103 s−1 and its lifetime 122 ± 5 μs (See Figure S7b.). Similarly, published lifetimes of other phenoxyl radicals are also longer than 100 μs.51
Radical formation increases with irradiation time and concentration of 2,6-DBP
The effect of irradiation time and concentration of 2,6-DBP in aqueous solution (pH 7.4) on the absolute concentration of bromophenoxyl radicals (280 nm cutoff) was determined by EPR using 3-carboxy-PROXYL as a standard (Table 1, Figure S8). The total amounts of 2,6-dibromophenoxyl radicals in eno and keto forms increased with irradiation time and with the concentration of 2,6-DBP. With an increase in either the time of exposure or the concentration of 2,6-DBP, an apparent steady-state level of eno or keto radical was attained (Figure S9A, B), which is consistent with an equilibrium between the formation and loss of the 2,6-dibromophenoxyl radicals. Previous theoretical and experimental studies showed that the keto form of phenoxyl or chlorophenoxyl radicals is more stable than the eno form.52 Consistent with these earlier studies, the amount of the keto form of the 2,6-bromophenoxyl radical formed at the same irradiation time (5.6 min) or exposure concentration (5 mM) was 5.3- or 5.8-fold higher than that of the eno form.
Table 1.
Free radical concentration from UV irradiation of 2,6-DBP with irradiation time and concentration of 2,6-DBP.
| Conditions | “keto” form (μmol) | “eno” form (μmol) | # Samples | |
|---|---|---|---|---|
| Reaction time (min) | 1.4 | 3.9±0.6 | 0.5±0.1 | 3 |
| 2.8 | 5.9±0.8 | 1.0±0.3 | 3 | |
| 4.2 | 6.5±0.5 | 1.2±0.5 | 3 | |
| 5.6 | 7.1±0.9 | 1.3±0.5 | 3 | |
| 7.0 | 7.1±0.4 | 1.3±0.3 | 3 | |
| 8.4 | 7.1±0.6 | 1.3±0.5 | 3 | |
|
| ||||
| 2,6-DBP concentration (mM)a | 1 | 0.3±0.1 | 0.1±0.1 | 3 |
| 5 | 5.8±0.9 | 1.0±0.6 | 3 | |
| 10 | 7.1±0.4 | 1.3±0.3 | 3 | |
The exposure time was 5.6 min for each concentration.
Formation mechanism of dimeric photoproducts
The 2,6-dibromophenoxyl radicals formed during UV irradiation are likely involved in the formation of dimeric photoproducts of 2,6-DBP (Figure 1). In general, phenoxyl radicals readily undergo C-C and C-O coupling reactions, whereas O-O coupling products are seldom found due to the instability of these products.29 FMO theory calculations demonstrate that the coupling of phenoxyl radicals occurs preferentially at the site of the highest spin density. SOMO electron-spin densities calculated by AM1/UHF show that the ortho and para positions of phenoxyl radicals have a high spin density.53 Since both ortho positions in 2,6-DBP are occupied by electron withdrawing bromine substituents, ortho-ortho C-C coupling, ortho-para C-C coupling and O-ortho-C coupling reactions are unlikely without loss of bromine. Therefore, the tetrabrominated, dimeric photoproducts formed during the irradiation of 2,6-DBP likely represent para-para C-C coupling and O-para-C coupling products.
