This was a randomized phase II study of chemotherapy (arm A: pemetrexed or docetaxel) versus chemotherapy plus erlotinib (ERL) (arm B) in 46 patients with progressive non-small cell lung cancer following clinical benefit from ERL. The primary endpoint was that continuation of erlotinib in this patient population could extend progression-free survival (PFS) by 50%. No significant difference in PFS was seen for continuing ERL beyond progression in mutation-positive patients, and patients in arm A experienced more toxicity.
Keywords: Epidermal growth factor receptor, Tyrosine kinase inhibitor, Non-small cell lung cancer, Erlotinib
Abstract
Background.
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) therapy is clearly beneficial in patients with advanced EGFR-mutated non-small cell lung cancer (NSCLC). However, acquired resistance develops uniformly and the benefit of continuation of EGFR TKI therapy beyond progression remains unclear.
Materials and Methods.
This was a randomized phase II study of chemotherapy (arm A: pemetrexed or docetaxel) versus chemotherapy plus erlotinib (ERL) (arm B) in patients with progressive NSCLC following clinical benefit from erlotinib. In arm B, chemotherapy was given with erlotinib at an oral daily dose of 150 mg on days 2–19 of each cycle to minimize negative pharmacodynamic interactions. The primary endpoint was that continuation of erlotinib in this patient population could extend progression-free survival (PFS) by 50%.
Results.
A total of 46 patients were randomized (arm A: 24; arm B: 22). Patient characteristics were well balanced except there were more female patients in arm A (p = .075). The median PFS of patients in arm A was 5.5 months and for those in arm B, 4.4 months (p = .699). The response rates were 13% and 16% in arms A and B, respectively (p = .79). EGFR status data were available for 39 of the 46 patients and no significant difference in PFS was seen for continuing ERL beyond progression in mutation-positive patients. Substantially more toxicity was seen in arm B than arm A.
Conclusion.
There was added toxicity but no benefit with the continuation of ERL beyond progression along with chemotherapy as compared with chemotherapy alone.
Implications for Practice:
The benefits of continuing erlotinib upon progression alongside conventional chemotherapy are unclear. This randomized phase II study, initiated prior to the establishment of routine epidermal growth factor receptor (EGFR) mutation testing, addressed this clinically relevant issue through randomizing patients with prior clinical benefit from erlotinib (thereby enriching for EGFR-mutated tumors) upon progression in the second- or third-line setting to conventional chemotherapy (single-agent pemetrexed or docetaxel) with or without continued erlotinib. The results showed no benefit to continuing erlotinib beyond progression, while significantly more side effects were noted in the combination arm. Along with other recently presented study findings, these results argue against the routine practice of continuing erlotinib in this setting.
Abstract
摘要
背景. 对于表皮生长因子受体 (EGFR) 突变的晚期非小细胞肺癌 (NSCLC) 患者, EGFR 酪氨酸激酶抑制剂 (TKI) 治疗有明确的获益。但患者均会发生获得性耐药,而进展后继续 EGFR TKI 治疗的获益尚不明确。
方法. 本研究为随机 II 期临床研究,在厄洛替尼治疗有临床获益的 NSCLC 患者发生进展后对化疗(A 组: 培美曲塞或多西他赛) 与化疗联合厄洛替尼 (ERL) (B 组) 进行比较。B 组在化疗的同时, 还给予厄洛替尼 150 mg 每日一次口服, 在每个治疗周期的 D2 ∼ D29 给药,以将不利药效动力学相互作用降至最小。主要终点为该患者人群继续厄洛替尼治疗可使无进展生存 (PFS) 增加 50%。
结果. 共 46 例患者进入随机分组(A 组: 24 例, B 组: 22 例)。除A组女性较多以外(P = 0.075), 两组患者特征分布均衡。A 组的中位 PFS 为 5.5 个月, B 组为 4.4 个月 (P = 0.699)。A 组缓解率为 13%, B 组为 16% (P = 0.79)。39/46 例患者有 EGFR 状态数据, 突变阳性患者进展后继续 ERL 治疗的 PFS 差异无统计学意义。B 组的毒性事件明显多于A 组。
结论. 与单用化疗相比,进展后继续ERL联合化疗毒性增加而未见获益。The Oncologist 2015;20:1298–1303
对临床实践的提示:进展后给予传统化疗的同时继续厄洛替尼治疗的获益尚不明确。本项随机 II 期研究开始于常规表皮生长因子 (EGFR) 突变检测确认阳性之前,通过将曾从厄洛替尼治疗中得到临床获益的患者 (因此聚集了 EGFR 突变肿瘤患者) 在发生进展后进行随机分配, 于之后的二线或三线治疗中在传统化疗 (培美曲塞或多西他赛单药) 基础上联合或不联合继续厄洛替尼治疗, 从而解决了前述的问题。结果显示进展后继续厄洛替尼治疗没有获益, 而联合组中还观察到明显较多的不良反应。考虑到近期发表的其他研究结果, 这些结果反对在该人群中继续使用厄洛替尼作为常规方案。
Introduction
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) therapy has proven value in patients with advanced non-small cell lung cancer. Results of the JBR.21 study demonstrated a survival benefit for erlotinib versus best supportive care in the second- or third-line therapy of advanced non-small cell lung cancer, leading to the approval of erlotinib for this indication [1]. It was later discovered that EGFR TKI therapy is of particular benefit for a subset of patients with tumors harboring activating EGFR gene mutations, such as exon 19 deletions and exon 21 L858R gene mutations. Based on a series of randomized studies demonstrating improved response rates and progression-free survival with EGFR TKI therapy (erlotinib, gefitinib, and afatinib) when compared with frontline chemotherapy, frontline EGFR TKI therapy has become the standard of care for this molecularly defined subgroup of patients [2].
