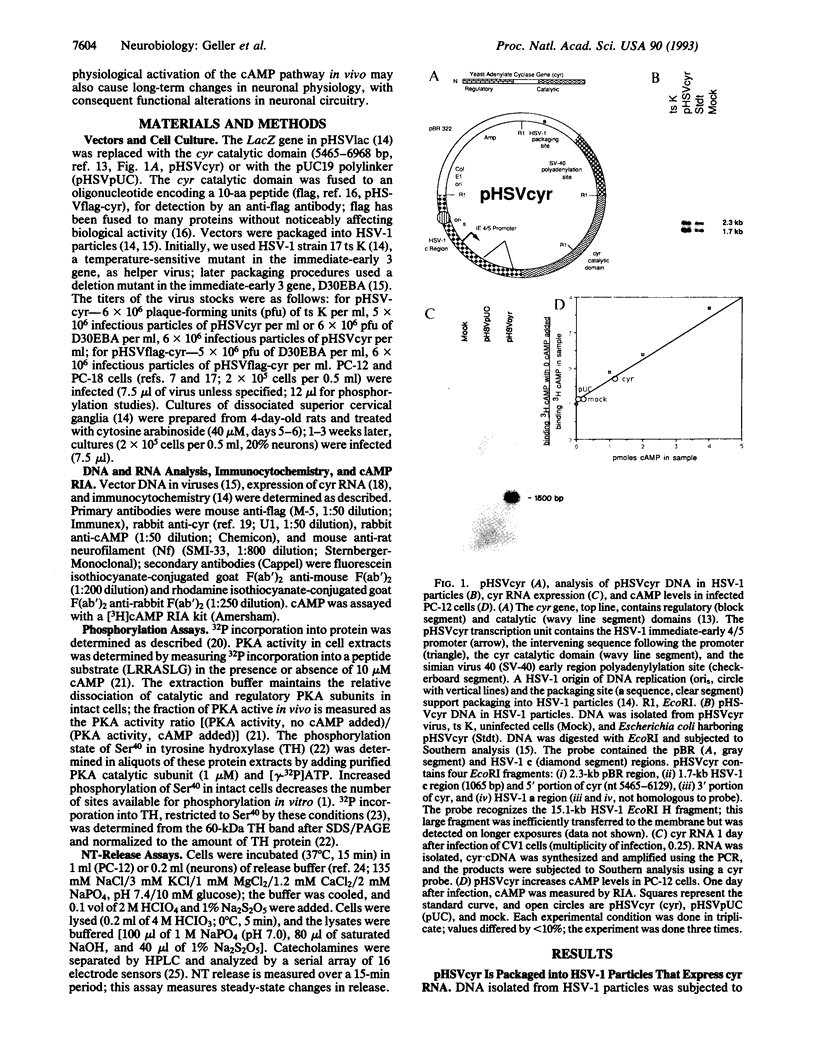
Abstract
Signal-transduction pathways mediate a wide range of short-term changes in the physiology of neuronal systems from invertebrates to mammals. However, examples of long-term changes in neuronal physiology mediated by these pathways have been limited to invertebrate systems. In this report, long-term changes in the physiology of mammalian neurons were studied by using genetic intervention to cause a long-lasting activation of the cAMP pathway. The catalytic domain of yeast adenylate cyclase (cyr), encoding a constitutive enzyme activity, was expressed in neuronal cells infected with a defective herpes simplex virus vector (pHSVcyr). In PC-12 cells infected with pHSVcyr, increases were seen in cAMP levels, protein kinase A activity, protein phosphorylation, phosphorylation of the tyrosine hydroxylase protein kinase A site (Ser40), and catecholamine release. Infection of sympathetic neurons with pHSVcyr increased cAMP levels, protein phosphorylation, and catecholamine release. Yeast adenylate cyclase immunoreactivity and elevated cAMP levels were localized to the cell bodies of sympathetic neurons. The increase in neurotransmitter release was both Ca(2+)- and activity-dependent and persisted for at least 1 week after infection of the sympathetic neurons, suggesting that sustained physiological activation of the cAMP pathway may mediate long-term changes in the neuronal physiology of mammalian systems.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baizer L., Weiner N. Regulation of dopamine release from PC12 pheochromocytoma cell cultures during stimulation with elevated potassium or carbachol. J Neurochem. 1985 Feb;44(2):495–501. doi: 10.1111/j.1471-4159.1985.tb05441.x. [DOI] [PubMed] [Google Scholar]
- Björklund A., Cegrell L., Falck B., Ritzén M., Rosengren E. Dopamine-containing cells in sympathetic ganglia. Acta Physiol Scand. 1970 Mar;78(3):334–338. doi: 10.1111/j.1748-1716.1970.tb04668.x. [DOI] [PubMed] [Google Scholar]
- Briggs C. A., McAfee D. A., McCaman R. E. Long-term regulation of synaptic acetylcholine release and nicotinic transmission: the role of cyclic AMP. Br J Pharmacol. 1988 Feb;93(2):399–411. doi: 10.1111/j.1476-5381.1988.tb11447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti G., Madeddu L., Pandiella A., Pozzan T., Meldolesi J. Second-messenger generation in PC12 cells. Interactions between cyclic AMP and Ca2+ signals. Biochem J. 1988 Nov 1;255(3):753–760. doi: 10.1042/bj2550753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller A. I., Breakefield X. O. A defective HSV-1 vector expresses Escherichia coli beta-galactosidase in cultured peripheral neurons. Science. 1988 Sep 23;241(4873):1667–1669. doi: 10.1126/science.241.4873.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller A. I., During M. J., Neve R. L. Molecular analysis of neuronal physiology by gene transfer into neurons with herpes simplex virus vectors. Trends Neurosci. 1991 Oct;14(10):428–432. doi: 10.1016/0166-2236(91)90040-2. [DOI] [PubMed] [Google Scholar]
