Abstract
Glial cell line-derived neurotrophic factor (GDNF) protects against high-fat diet (HFD)-induced hepatic steatosis in mice, however, the mechanisms involved are not known. In this study we investigated the effects of GDNF overexpression and nanoparticle delivery of GDNF in mice on hepatic steatosis and fibrosis and the expression of genes involved in the regulation of hepatic lipid uptake and de novo lipogenesis. Transgenic overexpression of GDNF in liver and other metabolically active tissues was protective against HFD-induced hepatic steatosis. Mice overexpressing GDNF had significantly reduced P62/sequestosome 1 protein levels suggestive of accelerated autophagic clearance. They also had significantly reduced peroxisome proliferator-activated receptor-γ (PPAR-γ) and CD36 gene expression and protein levels, and lower expression of mRNA coding for enzymes involved in de novo lipogenesis. GDNF-loaded nanoparticles were protective against short-term HFD-induced hepatic steatosis and attenuated liver fibrosis in mice with long-standing HFD-induced hepatic steatosis. They also suppressed the liver expression of steatosis-associated genes. In vitro, GDNF suppressed triglyceride accumulation in Hep G2 cells through enhanced p38 mitogen-activated protein kinase-dependent signaling and inhibition of PPAR-γ gene promoter activity. These results show that GDNF acts directly in the liver to protect against HFD-induced cellular stress and that GDNF may have a role in the treatment of nonalcoholic fatty liver disease.
Keywords: nonalcoholic fatty liver disease, de novo lipogenesis, transgenic, nanoparticles, peroxisome proliferator-activated receptor-γ
nonalcoholic fatty liver disease (NAFLD) is increasingly recognized as the leading cause of chronic liver disease, affecting 20–30% of the population in Western countries (1). NAFLD is a major complication of obesity and insulin resistance, and its prevalence is rapidly rising worldwide due to an increase in the incidence of the metabolic syndrome in adults and children (3, 29, 32).
NAFLD is a complex disease that in the early stages manifests as simple hepatic steatosis characterized by increased deposition of triglyceride as lipid droplets in the cytoplasm of hepatocytes (7). While hepatic steatosis is most often self-limiting and rarely causes significant damage to the liver, it occasionally can sensitize the liver to inflammatory injury from a variety of stimuli (10), leading to nonalcoholic steatohepatitis (NASH) (9). NASH can in turn progress to cirrhosis and hepatocellular carcinoma, resulting in significant morbidity and mortality (49). In spite of the increasing threat from NAFLD, there are currently no approved drugs for the treatment of the disease, and management of the disease mainly involves diet and lifestyle changes (5, 42).
Glial cell line-derived neurotrophic factor (GDNF) is a neurotrophic factor that plays an important role in the development of the enteric nervous system and the survival of enteric, midbrain catecholaminergic, motor, sensory, sympathetic, and parasympathetic neurons (40). GDNF has recently shown promise as a factor that cannot only increase β-cells mass and improve blood glucose control but also induce weight loss and protect against obesity in rodents (31, 37, 38, 50). In our previous study we showed that GDNF transgenic (GDNF Tg) mice that overexpresses GDNF globally in glia are protected against HFD-induced insulin resistance, hyperlipidemia, and obesity through increased β-adrenergic receptor-mediated energy expenditure in brown adipose tissue (38). These mice are also protected against HFD-induced hepatic steatosis, but the mechanisms involved have not been elucidated. Moreover, the pharmacological use of GDNF to treat HFD-induced hepatic steatosis has never been explored. In the present study we examined the effects of GDNF on key signaling pathways involved in the regulation of lipid metabolism in the liver. We also examined the efficacy of GDNF-loaded nanoparticles in accelerating recovery of mice with HFD-induced hepatic steatosis. The data demonstrate the ability of GDNF to induce suppression of signaling processes that promote lipid uptake and de novo lipogenesis and to increase autophagic clearance in the liver. The data also reveal the potential for the use of GDNF-loaded nanoparticles in accelerating recovery from hepatic steatosis.
METHODS
Animals.
Animal studies were conducted using GDNF Tg mice (on a CF-1 background) and CF-1 wild-type (WT) littermates. The generation of the GDNF Tg mice has been previously described (56). The mice were maintained on a 12:12-h light-dark cycle in a temperature-controlled barrier facility with free access to food and water. They were fed a regular laboratory rodent diet (RD) (2018SX; Teklad Global 18% Protein Extruded Rodent Diet; Harlan Laboratories, Madison, WI) containing 6.2% fat by weight or a HFD (TD.06414; Harlan) containing 34.3% fat by weight (38). Heterozygous GDNF family receptor α1 (GFRα1) knockout (GFRα1 KO) mice carried a GFRα1 GFP-knockin allele and were on a mixed 129/Sv × C57BL/6 background (51). All animal studies were approved by the Atlanta Veteran Affairs Medical Center Institutional Animal Care and Use Committee.
Histology and immunofluorescence staining.
Mice liver tissues were fixed in 10% formalin solution and embedded in paraffin or frozen in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA) using standard techniques. Oil Red-O staining was performed using a modification of a previously published protocol (http://bio.lonza.com/uploads/tx_mwaxmarketingmaterial/Lonza_ManualsProductInstructions_Oil_Red_O_Stain_for_In_Vitro_Adipogenesis.pdf). Briefly, frozen liver sections (7 μm thick) were fixed for 1 h at room temperature in paraformaldehyde (4%), washed briefly with deionized water, and incubated for 2–5 min in 60% isopropanol. The sections were then incubated for 5 min in a freshly prepared filtered 1.8 mg/ml solution of Oil Red-O (Sigma Aldrich, St. Louis, MO) in 60% isopropanol and washed thoroughly with running tap water until the water ran clear. They were counterstained for 15 s with Harleco Gill modified hematoxylin solution III (EMD Chemicals, Billerica, MA), washed thoroughly with warm running tap water, and mounted with glycerol vinyl alcohol aqueous mounting solution (Invitrogen). Oil Red-O staining areas were quantified using the MetaMorph Offline (version 7.0r3) software (Molecular Devices, Downingtown, PA). Immunofluorescence staining was performed on paraffin-embedded liver sections (5 μm thick) and frozen liver sections (6 μm thick). Deparaffinization, rehydration, and antigen unmasking with 10 mM sodium citrate buffer, pH 6.0, was performed according to suggested protocols (Cell Signaling Technologies, Danvers, MA). The sections were blocked in PBS containing 3% BSA and 0.02% Triton X-100 and incubated overnight at 4°C with anti-human GFRα1 (H-70) antibody (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:200, rabbit polyclonal antibody against peripherin (Chemicon International, Temecula, CA) diluted 1:100, and mouse monoclonal antibody to β-actin (Cell Signaling Technologies) diluted 1:500. Secondary detection was performed by 1 h incubation with anti-rabbit IgG (1:500) antibody conjugated to Alexa Fluor 594 (Molecular Probes, Eugene, OR) and anti-mouse IgG (1:500) conjugated to Alexa Fluor 488 (Molecular Probes). Sirius Red and hematoxylin and eosin (H&E) staining were also performed according to common protocols on paraffin-embedded liver sections. Images were acquired with the aid of an Olympus IX51 microscope (Olympus, Tokyo, Japan) equipped with the cellSens Standard 1.12 imaging software (Olympus). Scoring of histological features was performed by an expert pathologist (Dr. A. Farris, Emory University School of Medicine, Department of Pathology and Laboratory Medicine) who was blinded to the experimental groups. The scoring was based on the NASH Clinical Research Network histological scoring system (23) and included steatosis grade, steatosis location, microvesicular steatosis, fibrosis stage, lobular inflammation, microgranulomas, large lipogranulomas, portal inflammation, hepatocyte ballooning, acidophil bodies, megamitochondria, pigmented macrophages, Mallory's hyaline, and glycogenated nuclei. Quantification of liver fibrosis was performed with the aid of the Image J (version 1.48v; National Institutes of Health) software using a thresholding algorithm (http://rsbweb.nih.gov/ij/docs/examples/stained-sections/index.html), which was set to detect the red hue of fibrous tissue in Sirius Red-stained liver sections. Briefly, images acquired from Sirius Red-stained liver sections were split into red, blue, and green channels. The green channel, which showed the best separation between the darker fibrous tissue and light gray nonfibrous tissue, was thresholded in such a way that only the stained fibrous tissue was highlighted in red. Blood vessels and ducts were erased in order not to include them in the calculated area. The thresholded area (red) was then measured and expressed as a percentage of the total liver tissue surface area.
Serum chemistry and liver triglyceride analyses.
Serum chemistry and liver triglyceride analyses were performed as previously described (38). Blood was collected in serum separator tubes (BD, Franklin Lakes, NJ), and serum was separated by centrifugation at 3,000 g for 15 min at room temperature. Liver profiles were tested using the Abaxis mammalian liver profile kit (Abaxis, Union City, CA).
Preparation and administration of GDNF- and FITC-loaded nanoparticles.
