Abstract
Non-small-cell lung cancer (NSCLC) is the leading cause of cancer deaths. Erlotinib is the first-generation epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs), the National Comprehensive Cancer Network (NCCN) guidelines recommend it as a first-line agent in patients with sensitizing EGFR mutations.
We conducted a meta-analysis to compare the efficacy of erlotinib and chemotherapy for advanced NSCLC, and evaluated the efficacy of them to provide references for further clinical practice and research.
PubMed, EMBASE, CBM, CNKI, WanFang database, The Cochrane library, and Web of Science, as well as abstracts presented at ASCO conferences and ClinicalTrials.gov were searched to identify relevant studies. HR with 95% confidence intervals (CIs) for progression-free survival (PFS) and overall survival (OS), relative risk (RR) with 95% CIs for objective response rate (ORR) and 1-year survival rate (OSR) were all extracted. If the I2 was ≤40%, then the trial was considered to be heterogeneous, and a fixed-effects model was selected. Otherwise, a random-effects model was used. Meta-regression and sensitivity analyses were conducted to determine the possible heterogeneity causes and to further identify the influence of the various exclusion criteria on the overall risk estimate.
The pooled analysis demonstrated a PFS HR of 0.93 (95% CI = 0.73, 1.19) for erlotinib versus chemotherapy and an ORR of 18.43% versus 22.07%, respectively. The OS HR was 1.02 (95%CI = 0.93, 1.12). The HRs for PFS estimated based on 10 trials involving 1101 patients were 0.22 (95% CI = 0.15, 0.29) and 1.27 (95% CI = 1.04, 1.48) in EGFR mutation-type and wild-type patients, respectively. The HRs for OS calculated from 4 studies including 681 participants were 0.83 (95% CI = 0.61, 1.05) and 0.86 (95% CI = 0.68, 1.04) in EGFR mutation-type and wild-type patients, respectively. The 1-year survival rates were 31.31% and 32.41%, respectively.
Overall, the present meta-analysis suggested that erlotinib did not improve the ORR, PFS, OS or the 1-year survival rate for whole patients. However, erlotinib could benefit patients with EGFR mutation in terms of PFS, but the OS does not benefit from it for these patients. Further studies of erlotinib for these subgroup patients are warranted.
INTRODUCTION
Lung cancer is the leading cause of cancer deaths in China and over the world, and nearly 1 million new cases are expected annually by 2025.1–3 Non-small cell lung cancer (NSCLC) accounts for more than 85% of all lung tumors.4 Approximately 60% of diagnosed NSCLCs are in the terminal stage. The median overall survival of patients treated with first-line chemotherapy ranges from 7 to 12 months.5 Second- and third-line chemotherapy treatments have been used to further increase survival rates. Despite the use of a combination of all current therapies, patient survival remains unoptimistic.6
In 2013, the Food and Drug Administration (FDA) approved erlotinib (Tarceva®) as a first-line treatment for metastatic NSCLC patients with EGFR mutations.7 The NCCN also recommended erlotinib as a first-line therapy in patients with sensitizing EGFR mutations. However, it did not recommend that erlotinib be given as first therapy for patients with a negative or unknown EGFR status. As a second-line therapy, erlotinib is superior to the best available supportive care. However, as a third-line therapy, the efficacy of erlotinib is uncertain.8
Numerous clinical trials have been developed to evaluate the efficacy of erlotinib in the treatment of advanced NSCLC, either in combination with chemotherapy or alone; however, consistent results have not been identified, and our meta analysis showed that erlotinib combined with CT could increase PFS and objective response rate, but not benefit OS,9 our another meta analysis disclosed that erlotinib could decrease the incidence of neutropenia and leukopenia in patients with advanced NSCLC undergoing erlotinib regardless of whether combined with CT or not.10 In recent years, many published meta-analyses have been focusing on EGFR-TKIs for NSCLC11–14; however, all 4 studies explored a combination of EGFR-TKIs rather than the effects of single agent. However, some studies reported different antitumor activities and favorable toxicities for various oral EGFR-TKIs.15
Therefore, a pooled analysis of the currently available studies that were restricted to patients who used erlotinib alone compared with other chemotherapy, which may provide relevant information for the treatment of patients with advanced NSCLC, was performed to evaluate the efficacy of erlotinib compared with chemotherapy. Additionally, we performed meta-regression and subgroup analyses according to the treatment period, ECOG-PS, gender, EGFR mutation status, and smoking status. We also comprehensively appraised the quality of the evidence with GRADEpro to facilitate clinical decision-making.
METHODS
Ethical approval and patient written informed consent are not required due to that this is a systematic review and meta-analysis of previously published studies. This study was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.16 The protocol was published by Centre for Reviews and Dissemination PROSPERO (Registration No. CRD42014010347).
Search Strategy
Eligible trials were identified by electronically searching PubMed, EMBASE, ISI Web of Science (ISI), and The Cochrane Central Register of Controlled Trials (CENTRAL) with the following terms: (“non-small-cell lung carcinoma” OR “non-small cell lung cancer”) AND (“Erlotinib” OR “Tarceva”) (from inception to December 25, 2014, updated at October 28, 2015). The PubMed search strategy is summarized in Appendix 1. The abstracts indexed in ASCO and ESMO and search engines, including Baidu (Chinese), Google Scholar and DXY.com (Chinese), were also searched to include any potential studies. The reference lists of the included studies were also manually evaluated to improve the recall ratio, and precision ratio. No language restriction was imposed.
Selection Criteria
Using the PICOS acronym (population, intervention comparison, outcome, and study design), the following inclusion criteria were identified: Population: all the patients who were diagnosed as advanced NSCLC using pathology and cytology tests were eligible for the systematic review. The patients’ nationality was not limited, and the patients did not have any other complications, such as serious cardiopulmonary diseases and other severe basic diseases. Interventions and comparisons: the intervention is erlotinib alone, the comparison is conventional chemotherapy regardless any regimens or cycles. Outcomes: the overall survival (OS), objective response (ORR), progress-free survival (PFS), and 1-year survival rate (OSR) were evaluated.
