Supplemental Digital Content is available in the text.
Keywords: coronary artery disease, cystine, glutathione, inflammation, mortality, oxidative stress, prognosis, redox, risk
Abstract
Background—
Free radical scavengers have failed to improve patient outcomes, promoting the concept that clinically important oxidative stress may be mediated by alternative mechanisms. We sought to examine the association of emerging aminothiol markers of nonfree radical mediated oxidative stress with clinical outcomes.
Methods and Results—
Plasma levels of reduced (cysteine and glutathione) and oxidized (cystine and glutathione disulphide) aminothiols were quantified by high performance liquid chromatography in 1411 patients undergoing coronary angiography (mean age 63 years, male 66%). All patients were followed for a mean of 4.7±2.1 years for the primary outcome of all-cause death (n=247). Levels of cystine (oxidized) and glutathione (reduced) were associated with risk of death (P<0.001 both) before and after adjustment for covariates. High cystine and low glutathione levels (>+1 SD and <−1 SD, respectively) were associated with higher mortality (adjusted hazard ratio [HR], 1.63; 95% confidence interval [CI], 1.19–2.21; HR, 2.19; 95% CI, 1.50–3.19; respectively) compared with those outside these thresholds. Furthermore, the ratio of cystine/glutathione was also significantly associated with mortality (adjusted HR, 1.92; 95% CI, 1.39–2.64) and was independent of and additive to high-sensitivity C-reactive protein level. Similar associations were found for other outcomes of cardiovascular death and combined death and myocardial infarction.
Conclusions—
A high burden of oxidative stress, quantified by the plasma aminothiols, cystine, glutathione, and their ratio, is associated with mortality in patients with coronary artery disease, a finding that is independent of and additive to the inflammatory burden. Importantly, these data support the emerging role of nonfree radical biology in driving clinically important oxidative stress.
Oxidative stress (OS) is implicated in the pathophysiology of multiple conditions, including cardiovascular disease (CVD).1 Although the harmful cellular effects of free radical species in vitro remain undisputed, observational evidence along with clinical trials of free radical scavengers has been uniformly disappointing.2,3 This has promoted the concept that free radicals may not constitute clinically important sources of oxidants and that nonfree radical species may be of equal or greater importance.4
Clinical Perspective on p 369
Proteins are susceptible to oxidation through alterations of reactive aminothiol residues such as cysteine and glutathione. These covalent modifications serve to alter the cellular signaling activity of the proteins, thereby coupling redox modifications of aminothiols to functional activity.5 Importantly, these aminothiols can be quantified in plasma to assess the oxidant burden in vivo.6 Of these, cysteine constitutes the major aminothiol pool extracellularly that reacts readily with oxidants to form its oxidized disulphide cystine. Intracellularly, glutathione is a major antioxidant that helps eliminate peroxides and maintain cellular redox, and its oxidized form is glutathione disulfide.4 Increased OS, measured as higher levels of cystine, lower levels of glutathione, or altered ratios of oxidized to reduced aminothiols, is associated with cellular dysfunction, aging, risk factors for CVD, and subclinical vascular disease, and are likely to be reliable markers of systemic OS and antioxidant defense.7–13
However, there remains a need to determine whether oxidant burden as indicated by alterations in the levels or ratios of these aminothiols is clinically relevant and determines adverse outcomes. This would support the use of these aminothiols as biomarkers of OS and potentially promote development of novel antioxidant therapies. We thus sought to determine whether the major aminothiols and their respective ratios would be associated with increased mortality and cardiovascular events in a prospectively followed high-risk population.
Methods
Study Population
Study participants aged 20 to 90 years were recruited as part of the Emory Cardiovascular Biobank, an ongoing prospective cohort of patients enrolled prior to undergoing coronary angiography for investigation or management of coronary artery disease (CAD) across three Emory Healthcare sites with collection of extensive data on demographic characteristics, medical history, medication use, behavioral habits, and risk factor prevalence.14,15
Recruited patients were stable at the time of enrollment and undergoing an elective procedure, although stable patients with non–ST-segment–elevation myocardial infarction (MI), defined using international criteria were also included and classified as acute MI.16 CAD burden was quantified using the semiquantitative Gensini score, as previously described.16 Left ventricular function was expressed using ejection fraction.17 Finally glomerular filtration rate (GFR) was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.18 Subjects were excluded if they had a history of heart transplantation, recent transfusion, immunosuppressant use, malignancy, or significant infections or any vitamin supplements in the previous 6 weeks. Specific dietary intake patterns were not documented but blood samples and measurements were taken after an overnight fast before planned coronary angiography, except in <1% of patients who underwent angiography emergently on the same day. The study was approved by the Institutional Review Board at Emory University, and all subjects provided written informed consent.
Follow-Up and Outcomes
The cohort was prospectively followed for determination of the primary outcome of all-cause death and the secondary outcomes of cardiovascular death and the composite of death/or nonfatal MI. This was performed by personnel blinded to aminothiol data, through telephone interview, chart review, and linkage with the Social Security Death Index and state records. Cardiovascular death was defined as death attributable to an ischemic cardiovascular cause (fatal MI, ischemic stroke, peripheral arterial disease) or sudden death attributable to an unknown but presumed cardiovascular cause in high-risk CAD patients. Medical records were accessed or requested to validate all self-reported events including MI, which was defined using standard criteria as above.16 Fifteen patients (1%) were lost to follow-up and were excluded from analysis, leaving 1411 patients with complete biomarker and follow-up data.
