Abstract
OBJECTIVES
Prior reports have linked patient transmission of carbapenem-resistant Enterobacteriaceae (CRE, or “superbug”) to endoscopes used during endoscopic retrograde cholangiopancreatography (ERCP). We performed a decision analysis to measure the cost-effectiveness of four competing strategies for CRE risk management.
METHODS
We used decision analysis to calculate the cost-effectiveness of four approaches to reduce the risk of CRE transmission among patients presenting to the hospital for symptomatic common bile duct stones. The strategies included: (1) perform ERCP followed by U.S. Food and Drug Administration (FDA)-recommended endoscope reprocessing procedures; (2) perform ERCP followed by “endoscope culture and hold”; (3) perform ERCP followed by ethylene oxide (EtO) sterilization of the endoscope; and (4) stop performing ERCP in lieu of laparoscopic cholecystectomy (LC) with common bile duct exploration (CBDE). Our outcome was incremental cost per quality-adjusted life year (QALY) gained.
RESULTS
In the base-case scenario, ERCP with FDA-recommended endoscope reprocessing was the most cost-effective strategy. Both the ERCP with culture and hold ($4,228,170/QALY) and ERCP with EtO sterilization ($50,572,348/QALY) strategies had unacceptable incremental costs per QALY gained. LC with CBDE was dominated, being both more costly and marginally less effective versus the alternatives. In sensitivity analysis, ERCP with culture and hold became the most cost-effective approach when the pretest probability of CRE exceeded 24%.
CONCLUSIONS
In institutions with a low CRE prevalence, ERCP with FDA-recommended reprocessing is the most cost-effective approach for mitigating CRE transmission risk. Only in settings with an extremely high CRE prevalence did ERCP with culture and hold become cost-effective.
INTRODUCTION
Over 500,000 endoscopic retrograde cholangiopancreatographies (ERCP) are performed annually in the United States (U.S.) for diagnostic and therapeutic indications.(1) ERCP is the gold standard for the management of a variety of disorders, including symptomatic common bile duct (CBD) stones, biliary cholangitis, and pancreatic and biliary malignancy.(2) Unique to ERCP is the duodenoscope, an endoscope with an elevator channel that allows for the placement of guidewires, catheters and other endoscopic accessories into the operator’s visual field. While the design of this endoscope permits technically advanced and precise biliary procedures, the difficult to access elevator channel poses a challenge for effective duodenoscope reprocessing and decontamination.
Contaminated endoscopes cause more healthcare-associated infection outbreaks than any other medical device.(3, 4) In most cases, these infections are caused by intestinal flora, predominantly Enterobacteriaceae and Enterococcus.(4, 5) While some outbreaks have been associated with inadequate reprocessing of endoscopes, epidemics have occurred even without lapses in decontamination procedures.(6–14) The most serious of these epidemics are those caused by multidrug-resistant organisms (MDRO), including carbapenem-resistant Enterobacteriaceae (CRE), one of the resistant bacteria termed “superbugs” in the lay media. There are limited treatment options for MDRO and CRE infections, and multiple recent CRE outbreaks associated with contaminated duodenoscopes have been the focus of widespread media attention, including at our own institutions.(15, 16)
The most cost-effective approach for preventing CRE transmission remains uncertain. In March 2015, the U.S. Food and Drug Administration (FDA) released a safety communication detailing new reprocessing instructions for duodenoscopes, which includes additional brushing of the forceps elevator recess area with a new smaller bristle cleaning brush, among other steps.(17) Some medical centers have also adopted a “culture and hold” approach where duodenoscopes are cultured after ERCP and held until cultures are negative for 48 hours.(13) Others have turned to ethylene oxide (EtO) gas sterilization,(6, 14) a process that is believed to offer optimal endoscope sterilization, but is costly and typically requires outsourcing. Another potential option is to halt use of ERCP in favor of surgical and interventional radiology procedures. In this study, we performed a decision analysis to measure the cost-effectiveness and healthcare impact of these competing strategies for CRE risk management.
METHODS
Model Overview
We used decision analysis software (TreeAge Pro, version 2014, TreeAge Software, Inc, Williamstown, MA) to evaluate a hypothetical cohort of patients hospitalized for symptomatic CBD stones, the most common indication for ERCP.(2) To emulate a case mix in clinical practice, we assumed that some individuals with symptomatic CBD stones had concomitant obstructive jaundice and cholangitis. In 2012, 23% of U.S. hospitalizations principally for CBD stones were complicated by cholangitis.(18) Individuals entered the hypothetical model without previous intervention and underwent one of four competing strategies: (1) perform ERCP followed by FDA-recommended endoscope reprocessing procedures; (2) perform ERCP followed by “endoscope culture and hold”; (3) perform ERCP followed by EtO sterilization of the endoscope; or (4) stop ERCP and perform laparoscopic cholecystectomy (LC) with common bile duct exploration (CBDE). We then followed the cohort over the course of a one-year time horizon. During this time, it was assumed that there was no mortality from competing but unrelated causes.
