Micrographia is common in Parkinson's disease. Wu et al. show that consistent micrographia is related to dysfunction of the basal ganglia motor circuit, while progressive micrographia that worsens during writing is related to dysfunction in a more extensive circuit that includes rostral supplementary and cingulate motor areas.
Keywords: Parkinson’s disease, micrographia, attention, dopaminergic intervention, sequence effect
Micrographia is common in Parkinson's disease. Wu et al. show that consistent micrographia is related to dysfunction of the basal ganglia motor circuit, while progressive micrographia that worsens during writing is related to dysfunction in a more extensive circuit that includes rostral supplementary and cingulate motor areas.
Abstract
Micrographia is a common symptom in Parkinson’s disease, which manifests as either a consistent or progressive reduction in the size of handwriting or both. Neural correlates underlying micrographia remain unclear. We used functional magnetic resonance imaging to investigate micrographia-related neural activity and connectivity modulations. In addition, the effect of attention and dopaminergic administration on micrographia was examined. We found that consistent micrographia was associated with decreased activity and connectivity in the basal ganglia motor circuit; while progressive micrographia was related to the dysfunction of basal ganglia motor circuit together with disconnections between the rostral supplementary motor area, rostral cingulate motor area and cerebellum. Attention significantly improved both consistent and progressive micrographia, accompanied by recruitment of anterior putamen and dorsolateral prefrontal cortex. Levodopa improved consistent micrographia accompanied by increased activity and connectivity in the basal ganglia motor circuit, but had no effect on progressive micrographia. Our findings suggest that consistent micrographia is related to dysfunction of the basal ganglia motor circuit; while dysfunction of the basal ganglia motor circuit and disconnection between the rostral supplementary motor area, rostral cingulate motor area and cerebellum likely contributes to progressive micrographia. Attention improves both types of micrographia by recruiting additional brain networks. Levodopa improves consistent micrographia by restoring the function of the basal ganglia motor circuit, but does not improve progressive micrographia, probably because of failure to repair the disconnected networks.
Introduction
Micrographia, a common symptom in Parkinson’s disease, is characterized by small handwriting with further progressive reduction in size (McLennan et al., 1972; Jarzebska et al., 2006; Wagle Shukla et al., 2012). Micrographia has a high association with accurate diagnosis of Parkinson’s disease (Mutch et al., 1991; Duarte et al., 1995). Moreover, this problem can occur early in the disease and is one of the first symptoms (McLennan et al., 1972; Becker et al., 2002; Ponsen et al., 2008); thus, it may be useful for early diagnosis of Parkinson’s disease (Wagle Shukla et al., 2012; Rosenblum et al., 2013).
The neurophysiological mechanisms underlying micrographia in Parkinson’s disease remain unknown. It has been suggested that micrographia is a component of bradykinesia, as these two symptoms are correlated (Wagle Shukla et al., 2012). Inappropriate scaling of the dynamic muscle force to the movement parameters, which has been proposed to contribute to bradykinesia (Berardelli et al., 1986), may be a reason for micrographia (Van Gemmert et al., 1999; Wagle Shukla et al., 2012). However, the relationship between micrographia and bradykinesia remains controversial (McLennan et al., 1972). It is well known that micrographia can present at an early stage of Parkinson’s disease, even without significant bradykinesia. Other reasons like tremor, rigidity, or stress (McLennan et al., 1972), inability to properly control wrist and finger movements (Teulings et al., 1997; Van Gemmert et al., 2003), difficulty in maintaining a constant force (Teulings et al., 1991; Van Gemmert et al., 1999), increased cognitive or motor demands (Van Gemmert et al., 1998, 2001) and visuospatial perception (Van Gemmert et al., 2003; Broderick et al., 2009) have been suggested to contribute to micrographia. However, these mechanisms can only provide partial explanations. Most importantly, the neural correlates of this problem have not been identified.
Micrographia usually manifests in two forms: ‘consistent’ and ‘progressive’. Consistent micrographia is a global reduction in writing size compared with writing before the development of the disease, whereas progressive micrographia is a gradual reduction in size during writing (Wilson, 1925; Kim et al., 2005). Although most patients with micrographia have both consistent and progressive micrographia, some patients present consistent micrographia without clear progressive micrographia, and vice versa (Kim et al., 2005). Consistent micrographia is likely a manifestation of hypokinesia (smallness of movement), and can be alleviated by levodopa (McLennan et al., 1972) or high-frequency stimulation of the subthalamic nucleus (Siebner et al., 1999). Levodopa also improves kinematics of handwriting, such as velocity, acceleration, and stroke duration (Lange et al., 2006; Tucha et al., 2006). Thus, it is likely that consistent micrographia is a consequence of dysfunction of basal ganglia circuits secondary to dopaminergic depletion. In contrast, progressive micrographia is a manifestation of the sequence effect. The sequence effect is a description of progressive reduction in speed and amplitude of repetitive action, which is a common feature in Parkinson’s disease (Benecke et al., 1987; Agostino et al., 1992; Iansek et al., 2006; Kang et al., 2010). Unlike consistent micrographia, progressive micrographia is usually not improved by levodopa treatment (Ling et al., 2012), which suggests that progressive micrographia may be independent of dopaminergic pathways; the neural networks outside basal ganglia circuits may have a role in this phenomenon. Presumably, dysfunction of some areas that are important in controlling sequential movements, e.g. the rostral supplementary motor area (pre-SMA) or cerebellum (Nachev et al., 2008; D’Angelo, 2011), may be associated with progressive micrographia.
In the current study, we used functional MRI to investigate the neural correlates underlying micrographia in Parkinson’s disease. We supposed that different neural networks are involved in consistent and progressive micrographia. Moreover, we investigated the effects of attention and dopamine administration on micrographia. It has been commonly observed that external visual, auditory or verbal cues or attention can effectively increase the amplitude of handwriting in patients with Parkinson’s disease with consistent micrographia (Oliveira et al., 1997; Swinnen et al., 2000; Nieuwboer et al., 2009; Bryant et al., 2010; Ringenbach et al., 2011). In contrast, whether external cues or attention can improve progressive micrographia is unclear. We hypothesized that attention could recruit additional brain circuits (Redgrave et al., 2010) to improve consistent and progressive micrographia. Moreover, we hypothesized that dopamine could improve consistent micrographia by restoring function of the basal ganglia motor circuit, but would have no benefit to progressive micrographia. This work will be helpful to our understanding of neurophysiological mechanisms underlying micrographia, and provide new insights on our knowledge of hypokinesia and the sequence effect in Parkinson’s disease.
Materials and methods
Subjects
Forty-three patients with Parkinson’s disease diagnosed with micrographia were chosen from our dataset. The diagnosis of Parkinson’s disease was based on the UK Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria (Hughes et al., 1992). Patients were assessed with the Unified Parkinson’s Disease Rating Scale (UPDRS; Lang and Fahn, 1989), the Hoehn and Yahr Disability Scale (Hoehn and Yahr, 1967), Fatigue Severity Scale (Krupp et al., 1989) and Mini-Mental State Examination while OFF their medications. Bradykinesia/rigidity was the predominant symptom and was more severe on the right side in every patient. To avoid difficulty of handwriting and disturbance of the functional MRI signal, all patients were chosen to have at most a mild tremor.
Our patients with Parkinson’s disease were divided into two groups: consistent micrographia and progressive micrographia. The consistent micrographia group contained patients with global reduction but without significant progressive reduction in writing size; whereas patients in the progressive micrographia group had progressive reduction but without significant global reduction in writing size. Patients with mixed consistent and progressive micrographia were excluded. The criteria of consistent and progressive micrographia are shown in the ‘Behavioural data analysis’ section. There were 20 patients in the consistent micrographia group, and 23 patients in the progressive micrographia group. Two patients in the consistent micrographia group and five patients in the progressive micrographia group were excluded because of excessive head motion during the functional MRI acquisition. Each group had 18 patients remaining. Eighteen healthy subjects were included as the control group. All patients and control subjects were right-handed according to the Edinburgh Inventory (Oldfield, 1971). The demographics and clinical details from the controls and remaining Parkinson’s disease patients are shown in Table 1. The experiments were performed according to the Declaration of Helsinki and were approved by the Institutional Review Board. All subjects gave their written informed consent for the study.
Table 1.
Demographics and clinical details of the subjects
| Consistent micrographia group | Progressive micrographia group | Controls | |
|---|---|---|---|
| Age, yearsa (range) | 60.39 ± 3.47 (54–67) | 59.61 ± 3.34 (52–66) | 59.94 ± 3.13 (55–65) |
| Sexa | 6 female, 12 male | 5 female, 13 male | 6 female, 12 male |
| Disease duration (years)b | 4.38 ± 1.31 | 4.09 ± 1.27 | |
| UPDRS motor scoreb | 20.39 ± 5.30 | 19.06 ± 4.78 | |
| Hoehn and Yahr stagingb | 1.61 ± 0.40 | 1.53 ± 0.44 | |
| Fatigue Severity Scaleb | 3.83 ± 0.96 | 3.98 ± 1.03 | |
| Mini-Mental State Examinationa | 28.94 ± 1.11 | 29.28 ± 0.96 | 29.56 ± 0.70 |
| l-DOPA dose (mg/day)b | 338.89 ± 69.78 | 305.56 ± 80.24 |
Values are (mean ± SD).
aThe comparisons among the three groups were analysed with ANOVA (P > 0.05).
bThe comparisons between the consistent micrographia and progressive micrographia group were analysed with two-sample t-tests (P > 0.05).
