Supplemental Digital Content is available in the text
Abstract
Biological and epidemiological evidence have found that gestational diabetes mellitus (GDM) may be correlated with body iron status and dietary iron intake. Therefore, we investigated the relationship between dietary iron intake and body iron status and GDM risk.
We conducted a systematic search in Embase, PubMed, Web of Science, and Cochrane Library up to April 2015. Prospective cohort studies or case-control studies which appraised the relationship between body iron status, dietary iron intake, and GDM risk were included. Relative risks (RRs), standard mean difference (SMD), and 95% confidence intervals [CIs] were used to measure the pooled data.
A total of 8 prospective cohort studies and 7 case-control studies were in accordance with inclusive criteria, and 14 studies were included in meta-analysis. The overall RR comparing the highest and lowest levels of serum ferritin was 3.22 (95% CI: 1.73–6.00) for prospective cohort studies. Serum ferritin of GDM group is markedly higher than that of control (0.88 ng/mL; 95% CI: 0.40–1.35 ng/mL) for case-control studies. The comparison between the highest and the lowest serum ferritin levels and dietary total iron levels revealed pooled RRs of 1.53 (95% CI: 1.17–2.00) and 1.01 (95% CI: 1.00–1.01) for prospective cohort studies, respectively. The combined SMD comparing serum transferrin levels of cases and controls was −0.02 μmol/L (95% CI: −0.22 to 0.19 μmol/L) for case-control studies.
Increased higher ferritin levels were significantly correlated with higher risk of GDM, and higher heme iron levels may be correlated with higher risk of GDM; however, the present conclusion did not constitute definitive proof that dietary total iron or serum transferrin have relation to GDM.
INTRODUCTION
For humans, iron is an essential microelement. As a cofactor for several enzymes and a major component of oxygen transporter in the body, iron also has important metabolic functions; however, it is controversial that prophylactic iron supplement during pregnancy. On the one hand, during pregnancy, there is an increased requirement for iron, and body's iron store is often inadequate to satisfy the demands.1 It was showed that iron-deficiency anemia correlated with an increased risk of neonatal morbidity such as preterm delivery.2 On the other hand, it was considered that a high maternal hemoglobin level from iron supplement would reduce placental perfusion because of increased blood viscosity and cause adverse pregnancy outcomes such as low birthweight, stillbirths and preeclampsia.3
The etiology of gestational diabetes mellitus (GDM) is multifactorial, and has not completely been established yet. There are several known risk factors exist, such as age, race, and family history of diabetes mellitus. People have recognized that there is a connection between excess iron and GDM risk. Serum iron level is positively associated with the risk of GDM based on one case control study,4 while earlier research has found the opposite conclusion.5 One recent study indicated that elevated ferritin concentrations in mid-pregnancy are correlated with a greater risk of GDM independent of body mass index and C-reactive protein (CRP), whereas ferritin levels were not associated with oral glucose tolerance test results in early postpartum.6 However, mixed findings were reported for other populations.7,8 A study of Turkey indicated that there is no association between high ferritin, hemoglobin concentrations, and development of GDM.9
As these studies produced inconsistent findings on the relationship between body iron status, dietary total iron, and GDM risk, our aim is to summarize the available proof for correlation of body iron status, dietary total iron, and GDM risk in prospective cohort studies and case-control studies systematically in a meta-analysis.
METHODS
Literature Search Strategy
Two investigators (SF and FL) independently searched Embase, PubMed, Cochrane Library, and Web of Science for English-language prospective cohort studies or case-control studies that estimated relationship between body iron status, dietary iron intake, and GDM risk up to April 2015 using search queries “ferritin” or “iron” or “transferritin” or “sTfR” and “gestational diabetes” or “gestational diabetes mellitus” or “pregnancy diabetes” or “GDM”. We read the titles and abstracts of the retrieved records for eligibility to eliminate studies those were clearly irrelevant. Reference lists of retrieved articles were also hand-screened for relevant studies and review articles. We read full texts of all remaining articles to decide eligible studies.
Eligibility and Exclusion Criteria
Study was included if it meet the following criteria: investigated the association between ferritin, transferrin, heme iron, dietary total iron, and GDM in human subjects; had a prospective cohort or case-control design; relative risks (RRs) or standard mean difference (SMD) had to be reported in the article in accordance with the levels of respective iron parameter, be computed from reported data or got from authors.
Article would be excluded if it meet the following criteria: review articles; cases or the population of whole study suffered other diseases which may restrict generalizability of study findings due to a correlation with the surveyed iron indices or GDM. All articles were assessed by using the inclusion criterion described here and any disagreement regarding eligibility of an article was discussed and agreement reached by consensus with a third reviewer.
