Abstract
Single-cell analysis captures the heterogeneity of T-cell populations that target defined antigens. Human immunodeficiency virus (HIV) infection results in defects of antimycobacterial immunity, which remain poorly defined. We therefore recruited a small number of subjects, including those with latent and active M. tuberculosis infection, with or without concomitant HIV infection, and tracked the mycobacterial glycolipid-reactive T-cell repertoire by using CD1b tetramers. Glycolipid-reactive T cells expressed memory markers and the HIV coreceptors CD4 and CCR5; they were not detected in subjects with HIV-associated active M. tuberculosis infection. HIV infection may affect T cells that recognize mycobacterial glycolipids and influence immunity.
Keywords: tuberculosis, HIV, CD1, T cell, glycolipids, antigens, tetramer
Elucidating molecularly defined effectors of antimycobacterial immunity in vivo during human infection is a fundamental prerequisite for the development of a new, protective tuberculosis vaccine. Experimental infection in model organisms, as well as rare single gene defects in people predisposed to mycobacterial infection, have identified CD4+ T cells and the effector molecules they release as central to control of intracellular mycobacteria [1]. Most published studies have relied on functional measurements of bulk T-cell populations, which may mask significant heterogeneity and confound interpretation of complex pathogen-driven responses. Newer single-cell analytics based on flow cytometry with specific markers, including tetramer staining of T-cell receptors (TCRs), reveal unexpected aspects of cellular phenotype and function [2], as well as clonotypic differences in antimicrobial efficacy [3].
During mycobacterial infection, T cells target peptides [4], microbial metabolites [5] and lipid antigens presented by nonpolymorphic CD1 molecules [6]. The precise role of these T-cell subsets during natural infection in humans remains unknown and has been difficult to explore, owing to the lack of single-cell detection reagents. Whereas early studies suggested that CD1-reactive T cells typically express CD8 or no T-cell coreceptor [7], our recent study found that polyclonal CD1b-reactive cells express CD4, raising the possibility that CD1b-restricted T cells are targets of HIV infection [8]. The mycobacterial glycolipid-reactive repertoire identified by CD1b tetramers loaded with glucose monomycolate is composed of cells that express a conserved, high-affinity T-cell receptor, TRAV1-2 [9], and TCRs with a limited TRBV4-1 β chain repertoire or diverse T-cell receptors [10]. Longitudinal functional studies of T-cell responses to the related mycobacterial lipid mycolic acid suggest the possibility of immunological memory to mycobacterial glycolipids [11]. We therefore sought to evaluate potential T-cell memory to the mycobacterial glycolipid glucose monomycolate.
To obtain an approximate measurement of the mycobacterial glycolipid-reactive T-cell repertoire ex vivo, we used newly developed CD1b tetramers in a study of African patients with tuberculosis, with and without HIV coinfection. We found that glycolipid-reactive T cells persisted over time, expressed memory markers and the HIV coreceptor CCR5, and were disrupted by HIV infection.
METHODS
Subjects
Subjects were recruited into the iThimba study at McCord Hospital in Durban, South Africa, overseen by the University of KwaZulu-Natal Biomedical Research Ethics Committee (Protocol BFC 115/09), the Partners Healthcare Institutional Review Board (Protocol 2002-P-000061), and the Harvard Committee on Microbiologic Safety (Protocol 08–184). HIV status was assessed at baseline by means of an HIV-specific enzyme-linked immunosorbent assay and by measuring the HIV load; viral loads were measured quarterly, at each visit, for the duration of the study. Subjects received a diagnosis of active tuberculosis, based on symptom questionnaire and sputum analysis that revealed either positive results of acid-fast bacilli smear and/or growth of Mycobacterium tuberculosis on culture. Asymptomatic subjects were classified as being latently infected with M. tuberculosis, based on negative findings of sputum analysis and a positive interferon γ (IFN-γ) enzyme-linked immunospot (ELISPOT) response to the M. tuberculosis–specific peptides ESAT-6 and/or CFP-10. After informed consent was obtained from participants, 50 mL of blood was collected and cryopreserved; subjects returned quarterly for symptom review, repeat HIV load testing and tuberculosis ELISPOT analysis, and banking of peripheral blood mononuclear cells (PBMCs). Subjects with active M. tuberculosis infection were enrolled at the time of diagnosis for a single time point, after which they were referred for tuberculosis treatment and antiretroviral therapy.