Despite the consistent benefit of EGFR TKI therapy in EGFR-mutated lung cancer, disease progression is uniform and acquired resistance is a key clinical problem [3, 4]. It remains unclear whether continuation of EGFR TKI therapy at the time of disease progression is of benefit, but, in practice, it is commonly pursued, analogous to continuing antiandrogen or trastuzumab therapy beyond progression in prostate and ErbB2-positive breast cancer [5, 6]. At the time of progression, each patient likely harbors multiple tumor clones, such as ones with acquired resistance mechanisms responsible for the observed disease progression on imaging, as well as other clones that remain sensitive and suppressed through EGFR TKI therapy. Continued suppression of these clones could theoretically yield clinical benefits through EGFR TKI therapy beyond progression. In addition, anecdotal clinical observations suggestive of rapid tumor flares upon cessation of targeted therapy in approximately 20% of the patients have added to the common acceptance in clinical practice of continuing EGFR TKI therapy beyond progression [7]. However, pharmacodynamic interactions, added toxicity, and costs, as well as high-level molecular resistance, including the development of acquired T790M mutations, might limit the benefit of continued EGFR TKI therapy with a reversible EGFR TKI such as erlotinib [8]. Despite the intriguing basis for continued EGFR TKI therapy upon progression and its use in clinical practice, we continue to lack evidence of a clinical benefit from such an approach. Given the financial costs and associated toxicities of TKI therapy, randomized trials evaluating the role of continuing these drugs beyond progression are desperately needed.
In 2007, we initiated a randomized phase II study to assess the potential benefit for continued EGFR TKI therapy beyond progression. This study was initiated at a time when EGFR mutation testing was not uniformly accepted and frontline EGFR TKI therapy was not yet the standard of care. The study was designed, therefore, to allow patients to enter who had derived a significant clinical benefit from erlotinib therapy, and all patients entered this study directly from EGFR TKI therapy. The chemotherapy comparator was standard second-line chemotherapy with pemetrexed (with a later amendment allowing docetaxel, as well, for subjects who had previously received pemetrexed), as it was anticipated that the majority of patients would have received erlotinib following failure of frontline therapy. However, the study design permitted the participation of patients who had not yet received frontline chemotherapy.
Materials and Methods
Patient Selection
Eligible patients had pathologically confirmed stage IIIB (with pleural effusion) or stage IV non-small cell lung cancer (by American Joint Committee on Cancer 6th edition criteria), who showed signs of RECIST-defined disease progression after at least twelve weeks of erlotinib treatment that previously resulted in clinical benefit as assessed by physician and radiological assessments. Subjects were ≥18 years of age, had measurable disease by RECIST, had Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; had life expectancy of at least 12 weeks; had adequate hematologic, hepatic and renal functions; and agreed to practice appropriate contraception. Only patients who provided written informed consent were included.
Patients with history of more than one prior cytotoxic chemotherapy regimen for relapsed or metastatic disease (not including erlotinib) and any prior EGFR inhibitor (beside erlotinib) were excluded. Treatment with any systemic chemotherapy or experimental agent within 3 weeks and radiation therapy within 2 weeks of treatment were prohibited. All patients had their previous erlotinib held for a minimum of 2 weeks prior to study enrollment. Patients with known or suspected clinically active brain metastases were not included; however, patients with stable brain metastases were allowed. Patients with uncontrollable fluid in the pleural/peritoneal cavity, greater than grade 2 neuropathy, history of hypersensitivity to docetaxel or other drugs formulated with polysorbate, and pregnant or breast-feeding women were all excluded from the study. The protocol was approved by the institutional review board at each participating center.
Study Treatment
Stage IIIB (with pleural effusion) or stage IV EGFR TKI-responsive non-small cell lung cancer (NSCLC) patients were randomly assigned (1:1) to 1 of 2 treatment arms: arm A and arm B. Patients were stratified according to ECOG performance status (0–1 vs. 2) and smoking status (smokers vs. never-smokers). Patients randomized to arm A received pemetrexed 500 mg/m2 or docetaxel 75 mg/m2 on day 1, and then every 3 weeks. Patients randomized to arm B received the same treatment as arm A, with the addition of once-daily erlotinib 100–150 mg taken orally on days 2–19 of each treatment cycle. The dose of erlotinib was chosen based on the previous dose of erlotinib the patient was taking prior to study enrollment as long as it was at least 100 mg/day. This would thus prevent increasing the dose of erlotinib to 150 mg in a given patient if the previously tolerated dose was 100 mg. Patients treated with pemetrexed received appropriate vitamin B12 and folic acid supplementation and all patients received concomitant steroids according to institutional standards. Protocol allowed for a total of eight planned cycles of chemotherapy, with flexibility of increasing this number if a patient showed benefit from the treatment. Patients in the combination arm (arm B) were allowed to continue erlotinib alone after discontinuation of chemotherapy until disease progression, unacceptable toxicity, or withdrawal of consent.
