Abstract
Transcription factors (TFs) bind specific sequences in promoter-proximal and distal DNA elements in order to regulate gene transcription. RNA is transcribed from both of these DNA elements, and some DNA-binding TFs bind RNA. Hence, RNA transcribed from regulatory elements may contribute to stable TF occupancy at these sites. We show that the ubiquitously expressed TF YY1 binds to both gene regulatory elements and also to their associated RNA species genome-wide. Reduced transcription of regulatory elements diminishes YY1 occupancy whereas artificial tethering of RNA enhances YY1 occupancy at these elements. We propose that RNA makes a modest but important contribution to the maintenance of certain TFs at gene regulatory elements and suggest that transcription of regulatory elements produces a positive feedback loop that contributes to the stability of gene expression programs.
Active promoters and enhancer elements are transcribed bi-directionally (Fig. 1A) (1–3). Although various models have been proposed for the roles of RNA species produced from these regulatory elements, their functions are not fully understood (4–13). Evidence that some DNA-binding transcription factors (TFs) also bind RNA (14, 15) led us to consider the possibility that there might be a direct and general role for promoter-proximal and distal enhancer RNA in the binding and maintenance of TFs at regulatory elements.
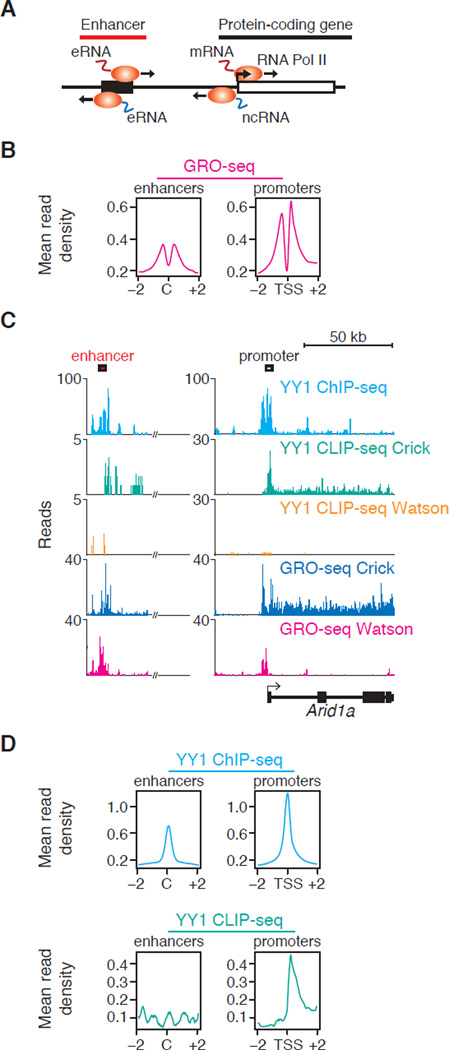
Fig. 1.
YY1 binds to DNA and RNA at transcriptional regulatory elements. (A) Cartoon depicting divergent transcription at enhancers and promoters in mammalian cells. (B) Alignment of GRO-seq reads at all enhancers and promoters in ESCs. Enhancers were defined as in (23). The x-axis indicates distance from either the enhancer center (C) or the transcription start site (TSS) in kilobases. The y-axis indicates average density of uniquely mapped GRO-seq reads per genomic bin. (C) Gene tracks for the Arid1a gene and enhancer showing ChIP-seq and CLIP-seq data for bio-YY1 cells, as well as GRO-seq reads for mESCs. (D) Mean read density of YY1 ChIP-seq and CLIP-seq reads at enhancers and promoters of all RefSeq genes in ESCs.
We sequenced nascent transcripts (GRO-seq) in murine embryonic stem cells (ESCs) at great depth, which confirmed that active promoters and enhancer elements are generally transcribed bi-directionally (Fig. 1B, fig. S1A, table S1). We then focused our studies on the TF Yin-Yang 1 (YY1) because it is ubiquitously expressed in mammalian cells, plays key roles in normal development, and can bind RNA species in vitro (15, 16). ChIP-seq analysis in ESCs revealed that YY1 binds to both active enhancers and promoters, with some preference for promoters (Fig. 1C, and D, fig. S1, table S2). In contrast, the pluripotency TF OCT4 preferentially occupies enhancers (fig. S1B). Consistent with this, YY1 sequence motifs were enriched at promoters, whereas OCT4 motifs were enriched at enhancers (fig. S1B). Neither YY1 nor OCT4 occupied the promoter-proximal sequences of inactive genes (fig. S2). These results establish that YY1 generally occupies active enhancer and promoter-proximal elements in ESCs.
