Abstract
Objective
This prospective investigation examined: 1) processing speed and working memory relative to other cognitive domains in non-demented medically managed idiopathic Parkinson’s disease, and 2) the predictive role of cortical/subcortical gray thickness/volume and white matter fractional anisotropy on processing speed and working memory.
Methods
Participants completed a neuropsychological protocol, Unified Parkinson’s Disease Rating Scale, brain MRI, and fasting blood draw to rule out vascular contributors. Within group a priori anatomical contributors included bilateral frontal thickness, caudate nuclei volume, and prefrontal white matter fractional anisotropy.
Results
Idiopathic Parkinson’s disease (n = 40; Hoehn & Yahr stages 1–3) and non-Parkinson’s disease ‘control’ peers (n = 40) matched on demographics, general cognition, comorbidity, and imaging/blood vascular metrics. Cognitively, individuals with Parkinson’s disease were significantly more impaired than controls on tests of processing speed, secondary deficits on working memory, with subtle impairments in memory, abstract reasoning, and visuoperceptual/spatial abilities. Anatomically, Parkinson’s disease individuals were not statistically different in cortical gray thickness or subcortical gray volumes with the exception of the putamen. Tract Based Spatial Statistics showed reduced prefrontal fractional anisotropy for Parkinson’s disease relative to controls. Within Parkinson’s disease, prefrontal fractional anisotropy and caudate nucleus volume partially explained processing speed. For controls, only prefrontal white matter was a significant contributor to processing speed. There were no significant anatomical predictors of working memory for either group.
Conclusions
Caudate nuclei volume and prefrontal fractional anisotropy, not frontal gray matter thickness, showed unique and combined significance for processing speed in Parkinson’s disease. Findings underscore the relevance for examining gray-white matter interactions and also highlight clinical processing speed metrics as potential indicators of early cognitive impairment in PD.
Introduction
The cognitive features of Parkinson’s disease (PD) are commonly attributed to frontostriatal dysfunction [1–4]. Frontostratial dysfunction can manifest as 1) slowness of thinking or information processing that reduces one’s ability to complete time based tasks efficiently; 2) mental inflexibility/working memory difficulty; 3) verbal or motor response suppression/disinhibition; and 4) abstract reasoning or problem solving difficulties. PD cognitive difficulties can, however, involve other cognitive realms such as the ability to learn new information (declarative memory; encoding/ retrieval; e.g., [5, 6]); language related operations, often measured with tests of verbal fluency and sentence comprehension [7]; and a wide variety of visuospatial skills [1]. Some researchers [1, 6, 7], yet not all [8], suggest these additional PD cognitive deficits (i.e., declarative memory, language, visuospatial) are subordinate to frontostriatal deficits. Further investigation into cognitive frontostriatal functions in PD and their relative contribution to the other cognitive domains will help clarify this issue.
Neuroanatomically, cognitive frontostriatal functions are linked to the frontostriatal circuitry that involves cortical gray matter, white matter, and subcortical gray matter regions [1]. Frontostriatal circuits originate in the frontal cortex, project to the striatum (caudate/ putamen; components of the basal ganglia), and then return to their respective cortical origin via the thalamus [9–11]. There are at least five circuits separated into ‘motor’ and ‘complex’ function, with the frontostriatal cognitive circuit involving projections from the dorsolateral prefrontal cortex to the dorsal portion of the caudate [11–15]. Dopaminergic, serotonergic, noradrenergic, and cholinergic cell groups along these pathways facilitate communication between the frontostriatal gray-white matter components [16–18].
Cognitive frontostriatal functions associate with separate regions of the frontostriatal circuitry. For example, among non-demented healthy adults, information processing speed abilities have largely been linked to prefrontal white matter integrity as measured by diffusion imaging [19, 20]. By contrast, animal/human lesion studies reveal working memory’s primary cortical site in the prefrontal cortex [21–23]. Fluid abstract reasoning, problem solving, and error monitoring involves various regions of the frontal cortex [24–26], although exceptions are noted [27].
For non-demented individuals with PD, the type and severity of frontostriatal cognitive deficit (e.g., processing speed, working memory, inhibition, abstract reasoning), relative to the integrity of other cognitive domains (e.g., language, visuospatial, memory) should speak to the location and burden of the disease. The six pathological stages of PD [28, 29] show that Lewy neurites and bodies progress from brainstem to neocortex. By stage three, there is disruption to the basal ganglia and associated dopaminergic pathways. Some investigators have shown that processing speed associates with caudate nucleus integrity [30–33]. Other investigators exclusively examining white matter integrity in PD report associations between white matter and processing speed, working memory, inhibition, and/or problem solving [34–38]. Abstract reasoning impairment as well as impairments in other cognitive domains (e.g., visuospatial) may signify cortical gray matter loss [39–44]. With minimal exception [37], however, these studies are limited in that they have examined PD cognition with either gray or white matter analyses. We propose that examining the relative contribution of gray and white matter integrity to the cognitive frontostriatal profile in PD will improve understanding of cognitive-neuroanatomical correspondence associated with disease progression.
The current study prospectively recruited individuals with idiopathic PD as well as non-PD “control” peers to assess hypotheses regarding 1) the dominance of frontal-striatal deficits in non-dementia PD and 2) the relative importance of cortical gray matter, white matter, and subcortical gray matter on these deficits. Using clinical neuropsychological tools, we expected individuals with PD to show dominant impairment in processing speed and working memory relative to non-PD peers, and for processing speed and working memory abilities to explain a portion of variability to other cognitive domains such as learning/memory, visuospatial, and motor abilities. Based on the rationale that frontostriatal deficits in PD are largely based on caudate nucleus to frontal cortical gray matter connections, we examined the relative contribution of cortical gray, white matter, and subcortical gray regions on the processing speed and working memory scores of both groups. Potential covariates that could confound white matter or frontostriatal functions (e.g., vascular risk factors) were also examined.
Materials and Methods
Participants
All participants had to be right-handed [45], show no signs of dementia (telephone screening for cognitive impairment had to be > 34; [46]; in-person completion of the Dementia Rating Scale-Revised (DRS-2; [47]) score > 130), and speak fluent English. Individuals with PD were diagnosed by a movement disorder trained neurologist, met criteria outlined by the UK Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria [48] and had a Hoehn and Yahr scale [49] ranging from 1–3. All participants were tested while on-medication. Medical exclusions included having any underlying disease likely to limit lifespan or outcome analysis: cancer requirement treatment in the past 5 months (exception; non-melanoma skin cancer), serious infectious diseases (e.g., self-reported HIV), myocardial infarction or cerebrovascular accident, congestive heart failure, chronic hepatitis, history of organ transplantation, and any other condition likely to limit lifespan, seizure disorders, head trauma resulting in intensive care. Surgical exclusions included having undergone Deep Brain Stimulation. Neurodegenerative exclusions included evidence of secondary/atypical Parkinsonism as suggested by the presence of any of the following: 1) history of major stroke(s) associated with cognitive sequelae, 2) exposure to toxins or neuroleptics, 3) history of encephalitis, 4) neurological signs of upper motor neuron disease, cerebellar involvement, supranuclear palsy, or significant orthostatic hypertension. Psychiatric exclusions included a major psychiatric disorder as assessed by the psychiatric or neurological team. We did not exclude for depression or anxiety because many PD patients report such symptoms. We excluded individuals with conditions likely to affect cognitive or MRI testing such as claustrophobia, non-medical bodily metal, pace-maker device, less than five years of education, inability to read or write, self-reported hearing difficulty that interferes with standardized test administration.
Procedures
The study was approved by the University of Florida Health Center Institutional Review Board (Protocol #472–2007). Written consent was obtained from all participants and the research followed the Declaration of Helsinki. Recruitment was conducted with a yoked procedure such that individuals were recruited first and non-PD “controls” peers second. Recruitment for individuals with PD involved a combination of: 1) brochure mailings to individuals identified through a clinical-research database within our local movement disorder center; 2) direct physician referrals, 3) advertisement at different PD support symposiums. Control participants were recruited through mail-outs to targeted individuals in two counties who met demographic inclusion criteria, community fliers, and free community memory screenings. All individuals were screened via telephone or in person, and then completed a baseline cognitive testing to ensure cognitive criteria. While on-medication participants completed neuropsychological testing, MRI, fasting blood draw, and the Unified Parkinson’s Disease Rating Scale (UPDRS; [50]). A spouse/friend completed participants’ instrumental activity of daily living questionnaire [51]. Measures of comorbidity [52], depression, apathy [53], and anxiety [54], were examined as potential covariates. Medications were reverted to a common metric (Levodopa Equivalency Dose; LED [55]). Raters blind to diagnosis double scored and entered all data.
Neuropsychological Test Measures by Domain
Raw test scores were standardized to z-scores based on demographically similar published norms [56–58]. Individual test z-scores were then averaged into z-score composites. The composite groupings were based on cognitive domains discussed within clinical neuropsychological literature [59]. General cognitive abilities–DRS-2 (total score [47]). Premorbid Intellectual Estimate -Wechsler Test of Adult Reading (WTAR; total score correct [60]). Attention—Wechsler Memory Scale-Third Edition WMS-III Digit Span forward (longest span forward) and Spatial Span forward (total score) subtests [61]; Processing speed–Trail Making Test Part A (time to completion [62]), WAIS-III Digit Symbol (score within 120 seconds [63]); Stroop Color Word Test—Word Reading test condition (score within 45seconds [64]); Working Memory–Wechsler Memory Scale-III [61] Digit Span Backward Span (longest span backward), Spatial Span Backward (total score), and Letter Number Sequencing (total score); Inhibition/set shifting- Trail Making Test Part B minus Part A (based on standardized scores created from time to completion [65–67]), Stroop Color Word Test—Color-Word Condition (total correct [64, 68]). Higher Abstract Reasoning/Problem Solving—WASI-III Matrix Reasoning subtest [58], Tower Test (total achievement score [69]); Wisconsin Card Sorting Test (total errors [70]); Language/ Lexical Retrieval—Boston Naming Test (total correct [71]); Animal Fluency (total correct); Letter Fluency (total correct [72, 73]); Visual Perceptual and Spatial—Benton Face Recognition (total correct [74, 75]); Judgment of Line Orientation (total correct [76, 77]); Learning and Memory—WMS-III Logical Memory [61] subtest (delay free recall) and Visual Reproduction subtest (delay free recall & recognition); Motor speed—Finger Tapping Test (dominant, non-dominant taps [57, 78]).
