Abstract
Background: Sickle cell anemia (SCA) is an inherited blood disease with known complications as a result of certain pathophysiological dysfunctions. It has been suggested that an increase in oxidative stress contributes to the incidence of these changes. Objectives: This study investigated the oxidant/antioxidant status of patients with SCA, and evaluated the effect of SCA on antioxidant enzymes and their cofactors. Methods: The study included 42 patients with SCA (in steady state), and a control group of 50 age-matched individuals without SCA. Serum malondialdehyde (MDA), copper, zinc, ferritin and iron levels, red blood cell (RBC) superoxide dismutase (SOD) and catalase levels were measured for the SCA and control groups. Results: Significantly lower levels of antioxidant enzymes (RBC SOD and catalase) and higher serum MDA levels (biomarker of oxidative stress) were found in SCA patients compared to the control group (all p < 0.001). Increased levels of serum ferritin, iron and copper and decreased zinc concentrations were also found in the SCA patients compared to the control group (all p < 0.001). In the SCA group, there were significant negative correlations between MDA levels and RBC SOD, RBC catalase, and serum zinc levels (p < 0.01), while a significant positive correlation between MDA with serum copper and iron levels (p < 0.01) was observed. Conclusion: SCA is associated with alterations in markers of oxidative stress including an increased MDA level, decreased antioxidant enzyme levels, and altered levels of enzyme cofactors (zinc, copper, and iron). This suggests that these antioxidant enzymes could be used as effective therapeutic targets for the treatment of this disease and supplementation of patients with substances with antioxidant properties may reduce the complications of this disease.
Keywords: malondialdehyde; superoxide dismutase; catalase, sickle cell anemia; oxidative stress; enzymes cofactors
Introduction
Sickle cell anemia (SCA) is a global disease with the highest prevalence in tropical Africa where approximately 20 percent of the population are carriers of the sickle gene. The sickle cell trait has a frequency of approximately 8 percent in the African-American population but it is found to a lesser extent in the Middle East.1
In Iraq, patients with sickle cell disease (SCD) live in two geographical areas, one among the Kurdish population in the extreme north and another among the Arabs in the extreme south.2 In Basrah Governorate in southern Iraq, 6.48 percent of the population are carriers of the hemoglobin S (HbS) gene, giving a gene frequency of 0.0324.3 Numerous red blood cell (RBC) membrane abnormalities have been described in SCA, leading to vaso-occlusive crises (VOC) and intravascular hemolysis.4
In the literature, there is a growing body of evidence suggesting that an increase in oxidative stress and abnormal oxidant/antioxidant balance are implicated in the pathophysiology of several dysfunctions observed in SCA patients,5–7 especially during VOC,8 resulting in various hematological and biochemical changes.9 Oxidative stress is increased when there is an elevated production of highly reactive by-products of metabolic pathways that are called reactive oxygen species (ROS), such as superoxide radical (O√ − ), hydrogen peroxide (H2O2), hydroxyl radical and nitric oxide.10 In the presence of hydrogen peroxide and transitional or redox-active metals ions like iron (Fe) and copper (Cu); superoxide radicals (O√ − ) are converted to the highly reactive hydroxyl radical through the Fenton and Haber-Weiss reactions.7,11,12 Therefore, iron released during hemolysis of the RBC is one of the major contributors to the increased ROS production in SCA.
HbS is reported to have an accelerated autoxidation rate, with increased generation of reactive oxygen species in sickled RBCs.7,13,14 ROS production was found to be 10–30 fold higher in the RBCs, platelets, and polymorphonuclear neutrophils of patients with SCA than in normal subjects.4
Denaturation of Hb releases iron that will be oxidized by H2O2 to form hydroxyl radicals by the Fenton's reaction which increases the levels of ROS, resulting in protein oxidation, lipid peroxidation, damage to cellular macromolecules such as DNA (breakage of the double helix), and mitochondrial dysfunction, thus disrupting their normal functions.7,15,16
Lipid peroxidation occurs by a radical chain reaction that involves the oxidation and destruction of the polyunsaturated lipid membrane structures leading to the loss of its normal cellular deformability and biological properties like degree of fluidity and increased tissue permeability, making the RBC membranes more susceptible to hemolysis.7,12 Lipid peroxidation is increased in situations where there are a high degree of unsaturated lipid molecules, the existence of transitional or redox active metal catalysts and rich supply of oxygen,17 all of which are found in the RBC. Products of lipid peroxidation such as malondialdehyde (MDA) are commonly used as biomarkers of oxidative stress and damage of lipid molecules.18
The body has several mechanisms to neutralize the increased oxidative stress by generating antioxidants, either produced naturally in the body (endogenous antioxidants), or externally via food supplementation (exogenous antioxidants). Antioxidants act to counteract additional formation of free radicals, to protect the cells against their destructive and noxious effects and to contribute to disease prevention.17
Antioxidant defense mechanisms against the harmful effects of ROS involve cellular and extracellular enzymes such as catalase, superoxide dismutase (SOD), glutathione reductase and peroxidase and free radical quenchers such as glutathione, vitamin C, vitamin E, carotenoids, albumin, and products of metabolism such as uric acid and bilirubin.
