Abstract
Bacterial keratitis is a major cause of corneal ulcers in developing and industrialized nations. In this study, we examined the host innate immune responses to Corynebacterium pseudodiphtheriticum, often overlooked as commensal, in human corneal epithelial cells. The expressions of innate immune mediators were determined by quantitative PCR from corneal ulcers of patients and immortalized human corneal epithelial cells (HCEC). We have found an elevated expression of Toll like receptors (TLRs) along with IL-6 and IL-1β from both ulcers and epithelial cells infected with C. pseudodiphtheriticum. Activation of NF-κB and MAPK signaling pathways were also observed in HCEC in response to C. pseudodiphtheriticum. In addition, we found a significant increase in the expression of antimicrobial peptides S100A8, S100A9 and human β-defensin 1 from both corneal ulcers and HCEC.
Keywords: antimicrobial peptides, cytokines, defensins, epithelial cells, toll like receptors
Introduction
Microbial keratitis is the most prevalent cause of corneal infection worldwide and is a painful disease with severe corneal opacity and visual impairment. Among microbial keratitis, bacterial infection is a major cause of corneal ulcers in India1,2 and other developing countries.3 Gram-positive bacteria are the most common cause of bacterial keratitis among Indian populations and are primarily associated with trauma related to agricultural work.1,2 Although traditionally S. aureus is the most commonly isolated Gram-positive organisms, increasing cases of Corynebacterium infections are also being reported. The number of cases of Corynebacterium keratitis diagnosed at our institute, L.V. Prasad Eye Institute (LVPEI), India has increased from less than 1% to more than 5% over last 5 years and Corynebacterium pseudodiphtheriticum consists of more than 28% of all Corynebacterium infection (unpublished data). A study on the microbial keratitis among the elderly also reported an increase in Corynebacterium infection from 0.7% to 11% over last 3 decades.4 Others have also reported several cases of Corynebacterium keratitis in last few years and the numbers of cases are on the rise.5-7
C. pseudodiphtheriticum is Gram-positive aerobic or facultative anaerobic bacilli found ubiquitously in food, water and soil. Although generally a harmless commensal, it is an opportunistic pathogen and can cause infection in people with predisposing medical conditions like HIV.8,9 However it can cause keratitis in fully immunocompetent individuals. C. pseudodiphtheriticum has been known to cause community based pneumonia10 and nonrespiratory infections including septic arthritis,11 endocarditis9 and dermal infections.12 The review of the literature indicates that C. pseudodiphtheriticum is largely overlooked, especially in developing countries, and should be regarded as an emerging pathogen. However, little is known about pathogenicity and our knowledge regarding host immune responses for C. pseudodiphtheriticum, particularly in corneal infections are very limited.
In this study, to understand the basic pathogenic mechanism underlying C. pseudodiphtheriticum infections, we looked into host responses in corneal tissues infected with C. pseudodiphtheriticum and immune responses of immortalized human corneal epithelial cells exposed to C. pseudodiphtheriticum. Our results show that there is significant elicitation of immune responses in the cornea to this bacterium which is characterized by upregulation of Toll like receptors (TLRs), expression of pro-inflammatory cytokines, and antimicrobial peptides and similar responses were also observed in immortalized human corneal epithelial cells. Thus our data will add to our understanding of the pathogenesis of Corynebacterium keratitis.
Materials and Methods
Statement of Ethics
The protocol for obtaining scrapings of corneal ulcer was reviewed and approved by Institutional Review Board of Hyderabad Eye Research Foundation, LVPEI, India and informed consent forms were obtained from patients. For controls, cadaver corneas unsuitable for transplant were obtained from Ramayamma International Eye Bank (LVPEI, India). Tissue procurement was approved by the Institutional Review Board, LVPEI.
Identification of bacterial strains
Corneal ulcer materials collected aseptically were placed on glass slides for Gram stain and inoculated in different specific media for bacterial cultures. The inoculated plates were incubated overnight and bacteria were identified by Gram stain and were confirmed by sequencing. For bacterial infection experiments, bacteria were grown overnight in thioglycollate media at 37°C, washed in 1X phosphate buffered saline (PBS) and resuspended in cell culture media (108 bacteria/ml).
