Abstract
Pertussis, a highly contagious respiratory infection, remains a public health priority despite the availability of vaccines for 70 years. Still a leading cause of mortality in developing countries, pertussis has re-emerged in several developed countries with high vaccination coverage. Resurgence of pertussis in these countries has routinely been attributed to increased awareness of the disease, imperfect vaccinal protection or high infection rates in adults. In this review, we first present 1980–2012 incidence data from 63 countries and show that pertussis resurgence is not universal. We further argue that the large geographical variation in trends probably precludes a simple explanation, such as the transition from whole-cell to acellular pertussis vaccines. Reviewing available evidence, we then propose that prevailing views on pertussis epidemiology are inconsistent with both historical and contemporary data. Indeed, we summarize epidemiological evidence showing that natural infection and vaccination both appear to provide long-term protection against transmission and disease, so that previously infected or vaccinated adults contribute little to overall transmission at a population level. Finally, we identify several promising avenues that may lead to a consistent explanation of global pertussis epidemiology and to more effective control strategies.
Keywords: pertussis, pertussis epidemiology, pertussis vaccines, pertussis resurgence, vaccine-derived immunity, infection-derived immunity
1. Introduction
Pertussis, or whooping cough, is a highly contagious respiratory disease, primarily caused by the bacterium Bordetella pertussis [1]. Historically, a prominent cause of mortality in young children [2], routine paediatric immunization with whole-cell pertussis (wP) vaccines brought about large (typically exceeding 90%) reductions in reported cases in most developed countries, such as the USA [3] and Canada [4]. Despite these indisputable successes, alarming statistics indicate that pertussis remains a public health challenge. According to 2008 estimates, pertussis caused 16 million cases and 195 000 deaths in children younger than 5 years old worldwide, despite a global 82% vaccine coverage [5,6]. While this burden remains overwhelmingly concentrated in developing countries, pertussis has also re-emerged in some developed countries that maintain high vaccine coverage, including the USA [3], the UK [7] and Australia [8]. Many candidate explanations have been advanced, but the causes of these resurgences remain enigmatic and contentious.
Clinically, pertussis first manifests in mild, non-specific symptoms (catarrhal phase), which progress to a cough of remarkably long duration, marked by paroxysms, inspiratory whoop and post-tussive vomiting [1,9]. Critically, the infection is most transmissible during the catarrhal phase, when it is least apparent, hampering early diagnosis, treatment and isolation of the bacterium [1]. Unlike other childhood diseases, pertussis exhibits no consistent pattern of seasonality [1,9]. The immunology of pertussis, however, remains its most obscure aspect. Despite considerable effort, no reliable serological correlates of protection have been identified, reflecting what is probably a complex immune response to the many virulence factors expressed by B. pertussis [10].
Surprisingly, while these complexities leave key questions in pertussis unanswered, a number of robust opinions are frequently expressed (illustrated in electronic supplementary material, figure S6). In particular, pertussis is resurgent universally [11,12]; whole-cell and acellular pertussis (aP) vaccines do not protect against transmission [13–15] and that waning of infection- or vaccine-derived immunity generates an endemic pool of adults, who act as a reservoir of transmission to young children [15–17]. In this review, we re-examine the evidence supporting these widespread opinions and propose that they are inconsistent with the body of evidence taken as a whole. Finally, we highlight promising ideas that may lead to a coherent picture of pertussis epidemiology.
2. Contentious topics in pertussis epidemiology
(a). Pertussis is re-emerging everywhere
Despite highly publicized instances of resurgence in well-vaccinated countries [3,8,18], the ubiquity of this phenomenon remains unclear. To assess this, we extended a previous analysis [19] and extracted from the WHO database yearly case counts (1980–2012) and pertussis vaccine coverage estimates for 63 countries that met our inclusion criteria (more than 80% complete case count and more than five million inhabitants; electronic supplementary material, text S1). For each country, we applied a series of segmented regressions to detect significant changes in trend [3,20].
The results reveal substantial temporal and spatial variability in pertussis incidence worldwide (figure 1; electronic supplementary material, figure S3). Of the 63 countries examined, 32 had at least one period of increase during 1980–2012, comprising zero in Africa, eight in the Americas, four in Eastern Mediterranean, 11 in Europe, five in southeast Asia and four in Western Pacific. Despite an uneven distribution between regions for these 32 countries (exact multinomial test, p < 0.01), no such evidence was found when considering countries outside Africa (exact multinomial test, p = 0.25). By contrast, 31 countries did not have any significant period of increase (Africa: eight; Americas: eight; Eastern Mediterranean: five; Europe: four; Southeast Asia: three; Western Pacific: three), with an even distribution across regions (exact multinomial test, p = 0.44). Even in the 32 countries with at least one period of increase, 28 also had at least one period marked by a decrease, whereas only four—Australia, Israel, the Netherlands and the USA—experienced no decrease in pertussis incidence over 1980–2012. Considering only contemporary epidemiological trends (i.e. the last time segment for each country), pertussis incidence had increased in 16 countries but decreased in 32 countries, with no significant trend in 15 countries; again, this pattern did not differ between regions (Fisher's exact test, p = 0.39).
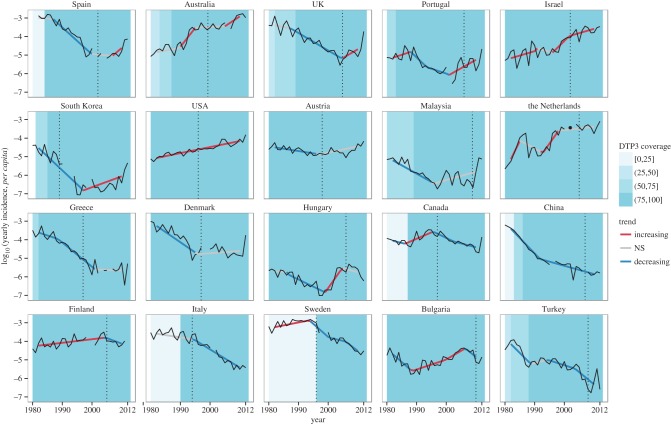
Figure 1.
Annual incidence data in 20 countries that switched to aP vaccines for primary immunization. We extracted 1980–2012 yearly case counts and pertussis vaccine coverage estimates from the WHO database (http://www.who.int/immunization/monitoring_surveillance/data/en/). For definiteness, we restricted our analysis to countries with more than 80% complete case count and more than 5 million inhabitants. Before analysis, incidence data were log10-transformed and a 5-year moving average was applied to remove the known 2–5 year cycles [21]. To detect long-term trends in pertussis reports, we proceeded in two steps. First, a series of segmented regression models with 0–3 breakpoints and time segments longer than 5 years were applied for each country [3,20]; of these, the most parsimonious model was selected according to the Bayesian information criterion. Second, to account for autocorrelation, we used generalized least-squares on each time segment identified in the first step, assuming the residuals autocorrelation structure followed an autoregressive process of order 1. For each country, the time segments were then classified according to their slope, as increasing (significantly positive slope), decreasing (significantly negative slope) or not significant. For each country, we represent the annual incidence (black solid lines), the fitted values from segmented regression, coloured according to the trend (red lines: significantly increasing; grey lines: no significant trend; blue lines: significantly decreasing), and the date of switch to aP vaccination (black vertical dotted lines). Coloured blue areas indicate the vaccine coverage for the third dose of DTaP vaccine. From left to right and top to bottom, countries are ranked by decreasing value of the last slope.