Catalase is a heme-containing enzyme that catalyzes the degradation of hydrogen peroxide to water and molecular oxygen, thus under normal physiological conditions, it controls the hydrogen peroxide levels so that this does not reach toxic and harmful levels that could result in oxidative damage to the cells.17 SOD is a copper and zinc-containing enzyme that converts superoxide radicals to hydrogen peroxides, which can subsequently be removed by catalase.10,11 Zinc and copper are required for full activity of SOD.15 Copper is essential for the enzyme's catalytic activity, whereas zinc confers structural stability to the active site of the enzymes. These antioxidant enzymes eliminate superoxide radicals, hydrogen peroxide and prevent hydroxyl radical formation that results in further cellular damage. The antioxidant action of zinc occurs through two mechanisms. Firstly, it protects the sulfhydryl groups of proteins from oxidation. Secondly, it competes with iron and copper for binding to cell membranes and some proteins. Consequently replacing these transition metal ions and making them further reachable to bind with ferritin and metallothionein, respectively. Thus it prevents the formation of ROS (hydroxyl radical and superoxide radical) that are harmful to the cell.19
Antioxidant capacity is an important cause of tissue injury, particularly in patients with elevated oxidative stress.20 However, no previous studies in Basrah have evaluated oxidative stress in terms of the RBC levels of antioxidant enzymes, or the correlations between these enzymes and their cofactors i.e., the serum levels of zinc, copper, and iron, in patients with SCA.
The aims of this study were to investigate the oxidant/antioxidant status of patients with SCA, and to evaluate the effects of SCA on antioxidant enzymes and their cofactors (zinc, copper, and iron). In addition, the correlation between the levels of antioxidant enzymes and their cofactors were also studied to investigate whether this relationship is unique to patients with SCA.
Subjects and methods
Subjects
The SCA patients included 42 cases with SCA (HbSS) in steady state21 diagnosed by high-performance liquid chromatography (HPLC) at the Center for Hereditary Blood Diseases in Basrah Governorate, southern Iraq, from January 2010 to May 2010. There were 20 males and 22 females aged between 6 and 28 years, including 21 patients aged < 18 years. Patients with VOC and those taking hydroxyurea or zinc supplements were excluded.
Fifty healthy, age-matched individuals with no history of relevant acute or chronic medical illness and normal Hb pattern confirmed with HPLC were recruited as a control group. Twenty-six individuals were < 18 years old and were relatives of the patients with SCA, while those aged ≥ 18 years were medical student volunteers. For all subjects, demographic data and clinical examination findings were recorded using a standardized form.
The details of the tests were explained to all individuals and/or their parents, and informed consent was obtained before enrollment in the study. The Ethical Committee of the College of Medicine, University of Basrah, approved the study protocol.
Materials and methods
All the reagents that had been used were analytical grade supplied from Fluka AG, Buchs, Switzerland and BDH Chemicals Ltd, Poole, England.
Approximately 3.0 ml of venous blood sample was obtained from all subjects in the SCA and control groups. A portion of the blood was added to an EDTA anticoagulant tube to measure the levels of the SOD and catalase antioxidant enzymes in RBCs. The remainder of the blood was left to clot in a clean plain tube for 20–30 minutes at room temperature. The serum was then separated by centrifugation at 3000 rpm for 15 minutes and divided into two portions. One portion of the serum was transferred into a plain tube and used to measure the level of the lipid peroxidation product MDA within 1–3 hours, and the second portion was transferred into another plain tube and stored at − 200C to be used for the measurements of the copper, zinc, iron, and ferritin levels within 2 to 3 weeks.
Biochemical parameters
Separation of red blood cells and preparation of hemolysate
The whole blood with EDTA as anticoagulant was centrifuged, the plasma and leucocytes layer was then removed and the packed erythrocyte sediments were washed three times with normal saline and hemolyzed by adding approximately 1.5 volumes of ice-cold distilled water. The hemoglobin (Hb) concentration in the hemolysate was measured by the Drabkin method and adjusted to 10 g/dl or 5 g/dl with distilled water for the estimation of RBC SOD or catalase activity, respectively.
SOD estimation in the RBC
A chloroform-ethanol extract was prepared by adding 0.5 ml hemolysate (containing 10 g Hb/dl) to 3–5 ml of ice-cold water, followed by 1 ml of ethanol then 0.6 ml of chloroform, mixing thoroughly after each addition. The tubes were centrifuged for 10 minutes at about 3000 rpm. The clear upper layer was retained for the measurement of SOD enzyme activity.
The RBC SOD level was measured using the method of Winterbourn et al..22 Briefly, this is based on the capability of SOD to prevent the reduction of nitroblue tetrazolium by superoxide, which is produced during the oxidation-reduction reaction of riboflavin and oxygen. SOD activity was expressed as units per gram of Hb.
Catalase estimation in the RBC
The stock hemolysate containing 5 g Hb/dl was diluted 1:500 with phosphate buffer (50 mM; pH 7.0) for the estimation of catalase enzyme activity.
The RBC catalase level was measured by using the method of Aebi,23 which is based on determination of the rate constant (s− 1, k) of hydrogen peroxide (H2O2) decomposition. Catalase activity was expressed in K per gram of Hb.
MDA estimation in serum
The serum MDA level was measured using the thiobarbituric acid (TBA) method of Buege and Aust.24 Under the acid and heating conditions of the reaction, the peroxides break down to form MDA, which complexes with thiobarbituric acid to form a red compound that can be measured spectrophotometrically at 535 nm. Results were expressed in μmol/l.