Cell culture
Human corneal epithelial cell line 10.014 pRSV-T13 was maintained in keratinocyte serum free media containing bovine pituitary extract and recombinant human epidermal growth factors (Invitrogen, Carlsbad, USA) at 37°C and 5% CO2. HCEC were grown overnight in 6 well plates (5 × 105 cells/well) and incubated with bacteria for 4 hours. The cells were then washed with 1X PBS and processed further.
RNA isolation, cDNA synthesis and quantitative PCR analysis
Quantitative real-time PCR was used to determine mRNA expression of different genes from corneal scrapings and human corneal epithelial cells. Total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA), quantity and A260/A280 value of RNA were determined by spectrophotometer. The quality of RNA was checked by agarose gel electrophoresis and was reverse transcribed using Reverse Transcriptase Kit (Eurogentec, Belgium) according to the manufacturer's protocol. Quantitative PCR was performed on ABI PRISM 7000HT Sequence Detection System (Applied Biosystems, Grand Island, NY) using the SYBR Green PCR Master Mix (Applied Biosystems, Grand Island, NY). The primer sequences are shown in Table 1. The optimum concentrations of primers used were standardized and was checked to eliminate primer-dimer formation. Relative quantities of mRNA expression of respective genes were normalized using the 2−ΔΔct method with GAPDH as the housekeeping gene. The final PCR products were analyzed by agarose gel electrophoresis and single band of desired size was obtained in each case.
Table 1.
Oligonucleotide Sequences
| Gene | Sequence (5′→3′) |
|---|---|
| TLR1 | FWD:TTCAAACGTGAAGCTACAGGG REV:CCGAACACATCGCTGACAACT |
| TLR2 | FWD:GCCAAAGTCTTGATTGATTGG REV:TTGAAGTTCTCCAGCTCCTG |
| TLR3 | FWD:CAAACACAAGCATTCGGAATC REV:AAGGAATCGTTACCAACCACAT |
| TLR4 | FWD:TGAGCAGTCGTGCTGGTATC REV:CAGGGCTTTTCTGAGTCGTC |
| TLR5 | FWD:TTCAACTTCCCAAATGAAGGA REV:TTGCATCCAGATGCTTTTCA |
| TLR6 | FWD:TGAATGCAAAAACCCTTCACC REV:CCAAGTCGTTTCTATGTGGTT |
| IL-6 | FWD:CTCACCTCTTCAGAACGAATTG REV:CCATCTTTGGAAGGTTCAGGT |
| IL-8 | FWD:GCAGTTTTGCCAAGGAGTGCT REV:GCATCTGGCAACCCTACAAC |
| IL-1b | FWD:CCACAGACCTTCCAGGAGAA REV:GTGCAGTTCAGTGATCGTACAG |
| BD-1 | FWD:GCCTCCAAAGGAGCCAGCCT REV:CTTCTGGTCACTCCCAGCTCA |
| BD-2 | FWD:CAGCCATCAGCCATGAGG REV:TGGCTTTTTGCAGCATTTT |
| BD-3 | FWD:TCTCAGCGTGGGGTGAAGC REV:CGGCCGCCTCTGACTCTG |
| S100A8 | FWD:CCGAGTGTCCTCAGTATATA REV:GCCCATCTTTATCACCAGAAT |
| S100A9 | FWD:TGGCTCCTCGGCTTTGG REV:CGACATTTTGCAAGTCATCGTC |
| LL-37 | FWD:TCGGATGCTAACCTCTACCG REV:ACAGGCTTTGGCGTGTCT |
| NLRP3 | FWD:CGTGAGTCCCATTAAGATGG REV:CCCGACAGTGGATATAGAACA |
| ASC | FWD:GACGGGGCCAATACCACAC REV:CTGTAACAAAAGTCGTGCTT |
| GAPDH | FWD:GATCCCTCCAAAATCAAGTG REV:GGCAGAGATGATGACCCTTTT |
Western blot analysis
HCEC were incubated with C. pseudodiphtheriticum at 10:1 ratio of bacteria to cells for indicated time points. The cells were washed and lysed and western blot analysis was done as described before14 using antibodies against p-IκBα, p-p38, p-ERK, p-JNK and β-actin (Cell Signaling Technology, Beverly, MA).