Measurement of Aminothiols and C-Reactive Protein
We measured plasma cysteine (CyS), its oxidized form cystine (CySS), glutathione (GSH), and its oxidized form glutathione disulphide (GSSG) in all subjects using high-performance liquid chromatography mass spectrometry. A full methods and protocol article has been published previously outlining sample collection, processing, and analysis steps in detail.6 Summary details are also presented in the online-only Data Supplement, but briefly, arterial blood samples were drawn via syringe immediately after placement of a femoral arterial sheath (prior to heparin or saline flush or any coronary intervention) and transferred into preprepared Eppendorf tubes containing preservatives to retard auto-oxidation, centrifuged, and stored at −80°C for no more than 2 months before transfer to the laboratory. Sample collection and storage conditions in this way have been previously verified.6 Analyses by high-performance liquid chromatography were performed after dansyl derivatization on a 3-aminopropyl column with fluorescence detection. Metabolites were identified by coelution with standards and quantified by integration relative to the internal standard, with validation relative to external standards as previously described.6 Ratios of oxidized to reduced aminothiols (cystine/cysteine and glutathione disulphide/glutathione) are expressed directly. The coefficients of variation for each of the aminothiols were as follows: cysteine 3.8%, cystine 3.2%, glutathione 5%, and glutathione disulphide 9.7%. High-sensitivity C-reactive protein (hsCRP) levels were quantified using a sandwich immunoassay (R&D Systems, Minneapolis, MN). Minimum detectable hsCRP concentrations were 0.1 mg/l.
Statistical Methods
Continuous variables are presented as means±SD or as median (interquartile range) and categorical variables as proportions (%) with one-way analysis of variance and chi-squared tests used to determine differences between groups.
Before analysis, aminothiol measures were non-normally distributed and were natural log +1 transformed. Furthermore, to make the effects comparable between markers, the log-transformed variables were standardized to have mean 0 and SD 1. They were assessed as continuous and categorical traits, initially by per unit log increase and per SD increment and then by a 1×SD cut-off to classify high and low values. Survival analysis was performed using Kaplan–Meier curves as well as Cox proportional-hazards regression in models adjusted first for age, gender, and then additionally for body mass index (kg/m2), GFR (l/min), presence of diabetes mellitus, hypertension, total cholesterol (mg/dL), high-density lipoprotein (mg/dL), current smoking, statin use, acute MI at enrollment, left ventricular function (ejection fraction; %), Gensini score, and plasma hsCRP at baseline. Given that inflammation and oxidative stress are biologically interrelated, an interaction term between hsCRP and each of the markers (including their ratios) was initially included in the model. Interaction between age and each marker was also considered to examine any potential age-modifying effects. Missing covariate data (range, 0% to 3%) were imputed and sensitivity analysis with unimputed data found results to be similar. The proportional hazards assumption for Cox models was evaluated by plots of Schoenfeld residuals and formal testing (a χ2 test calculated as the sum of Schoenfeld residuals), with no significant violations of the assumption found.
