Abstract
Recent studies suggest that medulloblastoma, the most common malignant brain tumor of childhood, is comprised of four disease variants. The WIP1 oncogene is overexpressed in Group 3 and 4 tumors, which contain medulloblastomas with the most aggressive clinical behavior. Our data demonstrate increased WIP1 expression in metastatic medulloblastomas, and inferior progression-free and overall survival of patients with WIP1 high-expressing medulloblastoma. Microarray analysis identified up-regulation of genes involved in tumor metastasis, including the G protein-coupled receptor CXCR4, in medulloblastoma cells with high WIP1 expression. Stimulation with the CXCR4 ligand SDF1ααactivated PI-3 kinase signaling, and promoted growth and invasion of WIP1 high-expressing medulloblastoma cells in a p53-dependent manner. When xenografted into the cerebellum of immunodeficient mice, medulloblastoma cells with stable or endogenous high WIP1 expression exhibited strong expression of CXCR4 and activated AKT in primary and invasive tumor cells. WIP1 or CXCR4 knock-down inhibited medulloblastoma growth and invasion. WIP1 knock-down also improved the survival of mice xenografted with WIP1 high-expressing medulloblastoma cells. WIP1 knock-down inhibited cell surface localization of CXCR4 by suppressing expression of the G protein receptor kinase 5, GRK5. Restoration of wild-type GRK5 promoted Ser339 phosphorylation of CXCR4 and inhibited the growth of WIP1-stable medulloblastoma cells. Conversely, GRK5 knock-down inhibited Ser339 phosphorylation of CXCR4, increased cell surface localization of CXCR4, and promoted the growth of medulloblastoma cells with low WIP1 expression. These results demonstrate cross-talk among WIP1, CXCR4, and GRK5, which may be important for the aggressive phenotype of a subclass of medulloblastomas in children.
Keywords: medulloblastoma, WIP1, PPM1D, CXCR4, GRK5
Introduction
Advances in neurosurgical techniques, combination chemotherapy, cranio-spinal radiation, and supportive care measures have led to dramatic overall improvements in survival from medulloblastoma.1,2 Yet, most survivors suffer long-term toxicities3-5, and up to one-third may develop recurrent disease.6,7 Improvements in treatment will likely require a better understanding of the molecular pathobiology of this disease. To that end, recent gene expression analyses suggest that medulloblastoma consists of 4 disease variants: WNT, SHH, Group 3, and Group 4.8,9
Approximately 10% of medulloblastomas demonstrate active Wingless (WNT) signaling. These tumors exhibit classic histology, cytogenetics with monosomy of chromosome 6, and mutations of CTNNB1 and DDX3X in 50-85% of cases.10 Retrospective studies suggest that the 5-year progression-free (PFS) and overall survival (OS) of patients with WNT-activated medulloblastoma is > 90%.9,11 Future clinical trials will likely reduce treatment intensity to prevent therapy-related toxicities.
Thirty percent of medulloblastomas exhibit active Sonic Hedgehog (SHH) signaling. These tumors display classic or nodular/desmoplastic histology, 9q deletion in 50% of cases9,12, and positive immunohistochemistry (IHC) for SFRP112 or GAB113. Studies suggest that upregulation of CXCR4 and mutation of the key tumor suppressor, p53, represent distinct subgroups within SHH medulloblastomas.14,15 The 5-year OS of children > 3 years-old with SHH-activated medulloblastomas is 68%.9 Attempts to salvage those patients with progressive disease with a targeted SHH inhibitor have yielded mostly transient responses.16,17 This suggests a need for combinations of SHH- and other molecularly-targeted therapies to avoid treatment resistance.
The biology of Group 3 and 4 medulloblastomas is less-well understood. There are currently no viable targeted treatments for these medulloblastoma variants. MYC amplification18 and a MYC target gene expression signature19 constitute hallmark oncogenic features of Group 3 tumors, which contain a high percentage of large or anaplastic cells, and a dismal 39% 10-year OS.9 Both Group 3 and 4 medulloblastomas have an increased incidence of clinically-relevant, poor prognostic features, including chromosome i17q by cytogenetics and metastasis at diagnosis.13,20
WIP1, on chromosome 17q22-q23, inhibits p53 activity and functions as an oncogene when expressed at high levels along with oncogenes, including Myc.21-23 WIP1 amplification or overexpression has been described in multiple cancers that are wild-type for p53.21,23,24 We and others have described amplification and overexpression of WIP1 in 64% of human medulloblastomas.18,25,26 We recently reported increased WIP1 expression in Group 3 and 4 medulloblastomas.27
We now demonstrate increased WIP1 expression in metastatic medulloblastomas, and inferior survival in patients with WIP1 high-expressing medulloblastoma. Gene expression demonstrated up-regulation of CXCR4 in WIP1 high-expressing medulloblastomas. CXCR4 activation promoted AKT phosphorylation, increased growth, and invasion of WIP1-stable medulloblastoma cells in vitro and in mouse models. WIP1 or CXCR4 knock-down inhibited AKT activation, growth, and migration of WIP1 high-expressing medulloblastoma cells. WIP1 knock-down inhibited cell membrane localization of CXCR4 due to suppression of the G protein receptor kinase 5, GRK5. Restoration of wild-type GRK5 promoted Ser339 phosphorylation of CXCR4 and inhibited the growth of WIP1 stable cells. Conversely, GRK5 knock-down in cells with low WIP1 expression inhibited CXCR4 phosphorylation, increased cell membrane expression of CXCR4, and promoted medulloblastoma growth. This suggests an important cross-talk among WIP1, CXCR4, and GRK5, which promotes tumor growth and invasion, and which may be responsible for the aggressive behavior of WIP1 high-expressing medulloblastomas.
Results
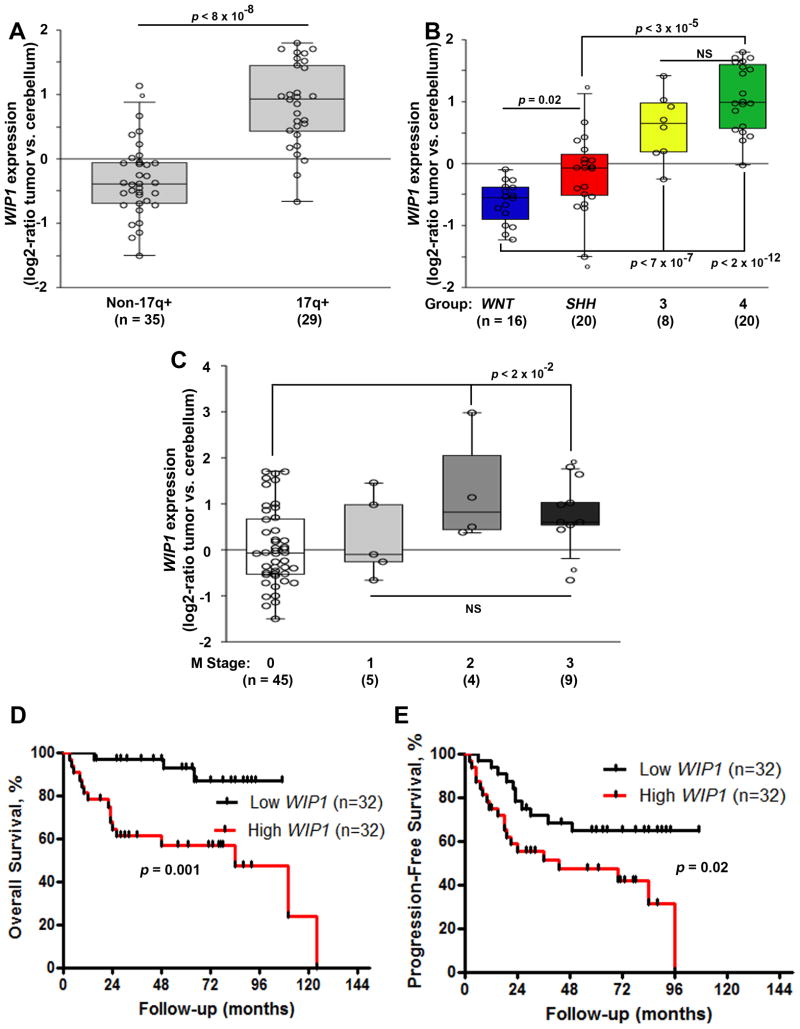
We validated increased WIP1 expression in a cohort of 64 medulloblastomas with gain of chromosome 17q, and in Group 3 and 4 medulloblastomas (Fig. 1A-B). Patient characteristics are shown in Table 1. We noted a significant association between high WIP1 expression and medulloblastomas classified as Chang stage M2-3, due to dissemination of medulloblastoma cells beyond the primary site (Fig. 1C). One patient did not have information available regarding Chang staging. Further analysis demonstrated inferior PFS and OS of patients with WIP1 high-expressing medulloblastomas (Fig. 1D-E).
Figure 1. High WIP1 expression in medulloblastoma is associated with adverse prognostic factors and inferior survival.
(A) WIP1 expression, based on gene expression profiling, in 64 pediatric medulloblastomas, with or without gain of chromosome 17q. Copy number status of chromosome 17q was determined using an Agilent-014850 Whole Human Genome Microarray 4×44K G4112F and array-based comparative genomic hybridization (CGH). (B) WIP1 expression among medulloblastoma subgroups. Subgroup affiliation was determined using unsupervised clustering approaches. (C) WIP1 expression, segregated by Chang M stage. R2 software was used to compare WIP1 expression according to 17q status, subgroup, and M stage. (D, E) Kaplan-Meier analysis of patient survival was based on median WIP1 expression. Survival was measured from diagnosis until death or last follow-up. Patient survival was analyzed according to the Kaplan-Meier method, using log-rank statistics. The median value, in panels A-C, is denoted by the middle line in each rectangle. Whiskers represent the bottom 10th and top 90th percentiles. The Y-axis denotes relative expression (log2-ratio tumor vs. cerebellum controls). NS, not significant.
Table 1. Patient characteristics.
| No. of patients | WIP1 High | WIP1 Low | |
|---|---|---|---|
| Age (years) | |||
| <3 | 3 | 2 | 1 |
| 3-18 | 37 | 20 | 17 |
| >18 | 24 | 10 | 14 |
| Gender | |||
| Male | 39 | 17 | 22 |
| Female | 25 | 15 | 10 |
| Chang stage | |||
| M0 | 45 | 18 | 28 |
| M1 | 5 | 2 | 3 |
| M2 | 4 | 4 | 0 |
| M3 | 9 | 8 | 1 |
| No information | 1 | 0 | 0 |
| Molecular subtype | |||
| WNT-activated | 16 | 0 | 16 |
| SHH-activated | 20 | 8 | 12 |
| Group 3 | 8 | 7 | 1 |
| Group 4 | 20 | 19 | 1 |
| 17q status | |||
| Copy number gain | 29 | 26 | 3 |
| Total | 64 |
WIP1 high versus low expression is based on median WIP1 expression. Age refers to age at diagnosis. Chang staging refers to published criteria for staging of medulloblastoma metastasis. Molecular subtyping was determined using unsupervised clustering approaches. Copy number status of 17q was determined using array-based comparative genomic hybridization. Abbreviations: WNT, wingless; SHH, sonic hedgehog; 17q, long arm of chromosome 17.