Serum copper, zinc, and iron measurements
The serum copper, zinc, and iron levels (as cofactors for the antioxidant enzyme reactions) were measured using a flame atomic absorption spectrophotometer (Pye Unicam SP 2900; Philips Scientific, Cambridge, UK) by direct aspiration of the serum after dilution with deionized water (1/10).25 Results were expressed in μg/dl.
Serum ferritin estimation
The serum ferritin level was measured by enzyme-linked immunosorbent assay using the mini VIDAS system (bioMérieux, Lyon, France). Results were expressed in ng/dl. All parameter samples were run in duplicates and their mean value was used in the statistical analyses.
Statistical analysis
All results are expressed as mean ± SD. Data were analyzed using the Statistical Package for Social Sciences, version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). The mean values of parameters were compared between groups using the independent samples t-test. The strength and direction of linear relationships between variables were evaluated using Pearson's correlation coefficient. Linear regression curve estimation was used to assess the association between the serum ferritin level (as an index of iron overload) and the serum MDA level (as an index of oxidative stress). A value of p < 0.05 was considered statistically significant.
Results
Comparisons of various parameters between the SCA and control groups are shown in Table 1. The serum levels of MDA, ferritin, iron, and copper were significantly higher in the SCA group than in the control group (all p < 0.001), and the RBC SOD, RBC catalase, and serum zinc levels were significantly lower in the SCA group than in the control group (all p < 0.001). Our results showed an increase of 71.4% in the level of serum MDA from SCA subjects versus 2% in the control groups, whereas iron increment was 52% versus 2% and copper 16.7% versus 2%. On the other hand, there was a 26.2% reduction in the concentration of RBC SOD from SCA subjects versus 8% in the control group, 16.7% versus 2% for RBC catalase and 26% versus 6% for zinc.
Table 1.
Selected oxidant and antioxidant variables in SCA and control groups.
| Parameter | SCA patients | Control group | |
|
| |||
| N = 42 | N = 50 | P-value | |
|
| |||
| Serum MDA (μmol/l) | 1.24 ± 0.12 | 0.67 ± 0.12 | < 0.001 |
|
| |||
| Erythrocytes SOD (U/g Hb) | 1158 ± 254 | 1800 ± 313 | < 0.001 |
|
| |||
| Erythrocytes CAT (k/g Hb) | 218 ± 30.3 | 275 ± 37.7 | < 0.001 |
|
| |||
| Serum iron (μg/dl) | 160 ± 14 | 102 ± 14.2 | < 0.001 |
|
| |||
| Serum ferritin (ng/ml) | 666.9 ± 557.9 | 35.6 ± 24.8 | < 0.001 |
|
| |||
| Serum copper (μg/dl) | 145.5 ± 14.3 | 100.9 ± 13.5 | < 0.001 |
|
| |||
| Serum zinc (μg/dl) | 62.2 ± 12.6 | 94.2 ± 12.5 | < 0.001 |
|
| |||
Values are shown as mean ± standard deviation.
CAT: catalase; SOD: superoxide dismutase; MDA: malondialdehyde; SCA: sickle cell anemia.
In the SCA group, there were significant negative correlations between the serum MDA level and the RBC SOD, RBC catalase, and serum zinc levels (all p < 0.01), and significant positive correlations between the serum MDA level and the serum copper level (p < 0.01) and serum iron level (p < 0.05) (Table 2). In the control group, there were no significant correlations between the serum MDA level and the other parameters.
Table 2.
Pearson's correlation between the serum MDA level and selected parameters in SCA and control groups.
| Parameters | MDA | |
|
| ||
| SCA patients (N = 42) | Control (N = 50) | |
|
| ||
| r | R | |
|
| ||
| SOD | − 0.546** | − 0.239 |
|
| ||
| CAT | − 0.5633** | − 0.029 |
|
| ||
| Iron | 0.391* | 0.065 |
|
| ||
| Copper | 0.574** | 0.01 |
|
| ||
| Zinc | − 0.575** | − 0.62 |
|
| ||
Values are Pearson's correlation coefficient (r).
* P < 0.05, ** P < 0.01.
CAT: catalase; SOD: superoxide dismutase; MDA: malondialdehyde; SCA: sickle cell anemia.
In SCA patients, Pearson's correlation (r) analyses showed significant positive correlations between the RBC SOD and catalase levels and the serum zinc level (both p < 0.01), and significant negative correlations between the RBC SOD, catalase, the serum copper (both p < 0.05) and serum iron levels (both p < 0.01). In the control group, there were similar significant correlations for the serum copper and zinc levels (all p < 0.01), but not for the serum iron level (Table 3).
Table 3.
Pearson's correlation analyses of the relationships between antioxidant enzyme levels and their cofactors in patients with SCA and control group.
| Antioxidant enzyme cofactor | SCA patients (N = 42) | Controls (N = 50) | ||
|
| ||||
| R | R | |||
|
| ||||
| SOD | CAT | SOD | CAT | |
|
| ||||
| Iron | − 0.608** | − 0.652** | − 0.22 | − 0.063 |
|
| ||||
| Copper | − 0.317* | − 0.345* | − 0.438** | − 0.501** |
|
| ||||
| Zinc | 0.734** | 0.467** | 0.531** | 0.417** |
|
| ||||
Values are Pearson's correlation coefficient (r).