The incremental value of the aminothiol markers for risk prediction was tested before and after their addition to a clinical model with traditional risk predictors (age, gender, body mass index, GFR, diabetes mellitus, hypertension, total cholesterol, high-density lipoprotein, current smoking, statin use, acute MI, left ventricular function, Gensini score). The C-statistic and category-free net reclassification improvement as well as integrated discrimination improvement that can account for censored data were calculated as a measure of risk discrimination.19–22 We set the truncation time at 5 years. The resulting risk discrimination metrics indicate the performance of the given model in predicting events that occurred in the time range from baseline to 5 years. P values <0.05 from 2-sided tests were considered to indicate statistical significance. Statistical analyses were performed using SPSS 20.0 (Chicago, IL), SAS (Cary, NC), and R (3.1.0).
Results
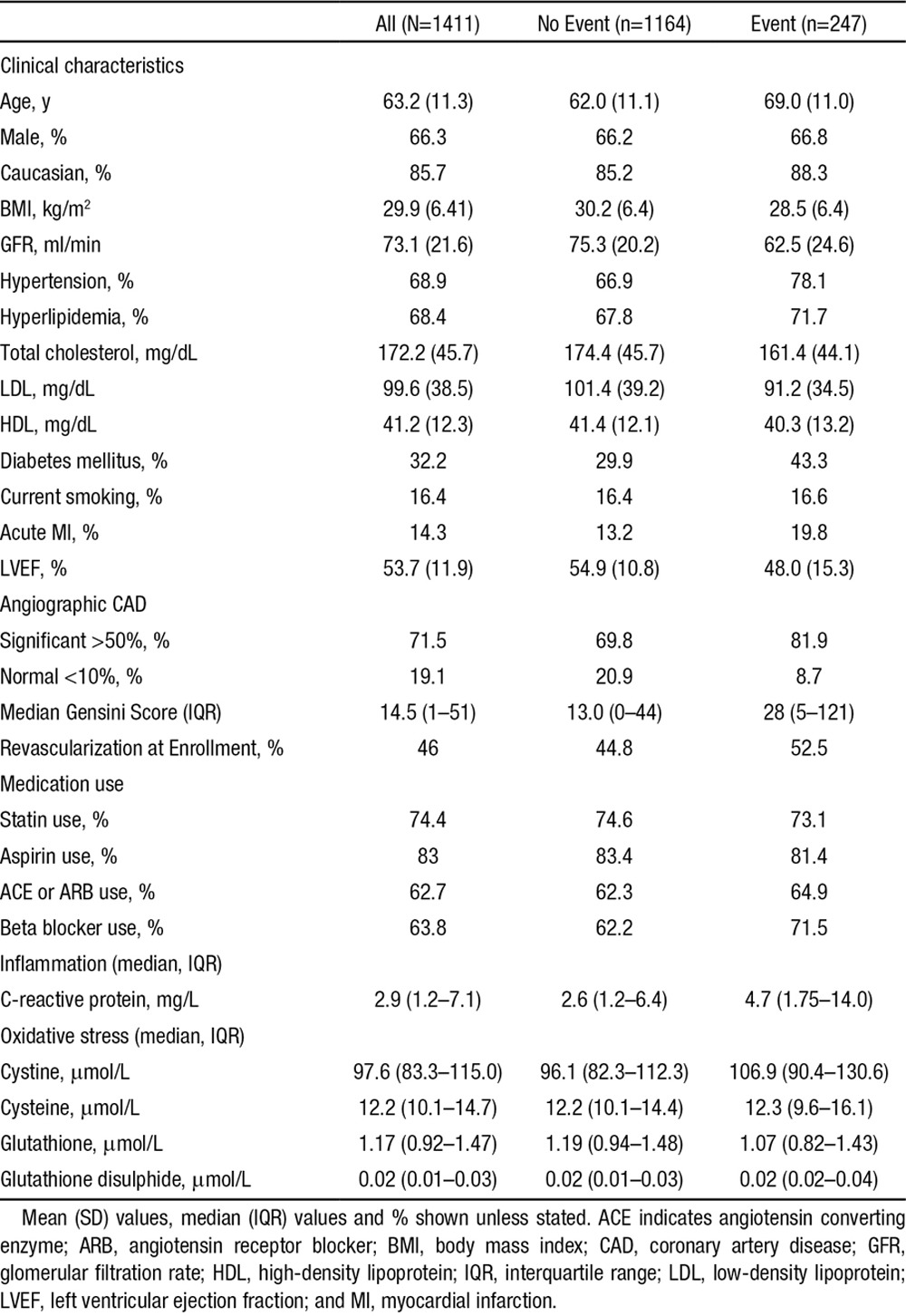
Baseline characteristics of the 1411 patients are presented in Table 1 and were reflective of a typical population recruited at coronary angiography. The mean age of the cohort was 63.2 (±11.3) years, 66% male, 32% with diabetes mellitus, 69% with hypertension or hyperlipidemia, and 16% were current smokers. Approximately 72% had significant CAD (>50% luminal stenosis) on angiography, 14% had presented with evidence of acute MI (all stable non–ST-segment–elevation MI), and 46% were treated with revascularization during the admission at which they were enrolled (Table 1).
Table 1.
Patient Characteristics
Relationship Between Aminothiols
The oxidized aminothiol, cystine was almost 8-fold more abundant than its reduced form cysteine, whereas the reduced aminothiol glutathione was 40 times more abundant than its oxidized form glutathione disulphide (Table 1). There were modest correlations between the various aminothiols, whereas hsCRP was only marginally associated with the aminothiol markers (Table I in the online-only Data Supplement).
Relationship Between Aminothiols and Demographic and Clinical Features
In univariate analyses, higher plasma cystine levels (high OS) were associated with older age, female gender, higher body mass index, impaired renal function (lower GFR), presence of diabetes mellitus, hypertension, lower total cholesterol levels, statin use, impaired left ventricular function (lower ejection fraction), greater CAD burden (Gensini), and greater inflammation (hsCRP). Of these, only age, sex, body mass index, GFR, diabetes mellitus, and hypertension were independently associated with plasma cystine in a multivariate model. Higher glutathione levels (less OS) were associated with younger age, lower GFR, absence of diabetes mellitus and hypertension, lower CAD burden, and higher total cholesterol. Of these only age, GFR, CAD burden, and total cholesterol remained independently associated with plasma glutathione. Higher cysteine levels also correlated independently with GFR, diabetes mellitus, CAD burden, and total cholesterol whereas glutathione disulfide did not show any associations aside from an inverse association with GFR (Table IIA and IIB in the online-only Data Supplement).
Relationships Between Individual Aminothiols and Outcomes
During a mean follow-up of 4.7 (±2.1) years (median, 5.3; interquartile range, 3.1–6.2), representing 6570 person-years of follow-up, 247 patients experienced the primary outcome of death, of which there were 169 cardiovascular deaths and 314 composite outcomes of death/MI. Patients who experienced the primary outcome were generally older and had more risk factors and disease burden as shown in Table 1. Independent clinical predictors of outcomes are presented in Table III in the online-only Data Supplement.
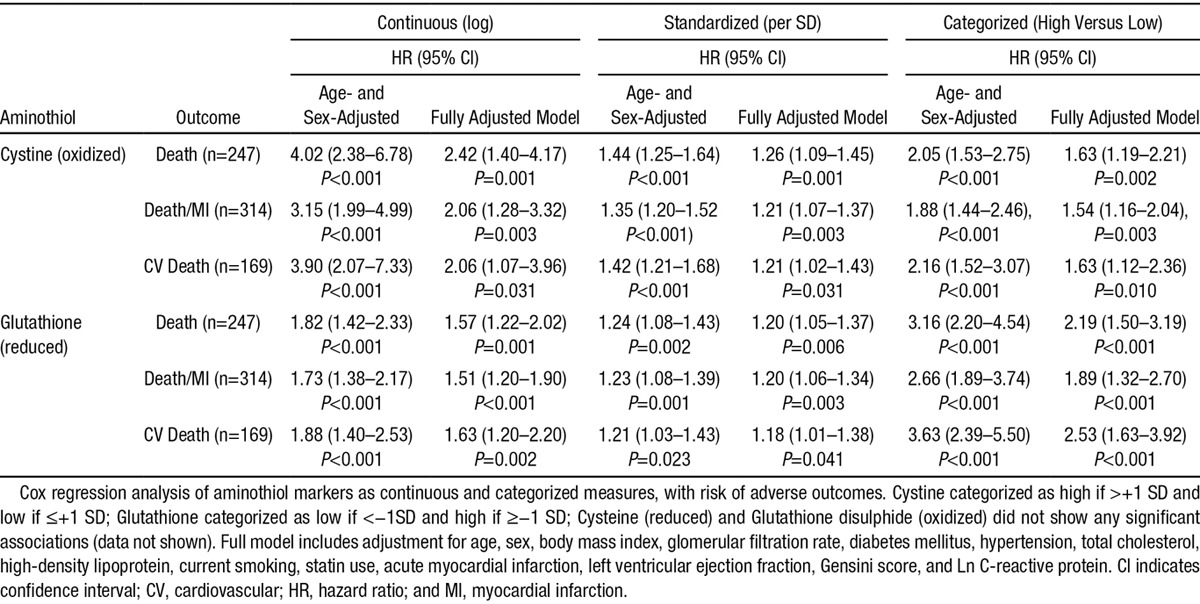
The baseline cystine (P<0.001) and glutathione (P=0.002) levels were both associated with risk of future death after adjustment for age and sex (log values, Table 2). These associations persisted after further adjustment for important covariates (see methods) including hsCRP (P=0.001 and P=0.006, respectively). After standardization, to permit marker comparisons, a 1-SD increment in cystine and a 1-SD decrease in glutathione was associated with a 26% and 20% increase in risk of death after adjustment for all risk factors, respectively (Table 2).