Data Extraction and Quality Assessment
The extraction of data and evaluation of trials quality were assessed in duplicate by 2 reviewers independently (SF and FL). We collected the following content: first author, year of publication, country, mean age, number of GDM patients and controls, reported iron indices, effect estimate and corresponding 95% confidence interval (CI), and adjustments or matched variables.
The qualities of included studies were evaluated according to the Newcastle-Ottawa Scale (NOS) (9-star) by using predefined criteria population representativeness, comparability (adjustment/matched variables), exposure and outcome factors, and follow-up.10 The level of evidence was assessed by using the GRADE system11–14 (GRADE version 3.6).
Statistical Analysis
We pooled the RRs and SMD with corresponding 95% CIs by using the random-effects model, when significant between-study heterogeneity exited. Alternatively, an inverse-variance fixed-effect model was used, when there was no significant heterogeneity across studies.15 Publication bias was assessed by using Begg test and Egger test (P < 0.05 was considered statistically significant for publication bias).
One study25 did not provide valuable data, and we did not get data from author, thus it was excluded from the meta-analysis. For prospective cohort studies, as no study in detail reported a multivariable model-adjusted ferritin-GDM RR, as long as ferritin-GDM RR was adjusted for age, it would be included, and we computed ferritin-GDM RR according to raw data provided. For heme iron and dietary total iron, several eligible studies reported a multivariable model-adjusted RR estimates of GDM for the comparison of the highest with the lowest heme iron and dietary total iron levels, thus we merged RR estimates from a multivariable model which adjusted for as many traditional covariates as possible (such as age, body mass index [BMI], and so on). For case-control studies, because all data collected were continuous, indicators were analyzed using the mean and SD. Heterogeneity between studies was assessed using Q test and I2 statistic.
All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
RESULTS
Literature Selection and Risk of Bias Assessment
We identified 220 potentially citations from PubMed, EMBASE, Cochrane Library, and Web of Science databases, screened titles and abstracts, and then detailed evaluated full texts. Finally, 8 prospective cohort studies16–23 and 7 case-control studies4–6,9,24–26 were accorded with our inclusion criteria (Figure 1). The median bias risk score of prospective cohort studies and case-control studies was 8.5 (range: 5–9) and 7.5 (range: 6–9), respectively. Study-specific results of the bias risk assessment are supplied in the supplementary material (Supplementary Table S1). This systemic review and meta-analysis was conducted following the PRISMA guidelines.
FIGURE 1.
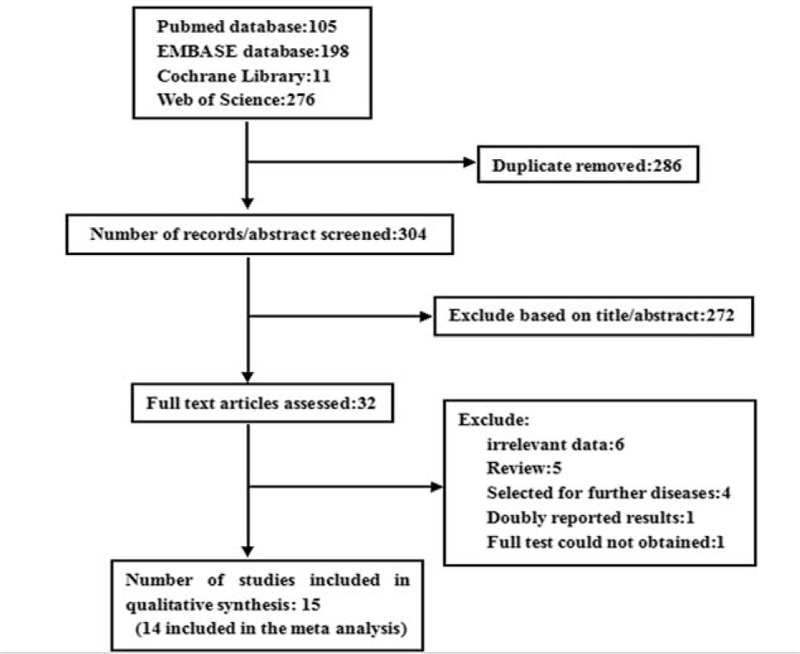
The literatures selection process of this meta-analysis.