We next investigated YY1 binding to RNA in vivo by using CLIP-seq in ESCs (fig. S3, S4, table S3). The results showed that YY1 binds RNA species at the active enhancer and promoter regions where it is bound to DNA (Fig. 1, C and D, fig. S1C). At promoters, YY1 preferentially occupied RNA downstream rather than upstream of transcription start sites (fig. S1B), consistent with YY1 motif distribution and evidence that upstream ncRNA is unstable (3, 17, 18). In similar experiments with OCT4, significant levels of RNA binding were not observed (fig. S5). These results suggest that YY1 generally binds to RNA species transcribed from enhancers and promoters in vivo.
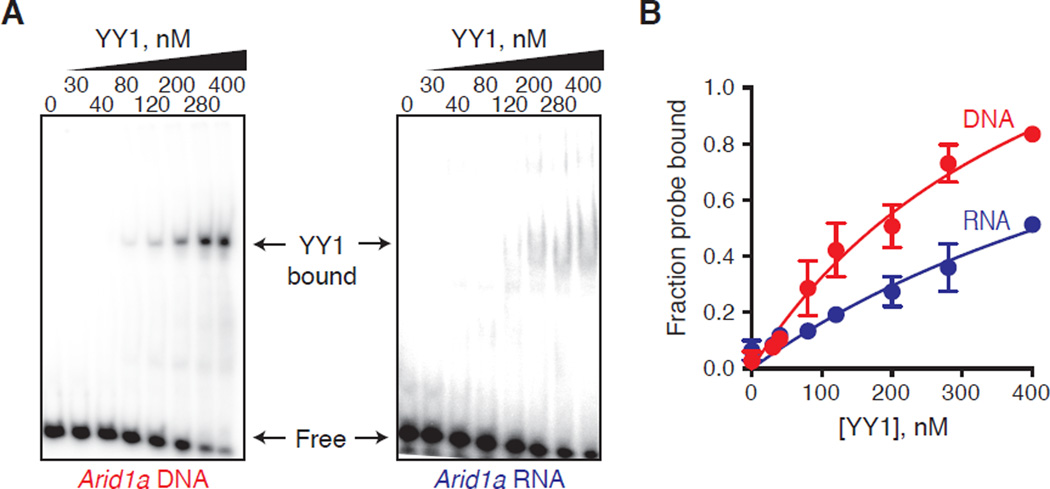
The DNA and RNA binding properties of YY1 were further investigated in vitro (Fig. 2, fig. S6 to S8). Recombinant murine YY1 protein bound both DNA and RNA probes in electrophoretic mobility shift essays (EMSA), showing higher affinity for DNA than RNA. There was variation in the affinity of YY1 for different RNA sequences (fig. S8). The four YY1 zinc-fingers can bind DNA (19), but the portion of YY1 that interacts with RNA is unknown. The zinc-finger –containing C-terminal region and the N-terminal region of YY1 were purified and their DNA and RNA binding properties were further investigated (fig. S9). The zinc-finger region of YY1 bound to DNA, but not to RNA, whereas the N-terminal region of YY1 bound to RNA (fig. S9). Furthermore, the DNA probe did not compete efficiently with the RNA probe for YY1 binding (fig. S7C, S8C). These results suggest that different regions of YY1 are responsible for binding to DNA and RNA.
Fig. 2.
YY1 binds to DNA and RNA in vitro. (A) Left panel: EMSA of YY1-DNA complexes at different concentrations of recombinant YY1. 5 nM of radioactively labelled 30-bp DNA probe derived from the promoter region of Arid1a gene containing a consensus YY1 binding motif (CTCTTCTCTCTTAAAATGGCTGCCTGTCTG) was incubated with increasing concentrations of recombinant murine YY1 protein. Right panel: EMSA of YY1-RNA complexes at different concentrations of recombinant YY1. 5 nM of radioactively labeled 30-nt RNA probe derived from the same region of the Arid1a gene was incubated with increasing concentrations of recombinant YY1 protein. (B) Graph depicting relationship between the fraction of radioactively labeled DNA or RNA probe bound and the concentration of recombinant YY1 in the binding reaction.