Task
All subjects wrote with their right hand. The Chinese character ‘Zheng’ (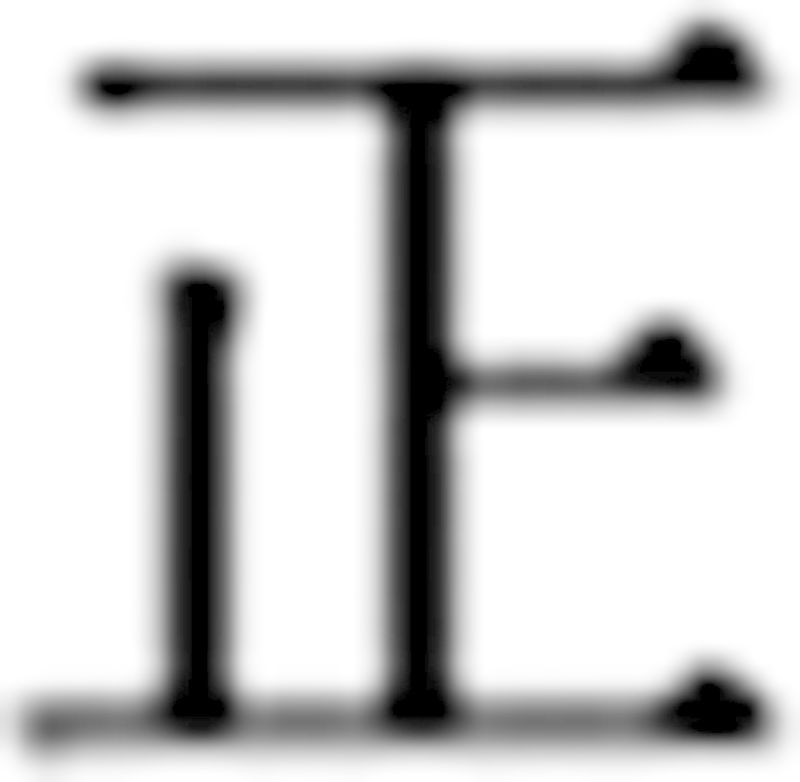 ) was used for handwriting in the current study (Ma et al., 2013; Supplementary Fig. 1). We chose this character as it is simple (has five distinct strokes) and is commonly used. All our subjects had no difficulty writing this character. Moreover, this character has a well-defined overall square shape, the size of each character can be easily measured (Ma et al., 2013). Subjects had ample time to practice the task before functional MRI scanning. The subjects first practiced outside the MRI scanner. They were asked to write the character horizontally as they would do naturally (free writing). Then, we asked the subjects to pay attention to the handwriting: patients in the consistent micrographia group were asked to write the characters larger (attention on size); whereas progressive micrographia group patients were required to write the characters with same size (attention on consistency). We did not provide any external visual or auditory cues to help them to improve micrographia.
) was used for handwriting in the current study (Ma et al., 2013; Supplementary Fig. 1). We chose this character as it is simple (has five distinct strokes) and is commonly used. All our subjects had no difficulty writing this character. Moreover, this character has a well-defined overall square shape, the size of each character can be easily measured (Ma et al., 2013). Subjects had ample time to practice the task before functional MRI scanning. The subjects first practiced outside the MRI scanner. They were asked to write the character horizontally as they would do naturally (free writing). Then, we asked the subjects to pay attention to the handwriting: patients in the consistent micrographia group were asked to write the characters larger (attention on size); whereas progressive micrographia group patients were required to write the characters with same size (attention on consistency). We did not provide any external visual or auditory cues to help them to improve micrographia.
In addition, we asked the subjects to write at a certain speed and force to avoid the influences of these factors on our results. They were required to write at approximately one stroke per second, and all subjects had no difficulty to write at this speed. We used an electronic pressure gauge to quantitatively measure the force of right hand writing, and required the subjects to write with ∼20% of maximal writing force. With this force, all subjects wrote the character clearly, and no subject reported fatigue during functional MRI scanning. We gave the subjects enough practice until they could write at the required speed and force. When the subjects could perform the tasks correctly, they practiced inside the MRI scanner until they could write comfortably, smoothly, and could execute the writing tasks as required without any difficulty while lying inside the scanner.
Functional magnetic resonance imaging
Functional MRIs were performed on a 3 T MR scanner (Trio system; Siemens Magnetom scanner). A standard head coil was used with foam padding to restrict head motion. High-resolution axial T1- and T2-weighted images were obtained in every participant to detect clinically silent lesions. High-resolution anatomical images were acquired with 3D-MPRAGE (magnetization-prepared rapid gradient-echo) sequence (repetition time = 2000 ms, echo time = 2.19 ms, 176 sagittal slices, 1 mm slice thickness, field of view = 224 mm × 256 mm). Blood oxygen level-dependent data were acquired with gradient-echo echo-planar sequences (repetition = 2000 ms, echo time = 40 ms, 33 axial slices, 3.5-mm thickness, no gap, flip angle = 90°, field of view = 256 mm × 256 mm, matrix size = 64 × 64).
During functional MRI scanning, subjects had a locally developed MRI-compatible graphic tablet placed on a cushion over their laps, and held a fibre optic pen in the right hand. The tablet could be oriented in the MRI scanner so that the writing posture could be comfortably adjusted. The subjects could see the tablet and what they have written on the tablet clearly through a mirror built into the head coil. Each functional MRI scan session lasted 8 min, was block designed and contained two conditions, which were defined as the ‘rest’ and ‘write’ condition, respectively. Each condition lasted 40 s and was repeated six times. In the ‘rest’ condition, the subjects were asked to relax and focus on the tablet. During the ‘write’ condition, the subjects performed following writing tasks as required.
Healthy controls had three functional MRI sessions, in which they performed free writing, attention on size, and attention on consistency tasks in separate sessions. Patients were first scanned after their medication had been withdrawn for at least 12 h (OFF condition). In the OFF condition, consistent micrographia patients performed free writing and attention on size tasks; while progressive micrographia patients performed free writing and attention on consistency tasks in separate sessions. We verbally informed each subject which task to be performed before the beginning of each session. Then, levodopa was administered orally as 250 mg Madopar® (200 mg levodopa/50 mg benserazide, Roche) in all patients. Sixty minutes after oral levodopa had been given, approximately when plasma levodopa achieved the highest level, the patients received the third functional MRI session and were asked to repeat the free writing task (free writing ON). No external cue was given to help the subjects write at the specified rate or size. The functional MRI scanning sessions are summarized in Supplementary Table 1.
Data analysis
Behavioural data analysis
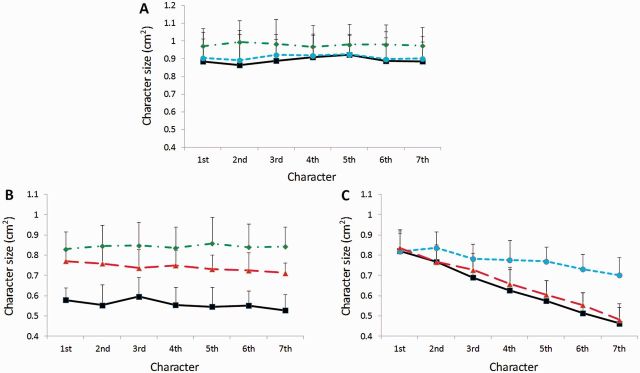
The characters that were written during functional MRI scanning were stored and analysed offline. The size of the character was defined as the area of the quadrilateral outlined by the top, bottom, left, and right margins of each character (Ma et al., 2013). Because all subjects could write at least seven characters in each ‘write’ block (40 s), we used sizes from the first seven characters in each block for behavioural data analysis. Consistent micrographia in each patient was defined by the mean size of the characters that was below the mean −2 SD (standard deviation) of the controls (Kim et al., 2005). We applied regression analysis to quantify progressive micrographia (Kim et al., 2005; Ling et al., 2012). The mean character sizes across the writing blocks were plotted from the first to the seventh and the b value (slope) of the regression line was obtained in each functional MRI session in each subject (Fig. 1). Progressive micrographia was defined by a b-value below the mean −2 SD of the controls (Kim et al., 2005). This b-value was termed Mb and was used to evaluate the severity of progressive micrographia in the current study. For the progressive micrographia group, the size of the first character in each ‘write’ block was compared to that in the controls to evaluate whether there was consistent micrographia in the patients, as the mean size of the characters across the ‘write’ block was contaminated with the sequence effect.
Figure 1.
Character size in each group. Mean character size (from the first to the seventh character) in the control subjects (A), Parkinson’s disease patients with consistent micrographia (B), and Parkinson’s disease patients with progressive micrographia (C) in each writing condition during functional MRI scanning. Values are shown as mean and SD. Black line = the free writing condition; green line = the attention on size condition; blue line = the attention on consistency condition; red line = the free writing ON condition.
We used two repeated-measures ANOVA to evaluate behavioural performances. The first ANOVA model contained two groups (controls and consistent micrographia group) and two conditions (free writing and attention on size). The values of the mean size of the characters in each subject and each condition were entered into this model. The second ANOVA model contained two groups (controls and progressive micrographia group) and two conditions (free writing and attention on consistency). The Mb values were entered into this model. The main effect of group and condition on writing size or consistency, and the group–condition interaction were calculated. Post hoc t-tests of between conditions or groups were investigated using polynominal contrasts and Tukey test. Then, two-sample t-tests were used to compare the character sizes before and after levodopa administration in the consistent micrographia group, as well as compare the Mb values before and after levodopa administration in the progressive micrographia group.
The speed of writing was calculated as total strokes in each session divided by 240 s (40 s/block × six blocks of writing). Similar analysis as that described above was used to explore the effects of groups and conditions on writing speed. The slope of change in speed of handwriting across the functional MRI scanning sessions was used to assess whether there was a progressive slowing of movement or ‘fatigue’ (regression analysis; Ling et al., 2012). A correlation analysis between the Fatigue Severity Scale and Mb values in the free writing condition was performed to evaluate whether progressive micrographia was related to fatigue. In addition, pen pressure on the tablet was recorded to measure the force of handwriting during functional MRI scanning in each group. All statistical analyses were performed with SPSS 17.0 software.