Data Extraction
Two reviewers (Jian-Guo Zhou and Yu Zhang) independently screened the titles and abstracts to exclude studies that failed to meet the inclusion criteria, and the full texts of the remaining studies were subsequently reviewed. Finally, data extraction was conducted with a premade data extraction form to collect information about the authors, the populations studied, publication year, country, and detailed information regarding the PICOs. The formula recommended by Tierney et al was adopted to calculate the corresponding HR of the missing data.17 Kaplan–Meier curves were produced with the Engauge Digitizer, version 4.1 (free software downloaded from http://sourceforge.net/) if the available data is not directly shown.9 Yu Zhang performed the data extraction and entry, and Jian-Guo Zhou examined the data. Any disagreement between the researchers concerning trial eligibility was resolved by consulting a third reviewer (Xu Tian). Each trial included in the study was independently evaluated for bias assessment risks according to the Cochrane Collaboration's tool by 2 reviewers (Fei Wang and Yi Wang).18
Level of Evidence
The GRADE profiler software (version 3.6) (available at: http://www.gradeworkinggroup.org/) was used to evaluate the level of evidence, and an evidence profile was developed to reveal the summary results.
The GRADE system identified the following four rating grades of evidence quality19: High: further research is very unlikely to change our confidence in the effect estimate; Moderate: further research is likely to have an important impact on our confidence in the effect estimate and may change the estimate; Low: further research is very likely to have an important impact on our confidence in the effect estimate and is likely to change the estimate; and Very low: any effect estimate is very uncertain.
Statistical Analysis
All data were pooled using STATA, version 12.0 (Stata Corp., College Station, TX). The effect size indicators, including HR, risk ratio (RR) and corresponding 95% CIs, were calculated. Heterogeneity among the included studies was evaluated with I2 statistics. I2 of 40%, 70%, and 100% were used to represent low, moderate, and high heterogeneity, respectively. If the I2 was ≤40%, then the trial was considered to be heterogeneous, and a fixed-effects model was selected. Otherwise, a random-effects model was used.20 Meta-regression and sensitivity analyses were conducted to determine the possible heterogeneity causes and to further identify the influence of the various exclusion criteria on the overall risk estimate. The influence of individual trials was also investigated with the leave-one-out cross validation method to test the robustness of the primary outcomes.21 Publication bias was assessed graphically using funnel plots and regression tests, according to the method reported by Egger,22 and by the Begg test.23 A P-value < 0.05 was considered statistically significant.
RESULTS
A total of 688 unfiled titles and abstracts were identified in the initial search, and 14 studies,13,24–39 which involved a total of 3559 participants, met the inclusion criteria and were thus included in the final analysis. A flow diagram of the literature that was searched and evaluated is presented in Figure 1.
FIGURE 1.
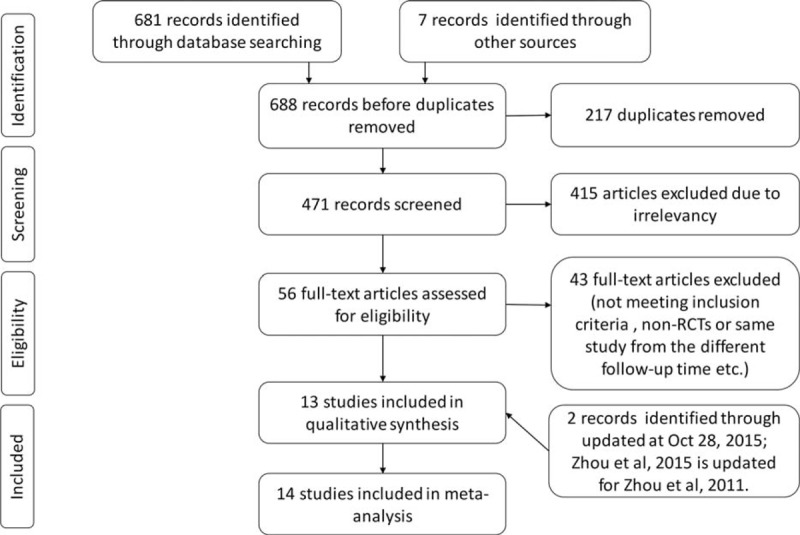
Flow diagram of the study details.
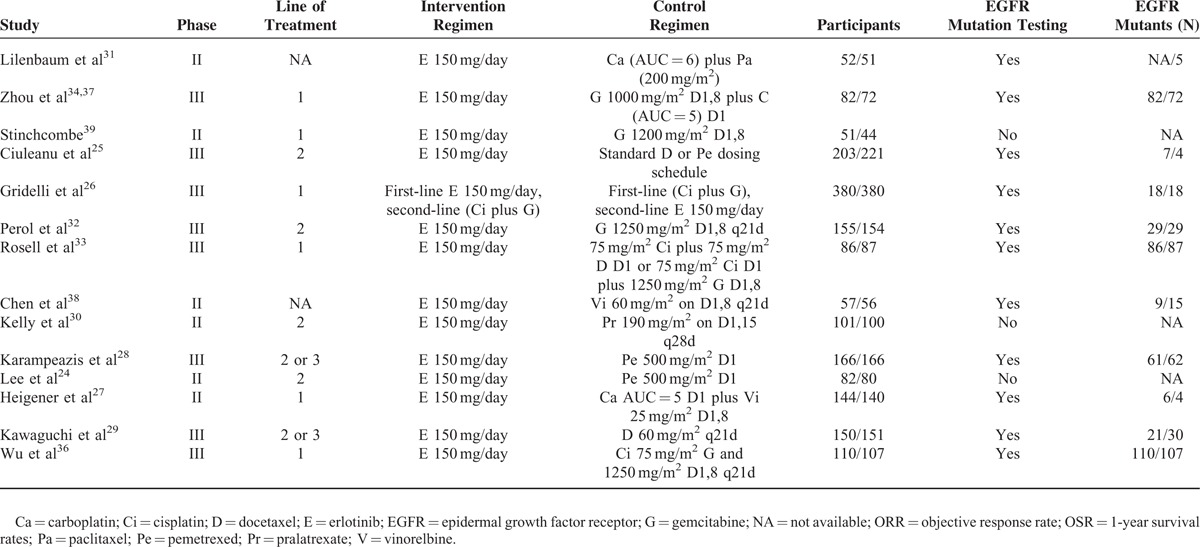
All the eligible studies were published between 2008 and 2015. In total, 13 trials provided PFS outcomes and 1 study reported the tumor progression time.29 The objective response rate and overall survival outcomes were available in 10 and 13 trials, respectively. The main characteristics of the included studies are recorded in Table 1.
TABLE 1.