p65 Translocation
p65 translocation assay was carried out in HCEC after incubation with C. pseudodiphtheriticum for indicated time points as described earlier.15
Statistical analysis
Statistical analysis was performed using an unpaired t test (Prism; GraphPad Software). P values less than 0.05 were considered significant.
Results
Characteristics of the study population
Corneal ulcers were collected from patients presented at LVPEI, India. LVPEI, a tertiary eye care facility, is routinely involved in diagnosis and treatment of corneal ulcers. The samples were taken from keratitis patients but without any systemic illness. Corneal ulcer scrapings were cultured and subsequent examinations of these isolates confirmed the identity of C. pseudodiphtheriticum. The scrapings were collected from 7 patients identified with C. pseudodiphtheriticum in a period of 10 months and were within 6 to 61 years old. Figure 1A is the representative corneas of a patient with culture proven C. pseudodiphtheriticum keratitis and a representative Gram stained corneal ulcer showing Gram-positive rods of C. pseudodiphtheriticum.
Figure 1.
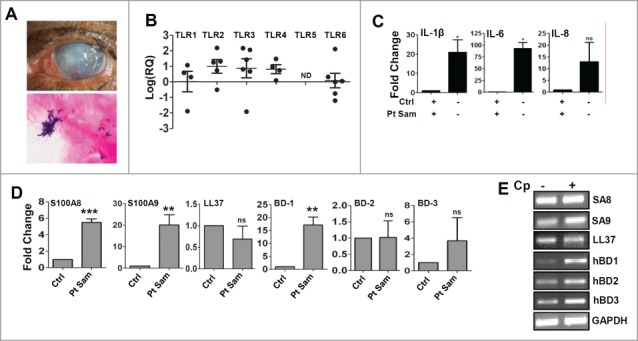
Gene expression of Toll like Receptors, cytokines and antimicrobial peptides in corneal ulcers from patients with C. pseudodiphtheriticum keratitis. (A) Representative corneal ulcer of patient with C. pseudodiphtheriticum keratitis and Gram staining of corneal ulcer material showing Gram-positive C. pseudodiphtheriticum. RNA was isolated from corneal ulcers, reverse transcribed and quantitative PCR was done. Data points represent individual patients infected with C. pseudodiphtheriticum, and the values are presented either as log of relative expression or fold change in relation to uninfected donor corneas. The expressions of Toll like Receptors (B), proinflammatory cytokines (C) and antimicrobial peptides are (D) are shown. The representative gel images of the amplified products of antimicrobial peptides are shown (E). (**) designates P < 0.005.
Innate immune responses during C. pseudodiphtheriticum corneal infections
Toll like receptors are expressed and activated in corneal ulcers caused by bacteria, or filamentous fungi leading to cytokine production. In order to study the innate immune responses during C. pseudodiphtheriticum corneal infection, RNA was isolated from corneal ulcer scrapings collected from Corynebacterium keratitis patients. mRNA expression levels of TLRs, pro-inflammatory cytokines and antimicrobial peptides were determined by quantitative PCR. Figure 1B shows significant elevated expression of TLR1, TLR2, TLR3 and TLR4 compared with uninfected control corneas. Cadaveric corneas unsuitable for transplant but free from any infection were used as controls. There is almost 30 fold increase in TLR2 expression in the infected corneal scrapings compared to the uninfected corneas. However, TLR5 was not detected in any of the samples and there was a significant decrease in the expression of TLR6. 100 fold increase in expression of IL-6 and more than 20 fold increases in IL-1β expression were observed, while no significant changes in IL-8 expression was determined (Fig. 1C). No expressions of IL-17, IL-22 or IFN-γ were either detected (data not shown). Since epithelial cells are known to express antimicrobial peptides, the expressions of human β-defensins and S100A proteins were determined. There was almost 6 fold increase in S100A8 and more than 20 fold increase in S100A9 expressions. Of the human β-defensins, increase of only β-defensin 1 (nearly 20 fold) was observed while no significant changes were seen for β-defensin 2 or β-defensin 3 compared to control (Fig. 1D). There were also no significant changes in the expression of cathelicidin or LL-37. The representative gel images of the antimicrobial peptides are shown (Fig. 1E).
