Abstract
Social information can profoundly influence behavior, but its effects are often explained in terms of “conformity,” implying effects on decision-making and communication rather than deeper sensory modulation. We examined whether information about other people’s pain reports affected both participants’ pain experience and skin conductance responses (SCR) during pain. Sixty volunteers experienced painful heat stimulation preceded by two kinds of informational cues: (1) non-reinforced social information indicating low or high pain ratings from previous participants; and (2) reinforced conditioned cues (CSlow, CShigh). Both high-pain social information and CShigh cues enhanced pain and SCRs relative to their respective controls, with particularly robust effects of social information. Effects of both manipulations on both pain and SCRs were mediated by trial-by-trial pain expectancies. These results demonstrate strong social influences on pain and autonomic responses, and suggest that expectations from multiple sources can influence pain physiology independent of reinforcement.
Keywords: social influence, heat pain, placebo, personality, learning
A large literature has demonstrated social influences on preferences and decision-making (Asch, 1951; Cialdini & Goldstein, 2004; for reviews see Wood, 2000). Individuals often change their judgments to be in agreement with what they perceive as group norms. Social influences are widely assumed to affect overt behavior and evaluative processes. Yet, it is unknown whether social conformity can also change the internal affective processes that give rise to experience and physiological responses (Cialdini & Goldstein, 2004). That is, if your friends tell you that jumping in an icy lake is not so painful, you might be influenced to jump in. However, it is not known whether your friends’ opinion also changes the painful sensation of the ice water on your skin and your physiological responses to the cold. Here, we test this question in a laboratory setting, using experimental heat pain stimulation manipulated by non-reinforced social feedback.
Previous studies have demonstrated that pain is strongly modulated by associations learned through reinforcement: Stimuli associated with intense pain subsequently become more painful, and those associated with less pain become less painful (Atlas, Bolger, Lindquist, & Wager, 2010; Colloca, Petrovic, Wager, Ingvar, & Benedetti, 2010; Price, Finniss, & Benedetti, 2008). Recent studies have shown that observing another person experiencing pain relief is also sufficient to serve as a reinforcer, and shapes pain perception (Colloca & Benedetti, 2009; Goubert, Vlaeyen, Crombez, & Craig, 2011; Hunter, Siess, & Colloca, 2013). Another, related study investigated how the vicarious experiences of others’ pain ratings—presented as simple displays of lines—affected pain perception (Yoshida, Seymour, Koltzenburg, & Dolan, 2013). This study showed an increase in pain when others’ pain ratings were higher in mean and had higher variance across individuals (Yoshida et al., 2013). However, because the social information was correlated with the actual stimulus intensity, it remains unknown whether social information can modulate pain ratings independent of learning, or whether social information may have served as a conditioned cue. The threat value of a stimulus can be learned by observational learning (Olsson, Nearing, & Phelps, 2007), and even by pure instructions (e.g. reviewed by Olsson & Phelps, 2007; Phelps et al., 2001), but it is unknown whether implicit, physiological measures of pain itself, such as skin conductance responses (SCR) to painful events, are modulated by social influence and whether unreinforced social influences on pain can remain stable over time.
A related, unresolved question concerns the role of conscious expectancies in mediating effects of both social influences and conditioned pain modulation. In many paradigms, expectancies appear to mediate the effects of conditioning and suggestion alike on pain reports (Kirsch, 2004). However, it is also possible that expectancies chiefly mediate effects on decision-making processes, and that effects on primary affective responses—e.g., early pain-related processing in the brain (Amanzio, Benedetti, Porro, Palermo, & Cauda, 2013; Atlas et al., 2010; Meissner et al., 2011), and spinal cord (Geuter & Büchel, 2013)—reflect conditioned associations independent of conscious expectations.
To address these questions and to disentangle the effects of experience-based learning from unreinforced social influences on pain, we examined the effects of both social information (without systematic reinforcement) and conditioned cues on two outcomes: pain ratings and SCR to noxious heat. Comparing pain ratings and SCRs to stimuli with medium intensity (48 °C) allowed us to assess the effects of social information and conditioned cues, and their potential interactions, on both explicit and implicit measures of pain experience. Expectancy ratings made before each painful stimulus allowed us to test whether expectancy effects mediated the effects of both social information and learned cues on pain and pain-evoked SCRs.
Method
Participants
60 healthy volunteers took part in the experiment (33 female, mean age = 23.0, age range 18–55 years). Screening instruments indicated that all participants were free of psychiatric, neurologic and pain conditions. Five additional participants opted to not complete the task due to high pain sensitivity and were excluded from further testing. All participants provided written informed consent and were paid for their participation. The study was conducted according to the Declaration of Helsinki and approved by the Institutional Review Board at the University of Colorado Boulder.
Materials and Procedures
Stimuli
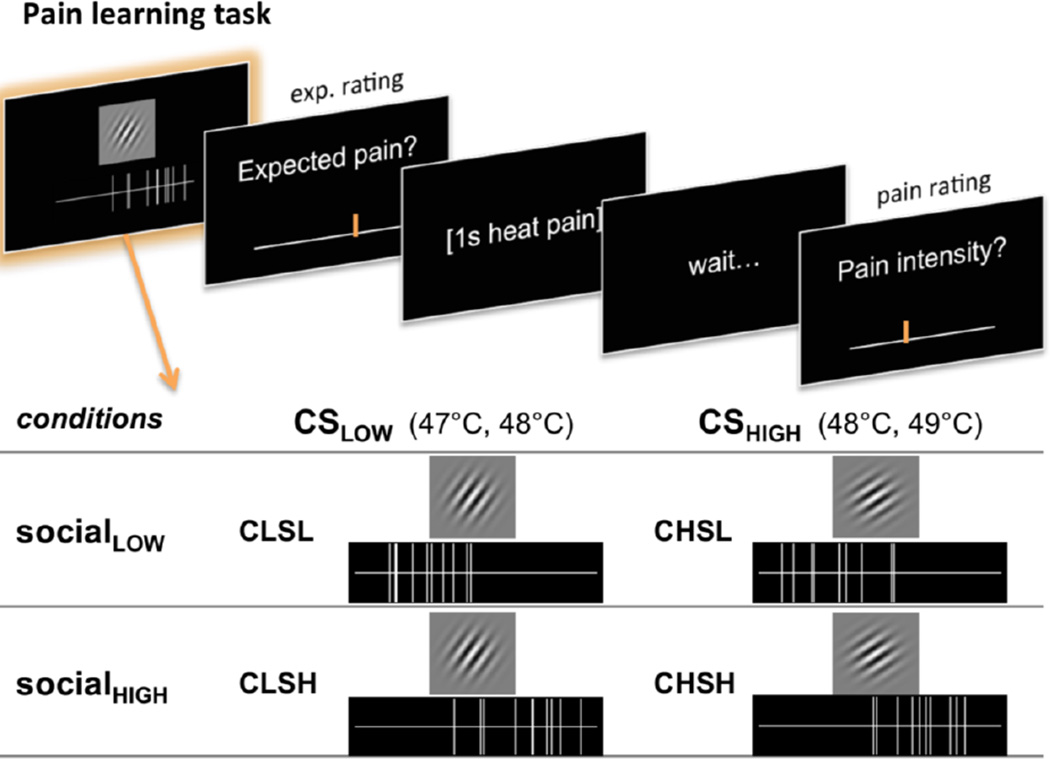
Participants experienced heat at three temperatures, preceded by two kinds of cues (see Fig. 1). Simple visual displays of putatively social information indicated the ratings of 10 fictitious, previous participants, which were either low (SocialLOW) or high (SocialHIGH) on average (vertical lines in Fig. 1). In addition, one of two visual cues was presented, partially reinforced with moderately painful heat (CueLOW, followed by 47 or 48 °C stimuli) or more intensely painful heat (CueHIGH, followed by 48 or 49 °C stimuli; Gabor patches in Fig. 1). Social information was completely non-predictive of pain, i.e. both social cue types were succeeded equally often by 47, 48, or 49 °C stimuli.