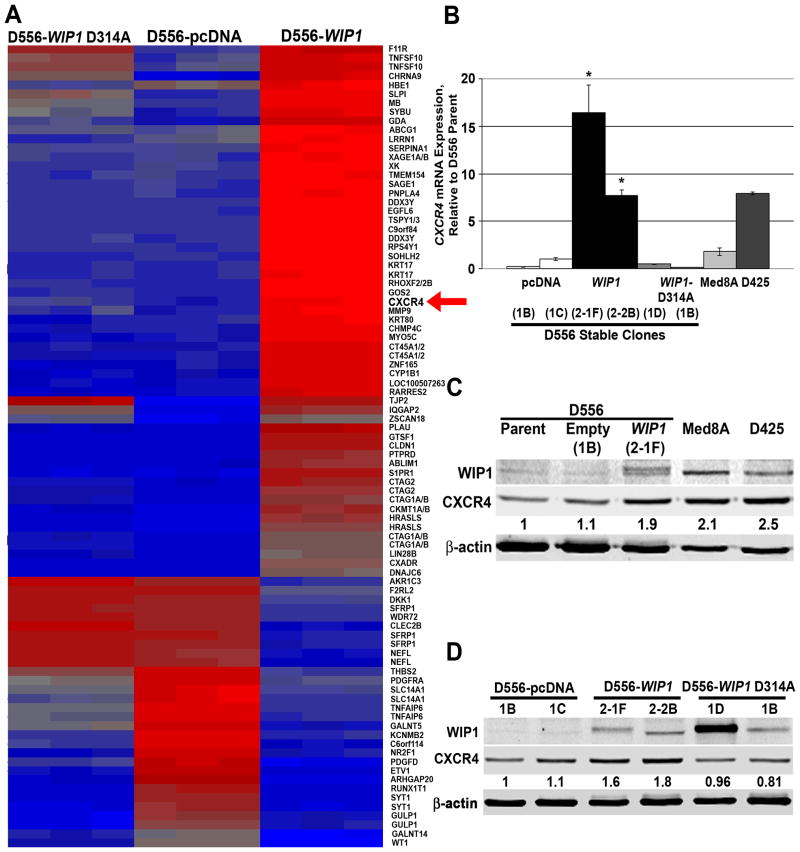
Since high MYC expression or amplification has been identified as a defining characteristic of Group 3 medulloblastomas8,18,19,28, we used MYC high-expressing D556, D425, and Med8A cells to model aggressive medulloblastoma variants.18,29 We have previously described high WIP1 expression in Group 3 and 4 human medulloblastomas, and in D425 and Med8A cells.27 In addition, we have shown that stable expression of WIP1 in D556 cells significantly enhances medulloblastoma growth.27 By Western blotting, we also found increased expression of the Group 3 and 4 markers, NPR3 and KCNA130, in D556-WIP1 stable clones (Supplementary Fig. S1).
Principal component analysis showed a clear separation in gene expression among D556 cells with stable expression of wild-type WIP1, empty vector, or mutant, phosphatase-deficient WIP1.27 Pathway analysis revealed up-regulation of genes involved in cell adhesion and migration in D556 WIP1-stable cells, including the G protein-coupled receptor CXCR4. Conversely, the gene for adenylate cyclase 1, ADCY1, was down-regulated in D556-WIP1 stable cells (Fig. 2A & S2). Others have shown that CXCR4 activation inhibits adenylate cyclase, which, in turn, reduces intracellular cAMP to promote growth of brain tumor cells.31 By real-time, RT-PCR, we confirmed increased CXCR4 in medulloblastoma cells with stable or endogenous high WIP1 expression (Fig. 2B). Western blotting and immunofluorescence (IF) confirmed increased CXCR4 protein expression in D556 WIP1-stable cells (Fig. 2C-D). Thus, high WIP1 expression correlated with up-regulation of CXCR4 in cell line models of aggressive medulloblastoma variants.
Figure 2. Association of WIP1 and CXCR4 expression in medulloblastoma cells.
(A) Heat map of gene expression in D556 cells with stable expression of empty vector (pcDNA3), WIP1 (D556-WIP1), or mutant, phosphatase-dead WIP1 (WIP1 D314A). Three clones from D556 stable cell lines were run in triplicate on an Affymetrix HG-U133 Plus 2.0 Array. Data were normalized using the Robust MultiChip Average algorithm. Differential gene analysis was performed using an ANOVA model, with an absolute fold change threshold of 6 and a p-value with an FDR threshold of 0.001. Up-regulated genes are shown in red (red arrow, CXCR4); down-regulated genes in blue. (B) CXCR4 mRNA expression by real-time, RT-PCR. Absolute gene expression was determined based on standard curves. Target gene expression was normalized to GAPDH expression. (C, D) Western blotting confirmed increased expression of CXCR4 protein in WIP1 high-expressing medulloblastoma cell lines. CXCR4 protein expression was quantified from near-infrared fluorescence and normalized to expression of β-actin, the loading control. The relative amount of CXCR4 (noted below each western blot for CXCR4) is expressed as a ratio of normalized CXCR4 expression in a particular lane, to normalized expression of CXCR4 in (C) D556 parental or (D) D556-pcDNA stable cells. Error bars, standard error of the mean. Experiments were repeated at least three times.
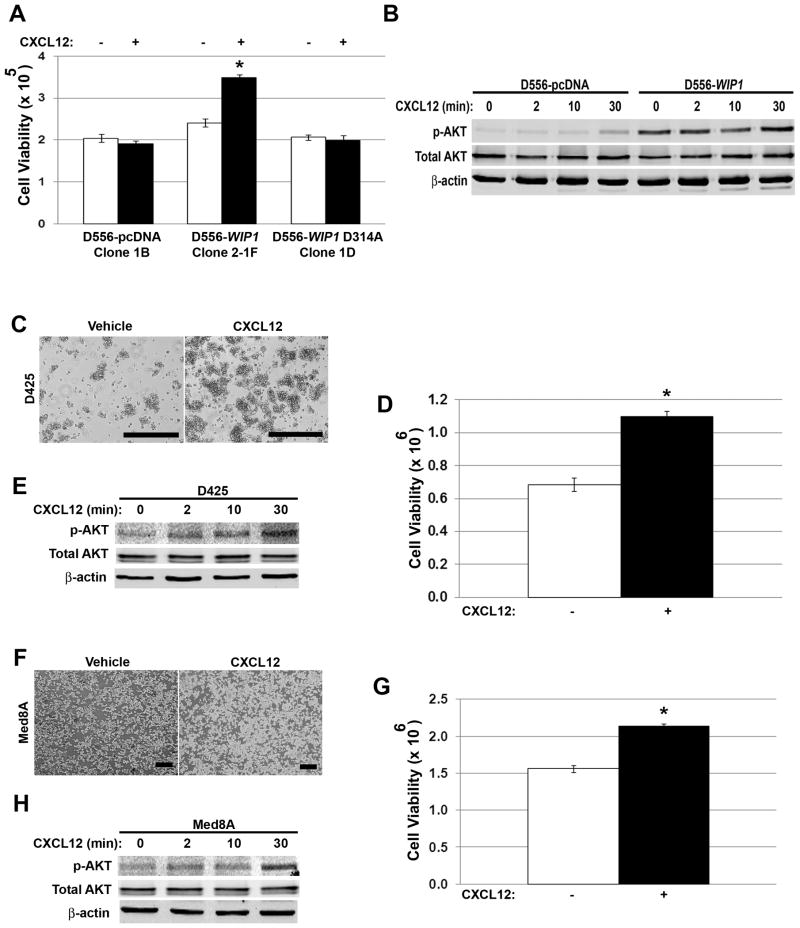
To determine the functional significance of increased CXCR4 expression, we serum-starved and stimulated D556-WIP1 clones with the CXCR4 ligand, CXCL12 (SDF1α). Only D556-WIP1 stable cells proliferated in response to SDF1α stimulation (Fig. 3A & S3A-B). Since SDF1α stimulation is known to activate downstream PI-3 kinase pathways, we examined phosphorylation of Ser473 on the PI-3 kinase target, AKT. Western blotting demonstrated increased baseline and SDF1α-stimulated phosphorylation of AKT in D556-WIP1 cells (Fig. 3B). Similarly, SDF1α increased Ser473 phosphorylation of AKT and increased growth of endogenous WIP1 high-expressing D425 (Fig. 3C-E & S3C) and Med8A (Fig. 3F-H & S3C) cells.
Figure 3. Stimulation with SDF1α activates PI-3 kinase signaling and promotes growth of WIP1 high-expressing medulloblastoma cells.
(A) Number of viable D556 stably-transfected cells by trypan blue exclusion (i.e. viability; Y-axis), 48 hours following serum starvation and stimulation with vehicle (-) or CXCL12 (+; SDF1α, 1 μg/mL). *, p < 0.0002. (B) Western blotting of whole cell lysates from (A) for Ser473-phosphorylated and total AKT. (C, F) Photomicrographs and (D, G) number of viable D425 and Med8A cells by trypan blue exclusion, respectively, 48 hours following serum starvation and SDF1α stimulation, as above. Scale bars, 400 μm. *, p < 0.0002. (E, H) Western blotting of whole cell lysates from (D, G) for serine 473-phosphorylated and total AKT. Error bars, standard deviation. Experiments were repeated at least three times.