Imaging data analysis
Data preprocessing
Image analysis was performed with SPM8 software (Wellcome Institute of Cognitive Neurology, London, UK). Functional MRI data were slice-time corrected and aligned to the first image of each run for motion correction. Functional images were co-registered to high-resolution anatomical images. After spatial normalization, all images were resampled into voxels that were 3 × 3 × 3 mm in size, and smoothed with a 6-mm Gaussian smoothing kernel. Each participant's movement parameters were examined. As described previously, seven patients had excessive head motion (>1.5 mm maximum translation in x, y or z, or 1.5° of maximum angular rotation about each axis), and their datasets were discarded. Root mean squared movement of translation and rotation parameters (Power et al., 2012) was used to evaluate head motion. There was no significant difference in head motion among the groups in the remaining subjects (ANOVA, P > 0.05; Supplementary Table 2).
Brain activity analysis
Data were first analysed for each single participant separately on a voxel-by-voxel basis using the general linear model approach for the time series. We defined a model using a fixed effect boxcar design convolved with a haemodynamic response function for analysis of task-dependent activation. We added the head motion parameters as regressors to optimally control for the motion effects. A contrast representing the effect of the ‘write’ condition compared with the ‘rest’ condition was calculated in each participant. These contrast images were used in the second level for random-effects analyses.
At the second level, first, a one-sample t-test model was used to identify the brain activity in each condition in each group. Then, three repeated-measures ANOVAs were used to determine the interaction between group and experimental conditions. The first ANOVA contained groups of control and consistent micrographia and conditions of free writing and attention on size. The second ANOVA had groups of control and progressive micrographia and conditions of free writing and attention on consistency. The third ANOVA contained groups of patients with consistent micrographia, and those with progressive micrographia and conditions of free writing and free writing ON. The contrast images from the first level analysis were modelled using a flexible factorial design for each ANOVA analysis. Post hoc tests were performed to explore the differences between conditions or groups.
In the free writing condition, regression analysis between brain activity and mean character size in the consistent micrographia group, and between brain activity and Mb values in the progressive micrographia group was performed to find out brain activations specifically relating to the severity of consistent or progressive micrographia. In addition, the differences of the character sizes between the free writing and attention on size, and between the free writing and free writing ON conditions in the consistent micrographia group (Δsize); as well as the differences of Mb values between the free writing and attention on consistency, and between the free writing and free writing ON conditions in the progressive micrographia group (ΔMb) were calculated in each patient. In the consistent micrographia group, regression analysis between brain activity in the attention on size condition or in the free writing ON condition and corresponding Δsize values was measured to find out brain activations specifically relating to the improvement of consistent micrographia. In the progressive micrographia group, regression analysis between brain activity and ΔMb values in the attention on consistency condition was performed to find out brain activations specifically relating to the improvement of progressive micrographia.
Functional connectivity analysis
We additionally investigated the functional connectivity of neural networks relating to micrographia. Because focal damage in the putamen can result in micrographia (Lewitt, 1983; Pullicino et al., 1994; Troyer et al., 2004), and the putamen is the most severely affected region in Parkinson’s disease (Brooks et al., 1990), we chose the putamen as a region of interest to assess micrographia-related network changes. As all patients were more affected in the right side, and all subjects wrote with their right hands, we focused on the left putamen in this study. The putamen is divided into anterior and posterior portions by the anterior commissure. As the anterior and posterior putamen have different functions and are differently affected in Parkinson’s disease (see ‘Discussion’ section), the left posterior and anterior putamen were chosen as two separate regions of interest. According to our brain activity results, we chose five more regions that may relate with micrographia as regions of interest, including the left rostral and caudal supplementary motor area (pre-SMA and caudal SMA, respectively), left premotor cortex (PMC), right rostral cingulate motor area (rCMA), and right cerebellum (anterior lobe, culmen). Among these regions of interest, the pre-SMA (Nachev et al., 2008) and cerebellum (D’Angelo, 2011) are important in controlling sequential movements; while volume reduction of the rCMA has been related to the sequence effect (Lee et al., 2014). We supposed that connectivity changes in these areas may be associated with progressive micrographia.
These seven regions of interest were centred at the voxels showing the maximum magnitude of activation within each area. The radius for the pre-SMA, caudal SMA, PMC, rCMA, and cerebellum was 5 mm, whereas the radius for the anterior and posterior putamen was 3 mm to avoid the regions of interest extending to the adjacent regions (e.g. globus pallidus; Wu et al., 2011). A seed reference time course was obtained within each region of interest. Correlation analysis was carried out between the seed reference and the whole brain in a voxel-wise manner in each region of interest. The nuisance covariates of head motion parameters, white matter signal, and CSF signal were regressed out. The individual results were entered into a random effect one-sample t-test to determine brain regions showing significant connectivity with each region of interest within each group in each condition.
Similar to the brain activity analysis, we used three ANOVAs with a flexible factorial design to measure the interaction of network connectivity between group and experimental conditions in each region of interest. Post hoc t-tests were performed to explore the differences of network connectivity between conditions or groups in each region of interest. In the free writing condition, regression analysis between network connectivity in each region of interest and mean character sizes in the consistent micrographia group, and between network connectivity in each region of interest and Mb values in the progressive micrographia group was performed to find out brain networks specifically relating to the severity of consistent or progressive micrographia. In the consistent micrographia group, regression analysis between network connectivity in each region of interest in the attention on size condition or in the free writing ON condition and corresponding Δsize values was measured to find out brain networks specifically relating to the improvement of consistent micrographia. In the progressive micrographia group, regression analysis between network connectivity in each region of interest and ΔMb values in the attention on consistency condition was performed to find out brain networks specifically relating to the improvement of progressive micrographia. A family-wise error-corrected threshold of P < 0.05 was used for all brain activity and connectivity analysis. Extent threshold was 10 voxels.
Results
Task performance
The results of task performance in each condition in each group are shown in Table 2. In the consistent micrographia group, the mean size of the characters was less than the mean −2 SD of the controls (0.636 cm2); whereas the Mb value was higher than the mean Mb –2 SD of the controls (−0.023) in the free writing condition in each patient. In the progressive micrographia group, the Mb value was below the mean Mb –2 SD of the controls; whereas the mean size of the first character was larger than the mean –2 SD of the controls (0.627 cm2) in the free writing condition in each patient. Thus, all patients in the consistent micrographia group had a significant consistent micrographia, but without a clear progressive micrographia; while patients in the progressive micrographia group had a significant progressive micrographia, but without a consistent micrographia (Fig. 1).
Table 2.
Task performance during functional MRI scanning
| Control |
Consistent micrographia |
Progressive micrographia |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Free writing | Attention on size | Attention on consistency | Free writing | Attention on size | Free writing ON | Free writing | Attention on consistency | Free writing ON | |
| Mean size of the characters (cm2) | 0.892 ± 0.128 | 0.978 ± 0.135 | 0.908 ± 0.132 | 0.558 ± 0.086a | 0.842 ± 0.106b | 0.740 ± 0.076c | 0.636 ± 0.153a | 0.773 ± 0.093b | 0.661 ± 0.139 |
| Mean size of the first character (cm2) | 0.885 ± 0.129 | 0.971 ± 0.148 | 0.903 ± 0.111 | 0.579 ± 0.060a | 0.829 ± 0.086b | 0.770 ± 0.054c | 0.821 ± 0.089 | 0.818 ± 0.102 | 0.837 ± 0.091 |
| Mb value | 0.003 ± 0.013 | -0.001 ± 0.011 | 0.001 ± 0.012 | − 0.007 ± 0.009 | 0.002 ± 0.009 | − 0.009 ± 0.013 | −0.061 ± 0.012a | − 0.020 ± 0.010b | − 0.058 ± 0.014 |
| Speed (stroke/s) | 0.946 ± 0.030 | 0.946 ± 0.030 | 0.946 ± 0.030 | 0.942 ± 0.028 | 0.938 ± 0.038 | 0.938 ± 0.025 | 0.929 ± 0.044 | 0.932 ± 0.032 | 0.940 ± 0.022 |
Values are (mean ± SD).
aSignificant difference between patients and controls (repeated-measures ANOVA, post hoc t-test, P < 0.0001).
bSignificant difference between attention and free writing condition within each group of patients (repeated-measures ANOVA, post hoc t-test, P < 0.0001).
cSignificant difference between free writing ON and free writing condition within each group of patients (two-sample t-tests, P < 0.0001).
The first repeated-measures ANOVA showed that the interaction between group (control and consistent micrographia group) and condition (free writing and attention on size) was statistically significant (P < 0.0001). Post hoc t-tests revealed significant differences of character sizes between the control and consistent micrographia group with the free writing condition, and between the attention on size and free writing conditions in the consistent micrographia group (P < 0.0001). The second repeated-measures ANOVA showed that the interaction between group (control and progressive micrographia group) and condition (free writing and attention on consistency) was statistically significant (P < 0.0001). The Mb values between the control and progressive micrographia group in the free writing condition, and between the attention on consistency and free writing condition in the progressive micrographia group were significantly different (post hoc t-test, P < 0.0001). Character size was significantly different between the free writing and free writing ON condition in the consistent micrographia group (two-sample t-test, P < 0.0001). In contrast, dopamine administration did not induce a significant difference of Mb values between the free writing ON and free writing condition in the progressive micrographia group (two-sample t-test, P = 0.451; Fig. 1 and Table 2).
There was no significant effect of group or experimental condition on writing speed (repeated-measures ANOVA, P > 0.05). In addition, we found no significant change of speed during the writing process in each condition in each group, all slopes of writing speed across functional MRI session were within ± 0.012 (regression analysis), which demonstrated that there was no progressive slowing of movement or ‘fatigue’ in our subjects. Additionally, there was no significant correlation between the Fatigue Severity Scale and Mb values at the free writing condition (r = −0.27). The average pen pressure in the control, consistent micrographia, and progressive micrographia groups was 121.28 ± 16.28, 113.61 ± 17.06, and 111.72 ± 18.04 pascals, respectively. There was no significant difference in force of writing among the groups.