The observation that YY1 binds to enhancer and promoter-proximal elements and to RNA transcribed from those regions led us to postulate that nascent RNA contributes to stable TF occupancy at these regulatory elements (Fig. 3A). If this model is correct, then reduced levels of nascent RNA at promoters and enhancers might lead to reduced YY1 occupancy at these sites. We briefly inhibited transcription elongation with the reversible inhibitor D-rybofuranosylbenzimidazole (DRB) to reduce RNA levels at promoters and enhancers without causing changes in the steady-state levels of YY1 (fig. S10, S11). DRB treatment reduced transcription at promoters and enhancers and this caused small but significant decrease in the levels of YY1 at these regions (fig. S10). Super-enhancers are clusters of enhancers that are highly transcribed (20), and DRB treatment had a profound effect on transcription at these sites (fig. S10). Similar results were observed with additional inhibitors (fig. S10). When transcription was allowed to resume after DRB removal, the levels of YY1 increased at promoters and enhancers (Fig. 3B, fig. S10A). These results suggest that nascent RNA produced at promoters and enhancers contributes to YY1 binding to these elements.
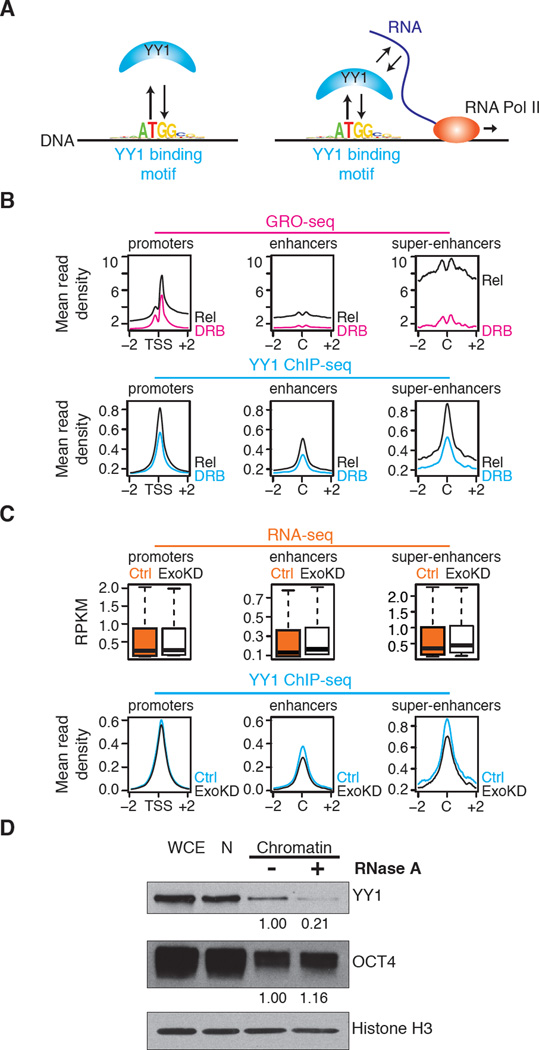
Fig. 3.
Perturbation of RNA levels affects YY1 binding to DNA. (A) Cartoon depicting hypothesis that RNA transcribed from regulatory elements enhances occupancy of these elements by TFs capable of binding both DNA and RNA. (B) (Top) GRO-seq reads (24) at promoters, enhancers, and super-enhancer constituents in cells before (DRB) and after release (Rel) from transcriptional inhibition by DRB. (Bottom) YY1 ChIP-seq reads at promoters, enhancers and super-enhancer constituents in cells before (DRB) and after release (Rel) from transcriptional inhibition by DRB. Increase in YY1 binding after release from DRB inhibition is significant: p-value < 3.6x10−207 for promoters, p-value < 1.6x10−214 for enhancers, p-value < 9.8x10−37 for super-enhancers. (C) (Top) Box plots depicting RNA-seq data for ribo-depleted total RNA at promoters, enhancers, and super-enhancers in ESCs after targeting with control (Ctrl) or Exosc3 (ExoKD) shRNA. (Bottom) Alignment of YY1 ChIP-seq reads at promoters, enhancers and super-enhancers in ESCs after targeting with control (Ctrl) or Exosc3 (ExoKD) shRNA. The decrease in YY1 binding in ExoKD ESCs is significant: p-value < 8.1x10−9 for promoters p-value < 1.8x10−27 for enhancers, p-value < 3.3x10−5 for super-enhancers. (D) Western blot analysis of YY1, OCT4, and histone H3 levels in whole-cell extracts (WCE), nuclei (N), and a nuclear chromatin preparation before and after RNase A treatment. Histone H3 serves as a loading control and OCT4 serves as a negative control. Quantitation of the relative levels of YY1 and OCT4 are noted.