Taken together, the experimental evidence suggests that the two dimeric, tetrabrominated photoproducts (P1, P2) formed during irradiation of an aqueous solution of 2,6-DBP are 4′-OH-BDE73 and 4,4′-di-OH-PBB80. As illustrated in Scheme 1a, the formation of 4′-OH-BDE73 occurs via O-para-C coupling of an eno with a keto 2,6-dibromophenoxyl radical. Initially, an ether adduct is formed, followed by hydrogen tautomerization to yield 4′-OH-BDE73. The formation 4,4′-di-OH-PBB80 is presented in Scheme 1b and involves the C-C self-condensation of two keto 2,6-dibromophenoxyl radicals in para position. The tetrabrominated biphenoxyl intermediate (with two ketone groups) subsequently undergoes hydrogen tautomerization to form the dihydroxylated photoproduct observed by HPLC-LTQ-Orbitrap MS and GC/MS analysis. Subsequently, 4′-OH-BDE73 and 4,4′-di-OH-PBB80 undergo debromination to yield the three tribrominated products observed by HPLC-LTQ-Orbitrap MS and GC/MS. This interpretation is supported by the lag time of their formation relative to the tetrabrominated compounds. These three tribrominated photoproducts were tentatively identified as 4,4′-di-OH-PBB36, 4-OH-PBDE34 and 4′-OH-PBDE27 (Scheme 1). Similar photodegradation pathways have been reported for decabromodiphenyl ether,54 which represents a typical photodegradation pathways for halogenated organic pollutants.55
Environmental Implications
Our findings demonstrate that both oxygen and carbon-centered 2,6-dibromophenoxyl radicals are generated during irradiation of 2,6-DBP with UV light. O-para-C coupling and C-C coupling reactions of these 2,6-dibromophenoxyl radicals result in the formation of a monohydroxylated PBDE and a dihydroxylated PBB. Both dimeric photoproducts were tentatively identified as 4′-OH-BDE73 and 4,4′-di-OH-PBB80. Subsequently, the primary photoproducts undergo debromination reactions, resulting in complex mixtures of photoproducts. Our findings demonstrate that OH-PBDEs can be formed via free radical mechanisms in aqueous solutions of BPs, such as 2,6-DBP, in during irradiation. Moreover, our study suggests that di-OH-PBBs are formed via the same mechanism.
Taken together, these observations are important because BPs are frequently found in surface water, with the average concentrations in the ng-μg/L range.19,20,26,27 For example, the reported concentrations of 2,6-DBP in surface water from Ganges and Yamuna rivers in the northern India are 2.5 and 3.3 μg/L, respectively;26 the concentration of 2,4,6-TBP in surface water from Saitama Prefecture in Japan is 0.3 μg/L;19 while the total maximum concentrations of BPs including 2,4-DBP and 2,4,6-TBP in surface water from the river and sea, Korea are 118 and 52.9 ng/L, respectively.27 Even higher BP levels are found in sediments and aquatic species. BP concentrations in sediments from the Rhone estuary, France, range from 26 μg/kg to 3.7 mg/kg,20 and concentration of 2,4,6-tribromophenol as high as 2.4 mg/kg has been reported in crustacean.56 Based on environmental levels of BPs in natural water, and assuming an apparent yield of 1‰ (e.g., apparent yield4,4′-di-OH-PBB80 = 1.9‰) and conversion that is independent of BP concentration, the production of OH-PBDEs and di-OH-PBBs from BP phototransformation is estimated to reach pg/L levels.
While levels of di-OH-PBBs have not been investigated in environmental samples, pg/L levels of OH-PBDEs have been reported by several studies.1,2,5,10 Our findings suggest that the transformation of BPs by sunlight irradiation may contribute to the production of OH-PBDEs and di-OH-PBBs in aqueous environments. Given that phenoxyl radicals may also react with dissolved organic matter (DOM) or other constituents in natural water, further studies are needed to determine to which extent the phototransformation of BP contributes to the contamination of surface waters with OH-PBDEs. Moreover, our mechanistic study suggests that di-OH-PBBs may represent a currently overlooked group of potentially (eco-)toxic contaminants in aquatic environments.
Supplementary Material
Acknowledgments
The study was supported by the National Natural Science Foundation of China (21277017, 21207013), the National Basic Research Program of China (2013CB430403), and the National Institute of Environmental Health Sciences/National Institutes of Health (ES05605, ES013661, ES017425 and CA169046).