The switch to aP vaccine for primary immunization has been proposed to be associated with resurgence [14,22]. To assess this, we reviewed the literature to identify the date of the switch to aP vaccines in the countries that met our inclusion criteria. For those 20 countries, we examined their primary immunization schedule and the timing of any paediatric and/or adolescent booster dose (electronic supplementary material, table S1). Although the switch to aP coincided with resurgence in some countries (e.g. Spain and the UK), in others the resurgence occurred much earlier, notably in Australia, the USA, Israel, the Netherlands, Finland and Bulgaria. In addition, the switch to aP, after a period of high, low or no vaccination, led to significant decreases in incidence in Finland, Italy and Sweden, respectively. Trends were similarly variable for the 43 countries that used whole-cell pertussis (wP) vaccines for primary immunization; for example, pertussis incidence increased with increasing vaccine coverage in Brazil and Columbia, but decreased with increasing coverage in Bolivia, Thailand and Vietnam (electronic supplementary material, figure S3). Considering only contemporary data, these trends did not differ between countries that used wP or aP for primary immunization (Fisher's exact test, p = 0.22). Because the number of aP components [23] and timing of primary immunization [24] may affect pertussis epidemiology, we also considered this possibility, but found no consistent association across countries (electronic supplementary material, table S1).
Despite ever-present incompleteness in notifications, several robust findings emerge from this analysis. First, contrary to previous claims [11,12], pertussis resurgence is not global, and a majority of countries experienced sustained decreases in incidence over the last 30 years. Second, except for countries in Africa, for which no significant periods of increase were estimated, we found no consistent geographical pattern in the data, both overall and in the most recent period. Finally, we found no simple association between epidemiological trends and country-specific differences in vaccination (i.e. type of vaccine, vaccine composition or vaccination schedule), although more complex interactions may be at work.
(b). Whole-cell pertussis vaccines do not block transmission
Understanding the nature of vaccine-induced protection is critical for predicting the benefits of a vaccine. In addition to the direct protection conferred to those vaccinated, vaccines that prevent transmissible infection also protect the unvaccinated, who benefit from a decreased risk of infection. Such indirect effects, called herd immunity, are crucial for the success of vaccination programmes [25]. Although these effects are rarely measured in vaccine trials, they may be inferred indirectly from epidemiological data, such as patterns of persistence, changes in periodicity or variations of incidence in unvaccinated populations [26–28].
A highly influential study by Fine & Clarkson [13] concluded that wP vaccines protect against disease, but not transmission. Analysing aggregate incidence data from England and Wales, these authors noted an increase in the interepidemic period following the inception of routine infant immunization, but interpreted its magnitude as insufficiently large to support a substantial decrease in transmission [13]. This view remains commonly held, is frequently stated and is now embedded in standard textbooks [1,9].
There is evidence contradicting this view, however. In a follow-up analysis of an extended dataset from England and Wales, it was found that, in contrast to Fine & Clarkson's study, mass vaccination had in fact resulted in a systematic increase in the interepidemic period, as predicted by theory [29]. Subsequent analyses also identified strong signatures of herd immunity after the rollout of wP vaccines [26,30], notably a comparative study comprising many countries that demonstrated an average 1.3-year increase in the interepidemic period [21]. These findings were supported by direct evidence from a longitudinal study that estimated high effectiveness of whole-cell vaccines in reducing transmission in vaccinated breakthrough cases [31]. Therefore, despite known variability in efficacy [23], good whole-cell vaccines can unquestionably provide excellent protection against both disease and transmission.
(c). Acellular pertussis vaccines do not block transmission
Widely publicized concerns over the safety and immunogenicity of whole-cell vaccines prompted the development of aP vaccines, which have progressively replaced wP vaccines in most developed countries [1]. Although vaccine trials demonstrated high efficacy against disease [23], the ability of aP vaccines to prevent transmission, and therefore to induce herd immunity, has been questioned. Specifically, studies of vaccinated children indicate that aP vaccines stimulate distinct immune response profiles from those induced by natural infection or wP vaccination [32]. Further, experimental studies in mice [22] and baboons [14] suggest limited effectiveness of aP vaccines in preventing transmissible infection. Empirical evidence for asymptomatic transmission in aP-vaccinated individuals is reviewed by Althouse & Scarpino [33].
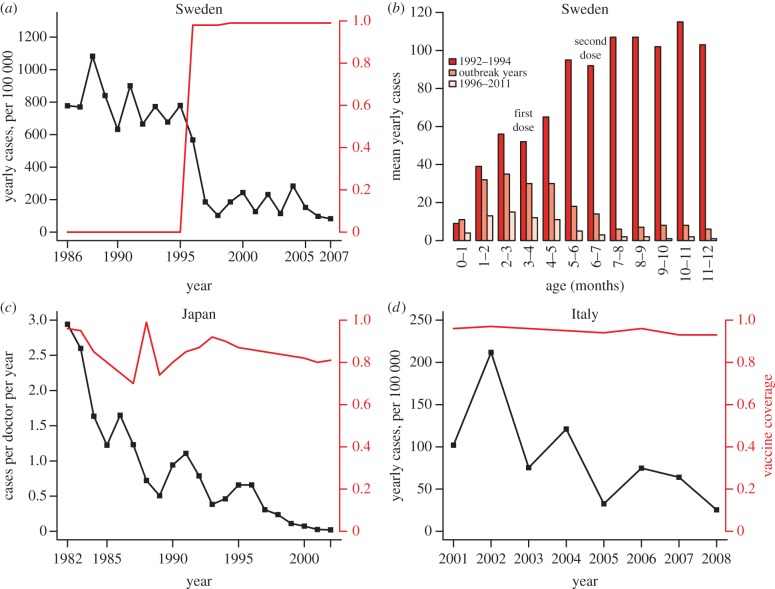
While intriguing, these conclusions are inconsistent with epidemiological trends in several countries that use aP vaccines (figure 2). In Sweden, resumption of aP vaccination, after a 17-year hiatus in pertussis immunization, clearly resulted in indirect protection of the unvaccinated population [37]. Indeed, the rollout of infant immunization was followed by substantial reductions of cases in all age groups, including both adults and infants too young to be vaccinated (figure 2) [34,38]. This finding confirmed earlier direct evidence of indirect protection of unvaccinated family members in a placebo-controlled trial of a pertussis toxoid vaccine [39]. Similar observations were made in Italy and Japan [35], where the first aP vaccine was developed (figure 2). To be sure, aP vaccines, like wP vaccines, vary in efficacy, with higher protection conferred by vaccines with three or more components [23]. The evidence from Sweden indicates that even monocomponent pertussis toxoid vaccines can effectively prevent transmission in addition to disease [39]. Therefore, simple extrapolation of recent experimental findings from animal models [14,22] to human populations is unwise [27].
Figure 2.
Epidemiological evidence of herd immunity induced by aP vaccines: decrease of cases in infants. (a) Yearly incidence (per 100 000) in infants less than 1 year old after the 1996 introduction of aP in Sweden (data redrawn from table 3 in [34]). (b) Mean yearly number of cases before (years 1992–1994) and after (overall period 1996–2011 or outbreak years) introduction of aP in Sweden, stratified by month of age in the first year of life. (c) Yearly cases per doctor in infants less than 1 year old after the 1981 introduction of aP in Japan (adapted from fig. 3 in [35]). (d) Yearly incidence (per 100 000) in infants less than 1 year old in Italy (adapted from fig. 5 in [36]).