The exosome reduces the levels of enhancer RNAs once they are released from Pol II (degradation is 3′ to 5′) (21), so knockdown of an exosome component will cause an increase in untethered enhancer RNA, which might titrate some YY1 away from enhancers. Indeed, exosome knockdown led to increased steady state levels of enhancer RNAs and a decrease in the levels of YY1 bound to enhancers (Fig. 3C, fig. S12). These results are consistent with the model that YY1 binding to DNA is stabilized by binding to nascent RNA.
If YY1 binding to DNA is stabilized by its binding to RNA, then RNase treatment of chromatin should reduce YY1 occupancy. Chromatin was extracted from ESC nuclei and the levels of YY1 in the chromatin preparation were compared with and without RNase A treatment (Fig. 3D). The results show that the levels of YY1 bound to chromatin were significantly decreased when the chromatin preparation was treated with RNase, consistent with the idea that RNA contributes to the stability of YY1 in chromatin.
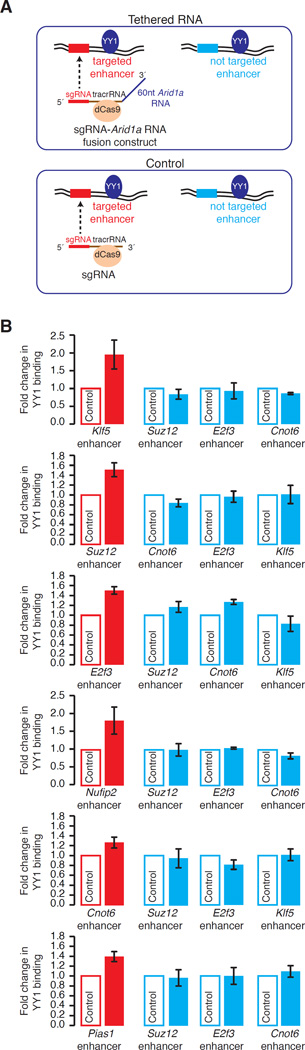
To test the idea that RNA near regulatory elements can contribute to stable TF occupancy in vivo, we tethered RNA in the vicinity of YY1 binding sites at six different enhancers in ESCs using the CRISPR/Cas9 system and determined whether the tethered RNA increases the occupancy of YY1 at these enhancers (Fig. 4). We generated stable murine ESC lines expressing both the catalytically inactive form of bacterial endonuclease Cas9 (dCas9) and a fusion RNA composed of guide RNA (sgRNA), tracrRNA, and a 60-nt RNA derived from the promoter sequence of Arid1a compatible with YY1 binding in vitro (fig. S8). For controls, stable cell lines were created that express dCas9 and sgRNA fused to tracrRNA for the six enhancers. Tethering the Arid1a RNA at each enhancer led to increased binding of YY1 to the targeted enhancer as measured by ChIP-qPCR (Fig. 4B). This elevation in YY1 binding was specific to the targeted locus and the sequence of tethered RNA as there was no observable increase in YY1 binding at the enhancers not targeted in the same cells (Fig. 4B) or targeted with tethered RNA not compatible with YY1 binding in vitro (fig. S13). These results show that RNA tethered near regulatory elements in vivo can enhance the level of YY1 occupancy at these elements.
Fig. 4.
