Abstract
Background
Surgery provides the best chance for cure and long-term survival in non-small cell cancer (NSCLC). Persistent symptoms following surgery are common, and they can negatively impact health-related quality of life (HRQOL). The purpose of this study was to examine the long-term effect of an interdisciplinary supportive care intervention to improve HRQOL, psychological distress, and symptoms in lung cancer survivors who were treated surgically.
Methods
Patients undergoing curative intent resection for NSCLC were enrolled in a prospective sequential design whereby the control group was accrued first followed by the intervention group. Patients in the intervention group were assessed and presented by nurses at weekly interdisciplinary care meetings prior to surgery, and received four educational sessions (physical, psychological, social, spiritual well-being) following surgery. Appropriate symptom management, social work, rehabilitation, and spiritual support interventions were coordinated by the study nurse. In both groups, HRQOL, psychological distress, and symptom severity were assessed at baseline and at 6, 12, 24, 36, and 52 weeks using surveys which included the validated FACT-L, Lung Cancer Subscale (LCS), and Distress Thermometer. Mean survey scores were analyzed using factorial Analysis of Covariance at 12 months.
Results
A total of 71 survivors (33=control, 38=intervention) were accrued. There was no difference in age, baseline performance status, or stage of disease between groups. Patients in the intervention group had significantly less distress (mean 1.0 vs 4.0, p<0.001, range 0–10) and more favorable mean FACT-L scores (126.1 vs 98.7, p<0.001, range 0–140) and LCS scores (29.4 vs 23.6, p<0.001, range 0–32) at 12 months. The mean scores of all categories of questions in FACT-L (physical, social/family, emotional, and functional well-being) were significantly more favorable in the intervention group at 12 months.
Conclusions
An interdisciplinary supportive care intervention improves psychological distress and HRQOL at 12 months after lung cancer surgery. This study has important implications in improving HRQOL of lung cancer survivors after surgery. Further study is warranted on incorporating the interdisciplinary personalized interventions used in this study into clinical practice for lung cancer survivors.
Keywords: Lung cancer surgery, quality of life, lung cancer clinical trials
Although surgery for early stage non-small cell lung cancer (NSCLC) is often curative, persistent physical symptoms and psychological distress are relatively common after treatment, and these adversely affect health related quality of life (HRQOL).1–4 Palliative care focuses on symptom improvement, access to interdisciplinary supportive care services, and supporting the best quality of life possible in patients with serious illnesses. Several studies have shown that early palliative and supportive care interventions improve HRQOL and even survival for patients with metastatic lung cancer.5–8 The Institute of Medicine (IOM) recommends palliative/supportive care interventions across the continuum of care.9 Yet, there is little data on the effect of palliative or supportive care interventions in survivors of early stage lung cancer. Multidisciplinary care planning in early stage NSCLC likely reduces delays in treatment and improves adherence to treatment guidelines.10 However the use of an interdisciplinary approach to focus on the survivor’s physical, emotional, and spiritual well-being during post-operative recovery has not been reported. An interdisciplinary team approach integrates various disciplines into holistic survivor-centered encounters.
In this study, we report the results of an interdisciplinary supportive care intervention in survivors who underwent surgical resection of stage I-III non-small cell lung cancer (NSCLC), comparing HRQOL, psychological distress, and symptoms of the usual care and the intervention groups at 12 months. We hypothesized that survivors receiving the interdisciplinary intervention would have improved HRQOL and lower distress scores than those survivors receiving usual care.
Patients and Methods
Study Design
This quasi-experimental, sequential enrollment trial aimed to test the effectiveness of an interdisciplinary supportive care intervention for NSCLC survivors who were treated surgically.. Survivors undergoing curative intent resection for NSCLC were sequentially enrolled into the control and intervention groups. Patient enrollment for the control group occurred from November 2009 to December 2010, and intervention group enrollment occurred between July 2011 and August 2014. Data collection for all outcomes ended in September 2014. The study was performed at an NCI-Designated Comprehensive Cancer Center located in Southern California. All study protocols and data safety monitoring plan were reviewed and approved by the institutional review board.
Patients
In this study, we included patients with pathologically confirmed stage I–III resectable NSCLC who were scheduled to undergo surgery and completed the baseline questionnaires and assessment preoperatively. We included only survivors who completed 12 month follow-up assessment. Only survivors able to read and understand English and willing to complete the assessment tools were eligible to participate. Survivors who had another cancer diagnosis within the last five years were excluded from the study. Written informed consents were obtained for all survivors prior to participating in the study.
