Abstract
Compromised dopamine signaling in the striatum has been associated with the expression of impulsive behaviors in addiction, obesity and alcoholism. In rodents, Intragastric infusion of the fatty acid amide oleoylethanolamide increases striatal extracellular dopamine levels via vagal afferent signaling. Here we tested whether supplementation with PhosphoLean™, a dietary supplement that contains the precursor of the fatty acid amide oleoylethanolamide (N-oleyl-phosphatidylethanolamine), would reduce impulsive responding and alcohol use in heavy drinking young adults. Twenty-two individuals were assigned to a three-week supplementation regimen with PhosphoLean™ or placebo. Impulsivity was assessed with self-report questionnaires and behavioral tasks pre- and post-supplementation. Although self-report measures of impulsivity did not change, supplementation with PhosphoLean™, but not placebo, significantly reduced false alarm rate on a Go/No-Go task. In addition, an association was found between improved sensitivity on the Go/No-Go task and reduced alcohol intake. These findings provide preliminary evidence that promoting fatty acid derived gut-brain dopamine communication may have therapeutic potential for reducing impulsivity in heavy drinkers.
Keywords: OEA, alcohol, impulsivity, Go/No-Go task, inhibitory control, dopamine
1. Introduction
Deficient dopamine signaling has been implicated as both a cause and a consequence of obesity, alcoholism and addiction [1, 2]. With respect to obesity, in rodent models a high fat diet increases adiposity, decreases extracellular striatal dopamine response to nutrients [3]; decreases D2 receptor density in the striatum and increases compulsive responding for food [4]. Neuroimaging studies suggest parallel effects in humans. Overweight/obese compared to healthy weight individuals show reduced change in striatal D2 receptor binding potential in response to glucose ingestion (consistent with reduced dopamine release) [5] and several studies have reported a negative association between body mass index (BMI) and the blood oxygen level dependent (BOLD) response to milkshake consumption in the caudate nucleus [6–8]. Although BOLD does not directly measure dopamine release, this effect is dependent upon the TaqIa A1 polymorphism, which affects D2 receptor density, thus linking the BOLD response to D2 receptor signaling [6]. Consistent with the rodent work, the decreased response also appears to be a consequence rather than a cause of obesity since it is associated with weight gain [9], but not risk for obesity [10], Finally, and critical for the aim of the current study, lower BOLD response to milkshake in the caudate nucleus is associated with increased impulsivity, especially in overweight/obese individuals [8]. Collectively these findings suggest that a diet high in fat and/or increased adiposity results in dopamine adaptions in the dorsal striatum that increase impulsive behaviors, which are themselves a risk-factor for obesity [11].
The exact mechanism by which this effect occurs in humans is unknown. However, recent work in the animal model suggests that compromised fatty-acid derived brain-gut communication plays a role. Nutrient infusion directly into the gut increases extracellular dopamine levels [3]. This response is compromised following a high fat diet, which concomitantly depletes intestinal levels of N-Acylethanolamines, a family of appetite-regulating fatty acid amides [12, 13]. One such amide [14], oleoylethanolamide (OEA), which is synthesized in the intestine in response to dietary oleic acid [15], not only acts as a powerful satiety messenger by signaling via a nuclear receptor peroxisome proliferator-activated receptor alpha (PPARα) [16], but also reverses diet-induced blunted striatal dopamine signaling [3]. Specifically, placing rats on a high fat diet decreases OEA levels in the intestine and blunts the rise in extracellular striatal dopamine normally observed in response to intragastric infusion of lipids. This blunting may then be reversed by intraperitoneal infusion of OEA. OEA infusion can also potentiate dopamine release in response to a low-fat IG infusion in lean animals on a low fat diet, an effect accompanied by a decrease in preference for high fatty foods demonstrating that the ability of OEA to influence striatal dopamine efflux is not restricted to the context of diet-induced dopamine adaptations [3]. Furthermore, intra-gastric injection of OEA in lean mice causes a decrease in preference for high fatty foods [17]. Whether similar effects can be observed in humans is unknown; however, plasma OEA levels are associated with brain responses to food images [18] and supplementation with the dietary supplement PhosphoLean™, which contains N-oleyl-phosphatidyl-ethanolamine (NOPE), the precursor for OEA, increases compliance with weight loss programs [19, 20], possibly indicating a beneficial effect of supplementation on self-control.
Similar to a high fat diet, prolonged heavy drinking of alcohol is associated with altered dopamine signaling [21] and impulsivity [11]. Alcohol abuse also clearly alters lipid metabolism [22] and liver function [23]. Similar to the effect of a high fat diet on OEA, alcohol intake releases OEA, and chronic ethanol administration decreased OEA levels in parallel with the onset of withdrawal symptoms [24]. Even more critically, OEA administration can block cue-induced reinstatement of alcohol-seeking behavior (the animal model for relapse). This suggests the intriguing possibility that OEA may be a novel therapeutic target for alcohol use disorders and alcoholism. With this in mind we set out to perform a preliminary study to test if dietary supplementation with a fatty acid amide could reduce alcohol intake and impulsivity in a group of young adult heavy drinkers. Twenty-two participants underwent three weeks of dietary supplementation with PhosphoLean™ (30 mg N-oleyl-phosphatidyl-ethanolamine (NOPE) + 20 mg epigallocatechin-3-gallate (EGCG) per capsule) or treatment with placebo. We predicted that impulsivity would decrease in the PhosphoLean™, but not placebo group, and that decreases in impulsivity would be associated with reduced alcohol intake. Given the animal data linking OEA administration with change in preference for fat [17] and to consider potential effects of OEA food choice, we also measured fat concentration preference.
2. Materials and methods
2.1 Participants
Twenty-two healthy human adults ranging in age from 21–45 years participated in the study. Participants had to meet NIAAA heavy drinking criteria [25] (5 or more drinks for men and 4 or more standard drinks for women on a drinking day) at least once per week for the prior 21 days, but they had to consume less than 40 drinks in total per week. Further criteria include right handedness, English speaking, and a body mass index (BMI expressed as kg/m2) within the range 18.5–35.
Participants were excluded if they had a past or current history of alcohol or drug abuse or dependence, or tested positive on any toxicology tests performed at each session (reported past or current use or positive test for Tetra Hydro Cannabinol (THC) was allowed), medical illness, psychiatric illness as defined by the DSM-IV criteria including eating disorders, medications that affect alertness, history of head trauma with loss of consciousness, diabetes, food allergy or ongoing pregnancy. Out of 29 recruited subjects seven did not complete the study: four because they were ineligible , two due to scheduling issues and one for not wanting to take the supplement.
Participants were recruited with flyers and online advertisements in the Yale University and the greater New Haven communities. Written informed consent was obtained and the protocol was approved by the Yale University Human Investigations Committee.
2.2 Supplement
PhosphoLean™ is a dietary supplement consisting of N-oleyl-phosphatidyl-ethanolamine (NOPE) and epigallocatechin-3-gallate (EGCG). NOPE is extracted from soy phospholipids and EGCG from standardized green tea extract. NOPE consists of OEA bound to phosphatidylethanolamine (PE). NOPE is a naturally occurring ethanolamine glycerol-phospholipid containing three fatty acid chains and is found in animal and vegetable foods that are part of the human diet. OEA activates peroxisome-proliferator-activated receptor-alpha found in the intestine tract through binding after oral admission [16]. EGCG polyphenols acts synergistically with OEA via sympathetic activation of thermogenesis and increases fat oxidation [26]. Every capsule, supplied by CHEMI Nutra (White Bear Lake, MN), contains 30 mg of NOPE and 20 mg of EGCG. PhosphoLean™ 40P is a dietary ingredient under the Dietary Supplement Health and Education Act (DSHEA) regulations of the US FDA (1994).
Participants were assigned in a double-blind manner to dietary supplementation with PhosphoLean™ or placebo. We instructed participants to take 6 capsules a day for three weeks (21 days); two capsules consumed one hour before lunch, two capsules one hour prior to dinner, and two capsules two hours after dinner. The dosage was chosen based on previous reports in the literature [19, 20] and recommendation from the supplier. We chose this schedule of delivery to maximize the effect of the supplement in the evening hours when most alcohol is consumed. The placebo was identical in appearance to the PhosphoLean™ capsules, but contained 100 mg of rice flour per capsule. Text message reminders were sent weekly to increase adherence with taking the dietary supplement or placebo, together with a weekly phone call to ask about adherence and experience of any side effects. Subjects were not explicitly instructed to change their alcohol consumption and were told that apart from taking their supplement they could continue their regular routine.
2.3 Study design
After phone screening, participants were invited to participate in three sessions; an intake session, followed by a pre-treatment session on a separate day and a post-treatment session after 3 weeks of supplementation.