MRI Protocol
The MRI protocol was designed to provide information regarding subcortical volume, cortical volume, and white matter integrity. The brain MRI was conducted within 24 hours of cognitive testing via Siemens 3T Verio and 8-channel head coil for: 1) Two T1-weighted scans (176 contiguous slices, 1mm3 voxels, TR/TE = 2500/3.77ms) optimized for gray/white matter segmentation; 2) Diffusion two separate single-shot EPI, gradients applied along 6 directions (b = 100s/ mm2) and 64 directions (b = 1000s/ mm2), 73 contiguous axial slices, 2mm3 voxels, TR/ TE = 17300/81ms); 3) T2-weighted 176 contiguous slices, 1mm3 voxels, TR/ TE = 3200/ 409ms; 4) Fluid Attenuated Inversion Recovery (FLAIR; 176 contiguous slices, 1mm3 voxels, TR/TE = 6000/395ms). Between-group registration and intensity-based metrics were examined with TRACULA [79]. For preprocessing, both T1-weighted sequences per participant were entered into the FreeSurfer processing stream in order to correct for motion artifacts and improve segmentation quality. Both diffusion sequences per participant were merged together to create an acquisition with 70 non-overlapping directions (64 b = 1000 and 6 b = 100) and two b = 0 images. Effects of motion were partially corrected for using eddy_correct and then DTI metrics and registration with FreeSurfer processed T1 images were calculated for each participant.
Total Intracranial Volume (TICV in mm3)
The inner surface of the skull was acquired through FSL version 4.1—Brain Extraction Tool (BET [80]). An expert rater (Dice Similarity Coefficient; DSC > 0.99) manually cleaned the output on every sagittal slice. The inferior termination was based on a straight line between the bottom of the occipital bone and clivus.
Volumetric segmentation
FreeSurfer [81] was applied to acquire caudate nuclei, putamen, and thalamic volumes; trained raters blind to diagnosis corrected output for extraction errors. Final volumes were also segmented with FSL/FIRST [82] subcortical segmentation tool for comparison, with intraclass coefficient values between FreeSurfer and FIRST values ≥ 0.85.
Cortical thickness
FreeSurfer’s QDEC tool was applied a priori to acquire cortical thickness values to examine the relative contribution of regional cortical thickness to processing speed and working memory deficits. The QDEC group z map was full-width/half-max (FHWM) smoothed at 10mm. The difference map was projected onto an average brain for visualization. Corrections for multiple comparisons were performed using a Monte Carlo simulation with thresholding set at p = 0.01. FreeSurfer average cortical thickness for bilateral frontal, temporal, and parietal cortices were extracted for regression analyses.
Group Fractional Anisotropy (FA)
Tract Based Spatial Statistics [83] was applied with nonlinear registration aligning FA images to a group-specific (n = 80) FA template [80] affine aligned to a template space (MNI152). Files were merged for a mean FA skeleton (threshold of 0.2). Two group t-test comparisons (PD < Controls and PD > Controls) used a Threshold-Free Cluster Enhancement with 20,000 Monte Carlo permutations. Significance was set at p≤0.05, corrected for multiple comparisons.
FA within group metrics
The cortical parcellations from FreeSurfer (Desikan-Killiany Atlas) were combined to create lobar parcellations by hemisphere. Then the FreeSurfer tool mri_aparc2aseg was used to segment temporal lobe white matter up to 5 mm away from the gray-white boundary. Left and right temporal lobe white matter were extracted separately and checked for segmentation accuracy. A similar process was repeated for left and right parietal lobe. White matter masks were then rigid body transformed into diffusion space. Finally, fslstats was used to calculate mean FA within the ROIs. For prefrontal white matter: White matter was extracted with FreeSurfer, transformed to MNI152 space using a linear transformation (12 degrees of freedom), and cleaned in ITK-SNAP to acquire pre-frontal regions using the ventricular surface of the rostrum of the corpus callosum as a guide. The rater went posterior one slice coronally and removed four whole coronal slices of the white matter mask. Using the scalpel tool in ITK-SNAP, a trained rater removed the posterior portion of the brain and remaining temporal lobe. The masks including frontal white matter anterior to the rostrum of the corpus callosum were back transformed into FreeSurfer original space and then transformed into diffusion space using the registration matrix produced by the script dt_recon. FSLstats was used to extract mean FA within the regions of interest for each participant.
Vascular Risk Markers
In order to characterize the relative contribution of potential vascular factors to the white matter and cognitive profile differences in the PD relative to the non-PD group we acquired the following: 1) Fasting Blood Draw–for cardiovascular and inflammatory markers (homocysteine, C-reactive protein; Uric Acid); 2) Blood Pressure–for systolic and diastolic metrics after a five-minute resting period; pulse pressure calculated from systolic BP minus diastolic BP; 3) Leukoaraiosis (LA [84])–white matter abnormalities were quantified by a reliable rater (DSC intra-rater range = 0.84–0.93; mean ± s.d. = 0.84 ± 0.12; Inter-rater range = 0.80–0.83) using an in-house macro within ImageJ [85, 86] on FLAIR scans; dependent variables = LA mm3 and LA relative to TICV.
Statistical Analysis Approaches
SPSS version 22 was used for all analyses. Subcortical structures were corrected to TICV (e.g., Caudate/ TICV) and averaged across hemispheres. Independent t-tests examined group demographics. An analysis of variance assessed for a Group by Domain differences with follow-up analyses completed with independent t-tests. Independent t-tests examined group differences in neuroanatomical regions of interest. Mann-Whitney U tests compared C-reactive protein and UPDRS scores. Two-tailed Pearson correlations examined relationships between cognitive composites, neuroanatomical, and mood variables. Bonferroni corrections applied and commented upon when appropriate. Spearman rho correlations examined UPDRS-III and disease duration. Linear regression analysis was used to examine the contribution of a priori selected variables on age and education standardized composites of processing speed and working memory. Age was entered as a covariate in step1 of the model, with step 2 including the neuroanatomical cortical gray, white matter FA, and subcortical structures of interest. To explore associations between total caudate nucleus and prefrontal white matter integrity, separate partial correlations controlling for age examined caudate FA relative to white matter FA within each group. Fisher r-to-z transformation was conducted to examine statistical difference between coefficient values. Significance was set at 0.05. The wording ‘not significantly different’ is used when there is no statistical significance.
Results
Of 186 people phone screened, 43 individuals with PD and 41 non-PD peers met criteria. Four enrolled participants could not complete MRI (i.e, claustrophobia, metal artifact). Our final sample involved 40 individuals with idiopathic PD and 40 non-PD “controls”.
Participant Demographic, General Cognitive, and Motor Characteristics
Table 1. Groups were not significantly different in demographics, comorbidity, premorbid intellect and general cognition estimates. All were independent in instrumental activities of daily living (i.e., telephone, financial management) with all but one PD individual independently managing medications. PD was largely unilateral tremor dominant (70% H&Y ≤ 1.5). PD reported more symptoms of depression, apathy, and anxiety (p values <0.01).
Table 1. Parkinson’s disease (PD) and non-PD “control” peers demographic, motor, general cognition, and mood characteristics.
| Measure | PD (n = 40) | Non-PD (n = 40) | t, u, x2 | p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age | 67.80 ± 5.44, 60/79 | 68.18 ± 4.64, 62/79 | -0.33 | 0.74 |
| Education | 16.28 ± 3.03, 10/20 | 16.75 ± 2.35, 12/20 | -0.78 | 0.44 |
| Sex (M:F) | 32:8 | 33:7 | 0.08 | 0.78 |
| Handedness | 0.45 ± 3.66, 12/24 | 1.20 ± 3.07, 13/24 | -0.99 | 0.32 |
| Charlson Comorbidity | 0.30 ±0.72, 0/4 | 0.28 ± .61, 0/2 | 0.12 | 0.91 |
| Motor | ||||
| UPDRS-III | 17.58 ± 10.74, 3/46 | 2.75 ± 3.36*; 0/15 | 83.50 | <0.001 |
| H&Y | 1.64±0.76, 1/3 | -- | -- | -- |
| Disease Duration (yrs) | 7.50 ± 5.15, 0/26 | -- | -- | -- |
| < 10 years duration | 33 of 40; 83% | -- | -- | -- |
| l-Dopa Equiv. Score | 685.79 ± 371.49; 0/1450 | 1.00 ± 6.32*, 0/40 | -- | -- |
| Side of Onset | 25 R / 14 L / 1 axial | -- | -- | -- |
| General Cognition | ||||
| WTAR Est. IQ | 107.35 ± 7.68, 81/118 | 108.80 ± 8.76, 86/119 | -0.79 | 0.43 |
| DRS-2 Total | 139.43 ± 3.13, 131/144 | 140.20± 2.49, 133/144 | -1.23 | 0.22 |
| Mood | ||||
| BDI-II raw | 2.33 ± 2.99, 0/28 | 9.03 ± 6.93, 0/16 | -5.61 | <0.001 |
| Apathy Scale | 19.18 ± 4.22, 2/20 | 11.90 ± 6.6, 2/26 | -2.20 | 0.03 |
| State Anxiety | 34.80 ± 11.00, 20/74 | 28.20 ± 6.46, 20/47 | -3.27 | <0.01 |
| Trait Anxiety | 33.33 ± 9.98, 20/54 | 30.30 ±7.29, 20/53 | -1.54 | 0.13 |
MMSE = Mini-Mental State Examination; DRS-2 = Dementia Rating Scale– 2nd Version; WTAR = Wechsler Test of Adult Reading; UPDRS Total = United Parkinson’s Disease Rating Scale Total score; l-Dopa Equiv. Score = Levodopa Equivalent Score = Total Daily levodopa dosage intake in milligrams.
