Abstract
BACKGROUND
Previous results from our lab indicate a tumor suppressor role for the transmembrane protein with epidermal growth factor and two follistatin motifs 2 (TMEFF2) in prostate cancer (PCa). Here, we further characterize this role and uncover new functions for TMEFF2 in cancer and adult prostate regeneration.
METHODS
The role of TMEFF2 was examined in PCa cells using Matrigel™ cultures and allograft models of PCa cells. In addition, we developed a transgenic mouse model that expresses TMEFF2 from a prostate specific promoter. Anatomical, histological and metabolic characterizations of the transgenic mouse prostate were conducted. The effect of TMEFF2 in prostate regeneration was studied by analyzing branching morphogenesis in the TMEFF2-expressing mouse lobes and alterations in branching morphogenesis were correlated with the metabolomic profiles of the mouse lobes. The role of TMEFF2 in prostate tumorigenesis in whole animals was investigated by crossing the TMEFF2 transgenic mice with the TRAMP mouse model of PCa and analyzing the histopathological changes in the progeny.
RESULTS
Ectopic expression of TMEFF2 impairs growth of PCa cells in Matrigel or allograft models. Surprisingly, while TMEFF2 expression in the TRAMP mouse did not have a significant effect on the glandular prostate epithelial lesions, the double TRAMP/TMEFF2 transgenic mice displayed an increased incidence of neuroendocrine type tumors. In addition, TMEFF2 promoted increased branching specifically in the dorsal lobe of the prostate suggesting a potential role in developmental processes. These results correlated with data indicating an alteration in the metabolic profile of the dorsal lobe of the transgenic TMEFF2 mice.
CONCLUSIONS
Collectively, our results confirm the tumor suppressor role of TMEFF2 and suggest that ectopic expression of TMEFF2 in mouse prostate leads to additional lobe-specific effects in prostate regeneration and tumorigenesis. This points to a complex and multifunctional role for TMEFF2 during PCa progression.
Keywords: TMEFF2, transgenic mouse, branching morphogenesis, neuroendocrine tumors, prostate cancer
INTRODUCTION
TMEFF2 is an evolutionarily conserved type I transmembrane protein expressed in the embryo (1,2) and selectively in the adult brain and prostate (3–5). TMEFF1, the only other member of the family, is also expressed in the embryo (6) and predominantly in the adult brain (1,7) but not prostate. The biological function of the TMEFF proteins remains unclear, although there are indications for their involvement in development and tumorigenesis. In fact, the domains in the TMEFF proteins and their expression in the embryo suggest a role in development. In support of this role, TMEFF1 can inhibit nodal and BMP signaling in Xenopus oocytes (8,9), and it is upregulated in the blastema during amphibian limb regeneration (7). TMEFF2 is one of the highest expressed genes in oocytes from primordial follicules (10), and although mice with a null allele of TMEFF2 do not demonstrate major structural abnormalities, TMEFF2 knockout pups exhibit retarded growth and die at weaning apparently from an impaired ability to feed, suggesting possible neurological defects (11,12).
As it is often the case for proteins with roles in developmental processes, the TMEFF proteins have also been implicated in oncogenic transformation. Gery et al. (13) proposed a tumor suppressor role for TMEFF1 based on the observation that its expression is downregulated in 96% of brain tumor samples relative to normal brain tissue, and that TMEFF1 overexpression in glioblastoma U118 cells had a cell growth inhibitory effect. Studies of TMEFF2 in cancer suggest that it can function as both a tumor suppressor and promoter. TMEFF2 is up-regulated in a significant fraction of primary and metastatic prostate tumors (3,5) and in leiomyomas (14), while it is downregulated in gliomas and gastric cancers (15–17). In addition, it’s promoter has been shown to be hypermethylated in several other cancers, also suggesting a tumor suppressor role ((15,18–22); and references therein). Data obtained using cell lines or mouse xenograft support the dual function of TMEFF2 in cancer in vitro and in vivo. While ectopic expression or addition of the purified extracellular domain of TMEFF2 promotes neuronal cell survival (23) and growth of several cell lines (24,25), ectopic expression of full length TMEFF2 protein demonstrates anti-proliferative effects in the same cell lines (4,25,26), and suppresses tumor growth in vivo in nude mouse xenografts (26). In addition, we have described that TMEFF2 modulates cellular invasion of benign prostate epithelial and cancer cells and that this function correlates with its ability to modulate one-carbon metabolism (25,27,28).
The transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse was developed by expressing the SV40 virus T/t antigens (Tag) under control of the androgen-dependent and prostate specific minimal rat probasin promoter. The Tag protein binds and inactivates the tumor suppressor proteins TRP53 and RB1; therefore, similar to human prostate cancer, the TRAMP mouse develops heterogeneous tumors that may contain regions of atypical hyperplasia, neuroendocrine differentiation and/or phylloides-like lesions (29,30). The spectrum of lesions in the TRAMP mouse underscores its utility to the study of PCa. In addition, several cell lines have been established from the primary tumor of TRAMP mice as a tool to complement studies in the mouse. Interestingly, however, the cell lines do not appear to express the SV40 T/t antigens (31).
To get further insights into the role of TMEFF2 in vivo, several studies were undertaken using TRAMP mouse derived cells, a newly generated prostate specific TMEFF2 transgenic mouse and a TRAMP/TMEFF2 double transgenic animal. The TMEFF2 transgenic mouse manifested no overt defects, however we observed increased ductal branching in the dorsal prostatic lobe that correlated with distinct metabolic alterations in response to TMEFF2. In addition, we demonstrated that while TMEFF2 overexpression inhibits ex vivo growth and allograft formation of TRAMP-C2 cells confirming its tumor suppressor role, expression of TMEFF2 in a double TMEFF2/TRAMP mouse did not prevent atypical hyperplasia normally observed in the TRAMP mouse. Surprisingly, however, we observed an increased incidence of neuroendocrine lesions in the double transgenic animals when compared to the TRAMP only mice. These results suggest that TMEFF2 modulates prostate regeneration after castration, and hint to a multifaceted role in tumorigenesis that encompasses a previously recognized tumor suppressor role and a complex effect on the neuroendocrine neoplasms.
MATERIALS AND METHODS
Cell culture and expression constructs
The TRAMPC2 cell line was purchased from American Type Culture Collection (ATCC, Manassas, VA) and cultured in DMEM supplemented with 0.005 mg/ml bovine insulin and 10 nM dehydroisoandrosterone, 90%; fetal bovine serum, 5%; Nu-Serum IV, 5% as recommended. For matrigel growth, cells were trypsinized, mixed 1:1 with culture medium containing 4% growth factor reduced Matrigel (BD Biosciences, San Jose, CA) and added to the wells of a 96-well plate coated with 35 μl of growth factor reduced Matrigel at low concentration (4 mg/ml) per well as described by Debnath et al. (32). Culture medium containing 2% Matrigel was replaced every 3 days. TMEFF2 full-length expression construct was as previously described (25). Development of a system for inducible expression of TMEFF2 (TMEFF2i) in TRAMPC2 cells was achieved using the Clontech’s Tet-On Advanced system as described before for other cell lines (25). To inducibly express TMEFF2, cultures were grown in the presence of doxycycline (250 ng/ml; SIGMA, St. Louis, MO). The probasin-TMEFF2 expression construct was generated by inserting the human full length TMEFF2 cDNA into the pTg1 vector downstream from the ARR2PB minimal rat probasin promoter and intervened by an intron sequence.
Transgenic Animal Studies
Animals were bred and maintained in accordance with the Institutional Animal Care and Use Committee (IACUC) of East Carolina University (ECU), where most of these studies were conducted, or at Oklahoma University Health Sciences (OUHSC) where the studies were completed. Mice were kept with ad libitum access to chow and water. Transgenic TMEFF2 mice were generated by injection of the transgene DNA into the pronuclei of C57BL/6 X DBA2 hybrid embryos (UNC Lineberger Cancer Center transgenic facility). Founder animals containing the transgene were identified by PCR genotyping of tail genomic DNA using the Terra PCR direct kit (Clontech Laboratories, Mountain View, CA) and the following primers: (sense 5′-CAGGGCACTACAGTTCAGACA-3′) or (sense 5′-GGAATTGCTCTGGTTATGATG-3′) and (antisense 5′-GGAATTGCTCTGGTTATGATG-3′). Founders were backcrossed to C57BL/6 mice for at least 7 generations before they were used for the studies. TRAMP mice were purchased from the Jackson laboratory (Bar Harbor, ME; stock number 008215) and screened for the presence of the SV40 large T-antigen by PCR, as detailed on the company website. TRAMP mice (FVB background) were crossed to C57Bl/6J (stock number 000664). The F1 derived from this cross, was then bred with the transgenic TMEFF2 mouse, and TRAMP/TMEFF2(+/+) mice and TRAMP/TMEFF2(+/−) male progeny were selected after genotyping.
