Abstract
Paramount among the challenges to our ability to address the role of food and nutrition in health promotion and disease prevention is how to design and implement context-specific interventions and guidance. The Integration to Effective Implementation (I-to-I) concept is intended to address the complexities of the global health context through engagement of the continuum of stakeholders involved in the food and nutrition enterprise. The 2014 Micronutrient Forum (MNF) Global Conference held in Addis Ababa, Ethiopia, in June 2014 offered the opportunity to apply the I-to-I approach with the use of current concerns about the safety and effectiveness of interventions to prevent and treat iron deficiency (ID) as a case study. ID is associated with a range of adverse outcomes, especially in pregnant and nonpregnant women, infants, and primary school-age children. Strategies to combat ID include iron supplementation, multiple micronutrient powders, and food-based interventions to enhance dietary iron intake. Recent reports indicate potential increased adverse risks when iron is provided in areas with high infection burdens (e.g., malaria). This paradox has weakened iron intervention programs. Furthermore, the selection and interpretation of available biomarkers for assessing iron nutrition have been found to be compromised by the inflammatory process. These issues highlight the need for a comprehensive approach that considers basic biology, assessment, interventions, and how these can be translated into appropriate programs and policies. The application of the I-to-I with the use of the MNF offered an opportunity to explore how that might be achieved.
Keywords: anemias, international nutrition, interventions, iron, public health
Introduction
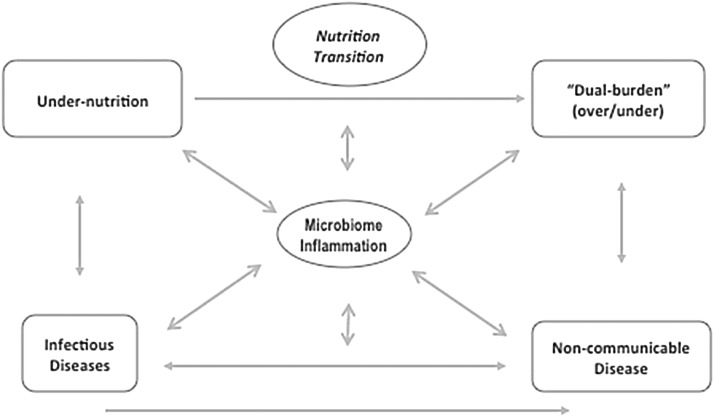
Malnutrition (overnutrition, undernutrition, or both) is increasingly recognized as a complex problem with multiple social, cultural, and biological determinants. The context in which programs to address malnutrition and the many associated health implications are becoming increasingly complex (1) with continuing pandemics of infectious disease; exploding epidemics of obesity, including the dual burden of overnutrition and undernutrition; and noncommunicable diseases (NCDs) all superimposed on an evolving environment of food insecurity (2), nutrition transition (3), and climate change (4). Figure 1 is a conceptualization of this complex global scenario, including 2 potential mediators of these interactions, the human gut microbiome (5), and inflammation (6). This conceptualization also includes a recognition of the significance of these relations across the life cycle, with specific reference to the emerging consensus about the importance of nutrition to the developmental origins of health and disease hypothesis (7).
FIGURE 1.
Nutrition transition, colliding epidemics, and health: a research agenda.
In addition to the biological complexity outlined in Figure 1 is the reality that all of these relations are superimposed on an additional layer of complicated social, behavioral, and economic factors that must also be considered in providing both patient care and implementation of context-relevant programs and policies. This complexity demands a more comprehensive view of nutrition as something more than too much or too little. Our ability to effectively respond to this complexity demands an integrated approach that is informed by evidence, through the continuum of biological mechanisms, to the many economic, food/agricultural, social, cultural, and health system factors that modify the potential for programs, implications for clinical care, and population-based policies and programs.
In response to a growing recognition of the importance of food and nutrition as integral components of health and global development targets, a number of efforts were initiated to address various aspects of this agenda. These include facilitating better prioritization and coordination at the global and country level (Scaling Up Nutrition) movement (8) and better integration of nutrition into the agriculture sector (Feed the Future) (9). Another priority that underlies many of these efforts is a better understanding and targeting of programs to address critical periods in the life course, most prominently, the 1000 Days, focused on needs during pregnancy and the first 2 y of life (10). The complexity of the problems being addressed, the need for effective solutions, and the growing number of organizations and groups linked to the nutrition field have created a need for a framework to structure our approach to addressing nutrition more holistically and a forum to bring together the breadth of the players who are or will need to be involved to substantially accelerate progress in nutrition.
The Integration to Effective Implementation (I-to-I)15 was developed in response to this growing need and was applied as a framework with the use of the issue of iron deficiency (ID) prevention and control strategies as a case study, via the opportunity provided by the 2014 Micronutrient Forum (MNF) Global Conference (11). In this review, an overview of the I-to-I framework, as applied to iron, is presented along with a summary of the key issues addressed during the MNF session series.
I-to-I
As highlighted in the section above, the global health community is confronted by a complex health context, particularly as it pertains to the role of food and nutrition (1). However, despite the relatively strong body of evidence on what works in nutrition, this evidence is not generally translated effectively into interventions to address the complex nature of these problems. In addition, the food/agricultural, social, cultural, health systems, and environmental (physical and economic) factors that modify the potential of efficacious interventions to improve nutrition are not adequately accounted for either clinically or programmatically. The I-to-I approach aims to frame the conversations necessary to engage the full continuum of expertise from sound biological principles to consideration of the contextual factors that influence food- and nutrition-related behaviors, programs/policy, and the systems through which safe and effective interventions are delivered.
Foundation of nutrition in biological systems.
The ability to fully integrate nutrition into all aspects of health promotion, disease prevention, and treatment and into efforts to improve food security requires a comprehensive view of nutrition that goes beyond exposure and includes interactions within a myriad of processes (e.g., digestion, absorption, transport, etc.) and biological systems (e.g., immune, neurologic, etc.). Inherently, the relations between nutrition and these systems are reciprocal, such that anomalies at any point in these processes or systems can have an impact on nutrition and vice versa (6, 12). An appreciation of this reciprocity is key to enable the development of safe and effective interventions. In addition, a fuller understanding of nutrition from a biological systems perspective will also enable us to identify better tools (biomarkers) to further explore nutrition both in terms of its role in health and as an outcome of interventions (13, 14).
Nutrition of individuals is influenced by multiple factors beyond biology.
Habicht et al. (15) conceptualized how we might incorporate a cultural-ecologic model as a framework for the determinants of nutrition. Their approach highlights the notion that comprehensive and sustainable success at improving nutrition must involve the primary systems by which individuals access nutritious foods and services, specifically the agriculture and food systems. This model also recognizes the critical role for the private sector as the primary food source for most of the population in most countries around the world. Failures in these systems and the elevated needs of individuals during specific ages and life stages also necessitate complementary actions across other social and health sectors. Information on food availability and access, the stability and consistency in the food supply to meet the needs of the population, and recent data on the nutrition and associated health problems are needed to adequately address nutritional challenges and to inform policy and program development. Because countries grapple with complex food and nutrition landscapes, a conversation is needed between technical agencies, academic/research leaders, funders, implementing agencies, nongovernmental organizations, and the private sector at country and global levels.
Health systems as the primary interface between individuals and potential targeted nutrition interventions
Because individuals access the health systems via diverse entry points, a need exists for both a better understanding of the importance of nutrition within segments of these systems and a coordination among these sectors such that individuals might receive the appropriate referrals or interventions as needed. The ability to provide safe and efficacious interventions and standards of care is contingent on knowledge of the nutrition issues that require attention, the importance of these issues, and potential interventions to address them at all levels of the health system (16). This dialogue must include a continual reassessment of the nutrition and health situation, relevance of existing programs, and an examination of whether a need exists for new programs in health promotion and disease prevention.