*P < 0.05, **P < 0.01.
CAT: catalase; SOD: superoxide dismutase; SCA: sickle cell anemia.
In patients with SCA, linear regression analyses showed a significant positive association between the serum MDA level and the serum ferritin level (r 2 = 0.164, p = 0.017, Figure 1).
Figure 1.
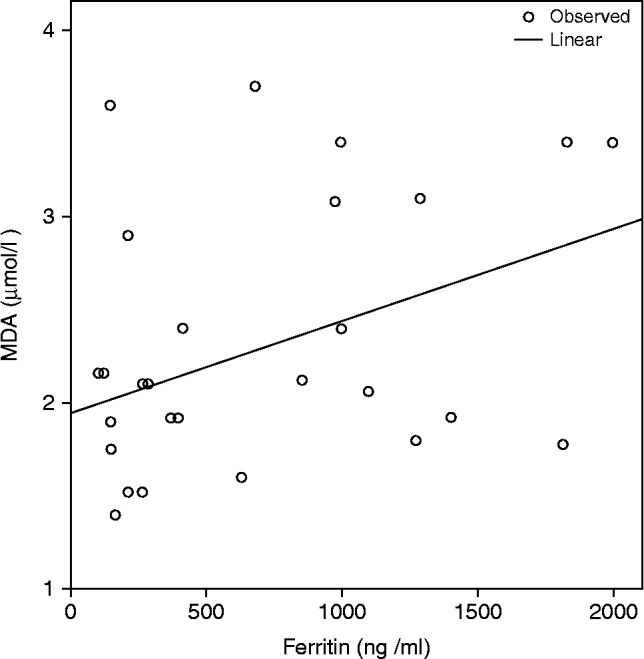
Linear regression analysis of the relationship between the serum ferritin and malondialdehyde (MDA) levels in patients with sickle cell anemia (SCA) r 2 = 0.164, P = 0.017.
Discussion
The pathophysiology of SCA is highly affected by the oxidant/antioxidant status.13,26,27 This may be a factor causing the progression of dense erythrocyte during sickling process, in addition to the formation of vaso-occlusion, and shortening of RBC life span. The oxidative damage in sickled RBCs is most likely the result of HbS instable character causing a rise in the formation of free radicals in association with reduced antioxidant defense mechanisms, leading to increased generation of oxidation products.13,26,27
In agreement with other studies,28–30 our study showed a significantly higher level of MDA in SCA patients than in the control group. This finding was suggested by other authors to be attributed to permanent structural membrane alterations in RBCs, and increased production of ROS in patients with SCA.31–33
The RBC SOD and catalase levels were significantly lower in the SCA group than in the control group. Previous studies reported conflicting results regarding the levels of these antioxidant enzymes in patients with SCA. While Adelakun et al., did not find significant differences in SOD and catalase levels between patients with SCA and the control group,34 Hundekar et al., reported an increased SOD level in patients with SCA,35 and other studies have reported decreased levels of SOD and catalase in patients with SCA.36,37 Reductions in RBC SOD and catalase levels may be related to the severity of oxidative stress, whereas increased RBC SOD and catalase levels may be a protective reaction to scavenge H2O2.35–37
The decreased RBC SOD level in patients with SCA may result from SOD being degraded by oxidants during sickling.17 In addition, endogenous H2O2 is not removed from sickled erythrocytes as readily as from normal erythrocytes.17 Excess H2O2 may react with Hb to form Heinz bodies, leading to the formation of insoluble hemichromes, release of ROS, and release of iron from the heme moiety of Hb, resulting in removal of RBCs containing Heinz bodies by cells of the reticuloendothelial system.38
In the present study, the serum zinc level was significantly lower in the SCA group than in the control group, which is consistent with the findings of previous studies.39–43 It has been postulated that low serum zinc levels in patients with SCA may be due to disturbed metabolism of zinc metalloenzymes, abnormal binding of zinc to tissue proteins, and abnormal renal tubular reabsorption of zinc due to sickling.39,40,44
In addition to other well-known important functions in the human body, zinc plays a role in the maintenance of copper/zinc SOD structural integrity.44 The strong positive correlation between the serum zinc and RBC SOD levels and the strong negative correlation between the serum zinc and MDA levels in the present study support the concept that the antioxidant role of RBC SOD requires zinc as a cofactor.
Copper plays an important role in the functions of mitochondrial cytochrome, oxidase, cytoplasmic, and SOD, some of which have antioxidant functions.44 The high serum copper levels found in patients with SCA in the current study are consistent with the results of previous studies.41,42 This could be explained by the effects of zinc deficiency in patients with SCA, which can greatly increase copper absorption from the gastrointestinal tract.44 The significant positive association between the serum copper and MDA levels, and the significant negative association between the serum copper and RBC SOD and catalase levels, indicate that the oxidant/antioxidant imbalance in patients with SCA is associated with alteration in the serum copper level.