Castrations were performed by scrotal incision and cauterization of the vas deferens and blood vessels. When specified, 25 mg 5α-dihydrotestosterone (5α-DHT) pellets (21 day release; Innovative Research of America, Sarasota, FL) were implanted subcutaneously in the lateral side of the neck, where there is maximal space between the skin and the muscle. Animals were sacrificed 7 or 20 days after pellet implantation, and the prostates were dissected, weighted and paraffin embedded for hematoxilin & eosin (H&E) staining and immunohistochemistry (IHC). When specified, 5α-DHT (SIGMA, St. Louis, MO) injections (50 mg/kg in sesame oil) were given subcutaneously instead of pellet implantation. The injections were administered every other day for 8 days, before sacrificing the animal for lobe microdissection and branching morphogenesis studies.
Tissue collections, histology and immunohistochemistry
Animals were euthanized at different ages, and their prostates were removed along with the genitourinary (GU) system for visual inspection of any potential changes associated with the genotype. The GU system was placed in cold PBS and prostates were then resected and weighted. Some of the prostates from the TMEFF2 transgenic and the non-transgenic mice were subjected to microdissection for individual lobe analyses including weight, metabolic or western blot analyses. Prostates and lungs were also collected and fixed in formalin overnight for paraffin embedding and 5-μm sections were prepared for hematoxilin & eosin (H&E) staining and immunohistochemistry (IHC). Other tissues were collected and processed for IHC or western blot analysis as necessary. Detailed IHC procedures are included in supplementary methods.
Prostate microdissection and branching morphogenesis
The prostate was removed along with the bladder and seminal vesicles, placed in ice cold phosphate-buffered saline solution (PBS; LifeTechnologies, Grand Island, NY) and microdissected under a dissection microscope. The ventral (VP) and dorsal (DP) lobes were then incubated for 10 min in 0.5% collagenase diluted in Hank’s buffered salt solution and branches were separated using fine forceps. Branch tips from the branched VP and DP were photographed, counted and plotted.
Subcutaneous tumors
TRAMPC2 cells carrying the TMEFF2i construct or the empty vector were grown to confluency in the presence of Doxycycline (250 ng/ml), trypsinized, and suspended in PBS. 2.5 × 106 cells in 0.15 ml of PBS were injected subcutaneously into the flanks of 8-week-old male C57Bl/6 mice (Jackson laboratory, Bar Harbor, ME). Animal protocols used for this study were approved by the IACUC at ECU and OUHSC. Mice were kept with ad libitum access to Doxycycline containing diet (200 mg/kg, BioServ) and water. Tumors were isolated after 10–13 weeks, weighed and sized with calipers. A piece of each tumor was fixed in formalin, paraffin embedded and analyzed by hematoxylin and eosin (H&E) staining and by immunohistochemistry (IHC) to detect TMEFF2 expression, while the rest was homogenized in RIPA buffer to obtain lysates.
Western blotting
Western blot analysis was conducted as described before (25) using cell lysates or lysates from tissues (liver, brain, lung, prostate) or from the different mouse prostatic lobes, prepared in RIPA buffer containing a protease inhibitor mixture (Roche, Basel, Switzerland). The following antibodies were used: anti-TMEFF2 for cell lines: ab50002 (Abcam, Cambridge, MA) and HPA015587 (SIGMA, St. Louis, MO) and anti-TMEFF2 for mouse tissues: custom antibody (SDIX, Newark, DE). Anti-GAPDH (2118) and anti-Caspase 3 (9662) from Cell signaling (Beverly, MA) and anti-Calnexin (ab22595) from Abcam, (Cambridge, MA).
Metabolome profiling
Prostate lobes were microdissected, weighed, quickly frozen and stored at −80°C following the recommendations of Metabolon Inc. (Durham, NC, USA) until submitted for analysis. At the time of analysis, samples were extracted and prepared using proprietary Metabolon’s standard solvent extraction method. The extracted samples were split into equal parts for analysis on the GC/MS and LC/MS/MS platforms. Also included were several technical replicate samples created from a homogeneous pool containing a small amount of all study samples. General platform methods are described in Supplementary Methods.
Transcript correlation and statistical analysis
Transcriptional correlation between TMEFF2 and other genes was analyzed as previously described (33) by global meta-analysis of all publicly available GEO two-channel human microarray datasets (75,000 experiments total) to identify genes with recurrent, reproducible patterns of co-regulation across different experimental conditions. Pearson’s correlation coefficients were calculated quantifying the global co-expression correlations for each gene pair. A Fisher’s exact test was used to analyze differences in subcutaneous tumor formation. Branching scoring was analyzed statistically for each genotype using a 2-tailed t test, where a P value less than 0.05 was considered statistically significant. The incidence of neuroendocrine tumors was analyzed using the chi-square goodness of fit test to compare the expected frequencies (TRAMP mice) with the observed frequencies in the TRAMP/TMEFF2 animals.
RESULTS
TMEFF2 impairs growth of mouse TRAMPC2 cells in matrigel
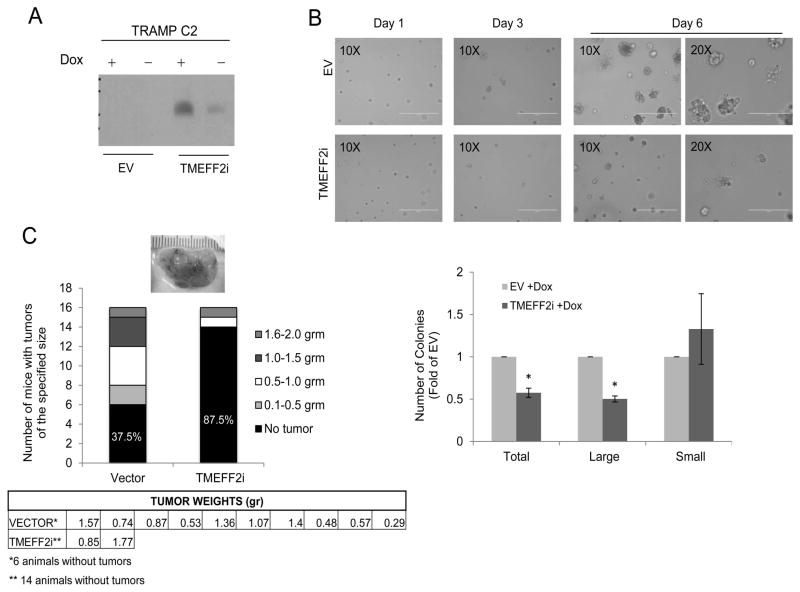
Before examining the role of TMEFF2 in mouse models, we first determined whether TMEFF2 is functional in mouse cells. We established a cell culture model using TRAMPC2, a cell line derived from the TRAMP transgenic mouse model of PCa, known to be tumorigenic when grafted into syngeneic C57BL/6 host (31). A TRAMPC2 cell subline was created that expresses TMEFF2 under the control of a doxycycline inducible promoter (TMEFF2i). Control cells were transduced with the transactivator construct only (vector) and do not express TMEFF2 (Fig. 1A). The effect of TMEFF2 overexpression on cell proliferation was examined on cells cultured in a reconstituted basement membrane gel (Matrigel™). Growth of vector containing or TMEFF2i–expressing TRAMPC2 cells was monitored after several days in doxycycline containing media including 2% matrigel. After 6 days in culture, cells proliferated and formed clusters. No significant differences were observed in the cell morphology of the two sublines, however, overexpression of TMEFF2 resulted in an observable decrease in cell number and cluster size after 3 days in culture and that effect became more prominent after 6 days (Fig.1B). Interestingly, using MTT analysis we did not observe changes in the proliferation rate of TRAMPC2 cells expressing TMEFF2 when grown in 2D cultures (supplementary Fig S1A). In addition, western blot analysis of lysates obtained from cells grown in 2D and 3D indicated that TMEFF2 does not enhance apoptosis as detected by caspase 3 cleavage. However, we observed a decrease in Ki67 (proliferation marker) when cells expressing TMEFF2 were grown in matrigel when compared to EV control cells (Supplementary Fig. S1B and S1C). These results indicate that TMEFF2 modulates proliferation of TRAMPC2 cells specifically in 3D cultures, suggesting that interactions with the extracellular matrix modulate the effect of TMEFF2 in cellular growth.
Figure 1. TMEFF2 impairs growth of TRAMPC2 cells in matrigel and mouse allograft formation.
A) TRAMPC2 cells expressingTMEFF2i or control cells were grown in the presence of 250 ng/ml doxycycline (Dox) and overexpression of TMEFF2 was assessed by western blot. B) Effect of TMEFF2 on growth of TRAMPC2 was examined 3 and 6 days after plating the cells in matrigel containing media with 250 ng/ml Dox. The experiment was repeated twice with individual quadruplicates (plates) per experiment each time. Colonies were counted by selecting 10 different fields per individual plate (from the 6 days group) and the results were graphed. C) TRAMPC2 cells expressingTMEFF2i or control cells were grown in the presence of 250 ng/ml dox and injected subcutaneously in the flanks of C57Bl/6 mice. Representative gross appearance of the tumors is shown. Number and weight of tumors formed were recorded, tabulated and graphed.