In essence, a need exists for a systems approach that includes biological systems, cultural-ecologic systems, and those systems within the public infrastructure intended to provide services throughout all components of the clinical and population-based care and service continuum.
In an attempt to develop a conceptual framework for such an effort, and to begin this conversation, the I-to-I concept was developed. The overarching goals of the I-to-I concept are to address critical aspects of the biology of nutrition as it pertains to specific clinical or population-level challenges; determine what is needed (e.g., identification of research gaps, new tools, new approaches/interventions) to address the challenge that are based on realities on the ground; foster a meaningful dialogue between countries, implementing agencies, private sector, donors, and civil society to address the many contextual factors that influence nutrition and the potential for effective programs; and initiate a process to develop effective strategies that integrates an appreciation of the evolving science of nutrition with contributions of the attributes from all the stakeholders that would be needed for successful implementation.
In application, I-to-I would identify situations and be able to suggest how to responsively move the science forward with an appreciation of the relevant contextual issues, along with how to best use the respective resources of different agencies/organizations to reach a common goal.
MNF Goals and Objectives
The newly revitalized MNF is intended to serve as an umbrella for global efforts to contribute to improved nutrition and health through improvements in micronutrient intakes, and so status, of the world’s populations. The MNF is intended to accomplish this by fostering the generation, dissemination, and utilization of evidence that responds to clinical, policy needs, and programmatic gaps and by advocating for the collaboration, commitments, and investments needed to ensure that effective programs reach all those in need and that filling the evidence gaps that limit the ability to do so are prioritized.
The overarching theme of the 2014 MNF Global Conference held in Addis Ababa, Ethiopia, in June 2014 was bridging, that is, recognizing the continuum of effort from research and translation of new data to evidence-informed programs and policies, and developing processes by which the requisite connections can be made among stakeholders in country and globally to most effectively move the nutrition agenda forward. To achieve this theme the MNF 2014 was divided into 4 tracks (Textbox 1).
TEXTBOX 1 . MICRONUTRIENT FORUM 2014 ORGANIZED INTO 4 LINKED TRACKS.
Track 1 focused on updating knowledge on currently recommended methods of defining the magnitude and distribution of micronutrient deficiencies and highlighted and explored advances in prevalence estimations and biomarkers for nutritional assessment and gaps in information.
Track 2 provided updates on evidence of the efficacy and effectiveness of interventions to improve micronutrient status and its health and functional consequences.
Track 3 focused on the challenges and experiences of taking evidence-based interventions and translating them into effective policies and programs at scale.
Track 4 focused on stakeholders and their role in creating and enabling environments, including issues related to political will, leadership, and capacity development; defining responsibilities and obligations; and innovative financing needed to ensure the sustainability of efforts to address micronutrient deficiencies in countries and globally.
Pulling It All Together: I-to-I
The synergy between the I-to-I concept and the bridging theme of the MNF forum presented an opportunity to dovetail these concepts. The goals of applying the I-to-I concept to the MNF were as follows:
Present the scientific evidence underpinning existing or new programs/policies (e.g., new WHO guidelines on specific aspects of the MNF agenda), including discussions of how to address overlap. For example:
Program or intervention development/implementation/evaluation in areas where food insecurity, micronutrient malnutrition co-exist with pandemic infection, NCDs, or,
Explore advances and additional need for coordination of efforts [e.g., President’s Emergency Plan for AIDS Relief (16), Feed the Future (9), rollout of new WHO guidelines], and integration of nutrition interventions into existing health/agriculture/economic development systems.
Discuss opportunities to support health promotion and disease prevention at point-of-care and population levels.
Discuss strategies for integration of these efforts at health system/country level.
Describe capacity needs and obstacles to implementation.
Identify needs to facilitate effective integration and implementation.
Case Study Application of the I-to-I Approach to the MNF: Iron
Background to the iron issues.
ID remains the most prevalent global nutritional deficiency, affecting >1 billion people (17). In addition to the primary causes of ID, which includes inadequate intake of iron-rich foods and high intake of absorption inhibitors, the elevated needs of specific groups, such as women, infants, young children, and adolescents, require adequate prevention and ID control. These present a number of additional challenges and exemplify the need for integration of what we know to support effective implementation strategies (Textbox 2).
TEXTBOX 2 . CHALLENGES PRESENTED BY IRON.
Biology
Incomplete/evolving understanding of iron homeostasis (e.g., the emerging importance of hepcidin)
Route of administration: oral supplements compared with food-based supplements (role of non–transferrin-bound iron)
Effect of infection and inflammation (acute compared with chronic) on iron homeostasis
Role of the microbiome in iron metabolism and vice versa
Anemia: continued use of anemia prevalence as proxy for iron deficiency, despite evidence for multiple causes beyond iron
What happens in the transition from deficient to replete in terms of risk and safety of iron, particularly in the context of malaria?
Assessment
Moving beyond anemia as an index of iron status or trigger for iron intervention programs
Better approaches to understand the other determinants of anemia to avoid the potential of providing iron to those who do not need it
Distinguishing between markers of status in otherwise healthy patients/populations and those that reflect function relative to status or the effect of iron interventions
Impact of inflammation on selection, use, and interpretation of iron biomarkers
Markers that reflect iron nutrition as opposed to iron physiology and response to other conditions on iron status, that is, malaria, HIV, noncommunicable diseases, etc.
Interventions
Appropriate uses for supplements (pills/liquids) for prevention and treatment of iron deficiency
Efficacy of food-based interventions
Safe approaches to prevention of iron deficiency, including improved appreciation of contextual and biological factors that might modify safety and efficacy
Implementation
How to determine risk/benefit to support best practices for addressing iron and health
How to develop evidence-informed guidance that includes an appreciation of all of the above
How to synthesize such guidance at regional and country levels in a manner that is not confusing and still implementable within the health context of specific countries and health delivery systems
Resource and capacity needs
Many of these issues coalesced in 2006 with the publication of the results of a large randomized controlled trial that challenged the view that iron supplementation to young children in malaria endemic settings was safe. The release of this report stimulated a series of responses to the challenge of delivering safe and effective interventions to prevent and treat ID in settings with endemic infection, most prominently malaria. The study documented a set of adverse outcomes in children who were not iron deficient but were administered iron supplements in a highly malaria endemic area of east Africa (18).
Although the question of adverse effects of supplementary iron given to those not iron deficient was not new (19–21), these results influenced both prevention and treatment programs and focused the global discussion on questions about the current approaches to iron. The response to this study included a reassessment by the WHO and UNICEF of the existing approaches to address ID. In essence, the revised guidance was to continue population-based preventive programs as recommended in non–malaria endemic regions and to move to a targeted approach on the basis of individual screening in areas of endemic malaria (22) or, at a minimum, integration of anemia control with malaria control programs.
This approach engendered great confusion at local, regional, and global levels both in terms of implementation (were supplements safe and if not what were the best alternatives?) and risk assessment (how to assess safety and benefit of iron in such settings?). Furthermore, the strategy relied on screening for risk of ID by assessing for anemia. Increasing data indicate that anemia is a nonspecific indicator of iron status, especially in the malaria endemic contexts in which this strategy was to be deployed.
In response to this conundrum, a partnership was initiated between the Bill and Melinda Gates Foundation and the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH, that was designed to address the following 3 core questions: 1) what are the mechanisms that could explain how iron might negatively affect the health of women, infants, and children in the context of malaria and other infections; 2) how best to assess iron status and function in the presence of infection/inflammation; and 3) what are the safest and most effective means of preventing and treating ID in such settings?
