Abstract
AIM: To prepare the conjugate of staphy lococcal enterotoxin A (SEA) protein which is a bacterial SAg and the F(ab’)2 fragment of mAb HAb18 against human hepatocellular carcinoma (HCC), and identify its activity in order to use SAg in the targeting therapy of HCC.
METHODS: MAb HAb18 was extracted from the abdominal dropsy of Balb/c mice, and was purified through chromatography column SP 40HR with Fast protein liquid chromatography (FPLC) system. The F(ab’)2 fragment of mAb HAb18 was prepared by papainic digestion method. The conjugate of mAb HAb18 F(ab’)2 fragment and SEA was prepared with chemical conjugating reagent N succinimidyl 3 (2pyridyldithio) propionate (SPDP) and purified through chromatography column Superose 12 with FPLC system. The molecular mass and purity of each collected peak were identified with SDS-PAGE assay. The protein content was assayed by Lowry’s method. The antibody activity of HAb18 F(ab’)2 against HCC in the conjugate was identified by indirect immunocytochemical ABC method, and the activity of SEA in the conjugate to activate peripheral blood mononuclear cells (PBMC) was identified with MTT assay.
RESULTS: The IgG mAb HAb18 was extracted, and purified successfully. Immunocytochemical staining demonstrated that it reacted with most of HHCC cells of human HCC cell line. There were two peaks in the process of purification of the prepared HAb18 F(ab’)2 SEA conjugate. SDS-PAGE assay demonstrated that the molecular mass of the first peak was about 130 ku, and the second peak was the mixture of about 45 ku and a little 100 ku proteins. The immunocytochemical staining was similar in HAb18 F(ab’)2 SEA conjugate and HAb18 F(ab’)2, i.e.the cytoplasm and/or cell membranes of most HHCC cells were positively stained. The MTT assay showed that the optical absorbance (A) value at 490 nm of HAb18 F(ab’)2 SEA conjugate was 0.182 ± 0.012, that of negative control was 0.033 ± 0.009, and there was significant difference between them (P < 0.05).
CONCLUSION: SPDP is a good protein conjugating reagent and can be used in preparing protein conjugate. The conjugate of mAb HAb18 F(ab’)2 fragment and SEA protein was prepared successfully in present study and can be used in the experimental study of HCC targeting therapy with the conjugate of SAg and anti HCC mAbs or their fragments.
Keywords: carcinoma, hepatocellular/immunology; liver neoplasms/immunology; superantigens; enterotoxins; antibodies, monoclonal
INTRODUCTION
Superantigen (SAg) is a group of proteins which can conjugate with some Vβ fragments of the α or β region of T lymphocytic receptor (TcR) when submitted by major histocompatibility complex (MHC) II molecules. SAg can activate T lymphocytes efficiently and make them release a lot of cytokines[1-5]. SAg has great potential anti-tumor effect, but single SAg has some severe side effects to normal cells expressing MHCII molecules[6]. Using the conjugates of SAg and anti-tumor monoclonal antibody (mAb) or their fusion proteins to treat tumor is a focus in tumor targeting therapy research currently[7-21]. There has been no report about anti-hepatoma with mAb-SAg conjugate until now. The conjugate of SAg-Staphylococcal enterotoxin- A (SEA) and the F(ab’)2 fragment of mAb HAb18 against human hepatocellular carcinoma (HCC) was prepared with heterotype bifunctional conjugating reagent N-succinimidyl-3-(2-pyridyldithio) propionate (SPDP), and the activities of the F(ab’)2 fragment of mAb HAb18 against HCC and SEA’s activating peripheral blood mononuclear cells (PBMC) in the conjugate of HAb18 F(ab’)2 and SEA was identified in this study, laying an experimental base for the research of HCC targeting therapy with mAb-SAg conjugate.
MATERIALS AND METHODS
Cells, animals and main reagents
The hybridoma cells which secrete mAb HAb18 against HCC-associated antigens was established by our department[22]. This hybridoma was prepared by taking the cell suspension of some fresh human HCC tissue as antigen, and the cells were fused according to normal procedures for mAb preparation. Human HCC cell line HHCC was purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). Balb/c mice (body weight of 20 g-25 g) that were used to prepare the abdominal dropsy of mAb HAb18 were provided by the Experimental Animals Center of our university. Papain, mitosis promoter PHA, Methabenzthiazuron (MTT) and dithiothreitol (DTT) were the products of Serva Co. (USA). SPDP was synthesized and kindly provided by Prof. Wen Xue Wang, Department of Toxicology of our university. SEA protein and rabbit mAb against the F(ab’)2 fragment of mouse IgG were purchased from the Academy of Military MedicalSciences(Beijing,China). Immunohistochemical ABC kit was the product of Vector Laboratories Inc. (USA). Dimethyl benzidine (DAB) and dimethyl sulfoxide (DMSO) were the products of Sigma Chemical Co. (USA).
Extract of mAb HAb18 and preparation of its F (ab’)2 fragment
Extract and purification of mAb HAb18 The hybridoma cells which secrete mAb HAb18 (its immunoglobulin type is IgG), was cloned and selected again. The abdominal dropsy of the antibody was prepared by injecting 1 × 106 hybridoma cells into the cavum abdominis of every Balb/c mouse and collected at the aseptic environment. After pretreated with 0.01 mol·L-1 phosphate-buffered saline (PBS) (pH 7.5), the collected abdominal dropsy was purified through chromatography column SP-40HR (column volume was 2 cm × 18 cm, and velocity of flow was 10 mL·min -1) with Fast protein liquid chromatography (FPLC) system (Waters 650E, Waters Co., USA), and the IgG part was collected. Then 227 g·L-1 solid ammonia sulfate was added at 4 °C, agitated for 15-20 min, stood at 4 °C for more than 1 h, and centrifuged at 1500 rpm for 5 min. The supernatant was aborted, while the deposit was dissolved in 0.01 mol·L-1 PBS (pH 7.4) and dialyzed with the same buffer for 12 h-20 h.
