Abstract
We report on a stroke patient who showed recovery of hypersomnia concurrent with the recovery of an injured ascending reticular activating system (ARAS), which was demonstrated by diffusion tensor tractography (DTT).
A 70-year-old female patient underwent coiling of the left ruptured posterior communicating artery after subarachnoid hemorrhage and both extraventricular drainage for management of an intraventricular hemorrhage. At 2 months after onset, when she started rehabilitation, she exhibited intact consciousness, with the full score on the Glasgow Coma Scale: 15. However, she showed severe hypersomnia: she always fell asleep without external stimulation and the Epworth Sleepiness Scale (EPS) score was 24 (full score: 24, cut off for hypersomnia: 10). She underwent comprehensive rehabilitative therapy, including neurotropic drugs, physical therapy, and occupational therapy. Her hypersomnia has shown improvement as 14 (3 months after onset), 11 (4 months after onset), 7 (12 months after onset), and 6 (24 months after onset), respectively.
On 2-month DTT, narrowing of both lower dorsal and ventral ARASs was observed on both sides: in particular, among 4 neural tracts of the lower ARAS, the right lower ventral ARAS was the narrowest. By contrast, on 24-month DTT, the 4 narrowed neural tracts of both lower dorsal and ventral ARASs were thickened compared with those of 2-month DTT.
Recovery of hypersomnia with recovery of an injured lower ARAS on DTT was observed in a stroke patient. Our results suggest that evaluation of the lower ARAS using DTT might be useful for stroke patients with hypersomnia.
INTRODUCTION
Hypersomnia (excessive daytime sleepiness) is a common sequela following stroke: persistent hypersomnia was reported in ∼5% of stroke patients.1 Hypersomnia in stroke patients is related to poor outcome; therefore elucidation of its pathogenetic mechanism is clinically important.1 Many previous studies have suggested that involvement of the ascending reticular activating system (ARAS) might be a pathogenetic mechanism of hypersomnia in stroke patients.2–5
Introduction of diffusion tensor tractography (DTT), derived from diffusion tensor imaging (DTI), has enabled 3-dimensional reconstruction and estimation of the ARAS in the live human brain.6 A few studies using DTT have reported that injury of the ARAS was the cause of hypersomnia in patients with brain injury.7,8 However, no study on the recovery of hypersomnia along with the recovery of an injured ARAS has been reported.
In the present study, we report on a stroke patient who showed recovery of hypersomnia concurrent with the recovery of an injured ARAS, which was demonstrated by DTT.
Case Report
A 70-year-old female patient underwent coiling of the left ruptured posterior communicating artery after subarachnoid hemorrhage and both extraventricular drainage for management of intraventricular hemorrhage and hydrocephalus at the neurosurgery department of a university hospital (Figure 1A). At 2 months after onset, she was transferred to the rehabilitation department of the same university hospital in order to undergo rehabilitation. The patient exhibited intact consciousness, with a full score on the Glasgow Coma Scale: 15. However, she showed severe hypersomnia: she always fell asleep without external stimulation and the Epworth Sleepiness Scale (EPS) score was 24 (full score: 24, cut off for hypersomnia: 10). She underwent comprehensive rehabilitative therapy, including neurotropic drugs, physical therapy, and occupational therapy. Her hypersomnia has shown improvement as 14 (3 months after onset), 11 (4 months after onset), 7 (12 months after onset), and 6 (24 months after onset), respectively.9 The patient provided signed, informed consent, and the study protocol was approved by Yeungnam University hospital Institutional Review Board.
FIGURE 1.
