Abstract
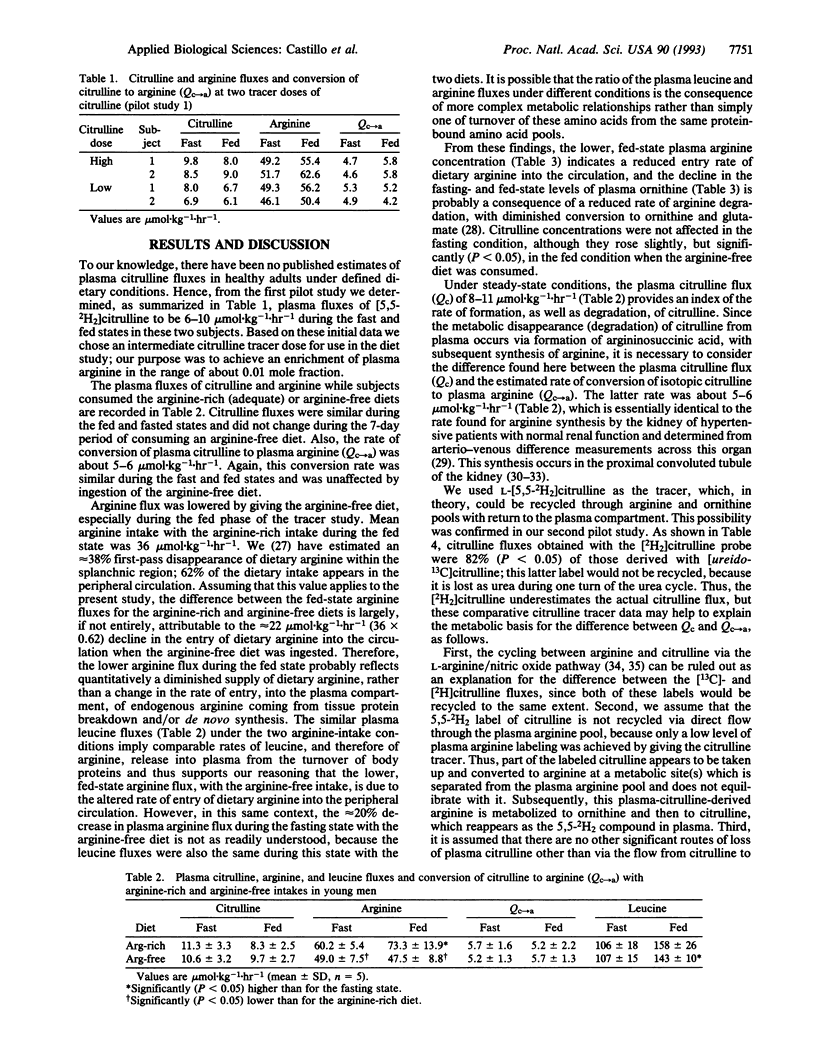
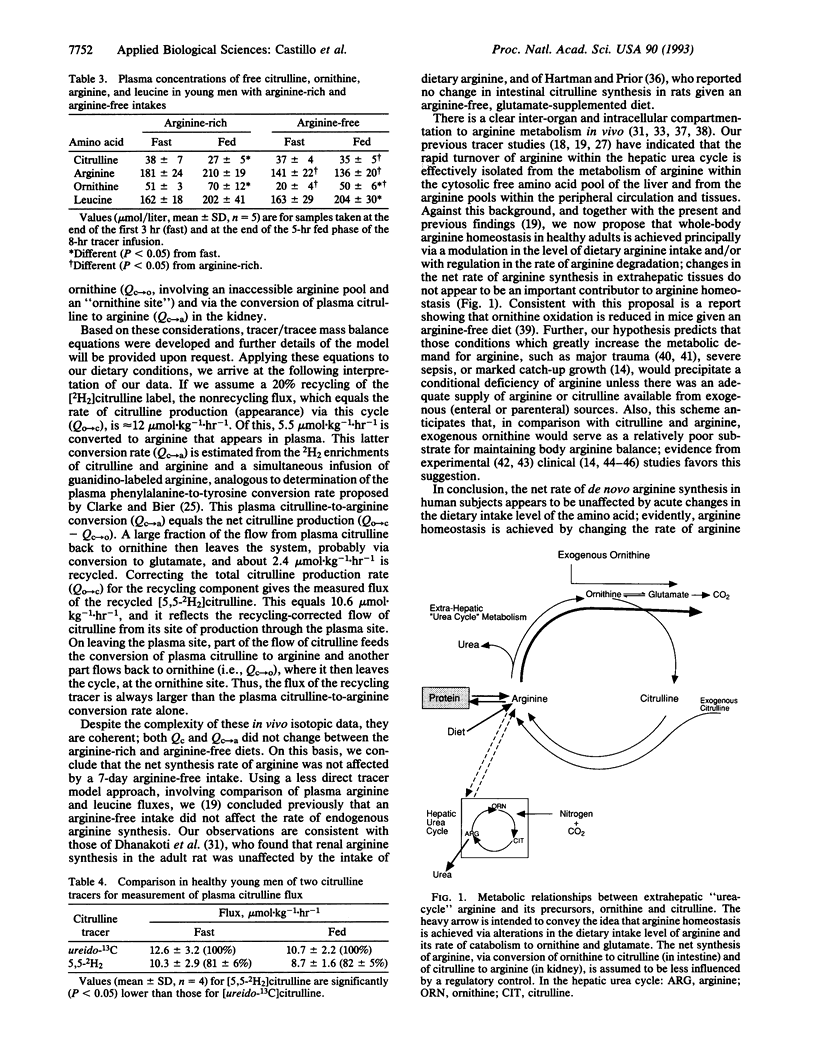
The fluxes of arginine and citrulline through plasma and the rate of conversion of labeled citrulline to arginine were estimated in two pilot studies (with a total of six adult subjects) and in a dietary study with five healthy young men. These latter subjects received an L-amino acid-based diet that was arginine-rich or arginine-free each for 6 days prior to conduct, on day 7, of an 8-hr (first 3 hr, fast; final 5 hr, fed) primed continuous intravenous infusion protocol using L-[guanidino-13C]arginine, L-[5,5-2H2]citrulline, and L-[5,5,5-2H3]leucine, as tracers. A pilot study indicated that citrulline flux was about 20% higher (P < 0.05) when determined with [ureido-13C]citrulline compared with [2H2]citrulline, indicating recycling of the latter tracer. Mean citrulline fluxes were about 8-11 mumol.kg-1.hr-1 for the various metabolic/diet groups and did not differ significantly between fast and fed states or arginine-rich and arginine-free periods. Arginine fluxes (mean +/- SD) were 60.2 +/- 5.4 and 73.3 +/- 13.9 mumol.kg-1.hr-1 for fast and fed states during the arginine-rich period, respectively, and were significantly lowered (P < 0.05), by 20-40%, during the arginine-free period, especially for the fed state, where this was due largely to reduced entry of dietary arginine into plasma. The conversion of plasma citrulline to arginine approximated 5.5 mumol.kg-1.hr-1 for the various groups and also was unaffected by arginine intake. Thus, endogenous arginine synthesis is not markedly responsive to acute alterations in arginine intake in healthy adults. We propose that arginine homeostasis is achieved largely via modulating arginine intake and/or the net rate of arginine degradation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. F. Determination of amino acid profiles in biological samples by gas chromatography. J Chromatogr. 1974 Aug 14;95(2):189–212. doi: 10.1016/s0021-9673(00)84078-9. [DOI] [PubMed] [Google Scholar]
- Adamson I., Fisher H. Amino acid requirement of the growing rabbits: an estimate of quantitative needs. J Nutr. 1973 Sep;103(9):1306–1310. doi: 10.1093/jn/103.9.1306. [DOI] [PubMed] [Google Scholar]
- Brusilow S. W. Arginine, an indispensable amino acid for patients with inborn errors of urea synthesis. J Clin Invest. 1984 Dec;74(6):2144–2148. doi: 10.1172/JCI111640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R. A., Milner J. A., Corbin J. E. Arginine: an indispensable amino acid for mature dogs. J Nutr. 1981 Jun;111(6):1020–1024. doi: 10.1093/jn/111.6.1020. [DOI] [PubMed] [Google Scholar]