Characteristics of Studies
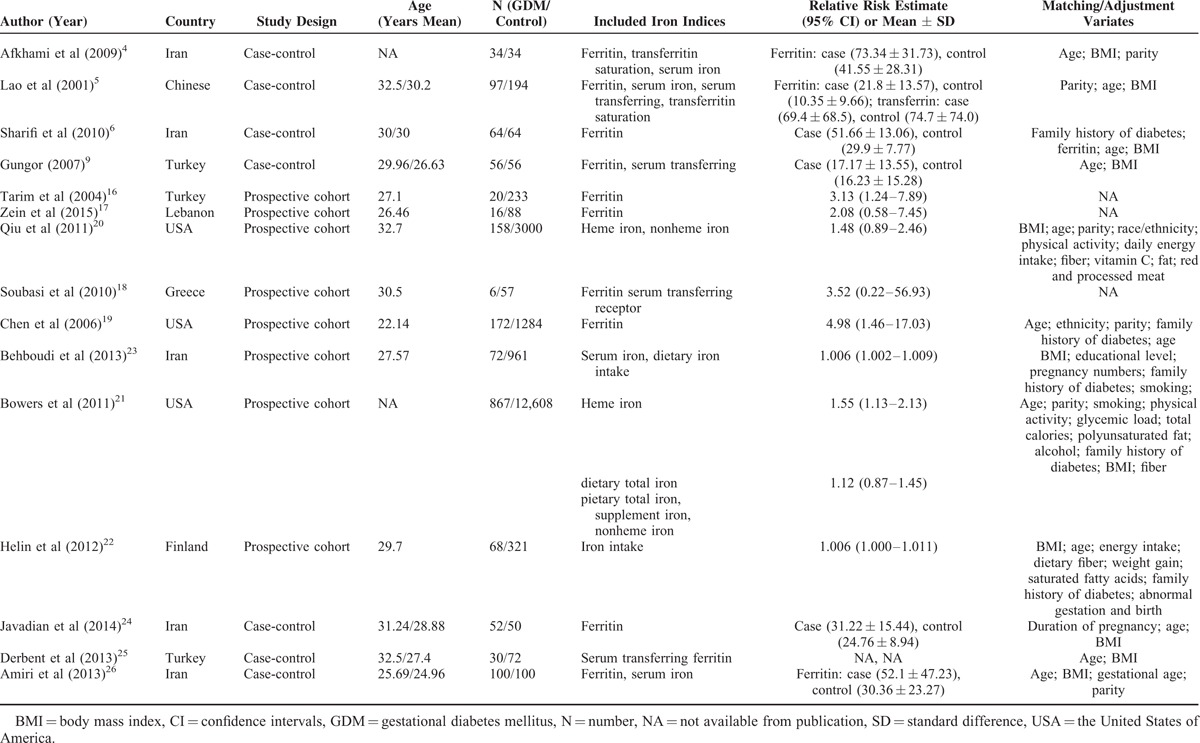
Four prospective cohort studies assessed ferritin levels (214 GDM patients and 1662 controls) and risk of GDM16,17,18,19; 6 case-control studies (403 GDM patients and 498 controls) compared ferritin levels of GDM women and non-GDM groups4–6,9,24,26; and 2 studies (153 GDM patients and 250 controls) compared serum transferrin levels of GDM women and non-GDM groups.5,9 Two studies assessed heme iron (1025 GDM patients and 15,608 controls) and risk of GDM,20,21 and 3 studies assessed dietary total iron (1007 GDM patients and 13,890 controls) and risk of GDM.21,22,23 Among 4 ferritin prospective cohort studies, 2 studies were conducted in western countries (1 in Europe18 and 1 in North America)19 and 2 in Asia,16,17 whereas the results of 3 studies16,17,18 were not adjusted for covariates (such as age, BMI, and so on). All ferritin and serum transferrin case-control studies were conducted in Asia,4–6,9,25,26 and all studies adjusted or matched age and BMI. The 2 heme iron studies20,21 were conducted in western countries (1 in Europe20 and 1 in North America),21 and directly provided a multivariate-adjusted RR estimates of GDM for the comparison of the highest with the lowest heme iron levels, and also 3 dietary total iron studies21,22,23 directly provided a multivariable model adjusted RR estimates of GDM for the comparison of the highest with the lowest dietary total iron levels, 3 studies were conducted in North America,21 Europe,22 and Asia,23 respectively. Table 1 shows the detailed characteristics of eligible studies regarding the relationship between body iron status, dietary total iron, and the risk of GDM.
TABLE 1.