Brain activation
In all groups, free writing was associated with activations in the left primary motor area, left pre-SMA and caudal SMA, right rCMA, bilateral PMC, left ventral PMC, right superior parietal lobule (SPL), bilateral inferior parietal lobule (IPL), left putamen, left thalamus, left fusiform gyrus and bilateral cerebellum (one-sample t-test, P < 0.05, FWE corrected; Supplementary Fig. 2). There was a significant interaction between group (control and consistent micrographia) and condition (free writing and attention on size) in the left anterior and posterior putamen, left thalamus, left pre-SMA, left caudal SMA, bilateral PMC, left ventral PMC, left dorsolateral prefrontal cortex (DLPFC), left IPL, right SPL, and bilateral cerebellum (repeated-measures ANOVA, P < 0.05, FWE corrected; Supplementary Table 3). There was a significant interaction between group (control and progressive micrographia) and condition (free writing and attention on consistency) in the left anterior and posterior putamen, left pre-SMA, bilateral PMC, left ventral PMC, left DLPFC, right rCMA, right SPL, and bilateral cerebellum (repeated-measures ANOVA, P < 0.05, FWE corrected; Supplementary Table 4). In addition, there was a significant interaction between group (consistent micrographia and progressive micrographia) and condition (free writing and free writing ON) in the left posterior putamen, left thalamus, left pre-SMA, left caudal SMA, right rCMA, left IPL and bilateral cerebellum (repeated-measures ANOVA, P < 0.05, FWE corrected).
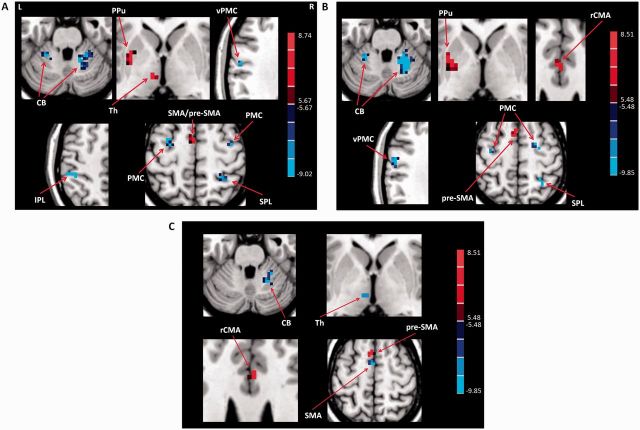
In the free writing condition, patients with consistent micrographia had less activity in the left posterior putamen, left pre-SMA, left caudal SMA, and left thalamus, but had more activity in the bilateral PMC, left ventral PMC, left IPL, right SPL and bilateral cerebellum; while progressive micrographia patients had less activity in the left posterior putamen, left pre-SMA, and right rCMA, but had more activity in the bilateral PMC, left ventral PMC, right SPL and bilateral cerebellum compared with controls. In addition, consistent micrographia patients had more activity in the left pre-SMA and right rCMA, but had less activity in the right cerebellum, left caudal SMA and left thalamus compared to patients with progressive micrographia (post hoc t-test, P < 0.05, FWE corrected; Fig. 2).
Figure 2.
Differences of brain activation between the groups. Differences of brain activation between Parkinson’s disease patients with consistent micrographia and controls (A), between Parkinson’s disease patients with progressive micrographia and controls (B), and between Parkinson’s disease patients with consistent micrographia and patients with progressive micrographia (C) in performing free writing task (post hoc t-test, P < 0.05, FWE corrected). Red and blue colours indicate increased and decreased activation in controls compared to patients, or in patients with consistent micrographia compared to patients with progressive micrographia, respectively. T-value bars are shown on the right. L = left; R = right; CB = cerebellum; PPu = posterior putamen; pre-SMA = rostral supplementary motor area; SMA = caudal supplementary motor area; Th = thalamus; vPMC = ventral premotor cortex.
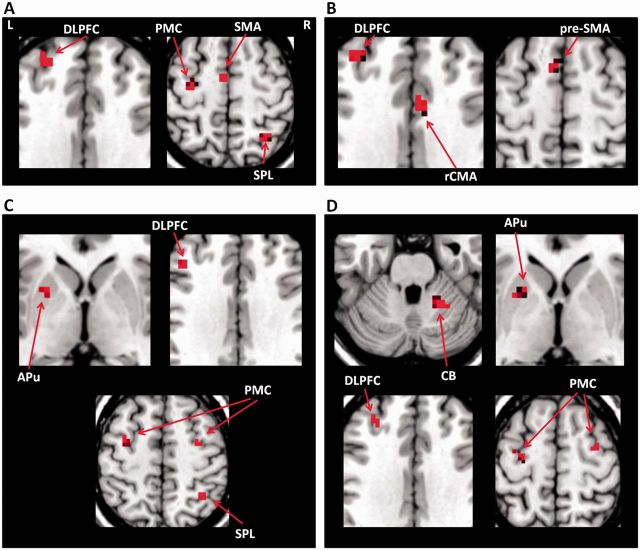
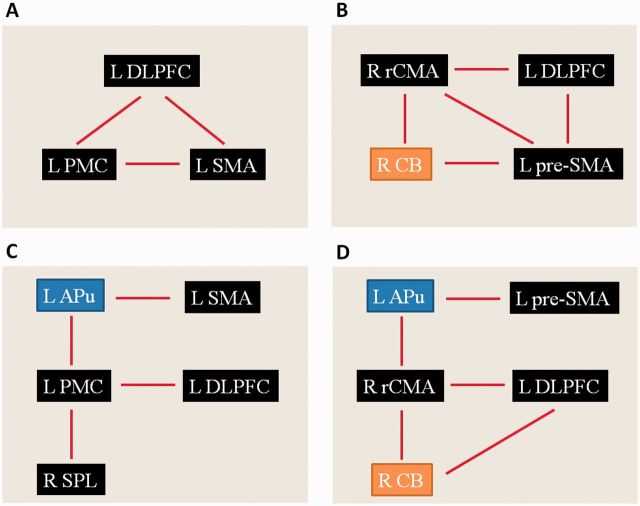
Focus of attention on size enhanced activity in the left caudal SMA, left DLPFC, right SPL, and left PMC in healthy controls; but increased the activity in the left anterior putamen, bilateral PMC, right SPL and left DLPFC in the consistent micrographia group compared to the free writing condition. Focus of attention on consistency enhanced activity in the left pre-SMA, right rCMA, and left DLPFC in controls; but increased activity in the left anterior putamen, bilateral PMC, left DLPFC and right cerebellum in the progressive micrographia group compared to the free writing condition (post hoc t-test, P < 0.05, FWE corrected; Fig. 3).
Figure 3.
The effects of attention on writing related brain activity. (A) Enhanced brain activations in the attention on size condition compared to the free writing condition in controls. (B) Enhanced brain activations in the attention on consistency condition compared to the free writing condition in controls. (C) Increased brain activations in the attention on size condition compared to the free writing condition in Parkinson’s disease patients with consistent micrographia. (D) Increased brain activations in the attention on consistency condition compared to the free writing condition in patients with Parkinson’s disease with progressive micrographia. L = left; R = right; APu = anterior putamen; CB = cerebellum; pre-SMA = rostral supplementary motor area; SMA = caudal supplementary motor area.
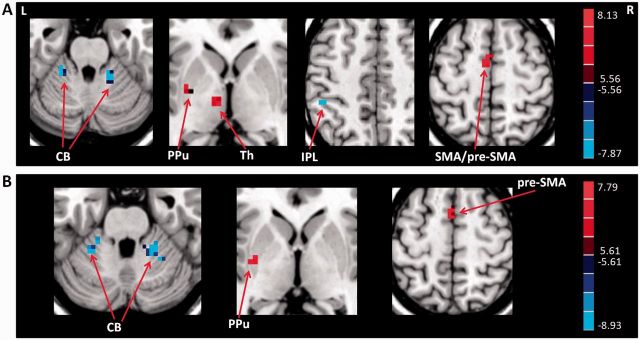
Compared to the free writing condition, free writing ON condition increased activity in the left posterior putamen, left thalamus, left pre-SMA, and left caudal SMA, but decreased activity in the bilateral cerebellum and left IPL in the consistent micrographia group; and increased activity in the left posterior putamen and left pre-SMA, but decreased activity in the bilateral cerebellum in the progressive micrographia group (post hoc t-test, P < 0.05, FWE corrected; Fig. 4). In addition, we checked the difference of dopaminergic effects between the consistent micrographia and progressive micrographia groups. We found that patients with consistent micrographia had more increased activity in the left thalamus and left caudal SMA compared to patients with progressive micrographia; whereas patients with progressive micrographia had more decreased activity in the right cerebellum and more increased activity in the left pre-SMA compared to patients with consistent micrographia with administration of levodopa (post hoc t-test, P < 0.05, FWE corrected).
Figure 4.
The effects of levodopa administration on brain activations. The effects of levodopa administration on brain activations in Parkinson’s disease patients with consistent (A) and progressive micrographia (B). Post hoc t-test, P < 0.05, FWE corrected. Red and blue colours indicate increased and decreased activation in the free writing ON condition compared to the free writing condition, respectively. T-value bars are shown on the right. L = left; R = right; CB = cerebellum; PPu = posterior putamen; pre-SMA = rostral supplementary motor area; SMA = caudal supplementary motor area; Th = thalamus.
In the consistent micrographia group, the activity in the left posterior putamen, left thalamus, and left caudal SMA had positive correlations with mean size of the characters in the free writing condition, as well as had positive correlations with the Δsize of the characters in the free writing ON condition. Additionally, the activity in the left PMC and left anterior putamen had positive correlations with the Δsize of the characters in the attention on size condition. In the progressive micrographia group, the activity in the left posterior putamen, left pre-SMA and right rCMA had positive correlations, while the activation in the right cerebellum had a negative correlation with the Mb values in the free writing condition. The activity in the right cerebellum and left anterior putamen had positive correlations with the ΔMb values in the attention on consistency condition (regression analysis, P < 0.05, FWE corrected; Supplementary Table 5).