- Castillo L., deRojas T. C., Chapman T. E., Vogt J., Burke J. F., Tannenbaum S. R., Young V. R. Splanchnic metabolism of dietary arginine in relation to nitric oxide synthesis in normal adult man. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):193–197. doi: 10.1073/pnas.90.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves Das Neves H. J., Vasconcelos A. M. Capillary gas chromatography of amino acids, including asparagine and glutamine: sensitive gas chromatographic-mass spectrometric and selected ion monitoring gas chromatographic-mass spectrometric detection of the N,O(S)-tert.-butyldimethylsilyl derivatives. J Chromatogr. 1987 Apr 17;392:249–258. doi: 10.1016/s0021-9673(01)94270-0. [DOI] [PubMed] [Google Scholar]
- Clarke J. T., Bier D. M. The conversion of phenylalanine to tyrosine in man. Direct measurement by continuous intravenous tracer infusions of L-[ring-2H5]phenylalanine and L-[1-13C] tyrosine in the postabsorptive state. Metabolism. 1982 Oct;31(10):999–1005. doi: 10.1016/0026-0495(82)90142-1. [DOI] [PubMed] [Google Scholar]
- Cynober L., Coudray-Lucas C., de Bandt J. P., Guéchot J., Aussel C., Salvucci M., Giboudeau J. Action of ornithine alpha-ketoglutarate, ornithine hydrochloride, and calcium alpha-ketoglutarate on plasma amino acid and hormonal patterns in healthy subjects. J Am Coll Nutr. 1990 Feb;9(1):2–12. doi: 10.1080/07315724.1990.10720343. [DOI] [PubMed] [Google Scholar]
- Czarnecki G. L., Baker D. H. Urea cycle function in the dog with emphasis on the role of arginine. J Nutr. 1984 Mar;114(3):581–590. doi: 10.1093/jn/114.3.581. [DOI] [PubMed] [Google Scholar]
- Dhanakoti S. N., Brosnan J. T., Brosnan M. E., Herzberg G. R. Net renal arginine flux in rats is not affected by dietary arginine or dietary protein intake. J Nutr. 1992 May;122(5):1127–1134. doi: 10.1093/jn/122.5.1127. [DOI] [PubMed] [Google Scholar]
- Dhanakoti S. N., Brosnan J. T., Herzberg G. R., Brosnan M. E. Renal arginine synthesis: studies in vitro and in vivo. Am J Physiol. 1990 Sep;259(3 Pt 1):E437–E442. doi: 10.1152/ajpendo.1990.259.3.E437. [DOI] [PubMed] [Google Scholar]