Figure 1.
Experimental design of the pain-learning task. Each trial started with the presentation of two cues. Participants were told that each of ten vertical lines on a horizontal visual analog scale (VAS) reflected the pain ratings of previous participants. This social information could either indicate low (SocialLOW) or high (SocialHIGH) others’ pain ratings. Unbeknown to participants, this information was actually not predictive of the actual stimulus temperature. Additionally, participants were presented with one of two Gabor patches with different orientation (35° and 55° angle). One gabor patch (CSLOW) was followed by low-to-medium (47 or 48°C), the other Gabor patch (CSHIGH) with medium-to-high (CH, 48 or 49°C) heat stimulation. Following the presentation of the two cues, participants had to indicate how much pain they expected and were then stimulated with short (1s) heat to the left forearm. After the heat stimulation, they had to rate on a VAS how much pain they actually felt.
For the social information, we generated 96 different stimuli (48 SocialLOW, 48 SocialHIGH). Each social rating stimulus depicted 10 vertical lines (“others’ ratings”) on a horizontal line that closely resembled the visual analog scale used for participants’ ratings (see Figure 1, cf. Yoshida et al. 2013). To generate a realistic and variable social rating stimuli set, each stimulus was generated using custom Matlab scripts by sampling 10 random values between 0 and 1 from one of two Gaussian distributions: N(0.3, 0.15) for SocialLOW and N(0.70, 0.15) for SocialHIGH. This sampling procedure resulted in stimuli that had some natural variation in the actual mean and standard deviation, making the stimuli realistic and plausible.
Conditioned cues (CSs) consisted of two Gabor patches with orientation angles of 35° and 55° (CueHIGH and CueLOW, counterbalanced across participants) and Gaussian envelopes (www.cogsci.nl/software/online-gabor-patch-generator).
Heat pain stimulation was applied to six different skin sites on the left volar forearm using a CHEPS thermode (27mm diameter) and controlled by a Pathway system and software (Medoc Advanced Medical Systems, Israel). Baseline temperature was set to 32°C. Heat pain stimulation was delivered in short epochs with 40°C/s ramp rate and 1s plateau duration at target temperatures.
Procedures
All participants first underwent a pain calibration procedure (total of 18 trials at all 6 skin sites, temperatures between 44–50°C) to ensure appropriate pain sensitivity and to familiarize the participants with the heat pain stimulation. Next, they were instructed that we were interested in their subjective experience of pain and how well they were able to predict pain by seeing the pain rating of several other participants as well as ‘abstract pattern’ cues. As an exploratory manipulation of the effects of instructions, half of the participants (N=30) were told that they should pay attention to both the social information as well as the ‘abstract patterns’ (Gabor patches), which also would contain information about the upcoming pain, without providing further instructions about the meaning of the patterns. The other half (N=30) was told that one of the cues was associated with higher average pain, and that their task was to learn which cue was predictive of high vs. low pain. No substantial differences between these two experimental groups were found (all between subject effect p-values > 0.1); therefore we collapsed across instruction types in all analyses reported here.
Participants then performed six blocks of the learning task, corresponding to 6 different skin sites chosen in randomized order. Each trial in the learning task (see Fig. 1) started with the presentation (4s) of one of two Gabor patches (CueHIGH or CueLOW) and social ratings, depicting either low or high vicarious pain ratings (SocialLOW or SocialHIGH). The position of social and conditioning cues on the screen (top or bottom) was counterbalanced across trials. Next, participants rated how much pain they expected on a horizontal visual analog scale (expectancy rating, average individual median RT 2358 ms, range of individual median RTs 1132–4774ms), and were then stimulated with low (47°C, 25% of trials), medium (48°C, 50% of trials), or high (49°C, 25% of trials) heat pain. Note that the CueHIGH was always followed by either medium or high pain and the CueLOW was always followed by either low or medium heat pain, whereas the social cues (SocialLOW and SocialHIGH) were—unbeknown to the participants—completely orthogonal to those learning cues, and therefore not predictive for the actual heat stimulation. After a jittered 3–5s delay, they were asked to rate how much pain they actually felt, again using a horizontal visual analog scale (pain rating, average individual median RT 2381 ms, range of individual median RTs 1164–4721 ms). The inter trial interval had a jittered duration of 6.5–9s. Following the learning task, participants performed an additional generalization task, which will be described elsewhere.
Skin conductance
Electrodermal activity was measured at the index and middle fingers of the left hand and recorded using a BIOPAC MP150 system and Acknowledge software at 500Hz sampling rate. Data was low pass filtered offline with a cutoff of 5Hz (61dB Butterworth).
Other measures
To examine which personality traits were associated with cue and social information effects on pain, we administered several questionnaires, which have previously been associated with individual differences in placebo analgesia and expectation effects (Koban, Ruzic, & Wager, 2013). Details regarding the measures used, the results, and their discussion are reported in the Supplementary Materials.