Polyvinyl alcohol (PVA)-covered GDNF- and FITC-loaded polylactic acid (PLA) nanoparticles were synthesized by double emulsion/solvent evaporation using previously described methods (27, 28). Nanoparticles size and charge were measured by Dynamic light scattering (DLS). The samples (nanoparticles) scatter incoming laser light. Because of the random motion of these particles, the scattered light intensity fluctuates in time. Processing the fluctuating signal yields the particle's diffusion coefficient from which the equivalent spherical particle size is calculated using the Stokes-Einstein equation. The same process allowed us to measure the zeta potential, a close approximation of the surface charges. The nanoparticles were found to have a charge of −27.1 ± 3.5 mV (enabled easy suspension of nanoparticles due to repulsive interactions) and a size of 376.8 ± 12.7 nm. They were suspended in normal saline (0.15 M NaCl) solution at a final GDNF concentration of 16.67 μg/ml before use. Groups of four to five mice each maintained on a regular diet or HFD were injected one time daily with 300 μl of either vehicle (GDNF-free nanoparticles) or GDNF-loaded nanoparticles (GDNF NP) containing 5.0 μg GDNF.
Cell culture.
The human hepatoma-derived Hep G2 cell line (24) was purchased from American Type Culture Collection (ATCC, Manassas, VA) and cultured according to recommended procedure in Minimum Essential Medium (ATCC) supplemented with fetal bovine serum added to a final concentration of 10%. Mouse hepatocytes were isolated as previously described (12) and cultured in Minimum Essential Medium supplemented with fetal bovine serum. The mouse pancreas-derived β-TC-6 (ATCC CRL-11506) β-cell insulinoma cells were cultured according to recommended procedure. Recombinant rat GDNF was produced as previously described (8) and used at a final concentration of 100 ng/ml. Water-soluble oleic acid (Sigma) was used at final concentration of 0.3 mM. The p38 mitogen-activated protein kinase (MAPK) inhibitor SB-203580 (Sigma-Aldrich) was used at a final concentration of 10 μM. Transfection of cells was performed as previously described (38) using On-TARGETplus SMARTpool GFRα1 siRNA (L-042178-01; Thermo Fisher Scientific, Lafayette, CO), siGENOME Control Pool nontargeting siRNA (D-001206-13-05; Thermo Fisher Scientific), and Lipofectamine RNAiMax (Invitrogen Life Technologies, Grand Island, NY).
Cellular toxicity assay.
To assess the potential toxicity of GDNF-loaded nanoparticles (GDNF NP), β-TC-6 cells were plated at a density of 2 × 104 cells/well in 96-well plates and cultured for 48 h as previously described (37) in culture medium supplemented with or without GDNF NP added to a final GDNF concentration of 100–200 ng/ml. Cell survival was assessed using the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay kit (Promega, Madison, WI) by measuring the quantity of aqueous, soluble formazan produced by the conversion of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium by dehydrogenases from viable cells.
Peroxisome proliferator-activated receptor-γ promoter activity assay.
Hep G2 cells were cotransfected with a previously described human peroxisome proliferator-activated receptor-γ (PPAR-γ) 1p3000 promoter/pGL3 luciferase reporter vector construct (4) and the phRL-TK Renilla luciferase vector (Promega) using Lipofectamine 2000 (Invitrogen). The cells were cultured for 48 h in normal medium followed by 24 h in medium supplemented with or without 0.3 mM oleic acid and GDNF (100 ng/ml). The cells were then lysed, and luciferase activity was determined using the Dual-Luciferase Reporter Assay System kit (Promega) reagents according to recommended procedure.
Western blotting.
Western blotting was performed as previously described (36, 38) using rabbit antibodies to phospho-p38 MAPK (Thr180/Tyr182) and PPAR-γ (C26H12) (Cell Signaling Technologies), GFRα1 (H-70) (Santa Cruz Biotechnology), p62/sequestosome 1 (SQSTM1) (Sigma-Aldrich), and CD36 (clone EPR6573, rabbit monoclonal) (EMD Millipore, Temecula, CA) diluted 1:1,000. Mouse monoclonal antibody to β-actin (A5441, clone AC-15) (Sigma-Aldrich) was diluted 1:5,000. Horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG (Cell Signaling Technologies) secondary antibodies were used at 1:2,000 dilution. A semiquantitative measurement of band density was performed using the Scion Image for Windows software (Scion).
Gene expression analysis.
Total RNA was isolated using the RNeasy Lipid Tissue and RNeasy Mini kits (Qiagen, Hilden, Germany) and first-strand cDNA synthesized using SuperScript VILO (Invitrogen, Carlsbad, CA). Real-time PCR reactions were set up using Fast SYBR Green Master Mix (Applied Biosystems, Foster City, CA), and thermal cycling was performed on a StepOnePlus Real-Time PCR System (Applied Biosystems). The sequences of the oligonucleotide primers used to assess the expression of the mouse PPAR-γ, PPAR-α, the PPARγ coactivator-1α (PGC-1α), CD36 (FAT), fatty acid synthase (Fasn), β3-adrenergic receptor (Adrb3), sterol regulatory element binding transcription factor 1 (Srebf1), fatty acid-binding protein (FABP4), and leptin genes, as well as the GAPDH and β2-microglobulin endogenous controls have been described before (38, 39). The sequences of the other primers used are presented in Table 1. The primers were designed such that at least one primer in each pair spanned an intron to prevent it from priming on genomic DNA. The inability of these primers to amplify genomic DNA was confirmed by PCR.
Table 1.
Sequences of oligonucleotide primers used
| Oligo Name | Oligo Sequence | Tm, °C | Product Size, bp | Gene Accession No. |
|---|---|---|---|---|
| Dgat2_FW | TCATCATCGTGGTGGGAGGT | 60.62 | 126 | NM_026384.3 |
| Dgat2_RV | TGGGAACCAGATCAGCTCCAT | 60.91 | ||
| Scd1_FW | GAAGTCCACGCTCGATCTCA | 59.55 | 87 | NM_009127.4 |
| Scd1_RV | TGG AGATCTCTTGGAGCATGTG | 59.83 | ||
| Srebf2_FW | AAGAAGGAGAGAGGCGGACA | 60.25 | 125 | NM_033218.1 |
| Srebf2_RV | GCCAGACTTGTGCATCTTGG | 59.48 | ||
| Cpt1a_FW | TGGCTTATCGTGGTGGTGGGTGT | 62.8 | 112 | NM_013495.2 |
| Cpt1a_RV | TTGACATGCGGCCAGTGGTGT | 62.2 | ||
| β2m_FW | ACCGGCCTGTATGCTATCCAGAAA | 63.80 | 124 | NM_009735.3 |
| β2m_RV | TTTCAATGTGAGGCGGGTGGAA | 62.73 | ||
| RNA18S5_FW | AACGGCTACCACATCCAAGGAA | 61.95 | 104 | NR_003286.2 (Human) |
| RNA18S5_RV | AGGGCCTCGAAAGAGTCCTGTATT | 63.20 | ||
| PPAR-γ_FW | TCAGTGGAGACCGCCCAGGTTT | 62.9 | 115 | NM_015869.4 (Human) |
| PPAR-γ_RV | AGCTGTGAGGACTCAGGGTGGT | 62.3 | ||
| PPARα _FW | AAGCTGTCACCACAGTAGCTTGGA | 60.3 | 188 | NM_001001928.2 (Human) |
| PPARα _RV | TCCTCGCCGATGGATTGCGAA | 61.4 | ||
| PGC1α_FW | AGCACGAGAGGCTGAAGAGGGA | 62.1 | 112 | NM_013261.3 (Human) |
| PGC1α _RV | ACACGGCGCTCTTCAATTGCCT | 62.0 | ||
| Srebf1_FW | TGCTGACCGACATCGAAGACATGC | 61.3 | 168 | NM_004176.4 (Human) |
| Srebf1_RV | AGCTCAATGTGGCAGGAGGTGGA | 62.7 | ||
| FASN_FW | ATCGTGGACGGAGGCATCAACC | 61.6 | 120 | NM_004104.4 (Human) |
| FASN_RV | CCATGCTGTAGCCCACGAGTGT | 61.6 |
Tm, melting temperature; Dgat2, diacylglycerol O-acyltransferase 2; Scd1, stearoyl-coenzyme A desaturase 1; Srebf, sterol regulatory element binding transcription factor; Cpt1a, carnitine palmitoyltransferase 1A; β2m, β2-microglobulin; PPAR, peroxisome proliferator-activated receptor; PGC1α, PPARγ coactivator-1α; FASN, fatty acid synthase; FW, upstream (sense) primer; RV, downstream (antisense) primer.
Statistical analysis.
Statistical analyses were conducted using the GraphPad Prism software version 3.00 for Windows (GraphPad Software, San Diego, CA). Data were tested for normality and subjected to unpaired t-test or one-way ANOVA with Tukey's posttest.
RESULTS
HFD-fed GDNF transgenic mice have normal liver histology and accelerated autophagic clearance.