- Dhanakoti S. N., Brosnan M. E., Herzberg G. R., Brosnan J. T. Cellular and subcellular localization of enzymes of arginine metabolism in rat kidney. Biochem J. 1992 Mar 1;282(Pt 2):369–375. doi: 10.1042/bj2820369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M. S., Lowry K. R., Baker D. H. Urea cycle metabolism: effects of supplemental ornithine or citrulline on performance, tissue amino acid concentrations and enzymatic activity in young pigs fed arginine-deficient diets. J Anim Sci. 1987 Sep;65(3):706–716. doi: 10.2527/jas1987.653706x. [DOI] [PubMed] [Google Scholar]
- Eriksson L. S., Reihnér E., Wahren J. Infusion of ornithine-alpha-ketoglutarate in healthy subjects: effects on protein metabolism. Clin Nutr. 1985 May;4(2):73–76. doi: 10.1016/0261-5614(85)90045-7. [DOI] [PubMed] [Google Scholar]
- Featherston W. R., Rogers Q. R., Freedland R. A. Relative importance of kidney and liver in synthesis of arginine by the rat. Am J Physiol. 1973 Jan;224(1):127–129. doi: 10.1152/ajplegacy.1973.224.1.127. [DOI] [PubMed] [Google Scholar]
- Ferber S., Ciechanover A. Role of arginine-tRNA in protein degradation by the ubiquitin pathway. Nature. 1987 Apr 23;326(6115):808–811. doi: 10.1038/326808a0. [DOI] [PubMed] [Google Scholar]
- HEINICKE H. R., HARPER A. E., ELVEHJEM C. A. Protein and amino acid requirements of the guinea pig. I. Effect of carbohydrate, protein level and amino acid supplementation. J Nutr. 1955 Dec 10;57(4):483–496. doi: 10.1093/jn/57.4.483. [DOI] [PubMed] [Google Scholar]
- Ha Y. H., Milner J. A., Corbin J. E. Arginine requirements in immature dogs. J Nutr. 1978 Feb;108(2):203–210. doi: 10.1093/jn/108.2.203. [DOI] [PubMed] [Google Scholar]
- Hecker M., Sessa W. C., Harris H. J., Anggård E. E., Vane J. R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun P., Rabier D., Bardet J., Parvy P. Citrulline concentrations in human plasma after arginine load. Clin Chem. 1991 Jul;37(7):1287–1287. [PubMed] [Google Scholar]
- Kirk S. J., Barbul A. Role of arginine in trauma, sepsis, and immunity. JPEN J Parenter Enteral Nutr. 1990 Sep-Oct;14(5 Suppl):226S–229S. doi: 10.1177/014860719001400514. [DOI] [PubMed] [Google Scholar]
- Levillain O., Hus-Citharel A., Morel F., Bankir L. Localization of arginine synthesis along rat nephron. Am J Physiol. 1990 Dec;259(6 Pt 2):F916–F923. doi: 10.1152/ajprenal.1990.259.6.F916. [DOI] [PubMed] [Google Scholar]
- MERTZ E. T., BEESON W. M., JACKSON H. D. Classification of essential amino acids for the weanling pig. Arch Biochem Biophys. 1952 Jul;38:121–128. doi: 10.1016/0003-9861(52)90015-5. [DOI] [PubMed] [Google Scholar]
- Matthews D. E., Motil K. J., Rohrbaugh D. K., Burke J. F., Young V. R., Bier D. M. Measurement of leucine metabolism in man from a primed, continuous infusion of L-[1-3C]leucine. Am J Physiol. 1980 May;238(5):E473–E479. doi: 10.1152/ajpendo.1980.238.5.E473. [DOI] [PubMed] [Google Scholar]