A debriefing questionnaire was administered to all but the first two participants (N=58) at the very end of the experimental session to assess subjective experiences during the task, asking open-ended questions about the experiences in the experiment, including what participants thought the purpose of the experiment was, how many different temperatures they perceived, and what strategies they used to make their expectation ratings. This debriefing questionnaire included two visual analog scales, on which participants were asked to rate the usefulness the social information and the cues for predicting the upcoming pain, as well as a forced-choice items that asked participants to pick the Gabor patch associated with higher pain (CueHIGH).
Analysis
Behavioral ratings were acquired on visual analog scales ranging from ‘absolutely no pain’ to ‘worst pain possible’ (in the context of the experiment), ranging from 0 to 100 (where 100 indicating highest pain or pain expectancy ratings).
Trial-wise SCR was analyzed using the SCRalyze toolbox (Bach, Flandin, Friston, & Dolan, 2009). SCRalyze uses a general linear model approach, similar to standard event-related fMRI analysis, to reliably estimate SCR to rapidly presented stimulus events (Bach, Flandin, Friston, & Dolan, 2010). The filtered skin conductance time series data was first normalized for each subject. Next, regressors for pain onsets in each trial in the learning task were convolved with a canonical SCR function (Bach et al., 2010) and fitted to the skin conductance time series, yielding estimates (in the form of beta weights) for the amplitude of pain-evoked SCRs for each trial. These beta weights reflect the size and direction of the SCR amplitude in each trial. As the conditioned cues and social information did not induce reliable positive SCR, we did not further analyze these events.
We used a multi-level robust general linear model to test how social and learning cues affected pain expectation ratings across all trials, as well as pain ratings and SCR to pain in medium temperature trials (48°C, therefore controlling for temperature). Further, multi-level mediation analysis (Atlas et al., 2010; Kenny, Korchmaros, & Bolger, 2003; Krull & MacKinnon, 2001) was employed to test whether the effects of learning cues (CueHIGH versus CueLOW) or social information (SocialLOW versus SocialHIGH) on the outcome (pain rating, pain SCR) were mediated by trial-wise and subject-wise differences in pain expectancy. Trial numbers were added as within-person covariates of no interest order to control for sequence (i.e. habituation and sensitization) effects (Jepma, Jones, & Wager, 2014). Further, experimental group was added as a 2nd level covariate of no interest to control for possible cohort effects.
The code for the multi-level GLM and the M3 multi-level mediation toolbox are available at wagerlab.colorado.edu/tools. Other statistical analyses were conducted in Matlab and SPSS software. A significance level of p < 0.05 was applied to all analyses unless otherwise stated.
Results
Effects of temperature
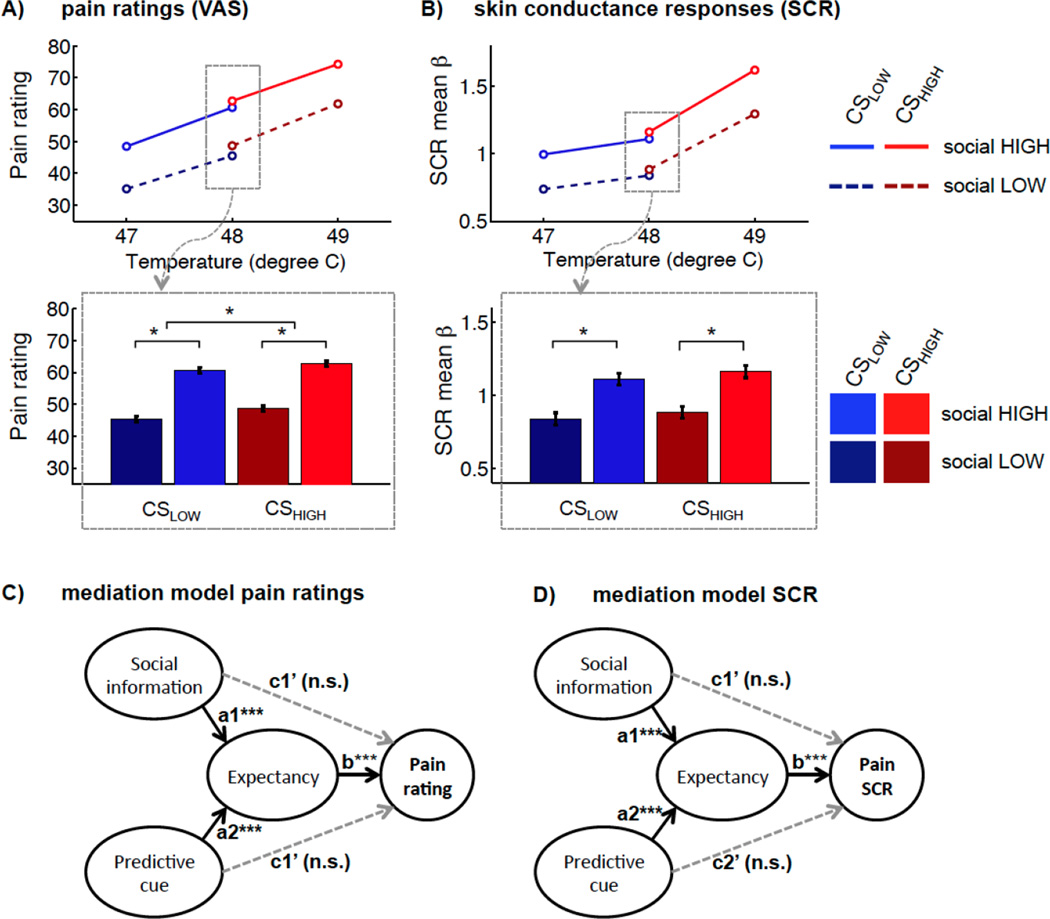
We first confirmed that participants were able to discriminate stimulations of different intensities, and that increasing temperatures in the noxious range produced clear increases in pain. Mean pain ratings for low (47°C), medium (48°C), and high temperature (49°C) were 41.8 (95%-CI ± 3.6), 54.4 (95%-CI ± 3.5), 68.0 (95%-CI ± 3.7), respectively. A multi-level robust regression (see Methods) confirmed a significant main effect of temperature on pain ratings (β = 13.1 (95%-CI ± 1.3), t(59) = 20.5, p < 0.0001), as shown in Figure 2A. Parallel effects of stimulus intensity were found on SCR (β = 0.30 (95%-CI ± 0.06), t(59) = 10.4, p < 0.0001), shown in Figure 2B.
Figure 2.