- Geller A. I., Keyomarsi K., Bryan J., Pardee A. B. An efficient deletion mutant packaging system for defective herpes simplex virus vectors: potential applications to human gene therapy and neuronal physiology. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8950–8954. doi: 10.1073/pnas.87.22.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson E. Accumulation of putative amino acid neurotransmitters, monoamines and D-Ala2-Met-enkephalinamide in primary astroglial cultures from various brain areas, visualized by autoradiography. Brain Res. 1983 Dec 19;289(1-2):189–196. doi: 10.1016/0006-8993(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Haycock J. W., Ahn N. G., Cobb M. H., Krebs E. G. ERK1 and ERK2, two microtubule-associated protein 2 kinases, mediate the phosphorylation of tyrosine hydroxylase at serine-31 in situ. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2365–2369. doi: 10.1073/pnas.89.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock J. W. Phosphorylation of tyrosine hydroxylase in situ at serine 8, 19, 31, and 40. J Biol Chem. 1990 Jul 15;265(20):11682–11691. [PubMed] [Google Scholar]
- Heideman W., Casperson G. F., Bourne H. R. Adenylyl cyclase in yeast: antibodies and mutations identify a regulatory domain. J Cell Biochem. 1990 Apr;42(4):229–242. doi: 10.1002/jcb.240420406. [DOI] [PubMed] [Google Scholar]
- Holz R. W., Senyshyn J., Bittner M. A. Mechanisms involved in calcium-dependent exocytosis. Ann N Y Acad Sci. 1991;635:382–392. doi: 10.1111/j.1749-6632.1991.tb36506.x. [DOI] [PubMed] [Google Scholar]
- Ivins K. J., Neve K. A., Feller D. J., Fidel S. A., Neve R. L. Antisense GAP-43 inhibits the evoked release of dopamine from PC12 cells. J Neurochem. 1993 Feb;60(2):626–633. doi: 10.1111/j.1471-4159.1993.tb03194.x. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Broek D., Wigler M. DNA sequence and characterization of the S. cerevisiae gene encoding adenylate cyclase. Cell. 1985 Dec;43(2 Pt 1):493–505. doi: 10.1016/0092-8674(85)90179-5. [DOI] [PubMed] [Google Scholar]
- Kuba K., Kato E., Kumamoto E., Koketsu K., Hirai K. Sustained potentiation of transmitter release by adrenaline and dibutyryl cyclic AMP in sympathetic ganglia. Nature. 1981 Jun 25;291(5817):654–656. doi: 10.1038/291654a0. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Cyclic adenosine 3',5'-monophosphate mediates beta-receptor actions of noradrenaline in rat hippocampal pyramidal cells. J Physiol. 1986 Mar;372:245–259. doi: 10.1113/jphysiol.1986.sp016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson W. R., Gamache P. G., Beal M. F., Bird E. D. EC array sensor concepts and data. Life Sci. 1987 Aug 17;41(7):905–908. doi: 10.1016/0024-3205(87)90192-5. [DOI] [PubMed] [Google Scholar]