(d). Changes in diagnostics and increased awareness explain pertussis resurgence
Because historically pertussis was regarded as a childhood disease, clinical diagnosis based on typical presentation in young children was considered reliable. For the same reason, the mild disease typical of infection in older children and adults was rarely reported [16,40]. For example, in the pre-vaccine era, out of 15 094 reported cases in Aberdeen, Scotland, only 0.59% were in individuals older than 15 years, with 0.26% identified as repeat attacks [41]. Although long-lasting infection-derived immunity explains this observation, it has been proposed that adult pertussis and repeat infections were, in fact, prevalent, but not reported because of lack of awareness and the reliance on clinical diagnosis [42]. Recently, more sensitive detection methods, including PCRs or serological assays of antibody titres against pertussis antigens, have allowed for a more sensitive (though not necessarily more specific [43]) measure of pertussis in adults. Using these methods, several studies identified pertussis as a frequent cause of prolonged coughs in adults [17], suggestive of an under-appreciated disease burden in this age group.
These observations have led some to assert that recent rises in pertussis reports merely reflected changes in observation instead of trends in epidemiology [44]. For example, it has been proposed that pertussis resurgence in the USA, where a shift in incidence to older age groups was observed, was mainly the result of better reporting of the disease in adults [44]. Although the positive correlation between surveillance effort and reported adult pertussis in US states superficially supports this claim [45], detailed observations suggest otherwise. Analyses of historical US data showed that the timing of the switch from a downward trend in incidence to an upward trend varied between states and mostly pre-dated the use of modern detection methods [3]. Furthermore, it was found that pertussis reports had declined over 2004–2007 in 24 out of 48 states [3]. These findings are inconsistent with an explanation of pertussis resurgence based on improved surveillance alone and point to changes in epidemiology.
(e). Natural infection and vaccination confer short-term immunity
Another commonly held view is that both natural infection and vaccination confer only transient protection [17,46]. This view stems from a highly cited review by Wendelboe et al. [15] that reported estimates of protective immunity of 4–20 years after natural infection and of 4–12 years after vaccination. As Wendelboe and co-workers acknowledged, however, most of the studies they reviewed were not designed to assess the duration of immunity. Consequently, they may measure a small and biased sample of the population, unrepresentative of those whose immunity did not fail. A closer examination of the studies reviewed to derive the reported range for the duration of infection-derived immunity makes these limitations apparent. Three empirical studies were reviewed [15]. The Aberdeen study [41] indicated near-lifelong immunity in a sample of 15 000 individuals, but was excluded by the authors from their estimates of duration of protection. The second study documented laboratory-confirmed reinfection in just four individuals, 3.5–12 years after first infection [40]. The third, a household contact study of 84 infected adults, found that 28 could recall having had pertussis at least 20 years before, though laboratory confirmation of the first infection was not possible [47]. We submit that this limited evidence is not a sound basis for claims of the brevity of infection-derived protection. By contrast, the evidence from the population dynamics of pertussis reports favours far longer durations of protection, consistent with epidemic periodicity and strong natural herd immunity observed in many countries [28,30,48,49]. Further, it has been shown that patterns of age-specific incidence during the 17-year vaccination hiatus in Sweden are inconsistent with even modest contributions to pathogen circulation from repeat infections [37].
Regarding wP vaccines, small-size cohort studies have estimated a diminished vaccine efficacy 4 [50] to 12 years [51] after completion of vaccination. However, to correctly interpret such estimates, one must overcome bias owing to case misclassification [52] and uncertainties surrounding the background force of infection and nature of vaccine failure (i.e. primary versus leaky) [53]. Epidemiological theory indicates that low durations of vaccine-derived immunity would result in low vaccinal herd immunity [54]. This is illustrated by a simple calculation of the reduction in the force of infection after vaccination, 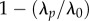 , where λp is the equilibrium force of infection at vaccine coverage p, and λ0 the equilibrium force of infection in the absence of vaccination. Following Magpantay et al. [55], we define the vaccine impact by
, where λp is the equilibrium force of infection at vaccine coverage p, and λ0 the equilibrium force of infection in the absence of vaccination. Following Magpantay et al. [55], we define the vaccine impact by 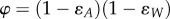 , where ɛA is the probability of primary vaccine failure and
, where ɛA is the probability of primary vaccine failure and 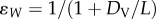 is the probability that vaccine-derived immunity wanes within a lifetime, with L the mean lifespan and DV the mean duration of vaccine-derived immunity. For a vaccinated–susceptible–infectious–recovered model with perfect infection-derived immunity (a plausible model of pertussis; cf. [48]),
is the probability that vaccine-derived immunity wanes within a lifetime, with L the mean lifespan and DV the mean duration of vaccine-derived immunity. For a vaccinated–susceptible–infectious–recovered model with perfect infection-derived immunity (a plausible model of pertussis; cf. [48]), 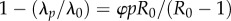 , where R0 is the basic reproduction number. Application of this formula with reasonable parameters (R0 = 15, ɛA = 0.15, L = 70 y, p = 0.8) and durations of immunity reported in reference [15] (DV range: 4–12 years) yields a modest 4–11% reduction in the force of infection. In other words, 80% vaccination coverage should produce a negligible impact on transmission and therefore fail to generate appreciable herd immunity, in contradiction to the historical experience following the rollout of wP vaccination in many countries [21,29,30,48].
, where R0 is the basic reproduction number. Application of this formula with reasonable parameters (R0 = 15, ɛA = 0.15, L = 70 y, p = 0.8) and durations of immunity reported in reference [15] (DV range: 4–12 years) yields a modest 4–11% reduction in the force of infection. In other words, 80% vaccination coverage should produce a negligible impact on transmission and therefore fail to generate appreciable herd immunity, in contradiction to the historical experience following the rollout of wP vaccination in many countries [21,29,30,48].
Although epidemiological datasets in the aP vaccine era are typically short, trends of increasing incidence among school-aged children have led many to speculate that aP vaccines provide only short-lived immunity, with efficacy waning within as little as 5 years [56,57]. Again, interpreting such observations requires a careful consideration of the mode of vaccine failure: one must distinguish waning immunity from primary vaccine failure. In Sweden, strong signatures of herd immunity [34] argue against a very short duration of aP-derived immunity. Careful accounting for age-specific contact rates explained much of the detailed age-specific incidence patterns in the Sweden data (including the observed increase in teenage cases), even under the assumption of lifelong aP-induced immunity [37].
The epidemiological evidence points to long-lasting vaccine- and infection-induced protection against transmissible infection. This is at odds with the clinical observation of disease in vaccinated and previously infected individuals. We suggest that it is possible to reconcile these discordant observations by recognizing the individual variability in the duration and mode of protection, which appears to be substantial in the case of pertussis [30]. Clearly, while pertussis immunity is imperfect, it is important to acknowledge the diversity of immunity characteristics across the population and that the average experience may significantly differ from that of a random individual.
(f). Adults are a reservoir of infection to young children
It has become conventional wisdom that adults are important in the modern epidemiology of pertussis [9,16,17,46]. According to this view, a short duration of infection- or vaccine-derived immunity results in an endemic pool of infected adults, who act as a hidden reservoir of infection to susceptible children [16,17]. This opinion has originated from studies that estimated high incidence in adults based on serological assays and PCR [17] (electronic supplementary material, table S2), and from household contact studies, in which adults and adolescents have been identified as the frequent source of infection for infants, although the source case frequently cannot be identified [58,59].