The Main Study Characteristics
All 14 trials were open-label. Random sequence generation and allocation concealment were performed adequately in most of the trials. However, 1 trial did not describe the reasons for incomplete outcome data.30 Under the assumption that the PFS outcome might not differ from the progression time, the PFS data were used and pooled.13 The blinding method was unclear for all the trials. However, it was unlikely to affect the quality assessment. Three references33,34,38 had small sample sizes and eventually included fewer than 150 cases. The overall methodological quality of the included trials was generally good and fair (Figure 2).
FIGURE 2.
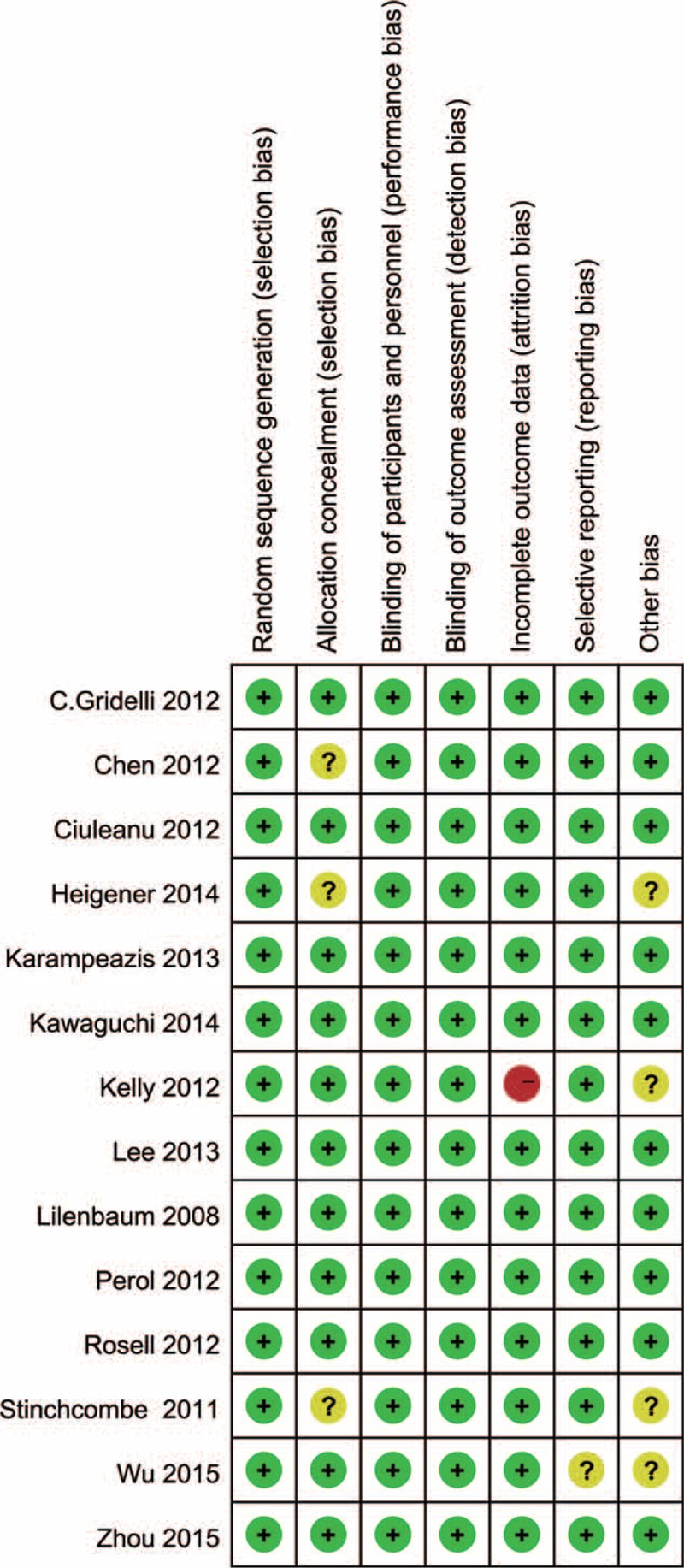
Appraisal of risk of bias of the included trials using the Cochrane risk-of-bias tool.
Objective Response Rate (10 Trials, 2560 Patients)
According to the heterogeneity test, the I2 was 77.5%, and the P-value was less than 0.05. Thus, a random-effects model was selected. The pooled RR for ORR showed that there were no significant differences between the erlotinib regimen and chemotherapy regimen groups (RR = 0.89; 95% CI = 0.60, 1.31, P = 0.560) (Figure 3, Table 2).
FIGURE 3.
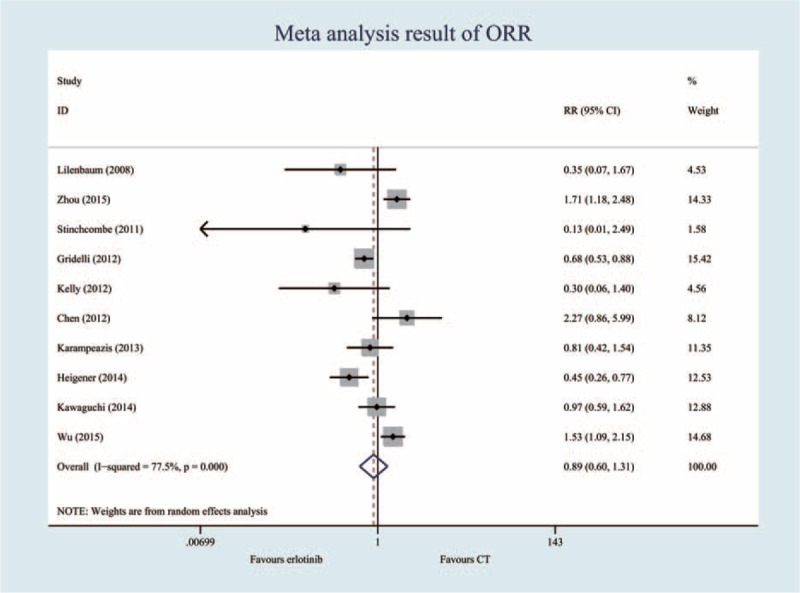
Meta-analysis results of the objective response rate.
TABLE 2.