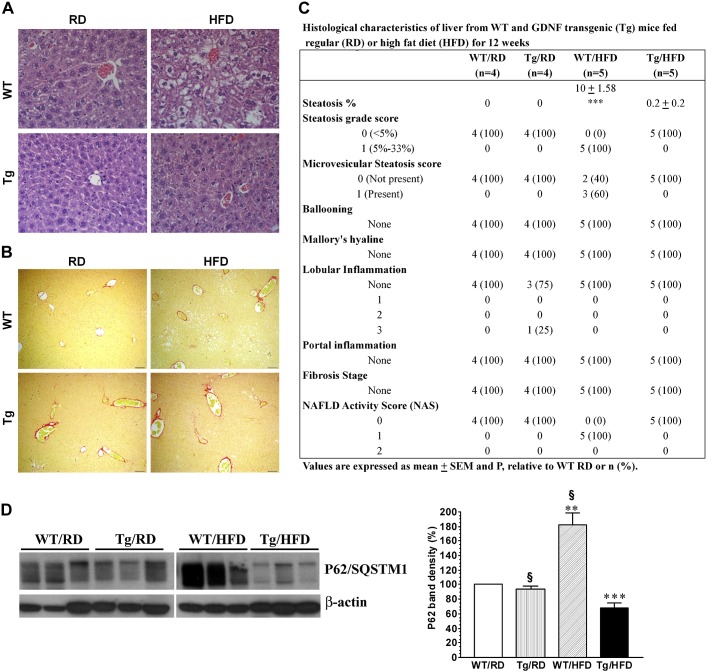
Liver tissue from GDNF Tg mice and WT littermates from our previous study (38) were used to further assess histological changes resulting from 12 wk of feeding on a HFD. As previously reported, all of the GDNF Tg mice overexpressed GDNF in various tissues, including the liver, were lean and insulin sensitive, and had normal serum alanine aminotransferase (ALT) and liver triglyceride levels while all of the HFD-fed WT mice were overweight and insulin resistant and had significantly elevated serum ALT and liver triglyceride levels. Liver sections were stained with H&E and Sirius Red stains (Fig. 1, A and B) and used to quantify the histological features present to understand to what extent GDNF was protective against HFD-induced fatty liver disease. Similar to liver from RD-fed WT and GDNF Tg mice, liver from the HFD-fed GDNF Tg mice showed no evidence of steatosis while liver from the HFD-fed WT mice had significant mixed steatosis (Fig. 1, A and C). No evidence of hepatocyte ballooning, Mallory's hyaline, inflammatory cell infiltration, and fibrosis was observed in liver from mice from all four treatment groups (Fig. 1, A–C).
Fig. 1.
Glial cell line-derived neurotrophic factor (GDNF) transgenic (Tg) mice are protected against high-fat diet (HFD)-induced hepatic steatosis. Representative photomicrographs of liver sections from regular diet (RD)- and HFD-fed wild-type (WT) mice and GDNF Tg littermates stained with hematoxylin and eosin stains (H&E, A) and Sirius Red stain (B). Scale bar, 50 μm. C: nonalcoholic fatty liver disease (NAFLD) histological feature scores of liver from RD- and HFD-fed WT mice and GDNF Tg littermates. D: Western blot analysis of P62/sequestosome 1 (SQSTM1) protein levels in liver from WT and GDNF Tg mice maintained on a RD or HFD and plot of P62/SQSTM1 band densities (relative to WT, RD). Plotted are means + SE from 3 separate experiments. **P < 0.01 and ***P < 0.001, relative to WT mice maintained on the RD; §P < 0.001, relative to GDNF Tg mice maintained on the HFD. n = 3 Mice/group.
P62/SQSTM1, a key autophagic protein, has been found to associate with Mallory's hyaline bodies in the liver of NASH patients (47), and excessive cellular accumulation of P62/SQSTM1 has been reported to be indicative of defective autophagic clearance, which is associated with cellular stress (25). We compared liver P62/SQSTM1 protein levels between the four groups of mice to see if GDNF Tg mice were protected against HFD-induced disruption in autophagy. P62/SQSTM1 levels were significantly decreased (32.06 ± 7.52%; P < 0.001) in liver from HFD-fed GDNF Tg mice and greatly increased (82.91 ± 21.22%; P < 0.01) in liver from HFD-fed WT mice relative to their levels in liver from RD-fed WT mice (Fig. 1D).
HFD-fed GDNF Tg mice have reduced liver expression of genes involved in regulating lipid transport and de novo lipogenesis.
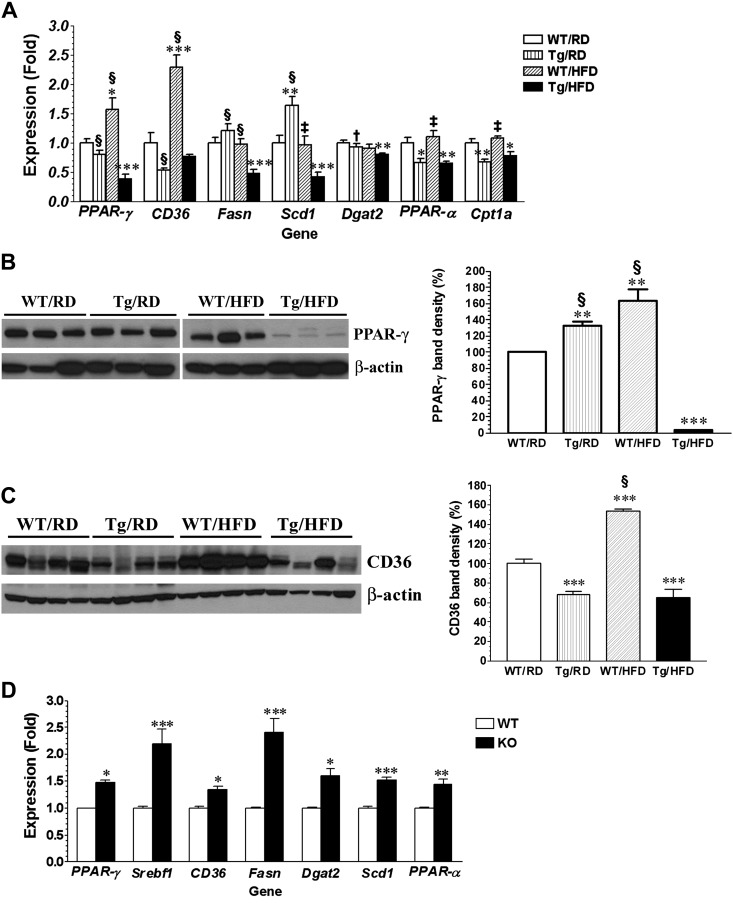
Triglycerides stored in hepatocytes come from the uptake of dietary fat, from the hydrolysis of white adipose tissue (WAT) fat, and from de novo lipogenesis (6). We assessed for changes in liver expression of genes coding for key proteins that regulate these processes to determine if their suppression could have contributed to the low triglyceride deposits seen in liver from GDNF Tg mice. Genes that showed significant differential expression between the four treatment groups are presented in Fig. 2A. The PPAR-γ gene codes for a transcription factor that enhances lipid accumulation in hepatocytes by inducing expression of genes coding for proteins involved in lipid uptake, lipid droplet formation, and de novo lipogenesis (20). Relative to its expression in the liver of RD-fed WT mice, the expression of the PPAR-γ gene was 61.51 ± 11.63% (P < 0.0001) lower in the liver of HFD-fed GDNF Tg mice and 57.27 ± 19.92% (P < 0.05) higher in the liver of HFD-fed WT mice (Fig. 2A). In line with the gene expression data, liver PPAR-γ protein levels were 96.2 + 0.52% (P < 0.0001) lower in HFD-fed GDNF Tg mice and 63.53 + 25.71% (P < 0.004) higher in HFD-fed WT mice compared with RD-fed WT mice (Fig. 2B). The CD36 (FAT) gene encodes a lipid transport protein that is induced by PPAR-γ. The liver expression of the CD36 gene was significantly increased (P < 0.001) in HFD-fed WT mice, while its expression in HFD-fed GDNF Tg mice was unchanged relative to RD-fed WT mice (Fig. 2A). CD36 protein levels were also significantly increased in liver from HFD-fed WT mice (P < 0.001 relative to RD-fed WT mice) but decreased in liver from HFD-fed GDNF Tg mice (P < 0001 relative to RD-fed and HFD-fed WT mice) (Fig. 2C). Fasn and stearoyl-CoA desaturase (Scd1) are key enzymes involved in the catalysis of de novo lipogenesis. Liver levels of mRNA encoding these enzymes were significantly lower in HFD-fed GDNF Tg mice relative to their levels in the other three groups of mice (Fig. 2A). Their levels in HFD-fed WT mice were, however, unchanged relative to the RD-fed WT mice (Fig. 2A). Diacylglycerol O-acyltransferase 2 (Dgat2) catalyzes the final step in the synthesis of triacylglycerides (TAG) and is a key determinant of the rate of de novo lipogenesis (53). The liver expression of the gene coding for this enzyme was also reduced significantly in HFD-fed GDNF Tg mice (P ≤ 0.05, relative both groups of RD-fed mice) but unchanged in HFD-fed WT mice (Fig. 2A). PPAR-α and carnitine palmitoyltransferase 1A (Cpt1a) genes code for proteins involved in lipid β-oxidation, and their expression is upregulated in NAFLD and animal models of NAFLD. The liver expression of these genes was significantly reduced in HFD-fed GDNF Tg mice relative to their expression in both groups of RD-fed mice and HFD-fed WT mice (Fig. 2A). The expression of these genes in the liver from HFD-fed WT was unchanged compared with their expression in the liver from RD-fed WT mice (Fig. 2A).