Results of the learning task. A) Pain ratings showed significant effects of stimulus temperature, of social information, as well as cue value (CSLOW vs. CSHIGH). The bar plot illustrates the effects of interest for medium temperature trials. B) SCR to pain were also characterized by effects of temperature, as well as social information. The lower panel bar plot illustrates these effects for medium temperature trials. C and D) Multi-level mediation models demonstrated that the effects of both social information and of CS on pain report (C) and on SCR (D) were fully mediated by expectancy ratings.
Effects of social information and learning cues on pain ratings and SCR
Figure 2 also shows how the experimental manipulations influenced pain ratings on intensity-matched medium temperature trials (selected for analysis to avoid any potential confounds with stimulus intensity). Social information (SocialHIGH versus SocialLOW) had large effects on pain reports, β = 7.3 (95%-CI ± 1.3), t(59) = 11.7, p < 0.0001, Figure 2A. A significant, albeit smaller, effect was also found for conditioned cues (CueHIGH versus CueLOW) on pain, β = 1.3 (95%-CI ± 0.7), t(59) = 3.7, p = 0.0004. No significant interaction was seen for CUE*SOCIAL effects on pain report, β = 0.25 (95%-CI ± 0.4), t(59) = 1.3, p = 0.20.
In parallel to the effects on behavioral pain reports, SCRs to medium-temperature painful events were significantly modulated by social information (SocialHIGH versus SocialLOW), β = 0.14 (95%-CI ± 0.04), t(59) = 6.4, p < 0.0001; Figure 2B. In contrast, the effects of conditioned cues (CueHIGH versus CueLOW) on SCR were not significant, β = 0.02 (95%-CI ± 0.04), t(59) = 1.2, p = 0.24. No effect was observed for the interaction between CUE*SOCIAL on SCR, β = 0.0 (95%-CI ± 0.04), t(59) = 0.1, p = 0.90. Trial-wise pain ratings and SCR estimates were significantly correlated for medium temperatures, β = 2.83 (95%-CI ± 1.02), t(59) = 5.6, p < 0.0001, indicating high consistency between self-report and physiological indices of pain. In addition, we tested, whether prediction errors, i.e. the difference between expected and experienced pain, had an influence on SCR to pain. There was a small negative effect of prediction error on SCR, β = −0.01 (95%-CI ± 0.005), t(59) =−2.0, p = 0.048, indicating that expected pain produces slightly higher SCRs, consistent with assimilation towards expected values (our main finding). When controlling for social information and CS, the effect of prediction error was no longer significant (p = 0.9).
Expectancy mediates effects on pain
We next used a multi-level meditation model (Atlas et al., 2010; Kenny et al., 2003; Krull & MacKinnon, 2001) to investigate whether cue and social effects on pain were mediated by expectations (Figure 2C). First, we tested how the two different types of information affected expectancy ratings (paths a1 and a2 in Figure 2C). SocialHIGH versus SocialLOW cues strongly increased pain expectancy ratings (path a1), β = 12.8 (95%-CI ± 1.6), t(59) = 16.1, p < 0.0001. In parallel, learning cues (CueHIGH versus CueLOW) also produced a highly significant, but smaller increase in pain expectancy (path a2), β = 2.7 (95%-CI ± 1.1), t(59) = 4.9, p < 0.0001. Further, expectancy strongly predicted pain ratings (path b, see Suppl. Fig. S1), β = 0.57, 95%-CI ± 0.08, t(59) = 14.6, p < 0.0001. Most importantly, in line with expectancy-based accounts, expectations completely mediated individual and trial-wise differences in social influence effects on pain, mediated path (a1*b) β = 6.6 (95%-CI ± 1.2), t(59) = 11.4, p = 0.0001 (see Figure 2C), as well as learning cue effects on pain, mediated path (a2*b) β = 1.1 (95%-CI ± 0.4), t(59) = 5.3, p = 0.0001 (Figure 2C). The residual direct path did not remain significant for both the social (path c’1), β = 0.2, (95%-CI ± 0.9), t(59) = 0.5, p = 0.65, as well as the conditioned cue effects (path c’2), β = −0.06 (95%-CI ± 0.7), t(59) = −0.3, p = 0.74.
Expectancy mediates effects on SCR
Next, we tested whether expectation also mediated social or cue effects on SCR. As described above, social information and conditioned cues significantly influenced expectancy of pain. Further, expectancy positively correlated with SCR (path b, see also Suppl. Fig. S1), β = 0.01 (95%-CI ± 0.0), t(59) = 3.5, p = 0.0002 (see also Suppl. Fig. S1). In parallel to the effects on pain reports, social information effects on SCR were fully mediated by expectations, mediated path (a*b) β = 0.13 (95%-CI ± 0.08), t(59) = 3.5, p < 0.0001. Even in the absence of a significant direct effect of learning cues on pain GSR, individual and trial-wise differences in expectancy had a significant mediating effect, β = 0.01 (95%-CI ± 0.0), t(59) = 3.1, p = 0.0009. In these models, the residual direct path c’ was not significant for both social information (path c’1), β = −0.01 (95%-CI ± 0.10), t(59) = −0.28, p = 0.81, and learning cues (path c’1), β = −0.02 (95%-CI ± 0.04), t(59) = −1.45 p = 0.18. This demonstrates that only when participants expected more or less pain based on social information or learning cues, they showed an increased autonomic response to painful stimulation.
Time course of effects on expectancy and pain
Given that participants had to learn the association between Gabor cues (CueHIGH versus CueLOW) and pain level by experience, we next investigated the role of TIME (trial number) and of interactions between TIME with CUE and SOCIAL effects (see Suppl. Fig S2). We found a significant TIME*CUE interaction effect on expectancy ratings, β = 0.02 (95%-CI ± 0.02), t(59) = 2.9, p = 0.0056, indicating that participants learned to expect more pain for the CueHIGH compared to the CueLOW over time. The TIME*CUE interaction effect on pain ratings also approached significance (β = 0.01 (95%-CI ± 0.01), t(59) = 1.92, p = 0.06). In parallel, the influence of social information on expectancy ratings decreased slightly over time, β = −0.03 (95%-CI ± 0.02), t(59) = −3.1, p = 0.0030, but remained strong across all trials (see Suppl. Fig S2). The TIME*SOCIAL interaction effect on pain ratings was not significant (p = 0.16), indicating that pain ratings were influenced by the social information across the entire experiment (see Suppl. Fig. S2).