An increased serum iron level can increase the risk of oxidative stress in patients with SCA. Iron overload is biochemically harmful, resulting in tissue damage through the conversion of H2O2 to free radical ions that attack cellular membrane lipids, proteins, and DNA.45 The results of the present study show that patients with SCA have significantly higher serum iron levels than individuals with normal Hb. The positive correlation between the serum iron and MDA levels in patients with SCA reflects the role of iron in lipid peroxidation. In addition, the significant positive correlation between the serum ferritin and MDA levels may indicate that iron overload plays a role in oxidative stress, because a high level of the oxidation product MDA was associated with low levels of antioxidant enzymes and their cofactors. Although Dos Santos et al., found a similar correlation;45 another study of patients with SCA who received transfusion did not observe this correlation.46 Studies of older patients,47 and patients who had received blood transfusion,46 found that patients with SCA had higher serum copper and iron levels and lower serum zinc levels than individuals with normal Hb. These biochemical changes were associated with high serum MDA levels, providing further support to our findings, even though our patients were younger.
Our study had certain limitations, first of which is the estimation of MDA by the TBA method. This assay was employed because it is easy to perform and widely available. Buege and Aust considered this assay of being a reasonably sensitive, simple and accurate index of lipid peroxidation in a number of in vitro oxidizing systems, but are less reliable as an index of lipid peroxidation in complex biological fluids or in vivo.24 Alternatively, MDA assessment using TBARS method is usually criticized in the literature by a lack of specificity and sensitivity. Nevertheless, our results showed that MDA levels are higher in SCA patients as compared to their age-matched control subjects despite the relative low sensitivity of the method. The results are also comparable to some of those cited in the literature.29,30
Secondly, the haplotype of beta-globin chain of hemoglobin S in our SCA patients has not been determined. In sickle cell anemia, the analysis of the beta S-globin haplotypes is vital because it designates the origin of the patients ethnic groups and their geographical distribution.48 It also contributes to better information, understanding and interpretation of the clinical variety and seriousness of SCA patients,48,49 as these distinctive haplotypes possess a diverse range of HbF levels that act to prevent or decrease the polymerization of hemoglobin S subunits and subsequent clinical manifestations.48,50,51 Globally, there are five main classes of beta S-globin haplotypes that are named after the location of which they were discovered. These are Senegal (SEN), Benin (BEN), Bantu or Central African Republic (CAR), Cameroon (CAM) and Arab-Indian (ARAB).48 Of these five, three constitute the major haplotypes among Arabs. These haplotypes are the Benin, the Arab-Indian and the Bantu haplotypes,52 with different frequency of occurrence in the Arab world. In northern Iraq, the predominant βS mutation found was the Benin haplotype (69.5%).2,52 The same high frequent haplotype was also found in Jordan (80%), Lebanon (73%), Syria (66.7%) and southwestern Saudi Arabia (98.5).52 While in Arab countries nearby southern Iraq, the highest frequent haplotype was the Arab-Indian haplotype being 80.8% in Kuwait, 94% in eastern Saudi Arabia and 52% in United Arab Emirates (UAE).52
Thus several questions arise, first: what is the predominant beta S-globin haplotype in the population of our region (southern Iraq); second, would similar patterns be observed in our SCA patients and/or different observations and correlations be obtained in this study according to the different types of haplotypes discovered in our area; third, how would this contribute to the clinical manifestation of the disease and how could such information develop a better understanding of the management and interventions of our SCA patients. These questions all warrant further investigation and research work to be done.
Of note, despite the significant reduction in the levels of antioxidant enzymes and alterations in the levels of their cofactors (copper, zinc, and iron) in patients with SCA compared with the control group, our findings were not unique to the SCA group with the exception of iron. There were significant correlations between the antioxidant enzyme levels and the copper and zinc levels in both groups, but significant correlations between the antioxidant enzyme levels and iron levels were observed only in the SCA group. These results indicate that copper and zinc are important for the optimal catalytic activity of antioxidant enzymes, especially SOD in cellular defense mechanisms of the human body. This highlights the occurrence of iron overload in patients with SCA. Our findings raise the question of whether supplementation with relevant antioxidant enzymes (SOD, catalase, or their mimics) or antioxidant compounds such as trace elements and vitamins may improve the health of patients with SCA, and prevent or reduce the occurrence of VOC and other complications of SCA.
Existing studies of antioxidant supplementation have found that administration of therapeutic doses of zinc to patients with SCA resulted in significant improvement in growth, reduction of other adverse effects of zinc deficiency, reduction in episodes of VOC,13,39,40 and increased zinc levels and antioxidant capacity compared with a placebo group.53 Zinc supplementation was also associated with a decrease in the serum level of copper, which may act as a pro-oxidant at high concentrations.13,53 On the other hand, supplementation with antioxidant vitamins (C and E) was not associated with changes in patients with SCA.54 Increased RBC SOD and catalase activity and decreased MDA levels were reported after 30 days of supplementation with tablets containing antioxidant vitamins and trace elements.35 The vitamins and trace elements included in these antioxidant tablets were not described, but the authors of that study35 supported use of a combined therapeutic strategy to reduce the oxidative stress in patients with SCA. Further studies are warranted to determine whether a combination of antioxidant supplements can improve or prevent certain complications of SCA.
Furthermore, Wood et al.,55 and Silva et al.,56 both advocated new therapeutic concepts based on the emphasis of the crucial role of RBC in the generation of ROS in SCA as the molecular approaches initiated by these cells could be important for the declining of oxidative stress in the blood. They stated that future research should be focused on new therapeutic strategies that reduce hemolytic episodes and production of ROS in SCA patients to prevent further complications and vasculopathy.