C. pseudodiphtheriticum activates of Toll like Receptors and mediate signaling via NF-κB and MAPK pathways in human corneal epithelial cells
As there is increase in expression of TLRs in corneal scrapings during Corynebacterium keratitis, we wanted to check the expression of these innate immune mediators in response to C. pseudodiphtheriticum in corneal epithelial cells in vitro. Immortalized human corneal epithelial cells were exposed to C. pseudodiphtheriticum for 4 h and expression of TLRs and pro-inflammatory cytokines were monitored by quantitative PCR. As seen in Figure 2A, there is 3-4 fold increase in expression of TLR1, TLR2, and TLR4, compared to uninfected control cells. Surprisingly, TLR3 that was elevated in corneal ulcers of infected patients was found to be down-regulated in immortalized corneal epithelial cell lines. Although there were 3 fold increases in TLR5 and TLR6 expression, the changes were not significant. To determine the pathways by which C. pseudodiphtheriticum mediates signaling, HCEC were exposed to C. pseudodiphtheriticum for defined time periods and p65 translocation and phosphorylation of IkBα, p38, ERK and JNK were examined. As shown in Figure 2B, phosphorylation of IkBα starts by 30 minutes but significantly increases at 60 minutes of exposure to C. pseudodiphtheriticum in HCEC. This is also evident from translocation of p65 subunit from the cytoplasm to the nucleus within 30 minutes of exposure to C. pseudodiphtheriticum, and is persistent after 60 minutes (Fig. 2C). C. pseudodiphtheriticum also causes phosphorylation of p38, ERK and JNK in HCEC within 30 minutes which was maximal after 60 minutes of infection (Fig. 2D). This clearly shows that C. pseudodiphtheriticum mediates cell signaling by both NF-κB and MAPK pathways in human corneal epithelial cells. As cytokines and chemokines transcriptions are induced by NF-κB and are important in TLR-induced corneal infection and inflammation, we examined expression of pro-inflammatory cytokines induced in HCEC by C. pseudodiphtheriticum. It is seen that C. pseudodiphtheriticum causes significant increase in expression of IL-6 (˜20 folds) and IL-1β (∼3 folds) whereas no change in IL-8 expression was observed, similar to the data obtained from patient's samples (Fig. 3A). Since there is an increase in IL-1β expression by C. pseudodiphtheriticum infection in human corneal epithelial cells, we also examined the expression of Nod like receptor protein 3 (NLRP3) together with the adaptor molecule, apoptosis associated speck like protein with caspase activation domain (ASC).These together are responsible for the recruitment of caspase 1 that processes IL-1β into mature and biologically active form. We found an increase in expression of both NLRP3 (˜4 fold) and ASC (∼3.5 fold) in HCEC by C. pseudodiphtheriticum (Fig. 3B).
Figure 2.
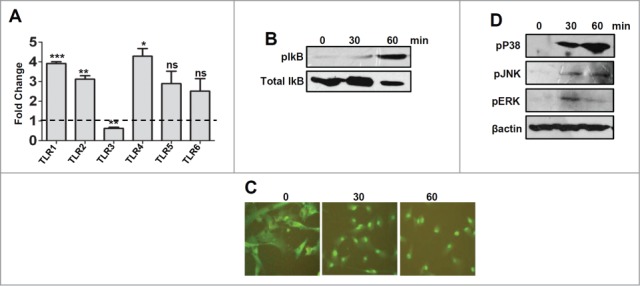
Expression and activation of Toll like Receptors by C. pseudodiphtheriticum in human corneal epithelial cells. Human corneal epithelial cells were infected with C. pseudodiphtheriticum for 4 hours, washed and RNA was isolated, reverse transcribed and quantitative PCR was done to determine the expression of Toll like Receptors in response to C. pseudodiphtheriticum (A); Western blot analysis of phosphorylation of IkBα in HCEC in response to C. pseudodiphtheriticum (B); p65 translocation from cytosol to nucleus in HCEC at 30 and 60 minutes postinfection with C. pseudodiphtheriticum was detected by immunocytochemistry (C). Activation of MAPK pathways in HCEC after incubation with C. pseudodiphtheriticum. At indicated times, cells were harvested and processed for Western blot analysis using antibodies to phospho-p38, phospho-ERK and phospho-JNK and β-actin (D).