*One control was on levodopa for restless leg syndrome; BDI-2 = Beck Depression Inventory-2.
Vascular Risk Considerations
Table 2. Consistent with literature [87, 88], PD systolic blood pressure and pulse pressure metrics were significantly lower than non-PD peer controls (both p values <0.001), with homocysteine levels significantly higher in PD and trending with LED (PD: r(40) = 0.296, p = 0.067). There were no group differences in LA controlling for total intracranial volume.
Table 2. Parkinson’s disease (PD) and non-PD ‘control’ peers vascular risk variables.
| Measure | PD (n = 40) | Non-PD (n = 40) | t, u, x2 | p-value |
|---|---|---|---|---|
| Homocysteine | 12.23±4.40, 2.7/22.2 | 10.65±1.69, 7.4/14.7 | 2.76 | 0.03 |
| C Reactive Protein | 1.42±1.47, 0.20/17.60 | 2.02±2.79, 0.20/8.1 | -1.62 | 0.11 |
| Uric Acid | 5.31±1.06, 2.0/8.2 | 5.78±1.10, 2.9/7.6 | -1.91 | 0.06 |
| BP Systolic | 128.16±10.46, 107/149 | 136.63±11.26, 112/157 | -3.49 | 0.001 |
| BP Diastolic | 76.29±7.17, 62/93 | 78.30±6.40, 66/97.50 | -1.32 | 0.19 |
| Pulse Pressure | 51.87±7.55, 37.50/64.67 | 58.33±9.26, 40/75.33 | -3.42 | 0.001 |
| Leukoaraiosis raw mm3 | 4950±6748, 497/29694 | 4032.15±4477.81, 0/19978 | 0.72 | 0.48 |
| Leukoaraiosis/TICV % | 0.29±0.40, 0/2 | 0.25±0.28, 0/1 | 0.57 | 0.57 |
Homocysteine—expected range 5–15 μmol/L, with 7 (17%) of PD participants having cut-offs above the recommended cut-point; C-reactive protein expected range = 0–3 mg/L; Uric Acid expected range 3–7 mg/dL. BP = blood pressure (average of measurements if multiple recordings acquired; results similar if based on the first BP recording; Systolic, p = 0.001; Diastolic, p = 0.14; Pulse Pressure, p = 0.001). Leukoaraiosis/TICV % = Percent of Leukoaraiosis as a proportion of total intracranial volume.
PD and Non-PD Neuropsychological Comparisons
Group comparisons by cognitive domain
For all domains, mean score composites were within the average range relative to published norms for both the PD and non-PD (Table 3; Fig 1). A repeated measures analysis of variance identified a significant difference between group (F(1, 78) = 14.65, p<0.001) with a trend for a group by domain interaction (F(8, 624) = 1.89, p = .06). Follow-up between group comparisons showed significantly reduced processing speed and working memory composite scores in the PD relative to non-PD (p values < 0.001), with significant differences for composite subtests (p values <0.001; S1 Table). Individual PD impairment relative to normative standards occurred for the processing speed composite, however (borderline impaired (5.00%; 2/40)); Low average (35.00%; 14/40). For working memory, all PD participants scored at average levels or higher. Reasoning, visual perceptual/spatial, and memory composite scores were also significantly reduced in PD but with variability by subtest (e.g., significantly reduced delay memory (p values < 0.02) but not recognition; see S1 Table).
Table 3. Standardized neuropsychological composites for PD and non-PD peers with mean, standard deviation, and minimum/maximum scores shown.
| PD (n = 40) | Non-PD (n = 40) | t | p-value | |
|---|---|---|---|---|
| Attention | 0.31± 0.69, -1.39/2.12 | 0.38±0.73, -1.39/1.51 | 0.40 | 0.69 |
| P. Speed | -0.48±0.61, -1.76/0.83 | 0.16±0.47, -0.58/1.27 | 5.25 | <0.001* |
| W. Memory | 0.34±0.51, -0.59/1.77 | 0.87±0.57, -0.45/1.92 | 4.42 | <0.001* |
| Inhibition | 0.07±0.68, -1.25/1.60 | 0.21/0.69, -1.10/1.45 | 1.12 | 0.27 |
| Reasoning | 0.43±0.84, -0.93/2.52 | 0.78/0.50, -0.36/1.76 | 2.28 | 0.03 |
| Language | 0.28±0.71, -1.17/1.73 | 0.55±0.67, -0.63/2.30 | 1.77 | 0.08 |
| Visual | 0.15±0.83, -2.50/1.34 | 0.50±0.59, -0.64/1.59 | 2.91 | 0.03 |
| Memory | 0.75±0.77, -0.67/2.33 | 1.15±0.55, 0.11/2.11 | 2.67 | 0.01 |
| Motor | -0.57±1.12, -2.50/1.85 | -0.47±0.67, -1.80/1.25 | 0.48 | 0.63 |
W. Memory = Working Memory; P. Speed = Processing Speed;
*Significant after Bonferroni correction. See S1 Table for the composite subtest raw and standardized scores by group.
Note: Further examination of the motor speed scores showed that group motor tapping scores were normally distributed (skewness; PD = 0.493; non-PD = 0.029), but different in range. For PD: although fifteen of the participants (38%) scored within the below average to impaired range, 10% (4 / 40) scored in the superior range. These superior scores associated with lower LED metrics (three had LED < 400; one of 700) and a disease range of 3 to 8 years. By contrast, for the non-PD peers 10% (4/40) scored in the below average with none in the superior range. Removing the four individuals with PD who had superior finger tapping scores showed a trend for finger tapping difference (t(73) = 1.78, p = .079; PD mean = -0.79).
Fig 1. Group comparison by neuropsychological domain composite score.
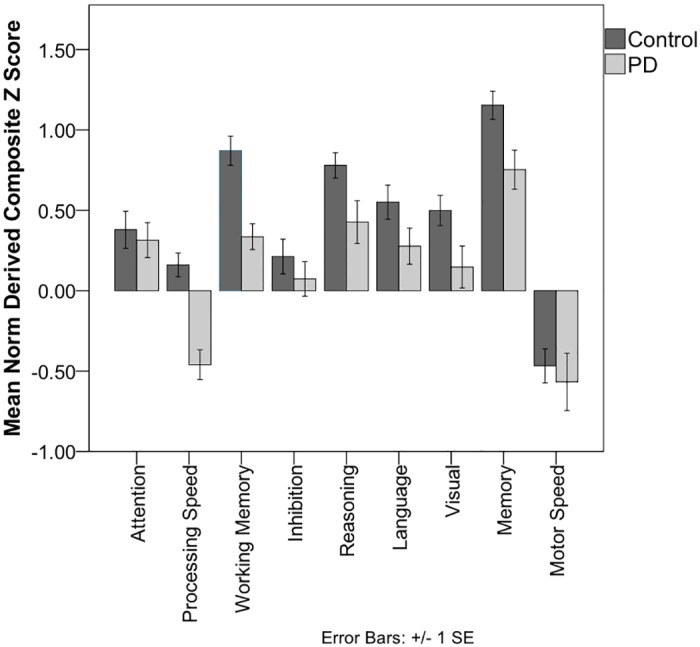
Each composite is based on the average of subtest standardized z-scores derived from published normative references. Average scores range from -0.67 to +0.67. Composite subtest scores are shown in S1 Table.
Processing speed and working memory composite correlations to other cognitive domains
S2 Table. Processing Speed: PD processing speed positively associated with basic attention, language, visual perceptual/spatial, and finger tapping motor speed. Working Memory: PD working memory positively associated with inhibition, memory composites, attention and reasoning.
For non-PD, only working memory positively associated with the attention composite.
Processing speed and working memory composite correlations to motor and disease metrics
Processing speed associated with PD motor speed finger tapping scores (r(40) = 0.44, p<0.01), but not UPDRS part III (spearman rho (40) = -0.29, p = 0.069). Neither processing speed nor working memory significantly associated with years of disease duration, LED, mood metrics, or vascular variables (p values > 0.13).
PD to Non-PD Neuroanatomical Comparisons
Between-group registration and intensity-based metrics demonstrated no significant group differences in diffusion sequence motion (Registration: average translation: t = 0.98, p = 0.33; average rotation: χ2 = 1.25, p = 0.26; Intensity: Percent bad slices χ2 = 0.26, p = 0.61; Average dropout score χ2 = 0.26, p = 0.61) suggesting data were appropriate for group comparisons.
Table 4. Fig 2. TICV—was significantly greater in PD (p<0.001). Cortical Gray Thickness Group Differences (Fig 2)—were statistically similar for whole brain comparisons. Individual lobe mean thickness comparisons showed a trend (Bonferroni uncorrected) for reduced temporal thickness in PD (t(78) = -1.97, p = 0.053). White Matter Fractional Anisotropy (Fig 2; S1 Video)—as measured by voxel by voxel comparisons showed PD with reduced FA in specific tracts (corpus callosum genu and body, forceps minor, anterior thalamic radiations, portions of inferior fronto-occipital fasciculus, and uncinate fasciculus). Averages across lobe region (frontal, temporal, parietal) were, however, were not statistically different between groups (p values >0.13). Subcortical Gray Volume—comparisons were only significant for bilateral putamen (PD< Non-PD, p<0.001; remaining significant after Bonferroni correction) with this associating with disease duration (spearman rho (40) = -0.454, p = 0.003).