Model Assumptions
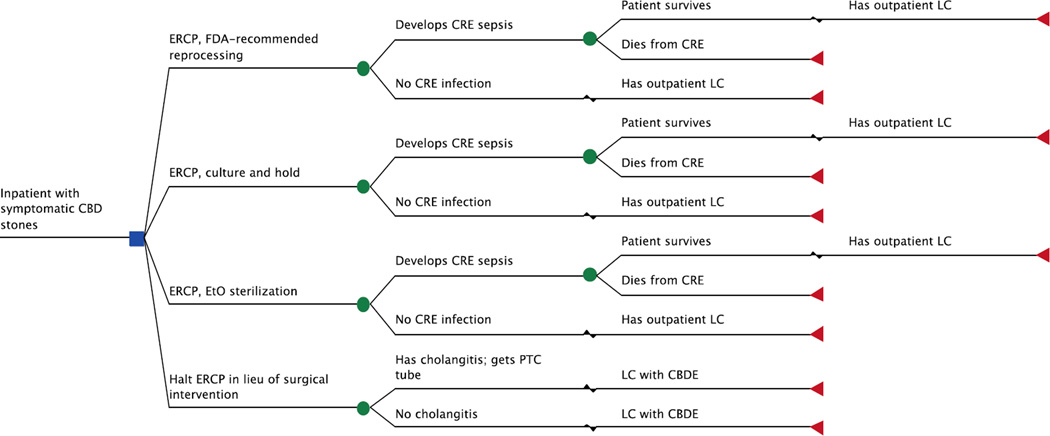
Figure 1 displays the truncated decision model. We assumed that all duodenoscopes were previously used in patients without a known, culture-confirmed history of CRE. In other words, our model assumed that some individuals were unrecognized CRE carriers and unknowingly transferred CRE to the duodenoscope. We chose to not include patients previously recognized as CRE carriers, as duodenoscopes used in these cases in real clinical practice are likely to undergo more aggressive reprocessing (i.e., EtO sterilization) and surveillance. This would subsequently lower the pretest probability of the duodenoscope harboring CRE.
Figure 1.
Truncated decision model. The base-case patient was hospitalized with symptomatic CBD stones and underwent 1 of 4 competing strategies. The model accounted for procedure-related complications and mortality. Patients who had an ERCP were at risk for acquiring CRE; those who did not develop an overt CRE infection had an outpatient LC 6 weeks after their initial hospitalization. Those who developed CRE sepsis consequently required readmission to the hospital; patients who survived were discharged and had an outpatient LC 6 weeks later. For the surgery-based approach, treatment was stratified by presence of cholangitis, as those with cholangitis first had PTC tube placement prior to LC with CBDE.
CBD = common bile duct; CBDE = common bile duct exploration; CRE = carbapenem-resistant Enterobacteriaceae; ERCP = endoscopic retrograde cholangiopancreatography; EtO = ethylene oxide; FDA = Food and Drug Administration; LC = laparoscopic cholecystectomy; PTC = percutaneous transhepatic cholangiography.
We assumed that all patients who underwent ERCP were at risk for acquiring CRE. Those who acquired CRE and developed clinical symptoms consequently required readmission to the hospital for management of CRE sepsis; all point sources of CRE sepsis (e.g., cholangitis, bacteremia, urinary, pulmonary, etc.) were considered equivalent. We assumed patients with a clinical CRE infection presented back to the hospital within 30 days of the ERCP.(6) Based on prior reports, we estimated that CRE sepsis had a case fatality rate of 43%.(7, 13,16, 19, 20) Because of the lack of data on the natural history and long-term consequences of CRE carriage, we used a one-year time horizon for this study.
We also assumed that all individuals who received an ERCP subsequently underwent an outpatient LC. Those who did not develop a symptomatic CRE infection had an outpatient LC six weeks after their initial hospitalization for symptomatic CBD stones.(21) Patients with CRE sepsis had an outpatient LC six weeks after their associated hospitalization for CRE.
Competing Strategies
ERCP With Only FDA-Recommended Reprocessing Procedures Strategy
This strategy served as our referent case for the analysis. For all ERCP-based strategies, we assumed that hospitalized patients with symptomatic CBD stones +/− cholangitis were managed similarly with an ERCP with clearance of the CBD and sphincterotomy. Duodenoscopes then underwent high-level disinfection (HLD) adhering to the FDA-recommended reprocessing instructions issued in March 2015.(17) Key changes include additional brushing of the forceps elevator recess area with a new smaller bristle cleaning brush, additional flushing of the forceps elevator recess area, and additional manual flushing steps and increased flushing volume of the channels, among other steps.(17)
Of note, the FDA released another safety communication in August 2015 that recommended repeat HLD (i.e., “double wash”) as a viable option for reprocessing duodenoscopes.(22) However, there is no data regarding whether repeat HLD is more efficacious than single HLD at clearing CRE and other MDRO. Because of this combined with the fact that repeat HLD costs minimally more than single HLD ($68.55 per HLD, see Supplementary Table 1) we chose to not include “double wash” as a separate arm in our decision analysis.
ERCP Followed By “Culture and Hold” Strategy
Hospitalized patients first underwent ERCP with clearance of the CBD and sphincterotomy. Duodenoscopes then had HLD using the new FDA-recommended reprocessing guidelines.(17) Afterwards, the duodenoscopes were cultured and sequestered until cultures were negative for growth after 48 hours.(13) Duodenoscopes with positive cultures underwent a second HLD and were then subsequently recultured and held pending negative culture results. For the purposes of this analysis, we assumed that duodenoscopes with two successive cultures positive for “high-concern” bacteria,(23) despite appropriate reprocessing, were sent to an outside facility for EtO sterilization. Given the minimum 48-hour turnaround time for culture results, additional duodenoscopes must be purchased at the outset of the study in order to maintain the same throughput in the endoscopy unit. Here we assumed that the base-case institution performed 1000 ERCPs per year and had four duodenoscopes (consistent with a high-volume center). Assuming four ERCPs were performed each weekday, we assumed that eight new duodenoscopes would need to be purchased to ensure that clinical flow was uninterrupted.
ERCP Followed By EtO Sterilization Strategy
Hospitalized patients in this arm first underwent ERCP with clearance of the CBD and sphincterotomy. Duodenoscopes were then disinfected using the FDA-recommended reprocessing guidelines.(17) Since most institutions do not offer in-house EtO sterilization, we assumed that EtO sterilization of the duodenoscope was outsourced and performed at an outside facility. We also assumed at least a 48-hour turnaround time (i.e., time required for the EtO sterilization and transport back and forth between the endoscopy unit and EtO sterilization provider) before the duodenoscopes could be used again; this requires eight additional duodenoscopes be purchased at the beginning of the study. Moreover, based on reports that EtO can damage duodenoscopes,(4) we performed a sensitivity analyses assuming that EtO exposure would require more frequent purchase of replacement scopes.