Study Endpoints
The primary endpoint was progression-free survival (PFS) and secondary endpoints were objective response rate, overall survival, and disease stabilization. Efficacy was evaluated by RECIST criteria. Overall response was recorded from the start of the treatment until disease progression/recurrence. The primary hypothesis of this study was that erlotinib beyond progression, in addition to standard of care (pemetrexed or docetaxel) chemotherapy, in patients who derived significant clinical benefit from erlotinib will lead to a significant prolongation of progression-free survival. The median progression-free survival in patients on second- and third-line chemotherapy was estimated in the range of 3 months and the outcome was expected to be identical in the aggregate regardless of whether pemetrexed or docetaxel was to be used for any individual patient. Our hypothesis was that erlotinib beyond progression in this select patient population could extend progression-free survival by 50%, from 3 to 4.5 months. By a randomized phase II screening design, with a follow-up period of 6 months after enrollment of the last patient, a two-group, one-sided exponential maximum likelihood estimate test with a 0.200 significance level would have 80% power to detect the difference between standard-of-care chemotherapy (pemetrexed or docetaxel) median PFS of 3 months and standard-of-care chemotherapy (pemetrexed or docetaxel) plus erlotinib median PFS of 4.5 months (hazard ratio: 1.5) when the sample size in each treatment arm is 39. Therefore, the planned sample size was 78 patients for the trial (39 patients per arm). Blocked randomization with a block size of 4 stratified by the status of smoking (i.e., lifetime nonsmoker vs. ever-smoker) and performance status (i.e., performance status 0–1 vs. 2) was used.
Survival analysis was performed following accrual of 46 patients. This decision was based on significant practice changes in the oncology community, where erlotinib was continued beyond progression in most patients, resulting in slowed patient accrual. By that time, 41 of the patients had had an event for PFS. The power conditioning of the data obtained to that point suggested that even if we were to finish the study by enrolling the remaining 32 patients (given the target enrollment of 39 patients per arm), at that point, the conditional power was calculated to be less than 1% to detect the originally stated objective (i.e., median PFS of 3 months for arm A vs. 4.5 months for arm B if another 32 patients were enrolled) and, therefore, the study was stopped prematurely in February 2013 because of poor enrollment/projected futility.
Safety Assessments
Adverse events were assessed using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Patients were evaluated for progression after every two treatment cycles. Treatment was administered on an outpatient basis and patients continued on protocol therapy until progression or unacceptable toxicity. At the start of every cycle, pemetrexed and docetaxel treatment was delayed for up to 2 weeks in both arms A and B if absolute neutrophil count (ANC) was less than 1,500/μL and platelet count less than 100,000/μL. Treatment was restarted at 75% of original dose for a platelet nadir of 50,000/µL or more and ANC nadir less than 500/µL, and 50% of original dose if the platelet nadir was less than 50,000/µL, regardless of ANC. For grade 3 or 4 myelosuppression, grade 3 or 4 diarrhea, grade 3 or 4 mucositis, grade 3 neuropathy (docetaxel only), and other toxicities of grade 3 or higher (with the exception of alopecia and grade 3 or 4 nausea/vomiting), treatment was delayed until resolution to grade 1 or equal to the patient’s original baseline grade. Treatment could be held for up to 2 weeks and was resumed at 75% of the previous dose. Patients were withdrawn from the study if toxicity did not resolve to lower than CTCAE grade 1 within 2 weeks.
Dose-modifying toxicities for erlotinib included grade 3 or 4 diarrhea, grade 3 rash, and all other grade 3 toxicities. Treatment was interrupted until resolution to grade 2 or lower and then restarted at a lower dose depending on the initial starting dose. The minimum dose was 50 mg/day; if additional reductions were required, the patient was taken off treatment. Patients were discontinued in the study if treatment needed to be delayed by more than 2 weeks. Any grade of interstitial lung disease, and all other grade 4 toxicities, resulted in permanent discontinuation of erlotinib.
Molecular Testing
Data on molecular testing for exon 19 and 21 alterations in patients were collected from participating sites, if available.
Statistical Analysis
PFS was measured from the date of onset of treatment to the date of disease progression or the date of death, whichever occurred earlier, and censored at the date of last follow-up for those alive without disease progression. The overall survival (OS) was measured from the date of onset of treatment to the date of death, and censored at the date of last follow-up for survivors. Survivor distribution was estimated using Kaplan-Meier methods and difference of OS and PFS between groups was examined by log-rank test. The effect of treatment on survival (OS, PFS) was estimated using the Cox model after controlling for effects of age, sex, nodal status and EGFR mutation results. The difference in age between treatment arms was examined by Student’s t test and the association between two categorical variables was examined using the chi-square test. All tests were two-sided and p ≤ .05 was considered statistically significant.