Figure 3.
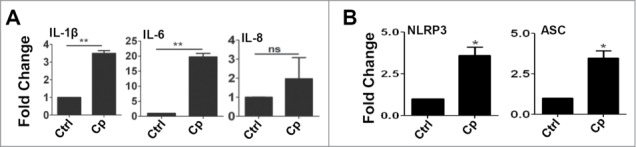
C. pseudodiphtheriticum causes expression of cytokines and activation of inflammasome. HCEC were infected with C. pseudodiphtheriticum for 4 hours, washed and RNA was isolated, reverse transcribed and expressions of cytokines (A) and inflammasomes (B) were determined by quantitative PCR. (*) designates P < 0.05.
C. pseudodiphtheriticum induces differential expression of antimicrobial peptides in human corneal epithelial cells
Epithelial cells are known to express repertoire of antimicrobial peptides in response to microbial insults. In order to determine the expressions of antimicrobial peptides in response to C. pseudodiphtheriticum infection of human corneal epithelial cells, cells were exposed to C. pseudodiphtheriticum for 4 h, RNA isolated and expressions were determined by quantitative PCR. Figure 4A shows 2-8 fold elevated expression of S100A8, S100A9, LL-37 and β-defensin 1 compared with uninfected control cells, however there was no significant difference in the levels of β-defensin 2 and 3. Representative gel images are shown in Figure 4B.
Figure 4.
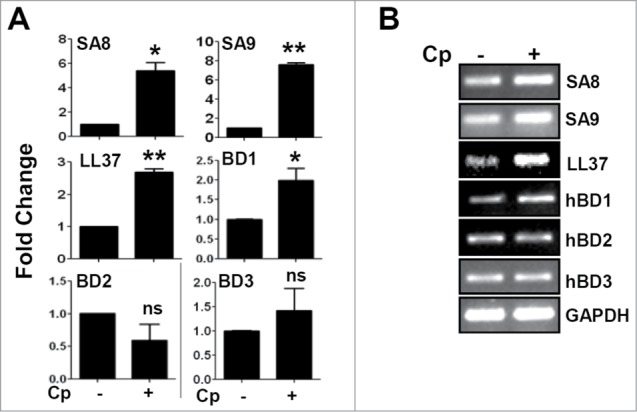
Up-regulation of antimicrobial peptide expression in response to C. pseudodiphtheriticum in human corneal epithelial cells. Immortalized human corneal epithelial cells were infected with C. pseudodiphtheriticum for 4 hours, washed and RNA was isolated, reverse transcribed and expressions of antimicrobial peptides were determined by quantitative PCR (A). The representative gel images of antimicrobial peptides are shown (B). (*) designates P < 0.05.
Taken together, we conclude that human corneal epithelial cells respond to C. pseudodiphtheriticum infection by activation of TLRs and by eliciting expression of pro-inflammatory cytokines and antimicrobial peptides.
Discussion
C. pseudodiphtheriticum, a common commensal of healthy individuals, is an opportunistic pathogen and usually detected in the skin and upper respiratory tract. It can cause respiratory infection in immunocompromised individuals, however keratitis can take place in fully immunocompetent person. In this study, we determined host responses in corneal scrapings from patients with C. pseudodiphtheriticum keratitis and detected increased expression of pattern recognition receptors TLR1, TLR2, TLR3 and TLR4, pro-inflammatory cytokines IL-6 and IL-1β and antimicrobial peptides S100A8, S100A9 and β-defensin1 compared with control uninfected cadaveric corneas. We have also demonstrated that human corneal epithelial cells respond to C. pseudodiphtheriticum infection in vitro that causes up-regulation of TLRs, cytokines and antimicrobial peptides.