Table 2.
Cox Regression Survival Analysis for the Individual Aminothiol Markers Showing Significant Association With Adverse Events
This association was also evident when cystine and glutathione levels were categorized into quartiles (Kaplan–Meier log rank P<0.001 and P=0.002, respectively; Figure I and Table IV in the online-only Data Supplement). Examination of the Kaplan–Meier plots revealed a possible threshold effect, especially for glutathione.
We further explored this by using a 1-SD cut point to define high and low levels of aminothiol markers (see Methods). Survival analysis confirmed a worse prognosis for patients with high cystine (>+1SD; >129.8 μmol/L) and for those with low glutathione (<−1SD, <0.68 μmol/L) levels (log rank P<0.001 for both; Figure 1). Both a high cystine level and low glutathione level were each associated with a 2- to 3-fold increase in age and sex adjusted risk of death (hazard ratio [HR], 2.05; 95% confidence interval [CI],1.53–2.75; HR, 3.16; 95% CI, 2.20–4.54; respectively). After adjustment for all covariates, a high cystine level was associated with a HR of 1.63 (95% CI, 1.19–2.21) and a low glutathione level of 2.19 (95% CI, 1.50–3.19).
Figure 1.
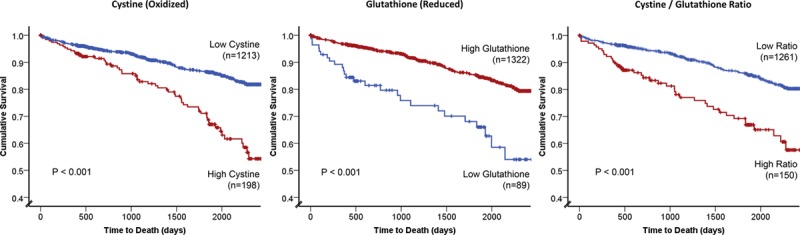
Kaplan–Meier curves for association between high vs low levels of cystine, glutathione, and the cystine/glutathione ratio. High vs low categorization was defined by SD cut off (see Methods). Log rank P values and number of patients within each category are shown.
Importantly, both cystine and glutathione were independently associated with the primary outcome of death, when entered into the same multivariate model. Furthermore, both of these aminothiols were also associated with the secondary outcomes of cardiovascular death and the composite of death and MI (Table 2). Glutathione in particular showed a greater effect size for cardiovascular death compared to all-cause death. However, their respective couples, cysteine (reduced) and glutathione disulphide (oxidized) were not associated with the outcomes examined (data not shown).
Relationship Between Aminothiol Ratios and Outcomes
We also examined the ratio of cystine to glutathione, as a novel measure of extracellular oxidation to intracellular reducing capacity and demonstrated a highly significant association with the primary outcome (P<0.001; Table 3, Figure 1). Patients with a >+1SD level of cystine/glutathione ratio, reflecting a high extracellular oxidant burden and low intracellular reducing capacity, demonstrated a HR of 1.92 (95% CI, 1.39–2.64) for death after full adjustment for all covariates (Table 3). Similar significant associations were noted for the secondary outcomes of cardiovascular death (HR, 1.91; 95% CI, 1.34–2.72) and the composite of death/MI (HR, 1.88; 95% CI, 1.40–2.52).
Table 3.
Cox Regression Survival Analysis for Oxidized to Reduced Ratios of Aminothiol Markers With the Primary Outcome of Death
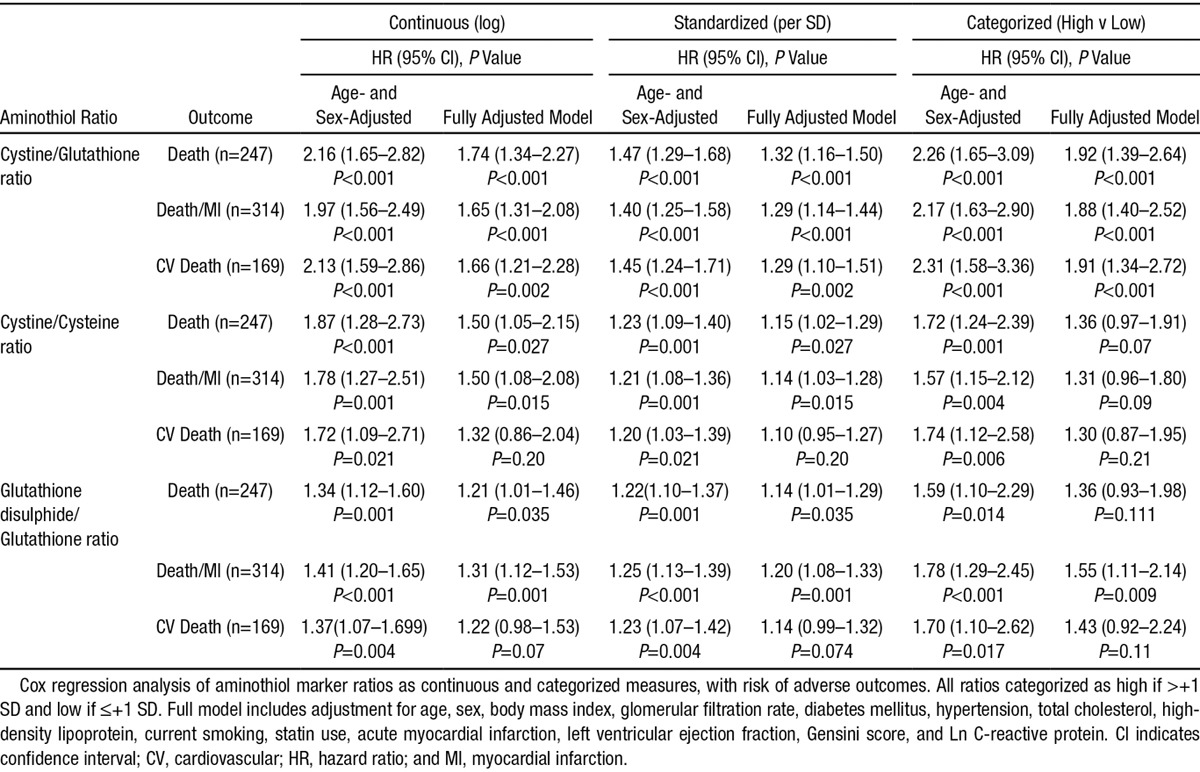
In contrast, although the direct ratios within the extracellular and intracellular compartments of cystine (oxidized) to cysteine (reduced) and glutathione disulphide (oxidized) to glutathione (reduced) were associated with the studied outcomes in adjusted models, the associations were attenuated in comparison with those for cystine, glutathione, or the cysteine/glutathione ratio (Table 3).