Several investigators, however, have questioned the overwhelming reliance on serology to detect cases in adults, a method prone to false-positives for which the association with transmission is unclear [43,60]. Indeed, despite imperfect sensitivity [61], culture from nasopharyngeal swabs remains the gold standard for the diagnosis of pertussis [1]. In contrast to the conventional wisdom, population-based models, challenged to explain incidence data in both the pre-vaccine and the vaccine era, have provided remarkably consistent evidence for a minimal impact of repeat infections (electronic supplementary material, table S4 [21,30,37,48]. This finding accords with epidemiological theory: the regular 2–5 year cycles in pertussis reports indicate periodic waves of infection interspersed by periods of slow build-up, via birth, of naive susceptibles; analyses of simple models show that constant input of infectives, for example endemic cases in adults, preclude oscillations in such systems [62].
Not surprisingly, direct observation of infection in adults is rare, but not unknown. Von König et al. [17] reviewed a number of studies that estimated prevalence of symptomatic infection in adults. Using culture, serology and PCR, the studies reviewed obtained estimates ranging from 0.05% to 0.5% incidence of symptomatic adult cases per year in highly vaccinated populations (electronic supplementary material, table S2). These may be compared with what would be expected if immunity were permanent. We formulated a basic age-structured SIR model, assuming different durations of infection- and vaccine-derived immunity (electronic supplementary material, text S2). Under the conservative assumptions of 90% vaccine coverage, 15% primary vaccine failure and an average duration of infection-derived immunity exceeding 30 years [30], we found the above-cited estimates to be inconsistent with durations of vaccine-induced protection shorter than 50 years (electronic supplementary material, figure S5). By contrast, durations of vaccine-derived immunity shorter than 20 years lead to incidences in excess of 1.5% per year in adults. While this intentionally simple model does not constitute empirical evidence, it nevertheless demonstrates that numbers reported in the literature are not inconsistent with long average durations of immunity, a finding confirmed using more elaborate models [21,30,37]. Finally, we remark on the possibility, rarely considered but consistent with epidemiological data from England and Wales, that symptomatic infections in adults represent primary infections in individuals who have escaped both previous vaccination and natural infection owing to incomplete coverage with imperfect vaccines [7].
Although transmissible infections in adults certainly can occur, the lines of above-described evidence incline us to concur with the conclusion of Preston [63] that there is ‘abundant evidence of the very limited role of adults' in pertussis epidemiology. This might be either because immunity wanes slowly (and thus adult infections are rare) or because adult infections transmit at lower rates, as some clinical evidence suggests [64]. Thus, observations, in adults, of frequent increases in serum titres of pertussis-associated antibodies (exceeding 5% per year in some broad serological surveys [65]) may represent anamnestic responses in the absence of transmissible infection [43,66]. In keeping with this hypothesis, serological studies in families have noted antibody-titre increases, without concomitant inception of clinical disease and probably without establishment of transmissible infection [67]. Intriguingly, the ability of B. pertussis to form biofilms in the respiratory tract [68] might help explain such responses, as might immunological cross-reactivity with other pathogens.
3. Promising ideas
In §2, we have shown that none of the frequently cited mechanisms proposed to explain pertussis epidemiology is consistent with all available. Here, we discuss additional candidate explanations.
(a). Bordetella pertussis populations evolve
The adaptation of B. pertussis to vaccination has been proposed as an explanation of recent epidemiological trends [69]. Indeed, many studies identified temporal variations in B. pertussis populations as a possible consequence of vaccine immune escape [70]. Serotyping—based on agglutination assays using antisera against antigens 1, 2 and 3—provided the first such evidence. In the UK, several studies documented a shift in the relative abundance of serotypes after the start of routine wP vaccination, from a predominance of serotypes containing antigen 2 (serotypes Fim2 and Fim2,3) in the 1940s to Fim3 in the 1960s [71]. Similar observations in other countries [72], particularly in Sweden during three consecutive periods with wP vaccination, no vaccination and aP vaccination [73], provided evidence of vaccine-driven evolution to serotype Fim3, hypothesized to be less antigenic and therefore at a selective advantage in vaccinated children [74].
Using newer molecular typing methods, many studies have also documented shifts in allele frequencies of major B. pertussis antigens following inception of vaccination [69]. A prominent example is the resurgence of pertussis in the Netherlands, which coincided with the emergence and subsequent spread of a novel allele of the pertussis toxin promoter, suggested to improve fitness by increasing pertussis toxin production and severity of infections [75]. In the USA, where similar epidemiological trends have been observed since the 1980s, pertussis resurgence was associated with a mutation in the gene coding for fimbrial proteins, although the functional role of that mutation remains unclear [76].
Based on the above observations, it has been proposed that vaccination has resulted in selection of more virulent strains that are more efficiently transmitted by previously primed hosts [69]. To assess the weight of evidence in support of this hypothesis, several key questions will need to be answered, with important implications for the design and the use of current and future vaccines [77]. For example, why have novel, beneficial variants not spread more broadly across the globe? What is the impact, if any, of these novel variants on vaccine effectiveness [73]? More importantly, a tentative test of this hypothesis will require a comprehensive description of variations in B. pertussis populations, notably in countries, such as Australia [78], where resurgence has not coincided with the timing of strain changes.
Answering the above-mentioned questions will require highly resolved, geographically distributed gene sequences from bacterial isolates. The phylodynamic analysis of such sequences would permit the identification of associations between transmission and genetic markers for virulence and antigenicity. Furthermore, such an analysis would permit the quantification of gene flow between geographical regions. Additionally, animal challenge experiments can be invaluable in quantifying the relative transmissibility of B. pertussis variants.
(b). Circulation of congeners is increasing
In addition to B. pertussis, the main aetiological agent of pertussis—other bacterial species from the genus Bordetella—can infect humans and may play a role in the epidemiology of pertussis-like illness [9]. Among these, B. parapertussis has been shown to cause symptoms very similar to those caused by B. pertussis, though shorter-lived [79,80]. Moreover, because they express two closely related surface proteins, filamentous haemagglutinin and pertactin, the two species induce quantitatively similar antibody response against these two antigens [79]. Although estimates of B. parapertussis incidence rates are low [80], experimental evidence suggests that aP fails to confer cross-protection and can even facilitate infection by this species [81]. This suggests, therefore, that a role for B. parapertussis in highly vaccinated populations should not be disregarded.
Another Bordetella species, B. holmesii, has recently attracted attention [82]. Although its epidemiology remains largely unstudied, the bacterium is known to cause invasive disease as well as pertussis-like respiratory infections, mostly in adolescents and adults [82]. Remarkably, current PCR-based methods do not discriminate between B. holmesii and B. pertussis, and several studies have identified B. holmesii in a substantial proportion of individuals clinically diagnosed with pertussis. For example, in a 2010 pertussis outbreak in Ohio, B. holmesii accounted for 43% of cases in the 11–18 age group and 30% of cases overall [83]. In addition, B. holmesii was retrospectively identified in 20% of samples from patients with suspected pertussis in a study in France [84]. These findings raise the possibility of frequent misdiagnosis of pertussis, with considerable implications for the estimation of vaccine efficacy [85]. While the pathogenicity and prevalence of B. pertussis congeners need to be established, their role will be important to consider in future studies. To properly quantify the contribution of B. pertussis congeners to reported incidence, there is a need for more sensitive diagnostic tools capable of distinguishing between different Bordetella species, in addition to more frequent bacterial isolation from suspected cases. Finally, animal challenge experiments aimed at determining heterospecific cross-immunity will be invaluable.