Expression of TMEFF2 impairs formation/growth of TRAMPC2 derived subcutaneous tumors
We next determined the effect of the TMEFF2 protein in the formation of subcutaneous tumors in mice. As mentioned above, TRAMPC2 cells form tumors when grafted into syngeneic C57BL/6 host. TRAMPC2-TMEFF2i and TRAMPC2-vector cells were grown in the presence of doxycycline for two days and then inoculated subcutaneously into the flanks of C57BL/6 mice that were pre-fed and kept in a doxycycline containing-diet for the duration of the experiment (n=8 per cell line; cells to be injected were grown in individual plates to represent iterations). Small subcutaneous tumors were palpable in 50% of the mice 3–5 weeks after the injection with control TRAMPC2-vector cells, but only in 1 of the 8 mice injected with TRAMPC2-TMEFF2i cells. Mice were sacrificed 10–13 weeks later and tumors, if present, were dissected, weighed and characterized by immunohistochemistry (Supplementary Fig. S1D). At this time, all tumors had grown beyond 1000 mm3 (Fig. 1C). Only 1 of the animals inoculated with TMEFF2 expressing cells grew tumors. In an independent repeat of this experiment, an additional 8 mice per cell line were injected subcutaneously with the TRAMPC2-TMEFF2i or TRAMPC2-vector cells, and similar results were obtained. The results of both experiments combined (n= 16; Fig. 1C) indicate that expression of TMEFF2 blocks tumor formation (62.5% vs. 12.5%). Importantly, the few tumors observed in the mice injected with the TMEFF2 expressing cells did not express TMEFF2 as determined by western blot or immunohistochemistry (IHC) analysis (not shown). As a control, TRAMPC2-TMEFF2i cells injected in seven mice kept under normal diet lacking doxycycline (low TMEFF2 expression) formed tumors in almost 43% of the animals. Note that, even in the absence of doxycycline, TRAMPC2-TMEFF2i cells express some TMEFF2 possibly as a consequence of a leaky promoter (Fig. 1A). Inducing TMEFF2 expression after tumor formation did not significantly impede growth or result in tumor regression (not shown), suggesting that TMEFF2 may be functioning in the early stages of the allograft establishment/progression. These results are consistent with a tumor suppressor role for TMEFF2 that we have previously reported (25,27,28).
Generation and characterization of prostate specific TMEFF2 transgenic mice
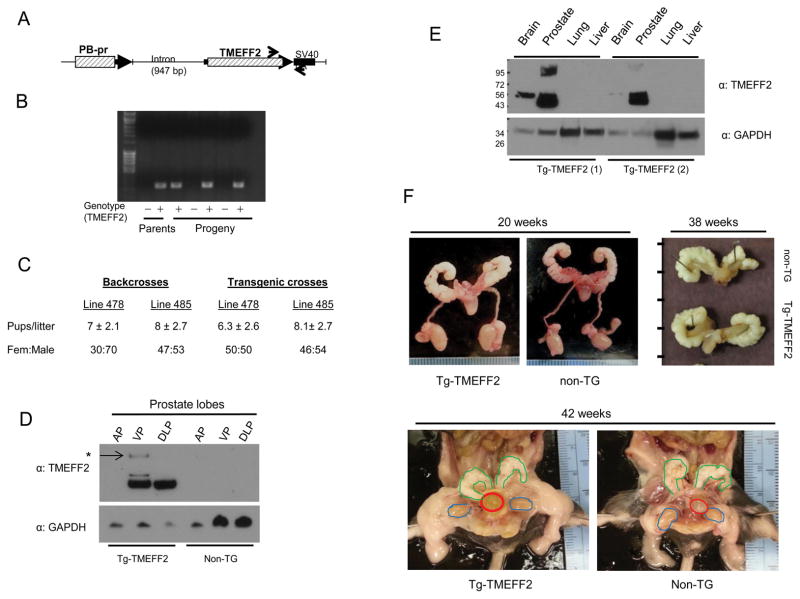
To further characterize the role of TMEFF2 in animal models, we generated a prostate specific TMEFF2 transgenic mouse model. TMEFF2 is not expressed in the normal adult mouse prostate (5,12), and therefore this mouse represents a TMEFF2 gain of function animal. To achieve prostate specific expression of TMEFF2, we cloned the full length TMEFF2 under the control of the ARR2PB (PB) composite probasin promoter (Fig. 2A), which drives androgen-dependent gene expression in the mouse prostate (34,35). Transgenic mice were then generated by injection of the transgene DNA into the pronuclei of C57BL/6 X DBA2 hybrid embryos. PCR analysis using transgene specific primers identified eight mice, all males, that expressed the transgene, but only two, #478 and #485, passed it on to the progeny when crossed to non-transgenic C57BL/6 females (Fig. 2B). PB-TMEFF2 transgenic males did not show any changes in overall appearance or size with respect to wild type (wt) males (not shown). Both transgenic lines were backcrossed to C57BL/6 mice for >7 generations and further characterized for size and composition of the litters. Both transgenic lines were fertile. Analysis of the progeny from the first 8 backcrosses indicated an average of 7 and 8 pups per litter for the 478 and 485 transgenic lines, not significantly different from the results obtained with the wt crosses (Fig. 2C and not shown). Notably, the female to male ratio, displayed a 30:70 distribution for the 478 line while it was nearly 50:50 for the wt and the 485 transgenic lines. Analysis of the progeny from the subsequent 7 crosses, in which both parents were positive for the TMEFF2 transgene, showed a 50:50 female:male ratio for both transgenic lines (Fig. 2C), while the size of the litters did not significantly change (not shown). TMEFF2 transgene expression was analyzed by western blot analysis (Fig. 2D) and RT-PCR (not shown). Because line #478 showed the most robust prostate-specific expression of the TMEFF2 transgene, it was chosen for further analysis.
Figure 2. Generation and characterization of a prostate specific TMEFF2 transgenic mouse.
A) Schematics of the transgene construct containing the ARR2PB probasin promoter (PB-pr), a β-globin derived intron and the TMEFF2 cDNA followed by the SV40 polyadenylation site. Arrows indicate approximate position of the primers used for genotyping. B) Example of a genotyping analysis from the progeny of one of the founders indicating transgene transmission to the progeny. C) Analysis of the size and sex distribution of the progeny from the two founder lines. D) Lobe-specific expression of TMEFF2. Lysates from the different prostate lobes from TMEFF2-transgenic and non-transgenic mice were analyzed by western blot for the presence of TMEFF2. Anterior, ventral and dorsolateral prostate lobes are indicated (AP, VP and DLP respectively). E) Prostate specific expression of the TMEFF2 transgene. Lysates from prostate and other organs form transgenic TMEFF2 mice were prepared and analyzed by western blot for the presence of TMEFF2. Expression of the endogenous TMEFF2 is shown in brain. The results for two different transgenic animals are presented. F) Gross anatomy of the transgenic-TMEFF2 mouse. Transgenic-TMEFF2 and non-transgenic siblings were sacrificed at different ages and the urogenital organs isolated and visually inspected. Representative pictures of the whole GU still in the mouse, dissected from the mouse, or just the prostate with the seminal vesicles attached are shown. (Bladder is circled in red, seminal vesicles in green and testes in blue). Tick marks in the right-hand side picture represent centimeters.
Western blotting of microdissected lobes of animals >6 weeks demonstrated that the TMEFF2 transgene was expressed strongly in the ventral prostate (VP) and dorsolateral prostate (DLP) lobes and very weakly or not expressed in the anterior prostate (AP) (Fig. 2D). This pattern of expression is characteristic of gene expression driven by the probasin promoter (34). Two slow mobility bands were prominent only in VP from transgenic TMEFF2 animals, but not in the DLP or in brain samples (Fig. 2D, arrow). Highly glycosylated forms of TMEFF2 have been previously described (3), however, whether they represent functionally different forms of the protein is presently unknown. No TMEFF2 expression was observed in any of the lobes obtained from non-transgenic siblings (Fig. 2D). Since the antibody used for western blotting reacts with mouse TMEFF2 protein (see below), this result further supports previous data indicating that TMEFF2 is not expressed in the adult mouse prostate (5,12). Using the same antibody, TMEFF2 expression was observed in the brain, known to endogenously express TMEFF2, of transgenic and non-transgenic siblings but not in other organs examined (lung, liver; Fig. 2E).
Gross anatomy, prostate size and histology are normal in the PB-TMEFF2 transgenic mouse
To determine the effect of TMEFF2 overexpression on the mouse prostate, we first analyzed the gross aspect of the prostates in transgenic TMEFF2 and non-transgenic age match siblings. No differences were observed in the size or appearance of the prostate, seminal vesicles, testes or other part of the urogenital system at different ages (Fig. 2F). The wet weight and histology of microdissected prostate lobes on samples obtained from two different age groups of TMEFF2 transgenic and non-transgenic animals were also analyzed. The relative weights of the microdissected AP, VP and DLP lobes from 10–14 or 24 weeks old transgenic TMEFF2 mice were slightly larger and demonstrated a larger data scatter than those of their non-transgenic mice (Supplementary Fig. S2). While those differences did not reach statistical significance, the Tg-TMEFF2 lobes demonstrated a trend towards significance at 24 weeks VP, (p=0.081). Histological analysis of H&E stained sections from prostates obtained at different ages demonstrated no major differences between the prostates of the transgenic TMEFF2 mice and the non-transgenic siblings (Supplementary Fig. S3).