Functional connectivity
As we were focused on micrographia-related network changes and the effects of attention and levodopa treatment on these networks, we only present the details of between condition or group differences and regression analysis here. In the free writing condition, all patients showed decreased connectivity between the left posterior putamen and several cortical and subcortical regions compared to controls. In the progressive micrographia group, the connectivity among the left pre-SMA, right rCMA and right cerebellum was also decreased compared with controls. Additionally, patients had some increased connections compared to controls (repeated-measures ANOVA and post hoc t-test, P < 0.05, FWE corrected; Supplementary Tables 6 and 7). The comparison between the consistent micrographia and progressive micrographia groups showed different patterns of connectivity (post hoc t-test, P < 0.05, FWE corrected; Supplementary Table 8).
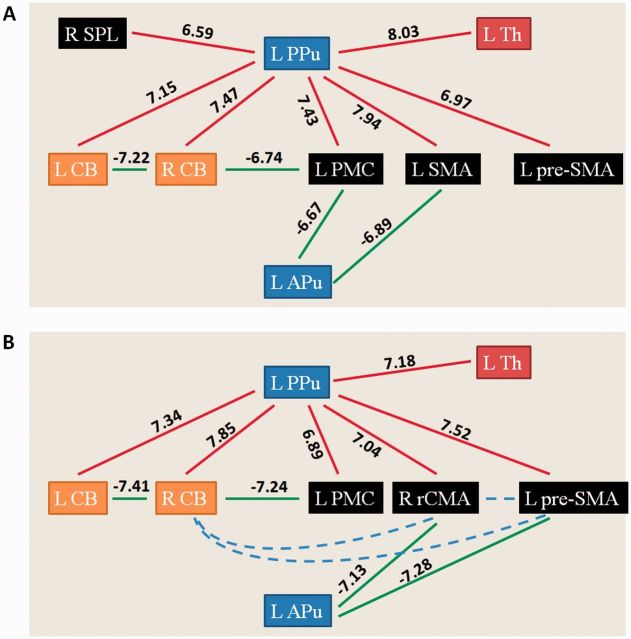
In healthy controls, focus of attention on size enhanced connectivity between the left caudal SMA and left PMC, left DLPFC and left caudal SMA, and between the left DLPFC and left PMC; while focus of attention on consistency increased the connectivity among the left pre-SMA, right rCMA, right cerebellum and left DLPFC compared to the free writing condition. In the consistent micrographia group, focus of attention on size increased connectivity between the left anterior putamen and left PMC, left anterior putamen and left caudal SMA, left PMC and left DLPFC, and between the left PMC and right SPL. In the progressive micrographia group, focus of attention on consistency strengthened connectivity between the left anterior putamen and left pre-SMA, left anterior putamen and right rCMA, right rCMA and right cerebellum, and between the left DLPFC and right cerebellum (post hoc t-test, P < 0.05, FWE corrected; Fig. 5). Administration of levodopa increased connectivity between the left posterior putamen and several cortical and subcortical regions in all patients. Additionally, some connections were weakened compared to the levodopa OFF condition, like between the left anterior putamen and left PMC/caudal SMA in the consistent micrographia group, and between the left anterior putamen and pre-SMA/rCMA in the progressive micrographia group (post hoc t-test, P < 0.05, FWE corrected; Fig. 6). The results of the regression analysis on network connectivity are shown in Supplementary Table 9.
Figure 5.
The effects of attention on writing related network. (A) Enhanced network connectivity in the attention on size condition compared to the free writing condition in controls. (B) Enhanced network connectivity in the attention on consistency condition compared to the free writing condition in controls. (C) Increased network connectivity in the attention on size condition compared to the free writing condition in Parkinson’s disease patients with consistent micrographia. (D) Increased network connectivity in the attention on consistency condition compared to the free writing condition in Parkinson’s disease patients with progressive micrographia. L = left; R = right; APu = anterior putamen; CB = cerebellum; pre-SMA = rostral supplementary motor area; SMA = caudal supplementary motor area.
Figure 6.
The effects of levodopa administration on writing related network. The effects of levodopa administration on writing related network connectivity in Parkinson’s disease patients with consistent (A) and progressive micrographia (B). Post hoc t-test, P < 0.05, FWE corrected. Red and green colours indicate increased and decreased connectivity in the free writing ON condition compared to the free writing condition, respectively. T-values of these connections are shown on each connection. Blue dashed line indicates disconnection among cerebellum, pre-SMA and rCMA. L = left; R = right; APu = anterior putamen; CB = cerebellum; PPu = posterior putamen; pre-SMA = rostral supplementary motor area; SMA = caudal supplementary motor area; Th = thalamus.
Discussion
The most important finding is that consistent micrographia and progressive micrographia are associated with different neural mechanisms. Dysfunction of the basal ganglia motor circuit appears to contribute to consistent micrographia, whereas dysfunction of the basal ganglia motor circuit plus disconnections among the pre-SMA, rCMA and cerebellum is likely involved in progressive micrographia. Attention recruited additional brain circuits to improve both consistent and progressive micrographia, while dopamine benefited only consistent micrographia by restoring the function of basal ganglia motor circuit.
All our subjects had considerable practice before functional MRI scanning. The writing tasks were overlearned, all subjects could perform the tasks correctly and at required speed and force. In addition, we found no clear fatigue in our patients. Thus, writing speed, force, fatigue, and motor learning should have no significant effect on our imaging results.
Consistent micrographia-related neural modulations
Parkinson’s disease patients and healthy controls activated similar brain regions during handwriting (Supplementary Fig. 2). This pattern of handwriting-related brain activity is largely consistent with previous reports (Matsuo et al., 2000; Katanoda et al., 2001; Sugihara et al., 2006; Roux et al., 2009; Horovitz et al., 2013; Planton et al., 2013). Patients with consistent micrographia had modulated activity and connectivity in several regions compared to controls during free writing (Fig. 2 and Supplementary Table 6). Among these regions, the activity and connectivity of the left posterior putamen, left thalamus and left caudal SMA had significantly positive correlation with the character sizes. All these areas are involved in the cortico-basal ganglia-thalamo-cortical motor circuit. The dysfunction of this circuit is linked to many motor difficulties in Parkinson’s disease, like akinesia and bradykinesia (DeLong and Wichmann, 2007). Our findings indicate that the disrupted basal ganglia motor circuit is involved in consistent micrographia (Gangadhar et al., 2008). As the dysfunction of basal ganglia motor circuit progresses, the global reduction of handwriting size becomes more severe.
Clinical studies have shown that focal damage in the putamen or thalamus can result in micrographia (Lewitt, 1983; Pullicino et al., 1994; Kim et al., 1998; Troyer et al., 2004; Sakurai et al., 2011). Studies on primates or human subjects suggest that putamen and internal globus pallidus are involved in controlling the amplitude of movement (DeLong et al., 1984; Turner et al., 1998, 2003; Desmurget et al., 2004; Spraker et al., 2007). In addition, it has been postulated that movement amplitude is regulated by phasic signals from the basal ganglia to the SMA (Alexander and Crutcher, 1990). Applying 5 Hz repetitive transcranial magnetic stimulation over the caudal SMA could increase the global size of handwriting in patients with Parkinson’s disease (Randhawa et al., 2013). These reports provide support regarding the involvement of the basal ganglia motor circuit in consistent micrographia.
Besides these areas, the connectivity between the left posterior putamen and left PMC had a significantly positive correlation with the character sizes. The part of the PMC that had decreased connectivity with the putamen in the current study was located in the posterior part of the middle frontal gyrus, which is considered as a ‘writing centre’, an area specifically involved in writing, and is usually referred to as Exner’s area (Roux et al., 2009, 2010; Planton et al., 2013). The role of this area in writing is suggested as an interface between orthographic or graphemic abstract representations and the generation of motor commands (Roux et al., 2009). The disconnection between the putamen and this ‘writing centre’ may be a reason contributing to the writing difficulties in Parkinson’s disease.
At the same time, the activation in the left PMC and the connectivity between the left anterior putamen and left PMC was increased in the consistent micrographia group compared to controls, and this activation and connectivity were negatively correlated with the character sizes. This indicates that as consistent micrographia becomes more severe, this activation and connectivity are more enhanced, possibly as a compensation for the basal ganglia dysfunction (Rascol et al., 1997; Catalan et al., 1999; Wu and Hallett, 2005; Wu et al., 2010, 2011).
Progressive micrographia-related neural modulations
Although patients with progressive micrographia and those with consistent micrographia activated the same neural network during handwriting, these two groups had different activity in several brain regions (Fig. 2). This finding demonstrates that the neural mechanism in progressive micrographia is different from that in consistent micrographia. The activity in the left posterior putamen, left pre-SMA and right rCMA, and the connectivity among these regions was positively correlated with the Mb values, which indicates that the dysfunction of basal ganglia motor circuit may contribute to the genesis of progressive micrographia in Parkinson’s disease. A previous report has shown that a patient with focal ischaemic lesion of the left striatum developed right hand progressive micrographia (Barbarulo et al., 2007). It has been suggested that the basal ganglia play important roles in matching and maintaining the amplitude of a cortically selected movement plan, and in running each component of the plan in a timely manner (Iansek et al., 2006).
The pre-SMA is known to be critical in learning, planning and initiation of a motor sequence (Nachev et al., 2008). Dysfunction of the pre-SMA may induce difficulty in generation and encoding motor representations in sustained activity prior to movement, and maintaining these representations in readiness for each action in a motor sequence (Cunnington et al., 2005). The rCMA connects with the pre-SMA, SMA, PMC, prefrontal areas, cerebellum and striatum (Takada et al., 2001; Beckmann et al., 2009; Hoffstaedter et al., 2014), and involves both motor and cognitive functions (Picard and Strick, 2001; Hoffstaedter et al., 2014). The functions that are traditionally attributed to the pre-SMA, such as programming and execution of movement sequences, actually may also involve the rCMA (Picard and Strick, 1997, 2001). Dysfunction of the rCMA may result in the difficulty in intentional movement initiation, outcome monitoring, and selection of action. A recent study (Lee et al., 2014) found that the degree of volume reduction of the anterior cingulate cortex, corresponding to the rCMA (Immisch et al., 2001), is correlated with the severity of the sequence effect in patients with Parkinson's disease.