Characteristic of Studies (N = 15) Contracting Data to Current Analysis
Serum Ferritin and Risk of GDM
The pooled RR comparing the highest and lowest ferritin levels for 4 prospective cohort studies in analysis unadjusted was 3.22 (95% CI: 1.73–6.00) using a inverse-variance fixed-effect model (result of random-effects model: 3.22 [95% CI: 1.73–6.00]). There is a significant link between ferritin levels and risk of GDM. Although there is little support of heterogeneity (I2 = 0%, P = 0.815) across the 4 ferritin studies (Figure 2), there is no evidence of publication bias (Begg test: P = 1.0, Egger test: P = 0.953) (Supplementary Figure S2). For case-control studies, data pooled from 6 studies that compared GDM women with non-GDM groups showed a significant increase in ferritin in GDM women (0.88 ng/mL; 95% CI: 0.40–1.35 ng/mL) using a random-effects model (result of inverse-variance fixed-effect model: 0.83 ng/mL; 95% CI: 0.69–0.97 ng/mL) (Figure 3). There is high heterogeneity (I2 = 90.6%, P < 0.0001) among the 6 studies; however, no publication bias was observed (Begg test: P = 0.573, Egger test: P = 0.744) (Supplementary Figure S2).
FIGURE 2.
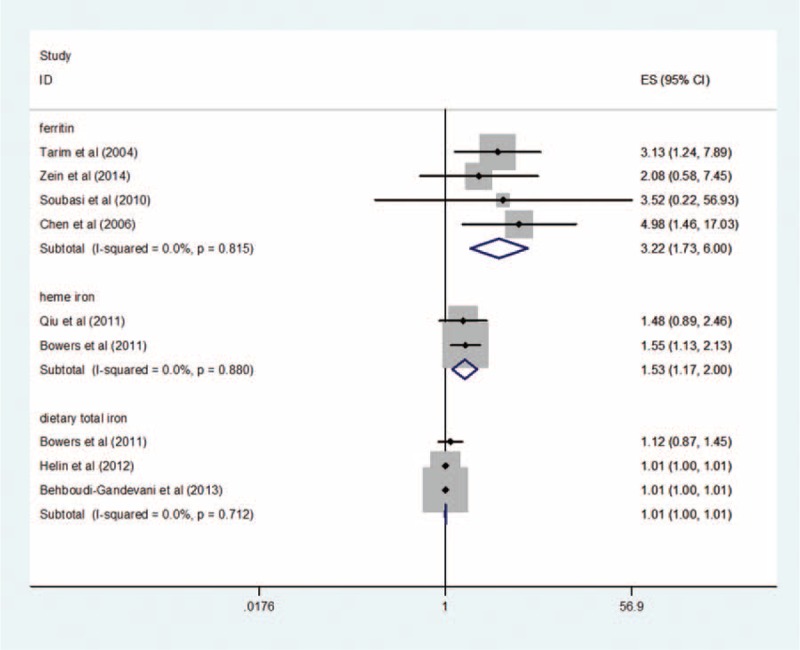
Forest plot of the relationship between ferritin, heme iron, dietary total iron levels, and GDM risk, comparing only the highest category with the lowest. The presented summary estimate was calculated using an inverse-variance fixed-effect model.
FIGURE 3.
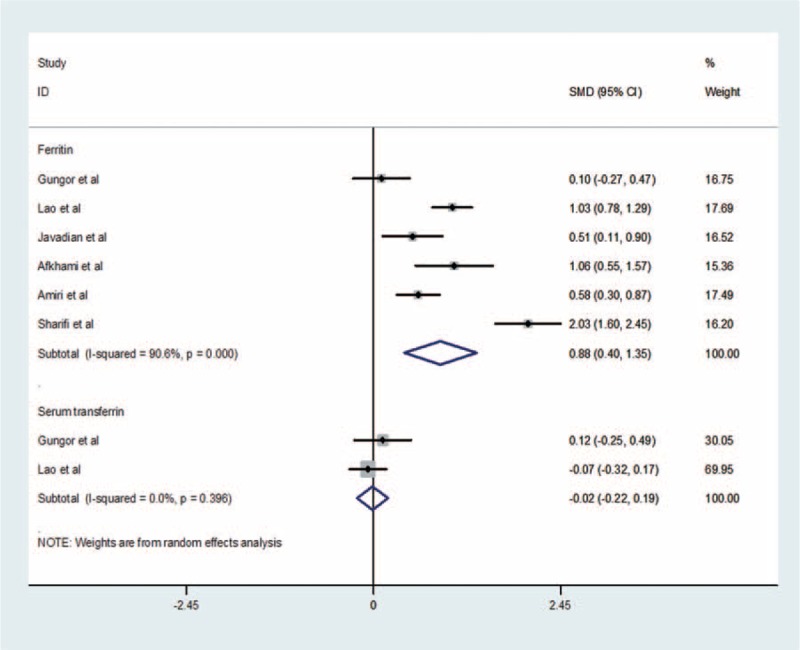
Forest plot of difference in ferritin and serum transferrin, comparing GDM women with non-GDM groups. The presented summary estimate was calculated using a random-effects model.
