Abstract
Diabetes is a chronic metabolic disease that affects a substantial part of the population around the world. Whether type I or type II, this disease has serious macro- and microvascular complications that constitute the primary cause of death in diabetic patients. Microvascular complications include diabetic retinopathy, nephropathy, and neuropathy. Although these complications are clinically and etiologically diverse, they share a common factor: glucose-induced damage. In the progression of diabetic complications, oxidative stress, inflammation, and the formation of glycation end products play an important role. Previous studies have shown that a healthy diet is vital in preventing these complications; in particular, the intake of antioxidants has been studied for their potential effect in ameliorating hyperglycemic injuries. Carotenoids are lipid-soluble pigments synthesized by plants, bacteria, and some kinds of algae that are responsible for the yellow, red, and orange colors in food. These compounds are part of the antioxidant machinery in plants and have also shown their efficacy in quenching free radicals, scavenging reactive oxygen species, modulating gene expression, and reducing inflammation in vitro and in vivo, showing that they can potentially be used as part of a preventive strategy for metabolic disorders, including diabetes and its related complications. This review highlights the potential protective effects of 4 non-provitamin A carotenoids—lutein, zeaxanthin, lycopene, and astaxanthin—in the development and progression of diabetic microvascular complications.
Keywords: carotenoids, diabetes, inflammation, microvascular complications, oxidative stress
Introduction
Diabetes mellitus (DM)3 is a chronic metabolic disorder that results from defects in insulin secretion, insulin signaling, or both (1). It is characterized by hyperglycemia and the consequent abnormalities in carbohydrate, lipid, and protein metabolism (2, 3). It is estimated that 366 million people worldwide had DM in 2011 and that it resulted in 4.6 million deaths. By 2030, the number of diabetic patients is expected to increase to 552 million (4).
Because of uncontrolled hyperglycemia and its consequent complications, DM is currently the seventh leading cause of death in the United States (5). Among diabetic complications, alterations to the vascular system are the main causes of mortality in both type I and type II DM (T2DM) (6, 7). These complications can be macrovascular (e.g., stroke, coronary artery disease, and atherosclerosis) when high-caliber vessels are affected or microvascular (e.g., diabetic nephropathy, neuropathy, and retinopathy) when there is damage to small vessels and capillaries (6, 8).
Although the etiology of each vascular symptom is different and dependent upon the type of diabetes, there are common risk factors among both type I and type II diabetic patients that make them more prone to having vascular complications, e.g., longer duration of diabetes, hypertension, smoking, obesity, poor glycemic control, and hyperlipidemia (2). All vascular complications also share some mechanisms by which hyperglycemia impairs cell and organ function (9). This explains why the main purpose of antidiabetic drugs is to achieve long-term glycemic control. However, this represents a challenge because current agents have treatment-limiting side effects and because diet is a very important factor in diabetes control, which means that in addition to pharmacological therapy adequate nutrition is crucial for preventing and controlling diabetes.
In addition to a controlled intake of macronutrients, specifically carbohydrates, there are other food components that are especially recommended for diabetic patients (e.g., fiber and antioxidants). Epidemiological data have consistently shown an inverse relation between fruit and vegetable intake and the risk of metabolic disorders, including T2DM (10). Among the bioactive components found in these 2 food groups, special attention is given to carotenoids for their antioxidant, anti-inflammatory, gene expression-modulating properties and their potential for preventing degenerative diseases such as atherosclerosis, cancer, and diabetic complications (11, 12). This review is focused on the effect of 4 non-provitamin A dietary carotenoids on several key mechanisms that lead to the development and progression of diabetic retinopathy, nephropathy, and neuropathy: lutein, zeaxanthin, lycopene, and astaxanthin.
Diabetic Microvascular Complications: Nephropathy, Retinopathy, and Neuropathy
Diabetic nephropathy (DN) is the leading cause of renal failure worldwide (2, 6, 13). This microvascular complication is characterized by the enlargement of the glomerular mesangium as a result of the accumulation of extracellular matrix proteins, microaneurysms, and mesangial nodule formation (6, 13). DN is clinically defined by proteinuria (>500 mg of urinary protein per 24 h), although this is preceded by microalbuminuria (30–299 mg of albumin per 24 h) that usually goes undetected (6). Epidemiological data report that ∼30–40% of patients with type I and 15% with T2DM develop end-stage renal disease (14).
Studies have shown that proximal tubular epithelial cells (PTECs) are the primary targets of hyperglycemia and that the chronic exposure of PTECs to glucose leads to cytotoxicity and the further initiation of DN (15). Furthermore, tubular dysfunction has been reported in DM in the absence of microalbuminuria, suggesting that the PTEC damage plays a pivotal role in the development of DN (14).
Diabetic retinopathy (DR) is 1 of the most common diabetic microvascular complications and fastest-growing causes of blindness and visual impairment for individuals aged 20–75 y (6, 8, 16). Almost every patient with type I diabetes and >60% of patients with uncontrolled T2DM are expected to develop DR within the first decade of diagnosis (17). This common eye disease is caused by changes in retinal ganglion cells (RGCs) (18) and presents with retinal vascular signs such as microaneurysms, hemorrhages, cotton wool spots, and hard exudates (16, 17, 19). If left untreated, DR will lead to advanced diabetic eye disease characterized by vitreous hemorrhages, retinal detachments, neovascular glaucoma, and eventually complete blindness (8).
The American Diabetes Association defines diabetic neuropathy as “the presence of symptoms and/or signs of peripheral nerve dysfunction after the exclusion of other causes” (6, 20, 21). Clinical manifestations are diverse because the diabetic neuropathies are heterogeneous (22). However, diabetic peripheral neuropathy (DPN) is the most common in long-standing diabetic neuropathy, affecting up to 50% of patients (23–25). DPN can manifest as pain but also as insensitivity, which increases the risk of burns, injuries, foot ulceration, and the need for amputations (25). Therefore, DPN can severely reduce a patient’s quality of life (9). Despite its high morbidity, DPN treatments are not very effective, probably because the mechanisms involved in its pathogenesis are diverse and complex; therefore, treatments usually target pain but not its cause. To date, glycemic control is the best way to prevent this complication (9, 26). Although all of these microvascular complications of diabetes are clinically diverse, they all share common characteristics in their pathogenesis, which is triggered by elevated concentrations of plasma glucose and oxidative stress, inflammation, and advanced glycation end products (AGEs).
Oxidative Stress and Inflammation
Oxidative stress is the result of an imbalance between oxygen-derived radicals and the organism’s antioxidant potential (3). Various studies have shown that DM can affect both factors, increasing reactive oxygen species (ROS) and decreasing antioxidant defense capacity. This leads to oxidative damage of cell components such as proteins, lipids, and nucleic acids and a chronic inflammatory response (3). There are multiple sources of oxidative stress in diabetes. Hyperglycemia can directly increase ROS generation by glucose auto-oxidation, which generates hydroxyl (·OH) radicals. In addition, during hyperglycemia, there is enhanced metabolism of glucose through the polyol (sorbitol) pathway, which also results in the elevated production of ·OH2−. The mitochondrial respiratory chain is another source of nonenzymatic generation of reactive oxygen and nitrogen species (7).This process is increased by the elevated production of the electron carriers nicotinamide adenine dinucleotide phosphate and flavin adenine dinucleotide in glucose oxidative metabolism (7, 9).