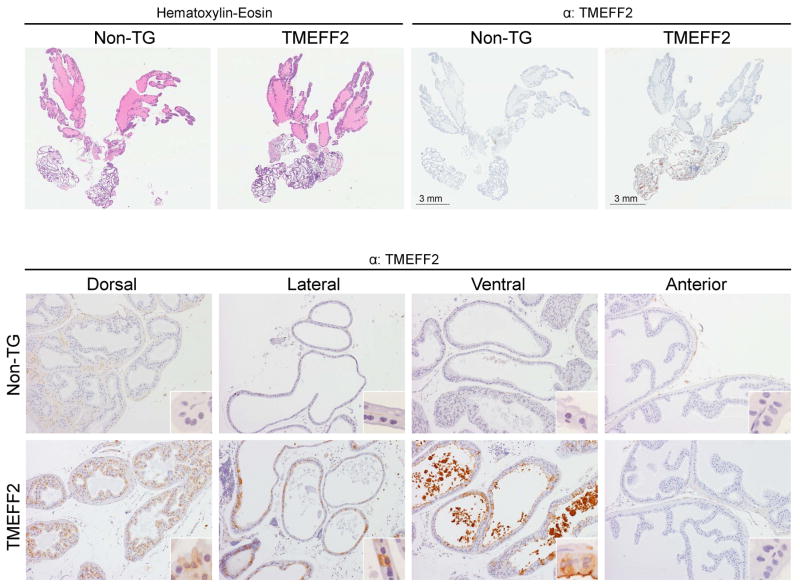
Immunohistochemical (IHC) analysis of TMEFF2 expression in the prostate lobes of the transgenic mice indicated that TMEFF2 is expressed in the ventral, dorsal and lateral lobes of the transgenic mouse, while it is missing in the anterior lobe (Fig. 3A and 3B). These results parallel the ones obtained with western blot analysis (Fig. 2D) and are characteristic of gene expression driven by the probasin promoter (34). Interestingly, the subcellular localization of the TMEFF2 protein was different in VP and DLP. In the VP, TMEFF2 was preferentially located in the luminal membrane and stained luminal secretions adjacent to the high TMEFF2 expressing cells, potentially suggesting that TMEFF2 is shed from the VP epithelium. However, in the DLP epithelium, TMEFF2 was mainly cytoplasmic (Fig. 3B). It is possible that the highly glycosylated form of the protein observed by western blot analysis in lysates prepared from the VP (but not from the DLP) corresponds with the membranous/secreted form observed by IHC. Since we observed a small tendency towards increased weight of the transgenic lobes, we stained the prostate sections with an antibody against the proliferative marker Ki67. We did not observe a significant difference in the Ki67 staining in the prostates of the transgenic animals when compared to the non-transgenic ones (Supplementary Fig S4).
Figure 3. Histological and immunohistochemical analysis of the transgenic TMEFF2 mouse.
Top: Representative images of whole prostates stained with H&E (left) or IHC with an antibody against TMEFF2 (top right). Bottom: Representative IHC images of the different prostatic lobes from non-transgenic or transgenic-TMEFF2 mice stained with an antibody against TMEFF2. Original magnification is 10x and in inserts is 60x.
TMEFF2 affects prostate branching morphogenesis in the transgenic mouse
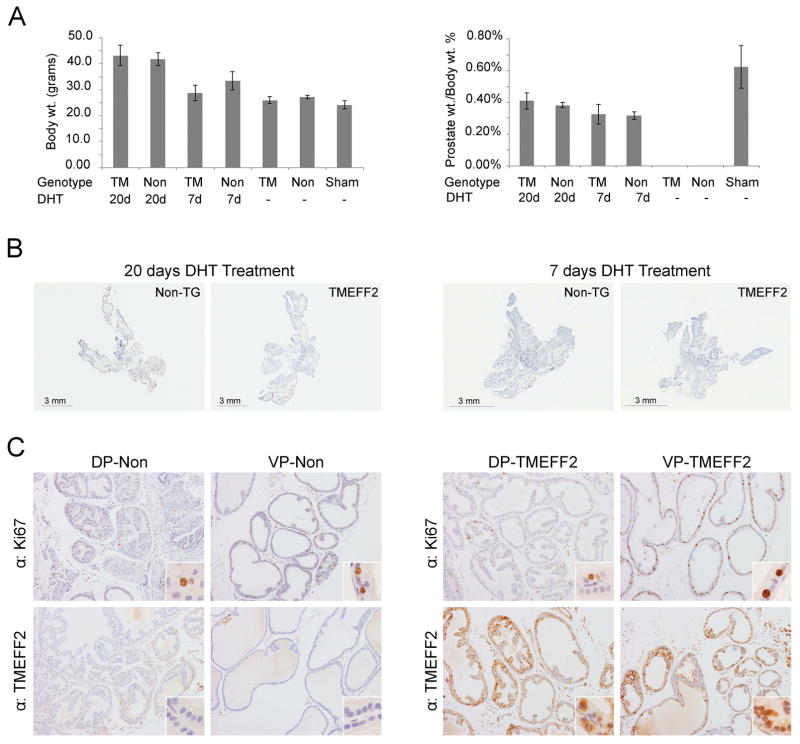
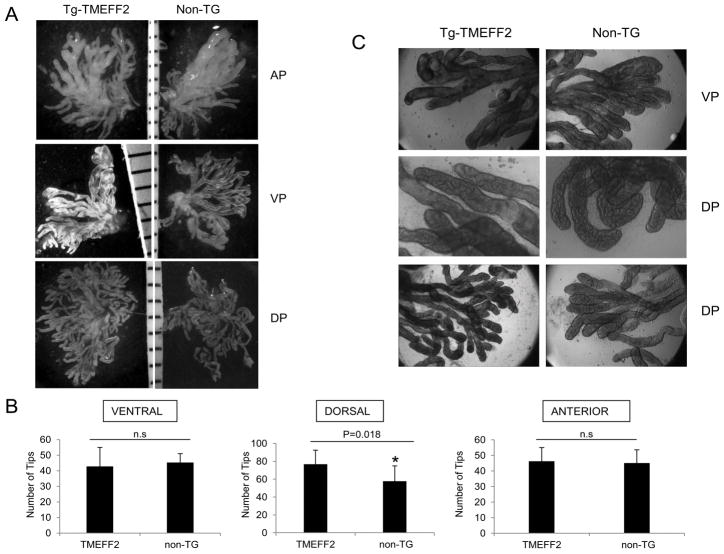
TMEFF2 and its family member, TMEFF1, are expressed in embryo. While the high degree of homology between these two genes may suggest a functional redundancy during development, TMEFF1 is predominantly expressed in the adult human brain while TMEFF2 is mainly expressed in brain and prostate. We therefore speculated that TMEFF2 may be affecting later stages of prostate development. Although we failed to observe a distinct phenotype in the transgenic TMEFF2 prostates, a likely explanation stems from the fact that the ARR2PB probasin promoter is developmentally regulated and its expression peaks 7–8 weeks after birth (34); therefore, TMEFF2 expression in the prostate of the transgenic animals occurs after branching and morphogenesis have been completed (36). To study the role of TMEFF2 overexpression in androgen-driven adult prostate morphogenesis, 10 week old TMEFF2-Tg mice and non-transgenic siblings were castrated, and after the prostates were allowed to regress for 20 days, a time-release testosterone pellet was implanted subcutaneously. Animals were sacrificed 7 or 20 days post pellet implantation and the wet weight and histology of the prostates were analyzed. By day 7, the prostates were regenerated and the lobes differentiated (Fig. 4B). There was no significant difference in body or prostate weight between the transgenic TMEFF2 mice and the non-transgenic siblings or in the gross anatomy or histology of the prostates (Fig. 4A and 4B). Cellular proliferation in the lobes was evaluated by immunostaining for the Ki-67 proliferation antigen. Only a few Ki-67 positive cells were observed in the lobes from prostates obtained 20 days after the testosterone pellet implantation (data not shown) consistent with a reported low rate of proliferation in normal mouse prostate epithelium (37). In general, Ki-67 staining was higher in all lobes from the TMEFF2-Tg and non-transgenic animals sacrificed 7 days after pellet implantation when compared to the 20 days post implantation. The VPs also had higher Ki-67 staining when compared to the DLPs. However, there were not significant differences between the lobes of TMEFF2-Tg animals when compared to the same lobes from non-transgenic animals (Fig. 4C). We then analyzed changes in branching morphogenesis. For this purpose, after castration and prostate regression, TMEFF2-Tg mice and non-transgenic animals were injected subcutaneously with DHT every other day for 8 days, followed by euthanasia and dissection of the prostate lobes, which were analyzed for the number of distal tips. Interestingly, this analysis revealed a significant increase in the number of distal tips exclusively in the dorsal lobes of the TMEFF2-Tg mice, indicating increased branching complexity upon regeneration of the adult prostate in the transgenic animals (Figs. 5A and 5B). No differences were seen in the diameter or architecture of the branches (Fig. 5C).
Figure 4. TMEFF2 expression does not affect androgen-dependent prostate growth.