Intervention
The interdisciplinary supportive care intervention consisted of the following key components: 1) comprehensive pre-operative QOL assessment, 2) interdisciplinary care planning, and 3) education sessions for patients. Upon enrollment, nurses completed a comprehensive assessment of QOL, symptoms, and psychological distress concerns using the study baseline evaluation data. The assessment was documented using a personalized supportive care plan, and categorized into the physical, psychological, social, and spiritual domains of QOL. Guided by the comprehensive QOL assessment, each survivor was presented at weekly, hour-long interdisciplinary team meetings. The meetings were consistently attended by thoracic surgeons, nurses, pain specialists, pulmonologist, social worker, chaplain, dietitian, physical therapist, and key members of the research team. Following each case presentation, the interdisciplinary care team provided recommendations on symptom management, psychosocial support, and referrals to supportive care services. Approximately 15 minutes time was devoted to discussions of each patient. Survivors also received four educational sessions, and teaching was focused on potential physical, psychological, social, and spiritual needs following surgery and during recovery. Any relevant supportive care resources that were identified and recommended by the interdisciplinary team were also discussed with survivors during the sessions. An educational manual that contained all teaching contents was provided to survivors. On average, each of the educational sessions lasted approximately 36 minutes. Survivors in the control group were treated and followed by the thoracic surgery team and were also allowed to access all supportive care services while participating in the study if needed. Our group’s follow-up routine involves clinical visits and chest CT every 3–6 months postoperatively for the first two years, then annually thereafter, depending on cancer stage and operation performed.
Outcome Measures
Quality of life and symptoms were assessed using the Functional Assessment of Cancer Therapy-Lung (FACT-L) tool. The FACT-L contains 27 items with questions divided into the physical, social/family, emotional, and functional well-being domains. An additional lung cancer subscale (LCS) is included to assess disease-specific symptoms. All items are scored on a 5-point Likert scale (0=not at all; 4=very much). Higher scores indicate better QOL, and the total score ranges from 0 to 140.11 The Functional Assessment of Chronic Illness Therapy-Spirituality Subscale (FACIT-Sp-12) was used to assess spiritual well-being. This is a 12-item, 5-point Likert scale measure that assesses sense of meaning, peace, and faith in illness. Total score ranges from 0 to 48, and a higher score indicates better spiritual well-being.12 Psychological distress was assessed using the Distress Thermometer (DT). The DT is an efficient, low burden method to evaluate distress, based on a scale of 0 to 10 (0=no distress; 10=extreme distress).13 Demographic and health status data (age, gender, race/ethnicity, education level, marital status, living situation, employment, religious preference, annual household income, co-morbidities, smoking history) were self-reported by patients at baseline. Disease and treatment characteristics, including stage of disease and type of surgical procedure were obtained through electronic medical records (EMR). All survivors completed baseline assessments prior to surgery, and were re-assessed at 6, 12, 24, 36, and 52 weeks (12 months) following surgery.
Data Analysis
Data processing included scanning demographic and outcome measures, entering chart audit data into a relational database, and exporting tracking data from an Access database. Data were analyzed using the Statistical Package for the Social Sciences, v. 21. (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.) Data were audited for accuracy, matched by ID, and scattered missing data were imputed using the SPSS Missing Values Analysis (MVA) procedure and the Estimation and Maximization (EM) method. Tracking and chart audit data were used to select the 71 early stage surgical patients who had completed their twelfth month in the study. Selected demographic data were compared by group (intervention vs. usual care) and by surgical method (open vs. minimally invasive (MIS)), using contingency table analysis and the chi square statistic or student’s t-tests, depending on the level of measurement. Because there was a significant association between group and surgical method, the factorial Analysis of Covariance (ANCOVA) was limited to examining two main effects without their interaction. The covariate was the baseline score and the outcomes were corresponding 12 month scores for psychological distress and six dimensions of quality of life for lung cancer patients (FACT-L and FACIT-Sp12). Finally, the 12-month trajectories of selected symptoms from the FACT-L were examined descriptively.
Results
239 patients were consented and enrolled in the main study in the early stage (stage I–IIIb) cohort, regardless of whether surgical treatment was planned. Selection for the cohort described in this manuscript is summarized in Supplementary Table 1. After excluding patients who did not undergo preoperative baseline assessment and patients who did not complete the 12 month assessment, we identified 71 patients who underwent surgical resection. 33 patients in the usual care and 38 patients in the intervention group were included in this analysis. Socio-demographics, smoking status, and disease stage were similar comparing survivors in the control and intervention groups (Table 1). A larger proportion of survivors in the intervention group underwent minimally invasive surgery (MIS) compared with the control group (57.9% vs. 30.3%, p=0.02). Survivors undergoing MIS had earlier stage disease, but otherwise there were no differences in socio-demographic or clinic characteristics comparing survivors who underwent MIS and open surgery (Table 1).
Table 1.