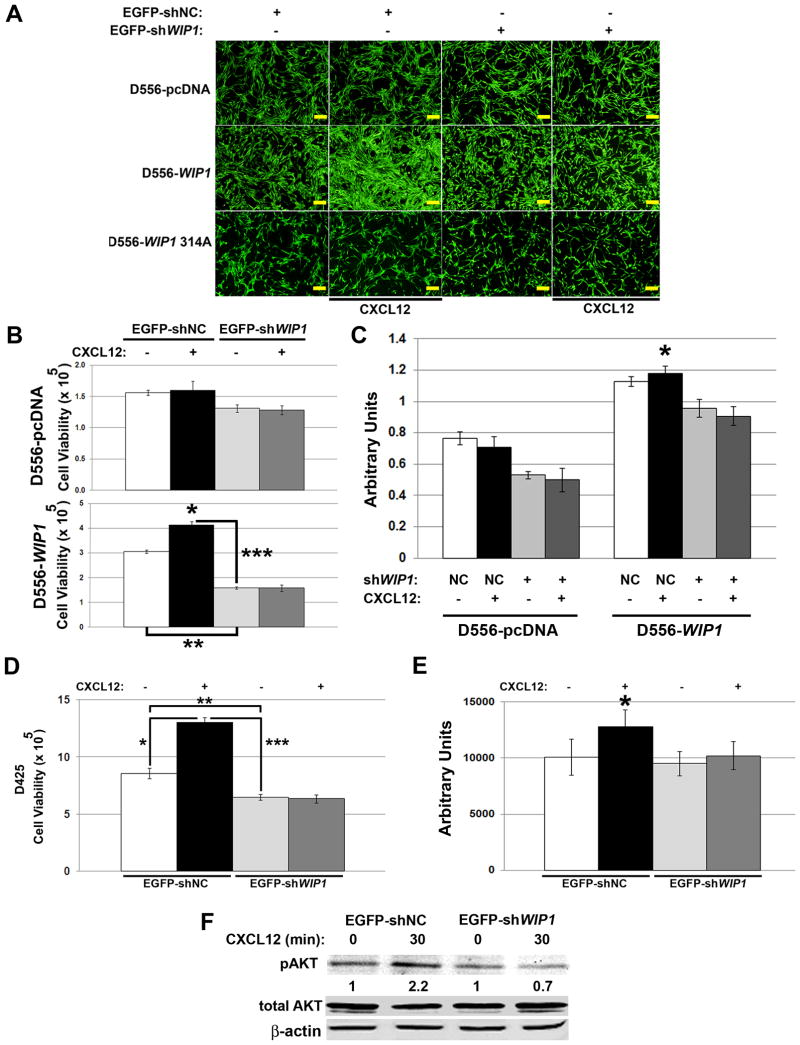
To examine the dependence of CXCR4 activity on WIP1, we transduced D556 and D425 cells with negative control or WIP1 shRNA. SDF1α stimulation enhanced the viability of D556-WIP1, but not D556-pcDNA or D556-WIP1 D314 stable cells in serum-free conditions (*, Fig. 4A-C). WIP1 knock-down inhibited the growth of D556-WIP1 stable cells by almost 50% (**, Fig. 4A-B). WIP1 knock-down also significantly inhibited the proliferative effect of SDF1α stimulation in D556-WIP1 stable cells (***, Fig. 4A-C). We observed similar effects in D425 cells, which have endogenous high WIP1 expression (Fig. 4D-E).
Figure 4. WIP1 knock-down suppresses SDF1α-stimulated growth of WIP1 high-expressing medulloblastoma cells.
(A) Representative photomicrographs of enhanced green fluorescent protein (EGFP) fluorescence, (B) number of viable cells by trypan blue exclusion (Y-axis), and (C) proliferation, as measured by absorbance following incubation with WST-1 reagent, of D556 stably-transfected cells in serum-free media 48 hours following infection with EGFP-tagged negative control (EGFP-shNC; NC) or WIP1 shRNA-encoding (EGFP-shWIP1) lentivirus, and stimulation with vehicle (-) or CXCL12 (+; SDF1α, 1 μg/mL) for 48 hours. Scale bars, 500 μm. *, p < 0.003; **, p < 0. 00001; ***, p < 0.00003. (D) Number of viable D425 cells by trypan blue exclusion and (E) proliferation, as measured by assayed by luminescence following incubation with CellTiter-Glo® reagent, following infection with EGFP-shNC or -shWIP1 lentivirus, and stimulation with vehicle or SDF1α for 48 hours. *, p < 0.0003; **, p < 0. 007; ***, p < 0.00004. (F) Western blotting of whole cell lysates from (D). Serine 473-phosphorylated AKT (pAKT) protein expression was quantified from near-infrared fluorescence and normalized to expression of β-actin, the loading control. The relative pAKT expression is shown as a ratio of normalized pAKT expression in SDF1α–stimulated to unstimulated D425 cells. Experiments were repeated at least twice.
Ser339-phosphorylation has previously been implicated in activation of CXCR4 and downstream activation of PI-3 kinase signaling.32 WIP1 knock-down did not change expression of total CXCR4. However, WIP1 knock-down increased Ser339 CXCR4 phosphorylation in the cytoplasmic fraction of D556 cells with stable expression of wild-type WIP1 (Supplementary Fig. S4A). WIP1 knock-down also increased Ser339 phosphorylation of CXCR4 (Supplementary Fig. S4B) and inhibited Ser473 phosphorylation of AKT in D425 cells (Fig. 4F). In two different D556-WIP1 stable clones, WIP1 knock-down inhibited membrane expression of CXCR4 (Supplementary Fig. S5). Thus, WIP1 knock-down altered CXCR4 localization, inhibited downstream phosphorylation of AKT, and prevented the growth-promoting effects of SDF1α in WIP1 high-expressing medulloblastoma cells.
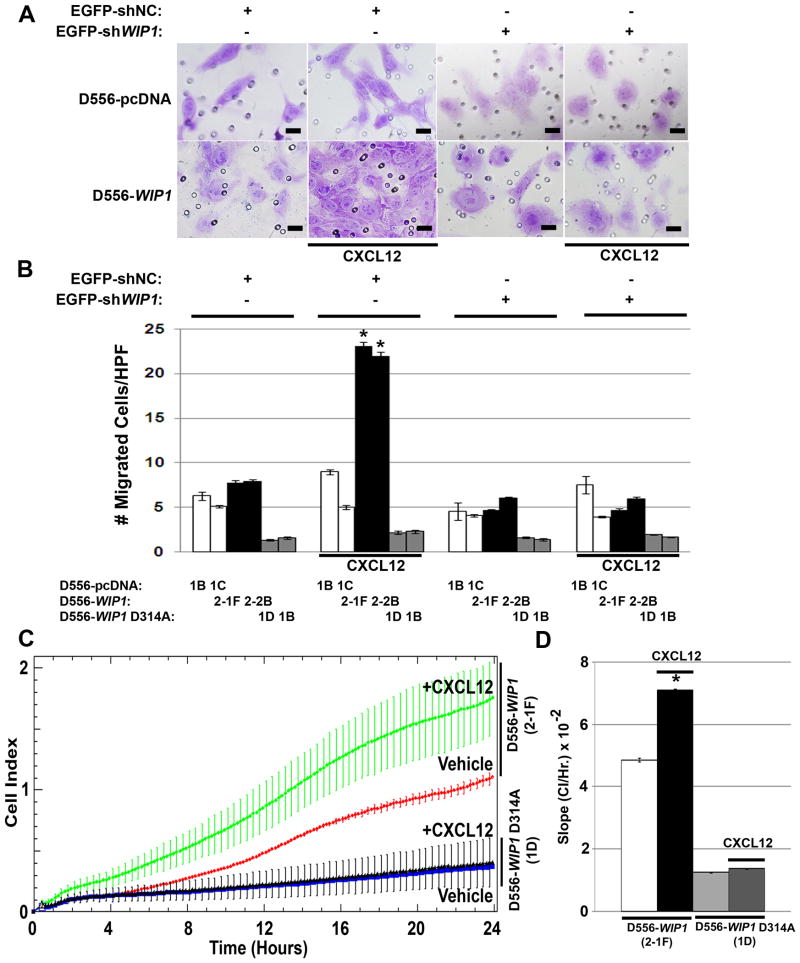
Since CXCR4 signaling has previously been implicated in invasion and metastasis, we examined invasion and migration of D556 stable clones in vitro. Neither D556-pcDNA nor D556-WIP1 D314A stable clones exhibited significant invasion. In contrast, D556-WIP1 stable clones demonstrated significant invasion through Boyden chambers that contain SDF1α as a chemo-attractant (*, Fig. 5A-B). WIP1 knock-down inhibited the ability of D556-WIP1 stable clones to invade through chambers exposed to SDF1α. We also monitored cell migration in real time. In the presence of SDF1α, D556-WIP1 stable cells demonstrated increased migration, as quantified by the rate of change of the cell index (CI)27. The CI slope was significantly higher in migration chambers containing D556-WIP1 cells and SDF1α (Fig. 5C-D). This suggests that high WIP1 expression facilitates medulloblastoma migration and invasion.
Figure 5. SDF1α stimulation promotes invasion and migration of D556-WIP1 stable clones.
(A) Representative photomicrographs of D556-pcDNA and D556-WIP1 stable clones that have migrated through a Boyden Chamber 48 hours following infection with WIP1 shRNA or empty-vector-containing lentiviral particles, and stimulation with vehicle or CXCL12 (SDF1α, 1 μg/mL). Scale bars, 50 μm. (B) Quantification of migrated D556 stable clones, as described in (A). Bar graphs represent the average number of migrated cells per high-power field (HPF) in triplicate measurements of 10 representative HPFs per treatment. Error bars, standard deviation. *, p < 5 × 10-22. (C) Invasion of D556-WIP1 or D556-WIP1 D314A cells through pores of a CIM plate, as measured by Cell Index (CI, arbitrary units), in response to stimulation with vehicle or SDF1α (1 μg/mL). Error bars denote SD among replicates. (D) Bar graphs represent the average change in CI per hour for invading D556-WIP1 or D556-WIP1 D314A cells stimulated with vehicle or SDF1α. Error bars denote SD among replicates of at least 3 per treatment and experiment. *, p < 0.005. Experiments in Panels A-B were repeated three times; those in Panels C-D were repeated twice.
Since most of the functions of WIP1 that have been described to date have required functional p53, we examined the p53 dependence of the interaction between WIP1 and CXCR4. As we have previously demonstrated, stimulation with SDF1α increased the number of viable D556-WIP1, but not D556-pcDNA, stable cells (Fig. 3). p53 knockdown did not affect the viability of unstimulated or SDF1α-stimulated D556-pcDNA cells (Supplementary Fig. S6A). However, p53 knockdown resulted in reduced serine 473 (Ser473) phosphorylation of AKT (Supplementary Fig. S6B) and inhibited the increase in viability of D556-WIP1 cells following SDF1α stimulation that was seen in D556-WIP1 cells transfected with scrambled, negative control siRNA (Supplementary Fig. S6A). We also examined p53 dependence of the interaction between WIP1 and CXCR4 on invasion using the previously characterized p53 mutant, WIP1 high-expressing Daoy cell line.27 Unlike D556-WIP1 stable clones, which demonstrated a significant increase in invasion compared to D556 cells with stable expression of an empty vector (Fig. 5), neither Daoy-pcDNA nor Daoy-WIP1 stable cells differed in the number of cells that invaded through a Matrigel Invasion Chamber when attracted with the chemokine SDF1α (Supplementary Fig. S7).
We further validated this p53 dependence by treating D556-pcDNA and D556-WIP1 stable cells with the small molecule inhibitors of p53 function, Nutlin-3a and RITA. Nutlin-3a is a cis-imidazoline analog that bind with high affinity to the endogenous p53 inhibitor HDM2. It inhibits the interaction between HDM2 and p53, which stabilizes p53 and promotes apoptosis or senescence of cancer cells that contain wild-type p53.33,34 The small molecule RITA binds with high affinity to the amino-terminal domain of p53.35 This results in activation of p53, transcriptional repression of anti-apoptotic proteins, such as Bcl-2 and survivin, and downregulation of oncogenic signaling pathways, including MYC and AKT.36,37 Treatment with Nutlin-3a or RITA did not affect the viability of unstimulated or SDF1α-stimulated D556-pcDNA cells (Supplementary Fig. S6C, E). However, treatment with Nutlin-3a resulted in reduced serine 473 (Ser473) phosphorylation of AKT (Supplementary Fig. S6D). Treatment with either Nutlin-3a or RITA also inhibited the increase in viability of D556-WIP1 cells following SDF1α stimulation that was seen in vehicle-treated D556-WIP1 cells (Supplementary Fig. S6C, E). Thus, either p53 knockdown, presence of mutant p53, or treatment with the small molecule p53-inhibiting drugs Nutlin-3a or RITA suppressed SDF1α-stimulated growth and migration of WIP1 high-expressing medulloblastoma cells. This suggests that the interaction between CXCR4 and WIP1 signaling is dependent on the presence of functional p53.