Ten week old transgenic-TMEFF2 and non-transgenic mice were subjected to bilateral orchiectomy and after 20 days, time-release testosterone pellets were subcutaneously implanted. Two groups per genotype (n=5 per group) were formed by randomly selecting animals that were euthanized at 20 or 7 days post-pellet implantation. Animals from the sham group (n=2) underwent surgery but were not castrated or subject to testosterone pellet implantation. A) Body and prostates weights were recorded. Dissected prostate weights are expressed as percentage of the total body weight. B and C) Prostates from animals euthanized 7 days post pellet implantation were formalin-fixed, paraffin embedded, H&E stained or subjected to IHC with an antibody against the proliferation marker Ki67 and TMEFF2. In C) original magnification is 10x and in inserts is 60x.
Figure 5. TMEFF2 modulates branching morphogenesis in the dorsal lobe.
Ten week old castrated TMEFF2-transgenic and non-transgenic mice were injected intraperitoneally with DHT every other day. After 8 days, the prostate lobes were dissected and the number and architecture of the branch tips were analyzed. A) Representative images of the different prostatic lobes from non-transgenic or transgenic-TMEFF2 mice after treatment with collagenase to separate the tips. B) Quantification of the results indicated a significant difference in the number of tips in the DP of the transgenic mouse. C) Representative images of the branch tips observed at high magnification.
TMEFF2 expression modulates prostate metabolism in the transgenic mouse
We next conducted global metabolomic profiling of the transgenic TMEFF2 mouse to gain insight into the metabolic actions of the TMEFF2 protein in mouse prostate tissue that could contribute to the observed changes in prostate morphogenesis. For this purpose, samples were submitted to Metabolon Inc. to be analyzed for compounds which differed significantly between the DLP or VP of the TMEFF2-Tg and the non-transgenic animals. As shown in table 1, expression of TMEFF2 significantly altered the levels of 47 out of 334 metabolites tested in the DLP (p<0.05); another 31 metabolites displayed altered levels trending toward significance (0.05<p<0.1). Pathway analyses indicated that TMEFF2 expression in the DLP lobe led to higher overall transmethylation and transulfuration activity (Supplemental Fig. S5) and also led to elevated sarcosine levels. This result is somewhat surprising since our previous results demonstrated decreased levels of sarcosine in certain cell lines engineered to overexpress the TMEFF2 protein. The transgenic DLP prostate also had altered glucose usage patterns that included potential changes in glycogen metabolism, elevated levels of pentose phosphate pathway metabolites, and an accumulation of TCA cycle metabolites. Finally, pyrimidine metabolites were also elevated in DLP lobes (data not shown). Interestingly, expression of TMEFF2 in the VP had a minimal effect on metabolism, with 8 metabolites statistically significant and 9 trending differences (Table I). This is consistent with the increased branching morphogenesis observed in the DLP vs. the VP of the transgenic TMEFF2 mice.
Table I.
Number of biochemicals that differ significantly (or that approach significance) between the transgenic TMEFF2 and non-transgenic prostate lobes (n=7 or 8 for the VP and DLP samples respectively).
| Changes in metabolites in response to TMEFF2 expression in the mouse prostate | ||
|---|---|---|
| Tg VP/non-Tg VP | Tg DLP/non-Tg DLP | |
| Total Biochemicals (p≤0.05) | 8 | 47 |
| Biochemicals (↑↓) | 3↑ 5↓ | 47↑ 0↓ |
| Total Biochemicals (0.05<p<0.1) | 9 | 31 |
| Biochemicals (↑↓) | 3↑ 6↓ | 31↑ 0↓ |
Transgenic TMEFF2 expression increases the incidence of NE-like tumors in the TRAMP prostate cancer model mouse
To determine whether TMEFF2 plays a role in PCa progression in mice, we interbred TMEFF2-Tg mice with transgenic adenocarcinoma of the mouse prostate (TRAMP) model mice, and analyzed primary tumor and metastasis formation in the double TRAMP/TMEFF2-Tg mice. The TRAMP mouse expresses the SV40 T/t antigens in the epithelial cells of the prostate under the control of the androgen responsive probasin promoter and develops heterogeneous tumors with various differentiation stages. Epithelial lesions have recently been named “atypical hyperplasias of Tag” to differentiate them from adenocarcinoma since they rarely were observed to invade adjacent tissues (38). This type of lesion mainly arises from the DP progressing from mild to severe with age. Phylloides-like lesions appear with low frequency in the TRAMP mouse and they are characterized by “branch-like” luminal patterns entrapping hypercellular stroma. Finally, poorly differentiated neuroendocrine (NE)-like aggressive carcinomas preferentially develop in the ventral lobes of the TRAMP mouse (39).
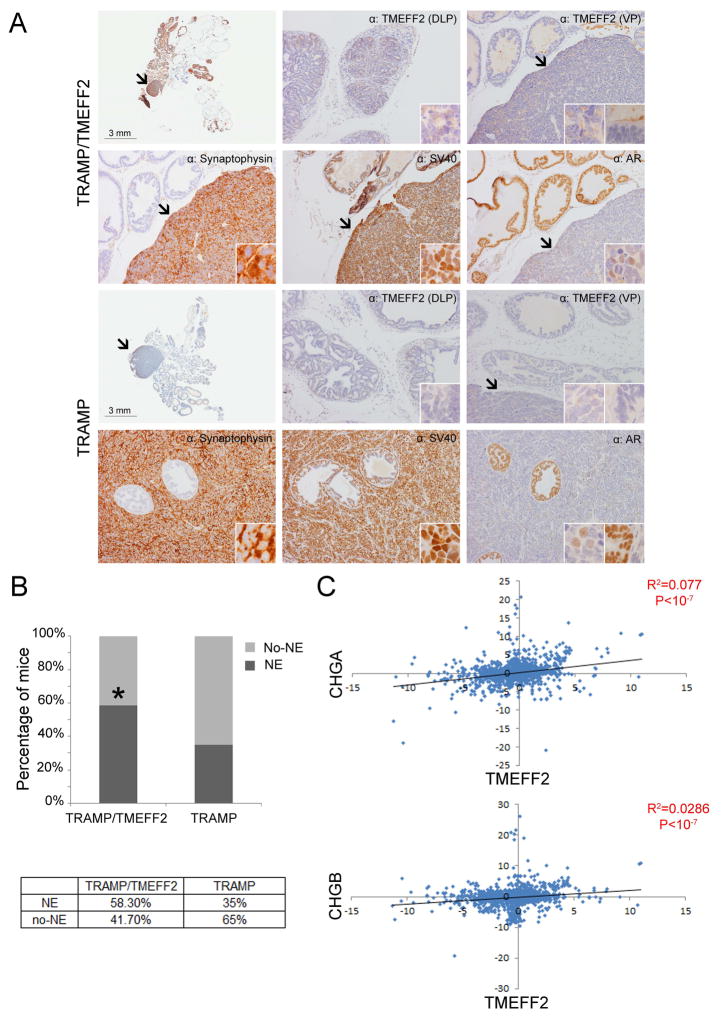
The male progeny from the TRAMP x TMEFF2-Tg crosses from a total of 48 litters were genotyped and TRAMP/TMEFF2-Tg and TRAMP/non-Tg mice were identified and sacrificed at different ages (from 6 to 24 weeks). The prostates were dissected, paraffin embedded and prepared for histological and IHC analysis. Tumor presence, type of lesion, and metastasis burden were analyzed in these animals. Mice that died prior to the time point of sacrifice were excluded from the study. Histological examination of the TRAMP/TMEFF2-Tg and TRAMP/non-Tg mice samples indicated the presence of atypical hyperplasia of Tag as early as 6 weeks characterized by hypercellularity, cell crowding, and focal piling. These lesions became more severe with age demonstrating focal and multi-focal cribriform structures, and thick, hypercelullar stroma (Supplementary Fig. S6). At 18–24 weeks old we observed evidence of local invasion and some examples of phylloide-like lesions in mice from both genotypes (Supplementary Fig. S7A). However, unexpectedly, there were no major differences in the severity, time of onset or incidence of these types of lesions between the TRAMP and the double TRAMP/TMEFF2 transgenic. Only 2 of the 24 week old double transgenic mice appear to have high grade comedocarcinoma (supplementary Fig. S7B), while we did not observe this type of lesion in the TRAMP mouse. We then examined the incidence of neuroendocrine (NE) lesions in animals 18–24 weeks old. NE tumors were present in both TRAMP/TMEFF2 and TRAMP mice and appeared to derive from the ventral lobe, as previously described (). They represented poorly differentiated areas of pleomorphic cells with scarce cytoplasm and were commonly very large and engulfed other glands (Fig. 6A and Supplementary Fig. 7).
Figure 6. TMEFF2 modulates NE tumor formation in the TRAMP/TMEFF2 mouse and its expression correlates with NE markers.
Twenty four week old TRAMP and TRAMP/TMEFF2 animals were sacrificed and prostates dissected and analyzed. A) Representative IHC images of the different areas of the prostate from TRAMP and TRAMP/TMEFF2 mice stained with antibody against different markers. Original magnification is 10x and in inserts is 60x B) Analysis of the presence/absence of NE lesions in the prostates (expressed as with or without NE tumors). C) Coexpression plot indicating a positive correlation between TMEFF2 mRNA level and expression of the NE markers chromogranin A and B (CHGA and CHGB).