Tetramer Staining of Human PBMCs
Glucose monomycolate with short alkyl chains (C32 GMM) was sonicated in 82 µL of 50 mM sodium citrate at pH 4.0 for 2 minutes. The mixture was then added at 100-fold molar excess to CD1b monomers and incubated in a 37°C water bath for 2 hours, with vortexing every 15 minutes, and neutralized to pH 7.4 with 6 µL of Tris (pH 9). Tetramers were generated and validated as previously described [8]. Approximately 5 million cryopreserved PBMCs per subject were thawed and incubated overnight at 37°C in T-cell medium. Cells were subsequently harvested and treated with human AB serum before staining with 1 µg of tetramer for 60 minutes at room temperature in the dark, after which they were washed and stained with live/dead fixable dyes (Invitrogen). Cells were washed again and then stained with monoclonal antibodies, including CD3 (BD), CD4, CD14, CD19, TRAV1-2, CCR5, and CD45RO (Biolegend), for 20 minutes at 4°C and then fixed in 2% formaldehyde prior to fluorescence-activated cell-sorting analysis. Subjects were considered positive for tetramer staining if there was a 3-fold higher frequency of T cells that stained with loaded as compared to control tetramers. Statistical analysis was performed using GraphPad Prism 6 software. The percentage of CD4+ tetramer–positive cells was compared between HIV-positive and HIV-negative subjects, using the Mann–Whitney test; the percentage of subjects with detectable tetramer-positive cells was compared between HIV-positive and HIV-negative subjects with active tuberculosis, using a 2-tailed Student t test.
RESULTS
Glycolipid-Reactive T Cells Are Memory T Cells That Express HIV Coreceptors
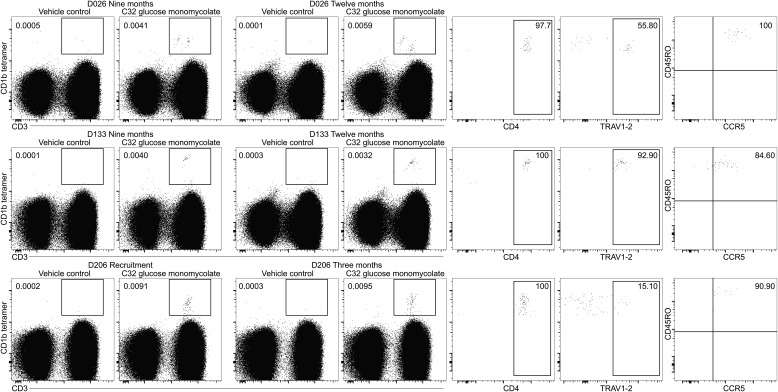
We sought to evaluate various aspects of T-cell memory to the mycobacterial glycolipid glucose monomycolate, using CD1b tetramers. Three HIV-negative subjects had distinct tetramer-positive T-cell populations at recruitment, as well as available specimens from multiple time points, and thus were studied longitudinally (Figure 1). Subjects D026 and D133 had evidence of latent M. tuberculosis infection by IFN-γ ELISPOT to ESAT-6/CFP-10; D206 was ELISPOT negative but may have received BCG or been exposed to environmental mycobacteria, possibly leading to sensitization to glucose monomycolate. Tetramer-positive cells were detected at 9 and 12 months after recruitment in subjects D026 and D133, as well as at the available repeat time point for subject D206 3 months after recruitment. Phenotyping results at the 2 tested time points per subject were similar and revealed uniform expression of the memory marker CD45RO. Thus, T cells that target the mycobacterial glycolipid glucose monomycolate persist over time and express a key cell surface marker associated with memory. Recent studies have shown that TCR expression by T cells reactive to CD1b and GMM is enriched for TRAV1-2, which define germ-line–encoded mycolyl-reactive (GEM) TCRs [9], as well as TCRs with conserved TRBV4-1 β chains or diverse TCRs [10]. The variable chain TRAV1-2 constitutes approximately 1.2% of αβ T cells on average; here TRAV1-2+ cells ranged from 15.10% to 92.9% of CD4+ tetramer–positive cells. These data confirm this TCR α chain as a marker of GEM T cells. In addition, the majority of CD1b tetramer–positive cells also expressed the HIV co-receptor CCR5 and are thus potential targets of HIV infection.