Table 4. Raw and TICV corrected neuroanatomical regions of interest for PD and non-PD peers with mean± standard deviation, and minimum/maximum scores shown).
Significance noted by *.
| PD (n = 40) | Non-PD (n = 40) | |
|---|---|---|
| Cortical | ||
| Prefrontal | 2.32±0.09, 2.11/2.56 | 2.31±0.08, 2.06/2.51 |
| Thickness (mm) | ||
| Temporal | 2.64±0.09, 2.39/2.82 | 2.67±0.07, 2.49/2.81* |
| Parietal | 2.24±0.09, 2.09/2.41 | 2.23±0.09, 2.03/2.38 |
| White Matter | ||
| Prefrontal | 0.33±0.03, 0.25/0.38 | 0.33±0.02, 0.28/0.37 |
| Mean FA | ||
| Temporal | 0.30±0.02, 0.24/0.34 | 0.30±0.02, 0.21/0.34 |
| Parietal | 0.34±0.02, 0.28/0.38 | 0.35±0.02, 0.30/0.38 |
| Subcortical | ||
| Caudate raw | 7367±1164, 4754/10082 | 7086±992, 5452/10455 |
| Raw mm3 | ||
| Putamen raw | 9875±1148, 7958/13563 | 10221±1057, 8359/12570 |
| Thalamus raw | 13937±1313, 11294/18378 | 13120±1319, 10286/16222** |
| Subcortical | ||
| % Caudate | 0.43± 0.06, 0.30/0.55 | 0.44±0.05, 0.34/0.58 |
| TICV correcteda | ||
| % Putamen | 0.507± 0.07, 0.44/0.72 | 0.64±0.06, 0.53/0.82** |
| % Thalamus | 0.81±0.07, 0.64/0.97 | 0.82±0.08, 0.64/0.99 |
| TICV cm3 | 1727±1698, 1416/2082 | 1603±1377, 1371/1881** |
**p < .01;
*trend at p = .05;
aBilateral subcortical structures corrected for total intracranial volume x 100. TICV = Total intracranial Volume in cm3. Using the non-PD mean and standard deviation TICV values, standardized z-scores indicated that 11/40 (27.5%) of the PD participants had a TICV at least one standard deviation above the control mean TICV, three (7.50%) individuals at least two standard deviations, and three (7.50%) at least three standard deviations larger than the control mean. For these reasons, subcortical volume structures are corrected for TICV. FSL/FIRST [82] subcortical values were not statistically different from FreeSurfer [81] with intraclass correlation > 0.85.
Fig 2. White matter areas with significantly decreased fractional anisotropy (FA) in PD (n = 40) versus non-PD peers (n = 40) corrected with threshold free cluster enhancement.
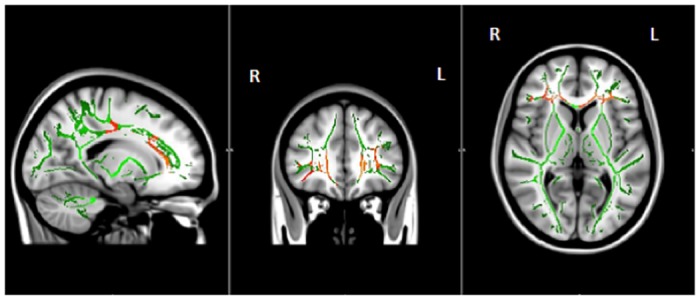
Areas with significantly decreased FA are shown in colors ranging from red to yellow (p < 0.05, corrected for multiple comparisons). Voxelwise group comparisons of FA were carried out using TBSS (Tract-Based Spatial Statistics, part of FSL). TBSS projects all participants’ FA data onto a mean FA tract skeleton (shown in green), before applying voxelwise cross-subject statistics. MRI conducted within 24 hours of cognitive testing. R = Right; L = Left.
Predicting Processing Speed and Working Memory in PD
Processing speed
Based on a priori hypotheses, we conducted regression analyses to explain the processing speed composite score in PD. The original model included total prefrontal gray thickness, prefrontal FA, and total caudate nucleus volume corrected for TICV as predictor variables. Due to potential associations with age on the anatomical structures, the variable of age in years was entered as a covariate in step 1 of each model.
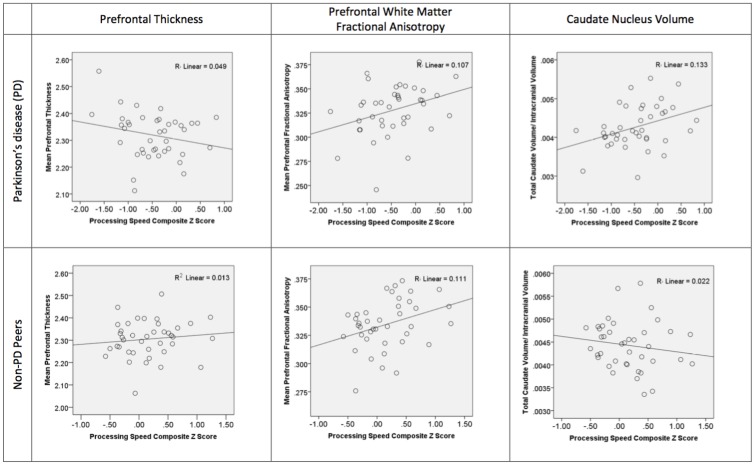
For PD: We controlled for age before examining the relative contribution of prefrontal cortical thickness, prefrontal white matter FA, and caudate nucleus volume on processing speed. Age was not a significant contributor (R = 0.006, p = 0.97) in step 1. The combined predictors for step 2 of the model was significant (R = 0.52, R2 = 0.27, F change = 4.27, p = 0.01) with positive significant beta weights only for caudate nucleus volume and prefrontal FA (beta weights: caudate nucleus volume = 0.32, p = 0.04; prefrontal FA = 0.32, p = 0.04; frontal thickness = -0.20, p = 0.16). We then explored the relative contribution of other structures. Excluding the caudate from step 2 weakened the overall model (R = 0.35, R2 = 0.12, p = 0.09). Replacing the caudate nucleus with total putamen volume (beta weight = 0.16, p = 0.31) or thalamus volume (beta weight = 0.10, p = 0.55) showed overall model reduction, supporting the caudate nucleus as the most relevant subcortical contributor. Replacing prefrontal FA with parietal FA (beta weight = -0.01, p = 0.96) weakened the model, although there was a trend for temporal FA contribution (beta weight = 0.33, p = 0.07). Replacing prefrontal gray thickness with temporal or parietal thickness did not change the model (beta weights < 0.12). Fig 3.
Fig 3. Scatter graphs showing group processing speed composite scores plotted against prefrontal gray thickness, prefrontal white matter fractional anisotropy, and caudate nucleus volume.
The top row shows scatter graphs for the individuals with PD (n = 40). The second row presents the non-PD peers (n = 40). The processing speed composite is based on the average of subtest standardized z-scores derived from published normative references. Note: The referenced r value is r squared.
For Non-PD: Age was not a significant predictor in step 1 (R = 0.08, p = 0.61). In step 2, only prefrontal white matter FA had a moderate beta weight (beta weight = 0.36, p = 0.03), but the overall model was not significant (R = 0.42, R2 = 0.17, F change = 2.33, p = 0.09; prefrontal thickness beta weight = 0.20, p = 0.23; caudate nucleus beta weight = -0.21, p = 0.21).
Vascular risk blood variables and LA did not significantly contribute to the models for either group (all p values >0.11).
Working memory
For PD, age was not a significant contributor to the model (R = 0.20, p = 0.22). The anatomical variables of interest also not significantly predicting over that of age (F change = -0.61, p = 0.61; R = 0.30, R2 = 0.09, p = 0.51; beta weights Prefrontal Thickness = 0.14, Prefrontal FA = 0.04, Caudate = 0.18, all p values >0.31). Non-PD analyses were similar (Age R = 0.02, p = 0.91; F change = 0.15, p = 0.94).
Age, LA and vascular risk variables did not significantly contribute to the models for either PD or non-PD (all p values >0.11).
Considerations for neuroanatomical structures on motor speed
Given the association between PD processing speed and motor speed (finger tapping), we examined the contribution of prefrontal cortical thickness, prefrontal FA, and caudate nucleus volume on the finger tapping motor speed composite. These neuroanatomical variables were not significant contributors (PD: R = 0.29; R2 = 0.01, p = 0.35; beta weights Prefrontal Thickness = -0.15; prefrontal FA = -0.04; caudate = 0.24, p = 0.14).
Discussion
Our prospective investigation identified processing speed as the dominant frontostriatal cognitive weakness in non-demented individuals diagnosed with PD, and that caudate nucleus volume and prefrontal white matter fractional anisotropy were significant contributors to their processing speed performance. For participants with PD, working memory was a secondary weakness relative to non-PD peers—yet no anatomical area of interest significantly contributed to their performance scores. The frontostriatal functions of processing speed and working memory abilities also explained a significant portion of variance (up to 24%) for scores of cognitive domains including learning/ memory, abstract reasoning, and perceptual/spatial matching. Our findings support the following assertions: 1) frontostriatal dysfunction and particularly processing speed is a primary contributor to other areas of cognition in PD [1, 6, 7]; 2) in order to understand PD cognition we need to examine both gray and white matter neuroimaging regions of interest [37].