The project has included the development of an evidence-informed technical report to support efforts of the WHO and others in their development of new guidance, (23) along with a number of research projects to address key elements of the core questions outlined in the previous paragraph. Another piece of the project was to develop an approach by which the larger community might be engaged in a consultative process to provide additional perspective to these issues. This element became the I-to-I project, and its application became the collaboration with the MNF.
The following are summaries of the sessions of the MNF through which the iron thread was woven. The summaries represent the key elements of the presentations and the discussions. They are not intended as comprehensive reviews of the subject of iron but rather the key issues identified by the speakers and the audience to iron across the spectrum of the 4 program tracks (Textbox 1).
Iron: from basic biology to policy and program implementation.
As outlined in Textbox 1, the structure of MNF offered a unique opportunity to follow the iron thread through each of the tracks, concluding with a candid conversation with country representatives, UN agencies, and key stakeholders. The following sections are summaries of those sessions, some suggested approaches, and next steps.
Session Summaries
Evidence, assessment, and interventions: why, what, and for whom?
Combined Tracks 1 and 2 session: Determining the risks and benefits of iron interventions, including recommended assessment methods and critical review of the need for individual level assessment
This was presented as a joint plenary session that combined key elements of Tracks 1 and 2. The talks included 2 focused on assessment/biology and function implications of iron and a talk on current strategies for intervention. The session concluded with an overview of program and policy implications, at clinical and population levels, of current approaches to prevent and treat ID.
Iron biology and assessment.
The biology and clinical consequences of ID continue to present a number of daunting challenges. Prominent among these are the complexity of anemia and the impact of inflammation on iron assessment, both of which have implications for solutions to iron as a clinical and public health problem.
Anemia contributes 8.8% to the global burden of years lived with disability (24). Yet, despite the existing knowledge about the biology and potential determinants of anemia, few studies were conducted in low-income settings to comprehensively address these issues. Daily iron supplementation as a strategy for controlling anemia was evaluated systematically via a meta-analysis of randomized controlled trials in children 4–23 mo of age (25). These analyses found that, although supplementation decreased anemia by 40% overall, in malaria endemic areas the impact is smaller (only 30%) (25), thereby emphasizing the likely variation in contribution of ID to the burden of anemia and highlighting concerns about efficacy of iron supplementation under specific conditions.
Studies have heightened attention to an evolving understanding of iron physiology and homeostasis. The master regulator of iron metabolism, hepcidin, exemplifies a complex interplay between nutrition, immunity, and erythropoiesis in iron metabolism. Elevated hepcidin prevents iron absorption and recycling, whereas suppressed hepcidin facilitates these processes. Hepcidin synthesis is induced not only by iron stores but also directly by inflammation (especially IL-6) (26) and represents a component of the innate immune response (27). Results from a recent study in Gambia support the potential value of hepcidin as a predictor of erythrocyte iron incorporation (28). For example, hepcidin concentrations may have utility in identifying children who were able to use and incorporate >20% of the administered iron into erythrocytes (29).
Because it reflects iron status, inflammation/infection, and erythropoeitic signals, measurement of hepcidin concentrations could be used to identify the window when iron might be safely given to individuals facing repeated infection (30). To most effectively explore these relations, a need exists to establish harmonized assays and to develop international reference material to enable reporting hepcidin concentrations along a single, non–assay-specific scale (similar to ferritin).
Future research could assess the potential to manipulate hepcidin to enhance recovery from ID anemia (IDA) (30), particularly in the context of infection, including malaria. Evidence now exists to support the potential utility of hepcidin as a marker for diagnosis and screening for the ability to distinguish between children with IDA and anemia of inflammation (29). However, further research is required to establish harmonized assays and international reference materials and to develop low-cost near-patient assays before presupplementation screening could be deployed.
Finally, it is incontrovertibly clear that humans, along with most other animals, have evolved an intricate system to withhold iron from the circulation during periods of infection (the hepcidin-ferroportin axis) as protection against the many iron-dependent pathogens that act as potent iron scavengers (27, 30). Although the essentiality of iron for human health is irrefutable, in settings of high infection burden and in light of what we know about the iron-pathogen nexus, an approach is needed to achieve optimal iron nutritional status that does not put people at risk by overriding an innate system intended to protect against infection. The evolution of our current understanding about iron thus raises the bar to use and expand the extant evidence to develop risk-benefit assessments that can account for both the importance of iron nutrition and the potential adverse outcomes, particularly in the context of infection.
Functional impact of iron malnutrition.
It is estimated that upward of 200 million children <5 y of age fail to reach their cognitive potential as a result of poverty and poor nutrition, resulting in an estimated 20% income reduction (31). ID and IDA were among the nutrition risk factors identified as affecting neurobiology (e.g., myelination, neurotransmitter metabolism, etc.), during early childhood and prenatally as well (32). The impact of iron may be better understood if viewed from the perspective of 3 development stages, prenatal, postnatal (infancy through adolescence), and in women of reproductive age. Some of the key issues relative to these critical periods of development are highlighted in Textbox 3.
TEXTBOX 3 . FUNCTIONAL IMPLICATIONS OF IRON MALNUTRITION ACROSS KEY DEVELOPMENTAL PERIODS.
Prenatal period
Evidence exists to support a positive association between iron status during the first trimester and temperament/behavior at 3 mo of age (LE Murray-Kolb, unpublished results, 2014).
Results from a large trial in Nepal indicate improved intellectual and fine motor functioning among 7- to 9-y old children whose mothers were given iron/folic acid supplementation compared with placebo (33).
Infancy
Lozoff et al. (34) reported that in children who were iron deficient during infancy and followed for 19 y, the effects of ID in infancy persisted in terms of lower cognition test scores in adolescence, despite remediation of the ID, and the impact was doubled for those with lower socioeconomic status.
These effects appear to persist into adulthood (35).
Potential for adverse effects on cognitive outcomes later in life of iron supplementation in infants who may not be iron deficient were reported (36).
Women of reproductive age
Murray-Kolb and Beard (37) reported that a decrease in 3 cognition domains (learning, memory, and attention) in young women with ID anemia, which improved with iron treatment.
ID in the absence of anemia was reported to affect some aspects of cognitive function (38).
Murray-Kolb and Beard (39) reported on the negative implications of these effects on mother-child interactions and subsequent adverse behavioral, developmental, and cognitive delays in infants.
Clearly, the challenge is to establish and implement guidance to prevent and treat ID and IDA and to support the maintenance of iron nutrition to optimize neurologic development for infants and school-age children. The literature is also replete with studies documenting the impact of IDA during childhood on lower scores on learning tasks, language learning, and motor development. Most individual studies document a reversal of these effects after iron supplementation, results appear to be age dependent (i.e., most consistent positive cognitive effects are seen in children >2 y). It is suggested that this inconsistency may be a result of population heterogeneity and that the positive findings more often reported in older children and adolescents indicate that, although there may be a critical period before which untreated ID/IDA may be irreversible, insults after that period may be more amenable to remediation. Thus, early detection of risk and prophylaxis becomes an even greater public health priority for infants and young children.
The overall message is that ID and IDA have important and long-term functional implications irrespective of when they occur during the life course. Clearly, the most vulnerable in terms of immediate and long-term impact are infants and young children faced with the prospect of irreversible deficits in cognitive/neurologic development in the absence of effective and safe remediation during critical periods of development.
Interventions to address ID and anemia: are they the same?