Figure 1.
Glycolipid-reactive T cells are memory T cells that express human immunodeficiency virus coreceptors. Peripheral blood mononuclear cells (PBMCs) from 3 subjects (D026, D133, and D206) were stained with CD1b tetramers at defined intervals. PBMCs were stained with CD3 FITC, CD4 brilliant violet 421, TRAV1-2 PE-Cy7, CCR5-PE, CD45RO-PercP-Cy5.5, CD14 APC-Cy7, CD19 APC-Cy7, near-infrared viability dye, and CD1b tetramers labeled with allophycocyanin and were gated on live lymphocytes. Each subject had detectable CD1b-tetramer–positive cells (for which positive staining was defined as a 3-fold increase in the percentage of T cells stained with glucose monomycolate–loaded tetramers, compared with vehicle-loaded control tetramers) at recruitment (D206, left panel; D026 and D133, data not shown), as well as at subsequent time points 3 months (D206) or 9 and 12 months later (D026 and D133). Phenotyping was performed at 2 time points for each subject and was similar at both; the later time point is shown.
HIV Disrupts the Mycobacterial Glycolipid-Reactive T-Cell Repertoire
T cells and natural killer T cells that express CD4 and the HIV coreceptor CCR5 can be infected in vitro with HIV, and their numbers are reduced in vivo during HIV infection [12]. Therefore, we sought to measure CD1b tetramer–positive cells among 8 HIV-negative and 7 HIV-positive patients. In HIV-negative patients, we detected tetramer-positive cells with very bright staining intensity (mean fluorescence intensity, 8641); subgating for CD4 expression revealed CD4 predominance in all cases and 100% CD4 positivity among patients with the brightest tetramer staining. With one exception (subject 126), HIV-positive patients showed a different pattern. Although some cells entered the tetramer-positive gate, these cells showed lower tetramer staining intensity (mean fluorescence intensity, 3932) and contained a much lower absolute prevalence of CD4+ T cells (45%; P = .0207, by the Mann–Whitney test; Supplementary Figure 1). This pattern might have resulted from a general depletion of CD4+ T cells, but the combined pattern of lower intensity and fewer CD4+ T cells was more consistent with a loss of GEM and other CD1b-restricted T cells during HIV infection. Therefore, we designed a cohort study to measure mycobacterial glycolipid-reactive T cells during HIV infection and assessed whether their absence might be associated with incident tuberculosis.
T Cells That Target Glycolipids Are Not Detected in HIV-Associated Tuberculosis
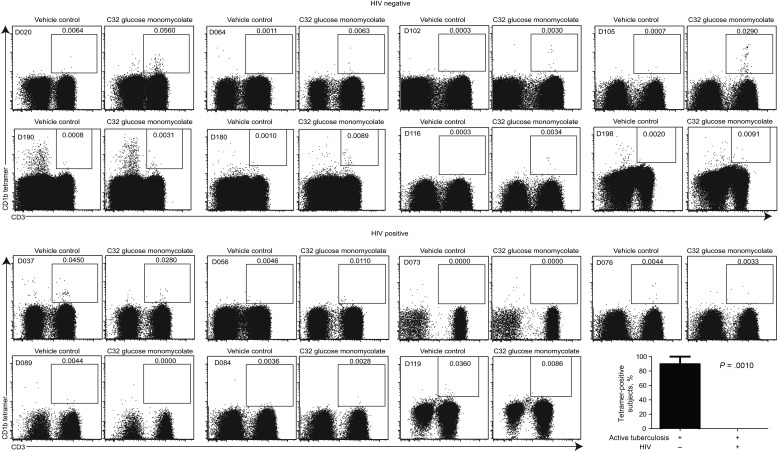
We studied 8 HIV-negative subjects with active tuberculosis and 7 HIV-positive subjects with active tuberculosis, all prior to the initiation of antimicrobial therapy. The 2 groups were similar with regard to ethnicity, sex (55% of the HIV-negative participants were women, as were 43% of the HIV-positive participants), and tuberculin status; the HIV-positive group was slightly younger (mean age, 30 years vs 36 years in the HIV-negative group) and had lower mean CD4+ T-cell counts (328 vs 776 cells/mm3 in the HIV-negative group; Supplementary Table 1). Whereas HIV-negative subjects with active tuberculosis at the time of diagnosis had tetramer-positive cells at a frequency ranging from 0.0030% to 0.0560% of total T cells, no staining above background with vehicle-loaded CD1b control tetramers was seen in HIV-positive subjects with active M. tuberculosis infection (Figure 2). The difference in the percentage of subjects with detectable glycolipid-reactive T cells between the 2 groups was compared using a 2-tailed Student t test (P = .0010). Thus, the mycobacterial glycolipid-reactive T-cell repertoire is composed of memory T cells that express HIV coreceptors and is impacted by HIV infection in vivo.