Sociodemographics and Clinical Characteristics by Group
| Control (N=33) |
Intervention (N=38) |
p-value | |
|---|---|---|---|
| Age – Mean (Range=34–91) | |||
| 65.6 | 69.4 | .167 | |
| Gender – N (%) | |||
| Male | 14 (42.4) | 14 (36.8) | .631 |
| Female | 19 (57.6) | 24 (63.2) | |
| Type of Procedure – N (%) | |||
| Open | 23 (69.7) | 16 (42.1) | .020b |
| aMIS | 10 (30.3) | 22 (57.9) | |
| Stage of Disease – N (%) | |||
| Stage I | 17 (51.5) | 20 (52.6) | .403 |
| Stage II | 7 (21.2) | 12 (31.6) | |
| Stage III | 9 (27.3) | 6 (15.8) | |
| Other Treatments – N (%) | |||
| Neoadjuvant | 2 (6.1) | 6 (15.8) | .270 |
| Adjuvant | 17 (51.5) | 13 (34.2) | .157 |
| Smoking History – N (%) | |||
| Current Smoker | 1 (3.0) | 2 (5.3) | .891 |
| Former Smoker | 27 (81.8) | 30 (78.9) | |
| Non-Smoker | 5 (15.2) | 6 (15.8) | |
| Race – N (%) | |||
| Asian | 3 (9.1) | 0 (0) | .141 |
| Black or African American | 2 (6.1) | 4 (10.5) | |