The global burden of anemia analyses indicate that IDA constitutes only about one-half of the burden of anemia in many low-income countries (24). Other important contributors to the burden of anemia include hemoglobinopathies and infection (40). The 3 primary iron intervention strategies are iron supplementation, food fortification, and food biofortification. Some selected examples of studies that have addressed the safety of iron interventions are provided in Textbox 4.
TEXTBOX 4 . EVIDENCE FOR SAFETY OF IRON INTERVENTIONS.
Food fortification
-
Efficacy of iron-fortified foods to decrease ID or IDA was reported for school-age children or young women in a wide variety of food vehicles, including salt, sugar, wheat flour, maize flour, rice, soy and fish sauces, and curry powder (41, 42).
Infections or widespread inflammation was not prevalent in these populations
-
Cercamondi et al. (43) examined the safety of iron-fortified sorghum porridge among Beninese women and reported the following:
Iron absorption decreased ∼40% in women with asymptomatic Plasmodium falciparum parasitemia and low-grade inflammation.
The effect resolved after treatment of malaria, the decrease of hepcidin, and resolution of inflammation as reflected by reduction of inflammatory biomarkers.
These investigators proposed that these findings may have resulted from the modulation of hepcidin in low-grade inflammation, representing a blunting effect on the efficacy of some iron-based interventions.
This was not confirmed, however, and efficacy studies in malaria-endemic Kenya and Cote d’Ivoire have reported that iron-fortified maize porridge (44) and iron-fortified salt (45) improved the iron status of young children and school-age children, respectively.
-
R Hurrell (R Hurrel, unpublished results, 2014) reported the following:
Iron-fortified maize-based complementary food virtually eliminated IDA when fed to young children for 9 mo.
These results may indicate that ID overrules inflammation in the control of iron absorption.
In this study, however, although IDA decreased from ∼40% to almost zero, total anemia prevalence remained unchanged, indicating an overlap between IDA and anemia of inflammation.
Zimmermann et al. (46) reported on a series of studies that involved children in Cote d’Ivoire which found a selective increase in pathogenic enterobacteria over gut commensal bacteria after consumption of iron-fortified biscuits.
-
Jaeggi et al. (47) corroborated these findings in a similar study of iron-fortified complementary foods for 6-mo-old infants in Kenya.
In this case, the addition of iron resulted in changes in intestinal microbiota, increased pathogenic bacteria, and intestinal inflammation.
In neither of the studies reported by Zimmermann et al. (46) was there an increase in diarrhea in the children; however, this was observed occasionally, most recently with MNPs [study by Soofi et al. (48) in Pakistan].
No evidence suggests that fortified foods or in-home fortification with MNPs increase the intensity of malaria infections (49).
Iron supplementation
A long history of safety concerns exist, particularly in the context of infections most prominently malaria (18, 50).
The 2009 Cochrane Systematic Review suggested an impact of iron on increased malarial parasitemia but not clinical malaria or mortality in areas with adequate surveillance and treatment (51). These findings are reflected in current recommendations that mandate iron status assessment and malaria treatment when giving iron supplementation in malaria endemic areas.
-
Recent reports have added to the following safety concerns:
The answers to 2 fundamental questions, 1 methodologic and 1 biological, may help weigh the cost/benefit of iron supplementation: 1) Can nutritional IDA be specifically identified in settings with inflammation/infection? and 2) How does iron status (reflecting stores plus circulating levels) predict either risk or benefit to health?
In terms of methods, a main challenge to our understanding of the role of various iron interventions is the incompatibility of studies because of the number of iron biomarkers used [e.g., serum ferritin, soluble transferrin receptor, and zinc protoporphrin], all of which are also affected by inflammation and infection. Formerly, serum ferritin was a recommended biomarker for assessing population iron status, because it showed large and consistent responses to iron interventions (53). However, in light of advances in understanding iron physiology and assessment, particularly in the context of inflammation and infections, its view as a recommended biomarker is being challenged.
In terms of the biology, the evidence from studies that involve iron interventions other than supplements [e.g., multiple micronutrient powders (MNPs) with iron, iron-fortified foods] is inconclusive. Although there is an assumption that these food-linked interventions may be safer than supplements in the context of higher stores, the safety of such interventions was questioned, particularly in high malaria endemic regions (53). A plausible mechanism that might explain improved health from increasing iron stores is that an increase in iron stores indicates that iron is absorbed and incorporated into hemoglobin and that a portion of the absorbed iron would also pass to the iron, requiring enzymes of the brain, energy, and immune systems and resulting in improved function of those systems.
Several mechanisms were proposed to explain relative effects of iron delivery with the use of different interventions. Among these hypotheses are 1) the possibility that iron supplements increase the amount of non–transferrin-bound iron (NTBI), thereby making more iron available to the parasite (53) and 2) that providing iron in food-based interventions improves safety by offering slower infusion of iron and thereby limiting the bolus of iron introduced into the system. These hypotheses were recently tested.
NTBI Hypothesis: results from a recent report provide support to this theory by indicating an increase in NTBI that was dose dependent and increased in subjects given supplements in the absence of food (54). The response was attenuated by the use of iron-fortified food (54).
Food-Based Interventions: a recent report on a trial in children 6–35 mo of age in Ghana documented no influence of iron-fortified MNPs on malaria incidence compared with children receiving non–iron-fortified MNPs. However, more hospitalizations were reported among children in the iron-fortified group during the intervention period (49).
One interpretation of the results of the Ghana study is that, although anemia was associated with a lower risk of malaria, ID without anemia was not (49). It might therefore be inferred that it might be more appropriate to treat ID but not anemia to minimize the malaria-related risk and to maintain adequate iron stores. Not treating anemia could result in less erythropoiesis and less young reticulocytes, which are the most favorable red blood cells to malaria infection (55). This can be achieved with fortification (56) but not with supplementation (25).
Implications for clinical care of individual- and population-targeted programs are as follows.
Great challenges confront the global community in making recommendations on the basis of the extant evidence which require a need to be adaptive, particularly because nutrition science will continue to evolve (57). The community must find a way to reach consensus when possible and ways to communicate an appreciation for the complexity of nutrition so that what appear to be contradictory results/recommendations do not undermine the credibility of existing recommendations/guidance or the field as a whole.
With specific regard to the core issues that confront our ability to move the iron agenda forward the primary questions to be addressed are summarized in Textbox 5.
TEXTBOX 5 . SOME KEY QUESTIONS RELATIVE TO IRON INTERVENTIONS/PROGRAMS/POLICY.
Are there functional consequences of insufficient iron intake in infants and other ages and life stages?
What are they, compromised immune function, neurologic, etc.?
Who is at greatest risk?
Do iron interventions (supplementation, fortification, others) improve functional outcomes in individuals with insufficient iron intake/ID?
Timing and screening will be critical both in terms of developmental stage when the insult occurs (e.g., early infancy) and assessment of the effectiveness of the intervention (e.g., too soon might miss the effect).
What is the ideal type of intervention?
Do we need interventions to prevent ID?
Type may be condition/risk group specific (e.g., supplements for pregnancy compared with food-based for infants and young children).
Health context (e.g., endemic infection)
Are there condition-specific risks associated with supplementation as an approach to treat ID? If so, do the benefits outweigh the risks?
Are there risks associated with supplementation as an approach to prevent ID? If so, do the benefits outweigh the risks?
What are the challenges for individual screening?
Hemoglobin = anemia, not ID
Serum ferritin, soluble transferrin receptor = potential but need to account for inflammation
Hepcidin: potential under certain circumstances (but much to be learned and need standards, sensitivity/specificity) and applicability in field settings
Can individual screening be done?
All depend on the timely and regular contact of individuals within the health system.