Heme Iron and Risk of GDM
The merged RR comparing the highest and lowest heme iron levels for 2 studies in multivariable adjusted analysis was 1.53 (95% CI: 1.17–2.00) using an inverse-variance fixed-effect model (result of random-effects model: 1.53 [95% CI: 1.17–2.00]). There was no proof of heterogeneity between 2 studies (I2 = 0%, P = 0.880) (Figure 2). It is not suitable to perform publication bias because of restrictions of literatures.
Dietary Total Iron and Risk of GDM
For the meta-analysis of studies using dietary total iron as the indicator, the combined multivariate-adjusted RR of GDM in the highest versus the lowest dietary total iron levels was 1.01 (95% CI: 1.00–1.01) (Figure 2) by using an inverse-variance fixed-effect model (result of random-effects model: 1.01 [95% CI: 1.00–1.01]). There was neither support of heterogeneity among these studies (I2 = 0%, P = 0.712) (Figure 2), nor evidence of publication bias (Begg test: P = 0.117, Egger test: P = 0.192) (Supplementary Figure S2).
Serum Transferrin and Risk of GDM
Two studies measured serum transferrin and provided the mean and SD; we pooled data and found no difference in serum transferritin between GDM women and non-GDM group (−0.02 μmol/L; 95% CI: −0.22–0.19 μmol/L) using a random-effects model (result of inverse-variance fixed-effect model: −0.02 μmol/L; 95% CI: −0.22–0.19 μmol/L)(Figure 3). No heterogeneity was detected between these 2 studies (I2 = 0%, P = 0.396). It is not suitable to perform publication bias because of restrictions of literatures.
Dose-Responsive Meta-Analysis
Two studies17,19 reported RR for ferritin exposure in at least 3 levels, also 2 studies20,21 reported RR for heme iron exposure in at least 3 levels, and 2 studies21,22 reported RR for dietary total iron exposure in at least 3 levels. Therefore, we performed dose–response analyses to quantitatively assess the potential relationship between ferritin, heme iron, dietary total iron, and risk of GDM. The summary RR for an increment of 5 μg/L in ferritin levels was 1.04 (95% CI: 0.9, 1.22), and an increment of 1 mg/d in heme iron levels was 1.35 ((95% CI: 0.78, 2.35). Although we found evidence of statistically departure from linearity of dietary total iron and GDM risk, a 1-mg/d-increment dietary total iron level conferred an RR of 0.99 (95% CI: 0.98, 1.02).
Subgroup Analysis
For the ferritin levels and GDM risk, because many factors influence the development of GDM, we performed subgroup analysis for prospective cohort studies according to location, age, BMI, and sample size (Figure 4). As BMI information was not be found in one study,18 3 studies were included in the BMI group. The summary assessment presented was calculated by using a fixed-effects model. Results showed that age was the most significant factor of GDM among these factors (results of random-effect model: 3.45 [95% CI: 1.84–6.46]; 7.34 [95% CI: 2.52–21.42]; 3.15 [95% CI: 1.66–5.95]; 3.21 [95% CI: 1.72–6.01]).
FIGURE 4.
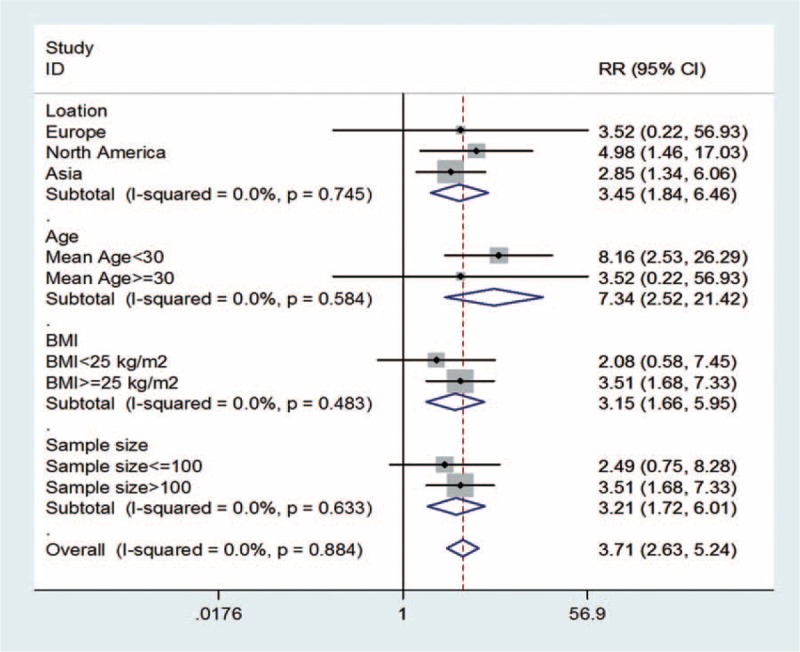
Forest plot of subgroup analysis of ferritin levels and GDM risk, grouped on the basis of 4 studies characteristics. The summary assessment presented was calculated by using a fixed-effects model. Information of BMI of individual could not be found in one study (Soubasi et al18). BMI = body mass index, CI = confidence interval, RR = relative risk.