For the processing speed metrics, nearly half (40%) of the PD sample scored in the below average or impaired range (less than the 9th %ile) and no PD participant scored greater than average levels (i.e., greater than 75th %ile). By contrast, each non-PD control participant scored at average levels or higher. Only PD processing speed scores positively associated with composites of basic attention, language (word retrieval and confrontation naming), perceptual/visuospatial abilities, and motor speed (finger tapping). These findings fit with expectations; the clinical processing speed measures we used in this investigation (Digit Symbol, Trail Making Test Part A, and Stroop Color Word Test—Word Reading) require sustained and selective attention, rapid integration of visual and verbal stimuli, and rapid hand/oral motor output [89, 90].
Processing speed has long been considered a fundamental component of the human cognitive architectural system [20, 91] and deficits on processing speed are considered sensitive markers to disease or brain damage [89, 90]. Working memory [92–95] and response inhibition [96–98] have additional dependence upon prefrontal and parietal gray matter. Abstract reasoning and decision-making are higher cortical functions. Although highly dependent upon prefrontal and the associative cortices [99, 100], abstract reasoning and decision making can be derailed by impaired processing speed, inhibitory functions, and working memory impairments [101, 102]. For PD, processing speed deficits in the confines of other relatively intact cognitive abilities, therefore, may mark a crucial time for cognitive intervention. Understanding white and gray matter contributions to processing speed has diagnostic and intervention relevance.
Neuroanatomically, fractional anisotropy of the prefrontal white matter explained a significant and similar portion of processing speed variance within both participant groups (such that higher prefrontal FA associated with better processing speed scores). The relationship between processing speed and white matter damage is well documented as a process of aging [19, 20]. While, reductions in processing speed has been observed with increasing amounts of white matter abnormalities which represents an ‘insult’ to white matter integrity (leukoaraioasis (LA) [84, 103, 104]), LA was not a contributor to processing speed within the current investigation. Prefrontal white matter appears therefore appears to be a foundational component of processing speed regardless of group disease status.
Tract based group comparisons, however, showed discrete anterior areas of prefrontal FA reduction in the PD relative to non-PD peers. Consistent with others’ reports (e.g., [35, 105, 106]), PD FA was reduced relative to their peers within regions of the bilateral forceps minor, anterior thalamic radiations, inferior fronto-occipital fasciculus, and uncinate fasciculus. These regions are intricately connected to the caudate nucleus—the only subcortical gray structure uniquely contributing to processing speed in PD.
Architectural studies of rhesus monkey show that the frontostriatal fibers have two major components [107]. Initially, the fibers course along with the long association fibers traveling within the frontal-occipital fasciculus lying just rostral to the lateral ventricle. Then, the set separates with one set traveling to the subcallosal fasciculus of Muratoff eventually ending within the caudate nucleus and putamen. The other set of striatal fibers enters the external capsule thereby targeting the ventral part of the caudate nucleus, the putamen and claustrum. Thus, the disease specific determinant of processing speed for PD may stem largely from the caudate nucleus and not reside within the prefrontal white matter alone. This interpretation would align with research supporting the caudate dopaminergic hypothesis of early cognitive impairment in PD [108–110].
Consistent with other reports [40, 43, 111], our study did not find group differences in cortical gray matter thickness and or associations between areas of cognition and prefrontal cortical thickness. Pereira and colleagues conducted a comprehensive examination of cognition and cortical thinning of individuals with PD relative to healthy adults who were enrolled in the Parkinson’s Progression Markers Initiative [112]. For cognitively well individuals with PD, there was limited reduced cortical thinning within the right inferior temporal gyrus, but no thinning in the frontal regions. Only the participants classified as PD-MCI via the Movement Disorder Society Task Force guidelines [113] had thinning in superior frontal, temporal, parietal, and occipital regions relative to their non-PD peers. Also consistent with our findings, Periera and colleagues’ assessment of processing speed (Symbol digit modalities test) did not associate with any cortical areas in either PD or non-PD peers. Segura and colleagues also show no associations between measures of (symbol digit modalities subtest, trail making test part A) and cortical thickness [43]. Cortical gray matter and subcortical gray matter thickness and volume differences appear minimal until individuals with PD have classified to have mild cognitive impairment [40, 43, 44].
Regarding other cognitive domains in our PD sample, we identified secondary difficulties in working memory. Despite scoring lower than their non-PD peers on working memory, no PD participant scored lower than average levels. No neuroanatomical region of interest explained PD working memory scores. This finding may be partially due to the sample’s limited working memory score range and otherwise average cognition relative to normative references and age matched peers. Between group analyses also showed PD weaknesses among sub-measures of learning/ memory, abstract reasoning, and spatial matching. Although PD group performance on learning/memory, abstract reasoning, perceptual/spatial matching was partially explained by frontostriatal deficits, we encourage further examination into individual profiles to rule out smaller PD heterogeneous subsamples (see [5]).
We recognize study limitations. First, we recognize the cognitive and neuroanatomical patterns may differ if participants were assessed off medication [114]. Second, two of our individuals had longer than 10 years of disease duration. Excluding these individuals from the analyses, however, did not change our cognitive or neuroanatomical findings. Third, PD individuals in our sample had larger TICV. Larger TICV in PD is not well known, although it has been reported [115] and referenced as a potential genetic association [116]. Head size may be a random effect of selection, but requires further consideration relative to interactions on white and gray matter metrics. Fifth, we recognize that our controls had slow motor speed (approximately a half standard deviation below published norms). Aside from acknowledging that some individuals within the non-PD control group had osteoarthritis of the hands, we are currently unable to explain this reduced finger tapping motor speed in our control group.
We encourage future investigations examining anatomical contributions to PD processing speed deficits. We conducted a post-hoc analysis examining gray-white matter contributors to processing speed in PD after additionally controlling for motor speed. This analysis showed that prefrontal white matter remained a significant contributor to processing speed, while the contribution of caudate nucleus volume reduced to a trend level (p = 0.10). We know, however, that caudate nucleus volume does not have a significant relationship to motor speed in our PD sample. For these reasons, we encourage additional statistical modeling to understand relationships between gray-white matter anatomical regions, processing speed, and motor speed in PD. Future investigators are also encouraged to examine frontal white matter with more sophisticated metrics, such as generalized anisotropy [117]. Fractional anisotropy, the white matter metric studied in the current investigation, may misrepresent white matter integrity due to the numerous kissing/ crossing fibers within the frontal forceps [118]. Finally, future studies are also encouraged to examine regions of caudate nucleus (ventral/dorsal; see [119]) on processing speed but also the relative integrity of specific white matter connections between these cortical to ventral/dorsal caudate regions on PD cognitive frontostriatal functions.
Despite study weaknesses, there were numerous study strengths including a prospective recruitment and assessment procedure for both PD and non-PD, an a priori structural neuroimaging methodology that incorporates white and gray matter regions of interest, and consideration for potential confounders such as vascular contributions. The neuropsychological protocol usedclinical measures that have normative references for age and/or education based peers. This has an advantage of providing a reference point of impairment for both the individuals with PD but also the non-PD peers. Finally, the findings demonstrate the probable value for coupling structural based white matter diffusion and gray matter region analyses with neuropsychological assessment for disease monitoring and prediction purposes.
Overall, this investigation demonstrated the dominance of frontostriatal deficits and particularly processing speed in non-demented individuals with idiopathic PD. It additionally showed unique and combined significance for caudate nucleus volume and prefrontal white matter FA on PD processing speed deficits. The findings improve awareness of gray and white matter interactions on the cognitive symptoms PD. It also highlights the value of clinical processing speed metrics as potential indicators of early cognitive impairment in PD.
Supporting Information
*PD<Non-PD peers p<0.05; **PD<non-PD peers p<0.01. DS = Digit Span Backward span length; SS = Spatial Span Backward total score; TMT = Trail Making Test; Tower Ach. = Tower Achievement score; WCST = Wisconsin Card Sorting Test; BNT = Boston Naming Test; FAS = Controlled Oral Word Association Test letters F, A, S; JLO = Judgment of Line Orientation; LM = Logical Memory; VR = Visual Reproductions; FT Dom = Finger Tapping Dominant hand; FT Nondom = Finger Tapping Non-dominant hand. aPD n = 39 due to missing data; bPD n = 39 and Control n = 38 due to color-blindness. All raw scores are unadjusted for age, sex, and education.
(DOCX)
*p<0.05, **p<0.01; two-tailed.
(DOCX)
Yellow depicts regions of reduced fractional anisotropy in PD. White depicts mean FA skeleton.
(MP4)
Acknowledgments
We sincerely thank the participants involved in the current investigation. We also acknowledge Glenn Stebbins, Ph.D., Professor of Neurological Sciences, Rush University Medical Center, Chicago, IL, for his mentorship related to study concept, Tony Mancuso, M.D., for assistance with securing the MR Siemens Verio, and the UF radiology team for their guidance. We are indebted to Irene Malaty, M.D., Ramon Rodriguez, M.D., Janet Romrell, ARNP, and Pam Zeilman, ARNP, all faculty within the UF Center for Movement Disorders and Neurorestoration, Gainesville, Florida, for referring individuals to the investigation and for their support of the study concept. We also acknowledge Ms. Lenora Stein for her excellent editorial assistance, Drs. Libon and Lamar for their peer review of a manuscript draft before submission.
Data Availability
Data are from the White Matter and Cognition in Parkinson's Disease study (NINDS K23NS60660) whose authors may be contacted at the Department of Clinical and Health Psychology, University of Florida, PO Box 100165, Gainesville, Florida, 32610, United States of America or by email: cep23@phhp.ufl.edu (CP). Data are available without limitations for researchers who meet the criteria for access to confidential data.