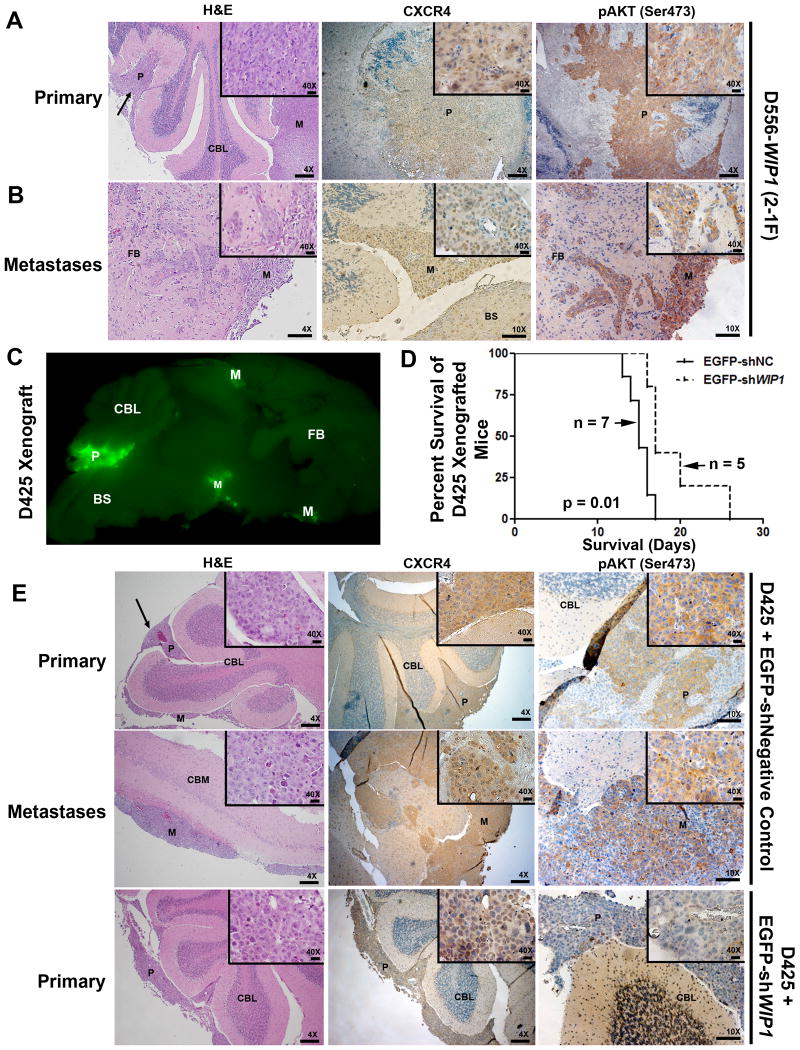
We have previously demonstrated increased tumor cell proliferation and reduced overall survival of mice bearing intra-cerebellar xenografts of D556-WIP1 stable cells, compared to mice bearing D556-pcDNA stable cells.27 By H&E staining, 7 of 9 D556-WIP1 xenografts showed evidence of local or distant brain metastasis (Fig. 6A). Neither parental (n = 2), nor D556-pcDNA (n = 9) xenografts, showed evidence of dissemination. By IHC, we identified increased cytoplasmic and membrane expression of CXCR4 and its downstream target, Ser473-phosphorylated AKT, in D556-WIP1 xenografted primary and metastatic cells (Fig. 6A-B).
Figure 6. Primary and metastatic tumors from WIP1-expressing medulloblastoma xenografts express CXCR4.
D556-WIP1 (5 × 105) or D425 (1 × 106) cells were infected with lentiviral particles (multiplicity of infection, MOI = 2) containing control or WIP1 shRNA. Twenty-four hours later, cells were harvested and injected into the cerebellum of SCID/Beige mice, 1mm posterior to the junction of the parietal and interparietal sutures, 1 mm lateral to midline, and at a 30° angle to the surface of the cerebellum, at a depth of 1 mm. Mice were sacrificed upon development of symptoms of medulloblastoma. Mouse brains were sectioned sagittally, fixed in 4% formalin, and paraffin embedded for pathological examination. (A) Representative hematoxylin and eosin (H&E)-stained primary and (B) metastatic tumors from intracerebellar xenografts of D556-WIP1 cells. IHC for CXCR4 and Ser473-phosphorylated AKT (pAKT) in primary and metastatic D556-WIP1 tumors (n = 4). (C) Representative GFP fluorescence, prior to fixation, and (D) Kaplan-Meier survival of EGFP-shNC or EGFP-shWIP1-infected D425 intracerebellar xenografted mice. (E) H&E and IHC of sagittally-sectioned mouse brains following orthotopic xenografting of EGFP-tagged lentivirus-infected D425 medulloblastoma cells. IHC for CXCR4 and pAKT in primary and metastatic D425 tumors (n = 4). BS, brain-stem; CBL, cerebellum; CBM, cerebrum; FB, forebrain; P, primary tumor; M, metastasis. Magnification 4×; Scale bars, 200 μm. Magnification 10×; Scale bars, 100 μm. Magnification 40×; Scale bars, 20 μm.
To confirm dissemination of xenografts, we examined 10 consecutive sagittal sections, with 10 mm spacing between sections, of each tumor for H&E staining and immunoreactivity against CXCR4 and phospho-AKT (Ser473). Phospho-AKT (Ser473) was expressed diffusely in all tumor cells and was moderate to strong in intensity in all D556-WIP1 xenografts (Table 2). At best, IHC for phospho-AKT (Ser473) was focal and weak (1+, <10%; Supplementary Fig. S8) in D556-pcDNA xenografts. In addition, D556-WIP1 xenografts had unequivocal evidence of invasion, almost exclusively as infiltration down the perivascular space (Supplementary Fig. S9). This is a pattern of tumor dissemination that is frequently observed in invasive human medulloblastomas. One D556-WIP1 xenograft had clear evidence of leptomeningeal metastasis out to the rostral cortex and olfactory bulb, which then invaded into the cortex along blood vessels. No such distant or local metastases were present in D556-pcDNA xenografted tumors.
Table 2. D556 Medulloblastoma Xenograft IHC for Phospho-AKT (Ser473).
| Xenografts | Phospho-AKT (Ser473) | Phospho-AKT (Ser473) | Distant Mets | Local Mets | Invasion |
|---|---|---|---|---|---|
| D556-pcDNA (1B) | Intensity | Extent | |||
| #1 | 1+ | 5-10% | No | No | Yes |
| #2 | 1+ | 5-10% | No | No | No |
| #3 | 0 | 0% | No | No | No |
| #4 | 0 | 0% | No | No | No |
| D556-WIP1 (2-1F) | |||||
| #1 | 2-3+ | Diffuse | No | Yes | Yes |
| #2 | 2-3+ | Diffuse | No | Yes | Yes |
| #3 | 2-3+ | Diffuse | Yes | Yes | Yes |
| #4 | 2+ | Diffuse | No | Yes | Yes |
We next confirmed this finding using intracerebellar xenografts of endogenous WIP1 high-expressing D425 cells, infected with control or WIP1 shRNA. Fluorescence microscopy of fresh sagittal brain sections from symptomatic animals xenografted with D425 cells infected with control lentivirus demonstrated GFP fluorescence both in the cerebellum, at the site of xenografting, and along the leptomeninges (Fig. 6C). A similar pattern of dissemination is often seen by MRI in children with metastatic medulloblastoma. As with D556-WIP1 stable xenografts, mice xenografted with D425 cells developed primary and metastatic tumors, both of which demonstrated positive cytoplasmic staining for CXCR4 and Ser473-phosphorylated AKT (Fig. 6E). In contrast, mice xenografted with WIP1 shRNA-infected D425 cells demonstrated significantly improved survival (Fig. 6D). Interestingly, tumors from shWIP1-infected D425 cells exhibited staining for CXCR4 that was predominantly nuclear. And, staining for Ser473-phosphorylated AKT was virtually absent in these tumors (Fig. 6E). These observations support our in vitro findings of increased CXCR4 and PI-3 kinase signaling in WIP1 high-expressing medulloblastomas. In fact, Wu et al. recently showed that PI-3 kinase signaling is a crucial driver of leptomeningeal dissemination of SHH medulloblastomas.38
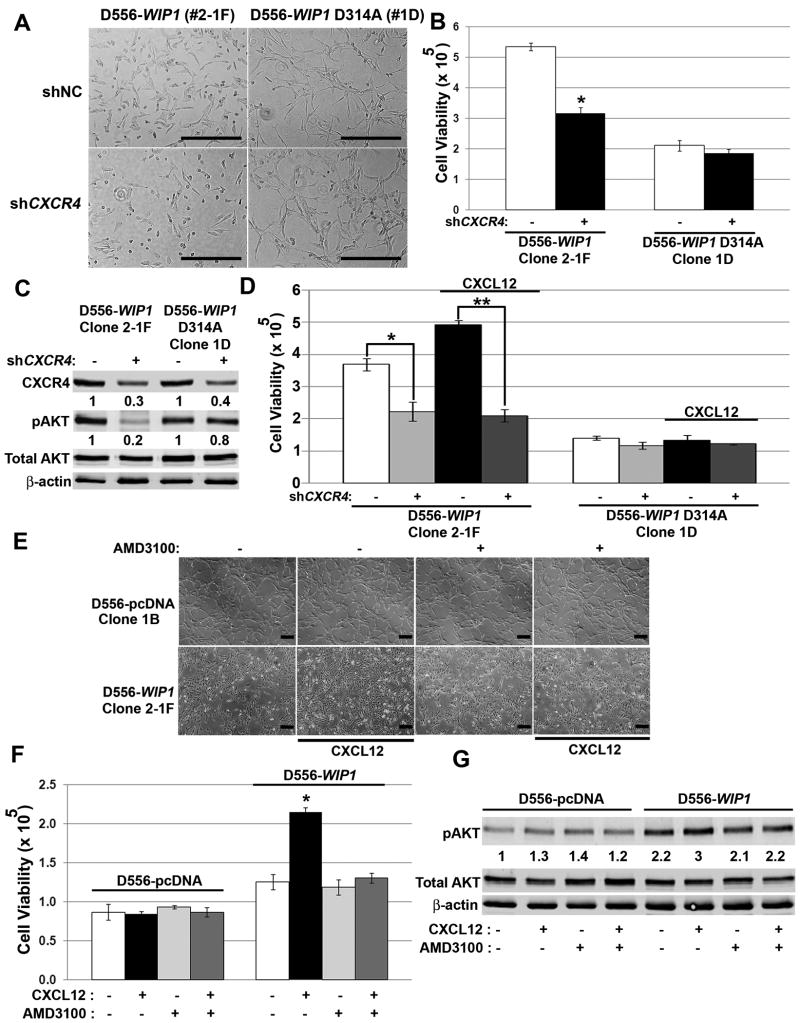
To confirm the interaction between WIP1 and CXCR4, we knocked-down CXCR4. Infection of medulloblastoma cells with CXCR4 shRNA resulted in a 70% reduction in CXCR4 expression (Fig. 7C) and a 40% reduction in viable D556-WIP1 cells (Fig. 7A-B). In serum-free conditions, CXCR4 knock-down inhibited the growth of SDF1α-stimulated D556-WIP1 cells by 60% (**, Fig. 7D). CXCR4 knock-down did not affect the viability of D556 cells with stable expression of a phosphatase-dead WIP1. By Western blotting, CXCR4 knock-down resulted in an 80% reduction in Ser473 phosphorylation of AKT in D556-WIP1, but not in WIP1 mutant D556-WIP1 D314A stable cells (Fig. 7C).
