Significance
Transmissible cancers are somatic cell lineages that are spread between individuals via the transfer of living cancer cells. Only three transmissible cancers have been reported in nature, suggesting that such diseases emerge rarely. One of the known transmissible cancers affects Tasmanian devils, and is threatening this species with extinction. Here we report the discovery of a second transmissible cancer in Tasmanian devils. This cancer causes facial tumors that are grossly indistinguishable from those caused by the first-described transmissible cancer in this species; however, tumors derived from this second clone are genetically distinct. These findings indicate that Tasmanian devils have spawned at least two different transmissible cancers, and suggest that transmissible cancers may arise more frequently in nature than previously considered.
Keywords: transmissible cancer, contagious cancer, Tasmanian devil facial tumor disease, Tasmanian devil
Abstract
Clonally transmissible cancers are somatic cell lineages that are spread between individuals via the transfer of living cancer cells. There are only three known naturally occurring transmissible cancers, and these affect dogs, soft-shell clams, and Tasmanian devils, respectively. The Tasmanian devil transmissible facial cancer was first observed in 1996, and is threatening its host species with extinction. Until now, this disease has been consistently associated with a single aneuploid cancer cell lineage that we refer to as DFT1. Here we describe a second transmissible cancer, DFT2, in five devils located in southern Tasmania in 2014 and 2015. DFT2 causes facial tumors that are grossly indistinguishable but histologically distinct from those caused by DFT1. DFT2 bears no detectable cytogenetic similarity to DFT1 and carries a Y chromosome, which contrasts with the female origin of DFT1. DFT2 shows different alleles to both its hosts and DFT1 at microsatellite, structural variant, and major histocompatibility complex (MHC) loci, confirming that it is a second cancer that can be transmitted between devils as an allogeneic, MHC-discordant graft. These findings indicate that Tasmanian devils have spawned at least two distinct transmissible cancer lineages and suggest that transmissible cancers may arise more frequently in nature than previously considered. The discovery of DFT2 presents important challenges for the conservation of Tasmanian devils and raises the possibility that this species is particularly prone to the emergence of transmissible cancers. More generally, our findings highlight the potential for cancer cells to depart from their hosts and become dangerous transmissible pathogens.
Clonally transmissible cancers are somatic cell lineages that are contagious between individuals via the transfer of living cancer cells. Only three transmissible cancers have been observed in nature, and these cause Tasmanian devil facial tumor disease (DFTD), canine transmissible venereal tumor (CTVT), and soft-shell clam disseminated neoplasia, respectively (1, 2). Each of these clones originated in a “founder animal” whose somatic cells acquired changes that drove carcinogenesis as well as adaptations for transmission and long-term survival (3). The rarity of transmissible cancer lineages in nature, despite the ubiquity of cancers that remain in one individual, suggests that the emergence of such clones is extraordinarily improbable.
Tasmanian devils (Sarcophilus harrisii) are iconic marsupial carnivores endemic to the Australian island state of Tasmania. DFTD is spread between Tasmanian devils by biting and causes tumors usually on the face or inside the mouth (Fig. 1) (4, 5). DFTD readily metastasises, and the disease usually causes death of affected animals within months of the appearance of symptoms (4, 5). Since it was first observed in 1996 in northeast Tasmania, DFTD has spread through most of Tasmania and has triggered widespread devil population declines (Fig. 1A) (4, 6, 7). The species was listed as endangered by the International Union for Conservation of Nature in 2008 (8).
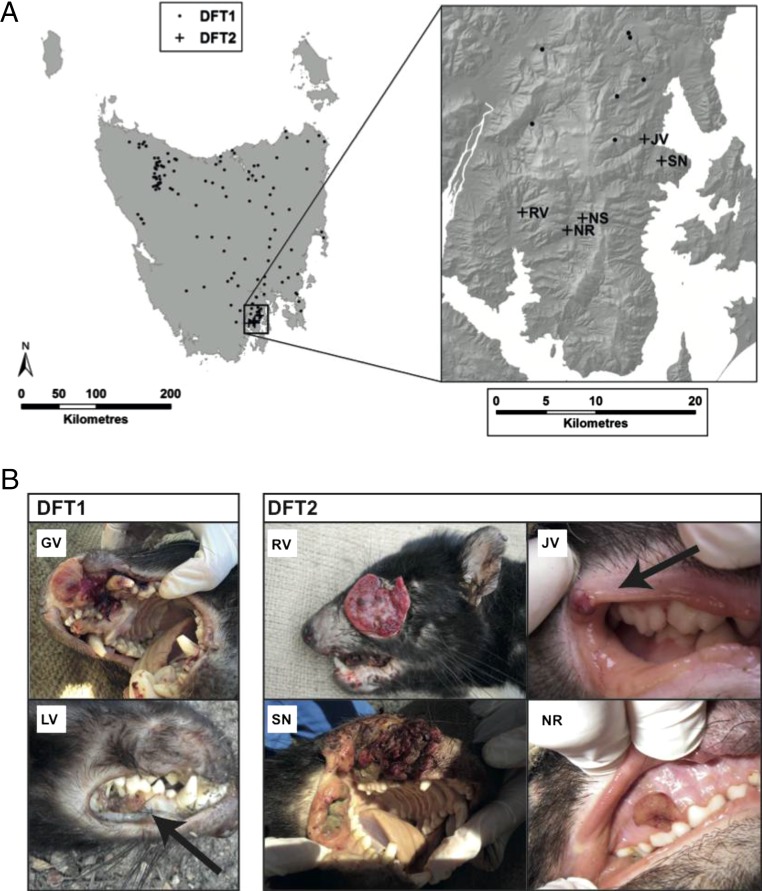
Fig. 1.
Geographical location and gross appearance of DFT2 tumors. (A) Locations of confirmed DFT1 and DFT2 tumors in Tasmania (Left) and the Channel Peninsula (Right). Each DFT1 location is represented with a single dot regardless of the number of tumors identified at this location. Tumor diagnosis was performed by histopathology, cytogenetics, and/or genetic analysis. (B) Gross appearance of two DFT1 tumors (Left) and four DFT2 tumors (Right). Tumors were identified in Tasmanian devils in the Channel region between 2012 and 2015. Further information about animals is available in Table S1.
DFTD has been associated with a single cancer clone that is genetically distinct from its hosts (9). This DFTD lineage carries a distinctive aneuploid karyotype notable for the presence of four rearranged marker chromosomes (9). DFTD tumors share identical alleles at major histocompatibility complex (MHC) loci (10), and the survival of this lineage as an allogeneic graft in MHC-discordant hosts is at least partly mediated by down-regulation of cell surface MHC molecules (11). DFTD tumors collected from different geographical locations in Tasmania share identical alleles at microsatellite loci (10, 12) and the whole genome sequences of two distantly located DFTD tumors were found to share the majority of their genetic variation (13). The DFTD clone has been closely monitored during its epidemic sweep through Tasmania (Fig. 1A) (13–16).
Here we report the discovery of a second transmissible cancer in Tasmanian devils. This second cancer, DFT2, manifests as facial tumors that are grossly indistinguishable from those caused by the original DFTD clone, now designated DFT1. However, DFT2 bears no detectable genetic or cytogenetic similarity to DFT1 and the tumors that it causes are histologically distinct. This finding indicates that DFTD tumors can be caused by at least two distinct transmissible cancer clones. Given the rarity of known transmissible cancer clones, it is remarkable that a second clone has emerged in Tasmanian devils. These findings suggest that Tasmanian devils may be particularly prone to this type of disease, or alternatively, that transmissible cancers are generally more common than previously detected. The discovery of DFT2 changes our perception of transmissible cancers as exceptionally rare and bizarre natural occurrences, and challenges our understanding of the processes that cause somatic cells to depart from their hosts and become transmissible cancer lineages.
Results
Gross and Histological Characteristics of DFT2.
DFTD was first observed in northeast Tasmania in 1996, and rapidly spread south and west across the island (Fig. 1A). Tasmanian devils with signs of DFTD were first recorded in the D'Entrecasteaux Channel area, a ∼550 km2 peninsula in Tasmania’s southeast, in December 2012. Since then, there have been 12 confirmed DFTD reports in the area (Fig. 1A and Table S1). Tumors in these animals ranged in appearance from small foci involving the oral mucosa and/or facial skin (e.g., JV, NR, LV; Fig. 1B), to locally extensive or disseminated masses deforming facial structures (e.g., RV, SN, GV; Fig. 1B). Metastases are common in DFTD (5), and were found in submandibular lymph node (SMLN) in NS, and in SMLN, lung and kidney in RV. Gross features of tumors in these animals were suggestive and typical of DFTD associated with the first described DFTD clone, DFT1.
Table S1.