In the development of T2DM, inflammation is thought to mediate the progression of insulin resistance and pancreatic cell dysfunction, but the activation of inflammatory processes could also explain microvascular damage in diabetic patients (27). There are many inflammatory pathways that can be described in the development of diabetes; however, the activation of NF-κB has been shown to be a key event in the early pathobiology of diabetes and its microvascular complications (16, 28, 29). The activation of the NF-κB pathway in diabetic patients is induced by oxidative stress, which can be caused by hyperglycemia and excess AGEs (29). AGEs are irreversible protein-bound compound products of glycation, an inevitable, nonenzymatic reaction between reducing sugars and the free amino groups of proteins, lipids, and DNA (30, 31). AGEs have specific properties such as fluorescence, browning, cross-linking, and production of ROS (32, 33). During hyperglycemia, the elevated concentration of glucose in the blood accelerates the formation of AGEs, which can accumulate in the capillaries where they can cross-link with other proteins, leading to arterial stiffness and vascular dysfunction (8, 34). In addition, AGEs can bind to specific receptors, such as the receptor for advanced glycation end products (RAGEs) that triggers a signaling cascade and the phosphorylation of protein kinases, including MAPKs and Janus kinase/signal transducers and activators of transcription that culminate in the production of proinflammatory and prosclerotic cytokines via the NF-κB pathway, including TNF-α and IL-1β, and create a loop that exacerbates the inflammatory response (16, 31, 35). In cell culture studies, AGEs were found to induce cellular activation associated with chemotaxis, oxidative stress, and cell proliferation or programmed cell death (36). It has been demonstrated that RBCs from diabetic patients have an abnormally increased ability to adhere to the vascular endothelium, which is mediated by the binding of N6-carboxymethyllysine (a very common AGE present in the red blood cell membrane) and the RAGEs located on endothelial cells. The involvement of RAGEs results in the activation of endothelial cells, which become proinflammatory and prothrombotic, leading to diabetic complications (37). Pharmacologic inhibition of AGE formation in long-term diabetic animals seems to prevent diabetic retinopathy, nephropathy, neuropathy, and arterial abnormalities in animal models (38).
Another inflammatory pathway that has been shown to mediate diabetic complications is the p38 MAPK signaling pathway (39, 40). Clinical and in vitro studies have shown that hyperglycemic conditions activate p38 MAPK in many tissues, including kidney, blood vessels, and heart (40). Studies in a hyperinsulinemic mouse model (db/db mice) have demonstrated that the p38 MAPK pathway is required for the progression of diabetic nephropathy (39) and that it contributes to the inflammatory response in diabetic eyes by increasing the production of cyclooxygenase 2 and inducible NO synthase and by upregulating the NF-κB pathway (40).
Many molecular signs of the inflammatory response, such as proinflammatory cytokines, adhesion molecules such as the intercellular adhesion molecule-1, and active immune cells, are increased in the retinas and vitreous humor of diabetic animals and humans (8, 16). Vascular endothelial growth factor (VEGF), an important angiogenic factor involved in vascular permeability, is elevated in the retina and vitreous humor of diabetic patients and animals (41). In addition to transforming growth factor-β and platelet-derived growth factor, VEGF has been shown to have a direct relation on the cell growth seen in diabetic nephropathy (27).
Carotenoids and Microvascular Diabetic Complications
The Diabetes Control and Complications Trial demonstrated that tight glycemic control could substantially reduce—but not completely prevent—clinical complications in the diabetic population (42). This suggests that, in addition to controlling blood glucose, alternative treatment strategies are needed. As stated previously, many diabetic complications originate by glucose-induced oxidative stress; therefore, controlling this stress could be a target for therapy, and the use of dietary antioxidants, such as carotenoids, is a promising option to be considered (43, 44).
Carotenoids are lipid-soluble phytochemicals that are mainly synthesized by plants, where they are pigments and antioxidants (45, 46) that protect cellular structures against oxidative damage by scavenging singlet molecular oxygen and peroxyl radicals usually caused from light and normal plant metabolism (10, 46–48). More than 700 naturally occurring carotenoids have been identified, but only around 50 are present in the human diet. Among these, 6 (β-carotene, β-cryptoxanthin, α-carotene, lycopene, lutein, and zeaxanthin) represent >95% of total blood carotenoids (49).
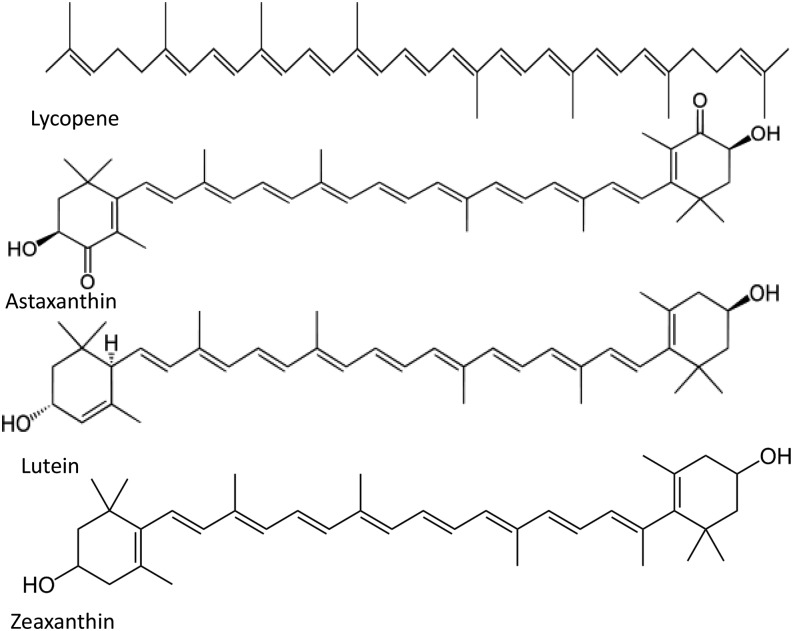
Structurally, carotenoids can be classified into oxygen-lacking carotenes and oxygen-containing xanthophylls. Functionally, they can be categorized into provitamin A if they can be metabolized by into retinal and retinol (50). Conversely, non-provitamin A carotenoids are those in which further metabolism does not yield a vitamin A vitamer. This group includes lycopene, lutein, zeaxanthin, and astaxanthin, among others (51). The structures of these 4 non-provitamin A carotenoids are depicted in Figure 1.
FIGURE 1.
Molecular structures of lycopene, astaxanthin, lutein, and zeaxanthin.
Because carotenoids do not meet the criteria for being considered vitamins, there is no daily recommended intake for them; however, lower serum concentrations of these compounds have been positively associated with increased risk of cardiovascular disease, T2DM, age-related macular degeneration, and certain types of cancer (12, 52–55). Dietary sources of carotenoids are orange, red, and yellow fruits and green leafy vegetables as well as egg yolk and some kinds of fish (49). However, 80–90% of human carotenoid intake comes from fruits and vegetables (49, 56). Given their beneficial health claims, carotenoids are also available in fortified foods and as dietary supplements, but their physiological effect in diabetic patients is not yet known.
A very important aspect to consider when evaluating the effects of carotenoids in humans is their low bioavailability (57). This term refers to the fraction of the ingested carotenoids that is available for utilization or storage in normal physiological conditions (58) and depends on bioaccessibility, which is the amount of carotenoids ingested that disrupts the food matrix and its availability for absorption in the intestine (59). Bioaccessibility can be influenced by many factors, such as the type of carotenoid, the food source, processing, storage, the amount and type of fat present in the meal, interaction with other nutrients such as fiber and other carotenoids, and individual differences such as nutrient status or genetics (57, 60, 61). Given the many components that determine carotenoid bioaccessibility, the amount of active compound that is bioavailable can vary from <10% to 50% of the amount consumed (62).
Once absorbed and delivered to target tissues, the main proposed mechanisms by which carotenoids may be beneficial in controlling diabetic microvascular complications are their antioxidant capacity, anti-inflammatory properties, impact on cellular signaling, and ability to modulate gene expression (10, 43, 48, 63–65). Given their particular structure, carotenoids are capable of quenching ROS and other free radicals of different origins (10, 43, 48). It has also been hypothesized that carotenoids or their derivatives can interact with cysteine residues of the IκB kinase and/or NF-κB subunits and inactivate the NF-κB pathway, thus decreasing inflammatory pathways (65). Another hypothesis regarding the action of carotenoids that is not related to antioxidant activity is the stimulatory effects they exert on gap junction communication, which is thought to be implicated in the regulation of cell growth, differentiation, and apoptosis (64). In hyperglycemic states, studies have reported impaired gap junction communication in vascular smooth muscle, endothelial cells, astrocytes, and retinal pericytes, suggesting a role of this glucose-induced gap closure in the development of diabetic microvascular dysfunctions (66, 67).