Tethering of RNA adjacent an YY1 DNA binding site enhances binding of YY1 to the genome in vivo. (A) Strategy for tethering of RNA in the vicinity of an YY1 binding site at enhancers in vivo. (B) ChIP-qPCR analysis of YY1 binding at six targeted (red) and three not targeted (blue) enhancers in three independent experiments. The y-axis indicates fold change in YY1 binding in ESCs expressing the sgRNA-Arid1a RNA fusion construct relative to cells expressing the control sgRNA targeted to the same locus. The difference in YY1 binding was significant for the targeted enhancers: Klf5 (p-value=0.03), Suz12 (p-value=0.01), E2f3 (p-value=0.01), Nufip2 (p-value=0.03), Cnot6 (p-value=0.03), and Pias1 (p-value=0.01), but not for the not targeted enhancers.
To corroborate the in vivo RNA tethering results, a competition EMSA was used to test whether tethered RNA increases the apparent binding affinity of YY1 to its motif in DNA (fig. S14, S15). A short 30-bp labeled DNA probe containing a consensus YY1 binding motif was incubated with recombinant murine YY1 protein in the presence of increasing concentrations of cold competitor DNA with tethered or untethered RNA, and the amount of radiolabeled DNA that remained bound was quantified (fig. S15). This analysis revealed that DNA containing tethered RNA outcompetes the DNA without tethered RNA for YY1 binding. These results indicate that tethering RNA near the YY1 binding motif in DNA leads to increased binding of YY1 to DNA in vitro.
In summary, our results are consistent with the proposal that RNA enhances the level of YY1 occupancy at active enhancer and promoter-proximal regulatory elements (Fig. 3A). We suggest that nascent RNA produced in the vicinity of enhancer and promoter elements captures dissociating YY1 via relatively weak interactions, which allows this TF to rebind to nearby DNA sequences, thus creating a kinetic sink that increases YY1 occupancy on the regulatory element. The observation that YY1 occupies active enhancers and promoters throughout the ESC genome where RNA is produced, coupled with evidence that YY1 is expressed in all mammalian cells, suggests that this model is general. There are additional DNA-binding TFs that can bind RNA (fig. S16) (14), so transcriptional control may generally involve a positive feedback loop, where YY1 and other TFs stimulate local transcription, and newly transcribed nascent RNA reinforces local TF occupancy. This model helps explain why TFs occupy only the small fraction of their consensus motifs in the mammalian genome where transcription is detected and suggests that bidirectional transcription of active enhancers and promoters evolved, in part, to facilitate trapping of TFs at specific regulatory elements. The model also suggests that transcription of regulatory elements produces a positive feedback loop that may contribute to the stability of gene expression programs in cells. The significance of this TF trapping mechanism in cellular regulation has yet to be established, but will be important for future study because much of disease-associated sequence variation occurs in enhancers (20, 22) and may thus affect both DNA and RNA sequences that interact with gene regulators.
Supplementary Material
Acknowledgments
We thank D. Orlando, C. Lin, V. Saint-André, Z. P. Fan, L. Zhang, and A. Chiu for help with computational analysis and advice. The lentiviral dCas9 and sgRNA expression plasmids are available from Addgene under the Uniform Biological Material Transfer Agreement. Supported by NIH HG002668 (R.A.Y.), the Hope Funds for Cancer Research (B.J.A.), the Cancer Research Institute (Y.E.G), and Biogen (R.A.Y.). The Whitehead Institute intends to file a patent application that relates to transcription factor trapping by RNA. R.A.Y. is a founder of Syros Pharmaceuticals.