(c). The nature of vaccine failure matters
Quantification of not only the probability of vaccine failure, but also the manner in which a vaccine fails, may prove critical in understanding pertussis epidemiology. Although pertussis immunity is complex [10], two simplified models of vaccine failure help clarify the issues by capturing opposite extremes [86]. A leaky vaccine confers equal, though incomplete, protection to all vaccinated individuals, by reducing the probability of infection at each exposure. By contrast, an all-or-nothing vaccine confers complete protection to a fraction of vaccinated individuals, but no protection to the others [86]. In addition, under both these models, vaccinal immunity can wane [53,87,88]. These alternative models lead to different interpretations of age-specific vaccine efficacy estimates from longitudinal studies [50,53,86], and to different predicted effectiveness of booster vaccination strategies [89].
Interestingly, these different models of vaccine failure leave distinct signatures in epidemiological dynamics. In particular, theory indicates that leakiness can give rise to a ‘reinfection threshold’ [90] separating a high-transmission regime—characterized by frequent, immunity-boosting reinfections, infrequent waning and severe infections concentrated in children—from a low-transmission regime marked by infrequent reinfections, waning immunity and high prevalence of severe infections in older age groups. Intriguingly, this pattern results in an overall increase in severe infections as a result of reduced transmission and was proposed as an explanation for recent epidemiological shifts in pertussis [90]. Nevertheless, because previous studies could not discriminate between the different modes of vaccine failure [53,88], key assumptions underlying this hypothesis remain unsupported by data. Recent analysis of models has revealed distinct epidemiological signatures for these different modes [55], suggesting that statistical inference on longitudinal incidence data may identify the mode of vaccine failure. Ideally, this question can be answered with longer-term vaccine trial designs [87]. Comparative epidemiological dynamics in the decades following changes in the vaccine regimen has the potential to resolve this issue.
(d). The honeymoon is over
For any infectious disease, transmission results from interactions between susceptible and infected individuals of a population. Because such interactions are inherently nonlinear, seemingly straightforward interventions can have unanticipated consequences. In particular, immediately following the roll-out of a vaccination programme, it is possible to observe a ‘honeymoon’ period, during which incidence is very low. This phenomenon arises via the combined effects of vaccination in newborns and initially high herd immunity from previously infected older individuals. As susceptibles slowly accumulate owing to incomplete immunization and herd immunity gradually dissipates through natural deaths of immune individuals, the honeymoon period eventually ends, leading to a rise in prevalence especially among older individuals who escaped both infection and vaccination. This effect, predicted by models [91] and documented for measles [92], has recently been shown to be consistent with pertussis resurgence in England and Wales [7]. Specifically, using an age-structured transmission model and assuming slowly waning vaccinal immunity, Riolo et al. interpreted the recent resurgence of pertussis in England and Wales as the inevitable consequence of a spillover of susceptible individuals into older age groups over decades of incomplete coverage with an imperfect vaccine [7]. Although the generality of this phenomenon is unknown, it demonstrates that recent epidemiological trends need not necessarily reflect recent changes in epidemiology or biology, but rather the slow-to-manifest effects of long-standing practice.
4. Conclusion
We have summarized empirical evidence showing that, contrary to the prevailing view, pertussis vaccines confer long-term protection against transmission and disease, so that previously infected or vaccinated adults play a minimal role in transmission. While this may appear at odds with the results of particular studies, we submit that the totality of the evidence is fully consistent with this conclusion. In general, because of the substantial heterogeneity among individuals, great care is needed in the extrapolation of clinical evidence to the population level, and vice versa.
We have highlighted several promising ideas that may explain the perplexing features of pertussis. Most of these ideas have implications that can be tested by integrating models with relevant data. While at present insufficient information is available on B. pertussis congeners, the growing interest in B. holmesii may soon yield enough data to inform a detailed transmission model, which would take into account the selective advantage imposed by widespread vaccination against B. pertussis. Similarly, estimation of key parameters from longitudinal incidence data, using modern inference techniques [93], will help elucidate the mechanisms of vaccinal immunity conferred by wP and aP vaccines. Pinpointing the vaccine impact will also be critical for the design of immunization strategies to protect newborns, such as cocooning or maternal immunization [94]. Finally, applying the concept of a honeymoon period to countries with resurging pertussis might help focus efforts on those characteristics of pertussis epidemiology most in need of further explanation.
In their 1951 review, Gordon & Hood [2, p. 334] noted that ‘the epidemiological behavior of whooping cough should be easy to predict; but whooping cough does not always behave according to expectation’. More than 60 years after this statement, our understanding of pertussis epidemiology remains far from perfect. Indeed, our analysis indicates considerable variability in trends across countries. These findings emphasize the complexity of pertussis population biology, arising from the dynamic interplay between country-specific vaccination practices, regional variations in sociodemographic factors and in the genetic make-up of the aetiological agents, and heterogeneities among individuals in transmission and disease. Nevertheless, the considerable burden owing to pertussis makes it worthwhile to reconsider long-held beliefs in the light of all available evidence.
Supplementary Material
Acknowledgements
We thank Maria Riolo for helpful comments on the manuscript.
Data accessibility
All data used for the time-series analysis are freely available from the WHO database (http://www.who.int/immunization/monitoring_surveillance/data/en; accessed 23 June 2014) and from the World Bank database (http://data.worldbank.org/data-catalog/world-development-indicators; accessed 23 June 2014).
Authors' contributions
M.D.d.C. performed the analyses and wrote a first draft of the manuscript. All authors contributed to the revision/final draft of the manuscript and approved the final version.
Competing interests
We have no competing interests.