- Melia K. R., Rasmussen K., Terwilliger R. Z., Haycock J. W., Nestler E. J., Duman R. S. Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase in the rat locus coeruleus: effects of chronic stress and drug treatments. J Neurochem. 1992 Feb;58(2):494–502. doi: 10.1111/j.1471-4159.1992.tb09748.x. [DOI] [PubMed] [Google Scholar]
- Mitts M. R., Grant D. B., Heideman W. Adenylate cyclase in Saccharomyces cerevisiae is a peripheral membrane protein. Mol Cell Biol. 1990 Aug;10(8):3873–3883. doi: 10.1128/mcb.10.8.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S., Kobayashi H., Libet B. Stimulation of adenylate cyclase in relation to dopamine-induced long-term enhancement (LTE) of muscarinic depolarization in the rabbit superior cervical ganglion. J Neurosci. 1987 Feb;7(2):311–318. doi: 10.1523/JNEUROSCI.07-02-00311.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B., Bunney B. S. Ionic composition of microdialysis perfusing solution alters the pharmacological responsiveness and basal outflow of striatal dopamine. J Neurochem. 1989 Aug;53(2):652–654. doi: 10.1111/j.1471-4159.1989.tb07383.x. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Kauer J. A., Malenka R. C. The current excitement in long-term potentiation. Neuron. 1988 Apr;1(2):97–103. doi: 10.1016/0896-6273(88)90193-6. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr, White L., Knowlton R., Roskoski L. M. Regulation of tyrosine hydroxylase activity in rat PC12 cells by neuropeptides of the secretin family. Mol Pharmacol. 1989 Dec;36(6):925–931. [PubMed] [Google Scholar]
- Rossie S., Catterall W. A. Cyclic-AMP-dependent phosphorylation of voltage-sensitive sodium channels in primary cultures of rat brain neurons. J Biol Chem. 1987 Sep 15;262(26):12735–12744. [PubMed] [Google Scholar]
- Sheng M., Greenberg M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990 Apr;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Westfall T. C., Kitay D., Wahl G. The effect of cyclic nucleotides on the release of 3H-dopamine from rat striatal slices. J Pharmacol Exp Ther. 1976 Oct;199(1):149–157. [PubMed] [Google Scholar]
- Wooten G. F., Thoa N. B., Kopin I. J., Axelrod J. Enhanced release of dopamine -hydroxylase and norepinephrine from sympathetic nerves by dibutyryl cyclic adenosine 3', 5'-monophosphate and theophylline. Mol Pharmacol. 1973 Mar;9(2):178–183. [PubMed] [Google Scholar]
- Yamamori T., Fukada K., Aebersold R., Korsching S., Fann M. J., Patterson P. H. The cholinergic neuronal differentiation factor from heart cells is identical to leukemia inhibitory factor. Science. 1989 Dec 15;246(4936):1412–1416. doi: 10.1126/science.2512641. [DOI] [PubMed] [Google Scholar]