Population screening presents similar issues about indicators plus criteria for program triggers that will ensure safety and efficacy (i.e., for those exposed but not iron deficient).
Individual-level screening is not possible at this time because of budgetary restraints, technical challenges (both in terms of consensus of which biomarker to use and capacity to use them), and limitations about the consistency of individual contact with the health care system.
However, population-level targeting may be achieved through the identification of high-risk populations (e.g., age, economic status, geographic location). Population-level targeting will still, however, imply that some individuals who are not iron deficient may receive the intervention. In this case, some individuals within a population may be at risk of adverse outcomes, whereas others will benefit, without a clear mechanism to identify and to weight the relative balance between these.
Track 3: scaling up MN interventions—bridging the gap between evidence and implementation: integrating iron guidelines to support programs
The overarching theme of this session, with the key stakeholders, was to determine where the obstacles lie in the development, delivery, and monitoring chain. The session objectives were to identify 1) effective policies and delivery strategies and 2) technical, capacity, and resource needs to ensure an integrated approach for iron interventions. The session began with a presentation outlining the existing global guidelines (Textbox 6) for iron interventions and a brief description of the WHO guideline development process (68).
TEXTBOX 6 . IRON-RELATED GUIDELINES.
WHO
Intermittent iron and folic acid supplementation in nonanemic pregnant women, 2012 (58)
Daily iron and folic acid supplementation in pregnant women, 2012 (59)
Intermittent iron supplementation in preschool and school-age children, 2011 (60)
Intermittent iron supplementation in menstruating women, 2011 (61)
Use of multiple MNPs for home fortification of foods consumed by pregnant women, 2011 (62)
Use of multiple MNPs for home fortification of foods consumed by infants and children 6–23 mo of age, 2011 (63)
Other publications
Hemoglobin concentrations for the diagnosis of anemia and assessment of severity, 2011 (summary statement) (64)
Priorities in the assessment of vitamin A and iron status in populations, 2012 (report) (65)
Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations, 2011 (summary statement) (66)
Weekly iron-folic acid supplementation in women of reproductive age, 2009 (67)
Existing overlap in these guidelines were highlighted along with the challenges in their interpretation, implementation, and effective integration of iron intervention programs. The intended use of prescriptive guidelines and adaptation within country contexts were emphasized for successful implementation of the guidelines to scale up programs. It was noted that the first step in the guidelines development process includes open consultations (country level and the broad nutrition and health community). One area in need of further development in the guideline process is for behavioral communication-focused interventions, with an understanding of the country-specific context.
Country experiences.
Representative country experiences were presented for India, Kenya, and Gambia. Key elements of each country’s experience are highlighted in Supplemental Tables 1–4. The speakers outlined the current national strategies in place in their respective countries to combat iron and anemia and the challenges that are often faced. It was clear that great accomplishments and efforts for integration were made, yet pivotal road blocks still needed to be removed to achieve what these policies and programs sought to accomplish.
The experiences in all countries provided great insight into those components of a successful approach that can effectively integrate nutrition-related interventions to achieve successful implementation of these interventions at scale. As outlined, successful elements of such programs include the political will to both inform and engage in a multisectoral, culturally acceptable manner at the community level. These efforts must be context specific (health, social/cultural) and, to the extent possible, dovetail on existing successful programs and infrastructure. The technical basis for such programs must be clearly stated and presented in a manner to convey a value-added message and not confuse recipients.
Although the problems of preventing and treating ID and IDA remain, the experiences presented, albeit diverse, clearly indicate great forward potential. The lessons learned can and should be used to inform future efforts to roll out and scale up programs to achieve national and global targets.
Clinical perspectives.
The clinical perspective is commonly overlooked in the development and implementation of public health programs/interventions, the latter being characterized by delivery platforms that are often not linked to clinical care. Moreover, in low- and middle-income countries, substantial constraints often exist to link public health to clinical care, given poor coverage and quality of regular and timely preventive and curative care. But ultimately, caregivers need to make decisions about what questions to ask and what to do in areas with endemic ID, infectious diseases, NCDs, and food insecurities, all of which may exist in the same patient. Some of the factors that need to be considered before a decision about iron intervention is made include the cause of anemia (Textbox 7) and the impact of anemia on the patient, the benefits compared with the risks of treating anemia.
TEXTBOX 7 . GENERAL CATEGORIES AND CAUSES OF ANEMIA.
Reduced red cell production
Bone marrow failure
Ineffective erythropoiesis
Iron restriction
Erythropoietin deficiency
Increased red cell loss
Hemolysis
Bleeding
Anemia of inflammation
Infection
Chronic/sterile inflammation: rheumatologic conditions, cancer, inflammatory bowel disease, burns
Other specific causes
Parasitic infection (e.g., helminthes)
Renal failure
Malaria (anemia of inflammation, hemolysis, sequestration, erythroid suppression)
Hemoglobinopathies [e.g., sickle cell crises (hemolysis, sequestration, aplastic)]
An important challenge to be considered is the balance between a public health perspective in which the goal is to improve the nutritional status of an entire population without exposing it to increased risks of infection and the inability of a public health program to conduct a holistic individual assessment and/or monitoring through the course of treatment. However, the ultimate goal needs to remain in sight, the critical balance of benefit without harm.
Track 4: iron and malaria sessions at the MNF debate: wrapping it up
In an effort to tie together the lessons learned in the previous sessions and to conclude the I-to-I iron case study, the final session was designed as a panel discussion. The panel included representatives from each of the primary target groups of the MNF, research/clinical, program, and policy. All of the speakers from the preceding I-to-I sessions were invited to participate in the conversation. The session focused on the 4 key elements represented in the MNF tracks, that is, evidence, interventions/guidance, programs, and policy.
Clinical/biology.
A consensus is emerging that the use of the different iron biomarkers and their response to implementation programs should be context specific. Although the evidence is still limited to define a gold standard approach to the correlation between iron and inflammation, efforts are ongoing to address this core issue (Biomarkers Reflecting Inflammation and Nutrition Determinants of Anemia). An additional driver is our ability to distinguish between ID and other potential causes of anemia. Our ability to make this distinction is not only important in the context of clinical care but also as a scale to prevent potential risks associated with provided iron when it is not needed. To support this agenda, a need exists for expanded epidemiologic studies to enable estimates of the contributions of the different causes of anemia (69). An important effort continues to develop modeling-based estimates of the determinants of anemia (24). With specific regard to iron, randomized clinical trials are needed to assess the functional effectiveness of current approaches to improve iron status clinically and at population levels. The challenges with these interventions include the interpretation of the findings and weighing the potential risk compared with benefit for certain populations.
It has become evident during the course of the iron discourse that the considerations of iron supplementation in conditions of inflammation have been broadened in the past few decades to include sources of oxidative stress such as NCDs (e.g., obesity, diabetes, cardiovascular disease risk factors, etc.), pollution, and other noninfectious conditions. Skepticism is growing about universal iron supplementation as a one size fits all in this complex health context. The use of inflammatory biomarkers for a more meaningful interpretation of iron biomarkers was a repeated theme, but, again, it is currently challenged by the lack of a consensus as to how best to account for this relation.
Program perspective.
From a country perspective, questions remain about how to implement current and future guidance. For example, if deemed safe, what is the most effective way of delivering iron? To date, many governments have embraced MNPs as a safer and more acceptable method for iron delivery compared with syrups or drops for children <5 y of age, yet challenges remain with regard to coverage and improving delivery, including the potential role of the private sector in supplying MNPs, and lacking evidence that multiple MNPs are indeed safer or better tolerated than iron supplements (70).