| White | 28 (84.8) | 34 (89.5) | |
| Ethnicity – N (%) | |||
| Hispanic/Latino | 1 (3.0) | 4 (10.5) | .218 |
| Non Hispanic/Latino | 32 (97) | 34 (89.5) | |
| Marital Status – N (%) | |||
| Married/Partnered | 12 (36.4) | 13 (34.2) | .850 |
| Other (Single, Separated, Widowed, Divorced) | 21 (63.6) | 25 (65.8) | |
| Living Situation: Live Alone? – N (%) | |||
| No | 23 (69.7) | 28 (73.7) | .710 |
| Yes | 10 (30.3) | 10 (26.3) | |
| Education Completed – N (%) | |||
| Secondary/High School | 15 (45.5) | 17 (44.7) | .952 |
| College | 18 (54.5) | 21 (55.3) | |
| Employment Status – N (%) | |||
| ≥ 32 hours per week | 9 (27.3) | 6 (15.8) | .237 |
| < 32 hours per week | 24 (72.7) | 32 (84.2) | |
| Annual Income – N (%) | |||
| </= $50K | 15 (45.5) | 11 (28.9) | .292 |
| > $50K | 14 (42.4) | 23 (60.5) | |
| Prefer not to answer | 4 (12.1) | 4 (10.5) | |
Minimally Invasive Surgery
Control group more likely to have open surgery