Funding Statement
This work was supported by National Institutes of Health National Institute of Neurological Disorders and Stroke K23NS60660 (CP) and R01NS082386 (CP), and in part by the Center for Movement Disorders and Neurorestoration, and the National Institutes of Health/National Center for Advancing Translational Sciences Clinical and Translational Science Award to the University of Florida UL1TR000064. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive and behavioral sequelae of Parkinson's disease: relationship to frontostriatal circuitry. Cognitive and behavioral neurology: official journal of the Society for Behavioral and Cognitive Neurology. 2003;16(4):193–210. Epub 2003/12/11. [DOI] [PubMed] [Google Scholar]
- 2.Zgaljardic DJ, Borod JC, Foldi NS, Mattis PJ, Gordon MF, Feigin A, et al. An examination of executive dysfunction associated with frontostriatal circuitry in Parkinson's disease. Journal of clinical and experimental neuropsychology. 2006;28(7):1127–44. Epub 2006/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Fuente-Fernandez R. Frontostriatal cognitive staging in Parkinson's disease. Parkinson's disease. 2012;2012:561046 Epub 2011/12/23. 10.1155/2012/561046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owen AM. Cognitive dysfunction in Parkinson disease: The role of frontostriatal circuitry. Neuroscientist. 2004;10(6):525–37. [DOI] [PubMed] [Google Scholar]
- 5.Tanner JJ, Mareci TH, Okun MS, Bowers D, Libon DJ, Price CC. Temporal Lobe and Frontal-Subcortical Dissociations in Non-Demented Parkinson's Disease with Verbal Memory Impairment. PLOS one. 2015;10(7):e0133792 Epub 2015/07/25. 10.1371/journal.pone.0133792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stebbins GT, Gabrieli JD, Masciari F, Monti L, Goetz CG. Delayed recognition memory in Parkinson's disease: a role for working memory? Neuropsychologia. 1999;37(4):503–10. [DOI] [PubMed] [Google Scholar]
- 7.Grossman M, Zurif E, Lee C, Prather P, Kalmanson J, Stern MB, et al. Information processing speed and sentence comprehension in Parkinson's disease. Neuropsychology. 2002;16(2):174–81. Epub 2002/04/13. [DOI] [PubMed] [Google Scholar]
- 8.Growdon JH, Corkin S, Rosen TJ. Distinctive aspects of cognitive dysfunction in Parkinson's disease. Advances in neurology. 1990;53:365–76. Epub 1990/01/01. [PubMed] [Google Scholar]
- 9.Alexander GE, Delong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–81. [DOI] [PubMed] [Google Scholar]
- 10.DeLong M, Wichmann T. Update on models of basal ganglia function and dysfunction. Parkinsonism & related disorders. 2009;15 Suppl 3:S237–40. Epub 2010/01/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42(2):183–200. Epub 2000/04/04. [DOI] [PubMed] [Google Scholar]
- 12.Middleton FA, Strick PL. A revised neuroanatomy of the fronto-striatal circuits In: Lichter DG, Cummings JL, editors. Frontal-subcortical circuits in psychiatric and neurological disorders. New York: Guildford Press; 2000. p. 44–58. [Google Scholar]
- 13.Zgaljardic DJ, Eidelberg D. Corpus striatum In: Aminoff M, Daroff R, editors. Encyclopedia of the neurological sciences. San Diego: Academic Press; 2003. p. 774–7. [Google Scholar]
- 14.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, "prefrontal" and "limbic" functions. Prog Brain Res. 1990;85:119–46. [PubMed] [Google Scholar]
- 15.Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Dev. 1987;58(3):601–22. [PubMed] [Google Scholar]
- 16.Abler B, Gron G, Hartmann A, Metzger C, Walter M. Modulation of frontostriatal interaction aligns with reduced primary reward processing under serotonergic drugs. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32(4):1329–35. Epub 2012/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohnen NI, Albin RL, Muller ML, Petrou M, Kotagal V, Koeppe RA, et al. Frequency of cholinergic and caudate nucleus dopaminergic deficits across the predemented cognitive spectrum of Parkinson disease and evidence of interaction effects. JAMA neurology. 2015;72(2):194–200. Epub 2014/12/17. 10.1001/jamaneurol.2014.2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jentsch JD, Taylor JR, Elsworth JD, Redmond DE Jr., Roth RH. Altered frontal cortical dopaminergic transmission in monkeys after subchronic phencyclidine exposure: involvement in frontostriatal cognitive deficits. Neuroscience. 1999;90(3):823–32. Epub 1999/04/28. [DOI] [PubMed] [Google Scholar]
- 19.Kerchner GA, Racine CA, Hale S, Wilheim R, Laluz V, Miller BL, et al. Cognitive processing speed in older adults: relationship with white matter integrity. PLOS one. 2012;7(11):e50425 Epub 2012/11/28. 10.1371/journal.pone.0050425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuznetsova KA, Maniega SM, Ritchie SJ, Cox SR, Storkey AJ, Starr JM, et al. Brain white matter structure and information processing speed in healthy older age. Brain structure & function. 2015. Epub 2015/08/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldman-Rakic PS. Circuity of primate prefrontal cortex and regulation of behavior by representational memory In: Plum F, editor. Handbook of physiology: The nervous system. Bethesda: American Physiological Society; 1987. p. 4787–98. [Google Scholar]
- 22.Petrides M. Frontal lobes and working memory: Evidence from investigations of the effects of cortical excisions in non-human primates In: Grafman FBJ, editor. Handbook of Neuropsychology. Amsterdam: Elsevier; 1994. p. 59–82. [Google Scholar]
- 23.Stuss DT, Eskes GA, Foster JK. Experimental neuropsychological studies of frontal lobe functions In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Amsterdam: Elsevier; 1994. [Google Scholar]
- 24.Babcock L, Vallesi A. The interaction of process and domain in prefrontal cortex during inductive reasoning. Neuropsychologia. 2015;67:91–9. Epub 2014/12/17. 10.1016/j.neuropsychologia.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yochim BP, Baldo JV, Kane KD, Delis DC. D-KEFS Tower Test performance in patients with lateral prefrontal cortex lesions: the importance of error monitoring. Journal of clinical and experimental neuropsychology. 2009;31(6):658–63. Epub 2008/11/26. 10.1080/13803390802448669 [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Benavides G, Gomez-Anson B, Quintana M, Vives Y, Manero RM, Sainz A, et al. Problem-solving abilities and frontal lobe cortical thickness in healthy aging and mild cognitive impairment. Journal of the International Neuropsychological Society: JINS. 2010;16(5):836–45. Epub 2010/07/06. 10.1017/S135561771000069X [DOI] [PubMed] [Google Scholar]
- 27.Tranel D, Manzel K, Anderson SW. Is the prefrontal cortex important for fluid intelligence? A neuropsychological study using Matrix Reasoning. The Clinical neuropsychologist. 2008;22(2):242–61. Epub 2007/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318(1):121–34. Epub 2004/09/01. [DOI] [PubMed] [Google Scholar]
- 29.Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson's disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. Epub 2009/02/24. [PubMed] [Google Scholar]
- 30.Bruck A, Portin R, Lindell A, Laihinen A, Bergman J, Haaparanta M, et al. Positron emission tomography shows that impaired frontal lobe functioning in Parkinson's disease is related to dopaminergic hypofunction in the caudate nucleus. Neuroscience letters. 2001;311(2):81–4. Epub 2001/09/25. [DOI] [PubMed] [Google Scholar]
- 31.Chou KL, Lenhart A, Koeppe RA, Bohnen NI. Abnormal MoCA and normal range MMSE scores in Parkinson disease without dementia: cognitive and neurochemical correlates. Parkinsonism & related disorders. 2014;20(10):1076–80. Epub 2014/08/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jokinen P, Karrasch M, Bruck A, Johansson J, Bergman J, Rinne JO. Cognitive slowing in Parkinson's disease is related to frontostriatal dopaminergic dysfunction. Journal of the neurological sciences. 2013;329(1–2):23–8. Epub 2013/04/09. 10.1016/j.jns.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 33.Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. The New England journal of medicine. 1988;318(14):876–80. Epub 1988/04/07. [DOI] [PubMed] [Google Scholar]
- 34.Matsui H, Nishinaka K, Oda M, Niikawa H, Komatsu K, Kubori T, et al. Wisconsin Card Sorting Test in Parkinson's disease: diffusion tensor imaging. Acta neurologica Scandinavica. 2007;116(2):108–12. Epub 2007/07/31. [DOI] [PubMed] [Google Scholar]
- 35.Gallagher C, Bell B, Bendlin B, Palotti M, Okonkwo O, Sodhi A, et al. White matter microstructural integrity and executive function in Parkinson's disease. Journal of the International Neuropsychological Society: JINS. 2013;19(3):349–54. Epub 2013/01/17. 10.1017/S1355617712001373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Auning E, Kjaervik VK, Selnes P, Aarsland D, Haram A, Bjornerud A, et al. White matter integrity and cognition in Parkinson's disease: a cross-sectional study. BMJ open. 2014;4(1):e003976 Epub 2014/01/23. 10.1136/bmjopen-2013-003976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duncan GW, Firbank MJ, Yarnall AJ, Khoo TK, Brooks DJ, Barker RA, et al. Gray and white matter imaging: A biomarker for cognitive impairment in early Parkinson's disease? Movement disorders: official journal of the Movement Disorder Society. 2015. Epub 2015/07/24. [DOI] [PubMed] [Google Scholar]