Debriefing questionnaire
Participants rated the usefulness of the social information as slightly higher (mean 0.46 on VAS from 0–1) than the usefulness of the cues (mean rating 0.35), t(57) = 2.1, p = 0.045. Interestingly, whereas most participants rated the social information as moderately useful, the usefulness ratings for the cues had a bimodal distribution (see Suppl. Fig. S5), indicating that most participants did not find them useful, whereas some participants (the ‘learners’) found them very useful. Individual usefulness ratings of social information correlated strongly with individual differences in social influence effects on pain (r = 0.44, p < 0.001), whereas usefulness ratings for the cues correlated very strongly with cue effects on pain (r = 0.55, p < 0.001). In line with these findings, only 47% of the participants correctly identified the CSHIGH in the forced choice question, indicating that only a subset of learners explicitly learnt about the cue contingency (see Supplementary Materials for more results and an in depth discussion of individual differences).
Discussion
Social influences are effects mediated by a person’s beliefs about the mental states or behaviors of others on one’s own behavior and experience. Here, we tested a social influence paradigm that manipulated beliefs about others’ experience using simple visual displays of lines, which indicated putative pain ratings of other individuals (Yoshida et al., 2013), and examined the effects of this manipulation on subjective experience ratings and SCR responses to pain. Our results show strong and persistent influences of social information on both pain reports and pain-evoked SCRs. We tested these effects alongside classically conditioned, cue-based pain modulation in the same paradigm, which has been shown previously to impact subjective, autonomic, and brain measures related to pain, at least when accompanied by instructions about the cue contingency (e.g., Atlas et al., 2010; Colloca, Sigaudo, & Benedetti, 2008). Here, social information without conditioning produced stronger effects on pain and pain-related SCRs than non-instructed conditioned cues. Furthermore, the effects of both social information and cues were fully mediated by reported expectancies, suggesting expectation as a common mediator underlying both conditioned and unconditioned social influences on pain.
These findings are informative regarding a longstanding debate; namely, whether social conformity effects reflect only changes in overt behavior or also internal affective processes (Cialdini & Goldstein, 2004; Deutsch & Gerard, 1955). The effects of both non-reinforced social and reinforced conditioned cues on pain report demonstrate effects on the ‘gold standard’ measure of pain experience in clinical and research settings. The effects on SCR demonstrate that social influences go beyond response biases or decision-making to directly affect processes mediated by descending brainstem-autonomic circuits.
Together, these findings converge with other evidence in suggesting three broad conclusions. First, the influences of non-reinforced social informational cues can originate purely from conceptual processes, rather than low-level plasticity or automatic, evolutionarily afforded responses to prepared stimuli. Second, conditioned cue effects on physiological responses to pain may depend (at least in this paradigm) on explicit, conceptual learning processes, as only participants who learnt the cue contingency, showed these effects. And third, ‘information-based’ influences, which originate in socially instructed belief, can influence internal affective responses, including pain-related processes critical in clinical settings and for avoidance learning across all areas of psychology. These ‘information-based’ social influences may even be more powerful in their effects on pain than the effects of purely experience-based learning (see, e.g., Kirsch, 2004).
Relationships with conformity, demand characteristics, and other social instruction effects
Many forms of social and cognitive influence are assumed to be caused by effects on decision-making and sociocultural display. Conformity refers to changes in one’s behavior in order to match the responses of others (Cialdini & Goldstein, 2004), and is thought to be driven by affiliation goals (e.g. to be more similar to and liked by others) and accuracy goals (when others provide useful information) (Cialdini & Goldstein, 2004; see also Deutsch & Gerard, 1955). Demand characteristics (Nichols & Maner, 2008; Orne, 1962) are related to conformity, and refer to a desire on the part of research subjects to alter their reports and behavior to meet the expectations of experimenters or the situation—i.e., participants want to be “good subjects” and so report what they think they should report. All of these effects are assumed to be effects on decision-making and behavior independent of underlying experience and physiology. In principle, any measures of self-reported subjective experience may be driven by demand characteristics. However, self-reports are the gold-standard measure used to probe subjective experiences in the vast majority of studies of emotion, attitudes, beliefs, and pain. Unless subjective ratings are collected, effects on experience cannot be inferred. The researcher is left with a conundrum: Subjective experience ratings are essential for demonstrating treatment effects on pain and emotion, but they provide limited information on how deep and physiologically meaningful those effects are. They may reflect fundamental changes in experience, or later-stage effects on communicative behavior.
One solution lies in the collection of other, objective measures that can corroborate treatment effects on reported experience. Our effects on SCRs are mediated by descending brainstem regulation of (primarily) the sympathetic nervous system, and are incompatible with demand characteristics as originally formulated and commonly used today. In addition, we conducted debriefing interviews (Suppl. Fig. S5) and administered the Marlowe Crowne social desirability scale to check for signs of demand characteristics, and did not find any relationships with social influence effects on pain (see Supplementary Materials).
The alternative, which is most plausible here, is that social information affected the processing of nociceptive signals that give rise to pain, and that these changes affected pain experience and thus pain reports. That does not mean that all of the very strong effects on pain reports we observed are mediated by changes in underlying experience, and some portion of the effect may still be decision- or communication-related, as we and others have found previously in studies of placebo effects (Martini, Lee, Valentini, & Iannetti, 2015; Wager, Matre, & Casey, 2006).
An effect on underlying experience is also consistent with the time course of expectation and pain ratings (see Suppl. Fig. S2), which indicated that the influence of social information remained very large throughout the experiment. In other words, despite the lack of any systematic reinforcement of social information with variations in actual stimulus intensity, participants did not learn to ignore or disbelieve the social information. This finding is in line with the idea that social information actually affects pain experience and the ‘teaching signals’ that update expectations. Similar confirmation effects on learning have been found in other domains (e.g. Biele, Rieskamp, & Gonzalez, 2009; Doll, Jacobs, Sanfey, & Frank, 2009) induced by experimenter instructions. Given the strong social influence effect on reported pain experience and physiological responses, the teaching signal might have been altered, potentially turning the social information into a self-fulfilling prophecy.
Relationship with placebo analgesia, learning, and other expectancy effects
Autonomic responses have been previously shown to be influenced by several expectancy-related effects, including placebo effects (caused by administration of a sham drug) and psychological stressors. However, it has not been clear that (a) expectations themselves are a critical and sufficient ingredient in these effects, and (b) that pain-related autonomic responses can be modified by purely informational manipulations.