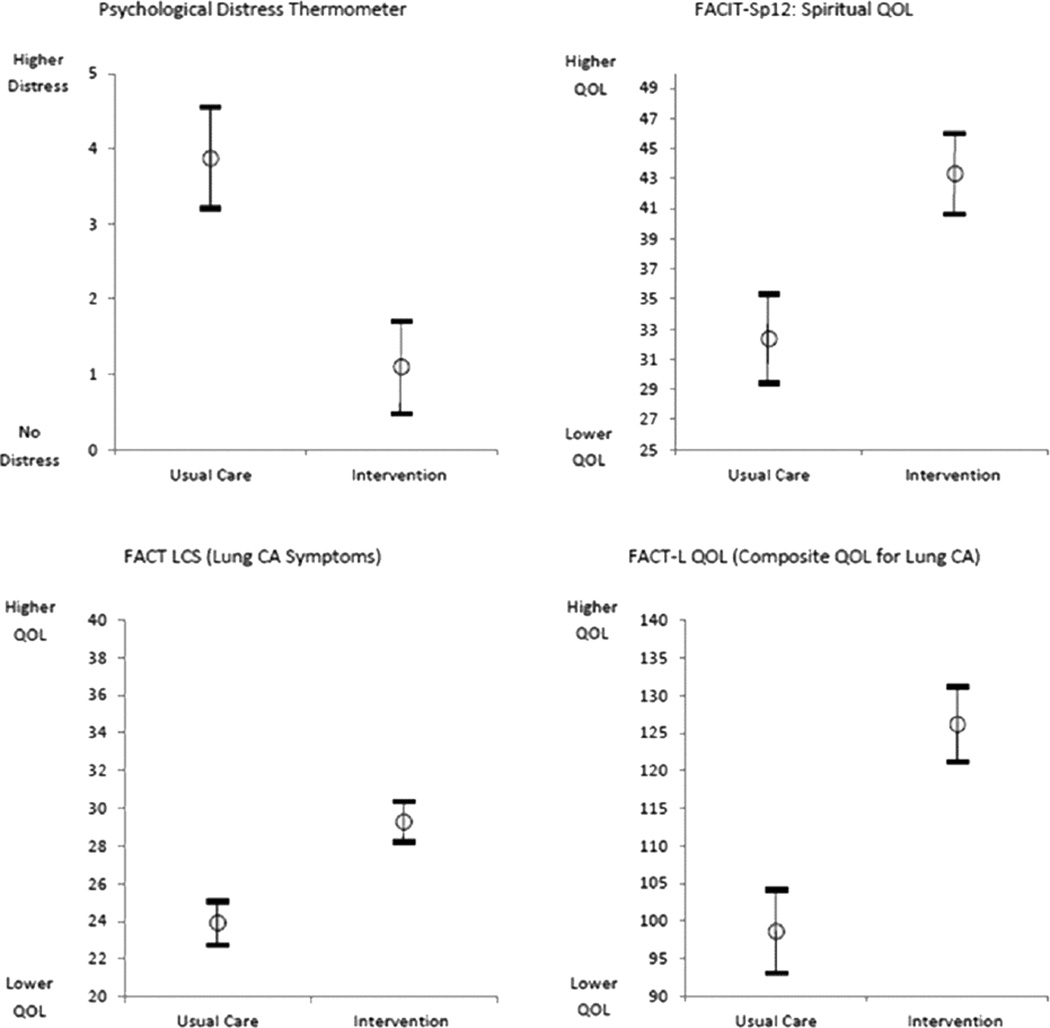
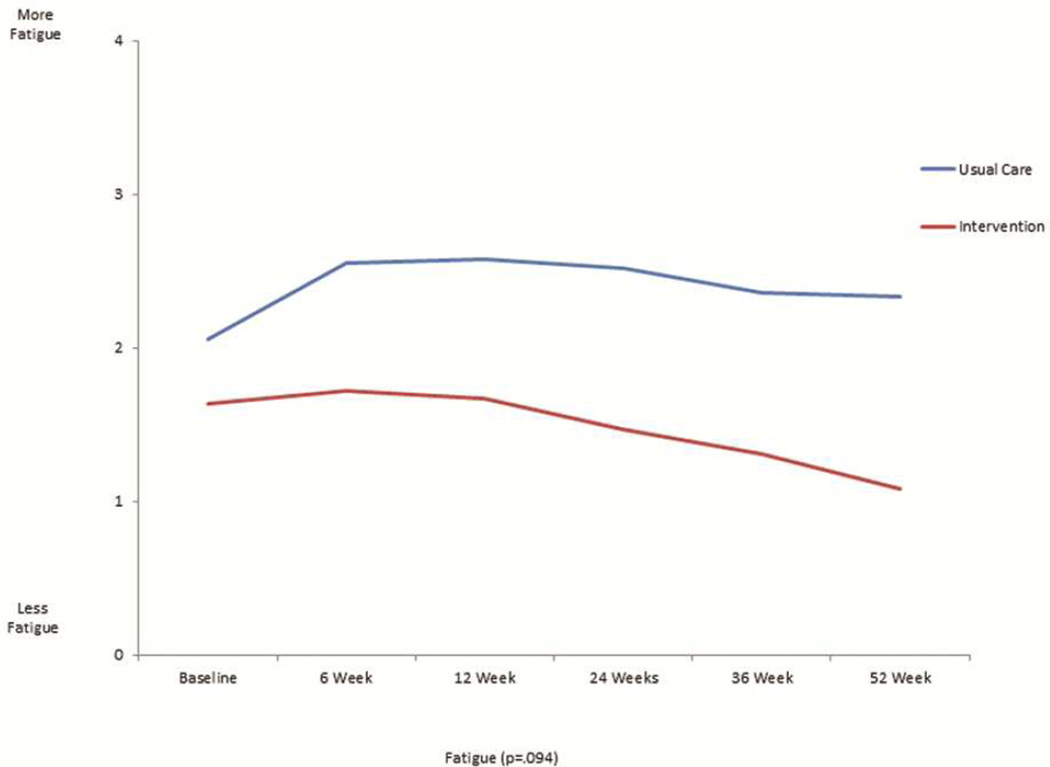
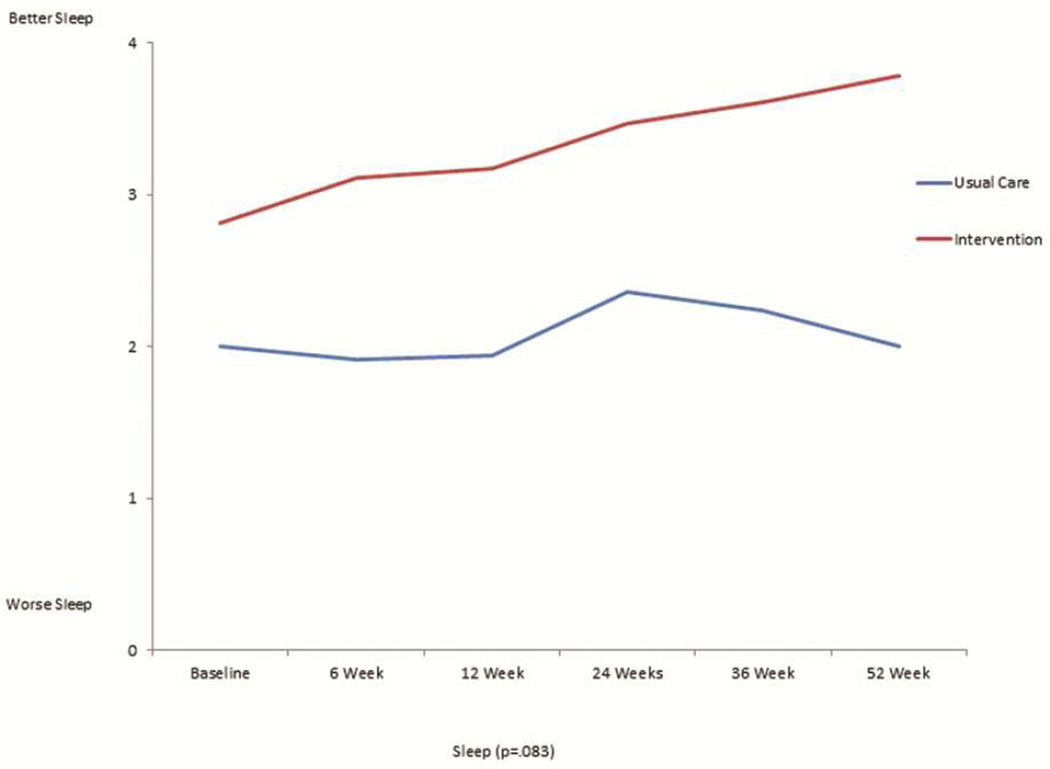
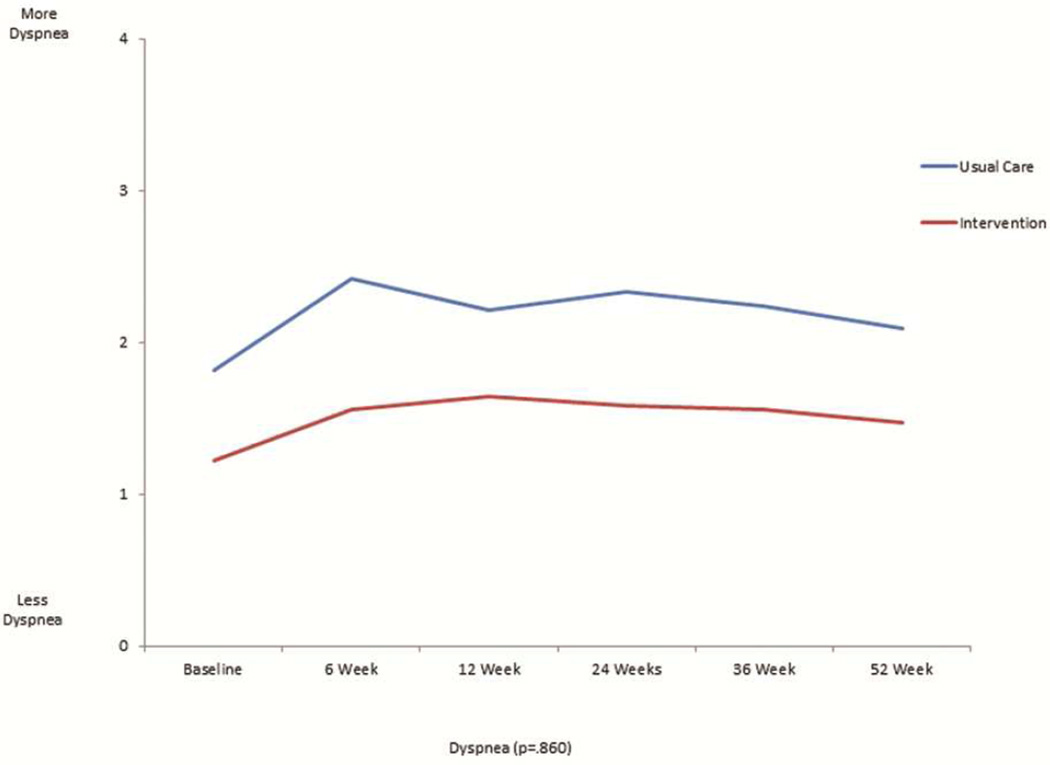
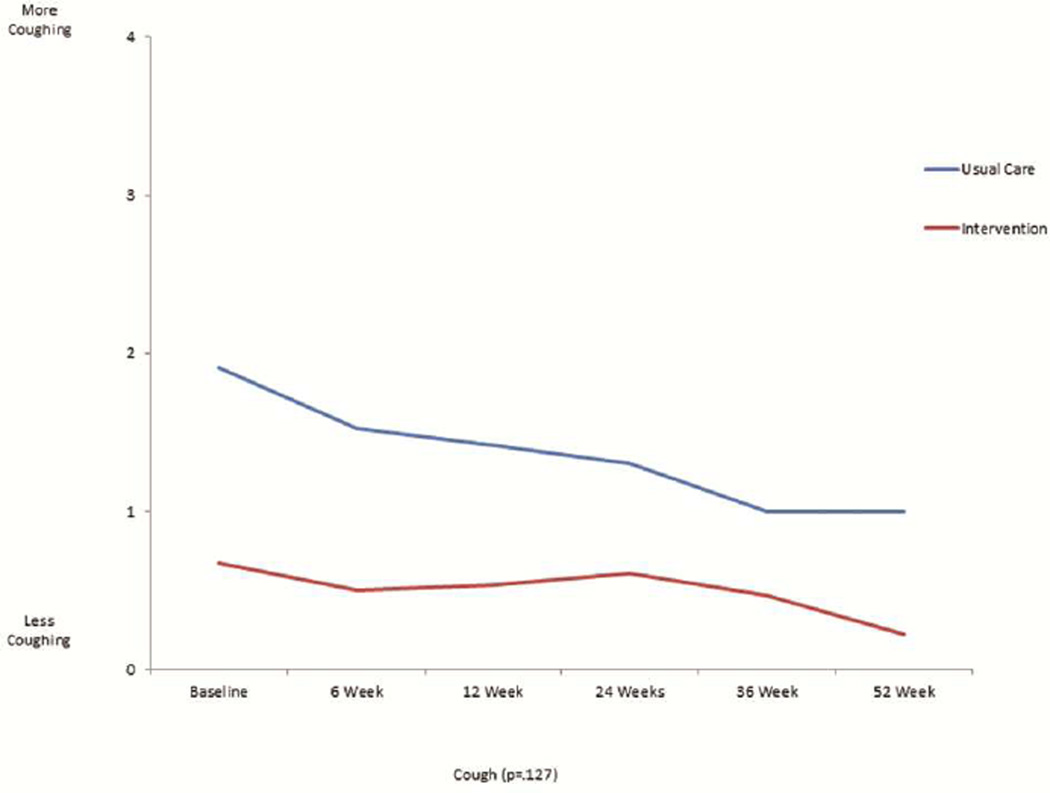
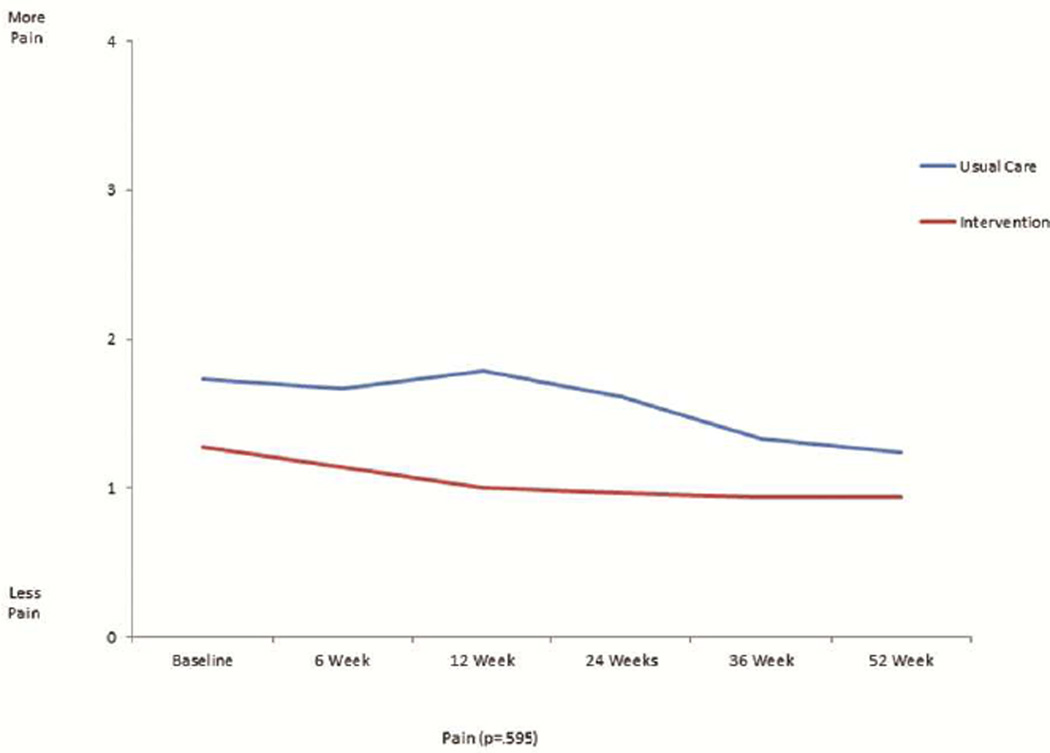
The mean scores for the FACT-L, the four QOL subsets of FACT-L, LCS, and FACITSP-12, adjusted for baseline scores, were all improved at 12 months in the intervention group compared to the control group (Table 2 and Figure 1). In addition, there was significantly less psychological distress in the intervention group compared with the control group (p<0.001). When stratifying by open or MIS, there were no differences in QOL scores or distress, with the exception of social/family well being which was slightly higher in the open surgery group (23.4±6.7 vs. 21.6±8.2, p=0.016). We analyzed the trajectory of specific symptom scores over time from the FACT-L and LCS, comparing the intervention group with the control group. There was no significant interaction by intervention over the 6 time points studied for each symptom (Figure 2). There appeared to be lower 12 months fatigue scores (1.1±1.0 vs 2.3±2.0, score range 0–4, 4= more fatigue, p=0.094), better sleep scores (3.8±0.5 vs 2.0±1.1; score range 0–4, 4=better sleep, p=0.083), lower dyspnea scores (3.8±0.4 vs 2.7±0.9; score range 0–4, 4=less dyspnea, p=0.86), and lower cough scores at 12 months (0.2±0.5 vs 1.0±1.1; score range 0–4, 4=worse cough, p=.127) in the intervention group, although none of these differences were statistically significant. However, baseline scores were generally more favorable in the intervention group compared with the control group. There did not seem to be a meaningful difference in pain at 12 months between groups (0.9±0.9 intervention vs. 1.2±1.2; score range 0–4, 4=worse pain).
Table 2.