DFTD in the Channel area
| Devil ID | Date | Location | Gender | Age (years) | Diagnosis | Clone |
| MG | Dec 2012 | Margate | Unknown | Unknown | DFTD | DFT1 |
| SF | Dec 2012 | Sandfly | Male | 3 | DFTD | DFT1 |
| RV | Mar 2014 | Cygnet | Male | 3 | DFTD | DFT2 |
| SN | Oct 2014 | Snug | Male | 3 | DFTD | DFT2 |
| GV | Feb 2015 | Grove | Female | 3 | DFTD | DFT1 |
| LV | Mar 2015 | Leslie Vale | Female | 2 | DFTD | DFT1 |
| JV | Mar 2015 | Coningham | Male | 2 | DFTD | DFT2 |
| SG | Mar 2015 | Snug Tiers | Male | 4 | DFTD | DFT1 |
| NR | Mar 2015 | Nicholls Rivulet | Male | 3 | DFTD | DFT2 |
| MZ | Mar 2015 | Kaoota | Female | 3 | DFTD | DFT1 |
| ST | Mar 2015 | Snug Tiers | Female | 2 | DFTD | DFT1 |
| NS | June 2015 | Nicholls Rivulet | Male | 2 | DFTD | DFT2 |
Details of the 12 confirmed DFTD cases (both DFT1 and DFT2) in the Channel area from the first reported case in December 2012 until June 2015. Date indicates the date when samples were collected from the animal, and age is the estimated age of the animal on that date.
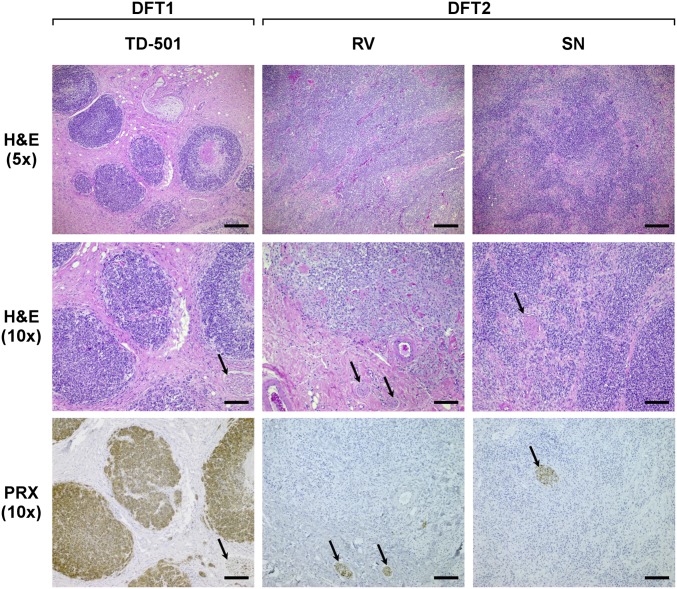
Initial histological assessment of two of the Channel DFTD cases from 2014, RV and SN, revealed that tumors from these animals presented atypical features. DFTD tumors associated with the DFT1 clone are generally composed of pleomorphic round cells arranged in distinct bundles, cords or packets (5). In contrast, the tumors from RV and SN were characterized by sheets of pleomorphic (amorphic to stellate and fusiform) cells arranged in a solid pattern (Fig. 2). Furthermore, tumors from RV and SN were negative for periaxin (PRX), an immunohistochemical marker that is diagnostic for DFT1 (12, 17) (Fig. 2). Although it was initially considered plausible that tumors from RV and SN were DFT1 variants or spontaneous tumors arising from the tissues of their hosts, further cytogenetic and genetic analyses were performed to confirm the nature of these two tumors and other tumors derived from devils in the Channel Peninsula.
Fig. 2.
DFT2 tumors are histologically distinct from DFT1. Representative images of H&E stained histological sections of DFT1 and DFT2 tumors (Upper and Middle). (Lower) Histological sections stained with DFT1 marker, PRX. Scale bars represent 200 μm (Upper) or 100 μm (Middle and Lower). Arrows indicate peripheral nerve bundles, which are positive for PRX.
Cytogenetic Profile of DFT2.
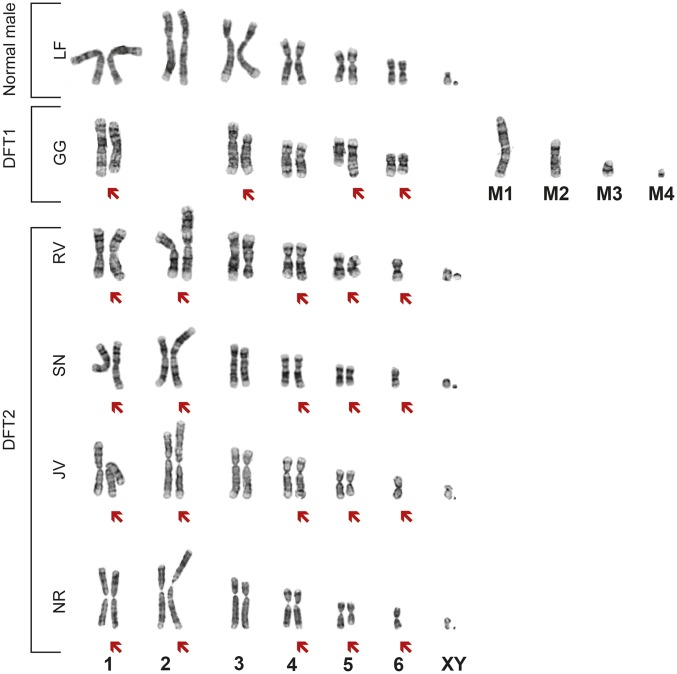
The clonal nature of DFTD was first suggested based on cytogenetic evidence indicating that DFTD tumors carry a distinctive aneuploid karyotype (9). This DFT1 cytogenetic profile differs markedly from the normal devil karyotype, and is characterized by the absence of identifiable chromosome 2 homologs, the presence of four marker chromosomes and missing sex chromosomes (Fig. 3). However, cytogenetic analysis of samples from the Channel revealed that tumors from five of the devils (those derived from RV, SN, JV, NR, and NS) shared an identical aneuploid karyotype that was clearly distinct from that of DFT1 (Fig. 3 and Fig. S1). Chromosomes from these tumors all exhibited identical complex structural abnormalities, including the presence of additional material on chromosomes 1, 2, and 4, a deletion involving chromosome 5 and monosomy for chromosome 6. Both X and Y sex chromosomes were present. This cytogenetic evidence presented the possibility that these five tumors were derived from a clone, which we have named DFT2, and that is distinct from DFT1.
Fig. 3.
DFT2 tumors are cytogenetically distinct from DFT1. Representative karyotypes of a normal male devil, a DFT1 tumor, and four DFT2 tumors. Red arrows indicate chromosomes carrying cytogenetic abnormalities. Four marker chromosomes found in DFT1 (9) are labeled M1 to M4. Karyotype for NS is presented in Fig. S1.
Fig. S1.
Representative karyotype for DFT2 tumor derived from animal NS.
Genetic Analysis of DFT2.
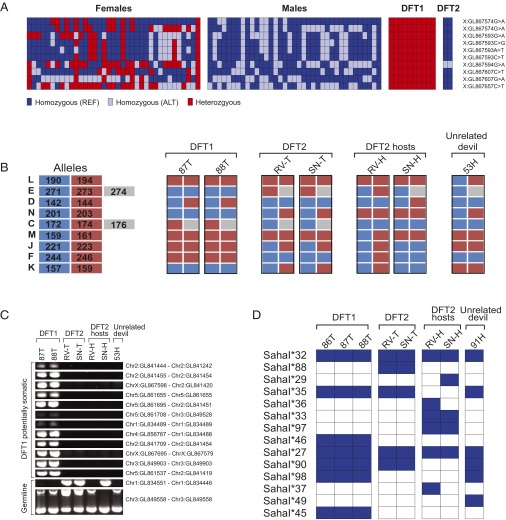
Despite the absence of cytogenetically identifiable sex chromosomes in DFT1, studies using fluorescence in situ hybridization and DNA sequencing have found evidence for two X chromosome copies in DFT1 (13, 14). Furthermore, the number of single point substitution variants mapping to the X chromosome in DFT1 suggested that the two X chromosomes were germ-line homologs rather than recent somatic duplicates (13). We further investigated the origins of the X chromosomes in DFT1 by genotyping a panel of 10 X chromosome variants that were heterozygous in DFT1 in a group of male and female devils. At each locus, we found that both alleles could be detected in the devil population, confirming that these variants are germ-line single nucleotide polymorphisms (SNPs) rather than somatic mutations (Fig. 4A). Furthermore, our analysis confirmed that these SNPs indeed map to the X chromosome as only females, and not males, were found to be heterozygous at these loci (Fig. 4A). These data confirm that DFT1 carries DNA from two homologous X chromosomes, indicating that this lineage probably arose in a female devil. The discovery that DFT2 carries a Y chromosome is thus incompatible with a single clonal origin for DFT1 and DFT2.
Fig. 4.