Immunohistochemical characterization of the prostate lesions was conducted with antibodies against TMEFF2, the androgen receptor (AR), SV40 Tag, the transcription factor FoxA1, and the NE differentiation marker synaptophysin. Both types of lesions, atypical hyperplasia of Tag and NE tumors, reacted with antibodies against the FoxA1 transcription factor and the SV40 Tag. Normal or hyperplastic prostatic epithelia demonstrated intense nuclear AR staining while the NE tumors were either negative or showed diffuse staining for AR (Fig. 6A and supplementary Figs. S7 and S8). Only the NE tumors, but not the areas of atypical hyperplasia or normal glandular epithelium, stained positive for the NE marker synaptophysin (Fig. 6 and supplementary Fig. S8). TMEFF2 reactivity in the double TRAMP/TMEFF2 mouse was diffuse but mainly cytoplasmic in the DLP and scarce and mainly membranous in the VP. No TMEFF2 reactivity was observed in the TRAMP mouse.
Surprisingly, we observed an increase in the incidence of NE tumors (58% vs. 35%) in the 18–24 weeks TRAMP/TMEFF2-Tg mice with respect to the single TRAMP mice, suggesting that TMEFF2 promotes or accelerates formation of this type of lesion (Fig. 6B). In support of this result, transcript correlation analysis using publicly available gene expression data obtained from 3,900 human 2-color expression microarray experiments indicate that TMEFF2 expression is highly correlated with the neuroendocrine markers, Chromogranin A and B (Figure 6C).
Although several reports have failed to observe conclusive evidence of metastasis in the TRAMP mouse (38,40), in our cohort, 30% and 37% of the 18–24 week TRAMP and TRAMP/TMEFF2 displayed SV40 Tag positive microscopic lung metastasis. The positive staining indicated that the tumors were of prostatic origin and the lack of synaptophysin staining ruled out neuroendocrine tumors (Supplementary Fig. S9). Only 1 TRAMP mouse and 2 TRAMP/TMEFF2 mice demonstrated macroscopic metastasis and they had the similar immunohistological characteristics as the micrometastasis, being positive for SV40 Tag and negative for synaptophysin (not shown). While in some rare cases the presence of mestastases in TRAMP mice has been attributed to lesions arising from seminal vesicle (SV) tumors (39), we did not observe a correlation between the presence of lung metastases and abnormalities of the SV.
DISCUSSION
This project was directed to analyze the in vivo roles of TMEFF2 in the prostate. Four studies were undertaken using TRAMP mouse derived cells, a newly generated prostate specific TMEFF2 transgenic mouse and a TRAMP/TMEFF2 double transgenic animal. The first two studies were aimed to determine the effect of TMEFF2 in growth of PCa cells ex-vivo and in allografts, while the other two sought to analyze the effect of TMEFF2 in mouse prostate regeneration and tumorigenesis. The observations reported here suggest that TMEFF2 plays role in branching morphogenesis in the prostate and as a negative modulator of prostate cancer cell growth. Unexpectedly, expression of TMEFF2 in the prostate of TRAMP mice led to increased incidence of NE tumors but had no effect on Tag-driven glandular adenocarcinoma (atypical hyperplasia) lesions or number of lung metastasis.
We and others have previously demonstrated a tumor suppressor role for TMEFF2. Expression of TMEFF2 in several benign and tumorigenic cell lines promotes growth inhibition and decreases cellular invasion (4,25–28). This role is supported by the fact that TMEFF2 is silenced by hypermethylation in several cancers ((15,18–22); and references therein) and reduced expression is associated with worse prognosis in gliomas and gastric cancers (15–17). The results described above using mouse PCa TRAMP-C2 cells indicating that TMEFF2 inhibits ex vivo growth and allograft formation are consistent with a tumor suppressor role for TMEFF2. However, unexpectedly, we did not observe reduced tumor growth, or incidence, or other changes in atypical hyperplasias or phylloide-like lesions in a double TRAMP/TMEFF2 transgenic mouse, expressing TMEFF2 specifically in the prostate, when compared with the single transgenic TRAMP mouse. There are several potential explanations for the lack of effect of TMEFF2 on the TRAMP mouse vs. the observed effect in the TRAMP-C2 cell lines. First, genotypic or tumor microenvironment differences can have an impact on tumor cell growth. Also, characterization of the TRAMP-C2 cell line indicated that although it remains tumorigenic, it does not express Tag mRNA or protein (31); it is possible that transformation may follow a different pathway of initiation and progression in the allografts than in the whole mouse prostates that express the Tag. Alternatively, differences in the level and/or timing of TMEFF2 expression could be responsible for the differences observed between the results obtained with cell lines/allografts and the transgenic mouse results. In the mouse, maximal expression from the probasin promoter does not occur until 6–10 weeks postnatally (34), while high expression of TMEFF2 in the TRAMP-C2 cells is achieved from the beginning of the experiment using a doxycycline inducible promoter.
The use of the TRAMP mouse model allowed uncovering of an unexpected role for TMEFF2. Expression of TMEFF2 in the TRAMP mouse promotes increased incidence of neuroendocrine (NE) tumors. While this effect is hard to reconcile with the tumor suppressor role of TMEFF2, it is not without precedent that under certain conditions, -- including tumor stage-- tumor suppressors can behave as positive modulators of tumorigenesis. In fact, the AR can have opposite roles promoting tumor suppression or tumor progression when expressed in prostate epithelial or stromal cells respectively (41,42). As another example, the tumor suppressor protein PML, functions as a tumor promoting factor in chronic myelogenic leukemia, as it is required to maintain quiescence of a stem-like cell population that is ultimately responsible for disease relapse (43). The role of TMEFF2 in NE differentiation is currently under investigation; however several possibilities can be taken into account based on previous literature. NE tumors in the TRAMP mouse originate from a population of cancer stem-like intermediate cells (44–46). It is possible that TMEFF2 marks this population of cells and maintain the cells in a quiescent state, preventing apoptosis as the cells wait for the signal to differentiate into epithelial or NE cells. Under conditions that drive NE differentiation –TRAMP background—the population of TMEFF2 positive cells may acquire the NE phenotype resulting in increased NE tumor incidence. Interestingly, TMEFF2 is known to be the second highest expressed mRNA in the oocyte of Drosophila melanogaster. It has been postulated that this high level of TMEFF2 maintains the oocyte in a quiescent state until the right cue signals maturation (10). Our results using human tissue arrays indicate that TMEFF2 expression is very variable in PCa even within the cells of the same gland, suggesting high specificity in expression (not shown). Alternatively, since we and others have described a growth promoting phenotype for the cleaved extracellular portion of TMEFF2 (ectodomain) (23–25), and TMEFF2 is mainly membranous in the VP epithelium, it is possible that increased cleavage of the ectodomain in the VP leads to ectodomain-induced paracrine growth effects on neuroendocrine cells. Finally, it has been described that the strain background contributes to malignancy of the TRAMP mouse (the FVB background being more malignant than the C57Bl/6; (38)). However, we do not believe that the changes in NE incidence observed between the TRAMP and the TRAMP/TMEFF2 mice are due to strain differences as a consequence of the mice crosses, since we did not observe any significant changes in the incidence, time of onset, lobe distribution or histology of the atypical hyperplasias between the two mice.
In addition to its role in tumorigenesis, data presented here indicate that TMEFF2 expression results in significant metabolic changes specifically in the dorsolateral lobe of the prostate. This lobe is also distinctly subjected to morphogenetic changes in response to TMEFF2. DLP lobes from transgenic TMEFF2 mice demonstrated a 37% increase in the number of bud tips in regenerating prostates. These results suggest that TMEFF2 may play a role in adult prostate regeneration by affecting cellular metabolism. In line with these results, previous data from our lab indicated that the role of TMEFF2 in PCa is mediated, at least in part, by its ability to modulate sarcosine and the folate-mediated one-carbon metabolism, and therefore, possibly the methylation potential of the cell (25,27). Based on these results we speculated that TMEFF2, by affecting one carbon metabolism, may epigenetically affect gene expression. In fact, we have observed decreased αv, β1 and β3 integrin expression in PCa and benign cell lines overexpressing TMEFF2 and in the double TMEFF2/TRAMP transgenic mouse when compared with the TRAMP mouse (28). Therefore, the role of TMEFF2 in prostate regeneration could be facilitated by its effect on integrin expression via its role in metabolism. It has been described that changes in integrin expression, specifically β1, not only affect cell migration but also glandular morphogenesis in different tissues (47–49). Alternative mechanisms related to the TMEFF2 signaling potential can also explain its role in prostate morphogenesis after castration. TMEFF2 interacts with PDGF-AA through its follistatin domain containing region and modulates PDGF-AA signaling (15). PDGF signaling is critical for morphogenesis in different tissues (50). In addition, follistatin regulates branching morphogenesis in prostate by binding and inhibiting activin (51). Based on this, follistatin-domain containing proteins may play roles in branching morphogenesis. Future studies are needed to test these ideas.