For example, several recent studies have shown placebo effects on SCRs, (Eippert et al., 2009; Geuter, Eippert, Attar, & Büchel, 2013; Nakamura et al., 2012), which might suggest influences of expectations. However, a closer look at these studies suggests that reliable effects of placebo treatment on SCR have mainly been produced by studies that use a combination of classical conditioning and experimenter instructions as part of the placebo manipulation. Classically conditioned autonomic effects can be produced by the brainstem alone, without the forebrain or cortex, and likely depend on learning in cerebellar-brainstem circuits (for review see Berntson & Micco, 1976). Thus, it may not be ‘expectation’ or related conceptual processes that affect SCR in these studies. In the present study, we independently manipulated socially instructed information and classical conditioning to disentangle socially transmitted instructions from experience-based learning. The results demonstrate that expectancies fully mediated social effects on both SCRs and pain reports. In addition, conditioned cue effects on SCRs were only apparent in participants who became consciously aware of the cue-heat contingency. Both effects suggest that expectation is a critical ingredient in modulating autonomic responses to pain.
Effects of social cues on pain physiology are similarly rare in the literature, and most of these may be explained in terms of basic affective responses to threatening cues. Previous studies have shown effects of social cues such as facial expressions on pain reports (Colloca & Benedetti, 2009; Reicherts, Gerdes, Pauli, & Wieser, 2013; Valentini, Martini, Lee, Aglioti, & Iannetti, 2014) and SCRs to threat cues (Olsson & Phelps, 2004). However, to our knowledge, no previous studies have shown clear effects of unreinforced social information on pain. Social cues like facial expressions may not be purely informational: These cues may serve as evolutionarily conserved, affective signals that trigger physiological responses in their own right (e.g., Jackson, Meltzoff, & Decety, 2005; Singer et al., 2004). By contrast, the social information presented in this study consisted of simple visual displays of lines, which are abstract representations of other people’s pain. Thus, in sum, we show that physiological responses to pain can be modulated by an informational social influence manipulation, even in the presence of more reliable conditioned cues.
The present findings are therefore important for the understanding of placebo and nocebo effects on pain, and other composite manipulations that influence both expectations and lower-level learning processes. They demonstrate that both unreinforced social information as well as experience-based learning can modulate expectations and experience of pain, independently of each other. Expectations may constitute a common pathway of social and experience-based effects on pain (Colloca & Miller, 2011; Kirsch, 1985), yet it remains an open question whether the neural mechanisms driving these two types of pain modulation are common or distinct (see Suppl. Materials for a discussion of different personality predictors of social influence and learning cue effects on pain).
Potential brain mechanisms of social modulation of pain
Recent brain imaging studies (Campbell-Meiklejohn, Bach, Roepstorff, Dolan, & Frith, 2010; Izuma & Adolphs, 2013; Klucharev, Hytönen, Rijpkema, Smidts, & Fernández, 2009) have demonstrated that detecting mismatch between one’s own and other people’s judgments evokes behavioral adjustments towards the social norm, and is paralleled by activity in dorsal mediofrontal cortex (dMFC), insula, and dorsolateral prefrontal cortex, suggesting a domain-general system for the detection and regulation of social conflict (Koban, Pichon, & Vuilleumier, 2014). Moreover, social adjustment also changes stimuli-related activation in brain regions related to reward processing and decision-making such as the ventral striatum and orbitofrontal cortex (Zaki, Schirmer, & Mitchell, 2011). One recent fMRI study demonstrated brainstem activity related to the uncertainty of partially reinforced social information (Yoshida et al., 2013), but so far, no study has investigated whether pain-specific neurophysiological activity is modulated by unreinforced social information and how these processes differ from conditioned-cue effects on pain. Future neuroimaging studies are needed to characterize the brain mediators of social compared to experience-based effects on pain report and physiology.
Limitations
There are several potential caveats and limitations to consider. We note that the relative strength of the social influence, compared to the conditioning, effects in our findings may be partially due to some of the characteristics of the experimental design. Presenting learning cues at the same time as the social information might increase processing load and reduce participants’ ability to accurately track social prediction errors and the value of the two abstract learning cues. The social information might have been more salient or easier to process than the difference between the two Gabor patches serving as conditioning cues. In addition, many demonstrations of conditioned pain modulation use conditioning reinforced by explicit instructions about which cues are associated with high vs. low pain (e.g., Atlas et al. 2010), thus potentially enhancing effects relative to those in the present experiment, which were learned solely through experience. Thus, we make no strong claims about the relative strength of conditioning vs. social influence in general.
We also note that we did not find clear SCRs in response to the presentation of the conditioned cues and the social information. Cue-evoked SCR might have indicated that either conditioned cues or social information (or both) were affectively arousing, with potential consequences for pain modulation. However, the absence of such effects may suggest that both types of cues may have had mainly informational value and that they were not highly arousing by themselves. In addition, they confirm that the pain-evoked SCR were evoked by the pain itself, and not confounded by residual cue-related SCR. More studies are needed to test whether the simultaneous presentation of two types or information may have influenced the results, e.g. by drawing attention to the potentially more salient social information and away from the abstract conditioning cues.
Further, an interesting possibility is that expectation ratings, as well as the social information itself, may serve as an anchor from which to adjust when performing pain ratings (Slovic & Lichtenstein, 1971; Tversky & Kahneman, 1974). However, high versus low social information also significantly predicted SCR, ruling out that the link between expectancy and pain ratings is purely driven by response biases. Further, a control experiment, which did not include expectation ratings, yielded highly similar results (see Suppl. Materials). Future studies may also test the effect of social information on SCR without measuring any behavioral pain ratings. More research is needed to understand the different levels on which social information influences perception, emotion, and behavior.
Conclusions
This study demonstrated strong effects of unreinforced social information on pain reports and physiology, even in the presence of more reliable and predictive information about upcoming pain. In parallel, experience-based learning cues modulated pain, but only for those participants who were explicitly aware of the cue-pain-contingency. This demonstrates a crucial mediating role of conceptual processes and consciously accessible expectations on endogenous pain regulation. Future brain imaging studies are needed to characterize the mechanisms underlying social and learning-based influences on pain.
Supplementary Material
Acknowledgments
We thank Megan Powell for help with data acquisition and Marieke Jepma and Marina López Solà for helpful discussions. This research was funded by a SNSF fellowship to LK (PBGEP1-142252) and NIH grants R01DA035484 and R01MH076136 (TDW).
Footnotes
Author Contributions
LK and TDW designed the experiment. LK acquired and analyzed the data. LK and TDW interpreted the results and wrote the manuscript.
References
References with asterisk indicate references from supplemental material.