Stop ERCP and Perform LC with CBDE Strategy
In this strategy, treatment for hospitalized patients was stratified by presence of cholangitis. Those presenting with symptomatic CBD stones without cholangitis underwent a LC with CBDE. Those with concomitant cholangitis first underwent percutaneous transhepatic cholangiography with biliary tube placement followed by an inpatient LC with CBDE the next day.
Clinical Probability Estimates
Our base-case model incorporated a wide range of estimates governing relevant clinical probabilities in the management of symptomatic CBD stones (Table 1). To derive these estimates, we primarily used data published in the Cochrane Database of Systematic Reviews. When such reviews were unavailable, we retrieved pertinent studies in MEDLINE and used a weighted average of estimates when multiple studies were identified.
Table 1.
Base-case probability and utility estimates.
| Variable | Base-case estimate (range tested in Monte Carlo analysis) |
|---|---|
| Clinical probabilities, % | |
| Pretest probability of CRE-infected endoscope* | 1 (0–10) |
| CRE transmission after ERCP with infected endoscope(6, 7, 14) | 31 (10–50) |
| Patient with CRE develops clinical symptoms(7, 14) | 73 (25–100) |
| CRE-related mortality(7, 13, 16, 19, 20) | 43 (10–75) |
| Proportion of initial admissions complicated by cholangitis requiring PTC tube placement(18)† | 23 (10–50) |
| ERCP minor complication(1) | 5.2 (1–10) |
| ERCP major complication(1) | 1.7 (1–7) |
| ERCP-related mortality(1) | 0.3 (0–2) |
| LC minor complication(34) | 5.6 (1–10) |
| LC major complication(34) | 4.1 (1–10) |
| LC-related mortality(34) | 0.1 (0–2) |
| LC/CBDE minor complication(35) | 14 (9–19) |
| LC/CBDE major complication(35) | 8.2 (3–13) |
| LC/CBDE-related mortality(36) | 0.5 (0–2) |
| FDA-recommended duodenoscope reprocessing strategy, % | |
| Effectiveness at eliminating CRE* | 90 (50–100) |
| Culture and hold strategy, % | |
| Sensitivity of duodenoscope culture for CRE* | 85 (50–95) |
| Duodenoscope with CRE-positive culture after one HLD(13) | 3.0 (1–10) |
| Duodenoscope with CRE-positive culture after two HLD* | 1.5 (1–10) |
| EtO sterilization strategy, % | |
| Effectiveness at eliminating CRE* | 99 (90–100) |
| Halt ERCP, perform LC with CBDE strategy, % | |
| CRE transmission with LC with CBDE strategy | 0 (0–0) |
| Utility estimates | |
| CRE-related sepsis(37) | 0.50 (0.40–0.60) |
| Post CRE-sepsis survival(37) | 0.80 (0.70–0.90) |
| ERCP without complication(38) | 0.89 (0.79–0.95) |
| ERCP minor complication(38) | 0.88 (0.78–0.95) |
| ERCP major complication(38) | 0.76 (0.66–0.86) |
| LC +/− CBDE without complication(39) | 0.90 (0.80–0.95) |
| LC +/− CBDE minor complication(39) | 0.80 (0.70–0.90) |
| LC +/− CBDE major complication(40) | 0.61 (0.51–0.71) |
CBDE = common bile duct exploration; CRE = carbapenem-resistant Enterobacteriaceae; ERCP = endoscopic retrograde cholangiopancreatography; EtO = ethylene oxide; HLD = high-level disinfection; FDA = Food and Drug Administration; LC = laparoscopic cholecystectomy; PTC = percutaneous transhepatic cholangiography.
No data supporting base-case estimate. The estimate is an assumption.
Only applicable for “halt ERCP, perform LC/CBDE” strategy.
Table 1 also lists our base-case CRE clinical and prevention strategy probability estimates. Because there is limited data and uncertainty regarding these estimates, we conducted a number of 1-way sensitivity analyses with these estimates, as described in detail in the Sensitivity Analyses section.
Outcomes
We used quality-adjusted life years (QALYs) as our main outcome measure, and our analysis reported the incremental cost per QALY gained among the four competing strategies. We also reported the 2.5th and 97.5th percentiles around the point estimates as generated by a Monte Carlo analysis of 1000 trials (see Sensitivity Analyses section for details). For our analyses, $100,000 per QALY gained was chosen as our cost-effective threshold; we opted to use $100,000 per QALY gained rather than the traditionally used $50,000 per QALY, as prior reports have found that the latter is too low for the U.S.(24–26)
Utility Estimates
The model included a wide range of relevant health state utilities for symptomatic CBD stones, procedure-related complications, and CRE infection. The Tufts Cost-Effectiveness Analysis Registry was searched to identify pertinent utilities (Table 1).(27) Since our study cohort was followed over a one-year period, discounting was not performed.
Cost Estimates
The model accounted for total healthcare costs attributable to the competing strategies, both from the payer and hospital perspective. Table 2 depicts all the base-case costs estimates used in our study, and Supplementary Table 1 describes these estimates in detail. Costs associated with hospital admissions were obtained from the Healthcare Cost and Utilization Project database.(18) We obtained costs for physician services and procedures using the 2015 American Medical Association Current Procedural Terminology codebook and the 2015 Medicare Physician Fee Schedule.(28, 29) We also incorporated the direct healthcare costs associated with each ERCP reprocessing strategy (e.g., FDA-recommended reprocessing, new and replacement duodenoscopes, cultures, and EtO sterilization). All estimates were updated to 2015 U.S. dollars by using the Consumer Price Index inflation calculator.(30) Since our study cohort was followed over a one-year period, discounting was not performed.
Table 2.