To exclude psychiatric conditions and control for post-hoc group differences, participants were interviewed, filled out questionnaires, and completed intelligence tests, during the intake session. To exclude psychiatric conditions and drug or alcohol abuse or dependence the Mini International Neuropsychiatric Interview (MINI) [27], the Beck Anxiety Inventory (BAI) [28] and the Beck Depression Inventory (BDI) [29] were given. Smoking, marijuana, and alcohol use in the past three weeks was measured with the timeline follow back (TLFB) [30] to ensure eligibility. Verbal intelligence was measured with the North American Adult Reading test (NAART) [31] and non-verbal intelligence with the Test of Nonverbal intelligence (TONI) [32]. Following the intake session, eligible participants were assigned to treatment conditions and participants scheduled for a pre- and post-treatment session. Assignment to treatment condition was performed by a simple coin toss for the first 10 participants and subsequent participants were allocated to treatment groups to minimize differences between the two groups on demographics and anthropometrics (age, gender, BMI, education level, depression, anxiety, verbal and non-verbal intelligence, smoking, alcohol and THC use at intake).
During both the pre- and post-treatment sessions, participants arrived at the lab between 8 AM and 2 PM for a 12-hour fasting blood draw (water was allowed). Participants then underwent a breath alcohol test (using the Alcohawk Elite Breathalyzer), urine toxicology tests (with the Integrated E-Z Split Key Cup II) for THC, benzodiazepines, cocaine, (meth) amphetamines and opiates, and if applicable, a urine pregnancy screening. Anthropometric measures including BMI, waist-hip ratio and relative body fat, measured with a BodPod body composition tracking system (Cosmed), which is an air displacement plethysmograph, were also obtained at each session Finally, participants filled out questionnaires, completed a TLFB interview, three behavioral tasks and a fat /sweet concentration preference task. We provide details for each of these questionnaires and tasks below. The study was approved by the Yale Medical School Institutional Review Board (Clinical trials registration number: NCT01902069).
2.4 Outcome measures
The effect of PhosphoLean™ on alcohol consumption as assessed by the TLFB interview and impulsivity were considered the main outcome measures. Secondary outcome measures were food intake, eating behavior, physical activity, anxiety, depressive symptoms, response inhibition, sensitivity to negative outcome learning, and fat concentration preference.
2.4.1 TLFB Interview
To have an accurate indication of the quantity and frequency of alcohol consumption during the prior 21 days the TLFB was administered by trained interviewers [30]. Participants were interviewed about their alcohol consumption for each day using a calendar and key dates as memory prompts. Samples of different cups and glasses in various sizes and shapes were provided to facilitate accurate reporting. Additionally, brand names were noted and percent alcohol verified. The TLFB has demonstrated good psychometric properties in numerous studies [33].
2.4.2 Questionnaires
To measure impulsivity, we asked participants to fill out the Barratt Impulsiveness Scale Version 11 (BIS-11) [34], which consists of motor (imprudent action), attentional (impatience with complexity and rapid shifts) and non-planning (lack of future orientation) impulsivity subscales. Cognitive control of eating (conscious considerations about food intake), ability to inhibit eating (uncontrolled food intake) and feelings about hunger (emotional influence on food intake) were measured with the Three Factor Eating Questionnaire (TFEQ) [35]. We estimated physical activity using the International Physical Activity Questionnaire (IPAQ) [36]. To evaluate overall fat intake participants completed the Dietary Fat and Free Sugar Questionnaire (DFS) [37], which collects information about the frequency of food eaten over the past 12 months. The DFS has four subscales to estimate consumption of saturated fats and free sugars: total score, saturated fat, free sugar intake and fat-sugar. Finally, to assess depression and anxiety participants filled out the BDI and the BAI.
2.4.3 Behavioral Tasks
We used the Go/No-Go task [38] to examine selective attention and response control. During the task the participant was instructed to press a response key (spacebar) as quickly as possible to a Go stimulus (X) and withhold their response to a No-Go stimulus (K), which appeared in different frequencies (87% × and 13% K) on a black computer screen (250 milliseconds (ms) display, 1000 ms inter trial interval). The appearance of Go and No-Go stimuli was pseudorandomized with intervals of 10–15 seconds between No-Go stimuli. The task consisted of two blocks with 246 trials each lasting min 21 seconds, producing a total of 492 total trials per participant. A break of approximately one min was provided between the blocks.
To assess the ability to learn from positive and negative outcomes we used The Probabilistic-Feedback Reward Task (PFRT) [39]. On each trial, the participant is presented with two unfamiliar symbols, asked to choose one symbol, and then receives feedback (“correct” or “incorrect”). The symbols are presented in three pairs: AB, CD and EF, counterbalanced across participants with a unique set of symbols used for each session. The probability of receiving “correct” feedback for each symbol pair is 80/20, 70/30, and 60/40 percent, respectively. The task begins with a training period of variable duration during which the participant receives feedback and must learn which stimulus is associated with positive feedback and which is associated with negative feedback. Pairs are presented in blocks of 60 choices until the participant reaches a threshold level of performance. After criterion is reached the participant continues with the same task, but the choices are presented in new pairs (AC, AD, AE, AF, BC, BD, BE, BF) and no feedback is given. The ability to learn from positive feedback is determined by examining the number of times the participant choses A, and the ability to learn from negative feedback was determined by counting the number of times that they successfully avoided choosing B. The ability to learn from positive outcomes has been postulated to be related to D1 receptor signaling, whereas ability to avoid negative outcomes has been postulated to be related D2 receptor signaling [40].
The Experiential Discounting Task (EDT) [41] measures delay discounting. The task consists of four blocks, each with a minimum of 16 choices between a delayed and immediate outcome. In each block the delayed option has a standard value ($0.30), is probabilistic (35% change of receiving), and delayed by a consistent interval (0, 15, 30, or 60 sec). The immediate option is an adjusting amount of money (starting at $0.20, range of $0.10 and $0.25) that is immediate and certain (100% chance of receiving). On each trial the participant chooses between the delayed and immediate option. They then “cash in” by clicking a button on a coin dispenser. If the participant chooses the delayed amount, the immediate amount for the next choice is increased by $0.05. If the participant chooses the immediate amount, the immediate amount for the next choice is decreased by $0.05. If a participant selects the same choice four consecutive times, they are forced to select the other choice (i.e., only one outcome button was presented). Between blocks there is an interblock interval to ensure a block could not be ended more quickly by any specific choice sequence. The total amount of money made after completing the whole task was added to the check amount for the participant payment.
2.4.4 Fat concentration preference assessment
To assess fat and sweet concentration preference participants rated puddings and Jell-O on a variety of attributes. The General Labeled Magnitude Scale (gLMS) [42] was used to assess intensity perception. It is a vertical line with quasi-logarithmic spaced labels that start at the bottom with ‘barely detectable’ to ‘strongest imaginable’ at the top. The Labeled Hedonic Scale (LHS) was used to assess liking [43]. It is similar to the gLMS, but the end labels are ‘most imaginable dislike’ to ‘most imaginable like’, with the label ‘neutral’ in the middle. Visual Analogue Scales (VAS) were used to assess hunger, fullness, thirst, oiliness, fattiness, creaminess and wanting. The VAS is a horizontal line anchored by ‘not at all’ at one end and ‘extremely’ at the other [44]. The puddings (Jell-O, Kraft Foods with milk Guida’s Dairy) were made with four different concentrations of fat: 0%, 3.1%, 6.9%, and 15.6% (with constant sugar content (w/w)) [45]. The Jell-O’s (flavored with Kool-Aid and deionized water) were made with four different concentrations of sucrose: 0, 0.1, 0.56, and 1 molar (M). Participants chose their preferred flavor prior to the start of the task (vanilla and chocolate pudding and orange or strawberry Jell-O).
The task started with the participant rating how hungry, full, and thirsty they felt. Then a stimulus was presented on a tasting spoon (± 1.5 mL) while the participant was blindfolded (to prevent differences in the color of the puddings from influencing ratings). After tasting and swallowing each stimulus, the participant removed the blindfold, rated the intensity, sweetness, liking, oiliness, fattiness, creaminess and wanting, then rinsed with deionized water and waited for 30 seconds before putting on the blindfold and receiving the next stimulus. Stimuli were delivered in blocks (pudding or Jello-O) and each stimulus was repeated three times in a randomized order.
2.5 Data analysis
All statistical analyses were done using IBM SPSS statistics 21. To evaluate group difference on intake measures, we used an independent sample t-test. To evaluate how supplementation with PhosphoLean™ affected outcome measures, we conducted a repeated measures MANOVA for each of the questionnaires and tasks, with group (PhosphoLean™ and placebo) as a between-participants factor, and time (pre and post) as a within-subjects factor, gender as a covariate, and subscales or sub measures as independent measures. We inspected the multivariate and univariate interaction effect of group and time. We report post-hoc pairwise comparisons of time within each group for significant and trending P-values. To investigate whether change from pre-supplementation to post-supplementation of any significant secondary outcomes measures correlated with the primary outcome measure of alcohol consumption, correlation analyses were performed with non-parametric Spearman’s coefficient to increase sensitivity due to low number of observations. We considered P-values < 0.05 as statistically significant and report P-values < 0.1 as a trend. We repeated all analyses with BMI as a covariate and the results were unchanged.