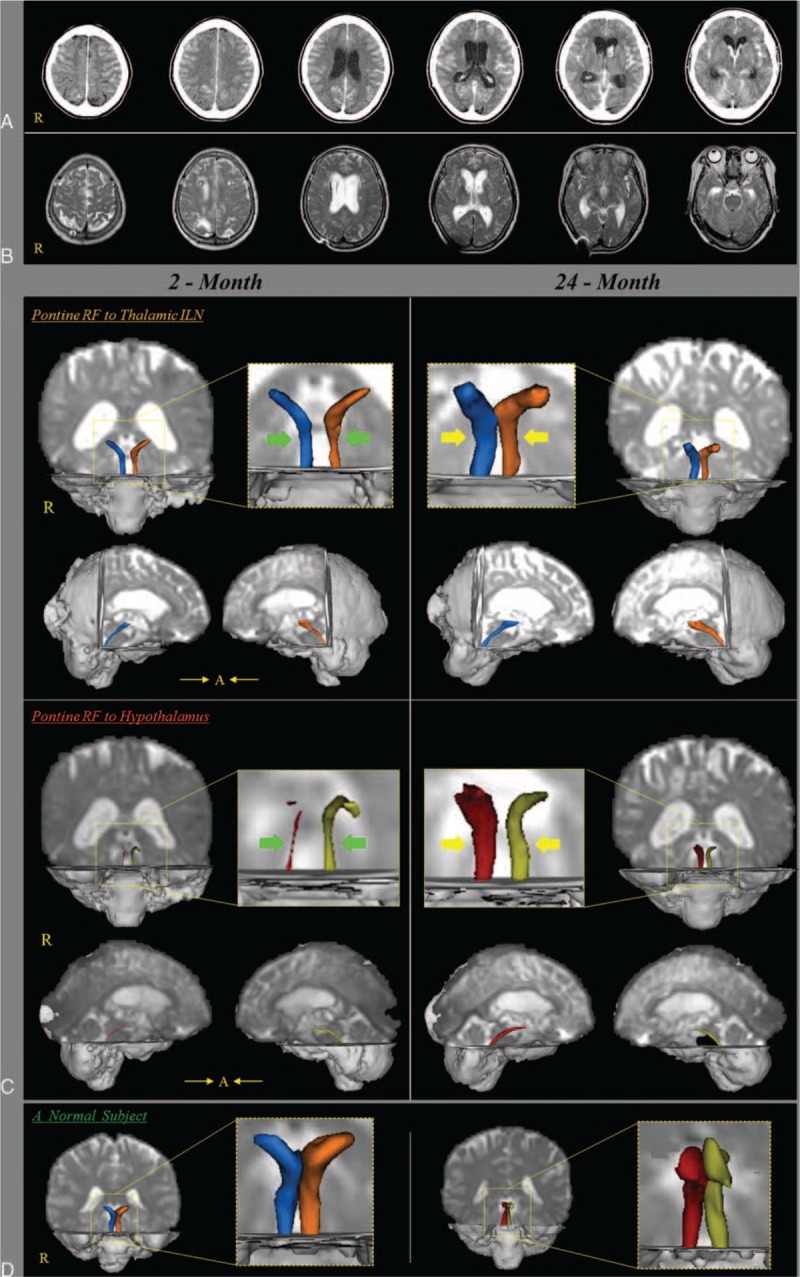
(A) Brain CT images at onset show a subarachnoid hemorrhage and intraventricular hemorrhage and hydrocephalus. (B) Brain MR images at 2 months after onset show a leukomalactic lesion in both fronto-parietal lobes. (C) Results of diffusion tensor tractography (DTT) for both lower dorsal and ventral ascending reticular activating system (ARAS). On 2-month DTT, narrowing (arrows) of both lower dorsal and ventral ARASs was observed on both sides: in particular, among 4 neural tracts of the lower ARAS, the right lower ventral ARAS was narrowest. By contrast, on 24-month DTT, the 4 narrowed neural tracts of both lower dorsal and ventral ARASs were thickened compared with those of 2-month DTT (arrows). (D) DTTs of the lower dorsal and ventral lower ARAS of a normal subject (65-year-old woman). ARAS = ascending reticular activating system, CT = computed tomography, DTT = diffusion tensor tractography, MR = magnetic resonance.