DFT2 tumors are genetically distinct from DFT1 and from their hosts. (A) Genotyping of X-linked SNPs. Genotypes of 37 female and 37 male devils at 10 X-linked SNP loci that are heterozygous in DFT1. Both homozygous and hemizygous genotypes are referred to as homozygous. Each individual is represented by a column, and chromosome and scaffold for each locus is shown. Further information about individuals and exact SNP coordinates are available in Tables S3 and S4. (B) Microsatellite genotypes at nine polymorphic microsatellite loci (L, E, D, N, C, M, J, F, and K). The lengths of the two (L, D, N, M, J, F, and K) or three (E and C) alleles found at each locus and their color codes are shown on the left, and their genotypes in DFT1 tumors, DFT2 tumors, DFT2 hosts, and in 53H, a representative unrelated devil, are shown. All tumors are presented as diploid, although true copy number at these loci is not known. Further information about allele sizes is found in Table S2. (C) Structural variant genotyping. PCRs spanning breakpoint junctions were performed to assess the presence or absence of twelve possibly somatic structural variants and two polymorphic germ-line variants. We cannot confirm if structural variants found only in DFT1 are somatic or rare germ-line variants, thus these are labeled potentially somatic. Chromosomes and scaffolds involved in each rearrangement are indicated, and full breakpoint coordinates are available in Table S5. (D) MHC class I exon 2 haplotypes detected in DFT1 tumors, DFT2 tumors, DFT2 hosts, and in 91H, a representative unrelated devil. Exon 2 haplotype names are indicated on the left, and their presence or absence in the panel of samples are indicated with blue and white squares respectively. All 14 haplotypes are predicted to encode a unique amino acid sequence. Complete sequences for each haplotype are available in Tables S6 and S7.
To investigate the genetic relationship between DFT1 and DFT2, we analyzed the genotypes of these two lineages at nine polymorphic microsatellite loci (10, 12, 18, 19). Our analysis confirmed that two DFT1 tumors, 87T and 88T, shared an identical genotype with each other and with a previously analyzed panel of 27 DFTD tumors collected from geographically dispersed areas of Tasmania (12) (Fig. 4B and Table S2). Analysis of DFT2, however, revealed that the tumors derived from RV and SN had different genotypes from DFT1 (Fig. 4B). Furthermore, these two tumors shared identical genotypes with each other and were genetically distinct from their hosts (Fig. 4B). Indeed, the microsatellite analysis indicated that DFT2 tumors were no more similar to DFT1 (identical genotypes at two of nine loci) than they were to their hosts (identical genotypes at four and three of nine loci for RV and SN, respectively), or to other devils in the population (e.g., 53H, a devil from northern Tasmania) (Fig. 4B).
Table S2.
Microsatellite primers and allele information
| Allele size (bp) | ||||||||
| This study | Murchison et al. (12) | |||||||
| Marker | Forward primer* | Reverse primer | Allele 1 | Allele 2 | Allele 3 | Allele 1 | Allele 2 | Allele 3 |
| L | AGGAAACAGCTATGACCATACACTCCATGTTTTAGTTTG | TCGGTATGTGTGTCTCTCAG | 190 | 194 | 190 | 194 | ||
| E | AGGAAACAGCTATGACCATAATGCTAGATTTCACTCCC | CCTCACATTTCTGGAACTG | 271 | 273 | 274 | 269 | 271 | 272 |
| D | AGGAAACAGCTATGACCATGAAATCCAAGCTCATTTTAG | AATCAACTCTGGAATGCATC | 142 | 144 | 141 | 143 | ||
| N | AGGAAACAGCTATGACCATTGAACCCCAAGCTCTATCA | CTTCCCCTGTAAGTGTATTTG | 201 | 203 | 200 | 202 | ||
| C | AGGAAACAGCTATGACCATAATAGCAGAGACTCGATCC | AGCCTTTATTACCTGGGAAG | 172 | 174 | 176 | 170 | 172 | 174 |
| M | AGGAAACAGCTATGACCATTGCCCCATCACACTTTCCTTG | GCAATCCTGGTCATGATGTAGTC | 159 | 161 | 156 | 158 | ||
| J | AGGAAACAGCTATGACCATGATTCTAGAAGGGATAGCAAGC | GACACTCCATAGAAATGCACTG | 221 | 223 | 220 | 222 | ||
| F | AGGAAACAGCTATGACCATGCTACTGCGGAGTCAGATTGC | GAAGTATACGTCTGCTATGTCCC | 244 | 246 | 242 | 244 | ||
| K | AGGAAACAGCTATGACCATAGATGGTCTGAGCATGTATCC | TAGTCCAGGTGTGAGGTGATG | 157 | 159 | 155 | 157 | ||
Concordance between alleles reported in a previous study (12) and those from the current study is indicated.
The 19-bp M13F sequence (5′-AGGAAACAGCTATGACCAT-3′) was added to the 5′ end of each forward primer in this study; this sequence is included in the size of the allele.
We further characterized DFT1 and DFT2 by analyzing panels of tumors for the presence of 12 putative somatic structural variants that had previously been identified in DFT1 (13), as well as for the presence of two polymorphic germ-line structural variants (Fig. 4C). None of the 12 putative somatic structural variants were found in DFT2 tumors, although they were all found in DFT1 tumors 87T and 88T (Fig. 4C). One of the polymorphic germ-line structural variants was present in DFT2, but was absent in DFT1 and host tissue derived from RV (Fig. 4C).
DFTD tumors are allogeneic grafts within their hosts. Previous studies have confirmed that DFT1 is able to colonize hosts carrying different genotypes at MHC loci (10, 20). However, MHC molecules are not expressed on the cell surface of most DFT1 cells, and this has been proposed as a mechanism whereby this lineage escapes destruction by host T cells (11). To investigate whether DFT2 is able to grow in hosts with disparate MHC genotype, we cloned and sequenced part of the polymorphic second exon of MHC class I loci from two DFT2 tumors and their corresponding hosts. We identified five MHC class I exon 2 haplotypes in DFT2, and confirmed that DFT2 has a different MHC class I genotype from DFT1 (Fig. 4D). It is important to note that the MHC class I genotype found in DFT2 was distinct from the genotypes found in DFT2 hosts, confirming that DFT2 is not restricted to hosts with an identical MHC class I genotype (Fig. 4D).
Discussion
The data presented here indicate that DFT2 is a transmissible cancer that is distinct from DFT1. Although DFT2 has so far been detected in only five male devils located on the Channel Peninsula in southeast Tasmania in 2014 and 2015, the extent of its current distribution in the devil population, and its location and time of origin, remain unknown.
Our analysis suggests that DFT2 may have arisen as a second independent transmissible cancer in Tasmanian devils manifesting as facial tumors that are outwardly indistinguishable from those caused by DFT1. This finding would challenge our current understanding that the emergence of transmissibility in cancer is an extraordinarily rare occurrence in nature. Thus, it is possible that the burden of transmissible cancers as pathogens in natural populations has been underestimated; alternatively, transmissible cancers may emerge rarely in most species, but species-specific vulnerabilities may promote their emergence within certain host populations.
The discovery of a second transmissible cancer in Tasmanian devils suggests that this species may be particularly at risk for the emergence of transmissible cancers. This could perhaps be mediated by this this species’ apparent elevated susceptibility to neoplasia (21, 22), low genetic diversity (10, 23–25), and/or biting behavior (26). However, if this is the case, it is surprising that tumors comparable with DFTD were not reported before 1996 (4, 5). It is possible, however, that additional Tasmanian devil transmissible cancers may currently exist, or previously occurred, but have remained undetected. It is also possible that exposure to novel pathogens or anthropogenic factors may have influenced the propensity of Tasmanian devils to develop transmissible cancers. The potential for new transmissible cancer clones to emerge in this species has important implications for Tasmanian devil conservation programs, the success of which largely depends on the long-term viability of isolated captive insurance populations.
Tasmanian devils have low levels of genetic diversity (10, 23–25), possibly caused by historical population declines driven by past climate change (27). Remaining genetic diversity was possibly further eroded by persecution following European settlement of Tasmania, and, more recently, by the DFTD epidemic (23, 27). Low genetic diversity may have contributed to risk for the emergence of transmissible cancers in this species.
The possibility that clonally transmissible cancers may arise more frequently in nature than previously considered warrants further investigation of the risk that such diseases could arise in humans. Although transfer of cancer cells between two humans has been reported in rare circumstances, involving injury, organ transplantation, experimental treatments, or pregnancy (3), no human cancer has been observed to naturally transmit between more than two human hosts.
An alternative explanation for the existence of two distinct transmissible cancer clones in Tasmanian devils is the possibility that DFT1 and/or DFT2 arose via hybridization of genetic material derived from ancestral DFT cells and host cells. Horizontal transfer of mitochondrial DNA between host cells and cancer cells has been documented in CTVT (28), indicating that, at least in CTVT, there are mechanisms that permit exchange of genetic material between host cells and cancer cells. However, our failure to find any significant evidence for shared DNA markers between DFT1 and DFT2 suggests that, if horizontal transfer has occurred, then host cell DNA largely replaced the ancestral DFT DNA in at least one of the DFT1 or DFT2. If this were the case, then DFTD may provide insight into somatic cell hybridization in cancer, a phenomenon that may be challenging to detect in cancers that remain in one host (29, 30).