Results
Patient Characteristics
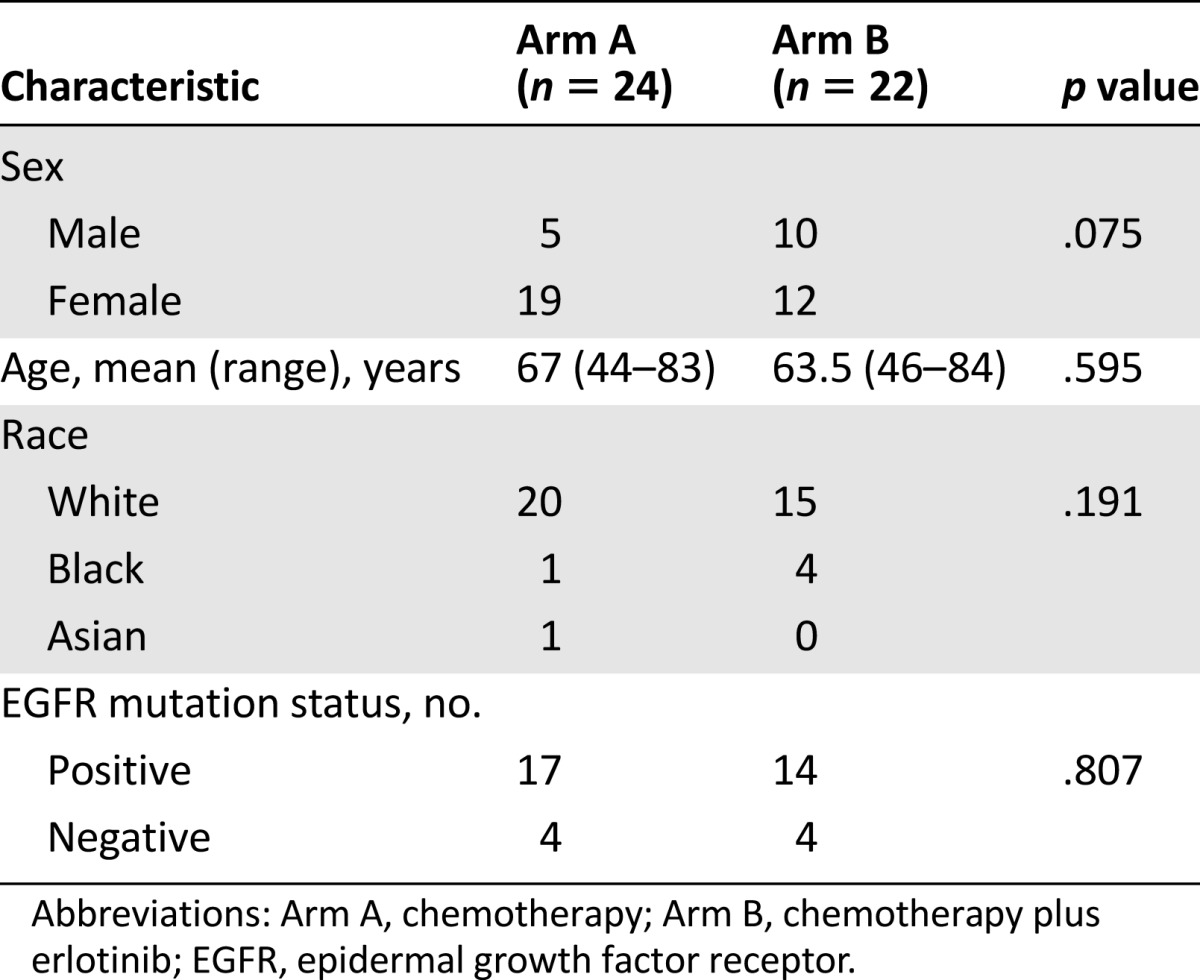
A total of 46 patients were randomized at 7 institutions between 2008 and 2012. Of these, 24 patients were randomized to arm A (chemotherapy alone) and 22 patients to arm B (chemotherapy plus erlotinib). Twenty-three patients from arm A and 20 patients from arm B received pemetrexed as their selected chemotherapy; the remaining received docetaxel (these patients received pemetrexed as part of their prior chemotherapy regimen). Patient characteristics were well balanced between study arms (Table 1) except more female patients were accrued to arm A (p = .075). Overall, as anticipated based on study criteria, there was a higher percentage of women (67%), the mean age was 65 years, the majority of patients were white (76%), and 5 patients were black. In arm A, 13 of 24 patients had received erlotinib alone previous to study enrollment (10 of 20 patients in arm B), while 11 of 24 received erlotinib after frontline chemotherapy (10 of 20 in arm B) prior to study treatment. However, no patient had received pemetrexed or docetaxel prior to study enrollment (depending on which chemotherapy was administered on this trial). The mean time on initial EGFR TKI was 18 months for arm A versus 16 months for arm B prior to study enrollment. In both arms, rates of partial response and stable disease during prior EGFR-TKI treatment were 65% and 35%, respectively. EGFR status was known for 39 of the 46 patients (85%) and 80% of the subjects with known EGFR status had tumors that harbored an activating EGFR mutation. Seventeen patients in arm A and 14 patients in arm B had documented EGFR-mutated tumors (all patients with documented mutations had classic exon 19 and 21 mutations). Of note is that the study was initiated at a time when EGFR mutation testing was not yet routine practice, accounting for the few subjects with unknown EGFR status.
Table 1.