Funding
P.R. and A.A.K. are supported by the Research and Policy in Infectious Disease Dynamics programme of the Science and Technology Directorate, Department of Homeland Security, the Fogarty International Center, National Institutes of Health, by a research grant from the National Institutes of Health (1R01AI101155) and by MIDAS, National Institute of General Medical Sciences U54-GM111274. The funding sources had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
References
- 1.Edwards KM, Decker MD. 2013. Pertussis vaccines. In Vaccines (eds SA Plotkin, WA Orenstein, PA Offit), pp. 447–492. Philadelphia, PA: Elsevier Saunders. [Google Scholar]
- 2.Gordon JE, Hood RI. 1951. Whooping cough and its epidemiological anomalies. Am. J. Med. Sci. 222, 333–361. ( 10.1097/00000441-195109000-00011) [DOI] [PubMed] [Google Scholar]
- 3.Rohani P, Drake JM. 2011. The decline and resurgence of pertussis in the US. Epidemics 3, 183–188. ( 10.1016/j.epidem.2011.10.001) [DOI] [PubMed] [Google Scholar]
- 4.Varughese P. 1985. Incidence of pertussis in Canada. Can. Med. Assoc. J. 132, 1041–1042. [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization 2010. Pertussis vaccines: WHO position paper. Wkly Epidemiol. Rec. 85, 385–400. [PubMed] [Google Scholar]
- 6.Black RE, et al. 2010. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375, 1969–1987. ( 10.1016/S0140-6736(10)60549-1) [DOI] [PubMed] [Google Scholar]
- 7.Riolo MA, King AA, Rohani P. 2013. Can vaccine legacy explain the British pertussis resurgence? Vaccine 31, 5903–5908. ( 10.1016/j.vaccine.2013.09.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheridan SL, Ware RS, Grimwood K, Lambert SB. 2012. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA 308, 454–456. ( 10.1001/jama.2012.6364) [DOI] [PubMed] [Google Scholar]
- 9.Cherry JD, Heininger U. 2014. Pertussis and other Bordetella infections. In Feigin and Cherry's textbook of pediatric infectious diseases (eds J Cherry, GJ Demmler-Harrison, SL Kaplan, WJ Steinbach, P Hotez), pp. 1616–1639. Philadelphia, PA: Saunders/Elsevier. [Google Scholar]
- 10.Higgs R, Higgins SC, Ross PJ, Mills KHG. 2012. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol. 5, 485–500. ( 10.1038/mi.2012.54) [DOI] [PubMed] [Google Scholar]
- 11.Libster R, Edwards KM. 2012. Re-emergence of pertussis: what are the solutions? Expert Rev. Vaccines 11, 1331–1346. ( 10.1586/erv.12.118) [DOI] [PubMed] [Google Scholar]
- 12.Plotkin SA. 2014. The pertussis problem. Clin. Infect. Dis. 58, 830–833. ( 10.1093/cid/cit934) [DOI] [PubMed] [Google Scholar]
- 13.Fine PE, Clarkson JA. 1982. The recurrence of whooping cough: possible implications for assessment of vaccine efficacy. Lancet 1, 666–669. ( 10.1016/S0140-6736(82)92214-0) [DOI] [PubMed] [Google Scholar]
- 14.Warfel JM, Zimmerman LI, Merkel TJ. 2014. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc. Natl Acad. Sci. USA 111, 787–792. ( 10.1073/pnas.1314688110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wendelboe AM, Van Rie A, Salmaso S, Englund JA. 2005. Duration of immunity against pertussis after natural infection or vaccination. Pediatr. Infect. Dis. J. 24, S58–S61. ( 10.1097/01.inf.0000160914.59160.41) [DOI] [PubMed] [Google Scholar]
- 16.Cherry JD. 1999. Epidemiological, clinical, and laboratory aspects of pertussis in adults. Clin. Infect. Dis. 28(Suppl 2), S112–S117. ( 10.1086/515058) [DOI] [PubMed] [Google Scholar]
- 17.von König CHW, Halperin S, Riffelmann M, Guiso N. 2002. Pertussis of adults and infants. Lancet Infect. Dis. 2, 744–750. ( 10.1016/S1473-3099(02)00452-8) [DOI] [PubMed] [Google Scholar]
- 18.van der Maas NAT, Mooi FR, de Greeff SC, Berbers GAM, Spaendonck MA.E.C.-V, de Melker HE. 2013. Pertussis in the Netherlands, is the current vaccination strategy sufficient to reduce disease burden in young infants? Vaccine 31, 4541–4547. ( 10.1016/j.vaccine.2013.07.060) [DOI] [PubMed] [Google Scholar]
- 19.Jackson DW, Rohani P. 2014. Perplexities of pertussis: recent global epidemiological trends and their potential causes. Epidemiol. Infect. 142, 672–684. ( 10.1017/S0950268812003093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muggeo VM. 2008. Segmented: an R package to fit regression models with broken-line relationships. R News 8, 20–25. [Google Scholar]
- 21.Broutin H, Viboud C, Grenfell BT, Miller MA, Rohani P. 2010. Impact of vaccination and birth rate on the epidemiology of pertussis: a comparative study in 64 countries. Proc. R. Soc. B 277, 3239–3245. ( 10.1098/rspb.2010.0994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smallridge WE, Rolin OY, Jacobs NT, Harvill ET. 2014. Different effects of whole-cell and acellular vaccines on Bordetella transmission. J. Infect. Dis. 209, 1981–1988. ( 10.1093/infdis/jiu030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Prietsch SOM, Axelsson I, Halperin SA. 2012. Acellular vaccines for preventing whooping cough in children. Cochrane Database Syst. Rev. 3, CD001478 ( 10.1002/14651858.cd001478.pub5) [DOI] [PubMed] [Google Scholar]
- 24.Taranger J, Trollfors B, Knutsson N, Sundh V, Lagergåard T, Ostergaard E. 1999. Vaccination of infants with a four-dose and a three-dose vaccination schedule. Vaccine 18, 884–891. ( 10.1016/S0264-410X(99)00341-2) [DOI] [PubMed] [Google Scholar]
- 25.Fine PE. 1993. Herd immunity: history, theory, practice. Epidemiol. Rev. 15, 265–302. [DOI] [PubMed] [Google Scholar]
- 26.Blackwood JC, Cummings DAT, Broutin H, Iamsirithaworn S, Rohani P. 2012. The population ecology of infectious diseases: pertussis in Thailand as a case study. Parasitology 139, 1888–1898. ( 10.1017/S0031182012000431) [DOI] [PubMed] [Google Scholar]
- 27.Domenech de Cellès M, Riolo MA, Magpantay FMG, Rohani P, King AA. 2014. Epidemiological evidence for herd immunity induced by acellular pertussis vaccines. Proc. Natl Acad. Sci. USA 111, E716–E717. ( 10.1073/pnas.1323795111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavine JS, Rohani P. 2012. Resolving pertussis immunity and vaccine effectiveness using incidence time series. Expert Rev. Vaccines 11, 1319–1329. ( 10.1586/erv.12.109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rohani P, Earn DJ, Grenfell BT. 2000. Impact of immunisation on pertussis transmission in England and Wales. Lancet 355, 285–286. ( 10.1016/S0140-6736(99)04482-7) [DOI] [PubMed] [Google Scholar]
- 30.Wearing HJ, Rohani P. 2009. Estimating the duration of pertussis immunity using epidemiological signatures. PLoS Pathog. 5, e1000647 ( 10.1371/journal.ppat.1000647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Préziosi M-P, Halloran ME. 2003. Effects of pertussis vaccination on transmission: vaccine efficacy for infectiousness. Vaccine 21, 1853–1861. ( 10.1016/S0264-410X(03)00007-0) [DOI] [PubMed] [Google Scholar]