Footnotes
Supporting Information. Detailed analytical conditions of GC-MS and HPLC-MS, degradation of 2,6-DBP, the mass spectra of the identified other products, the absorption spectra of 2,6-DBP, the microwave power (mW) dependence of the peak-to-peak intensity of radicals generated from irradiation of 2,6-DBP, the transient absorption spectrum of 2,6-dibromophenoxyl radical and its decay, free radical formation increase with time of light-exposure and concentration of 2,6-DBP, the apparent yield of six products from 2,6-DBP irradiation with different times of exposure. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kim UJ, Yen NTH, Oh JE. Hydroxylated, Methoxylated, and Parent Polybrominated Diphenyl Ethers (PBDEs) in the Inland Environment, Korea, and Potential OH- and MeO-BDE Source. Environ Sci Technol. 2014;48(13):7245–7253. doi: 10.1021/es5006972. [DOI] [PubMed] [Google Scholar]
- 2.Ueno D, Darling C, Alaee M, Pacepavicius G, Teixeira C, Campbell L, Letcher RJ, Bergman A, Marsh G, Muir D. Hydroxylated Polybrominated diphenyl ethers (OH-PBDEs) in the abiotic environment: Surface water and precipitation from Ontario, Canada. Environ Sci Technol. 2008;42(5):1657–1664. doi: 10.1021/es7021279. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Q, Zhao HM, Quan X, He X, Chen S. Photochemical Formation of Hydroxylated Polybrominated Diphenyl Ethers (OH-PBDEs) from Polybrominated Diphenyl Ethers (PBDEs) in Aqueous Solution under Simulated Solar Light Irradiation. Environ Sci Technol. 2015;49(15):9092–9099. doi: 10.1021/acs.est.5b01240. [DOI] [PubMed] [Google Scholar]
- 4.Sun J, Liu J, Liu Q, Ruan T, Yu M, Wang Y, Wang T, Jiang G. Hydroxylated polybrominated diphenyl ethers (OH-PBDEs) in biosolids from municipal wastewater treatment plants in China. Chemosphere. 2013;90(9):2388–2395. doi: 10.1016/j.chemosphere.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Chang H, Wu F, Jin F, Feng C, Zhao X, Liao H. Picogram per liter level determination of hydroxylated polybrominated diphenyl ethers in water by liquid chromatography–electrospray tandem mass spectrometry. J Chr A. 2012;1223(3):131–135. doi: 10.1016/j.chroma.2011.12.075. [DOI] [PubMed] [Google Scholar]
- 6.Zeng YH, Luo XJ, Zheng XB, Tang B, Wu JP, Mai BX. Species-Specific Bioaccumulation of Halogenated Organic Pollutants and Their Metabolites in Fish Serum from an E-Waste Site, South China. Arch Environ Con Tox. 2014;67(3):348–357. doi: 10.1007/s00244-014-0040-8. [DOI] [PubMed] [Google Scholar]
- 7.Kelly BC, Ikonomou MG, Blair JD, Gobas FAPC. Hydroxylated and methoxylated polybrominated diphenyl ethers in a Canadian Arctic marine food web. Environ Sci Technol. 2008;42(19):7069–7077. doi: 10.1021/es801275d. [DOI] [PubMed] [Google Scholar]
- 8.Haraguchi K, Kato Y, Ohta C, Koga N, Endo T. Marine Sponge: A Potential Source for Methoxylated Polybrominated Diphenyl Ethers in the Asia-Pacific Food Web. J Agric Food Chem. 2011;59(24):13102–13109. doi: 10.1021/jf203458r. [DOI] [PubMed] [Google Scholar]
- 9.Valters K, Li HX, Alaee M, D’Sa I, Marsh G, Bergman A, Letcher RJ. Polybrominated diphenyl ethers and hydroxylated and methoxylated brominated and chlorinated analogues in the plasma of fish from the Detroit River. Environ Sci Technol. 2005;39(15):5612–5619. doi: 10.1021/es0506410. [DOI] [PubMed] [Google Scholar]
- 10.Zota AR, Park JS, Wang Y, Petreas M, Zoeller RT, Woodruff TJ. Polybrominated Diphenyl Ethers, Hydroxylated Polybrominated Diphenyl Ethers, and Measures of Thyroid Function in Second Trimester Pregnant Women in California. Environ Sci Technol. 2011;45(18):7896–7905. doi: 10.1021/es200422b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan Y, Choi K, Kim S, Ji K, Chang H, Wiseman S, Jones PD, Khim JS, Park S, Park J, Lam MHW, Giesy JP. Hydroxylated Polybrominated Diphenyl Ethers and Bisphenol A in Pregnant Women and Their Matching Fetuses: Placental Transfer and Potential Risks. Environ Sci Technol. 2010;44(13):5233–5239. doi: 10.1021/es1002764. [DOI] [PubMed] [Google Scholar]