Patient characteristics
Efficacy Evaluation
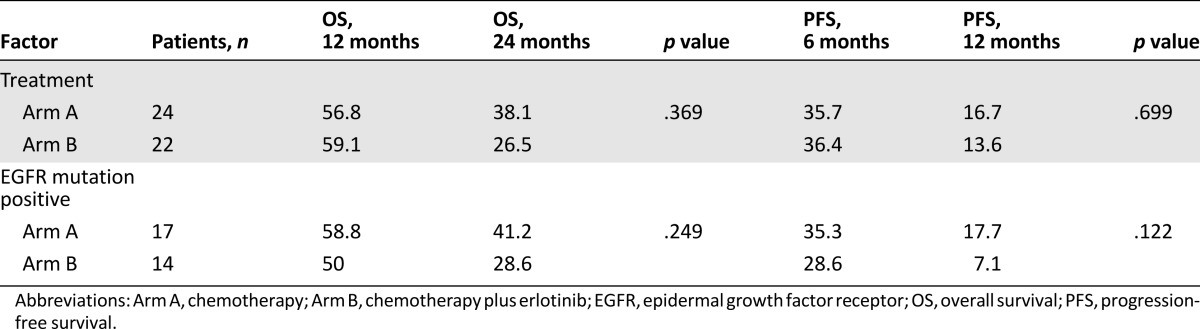
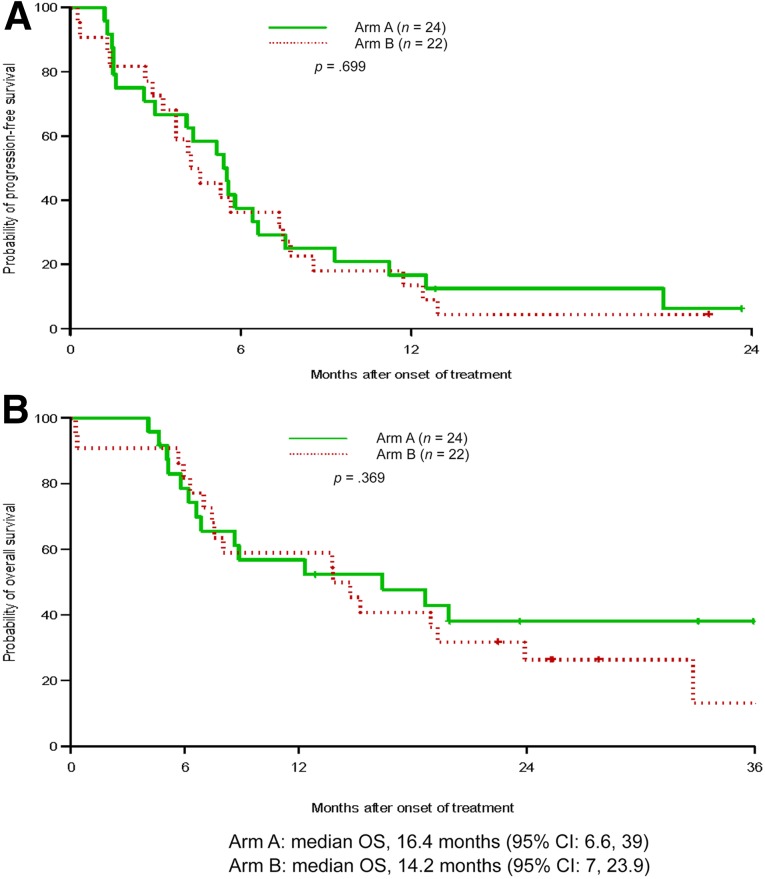
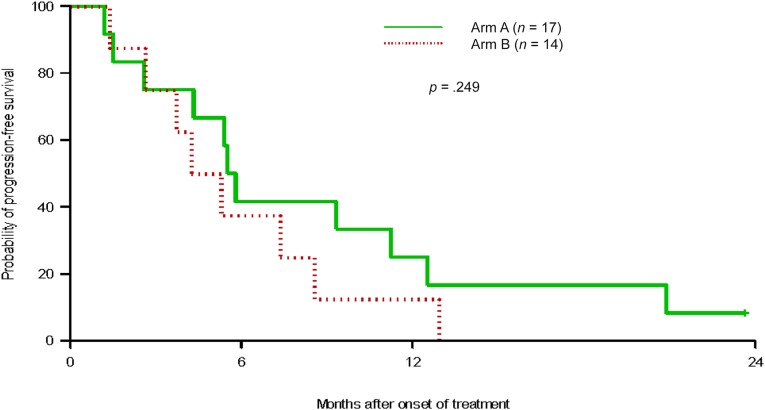
The median progression-free survival (the primary endpoint of the study) of patients in arm A was 5.5 months, while in arm B, it was 4.4 months; there was no statistically significant difference between the arms (p = .699) (Table 2, Fig. 1). The median overall survival in arm A was 16.4 months and for arm B, it was 14.2 months (p = .369). Subset analyses were limited to patients who were documented as EGFR-mutation positive and no difference in progression-free or overall survival (p = .332 [Fig. 2], and p = .346, respectively) was noted between the arms in this subset, either. In the mutation-positive patients, 6-month survival was 39% in arm A and 32% in arm B. The overall response rate was 15% for the entire study group and similar between the 2 groups: 13% for arm A and 17% for arm B (p = .37). Disease control rate (response plus stable disease) was 94% for the overall group, 100% for arm A, and 89% for arm B. Subgroup analysis of patients with known EGFR mutation status showed that the response rates for those positive for EGFR mutation and those negative for EGFR mutation were 14.3% and 16.7%, respectively (p = .885). No documented cases of tumor flare were noted in arm A of study therapy.