The discovery of a second transmissible cancer in Tasmanian devils changes our perception of the potential of cancer cells to adapt to new niches as parasitic clonal cell lineages. Regardless of whether the plight of Tasmanian devils represents the existence of a common pathological process that has previously been overlooked, is the consequence of an unfortunate species-specific vulnerability, or has arisen due to an exceptionally improbable concomitance of events, clarification of the biological basis of DFT2 promises to illuminate important concepts underpinning cancer evolution.
Methods
Animals.
Wild Tasmanian devils in the Channel area with signs of DFTD were either trapped or found dead from road trauma or other causes. Live devils with visible signs of distress were euthanized for welfare reasons. Tissue biopsies and tumor fine needle aspirates were either collected post mortem, or from live devils, which were subsequently released. All animal procedures were performed under a Standard Operating Procedure approved by the General Manager, Natural and Cultural Heritage Division, Tasmanian Government Department of Primary Industries, Parks, Water and the Environment (DPIPWE), in agreement with the DPIPWE Animal Ethics Committee.
Histology and Immunohistochemistry.
Standard hematoxylin and eosin (H&E) and immunohistochemical staining with a periaxin antibody (HPA001868; Sigma Aldrich) were performed on 3-μm paraffin sections from tumor tissues fixed in 10% neutral buffered formalin as previously described (17).
Cytogenetics.
Karyotyping was performed as previously described (31). Chromosomes were banded with Leishman’s stain (Sigma-Aldrich) according to standard methods. Analysis and imaging were performed using Metasystems karyotyping software.
X Chromosome Analysis.
Candidate SNPs mapping to the X chromosome that were heterozygous in DFT1 tumors as well as in the germ line of a female devil (91H) were identified from whole genome sequence reads (13). Ten of these SNPs were genotyped in 74 devils (37 males and 37 females), as well as in 10 DFT1 tumors and 2 DFT2 tumors, by PCR and capillary sequencing; DNA was only available for 2 DFT2 tumors (those from RV and SN) during preparation of the manuscript. Tables S3 and S4 have details of PCR primers, samples, and genotypes. All PCRs were performed under standard conditions with annealing temperature of 60 °C, extension time of 45 s and 31 cycles.
Table S3.
Genotype and sample summary for X chromosome SNP analysis
| Genotype (Chr:scaffold:coordinate:REF > ALT) | |||||||||||||||
| Position within group (left to right in Fig. 4A) | ID | Type | Gender | Location | Year of collection | ChrX:GL867574:1996160:G > A | ChrX:GL867574:2384835:G > A | ChrX:GL867593:1106751:G > A | ChrX:GL867593:1192257:C > G | ChrX:GL867593:1194386:A > T | ChrX:GL867593:1194433:C > T | ChrX:GL867594:938376:G > A | ChrX:GL867607:103606:C > T | ChrX:GL867607:963505:G > A | ChrX:GL867657:38331:C > T |
| F-1 | 232H | Devil germ line | Female | Bronte | 2014 | G | G | G/A | C | A | C | G/A | C/T | G/A | C/T |
| F-2 | 233H | Devil germ line | Female | Bronte | 2014 | G | G | G/A | C | A | C | A | T | G | C |
| F-3 | 236H | Devil germ line | Female | Bronte | 2014 | G | G | G/A | C | A | C | G | T | G | T |
| F-4 | 239H | Devil germ line | Female | Kempton/Elderslie | 2014 | G | G | G | C | A | C | G | C | A | C/T |
| F-5 | 241H | Devil germ line | Female | Kempton/Elderslie | 2014 | G | G | G | C | A | C | G/A | C | A | C/T |
| F-6 | 242H | Devil germ line | Female | Kempton/Elderslie | 2014 | G/A | G | A | G | A | C | A | C | A | T |
| F-7 | 245H | Devil germ line | Female | Kempton/Elderslie | 2014 | G/A | G/A | G | C | A | C | G/A | C | A | C |
| F-8 | 247H | Devil germ line | Female | Kempton/Elderslie | 2014 | G | G | G/A | C | T | C | G | C | A | C/T |
| F-9 | 248H | Devil germ line | Female | Kempton/Elderslie | 2014 | G/A | G/A | G/A | C | A | C | A | C | A | T |
| F-10 | 249H | Devil germ line | Female | Kempton/Elderslie | 2014 | G | G | G/A | C | A | C | G/A | C | A | C/T |
| F-11 | 250H | Devil germ line | Female | Kempton/Elderslie | 2014 | G/A | G/A | A | C/G | T | C/T | G/A | C/T | G/A | C/T |
| F-12 | 251H | Devil germ line | Female | Fentonbury | 2014 | G | G | G/A | C | A | C | G/A | C | A | C/T |
| F-13 | 252H | Devil germ line | Female | Fentonbury | 2014 | G/A | G/A | G | C | A | C | G/A | C | A | C/T |
| F-14 | 253H | Devil germ line | Female | Fentonbury | 2014 | G | G/A | G/A | C/G | A/T | C/T | G | C | A | C/T |
| F-15 | 254H | Devil germ line | Female | Fentonbury | 2014 | G | G | G/A | C/G | A/T | C/T | G | C | A | C/T |
| F-16 | 257H | Devil germ line | Female | Fentonbury | 2014 | G | G | G | C | A | C | G | C/T | A | C/T |
| F-17 | 258H | Devil germ line | Female | Fentonbury | 2014 | G | G | A | C/G | A/T | C/T | G/A | T | G/A | C |
| F-18 | 262H | Devil germ line | Female | Buckland | 2014 | G/A | G/A | G | C | A | C | G/A | C/T | A | C/T |
| F-19 | 264H | Devil germ line | Female | Buckland | 2014 | G/A | G/A | G | C | A | C | G/A | C | A | C/T |
| F-20 | 265H | Devil germ line | Female | Buckland | 2014 | G | G | G | C | A | C | G/A | C/T | A | C |
| F-21 | 266H | Devil germ line | Female | Buckland | 2014 | G/A | G/A | G/A | C/G | A/T | C/T | G | C/T | G | C/T |
| F-22 | 272H | Devil germ line | Female | Buckland | 2014 | G | G | G | C | A | C | G/A | C/T | A | C/T |
| F-23 | 273H | Devil germ line | Female | Buckland | 2014 | G/A | G/A | G | C | A | C | G/A | C/T | G/A | C/T |
| F-24 | 278H | Devil germ line | Female | Stony Head | 2014 | G | G | G | C | A | C | A | C | A | T |
| F-25 | 279H | Devil germ line | Female | Stony Head | 2014 | G | G | G | C | A | C | G/A | C | G/A | C/T |
| F-26 | 280H | Devil germ line | Female | Stony Head | 2014 | G | G | G | C | A | C | G/A | C | A | T |
| F-27 | 281H | Devil germ line | Female | Woolnorth | 2014 | G | G/A | A | G | T | T | G | C/T | G | C/T |
| F-28 | 283H | Devil germ line | Female | Woolnorth | 2014 | G | A | G | C | A | C | A | C | G | C/T |
| F-29 | 284H | Devil germ line | Female | Woolnorth | 2014 | G | A | A | G | T | T | G | T | G | T |
| F-30 | 285H | Devil germ line | Female | Woolnorth | 2014 | G | G/A | A | G | T | T | G/A | C | G | T |
| F-31 | 287H | Devil germ line | Female | Woolnorth | 2014 | G | G/A | A | G | T | T | G/A | C | A | T |
| F-32 | 289H | Devil germ line | Female | Woolnorth | 2014 | G | G/A | A | C/G | A/T | C/T | G/A | C | G | T |
| F-33 | 293H | Devil germ line | Female | Woolnorth | 2014 | G | G/A | A | G | T | T | G/A | C | G | T |
| F-34 | 298H | Devil germ line | Female | Woolnorth | 2014 | G | A | G | C | A | C | A | C | G | C/T |
| F-35 | 301H | Devil germ line | Female | Woolnorth | 2014 | G | G/A | A | C | T | C | G | C/T | G/A | T |
| F-36 | 302H | Devil germ line | Female | Woolnorth | 2014 | G | A | G | C | A | C | A | T | G | T |
| F-37 | 91H | Devil germ line | Female | Taronga Zoo | 2006 | G/A | G/A | G/A | C/G | A/T | C/T | G/A | C/T | G/A | C/T |