2.5.1 TLFB Interview
The answers on the TLFB were converted to standard alcoholic drinks per day. We then calculated: total drinks in the past 21 days (total dr), number of days drinking (drdy), the average amount of standard drinks per drinking day (dr/drdy), the maximum number of drinks per day (max dr/dy), and number of heavy drinking days per week (m≥5 and f≥4 drinks per day, heavy drdy). To evaluate how supplementation with PhosphoLean™ affected alcohol consumption, we conducted a repeated measures ANOVA, with group (Phosphlean and placebo) as a between-subject factor, and time (pre and post) as a within-subject factor, gender as a covariate, and total dr, drdy, dr/drdy, max dr/dy, and heavy drdy as independent measures.
2.5.2 Questionnaires
BIS11, TFEQ and DFS were recoded and scored as instructed in user manuals. From the IPAQ, the metabolic equivalent minutes per week (MET-minutes/WK) was calculated by multiplying metabolic intensity with the minutes for each activity over the last week. For the BDI and BAI scores were calculated by a simple sum of all answers.
2.5.3 Behavioral Tasks
In every trial of the Go/No-Go task, there are four possible stimulus/response combinations as an outcome; a response to a Go stimulus is classified a “hit”, a response to a No-Go stimulus is a “false alarm”, not responding to a Go stimulus is a “miss”, and no response to No-Go stimulus is a “correct rejection”. Proportion of hits (pH) and false alarms (pFA) were included in our analysis and proportion of misses and correct rejections were omitted since these are perfectly correlated to hit and false alarm rates. Besides these two measures, the reaction time of the hits (rtH) and the reaction time of the false alarms (rtFA) was registered. D’ (proportion of hits – proportion false alarms) was calculated to show the effectiveness of individual decision making. To evaluate how supplementation with PhosphoLean™ affected response inhibitions, we conducted a repeated measures ANOVA, with group (PhosphoLean™ and placebo) as a between-subject factor, and time (pre and post) as a within-subject factor, gender as a covariate, and pH, pFA, rtH, rtFA and d’ as independent measures.
Outcome measures on the PFRT were the proportion of times the participant chose A, avoided B, and the number of blocks needed to perform at criterion in the training phase.
For the EDT we calculated the indifference point; the point at which the participant evaluates the two choices as equally reinforcing. A titration procedure was designed to find a stable point of indifference between the delayed and immediate amounts. Stability was defined as the time that three out of the previous six free choices were made for the immediate option. We calculated the area under the discount curve (AUC) with the formula V = A/(1+kD), where V represents the value of the delayed reinforcer, A is the amount of the reinforcer and D the length of delay to its delivery, k indicates the steepness of the discount curve.
2.5.4 Fat concentration preference assessment
Ratings from the fat and sweet concentration preference test were averaged across the 3 presentations of the same stimulus concentration within each participant, and then entered into a repeated measures MANOVA with group (PhosphoLean™ and placebo) as a between-subject factor and time (pre and post) and concentration (1–4) as within-subject factors, gender as a covariate and rating scales for puddings and Jell-O’s as independent measures. We inspected the multivariate and univariate interaction effect of group and time, as well as the interaction effect of group, time and concentration.
We also coded variables for change from pre to post- supplementation in most preferred concentration for puddings and Jell-Os by using: 1) change in concentration steps (negative values for decreased preferred concentration and positive values for increased preferred concentration), 2) change in absolute concentration steps, and 3) a dummy variable with a value of 1 for a change in concentration and 0 for no change in concentration. To test for differences between treatment groups, these variables were each tested with a nonparametric independent-samples Mann-Whitney U test.
3. Results
3.1 Side effects
One participant reported feeling stomach discomfort after taking the supplement shortly before a meal on one occasion but remained in the study.
3.2 Demographics & Anthropometrics
As displayed in Table 1, the groups did not differ in age, gender, BMI, education level, depression, anxiety, verbal and non-verbal intelligence, smoking, alcohol and THC use at intake. Treatment had no effect on anthropometrics (Table 2).
Table 1.
Subject anthropometric & demographic data of two groups based on intake measuresa
| PhosphoLean (n=11) | Placebo (n=11) | p-value | |
|---|---|---|---|
| Age (years) | 27.3 ± 7.89 | 25.3 ± 4.35 | 0.47 |
| Male sex (n,%) | 7 (63.6%) | 6 (54.5%) | 0.68 |
| BMI (kg/m2) | 25.7 ± 4.99 | 24.7 ± 3.69 | 0.62 |
| Education (years) | 15.8 ± 1.83 | 15 ± 2.1 | 0.34 |
| BDI | 4.8 ± 4.21 | 2.7 ± 2.76 | 0.18 |
| BAI | 3.4 ± 4.37 | 2.5 ± 3.64 | 0.60 |
| NAART | 19.2 ± 6.84 | 18.5 ± 9.09 | 0.85 |
| TONI | 41.2 ± 8.93 | 41 ± 8.28 | 0.96 |
| Smoking (cig.) | 0.6 ± 1.57 | 1.4 ± 3.59 | 0.54 |
| Alcoholic drinks (avg. p.w.) | 14.9 ± 11.77 | 16.4 ± 8.97 | 0.74 |
| THC | 0.3 ± 0.48 | 0.4 ± 0.5 | 0.77 |
Values are expressed as mean ± standard deviation or n (%)
Table 2.
Data and statistics of anthropometrics
| PhosphoLean (n=11) a |
Placebo (n=9) a | Time × group interaction |
Pairwise comparison p | |||||
|---|---|---|---|---|---|---|---|---|
| pre-test | post- test |
pre- test |
post- test |
F | p | phospholean | placebo | |
| Multivariate | - | - | - | - | [3,15]b 1.388 |
.285 | - | - |
| Univariate | ||||||||
| BMI (kg/m2) |
26.2 ± 5.91 |
26.14 ± 5.69 |
25.58 ± 5.22 |
26.09 ± 5.45 |
[1,17] 2.489 |
.133 | .618 | .113 |
| BF (%) | 28.19 ± 12.62 |
26.86 ± 12.63 |
24.9 ± 11.76 |
24.15 ± 12.09 |
1.154 | .298 | .125 | .994 |
| W/H ratio | 0.91 ± 0.05 |
0.88 ± 0.08 |
0.88 ± 0.07 |
0.86 ± 0.09 |
1.444 | .246 | .040 | .700 |
Values are expressed as mean ± standard deviation or n (%)
Degrees of freedom [hypothesis, error]
3.3 TLFB interview
There was no effect of treatment on any of the alcohol intake measures (Table 3).
Table 3.
Data and statistics for alcohol consumption of the TLFB
| PhosphoLean (n=11) a |
Placebo (n=11) a | Time × group interaction |
Pairwise comparison p | |||||
|---|---|---|---|---|---|---|---|---|
| pre-test | post- test |
pre- test |
post- test |
F | p | phospholean | placebo | |
| Multivariate | - | - | - | - | [6,14] b .719 |
.641 | - | - |
| Univariate | ||||||||
| total dr | 49.31 ± 31.04 |
35.2 ± 26.12 |
49.07 ± 36.53 |
34 ± 16.06 |
[1,19] .034 |
.857 | .112 | .069 |
| drinking days (n) |
10 ± 5.93 |
8.27 ± 4.8 |
10.18 ± 3.66 |
8.45 ± 3.93 |
.049 | .827 | .209 | .123 |
| average dr/drdy |
4.75 ± 2.72 |
4.33 ± 2.35 |
5.01 ± 3.26 |
3.87 ± 1.98 |
.617 | .442 | .481 | .083 |
| heavy drdy | 3.36 ± 2.5 |
2.73 ± 2.2 |
3.36 ± 2.54 |
2.64 ± 1.75 |
.025 | .875 | .293 | .207 |
| max dr/dy | 12.34 ± 11.3 |
7.63 ± 3.97 |
13.34 ± 16.13 |
10.61 ± 6.36 |
.136 | .716 | .249 | .513 |
Values are expressed as mean ± standard deviation
Degrees of freedom [hypothesis, error]
3.4 Questionnaires
No significant effect of treatment was found for the BIS-11 (Table 4), TFEQ (Table 5), IPAQ (Table 6), DFS (Table 7) or BAI (Table 8). However, we observed a group by time interaction on the BDI with symptoms of depression tending to increase in the PhosphoLean™ group from pre to post-supplementation (Table 8).
Table 4.