Table 2.
Kaplan-Meier estimation of OS (%) and PFS (%)
Figure 1.
Graphs of Kaplan-Meier estimations. (A): Progression-free survival in treatment arms. (B): Overall survival in treatment arms.
Figure 2.
Kaplan-Meier estimation of progression-free survival in patients with documented epidermal growth factor receptor mutations.
Safety and Tolerability
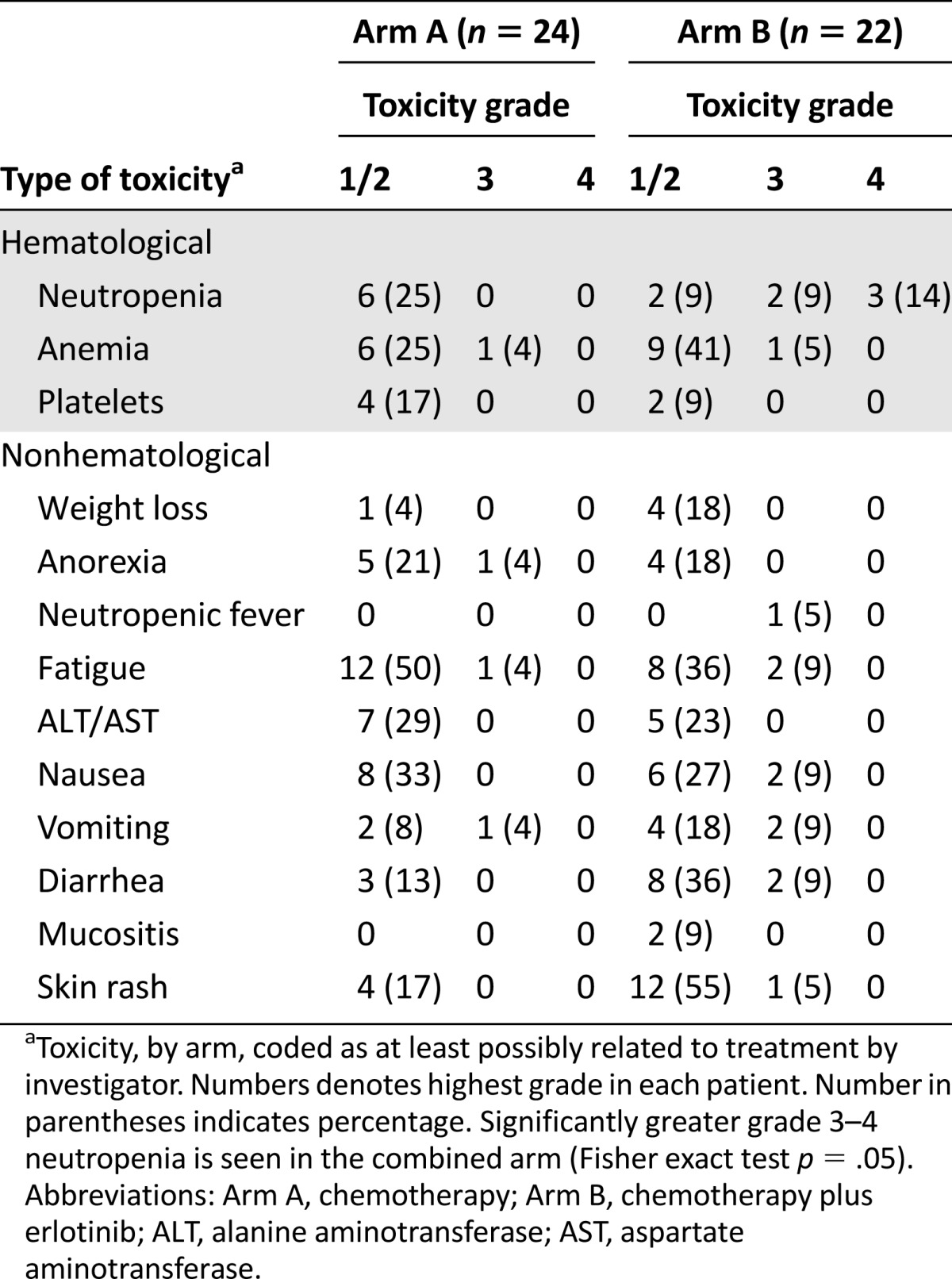
Toxicities were assessed by CTCAE 4.0 criteria and, overall, a significant increase in adverse events was noted in subjects in arm B as opposed to patients in arm A of the study (Table 3). A total of 7 grade 3 or 4 adverse events were reported in arm A, while 24 grade 3 or 4 events were noted in arm B. One grade 5 event occurred in each study arm. Overall, 7 of 24 patients in arm A suffered at least 1 grade 3 or higher toxicity while 16 of 22 patients (72.7%) had a grade 3 or higher event in arm B (p = .01). The increased toxicity principally appeared to be caused by hematological and gastrointestinal toxicities.
Table 3.