| M-1 | 237H | Devil germ line | Male | Bronte | 2014 | G | G | G | C | A | C | G | C | G | T |
| M-2 | 238H | Devil germ line | Male | Kempton/Elderslie | 2014 | G | G | A | G | T | T | G | C | A | C |
| M-3 | 240H | Devil germ line | Male | Kempton/Elderslie | 2014 | A | G | A | C | A | C | A | C | A | T |
| M-4 | 243H | Devil germ line | Male | Kempton/Elderslie | 2014 | A | A | G | C | A | C | G | C | A | C |
| M-5 | 244H | Devil germ line | Male | Kempton/Elderslie | 2014 | G | G | G | C | A | C | G | C | A | T |
| M-6 | 246H | Devil germ line | Male | Kempton/Elderslie | 2014 | G | G | G | C | A | C | G | T | G | T |
| M-7 | 255H | Devil germ line | Male | Fentonbury | 2014 | G | G | G | C | A | C | A | T | G | T |
| M-8 | 256H | Devil germ line | Male | Fentonbury | 2014 | A | A | A | G | T | T | A | T | G | C |
| M-9 | 259H | Devil germ line | Male | Fentonbury | 2014 | G | G | G | C | A | C | G | C | A | T |
| M-10 | 260H | Devil germ line | Male | Buckland | 2014 | A | A | A | C | A | C | A | C | A | C |
| M-11 | 261H | Devil germ line | Male | Buckland | 2014 | G | G | A | C | A | C | G | T | G | C |
| M-12 | 263H | Devil germ line | Male | Buckland | 2014 | A | A | A | G | T | C | G | C | A | T |
| M-13 | 267H | Devil germ line | Male | Buckland | 2014 | A | A | A | G | T | T | G | C | A | T |
| M-14 | 268H | Devil germ line | Male | Buckland | 2014 | G | G | G | C | A | C | G | C | G | T |
| M-15 | 269H | Devil germ line | Male | Buckland | 2014 | A | A | A | G | T | T | A | T | G | C |
| M-16 | 270H | Devil germ line | Male | Buckland | 2014 | G | G | G | C | A | C | A | C | A | C |
| M-17 | 271H | Devil germ line | Male | Buckland | 2014 | A | A | G | C | A | C | G | C | G | T |
| M-18 | 274H | Devil germ line | Male | Buckland | 2014 | G | G | A | G | T | C | G | C | A | C |
| M-19 | 275H | Devil germ line | Male | Stony Head | 2014 | G | G | G | C | A | C | G | C | A | C |
| M-20 | 276H | Devil germ line | Male | Stony Head | 2014 | G | G | G | C | A | C | A | C | A | T |
| M-21 | 282H | Devil germ line | Male | Woolnorth | 2014 | G | A | A | G | T | T | G | C | G | T |
| M-22 | 286H | Devil germ line | Male | Woolnorth | 2014 | G | A | G | C | A | C | A | C | G | C |
| M-23 | 288H | Devil germ line | Male | Woolnorth | 2014 | G | A | G | C | A | C | A | C | G | T |
| M-24 | 290H | Devil germ line | Male | Woolnorth | 2014 | G | A | G | C | A | C | A | C | G | C |
| M-25 | 291H | Devil germ line | Male | Woolnorth | 2014 | G | A | A | G | T | T | G | T | G | T |
| M-26 | 292H | Devil germ line | Male | Woolnorth | 2014 | G | G | A | G | T | T | G | C | G | T |
| M-27 | 294H | Devil germ line | Male | Woolnorth | 2014 | G | A | G | G | A | C | A | C | G | T |
| M-28 | 295H | Devil germ line | Male | Woolnorth | 2014 | G | A | G | C | A | C | A | T | G | T |
| M-29 | 296H | Devil germ line | Male | Woolnorth | 2014 | G | A | A | G | T | T | G | C | G | C |
| M-30 | 297H | Devil germ line | Male | Woolnorth | 2014 | G | G | A | G | T | T | G | T | G | C |
| M-31 | 299H | Devil germ line | Male | Woolnorth | 2014 | G | A | G | C | A | C | A | T | G | T |
| M-32 | 300H | Devil germ line | Male | Woolnorth | 2014 | G | A | G | C | A | C | A | C | G | T |
| M-33 | 303H | Devil germ line | Male | Woolnorth | 2014 | G | A | G | C | A | C | A | C | G | C |
| M-34 | 202H1 | Devil germ line | Male | Cygnet | 2014 | G | G | A | C | A | C | A | C | A | C |
| M-35 | 203H | Devil germ line | Male | Snug | 2014 | G | G | G | C | A | C | A | C | A | C |
| M-36 | 53H | Devil germ line | Male | Narawntapu | 2007 | G | A | A | G | T | T | G | T | G | T |
| M-37 | 31H | Devil germ line | Male | Forestier | 2007 | G | G | G | C | A | C | G | C | G | C |
| DFT1-1 | 86T2 | DFT1 (cell line) | N/A | Fentonbury | 2005 | G/A | G/A | G/A | C/G | A/T | C/T | G/A | C/T | G/A | C/T |
| DFT1-2 | 87T2 | DFT1 (cell line) | N/A | Forestier | 2007 | G/A | G/A | G/A | C/G | A/T | C/T | G/A | C/T | G/A | C/T |
| DFT1-3 | 88T2 | DFT1 (cell line) | N/A | Freycinet | 2007 | G/A | G/A | G/A | C/G | A/T | C/T | G/A | C/T | G/A | C/T |
| DFT1-4 | 90T2 | DFT1 (cell line) | N/A | Forestier | 2007 | G/A | G/A | G/A | C/G | A/T | C/T | G/A | C/T | G/A | C/T |
| DFT1-5 | 204T | DFT1 (cell line) | N/A | Forestier | 2012 | G/A | G/A | G/A | C/G | A/T | C/T | G/A | C/T | G/A | C/T |
| DFT1-6 | 85T | DFT1 (cell line) | N/A | Fentonbury | 2005 | G/A | G/A | G/A | C/G | A/T | C/T | G/A | C/T | G/A | C/T |
| DFT1-7 | 86T | DFT1 (cell line) | N/A | Fentonbury | 2005 | G/A | G/A | G/A | C/G | A/T | C/T | G/A | C/T | G/A | C/T |
| DFT1-8 | 87T | DFT1 (cell line) | N/A | Forestier | 2007 | G/A | G/A | G/A | C/G | A/T | C/T | G/A | C/T | G/A | C/T |
| DFT1-9 | 88T | DFT1 (cell line) | N/A | Freycinet | 2007 | G/A | G/A | G/A | C/G | A/T | C/T | G/A | C/T | G/A | C/T |
| DFT1-10 | 53T | DFT1 (cell line) | N/A | Narawntapu | 2007 | G/A | G/A | G/A | C/G | A/T | C/T | G/A | C/T | G/A | C/T |
| DFT2-1 | 202T1 (RV-T) | DFT2 (primary tumor) | N/A | Cygnet | 2014 | G | G | G | C | A | C | A | C | G | C |
| DFT2-2 | 203T1 (SN-T; upper lip) | DFT2 (primary tumor) | N/A | Snug | 2014 | G | G | G | C | A | C | A | C | G | C |
All coordinates are from genome build DEVIL7.0.
Table S4.
Genome coordinates and primers for X chromosome SNP analysis
| SNP genome coordinates (DEVIL7.0) | Primers | |||||
| Chromosome | Scaffold | Position | REF | ALT | Forward_primer | Reverse_primer |
| ChrX | GL867574.1 | 1996160 | G | A | TGGGATTTTTCTTGGTCTCC | GCAAAGATGGCAAATGACAG |
| ChrX | GL867574.1 | 2384835 | G | A | TCTCTGACAGGTGTTGCCTG | TTTAGAGCAGGGGCAGAAAG |
| ChrX | GL867593.1 | 1106751 | G | A | TGGAAGCCAGGCTAACATTT | ATGTCTCGCCACAGGTCTTT |
| ChrX | GL867593.1 | 1192257 | C | G | ACCTCTCTGGGTTTGCTTCA | CTCTTTCCTCTGTCCCCCTC |
| ChrX | GL867593.1 | 1194386 | A | T | CCACGATGTTGCCATAACTG | GGGTATGATTCCGTGACCAG |
| ChrX | GL867593.1 | 1194433 | C | T | GCCTTAACAAGACCAGCAGC | CCATCTTGCAGGGTAACAAGA |
| ChrX | GL867594.1 | 938376 | G | A | TGCGAATACATGGTAAAGAATGA | CCTTTGAATCCCCTTCCAGT |
| ChrX | GL867607.1 | 103606 | C | T | ATCTCCCTCTCATTCGCCTC | ATCTAGCAGGCAGTTCCCAA |
| ChrX | GL867607.1 | 963505 | G | A | ACCTTTCCCTTCCTTTCCCT | TTGTCCTTTCTGTACTGATGGC |
| ChrX | GL867657.1 | 38331 | C | T | GAAAGTCTAGCAAAGCAGCGA | TTTGTGGTCTGACACTTGGC |
Microsatellite Genotyping.
Microsatellite genotyping was performed as previously described (12). In brief, PCR was performed with primers listed in Table S2; each forward primer had a 19-bp M13F sequence (5′-AGG-AAA-CAG-CTA-TGA-CCA-T-3′) attached to its 5′ end. PCR was performed with 57 °C annealing temperature, reducing the annealing temperature by 1 °C per cycle for 6 cycles, followed by 31 cycles with annealing temperature 50 °C; at this step, 8 pmol of 5′-6FAM or 5′-HEX labeled M13F primer was added to each reaction. The reaction was then continued for eight additional cycles with annealing temperature 48 °C. Products were separated on an ABI 3730XL instrument and analyzed using GeneMarker software. Concordance of alleles shown in Fig. 4B with previously published microsatellite alleles (12) is provided in Table S2.