In addition to these modulations, the connectivity between the right cerebellum and pre-SMA/rCMA was decreased, possibly as a consequence of the dysfunction of pre-SMA/rCMA. These connections were positively correlated with the Mb values; which indicates that as progressive micrographia progresses, the disconnection of these regions becomes more significant. The cerebellum is involved in timing, detecting, generating, and in controlling the order and precise execution of motor sequences (Ivry and Keele, 1989; Leggio et al., 2008; D’Angelo, 2011). It may contain high-level cognitive function processing controlling the correctness of internal brain predictions (D’Angelo, 2011). The disconnection of the cerebellum and pre-SMA/rCMA pathway might impair the controlling of motor sequences. Our findings suggest that progressive micrographia is associated with the interaction of dysfunction of basal ganglia motor circuit and disconnection of the pre-SMA, rCMA and cerebellum.
We also found decreased connectivity between the posterior putamen and cerebellum in patients with progressive micrographia compared to controls (Supplementary Table 7). As the cerebellum receives a disynaptic projection from the basal ganglia (Bostan et al., 2010), this decreased connectivity is likely a reflection of the abnormal basal ganglia outflow to the cerebellum. Because this connectivity did not correlate with the severity of progressive micrographia, and normalization of this connectivity with levodopa (Fig. 6) did not improve progressive micrographia, we cannot prove that the disconnection of basal ganglia-cerebellar pathway is critical in the genesis of progressive micrographia. However, the abnormal outflow from the basal ganglia should disturb the function of cerebellum, which in turn might contribute to disconnection of the pre-SMA, rCMA and cerebellum.
In contrast to the weakened connections of the cerebellum, the activation in the cerebellum was enhanced in progressive micrographia patients compared to controls (Fig. 2). Additionally, the activity in the cerebellum was negatively correlated with the Mb values, which indicates that as progressive micrographia progresses, the activity in the cerebellum becomes stronger. The nature of the hyperactivation in the cerebellum in Parkinson’s disease remains unclear. One common explanation is that this phenomenon presents a functional compensation for the defective basal ganglia (Rascol et al., 1997; Catalan et al., 1999; Wu and Hallett, 2005, 2013; Yu et al., 2007). Presumably, this compensatory effort was not strong enough to overcome the dysfunction of the pre-SMA/rCMA; the disconnection between the cerebellum and pre-SMA/rCMA was still significant. However, it is also possible that the increased activity in the cerebellum reflects a primary pathophysiological change of Parkinson’s disease, as a consequence of the inability to inhibit contextually inappropriate circuits secondary to abnormal basal ganglia outflow (Mink, 1996; Grafton et al., 2006; Wu and Hallett, 2013). Then, hyperactivation in the cerebellum might be associated with the genesis of progressive micrographia instead of being compensatory.
Progressive micrographia is a manifestation of the sequence effect. The pathophysiological mechanisms underlying sequence effect have been unclear. It is controversial whether the sequence effect relates to fatigue (Agostino et al., 1992; Kang et al., 2010). Our patients did not show progressive slowing of movement or ‘fatigue’ during functional MRI scanning. Additionally, there was no significant correlation between the Fatigue Severity Scale and the severity of progressive micrographia. Thus, our findings suggest that the sequence effect is not related to fatigue (Kang et al., 2010). Another reason speculated to contribute to the sequence effect is dopaminergic deficit (Agostino et al., 1992). A recent study showed that the sequence effect is related to the reduced dopaminergic function of the caudate nucleus (Lee et al., 2014). However, as levodopa has no significant impact on the sequence effect (Iansek et al., 2006), neural networks outside the basal ganglia should be involved in the genesis of the sequence effect. Our findings that both dysfunction of basal ganglia motor circuit and disconnected network of pre-SMA, rCMA and cerebellum are associated with progressive micrographia provide new insights into neural correlates of the sequence effect. Whether the sequence effect in other motor deficits (e.g. parkinsonian gait) shares similar neural mechanisms needs further investigation.
Why attention improves micrographia
Attention significantly improved both consistent micrographia and progressive micrographia (Table 2), which is consistent with previous reports that external cues or attention could improve handwriting in Parkinson’s disease (Oliveira et al., 1997; Swinnen et al., 2000; Nieuwboer et al., 2009; Bryant et al., 2010; Ringenbach et al., 2011). In both controls and patients, attention to writing commonly enhanced activity in the DLPFC compared to the free writing condition (Fig. 3). The DLPFC is critical in attentional networks (Deiber et al., 1991; Owen et al., 1996; Jueptner et al., 1997). Greater activation of the DLPFC when attending to learned movements has been reported (Jueptner et al., 1997; Rowe et al., 2002; Wu et al., 2015).
Attention increased activation in the caudal SMA or pre-SMA in healthy subjects, but not in patients with Parkinson’s disease. This may be a consequence of the dysfunction of basal ganglia motor circuit. In healthy controls, the enhanced activity and connectivity was restricted to the cortical areas and cerebellum. In contrast, attention was accompanied by increased activity in the left anterior putamen in both consistent micrographia and progressive micrographia patients (Fig. 3). In addition, patients with consistent micrographia had increased connectivity between the anterior putamen and PMC/caudal SMA, whereas patients with progressive micrographia had strengthened connectivity between the anterior putamen and pre-SMA/rCMA (Fig. 5). Moreover, these connections were positively correlated with the improvement of micrographia. These findings demonstrate that attentional strategies recruit the anterior putamen-cortical motor circuit to improve handwriting in patients with Parkinson’s disease.
Handwriting is a well-habituated, coordinated motor skill which has been exercised for many years. Although writing can be considered a visually controlled motor task, it also consists of highly automatically performed features, including writing size and consistency (Longstaff and Heath, 1997; Blank et al., 1999; Nackaerts et al., 2013). A recent study evaluated the effect of a secondary cognitive task on the performance of handwriting in patients with Parkinson’s disease (Broeder et al., 2014), and showed that dual-tasking significantly reduced writing amplitude in patients, but not in healthy controls. It has been suggested that one reason underlying the difficulty in performing dual motor and cognitive tasks in Parkinson’s disease patients is the less automaticity in performing the motor task (Wu and Hallett, 2008). Thus, impaired motor automaticity is likely a reason contributing to micrographia in Parkinson’s disease.
The posterior putamen is a sensorimotor area, which appears critical for support in acquiring and running automatic programs (Miyachi et al., 1997, 2002; Lehéricy et al., 2005; Yin et al., 2005; Yin and Knowlton, 2006). Impairment of the sensorimotor putamen relates to the difficulty in advancing learned motor skills to the automatic stage, as well as in performing previously acquired automated movements (Wu and Hallett, 2005; Wu et al., 2010, 2015). In Parkinson’s disease, the dopamine neurons are heavily degenerated in the posterior putamen, but are relatively spared in the anterior putamen (Kish et al., 1988; Nurmi et al., 2001). Loss of dopaminergic neurons can trigger collateral sprouting of residual neurons (Finkelstein et al., 2000; Song and Haber, 2000), and thus spared dopaminergic fibres in the anterior putamen may compensate for severe dopamine depletion in the posterior putamen (Mounayar et al., 2007) helping to execute desired movements.
The anterior putamen is considered an association area, which is more involved in acquisition of new motor skills and in regulating attentionally controlled behaviours (Miyachi et al., 1997, 2002; Lehéricy et al., 2005; Yin et al., 2005; Yin and Knowlton, 2006). It is possible that patients need more involvement of the associative striatum with attentional control of handwriting. In addition, attention recruited the DLPFC in patients. Anatomical studies have demonstrated the connection between the associative striatum and prefrontal cortex (Middleton and Strick, 2000). Attention also recruited the right SPL in control and consistent micrographia group. The SPL is suggested as a major node of the goal-directed attention network (Corbetta and Shulman, 2002; Yantis et al., 2002); and may be involved in the representation, selection and production of letter shapes during writing (Rapp and Dufor, 2011). These findings suggest that using attentional strategies is to allow handwriting to be mediated less by impaired automatic processes and more by attentional control processes, in order to improve micrographia (Morris et al., 1996; Cunnington et al., 1999; Redgrave et al., 2010; Wu et al., 2015).
In patients with progressive micrographia, attention on writing consistency additionally increased activity in the right cerebellum and strengthened the connectivity between the right cerebellum and right rCMA, which was positively correlated with the improvement of progressive micrographia. This enhanced activity and connectivity in the cerebellum is likely a compensatory effort helping to overcome the dysfunction of the putamen-pre-SMA/rCMA pathway to improve progressive micrographia.
Effects of levodopa on micrographia
Administration of levodopa significantly improved writing size compared to the free writing state in the consistent micrographia group (Fig. 1 and Table 2). This finding is consistent with a previous observation (McLennan et al., 1972). Levodopa increased activity and connectivity of the posterior putamen-thalamus-caudal SMA pathway (Figs 4 and 6), which was positively correlated with the improvement of writing size. At the same time, the connectivity between the anterior putamen and PMC/caudal SMA was decreased (Fig. 6). These findings suggest that levodopa could relatively restore the function of cortico-basal ganglia-thalamo-cortical motor circuit, which in turn improves the micrographia. Functional restoration within the sensorimotor striatum might strengthen the automatic control of writing size; as a consequence, attentional control becomes less necessary.
Similar to that in the consistent micrographia group, administration of levodopa increased activity in the putamen and pre-SMA in the progressive micrographia group (Fig. 4). In contrast, levodopa did not increase the connections among the pre-SMA, rCMA and cerebellum (Fig. 6). Therefore, although the posterior putamen-cortical motor pathway was partially normalized, the disconnection of the pre-SMA, rCMA and cerebellum was not repaired. As an apparent consequence, progressive micrographia was not improved by dopamine (Ling et al., 2012). It has been demonstrated that there is a highly selective loss of cortico-cortical projecting pyramidal neurons in the pre-SMA (MacDonald and Halliday, 2002). Thus, even if the activation in the pre-SMA was partially normalized, the influence from the pre-SMA to other regions remained limited, which might be a reason restricting the repair of disconnection of the pre-SMA, rCMA and cerebellum.