In addition, animal studies have shown that some carotenoids are capable of inducing xenobiotic-metabolizing enzyme systems such as cytochrome P450-dependent enzymes and glutathione S-transferases (64, 68). Functional polymorphisms of these enzymes have been reported to be involved in the pathogenesis of T2DM (69) and in some complications, such as diabetic retinopathy (70).
Furthermore, carotenoids and their metabolites have been shown to affect gene expression and cell function through multiple mechanisms, including the interaction/interference with several transcription factors and the modulation of signaling pathways associated with inflammatory and oxidative stress responses (71).
Lutein and Zeaxanthin
Lutein and zeaxanthin are non-provitamin A carotenoids from the xanthophyll family. These carotenoids are differentiated from others by having hydroxyl groups on each end that provide an amphipathic character that allows xanthophylls to be inserted into the lipid bilayer of cell membranes and the outer monolayer of lipoproteins (59, 72, 73).
Lutein and zeaxanthin are preferentially accumulated in the macular region of the retina. Zeaxanthin is mostly found in the foveal region, whereas lutein is more abundant in the perifoveal region (74, 75). Together, these pigments are referred to as the macular pigment, where they provide protection to the retina against the oxidative blue light (59, 73, 75, 76). Given their selective and exclusive uptake by the retina, lutein and zeaxanthin are traditionally linked to eye health, specifically to the prevention of age-related macular degeneration (59, 75, 77, 78). However, numerous studies have linked these 2 xanthophylls to other eye diseases, such as cataracts, retinal ischemia, uveitis, retinitis pigmentosa, and DR (73, 79). As mentioned previously, antioxidant compounds can also be anti-inflammatory given the relation between oxidative stress and several inflammatory pathways, such as NF-κB and p38 MAPK (65, 80, 81). Both are pivotal in the development of DR (16, 40) and have been shown to be blocked or decreased by lutein in vitro and in vivo (82, 83).
To test the effect of lutein in hyperglycemic conditions, Muriach et al. (82) used an alloxan-induced diabetic mouse model. Alloxan is a glucose analogue that inhibits glucose-induced insulin secretion in pancreatic β-cells, mimicking insulin-dependent diabetes (84). After 4 d of inducing diabetes, the mice were given 0.2 mg lutein/(kg · d) via a stomach tube or 500 mU insulin/g body weight injected intraperitoneally. As expected, diabetic mice showed an increase in blood glucose, glycated hemoglobin, and malondialdehyde (MDA) compared with controls. Although both lutein and insulin restored serum MDA concentrations, lutein treatment did not alter the hyperglycemic status (plasma glucose and glycated hemoglobin) of alloxan-diabetic mice like insulin did. This suggests that lutein ameliorates oxidative stress in diabetic conditions but does not modify glycemic status (82). The ocular results showed that MDA concentration was higher in diabetic mice than in controls, whereas glutathione concentration and glutathione peroxidase (GPx) activity were decreased in the diabetic retina (75). The lutein-treated mice showed values of MDA, glutathione, and GPx activity similar to controls; however, insulin treatment only restored GPx activity. In terms of inflammation, NF-κB activity was increased in the retina of diabetic mice, and all treatments prevented this increase, denoting also the anti-inflammatory properties of lutein in this animal model (82).
Sasaki et al. (85) reported similar results in a study conducted with streptozotocin (STZ)-induced diabetic mice. Like alloxan, STZ causes a state of insulin-dependent DM by β-cell toxicity (84). In Sasaki’s study, mice were divided into 4 groups: diabetic, diabetic + lutein, nondiabetic, and nondiabetic + lutein. The mice allocated in the lutein groups were fed unpurified diet with and additional 0.1% (wt:wt) of lutein and were analyzed 1 and 4 mo after diabetic induction. The results indicated that supplementation did not alter the glycemic status of diabetic mice but did prevent the visual impairment generated by diabetes by reducing ROS, extracellular signal-regulated kinase activation, and the depletion of brain-derived neurotrophic factor in the diabetic retina (85). In addition, using STZ-treated rats and 0.02% or 0.01% zeaxanthin supplementation, Kowluru et al. (42) found that this carotenoid prevented diabetes-induced retinal damage and restored the concentrations of VEGF and intercellular adhesion molecule-1 concentrations to concentrations that were comparable to controls. Furthermore, the concentrations of lipid peroxides, oxidized DNA, electron transport complex III, nitrotyrosine, and mitochondrial superoxide dismutase (SOD) were also similar in the retinas of the zeaxanthin group compared with the controls, which means that the glucose-induced oxidative stress commonly seen in diabetes was restored to normal with zeaxanthin treatment. Similar to the results of other studies, these outcomes were independent of hyperglycemic status (41).
Hu et al. (86) explored the potential benefits of lutein and zeaxanthin together in mostly type 2 diabetic patients (26 of 30) with diagnosed nonproliferative diabetic retinopathy (NDR). In this study, plasma concentrations of these carotenoids were substantially lower in the diabetic group compared with normal subjects at baseline (86). As reported by Brazionis et al. (51), these different carotenoid plasma concentrations may play a role in the development of NDR, where a higher concentration of lycopene and lutein/zeaxanthin in plasma was associated with substantially lower odds of this diabetic complication. In the Hu et al. study, after 3 months of supplementation with 6 mg lutein/d and 0.5 mg zeaxanthin/d, the NDR group presented higher plasma concentrations than controls. The treatment group also improved their visual acuity and showed a decrease in foveal thickness associated with the severity of NDR (86). These findings suggest that lutein and zeaxanthin supplementation may be used to ameliorate NDR symptoms, but longer and larger clinical trials are needed to explore how the chronic intake of these carotenoids affects the diabetic population. It is important to mention that elevated doses of lutein and zeaxanthin could yield to cytotoxic carotenoid-derived aldehydes, and it has been reported that these molecules exacerbate oxidative stress in vitro on human retinal pigment epithelial cells (87); therefore, the adequate dose of supplementation should be carefully assessed and should focus on potential toxicity or the counterproductivity of the compounds used.
An important aspect to consider with lutein and zeaxanthin is that despite the claimed benefits that these compounds may give to human health, the intake of natural dietary sources of these carotenoids is very low. For example, the intake associated with a decreased risk of age-related macular degeneration is ∼6 mg lutein/d (88), which is far greater than the mean American consumption of 1.7 mg/d (59). Given the low consumption from dietary sources, purified lutein can also be found as a supplement, added to food products, or as part of multivitamins. However, even with an increased intake with supplement use, lutein has variable bioavailability given the many previously mentioned factors that affect its bioaccessibility. Different studies have reported lutein bioavailability to be from 2% to 20% (49, 59, 89) depending on the lutein source, how it was administered to the subjects, and the assessment method used. This suggests that to observe positive effects in diabetic patients, the delivery method should be consistent to reach a certain concentration of lutein in plasma. Further studies with humans are needed to establish this consistency.
Lycopene
Lycopene is a nonoxygenated acyclic carotenoid responsible for the red color in tomatoes (90). Epidemiological studies show that high consumption of tomatoes correlates to higher concentrations of lycopene in plasma and has been related to a lower risk of cancer and cardiovascular disease development (90–92). Lycopene is the most potent singlet oxygen quencher among carotenoids and is thus hypothesized to contribute to the attenuation of oxidative stress in metabolic diseases (93). Furthermore, it has been shown that this carotenoid can inhibit NF-κB activation and TNF-α expression, suggesting that it also has anti-inflammatory and gene expression-modulating properties (93, 94). Lycopene may protect against diabetic microvascular complications by interfering with the AGE/RAGE binding associated with oxidative stress and inflammatory responses that result in increased cytokine expression. Therefore, the inhibition of AGE formation and RAGE expression suggest a possible strategy for preventing the inflammatory complications in diabetic patients (32, 35).