Data and statistics of the BIS-11
| PhosphoLean (n=11) a |
Placebo (n=11) a | Time × group interaction |
Pairwise comparison p | |||||
|---|---|---|---|---|---|---|---|---|
| pre-test | post- test |
pre- test |
post- test |
F | p | phospholean | placebo | |
| Multivariate | - | - | - | - | [3,17] b .436 |
.730 | - | - |
| Univariate | ||||||||
| attentional | 17.09 ± 3.14 |
17.82 ± 4.49 |
17.45 ± 3.01 |
17.64 ± 4.13 |
[1,19] .282 |
.602 | .311 | .776 |
| motor | 22.45 ± 4.32 |
22.64 ± 4.57 |
23.45 ± 4.87 |
22.45 ± 3.45 |
1.266 | .275 | .774 | .208 |
| non- planning |
23.36 ± 2.91 |
23.91 ± 3.11 |
23.09 ± 4.48 |
23.45 ± 4.99 |
.003 | .953 | .375 | .419 |
| total | 62.91 ± 8.06 |
64.36 ± 8.94 |
64 ± 9.91 |
63.55 ± 10.5 |
1.062 | .316 | .274 | .741 |
Values are expressed as mean ± standard deviation
Degrees of freedom [hypothesis, error]
Table 5.
Data and statistics of the TFEQ
| PhosphoLean (n=10) a |
Placebo (n=10) a | Time × group interaction |
Pairwise comparison p | |||||
|---|---|---|---|---|---|---|---|---|
| pre-test | post- test |
pre- test |
post- test |
F | p | phospholean | placebo | |
| Multivariate | - | - | - | - | [3,15] b .292 |
.830 | - | - |
| Univariate | ||||||||
| restraint | 7.73 ± 5.5 |
7 ± 6.32 | 8.6 ± 3.92 |
9.36 ± 5.95 |
[1,17] .855 | .368 | ,395 | .660 |
| disinhibition | 6.09 ± 4.11 |
4.4 ± 1.58 |
5.3 ± 4.27 |
4.55 ± 4.25 |
.032 | .859 | .312 | .211 |
| hunger | 6.36 ± 3.11 |
5.6 ± 3.69 |
5.2 ± 2.74 |
5.36 ± 3.14 |
.093 | .765 | .516 | .821 |
Values are expressed as mean ± standard deviation
Degrees of freedom [hypothesis, error]
Table 6.
Data and statistics of the IPAQ
| PhosphoLean (n=11) a |
Placebo (n=11) a | Time × group interaction |
Pairwise comparison p | |||||
|---|---|---|---|---|---|---|---|---|
| pre- test |
post- test |
pre- test |
post- test |
F | p | phospholean | placebo | |
| MET- MINUTES/WK |
8066 ± 4487 |
9411 ± 6464 |
19332 ± 30621 |
14548 ± 18671 |
[1,19] b 2.824 |
.109 | .572 | .087 |
Values are expressed as mean ± standard deviation
Degrees of freedom [hypothesis, error]
Table 7.
Data and statistics of the DFS a
| PhosphoLean (n=9) a |
Placebo (n=10) a |
Time × group interaction |
Pairwise comparison p | |||||
|---|---|---|---|---|---|---|---|---|
| pre-test | post- test |
pre- test |
post- test |
F | p | phospholean | placebo | |
| Multivariate | - | - | - | - | [3,14]b.571 | .643 | - | - |
| Univariate | ||||||||
| total | 55.33 ± 9.67 |
51.64 ± 9.89 |
57.2 ± 8.84 |
53 ± 5.4 |
[1,16].751 | .339 | .028 | .224 |
| satfat | 29.33 ± 5.61 |
27.45 ± 7.24 |
29.9 ± 5.04 |
26.36 ± 3.59 |
.188 | .670 | .034 | .090 |
| sugar | 11.22 ± 4.06 |
10.45 ± 2.11 |
11 ± 3.09 |
10.91 ± 1.7 |
.082 | .778 | .855 | .809 |
| fat-sugar | 14.78 ± 3.42 |
13.73 ± 2.97 |
16.3 ± 3.16 |
15.73 ± 3.13 |
1.690 | .212 | .890 | .843 |
DFS, Dietary Fat and Free Sugar Question; satfat, saturated fat.
Values are expressed as mean ± standard deviation
Degrees of freedom [hypothesis, error]
Table 8.
Data and statistics of the BDI, BAI
| PhosphoLean (n=10) a |
Placebo (n=11) a | Time × group interaction |
Pairwise comparison p | |||||
|---|---|---|---|---|---|---|---|---|
| pre-test | post- test |
pre- test |
post- test |
F | p | phospholean | placebo | |
| Multivariate | - | - | - | - | [2,17] b 3.430 |
.056 | - | - |
| Univariate | ||||||||
| BDI | 5.18 ± 4 | 6.36 ± 5.9 |
3.91 ± 4.76 |
2.91 ± 3.48 |
[1,18] 4.501 |
.048 | .084 | .255 |
| BAI | 3.64 ± 2.94 |
3.2 ± 3.22 |
3.18 ± 4.49 |
3.55 ± 5.15 |
.131 | .721 | .826 | .772 |
Values are expressed as mean ± standard deviation
Degrees of freedom [hypothesis, error]
3.5 Behavioral tasks
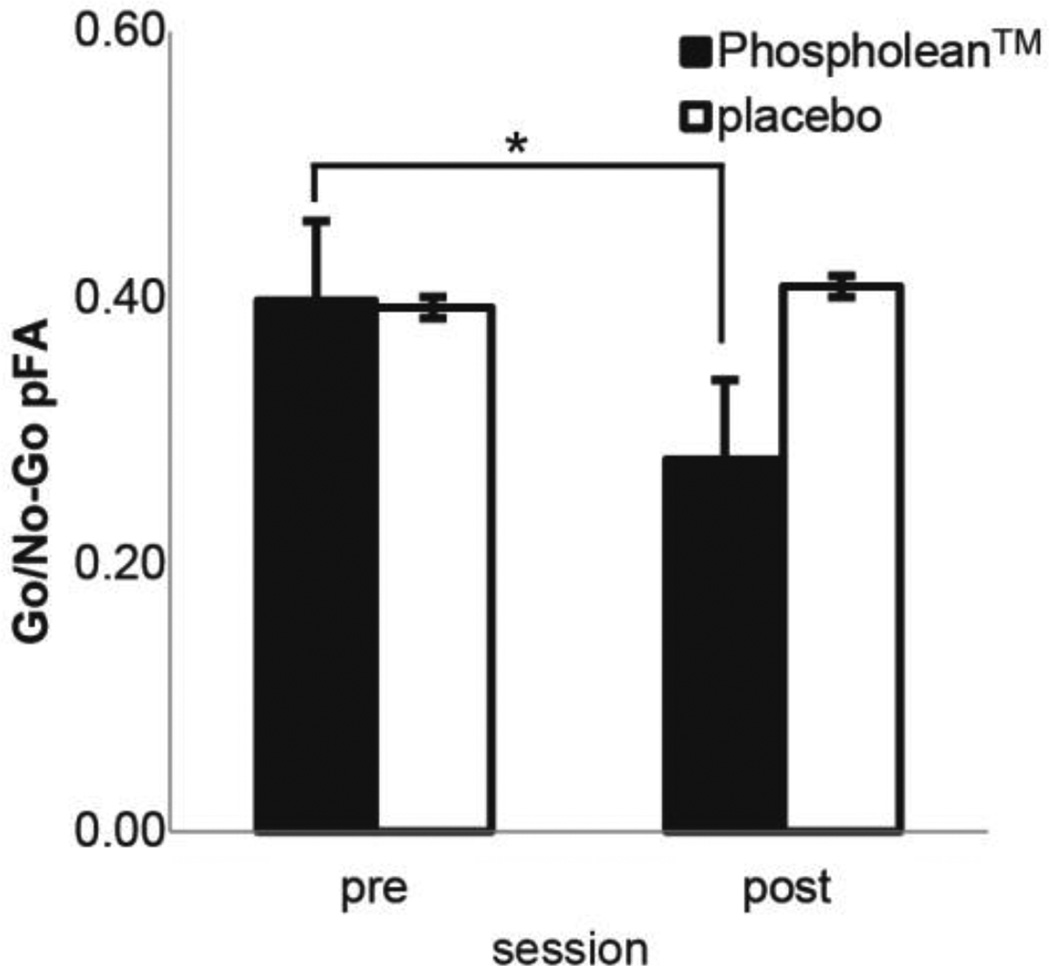
We observed a trend for an interaction between treatment group and time on the Go/ No-Go task, such that supplementation with PhosphoLean™, but not placebo, decreased the proportion of false alarms (Table 9 and Fig. 1) from pre to post-supplementation. We also observed a significant interaction between time and treatment group on the response times for false alarms, which was driven primarily by response times decreasing in the placebo group from pre to post-supplementation (Table 9). No effect of treatment was observed on the PFRT (Table 10) or EDT (Table 11).
Table 9.