A limitation of this study is that we chose patients with selective consistent micrographia or progressive micrographia. Most patients with micrographia present both consistent and progressive micrographia; thus, neural modulations in these patients will need to be investigated in future studies. Another limitation is the difference of character size between the controls and patients. However, we cannot control character size as it is a reflection of neural mechanisms underlying micrographia. This behavioural difference may have influence on our results.
Conclusion
Our findings demonstrate that consistent micrographia is related to dysfunction of the basal ganglia motor circuit; while a combination of dysfunction of the basal ganglia motor circuit and disconnection of the pre-SMA, rCMA and cerebellum is associated with progressive micrographia. Attention recruits additional brain circuits to bypass impaired automatic processes to improve micrographia. Levodopa intervention improves consistent micrographia by restoring the function of the basal ganglia motor circuit. In contrast, levodopa does not improve progressive micrographia, and this may be due to its failure to repair the disconnection of the pre-SMA, rCMA and cerebellum. This study provides further understanding of both hypokinesia and the sequence effect in Parkinson’s disease.
Funding
This work was supported by grants from the National Science Foundation of China (81071012 and 81271429), and Seed Grant of International Alliance of Translational Neuroscience (PXM2014_014226_000015). Dr Hallett is supported by the NINDS Intramural Program.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- DLPFC
dorsolateral prefrontal cortex
- IPL
inferior parietal lobule
- PMC
premotor cortex
- SMA
supplementary motor area
- rCMA
rostral cingulate motor area
- SPL
superior parietal lobule
References
- Agostino R, Berardelli A, Formica A, Accornero N, Manfredi M. Sequential arm movements in patients with Parkinson’s disease, Huntington’s disease and dystonia. Brain 1992; 115: 1481–95. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 1990; 13: 266–71. [DOI] [PubMed] [Google Scholar]
- Barbarulo AM, Grossi D, Merola S, Conson M, Trojano L. On the genesis of unilateral micrographia of the progressive type. Neuropsychologia 2007; 45: 1685–96. [DOI] [PubMed] [Google Scholar]
- Becker G, Müller A, Braune S, Büttner T, Benecke R, Greulich W, et al. Early diagnosis of Parkinson’s disease. J Neurol 2002; 249: 40–8. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci 2009; 29: 1175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benecke R, Rothwell JC, Dick JPR, Day BL, Marsden CD. Disturbance of sequential movements in patients with Parkinson's disease. Brain 1987; 110: 361–79. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Dick JP, Rothwell JC, Day BL, Marsden CD. Scaling of the size of the first agonist EMG burst during rapid wrist movements in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 1986; 49: 1273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank R, Miller V, von Voss H, von Kries R. Effects of age on distally and proximally generated drawing movements: a kinematic analysis of school children and adults. Dev Med Child Neurol 1999; 41: 592–6. [DOI] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA 2010; 107: 8452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick MP, Van Gemmert AWA, Shill HA, Stelmach GE. Hypometria and bradykinesia during drawing movements in individuals with Parkinson’s disease. Exp Brain Res 2009; 197: 223–33. [DOI] [PubMed] [Google Scholar]
- Broeder S, Nackaerts E, Nieuwboer A, Smits-Engelsman BCM, Swinnen SP, Heremans E. The effects of dual tasking on handwriting in patients with Parkinson’s disease. Neuroscience 2014; 263: 193–202. [DOI] [PubMed] [Google Scholar]
- Brooks DJ, Ibanez V, Sawle GV, Quinn N, Lees AJ, Mathias CJ, et al. Differing patterns of striatal 18F-dopa uptake in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol 1990; 28: 547–55. [DOI] [PubMed] [Google Scholar]
- Bryant MS, Rintala DH, Lai EC, Protas EJ. An investigation of two interventions for micrographia in individuals with Parkinson’s disease. Clin Rehabil 2010; 24: 1021–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan MJ, Ishii K, Honda M, Samii A, Hallett M. A PET study of sequential finger movements of varying length in patients with Parkinson’s disease. Brain 1999; 122: 483–95. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 2002; 3: 201–15. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Iansek R, Bradshaw JL. Movement-related potentials in Parkinson’s disease: external cues and attentional strategies. Mov Disord 1999; 14: 63–8. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Moser E. Premovement activity of the pre-supplementary motor area and the readiness for action: studies of time-resolved event-related functional MRI. Hum Mov Sci 2005; 24: 644–56. [DOI] [PubMed] [Google Scholar]
- D’Angelo E. Neural circuits of the cerebellum: hypothesis for function. J Integr Neurosci 2011; 10: 317–52. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res 1991; 84: 393–402. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Alexander GE, Georgopoulos AP, Crutcher MD, Mitchell SJ, Richardson RT. Role of basal ganglia in limb movements. Hum Neurobiol 1984; 2: 235–44. [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol 2007; 64: 20–4. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton ST, Vindras P, Gréa H, Turner RS. The basal ganglia network mediates the planning of movement amplitude. Eur J Neurosci 2004; 19: 2871–80. [DOI] [PubMed] [Google Scholar]
- Duarte J, Claveria LE, de Pedro-Cuesta J, Sempere AP, Coria F, Calne DB. Screening Parkinson's disease: a validated questionnaire of high specificity and sensitivity. Mov Disord 1995; 10: 643–9. [DOI] [PubMed] [Google Scholar]
- Finkelstein DI, Stanic D, Parish CL, Tomas D, Dickson K, Horne MK. Axonal sprouting following lesions of the rat substantia nigra. Neuroscience 2000; 97: 99–112. [DOI] [PubMed] [Google Scholar]
- Gangadhar G. Joseph D, Chakravarthy VS. Understanding Parkinsonian handwriting through a computational model of basal ganglia. Neural Comput 2008; 20: 2491–525. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Turner RS, Desmurget M, Bakay R, Delong M, Vitek J, et al. Normalizing motor-related brain activity: subthalamic nucleus stimulation in Parkinson disease. Neurology 2006; 66: 1192–9. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967; 17: 427–42. [DOI] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero-Gallagher N, Laird AR, et al. The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Hum Brain Mapp 2014; 35: 2741–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz SG, Gallea C, Najee-ullah M, Hallett M. Functional anatomy of writing with the dominant hand. Plos One 2013; e67931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55: 181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iansek R, Huxham F, McGinley J. The sequence effect and gait festination in Parkinson disease: contributors to freezing of gait? Mov Disord 2006; 21: 1419–24. [DOI] [PubMed] [Google Scholar]
- Immisch I, Waldvogel D, van Gelderen P, Hallett M. The role of the medial wall and its anatomical variations for bimanual antiphase and in-phase movements. NeuroImage 2001; 14: 674–84. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Keele SW. Timing functions of the cerebellum. J Cognit Neurosci 1989; 1: 136–52. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RSJ. Anatomy of motor learning. I. Frontal cortex and attention to action. J Neurophysiol 1997; 77: 1313–24. [DOI] [PubMed] [Google Scholar]
- Kang SY, Wasaka T, Shamim EA, Auh S, Ueki Y, Lopez GJ, et al. Characteristics of the sequence effect in Parkinson's disease. Mov Disord 2010; 25: 2148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katanoda K, Yoshikawa K, Sugishita M. A functional MRI study on the neural substrates for writing. Hum Brain Mapp 2001; 13: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Lee BH, Park KC, Lee WY, Na DL. Micrographia on free writing versus copying tasks in idiopathic Parkinson’s disease. Parkinsonism Relat Disord 2005; 11: 57–63. [DOI] [PubMed] [Google Scholar]
- Kim JS, Im JH, Kwon SU, Kang JH, Lee MC. Micrographia after thalamo-mesencephalic infarction: evidence of striatal dopaminergic hypofunction. Neurology 1998; 51: 625–7. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med 1988; 318: 876–80. [DOI] [PubMed] [Google Scholar]
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46: 1121–3. [DOI] [PubMed] [Google Scholar]
- Lang AE, Fahn S. Assessment of Parkinson’s disease. In: Munsat TL, editor. Quantification of neurological deficit. Boston: Butterworths; 1989, p. 285–309. [Google Scholar]
- Lange KW, Mecklinger L, Walitza S, Becker G, Gerlach M, Naumann M, et al. Brain dopamine and kinematics of graphomotor functions. Hum Mov Sci 2006; 25: 492–509. [DOI] [PubMed] [Google Scholar]
- Lee E, Lee JE, Yoo K, Hong JY, Oh J, Sunwoo MK, et al. Neural correlates of progressive reduction of bradykinesia in de novo Parkinson's disease. Parkinsonism Relat Disord 2014; 20: 1376–81. [DOI] [PubMed] [Google Scholar]
- Leggio MG, Tedesco AM, Chiricozzi FR, Clausi S, Orsini A, Molinari M. Cognitive sequencing impairment in patients with focal or atrophic cerebellar damage. Brain 2008; 131: 1332–43 [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Benali H, Van de Moortele P, Pélégrini-Issac M, Waechter T, Ugurbil K, et al. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA 2005; 102: 12566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitt PA. Micrographia as a focal sign of neurological disease. J Neurol Neurosurg Psychiatry 1983; 46: 1152–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H, Massey LA, Lees AJ, Brown P, Day BL. Hypokinesia without decrement distinguishes progressive supranuclear palsy from Parkinson’s disease. Brain 2012; 135: 1141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstaff MG, Heath RA. Space–time invariance in adult handwriting. Acta Psychologica 1997; 97: 201–14. [Google Scholar]
- Ma HI, Hwang WJ, Chang SH, Wang TY. Progressive micrographia shown in horizontal, but not vertical, writing in Parkinson’s disease. Behav Neurol 2013; 27: 169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald V, Halliday GM. Selective loss of pyramidal neurons in the pre-supplementary motor cortex in Parkinson's disease. Mov Disord 2002; 17: 1166–73. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Nakai T, Kato C, Moriya T, Isoda H, Takehara Y, et al. Dissociation of writing processes: functional magnetic resonance imaging during writing of Japanese ideographic characters. Brain Res Cogn Brain Res 2000; 9: 281–6. [DOI] [PubMed] [Google Scholar]