Lycopene has also been shown to have specific effects in selected tissues. For example, Pierine et al. (93) used obese rats fed with a high-fat, high-sucrose diet and showed that supplementation with 10 mg of lycopene per kilogram of body weight for 5 d/wk during 6 wk decreased RAGE concentration in the renal tissue. The authors also observed less TNF-α expression in the supplemented rat kidneys; however, this reduction was not observed in plasma, suggesting that the beneficial effect is localized to specific sites (93). Another piece of evidence that supports the selective action of lycopene on RAGE expression comes from a crossover placebo compared to treatment study conducted by Oborna et al. (85), in which 20 mg lycopene/d supplementation in both fertile and infertile men decreased soluble isoforms of RAGE in seminal plasma but not in blood (95). These findings point to the need of conducting more studies to determine whether these decreases in glycation products could be observed in other tissues or, as mentioned previously, are selective to certain organs.
To study the specific therapeutic prospect of lycopene against DN, Guo et al. (96) conducted a study with STZ-induced kidney injury in mice. In this study, supplementation with 40 and 80 mg lycopene/kg for 8 wk showed a dose-dependent alleviation of kidney lesions with reduced serum concentrations of glucose and LDL-C, increased HDL-C, and decreased proteinuria (96). A healthy lipid profile is very important for diabetic patients not only in preventing macrovascular complications but also microvascular damage. Epidemiological research has revealed that dyslipidemia is a risk factor for diabetic nephropathy development and progression (97). Moreover, supplemented mice showed augmented bioactivities of SOD and GPx, indicating an improved enzymatic antioxidant defense and decreased plasma MDA that means less lipid peroxidation. Immunohistochemical analysis demonstrated that lycopene intake also decreased NF-κB and TNF-α expressions in kidney tissue (96). Li et al. (98) obtained similar results using a smaller dose (20 mg/kg) given by oral gavage to STZ-induced diabetic rats. In this study, the treatment of diabetic rats with lycopene decreased blood urea nitrogen, 24-h urinary protein, and creatinine. Lycopene also improved the lipid profile, reducing total cholesterol, TGs, and LDL-C and increasing HDL-C compared with the values of nonsupplemented diabetic rats. Lycopene administration also decreased MDA concentration and the expression of connective tissue growth factor, which plays an important role in the fibrotic response. In addition, results showed increased protein kinase B phosphorylation and SOD activity in diabetic renal tissues (98). However, it is important to acknowledge that very high doses of lycopene, not relevant to human consumption, were used in this animal study. More clinical trials are needed that use nutritionally relevant consumptions of lycopene so that it can be extrapolated to humans.
Lycopene has also been tested for its potential in controlling diabetic neuropathy pain in animal models. Kuhad et al. (26) evaluated 3 different doses of orally injected lycopene (1, 2, and 4 mg/kg body weight) from the fourth to eighth week after STZ injection in male mice. Pain was assessed by thermal tolerance using warm water tail immersion and hot plate tests. A short time response for struggle, flicking response (tail withdrawal), or paw licking was considered hyperalgesia. In addition, serum TNF-α and NO production (calculated by serum nitrite concentrations) were measured. The lycopene treatment substantially inhibited thermal hyperalgesia and TNF-α and NO release in a dose-dependent manner, which may be the mechanism by which lycopene attenuated pain in this mouse model (25).
Although the bioprotective activity of lycopene is generally attributed to its antioxidant properties, there is also evidence that suggests that other nonoxidative mechanisms are also involved. These alternative mechanisms could be intercellular gap junction communication, hormonal and immune system modulation, and metabolic pathway modification (99, 100). In fact, some authors question the available data to support the “antioxidant hypothesis” as a major mechanism of the in vivo action of lycopene because most of the evidence comes from in vitro models. A suggested alternative hypothesis is that the lycopenoids—the metabolic products of lycopene—may be more bioactive and therefore responsible for the outcomes seen in vivo with lycopene. It has been proposed that lycopenoids affect health by altering gene expression (100, 101).
Another important point to consider when studying the potential benefits of lycopene in humans is that its applications in food are limited by the oxidation that occurs normally during processing and storage, because lycopene readily deteriorates in the presence of light and heat, thus affecting the bioactivity of the compound present in regular diets (102, 103). However, lycopene has been shown to be better absorbed from processed tomato products than from fresh tomatoes, the most important lycopene dietary source (104), suggesting that some processing is needed to make this carotenoid more bioaccessible.
To establish proper dietary guidelines or recommend supplementation for susceptible people, all of these proposed mechanisms of action and factors that affect the absorption and bioactivity of lycopene need to be considered when studying the relation between this carotenoid and diabetic microvascular complications in humans.
Astaxanthin
Astaxanthin is a ketocarotenoid oxidized from β-carotene and is a common pigment in algae, fish, and birds. It can also be extracted from crustacean byproducts, such as shrimp, crawfish, crabs, and lobsters (13, 44).
As with lutein and zeaxanthin, astaxanthin belongs to the xanthophyll family. One of the most important properties of astaxanthin is its potent antioxidant activity, which is thought to be 10 times higher than other known compounds such as polyphenols or β-carotene (44). Because of this property, astaxanthin has been studied, mainly in animal and cell studies, for its possible role in the treatment of chronic diseases that involve oxidative stress, including diabetes and its complications (13, 44, 105, 106).
Astaxanthin has been tested on DN by using an alloxan-induced diabetic rat model designed by Sila et al. (45), in which an increase in glycemia, creatinine, urea, and uric acid concentrations along with elevated plasma and kidney MDA and protein carbonyls were observed in diabetic animals compared with controls. Antioxidant mechanisms (catalase, SOD, glutathione) were, on the other hand, decreased in the diabetic group. These rats also showed glomerular hypertrophy and tubular dilatation in an examination of renal histology. The group supplemented with 20 mg astaxanthin/kg body weight via oral gavage for 21 d improved all measured parameters, including glucose and insulin concentrations, making them comparable to those observed in the controls. In addition to reducing hyperglycemia and oxidative damage, astaxanthin-treated animals showed a healthier kidney histology, reducing tubular dilation, glomerular space reduction, and necrosis (44). Naito et al. (14) obtained similar results with db/db mice, which is a rodent model of type 2 diabetes. After 12 wk of oral supplementation with 0.02% astaxanthin mixed in the food, the treatment group showed decreased blood glucose, although the concentrations were still substantially higher than controls. Urinary albumin was significantly reduced in the treatment group, as were the histological changes typically observed in diabetes. Moreover, both urinary and mesangial 8-oxo-2′-deoxyguanosine, which is a good marker to evaluate renal oxidative stress, were decreased by astaxanthin (13). Using proximal tubular epithelial cells, Kim et al. (15) showed that 0.5% (vol:vol) astaxanthin effectively suppressed lipid peroxidation, total reactive oxygen and nitrogen species, inducible NO synthase, cyclooxygenase 2 protein concentrations, NF-κB nuclear translocation, and the proapoptotic regulator Bcl-2-associated X protein and increased antiapoptotic B-cell lymphoma-2 protein concentrations. In this study, we concluded that astaxanthin protects PTECs against the damage caused by hyperglycemia, suggesting that this carotenoid could be used in further studies to determine whether it has a similar effect in vivo (15).