Data and statistics for response inhibition in Go/No-Go
| PhosphoLean (n=10) a |
Placebo (n=11) a | Time × group interaction |
Pairwise comparison p | |||||
|---|---|---|---|---|---|---|---|---|
| pre | post | pre | post | F | p | phospholean | placebo | |
| Multivariate | - | - | - | - | [5,14] b 1.744 |
.189 | - | - |
| Univariate | ||||||||
| pH | 0.98 ± 0.03 |
0.99 ± 0.02 |
0.97 ± 0.07 |
0.98 ± 0.05 |
[1,18] .126 |
.726 | .891 | .515 |
| pFA | 0.4 ± 0.2 | 0.28 ± 0.09 |
0.39 ± 0.17 |
0.41 ± 0.19 |
4.028 | .060 | .034 | .611 |
| rtH | 356.83 ± 39.62 |
364.5 ± 31.18 |
359.18 ± 54.14 |
363.05 ± 55.58 |
.085 | .774 | .926 | .713 |
| rtFA | 335.36 ± 62.51 |
330.44 ± 17.43 |
310.41 ± 34.79 |
339.44 ± 43.47 |
5.005 | .038 | .267 | .054 |
| d’ | 2.54 ± 1.09 |
3.09 ± 0.64 |
2.75 ± 1.12 |
2.7 ± 0.88 |
1.105 | .307 | .206 | .877 |
Values are expressed as mean ± standard deviation
Degrees of freedom [hypothesis, error]
Fig. 1.
Average proportion false alarms (+/− standard error of the mean) on the Go/No-Go Task for the PhosphoLean™ group (solid bars) versus placebo group (open bars) in pre versus post-test. *pairwise comparison p < 0.05.
Table 10.
Data and statistics for the PFRT
| PhosphoLean (n=10) a |
Placebo (n=11) a | Time × group interaction |
Pairwise comparison p | |||||
|---|---|---|---|---|---|---|---|---|
| pre-test | post- test |
pre-test | post- test |
F | p | phospholean | placebo | |
| Multivariate | - | - | - | - | [3,16] b .423 |
.739 | - | - |
| Univariate | ||||||||
| choose A | 61.36 ± 24.5 |
59.1 ± 21.36 |
71.03 ± 22.06 |
68.75 ± 21.83 |
[1,18] .008 |
.928 | .954 | .848 |
| avoid B | 61.37 ± 27.51 |
61.37 ± 29.56 |
57.95 ± 24.54 |
64.77 ± 28.4 |
.352 | .560 | .809 | .278 |
| blocks | 3.8 ± 2.2 | 4.18 ± 2.09 |
2.73 ± 2.05 |
3.91 ± 1.87 |
.731 | .404 | .824 | .157 |
Values are expressed as mean ± standard deviation
Degrees of freedom [hypothesis, error]
Table 11.
Data and statistics for the EDT
| PhosphoLean (n=11) a |
Placebo (n=9) a | Time × group interaction |
Pairwise comparison p | |||||
|---|---|---|---|---|---|---|---|---|
| pre-test | post-test | pre-test | post- test |
F | p | phospholean | placebo | |
| AUC | 1.22 ± 2.5 |
0.45 ± 0.19 |
0.52 ± 0.15 |
0.55 ± 0.14 |
[1,17] b 1.205 | .288 | .155 | .892 |
Values are expressed as mean ± standard deviation
Degrees of freedom [hypothesis, error]
3.6 Fat concentration preference assessment
Treatment had no effect on fat concentration preference (Table 12 and 13). However, we did observe a trend for an interaction between treatment group and time on sweetness ratings for both puddings and Jell-O’s, such that supplementation with PhosphoLean™, but not placebo decreased sweetness intensity ratings (Table 12). Treatment had no effect on any of the other variables in the fat concentration preference assessment.
Table 12.
Data and statistics for the fat preference concentration assessment
| PhosphoLe an (n=11) a |
Placebo (n=11) a |
Time × group interactio n |
Time × group × concentrati on interaction |
Pairwise comparison p |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pre- test |
post- test |
pre- test |
post -test |
F | p | F | p | phospholean | placebo | ||
| Multivariate | [16,4] 1.107 |
.515 | [48,132]. 741 |
.882 | - | - | |||||
| Univariate | |||||||||||
| LHS pudding |
0% | 12.74 ± 6.86 |
11.28 ± 6.01 |
6.36 ± 6.86 |
4.86 ± 6.01 |
[1,19] .000 |
.987 | [3,57] 1.130 |
.344 | .883 | .864 |
| 3.1% | 20.46 ± 6.35 |
17.93 ± 5.87 |
10.81 ± 6.35 |
11.05 ± 5.87 |
|||||||
| 6.9% | 22.02 ± 6.26 |
25.98 ± 6.10 |
16.28 ± 6.26 |
12.02 ± 6.10 |
|||||||
| 15.6% | 25.62 ± 6.66 |
23.17 ± 6.38 |
11.51 ± 6.66 |
14.17 ± 6.38 |
|||||||
| LMS pudding |
0% | 19.70 ± 3.21 |
14.28 ± 2.91 |
25.92 ± 3.21 |
22.64 ± 2.91 |
1.452 | .243 | .249 | .862 | .126 | .914 |
| 3.1% | 19.90 ± 3.73 |
14.22 ± 2.77 |
22.07 ± 3.73 |
22.36 ± 2.77 |
|||||||
| 6.9% | 23.27 ± 3.47 |
19.80 ± 3.05 |
21.81 ± 3.47 |
23.21 ± 3.05 |
|||||||
| 15.6 % | 23.25 ± 3.73 |
20.96 ± 3.24 |
20.86 ± 3.73 |
23.60 ± 3.24 |
|||||||
| Saltiness pudding |
0% | 1.75 ± 1.95 |
2.48 ± 3.42 |
8.51 ± 1.95 |
10.43 ± 3.42 |
.081 | .779 | .112 | .953 | .991 | .700 |
| 3.1% | 2.28 ± 1.87 |
1.49 ± 2.98 |
7.20 ± 1.87 |
7.83 ± 2.98 |
|||||||
| 6.9% | 1.10 ± 1.68 |
0.80 ± 1.77 |
7.22 ± 1.68 |
7.30 ± 1.77 |
|||||||
| 15.6 % | 1.09 ± 2.57 |
1.38 ± 2.89 |
10.98 ± 2.57 |
10.97 ± 2.89 |
|||||||
| Sweetness pudding |
0% | 18.31 ± 3.15 |
13.20 ± 3.50 |
20.18 ± 3.15 |
21.39 ± 3.50 |
3.160 | .091 | .596 | .620 | .052 | .661 |
| 3.1% | 22.30 ± 3.22 |
15.16 ± 3.43 |
21.02 ± 3.22 |
23.58 ± 3.43 |
|||||||
| 6.9% | 25.03 ± 3.12 |
18.96 ± 3.20 |
23.76 ± 3.12 |
22.30 ± 3.20 |
|||||||
| 15.6 % | 23.88 ± 3.61 |
20.33 ± 4.12 |
19.59 ± 3.61 |
21.98 ± 4.12 |
|||||||
| Creaminess pudding |
0% | 40.91 ± 4.78 |
40.10 ± 5.89 |
46.85 ± 4.78 |
38.41 ± 5.89 |
.173 | .682 | 1.434 | .242 | .227 | .082 |
| 3.1% | 50.05 ± 5.32 |
42.86 ± 5.98 |
55.34 ± 5.32 |
47.66 ± 5.98 |
|||||||
| 6.9% | 52.58 ± 4.63 |
45.43 ± 5.92 |
54.37 ± 4.63 |
49.80 ± 5.92 |
|||||||
| 15.6 % | 53.56 ± 5.63 |
48.16 ± 6.34 |
60.11 ± 5.63 |
50.55 ± 6.34 |
|||||||
| Fattiness pudding |
0% | 28.49 ± 5.88 |
22.86 ± 6.21 |
35.83 ± 5.88 |
33.93 ± 6.21 |
.084 | .775 | .092 | .964 | .329 | .561 |
| 3.1% | 34.78 ± 7.19 |
26.96 ± 6.58 |
44.17 ± 7.19 |
38.61 ± 6.58 |
|||||||
| 6.9% | 33.84 ± 6.20 |
30.54 ± 6.69 |
39.72 ± 6.20 |