Multivariate Analysis of Main Outcomes at 12 months (N=71)
| Outcome | Pre x̄±SD |
Usual Care (N=33) 12 months x̄±SD |
x̄1 | Pre x̄±SD |
Intervention (N=38) 12 months x̄±SD |
x̄1 | p-value |
|---|---|---|---|---|---|---|---|
| FACT-L (Range = 0–140; higher = better QOL) |
94.0±2.0 | 98.7±20.5 | 98.6 | 106.4±18.8 | 126.1±8.2 | 126.2 | <.001 |
| Lung Cancer Subscale2 (Range = 0–32; higher = better QOL) |
23.0±4.7 | 23.6±4.2 | 23.9 | 26.4±4.0 | 29.4±2.1 | 29.3 | <.001 |
| Physical Well Being2 (Range = 0–28; higher = better QOL) |
20.5±6.2 | 22.2±4.1 | 22.5 | 23.0±4.7 | 25.2±2.3 | 25.1 | .001 |
| Social/Family Well Being2 (Range = 0–28; higher = better QOL) |
19.2±9.0 | 19.1±9.1 | 18.3 | 22.6±6.5 | 25.6±3.6 | 26.0 | <.001 |
| Emotional Well Being2 (Range = 0–24; higher = better QOL) |
16.9±5.0 | 19.4±3.6 | 19.5 | 18.2±5.2 | 23.2±1.8 | 23.1 | <.001 |
| Functional Well Being2 (Range = 0–28; higher = better QOL) |
14.4±5.4 | 14.4±5.1 | 14.3 | 16.1±5.3 | 22.6±2.6 | 22.7 | <.001 |
| FACIT-Sp-12 (Range = 0–48; higher = better spiritual well-being) |
33.5±8.6 | 32.7±9.5 | 32.3 | 39.0±6.9 | 43.1±6.8 | 43.3 | <.001 |
| Psychological Distress (Range = 0–10; higher = more distress) |
3.9±2.8 | 4.0±2.3 | 3.9 | 4.1±2.4 | 1.0±1.4 | 1.1 | <.001 |
Adjusted means
Pillai’s Trace Multivariate p value <.001 for group and type of procedure
Minimally Invasive Surgery
Figure 1.
This is a graphical representation of 12 month scores for the psychological distress thermometer, FACT LCS, FACIT-Sp12, and FACT-L for the usual care and intervention groups. Error bars represent standard deviation.
Figure 2.
This is a graphical representation of the trajectory of individual symptom scores for fatigue (figure 2a), sleep (figure 2b), dyspnea (figure 2c), cough (figure 2d), and pain (figure 2e) from the FACT-L across the 12 month study period for the usual care and intervention groups.
Seven patients (54%) in the intervention group and 6 patients (47%) in the control group received pulmonary rehabilitation consultations (p=0.98), while 3 patients (8%) in the intervention group and 2 patients (6%) in the control group received pain management consultations (p=0.76).
Comment
We found that an interdisciplinary supportive care intervention significantly improved HRQOL and distress 12 months after lung cancer surgery. Our study highlights the changes in HRQOL that may continue months after early stage lung cancer diagnosis and treatment. We consider the changes in HRQOL scores we observed to be clinically meaningful. The minimally important difference (MID) is the smallest difference in score in the domain of interest that patients perceive as important and which would lead the clinician to change treatment. A two to three point change in the LCS or any of the FACT-L subscales is considered to be clinically meaningful, and this estimate has been validated in the LCS.14
In this non-randomized trial, a significantly larger proportion of survivors in the intervention group underwent more minimally invasive surgery compared with the control group. This was because over time our group has performed a larger proportion of operations using minimally invasive techniques. There was no significant difference in QOL, psychological distress, or symptoms comparing survivors who had open or minimally invasive surgery, with the exception of social/family wellbeing, which had more favorable adjusted mean scores in the open group. These findings are consistent with other groups, including a recent report by Rizk and colleagues that demonstrated no significant differences between minimally invasive and open surgery QOL or pain scores at 12 months with the exception of emotional well-being, which was better in the open surgery group.15
This is the first study to our knowledge to test an interdisciplinary supportive care intervention in early stage lung cancer survivors. Our findings validate the IOM recommendation that an interdisciplinary approach to palliative/supportive care be incorporated into the care of survivors with early stage lung cancer.9 The improvements in quality of life and physical symptoms that we observed in survivors receiving the intervention were likely due to a number of factors including improved education, reassurance, and early symptom management. We were unable with this analysis to determine which component of the intervention had a direct impact on specific outcomes. Many surgeons, including our own group, provide preoperative teaching and counseling in conjunction with nurses or physician extenders which probably improve postoperative QOL and anxiety through education and establishing expectations. Future studies are needed to determine the specific causal effects of key components of the intervention in order to inform the potential generalizability of this approach to palliative/supportive care.
This study has several potential limitations. First, this was a sequential trial of usual care followed by experimental versus a randomized trial and as a result there may have been unmeasured variables that contributed to the findings. The groups were well-balanced with respect to socio-demographic and clinical characteristics, with the exception of minimally invasive surgery. There were some personnel changes during the study period, and it is possible that these changes accounted for some of the difference observed between the two groups. This is a limitation of the sequential study design and may have introduced bias. This may in part be a reason for somewhat higher baseline QOL scores, although when adjusted for baseline scores, the scores in the intervention group were still significantly more favorable than in the usual care group. Moreover, because of strict eligibility criteria, a large proportion of patients undergoing lung resection were excluded from this study and this may limit the generalizability of the results. Specifically, patients whose primary language was not English, those with a personal history of cancer, and patients not willing to commit the necessary time to complete the assessment tools were not included. Next, this study compares the intervention to usual care at our institution which may vary from the usual care at other institutions. We followed National Comprehensive Cancer Network (NCCN) guidelines for follow-up of survivors who underwent lung cancer resection16, seeing survivors every 3–6 months during the first postoperative year, depending on stage and operation performed. Finally, the weekly interdisciplinary meetings and educational sessions employed in the study intervention may not be feasible in all practice settings. A clinical trial testing the implementation of key components of the intervention described in this study in lung cancer patients in a community practice setting is currently underway by our group. Results of that study will be important to guide translation of this intervention into clinical practice.