Figure 7. CXCR4 inhibition suppresses the growth of WIP1 high-expressing medulloblastoma cells.
(A) Representative photomicrographs and (B) number of viable D556-WIP1 and D556-WIP1 D314A cells (Y-axis) in serum-containing media, by trypan blue exclusion, 72 hours following lentiviral-mediated CXCR4 knock-down. *, p < 0.0005. (C) Western blotting of whole cell lysates from (B) for CXCR4, Ser473 phosphorylated-, and total AKT, 72 hours following lentiviral-mediated CXCR4 knock-down. CXCR4 and Ser473-phosphorylated AKT (pAKT) protein expression was quantified from near-infrared fluorescence and normalized to expression of β-actin. The relative amount of protein in either cell clone is expressed as a ratio of normalized CXCR4 or pAKT, to normalized expression following infection with control shRNA-containing lentivirus. (D) Number of viable cells (Y-axis) in serum-free media 72 hours following lentiviral-mediated knock-down of CXCR4, and stimulation with either vehicle or CXCL12 (SDF1α, 1 μg/mL). *, p < 0.005; **, p < 0.0001. (E) Representative photomicrographs and (F) number of viable (Y-axis) D556-WIP1 and D556-pcDNA cells in serum-free media 72 hours following stimulation with vehicle or CXCL12, and treatment with vehicle or 20 μM AMD3100. *, p < 0.0007. (G) Western blotting of whole cell lysates, as described in (F). Expression is quantified from near-infrared fluorescence, relative to expression in CXCL12-unstimulated, AMD3100-untreated D556-pcDNA cells, and normalized to β-actin. Scale bars, 400 μm. Error bars, standard deviation. Experiments were repeated at least three times.
We validated the results of CXCR4 knock-down using the small molecule CXCR4 inhibitor, AMD3100. Alone, AMD3100 did not affect cell viability. However, similar to the effects of CXCR4 knock-down, AMD3100 prevented the effects of SDF1α stimulation on growth of D556-WIP1 stable cells. (Fig. 7E-F). Western blotting confirmed inhibition of Ser473 AKT phosphorylation in D556-WIP1 cells treated with AM3100 and stimulated with SDF1α (Fig. 7G).
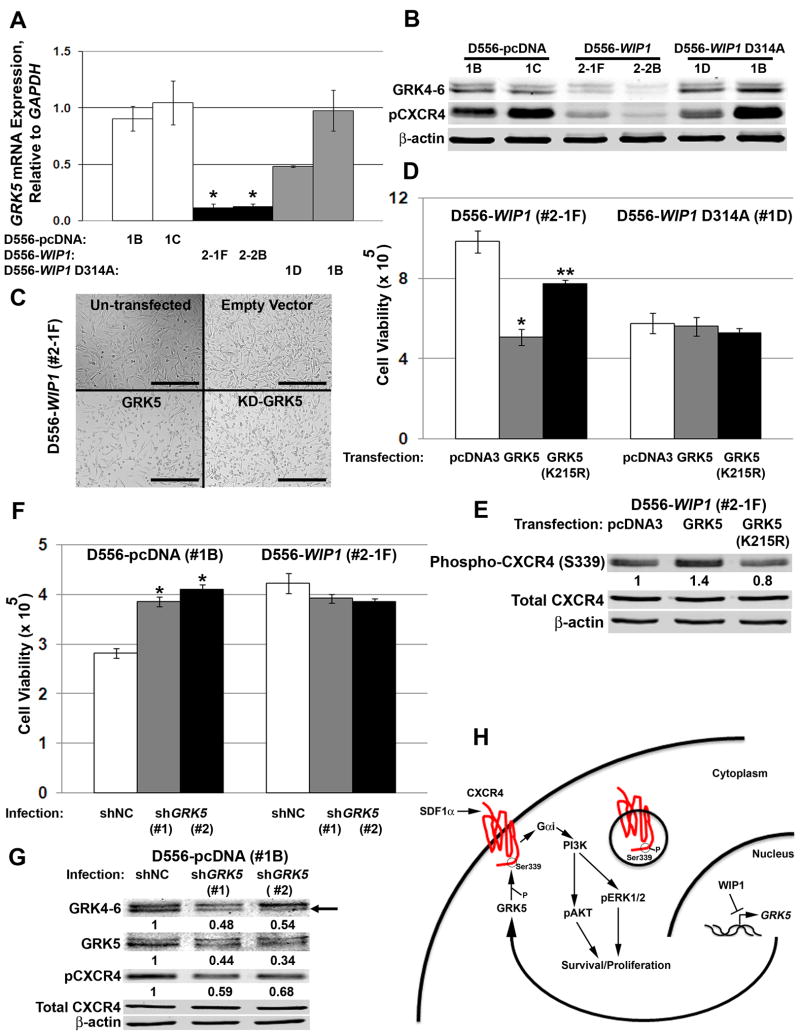
Since WIP1 knock-down failed to alter total CXCR4 expression, but did affect CXCR4 localization in vitro as well as in intra-cerebellar xenografts, we examined the role of G-protein receptor kinases in trafficking of CXCR4. Others have shown that the G-protein receptor kinase 5, GRK5, promotes CXCR4 phosphorylation and internalization.39 Our analysis suggests a high degree of co-regulated expression of WIP1 and GRK5 in our cohort of human medulloblastomas (R = 0.44, p < 0.005). Our microarray data demonstrated significant down-regulation of GRK5 in D556-WIP1 stable clones. By real-time, RT-PCR and Western blotting, we validated significantly lower levels of GRK5 mRNA and protein in D556-WIP1stable clones (Fig. 8A-B). Reduced GRK5 levels corresponded with reduced Ser339-phosphorylated CXCR4 in D556-WIP1 stable clones (Fig. 8B). To study the functional implications of altered GRK5 expression, we transfected expression plasmids containing an empty vector, wild-type, or kinase-dead GRK5 (GRK5 K215R) into D556-WIP1 or D556-WIP1 D314A stable cells. Neither wild-type nor kinase-dead GRK5 had a significant effect on the growth of D556 cells that stably express phosphatase-dead WIP1. However, transfection with wild-type GRK5 reduced the number of viable D556-WIP1 stable cells by 50% (Fig. 8C-D). Transfection of kinase-dead GRK5 also inhibited the growth of D556-WIP1 stable cells, but to a lesser degree. Western blotting revealed an increase in Ser339 CXCR4 phosphorylation, in GRK5-transfected D556-WIP1 cells (Fig. 8E). There was no difference in Ser339-phosphorylated CXCR4 in D556-WIP1 D314A stable cells following transfection with empty vector, wild-type, or kinase-dead GRK5. Moreover, GRK5 transfection rescued D556-WIP1 cells from the effects of GRK5 knock down. GRK5 knock down reduced Ser339 phosphorylation of CXCR4 in D556-WIP1 stable cells transfected wild-type GRK5, and increased the number of viable D556-WIP1 stable cells to near baseline levels (Supplementary Fig. S10). Thus, GRK5 may suppress the growth of WIP1 high-expressing medulloblastoma cells by promoting Ser339 phosphorylation of CXCR4.
Figure 8. WIP1 promotes medulloblastoma growth by inhibiting Ser339 phosphorylation of CXCR4 through suppression of GRK5.
(A) Real-time, RT-PCR for GRK5, relative to GAPDH, in D556 stable clones. *, p < 0.0005. Error bars, standard error of the mean. (B) Western blotting of whole cell lysates for GRK5, Ser339-phosphorylated CXCR4, and β-actin. The GRK4-6 antibody is specific for GRK4, GRK5, and GRK6. (C) Representative photomicrographs and (D) number of viable cells (Y-axis) by trypan blue exclusion following transfection with empty vector (pcDNA3), wild-type, or kinase-dead GRK5 (KD-GRK5, GRK5 K215R). *, p < 0.0005; **, p < 0.02. Error bars, standard deviation (SD). (E) Western blotting of whole cell lysates following transfection, as in (D). Ser339-phosphorylated CXCR4 protein expression was quantified from near-infrared fluorescence and normalized to expression of β-actin. The relative amount of protein is expressed as a ratio of normalized Ser339-phosphorylated CXCR4, to normalized expression following transfection with an empty vector control (pcDNA3). (F) Number of viable cells (Y-axis), 48 hours following infection with negative control pLKO.1 or GRK5 shRNA-containing lentivirus. *, p < 0.0003. Error bars, SD. (G) Western blotting of whole cell lysates, as in (F). GRK5 is the lower band (black arrow). Protein expression was normalized to expression of β-actin, and shown relative to expression following transduction with an empty vector-containing shRNA lentivirus (shNC). (H) Model: GRK5 promotes Ser339 phosphorylation and internalization of CXCR4. WIP1 suppresses GRK5 expression, which in turn permits membrane localization of CXCR4 and medulloblastoma growth and invasion in response to SDF1α stimulation.
We also examined the role of CXCR4 phosphorylation at serine 339 in D425 medulloblastoma cells, which have high endogenous WIP1 expression. The HA-tagged CXCR4-S339A lentivirus expresses a mutant CXCR4 in which serine has been mutated to alanine at amino acid 339. This results in a form of CXCR4 that is resistant to phosphorylation at serine 339. Infection of D425 cells with HA-tagged CXCR4-S339A lentivirus inhibited cell viability and responsiveness to SDF1α stimulation. Co-infection of D425 cells with WIP1 shRNA and HA-tagged CXCR4-S339A further suppressed cell viability and also inhibited responsiveness to SDF1α stimulation (Supplementary Fig. S11). This suggests that phosphorylation of serine 339 on CXCR4 is important for cell viability. We have shown that activation of CXCR4 induces signaling AKT, which promotes cell growth. What is not clear from this experiment is the localization of mutant CXCR4-S339A protein. It is possible that the mutant protein is not being expressed at the plasma membrane, which would explain the reduced cell viability and lack of response to SDF1α stimulation.