Base-case cost estimates.
| Cost | Base-case estimate (range tested in Monte Carlo analysis) |
|---|---|
| Procedure and admission costs, $ | |
| Admission for ERCP without complication | 7520 (3000–13,000) |
| Admission for ERCP with minor complication | 10,291 (5000–15,000) |
| Admission for ERCP with major complication* | 14,568 (10,000–20,000) |
| Admission for PTC and LC/CBDE without complication | 16,892 (12,000–22,000) |
| Admission for PTC and LC/CBDE with minor complication | 22,896 (18,000–28,000) |
| Admission for PTC and LC/CBDE with major complication* | 33,350 (28,000–38,000) |
| Admission for LC/CBDE without complication | 15,924 (11,000–21,000) |
| Admission for LC/CBDE with minor complication | 21,928 (17,000–27,000) |
| Admission for LC/CBDE with major complication* | 32,382 (27,000–37,000) |
| Outpatient LC without complication | 4752 (2000–10,000) |
| Admission for LC with minor complication | 14,472 (10,000–20,000) |
| Admission for LC with major complication* | 19,595 (15,000–25,000) |
| Admission for CRE* | 32,915 (28,000–38,000) |
| Duodenoscope reprocessing strategy costs, $ | |
| FDA-recommended duodenoscope reprocessing | 69 (20–150) |
| Culture and hold† | 400 (200–800) |
| EtO sterilization‡ | 1044 (500–1500) |
CBDE = common bile duct exploration; ERCP = endoscopic retrograde cholangiopancreatography; EtO = ethylene oxide; FDA = Food and Drug Administration; LC = laparoscopic cholecystectomy; PTC = percutaneous transhepatic cholangiography.
Cost was also used for admissions with patient mortality.
Includes cost of new duodenoscopes, FDA-recommended reprocessing, cultures, EtO sterilization for duodenoscopes with two successive positive cultures, and cost of replacing duodenoscopes damaged by EtO.
Includes cost of new duodenoscopes, FDA-recommended reprocessing, EtO sterilization, and cost of replacing duodenoscopes damaged by EtO.
Sensitivity Analyses
Because there are limited data regarding the CRE-related clinical probability estimates as well as the effectiveness of the proposed CRE-prevention strategies, we conducted extensive 1-way sensitivity analyses for each estimated probability, ranging variables from 0% to 100%. In our base-case model we assumed that endoscopes in the EtO sterilization strategy were sent to an outside facility, but some institutions have in-house facilities that perform such sterilization. Thus, we also performed a sensitivity analysis accounting for this scenario as it precludes the need to purchase additional endoscopes at the outset and for courier costs.
We also conducted a Monte Carlo simulation, assuming that all variables followed a triangular distribution with base-case, minimum, and maximum values listed in Tables 1 and 2. One thousand trials were simulated and we analyzed the base-case cohort to find the 2.5th and 97.5th percentiles for our estimate of incremental cost per QALY gained among competing strategies. We also used results from the Monte Carlo simulation to generate a cost-effectiveness acceptability curve plot of the four competing strategies and tested willingness to pay thresholds ranging from $0 to $200,000 per QALY gained.
RESULTS
Base-Case Results
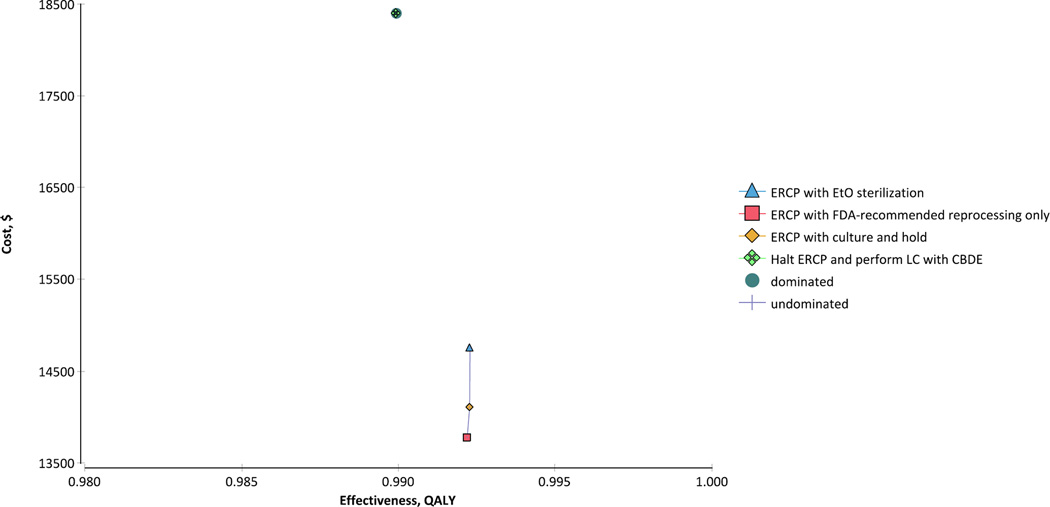
Among the four competing strategies, the least expensive was ERCP with FDA-recommended reprocessing procedures (Figure 2). Compared with ERCP with FDA-recommended endoscope reprocessing, the ERCP with culture and hold strategy cost an incremental $4,228,170 per additional QALY gained (2.5th and 97.5th percentiles, $93,051 and $23,656,027, respectively). Compared with ERCP with culture and hold, the ERCP with EtO sterilization strategy cost an incremental $50,572,348 to gain one additional QALY (2.5th and 97.5th percentiles, $224,167 and $200,183,527, respectively). The strategy in which ERCP is discontinued in lieu of performing LC with CBDE was dominated by the alternative strategies, as it was both more expensive and marginally less effective.
Figure 2.
Results of cost-effectiveness analysis. The ERCP followed by FDA-recommended reprocessing strategy was the least expensive of the four competing strategies. Both the ERCP with culture and hold and ERCP with EtO sterilization strategies were marginally more effective, but had incremental costs per QALY gained significantly greater than $100,000. The LC with CBDE strategy was dominated by the other strategies, as it was both more costly and slightly less effective.
CBDE = common bile duct exploration; ERCP = endoscopic retrograde cholangiopancreatography; EtO = ethylene oxide; FDA = Food and Drug Administration; LC = laparoscopic cholecystectomy; QALY = quality-adjusted life year.