We have earlier reported that in murine model of bacterial keratitis, TLRs are activated on resident corneal epithelial cells and macrophages that induce the production of chemotactic and pro-inflammatory cytokines and mediate neutrophil recruitment to corneal stroma.14 Epithelial cells are the first line of defense against pathogens and responds through identification of pathogen associated molecular patterns (PAMPs). It has already known for corneal epithelial cells and epithelial cells of other origin that cells respond to several bacterial components including lipopolysachharides, lipoproteins and flagellin of Gram positive and Gram-negative bacteria. We have also shown that HCEC responds to LPS in presence of IFNγ by upregulation of MD-2.15 TLR2 has shown to be activated by S. aureus leading to β-defensins production in human corneal epithelial cells.16 Activation of TLRs and pro-inflammatory cytokines has been recently reported in case of Pseudomonas and Streptococcus keratitis.17 Gram-positive bacteria are known to activate and mediate downstream signaling through heterodimerization of either TLR2/1 or TLR2/6.18 Since TLR6 is downregulated in ulcers obtained from patients, C. pseudodiphtheriticum might initiate cell signaling via TLR2/1. However, the case is little different in immortalized cell lines, where both TLR1 and TLR6 is up-regulated and thus both TLR2/1 and TLR2/6 might be activated.
Antimicrobial peptides have been identified to play an important role in innate host defense at mucosal barriers and also in ocular infections.19,20 These often have immunomodulatory properties along with antimicrobial activities. There are several reports of antimicrobial peptides successfully preventing invasion by various bacterial infection. However, the knowledge of antimicrobial peptides elicited during keratitis particularly in case of bacterial keratitis is still very limited. S100 proteins are low molecular weight cationic proteins and involved in a variety of cellular processes. So far, microbicidal activity has been described for S100A7, S100A8, S100A9 and S100A12. Calprotectin, dimer formed by S100A8 and S100A9, are expressed by neutrophils, monocytes and activated epithelial cells and have been shown to exert antimicrobial activity against bacteria such as E. coli, Klebsiella spp., S. aureus and S. epidermis.21,22 This suggests that our finding of up regulation of S100A8 and S100A9 by C. pseudodiphtheriticum is consistent with the earlier reports. LL-37 has also been reported earlier to be upregulated during both bacterial and fungal infections and expressed by epithelial cells of ocular surface, oral cavity, respiratory and gastrointestinal tracts.23,24 C. pseudodiphtheriticum also causes increased expression of the lone member of human cathelicidin antimicrobial peptide. Human β-defensins (hBDs), a family of epithelial cell derived cationic peptides, has been demonstrated to have activity against Gram-positive and negative bacteria, mycobacterium and even fungi at low concentration.25 Although human β-defensin 1 is known to be constitutively expressed by epithelial cells, we found that C. pseudodiphtheriticum infection induces increased expression of hBD-1.
In conclusion, the data from this study shows that C. pseudodiphtheriticum activates Toll like Receptors and induce pro-inflammatory cytokine production by human corneal epithelial cells both during corneal infection and in vitro. It also induces the expression of antimicrobial peptides of at least 3 different groups, S100 proteins, cathelicidins and β-defensins, as a host defense mechanism. Further studies on the interactions between corneal epithelial cells and C. pseudodiphtheriticum will help us understand the host responses better and what makes these commensal bacteria a virulent one.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Dr. Savitri Sharma for the Corynebacterium pseudodiphtheriticum strain and Dr. Prashant Garg for help with clinical samples. We sincerely thank Dr. Eric Pearlman for helpful discussions and critical reading of the manuscript. The technical assistance of Guru Vaidu is duly acknowledged.