CAD and MI Subgroup Analyses
There was no significant heterogeneity in the association between the cystine/glutathione ratio and adverse events based on baseline characteristics. Thus, among patients with obstructive CAD, those with high cystine/glutathione ratio had a HR of 1.80 (95% CI, 1.27–2.54) in comparison with 3.11 (95% CI, 1.19–8.15) for those with nonobstructive CAD. Although there was no significant interaction, the risk of a high cystine/glutathione ratio was higher in those with acute MI in comparison with those without, HR 3.87 (95% CI, 1.74–8.63) and HR 1.82 (95% CI, 1.26–2.62), respectively.
Inflammation and Oxidant Stress
Given that no significant interaction between hsCRP and each of the markers (including their ratios) was found (results not shown) and both hsCRP and the cystine/glutathione ratio were independently associated with risk of death, we devised a simple multi-marker score, using high/low categories to identify the potential value of combining inflammation and OS measures for predicting future events. A score of 0 (n=647) represented both low inflammation (low hsCRP, defined as <3 mg/L [median])23 and low OS (low cystine/glutathione ratio [by SD as above]), whereas a score of 2 reflected both high hsCRP and high cystine/glutathione (n=84). A score of 1 was given to the remaining 661 subjects (Figure 2). In comparison with those with a score of 0, those with a score of 1 had a covariate adjusted HR of 1.46 (95% CI, 1.08–1.97) for risk of death, whereas those with a score of 2 had a HR of 3.26 (95% CI, 2.17–4.90). Thus, patients with a score of 0, 1, or 2 experienced a 1-year death rate of 1.1%, 4.9%, or 14.5% or a 5-year event rate of 9.7%, 17.3%, and 41.5%, respectively.
Figure 2.
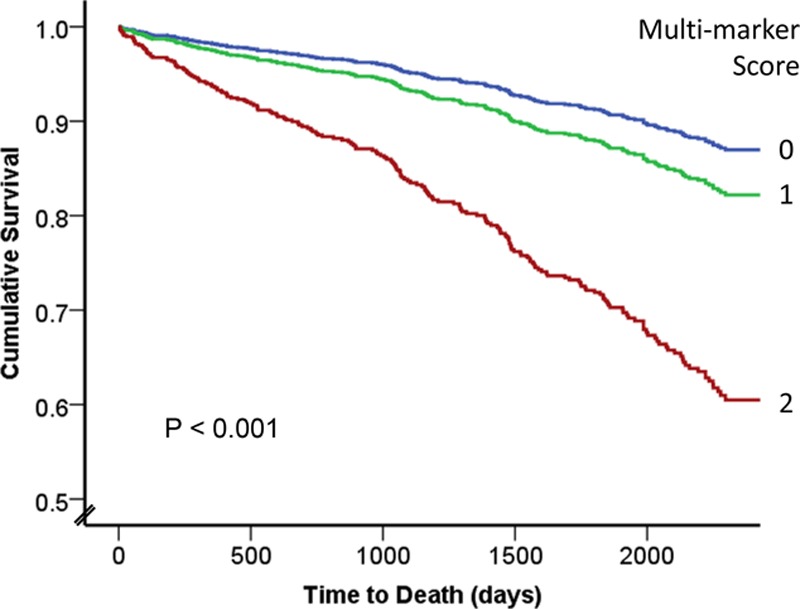
Covariate adjusted survival analysis for the multi-marker score by Cox regression, combining low vs high hsCRP (inflammation) and low vs high cystine/glutathione ratio (oxidative stress [OS]). A score of 0 (blue line, n=647) represents low inflammation and low OS, whereas a score of 2 (red line, n=84) represents high inflammation and high OS. A score of 1 (green line, n=661) represents either high inflammation or high OS only. hsCRP indicates high-sensitivity C reactive protein.