- 38.Theilmann RJ, Reed JD, Song DD, Huang MX, Lee RR, Litvan I, et al. White-matter changes correlate with cognitive functioning in Parkinson's disease. Frontiers in neurology. 2013;4:37 Epub 2013/05/01. 10.3389/fneur.2013.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagano-Saito A, Washimi Y, Arahata Y, Kachi T, Lerch JP, Evans AC, et al. Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology. 2005;64(2):224–9. Epub 2005/01/26. [DOI] [PubMed] [Google Scholar]
- 40.Pereira JB, Svenningsson P, Weintraub D, Bronnick K, Lebedev A, Westman E, et al. Initial cognitive decline is associated with cortical thinning in early Parkinson disease. Neurology. 2014;82(22):2017–25. Epub 2014/05/09. 10.1212/WNL.0000000000000483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanganu A, Bedetti C, Jubault T, Gagnon JF, Mejia-Constain B, Degroot C, et al. Mild cognitive impairment in patients with Parkinson's disease is associated with increased cortical degeneration. Movement disorders: official journal of the Movement Disorder Society. 2013;28(10):1360–9. Epub 2013/06/27. [DOI] [PubMed] [Google Scholar]
- 42.Hanganu A, Bedetti C, Degroot C, Mejia-Constain B, Lafontaine AL, Soland V, et al. Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson's disease longitudinally. Brain. 2014;137(Pt 4):1120–9. Epub 2014/03/13. 10.1093/brain/awu036 [DOI] [PubMed] [Google Scholar]
- 43.Segura B, Baggio HC, Marti MJ, Valldeoriola F, Compta Y, Garcia-Diaz AI, et al. Cortical thinning associated with mild cognitive impairment in Parkinson's disease. Movement disorders: official journal of the Movement Disorder Society. 2014;29(12):1495–503. Epub 2014/08/08. [DOI] [PubMed] [Google Scholar]
- 44.Pagonabarraga J, Corcuera-Solano I, Vives-Gilabert Y, Llebaria G, Garcia-Sanchez C, Pascual-Sedano B, et al. Pattern of regional cortical thinning associated with cognitive deterioration in Parkinson's disease. PLOS one. 2013;8(1):e54980 Epub 2013/01/30. 10.1371/journal.pone.0054980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex. 1975;11(3):230–8. Epub 1975/09/01. [DOI] [PubMed] [Google Scholar]
- 46.Cook SE, Marsiske M, McCoy KJ. The use of the Modified Telephone Interview for Cognitive Status (TICS-M) in the detection of amnestic mild cognitive impairment. Journal of geriatric psychiatry and neurology. 2009;22(2):103–9. Epub 2009/05/07. 10.1177/0891988708328214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matteau E, Dupre N, Langlois M, Jean L, Thivierge S, Provencher P, et al. Mattis Dementia Rating Scale 2: screening for MCI and dementia. Am J Alzheimers Dis Other Demen. 2011;26(5):389–98. Epub 2011/06/24. 10.1177/1533317511412046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 1992;42(6):1142–6. Epub 1992/06/01. [DOI] [PubMed] [Google Scholar]
- 49.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–42. [DOI] [PubMed] [Google Scholar]
- 50.Fahn S, Elton RL, UPDRA Development Committee. Unified Parkinson's Disease Rating Scale In: Fahn S, Marsden CD., Goldstein M., Calne D. B., editor. Recent Developments in Parkinson's Disease. New York: Macmillan Publishing Company; 1987. p. 153–64. [Google Scholar]
- 51.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9(3):179–86. [PubMed] [Google Scholar]
- 52.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 53.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry research. 1991;38(2):143–62. Epub 1991/08/01. [DOI] [PubMed] [Google Scholar]
- 54.Speilberger CD. Self-Evaluation Questionnaire. Palo Alto: Cons. Psych. Press; 1968. [Google Scholar]
- 55.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Movement disorders: official journal of the Movement Disorder Society. 2010;25(15):2649–53. Epub 2010/11/12. [DOI] [PubMed] [Google Scholar]
- 56.Brzezicka A, Sedek G, Marchewka A, Gola M, Jednorog K, Krolicki L, et al. A role for the right prefrontal and bilateral parietal cortex in four-term transitive reasoning: an fMRI study with abstract linear syllogism tasks. Acta Neurobiol Exp (Wars). 2011;71(4):479–95. Epub 2012/01/13. [DOI] [PubMed] [Google Scholar]
- 57.Heaton RK, Psychological Assessment Resources Inc. Revised comprehensive norms for an expanded Halstead-Reitan battery: demographically adjusted neuropsychological norms for African American and Caucasian adults, professional manual. [Updated ed. Lutz, Fla.: Psychological Assessment Resources; 2004. vi, 638 p. p.
- 58.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Assessment; 1999. February 1. [Google Scholar]
- 59.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment: Fourth Edition New York: Oxford University Press; 2004. [Google Scholar]
- 60.Aita Y, Kasahara T, Isobe K, Kawakami Y, Takekoshi K. Effect of urotensin II on PC12 rat pheochromocytoma cells. J Neuroendocrinol. 2010;22(2):83–91. Epub 2009/12/23. 10.1111/j.1365-2826.2009.01944.x [DOI] [PubMed] [Google Scholar]
- 61.Wechsler D. Wechsler Memory Scale—Third Edition San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 62.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists. 2004;19(2):203–14. [DOI] [PubMed] [Google Scholar]
- 63.Wechsler D. Wechsler Adult Intelligence Scale—Third Edition San Antonio, Texas: The Psychological Corporation; 1997. [Google Scholar]
- 64.Stroop JR. Studies of Interference in Serial Verbal Reactions. Journal of Experimental Psych. 1935;18(6):643–60. [Google Scholar]
- 65.Stuss DT, Bisschop SM, Alexander MP, Levine B, Katz D, Izukawa D. The trail making test: A study in focal lesion patients. Psychological assessment. 2001;13(2):230–9. [PubMed] [Google Scholar]
- 66.Choi SA, Evidente VG, Caviness JN. Comparing Cerebral White Matter Lesion Burdens between Parkinson's Disease with and without Dementia. Journal of movement disorders. 2010;3(1):6–10. Epub 2010/05/01. 10.14802/jmd.10002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi SA, Evidente VG, Caviness JN, Shill HA, Sabbagh MN, Connor DJ, et al. Are there differences in cerebral white matter lesion burdens between Parkinson's disease patients with or without dementia? Acta neuropathologica. 2010;119(1):147–9. Epub 2009/12/04. 10.1007/s00401-009-0620-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JE, Park B, Song SK, Sohn YH, Park HJ, Lee PH. A comparison of gray and white matter density in patients with Parkinson's disease dementia and dementia with Lewy bodies using voxel-based morphometry. Movement disorders: official journal of the Movement Disorder Society. 2010;25(1):28–34. Epub 2009/11/13. [DOI] [PubMed] [Google Scholar]
- 69.Dalaker TO, Larsen JP, Dwyer MG, Aarsland D, Beyer MK, Alves G, et al. White matter hyperintensities do not impact cognitive function in patients with newly diagnosed Parkinson's disease. NeuroImage. 2009;47(4):2083–9. Epub 2009/06/23. 10.1016/j.neuroimage.2009.06.020 [DOI] [PubMed] [Google Scholar]
- 70.Slawek J, Wieczorek D, Derejko M, Dubaniewicz M, Brockhuis B, Sitek E, et al. The influence of vascular risk factors and white matter hyperintensities on the degree of cognitive impairment in Parkinson's disease. Neurologia i neurochirurgia polska. 2008;42(6):505–12. Epub 2009/02/24. [PubMed] [Google Scholar]
- 71.Kaplan E, Goodglass H, Weintrab S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. February 1. [Google Scholar]
- 72.Gourovitch ML, Kirkby BS, Goldberg TE, Weinberger DR, Gold JM, Esposito G, et al. A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology. 2000;14(3):353–60. Epub 2000/08/06. [DOI] [PubMed] [Google Scholar]
- 73.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists. 1999;14(2):167–77. [PubMed] [Google Scholar]
- 74.Benton AL, Van Allen MW. Impairment in facial recognition in patients with cerebral disease. Transactions of the American Neurological Association. 1968;93:38–42. Epub 1968/01/01. [PubMed] [Google Scholar]
- 75.Benton AL. Contributions to neuropsychological assessment: a clinical manual. 2nd ed New York: Oxford University Press; 1994. 159 p. p. [Google Scholar]
- 76.Tranel D, Vianna E, Manzel K, Damasio H, Grabowski T. Neuroanatomical correlates of the Benton Facial Recognition Test and Judgment of Line Orientation Test. Journal of clinical and experimental neuropsychology. 2009;31(2):219–33. Epub 2008/12/04. 10.1080/13803390802317542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benton A, Hannay HJ, Varney NR. Visual perception of line direction in patients with unilateral brain disease. Neurology. 1975;25(10):907–10. Epub 1975/10/01. [DOI] [PubMed] [Google Scholar]
- 78.Wurster CD, Graf H, Ackermann H, Groth K, Kassubek J, Riecker A. Neural correlates of rate-dependent finger-tapping in Parkinson's disease. Brain structure & function. 2015;220(3):1637–48. Epub 2014/03/22. [DOI] [PubMed] [Google Scholar]
- 79.Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B. Spurious group differences due to head motion in a diffusion MRI study. NeuroImage. 2013;88C:79–90. Epub 2013/11/26. 10.1016/j.neuroimage.2013.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith SM, editor. Fast robust automated brain extraction Human brain mapping; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fujibuchi T, Tanabe Y, Sakae T, Terunuma T, Isobe T, Kawamura H, et al. Feasibility study on using imaging plates to estimate thermal neutron fluence in neutron-gamma mixed fields. Radiation protection dosimetry. 2011;147(3):394–400. Epub 2011/01/05. 10.1093/rpd/ncq493 [DOI] [PubMed] [Google Scholar]
- 82.Nugent AC, Luckenbaugh DA, Wood SE, Bogers W, Zarate CA Jr., Drevets WC. Automated subcortical segmentation using FIRST: test-retest reliability, interscanner reliability, and comparison to manual segmentation. Human brain mapping. 2013;34(9):2313–29. Epub 2012/07/21. 10.1002/hbm.22068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–505. Epub 2006/04/21. [DOI] [PubMed] [Google Scholar]