Conclusions
The results of this study provide clear evidence that the SCA population of southern Iraq exhibit an alteration in the levels of various oxidative stress markers (depletion of antioxidant enzymes and increased MDA level), and that increased levels of ROS are associated with decreased levels of antioxidants. Thus, these antioxidant enzymes can be recognized as potential and effective therapeutic targets or strategies for the treatment of this disease. Supplementation of substances with antioxidant properties or those that decrease hemolytic episodes or the production of ROS may alter the course of the disease and decrease the incidence of the associated complication and vasculopathy. Further studies are recommended to support these effects.
Patient consent and ethical approval
The study protocol was approved by the Ethical Committee of the College of Medicine, University of Basrah and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Written informed consent was obtained from all subjects or their parents prior to inclusion in the study.
Conflict of interest
The authors state that they have no conflict of interest.
Funding sources
None.
References
- 1.Beutler E. Disorders of hemoglobin structure: Sickle cell anemia and related abnormalities: Overview. In: Lichtman MA, Beutler E, Kipps TJ, Seligsohn U, Kaushansky K, Prchal J, editors. Williams Hematology. 7th ed. McCraw-Hill Medical Education: New York, NY; 2006. pp. 669–684. [Google Scholar]
- 2.Al-Allawi NA, Jalal SD, Nerwey FF, Al-Sayan GO, Al-Zebari SS, Alshingaly AA, Markous RD, Jubrael JM, Hamamy H. Sickle cell disease in the Kurdish population of northern Iraq. Hemoglobin. 2012;36(4):333–342. doi: 10.3109/03630269.2012.692344. [DOI] [PubMed] [Google Scholar]
- 3.Hassan MK, Taha JY, Al-Naama LM, Widad NM, Jasim SN. Frequency of haemoglobinopathies and glucose-6-phosphate dehydrogenase deficiency in Basra. East Mediterr Health J. 2003;9(1-2):45–54. [PubMed] [Google Scholar]
- 4.Amer J, Ghoti H, Rachmilewitz E, Koren A, Levin A, Fibach E. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. Br J Haematol. 2006;132(1):108–111. doi: 10.1111/j.1365-2141.2005.05834.x. [DOI] [PubMed] [Google Scholar]
- 5.Fasola F, Adedapo K, Anetor J, Kuti M. Total antioxidants status and some hematological values in sickle cell disease patients in steady state. J Natl Med Assoc. 2007;99(8):891–894. [PMC free article] [PubMed] [Google Scholar]
- 6.Nur E, Biemond BJ, Otten HM, Brandjes DP, Schnog JJ, CURAMA Study Group Oxidative stress in sickle cell disease; pathophysiology and potential implications for disease management. Am J Hematol. 2011;86(6):484–489. doi: 10.1002/ajh.22012. [DOI] [PubMed] [Google Scholar]
- 7.Voskou S, Aslan M, Fanis P, Phylactides M, Kleanthous M. Oxidative stress in β-thalassemia and sickle cell disease. Redox Biol. 2015;6:226–239. doi: 10.1016/j.redox.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klings ES, Farber HW. Role of free radicals in the pathogenesis of acute chest syndrome in sickle cell disease. Respir Res. 2001;2(5):280–285. doi: 10.1186/rr70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odièvre MH, Verger E, Silva-Pinto AC, Elion J. Pathophysiological insights in sickle cell disease. Indian J Med Res. 2011;134(4):532–537. [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshikawa T, Toyokuni S, Yamamoto Y, Naito Y. Free Radicals in Chemistry, Biology and Medicine. London, UK: OICA International; 2000. p. 580. [Google Scholar]
- 11.Lunec J. Free radicals: their involvement in disease processes. Ann Clin Biochem. 1990;27:173–182. doi: 10.1177/000456329002700301. [DOI] [PubMed] [Google Scholar]
- 12.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 13.Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life. 2012;64(1):72–80. doi: 10.1002/iub.584. [DOI] [PubMed] [Google Scholar]
- 14.Sheng K, Shariff M, Hebbel RP. Comparative oxidation of hemoglobins A and S. Blood. 1998;91(9):3467–3470. [PubMed] [Google Scholar]
- 15.Chan AC, Chow CK, Chiu D. Interaction of antioxidants and their implication in genetic anemia. Proc Soc Exp Biol Med. 1999;222(3):274–282. doi: 10.1177/153537029922200310. [DOI] [PubMed] [Google Scholar]
- 16.Vichinsky E. Emerging ‘A’ therapies in hemoglobinopathies: Agonists, antagonists, antioxidants, and arginine. Hematology Am Soc Hematol Educ Program. 2012;2012:271–275. doi: 10.1182/asheducation-2012.1.271. [DOI] [PubMed] [Google Scholar]
- 17.Aslan M, Thornley-Brown D, Freeman BA. Reactive species in sickle cell disease. Ann New York Acad Sci. 2000;899:375–391. doi: 10.1111/j.1749-6632.2000.tb06201.x. [DOI] [PubMed] [Google Scholar]