38.55 ± 6.69 |
|||||||
| 15.6 % | 35.89 ± 6.78 |
31.19 ± 6.95 |
48.18 ± 6.78 |
44.15 ± 6.95 |
|||||||
| Oiliness pudding |
0% | 4.51 ± 2.98 |
3.95 ± 3.51 |
31.26 ± 2.98 |
24.53 ± 3.51 |
1.466 | .241 | 2.027 | .120 | .958 | .093 |
| 3.1% | 3.27 ± 3.15 |
4.46 ± 3.98 |
31.28 ± 3.15 |
28.26 ± 3.98 |
|||||||
| 6.9% | 5.50 ± 3.14 |
4.83 ± 3.95 |
27.41 ± 3.14 |
29.01 ± 3.95 |
|||||||
| 15.6 % | 5.20 ± 3.68 |
4.81 ± 3.64 |
34.37 ± 3.68 |
28.49 ± 3.64 |
|||||||
| Wanting pudding |
0% | 33.53 ± 7.27 |
34.49 ± 5.85 |
24.57 ± 7.27 |
20.01 ± 5.85 |
.598 | .449 | .684 | .566 | .420 | .789 |
| 3.1% | 37.48 ± 7.85 |
40.63 ± 6.32 |
30.23 ± 7.85 |
29.45 ± 6.32 |
|||||||
| 6.9% | 37.69 ± 8.23 |
46.51 ± 6.33 |
35.59 ± 8.23 |
32.08 ± 6.33 |
|||||||
| 15.6% | 42.39 ± 8.50 |
47.90 ± 7.80 |
25.21 ± 8.50 |
27.96 ± 7.80 |
|||||||
| LHS Jell-O | 0 M | −39.73 ± 5.79 |
−42.04 ± 7.91 |
−36.82 ± 5.79 |
−33.31 ± 7.91 |
.006 | .938 | .407 | .748 | .426 | .491 |
| 0.1 M | −18.41 ± 5.34 |
-17.38 ± 5.75 |
−15.77 ± 5.34 |
−17.05 ± 5.75 |
|||||||
| 0.56 M | 16.31 ± 4.39 |
14.63 ± 6.54 |
11.68 ± 4.39 |
6.41 ± 6.54 |
|||||||
| 1 M | 14.45 ± 7.10 |
9.04 ± 7.08 |
10.29 ± 7.10 |
6.11 ± 7.08 |
|||||||
| LMS Jell-O | 0 M | 32.29 ± 4.46 |
29.77 ± 5.05 |
29.37 ± 4.46 |
31.04 ± 5.05 |
1.472 | .240 | .708 | .551 | .491 | .322 |
| 0.1 M | 19.76 ± 3.88 |
16.70 ± 2.89 |
17.22 ± 3.88 |
22.97 ± 2.89 |
|||||||
| 0.56 M | 24.45 ± 3.34 |
24.47 ± 2.88 |
20.41 ± 3.34 |
22.41 ± 2.88 |
|||||||
| 1 M | 34.65 ± 3.92 |
32.53 ± 4.08 |
26.06 ± 3.92 |
27.80 ± 4.08 |
|||||||
| Saltiness Jell-O |
0 M | 14.13 ± 4.71 |
9.63 ± 6.26 |
14.76 ± 4.71 |
14.94 ± 6.26 |
.271 | .608 | .262 | .853 | .850 | .591 |
| 0.1 M | 5.30 ± 2.94 |
6.11 ± 3.92 |
9.96 ± 2.94 |
10.34 ± 3.92 |
|||||||
| 0.56 M | 0.21 ± 0.70 |
0.95 ± 2.55 |
2.83 ± 0.70 |
5.82 ± 2.55 |
|||||||
| 1 M | 0.85 ± 0.84 |
1.52 ± 2.35 |
2.79 ± 0.84 |
5.73 ± 2.35 |
|||||||
| Sweetness Jell-O |
0 M | 2.02 ± 1.28 |
1.52 ± 1.41 |
4.57 ± 1.28 |
6.23 ± 1.41 |
3.745 | .068 | .198 | .898 | .082 | .378 |
| 0.1 M | 3.15 ± 1.01 |
3.82 ± 2.12 |
6.82 ± 1.01 |
10.94 ± 2.12 |
|||||||
| 0.56 M | 26.89 ± 3.64 |
21.43 ± 2.73 |
22.31 ± 3.64 |
22.77 ± 2.73 |
|||||||
| 1 M | 35.54 ± 3.68 |
30.62 ± 5.57 |
32.63 ± 3.68 |
31.40 ± 5.57 |
|||||||
| Creaminess Jell-O |
0 M | 1.66 ± 2.61 |
1.59 ± 2.66 |
8.73 ± 2.61 |
9.38 ± 2.66 |
.906 | .353 | 1.174 | .328 | .980 | .201 |
| 0.1 M | 2.11 ± 2.52 |
1.53 ± 3.01 |
7.04 ± 2.52 |
9.58 ± 3.01 |
|||||||
| 0.56 M | 3.25 ± 3.14 |
2.22 ± 3.72 |
8.65 ± 3.14 |
14.35 ± 3.72 |
|||||||
| 1 M | 3.83 ± 4.13 |
5.27 ± 4.48 |
12.32 ± 4.13 |
16.16 ± 4.48 |
|||||||
| Fattiness Jell-O |
0 M | 4.27 ± 4.87 |
3.78 ± 4.72 |
14.65 ± 4.87 |
12.03 ± 4.72 |
.002 | .965 | .968 | .414 | .309 | .281 |
| 0.1 M | 5.41 ± 4.73 |
4.06 ± 4.51 |
14.68 ± 4.73 |
11.27 ± 4.51 |
|||||||
| 0.56 M | 8.04 ± 5.48 |
5.47 ± 5.16 |
16.66 ± 5.48 |
13.98 ± 5.16 |
|||||||
| 1 M | 11.24 ± 6.15 |
6.11 ± 5.23 |
16.71 ± 6.15 |
15.30 ± 5.23 |
|||||||
| Oiliness Jell-O |
0 M | 3.21 ± 3.55 |
1.75 ± 5.17 |
14.29 ± 3.55 |
21.38 ± 5.17 |
3.045 | .097 | .606 | .614 | .821 | .037 |
| 0.1 M | 3.30 ± 3.45 |
2.70 ± 4.78 |
11.33 ± 3.45 |
19.64 ± 4.78 |
|||||||
| 0.56 M | 2.75 ± 2.96 |
2.80 ± 4.38 |
11.06 ± 2.96 |
17.49 ± 4.38 |
|||||||
| 1 M | 2.93 ± 3.22 |
1.74 ± 4.67 |
10.21 ± 3.22 |
19.72 ± 4.67 |
|||||||
| Wanting Jell-O |
0 M | 4.70 ± 2.31 |
7.12 ± 2.43 |
5.82 ± 2.31 |
3.43 ± 2.43 |
3.511 | .076 | .383 | .766 | .346 | .107 |
| 0.1 M | 11.61 ± 3.70 |
14.65 ± 4.15 |
9.82 ± 3.70 |
10.79 ± 4.15 |
|||||||
| 0.56 M | 30.04 ± 7.12 |
35.16 ± 7.09 |
26.13 ± 7.12 |
22.19 ± 7.09 |
|||||||
| 1 M | 37.75 ± 8.66 |
35.87 ± 7.06 |
26.83 ± 8.66 |
16.97 ± 7.06 |
|||||||
Values are expressed as mean ± standard deviation
Degrees of freedom [hypothesis, error]
Table 13.
Data and statistics for non-parametric analyses of most liked fat and sweet concentration
| PhosphoLean (n=11) |
Placebo (n=11) |
Variable | Mann-Whitney U p | ||||
|---|---|---|---|---|---|---|---|
| pre- test |
post- test |
pre- test |
post- test |
Pre-test pudding | .332 | ||
| conc | n | n | n | n | Post-test pudding | .300 | |
| Most liked pudding |
0% | 2 | 2 | 0 | 2 | Pre-test Jell-O | .519 |
| 3.1% | 3 | 2 | 2 | 2 | Post-test Jell-O | .116 | |
| 6.9% | 2 | 4 | 4 | 1 | Δ conc steps pudding | .699 | |
| 15.6% | 4 | 3 | 5 | 6 | Δ conc steps Jell-O | .797 | |
| Most liked Jell-O |
0 M | 0 | 0 | 0 | 0 | |Δ| conc steps pudding | .652 |
| 0.1 M | 0 | 0 | 0 | 1 | |Δ| conc steps Jell-O | .365 | |
| 0.56M | 6 | 4 | 3 | 7 | Dummy Δ pudding | .748 | |
| 1 M | 5 | 7 | 8 | 3 | Dummy Δ Jell-O | .748 | |
3.7 Relation between alcohol intake and Go/No-Go task
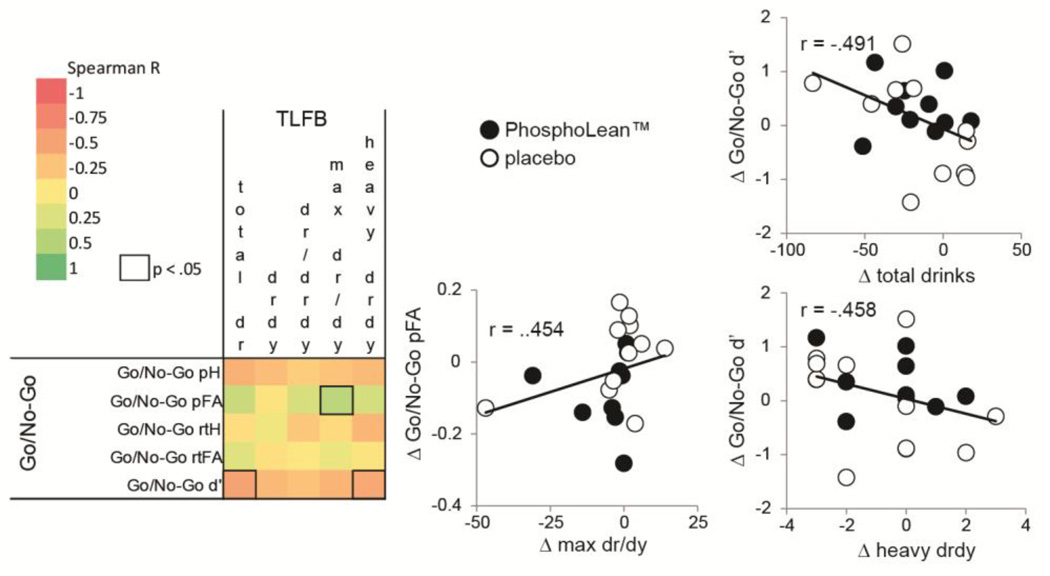
We observed a relation between change in alcohol intake from pre to post-supplementation and change in performance on the Go/No-Go task from pre to post test when considering the entire sample (control and PhosphoLean) (Fig. 2). Specifically, the change of proportion of false alarms was positively associated with change in maximum drinks per drinking day, and change in d’ was negatively associated with change in total drinks consumed as well as change in number of heavy drinking days. These observations for the Go/No-Go task indicate that consumption of fewer drinks is associated with greater sensitivity (false alarms relative to hits), indicative of less impulsivity.