- 84.Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Archives of neurology. 1987;44(1):21–3. [DOI] [PubMed] [Google Scholar]
- 85.Abramaoff M, Magelhaes P, Ram S. Image processing with ImageJ. Biophotonics International. 2004;11(7):36–42. [Google Scholar]
- 86.Price CC, Mitchell SM, Brumback B, Tanner JJ, Schmalfuss I, Lamar M, et al. MRI-Leukoaraiosis Thresholds and the Phenotypic Expression of Dementia. Neurology. 2012;79:734–40. 10.1212/WNL.0b013e3182661ef6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suzuki H, Shibata R, Kito T, Ishii M, Li P, Yoshikai T, et al. Therapeutic angiogenesis by transplantation of induced pluripotent stem cell-derived Flk-1 positive cells. BMC cell biology. 2010;11:72 Epub 2010/09/24. 10.1186/1471-2121-11-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Isobe K. Enzymes responsible for metabolism of Nalpha-benzyloxycarbonyl-L-lysine in microorganisms. New biotechnology. 2010;27(6):751–4. Epub 2010/05/13. 10.1016/j.nbt.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 89.Lezak MD. Neuropsychological assessment. 5th ed Oxford; New York: Oxford University Press; 2012. xxv, 1161 p. p. [Google Scholar]
- 90.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. New York: Oxford University Press, Inc.; 2004. [Google Scholar]
- 91.Kail R, Salthouse TA. Processing speed as a mental capacity. Acta psychologica. 1994;86(2–3):199–225. Epub 1994/08/01. [DOI] [PubMed] [Google Scholar]
- 92.Muller NG, Knight RT. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience. 2006;139(1):51–8. Epub 2005/12/15. [DOI] [PubMed] [Google Scholar]
- 93.Bedwell JS, Horner MD, Yamanaka K, Li X, Myrick H, Nahas Z, et al. Functional neuroanatomy of subcomponent cognitive processes involved in verbal working memory. Int J Neurosci. 2005;115(7):1017–32. Epub 2005/07/30. [DOI] [PubMed] [Google Scholar]
- 94.Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133(1):23–32. Epub 2000/08/10. [DOI] [PubMed] [Google Scholar]
- 95.Gruber O, von Cramon DY. The functional neuroanatomy of human working memory revisited. Evidence from 3-T fMRI studies using classical domain-specific interference tasks. NeuroImage. 2003;19(3):797–809. Epub 2003/07/26. [DOI] [PubMed] [Google Scholar]
- 96.Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11(9):825–36. Epub 2001/09/05. [DOI] [PubMed] [Google Scholar]
- 97.Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage. 2003;20(1):351–8. Epub 2003/10/07. [DOI] [PubMed] [Google Scholar]
- 98.Forstmann BU, Jahfari S, Scholte HS, Wolfensteller U, van den Wildenberg WP, Ridderinkhof KR. Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: a model-based approach. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28(39):9790–6. Epub 2008/09/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aichelburg C, Urbanski M, Thiebaut de Schotten M, Humbert F, Levy R, Volle E. Morphometry of Left Frontal and Temporal Poles Predicts Analogical Reasoning Abilities. Cereb Cortex. 2014. Epub 2014/10/22. [DOI] [PubMed] [Google Scholar]
- 100.Ebisch SJ, Mantini D, Romanelli R, Tommasi M, Perrucci MG, Romani GL, et al. Long-range functional interactions of anterior insula and medial frontal cortex are differently modulated by visuospatial and inductive reasoning tasks. NeuroImage. 2013;78:426–38. Epub 2013/04/30. 10.1016/j.neuroimage.2013.04.058 [DOI] [PubMed] [Google Scholar]
- 101.Fitoussi A, Le Moine C, De Deurwaerdere P, Laqui M, Rivalan M, Cador M, et al. Prefronto-subcortical imbalance characterizes poor decision-making: neurochemical and neural functional evidences in rats. Brain structure & function. 2015;220(6):3485–96. Epub 2014/08/20. [DOI] [PubMed] [Google Scholar]
- 102.Shuvaev VT, Shefer VI. Structure of neuronal activity of the caudate nucleus of monkeys during decision-making and the realization of the motor program in different variants of a delayed spatial choice task. Neuroscience and behavioral physiology. 1995;25(1):63–70. Epub 1995/01/01. [DOI] [PubMed] [Google Scholar]
- 103.O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical "disconnection" as a mechanism of age-related cognitive decline. Neurology. 2001;57(4):632–8. [DOI] [PubMed] [Google Scholar]
- 104.Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O'Sullivan M, Howe FA, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66(2):217–22. Epub 2006/01/26. [DOI] [PubMed] [Google Scholar]
- 105.Huang P, Xu X, Gu Q, Xuan M, Yu X, Luo W, et al. Disrupted white matter integrity in depressed versus non-depressed Parkinson's disease patients: a tract-based spatial statistics study. Journal of the neurological sciences. 2014;346(1–2):145–8. Epub 2014/09/10. 10.1016/j.jns.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 106.Agosta F, Canu E, Stojkovic T, Pievani M, Tomic A, Sarro L, et al. The topography of brain damage at different stages of Parkinson's disease. Human brain mapping. 2013;34(11):2798–807. Epub 2012/04/25. 10.1002/hbm.22101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. New York: Oxford University Press; 2006. [Google Scholar]
- 108.Marklund P, Larsson A, Elgh E, Linder J, Riklund KA, Forsgren L, et al. Temporal dynamics of basal ganglia under-recruitment in Parkinson's disease: transient caudate abnormalities during updating of working memory. Brain. 2009;132(Pt 2):336–46. Epub 2008/11/28. 10.1093/brain/awn309 [DOI] [PubMed] [Google Scholar]
- 109.Bohnen NI, Albin RL, Muller ML, Petrou M, Kotagal V, Koeppe RA, et al. Frequency of Cholinergic and Caudate Nucleus Dopaminergic Deficits Across the Predemented Cognitive Spectrum of Parkinson Disease and Evidence of Interaction Effects. JAMA neurology. 2014. Epub 2014/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Helie S, Ell SW, Ashby FG. Learning robust cortico-cortical associations with the basal ganglia: An integrative review. Cortex. 2015;64C:123–35. Epub 2014/12/03. [DOI] [PubMed] [Google Scholar]
- 111.Filoteo JV, Reed JD, Litvan I, Harrington DL. Volumetric correlates of cognitive functioning in nondemented patients with Parkinson's disease. Movement disorders: official journal of the Movement Disorder Society. 2014;29(3):360–7. Epub 2013/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parkinson Progression Marker I. The Parkinson Progression Marker Initiative (PPMI). Progress in neurobiology. 2011;95(4):629–35. Epub 2011/09/21. 10.1016/j.pneurobio.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Movement disorders: official journal of the Movement Disorder Society. 2011;27(3):349–56. Epub 2012/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vaillancourt DE, Schonfeld D, Kwak Y, Bohnen NI, Seidler R. Dopamine overdose hypothesis: evidence and clinical implications. Movement disorders: official journal of the Movement Disorder Society. 2013;28(14):1920–9. Epub 2013/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Song IU, Kim JS, Park IS, Kim YD, Cho HJ, Chung SW, et al. Clinical significance of homocysteine (hcy) on dementia in Parkinson's disease (PD). Arch Gerontol Geriatr. 2013;57(3):288–91. Epub 2013/05/28. 10.1016/j.archger.2013.04.015 [DOI] [PubMed] [Google Scholar]
- 116.Taal HR, St Pourcain B, Thiering E, Das S, Mook-Kanamori DO, Warrington NM, et al. Common variants at 12q15 and 12q24 are associated with infant head circumference. Nature genetics. 2012;44(5):532–8. Epub 2012/04/17. 10.1038/ng.2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bublak P, Muller U, Gron G, Reuter M, von Cramon DY. Manipulation of working memory information is impaired in Parkinson's disease and related to working memory capacity. Neuropsychology. 2002;16(4):577–90. Epub 2002/10/18. [DOI] [PubMed] [Google Scholar]
- 118.Colon-Perez LM, Spindler C, Goicochea S, Triplett W, Parekh M, Montie E, et al. Dimensionless, Scale Invariant, Edge Weight Metric for the Study of Complex Structural Networks. PLOS one. 2015;10(7):e0131493 Epub 2015/07/15. 10.1371/journal.pone.0131493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nemmi F, Sabatini U, Rascol O, Peran P. Parkinson's disease and local atrophy in subcortical nuclei: insight from shape analysis. Neurobiology of aging. 2015;36(1):424–33. Epub 2014/09/02. 10.1016/j.neurobiolaging.2014.07.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
*PD<Non-PD peers p<0.05; **PD<non-PD peers p<0.01. DS = Digit Span Backward span length; SS = Spatial Span Backward total score; TMT = Trail Making Test; Tower Ach. = Tower Achievement score; WCST = Wisconsin Card Sorting Test; BNT = Boston Naming Test; FAS = Controlled Oral Word Association Test letters F, A, S; JLO = Judgment of Line Orientation; LM = Logical Memory; VR = Visual Reproductions; FT Dom = Finger Tapping Dominant hand; FT Nondom = Finger Tapping Non-dominant hand. aPD n = 39 due to missing data; bPD n = 39 and Control n = 38 due to color-blindness. All raw scores are unadjusted for age, sex, and education.
(DOCX)
*p<0.05, **p<0.01; two-tailed.
(DOCX)
Yellow depicts regions of reduced fractional anisotropy in PD. White depicts mean FA skeleton.
(MP4)
Data Availability Statement
Data are from the White Matter and Cognition in Parkinson's Disease study (NINDS K23NS60660) whose authors may be contacted at the Department of Clinical and Health Psychology, University of Florida, PO Box 100165, Gainesville, Florida, 32610, United States of America or by email: cep23@phhp.ufl.edu (CP). Data are available without limitations for researchers who meet the criteria for access to confidential data.