Diffusion Tensor Imaging
DTI data were acquired 2 times (2 and 24 months after onset) using a 6-channel head coil on a 1.5 T Philips Gyroscan Intera (Philips, Best, Netherlands) with single-shot echo-planar imaging. For each of the 32 noncollinear diffusion sensitizing gradients, 67 contiguous slices were acquired parallel to the anterior commissure-posterior commissure line. Imaging parameters were as follows: acquisition matrix = 96 × 96; reconstructed matrix = 192 × 192; field of view = 240 × 240 mm2; TR = 10,726 ms; TE = 76 ms; b = 1000 s/mm2; NEX = 1; and a slice thickness of 2.5 mm with no gap.
Probabilistic Fiber Tracking
The Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library was used for analysis of the diffusion-weighted imaging data. Affine multiscale 2-dimensional registration was used for correction of head motion effect and image distortion due to eddy current. FMRIB Diffusion Software with routines option (0.5 mm step lengths, 5000 streamline samples, curvature thresholds = 0.2) was used for fiber tracking.10,11 Two portions of the ARAS were reconstructed by selection of fibers passing through regions of interest (ROI). For analysis of the lower dorsal ARAS, the seed ROI was placed on the pontine reticular formation (RF) and the target ROI was placed on the intralaminar thalamic nucleus.12 For reconstruction of the lower ventral ARAS, the seed ROI was placed on the pontine RF and the target ROI was placed on the hypothalamus.13 Out of 5000 samples generated from a seed voxel, results were visualized at the threshold of 2 streamlines through each voxel for analysis.
On 2-month DTT, narrowing of both lower dorsal and ventral ARASs was observed on both sides: in particular, among 4 neural tracts of the lower ARAS, the right lower ventral ARAS was narrowest. By contrast, on 24-month DTT, the 4 narrowed neural tracts of both lower dorsal and ventral ARASs were thickened compared with those of 2-month DTT.
DISCUSSION
In the present study, 4 neural tracts of the lower dorsal and ventral lower ARAS were evaluated using DTT: the lower dorsal ARAS between the pontine RF and the thalamic ILN, and the lower ventral ARAS between the pontine RF and the hypothalamus. Narrowing of the 4 neural tracts of the lower dorsal and ventral ARAS was observed on 2-month DTT and these were thickened on 24-month DTT compared with those of 2-month DTT concurrent with the recovery of hypersomnia. The change of findings indicated recovery of 4 injured neural tracts of the lower dorsal and ventral ARAS. Many studies have reported close association of the hypothalamus with hypersomnia; thus it appeared that the recovery of this patient's hypersomnia was attributed to the recovery of an injured lower ARAS, particularly the recovery of an injured right ventral lower ARAS which appeared to be most severely injured on 2-month DTT.5,14,15
Previous studies have demonstrated injury of the lower ARAS by intraventricular hemorrhage and subarachnoid hemorrhage, respectively, using DTT.16,17 A few studies using DTT have reported on the association of injury of the ARAS with hypersomnia in patients with brain injury.7,8 In 2015, Jang et al reported on injury of the lower ARAS, particularly injury of the ventral lower ARAS in a patient with a pontine hemorrhage. A recent case study reported that narcolepsy was ascribed to injury of the ventral ARAS in a patient with mild traumatic brain injury.8 Therefore, our results appear to be consistent with those of the above-mentioned previous studies in terms of the pathogenetic mechanism of injury of the lower ARAS by intraventricular hemorrhage and subarachnoid hemorrhage, and the association of injury of the ventral ARAS with hypersomnia.7,8,16,17 In addition, to the best of our knowledge, this is the first study to demonstrate recovery of hypersomnia concurrent with recovery of an injured lower ARAS in stroke patients.