Fig. 2.
Analyses of gene expression and protein levels of steatosis-associated genes in liver from WT and GDNF Tg mice. A: PCR analyses of gene expression in liver from WT mice and GDNF Tg littermates fed a RD or HFD for 12 wk. Plotted are means + SE. *P < 0.05, **P < 0.01, and ***P < 0.001 relative to WT mice maintained on the RD; †P < 0.05, ‡P < 0.01, and §P < 0.001 relative to GDNF Tg mice maintained on the HFD. n = 5–7 Mice/group. Western blot analyses and plot of band densities (relative to WT mice fed RD) of peroxisome proliferator-activated receptor-γ (PPAR-γ, B) and CD36 protein levels (C) in liver from WT mice and GDNF Tg littermates fed a RD or HFD for 12 wk. Plotted are means + SE. **P < 0.01 and ***P < 0.001 relative to WT mice maintained on the RD; §P < 0.001 relative to GDNF Tg mice maintained on the HFD. n = 3–4 Mice/group. D: analyses of gene expression in liver from heterozygous GDNF family receptor α1 (GFRα1) knockout (GFRα1GFP/+) mice and WT controls. Plotted are means + SE. *P < 0.05, **P < 0.01, and ***P < 0.001 relative to WT controls.
To determine the effect of loss of GDNF signaling on the expression of genes associated with increased hepatic steatosis, we assessed liver gene expression in the GFRα1 KO mouse, which has a global knockdown of one allele of the gene coding for the GDNF-specific GFRα1 receptor. The liver expression of the PPAR-γ, CD36, Dgat2, Scd1, Srebf1, Fasn, and PPAR-α genes was significantly increased in liver from GFRα1 KO mice compared with liver from age-matched WT mice (Fig. 2D).
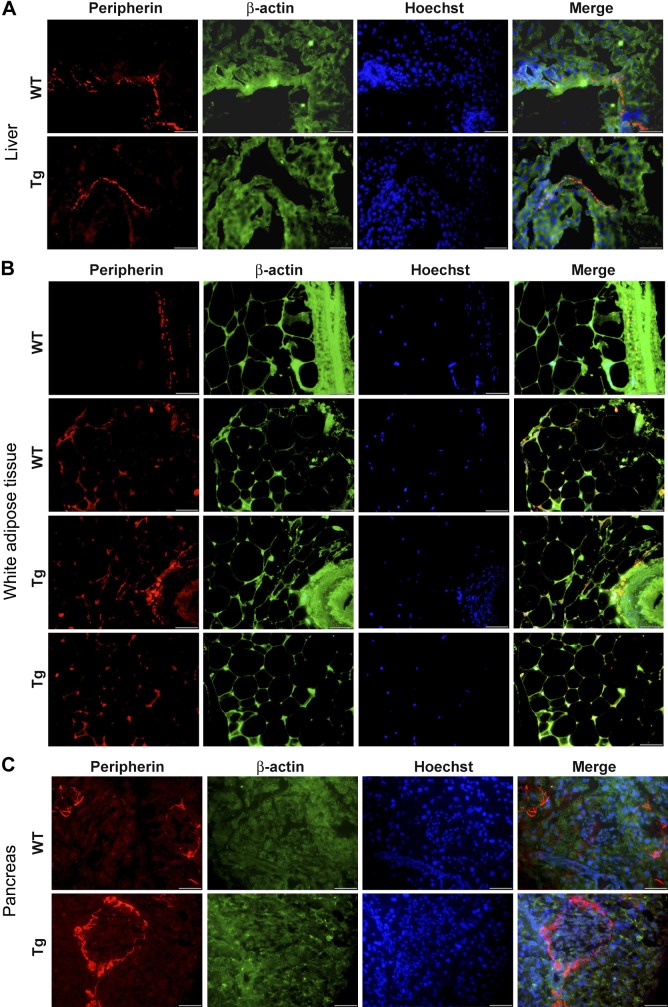
GDNF Tg mice have similar level of neuronal innervation of the liver, WAT, and pancreas as WT mice.
We investigated the possibility that the superior ability of GDNF Tg mice to resist HFD-induced hepatic steatosis, weight gain, and insulin resistance could be due to increased neuronal innervation in the liver, WAT, and pancreas driven by the overexpression of GDNF in these tissue. After tissue staining for the neuronal marker peripherin, we observed similar staining in both strains of mice in liver ductal tissue and no clear staining in the liver parenchyma (Fig. 3A). We also observed similar staining in the WAT and pancreatic islet and acinar tissue in both WT and GDNF Tg mice (Fig. 3, B and C).
Fig. 3.
Analyses of effects of GDNF overexpression on nervous innervation in the liver, white adipose tissue, and pancreas. Immunofluorescence staining for peripherin (red) and counterstaining for β-actin (green) with Hoechst nuclear staining (blue) in the liver (A), white adipose tissue (B), and pancreas (C) from RD-fed WT and GDNF Tg mice. Scale, 50 μm.
GDNF suppresses triglyceride accumulation and the expression of hepatic steatosis-linked genes in vitro.
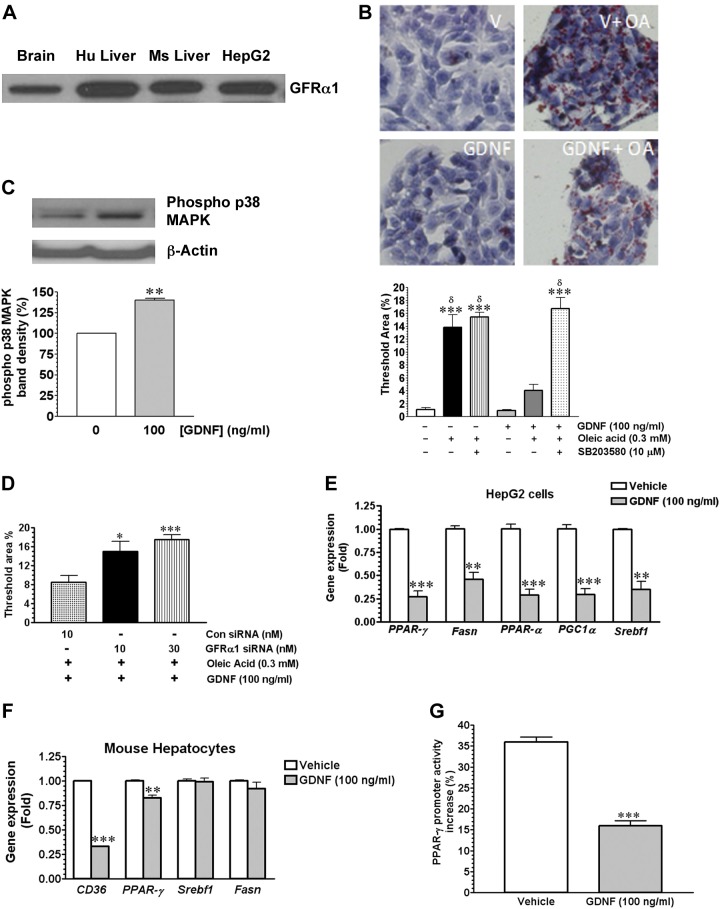
To confirm the in vivo observations, we performed in vitro studies using the Hep G2 cell line and isolated mouse hepatocytes. Hep G2 cells express the GDNF-specific GFRα1 receptor, which is also expressed abundantly in the mouse and human liver (Fig. 4A). We first assessed the effects of GDNF on triglyceride accumulation in the Hep G2 cell line and whether or not the MAPK signaling pathway played any role in the observed effects of GDNF on triglyceride accumulation. We cultured Hep G2 cells for 24 h in medium supplemented with or without 0.3 mM oleic acid in the presence or absence of GDNF (100 ng/ml) and the p38 MAPK (p38 MAPK) inhibitor SB-203580 (10 μM) and assessed triglyceride content by Oil Red-O staining. Hep G2 cells cultured in medium supplemented with oleic acid and GDNF had lower triglyceride content than cells cultured in the absence of GDNF (Fig. 4B). The inhibitory effects of GDNF on triglyceride accumulation, however, were abolished in the presence of SB-203580. The ability of GDNF to activate p38 MAPK signaling in Hep G2 cells was confirmed by assessing p38 MAPK phosphorylation by Western blotting (Fig. 4C). To preclude the possibility that the observed effects were nonspecific and not GDNF-mediated we knocked down the GFRα1 receptor using a GFRα1 siRNA and assessed triglyceride accumulation. Following the knock down of the GFRα1 receptor, the ability of GDNF to inhibit triglyceride accumulation in Hep G2 cells was abolished (Fig. 4D).