- Milner J. A., Wakeling A. E., Visek W. J. Effect of arginine deficiency on growth and intermediary metabolism in rats. J Nutr. 1974 Dec;104(12):1681–1689. doi: 10.1093/jn/104.12.1681. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Morris J. G., Rogers Q. R. Arginine: an essential amino acid for the cat. J Nutr. 1978 Dec;108(12):1944–1953. doi: 10.1093/jn/108.12.1944. [DOI] [PubMed] [Google Scholar]
- Morris J. G., Rogers Q. R., Winterrowd D. L., Kamikawa E. M. The utilization of ornithine and citrulline by the growing kitten. J Nutr. 1979 Apr;109(4):724–729. doi: 10.1093/jn/109.4.724. [DOI] [PubMed] [Google Scholar]
- Nissim I., Yudkoff M., Terwilliger T., Segal S. Rapid determination of [guanidino-15N]arginine in plasma with gas chromatography--mass spectrometry: application to human metabolic studies. Anal Biochem. 1983 May;131(1):75–82. doi: 10.1016/0003-2697(83)90137-9. [DOI] [PubMed] [Google Scholar]
- Pelletier V., Marks L., Wagner D. A., Hoerr R. A., Young V. R. Branched-chain amino acid interactions with reference to amino acid requirements in adult men: valine metabolism at different leucine intakes. Am J Clin Nutr. 1991 Aug;54(2):395–401. doi: 10.1093/ajcn/54.2.395. [DOI] [PubMed] [Google Scholar]
- Stewart P. M., Batshaw M., Valle D., Walser M. Effects of arginine-free meals on ureagenesis in cats. Am J Physiol. 1981 Oct;241(4):E310–E315. doi: 10.1152/ajpendo.1981.241.4.E310. [DOI] [PubMed] [Google Scholar]
- Thompson G. N., Pacy P. J., Merritt H., Ford G. C., Read M. A., Cheng K. N., Halliday D. Rapid measurement of whole body and forearm protein turnover using a [2H5]phenylalanine model. Am J Physiol. 1989 May;256(5 Pt 1):E631–E639. doi: 10.1152/ajpendo.1989.256.5.E631. [DOI] [PubMed] [Google Scholar]
- Visek W. J. Arginine and disease states. J Nutr. 1985 Apr;115(4):532–541. doi: 10.1093/jn/115.4.532. [DOI] [PubMed] [Google Scholar]
- Visek W. J. Arginine needs, physiological state and usual diets. A reevaluation. J Nutr. 1986 Jan;116(1):36–46. doi: 10.1093/jn/116.1.36. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Source and fate of circulating citrulline. Am J Physiol. 1981 Dec;241(6):E473–E480. doi: 10.1152/ajpendo.1981.241.6.E473. [DOI] [PubMed] [Google Scholar]
- Zieve L. Conditional deficiencies of ornithine or arginine. J Am Coll Nutr. 1986;5(2):167–176. doi: 10.1080/07315724.1986.10720123. [DOI] [PubMed] [Google Scholar]