Figure 2.
T cells that target glycolipids are not detected in human immunodeficiency virus (HIV)–associated tuberculosis. Peripheral blood mononuclear cells from 8 HIV-negative subjects with active Mycobacterium tuberculosis infection (D020, D064, D102, D105, D190, D180, D116, and D198) and 7 HIV-positive subjects with active tuberculosis (D037, D056, D073, D076, D089, D084, and D119) were stained with CD3 FITC, CD14 PerCP-Cy5.5, CD19 PerCP-Cy5.5, violet viability dye, and APC-labeled CD1b tetramers. The percentage of tetramer-positive subjects in each group was compared using a 2-tailed Student t test (GraphPad Prism 6).
DISCUSSION
In this small study of African subjects, we have found that the mycobacterial glycolipid-reactive T-cell repertoire is composed of memory CD4+ T cells that express the HIV coreceptors CD4 and CCR5. In contrast, CD3+ tetramer-positive CD4+ cells are essentially undetectable in HIV-positive subjects (Supplementary Figure 1) and subjects coinfected with HIV and M. tuberculosis (Figure 2). Previously reported CD4− T cells that respond to M. tuberculosis in the presence of CD1b and can be derived from HIV-positive subjects [13] may be present at a precursor frequency below the detection threshold of CD1b tetramers. Bulk T-cell populations that respond to mycobacterial antigens in functional assays cannot be detected early after HIV infection, suggesting that the dysfunction of antigen-specific CD4+ T cells may contribute to the increased rates of incident tuberculosis in this group [14]. Functional assays, however, cannot distinguish between T-cell anergy (antigen unresponsiveness) and T-cell deletion, an important distinction when considering whether a particular antigen specificity and/or functional phenotype is critical to the maintenance of latency. Major histocompatibility complex tetramers overcome this limitation but are themselves limited by both genetic polymorphism and epitope diversity. CD1b tetramers that identify human T cells reactive to mycobacterial glycolipids overcome these barriers because CD1b is nonpolymorphic and mycobacterial glycolipids are synthesized via conserved pathways not subject to immune escape. Here we show that a high-affinity T-cell population that targets mycobacterial glycolipids is disrupted by HIV infection. Whether these cells are mere bystanders of bulk CD4 depletion or whether their loss either predicts incident tuberculosis or contributes to it remain important questions for future study.
Tetramers might have failed to detect antigen-specific T cells during active infection, owing to downregulation of the T-cell receptor; our findings, as well as those from studies of bacille Calmette-Guerin infection in mice [15], suggest that is not the case. We had considered that antigen-specific T cells might home to infected tissues and not be detectable in the peripheral circulation; our findings and those from studies of mycolic acid–reactive T cells [11] show that these cells can in fact be detected in the periphery during active infection. Panels of clones have shown that GEM T cells express TRAV1-2 TCRs with high affinity for CD1b and coexpress CD4. Other GMM-reactive T cells, known as LDN5-like T cells, do not express TRAV1-2, have lower affinity binding to CD1b-GMM complexes, and have diverse coreceptor expression. Here polyclonal T cells studied ex vivo show similar linkage of TRAV1-2+ TCRs to higher-avidity CD1b binding and higher rates of CD4 expression. Whether TCR use or other aspects of surface phenotype [2] or effector function [7] correlate with latency or active disease are fascinating questions that remain to be answered.