- McLennan JE, Nakano K, Tyler HR, Schwab RS. Micrographia in Parkinson's disease. J Neurol Sci 1972; 15: 141–52. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Rev 2000; 31: 236–50. [DOI] [PubMed] [Google Scholar]
- Mink J. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 1996; 50: 381–425. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Lu XF. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res 2002; 146: 122–6. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Miyashita K, Karadi Z, Rand MK. Differential roles of monkey striatum in learning of sequential hand movement. Exp Brain Res 1997; 115: 1–5. [DOI] [PubMed] [Google Scholar]
- Mounayar S, Boulet S, Tande D, Jan C, Pessiglione M, Hirsch EC, et al. A new model to study compensatory mechanisms in MPTP-treated monkeys exhibiting recovery. Brain 2007; 130: 2898–2914. [DOI] [PubMed] [Google Scholar]
- Morris ME, Lansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain 1996; 119: 551–68. [DOI] [PubMed] [Google Scholar]
- Mutch WJ, Smith WC, Scott RF. A screening and alerting questionnaire for parkinsonism. Neuroepidemiology 1991; 10: 150–6. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 2008; 9: 856–69. [DOI] [PubMed] [Google Scholar]
- Nackaerts E, Vervoort G, Heremans E, Smits-Engelsman BC, Swinnen SP, Nieuwboer A. Relearning of writing skills in Parkinson's disease: a literature review on influential factors and optimal strategies. Neurosci Biobehav Rev 2013; 37: 349–57. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Vercruysse S, Feys P, Levin O, Spildooren J, Swinnen S. Upper limb movement interruptions are correlated to freezing of gait in Parkinson’s disease. Eur J Neurosci 2009; 29: 1422–30. [DOI] [PubMed] [Google Scholar]
- Nurmi E, Ruottinen HM, Bergman J, Haaparanta M, Solin O, Sonninen P, et al. Rate of progression in Parkinson's disease: a 6-[18F]fluoro-L-dopa PET study. Mov Disord 2001; 16: 608–15. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971; 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Owen AM, Evans AC, Petrides M. Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cereb Cortex 1996; 6: 31–8. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol 2001; 11: 663–72. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Activation on the medial wall during remembered sequences of reaching movements in monkeys. J. Neurophysiol 1997; 77: 2197–201. [DOI] [PubMed] [Google Scholar]
- Planton S, Jucla M, Roux F, Démonet J. The “handwriting brain”: a meta-analysis of neuroimaging studies of motor versus orthographic processes. Cortex 2013; 49: 2772–87. [DOI] [PubMed] [Google Scholar]
- Ponsen MM, Daffertshofer A, Wolters EC, Beek PJ, Berendse HW. Impairment of complex upper limb motor function in de novo Parkinson’s disease. Parkinsonism Relat Disord 2008; 14: 199–204. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012; 59: 2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullicino P, Lichter D, Benedict R. Micrographia with cognitive dysfunction: ‘‘minimal’’ sequelae of a putaminal infarct. Mov Disord 1994; 9: 371–3. [DOI] [PubMed] [Google Scholar]
- Randhawa BK, Farley BG, Boyd LA. Repetitive transcranial magnetic stimulation improves handwriting in Parkinson's disease. Parkinsons Dis 2013; 2013: 751925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B, Dufor O. The neurotopography of written word production: an FMRI investigation of the distribution of sensitivity to length and frequency. J Cogn Neurosci 2011; 23: 4067–81. [DOI] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Fabre N, Brefel C, Loubinoux I, Celsis P, et al. The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain 1997; 120: 103–10. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat Rev Neurosci 2010; 11: 760–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringenbach SD, van Gemmert AW, Shill HA, Stelmach GE. Auditory instructional cues benefit unimanual and bimanual drawing in Parkinson’s disease patients. Hum Mov Sci 2011; 30: 770–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum S, Samuel M, Zlotnik S, Erikh I, Schlesinger I. Handwriting as an objective tool for Parkinson’s disease diagnosis. J Neurol 2013; 260: 2357–61. [DOI] [PubMed] [Google Scholar]
- Roux FE, Draper L, Kopke B, Demonet JF. Who actually read Exner? Returning to the source of the frontal “writing centre” hypothesis. Cortex 2010; 46: 1204–10. [DOI] [PubMed] [Google Scholar]
- Roux FE, Dufor O, Giussani C, Wamain Y, Draper L, Longcamp M, et al. The graphemic/motor frontal area Exner’s area revisited. Ann Neurol 2009; 66: 537–45. [DOI] [PubMed] [Google Scholar]
- Rowe J, Stephan KE, Friston K, Frackowiak R, Lees A, Passingham R. Attention to action in Parkinson's disease: impaired effective connectivity among frontal cortical regions. Brain 2002; 125: 276–289. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Yoshida Y, Sato K, Sugimoto I, Mannen T. Isolated thalamic agraphia with impaired grapheme formation and micrographia. J Neurol 2011; 258: 1528–37. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Ceballos-Baumann A, Standhardt H, Auer C, Conrad B, Alesch F. Changes in handwriting resulting from bilateral high-frequency stimulation of the subthalamic nucleus in Parkinson's disease. Mov Disord 1999; 14: 964–71. [DOI] [PubMed] [Google Scholar]
- Song DD, Haber SN. Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting. J Neurosci 2000; 20: 5102–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spraker MB, Yu H, Corcos DM, Vaillancourt DE. Role of individual basal ganglia nuclei in force amplitude generation. J Neurophysiol 2007; 98: 821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara G, Kaminaga T, Sugishita M. Interindividual uniformity and variety of the “Writing center”: a functional MRI study. Neuroimage 2006; 32: 1837–49. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Steyvers M, Van Den Bergh L, Stelmach GE. Motor learning and Parkinson’s disease: refinement of within-limb and between-limb coordination as a result of practice. Behav Brain Res 2000; 111: 45–59. [DOI] [PubMed] [Google Scholar]
- Takada M, Tokuno H, Hamada I, Inase M, Ito Y, Imanishi M, et al. Organization of inputs from cingulate motor areas to basal ganglia in macaque monkey. Eur J Neurosci 2001; 14: 1633–50. [DOI] [PubMed] [Google Scholar]
- Teulings HL, Contreras-Vidal JL, Stelmach GE, Adler CH. Parkinsonism reduces coordination of fingers, wrist, and arm in fine motor control. Exp Neurol 1997; 146: 159–70. [DOI] [PubMed] [Google Scholar]
- Teulings HL, Stelmach GE. Control of stroke size, peak acceleration, and stroke duration in Parkinsonian handwriting. Hum Mov Sci 1991; 10: 315–34. [Google Scholar]
- Troyer AK, Black SE, Armilio ML, Moscovitch M. Cognitive and motor functioning in a patient with selective infarction of the left basal ganglia: evidence for decreased non-routine response selection and performance. Neuropsychologia 2004; 42: 902–11. [DOI] [PubMed] [Google Scholar]
- Tucha O, Mecklinger L, Thome J, Reiter A, Alders GL, Sartor H, et al. Kinematic analysis of dopaminergic effects on skilled handwriting movements in Parkinson's disease. J Neural Transm 2006; 113: 609–23. [DOI] [PubMed] [Google Scholar]
- Turner RS, Desmurget M, Grethe J, Crutcher MD, Grafton ST. Motor subcircuits mediating the control of movement extent and speed. J Neurophysiol 2003; 90: 3958–66. [DOI] [PubMed] [Google Scholar]
- Turner RS, Grafton ST, Votaw JR, Delong MR, Hoffman JM. Motor subcircuits mediating the control of movement velocity: a PET study. J Neurophysiol 1998; 80: 2162–76. [DOI] [PubMed] [Google Scholar]
- Van Gemmert AW, Adler CH, Stelmach GE. Parkinson’s disease patients undershoot target size in handwriting and similar tasks. J Neurol Neurosurg Psychiatry 2003; 74: 1502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gemmert AW, Teulings HL, Contreras-Vidal JL, Stelmach GE. Parkinson’s disease and the control of size and speed in handwriting. Neuropsychologia 1999; 37: 685–94. [DOI] [PubMed] [Google Scholar]
- Van Gemmert AW, Teulings HL, Stelmach GE. Parkinsonian patients reduce their stroke size with increased processing demands. Brain Cogn 2001; 47: 504–12. [DOI] [PubMed] [Google Scholar]
- Van Gemmert AW, Teulings HL, Stelmach GE. The influence of mental and motor load on handwriting movements in Parkinsonian patients. Acta Psychol 1998; 100: 161–75. [DOI] [PubMed] [Google Scholar]
- Wagle Shukla A, Ounpraseuth S, Okun MS, Gray V, Schwankhaus J, Metzer WS. Micrographia and related deficits in Parkinson's disease: a cross-sectional study. BMJ 2012; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SK. The Croonian Lectures. On some disorder of motility and of muscle tone with special reference to the corpus. Lancet 1925; 2: 1–10. [Google Scholar]
- Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain 2005; 128: 2250–9. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. Neural correlates of dual task performance in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 2008; 79: 760–6. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. The cerebellum in Parkinson's disease. Brain 2013; 136: 696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Liu J, Zhang H, Hallett M, Zheng Z, Chan P. Attention to automatic movements in Parkinson’s disease: modified automatic mode in the striatum. Cereb Cortex 2015; 25: 3330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson's disease. NeuroImage 2011; 55: 204–15. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Li K, Chan P. Neural correlates of bimanual anti-phase and in-phase movements in Parkinson's disease. Brain 2010; 133: 2394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, et al. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci 2002; 5: 995–1002. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature Rev Neurosci 2006; 7: 464–76. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Blockade of NMDA receptors in the dorsomedial striatum prevents action–outcome learning in instrumental conditioning. Eur J Neurosci 2005; 22: 505–12. [DOI] [PubMed] [Google Scholar]
- Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. Neuroimage 2007; 35: 222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.