Structural Variant Analysis.
Whole genome sequence reads derived from two DFT1 tumors and a male and female devil (31H and 91H) were analyzed using an algorithm that uses discordantly mapped read pairs to identify putative structural variants, as previously described (13). A set of 14 putative structural variants were analyzed by PCR with DNA from DFT1 and DFT2 tumors and as well as with germ-line DNA from devils. PCRs were performed under standard conditions with annealing temperature of 60 °C and 35 cycles. Primer sequences can be found in Table S5.
Table S5.
Structural variant breakpoint coordinates and primers
| Lower breakpoint scaffold | Lower breakpoint strand | Lower breakpoint coordinate range | Upper breakpoint scaffold | Upper breakpoint strand | Upper breakpoint coordinate range | Forward oligo | Reverse oligo | ||
| Chr2:GL841444 | + | 373844 | 374130 | Chr2:GL841242 | + | 200819 | 201085 | AGGAGCGAGTGTTTCTGCTTATAC | CCTTGCTATCACAAAAAGAGCTG |
| Chr2:GL841455 | + | 26550 | 26748 | Chr2:GL841454 | + | 239574 | 239822 | GCAAGAGTTCTTCATCAGAAGATAACC | GATCAGTTAAAGGAAGTGGAGAGATTG |
| ChrX:GL867598 | — | 2128177 | 2128435 | Chr2:GL841420 | + | 441752 | 441984 | AGTAAAAATGCAATAGGCCCAGG | GGTGATGGCAGATTTCAGCTAAG |
| Chr5:GL861655 | — | 2377162 | 2377364 | Chr5:GL861655 | + | 2363778 | 2364045 | GGAGGATCAGGAAGGACAGTAAA | CTTACTTTTCATTAGGCCACTCAAC |
| Chr5:GL861695 | — | 228625 | 228899 | Chr2:GL841451 | — | 4043144 | 4043428 | AAGATGGGAAGACAGGTAAGACAG | CTAAAGTTCTGGAGCAAACCCTT |
| Chr5:GL861708 | + | 729973 | 730179 | Chr3:GL849528 | + | 255127 | 255416 | GGTATGTGTGTGGACAAGAGCAC | CTAAGACACCCTCAATTCTCACTTT |
| Chr1:GL834489 | + | 324236 | 324463 | Chr1:GL834489 | — | 320634 | 320880 | GGAATAAAGGAGTCCTACCAAATTCTC | ACTTCAGTTAGGTACAAGGAGTCTGTG |
| Chr4:GL856767 | — | 219206 | 219478 | Chr1:GL834488 | — | 1096487 | 1096770 | GTACTGATTTCCTGCCAGTCTCTT | GGAACAATAGCATTGGTAAAGGG |
| Chr2:GL841709 | + | 293121 | 293343 | Chr2:GL841454 | + | 756307 | 756590 | ACATTGTTACCTCACTTAAACCCTG | AAGTGGAAGCTACAATTCATCGAG |
| ChrX:GL867695 | — | 14770 | 15030 | ChrX:GL867579 | + | 365807 | 366051 | AGTTAGTGTTCTAAGGATCTTCCCAG | CAGCTCATTTTACAGAGGAGAAAAC |
| Chr3:GL849903 | — | 2332457 | 2332734 | Chr3:GL849903 | + | 2329315 | 2329563 | AGAAGACAGAAAGATTTCCAAGGAG | GTGCTAACTGTTTTACCTCTCTTCATC |
| Chr5:GL861537 | — | 231027 | 231294 | Chr2:GL841419 | + | 404988 | 405201 | GACCATGGAGCTGAATTTTTATCC | GCTAACTATGAATTCTAACCAAACCCC |
| Chr1:GL834551 | — | 1036871 | 1037215 | Chr1:GL834446 | — | 378385 | 378698 | AGATAGGTGAAGGTAGAGATAAGGAGG | GAGAGAGCAAGAGAAAGAGAGAAAA |
| Chr3:GL849558 | — | 1424405 | 1424672 | Chr3:GL849558 | + | 1423958 | 1424197 | TACTGTCCAACACACATGCCTATTT | GGCATCCATTTGAATTTGACACTAC |
All coordinates are from genome build DEVIL7.0.
MHC Analysis.
A 274-bp DNA segment was amplified from genomic DNA using primers recognizing the polymorphic exon 2 of devil MHC class I loci. Primer sequences, Sahaα1F (5′-TCT CAC TCC TTG AGG TAC TTC G-3′) and Sahaα1R2 (5′-CTC GCT CTG GTT GTA GTA GCC G-3′) were modified from ref. 32; modifications to Sahaα1R (32) were made based on sequence alignments available in ref. 25. The number of loci amplified by these primers is not known, and may vary between individuals, but is predicted to be between four and five (33, 34); furthermore, we cannot exclude the possibility that these primer sequences select for only a subset of haplotypes due to polymorphisms present within primer binding sites. Libraries were prepared from amplicons from each individual and sequenced on the Illumina MiSeq platform with 150-bp paired end reads. Between 100,000 and 900,000 read pairs were sequenced per individual. Forward and reverse sequences were merged for each read pair, discarding any read pairs with mismatches in the overlapping region as well as any read pair that did not exactly match both primer sequences, yielding between 10,000 and 23,000 unique haplotypes per individual. Of these, only 17 haplotypes that were present with at least 1% frequency in at least one individual were considered further. We further validated these haplotypes by searching for reads exactly matching the haplotypes within the available whole genome sequences from 87T and 91H (13). One haplotype could not be validated using this approach and was discarded. The sequence composition of two further haplotypes meant that they could only be validated in conjunction with another haplotype, and the pattern of haplotype read counts made it uncertain if these two haplotypes were present in some individuals; thus these two haplotypes were excluded from Fig. 4D. Tables S6 and S7 contain nucleotide and predicted amino acid sequences for the MHC class I haplotypes presented in Fig. 4D. Each of the 14 haplotypes in Fig. 4D are predicted to encode a unique peptide sequence. Two haplotypes that had not previously been described were named SahaI*97 and SahaI*98 and their sequences submitted to GenBank with accession numbers KT188437 and KT188438, respectively.
Table S6.
MHC class I exon 2 haplotypes (nucleotide sequence)
| Haplotype | Accession | Nucleotide sequence |