Fig. 4.
GDNF suppresses triglyceride accumulation in Hep G2 cells. A: Western blot analysis of GFRα1 receptor expression in human and mouse liver tissue and the Hep G2 cell line. B: Oil Red-O staining in Hep G2 cells after culture in medium alone (V) or medium supplemented with 0.3 mM oleic acid (OA) with or without GDNF (100 ng/ml) and the p38 mitogen-activated protein kinase (MAPK) inhibitor SB-203580 (SB). Scale bar, 50 μm. Histogram shows comparison of Oil Red-O staining intensity between the treatments. Plotted are means + SE from 3 separate experiments. ***P < 0.001 relative to vehicle (V); δP < 0.001 relative to GDNF without OA and SB. C: Western blot analysis of effect of GDNF on P38 MAPK phosphorylation in Hep G2 cells cultured in medium alone (Vehicle) or medium supplemented with GDNF (100 ng/ml) and densitometric analysis of band density. Plotted are means + SE. **P < 0.01 relative to vehicle. D: effect of knockdown of the GDNF-specific receptor GFRα1 by a GFRα1 siRNA on Oil Red-O staining intensity in Hep G2 cells cultured in medium supplemented with GDNF (100 ng/ml) and oleic acid (0.3 mM). Plotted are means + SE. ***P < 0.001 and *P < 0.05 relative to control scrambled (Con) siRNA. Analyses of gene expression in Hep G2 cells (E) and primary mouse hepatocytes (F) cultured, respectively, for 48 and 24 h in medium supplemented with 0.3 mM oleic acid with or without GDNF (100 ng/ml). Plotted are means + SE. ***P < 0.001, **P < 0.01, and *P < 0.05 relative to vehicle. G: effects of GDNF (100 ng/ml) on oleic acid-induced PPAR-γ gene promoter activity in Hep G2 cells transfected with a pGL3-PPAR-γ gene promoter. Plotted are means + SE. ***P < 0.001 relative to vehicle.
We then assessed the effects of GDNF on the expression of genes associated with increased hepatic steatosis. The expression of mRNA for Fasn, PGC1α, PPAR-γ, PPAR-α, and Srebf1 in Hep G2, and of PPAR-γ and CD36 in primary mouse hepatocytes was reduced significantly in cells cultured, respectively, for 48 and 24 h in the presence of GDNF compared with cells cultured in vehicle (Fig. 4, E and F).
GDNF suppresses the promoter activity of the PPAR-γ gene.
After observing GDNF-induced suppression of PPAR-γ expression both in vivo and in vitro, we wanted to know if GDNF can directly regulate promoter activity of the PPAR-γ gene. We transfected Hep G2 cells with a pGL3 luciferase reporter vector carrying the human PPAR-γ gene promoter and measured oleic acid-induced luciferase activity. We observed a 36.0 ± 1.16% increase in PPAR-γ gene promoter activity when transfected Hep G2 cells were stimulated with oleic acid alone for 24 h (Fig. 4F). However, when the transfected cells were stimulated with oleic acid in the presence of GDNF we saw a significant (55.56 ± 4.54%, P < 0.001, relative to vehicle) reduction in promoter activity (Fig. 4G).
GDNF-loaded nanoparticles accelerate recovery of mice with short-term HFD-induced hepatic steatosis.
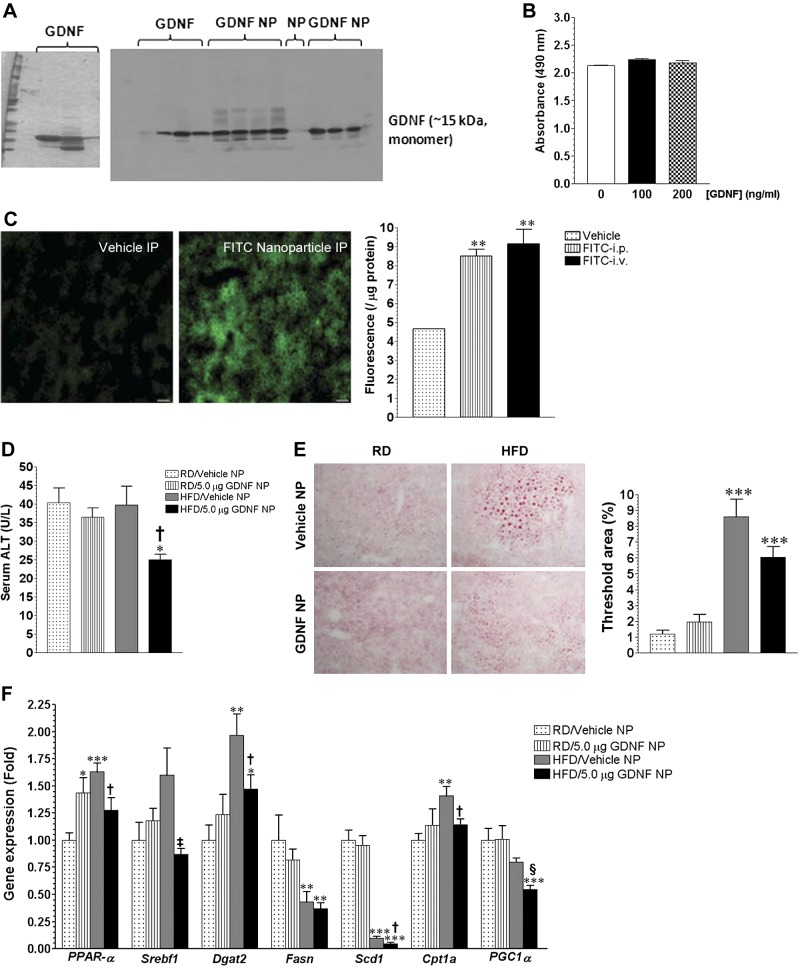
We investigated if GDNF-loaded, PVA-coated PLA nanoparticles (GDNF NP) could be used therapeutically to treat mice with HFD-induced hepatic steatosis. We loaded recombinant rat GDNF in PVA-coated PLA nanoparticles and confirmed the purity and loading of the protein by Western blotting (Fig. 5A). We then assessed the potential cytotoxicity of the synthesized GDNF NP by exposing β-TC-6 cells to GDNF NP for 48 h and comparing cell survival with that of cells cultured in medium alone. Cells cultured in medium supplemented with GDNF NP showed the same survival rate as cells cultured in medium without GDNF (Fig. 5B). Because the liver is a major site for first-pass metabolism of intravenously, intraperitoneally, and orally administered drugs, we injected a group of mice with dextran-FITC-loaded-PVA coated PLA nanoparticles and assessed fluorescence levels in the liver to determine which between the intravenous and intraperitoneal routes of nanoparticle administration would target the most nanoparticles to the liver. Our analysis showed that there was significant accumulation of the nanoparticles in the liver within 1 h of both injections with the intraperitoneal and intravenous routes of administration showing nearly equal levels of fluorescence (Fig. 5C).
Fig. 5.
GDNF-loaded nanoparticles improve recovery of mice with short-term HFD-induced hepatic steatosis. A: Coomassie Blue-stained SDS-PAGE showing purified recombinant rat GDNF and Western blot showing presence of GDNF in GDNF-loaded nanoparticles (GDNF NP). B: analysis of effect of GDNF NP on β-TC-6 cell survival. C: assessment of green fluorescence and comparison of fluorescence intensity in mice liver 1 h after ip or iv injection with dextran-FITC-loaded nanoparticles or vehicle. **P < 0.01. D: serum alanine aminotransferase (ALT) levels of WT mice maintained for 6 wk on a RD or HFD followed by 14 days on RD with daily injections of GDNF NP or Vehicle NP. Plotted are means + SE. *P < 0.05 relative to mice maintained on the RD and injected with vehicle; †P < 0.05 relative to mice maintained on the HFD and injected with Vehicle NP. n = 4 Mice/group. E: Oil Red-O-stained liver sections from mice maintained for 6 wk on a RD or HFD followed by 14 days on RD with daily injections of GDNF NP or Vehicle NP and comparison of Oil Red-O staining area. Plotted are means + SE. ***P < 0.001 relative to mice maintained on the RD and injected with Vehicle NP. F: analyses of gene expression in liver from WT mice maintained for 6 wk on a RD or HFD followed by 14 days on RD with daily injections of GDNF NP or Vehicle NP. Plotted are means + SE. ***P < 0.001, **P < 0.01, and *P < 0.05 relative to mice maintained on the RD and injected with Vehicle NP; †P < 0.05, ‡P < 0.01, and §P < 0.001 relative to mice maintained on the HFD and injected daily with Vehicle NP.