- 12.Kim KH, Bose DD, Ghogha A, Riehl J, Zhang R, Barnhart CD, Lein PJ, Pessah IN. Para- and Ortho-Substitutions Are Key Determinants of Polybrominated Diphenyl Ether Activity toward Ryanodine Receptors and Neurotoxicity. Environ Health Persp. 2011;119(4):519–526. doi: 10.1289/ehp.1002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai YQ, Lu MH, Gao X, Wu HZ, Cai ZW. New Evidence for Toxicity of Polybrominated Diphenyl Ethers: DNA Adduct Formation from Quinone Metabolites. Environ Sci Technol. 2011;45(24):10720–10727. doi: 10.1021/es203068f. [DOI] [PubMed] [Google Scholar]
- 14.Erickson PR, Grandbois M, Arnold WA, McNeill K. Photochemical Formation of Brominated Dioxins and Other Products of Concern from Hydroxylated Polybrominated Diphenyl Ethers (OH-PBDEs) Environ Sci Technol. 2012;46(15):8174–8180. doi: 10.1021/es3016183. [DOI] [PubMed] [Google Scholar]
- 15.Gross MS, Butryn DM, McGarrigle BP, Aga DS, Olson JR. Primary Role of Cytochrome P450 2B6 in the Oxidative Metabolism of 2, 2′, 4, 4′, 6-Pentabromodiphenyl Ether (BDE-100) to Hydroxylated BDEs. Chem Res Toxicol. 2015;28(4):672–681. doi: 10.1021/tx500446c. [DOI] [PubMed] [Google Scholar]
- 16.Raff JD, Hites RA. Gas-phase reactions of brominated diphenyl ethers with OH radicals. J Phys Chem A. 2006;110(37):10783–10792. doi: 10.1021/jp0630222. [DOI] [PubMed] [Google Scholar]
- 17.Reddy CM, Xu L, Eglinton TI, Boon JP, Faulkner DJ. Radiocarbon content of synthetic and natural semi-volatile halogenated organic compounds. Environ Pollut. 2002;120(2):163–168. doi: 10.1016/s0269-7491(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 18.Teuten EL, Xu L, Reddy CM. Two abundant bioaccumulated halogenated compounds are natural products. Science. 2005;307(5711):917–920. doi: 10.1126/science.1106882. [DOI] [PubMed] [Google Scholar]
- 19.OECD SIDS. [accessed on 13.04.2010];2004 http://www.inchem.org/documents/sids/sids/118796.pdf.
- 20.World Health Organization (WHO) Concise International Chemical Assessment Document (CICAD) Vol. 66 WHO; Geneva, Switzerland: 2005. 2,4,6-Tribromophenol and Other Simple Brominated Phenols. [Google Scholar]
- 21.IUCLID. Data Set for 2,4,6-Tribromophenol. Ispra: European Chemicals Bureau, International Uniform Chemical Information Database; 2003. 2,4,6-tribromophenol and other simple brominated phenols. [Google Scholar]
- 22.Walter V, Dorte J. Halogenated Natural Products in Five Species of Antarctic Sponges: Compounds with POP-like Properties? Environ Sci Technol. 2005;39(11):3889–3895. doi: 10.1021/es0484597. [DOI] [PubMed] [Google Scholar]
- 23.Tolosa I, Bayona JM, Albaiges J. Identification and Occurrence of Brominated and Nitrated Phenols in Estuarine Sediments. Mar Pollut Bull. 1991;22(12):603–607. [Google Scholar]
- 24.Whitfield FB, Helidoniotis F, Shaw KJ, Svoronos D. Distribution of bromophenols in species of marine algae from eastern Australia. J Agric Food Chem. 1999;47(6):2367–2373. doi: 10.1021/jf981080h. [DOI] [PubMed] [Google Scholar]
- 25.Reineke N, Biselli S, Franke S, Francke W, Heinzel N, Huhnerfuss H, Iznaguen H, Kammann U, Theobald N, Vobach M, Wosniok W. Brominated indoles and phenols in marine sediment and water extracts from the North and Baltic Seas-concentrations and effects. Arch Environ Contam Toxicol. 2006;51(2):186–196. doi: 10.1007/s00244-005-0135-3. [DOI] [PubMed] [Google Scholar]