| SahaI*32 | GQ411440 | GCACCACCGTGTCCCGGCCCGGACTCGGGGAGCCGCGATTCTTCTCCGTGGGCTACGTGGACGATCAGCAGTTCGTGGGCTTCGACAGCGACAGTGCGAGTCAGAGGGTGGAGCCGCGGGCACCATGGATAGAGAAGATGGAGAATGTGGACCGGGACTACTGGGAGCGGAACACGCAGAACAGTAAGAGGAATGCACAAATTTCCCGAGAGGACCTGCAGACCCTACA |
| SahaI*88 | JN389436 | ACACCGCCGTGTCCCGGCCCGGGCTCGGGGAGCCGCGGTTCCTCTCCGTGGGCTACGTGGACGATCAGCAGTTCGTGCGCTTCGACAGCGACAGCGCGAGTCAGAGTGAGGAGCCGCGGGCGCCGTGGATGGAGAAGGTGCAGGACGTGGACCCGGGATACTGGGAGCGGAACACACAGATCAGTAAGGAGAACGCACAGAGTTCCCGAGTGAGCCTGCAGACCCTGCG |
| SahaI*29 | GQ411437 | ACACCGCCGTGTCCCGGCCCGGGCTCGGGGAGCCGCGGTTCCTCTCCGTGGGCTACGTGGACGATCAGCAGTTCGTGCGCTTCGACAGCGACAGCGCGAGTCAGAGTGAGGAGCCGCGGGCGCCGTGGATGGAGAAGGTGCAGGACGTGGACCCGGGATACTGGGAGCAGGAGACACAGATCATTAAGGAGACTGCACAGATTTCCCGAGTGGACCTGCAGACCCTGCG |
| SahaI*35 | GQ411443 | ACACCGCCGTGTCCCGGCCCGGGCTCGGGGAGCCGCGGTTCCTCTCCGTGGGCTACGTGGACGATCAGCAGTTCGTGCGCTTCGACAGCGACAGCGCGAGTCAGAGTGAGGAGCCGCGGGCGCCGTGGATGGAGAAGGTGAAGGACGTGGACCCGGGATACTGGGAGCAGGAGACACAGATCATTAAGGAGACTGCACAGATTTCCCGAGTGGACCTGCAGACCCTGCG |
| SahaI*36 | GQ411444 | ACACCGCCGTGTCCCGGCCCGGGCTCGGGGAGCCGCGGTTCCTCTCCGTGGGCTACGTGGACGATCAGCAGTTCGTGCGCTTCGACAGCGACAGCGCGAGTCAGAGTGAGGAGCCGCGGGCGCCGTGGATGGAGAAGGTGAAGGACGTGGACCCGGGATACTGGGAGCAGGAGACACAGATCAGTAAGGAGACTGCACAGATTTACCGAGTGGGCCTGCAGACCCTGCG |
| SahaI*33 | GQ411441 | ACACCGCCGTGTCCCGGCCCGGGCTCGGGGAGCCGCGGTTCCTCTCCGTGGGCTACGTGGACGATCAGCAGTTCGTGCGCTTCGACAGCGACAGCGCGAGTCAGAGTGAGGAGCCGCGGGCGCCGTGGATGGAGAAGGTGAAGGACGTGGACCCGGGATACTGGGAGCAGCAGACACAGATCATTAAGGAGACTGCACAGATTTACCGAGTGGGCCTGCAGACCCTGCG |
| SahaI*97 | KT188437 | ACACCGCCGTGTCCCGGCCCGGGCTCGGGGAGCCGCGGTTCCTCTCCGTGGGCTACGTGGACGATCAGCAGTTCGTGCGCTTCGACAGCGACAGCGCGAGTCAGAGGCAGGAGCCGCGGGCGCCGTGGATGGAGAAGGTGAAGGACGTGGACCCGGGATACTGGGAGCAGCAGACACAGATCATTAAGGAGACTGCACAGATTTCCCGAGTGGACCTGCAGACCCTGCG |
| SahaI*27 | GQ411435 | ACACCGCCGTGTCCCGGCCCGGGCTCGGGGAGCCGCGGTTCCTCGCCGTGGGCTACGTGGACGATCAGCAGTTCGTGCGCTTCGACAGCGACAGCGCGAGTCAGAGTGAGGAGCCGCGGGCGCCGTGGATGGAGAAGGTGCAGGACGTGGACCCGGGATACTGGGAGCGGAACACACAGATCAGTAAGGAGAACGCACAGAGTTCCCGAGTGAGCCTGCAGAACCTGCG |
| SahaI*46 | GQ411454 | ACACCGCCGTGTCCCGGCCCGGGCTCGGGGAGCCGCGGTTCCTCGCCGTGGGCTACGTGGACGATCAGCAGTTCGTGCGCTTCGACAGCGACAGCGCGAGTCAGAGTGAGGAGCCGCGGGCGCCGTGGATGGAGAAGGTGCAGGACGTGGACCCGGGATACTGGGAGCAGGAGACACAGATCATTAAGGAGAACGCACAGAGTTCCCGAGTGGACCTGCAGACCCTGCG |
| SahaI*90 | JN389438 | ACACCGCCGTGTCCCGGCCCGGGCTCGGGGAGCCGCGGTTCCTCGCCGTGGGCTACGTGGACGATCAGCAGTTCGTGCGCTTCGACAGCGACAGCGCGAGTCAGAGTGAGGAGCCGCGGGCGCCGTGGATGGAGAAGGTGCAGGACGTGGACCCGGGATACTGGGAGCAGCAGACACAGAACAGTAAGGGGAATGCACAGATTTACCGAGTGGGCCTGCAGACCCTGCG |
| SahaI*98 | KT188438 | ACACCGCCGTGTCCCGGCCCGGGCTCGGGGAGCCGCGGTTCCTCGCCGTGGGCTACGTGGACGATCAGCAGTTCGTGCGCTTCGACAGCGACAGCGCGAGTCAGAGTGAGGAGCCGCGGGCGCCGTGGATGGAGAAGGTGAAGGACGTGGACCCGGGATACTGGGAGCAGGAGACACAGATCATTAAGGAGACTGCACAGATTTCCCGAGTGGACCTGCAGACCCTGCG |
| SahaI*37 | GQ411445 | ACACCGCCGTGTCCCGGCCCGGGCTCGGGGAGCCGCGGTTCCTCGCCGTGGGCTACGTGGACGATCAGCAGTTCGTGCGCTTCGACAGCGACAGCGCGAGTCAGAGTGAGGAGCCGCGGGCGCCGTGGATGGAGAAGGTGAAGGACGTGGACCCGGGATACTGGGAGCAGGAGACACAGATCAGTAAGGAGAACGCACAGATTTACCGAGTGGGCCTGCAGACCCTGCG |
| SahaI*49 | GQ411457 | ACACCGCCGTGTCCCGGCCCGGGCTCGGGGAGCCGCGGTTCCTCGCCGTGGGCTACGTGGACGATCAGCAGTTCGTGCGCTTCGACAGCGACAGCGCGAGTCAGAGTGAGGAGCCGCGGGCGCCGTGGATGGAGAAGGTGAAGGACGTGGACCCGGGATACTGGGAGCAGCAGACACAGATCAGTAAGGAGAACGCACAGATTTACCGAGTGGGCCTGCAGACCCTGCG |
| SahaI*45 | GQ411453 | ACACCGCCGTGTCCCGGCCCGGGCTCGGGGAGCCGCGGTTCCTCTCCGTGGGCTACGTGGACGATCAGCAGTTCGTGCGCTTCGACAGCGACAGCGCGAGTCAGAGTGAGGAGCCGCGGGCGCCGTGGATGGAGAAGGTGCAGGACGTGGACCCGGGATACTGGGAGCAGGAGACACAGATCATTAAGGAGAACGCACAGAGTTCCCGAGTGGACCTGCAGACCCTGCG |
Table S7.
MHC class I exon 2 haplotypes (predicted amino acid sequence)
| Haplotype | Accession | Predicted amino acid sequence |
| SahaI*32 | GQ411440 | TTVSRPGLGEPRFFSVGYVDDQQFVGFDSDSASQRVEPRAPWIEKMENVDRDYWERNTQNSKRNAQISREDLQTL |
| SahaI*88 | JN389436 | TAVSRPGLGEPRFLSVGYVDDQQFVRFDSDSASQSEEPRAPWMEKVQDVDPGYWERNTQISKENAQSSRVSLQTL |
| SahaI*29 | GQ411437 | TAVSRPGLGEPRFLSVGYVDDQQFVRFDSDSASQSEEPRAPWMEKVQDVDPGYWEQETQIIKETAQISRVDLQTL |
| SahaI*35 | GQ411443 | TAVSRPGLGEPRFLSVGYVDDQQFVRFDSDSASQSEEPRAPWMEKVKDVDPGYWEQETQIIKETAQISRVDLQTL |
| SahaI*36 | GQ411444 | TAVSRPGLGEPRFLSVGYVDDQQFVRFDSDSASQSEEPRAPWMEKVKDVDPGYWEQETQISKETAQIYRVGLQTL |
| SahaI*33 | GQ411441 | TAVSRPGLGEPRFLSVGYVDDQQFVRFDSDSASQSEEPRAPWMEKVKDVDPGYWEQQTQIIKETAQIYRVGLQTL |
| SahaI*97 | KT188437 | TAVSRPGLGEPRFLSVGYVDDQQFVRFDSDSASQRQEPRAPWMEKVKDVDPGYWEQQTQIIKETAQISRVDLQTL |
| SahaI*27 | GQ411435 | TAVSRPGLGEPRFLAVGYVDDQQFVRFDSDSASQSEEPRAPWMEKVQDVDPGYWERNTQISKENAQSSRVSLQNL |
| SahaI*46 | GQ411454 | TAVSRPGLGEPRFLAVGYVDDQQFVRFDSDSASQSEEPRAPWMEKVQDVDPGYWEQETQIIKENAQSSRVDLQTL |
| SahaI*90 | JN389438 | TAVSRPGLGEPRFLAVGYVDDQQFVRFDSDSASQSEEPRAPWMEKVQDVDPGYWEQQTQNSKGNAQIYRVGLQTL |
| SahaI*98 | KT188438 | TAVSRPGLGEPRFLAVGYVDDQQFVRFDSDSASQSEEPRAPWMEKVKDVDPGYWEQETQIIKETAQISRVDLQTL |
| SahaI*37 | GQ411445 | TAVSRPGLGEPRFLAVGYVDDQQFVRFDSDSASQSEEPRAPWMEKVKDVDPGYWEQETQISKENAQIYRVGLQTL |
| SahaI*49 | GQ411457 | TAVSRPGLGEPRFLAVGYVDDQQFVRFDSDSASQSEEPRAPWMEKVKDVDPGYWEQQTQISKENAQIYRVGLQTL |
| SahaI*45 | GQ411453 | TAVSRPGLGEPRFLSVGYVDDQQFVRFDSDSASQSEEPRAPWMEKVQDVDPGYWEQETQIIKENAQSSRVDLQTL |
Nucleotide and predicted amino acid sequences for 14 MHC class I (SahaI) exon 2 haplotypes included in Fig. 4D.