Astaxanthin has additional benefits to those observed in nephropathy. Dong et al. (19) used a genetically diabetic db/db mouse to evaluate the potential of astaxanthin in decreasing oxidative stress and reducing RGC apoptosis. After 8 wk of treatment with 25 or 50 mg astaxanthin (kg · d), the supplemented mice showed an increase manganese dismutase activity and reduced hyperglycemia-induced increase in MDA and 8-oxo-2′-deoxyguanosine. Furthermore, astaxanthin supplementation reduced hydrogen peroxide-induced apoptosis in the transformed rat retinal ganglion cell line RGC-5 (18). These molecular studies suggest that astaxanthin could be a potential dietary aid in treating diabetic microvascular complications; however, more animal studies and human trials are needed to corroborate these beneficial effects in diabetic patients.
The proposed mechanism by which astaxanthin may help to prevent and treat diabetic microvascular complications is by modulating oxidative stress, inflammation, and apoptosis by quenching free radicals (15). The capacity of astaxanthin to inhibit lipid peroxidation may be helpful for increasing survival in pancreatic β-cells because these cells are sensitive to ROS under oxidative conditions; therefore, oxidative stress may play an important role in β-cell deterioration in type 2 diabetes (107). This increased survival of β-cells could explain improved glycemic values with astaxanthin supplementation (44). Another mechanism could be that astaxanthin prevents the formation of AGEs. A study done with STZ-induced type 1 diabetic rats showed that 50 mg astaxanthin/(kg · d) for 18 d effectively attenuated the formation of hepatic AGEs, whereas RAGE expression did not change after treatment (105).
It is important to consider that although cell and animals studies have provided evidence that astaxanthin may be useful in ameliorating some symptoms of diabetic microvascular complications, human dietary sources are almost exclusively seafood (108). The tested doses in these studies are therefore very difficult to achieve through diet. The data suggest that the potential benefits from this carotenoid could only be perceived in humans through the consumption of supplements in doses not achievable by diet. Further clinical trials are therefore needed regarding the potential of astaxanthin in the diabetic population.
A summary of all the protective effects of carotenoids against diabetic complications is presented in Table 1, and the mechanisms by which these carotenoids reduce diabetic complications are depicted in Figure 2.
TABLE 1.
Carotenoid effects on diabetic complications1
| Carotenoid | Complication | Model | Dose | Duration | Outcome | Reference |
| Lutein and zeaxanthin | DR | Human/case control | 6 mg lutein/d | 3 mo | ↑Serum lutein/zeaxanthin | (70) |
| 0.5 mg zeaxanthin/d | ↑Visual acuity | |||||
| ↓Foveal thickness | ||||||
| Lutein | DR | Diabetic mice | 0.1% | 1 and 4 mo | ↓ROS generation in retina | (69) |
| ↓Visual impairment | ||||||
| ↓ERK activation | ||||||
| Lutein | DR | Diabetic mice | 0.2 mg/(kg · d) | 10 d | ↓Plasma/retina MDA | (68) |
| ↓NFκB activity | ||||||
| ↑GSH/GPx activity | ||||||
| Zeaxanthin | DR | Diabetic rats | 0.02% and 0.1% | 8 wk | ↓NFκB activity | (42) |
| ↑GSH/GPx activity | ||||||
| ↓VEGF | ||||||
| ↓ICAM-1 | ||||||
| ↑Mitochondrial SOD | ||||||
| ↓Lipid peroxidation | ||||||
| Lycopene | DN | Diabetic rats | 20 mg/(kg · d) | 8 wk | ↓Blood urea nitrogen | (78) |
| ↓24-h urea protein | ||||||
| ↓24-h creatinine | ||||||
| ↑AKT/PKB phosphorylation | ||||||
| ↓MDA and CTFG expression | ||||||
| ↑SOD activity | ||||||
| Lycopene | DN | Diabetic mice | 40 and 80 mg/(kg · d) | 8 wk | ↓NFκB/TNF-α | (77) |
| ↓LDL-C, | ||||||
| ↑HDL-C | ||||||
| ↓Blood glucose | ||||||
| ↑SOD | ||||||
| ↑GPx | ||||||
| ↑MDA | ||||||
| Lycopene | DPN | Diabetic mice | 1.2 and 4 mg/kg | 4 wk | ↓TNF-α/NO release | (26) |
| ↓Thermal hyperalgesia | ||||||
| Astaxanthin | DN | Diabetic db/db mice | 0.02% | 12 wk | ↓Blood glucose | (14) |
| ↓Urinary albumin | ||||||
| ↓Renal/mesangial 8-OHdG | ||||||
| ↓Relative mesangial | ||||||
| Astaxanthin | DN | Diabetic rats | — | 21 d | ↑Antioxidant enzymes activity (catalase, SOD) | (15) |
| ↓Plasma/kidney MDA | ||||||
| ↓Plasma/kidney PCO | ||||||
| Astaxanthin | DN | Epithelial cells | 5 and 10 μg/mL | — | ↓Lipid peroxidation | (16) |
| ↓Total reactive species | ||||||
| ↓iNOS/COX-2 protein concentrations | ||||||
| ↓NF-κB nuclear translocation | ||||||
| ↓Apoptosis | ||||||
| Astaxanthin | DR | Diabetic db/db mice | 25 and 50 mg/(kg · d) | 8 wk | ↓Apoptosis of RGCs | (19) |
| ↓MDA, | ||||||
| ↓8-OHdG | ||||||
| ↑MnSOD | ||||||
| ↓H2O2-induced apoptosis in RGC-5 |
AKT/PKB, protein kinase B; COX-2, cyclooxygenase 2; CTFG, connective tissue growth factor; DN, diabetic nephropathy; DPN, diabetic peripheral neuropathy; DR, diabetic retinopathy; ERK, extracellular signal-regulated kinase; GSH/GPx, glutathione/glutathione peroxidase; ICAM-1, intercellular adhesion molecule 1; iNOS, inducible NO synthase; MDA, malondialdehyde; MnSOD, manganese superoxide dismutase; PCO, protein carbonyl; RGC, retinal ganglion cell; ROS, reactive oxygen species; SOD, superoxide dismutase; VEGF: vascular endothelial growth factor; 8-OHdG, 8-hydroxy-2-deoxyguanosine; ↑, increase; ↓, decrease.
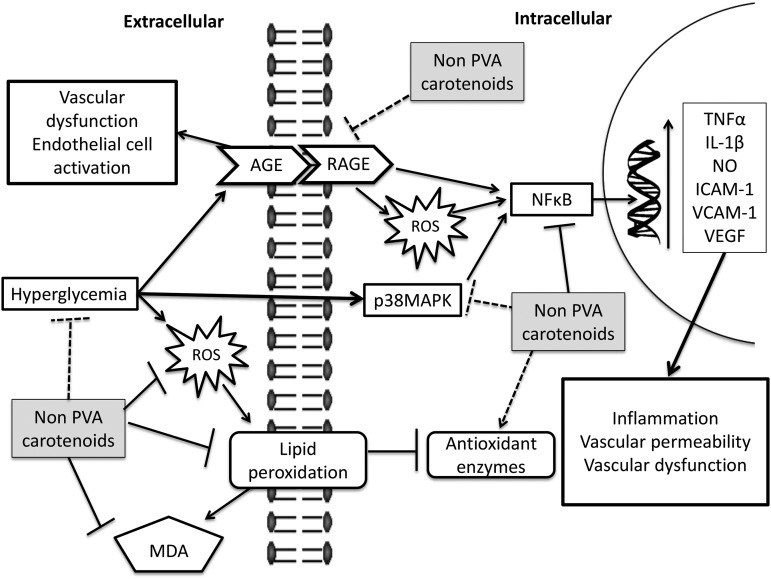
FIGURE 2.