Fig. 2.
Correlation matrix for TLFB and Go/No-Go task (change in pre versus post-test). Color gradient indicates Spearman correlation coefficient. Cells with a solid outline indicate significant correlations (p < .05). Scatterplots illustrate significant correlations, with solid circles depicting values for participants in the PhosphoLean™ group and open circles depicting values for participants in the placebo group.
4. Discussion
The primary goal of this study was to test the prediction that increasing gut fatty acid amide levels by three weeks of dietary supplementation with PhosphoLean™ can reduce impulsivity and alcohol intake in heavy drinkers. Our findings provide partial support for this possibility. While we did not observe a main effect of the supplement on self-reported alcohol intake, we did find that supplementation with PhosphoLean, but not placebo, led to improvements on a task of motor impulsivity. Motor impulsivity was, in turn, related to reductions in alcohol intake. Collectively, the results provide preliminary support for a novel mechanism to reduce impulsivity that may translate into reduced alcohol consumption in heavy drinkers.
Alcohol use disorders and alcoholism are associated with impulsive behavior and compromised dopamine signaling [11, 21]. Recent data from rodents demonstrates that the compromised dopamine signaling observed following a high fat diet is associated with the depletion of a family of appetite-regulating fatty acid amides from the gut, which when replenished rescues dopamine signaling and shifts preferences towards lower fat foods [3]. With respect to alcohol, ethanol administration increases OEA levels in variety of tissues including the nucleus accumbens [24]. Following chronic ethanol administration OEA levels then drop in parallel with the onset of withdrawal behaviors. Resembling effects with the high fat diet acute OEA administration can then reduce withdrawal symptoms and block cue-induced re-instatement of alcohol seeking behavior - the animal model of relapse. This raises the possibility that OEA may have therapeutic effects for alcohol use disorders and alcoholism.
Our data provide preliminary support for this possibility. After only three weeks of supplementation with PhosphoLean, which contains the precursor to OEA and is converted to OEA in the gut [16], we observed an improvement on a standard behavioral measure of impulsivity in heavy drinking young adults. Specifically, false alarm responses on the Go/No-Go task decreased significantly in the PhosphoLean™, but not the placebo control group. Whether this improvement reflects a rescuing of gut-brain communication is unknown. However, false alarm rate on this same task has been linked to the dorsal striatal dopamine adaptation observed in obesity [8], suggesting that improved performance might have resulted from rescuing dorsal striatal dopamine signaling compromised by heavy drinking. Future studies are warranted to test this possibility.
We also found that reductions in motor impulsivity correlated with reductions in maximal drinks consumed per day. Although this occurred irrespective of group it supports the possibility that supplementation may influence core functions (i.e. motor impulsivity), which in turn relate to positive behavioral change. As such, studies assessing whether longer supplementation in a larger sample might translate into a reduction of adverse consequences of decision-making are called for. For example, participants on the dietary supplement may make fewer bad decisions, such as drinking and driving. It may also be that the effects of the supplement would have been more pronounced in heavy drinkers who are looking to reduce drinking and when looking at the effects of the supplement on maintenance of abstinence (for example, with reduced impulsivity, participants may be less likely to relapse to drinking).
Contrary to our prediction, supplementation with PhosphoLean™ did not change self-reported impulsivity (BIS-11). A possible explanation for the discrepant findings in the behavioral task and self-report measure may be related to the length of our trial. The BIS-11 measures trait impulsivity [34], with questions designed to have the respondent reflect upon their typical behaviors in a number of situations. It may well be that three weeks is too short a period of time for individuals to change self-perception. An alternative explanation is that the BIS-11 and Go/No-Go measure different aspects of impulsivity, which are differentially influenced by alcohol use. For example, alcohol use does not generally associate with “lack of planning” impulsivity, on which all BIS-11 subscales load highly [46]. In contrast, prepotent response inhibition is often impaired in heavy drinkers) generally do not correlate with alcohol use [47].
We also failed to observe a shift in fat concentration preference, as was reported in rodents [17]. While the short length of the trial may also account for this null result, another possibility is that fat preference shifts are specific to dopamine disruption by a high fat diet. Along these lines, it would be interesting to assess whether alcohol perception is influenced by OEA supplementation. In fact we did find a trend for sweetness perception of the Jell-O’s to decrease after PhosphoLean™ supplementation. This finding is intriguing given reports of a positive association between excessive alcohol intake and increased sweetness preference [48].
Although the findings of this preliminary study are encouraging there are a number caveats and limitations. First, the sample size was small (n=22) and the trial length short, both of which could contribute to our inability to find effects on alcohol intake. We suggest an additional evaluation of outcomes after longer supplementation for future studies. Second, although we asked participants if they adhered to the supplementation instructions, we cannot rule out non-adherence. Third, OEA is synthesized in response to dietary oleic acid [15]. Since we did not administer food diaries we cannot rule out the possibility of dietary influences on OEA levels. This might have influenced our results by increasing variance and negatively impacting sensitivity. However, it is also possible that OEA supplementation increased OEA levels, leading to preference shifts towards lower fat foods, which would potentiate the effects of supplementation. Although shifts in fat concentration preferences were not observed in our laboratory test, it is possible that food choices were influenced outside the laboratory. Such interactions would be interesting to investigate in future studies. Fourth, another source of intra and inter-individual variance may be the amount of NOPE in the dietary supplement. We did not evaluate the consistency and quality of NOPE in the dietary supplement. Future investigations should independently assess properties of PhosphoLean with liquid chromatograph or mass spectrometry. Fifth, we observed a trend towards an increase in depressive symptoms, in participants supplementing with PhosphoLean™. Although the increase was small, it raises the possibility that mood disturbance could be a side effect of long-term supplementation in heavy drinkers. An increase in depressive symptoms has not been shown before, and was unexpected. In fact in the rodent model OEA decreases immobility in a model of behavioral despair [49] and in humans has been shown to reduce depressive symptoms [19]. One possible explanation for our discrepant finding is our use of a dosage that was slightly higher and a regimen that was slightly more frequent (180 mg NOPE across three equal doses daily, before lunch and dinner, and after dinner) than previous reports [19, 20]. It is therefore possible that benefits could be achieved with lower doses that may have less chance of influencing depressive symptoms. These caveats notwithstanding, the findings we report nevertheless suggest a novel mechanism for improving impulsivity and support further studies to evaluate OEA as a therapeutic target for alcohol disorders and alcoholism.
HIGHLIGHTS.
False alarm rate on Go/No-Go has been linked to dorsal striatal dopamine adaptation
OEA supplementation does not change self-reported impulsivity (BIS-11)
OEA supplementation reduces false alarms on a Go/No-Go task in heavy drinkers
Improved sensitivity on a Go/No-Go task is associated with reduced alcohol intake
Acknowledgments
This work was funded by grants from the National Institute for Alcohol Abuse and Alcoholism (NIAAA) P50 K05AA014715, P50AA012870). The content is solely the responsibilities of the authors and does not necessarily represent the official views of the NIAAA or the National Institutes of Health. We also thank ChemiNutra for supplying the PhosphoLean™ and placebo supplements. Dr. O’Malley reports the following activities: Member of the ASCP workgroup, the Alcohol Clinical Trials Initiative, supported with funding from Abbott Laboratories, Eli Lilly, Lundbeck, Pfizer, Ethyphama; Consultant/advisory board member, Alkermes; Contract as a site for a multi-site study, Eli Lilly; Medication supplies, Pfizer; Scientific Panel Member, Hazelden Betty Ford Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors declare no conflict of interest with the supplier of ChemiNutra.