A desperate need exists for innovative approaches to integrate malaria treatment and iron interventions into existing community health strategies. So far, health systems have been a great platform that allow the coupling of malaria treatment interventions such as bed nets and intermittent preventive treatment with ID treatment delivery to pregnant women in countries such as Kenya. However, the implementation of some recommendations such as screening and risk screening of nonpregnant women remain challenges. Additional challenges highlighted include ideal platforms for delivery of interventions, relative benefit/risk of weekly compared with daily supplementation, the cost-benefit of such programs, and effective methods for incentivizing the use of interventions at scale. Other challenges highlighted include how best to bring nutrition to the forefront of national policies, integrate nutrition interventions into existing platforms, and involve other sectors.
Strategies to monitor effectiveness and safety of anemia programs for infection do not exist. A priority is to determine the importance of timing of interventions in the context of existing prophylactic or treatment programs. For example, what is the best timing for iron interventions relative to malaria treatment?
Policy/guideline development.
Perspectives on global health policy and guidelines development were an essential part of the I-to-I discussions. It was suggested that decisions do need to be made on a population level; hence, a risk-benefit assessment with the use of state-of-the-art evidence would be helpful. That said, due consideration must be given to such potential sources of variability, such as geographic location, health context, resources, capacity, and the enabling environment.
Although risk does exist in areas of high infectious disease burden, the discussion in this session revealed that agencies such as UNICEF follow the technical guidance provided by the WHO. In the absence of compelling evidence to the contrary, these agencies continue to operate under the assumption that on balance and in most settings the benefits of MNPs compared with oral iron supplementation provide sufficient support to continue these programs, albeit with caution and an eye toward the evolving evidence. This approach would still require an integrated health system to incorporate measures for malaria screening, control, and treatment and ID treatment with the use of existing public health approaches such as those for sanitation or complementary feeding. Many of these approaches are and continue to be successfully used by such agencies as UNICEF. From a policy and program perspective, research needs to identify all possible populations at risk of either the condition of interest, in this case ID, or at risk of potential adverse effects from an intervention, for example, iron supplementation or MNPs, and to support the development of a better matrix to address these risks. Such an approach was successfully used in areas such as vaccine deployment. As the evidence evolves, the challenge will continue to be how best to provide critical programs and policies without disrupting systems and the provision of care/services.
Many questions remain about implementation of global guidance. These include 1) are there protocols for how to successfully implement an iron intervention program in the current health context? and 2) are there guidelines about the timing of interventions in the context of infection control programs?
About the latter, UNICEF currently suggests linking delivery of MNPs to malaria interventions (e.g., bed nets, medication) to improve access. Such efforts rely on existing micronutrient programs whereby there is a level of comfort about the safety of such interventions.
In addition to the operational guidelines, country officials are also concerned about the implications of increasing infection, and so having a protocol to follow on how to provide and manage nutritional care in the context of possible infection would be useful. An example of such a protocol is exemplified by the publication of the WHO guidelines for nutritional care of HIV-infected infants >6 mo and children (<14 y) which provides a stepwise approach to prevention, care, and treatment of both nutritional and HIV-related issues (71). Similar manuals for care of infections, including malaria, would serve as tools to strengthen the monitoring and evaluation of any iron intervention.
Even in the presence of evidence-informed practice guidelines and supporting materials, such as the integrated manuals for prevention and care, perfect coverage by any public health intervention is an unrealized ideal. The presence of individuals who are not reached must be a recognized contingency for clinicians and program providers alike. Moreover, in the case of pandemic infections such as malaria, the goal of concomitant prophylaxis along with nutritional interventions such as iron supplementation has clear budget implications that need to be considered.
The WHO will continue to work to develop new evidence-informed guidelines on iron supplementation for children (due in 2015) that will consider malaria endemic areas. However, these discussions highlighted the need to include other infections and causes of inflammation such as HIV, diarrhea, NCDs, etc. It was noted that such a comprehensive approach is desirable but limited by the absence of evidence to support systematic review processes leading to guideline development. As the guideline priorities evolve, so will the evidence to enable consideration of the issues raised in the I-to-I discussions.
Concluding remarks.
ID in all its forms remains a major global challenge. Policy makers are faced with marked complexity in devising strategies to address this burden. ID likely results in considerable short- and long-term adverse consequences for health, especially cognitive development. However, ID may also be a physiologic response to infection and may protect against malarial disease. Furthermore, interventions to control IDA by administering iron appear to exacerbate the risk and/or severity of infection. Data on functional benefits for controlling ID and IDA are surprisingly sparse. Modern guideline processes and efforts to implement strategies in the field struggle to cope with this complexity. The evidence reviewed and experiences conveyed over the course of the I-to-I sessions make a compelling case for how we may proceed, including pitfalls to avoid where possible.
Most guidelines developed by normative agencies intended to address specific nutritional issues such as prevention of ID focus on individual interventions, for example, use of iron supplements for pregnant women, rather than on a comprehensive approach to addressing the nutritional problem. Guidelines for choosing one type of intervention over another do not exist. Ideally, the best option is the consumption of iron-rich foods to meet population requirements, yet substantial evidence exists and it may be difficult to meet those needs with accessible and affordable foods during specific ages and life stages. The general consensus from the discussions is that food-based solutions are safer options than supplementation in children, but the need for complementary strategies may persist in some populations. The evidence presented throughout these sessions indicated clear benefits but also risks in areas with inflammation and infection, but, although researchers are working on balancing the risk/benefit, countries need to continue moving forward.
A disconnect often exists between the goals of different components of health systems. Of particular relevance to the iron agenda is the disconnect between efforts to address nutrition, that is, programs/interventions to prevent and treat ID and those addressing infectious disease such as malaria. A priority must be placed on the creation of a bridging dialogue that effectively links the problems (safe and effective use of interventions to prevent and treat ID in the context of infections such as malaria) with the solution (implementation of interventions). This could be through a risk-benefit analysis for treatment or preventive strategies done in infectious areas to answer some of the questions such as what is the lowest effective dose.
Conclusions and Next Steps
The overarching goal of the global nutrition and health community is to improve the health of the individuals and populations we serve. This can only be achieved with due recognition of the balance between the need for timely, evidence-informed standards of care and programs and the challenges of developing and implementing nutritional interventions, for either prevention or treatment, which are responsive to a complex public health scenario. The sessions clearly outlined many of the issues that contribute to that complexity. The I-to-I approach is intended to incorporate a full understanding of that complexity to allow for safe and effective implementation of technical guidance at both the individual and population levels. The ability to develop and implement that guidance will be contingent on an appreciation of the relative strengths of the full spectrum of stakeholder communities from research to clinicians, program developers, and policy makers. It is only through the engagement of these communities in a continuum of effort that our goals of health promotion and disease prevention will be achieved.
The I-to-I approach is a call to action for the entire food and nutrition community to become more engaged in the kinds of dialogue that will lead to more effective, safe, and contextually relevant programs and interventions. It is also essential to ensure that nutrition and all its biological and contextual complexities must be fully integrated throughout the systems intended to provide health services to individuals and populations. The MNF is now well positioned to lead this dialogue, and this review along with the iron case study was an effort to suggest how this process may be applied across a range of issues that face the global nutrition and health community.
The materials and discussions outlined in this review will be used to inform a new task force initiated by the International Union of Nutrition Sciences. The focus of this effort will be to build on existing and evolving evidence to conduct a risk-benefit analysis that was highlighted and endorsed throughout the I-to-I process.