We then assessed the efficacy of GDNF NP in treating mice with short-term hepatic steatosis. To this end, male CF-1 mice were fed RD or HFD for 6 wk followed by 14 days of feeding on RD with daily intraperitoneal injections of GDNF-free nanoparticles (Vehicle NP) or GDNF NP delivering 5.0 μg of GDNF daily. This dose of GDNF was calculated to deliver the same amount of GDNF used in vitro (100 ng/ml). Assessment of serum ALT levels showed that mice fed the HFD and injected with GDNF NP had significantly lower levels than mice from the other groups (Fig. 5D). Oil Red-O staining of liver sections from these mice also showed that mice fed the HFD and injected with GDNF NP had less hepatic steatosis than HFD-fed mice injected with GDNF-free NP (Fig. 5E). Figure 5F shows results of gene expression analyses of some of the genes that were found to be differentially expressed in liver from these mice. The analyses revealed no significant changes in the expression of PPAR-γ and CD36 (data not shown) and significant reductions in the expression of Scd1 and Fasn in both groups of HFD-fed mice (Fig. 5F). They also showed a larger increase in Dgat2 gene expression in the liver of HFD-fed, Vehicle NP-injected mice than in the liver of HFD-fed, GDNF NP-injected mice. Liver PPAR-α and Cpt1a gene expression, on the other hand, was significantly increased in Vehicle NP-injected HFD-fed mice but unchanged in GDNF NP-injected HFD-fed mice, while Srebf1 gene expression was unchanged in both groups of HFD-fed mice.
GDNF-loaded nanoparticles suppress the expression of hepatic steatosis-linked genes and protect against fibrosis in mice with longstanding hepatic steatosis.
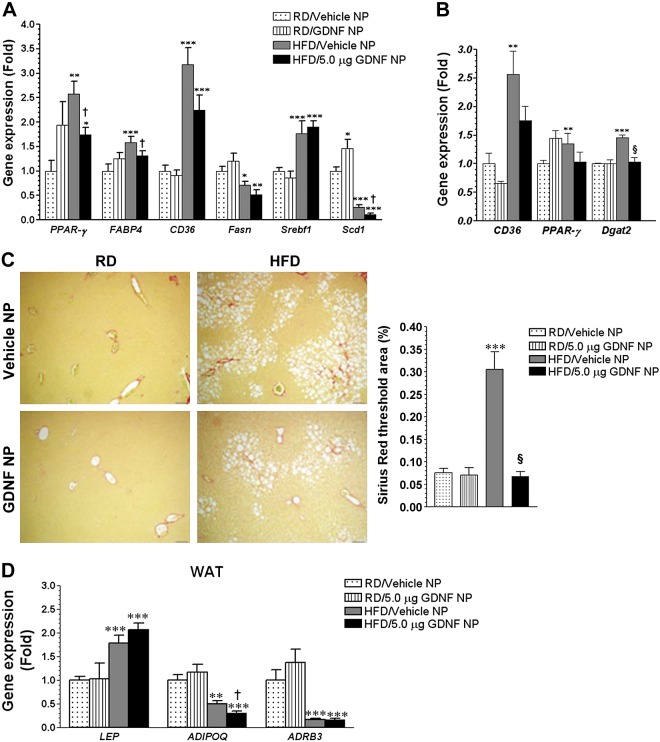
We also investigated the efficacy of GDNF NP in treating mice with longstanding HFD-induced hepatic steatosis. One cohort of CF-1 mice was maintained on the RD or HFD for 16 wk and then daily injected with Vehicle NP or GDNF NP during the last 5 wk. Another cohort of mice was maintained on the RD or HFD for 19 wk and then daily injected with Vehicle NP or GDNF NP during the last 13 wk. Analyses of liver gene expression in the first cohort of mice injected with Vehicle or GDNF NP during the last 5 wk revealed significant increases in PPAR-γ, CD36, and Srebf1 gene expression in Vehicle NP and GDNF NP-injected, HFD-fed mice, with PPAR-γ gene expression levels in the GDNF NP-injected group being significantly lower than those in the Vehicle NP-injected group (Fig. 6A). Liver FABP4 gene expression, on the other hand, was significantly increased in Vehicle NP-injected HFD-fed mice but unchanged in GDNF NP-injected HFD-fed mice (Fig. 6A). Liver Fasn and Scd1 gene expression was significantly reduced in both groups of HFD-fed mice, with Scd1 levels in the GDNF NP-injected group being significantly lower than those in the Vehicle NP-injected group (Fig. 6A). Analyses of gene expression in liver from the second cohort of mice that had been fed the RD and HFD for 19 wk and injected with Vehicle or GDNF NP for 13 wk revealed significant increases in PPARγ, CD36, and Dgat2 gene expression in liver from Vehicle NP-injected, HFD-fed mice but no significant changes in liver from GDNF NP-injected, HFD-fed mice (Fig. 6B). We also assessed for fibrosis and inflammatory cell infiltration in liver from these mice. Both groups of HFD-fed mice had mild hepatic fibrosis; however, Vehicle NP-injected, HFD-fed mice had 4.5-fold (P < 0.001) higher fibrosis than GDNF NP-injected, HFD-fed mice (Fig. 6C). No inflammatory cell infiltration was observed in liver from both groups of RD-fed, NP-injected mice (data not shown). Several inflammatory foci comprising mainly of lymphocytic cells were, however, observed in liver from all four Vehicle NP-injected, HFD-fed mice (lobular inflammation, with one also showing evidence of portal inflammation) and three out of four GDNF NP-injected, HFD-fed mice (lobular inflammation only) (data not shown). To assess for potential off-target effects of the nanoparticles, we analyzed the expression of key obesity-associated genes in the WAT. The expression of the leptin (LEP) gene was equally significantly increased in the WAT of both groups of mice maintained on the HFD while the expression of the adiponectin (ADIPOQ) and ADRB3 genes was significantly reduced (Fig. 6D).
Fig. 6.
GDNF-loaded nanoparticles suppress the expression of steatosis-associated genes in mice with longstanding hepatic steatosis. A: assessment of gene expression in liver from WT mice fed RD or HFD for 16 wk and daily injected with Vehicle NP or GDNF NP during the final 5 wk. Plotted are means + SE. ***P < 0.001, **P < 0.01, and *P < 0.05 relative to mice fed RD and injected with Vehicle NP; †P < 0.05 relative to Vehicle NP-injected, HFD-fed mice. B: analysis of gene expression in liver from WT mice maintained on a RD or HFD for 19 wk and daily injected with Vehicle NP or GDNF NP during the last 13 wk of the study. Plotted are means + SE. ***P < 0.001 and **P < 0.01 relative to mice maintained on the RD and injected with Vehicle NP; §P < 0.001 relative to mice maintained on the HFD and injected with Vehicle NP. C: Sirius Red-stained liver sections from WT mice fed the RD or HFD for 19 wk and daily injected with Vehicle NP or GDNF NP during the last 13 wk of the study, and comparison of staining area. Scale, 50 μm. Plotted are means + SE. ***P < 0.001 relative to mice maintained on the RD and injected daily with Vehicle NP; §P < 0.001 relative to mice maintained on the HFD and injected with Vehicle NP. D: analysis of gene expression in white adipose tissue (WAT) from mice maintained on a RD or HFD for 19 wk and daily injected with Vehicle NP or GDNF NP during the last 13 wk of the study. Plotted are means + SE. ***P < 0.001 and **P < 0.01 relative to mice maintained on the RD and injected with Vehicle NP; †P < 0.05 relative to mice maintained on the HFD and injected with Vehicle NP.
DISCUSSION
The goal of this study was to understand how GDNF protects against HFD-induced hepatic steatosis and to evaluate the therapeutic value of GDNF-loaded nanoparticles for the treatment of HFD-induced hepatic steatosis.
Data from the present study revealed an important new role for GDNF in liver metabolism and the potential for the use of GDNF in protecting against and in the treatment of NAFLD. GDNF protected against HFD-induced hepatic steatosis in transgenic mice overexpressing GDNF under the control of the glial fibrillary acidic protein promoter and in vitro prevented fatty acid uptake and triglyceride accumulation in hepatocytes. When administered in vivo through GDNF-loaded nanoparticles, GDNF accelerated recovery in mice with short-term hepatic steatosis and attenuated the disease severity in mice with long-term HFD-induced hepatic steatosis. GDNF-loaded nanoparticles were protective against HFD-induced liver pathology without inducing hepatotoxicy. The effects of the nanoparticles were specific to the liver, since no effects on weight gain or gene expression in other tissues, including the WAT, were observed in mice injected with GDNF NP.
Our previous studies have shown that GDNF overexpression increases β-cell mass and improves insulin sensitivity in mice and protects against HFD-induced obesity through increased energy expenditure and β-adrenergic receptor-mediated lipolysis in brown adipose tissue and WAT (37, 38). While it is clear that the later processes help to reduce circulating triglyceride levels and adipose tissue fat deposits in GDNF Tg mice, data from the present study also indicate an important role for GDNF-mediated liver-specific gene expression changes in the prevention of hepatic steatosis in mice. These effects were, however, not due to increased neuronal innervation in the liver.