CONCLUSIONS
The results presented here support the notion that TMEFF2 plays a role in tumorigenesis and adult prostate regeneration after castration. This last observation may reflect a potential role for TMEFF2 in prostate developmental processes. Changes in TMEFF2 expression during PCa progression and the fact that it is expressed in the embryo are consistent with this statement. How can we reconcile this data with the finding that mice with a null allele of TMEFF2 do not demonstrate major structural abnormalities? The high level of sequence similarity between TMEFF1 and TMEFF2, and the fact that both are expressed in embryo suggests that functional compensation during mouse embryonic development, could account for the lack of structural abnormalities in TMEFF2-null mice. Expression changes later in development likely promote specificity and divergent roles in the adult. Alternatively, since embryonic prostate development differs from adult prostate regeneration, it is possible that TMEFF2 only plays a role in the latter form of growth/morphogenesis. Importantly, changes in the prostate branching patterns in the adult are associated with benign prostatic hyperplasia (BPH), a disease that affects 50% of older men. Finally, TMEFF2 knockout (KO) mice or heterozygous mice with a null allele of TMEFF2 did not demonstrate the presence of tumors. While it was concluded that these results did not support a tumor suppressor role for TMEFF2, the TMEFF2-KO mice died around weaning age, not old enough to demonstrate tumors. In addition, the eight heterozygous mice analyzed for tumors should still express TMEFF2, although at reduced levels. The results presented here indicating a tumor suppressor role for TMEFF2 in allografts but not in a TRAMP mouse model, and an involvement in NE tumor formation, suggest a very complex role for TMEFF2 in tumorigenesis that is influenced by background and therefore, specific oncogenic signals.
Supplementary Material
Acknowledgments
The authors are thankful to Dr. Matusik (Vanderbilt-Ingram Center) and to Dr. Thresher (University of North Carolina, Chapel Hill) for providing the probasin promoter and the pTg1 vectors respectively. The authors acknowledge Tom Green (East Carolina University, ECU) for his help with the characterization of the custom TMEFF2 antibody, Joani Zari (ECU) for help with the preparation of the tissue paraffin blocks and H&E staining and Dr. D. Zhao (OUHSC) for guidance with statistical analysis. We thank the Stephenson Cancer Center at the University of Oklahoma, Oklahoma City, OK and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the NIH under grant number P20 GM103639 for the use of Histology and Immunohistochemistry Core, which provided immunohistochemistry and image analysis services.
This work was supported in part by a grant from the National Cancer Institute (1R15CA155873). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Footnotes
The results presented here were obtained at East Carolina University, the University of North Carolina at Chapel Hill, the Oklahoma Medical Research Foundation and the Oklahoma University Health Sciences Center.
The authors declare no conflict of interest.
References
- 1.Uchida T, Wada K, Akamatsu T, Yonezawa M, Noguchi H, Mizoguchi A, Kasuga M, Sakamoto C. A novel epidermal growth factor-like molecule containing two follistatin modules stimulates tyrosine phosphorylation of erbB-4 in MKN28 gastric cancer cells. Biochemical and biophysical research communications. 1999;266(2):593–602. doi: 10.1006/bbrc.1999.1873. [DOI] [PubMed] [Google Scholar]
- 2.Heanue TA, Pachnis V. Expression profiling the developing mammalian enteric nervous system identifies marker and candidate Hirschsprung disease genes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(18):6919–6924. doi: 10.1073/pnas.0602152103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glynne-Jones E, Harper ME, Seery LT, James R, Anglin I, Morgan HE, Taylor KM, Gee JM, Nicholson RI. TENB2, a proteoglycan identified in prostate cancer that is associated with disease progression and androgen independence. International journal of cancer Journal international du cancer. 2001;94(2):178–184. doi: 10.1002/ijc.1450. [DOI] [PubMed] [Google Scholar]
- 4.Gery S, Sawyers CL, Agus DB, Said JW, Koeffler HP. TMEFF2 is an androgen-regulated gene exhibiting antiproliferative effects in prostate cancer cells. Oncogene. 2002;21(31):4739–4746. doi: 10.1038/sj.onc.1205142. [DOI] [PubMed] [Google Scholar]
- 5.Afar DE, Bhaskar V, Ibsen E, Breinberg D, Henshall SM, Kench JG, Drobnjak M, Powers R, Wong M, Evangelista F, O’Hara C, Powers D, DuBridge RB, Caras I, Winter R, Anderson T, Solvason N, Stricker PD, Cordon-Cardo C, Scher HI, Grygiel JJ, Sutherland RL, Murray R, Ramakrishnan V, Law DA. Preclinical validation of anti-TMEFF2-auristatin E-conjugated antibodies in the treatment of prostate cancer. Molecular cancer therapeutics. 2004;3(8):921–932. [PubMed] [Google Scholar]
- 6.Eib DW, Holling TM, Zwijsen A, Dewulf N, de Groot E, van den Eijnden-van Raaij AJ, Huylebroeck D, Martens GJ. Expression of the follistatin/EGF-containing transmembrane protein M7365 (tomoregulin-1) during mouse development. Mechanisms of development. 2000;97(1–2):167–171. doi: 10.1016/s0925-4773(00)00426-3. [DOI] [PubMed] [Google Scholar]
- 7.Morais da Silva S, Gates PB, Eib DW, Martens GJ, Brockes JP. The expression pattern of tomoregulin-1 in urodele limb regeneration and mouse limb development. Mechanisms of development. 2001;104(1–2):125–128. doi: 10.1016/s0925-4773(01)00362-8. [DOI] [PubMed] [Google Scholar]
- 8.Chang C, Eggen BJ, Weinstein DC, Brivanlou AH. Regulation of nodal and BMP signaling by tomoregulin-1 (X7365) through novel mechanisms. Developmental biology. 2003;255(1):1–11. doi: 10.1016/s0012-1606(02)00075-1. [DOI] [PubMed] [Google Scholar]
- 9.Harms PW, Chang C. Tomoregulin-1 (TMEFF1) inhibits nodal signaling through direct binding to the nodal coreceptor Cripto. Genes & development. 2003;17(21):2624–2629. doi: 10.1101/gad.1127703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markholt S, Grondahl ML, Ernst EH, Andersen CY, Ernst E, Lykke-Hartmann K. Global gene analysis of oocytes from early stages in human folliculogenesis shows high expression of novel genes in reproduction. Molecular human reproduction. 2012;18(2):96–110. doi: 10.1093/molehr/gar083. [DOI] [PubMed] [Google Scholar]
- 11.Kanemoto N, Horie M, Omori K, Nishino N, Kondo M, Noguchi K, Tanigami A. Expression of TMEFF1 mRNA in the mouse central nervous system: precise examination and comparative studies of TMEFF1 and TMEFF2. Brain research Molecular brain research. 2001;86(1–2):48–55. doi: 10.1016/s0169-328x(00)00257-6. [DOI] [PubMed] [Google Scholar]
- 12.Chen TR, Wang P, Carroll LK, Zhang YJ, Han BX, Wang F. Generation and characterization of Tmeff2 mutant mice. Biochemical and biophysical research communications. 2012;425(2):189–194. doi: 10.1016/j.bbrc.2012.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gery S, Yin D, Xie D, Black KL, Koeffler HP. TMEFF1 and brain tumors. Oncogene. 2003;22(18):2723–2727. doi: 10.1038/sj.onc.1206351. [DOI] [PubMed] [Google Scholar]
- 14.Wu X, Blanck A, Norstedt G, Sahlin L, Flores-Morales A. Identification of genes with higher expression in human uterine leiomyomas than in the corresponding myometrium. Molecular human reproduction. 2002;8(3):246–254. doi: 10.1093/molehr/8.3.246. [DOI] [PubMed] [Google Scholar]
- 15.Lin K, Taylor JR, Jr, Wu TD, Gutierrez J, Elliott JM, Vernes JM, Koeppen H, Phillips HS, de Sauvage FJ, Meng YG. TMEFF2 is a PDGF-AA binding protein with methylation-associated gene silencing in multiple cancer types including glioma. PloS one. 2011;6(4):e18608. doi: 10.1371/journal.pone.0018608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun T, Du W, Xiong H, Yu Y, Weng Y, Ren L, Zhao H, Wang Y, Chen Y, Xu J, Xiang Y, Qin W, Cao W, Zou W, Chen H, Hong J, Fang JY. TMEFF2 deregulation contributes to gastric carcinogenesis and indicates poor survival outcome. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(17):4689–4704. doi: 10.1158/1078-0432.CCR-14-0315. [DOI] [PubMed] [Google Scholar]
- 17.Sun TT, Tang JY, Du W, Zhao HJ, Zhao G, Yang SL, Chen HY, Hong J, Fang JY. Bidirectional regulation between TMEFF2 and STAT3 may contribute to Helicobacter pylori-associated gastric carcinogenesis. International journal of cancer Journal international du cancer. 2015;136(5):1053–1064. doi: 10.1002/ijc.29061. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki M, Shigematsu H, Shames DS, Sunaga N, Takahashi T, Shivapurkar N, Iizasa T, Frenkel EP, Minna JD, Fujisawa T, Gazdar AF. DNA methylation-associated inactivation of TGFbeta-related genes DRM/Gremlin, RUNX3, and HPP1 in human cancers. British journal of cancer. 2005;93(9):1029–1037. doi: 10.1038/sj.bjc.6602837. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Brucher BL, Geddert H, Langner C, Hofler H, Fink U, Siewert JR, Sarbia M. Hypermethylation of hMLH1, HPP1, p14(ARF), p16(INK4A) and APC in primary adenocarcinomas of the small bowel. International journal of cancer Journal international du cancer. 2006;119(6):1298–1302. doi: 10.1002/ijc.21990. [DOI] [PubMed] [Google Scholar]