Acknowledgments
We thank the staff of the Pediatric Growth and Nutrition Branch/NICHD, Dr. Gilman Grave (Branch Chief) and Ms. Alexandra Porter, for their support and contributions in planning and execution of the I-to-I project. We also thank Ms. Lucie Bohac and Ms. Katie Birks, both of the Micronutrient Initiative, for their help in manuscript preparation and revision. LMN, L-MD-R, S-RP, ID-H, RK, and FASA contributed to the planning and implementation of the I-to-I process and to the review and revision of the manuscript. In addition, LMN, L-MD-R, S-RP, RH, LM-K, KMN, TW, and MCP were presenters at the sessions discussed and contributed editorially to ensure accuracy of their respective sections. DJR was the primary author of the paper. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ID, iron deficiency; IDA, iron deficiency anemia; I-to-I, Integration to Effective Implementation; MNF, Micronutrient Forum; MNP, micronutrient powder; NTBI, non–transferrin-bound iron.
References
- 1.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382:427–51. [DOI] [PubMed] [Google Scholar]
- 2.Johnston JL, Fanzo JC, Cogill B. Understanding sustainable diets: a descriptive analysis of the determinants and processes that influence diets and their impact on health, food security, and environmental sustainability. Adv Nutr 2014;5:418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 2012;70:3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMichael T, Montgomery H, Costello A. Health risks, present and future, from global climate change. BMJ 2012;344:e1359. [DOI] [PubMed] [Google Scholar]
- 5.Duffy LC, Raiten DJ, Hubbard VS, Starke-Reed P. Progress and challenges in developing metabolic footprints from diet in human gut microbial cometabolism. J Nutr 2015;145:1123S–30S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raiten DJ, Ashour FAS, Ross AC, Meydani SN, Dawson HD, Stephensen CB, Brabin BJ, Suchdev PS, van Ommen B; INSPIRE Consultancy Group. Inflammation and nutritional science for programs/policies and interpretation of research evidence (INSPIRE). J Nutr 2015;145:1039S–108S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker DJ. Sir Richard Doll Lecture: Developmental origins of chronic disease. Public Health 2012;126:185–9. [DOI] [PubMed] [Google Scholar]
- 8.Scaling Up Nutrition [Internet]. New York: United Nations; 2013 [updated 2013 Dec 26; cited 2015 Jan 6]. Available from: http://scalingupnutrition.org/.
- 9.Feed the Future [Internet]. Washington (DC): The Organization [updated 2015 Apr 30; cited 2015 Jan 6]. Available from: http://www.feedthefuture.gov/.
- 10.Thousand Days [Internet]. Washington (DC): The Organization [updated 2015 Jun 16; cited 2015 Jan 6]. Available from: http://www.thousanddays.org/.
- 11.Micronutrient Forum [Internet]. Ottawa, Canada: The Organization [updated 2014 Dec 21; cited 2015 Jan 6]. Available from: http://micronutrientforum.org/.
- 12.Raiten DJ. Nutrition and pharmacology: general principles and implications for HIV. Am J Clin Nutr 2011;94:1697S–702S 10.3945/ajcn.111.019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Ommen B. Nutrigenomics: exploiting systems biology in the nutrition and health arenas. Nutrition 2004;20:4–8. [PubMed] [Google Scholar]
- 14.van Ommen B, Keijer J, Kleemann R, Elliott R, Drevon CA, McArdle H, Gibney M, Müller M. The challenges for molecular nutrition research 2: quantification of the nutritional phenotype. Genes Nutr 2008;3:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habicht JP, Pelto GH. From biological to program efficacy: promoting dialogue among the research, policy, and program communities. Adv Nutr 2014;5:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.PEPFAR [Internet]. Washington (DC): The Organization [updated 2015; cited 2015 Feb 10]. Available from: http://www.pepfar.gov/.
- 17.WHO. The World Health Report. Geneva (Switzerland): WHO; 2002. [Google Scholar]
- 18.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 2006;367:133–43. [DOI] [PubMed] [Google Scholar]
- 19.Oppenheimer SJ, Gibson FD, Macfarlane SB, Moody JB, Harrison C, Spencer A, Bunari O. Iron supplementation increases prevalence and effects of malaria: report on clinical studies in Papua New Guinea. Trans R Soc Trop Med Hyg 1986;80:603–12. [DOI] [PubMed] [Google Scholar]
- 20.Bates CJ, Powers HJ, Lamb WH, Gelman W, Webb E. Effect of supplementary vitamins and iron on malaria indices in rural Gambian children. Trans R Soc Trop Med Hyg 1987;81:286–91. [DOI] [PubMed] [Google Scholar]
- 21.Smith AW, Hendrickse RG, Harrison C, Hayes RJ, Greenwood BM. The effects on malaria of treatment of iron-deficiency anaemia with oral iron in Gambian children. Ann Trop Paediatr 1989;9:17–23. [DOI] [PubMed] [Google Scholar]
- 22.WHO UNICEF. Iron supplementation of young children in regions where malaria transmission is intense and infectious disease highly prevalent. Geneva (Switzerland): WHO; 2006. [Google Scholar]
- 23. Iron and Malaria Technical Working Group. Considerations for the safe and effective use of iron interventions in areas of malaria burden: full technical report. Bethesda (MD): NIH; 2009 September. [Google Scholar]
- 24.Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TP, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014;123:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasricha SR, Hayes E, Kalumba K, Biggs BA. Effect of daily iron supplementation on health in children aged 4–23 months: a systematic review and meta-analysis of randomised controlled trials. Lancet Glob Health 2013;1:e77–86. [DOI] [PubMed] [Google Scholar]
- 26.Ganz T. Systemic iron homeostasis. Physiol Rev 2013;93:1721–41. [DOI] [PubMed] [Google Scholar]
- 27.Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, Ho LP, Townsend AR, Drakesmith H. Hepcidin regulation by innate immune and infectious stimuli. Blood 2011;118:4129–39. [DOI] [PubMed] [Google Scholar]
- 28.Prentice AM, Doherty CP, Abrams SA, Cox SE, Atkinson SH, Verhoef H, Armitage AE, Drakesmith H. Hepcidin is the major predictor of erythrocyte iron incorporation in anemic African children. Blood 2012;119:1922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasricha SR, Atkinson SH, Armitage AE, Khandwala S, Veenemans J, Cox SE, Eddowes LA, Hayes T, Doherty CP, Demir AY, et al. Expression of the iron hormone hepcidin distinguishes different types of anemia in African children. Sci Transl Med 2014;6:235re3. [DOI] [PubMed] [Google Scholar]
- 30.Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science 2012;338:768–72. [DOI] [PubMed] [Google Scholar]
- 31.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B; International Child Development Steering Group. Developmental potential in the first 5 years for children in developing countries. Lancet 2007;369:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, Carter JA; International Child Development Steering Group. Child development: risk factors for adverse outcomes in developing countries. Lancet 2007;369:145–57. [DOI] [PubMed] [Google Scholar]
- 33.Christian P, Murray-Kolb LE, Khatry SK, Katz J, Schaefer BA, Cole PM, Leclerq SC, Tielsch JM. Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. JAMA 2010;304:2716–23. [DOI] [PubMed] [Google Scholar]