The Overall Survival Result of Erlotinib Versus Conventional Chemotherapy for Advanced NSCLC
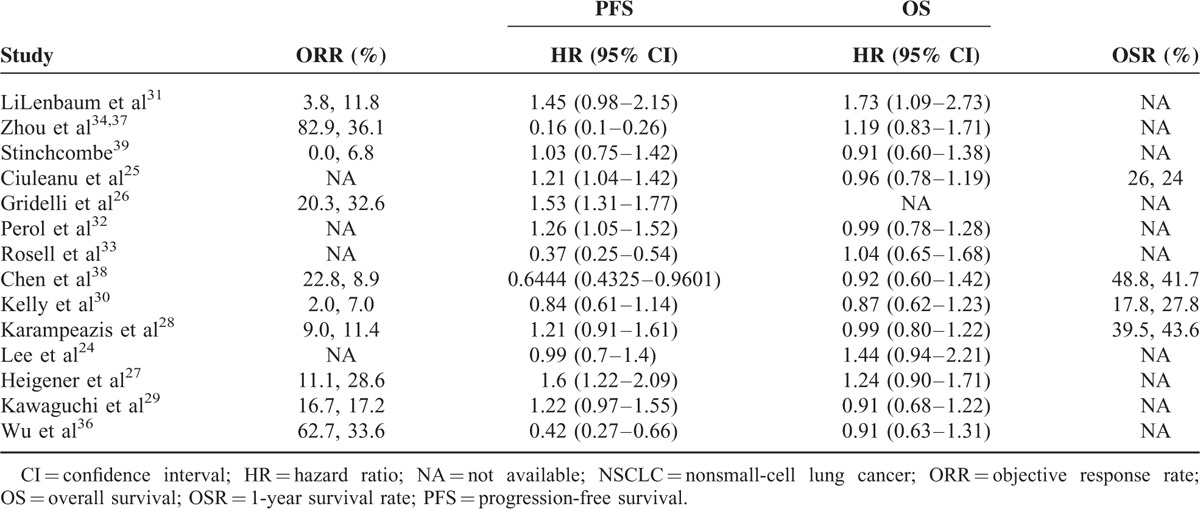
Progression-Free Survival (14 Trials, 3559 Patients)
The PFS of the erlotinib arm ranged from 1.6 to 13.1 months, and the PFS of the chemotherapy arm ranged from 1.2 to 5.2 months. The meta-analysis showed that the pooled HR was 0.98 (95% CI = 0.69, 1.27; P = 0.330), without statistical significance when the erlotinib regimen patients were compared with the chemotherapy regimen patients (Figure 4, Table 2).
FIGURE 4.
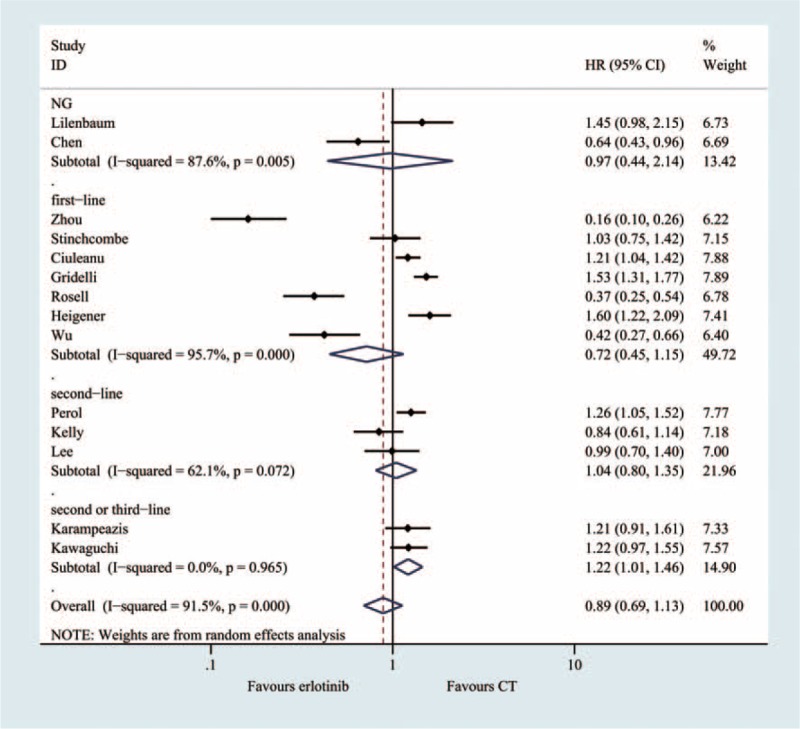
Meta-analysis results of the progression-free survival.
Overall Survival (13 Trials, 2868 Patients)
A total of 13 RCTs were included in the meta-analysis that was used to evaluate overall survival. The heterogeneity test indicated that a fixed-effect model could be selected (I2 = 3.7%, P = 0.410). The pooled results of the meta-analysis showed that there was no significant difference between the 2 groups (HR = 1.02; 95% CI = 0.94, 1.12; P = 0.609) (Figure 5, Table 2).
FIGURE 5.
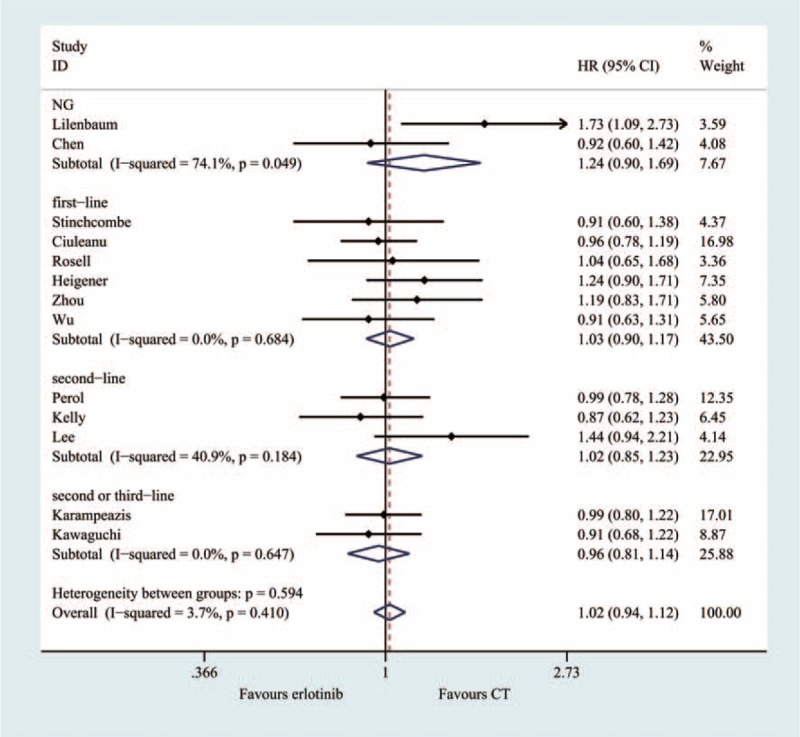
Meta-analysis results of the overall survival.