Toxicity assessment
Discussion
Our study evaluated the potential benefit of EGFR TKI therapy with erlotinib beyond progression, in addition to standard chemotherapy, in patients with erlotinib-responsive advanced non-small cell lung cancer (predominantly patients with EGFR-mutated lung adenocarcinomas). This study was terminated because of slowed accrual as a result of significant practice changes; 46 of the planned 78 patients were enrolled at the time of study termination. At that point, statistical modeling suggested that even if the study were to be completed, it was highly unlikely that positive results demonstrating the benefit of continued erlotinib treatment would be seen. Despite early termination and poor accrual, this study still is of significant value to guide practical management of patients. Contrary to the perceived utility of continuing erlotinib beyond progression, our study showed no significant benefit for erlotinib beyond progression, as measured by response rate and progression-free survival. Although not all patients in this study had EGFR testing, strict study eligibility led to a highly enriched patient population. Importantly, results were no different in those patients harboring EGFR mutations (80% of those tested). In addition, we observed a significant increase in toxicities in the combination arm, overall arguing against the widespread adoption of this approach.
Limited retrospective experiences have been reported on this subject, with mixed results and great limitations because of the retrospective nature of the studies and high potential for bias. In their retrospective review of 64 patients with advanced non-small cell lung cancer with an activating EGFR gene mutation [9], Nishie et al. reported that all patients received upfront gefitinib therapy and, upon progression, 39 of them continued gefitinib (without added chemotherapy) and 25 switched to chemotherapy. In the group that continued gefitinib, overall survival was 32 months compared with 23 months in the chemotherapy group (p = .005), suggesting a benefit for continued EGFR TKI therapy. One major concern of this analysis was that patients with slow progression/more indolent disease were selected by their treating physicians for the continued EGFR TKI therapy, leading to potential selection bias and limiting the ability to draw firm conclusions. Nishino et al. performed a retrospective evaluation of patients with advanced NSCLC who were treated with and responded to gefitinib between 2002 and 2010 [10]. This study found that those patients defined as long-term survivors were more likely to have been rechallenged by gefitinb or receive gefitinib beyond progression, leading the authors to speculate that the longer EGFR TKI exposure led to improved outcomes. However, without randomization, one cannot be certain that the differences demonstrated might not be confounded by more indolent biology in this group.
Asami et al. reported on a similarly limited retrospective experience in which it was found that overall survival seemed improved in the group of patients continuing gefitinib beyond progression for at least 3 months [11]. The most clinically useful data come from a retrospective U.S. experience reported by Goldberg et al., who performed a retrospective analysis of their institutional database for patients with EGFR-mutated non-small cell lung cancer who developed acquired resistance to an EGFR TKI (erlotinib or gefitinib) [12]. This study analyzed outcomes based on postprogression treatment with chemotherapy with or without continued EGFR TKI (principally erlotinib) [12]. Of the 78 patients included in this study, 34 received chemotherapy plus erlotinib and 44 received chemotherapy alone. Objective response rates were evaluable in only 57 and were higher in the chemotherapy plus EGFR TKI group (41% vs. 18%). However, median progression-free survival was not different (4.4 vs. 4.2 months), nor was overall survival (14.2 vs. 15.0) months. The authors concluded that EGFR TKI therapy beyond progression, based on this experience, could be beneficial. However, the fact that there was no difference in PFS and OS makes this statement somewhat questionable.
Preliminary findings of the LUX-Lung 5 study also have been reported [13]. This study randomized a clinically enriched patient population with advanced non-small cell lung cancer whose disease progressed on chemotherapy as well as while receiving at least 12 weeks of EGFR TKI (gefitinib or erlotinib). Patients were then randomized to afatinib, and patients who progressed after at least 12 weeks of afatinib monotherapy were then randomized again to afatinib plus paclitaxel chemotherapy versus investigator’s choice chemotherapy alone. Longer PFS (5.6 vs. 2.8 months) and higher response rates (32.1 vs. 13.2 months) favored the combination group, while overall survival was not different between groups.
Last, in an abstract, preliminary results of the more definitive Asian IMPRESS study demonstrate no benefit for gefitinib beyond progression in a molecularly defined subset of patients treated with frontline EGFR TKI therapy [14], corroborating our results. In this randomized phase III study, 265 predominantly Asian patients with EGFR-mutated NSCLC and who received frontline gefitinib with a clinical benefit were randomized to doublet chemotherapy with cisplatinum/pemetrexed versus same chemotherapy with continued gefitinib. Although the data at the time of presentation were immature for OS, no benefit of continued gefitinib was noted neither in overall response rate, nor in PFS [14].
In light of the data from these studies, we believe that our study provides the most robust, mature information yet reported in this area. Overall, our study strongly suggests that erlotinib beyond progression does not provide a significant clinical benefit and is associated with increased toxicity in patients representative of a usual North American patient population clinically enriched for EGFR mutations. While further data are awaited to more conclusively answer this question, caution is advised in patient selection and side-effect management. A number of studies are ongoing that will address this question more definitively (NCT01544179, NCT01928160, NCT01310036), and results are eagerly awaited. These results, however, may be overshadowed by the recent introduction and promise of the third-generation EGFR T790M targeting inhibitors (CO-1686 and AZD9291), which have demonstrated response rates of up to 60% for EGFR patients who progress on frontline TKI [15]. Overall, our data strongly suggest that continuing erlotinib beyond progression adds no clinical benefit but leads to an increase in adverse events and potential financial costs. More effective strategies will be needed to overcome acquired resistance and synergize with ongoing chemotherapy strategies.