Base-Case Sensitivity Analyses
Because of limited data regarding the probability estimates for CRE clinical and prevention strategies, we performed extensive sensitivity analyses (Table 3). Interestingly, the model was insensitive to most of the variables for which an accurate estimate was not available. For example, when setting the effectiveness of FDA-recommended reprocessing at 0% (i.e., testing the status quo prior to implementation of FDA-recommended reprocessing procedures), we found that the other competing approaches were still cost-prohibitive.
Table 3.
Results of 1-way sensitivity analyses.
| Variable | Base-case estimate |
Threshold | Comment |
|---|---|---|---|
| Pretest probability of CRE, % | 1 | 24 | If probability exceeds this threshold, then culture and hold becomes the most cost-effective strategy |
| Probability of patient CRE transmission from a CRE-infected duodenoscope, % | 31 | – | FDA-recommended reprocessing is the most cost-effective strategy across the tested range (0% to 100%) |
| Probability patient with CRE develops clinical symptoms, % | 73 | – | FDA-recommended reprocessing is the most cost-effective strategy across the tested range (0% to 100%) |
| Probability patient with CRE dies, % | 43 | – | FDA-recommended reprocessing is the most cost-effective strategy across the tested range (0% to 100%) |
| Effectiveness of FDA-recommended reprocessing at eliminating CRE, % | 90 | – | FDA-recommended reprocessing is the most cost-effective strategy across the tested range (0% to 100%) |
| Sensitivity of duodenoscope culture for CRE, % | 85 | – | FDA-recommended reprocessing is the most cost-effective strategy across the tested range (0% to 100%) |
| Effectiveness of EtO sterilization at eliminating CRE, % | 99 | – | FDA-recommended reprocessing is the most cost-effective strategy across the tested range (0% to 100%) |
| Probability endoscope damaged by EtO, requiring replacement, % | 1 | – | FDA-recommended reprocessing is the most cost-effective strategy across the tested range (0% to 100%) |
| Cost culture and hold*, $ | 400 | 82 | If cost is below this threshold, then culture and hold is the most cost-effective strategy |
| Cost EtO reprocessing†, $ | 1044 | 84 | If cost is below this threshold, then EtO reprocessing is the most cost-effective strategy |
CRE = carbapenem-resistant Enterobacteriaceae; EtO = ethylene oxide; FDA = Food and Drug Administration.
Includes cost of new duodenoscopes, FDA-recommended reprocessing, cultures, EtO sterilization on duodenoscopes with two successive positive cultures, and cost of replacing duodenoscopes damaged by EtO.
Includes cost of new duodenoscopes, FDA-recommended reprocessing, EtO sterilization, and cost of replacing duodenoscopes damaged by EtO.
However, a few thresholds were noted. For instance, when the pretest probability of CRE exceeded 24%, ERCP with culture and hold became the most cost-effective strategy (i.e., incremental cost per QALY gained <$100,000) compared to ERCP with FDA-recommended reprocessing. If the total cost associated with EtO reprocessing was less than $84 per ERCP, then ERCP with EtO sterilization became the most cost-effective strategy. Similarly, if the total cost related to the culture and sequester protocol was less than $82 per ERCP, then ERCP with culture and hold became the most cost-effective strategy. ERCP with FDA-recommended reprocessing remained the most cost-effective strategy across the tested range for the remaining variables listed in Table 3.
Because some institutions have in-house facilities that perform EtO sterilization, we performed a sensitivity analysis that accounted for a 24-hour endoscope turnaround time. This obviates the need to purchase additional endoscopes at the outset as well as courier costs. Even in this scenario, in-house EtO sterilization was still cost-prohibitive ($7,478,728 per QALY gained; 2.5th and 97.5th percentiles, $176,221 and $26,051,419, respectively) compared to ERCP with FDA-recommended reprocessing.
Monte Carlo Analyses
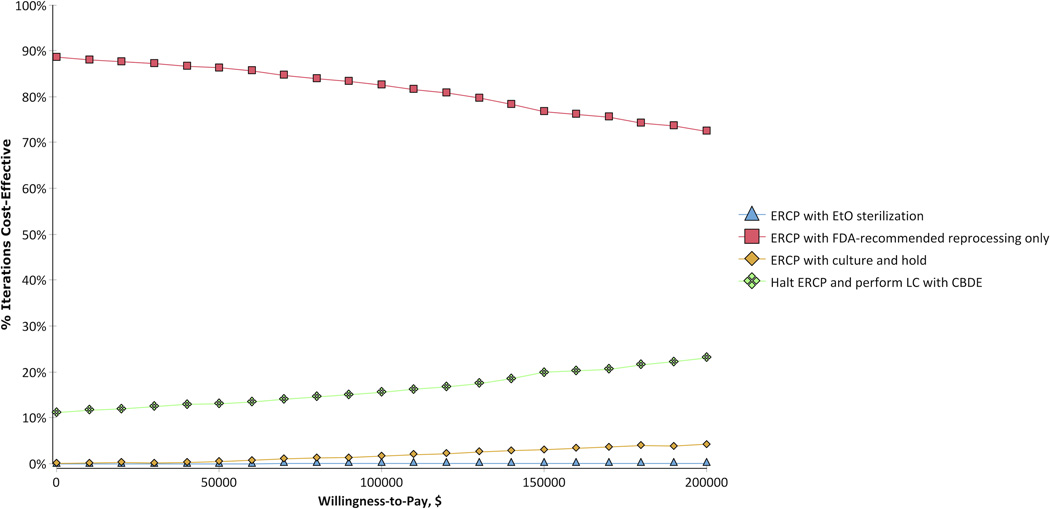
Figure 3 depicts the cost-effectiveness acceptability curve plot for the four competing approaches in 1000 simulations across a tested willingness to pay range from $0 to $200,000 per additional QALY. At a willingness to pay threshold of $100,000 per QALY gained, most trials favored ERCP with FDA-recommended reprocessing as the most cost-effective approach (82.6%), followed by LC with CBDE (15.6%), ERCP with culture and hold (1.7%), and ERCP with EtO sterilization (0.1%). Results were similar when using a higher willingness to pay threshold of $200,000 per QALY (ERCP with FDA-recommended reprocessing, 72.5%; LC with CBDE, 23.1%; ERCP with culture and hold, 4.3%; and ERCP with EtO sterilization, 0.1%).