One-Year Survival Rate (4 Trials, 1070 Patients)
Four RCTs evaluated the 1-year survival rate. There was no significant heterogeneity (I2 = 27.8%, P = 0.245), therefore, a fixed-effect model was used. The result of the meta-analysis suggested that there was no significant difference between the erlotinib and conventional chemotherapy groups (RR = 0.96; 95% CI = 0.81, 1.14, P = 0.632) (Figure 6, Table 2).
FIGURE 6.
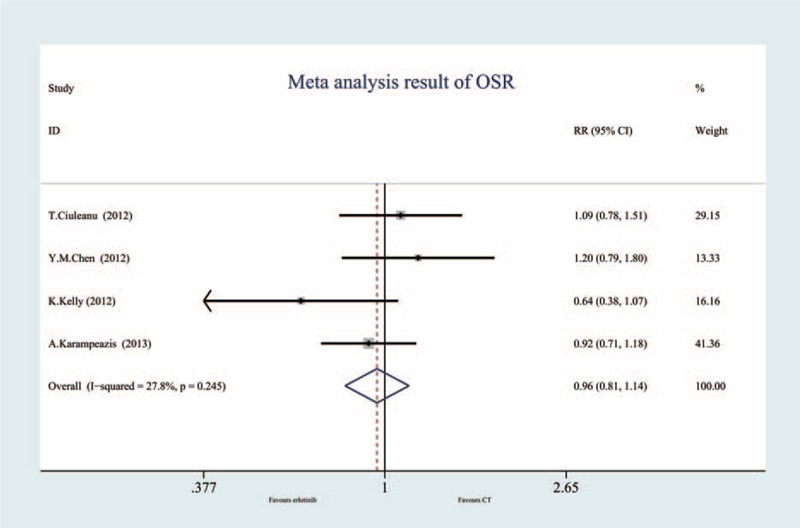
Meta-analysis results of the 1-year survival rate.
Subgroup Analyses
A subgroup analysis was adopted to determine the heterogeneity causes for the PFS (Figure 7) and OS (Figure 8) analyses. The effect sizes were similar between the subgroups, which were divided into 8 predefined subgroups according to gender, smoking status, histology and patient year, ECOG-PS, anatomic stage, and treatment status. No statistical significance was identified regarding treatment effect differences in the various subgroups, and the P values for gender, smoking status, histology and patient year, ECOG-PS, anatomic stage were 0.618, 0.443, 0.626, 0.395, 0.582, and 0.555 in PFS, respectively. The subgroup analysis based on EGFR mutation status appeared to be discordant, as the patients without EGFR mutations showed significantly prolonged PFS with chemotherapy (HR, 0.22; 95%CI = 0.15–0.30, P < 0.001). However, among the patients without EGFR mutations, conventional chemotherapy demonstrated decreased PFS (HR = 1.27; 95% CI = 1.04, 1.45) compared with erlotinib.
FIGURE 7.
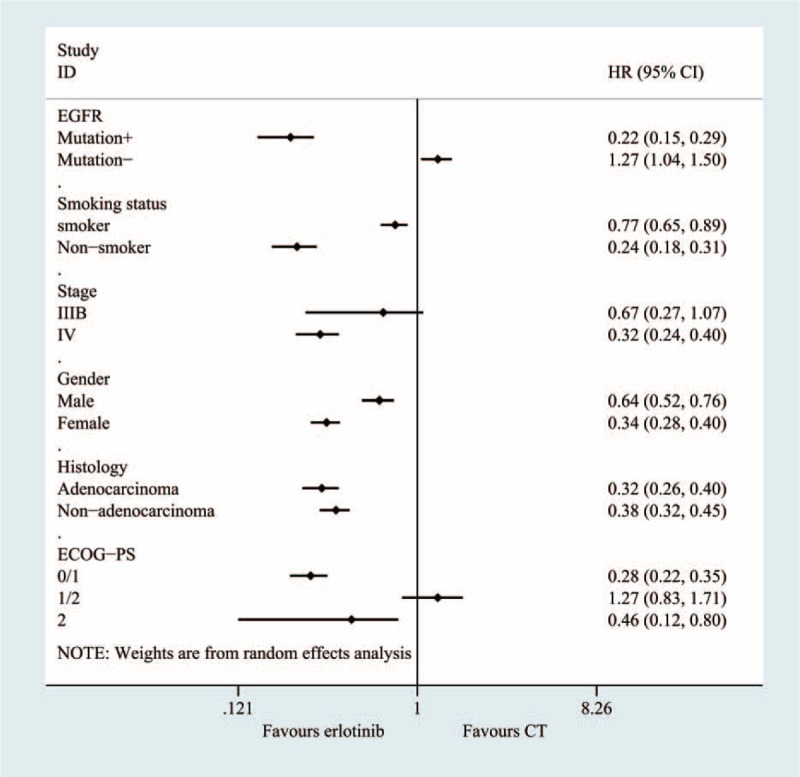
Subgroup and meta-regression analyses of the PFS.
FIGURE 8.
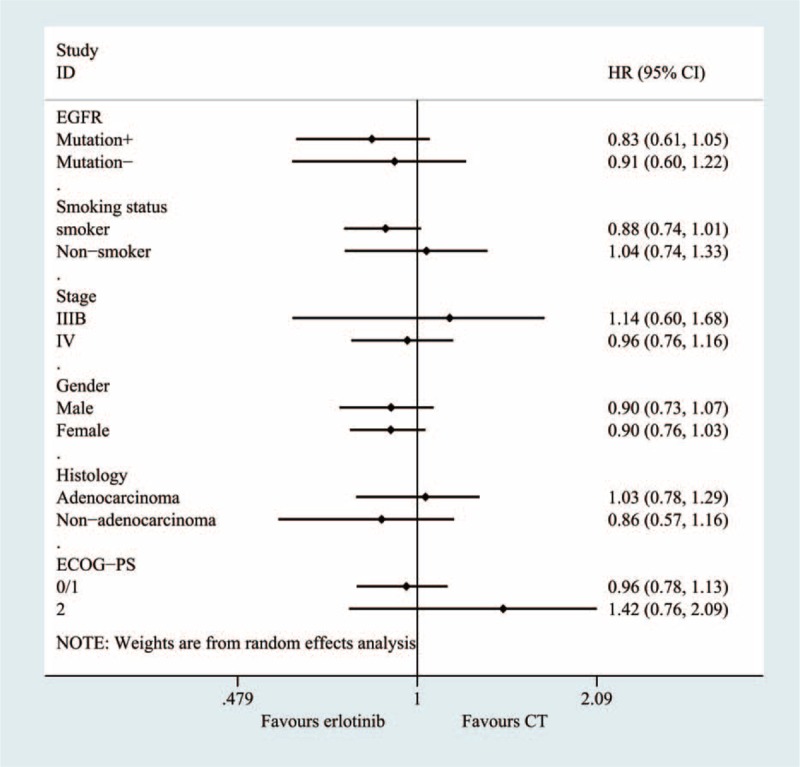
Subgroup and meta-regression analyses of the overall survival.