To determine if GRK5 knock-down could recapitulate the proliferative effects of increased WIP1 expression, we infected D556-pcDNA and D556-WIP1 stable cells with empty vector, negative control or GRK5 shRNA-encoding lentivirus. GRK5 knock-down resulted in a 50% reduction in expression of GRK5 mRNA or protein (Fig. 8G & S12), and a corresponding increase in the viability of empty vector-containing D556 cells (Fig. 8F). GRK5 knock-down did not significantly affect the viability of D556-WIP1 stable cells. Western blotting revealed reduced Ser339 phosphorylation of CXCR4 in shGRK5-infected D556-pcDNA cells (Fig. 8G). Immunofluorescence validated increased CXCR4 expression and membrane localization following GRK5 knock-down in D556-pcDNA stable cells (Supplementary Fig. S13). This suggests that GRK5 promotes internalization of CXCR4. Conversely, WIP1 suppresses GRK5 expression, which in turn permits membrane localization of CXCR4 and medulloblastoma growth, and invasion in response to SDF1α stimulation (Fig. 8H). Thus, strategies that inhibit CXCR4 or promote GRK5 expression may be useful, especially in the treatment of WIP1 high-expressing medulloblastomas.
Discussion
A number of retrospective studies suggest a prognostic relevance for genes on chromosome 17q in medulloblastoma.40-43 Group 3 and 4 medulloblastomas exhibit an increased frequency of chromosome 17 alterations and increased expression of WIP1.9,12,20 In a multivariate analysis, either loss of chromosome 17p or gain of 17q was prognostic of poor OS.9 One publication suggests a role for the chromosome 17q gene LIM and SH3 protein 1 (LASP1) in medulloblastoma metastasis.44 We have now identified an association between high WIP1 expression and inferior PFS and OS in medulloblastoma. We have also identified significantly higher WIP1 expression in metastatic Chang-stage M2-3 medulloblastomas.
Metastatic disease at diagnosis has long been considered a poor prognostic factor in medulloblastoma. Recent studies report an overall frequency of medulloblastoma metastasis (M+) of 24%. M+ disease is least common in WNT-activated (0-18%) and most common in Group 3 (30-75%) and 4 (30-31%) tumors.9,12,20 Kaplan-Meier analysis demonstrates significantly worse OS in children > 3 years of age and in group 4 tumors with M+ disease.9 Sadly, the mechanisms that drive medulloblastoma invasion and metastasis remain poorly understood.
WIP1, located on chromosome 17q22-23, is a PP2C-like serine/threonine protein phosphatase that is a direct target of the tumor suppressor, p53.45 It, in turn, regulates the cell cycle through p53-dependent mechanisms. WIP1 directly dephosphorylates and inactivates p53 at Ser15. It also inactivates important regulators of p53 function, dephosphorylating p38MAPK at Thr18046 and MDM2 at Ser395.47 Upstream of p53, WIP1 inactivates an early step in DNA-damage response by dephosphorylating the ataxia-telangiectasia mutated (ATM) kinase on Ser1981.48 WIP1 also binds and dephosphorylates the serine/threonine kinase, Chk1, at Ser345.49 And, it inactivates Chk2 at Thr68.50,51 p53-independent WIP1 regulatory roles are not as well characterized, but WIP1 is thought to play an important role, independent of p53, in the maintenance of mouse intestinal stem cells52 and, with hedgehog signaling, in the growth of human cancer cell lines.53
Wip1 is best understood for its important role in DNA damage response and tumorigenesis. While evidence from mouse models suggests that Wip1 overexpression alone is insufficient for cancer formation, Wip1 can cooperate with other “classic” oncogenes to transform mouse embryo fibroblasts.54 WIP1 amplification or overexpression has been described in multiple cancers that are wild-type for p53.21,23,24 Recent publications have reported activating mutations in the C-terminus of WIP1 in some cancers.55-57 However, to date, there have been no reports implicating WIP1 in cancer invasion or metastasis.
G protein-coupled receptors have been implicated both in brain development and cancer metastasis.58 CXCR4 and its ligand, SDF1α, are required for appropriate cerebellar development.59 CXCR4 is also the most common chemokine receptor expressed on cancer cells, and promotes cancer growth and metastasis.60 Other groups have shown that increased CXCR4 expression in primary CNS malignancies, including medulloblastoma, is associated with inferior survival.15,31,61,62 Sengupta et al. reported high CXCR4 expression in infant and adult medulloblastomas with classic or desmoplastic histology. SHH activation promoted cell surface accumulation of CXCR4, increased downstream signaling, and cell growth in culture.15
Using human cell line models of aggressive medulloblastoma variants, with increased expression of MYC and either stable or endogenous high WIP1 expression, we identified increased expression of CXCR4 and activation of downstream targets of PI-3 kinase signaling in response to SDF1α stimulation. We observed increased growth and invasion of WIP1-stable medulloblastoma cells in vitro and in orthotopic, xenografted mouse models. WIP1 high-expressing xenografts demonstrated invasion as well as local and distant metastases, which stained strongly for CXCR4 and Ser473-phosphorylated AKT. Conversely, WIP1 or CXCR4 knock-down inhibited the effects of SDF1α on PI-3 kinase signaling, growth, and migration. SCID mice xenografted with shWIP1-infected D425 cells demonstrated increased survival. This suggests an important interaction between WIP1 and CXCR4 which promotes growth and dissemination of aggressive medulloblastoma variants.
G protein-coupled receptors (GPCRs) are regulated by desensitization, internalization, and degradation. This process is initiated by G protein-coupled receptor kinases (GRKs), which phosphorylate serine or threonine residues in the cytoplasmic tail following GPCR activation.63 Woerner et al. showed that SDF1α stimulation promotes Ser339 phosphorylation of CXCR4 in human astrocytomas.32 Others have shown that mutation of Ser338 and Ser339 results in reduced SDF1α-stimulated phosphorylation of these sites.64 GRK5 phosphorylation of Hsp70 interacting protein promotes internalization of CXCR4.39 We found that D556-WIP1 cells had reduced levels of GRK5. Transfection of GRK5 into D556-WIP1 cells increased Ser339 phosphorylation of CXCR4 and reduced proliferation. Conversely, knock-down of GRK5 resulted in reduced Ser339 phosphorylation of CXCR4 and increased proliferation of D556 containing an empty vector. Thus, modulation of GRK5 expression is an important mechanism through which WIP1 alters phosphorylation and function of CXCR4 to promote medulloblastoma growth and dissemination.
Using mouse models, Wu et al. recently demonstrated mechanisms of metastasis by inserting the Sleeping Beauty (SB) transposon into Patched +/- mice. Dysregulation of Shh signaling in granule neuron precursor (GNP) cells leads to medulloblastoma that is localized to the cerebellum in 15-39% of Patched +/- mice.38,65,66 Expression of SB transposase from the Math1 promoter resulted in 97% of Patched +/- mice with metastatic medulloblastoma by 10 weeks of age. Interestingly, PI-3 kinase signaling was a significant driver of leptomeningeal dissemination in Shh-activated, Patched +/-/Math1-SB11/T2Onc mouse medulloblastomas.38
Mumert et al. used the RCAS/tv-a system in cerebellar neural progenitor cells to validate candidate genes (Eras, Lhx1, Ccrk, and Akt) identified by Wu et al. The incidence of spinal metastases was most significant in mice with co-expression of Shh and either Ccrk or Akt.67 It is possible that Eras, Lhx1, Ccrk, and Akt are of greatest significance in SHH-activated human medulloblastomas while genes on chromosome 17q, such as WIP1, have a more significant role in dissemination of non-SHH-activated medulloblastomas.
Materials and Methods
Materials
SDF1α (CXCL12) (PeproTech, Rocky Hill, NJ) was prepared in H2O and used at 0.1-1 μg/mL. AMD3100 (Sigma-Aldrich, St. Louis, MO) was prepared in H2O and diluted in media to 20 μM. Nutlin 3A/B (Cayman Chemical Company, Ann Arbor, MI) was prepared in EtOH at a stock concentration of 3200μM and used at a concentration of 8μM (4μM effective). RITA (Selleck Chemicals, Houston, TX) was prepared in DMSO at a stock concentration of 50μM and was diluted in media to 25nM for experiments.
Cell Culture
D556, Med8A, and D425 cell lines, and D556 stable clones were derived and maintained as previously described.27
Proliferation Assays
2 × 104 cells were plated in 96-well plates, serum-starved, and cultured in phenol red-free MEM media (Mediatech, Inc., Manassas, VA). For knockdown, cells were infected with control or shWIP1 lentivirus during starvation, and treated with vehicle or 1μg/mL SDF1α. 48 hours post-treatment, WST-1 (Roche Applied Science, Indianapolis, IN) was added at a 1:100 dilution, or an equal volume of Cell Titer-Glo (Promega, Madison, WI). For WST-1 assays, plates were analyzed using a Synergy MX plate reader (wavelength-450nm/reference-620nm; BioTek, Winooski, VT). For Cell Titer-Glo assays, luminescence was measured using a 1 second integration time. Alternatively, the number of viable cells in an experiment was counted by trypan blue exclusion, using standard methods.
Gene Expression Microarray Analysis
Patients were partitioned using median WIP1 expression as a cutoff. Survival was measured from diagnosis until death or last follow-up. Patient survival was analyzed according to the Kaplan-Meier method, using log-rank statistics. Copy number status of chromosome 17q was determined using array-based comparative genomic hybridization (CGH), as previously published. Gene expression profiling data were performed and analyzed as previously described.28,68 Using R2 software (http://r2.amc.nl), we compared WIP1 expression patterns according to 17q status, subgroup, and M-stage, as previously described.28,68 Subgroup affiliation was determined using unsupervised clustering approaches.28,68
Quantitative Real-Time, RT-PCR
Total cellular RNA was extracted, as previously described.27 Quantitative real-time, RT-PCR reactions containing cDNA, Syber Green PCR Master Mix (Life Technologies, Grand Island, NY) and primers for human WIP1, CXCR4, GRK5, and/or Glyceraldehyde-3 Phosphate Dehydrogenase (GAPDH) were run on an ABI 7500 Real-Time PCR Cycler (Life Technologies), using absolute quantification with a standard curve. Primer sequences are available upon request. Amplification products were verified by analysis of melting curves. Serial cDNA dilutions were used to determine standard curves for each primer. Analysis of housekeeping gene expression was included with each run. Absolute gene expression was determined based on standard curves. Target gene expression was normalized to GAPDH expression.