Sensitivity Analysis
As high heterogeneity (I2 = 90.6%, P < 0.0001) was detected for ferritin case-control studies, we performed a sensitivity analysis by omitting one study at a time and calculating the pooled SMD for remaining studies to identify any source of heterogeneity. Results suggested that there are 2 studies reporting the 2 most estimates dramatically influenced the pooled SMD6,9 (Supplementary Figure S1). The heterogeneity reduced (I2 = 64.5%, P = 0.038) after these 2 studies were excluded.
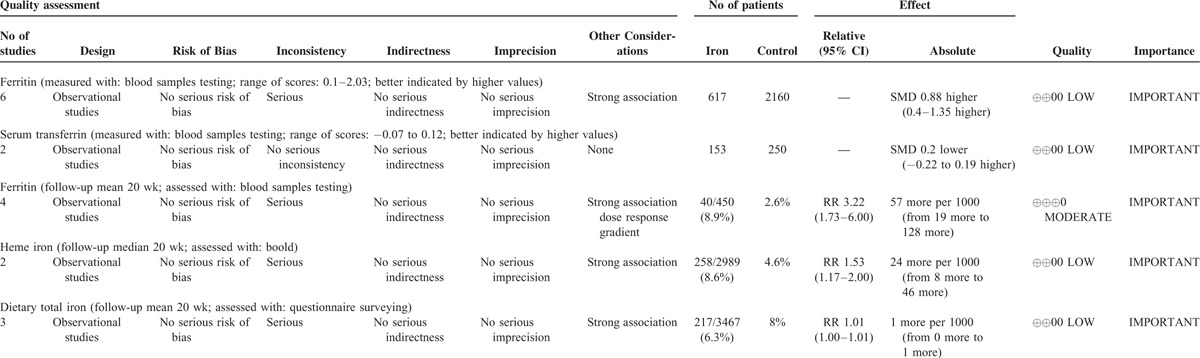
Evidence Quality Based on GRADE System
The level of evidence was evaluated by GRADE system. Based on GRADE system, the evidence of ferritin-GDM risk based on prospective cohort studies was at level B and moderate recommendation, whereas other evidence were all at level C and weak recommendation. The level of evidence and other characteristics were shown in Table 2.
TABLE 2.
GRADE Evidence Profile for the Relationship Between Body Iron Status, Iron Intake, and GDM Risk
DISCUSSION
We conducted a systematic review and meta-analysis to assess the relationship between dietary iron intake, body iron status, and GDM risk. In this study, we found positive correlation between ferritin levels and GDM based on prospective cohort studies and case-control studies. Although heme iron intake was positively related with an increased risk of GDM after adjustment for known potential confounders, considering the small amount of studies, the reliability of the result can be affected. Although dietary total iron intake has been associated with GDM in a cohort study,22 our study found no statistically significant relationship between dietary total iron and the risk of GDM, nor significantly relationship between serum transferrin and the risk of GDM. Because heme iron intake was found to be related with higher body iron stores in previous studies,27,31 our findings indicated that high levels of body iron may increase the risk of GDM. As for the relationship between ferritin levels and GDM risk, we further performed subgroup analysis, and the result suggested that age was the most significant factor of GDM among these factors.
Researchers hypothesized that elevated ferritin levels may represent elevated body iron stores, and also as an acute-phase reactant, elevated ferritin may reflect inflammation.28,29 Some researchers found that CRP at mid-pregnancy is correlated with GDM, and inflammation may play an important role in the development of GDM.30 Increasing evidence suggested that GDM might be part of an inflammatory process, and elevated ferritin could act on GDM may be through causing inflammation.