Meta-Regression and Sensitivity Analysis
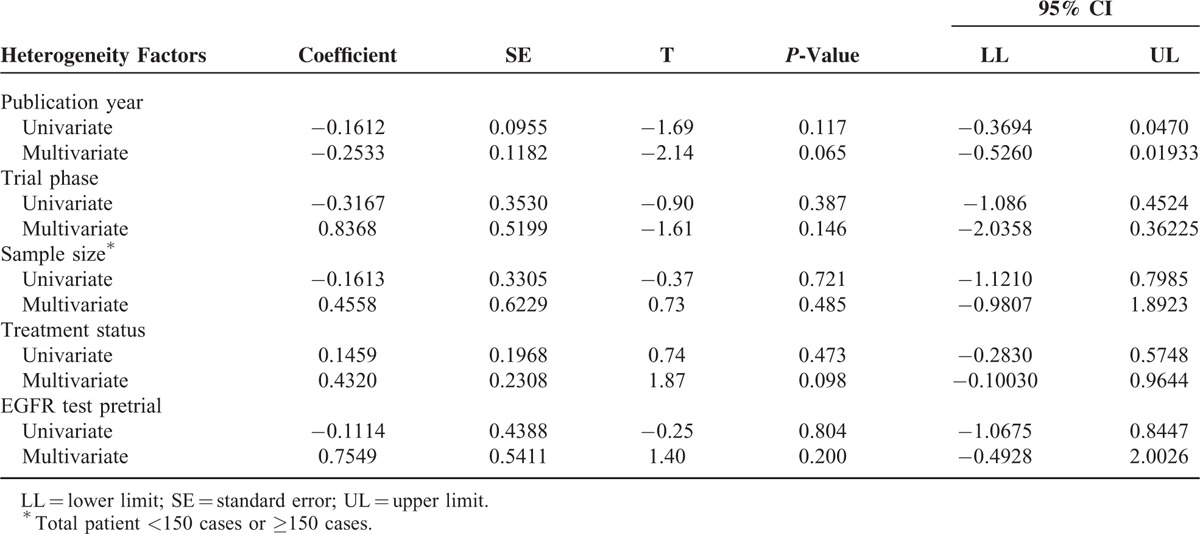
To investigate the effects of various study characteristics on HR estimates, a meta-regression analysis was conducted (only for the PFS results) by grouping the studies according to specific characteristics, such as pretrial EGFR test, sample size, trial phase, treatment status, and publication year. However, the univariate and multivariate meta-regression analyses did not detect a borderline significant association between PFS and pretrial EGFR test, or other characteristics33,34 (Table 3).
TABLE 3.
Univariate and Multivariate Meta-Regression Analyses of Potential Sources of Heterogeneity in PFS
The sensitivity analysis indicated that the pooled PFS results were affected by the exclusion of certain individual trials, specifically the trials of Zhou et al,34,37 Chen et al,38 and Rosell et al33 (Figure 9). However, the pooled OS results were not affected by the exclusion of individual trials.
FIGURE 9.
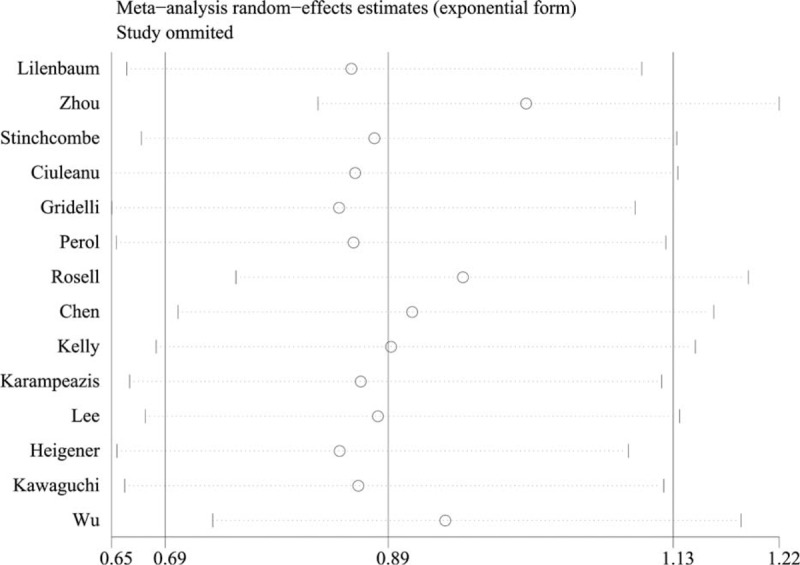
Sensitivity analysis result of progression-free survival.
Publication Bias
A funnel plot was performed on all the included studies that investigated the OS and PFS efficacies to determine the publication bias from the literature. The analysis outcome showed asymmetry, which suggests that a publication bias possibly existed in the included trials. Begg and Egger tests were performed to quantitatively test the asymmetry of the funnel plot, and no bias was determined for the OS (PEgger = 0.194, PBegg = 0.194) and PFS rates (PEgger = 0.066, PBegg = 0.066).