In this study we showed for the first time that hepatocytes express the GDNF-specific GFRα1 receptor and that GDNF is able to act directly on these cells to suppress the accumulation of fat. Through in vivo and in vitro experiments, we showed that GDNF acts to downregulate the expression in hepatocytes of genes coding for transcription factors, including PPAR-γ, PPAR-α, and Srebf1, and of their target genes, including CD36, Fasn, Scd1, Dgat2, and Cpt1a, that code for proteins that regulate lipid uptake, transport, triglyceride synthesis through de novo lipogenesis, and lipid β-oxidation. We also showed an ability of GDNF to act on hepatocytes to inhibit the activity of the PPAR-γ gene promoter. In addition, we showed an ability of GDNF to prevent fatty acid uptake and triglyceride accumulation by activating the p38 MAPK signaling pathway that has previously been shown to be inhibitory to hepatic lipogenesis (54).
The liver is a key organ in the maintenance of lipid homeostasis. Storage of hepatic triglyceride requires both free fatty acids (FFA) and glycerol and critically depends on the hepatocellular FFA pool size, which results from the balance between FFA formation (circulating FFA, de novo lipogenesis) and lipid usage or disposal (through β-oxidation and export) (45). Increased lipolysis, mitochondrial oxidative metabolism, and de novo lipogenesis are hallmarks of NAFLD (6, 13, 14, 48). The expression in the liver of genes that regulate these processes is increased in NAFLD and experimental models of NAFLD while the absence of these factors is protective against hepatic steatosis (15, 19, 22, 26, 34, 41, 44). In mammals, fatty acid synthesis is catalyzed by acetyl-CoA carboxylase 1 (ACC1) and fatty acid synthase. SREBP-1c induces the expression of fatty acid synthase and ACC1 (11). Studies have shown that genes encoding lipogenic enzymes are elevated in livers of ob/ob mice, and the transcription factor SREBP-1c was shown to contribute to high rates of lipogenesis in the livers of these mice (46). Mice treated with an antisense oligonucleotide to SREBP-1c showed a reduction in proteins involved in lipogenesis in the liver, including ACC, Fasn, and Scd1. They also had significant reduction in hepatic steatosis (16). Nuclear receptors such as the PPARs also play an important role in the pathophysiology of hepatic steatosis (30). In contrast to healthy livers, upregulation of PPAR-γ expression is a general property of steatotic livers (2, 43, 52, 55). Consistent with these findings, hepatic PPAR-γ expression has been linked to exacerbated steatosis by mechanisms involving activation of lipogenic genes and de novo lipogenesis and increased hepatic triglyceride concentrations (21). Mice with hepatocyte-specific PPAR-γ knockout have decreased hepatic lipid accumulation (33, 35). In our study, liver levels of PPAR-γ and its downstream target CD36 were significantly decreased both at the mRNA and protein level in HFD-fed GDNF Tg mice. We, therefore, believe that GDNF-mediated suppression of lipid uptake and of de novo lipogenesis in the liver combined with increased lipolysis and β-oxidation in brown adipose tissue and skeletal muscle served to reduce triglyceride levels in the liver of GDNF transgenic mice.
We observed significant reductions in liver P62/SQSTM1 levels in GDNF Tg mice fed a HFD, which was indicative of increased autophagic flux. P62/SQSTM1 is involved in the packaging of cellular waste for destruction in lysosomes through autophagy and is a major component of the Mallory's hyaline seen in liver of patients with advanced NAFLD (47). Autophagy is beneficial for protecting against fatty acid-induced hepatocyte death, and disruption of autophagy is associated with increased cellular stress and high P62/SQSTM1 levels in the liver (17, 18, 25). Hence, we believe that GDNF accelerates autophagic clearance in hepatocytes and thus provides protection against cellular stress and hepatocytes cell death.
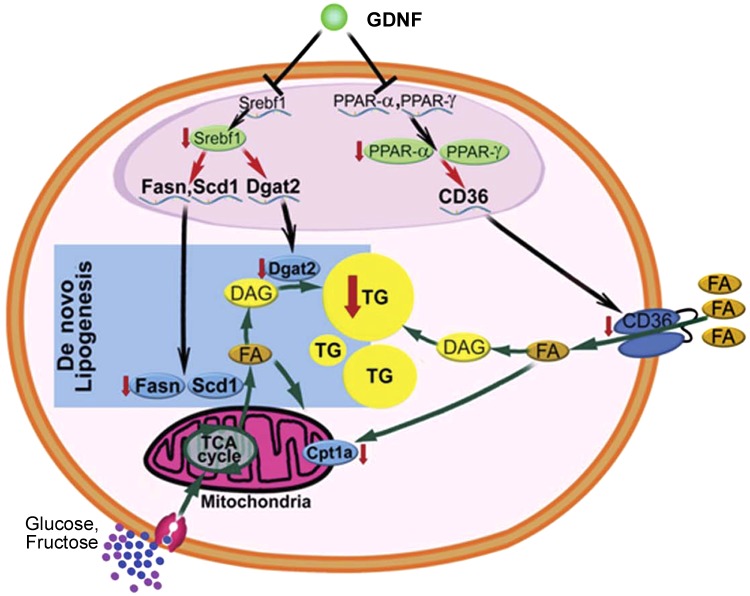
In conclusion, our data suggest that GDNF has the ability to inhibit hepatic steatosis by suppressing lipogenesis and lipid uptake in the liver and by preserving beneficial signaling pathways, including autophagy. Thus, we propose that GDNF acts primarily by targeting key transcription factors, including PPAR-γ and PPAR-α, which play an important regulatory role in lipid uptake in the liver and lipid synthesis (Fig. 7). Future studies may aim to investigate how GDNF regulates the expression levels of these genes by looking at the possible involvement of a select group of micro-RNAs.
Fig. 7.
Mechanism of GDNF prevention of hepatic steatosis. GDNF signaling in hepatocytes through its receptors supressess gene expression and protein levels of transcription factors, including PPAR-γ, PPAR-α, and Srebf1. This leads to reductions in gene expression and protein levels of their targets, which include lipid membrane transporters such as CD36 and carnitine palmitoyl-transferase 1a (Cpt1a) and enzymes that catalyze triglyceride synthesis during de novo lipogenesis such as fatty acid synthase (Fasn), stearoyl-coenzyme A desaturase 1 (Scd1), and diacylglycerol O-acyltransferase 2 (Dgat2). Green arrows indicate pathways that increase or decrease intracellular fatty acid and triglyceride levels in hepatocytes. Black arrows indicate gene transcriptional processes. Processes that are downregulated by GDNF are shown by a red arrow facing downward. DAG, diacylglycerol; FA, fatty acid.
GRANTS
This study was supported by the following grants: National Institute of Diabetes and Digestive and Kidney Diseases Grants NIH-RO1 DK-080684 to S. Srinivasan, DK-062092 to F. Anania, DK-097192 to H. Laroui, and VA-MERIT award to S. Srinivasan, FAA and DM.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.M.M., H.L., D.M., F.A.A., and S.S. conception and design of research; S.M.M., S.P., B.G.N., N.T., and H.L. performed experiments; S.M.M., S.P., and A.B.F. analyzed data; S.M.M., A.B.F., S.J., D.M., F.A.A., and S.S. interpreted results of experiments; S.M.M. prepared figures; S.M.M. drafted manuscript; S.M.M., B.G.N., A.B.F., S.J., H.L., D.M., F.A.A., and S.S. edited and revised manuscript; S.M.M., S.P., B.G.N., N.T., A.B.F., S.J., H.L., D.M., F.A.A., and S.S. approved final version of manuscript.