- 26.Nomani AA, Ajmal M, Ahmad S. Gas chromatography—mass spectrometric analysis of four polluted river waters for phenolic and organic compounds. Environ Monit Assess. 1996;40(1):1–9. doi: 10.1007/BF00395164. [DOI] [PubMed] [Google Scholar]
- 27.Sim WJ, Lee SH, Lee IS, Ch’oi SD, Oh JE. Distribution and formation of chlorophenols and bromophenols in marine and riverine environments. Chemosphere. 2009;77(4):552–558. doi: 10.1016/j.chemosphere.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Zhao HM, Quan X, Zhang YB, Chen S, Zhao HX. Formation of 2′-hydroxy-2,3′,4,5′-tetrabromodipheyl ether (2′-HO-BDE68) from 2,4-dibromophenol in aqueous solution under simulated sunlight irradiation. Chemosphere. 2011;84(4):512–518. doi: 10.1016/j.chemosphere.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Yu WN, Hu JT, Xu F, Sun XY, Gao R, Zhang QZ, Wang WX. Mechanism and Direct Kinetics Study on the Homogeneous Gas-Phase Formation of PBDD/Fs from 2-BP, 2,4-DBP, and 2,4,6-TBP as Precursors. Environ Sci Technol. 2011;45(5):1917–1925. doi: 10.1021/es103536t. [DOI] [PubMed] [Google Scholar]
- 30.Gao R, Xu F, Li SQ, Hu JT, Zhang QZ, Wang WX. Formation of bromophenoxyl radicals from complete series reactions of bromophenols with H and OH radicals. Chemosphere. 2013;92(4):382–390. doi: 10.1016/j.chemosphere.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 31.Whitfield FB, Helidoniotis F, Shaw KJ, Svoronos D. Distribution of bromophenols in species of marine algae from eastern Australia. J Agric Food Chem. 1999;47(6):2367–2373. doi: 10.1021/jf981080h. [DOI] [PubMed] [Google Scholar]
- 32.Norrgran J, Jones B, Bignert A, Athanassiadis I, Bergman A. Higher PBDE Serum Concentrations May Be Associated with Feline Hyperthyroidism in Swedish Cats. Environ Sci Technol. 2015;49(8):5107–5114. doi: 10.1021/acs.est.5b00234. [DOI] [PubMed] [Google Scholar]
- 33.Evans CS, Dellinger B. Mechanisms of dioxin formation from the high-temperature oxidation of 2-bromophenol. Environ Sci Technol. 2005;39(7):2128–2134. doi: 10.1021/es048461y. [DOI] [PubMed] [Google Scholar]
- 34.Venkataraman S, Martin SM, Schafer FQ, Buettner GR. Detailed methods for the quantification of nitric oxide in aqueous solutions using either an oxygen monitor or EPR. Free Radical Biol Med. 2000;29(6):580–585. doi: 10.1016/s0891-5849(00)00404-4. [DOI] [PubMed] [Google Scholar]
- 35.Li ASW, Cummings KB, Roethling HP, Buettner GR, Chignell CF. A Spin-Trapping Database Implemented on the Ibm Pc/At. J Magn Reson. 1988;79(1):140–142. [Google Scholar]
- 36.Buettner GR. Spin Trapping - Electron-Spin-Resonance Parameters of Spin Adducts. Free Radical Biol Med. 1987;3(4):259–303. doi: 10.1016/s0891-5849(87)80033-3. [DOI] [PubMed] [Google Scholar]
- 37.Joschek HI, Miller SI. Photooxidation of Phenol, Cresols, and Dihydroxybenzenes1, 2. J Am Chem Soc. 1966;88(14):3273–3281. doi: 10.1021/ja00966a019. [DOI] [PubMed] [Google Scholar]
- 38.Hites RA. Electron impact and electron capture negative ionization mass spectra of polybrominated diphenyl ethers and methoxylated polybrominated diphenyl ethers. Environ Sci Technol. 2008;42(7):2243–2252. doi: 10.1021/es072064g. [DOI] [PubMed] [Google Scholar]
- 39.Marsh G, Athanasiadou M, Athanassiadis I, Bergman A, Endo T, Haraguchi K. Identification, quantification, and synthesis of a novel dimethoxylated polybrominated biphenyl in marine mammals caught off the coast of Japan. Environ Sci Technol. 2005;39(22):8684–8690. doi: 10.1021/es051153v. [DOI] [PubMed] [Google Scholar]
- 40.Khachatryan L, Adounkpe J, Dellinger B. Formation of phenoxyl and cyclopentadienyl radicals from the gas-phase pyrolysis of phenol. J Phys Chem A. 2008;112(3):481–487. doi: 10.1021/jp073999m. [DOI] [PubMed] [Google Scholar]