Our meta-analysis has several strengths. First, it is the first meta-analysis focused on the relationship between body iron status, dietary iron intake, and GDM risk. Compared with the former reviews about the correlation between iron and GDM,31–35 we conducted a systematic review and meta-analysis to appraise the relationship between iron and the GDM risk, and performed supplementary analysis including subgroup analysis, sensitivity analysis, and dose–response meta-analysis. Second, all the included studies had high qualities according to the methodological quality assessment by the NOS.
In spite of the considerable efforts to explore the possible relationship between iron and GDM risk, there are some limitations. First, the number of included studies limited further analysis, and the reliability of the conclusion may be affected. Additionally, the efficiency of the detection of publication bias may be affected by the small number of literature. Owing to the limited quantity of the included studies, more researches with high-grade evidence are needed.
Based on GRADE system, observational study is a low level of evidence. Although ferritin-GDM risk results are moderate level of evidence according to the evaluation of GRADE system, the reasons are as follows: literatures included are methodologically strong observational studies; studies yield large and are confident about the results. The weak study design is unlikely to explain all of the obvious benefits, although the observational studies are likely to provide an overestimate of the true effect. Therefore, increased higher ferritin levels were significantly correlated with higher risk of GDM, and higher heme iron levels may be correlated with higher risk of GDM. As for dietary total iron-GDM risk and serum transferrin-GDM risk, because the number of literature is too little, the reliability of the conclusion needs further validation.
CONCLUSIONS
In summary, higher ferritin levels had significant association with greater risk of GDM in this meta-analysis of prospective cohort studies and case-control studies. Moderately increased ferritin levels may be useful for clinical and public identification of high-risk group for GDM. And higher heme iron levels may be correlated with higher risk of GDM; however, the present conclusion did not constitute definitive proof that dietary total iron or serum transferrin has relation to GDM.
Supplementary Material
Acknowledgments
We thank authors who contributed data of their studies and also thank Dr Jinhui Tian for this statistical advice.
Footnotes
Abbreviations: BMI = body mass index; CI = confidence intervals; CRP = C-reactive protein; GDM = gestational diabetes mellitus; NOS = Newcastle-Ottawa Scale; RR = relative risk; SD = standard difference; SMD = standard mean difference; sTfR = serum transferrin receptor.
This study was supported by Chongqing Municipal Health Bureau Research Fund (Fund No.: 2010-2-078). The 2010 national key clinical nurse project (Fiscal Club 305 [2010]). And the National Key Clinical Specialties Construction Program of china. The funding source of the study was not part of the design of study, collection of information, analysis of data, nor preparation of this manuscript.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr 2000; 72:257S–264S. [DOI] [PubMed] [Google Scholar]
- 2.Klebanoff MA, Shiono PH, Selby JV, et al. Anemia and spontaneous preterm birth. Am J Obstet Gynecol 1991; 164:59–63. [DOI] [PubMed] [Google Scholar]
- 3.Scholl TO, Hediger M, Fischer RL, et al. Anemia vsiron deficency: increased risk of pretem delivery in a prospective study. Am J Clin Nutr 1992; 55:985–988. [DOI] [PubMed] [Google Scholar]
- 4.Afkhami-Ardekani M, Rashidi M. Iron status in women with and without gestational diabetes mellitus. J Diabetes Complications 2009; 23:194–198. [DOI] [PubMed] [Google Scholar]
- 5.T.T Lao, P.L Chan, K.F Tam. Gestational diabetes mellitus in the last trimester—a feature of maternal iron excess? Diabet Med 2001; 18:218–223. [DOI] [PubMed] [Google Scholar]
- 6.Sharifi F, Ziaee A, Feizi A, et al. Serum ferritin concentration in gestational diabetes mellitus and risk of subsequent development of early postpartum diabetes mellitus. Diabetes Metab Syndr Obes 2010; 3:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan K, Chan B, Lam K, et al. Iron supplement in pregnancy and development of gestational diabetes—a randomised placebo-controlled trial. BJOG 2009; 116:789–798. [DOI] [PubMed] [Google Scholar]
- 8.Kinnunen TI, Luoto R, Helin A, et al. Supplemental iron intake and the risk ofglucose intolerance in pregnancy: re-analysis of a randomised controlled trial in Finland. Matern Child Nutr 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gungor ES, Danisman N, Mollamahmutoglu L. Maternal serum ferritin and hemoglobin values in patients with gestational diabetes mellitus. Saudi Med J 2007; 28:478–480. [PubMed] [Google Scholar]