Level of Evidence
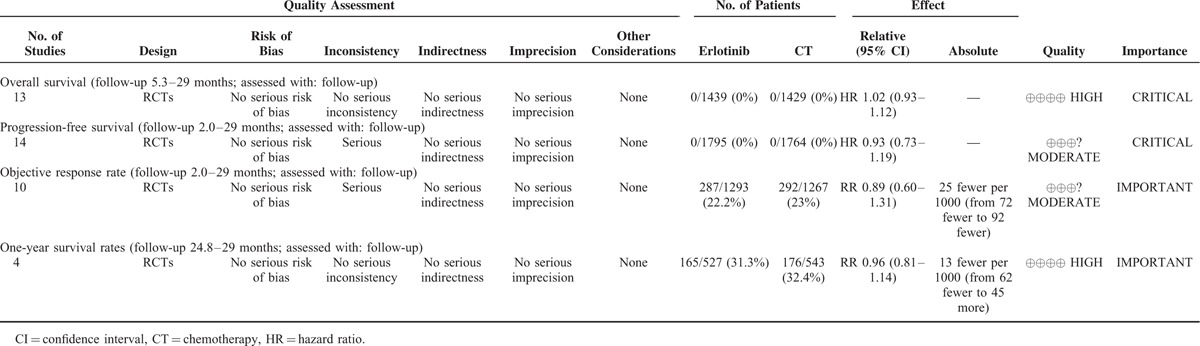
There were 4 efficacy outcomes in this meta-analysis. The OS and PFS rates were critical results, and the 1-year survival and ORR were important results. The quality of the evidence of each result is reported in Table 4.
TABLE 4.
GRADE Profile Evidence of the Included Studies
DISCUSSION
The pooled results of the meta-analysis that utilized 14 RCTs to compare erlotinib and chemotherapy treatment groups demonstrated that no significant difference existed regarding most outcomes, including the OS, PFS, 1-year survival and objective response rates. In this review, subgroup analyses were conducted according to EGFR status, drug condition, histology condition, length of illness and other factors. The subgroup analysis, which was based on EGFR mutations, suggested that erlotinib could greatly increase the PFS rate in patients with an EGFR mutation (HR, 0.22; 95% CI = 0.15–0.30, P < 0.0001). For patients without an EGFR mutation, the usage of erlotinib inversely varied with the PFS rate. In other words, the PFS time could increase with erlotinib use. The pooled results showed that erlotinib use was unrelated to OS among patients with and without an EGFR mutation. The sample size and mutation condition tests can explain most of the heterogeneity observed, according to the results of the sensitivity analysis and meta-regression. The sensitivity analysis using the leave-one-out method revealed that the heterogeneity was decreased to 67% when the 2 trials that evaluated mutation conditions were eliminated.
Several meta-analyses on EGFR-TKIs have been published in recent years, most of which employed trials with varying drug priorities. A majority of the published studies focused on efficacy, while the correlation between EGFR mutation and efficacy was reported in 4 meta-analyses.11–14 Additionally, of these meta-analyses, 3 focused on the relationship between the mutation type and efficacy; however, 1 study, which was published in JAMA,13 did not assess this correlation. Another major point that was not presented in all 4 of these meta-analyses is that the efficacy may be affected by sample size, the eligible patient age, treatment duration and other factors. Deficiencies in some published studies were managed by planning in the meta-analysis, in which some stage 2 clinical trials were included. The meta-analysis showed that no difference existed between stage 2 and 3 of the clinical drug groups. Importantly, the GRADE system was performed to assess the level of evidence summarized in the meta-analysis.
There are a number of limitations to this meta-analysis that need to be acknowledged. First, only English and Chinese language literature articles were considered in the analysis. If the search had been extended to include literature published in other languages, it is possible that additional relevant trials may have been identified. Second, on-going studies were ineligible for inclusion, although this meta-analysis included in 14 studies, but the sample is not very enough, some studies were small samples. Limitations in quality, even though most of the studies were of high quality, cannot be ignored, and the pooled results of this meta-analysis may have been slightly affected. Moreover, only a small number of trials met the subgroup analysis criteria, thus reducing the power of the analysis. Additionally, some parameters are not coming from the real data, we used the Tierney et al's17 formula to calculate the missing hazard rate, although our previous research used this method,9 but this formula might reduce the credibility of the analysis results. Furthermore, as the studies included in the meta-analysis were carried out in various countries, oncologists should carefully and judiciously assess the feasibility of applying the results to the clinical setting in China.
In conclusion, the present systematic review and meta-analysis suggested that erlotinib did not improve the ORR, PFS, OS, or the 1-year survival rate for whole patients with or without EGFR mutation test. Nevertheless, the subgroup analysis revealed that erlotinib did not affect the OS regardless of EGFR mutation status, however, the agent prolonged PFS in subjects with EGFR mutation, but not in those without EGFR mutation. The GRADE system suggested our evidences are of good quality, however, our finding partly relies on data from Kaplan–Meier curves by Engauge Digitizer (version 4.1), potentially subject to other bias, this conclusion should be interpreted cautiously, and thus this conclusion should be interpreted cautiously, and the meta-regression did not find significant association between PFS and characteristics. Therefore, high-quality and adequately powered RCTs for this subgroup patients are warranted.
Acknowledgments
The authors would like to appreciate the work of editors and anonymous referees. Prof. Jinhui Tian of the Evidence-Based Medicine Center, Lanzhou University, for information retrieval. Dr. Meng-Meng Han of College of traditional Chinese medicine, Tianjin University of Traditional Chinese Medicine, critically edited language.
Footnotes
Abbreviations: Ca = carboplatin; Ci = cisplatin; CI = confidence interval; CT = chemotherapy; D = docetaxel; E = erlotinib; ECOG PS = Eastern Cooperative Oncology Group performance status; EGFR = epidermal growth factor receptor; EGFR-TKI = epidermal growth factor receptor-tyrosine kinase inhibitor; G = gemcitabine; HR = hazard ratio; NA = not available; NCCN = The National Comprehensive Cancer Network; NSCLC = nonsmall-cell lung cancer; ORR = objective response rate; OS = overall survival; Pa = paclitaxel; Pe = pemetrexed; PFS = progression-free survival; Pr = pralatrexate; RCTs = Randomized Controlled Trials; RR = relative risk, risk ratio; TTP = tumor progression time; V = vinorelbine.
All the authors reviewed the ICMJE criteria for authorship and agreed with manuscript results and conclusions. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
HM, XT, and XTZ contributed equally to this work as first author.
Authors’ contributions: J-GZ conceived and designed the experiments; JGZ, XT, and HM performed the experiments; JGZ, XT, XTZ, and YZ analyzed the data; JGZ, XTZ, YW, and FW contributed reagents/materials/analysis tools; JGZ, HM, YZ, and FW wrote the first draft of the manuscript. All the authors contributed to the writing of the manuscript.