- 41.Wiese FW, Chang HC, Lloyd RV, Freeman JP, Samokyszyn VM. Peroxidase-catalyzed oxidation of 2,4,6-trichlorophenol. Arch Environ Con Tox. 1998;34(3):217–222. doi: 10.1007/s002449900308. [DOI] [PubMed] [Google Scholar]
- 42.Delhaes P, Marchand AA. Analize de la forme et de la positionde signaux RPE observes sur des carbons graphitiques pulverulents. Carbon. 1968;6(2):257–266. [Google Scholar]
- 43.Barclay LRC, Cromwell GR, Hilborn J. Photochemistry of a model lignin compound. Spin trapping of primary products and properties of an oligomer. Can J Chem. 1994;72(1):35–41. [Google Scholar]
- 44.Allard P, Barra A, Andersson K, Schmidt P, Atta M, Gräslund A. Characterization of a new tyrosyl free radical in Salmonella typhimurium ribonucleotide reductase with EPR at 9.45 and 245 GHz. J Am Chem Soc. 1996;118(4):895–896. [Google Scholar]
- 45.Liu A, Potsch S, Davydov A, Barra AL, Rubin H, Graslund A. The tyrosyl free radical of recombinant ribonucleotide reductase from Mycobacterium tuberculosis is located in a rigid hydrophobic pocket. Biochemistry-Us. 1998;37(46):16369–16377. doi: 10.1021/bi981471p. [DOI] [PubMed] [Google Scholar]
- 46.Sidhu S, Edwards P. Role of phenoxyl radicals in PCDD/F formation. Int J Chem Kinet. 2002;34(9):531–541. [Google Scholar]
- 47.Li ASW, Chignell CF. The NoH value in EPR spin trapping: a new parameter for the identification of 5,5-dimethyl-1-pyrroline-N-oxide spin adducts. J Biochem Bioph Methods. 1991;22(1):83–87. doi: 10.1016/0165-022x(91)90084-a. [DOI] [PubMed] [Google Scholar]
- 48.Motten AG, Buettner GR, Chignell CF. Spectroscopic studies of cutaneous photosensitizing agents--VIII. A spin-trapping study of light induced free radicals from chlorpromazine and promazine. Photochem Photobiol. 1985;42(1):9–15. doi: 10.1111/j.1751-1097.1985.tb03540.x. [DOI] [PubMed] [Google Scholar]
- 49.Yang MT, Zhang XR. Comparative Developmental Toxicity of New Aromatic Halogenated DBPs in a Chlorinated Saline Sewage Effluent to the Marine Polychaete Platynereis dumerilii. Environ Sci Technol. 2013;47(19):10868–10876. doi: 10.1021/es401841t. [DOI] [PubMed] [Google Scholar]
- 50.Peller J, Kamat PV. Radiolytic transformations of chlorinated phenols and chlorinated phenoxyacetic acids. J Phys Chem A. 2005;109(42):9528–9535. doi: 10.1021/jp053001s. [DOI] [PubMed] [Google Scholar]
- 51.Joschek HI, Grosswei Li. Optical Generation of Hydrated Electrons from Aromatic Compounds .2. J Am Chem Soc. 1966;88(14):3261–3268. doi: 10.1021/ja00966a017. [DOI] [PubMed] [Google Scholar]
- 52.Mackie JC, Doolan KR, Nelson PF. Kinetics of the Thermal-Decomposition of Methoxybenzene (Anisole) J Phys Chem. 1989;93(2):664–670. [Google Scholar]
- 53.Tuppurainen K, Halonen I, Tarhanen J, Ruuskanen J. Chlorinated Phenoxyl Radicals and Phenolate Anions as Precursors of Polychlorinated Dibenzo-P-Dioxins (PCDDs) and Dibenzofurans (PCDFs) in Municipal Waste Incineration - a Semiempirical Am1 Molecular-Orbital Study. Theochem. 1994;118(2):139–144. [Google Scholar]
- 54.Suh YW, Buettner GR, Venkataraman S, Treimer SE, Robertson LW, Ludewig G. UVA/B-Induced Formation of Free Radicals from Decabromodiphenyl Ether. Environ Sci Technol. 2009;43(7):2581–2588. doi: 10.1021/es8022978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matykiewiczova N, Klanova J, Klan P. Photochemical degradation of PCBs in snow. Environ Sci Technol. 2007;41(24):8308–8314. doi: 10.1021/es0714686. [DOI] [PubMed] [Google Scholar]
- 56.Chung HY, Ma WCJ, Kim TS. Seasonal distribution of bromophenols in selected Hong Kong seafood. J Agr Food Chem. 2003;51(23):6752–6760. doi: 10.1021/jf034632r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.