Funding
This work was supported in part from a grant by Science and Engineering Research Board, DST, Government of India (S.R.) and Hyderabad Eye Research Foundation, India.
References
- 1.Kaliamurthy J, Kalavathy CM, Parmar P, Nelson Jesudasan CA, Thomas PA. Spectrum of bacterial keratitis at a tertiary eye care centre in India. Biomed Res Int 2013; 2013:181564; PMID:24066286; http://dx.doi.org/ 10.1155/2013/181564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gopinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: experience of over a decade. Indian J Ophthalmol 2009; 57:273-9; PMID:19574694; http://dx.doi.org/ 10.4103/0301-4738.53051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah A, Sachdev A, Coggon D, Hossain P. Geographic variations in microbial keratitis: an analysis of the peer-reviewed literature. Br J Ophthalmol 2011; 95:762-7; PMID:21478201; http://dx.doi.org/ 10.1136/bjo.2009.169607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passos RM, Cariello AJ, Yu MC, Hofling-Lima AL. Microbial keratitis in the elderly: a 32-year review. Arq Bras Oftalmol 2010; 73:315-9; PMID:20944931; http://dx.doi.org/ 10.1590/S0004-27492010000400002 [DOI] [PubMed] [Google Scholar]
- 5.Eguchi H. Ocular infections caused by Corynebacterium species. In: (Basak S. ed. City: In Tech; 2013) [Google Scholar]
- 6.Li A, Lal S. Corynebacterium pseudodiphtheriticum keratitis and conjunctivitis: a case report. Clin Exp Ophthalmol 2000; 28:60-1; PMID:11345349; http://dx.doi.org/ 10.1046/j.1442-9071.2000.00268.x [DOI] [PubMed] [Google Scholar]
- 7.Ramesh RRS, Jayahar Bharathi M, Amuthan M, Viswanathan S. Prevalence of bacterial pathogens causing ocular infections in South India. Indian J Pathol Microbiol 2010; 53:281-6; PMID:20551533; http://dx.doi.org/ 10.4103/0377-4929.64336 [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez-Rodero F, Ortiz de la Tabla V, Martinez C, Masia MM, Mora A, Escolano C, Gonzalez E, Martin-Hidalgo A. Corynebacterium pseudodiphtheriticum: an easily missed respiratory pathogen in HIV-infected patients. Diagn Microbiol Infect Dis 1999; 33:209-16; PMID:10212746; http://dx.doi.org/ 10.1016/S0732-8893(98)00163-1 [DOI] [PubMed] [Google Scholar]
- 9.Camello TC, Souza MC, Martins CA, Damasco PV, Marques EA, Pimenta FP, Pereira GA, Hirata R Jr., Mattos-Guaraldi AL. Corynebacterium pseudodiphtheriticum isolated from relevant clinical sites of infection: a human pathogen overlooked in emerging countries. Lett Appl Microbiol 2009; 48:458-64; PMID:19228291; http://dx.doi.org/ 10.1111/j.1472-765X.2009.02553.x [DOI] [PubMed] [Google Scholar]
- 10.Chudnicka A, Szmygin-Milanowska K, Kieszko R, Milanowski J, Koziol-Montewka M. The role of opportunistic species of Corynebacterium pseudodiphtheriticum in the pathogenesis of CAP(Community Accquired Pneumonia). Ann Univ Mariae Curie Sklodowska 2003; 58:142-8 [PubMed] [Google Scholar]
- 11.Erturan G, Holme H, Iyer S. Corynebacterium pseudodiphtheriticum septic arthritis secondary to intra-articular injection–a case report and literature review. J Med Microbiol 2012; 61:860-3; PMID:22361458; http://dx.doi.org/ 10.1099/jmm.0.037937-0 [DOI] [PubMed] [Google Scholar]
- 12.Cantarelli VV, Brodt TC, Secchi C, Inamine E, Pereira Fde S. Cutaneous infection caused by Corynebacterium pseudodiphtheriticum: a microbiological report. Rev Inst Med Trop Sao Paulo 2008; 50:51-2; PMID:18327488; http://dx.doi.org/ 10.1590/S0036-46652008000100011 [DOI] [PubMed] [Google Scholar]