Figure 3.
Cost-effectiveness acceptability curve plot of the competing strategies. Across the tested willingness to pay range, the majority of trials found ERCP with FDA-recommended reprocessing to be the most cost-effective approach.
CBDE = common bile duct exploration; ERCP = endoscopic retrograde cholangiopancreatography; EtO = ethylene oxide; FDA = Food and Drug Administration; LC = laparoscopic cholecystectomy.
DISCUSSION
In light of several recent ERCP-associated CRE (“superbug”) outbreaks across the U.S.,(6, 13–16) we performed a comprehensive decision analysis to identify the most cost-effective approach to endoscope reprocessing. Our analysis has four key findings. First, we found that use of FDA-recommended reprocessing procedures after ERCP was the most cost-effective approach for mitigating CRE transmission. As the overall incidence of clinically overt CRE transmission via ERCP appears to be rare, the differences in QALYs between the four competing strategies were extremely small. Because the overall effectiveness among strategies was similar, differential cost is the principal factor driving the health economic interpretation.
Second, we demonstrated that results were highly contingent on the pretest probability for CRE. The FDA-recommended reprocessing strategy was the most cost-effective strategy until the CRE pretest probability exceeded 24%. At that point, the culture and hold strategy became more cost-effective. Given the low positive-culture rate for “high-concern” bacteria in endoscope studies,(13) it is highly unlikely that CRE carriage rates will exceed 24% in the near future. However, as the usage of potent antibiotics continues, it is possible that the prevalence of CRE and other “superbugs” may approach this threshold, particularly in high-volume, quaternary-care centers. At that time, these healthcare centers and endoscopy units may benefit from utilizing culture and hold as a more cost-effective approach to reducing the risk of CRE transmission. Yet, we should note that the culture and hold strategy may pose potential logistic challenges, particularly at stand-alone endoscopy centers. Namely, these units may not have in-house laboratory facilities or may only have access to a facility that lacks on-site experience with the duodenoscope culturing and interpretation of results.(22)
Third, we found that using EtO gas sterilization was unlikely to be cost-effective under any circumstances. While EtO gas sterilization is believed to offer optimal endoscope sterilization, its impact on reducing CRE transmission has not been formally tested in a clinical trial. Yet, there is anecdotal evidence that it is effective, as no additional cases of CRE were found – to date – in hospitals that implemented EtO sterilization after having had CRE outbreaks linked to ERCPs.(6, 14) Along the same lines, there are currently no published, randomized controlled data demonstrating the effectiveness of the FDA-recommended reprocessing and culture and hold strategies for preventing CRE transmission. So, while awaiting additional data, endoscopy centers that opt out of EtO sterilization may need to assume an unavoidable, low level of CRE risk. This may be unacceptable, though, for institutions with a known, relatively high CRE prevalence or that had patients infected with CRE linked to recently performed ERCPs.
Fourth, while we found that LC with CBDE was dominated in the base-case scenario, Monte Carlo analyses revealed that it was cost-effective in approximately 15% of trials at a willingness to pay threshold of $100,000 per QALY. This is likely a reflection of the greater uncertainty in the clinical probability estimates used in the ERCP-based strategies versus those used in the surgical arm. However, in real clinical practice where the prevalence of CRE remains low, it is unlikely that the more invasive LC with CBDE will supplant ERCP as the preferred modality for managing symptomatic choledocholithiasis. There have also been concerns about insufficient exposure and training among general surgery residents in performing CBDE.(31)
This analysis has limitations. First, the CRE-related clinical and prevention strategy estimates used in the model were based on limited data. Specifically, the pretest probability for CRE and the effectiveness of FDA-recommended reprocessing, culture and hold, and EtO sterilization at preventing CRE transmission are unknown at this time, yet strongly impact the results. To address this, we tested a wide range of potential values for the CRE-related probability estimates in both 1-way sensitivity and Monte Carlo analyses. Here we found that the cost-effectiveness rankings were robust to sensitivity analyses. Second, our model did not account for legal costs related to CRE cases from infected duodenoscopes. Namely, lawsuits have been filed against some institutions with such cases.(32, 33) Factoring in legal costs is problematic given that legal fees, settlements, and patient compensation vary widely. Legal costs are also not exclusive to cases of CRE, as lawsuits can be filed after complications from ERCPs and surgical interventions. Our model also did not account for costs associated with negative publicity from cases of CRE transmission. Similar to legal issues, it is difficult to assign a cost to negative press as its impact (e.g., decreased referrals, cancellation of elective procedures, etc.) will vary widely between institutions. Because of these inherent issues, we opted to not include costs associated with litigation or poor publicity in our decision analysis. However, for institutions that anticipate exorbitant legal costs or damaging negative press from CRE cases, employing FDA-recommended reprocessing (the “least effective” ERCP-based approach) may not be a reasonable option, particularly among institutions with prior cases of CRE. Rather, culture and hold or EtO sterilization may be the only viable strategies for these establishments. Third, our findings may not be generalizable to all indications for ERCP. Our hypothetical cohort was inpatients with symptomatic CBD stones, only one of many conditions where ERCP is strongly indicated. Nonetheless, our base-case cohort reflects the most common indication for ERCP(2); therefore, it is the most appropriate condition on which to base a cost-effectiveness analysis. By using a common condition in the base-case model, we have tried to generate results that are relevant to most settings where ERCP is performed. Fourth, our study focuses primarily on CRE and does not assess the impact of other MDROs. Just as there are limited data for CRE, there is even less data on the clinical impact of other MDROs transmitted during ERCP. This is an area worth investigating further. Lastly, our study does not account for screening of patients for CRE/MDRO prior to ERCP; this may identify duodenoscopes at “high-risk” for harboring such organisms thereby singling them out for more aggressive reprocessing and surveillance. However, the FDA has not yet recommended this as a viable approach for mitigating MDRO risk, and it is not clear as to which MDROs should be tested for and which are clinically relevant.