Discrimination Testing
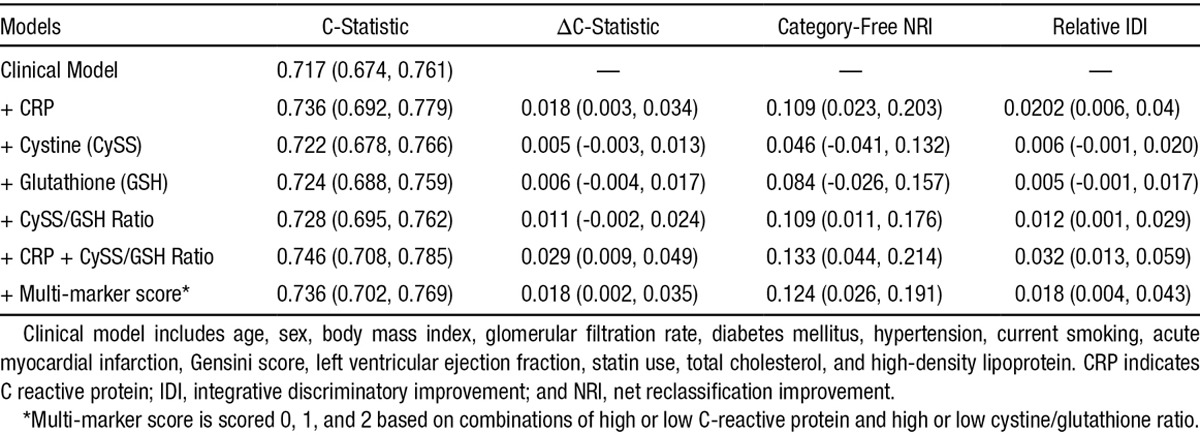
When compared with a standard model for risk of death, consisting of traditional risk factors (see Methods), the addition of hsCRP, hsCRP + the ratio of cystine/glutathione, and the combination of these 2 biomarkers as a multi-marker score improved the risk discrimination significantly, including C-statistic, net reclassification improvement, and integrated discrimination improvement (Table 4). Specifically, whereas addition of the individual aminothiols cystine or glutathione did not improve risk discrimination, the ratio of cystine/glutathione improved both the net reclassification improvement (HR, 0.109; 95% CI, 0.011–0.176) and integrated discrimination improvement (HR, 0.012; 95% CI, 0.001–0.029; Table 4).
Table 4.
Estimates and the Corresponding 95% Confidence Limits for the Risk Discrimination Metrics (Change in C-Statistic, NRI, and IDI) for the Primary Outcome of Death Within Five Years of Follow-Up
Discussion
Herein we demonstrate that the plasma aminothiols cystine and glutathione associate with risk of future death in a high-risk population with CAD. This effect is independent of, and additive to, that of inflammation as assessed by hsCRP. Quantification of plasma aminothiol markers may thus represent an important advance for in vivo assessment of clinically important OS.
Specifically, we show that patients with high OS captured as (1) a high level of oxidized cystine, representing greater extracellular oxidant burden, (2) a low level of reduced glutathione, representing low intracellular reducing capacity, or (3) a high ratio of the 2, have a 2-fold increase in risk of mortality over a mean of 5 years independent of age and other risk factors including inflammation. Whereas previous attempts at quantifying aminothiol mediated OS have used the redox potential of cystine or glutathione disulphide using the Nernst equation,6 we found that the directly combined, cross compartment ratio of cystine to glutathione is simple and practical to calculate and able to discriminate risk, thus representing an improved approach to capturing the overall burden of OS in vivo.
Control of protein redox state via thiol-disulfide switching is critical for normal cellular activities and for maintaining physiological and pathophysiological functions including promotion of CVD. This includes (1) experimental evidence for effects on proinflammatory signaling, mitochondrial oxidation, nuclear factor KB activation, and elevated expression of genes for monocyte recruitment to endothelial cells5,7,8,24; (2) association with clinical risk factors such as aging, obesity, and smoking9,25,26; (3) translational studies confirming association between aminothiols and worse endothelial function, carotid intima media thickness, and arterial stiffness11,27,28; and (4) prospective outcome data in patients at high CVD risk as presented here. In totality, these findings support the use of plasma levels of oxidized and reduced aminothiols as key biomarkers of OS and cellular health and potentially as new therapeutic targets.
The utility of these aminothiols in clinical practice requires further testing. Whereas addition of cystine and glutathione individually did not improve risk discrimination beyond a standard clinical model, addition of the cystine/glutathione ratio did improve both risk reclassification metrics. Given the interplay between inflammation and OS at a molecular level, and with additive effect on risk, we devised and tested a simple multi-marker score combining the biomarkers of each, for ease of clinical use. This simple 3-point score clearly stratified risk and when added to a clinical model also improved the C-statistic and metrics of risk reclassification. Thus, a combination of biomarkers, in this case reflecting aminothiol-mediated OS and inflammation-quantified by CRP, may offer a valuable approach for clinical risk stratification as has been recently described.14
Mechanistically, these findings may have implications for understanding other observations. Homocysteine, an important aminothiol, is biosynthesized from dietary methionine, and in the presence of folate and B vitamins converts to cysteine by cystathione synthase (Figure II in the online-only Data Supplement). While patients with genetic hyperhomocystinemia are prone to severe atherosclerosis, folate supplementation and homocysteine reduction does not appear to reduce CVD risk.29,30 This may be because cysteine is independently maintained from homocysteine and represents a more abundant and reactive aminothiol that on oxidation forms cystine, which is 30 times more abundant than homocysteine and perhaps is a more pathological component. Although some studies have shown association between total cysteine and CVD,31,32 none until now has examined the individual oxidized and reduced components or their respective contribution to the oxidant burden. These data may thus offer a partial explanation for the failure of homocysteine targeted therapy and possible new treatment opportunities.