In conclusion, CD1b tetramer–positive cells express surface receptors that make them targets of HIV infection and are difficult to detect during chronic HIV infection. These findings provide an unexpected connection between HIV and a newly defined population of mycobacteria-specific T cells. Whether these cells impact antimycobacterial immunity and whether their effects are direct, owing to cognate interactions with CD1b-expressing targets, or indirect, via cytokine secretion and activation of other cellular subsets, including peptide-specific T cells, is an area to be actively explored prior to harnessing glycolipid-reactive T cells in future tuberculosis vaccines.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the study subjects, for their generous participation; the study staff, for their hard work; and the Harvard CFAR, for input into statistical analysis.
Financial support. This work was supported by the KwaZulu-Natal Research Institute for Tuberculosis and HIV (to V. O. K., T. N., A. G. K., and D. B. M.), the Harvard University Global Health Initiative (to A. G. K.), the Harvard University CFAR (P30 AI060354 to V. O. K.), the Burroughs Wellcome Fund program in Translational Research (to D. B. M.), the National Institutes of Health (T-32 AI 007306-22, T-32 AR 007530-23, and K08 AI089858 to A. G. K. and R01 AI49313 and R01 AR048632 to D. B. M.), the South African Department of Science and Technology/National Research Foundation Research Chairs Initiative (to T. N.), and the Victor Daitz Foundation (to T. N.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Nunes-Alves C, Booty MG, Carpenter SM, Jayaraman P, Rothchild AC, Behar SM. In search of a new paradigm for protective immunity to TB. Nat Rev Micro 2014; 12:289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Day CL, Kaufmann DE, Kiepiela P et al. . PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006; 443:350–4. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Ndhlovu ZM, Liu D et al. . TCR clonotypes modulate the protective effect of HLA class I molecules in HIV-1 infection. Nat Immunol 2012; 13:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lalvani A, Brookes R, Wilkinson RJ et al. . Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 1998; 95:270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gapin L. Check MAIT. J Immunol 2014; 192:4475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulrichs T, Moody DB, Grant E, Kaufmann SH, Porcelli SA. T-cell responses to CD1-presented lipid antigens in humans with Mycobacterium tuberculosis infection. Infect Immun 2003; 71:3076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stenger S, Mazzaccaro RJ, Uyemura K et al. . Differential effects of cytolytic T cell subsets on intracellular infection. Science 1997; 276:1684–7. [DOI] [PubMed] [Google Scholar]
- 8.Kasmar AG, van Rhijn I, Cheng TY et al. . CD1b tetramers bind alphabeta T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. J Exp Med 2011; 208:1741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Rhijn I, Kasmar A, de Jong A et al. . A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat Immunol 2013; 14:706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Rhijn I, Gherardin NA, Kasmar A et al. . TCR bias and affinity define two compartments of the CD1b-glycolipid-specific T Cell repertoire. J Immunol 2014; 192:4054–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montamat-Sicotte DJ, Millington KA, Willcox CR et al. . A mycolic acid-specific CD1-restricted T cell population contributes to acute and memory immune responses in human tuberculosis infection. J Clin Invest 2011; 121:2493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motsinger A, Haas DW, Stanic AK, Van Kaer L, Joyce S, Unutmaz D. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J Exp Med 2002; 195:869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong J, Stenger S, Zack JA et al. . Isolation of mycobacterium-reactive CD1-restricted T cells from patients with human immunodeficiency virus infection. J Clin Invest 1998; 101:383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geldmacher C, Ngwenyama N, Schuetz A et al. . Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med 2010; 207:2869–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiba A, Dascher CC, Besra GS, Brenner MB. Rapid NKT cell responses are self-terminating during the course of microbial infection. J Immunol 2008; 181:2292–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.