- 18.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52(4):601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 19.Bray TM, Bettger WJ. The physiological role of zinc as antioxidant. Free Radic Biol Med. 1990;8:281–291. doi: 10.1016/0891-5849(90)90076-u. [DOI] [PubMed] [Google Scholar]
- 20.Walter PB, Fung EB, Killilea DW, Jiang Q, Hudes M, Madden J, Porter J, Evans P, Vichinsky E, Harmatz P. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol. 2006;135(2):254–263. doi: 10.1111/j.1365-2141.2006.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Animasahun BA, Temiye EO, Ogunkunle OO, Izuora AN, Njokanma OF. The influence of socioeconomic status on the hemoglobin level and anthropometry of sickle cell anemia patients in steady state at the Lagos University Teaching Hospital. Niger J Clin Pract. 2011;14(4):422–427. doi: 10.4103/1119-3077.91748. [DOI] [PubMed] [Google Scholar]
- 22.Winterbourn CC, Hawkins RE, Brian M, Carrell RW. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975;85(2):337–341. [PubMed] [Google Scholar]
- 23. Aebi H. Catalase Bergmeyer HU, ed. Methods of Enzymatic Analysis New York: Academic Press; 1974. 647 683 [Google Scholar]
- 24.Buege JA, Aust SD. Microsomal lipid peroxidation. In: Fleischer S, Packer L, editors. Methods in Enzymology, Vol 52, Biomembranes, Part C, Biological Oxidations: Microsomal, Cytochrome P-450, and Other Homoprotein Systems. New York: Academic Press; 1978. pp. 302–310. [DOI] [PubMed] [Google Scholar]
- 25.Whiteside PJ. Pye Unicam Atomic Absorption Data Book. 2nd ed. England: Pye Unicam Ltd; 1976. [Google Scholar]
- 26.Rogers SC, Ross JG, d'Avignon A, Gibbons LB, Gazit V, Hassan MN, McLaughlin D, Griffin S, Neumayr T, Debaun M, DeBaun MR, Doctor A. Sickle hemoglobin disturbs normal coupling among erythrocyte O2 content, glycolysis, and antioxidant capacity. Blood. 2013;121(9):1651–1662. doi: 10.1182/blood-2012-02-414037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood KC, Granger DN. Sickle cell disease: Role of reactive oxygen and nitrogen metabolites. Clin Exp Pharmacol Physiol. 2007;34(9):926–932. doi: 10.1111/j.1440-1681.2007.04639.x. [DOI] [PubMed] [Google Scholar]
- 28.Manfredini V, Lazzaretti LL, Griebeler IH, Santin AP, Brandão VD, Wagner S, Castro SM, Peralba Mdo C, Benfato MS. Blood antioxidant parameters in sickle cell anemia patients in steady state. J Natl Med Assoc. 2008;100(8):897–902. doi: 10.1016/s0027-9684(15)31402-4. [DOI] [PubMed] [Google Scholar]
- 29.Hundekar P, Suryakar A, Karnik A, Ghone R, Vasaikar M. Antioxidant status and lipid peroxidation in sickle cell anemia. Biomed Res. 2010;21(4):461–464. [Google Scholar]
- 30.Emokpae AM, Uadia PO, Kuliya-Gwarzo A. Antioxidant enzymes and acute phase proteins correlate with marker of lipid peroxide in adult Nigerian sickle disease patients. Iranian J of Basic Medical Science. 2010;13(4):177–182. [Google Scholar]
- 31.Moore RB, Hulgan TM, Green JW, Jenkins LD. Increased susceptibility of the sickle cell membrane Ca2++ Mg2+ -ATPase to t-butylhydroperoxide: protective effects of ascorbate and desferal. Blood. 1992;79(5):1334–1341. [PubMed] [Google Scholar]
- 32.Kumerova A, Lece A, Skesters A, Silova A, Petuhovs V. Anemia and antioxidant defence of the red blood cells. Mater Med Pol. 1998;30(1-2):12–15. [PubMed] [Google Scholar]
- 33.Tamer L, Polat G, Yücebilgiç G, Güvenç B, Bas¸lamıs¸lı F. The levels of sera malondialdehyde, erythrocyte membrane Na+-K+/Mg++ and Ca++/Mg++ adenosine 5’ triphosphatase in patients with sickle cell anemia. Turk J Haematol. 2000;17:23–26. [PubMed] [Google Scholar]
- 34.Adelakun A, Ajani O, Ogunleye T, Disu E, Kosoko A, Arinola G. Respiratory burst enzymes and oxidant-antioxidant status in Nigerian children with sickle cell disease. Br Biotechnology J. 2014;4(3):270–278. [Google Scholar]
- 35.Hundekar P, Suryakar AN, Karnik AC, Valvi R, Ghone RA, Bhagat SS. The effect of antioxidant supplementation on the oxidant and antioxidant status in sickle cell anemia. J Clin Diag Res. 2011;5(7):1339–1342. [Google Scholar]
- 36.Al-Sultan AI, Seif MA, Amin TT, Naboli M, Alsuliman AM. Relationship between oxidative stress, ferritin and insulin resistance in sickle cell disease. Eur Rev Med Pharmacol Sci. 2010;14(6):527–538. [PubMed] [Google Scholar]