- Amanzio Martina, Benedetti Fabrizio, Porro Carlo A, Palermo Sara, Cauda Franco. Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Human brain mapping. 2013;34(3):738–752. doi: 10.1002/hbm.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch Solomon E. Effects of group pressure upon the modification and distortion of judgments. Groups, Leadership, and Men. S. 1951:222–236. [Google Scholar]
- Atlas Lauren Y, Bolger Niall, Lindquist Martin A, Wager Tor D. Brain mediators of predictive cue effects on perceived pain. The Journal of Neuroscience. 2010;30(39):12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach Dominik R, Flandin Guillaume, Friston Karl J, Dolan Raymond J. Time-series analysis for rapid event-related skin conductance responses. Journal of neuroscience methods. 2009;184(2):224–234. doi: 10.1016/j.jneumeth.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach Dominik R, Flandin Guillaume, Friston Karl J, Dolan Raymond J. Modelling event-related skin conductance responses. International Journal of Psychophysiology. 2010;75(3):349–356. doi: 10.1016/j.ijpsycho.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson Gary G, Micco David J. Organization of brainstem behavioral systems. Brain Research Bulletin. 1976;1(5):471–483. doi: 10.1016/0361-9230(76)90117-9. [DOI] [PubMed] [Google Scholar]
- Biele Guido, Rieskamp Jörg, Gonzalez Richard. Computational models for the combination of advice and individual learning. Cognitive science. 2009;33(2):206–242. doi: 10.1111/j.1551-6709.2009.01010.x. [DOI] [PubMed] [Google Scholar]
- Campbell-Meiklejohn Daniel K, Bach Dominik R, Roepstorff Andreas, Dolan Raymond J, Frith Chris D. How the opinion of others affects our valuation of objects. Current Biology. 2010;20(13):1165–1170. doi: 10.1016/j.cub.2010.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialdini Robert B, Goldstein Noah J. Social influence: Compliance and conformity. Annu. Rev. Psychol. 2004;55:591–621. doi: 10.1146/annurev.psych.55.090902.142015. [DOI] [PubMed] [Google Scholar]
- Colloca Luana, Benedetti Fabrizio. Placebo analgesia induced by social observational learning. Pain. 2009;144(1–2):28–34. doi: 10.1016/j.pain.2009.01.033. [DOI] [PubMed] [Google Scholar]
- Colloca Luana, Miller Franklin G. How placebo responses are formed: a learning perspective. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2011;366(1572):1859–1869. doi: 10.1098/rstb.2010.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca Luana, Petrovic Predrag, Wager Tor D, Ingvar Martin, Benedetti Fabrizio. How the number of learning trials affects placebo and nocebo responses. Pain. 2010;151(2):430–439. doi: 10.1016/j.pain.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca Luana, Sigaudo M, Benedetti F. The role of learning in nocebo and placebo effects. Pain. 2008;136(1–2):211–218. doi: 10.1016/j.pain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Deutsch Morton, Gerard Harold B. A study of normative and informational social influences upon individual judgment. The Journal of Abnormal and Social Psychology. 1955;51(3) doi: 10.1037/h0046408. [DOI] [PubMed] [Google Scholar]
- Doll Bradley B, Jacobs W Jake, Sanfey Alan G, Frank Michael J. Instructional control of reinforcement learning: a behavioral and neurocomputational investigation. Brain research. 2009;1299:74–94. doi: 10.1016/j.brainres.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63(4):533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- *.Geers Andrew L, Wellman Justin A, Fowler Stephanie L, Helfer Suzanne G, France Christopher R. Dispositional optimism predicts placebo analgesia. The Journal of Pain. 2010;11(11):1165–1171. doi: 10.1016/j.jpain.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuter Stephan, Büchel Christian. Facilitation of pain in the human spinal cord by nocebo treatment. The Journal of Neuroscience. 2013;33(34):13784–13790. doi: 10.1523/JNEUROSCI.2191-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuter Stephan, Eippert Falk, Attar Catherine Hindi, Büchel Christian. Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage. 2013;67(C):227–236. doi: 10.1016/j.neuroimage.2012.11.029. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubert Liesbet, Vlaeyen Johan WS, Crombez Geert, Craig Kenneth D. Learning about pain from others: an observational learning account. The journal of pain: official journal of the American Pain Society. 2011;12(2):167–174. doi: 10.1016/j.jpain.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Hunter T, Siess F, Colloca L. Socially induced placebo analgesia: A comparison of a pre-recorded versus live face-to-face observation. European journal of pain (London, England) 2013 doi: 10.1002/j.1532-2149.2013.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma Keise, Adolphs Ralph. Social Manipulation of Preference in the Human Brain. Neuron. 2013;78(3):563–573. doi: 10.1016/j.neuron.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson Philip L, Meltzoff Andrew N, Decety Jean. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24(3):771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jepma Marieke, Jones Matt, Wager Tor D. The dynamics of pain: Evidence for simultaneous site-specific habituation and site-nonspecific sensitization in thermal pain. The Journal of Pain. 2014 doi: 10.1016/j.jpain.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny David A, Korchmaros Josephine D, Bolger Niall. Lower level mediation in multilevel models. Psychological methods. 2003;8(2) doi: 10.1037/1082-989x.8.2.115. [DOI] [PubMed] [Google Scholar]
- Kirsch Irving. Response expectancy as a determinant of experience and behavior. American Psychologist. 1985;40(11):1189–1202. [Google Scholar]
- Kirsch Irving. Conditioning, expectancy, and the placebo effect: comment on Stewart-Williams and Podd (2004) Psychological Bulletin. 2004;130(2):341–343. doi: 10.1037/0033-2909.130.2.341. discussion 344–345. [DOI] [PubMed] [Google Scholar]
- Klucharev Vasily, Hytönen Kaisa, Rijpkema Mark, Smidts Ale, Fernández Guillén. Reinforcement learning signal predicts social conformity. Neuron. 2009;61(1):140–151. doi: 10.1016/j.neuron.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Koban Leonie, Pichon Swann, Vuilleumier Patrik. Responses of medial and ventrolateral prefrontal cortex to interpersonal conflict for resources. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nst020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koban Leonie, Ruzic Luka, Wager Tor D. Brain Predictors of Individual Differences in Placebo Responding. In: Colloca L, Flaten MA, Meissner K, editors. Placebo and Pain: From Bench to Bedside. San Diego: Academic Press; 2013. pp. 89–102. [Google Scholar]