- 20.Tsunoda S, Smith E, De Young NJ, Wang X, Tian ZQ, Liu JF, Jamieson GG, Drew PA. Methylation of CLDN6, FBN2, RBP1, RBP4, TFPI2, and TMEFF2 in esophageal squamous cell carcinoma. Oncology reports. 2009;21(4):1067–1073. doi: 10.3892/or_00000325. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalo V, Lozano JJ, Munoz J, Balaguer F, Pellise M, Rodriguez de Miguel C, Andreu M, Jover R, Llor X, Giraldez MD, Ocana T, Serradesanferm A, Alonso-Espinaco V, Jimeno M, Cuatrecasas M, Sendino O, Castellvi-Bel S, Castells A. Aberrant gene promoter methylation associated with sporadic multiple colorectal cancer. PloS one. 2010;5(1):e8777. doi: 10.1371/journal.pone.0008777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Zhao H, Li J, Liu H, Wang F, Wei Y, Su J, Zhang D, Liu T, Zhang Y. The identification of specific methylation patterns across different cancers. PloS one. 2015;10(3):e0120361. doi: 10.1371/journal.pone.0120361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horie M, Mitsumoto Y, Kyushiki H, Kanemoto N, Watanabe A, Taniguchi Y, Nishino N, Okamoto T, Kondo M, Mori T, Noguchi K, Nakamura Y, Takahashi E, Tanigami A. Identification and characterization of TMEFF2, a novel survival factor for hippocampal and mesencephalic neurons. Genomics. 2000;67(2):146–152. doi: 10.1006/geno.2000.6228. [DOI] [PubMed] [Google Scholar]
- 24.Ali N, Knauper V. Phorbol ester-induced shedding of the prostate cancer marker transmembrane protein with epidermal growth factor and two follistatin motifs 2 is mediated by the disintegrin and metalloproteinase-17. The Journal of biological chemistry. 2007;282(52):37378–37388. doi: 10.1074/jbc.M702170200. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Overcash R, Green T, Hoffman D, Asch AS, Ruiz-Echevarria MJ. The tumor suppressor activity of the transmembrane protein with epidermal growth factor and two follistatin motifs 2 (TMEFF2) correlates with its ability to modulate sarcosine levels. The Journal of biological chemistry. 2011;286(18):16091–16100. doi: 10.1074/jbc.M110.193805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elahi A, Zhang L, Yeatman TJ, Gery S, Sebti S, Shibata D. HPP1-mediated tumor suppression requires activation of STAT1 pathways. International journal of cancer Journal international du cancer. 2008;122(7):1567–1572. doi: 10.1002/ijc.23202. [DOI] [PubMed] [Google Scholar]
- 27.Green T, Chen X, Ryan S, Asch AS, Ruiz-Echevarria MJ. TMEFF2 and SARDH cooperate to modulate one-carbon metabolism and invasion of prostate cancer cells. The Prostate. 2013;73(14):1561–1575. doi: 10.1002/pros.22706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Corbin JM, Tipton GJ, Yang LV, Asch AS, Ruiz-Echevarria MJ. The TMEFF2 tumor suppressor modulates integrin expression, RhoA activation and migration of prostate cancer cells. Biochimica et biophysica acta. 2014;1843(6):1216–1224. doi: 10.1016/j.bbamcr.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(8):3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huss WJ, Maddison LA, Greenberg NM. Autochthonous mouse models for prostate cancer: past, present and future. Seminars in cancer biology. 2001;11(3):245–260. doi: 10.1006/scbi.2001.0373. [DOI] [PubMed] [Google Scholar]
- 31.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer research. 1997;57(16):3325–3330. [PubMed] [Google Scholar]
- 32.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30(3):256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 33.Wren JD. A global meta-analysis of microarray expression data to predict unknown gene functions and estimate the literature-data divide. Bioinformatics. 2009;25(13):1694–1701. doi: 10.1093/bioinformatics/btp290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenberg NM, DeMayo FJ, Sheppard PC, Barrios R, Lebovitz R, Finegold M, Angelopoulou R, Dodd JG, Duckworth ML, Rosen JM, Matusik RJ. The rat probasin gene promoter directs hormonally and developmentally regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Molecular endocrinology. 1994;8(2):230–239. doi: 10.1210/mend.8.2.8170479. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Thomas TZ, Kasper S, Matusik RJ. A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology. 2000;141(12):4698–4710. doi: 10.1210/endo.141.12.7837. [DOI] [PubMed] [Google Scholar]
- 36.Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biology of reproduction. 1986;34(5):961–971. doi: 10.1095/biolreprod34.5.961. [DOI] [PubMed] [Google Scholar]
- 37.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nature genetics. 1998;19(4):348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 38.Chiaverotti T, Couto SS, Donjacour A, Mao JH, Nagase H, Cardiff RD, Cunha GR, Balmain A. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. The American journal of pathology. 2008;172(1):236–246. doi: 10.2353/ajpath.2008.070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Wang L, Zhang Y, Lu J. In: Lobe-Specific Carcinogenesis in the Transgenic Adenocarcinoma of Mouse Prostate (TRAMP) Mouse Model. Tonissen DK, editor. InTech; 2013. [Google Scholar]
- 40.Tani Y, Suttie A, Flake GP, Nyska A, Maronpot RR. Epithelial-stromal tumor of the seminal vesicles in the transgenic adenocarcinoma mouse prostate model. Veterinary pathology. 2005;42(3):306–314. doi: 10.1354/vp.42-3-306. [DOI] [PubMed] [Google Scholar]
- 41.Niu Y, Altuwaijri S, Lai KP, Wu CT, Ricke WA, Messing EM, Yao J, Yeh S, Chang C. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(34):12182–12187. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niu Y, Chang TM, Yeh S, Ma WL, Wang YZ, Chang C. Differential androgen receptor signals in different cells explain why androgen-deprivation therapy of prostate cancer fails. Oncogene. 2010;29(25):3593–3604. doi: 10.1038/onc.2010.121. [DOI] [PubMed] [Google Scholar]
- 43.Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y, Rosenblatt J, Avigan DE, Teruya-Feldstein J, Pandolfi PP. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453(7198):1072–1078. doi: 10.1038/nature07016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan T-C, Veeramani S, Lin M-F. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocrine-Related Cancer. 2007;14(3):531–547. doi: 10.1677/ERC-07-0061. [DOI] [PubMed] [Google Scholar]
- 45.Huss WJ, Gray DR, Tavakoli K, Marmillion ME, Durham LE, Johnson MA, Greenberg NM, Smith GJ. Origin of Androgen-Insensitive Poorly Differentiated Tumors in the Transgenic Adenocarcinoma of Mouse Prostate Model. Neoplasia (New York, NY) 2007;9(11):938–950. doi: 10.1593/neo.07562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palapattu GS, Wu C, Silvers CR, Martin HB, Williams K, Salamone L, Bushnell T, Huang L-S, Yang Q, Huang J. Selective expression of CD44, a putative prostate cancer stem cell marker, in neuroendocrine tumor cells of human prostate cancer. The Prostate. 2009;69(7):787–798. doi: 10.1002/pros.20928. [DOI] [PubMed] [Google Scholar]
- 47.Castoria G, D’Amato L, Ciociola A, Giovannelli P, Giraldi T, Sepe L, Paolella G, Barone MV, Migliaccio A, Auricchio F. Androgen-induced cell migration: role of androgen receptor/filamin A association. PloS one. 2011;6(2):e17218. doi: 10.1371/journal.pone.0017218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bryant DM, Roignot J, Datta A, Overeem AW, Kim M, Yu W, Peng X, Eastburn DJ, Ewald AJ, Werb Z, Mostov KE. A molecular switch for the orientation of epithelial cell polarization. Developmental cell. 2014;31(2):171–187. doi: 10.1016/j.devcel.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plosa EJ, Young LR, Gulleman PM, Polosukhin VV, Zaynagetdinov R, Benjamin JT, Im AM, van der Meer R, Gleaves LA, Bulus N, Han W, Prince LS, Blackwell TS, Zent R. Epithelial beta1 integrin is required for lung branching morphogenesis and alveolarization. Development. 2014;141(24):4751–4762. doi: 10.1242/dev.117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoch RV, Soriano P. Roles of PDGF in animal development. Development. 2003;130(20):4769–4784. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- 51.Cancilla B, Jarred RA, Wang H, Mellor SL, Cunha GR, Risbridger GP. Regulation of prostate branching morphogenesis by activin A and follistatin. Developmental biology. 2001;237(1):145–158. doi: 10.1006/dbio.2001.0364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.