Acknowledgments
We thank Bill Brown, Phil Iles, Billie Lazenby, Jacinta Marr, Jane McGee, Sarah Peck, Holly Wiersma, and Phil Wise for assistance with sample collection and curation. Adrian Baez-Ortega, Andrew Davis, Jo Hanuszewicz, Gina Kalodimos, Amanda Patchett, Narelle Phillips, Elizabeth Reid Swainscoat, Jim Richley, Rachel Stivicic, and Jim Taylor assisted with surveying, laboratory analysis, data processing, and display. We are grateful for support received from Michael Stratton, the Wellcome Trust Sanger Institute (WTSI) sequencing and informatics teams, and the WTSI Cancer Genome Project. This work was supported by a Wellcome Trust Investigator Award (102942/Z/13/Z) and by grants from the Australian Research Council (ARC-DP130100715; ARC-LP130100218). Support was provided by Dr. Eric Guiler Tasmanian Devil Research Grants and by the Save the Tasmanian Devil Program. J.M.C.T. was partly supported by a Marie Curie Fellowship (FP7-PEOPLE-2012-IEF, 328364).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. KT188437 and KT188438).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1519691113/-/DCSupplemental.
References
- 1.Metzger MJ, Reinisch C, Sherry J, Goff SP. Horizontal transmission of clonal cancer cells causes leukemia in soft-shell clams. Cell. 2015;161(2):255–263. doi: 10.1016/j.cell.2015.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murchison EP. Clonally transmissible cancers in dogs and Tasmanian devils. Oncogene. 2008;27(Suppl 2):S19–S30. doi: 10.1038/onc.2009.350. [DOI] [PubMed] [Google Scholar]
- 3.Strakova A, Murchison EP. The cancer which survived: Insights from the genome of an 11000 year-old cancer. Curr Opin Genet Dev. 2015;30:49–55. doi: 10.1016/j.gde.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins CE, et al. Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii. Biol Conserv. 2006;131:307–324. [Google Scholar]
- 5.Loh R, et al. The pathology of devil facial tumor disease (DFTD) in Tasmanian Devils (Sarcophilus harrisii) Vet Pathol. 2006;43(6):890–895. doi: 10.1354/vp.43-6-890. [DOI] [PubMed] [Google Scholar]
- 6.Lachish S, Jones M, McCallum H. The impact of disease on the survival and population growth rate of the Tasmanian devil. J Anim Ecol. 2007;76(5):926–936. doi: 10.1111/j.1365-2656.2007.01272.x. [DOI] [PubMed] [Google Scholar]
- 7.Hamede R, et al. Reduced effect of Tasmanian devil facial tumor disease at the disease front. Conserv Biol. 2012;26(1):124–134. doi: 10.1111/j.1523-1739.2011.01747.x. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins CE, McCallum H, Mooney N, Jones M, Holdsworth M. 2008. Sarcophilus harrisii. The IUCN Red List of Threatened Species 2008: e.T40540A10331066. Available at dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T40540A10331066.en. Accessed December 14, 2015.
- 9.Pearse AM, Swift K. Allograft theory: Transmission of devil facial-tumour disease. Nature. 2006;439(7076):549. doi: 10.1038/439549a. [DOI] [PubMed] [Google Scholar]
- 10.Siddle HV, et al. Transmission of a fatal clonal tumor by biting occurs due to depleted MHC diversity in a threatened carnivorous marsupial. Proc Natl Acad Sci USA. 2007;104(41):16221–16226. doi: 10.1073/pnas.0704580104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddle HV, et al. Reversible epigenetic down-regulation of MHC molecules by devil facial tumour disease illustrates immune escape by a contagious cancer. Proc Natl Acad Sci USA. 2013;110(13):5103–5108. doi: 10.1073/pnas.1219920110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murchison EP, et al. The Tasmanian devil transcriptome reveals Schwann cell origins of a clonally transmissible cancer. Science. 2010;327(5961):84–87. doi: 10.1126/science.1180616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murchison EP, et al. Genome sequencing and analysis of the Tasmanian devil and its transmissible cancer. Cell. 2012;148(4):780–791. doi: 10.1016/j.cell.2011.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deakin JE, et al. Genomic restructuring in the Tasmanian devil facial tumour: Chromosome painting and gene mapping provide clues to evolution of a transmissible tumour. PLoS Genet. 2012;8(2):e1002483. doi: 10.1371/journal.pgen.1002483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearse AM, et al. Evolution in a transmissible cancer: A study of the chromosomal changes in devil facial tumor (DFT) as it spreads through the wild Tasmanian devil population. Cancer Genet. 2012;205(3):101–112. doi: 10.1016/j.cancergen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Ujvari B, et al. Anthropogenic selection enhances cancer evolution in Tasmanian devil tumours. Evol Appl. 2014;7(2):260–265. doi: 10.1111/eva.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tovar C, et al. Tumor-specific diagnostic marker for transmissible facial tumors of Tasmanian devils: Immunohistochemistry studies. Vet Pathol. 2011;48(6):1195–1203. doi: 10.1177/0300985811400447. [DOI] [PubMed] [Google Scholar]
- 18.Firestone KB. Isolation and characterization of microsatellites from carnivorous marsupials (Dasyuridae: Marsupialia) Mol Ecol. 1999;8(6):1084–1086. doi: 10.1046/j.1365-294x.1999.00655_6.x. [DOI] [PubMed] [Google Scholar]
- 19.Jones ME, Paetkau D, Geffen E, Moritz C. Microsatellites for the Tasmanian devil (Sarcophilus laniarius) Mol Ecol Notes. 2003;3:277–279. [Google Scholar]
- 20.Lane A, et al. New insights into the role of MHC diversity in devil facial tumour disease. PLoS One. 2012;7(6):e36955. doi: 10.1371/journal.pone.0036955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canfield PJ, Hartley WJ, Reddacliff GL. Spontaneous proliferations in Australian marsupials--A survey and review. 2. Dasyurids and bandicoots. J Comp Pathol. 1990;103(2):147–158. doi: 10.1016/s0021-9975(08)80171-5. [DOI] [PubMed] [Google Scholar]
- 22.Griner LA. Neoplasms in Tasmanian devils (Sarcophilus harrisii) J Natl Cancer Inst. 1979;62(3):589–595. doi: 10.1093/jnci/62.3.589. [DOI] [PubMed] [Google Scholar]
- 23.Jones ME, Paetkau D, Geffen E, Moritz C. Genetic diversity and population structure of Tasmanian devils, the largest marsupial carnivore. Mol Ecol. 2004;13(8):2197–2209. doi: 10.1111/j.1365-294X.2004.02239.x. [DOI] [PubMed] [Google Scholar]
- 24.Miller W, et al. Genetic diversity and population structure of the endangered marsupial Sarcophilus harrisii (Tasmanian devil) Proc Natl Acad Sci USA. 2011;108(30):12348–12353. doi: 10.1073/pnas.1102838108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddle HV, Marzec J, Cheng Y, Jones M, Belov K. MHC gene copy number variation in Tasmanian devils: Implications for the spread of a contagious cancer. Proc Biol Sci Roy Soc. 2010;277(1690):2001–2006. doi: 10.1098/rspb.2009.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamede RK, McCallum H, Jones M. Biting injuries and transmission of Tasmanian devil facial tumour disease. J Anim Ecol. 2013;82(1):182–190. doi: 10.1111/j.1365-2656.2012.02025.x. [DOI] [PubMed] [Google Scholar]
- 27.Brüniche-Olsen A, Jones ME, Austin JJ, Burridge CP, Holland BR. Extensive population decline in the Tasmanian devil predates European settlement and devil facial tumour disease. Biol Lett. 2014;10(11):20140619. doi: 10.1098/rsbl.2014.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rebbeck CA, Leroi AM, Burt A. Mitochondrial capture by a transmissible cancer. Science. 2011;331(6015):303. doi: 10.1126/science.1197696. [DOI] [PubMed] [Google Scholar]
- 29.Lu X, Kang Y. Cell fusion as a hidden force in tumor progression. Cancer Res. 2009;69(22):8536–8539. doi: 10.1158/0008-5472.CAN-09-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohr M, Zaenker KS, Dittmar T. Fusion in cancer: An explanatory model for aneuploidy, metastasis formation, and drug resistance. Methods Mol Biol. 2015;1313:21–40. doi: 10.1007/978-1-4939-2703-6_2. [DOI] [PubMed] [Google Scholar]
- 31.Kreiss A, Tovar C, Obendorf DL, Dun K, Woods GM. A murine xenograft model for a transmissible cancer in Tasmanian devils. Vet Pathol. 2011;48(2):475–481. doi: 10.1177/0300985810380398. [DOI] [PubMed] [Google Scholar]
- 32.Morris K, Austin JJ, Belov K. Low major histocompatibility complex diversity in the Tasmanian devil predates European settlement and may explain susceptibility to disease epidemics. Biol Lett. 2013;9(1):20120900. doi: 10.1098/rsbl.2012.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng Y, et al. Antigen-presenting genes and genomic copy number variations in the Tasmanian devil MHC. BMC Genomics. 2012;13:87. doi: 10.1186/1471-2164-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddle HV, Sanderson C, Belov K. Characterization of major histocompatibility complex class I and class II genes from the Tasmanian devil (Sarcophilus harrisii) Immunogenetics. 2007;59(9):753–760. doi: 10.1007/s00251-007-0238-2. [DOI] [PubMed] [Google Scholar]