- Krull Jennifer L, MacKinnon David P. Multilevel modeling of individual and group level mediated effects. Multivariate behavioral research. 2001;36(2):249–277. doi: 10.1207/S15327906MBR3602_06. [DOI] [PubMed] [Google Scholar]
- *.Leknes Siri, Tracey Irene. A common neurobiology for pain and pleasure. Nature Reviews. Neuroscience. 2008;9(4):314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- *.Loggia Marco L, Mogil Jeffrey S, Catherine Bushnell M. Empathy hurts: compassion for another increases both sensory and affective components of pain perception. Pain. 2008;136(1):168–176. doi: 10.1016/j.pain.2007.07.017. [DOI] [PubMed] [Google Scholar]
- *.Lyby Peter Solvoll, Aslaksen Per M, Flaten Magne Arve. Variability in placebo analgesia and the role of fear of pain--an ERP study. Pain. 2011;152(10):2405–2412. doi: 10.1016/j.pain.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Martini M, Lee MCH, Valentini E, Iannetti GD. Intracortical modulation, and not spinal inhibition, mediates placebo analgesia. The European journal of neuroscience. 2015;41(4):498–504. doi: 10.1111/ejn.12807. [DOI] [PubMed] [Google Scholar]
- Meissner Karin, Bingel Ulrike, Colloca Luana, Wager Tor D, Watson Alison, Flaten Magne Arve. The placebo effect: advances from different methodological approaches. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(45):16117–16124. doi: 10.1523/JNEUROSCI.4099-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Morton Debbie L, Watson Alison, El-Deredy Wael, Jones Anthony KP. Reproducibility of placebo analgesia: Effect of dispositional optimism. Pain. 2009;146(1):194–198. doi: 10.1016/j.pain.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Donaldson GW, Kuhn R, Bradshaw DH, Jacobson RC, Chapman CR. Investigating dose-dependent effects of placebo analgesia: a psychophysiological approach. Pain. 2012;153(1):227–237. doi: 10.1016/j.pain.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Nichols AL, Maner JK. The good-subject effect: Investigating participant demand characteristics. Journal of General Psychology. 2008;135(2):151–165. doi: 10.3200/GENP.135.2.151-166. doi: [DOI] [PubMed] [Google Scholar]
- Olsson Andreas, Nearing Katherine I, Phelps Elizabeth A. Learning fears by observing others: the neural systems of social fear transmission. Social Cognitive and Affective Neuroscience. 2007 doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson Andreas, Phelps Elizabeth A. Learned fear of "unseen" faces after Pavlovian, observational, and instructed fear. Psychological Science. 2004;15(12):822–828. doi: 10.1111/j.0956-7976.2004.00762.x. [DOI] [PubMed] [Google Scholar]
- Olsson Andreas, Phelps Elizabeth A. Social learning of fear. Nature Neuroscience. 2007;10(9):1095–1102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- Orne MT. On the Social-Psychology of the Psychological Experiment - with Particular Reference to Demand Characteristics and Their Implications. American Psychologist. 1962;17(11):776–783. doi: [Google Scholar]
- *.Peciña Marta, Azhar Hamdan, Love Tiffany M, Lu Tingting, Fredrickson Barbara L, Stohler Christian S, Zubieta Jon-Kar. Personality Trait Predictors of Placebo Analgesia and Neurobiological Correlates. Neuropsychopharmacology. 2013;38(4):639–646. doi: 10.1038/npp.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps Elizabeth A, O'Connor Kevin J, Gatenby J Christopher, Gore John C, Grillon Christian, Davis Michael. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4(4):437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Price Donald D, Finniss Damien G, Benedetti Fabrizio. A Comprehensive Review of the Placebo Effect: Recent Advances and Current Thought. Annual review of psychology. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- Reicherts Philipp, Gerdes Antje BM, Pauli Paul, Wieser Matthias J. On the mutual effects of pain and emotion: facial pain expressions enhance pain perception and vice versa are perceived as more arousing when feeling pain. Pain. 2013;154(6):793–800. doi: 10.1016/j.pain.2013.02.012. [DOI] [PubMed] [Google Scholar]
- *.Scott David J, Stohler Christian S, Egnatuk Christine M, Wang Heng, Koeppe Robert A, Zubieta Jon-Kar. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Archives of general psychiatry. 2008;65(2):220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- *.Sharot Tali, Korn Christoph W, Dolan Raymond J. How unrealistic optimism is maintained in the face of reality. Nature Neuroscience. 2011;14(11):1475–1479. doi: 10.1038/nn.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer Tania, Seymour Ben, O'Doherty John, Kaube Holger, Dolan Raymond J, Frith Chris D. Empathy for pain involves the affective but not sensory components of pain. Science (New York, N.Y.) 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Slovic Paul, Lichtenstein Sarah. Comparison of Bayesian and regression approaches to the study of information processing in judgment. Organizational Behavior and Human Performance. 1971;6(6):649–744. [Google Scholar]
- Tversky Amos, Kahneman Daniel. Judgment under Uncertainty: Heuristics and Biases. Science. 1974;185(4157):1124–1131. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- Valentini Elia, Martini Matteo, Lee Michael, Aglioti Salvatore M, Iannetti Giandomenico. Seeing facial expressions enhances placebo analgesia. Pain. 2014;155(4):666–673. doi: 10.1016/j.pain.2013.11.021. [DOI] [PubMed] [Google Scholar]
- *.Wager Tor D, Atlas Lauren Y, Leotti Lauren A, Rilling James K. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. The Journal of Neuroscience. 2011;31(2):439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager Tor D, Matre Dagfinn, Casey Kenneth L. Placebo effects in laser-evoked pain potentials. Brain, behavior, and immunity. 2006;20(3):219–230. doi: 10.1016/j.bbi.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. Attitude change: persuasion and social influence. Annual review of psychology. 2000;51:539–570. doi: 10.1146/annurev.psych.51.1.539. [DOI] [PubMed] [Google Scholar]
- Yoshida Wako, Seymour Ben, Koltzenburg Martin, Dolan Raymond J. Uncertainty increases pain: evidence for a novel mechanism of pain modulation involving the periaqueductal gray. The Journal of Neuroscience. 2013;33(13):5638–5646. doi: 10.1523/JNEUROSCI.4984-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki Jamil, Schirmer Jessica, Mitchell Jason P. Social influence modulates the neural computation of value. Psychological Science. 2011;22(7):894–900. doi: 10.1177/0956797611411057. [DOI] [PubMed] [Google Scholar]
- *.Zubieta Jon-Kar, Yau Wai-Ying, Scott David J, Stohler Christian S. Belief or Need? Accounting for individual variations in the neurochemistry of the placebo effect. Brain, behavior, and immunity. 2006;20(1):15–26. doi: 10.1016/j.bbi.2005.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.