Proposed mechanisms by which non-provitamin A carotenoids may suppress glucose-induced damage and prevent microvascular complications in diabetes derived from cell and animal studies. Detrimental effects of hyperglycemia lead to microvascular complications in diabetes. High blood sugar causes oxidative stress, inflammation, and an uncontrolled production of advanced glycation products, all of which create a proinflammatory loop and cause the vascular permeability and dysfunction commonly seen in diabetic patients with retinopathy, nephropathy, or neuropathy. The carotenoids lutein, lycopene, zeaxanthin, and astaxanthin may ameliorate this glucose-induced damage by quenching free radicals, scavenging reactive oxygen species, blocking inflammatory pathways, AGE-RAGE engagement/signaling, and even decreasing plasma glucose concentrations. Arrows indicate a positive interaction, whereas bar-headed lines denote a blockage of a pathway or a decrease of a compound. Note that the mechanisms that are more strongly supported by the published evidence are shown with a dark arrow, whereas the ones shown with dotted lines represent weaker evidence. AGE, advanced glycation end product; ICAM, intercellular adhesion molecule 1; MDA, malondialdehyde; PVA, provitamin A; RAGE: receptor for advanced glycation end product; ROS, reactive oxygen species; VCAM-1, vascular adhesion protein 1; VEGF, vascular endothelial growth factor.
Conclusions
Given their capacity for modulating gene transcription and the antioxidant and anti-inflammatory properties shown in vitro, non-provitamin A carotenoids such as lutein, zeaxanthin, lycopene, and astaxanthin have been studied for the reduction of glucose-induced damage in the microvascular system of diabetic animal models and have shown some positive results. This suggests that these compounds or their metabolites may also be useful in humans by quenching free radicals, decreasing AGE formation, and reducing oxidative stress and inflammation caused by hyperglycemia.
Nevertheless, the physiological effect of these carotenoids in diabetic patients is yet to be determined. The right dose for observing positive outcomes with microvascular complications should be addressed with adequate human trials that evaluate efficacy and safety considering bioavailability, individual response, metabolism, tissue delivery, and possible toxicity. The available evidence indicates that the concentrations of plasma carotenoids and other antioxidants are good predictors of the incidence of microvascular complications such as DR, DN, and DPN; however, the effects of these individual carotenoids on the treatment or direct prevention of these complications are not yet known. Therefore, an adequate intake of dietary non-provitamin A carotenoids could be beneficial as part of a healthy diet for treating diabetes-related complications. However, this treatment should be combined with proper glycemic control and the correct use of medication. Furthermore, supplementation has not been assessed adequately enough in human trials to recommend it to diabetic patients.
Acknowledgments
Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: AGE, advanced glycation end product; DM, diabetes mellitus; DN, diabetic nephropathy; DPN, diabetic peripheral neuropathy; DR, diabetic retinopathy; GPx, glutathione peroxidase; MDA, malondialdehyde; NDR, nonproliferative diabetic retinopathy; PTEC, proximal tubular epithelial cell; RAGE, receptor for advanced glycation end product; RGC, retinal ganglion cell; ROS, reactive oxygen species; SOD, superoxide dismutase; STZ, streptozotocin; T2DM, type II diabetes mellitus; VEGF, vascular endothelial growth factor.
References
- 1.Maritim AC, Sanders RA, Watkins JB III. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 2003;17:24–38. [DOI] [PubMed] [Google Scholar]
- 2.Dabla PK. Renal function in diabetic nephropathy. World J Diabetes 2010;1:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother 2005;59:365–73. [DOI] [PubMed] [Google Scholar]
- 4.Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J 2012;27:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang W, Dall TM, Halder P, Gallo P, Kowal SL, Hogan PF, Petersen M. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 2008;26:77–82. [Google Scholar]
- 7.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol 2005;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chistiakov DA. Diabetic retinopathy: pathogenic mechanisms and current treatments. Diabetes Metab Syndr 2012;5:165–72. [DOI] [PubMed] [Google Scholar]
- 9.Calcutt NA, Cooper ME, Kern TS, Schmidt AM. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov 2009;8:417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stahl W, Sies H. Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta 2005;1740:101–7. [DOI] [PubMed] [Google Scholar]
- 11.Naguib YM. Antioxidant activities of astaxanthin and related carotenoids. J Agric Food Chem 2000;48:1150–4. [DOI] [PubMed] [Google Scholar]
- 12.Ciccone MM, Cortese F, Gesualdo M, Carbonara S, Zito A, Ricci G, De Pascalis F, Scicchitano P, Riccioni G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediators Inflamm 2013;2013:782137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naito Y, Uchiyama K, Aoi W, Hasegawa G, Nakamura N, Yoshida N, Maoka T, Takahashi J, Yoshikawa T. Prevention of diabetic nephropathy by treatment with astaxanthin in diabetic db/db mice. Biofactors 2004;20:49–59. [DOI] [PubMed] [Google Scholar]
- 14.Allen DA, Harwood S, Varagunam M, Raftery MJ, Yaqoob MM. High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J 2003;17:908–10. [DOI] [PubMed] [Google Scholar]
- 15.Kim YJ, Kim YA, Yokozawa T. Protection against oxidative stress, inflammation, and apoptosis of high-glucose-exposed proximal tubular epithelial cells by astaxanthin. J Agric Food Chem 2009;57:8793–7. [DOI] [PubMed] [Google Scholar]
- 16.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res 2011;30:343–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol 2013;2013:343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong LY, Jin J, Lu G, Kang XL. Astaxanthin attenuates the apoptosis of retinal ganglion cells in db/db mice by inhibition of oxidative stress. Mar Drugs 2013;11:960–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen TT, Wong TY. Retinal vascular manifestations of metabolic disorders. Trends Endocrinol Metab 2006;17:262–8. [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care 2007;30(Suppl 1):S4–41. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care 2015;38(Suppl 1):S5–89. [PubMed] [Google Scholar]
- 22.American Diabetes Association. Microvascular complications and foot care. Diabetes Care 2015;38(Suppl 1):S58–66. [DOI] [PubMed] [Google Scholar]
- 23.Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev 2012;28:8–14. [DOI] [PubMed] [Google Scholar]
- 24.Boulton JM. Management of diabetic peripheral neuropathy. Clin Diabetes 2005;23:9–15. [Google Scholar]
- 25.Kuhad A, Sharma S, Chopra K. Lycopene attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pain 2008;12:624–32. [DOI] [PubMed] [Google Scholar]
- 26.Pop-Busui R, Lu J, Lopes N, Jones TLZ. Prevalence of diabetic peripheral neuropathy and relation to glycemic control therapies at baseline in the BARI 2D cohort. J Peripher Nerv Syst 2009;14:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams MD, Nadler JL. Inflammatory mechanisms of diabetic complications. Curr Diab Rep 2007;7:242–8. [DOI] [PubMed] [Google Scholar]
- 28.Patel S, Santani D. Role of NF- k B in the pathogenesis of diabetes and its associated complications. Pharmacol Rep 2009;61:595–603. [DOI] [PubMed] [Google Scholar]