In summary, this study demonstrates the importance of integrating routine supportive care and educational interventions in the care of early stage lung cancer survivors. Further research on early implementation of palliative/supportive care and educational interventions after lung cancer resection is needed.
Supplementary Material
Acknowledgements
The authors thank Gwen Uman, PhD for assistance with statistical analysis and consultation. The research described was supported by grant P01 CA136396 (Ferrell) and 5K12CA001727-20 (Raz) from the National Cancer Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Poghosyan H, Sheldon LK, Leveille SG, Cooley ME. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: A systematic review. Lung cancer. 2013;81:11–26. doi: 10.1016/j.lungcan.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Koczywas M, Williams AC, Cristea M, Reckamp K, Grannis FW, Jr, Tiep BL, Uman G, Ferrell B. Longitudinal changes in function, symptom burden, and quality of life in patients with early-stage lung cancer. Annals of surgical oncology. 2013;20:1788–1797. doi: 10.1245/s10434-012-2741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarna L, Cooley ME, Brown JK, Chernecky C, Elashoff D, Kotlerman J. Symptom severity 1 to 4 months after thoracotomy for lung cancer. American journal of critical care : an official publication, American Association of Critical-Care Nurses. 2008;17:455–467. quiz 468. [PMC free article] [PubMed] [Google Scholar]
- 4.Sarna L, Padilla G, Holmes C, Tashkin D, Brecht ML, Evangelista L. Quality of life of long-term survivors of non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:2920–2929. doi: 10.1200/JCO.2002.09.045. [DOI] [PubMed] [Google Scholar]
- 5.Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J, Hull JG, Li Z, Tosteson TD, Byock IR, Ahles TA. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The project enable ii randomized controlled trial. JAMA. 2009;302:741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brumley R, Enguidanos S, Jamison P, Seitz R, Morgenstern N, Saito S, McIlwane J, Hillary K, Gonzalez J. Increased satisfaction with care and lower costs: Results of a randomized trial of in-home palliative care. Journal of the American Geriatrics Society. 2007;55:993–1000. doi: 10.1111/j.1532-5415.2007.01234.x. [DOI] [PubMed] [Google Scholar]
- 7.Gade G, Venohr I, Conner D, McGrady K, Beane J, Richardson RH, Williams MP, Liberson M, Blum M, Della Penna R. Impact of an inpatient palliative care team: A randomized control trial. Journal of palliative medicine. 2008;11:180–190. doi: 10.1089/jpm.2007.0055. [DOI] [PubMed] [Google Scholar]
- 8.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ. Early palliative care for patients with metastatic non-small-cell lung cancer. The New England journal of medicine. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 9.Institute of Medicine. Improving palliative care for cancer. Washington, DC: The National Academies Press; 2001. [PubMed] [Google Scholar]
- 10.Ellis PM. The importance of multidisciplinary team management of patients with non-small-cell lung cancer. Current oncology. 2012;19:S7–S15. doi: 10.3747/co.19.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the functional assessment of cancer therapy-lung (fact-l) quality of life instrument. Lung Cancer. 1995;12:199–220. doi: 10.1016/0169-5002(95)00450-f. [DOI] [PubMed] [Google Scholar]
- 12.Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual wellbeing in people with cancer: The functional assessment of chronic illness therapy--spiritual well-being scale (facit-sp) Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2002;24:49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 13.Graves KD, Arnold SM, Love CL, Kirsh KL, Moore PG, Passik SD. Distress screening in a multidisciplinary lung cancer clinic: Prevalence and predictors of clinically significant distress. Lung Cancer. 2007;55:215–224. doi: 10.1016/j.lungcan.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cella D, Eton DT, Fairclough DL, Bonomi P, Heyes AE, Silberman C, Wolf MK, Johnson DH. What is a clinically meaningful change on the functional assessment of cancer therapy-lung (fact-l) questionnaire? Results from eastern cooperative oncology group (ecog) study 5592. Journal of clinical epidemiology. 2002;55:285–295. doi: 10.1016/s0895-4356(01)00477-2. [DOI] [PubMed] [Google Scholar]
- 15.Rizk NP, Ghanie A, Hsu M, Bains MS, Downey RJ, Sarkaria IS, Finley DJ, Adusumilli PS, Huang J, Sima CS, Burkhalter JE, Park BJ, Rusch VW. A prospective trial comparing pain and quality of life measures after anatomic lung resection using thoracoscopy or thoracotomy. Ann Thorac Surg. 2014;98:1160–1166. doi: 10.1016/j.athoracsur.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer N. Non-small cell lung cancer. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) 2014 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.