Acknowledgment
Support for this study was provided by Astellas Pharmaceuticals, Inc.
Footnotes
Editor's Note: See the related commentary, “Continued EGFR Inhibition With Postprogression Chemotherapy: Where Do We Stand?” on page 1230 of this issue.
Author Contributions
Conception/Design: Balazs Halmos, Afshin Dowlati
Provision of study material or patients: Balazs Halmos, Nathan A. Pennell, Shirish Gadgeel, Gregory A. Otterson, Tarek Mekhail, Michael Snell, J. Philip Kuebler, Neelesh Sharma, Afshin Dowlati
Collection and/or assembly of data: Shumaila Saad
Data analysis and interpretation: Pingfu Fu
Manuscript writing: Balazs Halmos, Shumaila Saad, Afshin Dowlati
Final approval of manuscript: Balazs Halmos, Nathan A. Pennell, Shumaila Saad, Shirish Gadgeel, Gregory A. Otterson, Tarek Mekhail, Michael Snell, J. Philip Kuebler, Neelesh Sharma, Afshin Dowlati
Disclosures
Balazs Halmos: Roche, AstraZeneca, Clovis, Boehringer Ingelheim (C/A), AstraZeneca, Boehringer Ingelheim, Roche, Novartis, Pfizer, Health Care Ventures, Merck, Eli Lilly (RF); Nathan A. Pennell: Celgene, Clovis, New Link Genetics, Bayer, Astex, Genentech (RF); Shirish Gadgeel: Genentech/Roche (C/A, H); Gregory A. Otterson: Genentech, Boehringer (C/A), Pfizer, Genentech, Clovis, Boehringer Ingelheim, Celgene, GlaxoSmithKline (RF); Tarek Mekhail: Genentech (RF, H); Afshin Dowlati: Boehringer Ingelheim (C/A), Eli Lilly, Takeda, AstraZeneca, EMD Serono (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 2.Mok TS, Lee K, Leung L. Targeting epidermal growth factor receptor in the management of lung cancer. Semin Oncol. 2014;41:101–109. doi: 10.1053/j.seminoncol.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: Learning from lung cancer. Nat Rev Clin Oncol. 2014;11:473–481. doi: 10.1038/nrclinonc.2014.104. [DOI] [PubMed] [Google Scholar]
- 4.Saad S, Huang K, Halmos B. Overcoming resistance to EGF receptor tyrosine kinase inhibitors in EGFR-mutated NSCLC. Lung Cancer Manag. 2014;3:459–476. [Google Scholar]
- 5.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: A German Breast Group 26/Breast International Group 03-05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 6.Kluth LA, Shariat SF, Kratzik C, et al. The hypothalamic-pituitary-gonadal axis and prostate cancer: Implications for androgen deprivation therapy. World J Urol. 2014;32:669–676. doi: 10.1007/s00345-013-1157-5. [DOI] [PubMed] [Google Scholar]
- 7.Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res . 2011;17:6298–6303. doi: 10.1158/1078-0432.CCR-11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 9.Nishie K, Kawaguchi T, Tamiya A, et al. Epidermal growth factor receptor tyrosine kinase inhibitors beyond progressive disease: A retrospective analysis for Japanese patients with activating EGFR mutations. J Thorac Oncol. 2012;7:1722–1727. doi: 10.1097/JTO.0b013e31826913f7. [DOI] [PubMed] [Google Scholar]
- 10.Nishino K, Imamura F, Morita S, et al. A retrospective analysis of 335 Japanese lung cancer patients who responded to initial gefitinib treatment. Lung Cancer. 2013;82:299–304. doi: 10.1016/j.lungcan.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Asami K, Okuma T, Hirashima T, et al. Continued treatment with gefitinib beyond progressive disease benefits patients with activating EGFR mutations. Lung Cancer. 2013;79:276–282. doi: 10.1016/j.lungcan.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg SB, Oxnard GR, Digumarthy S, et al. Chemotherapy with erlotinib or chemotherapy alone in advanced non-small cell lung cancer with acquired resistance to EGFR tyrosine kinase inhibitors. The Oncologist. 2013;18:1214–1220. doi: 10.1634/theoncologist.2013-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuler MH, Yang C-H, Park K, et al. Continuation of afatinib beyond progression: Results of a randomized, open-label, phase III trial of afatinib plus paclitaxel (P) versus investigator’s choice chemotherapy (CT) in patients (pts) with metastatic non-small cell lung cancer (NSCLC) progressed on erlotinib/gefitinib (E/G) and afatinib—LUX-Lung 5 (LL5) J Clin Oncol. 2014;32(5s):8019a. [Google Scholar]
- 14.Mok TSK, Wu Y, Nakagawa K, et al. Gefitinib/chemotherapy vs chemotherapy in epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer (NSCLC) after progression on first-line gefitinib: The phase III, randomized IMPRESS study. Ann Oncol. 2014;25(suppl 4):LBA2_PR. [Google Scholar]
- 15.Steuer CE, Khuri FR, Ramalingam SS. The next generation of epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of lung cancer. Cancer. 2015;121:E1–E6. doi: 10.1002/cncr.29139. [DOI] [PubMed] [Google Scholar]