REFERENCES
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 346: 1221–1231, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Bedoucha M, Atzpodien E, Boelsterli UA. Diabetic KKAy mice exhibit increased hepatic PPARgamma1 gene expression and develop hepatic steatosis upon chronic treatment with antidiabetic thiazolidinediones. J Hepatol 35: 17–23, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 59: 713–723, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanquicett C, Kang BY, Ritzenthaler JD, Jones DP, Hart CM. Oxidative stress modulates PPAR gamma in vascular endothelial cells. Free Radic Biol Med 48: 1618–1625, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The Diagnosis and Management of Non-alcoholic Fatty Liver Disease: Practice Guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 142: 1592–1609, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab 22: 353–363, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science 332: 1519–1523, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creedon DJ, Tansey MG, Baloh RH, Osborne PA, Lampe PA, Fahrner TJ, Heuckeroth RO, Milbrandt J, Johnson EM Jr. Neurturin shares receptors and signal transduction pathways with glial cell line-derived neurotrophic factor in sympathetic neurons. Proc Natl Acad Sci USA 94: 7018–7023, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology 54: 133–144, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 114: 842–845, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Dentin R, Girard J, Postic C. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie 87: 81–86, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Dentin R, Pégorier JP, Benhamed F, Foufelle F, Ferré P, Fauveau V, Magnuson MA, Girard J, Postic C. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J Biol Chem 279: 20314–20326, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab 29: 478–485, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115: 1343–1351, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorn C, Riener MO, Kirovski G, Saugspier M, Steib K, Weiss TS, Gabele E, Kristiansen G, Hartmann A, Hellerbrand C. Expression of fatty acid synthase in nonalcoholic fatty liver disease. Int J Clin Exp Pathol 3: 505–514, 2010. [PMC free article] [PubMed] [Google Scholar]
- 16.Frederico MJ, Vitto MF, Cesconetto PA, Engelmann J, De Souza DR, Luz G, Pinho RA, Ropelle ER, Cintra DE, De Souza CT. Short-term inhibition of SREBP-1c expression reverses diet-induced non-alcoholic fatty liver disease in mice. Scand J Gastroenterol 46: 1381–1388, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, Lin X, Watkins SM, Ivanov AR, Hotamisligil GS. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 473: 528–531, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuo Y, Yamashina S, Sonoue H, Arakawa A, Nakadera E, Aoyama T, Uchiyama A, Kon K, Ikejima K, Watanabe S. Abnormality of autophagic function and cathepsin expression in the liver from patients with non-alcoholic fatty liver disease. Hepatol Res 44: 1026–1036, 2014. [DOI] [PubMed] [Google Scholar]
- 19.García-Monzón C, Lo Iacono O, Crespo J, Romero-Gómez M, García-Samaniego J, Fernández-Bermejo M, Domínguez-Díez A, Rodríguez de Cía J, Sáez A, Porrero JL, Vargas-Castrillón J, Chávez-Jiménez E, Soto-Fernández S, Díaz A, Gallego-Durán R, Madejón A, Miquilena-Colina ME. Increased soluble CD36 is linked to advanced steatosis in nonalcoholic fatty liver disease. Eur J Clin Invest 44: 65–73, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, Vinson C, Gonzalez FJ, Reitman ML. Liver peroxisome proliferator-activated receptor γ contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem 278: 34268–34276, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Hasenfuss SC, Bakiri L, Thomsen MK, Williams EG, Auwerx J, Wagner EF. Regulation of steatohepatitis and PPARgamma signaling by distinct AP-1 dimers. Cell Metab 19: 84–95, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue M, Ohtake T, Motomura W, Takahashi N, Hosoki Y, Miyoshi S, Suzuki Y, Saito H, Kohgo Y, Okumura T. Increased expression of PPARγ in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun 336: 215–222, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science 209: 497–499, 1980. [DOI] [PubMed] [Google Scholar]
- 25.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura Si Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 12: 213–223, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Kotronen A, Seppanen-Laakso T, Westerbacka J, Kiviluoto T, Arola J, Ruskeepaa AL, Oresic M, Yki-Jarvinen H. Hepatic stearoyl-CoA desaturase (SCD)-1 activity and diacylglycerol but not ceramide concentrations are increased in the nonalcoholic human fatty liver. Diabetes 58: 203–208, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laroui H, Dalmasso G, Nguyen HT, Yan Y, Sitaraman SV, Merlin D. Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. Gastroenterology 138: 843–853, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Laroui H, Geem D, Xiao B, Viennois E, Rakhya P, Denning T, Merlin D. Targeting Intestinal Inflammation With CD98 siRNA/PEI-loaded Nanoparticles. Mol Ther 22: 69–80, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 10: 686–690, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Velazquez JA, Carrillo-Cordova LD, Chavez-Tapia NC, Uribe M, Mendez-Sanchez N. Nuclear receptors in nonalcoholic fatty liver disease. J Lipids 2012: 139875, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manfredsson FP, Tumer N, Erdos B, Landa T, Broxson CS, Sullivan LF, Rising AC, Foust KD, Zhang Y, Muzyczka N, Gorbatyuk OS, Scarpace PJ, Mandel RJ. Nigrostriatal rAAV-mediated GDNF overexpression induces robust weight loss in a rat model of age-related obesity. Mol Ther 17: 980–991, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masuoka HC, Chalasani N. Nonalcoholic fatty liver disease: an emerging threat to obese and diabetic individuals. Ann NY Acad Sci 1281: 106–122, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsusue K, Haluzik M, Lambert G, Yim SH, Gavrilova O, Ward JM, Brewer B Jr, Reitman ML, Gonzalez FJ. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest 111: 737–747, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miquilena-Colina ME, Lima-Cabello E, Sánchez-Campos S, García-Mediavilla MV, Fernández-Bermejo M, Lozano-Rodríguez T, Vargas-Castrillón J, Buqué X, Ochoa B, Aspichueta P, González-Gallego J, García-Monzón C. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut 60: 1394–1402, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Moran-Salvador E, Lopez-Parra M, Garcia-Alonso V, Titos E, Martinez-Clemente M, Gonzalez-Periz A, Lopez-Vicario C, Barak Y, Arroyo V, Claria J. Role for PPARgamma in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J 25: 2538–2550, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Mwangi S, Anitha M, Fu H, Sitaraman SV, Srinivasan S. Glial cell line-derived neurotrophic factor-mediated enteric neuronal survival involves glycogen synthase kinase-3beta phosphorylation and coupling with 14-3-3. Neuroscience 143: 241–251, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Mwangi S, Anitha M, Mallikarjun C, Ding X, Hara M, Parsadanian A, Larsen CP, Thule P, Sitaraman SV, Anania F, Srinivasan S. Glial cell line-derived neurotrophic factor increases beta-cell mass and improves glucose tolerance. Gastroenterology 134: 727–737, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mwangi SM, Nezami BG, Obukwelu B, Anitha M, Marri S, Fu P, Epperson MF, Le NA, Shanmugam M, Sitaraman SV, Tseng YH, Anania FA, Srinivasan S. Glial cell line-derived neurotrophic factor protects against high fat diet-induced obesity. Am J Physiol Gastrointest Liver Physiol 306: G515–G525, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mwangi SM, Usta Y, Raja SM, Anitha M, Chandrasekharan B, Parsadanian A, Sitaraman SV, Srinivasan S. Glial cell line-derived neurotrophic factor enhances neurogenin3 gene expression and beta-cell proliferation in the developing mouse pancreas. Am J Physiol Gastrointest Liver Physiol 299: G283–G292, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paratcha G, Ledda F. GDNF and GFRα: a versatile molecular complex for developing neurons. Trends Neurosci 31: 384–391, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Patsouris D, Reddy JK, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Endocrinology 147: 1508–1516, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510: 84–91, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahimian R, Masih-Khan E, Lo M, van Breemen C, McManus BM, Dube GP. Hepatic over-expression of peroxisome proliferator activated receptor gamma2 in the ob/ob mouse model of non-insulin dependent diabetes mellitus. Mol Cell Biochem 224: 29–37, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Memon RA, Tecott LH, Nonogaki Katsunori, Beigneux Anne, Moser Arthur, Carl Grunfeld, Feingold Kenneth R. Up-Regulation of peroxisome proliferator-activated receptors (PPAR-α) and PPAR-γ messenger ribonucleic acid expression in the liver in murine obesity: troglitazone induces expression of PPAR-γ-responsive adipose tissue-specific genes in the liver of obese diabetic mice. Endocrinology 141: 4021–4031, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Roden M. Mechanisms of Disease: hepatic steatosis in type 2 diabetes–pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab 2: 335–348, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem 274: 30028–30032, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Stumptner C, Fuchsbichler A, Heid H, Zatloukal K, Denk H. Mallory body-A disease-associated type of sequestosome. Hepatology 35: 1053–1062, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Sunny Nishanth E, Parks Elizabeth J, Browning Jeffrey D, Burgess Shawn C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab 14: 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torres DM, Williams CD, Harrison SA. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 10: 837–858, 2012. [DOI] [PubMed] [Google Scholar]
- 50.Tumer N, Scarpace PJ, Dogan MD, Broxson CS, Matheny M, Yurek DM, Peden CS, Burger C, Muzyczka N, Mandel RJ. Hypothalamic rAAV-mediated GDNF gene delivery ameliorates age-related obesity. Neurobiol Aging 27: 459–470, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Uesaka T, Jain S, Yonemura S, Uchiyama Y, Milbrandt J, Enomoto H. Conditional ablation of GFRα1 in postmigratory enteric neurons triggers unconventional neuronal death in the colon and causes a Hirschsprung's disease phenotype. Development 134: 2171–2181, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Vidal-Puig A, Jimenez-Linan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest 97: 2553–2561, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wurie HR, Buckett L, Zammit VA. Diacylglycerol acyltransferase 2 acts upstream of diacylglycerol acyltransferase 1 and utilizes nascent diglycerides and de novo synthesized fatty acids in HepG2 cells. FEBS J 279: 3033–3047, 2012. [DOI] [PubMed] [Google Scholar]
- 54.Xiong Y, Collins QF, An J, Lupo E, Liu HY, Liu D, Robidoux J, Liu Z, Cao W. p38 Mitogen-activated Protein Kinase Plays an Inhibitory Role in Hepatic Lipogenesis. J Biol Chem 282: 4975–4982, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV, Rao MS, Gonzalez FJ, Reddy JK. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J Biol Chem 278: 498–505, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Zhao Z, Alam S, Oppenheim RW, Prevette DM, Evenson A, Parsadanian A. Overexpression of glial cell line-derived neurotrophic factor in the CNS rescues motoneurons from programmed cell death and promotes their long-term survival following axotomy. Exp Neurol 190: 356–372, 2004. [DOI] [PubMed] [Google Scholar]