Footnotes
References
- 1.Core LJ, Waterfall JJ, Lis JT. Science. 2008;322:1845–1848. doi: 10.1126/science.1162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seila AC, et al. Science. 2008;322:1849–1851. doi: 10.1126/science.1162253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigova AA, et al. Proc Natl Acad Sci U S A. 2013;110:2876–2881. doi: 10.1073/pnas.1221904110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim TK, et al. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, et al. Nature. 2011;474:390–394. doi: 10.1038/nature10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melo CA, et al. Mol Cell. 2013;49:524–535. doi: 10.1016/j.molcel.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 7.Lai F, et al. Nature. 2013;494:497–501. doi: 10.1038/nature11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam MT, et al. Nature. 2013;498:511–515. doi: 10.1038/nature12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, et al. Nature. 2013;498:516–520. doi: 10.1038/nature12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaikkonen MU, et al. Mol Cell. 2013;51:310–325. doi: 10.1016/j.molcel.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mousavi K, et al. Mol Cell. 2013;51:606–617. doi: 10.1016/j.molcel.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Ruscio A, et al. Nature. 2013;503:371–376. doi: 10.1038/nature12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaukowitch K, et al. Mol Cell. 2014;56:29–42. doi: 10.1016/j.molcel.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassiday LA, Maher LJ., 3rd Nucleic Acids Res. 2002;30:4118–4126. doi: 10.1093/nar/gkf512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon Y, Lee JT. Cell. 2011;146:119–133. doi: 10.1016/j.cell.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon S, Akopyan G, Garban H, Bonavida B. Oncogene. 2006;25:1125–1142. doi: 10.1038/sj.onc.1209080. [DOI] [PubMed] [Google Scholar]
- 17.Flynn RA, Almada AE, Zamudio JR, Sharp PA. Proc Natl Acad Sci U S A. 2011;108:10460–10465. doi: 10.1073/pnas.1106630108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preker P, et al. Science. 2008;322:1851–1854. doi: 10.1126/science.1164096. [DOI] [PubMed] [Google Scholar]
- 19.Houbaviy HB, Usheva A, Shenk T, Burley SK. Proc Natl Acad Sci U S A. 1996;93:13577–13582. doi: 10.1073/pnas.93.24.13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hnisz D, et al. Cell. 2013;155:934–947. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lubas M, et al. Cell Rep. 2015;10:178–192. doi: 10.1016/j.celrep.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 22.Maurano MT, et al. Science. 2012;337:1190–1195. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whyte WA, et al. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, et al. Nature. 2015;523:621–625. doi: 10.1038/nature14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vella P, Barozzi I, Cuomo A, Bonaldi T, Pasini D. Nucleic Acids Res. 2012;40:3403–3418. doi: 10.1093/nar/gkr1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Chu J, Shen X, Wang J, Orkin SH. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyer LA, et al. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 28.Schmieder R, Edwards R. Bioinformatics. 2011;27:863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J, Cantor AB, Orkin SH, Wang J. Nature Protocols. 2009;4:506–517. doi: 10.1038/nprot.2009.23. [DOI] [PubMed] [Google Scholar]
- 30.Langmead B, Trapnell C, Pop M, Salzberg SL. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, et al. Genome biology. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kent WJ, et al. Genome research. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grant CE, Bailey TL, Noble WS. Bioinformatics. 2011;27:1017–1018. doi: 10.1093/bioinformatics/btr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bryne JC, et al. Nucleic acids research. 2008;36:D102–D106. doi: 10.1093/nar/gkm955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jolma A, et al. Cell. 2013;152:327–339. doi: 10.1016/j.cell.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Trapnell C, Pachter L, Salzberg SL. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Wang S, Li W. Bioinformatics. 2012;28:2184–2185. doi: 10.1093/bioinformatics/bts356. [DOI] [PubMed] [Google Scholar]
- 38.Loven J, et al. Cell. 2012;151:476–482. doi: 10.1016/j.cell.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin CY, et al. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinlan AR, Hall IM. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sigova AA, et al. Proc Natl Acad Sci U S A. 2013;110:2876–2881. doi: 10.1073/pnas.1221904110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D, et al. Nature. 2011;474:390–394. doi: 10.1038/nature10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kopylova E, Noe L, Touzet H. Bioinformatics. 2012;28:3211–3217. doi: 10.1093/bioinformatics/bts611. [DOI] [PubMed] [Google Scholar]
- 44.Kent WJ, Zweig AS, Barber G, Hinrichs AS, Karolchik D. Bioinformatics. 2010;26:2204–2207. doi: 10.1093/bioinformatics/btq351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jangi M, Boutz PL, Paul P, Sharp PA. Genes & development. 2014;28:637–651. doi: 10.1101/gad.235770.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin M. EMBnet journal. 2011;17:10–12. [Google Scholar]
- 47.Langmead B, Salzberg SL. Nature Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, et al. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cernilogar FM, et al. Nature. 2011;480:391-U151. doi: 10.1038/nature10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mullen AC, et al. Cell. 2011;147:565–576. doi: 10.1016/j.cell.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H, et al. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kearns NA, et al. Development. 2014;141:219–223. doi: 10.1242/dev.103341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.