Although experimental data support the role of free radical biology in OS, clinical attempts at improving outcomes with free radical scavengers (vitamins C, E, etc) have been uniformly disappointing.2,33 Our findings support the hypothesis that in vivo, OS may also be driven by nonfree radical processes, raising the possibility that alternative antioxidative therapies may be more effective. In humans there is no currently known pathway to reduce cystine to cysteine, although cystine levels are in part controlled by the Xc system acting as a highly efficient glutamate-cystine transporter.34 Cellular expression of this transporter declines with age, potentially explaining the association with cystine and age that we and others have observed. In contrast zinc enhances expression of this system and could represent a therapeutic option to reduce plasma cystine and OS. Indeed a recent pilot study in patients with macular degeneration has revealed reductions in plasma levels of oxidized cystine with zinc supplementation, suggesting that levels can be manipulated with therapeutic interventions.35
Strengths and Limitations
Strengths of our study include its prospective design, large sample size, exploration of both reduced and oxidized aminothiols, long follow-up, use of robust clinical outcomes, and exploration of the interaction with inflammation assessed by hsCRP. We did not have dietary information on our subjects, and ingestion of sulfur-rich amino acids may influence plasma aminothiol levels. However, after a meal, cysteine shows rapid distribution and cystine levels increase for 2 to 3 hours and almost all of our patients were fasting for >8 hours, which minimized the likely dietary changes on aminothiol levels. We did not have detailed drug information to explore whether thiol containing medications impacted on measured levels, and it is possible that some patients taking these drugs may have nonrepresentative levels. Our population is also not representative of all populations, and thus our findings may not be generalizable, and require further validation in different groups.
Finally, as an observational study we cannot infer causality, and confounding by CAD remains possible. Nonetheless, oxidative stress is an accepted mechanism for plaque development and previous associations with upstream risk factors and subclinical phenotypes in those without CAD, as well as sensitivity analysis in those with and without CAD showing a consistent effect in both groups, suggests reverse causation is less likely. However confounding can never be fully excluded without interventional studies, but even if causality is not confirmed, this does not limit the value of these thiols for use as biomarkers of intracellular and extracellular oxidative stress, analogous to use of CRP as a biomarker of systemic inflammation.
Conclusions and Implications
A high extracellular oxidant burden or reduced intracellular antioxidant capacity quantified through assessment of plasma aminothiols is associated with higher mortality in patients with CAD. As well as representing potentially novel therapeutic targets, OS measured in this way could complement risk stratification in conjunction with assessment of inflammation assessed by hsCRP. Further studies will evaluate non–high-performance liquid chromatography methods of aminothiol assessments to facilitate their wider use as biomarkers and to investigate whether therapies such as zinc supplementation, seeking to reduce plasma cystine can alter OS and improve outcomes.
Acknowledgments
We thank the many study coordinators and volunteers along with the cath laboratory nurses and physicians who helped facilitate patient enrolment and sample collections. R.S.P. and A.Q. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
R.S.P. was supported by an American Heart Association postdoctoral fellowship while data were being collected and currently by a British Heart Foundation intermediate clinical fellowship (UK); A.A.Q. has been supported by National Institutes of Health (NIH) grants 5P20HL113451-01, 5P01HL101398-02, 1R56HL126558-01, 1U10HL110302-01, and U01 HL-079156; D.P.J. was supported by HL113451, ES 009047, AG038746, ES019776, and HHSN272201200031C. Funding for collection and management of samples was received from the Robert W. Woodruff Health Sciences Center Fund (Atlanta, GA), Emory Heart and Vascular Center (Atlanta, GA), Katz Family Foundation Preventive Cardiology Grant (Atlanta, GA), and in part by NIH grants UL1 RR025008 and R01HL089650-02 from the Clinical and Translational Science Award program.
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.115.019790/-/DC1.
CLINICAL PERSPECTIVE
Although oxidative stress is a critically important process in atherosclerosis, observational evidence and clinical trials of free radical scavengers have proven uniformly disappointing. This has promoted the concept that clinically important oxidative stress may be mediated by nonfree radical species. Proteins with reactive aminothiols are susceptible to oxidation, and quantification of these reduced and oxidized (redox) residues offers an alternative means of quantifying in vivo oxidative stress and oxidant burden. Having developed means to reliably collect and quantify these markers in plasma, we have previously shown associations with multiple risk factors for cardiovascular disease as well as with subclinical markers such as arterial stiffness and intima media thickness. However, whether these markers are clinically relevant has remained unknown. In this study, we now present long-term outcome data demonstrating association between these redox markers and adverse cardiovascular outcomes and mortality. These findings are important as they support the use of these aminothiols as novel and reliable biomarkers of oxidative stress. Importantly, given that oxidation of these aminothiols leads to altered cellular signaling, these findings may offer new opportunities for therapeutic interventions for reducing the adverse clinical impact of oxidative stress in vivo.
References
- 1.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91(3A):7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 2.Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol. 2008;101(10A):14D–19D. doi: 10.1016/j.amjcard.2008.02.003. doi: 10.1016/j.amjcard.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 4.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Go YM, Jones DP. Thiol/disulfide redox states in signaling and sensing. Crit Rev Biochem Mol Biol. 2013;48:173–181. doi: 10.3109/10409238.2013.764840. doi: 10.3109/10409238.2013.764840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009;47:1329–1338. doi: 10.1016/j.freeradbiomed.2009.08.021. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Go YM, Jones DP. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation. 2005;111:2973–2980. doi: 10.1161/CIRCULATIONAHA.104.515155. doi: 10.1161/CIRCULATIONAHA.104.515155. [DOI] [PubMed] [Google Scholar]