- 10.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa: Ottawa Hospital Research Institute; 2011. [Google Scholar]
- 11.Swiglo BA, Murad MH, Schunemann HJ, et al. A case for clarity, consistency, helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J Clin Endocrinal Metab 2008; 93:666–673. [DOI] [PubMed] [Google Scholar]
- 12.Guyatt GH, Oxman AD, Kunz R, et al. What is “quality of evidence” and why is it important to clinicians? Bmj 2008; 336:995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommen dations. BMJ 2008; 336:1049–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 16.Tarim E, Kilicdag E, Bagis T, et al. High maternal hemoglobin and ferritin values as risk factors for gestational diabetes. Int J Gynaecol Obstet 2004; 84:259–261. [DOI] [PubMed] [Google Scholar]
- 17.Salam Zein, Samar Rachidi, Sanaa Awada, et al. High iron level in early pregnancy increased glucose intolerance. J Trace Elem Med Biol 2015; 30:220–225. [DOI] [PubMed] [Google Scholar]
- 18.Soubasi V, Petridou S, Sarafidis K, et al. Association of increased maternal ferritin levels with gestational diabetes and intra-uterine growth retardation. Diabetes Metab 2010; 36:58–63. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Scholl TO, Stein TP. Association of elevated serum ferritin levels and the risk of gestational diabetes mellitus in pregnant women: the Camden study. Diabetes Care 2006; 29:1077–1082. [DOI] [PubMed] [Google Scholar]
- 20.Qiu C, Zhang C, Gelaye B, et al. Gestational diabetes mellitus in relation to maternal dietary heme iron and nonheme iron in take. Diabetes Care 2011; 34:1564–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowers K, Yeung E, Williams MA, et al. A prospective study of prepregnancy dietary iron intake and risk for gestational diabetes mellitus. Diabetes Care 2011; 34:1557–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helin A, Kinnunen TI, Raitanen J, et al. Iron intake, haemoglobin and risk of gestational diabetes: a prospective cohort study. BMJ Open 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behboudi-Gandevani S, Safary K, Moghaddam-Banaem L, et al. The relationship between maternal serum iron and zinc levels and their nutritional intakes in early pregnancy with gestational diabetes. Biol Trace Elem Res 2013; 154:7–13. [DOI] [PubMed] [Google Scholar]
- 24.Javadian P, Alimohamadi S, Gharedaghi MH, et al. Gestational diabetes mellitus and iron supplement; effects on pregnancy outcome. Acta Med Iran 2014; 52:385–389. [PubMed] [Google Scholar]
- 25.Aysel Uysal Derbent, Serap Aynur Simavli, Ikbal Kaygusuz, et al. Serum hepcidin is associated with parameters of glucose metabolism in women with gestational diabetes mellitus. J Matern Fetal Neonatal Med 2013; 26:1112–1115. [DOI] [PubMed] [Google Scholar]
- 26.Amiri F, Basirat N, Omidvar Z, et al. Comparison of the serum iron, ferritin levels and total iron-binding capacity between pregnant women with and without gestational diabetes. J Nat Sci Biol Med 2013; 4:302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleming DJ, Jacques PF, Dallal GE, et al. Dietary determinants of iron stores in a free-living elderly population: the Framingham Heart Study. Am J Clin Nutr 1998; 67:722–733. [DOI] [PubMed] [Google Scholar]
- 28.Ford ES, Cogswell ME. Diabetes and serum ferritin concentrations among US adults. Diabetes Care 1999; 22:1978–1983. [DOI] [PubMed] [Google Scholar]
- 29.Cauza E, Hanusch-Enserer U, Bischof M, et al. Increased C282Y heterozygosity in gestational diabetes. Fetal Diagn Ther 2005; 20:349–354. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Lu X. Study on correlation between C-reactive protein and gestational diabetes mellitus. JNMU 2007; 21:382–385. [Google Scholar]
- 31.Liu JM, Hankinson SE, Stampfer MJ, et al. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr 2003; 78:1160–1167. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang T, Han H, Yang Z. Iron, oxidative stress and gestational diabetes. Nutrients 2014; 6:3968–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scholl TO. Iron status during pregnancy: setting the stage for mother and infant. Am J Clin Nutr 2005; 81:1218S–1222S. [DOI] [PubMed] [Google Scholar]
- 34.Hassanzadeh Rad A, Hassanzadeh Rad Z. Association between pregnancy dietary iron intake and gestational diabetes mellitus. Int J Fertil Steril 2012; 6:1–164.25505505 [Google Scholar]
- 35.Weinberg ED. Are iron supplements appropriate for iron replete pregnant women? Med Hypotheses 2009; 73:714–715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