- 32.Ryan M, et al. 1998. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology 93, 1–10. ( 10.1046/j.1365-2567.1998.00401.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Althouse BM, Scarpino SV. 2015. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Med. 13, 146 ( 10.1186/s12916-015-0382-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlsson R-M, Trollfors B. 2009. Control of pertussis-lessons learnt from a 10-year surveillance programme in Sweden. Vaccine 27, 5709–5718. ( 10.1016/j.vaccine.2009.07.092) [DOI] [PubMed] [Google Scholar]
- 35.Kuno-Sakai H, Kimura M. 2004. Safety and efficacy of acellular pertussis vaccine in Japan, evaluated by 23 years of its use for routine immunization. Pediatr. Int. 46, 650–655. ( 10.1111/j.1442-200x.2004.01970.x) [DOI] [PubMed] [Google Scholar]
- 36.Gonfiantini M, et al. 2014. Epidemiology of pertussis in Italy: disease trends over the last century. Euro Surveill. 19, 20291. [DOI] [PubMed] [Google Scholar]
- 37.Rohani P, Zhong X, King AA. 2010. Contact network structure explains the changing epidemiology of pertussis. Science 330, 982–985. ( 10.1126/science.1194134) [DOI] [PubMed] [Google Scholar]
- 38.Taranger J, et al. 2001. Mass vaccination of children with pertussis toxoid-decreased incidence in both vaccinated and nonvaccinated persons. Clin. Infect. Dis. 33, 1004–1010. ( 10.1086/322639) [DOI] [PubMed] [Google Scholar]
- 39.Trollfors B, Taranger J, Lagergåard T, Sundh V, Bryla DA, Schneerson R, Robbins JB. 1998. Immunization of children with pertussis toxoid decreases spread of pertussis within the family. Pediatr. Infect. Dis. J. 17, 196–199. ( 10.1097/00006454-199803000-00005) [DOI] [PubMed] [Google Scholar]
- 40.Versteegh FGA, Schellekens JFP, Nagelkerke AF, Roord JJ. 2002. Laboratory-confirmed reinfections with Bordetella pertussis. Acta Paediatr. 91, 95–97. ( 10.1111/j.1651-2227.2002.tb01648.x) [DOI] [PubMed] [Google Scholar]
- 41.Laing JS, Hay M. 1902. Whooping-cough: its prevalence and mortality in Aberdeen. Public Health 14, 584–599. ( 10.1016/S0033-3506(01)80186-4) [DOI] [Google Scholar]
- 42.Cherry JD. 1999. Pertussis in the preantibiotic and prevaccine era, with emphasis on adult pertussis. Clin. Infect. Dis. 28(Suppl 2), S107–S111. ( 10.1086/515057) [DOI] [PubMed] [Google Scholar]
- 43.Fine PE. 1997. Adult pertussis: a salesman's dream-and an epidemiologist's nightmare. Biologicals 25, 195–198. ( 10.1006/biol.1997.0083) [DOI] [PubMed] [Google Scholar]
- 44.Cherry JD. 2003. The science and fiction of the ‘resurgence’ of pertussis. Pediatrics 112, 405–406. ( 10.1542/peds.112.2.405) [DOI] [PubMed] [Google Scholar]
- 45.Güris D, Strebel PM, Bardenheier B, Brennan M, Tachdjian R, Finch E, Wharton M, Livengood JR. 1999. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990–1996. Clin. Infect. Dis. 28, 1230–1237. ( 10.1086/514776) [DOI] [PubMed] [Google Scholar]
- 46.Zepp F, Heininger U, Mertsola J, Bernatowska E, Guiso N, Roord J, Tozzi AE, Van Damme P. 2011. Rationale for pertussis booster vaccination throughout life in Europe. Lancet Infect. Dis. 11, 557–570. ( 10.1016/S1473-3099(11)70007-X) [DOI] [PubMed] [Google Scholar]
- 47.Wirsing von König CH, Postels-Multani S, Bock HL, Schmitt HJ. 1995. Pertussis in adults: frequency of transmission after household exposure. Lancet 346, 1326–1329. ( 10.1016/S0140-6736(95)92343-8) [DOI] [PubMed] [Google Scholar]
- 48.Blackwood JC, Cummings DAT, Broutin H, Iamsirithaworn S, Rohani P. 2013. Deciphering the impacts of vaccination and immunity on pertussis epidemiology in Thailand. Proc. Natl Acad. Sci. USA 110, 9595–9600. ( 10.1073/pnas.1220908110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lavine JS, King AA, Andreasen V, Bjørnstad ON. 2013. Immune boosting explains regime-shifts in prevaccine-era pertussis dynamics. PLoS ONE 8, e72086 ( 10.1371/journal.pone.0072086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jenkinson D. 1988. Duration of effectiveness of pertussis vaccine: evidence from a 10 year community study. Br. Med. J. (Clin. Res. Ed.) 296, 612–614. ( 10.1136/bmj.296.6622.612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lambert HJ. 1965. Epidemiology of a small pertussis outbreak in Kent county, Michigan. Public Health Rep. 80, 365–369. ( 10.2307/4592424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farrington CP. 1990. Quantifying misclassification bias in cohort studies of vaccine efficacy. Stat. Med. 9, 1327–1337. ( 10.1002/sim.4780091110) [DOI] [PubMed] [Google Scholar]
- 53.Farrington CP. 1992. The measurement and interpretation of age-specific vaccine efficacy. Int. J. Epidemiol. 21, 1014–1020. ( 10.1093/ije/21.5.1014) [DOI] [PubMed] [Google Scholar]
- 54.Keeling M, Rohani P. 2008. Modeling infectious diseases in humans and animals. Princeton, NJ: Princeton University Press. [Google Scholar]
- 55.Magpantay FMG, Riolo MA, Domenech de Cellès M, King AA, Rohani P. 2014. Epidemiological consequences of imperfect vaccines for immunizing infections. SIAM J. Appl. Math. 74, 1810–1830. ( 10.1137/140956695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gustafsson L, Hessel L, Storsaeter J, Olin P. 2006. Long-term follow-up of Swedish children vaccinated with acellular pertussis vaccines at 3, 5, and 12 months of age indicates the need for a booster dose at 5 to 7 years of age. Pediatrics 118, 978–984. ( 10.1542/peds.2005-2746) [DOI] [PubMed] [Google Scholar]
- 57.Witt MA, Katz PH, Witt DJ. 2012. Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a North American outbreak. Clin. Infect. Dis. 54, 1730–1735. ( 10.1093/cid/cis287) [DOI] [PubMed] [Google Scholar]
- 58.Baron S, Njamkepo E, Grimprel E, Begue P, Desenclos JC, Drucker J, Guiso N. 1998. Epidemiology of pertussis in French hospitals in 1993 and 1994: thirty years after a routine use of vaccination. Pediatr. Infect. Dis. J. 17, 412–418. ( 10.1097/00006454-199805000-00013) [DOI] [PubMed] [Google Scholar]
- 59.Wendelboe AM, et al. 2007. Transmission of Bordetella pertussis to young infants. Pediatr. Infect. Dis. J. 26, 293–299. ( 10.1097/01.inf.0000258699.64164.6d) [DOI] [PubMed] [Google Scholar]
- 60.The Lancet 1992. Pertussis: adults, infants, and herds. Lancet 339, 526–527. ( 10.1016/0140-6736(92)90343-2) [DOI] [PubMed] [Google Scholar]
- 61.Loeffelholz MJ, Thompson CJ, Long KS, Gilchrist MJ. 1999. Comparison of PCR, culture, and direct fluorescent-antibody testing for detection of Bordetella pertussis. J. Clin. Microbiol. 37, 2872–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anderson RM, May RM. 1991. Infectious diseases of humans: dynamics and control. Oxford, UK: Oxford University Press. [Google Scholar]
- 63.Preston NW. 2006. Diagnosis and prevention of pertussis. Lancet 368, 1769.; author reply 1769–1770 ( 10.1016/S0140-6736(06)69732-8) [DOI] [PubMed] [Google Scholar]
- 64.Thomas MG, Lambert HP. 1987. From whom do children catch pertussis? Br. Med. J. (Clin. Res. Ed.) 295, 751–752. ( 10.1136/bmj.295.6601.751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Melker HE, Versteegh FGA, Schellekens JFP, Teunis PFM, Kretzschmar M. 2006. The incidence of Bordetella pertussis infections estimated in the population from a combination of serological surveys. J. Infect. 53, 106–113. ( 10.1016/j.jinf.2005.10.020) [DOI] [PubMed] [Google Scholar]