In conclusion, recovery of hypersomnia with recovery of an injured lower ARAS was demonstrated in a stroke patient using DTT. Our results suggest that evaluation of the lower ARAS using DTT might be useful for stroke patients with hypersomnia. This study is limited because it is a case report; therefore, conduct of further studies comprising a large number of patients would be necessary. In addition, the critical region of the ARAS for hypersomnia should be elucidated by further studies.
Footnotes
Abbreviations: ARAS = ascending reticular activating system; DTI = diffusion tensor imaging; DTT = diffusion tensor tractography; EPS = Epworth sleepiness Scale; RF = reticular formation; ROI = region of interest.
Funding: this work was supported by the National Research Foundation (NRF) of Korea Grant funded by the Korean Government (MSIP) (2015R1A2A2A01004073).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Harris AL, Elder J, Schiff ND, et al. Post-stroke apathy and hypersomnia lead to worse outcomes from acute rehabilitation. Transl Stroke Res 2014; 5:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arpa J, Rodriguez-Albarino A, Izal E, et al. Hypersomnia after tegmental pontine hematoma: case report. Neurologia 1995; 10:140–144. [PubMed] [Google Scholar]
- 3.Forcadas MI, Zarranz JJ. Hypersomnia after tegmental pontine hematoma. Neurologia 1995; 10:307–310. [PubMed] [Google Scholar]
- 4.Blanco M, Espinosa M, Arpa J, et al. Hypersomnia and thalamic and brain stem stroke: a study of seven patients. Neurologia 1999; 14:307–314. [PubMed] [Google Scholar]
- 5.Tezer FI, Pektezel MY, Gocmen R, et al. Unusual presentation of hypothalamic hamartoma with hypersomnia in an adult patient. Epileptic Disord 2014; 16:366–369. [DOI] [PubMed] [Google Scholar]
- 6.Edlow BL, Takahashi E, Wu O, et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol 2012; 71:531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang SH, Chang CH, Jung YJ, et al. Hypersomnia due to injury of the ascending reticular activating system in a patient with pontine hemorrhage. Int J Stroke 2015; in press. [Google Scholar]
- 8.Jang SH, Seo WS, Kwon HG. Post-traumatic narcolepsy and injury of the ascending reticular activating system. Sleep Med 2016; 17:1–2. [DOI] [PubMed] [Google Scholar]
- 9.Bloch KE, Schoch OD, Zhang JN, et al. German version of the Epworth Sleepiness Scale. Respiration 1999; 66:440–447. [DOI] [PubMed] [Google Scholar]
- 10.Behrens TEJ, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 2003; 6:750–757. [DOI] [PubMed] [Google Scholar]
- 11.Behrens TE, Berg HJ, Jbabdi S, et al. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 2007; 34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeo SS, Chang PH, Jang SH. The ascending reticular activating system from pontine reticular formation to the thalamus in the human brain. Front Hum Neurosci 2013; 7:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang SH, Kwon HG. The ascending reticular activating system from pontine reticular formation to the hypothalamus in the human brain: a diffusion tensor imaging study. Neurosci Lett 2015; 590:58–61. [DOI] [PubMed] [Google Scholar]
- 14.Menzler K, Belke M, Unger MM, et al. DTI reveals hypothalamic and brainstem white matter lesions in patients with idiopathic narcolepsy. Sleep Med 2012; 13:736–742. [DOI] [PubMed] [Google Scholar]
- 15.Yassin W, Sugihara G, Oishi N, et al. Hypothalamic-amygdalar-brainstem volume reduction in a patient with narcolepsy secondary to diffuse axonal injury. J Clin Sleep Med 2015; 11:581–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang SH, Kim HS. Aneurysmal subarachnoid hemorrhage causes injury of the ascending reticular activating system: relation to consciousness. AJNR Am J Neuroradiol 2015; 36:667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang SH, Yeo SS. Injury of the lower portion of the ascending reticular activating system in a patient with intraventricular hemorrhage. Int J Stroke 2015; 10:162–163. [DOI] [PubMed] [Google Scholar]