In conclusion, our analysis suggests that of the four strategies examined, ERCP with FDA-recommended reprocessing is the most cost-effective approach for mitigating CRE risk. Only when the CRE pretest probability exceeds rates much higher than currently reported does ERCP with culture and hold become potentially cost-effective. Future research should formally investigate the effectiveness of CRE-elimination using these methods and determine whether other reprocessing strategies should be considered. CRE infections have become the object of intensive popular media scrutiny and a frequent source of patient concern. In light of the current uncertainty regarding the best management of CRE risk, limited treatment options for CRE-infected patients, and high mortality associated with CRE infection, our results may assist healthcare administrators, healthcare centers, and endoscopy clinics in deciding how to reduce CRE risk for what can otherwise be a life-saving procedure.
Supplementary Material
STUDY HIGHLIGHTS.
What is current knowledge?
Recent outbreaks of carbapenem-resistant Enterobacteriaceae (CRE, or “superbug”) have been linked to duodenoscopes used during endoscopic retrograde cholangiopancreatography (ERCP).
What is new here?
ERCP with U.S. Food and Drug Administration-recommended duodenoscope reprocessing is the most cost-effective strategy for mitigating CRE transmission risk.
ERCP followed by a duodenoscope culture and hold approach may be cost-effective in settings with a high CRE prevalence.
Strategies involving ethylene oxide gas sterilization of the duodenoscope are unlikely to be cost-effective under most circumstances.
Acknowledgments
STUDY SUPPORT
Financial Support:
Drs. Almario and May were supported by a National Institutes of Health T32 training grant (NIH T32DK07180-40) during their gastroenterology and health services research training at UCLA.
Footnotes
CONFLICTS OF INTEREST
Potential Competing Interests:
All authors do not have any relevant disclosures or conflicts of interest.
Guarantor of the Article:
Brennan M.R. Spiegel, MD, MSHS
- Christopher V. Almario, MD, MSHPM: Planning and conducting the study, collecting and interpreting data, drafting the manuscript, approval of final draft submitted.
- Folasade P. May, MD, PhD: Planning and conducting the study, collecting and interpreting data, drafting the manuscript, approval of final draft submitted.
- Nicholas J. Shaheen, MD, MPH: Planning and conducting the study, interpreting data, drafting the manuscript, approval of final draft submitted.
- Rekha Murthy, MD: Planning and conducting the study, interpreting data, drafting the manuscript, approval of final draft submitted.
- Kapil Gupta, MD, MPH: Planning and conducting the study, interpreting data, approval of final draft submitted.
- Laith H. Jamil, MD: Planning and conducting the study, interpreting data, approval of final draft submitted.
- Simon K. Lo, MD: Planning and conducting the study, interpreting data, approval of final draft submitted.
- Brennan M.R. Spiegel, MD, MSHS: Planning and conducting the study, interpreting data, drafting the manuscript, approval of final draft submitted.
REFERENCES
- 1.Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781–1788. doi: 10.1111/j.1572-0241.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 2.Moffatt DC, Yu BN, Yie W, et al. Trends in utilization of diagnostic and therapeutic ERCP and cholecystectomy over the past 25 years: a population-based study. Gastrointest Endosc. 2014;79:615–622. doi: 10.1016/j.gie.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Rutala WA, Weber DJ. Gastrointestinal endoscopes: a need to shift from disinfection to sterilization? JAMA. 2014;312:1405–1406. doi: 10.1001/jama.2014.12559. [DOI] [PubMed] [Google Scholar]
- 4.Kovaleva J, Peters FT, van der Mei HC, et al. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin Microbiol Rev. 2013;26:231–254. doi: 10.1128/CMR.00085-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson DJ, Shimpi RA, McDonald JR, et al. Infectious complications following endoscopic retrograde cholangiopancreatography: an automated surveillance system for detecting postprocedure bacteremia. Am J Infect Control. 2008;36:592–594. doi: 10.1016/j.ajic.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Epstein L, Hunter JC, Arwady MA, et al. New Delhi metallo-beta-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA. 2014;312:1447–1455. doi: 10.1001/jama.2014.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alrabaa SF, Nguyen P, Sanderson R, et al. Early identification and control of carbapenemase-producing Klebsiella pneumoniae, originating from contaminated endoscopic equipment. Am J Infect Control. 2013;41:562–564. doi: 10.1016/j.ajic.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Gastmeier P, Vonberg RP. Klebsiella spp. in endoscopy-associated infections: we may only be seeing the tip of the iceberg. Infection. 2014;42:15–21. doi: 10.1007/s15010-013-0544-6. [DOI] [PubMed] [Google Scholar]
- 9.Alfa MJ. Monitoring and improving the effectiveness of cleaning medical and surgical devices. Am J Infect Control. 2013;41:S56–S59. doi: 10.1016/j.ajic.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Aumeran C, Poincloux L, Souweine B, et al. Multidrug-resistant Klebsiella pneumoniae outbreak after endoscopic retrograde cholangiopancreatography. Endoscopy. 2010;42:895–899. doi: 10.1055/s-0030-1255647. [DOI] [PubMed] [Google Scholar]
- 11.Doherty DE, Falko JM, Lefkovitz N, et al. Pseudomonas aeruginosa sepsis following retrograde cholangiopancreatography (ERCP) Dig Dis Sci. 1982;27:169–170. doi: 10.1007/BF01311712. [DOI] [PubMed] [Google Scholar]
- 12.Muscarella LF. Investigation and prevention of infectious outbreaks during endoscopic retrograde cholangiopancreatography. Endoscopy. 2010;42:957–959. doi: 10.1055/s-0030-1255871. [DOI] [PubMed] [Google Scholar]
- 13.Wendorf KA, Kay M, Baliga C, et al. Endoscopic Retrograde Cholangiopancreatography-Associated AmpC Escherichia coli Outbreak. Infect Control Hosp Epidemiol. 2015:1–9. doi: 10.1017/ice.2015.66. [DOI] [PubMed] [Google Scholar]
- 14.Smith ZL, Oh YS, Saeian K, et al. Transmission of carbapenem-resistant Enterobacteriaceae during ERCP: time to revisit the current reprocessing guidelines. Gastrointest Endosc. 2015 doi: 10.1016/j.gie.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Cedars-Sinai. Media Statement Regarding CRE and Duodenoscope. [cited 2015 March 4];2015 Available from: http://cedars-sinai.edu/About-Us/News/News-Releases-2015/Media-Statement-Regarding-CRE-and-Duodenoscope.aspx. [Google Scholar]
- 16.UCLA Health System. UCLA notifies patients who received endoscopic procedures. [cited 2015 March 1];2015 Available from: http://newsroom.ucla.edu/releases/ucla-notifies-patients-who-received-endoscopic-procedures. [Google Scholar]
- 17.U.S. Food and Drug Administration. Olympus Validates New Reprocessing Instructions for Model TJF-Q180V Duodenoscopes. [cited 2015 March 26];2015 Available from: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm439999.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery&zbrandid=439&zidType=CH&zid=9415155&zsubscriberId=328595175&zbdom=http://www.informz.net.