- 29.Romeo G, Liu W, Asnaghi V, Kern TS, Lorenzi M. Activation of nuclear factor-kappa B induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes 2002;51:2241–8. [DOI] [PubMed] [Google Scholar]
- 30.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 2006;114:597–605. [DOI] [PubMed] [Google Scholar]
- 31.Stitt AW. AGEs and diabetic retinopathy. Invest Ophthalmol Vis Sci 2010;51:4867–74. [DOI] [PubMed] [Google Scholar]
- 32.Kiho T, Usui S, Hirano K, Aizawa K, Inakuma T. Tomato paste fraction inhibiting the formation of advanced glycation end-products. Biosci Biotechnol Biochem 2004;68:200–5. [DOI] [PubMed] [Google Scholar]
- 33.Ramasamy R, Vannucci SJ, Du Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 2005;15:16R–28R. [DOI] [PubMed] [Google Scholar]
- 34.Ola MS, Nawaz MI, Siddiquei MM, Al-Amro S, Abu El-Asrar AM. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J Diabetes Complications 2012;26:56–64. [DOI] [PubMed] [Google Scholar]
- 35.Rahbar S, Figarola JL. Novel inhibitors of advanced glycation endproducts. Arch Biochem Biophys 2003;419:63–79. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt A, Nöller J, Schmitt J. The binding of advanced glycation end products to cell surfaces can be measured using bead-reconstituted cellular membrane proteins. Biochim Biophys Acta 2007;1768:1389–99. [DOI] [PubMed] [Google Scholar]
- 37.Wautier MP, Khodabandehlou T, Le C. Modulation of RAGE expression influences the adhesion of red blood cells from diabetic patients. Clin Hemorheol Microcirc 2006;35:379–86. [PubMed] [Google Scholar]
- 38.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med 1995;46:223–34. [DOI] [PubMed] [Google Scholar]
- 39.Coulthard LR, White DE, Jones DL, McDermott MF. Burchill S a. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol Med 2009;15:369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du Y, Tang J, Li G, Li G, Berti-Mattera L, Lee CA, Bartkowski D, Gale D, Monahan J, Niesman MR, et al. . Effects of p38 MAPK inhibition on early stages of diabetic retinopathy and sensory nerve function. Invest Ophthalmol Vis Sci 2010;51:2158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kowluru RA, Menon B, Gierhart DL. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Invest Ophthalmol Vis Sci 2008;49:1645–51. [DOI] [PubMed] [Google Scholar]
- 42.Etzioni A, Frydman M, Pollack S, Avidor I, Phillips ML, Paulson JC, Gershoni-Baruch R. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin- dependent diabetes mellitus. N Engl J Med 1992;327:1789–92. [DOI] [PubMed] [Google Scholar]
- 43.Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014;6:466–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sila A, Ghlissi Z, Kamoun Z, Makni M, Nasri M, Bougatef A, Sahnoun Z. Astaxanthin from shrimp by-products ameliorates nephropathy in diabetic rats. Eur J Nutr 2015;54:301–7. [DOI] [PubMed] [Google Scholar]
- 45.Johnson EJ. The role of carotenoids in human health. Nutr Clin Care 2002;5:56–65. [DOI] [PubMed] [Google Scholar]
- 46.Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res 2007;55:207–16. [DOI] [PubMed] [Google Scholar]
- 47.Latowski D, Kuczyńska P, Strzałka K. Xanthophyll cycle—a mechanism protecting plants against oxidative stress. Redox Rep 2011;16:78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stahl W, Sies H. Antioxidant activity of carotenoids. Mol Aspects Med 2003;24:345–51. [DOI] [PubMed] [Google Scholar]
- 49.Maiani G, Castón MJP, Catasta G, Toti E, Cambrodón IG, Bysted A, Granado-Lorencio F, Olmedilla-Alonso B, Knuthsen P, Valoti M, et al. . Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res 2009;53 (Suppl 2):S194–218. [DOI] [PubMed] [Google Scholar]
- 50.Tang G. Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am J Clin Nutr 2010;91:1468S–73S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brazionis L, Rowley K, Itsiopoulos C, O’Dea K. Plasma carotenoids and diabetic retinopathy. Br J Nutr 2009;101:270–7. [DOI] [PubMed] [Google Scholar]
- 52.Rissanen TH, Voutilainen S, Salonen R, Kaplan GA, Salonen JT. Serum lycopene concentrations and carotid atherosclerosis: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 2003;77:133–38. [DOI] [PubMed] [Google Scholar]
- 53.Moeller SM, Voland R, Tinker L, Blodi BA, Klein L, Gehrs KM, Johnson EJ, Snodderly DM, Wallace B, Chappell RJ, et al. . Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in Age-Related Eye Disease Study (CAREDS), an ancillary study of the Women’s Health Initiative. Arch Ophthalmol 2008;126:354–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy MM, Barraj LM, Herman D, Bi X, Cheatham R, Randolph RK. Phytonutrient intake by adults in the United States in relation to fruit and vegetable consumption. J Acad Nutr Diet 2012;112:222–9. [DOI] [PubMed] [Google Scholar]
- 55.Sluijs I, Cadier E, Beulens JWJ, van der A DL, Spijkerman AMW, van der Schouw YT. Dietary intake of carotenoids and risk of type 2 diabetes. Nutr Metab Cardiovasc Dis 2015;25:376–81. [DOI] [PubMed] [Google Scholar]
- 56.Perry A, Rasmussen H, Johnson EJ. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J Food Compost Anal 2009;22:9–15. [Google Scholar]
- 57.Kotake-Nara E, Nagao A. Absorption and metabolism of xanthophylls. Mar Drugs 2011;9:1024–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lemmens L, Colle I, Van Buggenhout S, Palmero P, Van Loey A, Hendrickx M. Carotenoid bioaccessibility in fruit- and vegetable-based food products as affected by product (micro)structural characteristics and the presence of lipids: A review. Trends Food Sci Technol 2014;38:125–35. [Google Scholar]
- 59.Alves-Rodrigues A, Shao A. The science behind lutein. Toxicol Lett 2004;150:57–83. [DOI] [PubMed] [Google Scholar]
- 60.Yonekura L, Nagao A. Intestinal absorption of dietary carotenoids. Mol Nutr Food Res 2007;51:107–15. [DOI] [PubMed] [Google Scholar]
- 61.Castenmiller JJ, West CE. Bioavailability and bioconversion of carotenoids. Annu Rev Nutr 1998;18:19–38. [DOI] [PubMed] [Google Scholar]
- 62.Odeberg J, Lignell Å, Pettersson A, Höglund P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur J Pharm Sci 2003;19:299–304. [DOI] [PubMed] [Google Scholar]
- 63.Kawada T, Kamei Y. Fujita a, Hida Y, Takahashi N, Sugimoto E, Fushiki T. Carotenoids and retinoids as suppressors on adipocyte differentiation via nuclear receptors. Biofactors 2000;13:103–9. [DOI] [PubMed] [Google Scholar]
- 64.Stahl W, Ale-Agha N, Polidori MC. Non-antioxidant properties of carotenoids. Biol Chem 2002;383:553–8. [DOI] [PubMed] [Google Scholar]
- 65.Kaulmann A, Bohn T. Carotenoids, inflammation, and oxidative stress—implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res 2014;34:907–29. [DOI] [PubMed] [Google Scholar]
- 66.Oku H, Kodama T, Sakagami K, Puro DG. Diabetes-induced disruption of gap junction pathways within the retinal microvasculature. Invest Ophthalmol Vis Sci 2001;42:1915–20. [PubMed] [Google Scholar]
- 67.Gandhi GK, Ball KK, Cruz NF, Dienel GA. Hyperglycaemia and diabetes impair gap junctional communication among astrocytes. ASN Neuro 2010;2:e00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jewell C, Brien NMO. Effect of dietary supplementation with carotenoids on xenobiotic metabolizing enzymes in the liver, lung, kidney and small intestine of the rat. Br J Nutr 1999;81:235–42. [PubMed] [Google Scholar]