- 37.Gizi A, Papassotiriou I, Apostolakou F, Lazaropoulou C, Papastamataki M, Kanavaki I, Kalotychou V, Goussetis E, Kattamis A, Rombos I, Kanavakis E. Assessment of oxidative stress in patients with sickle cell disease: The glutathione system and the oxidant-antioxidant status. Blood Cells Mol Dis. 2011;46(3):220–225. doi: 10.1016/j.bcmd.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Liu SC, Yi SJ, Mehta JR, Nichols PE, Ballas SK, Yacono PW, Golan DE, Palek J. Red cell membrane remodeling in sickle cell anemia. Sequestration of membrane lipids and proteins in Heinz bodies. J Clin Invest. 1996;97(1):29–36. doi: 10.1172/JCI118402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasad AS. Zinc deficiency in patients with sickle cell disease. Am J Clin Nutr. 2002;75(2):181–182. doi: 10.1093/ajcn/75.2.181. [DOI] [PubMed] [Google Scholar]
- 40.Prasad AS. Discovery of human zinc deficiency: Its impact on human health and disease. Adv Nutr. 2013;4:176–190. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahdi JK. Plasma zinc level in patients with sickle cell anemia. Tech Res J. 2001;78:7–13. [Google Scholar]
- 42.Akenami FO, Aken'Ova YA, Osifo BO. Serum zinc, copper and magnesium in sickle cell disease at Ibadan, South western Nigeria. Afr J Med Sci. 1999;28(3–4):137–139. [PubMed] [Google Scholar]
- 43.Bashir NA. Serum zinc and copper levels in sickle cell anemia and beta-thalassemia in North Jordan. Ann Trop Paediatr. 1995;15(4):291–293. doi: 10.1080/02724936.1995.11747786. [DOI] [PubMed] [Google Scholar]
- 44.Osredkar J, Sustar N. Copper and zinc, biological role and significance of copper/zinc imbalance. J Clinic Toxicol. 2011;S3:001. doi: 10.4172/2161-0495.S3-001. [Google Scholar]
- 45.Dos Santos TE, de Sousa GF, Barbosa MC, Gonçalves RP. The role of iron overload on oxidative stress in sickle cell anemia. Biomark Med. 2012;6(6):813–819. doi: 10.2217/bmm.12.71. [DOI] [PubMed] [Google Scholar]
- 46.Abiodun EM, Aisha KG. The association of transfusion status with antioxidant enzymes and malondialdehyde level in Nigerians with sickle cell disease. Asian J Tranfus Sci. 2014;8(1):47–50. doi: 10.4103/0973-6247.126692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canellas CG, Carvalho SM, Anjos MJ, Lopes RT. Determination of Cu/Zn and Fe in human serum of patients with sickle cell anemia using radiation synchrotron. Appl Radiat Isot. 2012;70(7):1277–1280. doi: 10.1016/j.apradiso.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 48.Gabriel A, Przybylski J. Sickle-Cell anemia: A look at global haplotype distribution. Nature Education. 2010;3(3):2. [Google Scholar]
- 49.Loggetto SR. Sickle cell anemia: Clinical diversity and beta S-globin haplotypes. Rev Bras Hematol Hemoter. 2013;35(3):155–157. doi: 10.5581/1516-8484.20130048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flint J, Harding RM, Boyce AJ, Clegg JB. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol. 1998;11(1):1–51. doi: 10.1016/s0950-3536(98)80069-3. [DOI] [PubMed] [Google Scholar]
- 51.Inati A, Taher A, Bou Alawi W, Koussa S, Kaspar H, Shbaklo H, Zalloua PA. Beta-globin gene cluster haplotypes and HbF levels are not the only modulators of sickle cell disease in Lebanon. Eur J Haematol. 2003;70(2):79–83. doi: 10.1034/j.1600-0609.2003.00016.x. [DOI] [PubMed] [Google Scholar]
- 52.Hamamy HA, Al-Allawi NA. Epidemiological profile of common haemoglobinopathies in Arab countries. J Community Genet. 2013;4(2):147–167. doi: 10.1007/s12687-012-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bao B, Prasad AS, Beck FWJ, Snell D, Suneja A, Sarkar FH, Doshi N, Fitzgerald JT, Swerdlow P. Zinc supplementation decreases oxidative stress, incidence of infection and generation of inflammatory cytokines in sickle cell disease patients. Transl Res. 2008;152(2):67–80. doi: 10.1016/j.trsl.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Arruda MM, Mecabo G, Rodrigues CA, Matsuda SS, Rabelo IB, Figueiredo MS. Antioxidant vitamins C and E supplementation increases markers of haemolysis in sickle cell anaemia patients: a randomized, double-blind, placebo-controlled trial. Br J Haematol. 2013;160(5):688–700. doi: 10.1111/bjh.12185. [DOI] [PubMed] [Google Scholar]
- 55.Wood KC, Hsu LL, Gladwin MT. Sickle cell disease vasculopathy: A state of nitric oxide resistance. Free Radic Biol Med. 2008;44:1506–1528. doi: 10.1016/j.freeradbiomed.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Silva DG, Belini Junior E, de Almeida EA, Bonini-Domingos CR. Oxidative stress in sickle cell disease: An overview of erythrocyte redox metabolism and current antioxidant therapeutic strategies. Free Radic Biol Med. 2013;65:1101–1109. doi: 10.1016/j.freeradbiomed.2013.08.181. [DOI] [PubMed] [Google Scholar]