References
- 1.Volkow NDWG, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry DCM, M E, Petry NM. Obesity and Its Relationship to Addictions: Is Overeating a Form of Addictive Behavior? Am J Addict. 2009;18:439–451. doi: 10.3109/10550490903205579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tellez LA, Medina S, Han W, Ferreira JG, Licona-Limón P, Ren X, et al. A Gut Lipid Messenger Links Excess Dietary Fat to Dopamine Deficiency. Science. 2013;341:800–802. doi: 10.1126/science.1239275. [DOI] [PubMed] [Google Scholar]
- 4.Johnson PMKP. Addiction-like reward dysfunction and compulsive eating in obese rats: Role for dopamine D2 receptors. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang G-J, Dardo T, Convit A, Volkow N, Logan J, Wong C, et al. Peripheral insulin resistance affects brain dopaminergic signaling after glucose ingestion. Journal of Nuclear Medicine. 2013;54:29. [Google Scholar]
- 6.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babbs RK, Sun X, Felsted J, Chouinard-Decorte F, Veldhuizen MG, Small DM. Decreased caudate response to milkshake is associated with higher body mass index and greater impulsivity. Physiol Behav. 2013 doi: 10.1016/j.physbeh.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stice EYS, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J. Neurosci. 2010;30:13105–13019. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stice EYS, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J. Neurosci. 2011;31:4360–4366. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granö N, Virtanen M, Vahtera J, Elovainio M, Kivimäki M. Impulsivity as a predictor of smoking and alcohol consumption. Personality and Individual Differences. 2004;37:1693–1700. [Google Scholar]
- 12.Artmann APG, Hellgren LI, Boberg J, Skonberg C, Nellemann C, Hansen SH, Hansen HS. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim Biophys Acta. 2008;1781:200–212. doi: 10.1016/j.bbalip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Jones PJLL, Gillingham LG, Yang H, Omar JM. Modulation of plasma N-acylethanolamine levels and physiological parameters by dietary fatty acid composition in humans. Journal of lipid research. 2014;55:2655–2664. doi: 10.1194/jlr.P051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J, et al. An anorexic lipid mediator regulated by feeding. Nature. 2001;414:209–212. doi: 10.1038/35102582. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GJ, Fu J, Astarita G, Li X, Gaetani S, Campolongo P, et al. The Lipid Messenger OEA Links Dietary Fat Intake to Satiety. Cell Metabolism. 2008;8:281–288. doi: 10.1016/j.cmet.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez de Fonseca F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-[alpha] Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 17.Hansen H. Role of anorectic N-acylethanolamines in intestinal physiology and satiety control with respect to dietary fat. Pharmacological Research. 2014;86:18–25. doi: 10.1016/j.phrs.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Grosshans MSE, Bumb JM, Schaefer C, Rohleder C, Vollmert C, Vollstädt-Klein S, Tost H, Meyer-Lindenberg A, Kiefer F, Leweke M. Oleoylethanolamide and Human Neural Responses to Food Stimuli in Obesity. JAMA Psychiatry. 2014;71:1254–1261. doi: 10.1001/jamapsychiatry.2014.1215. [DOI] [PubMed] [Google Scholar]
- 19.Rondanelli M, Opizzi A, Solerte SB, Trotti R, Klersy C, Cazzola R. Administration of a dietary supplement ( N-oleyl-phosphatidylethanolamine and epigallocatechin-3-gallate formula) enhances compliance with diet in healthy overweight subjects: a randomized controlled trial. Br J Nutr. 2009;101:457–464. doi: 10.1017/S0007114508024008. [DOI] [PubMed] [Google Scholar]
- 20.Mangine GT, Gonzalez AM, Wells AJ, McCormack WP, Fragala MS, Stout JR, et al. The effect of a dietary supplement (N-oleyl-phosphatidyl-ethanolamine and epigallocatechin gallate) on dietary compliance and body fat loss in adults who are overweight: a double-blind, randomized control trial. Lipids in health and disease. 2012;11:127. doi: 10.1186/1476-511X-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laine T, Ahonen A, Torniainen P, Heikkilä J, Pyhtinen J, Räsänen P, et al. Dopamine transporters increase in human brain after alcohol withdrawal. Mol Psychiatry. 1999;4:189–191. doi: 10.1038/sj.mp.4000514. [DOI] [PubMed] [Google Scholar]
- 22.Baraona ELC. Effects of ethanol on lipid metabolism. Journal of lipid research. 1979;20:289–315. [PubMed] [Google Scholar]
- 23.Maher J. Exploring Alcohol’s Effects on Liver Function. Alcohol Health & Research World. 1997;21:5–12. [PMC free article] [PubMed] [Google Scholar]
- 24.Bilbao A, Serrano A, Cippitelli A, Pavón FJ, Giuffrida A, Suárez J, et al. Role of the satiety factor oleoylethanolamide in alcoholism. Addiction Biology. 2015 doi: 10.1111/adb.12276. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NIAAA Council approves definition of binge drinking. NIAAA Newslett. National Institute on Alcohol Abuse and Alcoholism; 2004. http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. [Google Scholar]
- 26.Dulloo A, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. The American journal of clinical nutrition. 1999;70:1040–1045. doi: 10.1093/ajcn/70.6.1040. [DOI] [PubMed] [Google Scholar]
- 27.Sheehan D, Lecrubier Y, Sheehan K, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;20:22–33. [PubMed] [Google Scholar]
- 28.Beck A, Steer R. Manual for the beck anxiety inventory. Psychological Corporation. 1993 [Google Scholar]
- 29.Beck A, Steer R. Beck depression inventory. Psychological Corporation. 1993 [Google Scholar]
- 30.Sobell L, Maisto S, Sobell M, Cooper A. Reliability of alcohol abusers’ self-reports of drinking behavior. Behaviour Research and Therapy. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- 31.Uttl B. North American Adult Reading Test: age norms, reliability, and validity. Journal of Clinical and Experimental Neuropsychology. 2002;24:1123–1137. doi: 10.1076/jcen.24.8.1123.8375. [DOI] [PubMed] [Google Scholar]
- 32.Brown L, Sherbenou R, Johnsen S. Test of Nonverbal Intelligence-4 [Google Scholar]
- 33.Sobell L, Sobell M. Measuring alcohol consumption: Psychosocial and biochemical methods. In: Allen J, Litten Z, editors. Timeline follow-back: a technique for assessing self-reported ethanol consumption. Totowa, NJ: Humana Press, Inc; 1992. pp. 41–472. [Google Scholar]
- 34.Patton J, Stanford M, Barratt E. Factor structure of the Barratt Impulsiveness Scale. Journal of clinical psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Stunkard A, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 36.Craig C, Marshall A, Sjostrom M, Bauman M, Booth M, Ainsworth B, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 37.Francis H, Stevenson R. Validity and test-retest reliability of a short dietary. Journal of Human Nutrition and Dietetics. 2013;26:234–242. doi: 10.1111/jhn.12008. [DOI] [PubMed] [Google Scholar]
- 38.Eagle D, Bari A, Robbin T. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology. 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- 39.Frank MJ, Seeberger LC, O’Reilly RC. By Carrot or by Stick: Cognitive Reinforcement Learning in Parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 40.Cohen M, Frank M. Neurocomputational models of basal ganglia function in learning, memory and choice. Behav Brain Res. 2009;199:141–156. doi: 10.1016/j.bbr.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynolds B, Schiffbauer R. Measuring state changes in human delay discounting: an experiential discounting task. Behavioural Processes. 2004;67:343–356. doi: 10.1016/j.beproc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Green B, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the “Labeled Magnitude Scale” for measuring sensations of taste and smell. Chem Senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- 43.Lim J, Wood A, Green B. Derivation and evaluation of a labeled hedonic scale. Chem Senses. 2009;34:739–751. doi: 10.1093/chemse/bjp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoner S, Fedoroff I, Andersen A, Rolls B. Food preferences and desire to eat in anorexia and bulimia nervosa. Int. J. Eat. Disord. 1996;19:13–22. doi: 10.1002/(SICI)1098-108X(199601)19:1<13::AID-EAT3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 45.Geha P, deAraujo I, Green B, Small D. Decreased food pleasure and disrupted satiety signals in chronic low back pain. Pain. 2014;155:712–722. doi: 10.1016/j.pain.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 46.Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. [Google Scholar]
- 47.Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, et al. REVIEW: Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kampov-Polevoy AB, Garbutt JC, Janowsky DS. Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol and alcoholism. 1999;34:386–395. doi: 10.1093/alcalc/34.3.386. [DOI] [PubMed] [Google Scholar]
- 49.Yu H-L, Sun L-P, Li M-M, Quan Z-S. Involvement of norepinephrine and serotonin system in antidepressant-like effects of oleoylethanolamide in the mice models of behavior despair. Neuroscience Letters. 2015;593:24–28. doi: 10.1016/j.neulet.2015.03.019. [DOI] [PubMed] [Google Scholar]