- 8.Nkabyo YS, Ziegler TR, Gu LH, Watson WH, Jones DP. Glutathione and thioredoxin redox during differentiation in human colon epithelial (Caco-2) cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1352–G1359. doi: 10.1152/ajpgi.00183.2002. doi: 10.1152/ajpgi.00183.2002. [DOI] [PubMed] [Google Scholar]
- 9.Dröge W. The plasma redox state and ageing. Ageing Res Rev. 2002;1:257–278. doi: 10.1016/s1568-1637(01)00008-3. [DOI] [PubMed] [Google Scholar]
- 10.Samiec PS, Drews-Botsch C, Flagg EW, Kurtz JC, Sternberg P, Jr, Reed RL, Jones DP. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 11.Ashfaq S, Abramson JL, Jones DP, Rhodes SD, Weintraub WS, Hooper WC, Vaccarino V, Harrison DG, Quyyumi AA. The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. J Am Coll Cardiol. 2006;47:1005–1011. doi: 10.1016/j.jacc.2005.09.063. doi: 10.1016/j.jacc.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 12.Ashfaq S, Beinart SC, Abramson JL, Rhodes SD, Jurkovitz C, Vaccarino V, Williams JK, Jones DP, Quyyumi AA, Weintraub WS, Harrison DG. Plasma glutathione redox state: A novel marker of oxidative stress, correlates with early atherosclerosis in humans. J Am Coll Cardiol. 2003;41(Suppl. A):293A–294A. [Google Scholar]
- 13.Patel RS, Al Mheid I, Morris AA, Ahmed Y, Kavtaradze N, Ali S, Dabhadkar K, Brigham K, Hooper WC, Alexander RW, Jones DP, Quyyumi AA. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis. 2011;218:90–95. doi: 10.1016/j.atherosclerosis.2011.04.033. doi: 10.1016/j.atherosclerosis.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eapen DJ, Manocha P, Patel RS, Hammadah M, Veledar E, Wassel C, Nanjundappa RA, Sikora S, Malayter D, Wilson PW, Sperling L, Quyyumi AA, Epstein SE. Aggregate risk score based on markers of inflammation, cell stress, and coagulation is an independent predictor of adverse cardiovascular outcomes. J Am Coll Cardiol. 2013;62:329–337. doi: 10.1016/j.jacc.2013.03.072. doi: 10.1016/j.jacc.2013.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel RS, Su S, Neeland IJ, Ahuja A, Veledar E, Zhao J, Helgadottir A, Holm H, Gulcher JR, Stefansson K, Waddy S, Vaccarino V, Zafari AM, Quyyumi AA. The chromosome 9p21 risk locus is associated with angiographic severity and progression of coronary artery disease. Eur Heart J. 2010;31:3017–3023. doi: 10.1093/eurheartj/ehq272. doi: 10.1093/eurheartj/ehq272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72; discussion 207. doi: 10.1002/sim.2929. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 20.Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30:1105–1117. doi: 10.1002/sim.4154. doi: 10.1002/sim.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uno H, Tian L, Cai T, Kohane IS, Wei LJ. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med. 2013;32:2430–2442. doi: 10.1002/sim.5647. doi: 10.1002/sim.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 24.Jiang S, Moriarty-Craige SE, Orr M, Cai J, Sternberg P, Jr, Jones DP. Oxidant-induced apoptosis in human retinal pigment epithelial cells: dependence on extracellular redox state. Invest Ophthalmol Vis Sci. 2005;46:1054–1061. doi: 10.1167/iovs.04-0949. doi: 10.1167/iovs.04-0949. [DOI] [PubMed] [Google Scholar]
- 25.Kaur S, Zilmer K, Kairane C, Kals M, Zilmer M. Clear differences in adiponectin level and glutathione redox status revealed in obese and normal-weight patients with psoriasis. Br J Dermatol. 2008;159:1364–1367. doi: 10.1111/j.1365-2133.2008.08759.x. doi: 10.1111/j.1365-2133.2008.08759.x. [DOI] [PubMed] [Google Scholar]
- 26.Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, Sternberg P. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic Biol Med. 2003;35:1582–1588. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Ashfaq S, Abramson JL, Jones DP, Rhodes SD, Weintraub WS, Hooper WC, Vaccarino V, Alexander RW, Harrison DG, Quyyumi AA. Endothelial function and aminothiol biomarkers of oxidative stress in healthy adults. Hypertension. 2008;52:80–85. doi: 10.1161/HYPERTENSIONAHA.107.097386. doi: 10.1161/HYPERTENSIONAHA.107.097386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel RS, Al Mheid I, Morris AA, Ahmed Y, Kavtaradze N, Ali S, Dabhadkar K, Brigham K, Hooper WC, Alexander RW, Jones DP, Quyyumi AA. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis. 2011;218:90–95. doi: 10.1016/j.atherosclerosis.2011.04.033. doi: 10.1016/j.atherosclerosis.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Sleight P, Peto R, Collins R. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA. 2010;303:2486–94. doi: 10.1001/jama.2010.840. [DOI] [PubMed] [Google Scholar]
- 30.Zhang SM, Cook NR, Albert CM, Gaziano JM, Buring JE, Manson JE. Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial. JAMA. 2008;300:2012–2021. doi: 10.1001/jama.2008.555. doi: 10.1001/jama.2008.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Khairy L, Ueland PM, Refsum H, Graham IM, Vollset SE European Concerted Action Project. Plasma total cysteine as a risk factor for vascular disease: The European Concerted Action Project. Circulation. 2001;103:2544–2549. doi: 10.1161/01.cir.103.21.2544. [DOI] [PubMed] [Google Scholar]
- 32.Focks JJ, van Schaik A, Clappers N, van Dijk EG, van Oijen MG, Verheugt FW, Peters WH. Assessment of plasma aminothiol levels and the association with recurrent atherothrombotic events in patients hospitalized for an acute coronary syndrome: a prospective study. Clin Chem Lab Med. 2013;51:2187–2193. doi: 10.1515/cclm-2013-0103. doi: 10.1515/cclm-2013-0103. [DOI] [PubMed] [Google Scholar]
- 33.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banjac A, Perisic T, Sato H, Seiler A, Bannai S, Weiss N, Kölle P, Tschoep K, Issels RD, Daniel PT, Conrad M, Bornkamm GW. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27:1618–1628. doi: 10.1038/sj.onc.1210796. doi: 10.1038/sj.onc.1210796. [DOI] [PubMed] [Google Scholar]
- 35.Brantley MA, Jr, Osborn MP, Sanders BJ, Rezaei KA, Lu P, Li C, Milne GL, Cai J, Sternberg P., Jr The short-term effects of antioxidant and zinc supplements on oxidative stress biomarker levels in plasma: a pilot investigation. Am J Ophthalmol. 2012;153:1104–9.e2. doi: 10.1016/j.ajo.2011.12.010. doi: 10.1016/j.ajo.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