Western Blotting
Protein was extracted, quantified, and probed with antibody, as previously described.69 Whole cell lysates were extracted using RIPA buffer (Cell Signaling). Nuclear and cytoplasmic lysates were extracted using the NE-PER Nuclear and Cytoplasmic Extraction Kit, according to the manufacturer (Thermo Scientific). Antibodies included AKT (Cell Signaling, Danvers, MA), phospho-AKT (S473) (Cell Signaling), CXCR4 (Abcam, Cambridge, MA), phospho-CXCR4 (S339) (Abcam), eEF2 (Cell Signaling), GRK4-6 (EMD Millipore, Billerica, MA), GRK5 (C-20) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), KCNA1 (Abcam), Lamin B1 (Cell Signaling), NPR3 (Abcam), WIP1 (Bethyl, Montgomery, TX), and β-actin (Sigma-Aldrich, St. Louis, MO). Secondary antibodies Alexa Fluor 680 goat anti-mouse IgG (Life Technologies) or IRDye 800 goat anti-rabbit IgG (Rockland, Gilbertsville, PA) were used at a dilution of 1:5,000. Immunoblots were imaged and quantified, as previously described.27
Immunofluorescence
Cells were plated at 3.5 × 104 on Nunc Lab Tek Chamber Slides (Nalge Nunc, Rochester, NY), fixed with 4% paraformaldehyde, incubated with 50 mM NH4Cl, and permeabilized with 0.3% Triton-X. To quench endogenous peroxidases, cells were incubated in 0.3% H2O2 in MeOH. Cells were subsequently incubated with 10% normal goat serum (Invitrogen) containing 0.3% Triton-X, blocked with 5% BSA, and incubated overnight at 4°C with primary antibody. Cells were then washed with TNT (1 mM Tris/HCl, 5 mM NaCl, 1%Tween-20) and incubated with goat, anti-rabbit Alexa Fluor 555 (Invitrogen) secondary antibody. Slides were mounted using Hard Set Mounting Media with DAPI (Vectashield, Vector Laboratories, Burlingame, CA). Images were acquired with an EVOS-fl fluorescent microscope (Advanced Microscopy Group, Bothell, WA).
SDF1α Stimulation
Cells were plated at 1 × 105 - 1×106 in 6 well plates and either stimulated with 100 ng/mL SDF1α (PeproTech) for 0, 2, 10 and 30 minutes, or with 1 μg/mL SDF1α for 48 hours in serum-free media.
Invasion Chamber Assays
Media containing 1 μg/mL SDF1α or vehicle was added to each well in a Matrigel Invasion Chamber (BD Biosciences, San Jose, CA), followed by placement of inserts. 2.5 × 104 cells were added to each insert and infected with lentivirus in the presence of 8 μg/μl polybrene. Inserts were fixed in 100% methanol and stained with crystal violet.
Invasion Assays using xCelligence
Media containing vehicle or 1 μg/mL SDF1α was placed in the lower chambers of a CIM plate-16 (Roche Applied Science, Indianapolis, IN). 2 ×104 cells were seeded in the upper chamber of a CIM plate and monitored for real-time changes in cell index (CI). Error bars represent standard deviation. Slope of the CI was computed using RTCA Software.
Short Hairpin RNA Lentivirus Production and Infection
EGFP-tagged negative control and shWIP1 lentiviral expression constructs were gifts from Dr. Lawrence Donehower (Baylor College of Medicine).27 psPAX2 and pVSVG plasmids were gifts from Dr. H. Trent Spencer (Emory University). The pLKO.1 empty vector control plasmid was a gift from Dr. Rita Nahta (Emory University). shCXCR4 and shGRK5 lentiviral expression constructs were purchased (Thermo Scientific). Production and infection with lentiviral particles were as previously described.27
CXCR4 Inhibition Assays
1× 105 cells were plated in 6 well plates, serum starved for 24 hours, and treated with vehicle, 100 ng/mL SDF1α, and/or 20 μM AMD3100. 48 hours post-treatment, cells were counted by trypan blue exclusion.
GRK5 Transfection
5 × 105 cells were plated in 6 well plates and, 24 hours later, were transfected with 1μg of pcDNA3, GRK5, or K215R GRK5 plasmids (gifts from Dr. Jeffrey Benovic, Jefferson University) using Lipofectamine 2000 (Invitrogen), according to the manufacturer.
Mouse Handling
All mice were housed in an American Association of Laboratory Animal Care–accredited facility and were maintained in accordance with NIH guidelines. All animal care and experiments were approved by the Institutional Animal Care and Use Committee of Emory University (Protocol # DAR-2002073).
Xenografting of Medulloblastoma Cells
SCID mice were anesthetized and prepared, as described.27 Mice were followed for symptoms and tissues processed, as described.27
Tissue Handling and Immunohistochemistry
The brains of mice were excised in total, examined under a fluorescence stereomicroscope for evidence of GFP expression, sectioned sagittally down the midline, photographed for expression of GFP, and fixed in formalin. Tissue blocks were paraffin-embedded and cut into 5 mm sections. Antigen retrieval was performed by heating slides in sodium citrate for 20 minutes. After blocking endogenous peroxidases, slides were incubated with primary antibody. Secondary antibodies were applied according to the manufacturer (Vector Laboratories). Slides were stained with hematoxylin and mounted using VectaMount permanent mounting media (Vector Laboratories).
Statistical Analysis
Unless stated otherwise, all bar graphs display mean values of triplicate measurements. Error bars denote standard deviation (SD) among replicates. And, experiments were repeated at least three times with reproducible results. Results were analyzed using a two-tailed Student's t-test or one-way ANOVA in Microsoft Excel or Graphpad Prism 4 software to assess statistical significance. Values of p < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Drs. Jeffrey Benovic, Lawrence Donehower, H. Trent Spencer, Laurent Brault, Jürg Schwaller, and Rita Nahta for gene expression constructs, Gregory Doho for assistance with analysis of microarrays, and Dr. Rita Nahta for editorial assistance. This work was supported by grants from the NIH (1R01CA172392-01, R.C.C.; CA159859, M.D. Taylor), St. Baldrick's Foundation (R.C.C.), CURE Childhood Cancer Foundation (R.C.C.), Southeastern Brain Tumor Foundation (R.C.C.), the Emory Egleston Children's Research Center (R.C.C.), and the Dr. Mildred-Scheel Foundation (M.R.).
Footnotes
Conflict of Interest: Dr. Castellino's and Dr. Taylor's work has been funded by the NIH. Other authors declare no conflict of interest.
References
- 1.McNeil DE, Cote TR, Clegg L, Rorke LB. Incidence and trends in pediatric malignancies medulloblastoma/primitive neuroectodermal tumor: a SEER update. Surveillance Epidemiology and End Results. Med Pediatr Oncol. 2002;39:190–194. doi: 10.1002/mpo.10121. [DOI] [PubMed] [Google Scholar]
- 2.Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 3.Dhall G. Medulloblastoma. J Child Neurol. 2009;24:1418–1430. doi: 10.1177/0883073809341668. [DOI] [PubMed] [Google Scholar]
- 4.von Hoff K, Hinkes B, Gerber NU, Deinlein F, Mittler U, Urban C, et al. Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT'91. Eur J Cancer. 2009;45:1209–1217. doi: 10.1016/j.ejca.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Mabbott DJ, Spiegler BJ, Greenberg ML, Rutka JT, Hyder DJ, Bouffet E. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23:2256–2263. doi: 10.1200/JCO.2005.01.158. [DOI] [PubMed] [Google Scholar]
- 6.Belza MG, Donaldson SS, Steinberg GK, Cox RS, Cogen PH. Medulloblastoma: freedom from relapse longer than 8 years--a therapeutic cure? J Neurosurg. 1991;75:575–582. doi: 10.3171/jns.1991.75.4.0575. [DOI] [PubMed] [Google Scholar]
- 7.Torres CF, Rebsamen S, Silber JH, Sutton LN, Bilaniuk LT, Zimmerman RA, et al. Surveillance scanning of children with medulloblastoma. N Engl J Med. 1994;330:892–895. doi: 10.1056/NEJM199403313301303. [DOI] [PubMed] [Google Scholar]
- 8.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123:473–484. doi: 10.1007/s00401-012-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pugh TJ, Weeraratne SD, Archer TC, Pomeranz Krummel DA, Auclair D, Bochicchio J, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488:106–110. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellison DW, Kocak M, Dalton J, Megahed H, Lusher ME, Ryan SL, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29:1400–1407. doi: 10.1200/JCO.2010.30.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C, Neale G, et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121:381–396. doi: 10.1007/s00401-011-0800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gessi M, von Bueren AO, Rutkowski S, Pietsch T. p53 expression predicts dismal outcome for medulloblastoma patients with metastatic disease. J Neurooncol. 2011;106:135–141. doi: 10.1007/s11060-011-0648-8. [DOI] [PubMed] [Google Scholar]
- 15.Sengupta R, Dubuc A, Ward S, Yang L, Northcott P, Woerner BM, et al. CXCR4 activation defines a new subgroup of Sonic hedgehog-driven medulloblastoma. Cancer Res. 2012;72:122–132. doi: 10.1158/0008-5472.CAN-11-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–574. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gajjar AJ, Stewart CF, Ellison DW, Curran T, Phillips P, Goldman S, et al. A phase I pharmacokinetic trial of sonic hedgehog (SHH) antagonist GDC-0449 in pediatric patients with recurrent of refractory medulloblastoma: A Pediatric Brain Tumor Consortium study (PBTC 25) J Clin Oncol. 2010;28(suppl; abstr CRA9501):18s. [Google Scholar]
- 18.Northcott PA, Shih DJ, Peacock J, Garzia L, Morrissy AS, Zichner T, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488:49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29:1424–1430. doi: 10.1200/JCO.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS ONE. 2008;3:e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bulavin DV, Demidov ON, Saito S, Kauraniemi P, Phillips C, Amundson SA, et al. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet. 2002;31:210–215. doi: 10.1038/ng894. [DOI] [PubMed] [Google Scholar]
- 22.Nannenga B, Lu X, Dumble M, Van Maanen M, Nguyen TA, Sutton R, et al. Augmented cancer resistance and DNA damage response phenotypes in PPM1D null mice. Mol Carcinog. 2006;45:594–604. doi: 10.1002/mc.20195. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Yang Y, Peng Y, Austin RJ, van Eyndhoven WG, Nguyen KC, et al. Oncogenic properties of PPM1D located within a breast cancer amplification epicenter at 17q23. Nat Genet. 2002;31:133–134. doi: 10.1038/ng888. [DOI] [PubMed] [Google Scholar]
- 24.Saito-Ohara F, Imoto I, Inoue J, Hosoi H, Nakagawara A, Sugimoto T, et al. PPM1D is a potential target for 17q gain in neuroblastoma. Cancer Res. 2003;63:1876–1883. [PubMed] [Google Scholar]