- 66.Preston NW, Matthews RC. 1996. Transmission of pertussis: do adults have an important role? Lancet 347, 129–130. ( 10.1016/S0140-6736(96)90260-3) [DOI] [PubMed] [Google Scholar]
- 67.Mertsola J, Ruuskanen O, Eerola E, Viljanen MK. 1983. Intrafamilial spread of pertussis. J. Pediatr. 103, 359–363. ( 10.1016/S0022-3476(83)80403-X) [DOI] [PubMed] [Google Scholar]
- 68.Serra DO, Conover MS, Arnal L, Sloan GP, Rodriguez ME, Yantorno OM, Deora R. 2011. FHA-mediated cell-substrate and cell-cell adhesions are critical for Bordetella pertussis biofilm formation on abiotic surfaces and in the mouse nose and the trachea. PLoS ONE 6, e28811 ( 10.1371/journal.pone.0028811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mooi FR. 2010. Bordetella pertussis and vaccination: the persistence of a genetically monomorphic pathogen. Infect. Genet. Evol. 10, 36–49. ( 10.1016/j.meegid.2009.10.007) [DOI] [PubMed] [Google Scholar]
- 70.Bart MJ, et al. 2014. Global population structure and evolution of Bordetella pertussis and their relationship with vaccination. mBio 5, e01074 ( 10.1128/mBio.01074-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Preston NW. 1965. Effectiveness of pertussis vaccines. Br. Med. J. 2, 11–13. ( 10.1136/bmj.2.5452.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Preston NW. 1976. Prevalent serotypes of Bordetella pertussis in non-vaccinated communities. J. Hyg. (Lond.) 77, 85–91. ( 10.1017/S0022172400055546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hallander HO, Advani A, Donnelly D, Gustafsson L, Carlsson R-M. 2005. Shifts of Bordetella pertussis variants in Sweden from 1970 to 2003, during three periods marked by different vaccination programs. J. Clin. Microbiol. 43, 2856–2865. ( 10.1128/JCM.43.6.2856-2865.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Preston NW. 1985. Essential immunogens in human pertussis: the role of fimbriae. Dev. Biol. Stand. 61, 137–141. [PubMed] [Google Scholar]
- 75.Mooi FR, et al. 2009. Bordetella pertussis strains with increased toxin production associated with pertussis resurgence. Emerg. Infect. Dis. 15, 1206–1213. ( 10.3201/eid1508.081511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmidtke AJ, Boney KO, Martin SW, Skoff TH, Tondella ML, Tatti KM. 2012. Population diversity among Bordetella pertussis isolates, United States, 1935–2009. Emerg. Infect. Dis. 18, 1248–1255. ( 10.3201/eid1808.120082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Boven M, Mooi FR, Schellekens JF, de Melker HE, Kretzschmar M. 2005. Pathogen adaptation under imperfect vaccination: implications for pertussis. Proc. R. Soc. B 272, 1617–1624. ( 10.1098/rspb.2005.3108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Poynten M, McIntyre PB, Mooi FR, Heuvelman KJ, Gilbert GL. 2004. Temporal trends in circulating Bordetella pertussis strains in Australia. Epidemiol. Infect. 132, 185–193. ( 10.1017/S095026880300164X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bergfors E, Trollfors B, Taranger J, Lagergaard T, Sundh V, Zackrisson G. 1999. Parapertussis and pertussis: differences and similarities in incidence, clinical course, and antibody responses. Int. J. Infect. Dis. 3, 140–146. ( 10.1016/S1201-9712(99)90035-8) [DOI] [PubMed] [Google Scholar]
- 80.Mastrantonio P, Stefanelli P, Giuliano M, Herrera Rojas Y, Ciofi degli Atti M, Anemona A, Tozzi AE. 1998. Bordetella parapertussis infection in children: epidemiology, clinical symptoms, and molecular characteristics of isolates. J. Clin. Microbiol. 36, 999–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Long GH, Karanikas AT, Harvill ET, Read AF, Hudson PJ. 2010. Acellular pertussis vaccination facilitates Bordetella parapertussis infection in a rodent model of bordetellosis. Proc. R. Soc. B 277, 2017–2025. ( 10.1098/rspb.2010.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pittet LF, Emonet S, Schrenzel J, Siegrist C-A, Posfay-Barbe KM. 2014. Bordetella holmesii: an under-recognised Bordetella species. Lancet Infect. Dis. 14, 510–519. ( 10.1016/S1473-3099(14)70021-0) [DOI] [PubMed] [Google Scholar]
- 83.Rodgers L, et al. 2013. Epidemiologic and laboratory features of a large outbreak of pertussis-like illnesses associated with cocirculating Bordetella holmesii and Bordetella pertussis—Ohio, 2010–2011. Clin. Infect. Dis. 56, 322–331. ( 10.1093/cid/cis888) [DOI] [PubMed] [Google Scholar]
- 84.Njamkepo E, Bonacorsi S, Debruyne M, Gibaud SA, Guillot S, Guiso N. 2011. Significant finding of Bordetella holmesii DNA in nasopharyngeal samples from French patients with suspected pertussis. J. Clin. Microbiol. 49, 4347–4348. ( 10.1128/JCM.01272-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guiso N. 2012. Specific biological diagnoses are needed to determine the durability of pertussis vaccine-induced immunity. Clin. Infect. Dis. 55, 1433–1434; author reply 1435–1436 ( 10.1093/cid/cis671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith PG, Rodrigues LC, Fine PE. 1984. Assessment of the protective efficacy of vaccines against common diseases using case-control and cohort studies. Int. J. Epidemiol. 13, 87–93. ( 10.1093/ije/13.1.87) [DOI] [PubMed] [Google Scholar]
- 87.Halloran ME, Longini IM, Struchiner CJ. 2010. Design and analysis of vaccine studies. New York, NY: Springer. [Google Scholar]
- 88.Kanaan MN, Farrington CP. 2002. Estimation of waning vaccine efficacy. J. Am. Stat. Assoc. 97, 389–397. ( 10.1198/016214502760046943) [DOI] [Google Scholar]
- 89.Riolo MA, Rohani P. 2015. Combating pertussis resurgence: one booster vaccination schedule does not fit all. Proc. Natl Acad. Sci. USA 112, E472–E477. ( 10.1073/pnas.1415573112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aguas R, Gonçalves G, Gomes MGM. 2006. Pertussis: increasing disease as a consequence of reducing transmission. Lancet Infect. Dis. 6, 112–117. ( 10.1016/S1473-3099(06)70384-X) [DOI] [PubMed] [Google Scholar]
- 91.McLean AR, Anderson RM. 1988. Measles in developing countries. Part II. The predicted impact of mass vaccination. Epidemiol. Infect. 100, 419–442. ( 10.1017/S0950268800067170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen RT, Weierbach R, Bisoffi Z, Cutts F, Rhodes P, Ramaroson S, Ntembagara C, Bizimana F. 1994. A ‘post-honeymoon period’ measles outbreak in Muyinga sector, Burundi. Int. J. Epidemiol. 23, 185–193. ( 10.1093/ije/23.1.185) [DOI] [PubMed] [Google Scholar]
- 93.Ionides EL, Bretó C, King AA. 2006. Inference for nonlinear dynamical systems. Proc. Natl Acad. Sci. USA 103, 18 438–18 443. ( 10.1073/pnas.0603181103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Swamy GK, Wheeler SM. 2014. Neonatal pertussis, cocooning and maternal immunization. Expert Rev. Vaccines 13, 1107–1114. ( 10.1586/14760584.2014.944509) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used for the time-series analysis are freely available from the WHO database (http://www.who.int/immunization/monitoring_surveillance/data/en; accessed 23 June 2014) and from the World Bank database (http://data.worldbank.org/data-catalog/world-development-indicators; accessed 23 June 2014).