- 34.Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Arch Pediatr Adolesc Med 2006;160:1108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lozoff B, Smith JB, Kaciroti N, Clark KM, Guevara S, Jimenez E. Functional significance of early-life iron deficiency: outcomes at 25 years. J Pediatr 2013;163:1260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lozoff B, Castillo M, Clark KM, Smith JB. Iron-fortified vs low-iron infant formula: developmental outcome at 10 years. Arch Pediatr Adolesc Med 2012;166:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr 2007;85:778–87. [DOI] [PubMed] [Google Scholar]
- 38.Blanton CA, Green MW, Kretsch MJ. Body iron is associated with cognitive executive planning function in college women. Br J Nutr 2013;109:906–13. [DOI] [PubMed] [Google Scholar]
- 39.Murray-Kolb LE, Beard JL. Iron deficiency and child and maternal health. Am J Clin Nutr 2009;89:946S–50S. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen PH, Gonzalez-Casanova I, Nguyen H, Pham H, Truong TV, Nguyen S, Martorell R, Ramakrishnan U. Multicausal etiology of anemia among women of reproductive age in Vietnam. Eur J Clin Nutr 2015;69:107–13. [DOI] [PubMed] [Google Scholar]
- 41.Hurrell R, Ranum P, de Pee S, Biebinger R, Hulthen L, Johnson Q, Lynch S. Revised recommendations for iron fortification of wheat flour and an evaluation of the expected impact of current national wheat flour fortification programs. Food Nutr Bull 2010;31(1 Suppl):S7–21. [DOI] [PubMed] [Google Scholar]
- 42.Martorell R, Ascencio M, Tacsan L, Alfaro T, Young MF, Addo OY, Dary O, Flores-Ayala R. Effectiveness evaluation of the food fortification program of Costa Rica: impact on anemia prevalence and hemoglobin concentrations in women and children. Am J Clin Nutr 2015;101:210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cercamondi CI, Egli IM, Ahouandjinou E, Dossa R, Zeder C, Salami L, Tjalsma H, Wiegerinck E, Tanno T, Hurrell RF, et al. Afebrile Plasmodium falciparum parasitemia decreases absorption of fortification iron but does not affect systemic iron utilization: a double stable-isotope study in young Beninese women. Am J Clin Nutr 2010;92:1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andang’o PE, Osendarp SJ, Ayah R, West CE, Mwaniki DL, De Wolf CA, Kraaijenhagen R, Kok FJ, Verhoef H. Efficacy of iron-fortified whole maize flour on iron status of schoolchildren in Kenya: a randomised controlled trial. Lancet 2007;369:1799–806. [DOI] [PubMed] [Google Scholar]
- 45.Wegmüller R, Camara F, Zimmermann MB, Adou P, Hurrell RF. Salt dual-fortified with iodine and micronized ground ferric pyrophosphate affects iron status but not hemoglobin in children in Cote d’Ivoire. J Nutr 2006;136:1814–20. [DOI] [PubMed] [Google Scholar]
- 46.Zimmermann MB, Chassard C, Rohner F, N’Goran EK, Nindjin C, Dostal A, Utzinger J, Ghattas H, Lacroix C, Hurrell RF. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d’Ivoire. Am J Clin Nutr 2010;92:1406–15. [DOI] [PubMed] [Google Scholar]
- 47.Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, Boekhorst J, Timmerman HM, Swinkels DW, Tjalsma H, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015;64:731–42. [DOI] [PubMed] [Google Scholar]
- 48.Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I, Zaidi AK, Bhutta ZA. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet 2013;382:29–40. [DOI] [PubMed] [Google Scholar]
- 49.Zlotkin S, Newton S, Aimone AM, Azindow I, Amenga-Etego S, Tchum K, Mahama E, Thorpe KE, Owusu-Agyei S. Effect of iron fortification on malaria incidence in infants and young children in Ghana: a randomized trial. JAMA 2013;310:938–47. [DOI] [PubMed] [Google Scholar]
- 50.Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr 2001;131(2S–2):616S–33S. [DOI] [PubMed] [Google Scholar]
- 51.Ojukwu JU, Okebe JU, Yahav D, Paul M. Oral iron supplementation for preventing or treating anaemia among children in malaria-endemic areas. Cochrane Database Syst Rev 2009;(3):CD006589. [DOI] [PubMed] [Google Scholar]
- 52.Salam RA, MacPhail C, Das JK, Bhutta ZA. Effectiveness of Micronutrient Powders (MNP) in women and children. BMC Public Health 2013;13(Suppl 3):S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.WHO. Conclusions and recommendations of the WHO Consultation on prevention and control of iron deficiency in infants and young children in malaria-endemic areas. Food Nutr Bull 2007;28(4 Suppl):S621–7. [DOI] [PubMed] [Google Scholar]
- 54.Brittenham GM, Andersson M, Egli I, Foman JT, Zeder C, Westerman ME, Hurrell RF. Circulating non-transferrin-bound iron after oral administration of supplemental and fortification doses of iron to healthy women: a randomized study. Am J Clin Nutr 2014;100:813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Novelli EM, Hittner JB, Davenport GC, Ouma C, Were T, Obaro S, Kaplan S, Ong’echa JM, Perkins DJ. Clinical predictors of severe malarial anaemia in a holoendemic Plasmodium falciparum transmission area. Br J Haematol 2010;149:711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glinz D, Kamiyango M, Phiri KS, Munthali F, Zeder C, Zimmermann MB, Hurrell RF, Wegmuller R. The effect of timing of iron supplementation on iron absorption and haemoglobin in post-malaria anaemia: a longitudinal stable isotope study in Malawian toddlers. Malar J 2014;13:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harding KB, Neufeld LM. Iron deficiency and anemia control for infants and young children in malaria-endemic areas: a call to action and consensus among the research community. Adv Nutr 2012;3:551–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.WHO. Intermittent iron and folic acid supplementation in non-anaemic pregnant women. Geneva (Switzerland): WHO; 2012. [PubMed] [Google Scholar]
- 59.WHO. Daily iron and folic acid supplementation in pregnant women. Geneva (Switzerland): WHO; 2012. [PubMed] [Google Scholar]
- 60.WHO. Intermittent iron supplementation in preschool and school-age children. Geneva (Switzerland): WHO; 2011. [PubMed] [Google Scholar]
- 61.WHO. Intermittent iron and folic acid supplementation in menstruating women. Geneva (Switzerland): WHO; 2011. [PubMed] [Google Scholar]
- 62.WHO. Use of multiple micronutrient powders for home fortification of foods consumed by pregnant women. Geneva (Switzerland): WHO; 2011. [PubMed] [Google Scholar]
- 63.WHO. Use of multiple micronutrient powders for home fortification of foods consumed by infants and children 6–23 months of age. Geneva (Switzerland): WHO; 2011. [PubMed] [Google Scholar]
- 64.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva (Switzerland): WHO; 2011. [Google Scholar]
- 65.WHO. Priorities in the assessment of vitamin A and iron status in populations. Geneva (Switzerland): WHO; 2012. [Google Scholar]
- 66.WHO. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Geneva (Switzerland): WHO; 2011. [Google Scholar]
- 67.WHO. Weekly Iron-Folic Acid Supplementation (WIFS) in women of reproductive age. Geneva (Switzerland): WHO; 2009. [PubMed] [Google Scholar]
- 68.Pena-Rosas JP, De-Regil LM, Rogers LM, Bopardikar A, Panisset U. Translating research into action: WHO evidence-informed guidelines for safe and effective micronutrient interventions. J Nutr 2012;142:197S–204S. [DOI] [PubMed] [Google Scholar]
- 69.Pasricha SR. Anemia: a comprehensive global estimate. Blood 2014;123:611–2. [DOI] [PubMed] [Google Scholar]
- 70.De-Regil LM, Suchdev PS, Vist GE, Walleser S, Pena-Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database Syst Rev 2011;(9):CD008959. [DOI] [PubMed] [Google Scholar]
- 71.WHO. Integrated Approach to the Nutritional Care of HIV-Infected Children (6 months-14 years). Geneva (Switzerland): WHO; 2009. [PubMed] [Google Scholar]