This research was supported by Natural Science Foundation of China (NSFC) (81360351), The Department of Science and Technology of Guizhou Province (grant no. Qian Ke He SY [2013] 3003), High-Level Innovative Talents Cultivation Program of Guizhou Province, Start-Up Fund for Doctor of Zunyi Medical University, and The Social Practice Program for Postgraduate of Zunyi Medical University (grant no. zyyjs2015004).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Zhang S, et al. Annual report on status of cancer in China, 2010. Chin J Cancer Res 2014; 26:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29.doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 5.Bezjak A, Tu D, Seymour L, et al. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 2006; 24:3831–3837. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/417/CN-00567417/frame.html [DOI] [PubMed] [Google Scholar]
- 6.Shepherd FA, Pereira JR, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005; 353:123–132. [DOI] [PubMed] [Google Scholar]
- 7.Zhou JN, Zeng Q, Wang HY, et al. MicroRNA-125b attenuates epithelial-mesenchymal transitions and targets stem-like liver cancer cells through small mothers against decapentaplegic 2 and 4. Hepatology 2015; 62:801–815.doi: 10.1002/hep.27887. [DOI] [PubMed] [Google Scholar]
- 8.NCCN org. NCCN Guidelines Non-Small Cell Lung Cancer Guidelines (2014). Available at:, http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#nscl,version (Accessed: 14th September 2014) [Google Scholar]
- 9.Zhou JG, Tian X, Wang X, et al. Treatment on advanced NSCLC: platinum-based chemotherapy plus erlotinib or platinum-based chemotherapy alone? A systematic review and meta-analysis of randomised controlled trials. Med Oncol 2015; 32:471.doi: 10.1007/s12032-014-0471-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhou JG, Tian X, Cheng L, et al. The risk of neutropenia and leukopenia in advanced non-small cell lung cancer patients treated with erlotinib: a prisma-compliant systematic review and meta-analysis. Medicine 2015; 94:e1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tassinari D, Scarpi E, Sartori S, et al. Second-line treatments in non-small cell lung cancer. Chest 2009; 135:1596–1609. [DOI] [PubMed] [Google Scholar]
- 12.Haaland B, Tan PS, de Castro G, Jr, et al. Meta-analysis of first-line therapies in advanced non-small-cell lung cancer harboring EGFR-activating mutations. J Thorac Oncol 2014; 9:805–811.doi: 10.1097/JTO.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JK, Hahn S, Kim DW, et al. Epidermal growth factor receptor tyrosine kinase inhibitors vs conventional chemotherapy in non-small cell lung cancer harboring wild-type epidermal growth factor receptor: a meta-analysis. JAMA 2014; 311:1430–1437. [DOI] [PubMed] [Google Scholar]
- 14.Zhao N, Zhang XC, Yan HH, et al. Efficacy of epidermal growth factor receptor inhibitors versus chemotherapy as second-line treatment in advanced non-small-cell lung cancer with wild-type EGFR: a meta-analysis of randomized controlled clinical trials. Lung Cancer 2014. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol 2013; 14:953–961. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8:16.doi:10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 24.Lee DH, Lee JS, Kim SW, et al. Three-arm randomised controlled phase 2 study comparing pemetrexed and erlotinib to either pemetrexed or erlotinib alone as second-line treatment for never-smokers with non-squamous non-small cell lung cancer. Eur J Cancer 2013; 49:3111–3121. [DOI] [PubMed] [Google Scholar]
- 25.Ciuleanu T, Stelmakh L, Cicenas S, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol 2012; 13:300–308. [DOI] [PubMed] [Google Scholar]
- 26.Gridelli C, Ciardiello F, Gallo C, et al. First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: the TORCH randomized trial. J Clin Oncol 2012; 30:3002–3011. [DOI] [PubMed] [Google Scholar]
- 27.Heigener DF, Deppermann KM, Pawel J, et al. Open, randomized, multi-center phase II study comparing efficacy and tolerability of Erlotinib vs. Carboplatin/Vinorelbin in elderly patients (>70 years of age) with untreated non-small cell lung cancer. Lung Cancer 2014; 84:62–66. [DOI] [PubMed] [Google Scholar]
- 28.Karampeazis A, Voutsina A, Souglakos J, et al. Pemetrexed versus erlotinib in pretreated patients with advanced non-small cell lung cancer: A Hellenic Oncology Research Group (HORG) randomized phase 3 study. Cancer 2013; 119:2754–2764. [DOI] [PubMed] [Google Scholar]
- 29.Kawaguchi T, Ando M, Asami K, et al. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol 2014; 32:1902–1908. [DOI] [PubMed] [Google Scholar]
- 30.Kelly K, Azzoli CG, Zatloukal P, et al. Randomized phase 2b study of pralatrexate versus erlotinib in patients with stage IIIB/IV non-small-cell lung cancer (NSCLC) after failure of prior platinum-based therapy. J Thorac Oncol 2012; 7:1041–1048. [DOI] [PubMed] [Google Scholar]
- 31.Lilenbaum R, Axelrod R, Thomas S, et al. Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol 2008; 26:863–869. [DOI] [PubMed] [Google Scholar]
- 32.Perol M, Chouaid C, Perol D, et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2012; 30:3516–3524. [DOI] [PubMed] [Google Scholar]
- 33.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13:239–246. [DOI] [PubMed] [Google Scholar]
- 34.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011; 12:735–742. [DOI] [PubMed] [Google Scholar]
- 35.Boutsikou E, Kontakiotis T, Zarogoulidis P, et al. Docetaxel-carboplatin in combination with erlotinib and/or bevacizumab in patients with non-small cell lung cancer. Onco Targets Ther 2013; 6:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE studydagger. Ann Oncol 2015; 26:1883–1889. [DOI] [PubMed] [Google Scholar]
- 37.Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015; 26:1877–1883. [DOI] [PubMed] [Google Scholar]
- 38.Chen YM, Tsai CM, Fan WC, et al. Phase II randomized trial of erlotinib or vinorelbine in chemonaive, advanced, non-small cell lung cancer patients aged 70 years or older, Journal of Thoracic Oncology. J Thorac Oncol 2012; 7:412–418. [DOI] [PubMed] [Google Scholar]
- 39.Stinchcombe TE, Peterman AH, Lee CB, et al. A randomized phase II trial of first-line treatment with gemcitabine, erlotinib, or gemcitabine and erlotinib in elderly patients (age >/=70 years) with stage IIIB/IV non-small cell lung cancer. J Thoracic Oncology : Official Publication International Association Study Lung Cancer 2011; 6:1569–1577. [DOI] [PubMed] [Google Scholar]