- 25.Castellino RC, De Bortoli M, Lu X, Moon SH, Nguyen TA, Shepard MA, et al. Medulloblastomas overexpress the p53-inactivating oncogene WIP1/PPM1D. J Neurooncol. 2008;86:245–256. doi: 10.1007/s11060-007-9470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendrzyk F, Radlwimmer B, Joos S, Kokocinski F, Benner A, Stange DE, et al. Genomic and protein expression profiling identifies CDK6 as novel independent prognostic marker in medulloblastoma. J Clin Oncol. 2005;23:8853–8862. doi: 10.1200/JCO.2005.02.8589. [DOI] [PubMed] [Google Scholar]
- 27.Buss MC, Read TA, Schniederjan MJ, Gandhi K, Castellino RC. HDM2 promotes WIP1-mediated medulloblastoma growth. Neuro Oncol. 2012;14:440–458. doi: 10.1093/neuonc/nos001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remke M, Hielscher T, Northcott PA, Witt H, Ryzhova M, Wittmann A, et al. Adult medulloblastoma comprises three major molecular variants. J Clin Oncol. 2011;29:2717–2723. doi: 10.1200/JCO.2011.34.9373. [DOI] [PubMed] [Google Scholar]
- 29.Siu IM, Lal A, Blankenship JR, Aldosari N, Riggins GJ. c-Myc promoter activation in medulloblastoma. Cancer Res. 2003;63:4773–4776. [PubMed] [Google Scholar]
- 30.Pfister S. Medulloblastoma: a potpourri of distinct entities. Acta Neuropathol. 2012;123:463–464. doi: 10.1007/s00401-012-0966-8. [DOI] [PubMed] [Google Scholar]
- 31.Rubin JB, Kung AL, Klein RS, Chan JA, Sun Y, Schmidt K, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A. 2003;100:13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woerner BM, Warrington NM, Kung AL, Perry A, Rubin JB. Widespread CXCR4 activation in astrocytomas revealed by phospho-CXCR4-specific antibodies. Cancer Res. 2005;65:11392–11399. doi: 10.1158/0008-5472.CAN-05-0847. [DOI] [PubMed] [Google Scholar]
- 33.Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 34.Shangary S, Wang S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol. 2009;49:223–241. doi: 10.1146/annurev.pharmtox.48.113006.094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Issaeva N, Bozko P, Enge M, et al. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med. 2004;10:1321–1328. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- 36.Grinkevich VV, Nikulenkov F, Shi Y, et al. Ablation of key oncogenic pathways by RITA-reactivated p53 is required for efficient apoptosis. Cancer Cell. 2009;15:441–453. doi: 10.1016/j.ccr.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Sun Y. Targeting p53 for Novel Anticancer Therapy. Transl Oncol. 2010;3:1–12. doi: 10.1593/tlo.09250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu X, Northcott PA, Dubuc A, Dupuy AJ, Shih DJ, Witt H, et al. Clonal selection drives genetic divergence of metastatic medulloblastoma. Nature. 2012;482:529–533. doi: 10.1038/nature10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barker BL, Benovic JL. G protein-coupled receptor kinase 5 phosphorylation of hip regulates internalization of the chemokine receptor CXCR4. Biochemistry. 2011;50:6933–6941. doi: 10.1021/bi2005202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfister S, Remke M, Benner A, Mendrzyk F, Toedt G, Felsberg J, et al. Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J Clin Oncol. 2009;27:1627–1636. doi: 10.1200/JCO.2008.17.9432. [DOI] [PubMed] [Google Scholar]
- 41.Clifford SC, Lusher ME, Lindsey JC, Langdon JA, Gilbertson RJ, Straughton D, et al. Wnt/Wingless pathway activation and chromosome 6 loss characterize a distinct molecular sub-group of medulloblastomas associated with a favorable prognosis. Cell Cycle. 2006;5:2666–2670. doi: 10.4161/cc.5.22.3446. [DOI] [PubMed] [Google Scholar]
- 42.Lamont JM, McManamy CS, Pearson AD, Clifford SC, Ellison DW. Combined histopathological and molecular cytogenetic stratification of medulloblastoma patients. Clin Cancer Res. 2004;10:5482–5493. doi: 10.1158/1078-0432.CCR-03-0721. [DOI] [PubMed] [Google Scholar]
- 43.Pan E, Pellarin M, Holmes E, Smirnov I, Misra A, Eberhart CG, et al. Isochromosome 17q is a negative prognostic factor in poor-risk childhood medulloblastoma patients. Clin Cancer Res. 2005;11:4733–4740. doi: 10.1158/1078-0432.CCR-04-0465. [DOI] [PubMed] [Google Scholar]
- 44.Traenka C, Remke M, Korshunov A, Bender S, Hielscher T, Northcott PA, et al. Role of LIM and SH3 protein 1 (LASP1) in the metastatic dissemination of medulloblastoma. Cancer Res. 2010;70:8003–8014. doi: 10.1158/0008-5472.CAN-10-0592. [DOI] [PubMed] [Google Scholar]
- 45.Fiscella M, Zhang H, Fan S, Sakaguchi K, Shen S, Mercer WE, et al. Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner. Proc Natl Acad Sci U S A. 1997;94:6048–6053. doi: 10.1073/pnas.94.12.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takekawa M, Adachi M, Nakahata A, Nakayama I, Itoh F, Tsukuda H, et al. p53-inducible wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. Embo J. 2000;19:6517–526. doi: 10.1093/emboj/19.23.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu X, Ma O, Nguyen TA, Jones SN, Oren M, Donehower LA. The Wip1 Phosphatase acts as a gatekeeper in the p53-Mdm2 autoregulatory loop. Cancer Cell. 2007;12:342–354. doi: 10.1016/j.ccr.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 48.Shreeram S, Demidov ON, Hee WK, Yamaguchi H, Onishi N, Kek C, et al. Wip1 phosphatase modulates ATM-dependent signaling pathways. Mol Cell. 2006;23:757–764. doi: 10.1016/j.molcel.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Lu X, Nannenga B, Donehower LA. PPM1D dephosphorylates Chk1 and p53 and abrogates cell cycle checkpoints. Genes Dev. 2005;19:1162–1174. doi: 10.1101/gad.1291305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujimoto H, Onishi N, Kato N, Takekawa M, Xu XZ, Kosugi A, et al. Regulation of the antioncogenic Chk2 kinase by the oncogenic Wip1 phosphatase. Cell Death Differ. 2006;13:1170–1180. doi: 10.1038/sj.cdd.4401801. [DOI] [PubMed] [Google Scholar]
- 51.Yoda A, Xu XZ, Onishi N, Toyoshima K, Fujimoto H, Kato N, et al. Intrinsic kinase activity and SQ/TQ domain of Chk2 kinase as well as N-terminal domain of Wip1 phosphatase are required for regulation of Chk2 by Wip1. J Biol Chem. 2006;281:24847–24862. doi: 10.1074/jbc.M600403200. [DOI] [PubMed] [Google Scholar]
- 52.Demidov ON, Timofeev O, Lwin HN, Kek C, Appella E, Bulavin DV. Wip1 phosphatase regulates p53-dependent apoptosis of stem cells and tumorigenesis in the mouse intestine. Cell Stem Cell. 2007;1:180–190. doi: 10.1016/j.stem.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 53.Pandolfi S, Montagnani V, Penachioni JY, Vinci MC, Olivito B, Borgognoni L, et al. WIP1 phosphatase modulates the Hedgehog signaling by enhancing GLI1 function. Oncogene. 2012 doi: 10.1038/onc.2012.502. e-pub ahead of print 12 November 2012. [DOI] [PubMed] [Google Scholar]
- 54.Demidov ON, Kek C, Shreeram S, Timofeev O, Fornace AJ, Appella E, et al. The role of the MKK6/p38 MAPK pathway in Wip1-dependent regulation of ErbB2-driven mammary gland tumorigenesis. Oncogene. 2007;26:2502–2506. doi: 10.1038/sj.onc.1210032. [DOI] [PubMed] [Google Scholar]
- 55.Kleiblova P, Shaltiel IA, Benada J, Sevcik J, Pechackova S, Pohlreich P, et al. Gain-of-function mutations of PPM1D/Wip1 impair the p53-dependent G1 checkpoint. J Cell Biol. 2013;201:511–521. doi: 10.1083/jcb.201210031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dudgeon C, Shreeram S, Tanoue K, Mazur SJ, Sayadi A, Robinson RC, et al. Genetic variants and mutations of PPM1D control the response to DNA damage. Cell Cycle. 2013;12:2656–2664. doi: 10.4161/cc.25694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruark E, Snape K, Humburg P, Loveday C, Bajrami I, Brough R, et al. Mosaic PPM1D mutations are associated with predisposition to breast and ovarian cancer. Nature. 2013;493:406–410. doi: 10.1038/nature11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 59.Klein RS, Rubin JB, Gibson HD, DeHaan EN, Alvarez-Hernandez X, Segal RA, et al. SDF-1 alpha induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development. 2001;128:1971–1981. doi: 10.1242/dev.128.11.1971. [DOI] [PubMed] [Google Scholar]
- 60.Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EG, et al. A review on CXCR4/CXCL12 axis in oncology: No place to hide. Eur J Cancer. 2013;49:219–230. doi: 10.1016/j.ejca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 61.Bian XW, Yang SX, Chen JH, Ping YF, Zhou XD, Wang QL, et al. Preferential expression of chemokine receptor CXCR4 by highly malignant human gliomas and its association with poor patient survival. Neurosurgery. 2007;61:570–578. doi: 10.1227/01.NEU.0000290905.53685.A2. discussion 578-579. [DOI] [PubMed] [Google Scholar]
- 62.Calatozzolo C, Maderna E, Pollo B, Gelati M, Marras C, Silvani A, et al. Prognostic value of CXCL12 expression in 40 low-grade oligodendrogliomas and oligoastrocytomas. Cancer Biol Ther. 2006;5:827–832. doi: 10.4161/cbt.5.7.2838. [DOI] [PubMed] [Google Scholar]
- 63.Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- 64.Orsini MJ, Parent JL, Mundell SJ, Marchese A, Benovic JL. Trafficking of the HIV coreceptor CXCR4. Role of arrestins and identification of residues in the c-terminal tail that mediate receptor internalization. J Biol Chem. 1999;274:31076–31086. doi: 10.1074/jbc.274.43.31076. [DOI] [PubMed] [Google Scholar]
- 65.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 66.Rao G, Pedone CA, Coffin CM, Holland EC, Fults DW. c-Myc enhances sonic hedgehog-induced medulloblastoma formation from nestin-expressing neural progenitors in mice. Neoplasia. 2003;5:198–204. doi: 10.1016/S1476-5586(03)80052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mumert M, Dubuc A, Wu X, Northcott PA, Chin SS, Pedone CA, et al. Functional genomics identifies drivers of medulloblastoma dissemination. Cancer Res. 2012;72:4944–4953. doi: 10.1158/0008-5472.CAN-12-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Remke M, Hielscher T, Korshunov A, Northcott PA, Bender S, Kool M, et al. FSTL5 is a marker of poor prognosis in non-WNT/non-SHH medulloblastoma. J Clin Oncol. 2011;29:3852–3861. doi: 10.1200/JCO.2011.36.2798. [DOI] [PubMed] [Google Scholar]
- 69.Castellino RC, De Bortoli M, Lin LL, Skapura DG, Rajan JA, Adesina AM, et al. Overexpressed TP73 induces apoptosis in medulloblastoma. BMC Cancer. 2007;7:127. doi: 10.1186/1471-2407-7-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.