- 13.Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci 1995; 36:614-21; PMID:7534282 [PubMed] [Google Scholar]
- 14.Sun Y, Karmakar M, Roy S, Ramadan RT, Williams SR, Howell S, Shive CL, Han Y, Stopford CM, Rietsch A, et al: TLR4 and TLR5 on corneal macrophages regulate Pseudomonas aeruginosa keratitis by signaling through MyD88-dependent and -independent pathways. J Immunol 2010; 185:4272-83; PMID:20826748; http://dx.doi.org/ 10.4049/jimmunol.1000874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy S, Sun Y, Pearlman E. Interferon-gamma-induced MD-2 protein expression and lipopolysaccharide (LPS) responsiveness in corneal epithelial cells is mediated by Janus tyrosine kinase-2 activation and direct binding of STAT1 protein to the MD-2 promoter. J Biol Chem 2011; 286:23753-62; PMID:21572044; http://dx.doi.org/ 10.1074/jbc.M111.219345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar A, Zhang J, Yu FS. Toll-like receptor 2-mediated expression of beta-defensin-2 in human corneal epithelial cells. Microbes Infect 2006; 8:380-9; PMID:16242370; http://dx.doi.org/ 10.1016/j.micinf.2005.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karthikeyan RS, Priya JL, Leal SM Jr., Toska J, Rietsch A, Prajna V, Pearlman E, Lalitha P. Host response and bacterial virulence factor expression in pseudomonas aeruginosa and streptococcus pneumoniae corneal Ulcers. PLoS One 2013; 8:e64867; PMID:23750216; http://dx.doi.org/ 10.1371/journal.pone.0064867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farhat K, Riekenberg S, Heine H, Debarry J, Lang R, Mages J, Buwitt-Beckmann U, Roschmann K, Jung G, Wiesmuller KH, et al: Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J Leukoc Biol 2008; 83:692-701; PMID:18056480; http://dx.doi.org/ 10.1189/jlb.0807586 [DOI] [PubMed] [Google Scholar]
- 19.Yuan X, Hua X, Wilhelmus KR. The corneal expression of antimicrobial peptides during experimental fungal keratitis. Curr Eye Res 2010; 35:872-9; PMID:20858107; http://dx.doi.org/ 10.3109/02713683.2010.495812 [DOI] [PubMed] [Google Scholar]
- 20.Deng Q, Sun M, Yang K, Zhu M, Chen K, Yuan J, Wu M, Huang X. MRP8/14 enhances corneal susceptibility to Pseudomonas aeruginosa Infection by amplifying inflammatory responses. Invest Ophthalmol Vis Sci 2013; 54:1227-34; PMID:23299480; http://dx.doi.org/ 10.1167/iovs.12-10172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee KC, Eckert RL. S100A7 (Psoriasin)–mechanism of antibacterial action in wounds. J Invest Dermatol 2007; 127:945-57; PMID:17159909; http://dx.doi.org/ 10.1038/sj.jid.5700663 [DOI] [PubMed] [Google Scholar]
- 22.Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schroder JM. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol 2005; 6:57-64; PMID:15568027; http://dx.doi.org/ 10.1038/ni1142 [DOI] [PubMed] [Google Scholar]
- 23.Mendez-Samperio P, Miranda E, Trejo A. Expression and secretion of cathelicidin LL-37 in human epithelial cells after infection by Mycobacterium bovis Bacillus Calmette-Guerin. Clin Vaccine Immunol 2008; 15:1450-5; PMID:18579695; http://dx.doi.org/ 10.1128/CVI.00178-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon YJ, Huang LC, Romanowski EG, Yates KA, Proske RJ, McDermott AM. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr Eye Res 2005; 30:385-94; PMID:16020269; http://dx.doi.org/ 10.1080/02713680590934111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, et al: Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999; 286:525-8; PMID:10521347; http://dx.doi.org/ 10.1126/science.286.5439.525 [DOI] [PubMed] [Google Scholar]