- 18.Agency for Healthcare Research and Quality. HCUPnet. [cited 2015 March 26];2015 Available from: http://hcupnet.ahrq.gov/HCUPnet.jsp.
- 19.Ducomble T, Faucheux S, Helbig U, et al. Large hospital outbreak of KPC-2-producing Klebsiella pneumoniae: investigating mortality and the impact of screening for KPC-2 with polymerase chain reaction. J Hosp Infect. 2015;89:179–185. doi: 10.1016/j.jhin.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis. 2011;69:357–362. doi: 10.1016/j.diagmicrobio.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li VK, Yum JL, Yeung YP. Optimal timing of elective laparoscopic cholecystectomy after acute cholangitis and subsequent clearance of choledocholithiasis. Am J Surg. 2010;200:483–488. doi: 10.1016/j.amjsurg.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration. Supplemental Measures to Enhance Duodenoscope Reprocessing: FDA Safety Communication. [cited 2015 August 10];2015 Available from: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm454766.htm.
- 23.Centers for Disease Control and Prevention. Interim duodenoscope surveillance protocol. [cited 2015 April 9];2015 Available from: http://www.cdc.gov/hai/organisms/cre/cre-duodenoscope-surveillance-protocol.html.
- 24.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 25.Braithwaite RS, Meltzer DO, King JT, Jr, et al. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 26.Hirth RA, Chernew ME, Miller E, et al. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20:332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 27.The Institute for Clinical Research and Health Policy Studies. Cost-Effectiveness Analysis Registry. [cited 2015 March 26];2015 Available from: https://research.tufts-nemc.org/cear4/Home.aspx. [Google Scholar]
- 28.American Medical Association. CPT Code/Relative Value Search. [cited 2015 March 23];2015 Available from: https://ocm.ama-assn.org/OCM/CPTRelativeValueSearch.do. [Google Scholar]
- 29.Centers for Medicare & Medicaid Services. Physician Fee Schedule Search. [cited 2015 March 26];2015 Available from: http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx.
- 30.Bureau of Labor Statistics. CPI Inflation Calculator. [cited 2015 April 6];2015 Available from: http://www.bls.gov/data/inflation_calculator.htm.
- 31.Helling TS, Khandelwal A. The challenges of resident training in complex hepatic, pancreatic, and biliary procedures. J Gastrointest Surg. 2008;12:153–158. doi: 10.1007/s11605-007-0378-6. [DOI] [PubMed] [Google Scholar]
- 32.Novak D, Amin R. Lawsuit says woman died a year after contracting 'superbug' CRE at Lutheran General. Chicago Sun-Times. 2015 Mar 23; [Google Scholar]
- 33.Aleccia J. Widow sues Virginia Mason; hospital begins notifying ‘superbug’ victims. The Seattle Times. 2015 Mar 4; [Google Scholar]
- 34.Keus F, Gooszen HG, van Laarhoven CJ. Open, small-incision, or laparoscopic cholecystectomy for patients with symptomatic cholecystolithiasis. An overview of Cochrane Hepato-Biliary Group reviews. Cochrane Database Syst Rev. 2010:Cd008318. doi: 10.1002/14651858.CD008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexakis N, Connor S. Meta-analysis of one- vs. two-stage laparoscopic/endoscopic management of common bile duct stones. HPB (Oxford) 2012;14:254–259. doi: 10.1111/j.1477-2574.2012.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dasari BV, Tan CJ, Gurusamy KS, et al. Surgical versus endoscopic treatment of bile duct stones. Cochrane Database Syst Rev. 2013;12:Cd003327. doi: 10.1002/14651858.CD003327.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fowler RA, Hill-Popper M, Stasinos J, et al. Cost-effectiveness of recombinant human activated protein C and the influence of severity of illness in the treatment of patients with severe sepsis. J Crit Care. 2003;18:181–191. doi: 10.1016/j.jcrc.2003.08.009. discussion 191-4. [DOI] [PubMed] [Google Scholar]
- 38.Howard K, Lord SJ, Speer A, et al. Value of magnetic resonance cholangiopancreatography in the diagnosis of biliary abnormalities in postcholecystectomy patients: a probabilistic cost-effectiveness analysis of diagnostic strategies. Int J Technol Assess Health Care. 2006;22:109–118. doi: 10.1017/s0266462306050902. [DOI] [PubMed] [Google Scholar]
- 39.Wilson E, Gurusamy K, Gluud C, et al. Cost-utility and value-of-information analysis of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg. 2010;97:210–219. doi: 10.1002/bjs.6872. [DOI] [PubMed] [Google Scholar]
- 40.Johner A, Raymakers A, Wiseman SM. Cost utility of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Surg Endosc. 2013;27:256–262. doi: 10.1007/s00464-012-2430-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.