- 69.Banerjee M, Vats P. Reactive metabolites and antioxidant gene polymorphisms in type 2 diabetes mellitus. Redox Biol 2013;2C:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dadbinpour A, Sheikhha MH, Darbouy M, Afkhami-Ardekani M. Investigating GSTT1 and GSTM1 null genotype as the risk factor of diabetes type 2 retinopathy. J Diabetes Metab Disord 2013;12:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonet ML, Canas JA, Ribot J, Palou A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch Biochem Biophys 2015;572:112–25. [DOI] [PubMed] [Google Scholar]
- 72.Roberts RL, Green J, Lewis B. Lutein and zeaxanthin in eye and skin health. Clin Dermatol. 2009;27:195–201. [DOI] [PubMed] [Google Scholar]
- 73.Kijlstra A, Tian Y, Kelly ER, Berendschot TTJM. Lutein: more than just a filter for blue light. Prog Retin Eye Res 2012;31:303–15. [DOI] [PubMed] [Google Scholar]
- 74.Loane E, Nolan JM, O’Donovan O, Bhosale P, Bernstein PS, Beatty S. Transport and retinal capture of lutein and zeaxanthin with reference to age-related macular degeneration. Surv Ophthalmol 2008;53:68–81. [DOI] [PubMed] [Google Scholar]
- 75.Mozaffarieh M, Sacu S, Wedrich A. The role of the carotenoids, lutein and zeaxanthin, in protecting against age-related macular degeneration: a review based on controversial evidence. Nutr J 2003;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson EJ, Maras JE, Rasmussen HM, Tucker KL. Intake of leutein and zeaxathin differ with age, sex, and ethnicity. J Am Diet Assoc 2010;110:1357–62. [DOI] [PubMed] [Google Scholar]
- 77.Neuringer M, Sandstrom MM, Johnson EJ, Snodderly DM. Nutritional manipulation of primate retinas, I: effects of lutein or zeaxanthin supplements on serum and macular pigment in xanthophyll-free rhesus monkeys. Invest Ophthalmol Vis Sci 2004;45:3234–43. [DOI] [PubMed] [Google Scholar]
- 78.Kalariya NM, Ramana KV. Vankuijk FJGM. Focus on molecules: lutein. Exp Eye Res 2012;102:107–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berson EL, Rosner B, Sandberg MA, Weigel C, Brockhurst RJ, Hayes KC, Johnson EJ, Anderson EJ, Johnson CA, Gaudio AR, et al. . Clinical trial of lutein in patients with retinitis pigmentosa receiving vitamin A. Arch Ophthalmol 2010;128:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim EK, Choi E-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 2010;1802:396–405. [DOI] [PubMed] [Google Scholar]
- 81.Chew BP, Park JS. Functions and actions of retinoids and carotenoids: building on the vision on James Allen Olson. Proceedings of a symposium to honor the memory of James Allen Olson. June 21–24, 2001, Ames, Iowa, USA. J Nutr 2004;134:220S–93S. [DOI] [PubMed] [Google Scholar]
- 82.Muriach M, Bosch-Morell F, Alexander G, Blomhoff R, Barcia J, Arnal E, Almansa I, Romero FJ, Miranda M. Lutein effect on retina and hippocampus of diabetic mice. Free Radic Biol Med 2006;41:979–84. [DOI] [PubMed] [Google Scholar]
- 83.Kim JE, Clark RM, Park Y, Lee J, Fernandez ML. Lutein decreases oxidative stress and inflammation in liver and eyes of guinea pigs fed a hypercholesterolemic diet. Nutr Res Pract 2012;6:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008;51:216–26. [DOI] [PubMed] [Google Scholar]
- 85.Sasaki M, Ozawa Y, Kurihara T, Kubota S, Yuki K, Noda K, Kobayashi S, Ishida S, Tsubota K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 2010;53:971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu B-J, Hu Y-N, Lin S, Ma W-J, Li X-R. Application of lutein and zeaxanthin in nonproliferative diabetic retinopathy. Int J Ophthalmol 2011;4:303–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalariya NM, Ramana KV, Srivastava SK, van Kuijk FJ. Carotenoid derived aldehydes-induced oxidative stress causes apoptotic cell death in human retinal pigment epithelial cells. Exp Eye Res 2008;29:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seddon J, Ajani U, Sperduto R, Hiller R, Blair N, Burton T, Farber M. Dietary carotenoids, vitamins A, C and E, and advanced age-related macular degeneration. JAMA 1994;35:21–41. [PubMed] [Google Scholar]
- 89.Tanumihardjo SA, Li J, Dosti MP. Lutein absorption is facilitated with cosupplementation of ascorbic acid in young adults. J Am Diet Assoc 2005;105:114–8. [DOI] [PubMed] [Google Scholar]
- 90.Rissanen T, Voutilainen S, Nyyssönen K, Salonen JT. Lycopene, atherosclerosis, and coronary heart rt disease. Exp Biol Med (Maywood) 2002;227:900–7. [DOI] [PubMed] [Google Scholar]
- 91.Giovannucci E. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst 2002;94:391–8. [DOI] [PubMed] [Google Scholar]
- 92.Rao AV, Agarwal S. Role of antioxidant lycopene in cancer and heart disease. J Am Coll Nutr 2000;19:563–9. [DOI] [PubMed] [Google Scholar]
- 93.Pierine DT, Navarro MEL, Minatel IO, Luvizotto RAM, Nascimento AF, Ferreira ALA, Yeum K-J, Corrêa CR. Lycopene supplementation reduces TNF-α via RAGE in the kidney of obese rats. Nutr Diabetes 2014;4:e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bae JW, Bae J-S. Barrier protective effects of lycopene in human endothelial cells. Inflamm Res 2011;60:751–8. [DOI] [PubMed] [Google Scholar]
- 95.Oborna I, Malickova K, Fingerova H, Brezinova J, Horka P, Novotny J, Bryndova H, Filipcikova R, Svobodova M. A randomized controlled trial of lycopene treatment on soluble receptor for advanced glycation end products in seminal and blood plasma of normospermic men. Am J Reprod Immunol 2011;66:179–84. [DOI] [PubMed] [Google Scholar]
- 96.Guo Y, Liu Y, Wang Y. Beneficial effect of lycopene on anti-diabetic nephropathy through diminishing inflammatory response and oxidative stress. Food Funct 2015;6:1150–6. [DOI] [PubMed] [Google Scholar]
- 97.Toyama T, Shimizu M, Furuichi K, Kaneko S, Wada T. Treatment and impact of dyslipidemia in diabetic nephropathy. Clin Exp Nephrol 2014;18:201–5. [DOI] [PubMed] [Google Scholar]
- 98.Li W, Wang G, Lu X, Jiang Y, Xu L, Zhao X. Lycopene ameliorates renal function in rats with streptozotocin-induced diabetes. Int J Clin Exp Pathol 2014;7:5008–15. [PMC free article] [PubMed] [Google Scholar]
- 99.Rao V, Agarwal S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: a review. Nutr Res 1999;19:305–23. [Google Scholar]
- 100.Erdman JW, Ford NA, Lindshield BL. Are the health attributes of lycopene related to its antioxidant function? Arch Biochem Biophys 2009;483:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lindshield BL, Canene-Adams K, Erdman JW. Lycopenoids: are lycopene metabolites bioactive? Arch Biochem Biophys 2007;458:136–40. [DOI] [PubMed] [Google Scholar]
- 102.Shi J, Dai Y, Kakuda Y, Mittal G, Xue SJ. Effect of heating and exposure to light on the stability of lycopene in tomato puree. Food Control 2008;19:514–20. [Google Scholar]
- 103.Xianquan S, Shi J, Kakuda Y, Yueming J. Review stability of lycopene during food processing and storage. J Med Food 2005;8:413–22. [DOI] [PubMed] [Google Scholar]
- 104.Rao AV. Lycopene, tomatoes, and the prevention of coronary heart disease. Exp Biol Med (Maywood) 2002;227:908–13. [DOI] [PubMed] [Google Scholar]
- 105.Park CH, Xu FH, Roh S-S, Song YO, Uebaba K, Noh JS, Yokozawa T. Astaxanthin and corni fructus protect against diabetes-induced oxidative Stress, inflammation, and advanced glycation end product in livers of streptozotocin-induced diabetic rats. J Med Food 2015;18:337–44. [DOI] [PubMed] [Google Scholar]
- 106.Nakajima Y, Inokuchi Y, Shimazawa M, Otsubo K, Ishibashi T, Hara H. Astaxanthin, a dietary carotenoid, protects retinal cells against oxidative stress in-vitro and in mice in-vivo. J Pharm Pharmacol 2008;60:1365–74. [DOI] [PubMed] [Google Scholar]
- 107.Kajimoto Y, Kaneto H. Role of oxidative stress in pancreatic β-cell dysfunction. Ann N Y Acad Sci 2004;1011:168–76. [DOI] [PubMed] [Google Scholar]
- 108.Kidd P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern Med Rev 2011;16:355–64. [PubMed] [Google Scholar]