Identification of mAb HAb18 The antibody specialty of mAb HAb18 was identified by normal immunocytochemical ABC method. The procedures were as follows: ①HHCC cells were cultured in 6-well culture plates which contained some coverslips in RPMI1640 medium containing 150 mL·L-1 new born bovine serum at 37 °C until the cells grew into log phase; ②the cells were fixed in 950 mL·L-1 ethanol for 15 min; ③7.5 mL·L-1 H2O2 was added and incubated at 37 °C for 10 min; ④normal sheep serum was added and incubated at 37 °C for 30 min; ⑤ MAb HAb18 was added, while PBS was used as negative control, and incubated at 37°C for 1 h; ⑥ biotin-labeled sheep anti-mouse IgG mAb was added and incubated at 37 °C for 30 min; ⑦ ABC complex was added and incubated at 37 °C for 30 min; the color was developed with DAB for 10-20 min; ⑨the slides were counterstained with hematoxylin, dehydrated, cleared and mounted.
Preparation of the F(ab’)2 fragment of mAb HAb18 The F(ab’)2 fragment of mAb HAb18 was prepared by papainic digestion method. The procedures were: ① MAb HAb18 was dialyzed with 1 mol·L-1 NaAc (pH 5.5) for 12 h; ② papain was dissolved in NaAc buffer containing 3 mol·L-1 EDTA and 2 mol·L-1 DTT; ③ the dissolved papain whose mass was about 5% of the pre-digested mAb, was added into the mAb solution and incubated at 37 °C for 10 h; ④ the same concentration of papain was added again and incubated at 37 °C for 10 h-12 h; ⑤ 2 mmol·L-1 iodinate acetamide was added to terminate the reaction; ⑥ at last, the proteins were dialyzed with 0.01 mol·L-1 phosphate buffer (PB) (pH 7.5).
Preparation and purification of HAb18 F(ab’)2-SEA conjugate
SEA protein and mAb HAb18 F(ab’)2 purified through chromatography column with FPLC were respectively dissolved and dialyzed in 0.1 mol·L-1 PB (pH 7.5, containing 0.1 mol·L-1 NaCl). Then they reacted with SPDP, which was dissolved in DMSO and whose molecular value was twice that of HAb18 F(ab’)2 or SEA protein, for 30 min. SEA was dialyzed again in 0.1 mol·L-1 PB (pH 7.5, containing 0.1 mol·L-1 NaCl), and HAb18 F(ab’)2 was dialyzed again in 0.1 mol·L-1 PB (pH 4.6, containing 0.1 mol·L-1 NaCl). DTT whose final concentration was 50 m mol·L-1 was added into HAb18 F(ab’)2. Thirty min later, they were dialyzed in 0.1 mol·L-1 PBS (pH 7.5) in nitrogenous environment and at room temperature. Finally, the complex of SPDP and SEA was mixed with the reductive complex of HAb18 F(ab’)2 and SPDP, and they were stirred for 24 h. The conjugate was purified through chromatography Superose 12 column (column volume was 1 cm × 65 cm, and velocity of flow was 0.2 mL·min-1) with FPLC system. The molecular mass and purity of the protein of each collected peak were identified with SDS-PAGE assay (the concentration of separation gel was 120 g·L-1). The protein content was assayed by Lowry’s method.
Identification of the activity of HAb18 F(ab’)2 in the conjugate
The antibody specialty of HAb18 F(ab’)2-SEA conjugate against HCC was identified by indirect immunocytochemical ABC method. The procedures were: ① HHCC cells were cultured in 6-well culture plates which contained some coverslips in RPMI1640 medium containing 150 mL·L-1 new born bovine serum at 37 °C until the cells grew into log phase; ② the cells were fixed in 950 mL·L-1 ethanol for 15 min; ③ 7.5 mL·L-1 H2O2 was added and incubated at 37 °C for 10 min; ④ normal sheep serum was added and incubated at 37 °C for 30 min; ⑤ HAb18 F(ab’)2-SEA conjugate was added, while HAb18 F(ab’)2 and PBS was respectively used as positive and negative controls, and incubated at 37 °C for 1 h; ⑥ rabbit anti-mouse IgG F(ab’)2 mAb was added and incubated at 37 °C for 30 min; ⑦ biotin-labeled sheep anti-rabbit IgG mAb was added and incubated at 37 °C for 30 min; ⑧ ABC complex was added and incubated at 37 °C for 30 min; ⑨ the color was developed with DAB for 10-20 min; ⑩ the slides were counterstained with hematoxylin, dehydrated, cleared and mounted.
Identification of the activity of SEA in the conjugate to activate PBMC
PBMC was extracted from fresh human peripheral blood that had been treated with anticoagulant reagent heparin by Ficoll’s density gradient centrifugation method, and 2 × 105·well-1 PBMC were added into 96-well flat-bottom culture plates. Then 0.1 mg·L-1 HAb18 F(ab’)2-SEA conjugate was added, while RPMI1640 medium containing 150 mL·L-1 new born bovine serum was used as negative control, 0.1 g·L-1 PHA and 0.02 g·L-1 SEA as positive controls. Six wells were used for each group of cells. They were cultured in CO2 incubator at 37 °C for 60 h, then MTT (2 g·L-1, 50 μL·well-1) was added and incubated continually at 37 °C for 4 h. The medium was centrifuged and discarded, and 100 μL·well-1 DMSO was added. The culture plate was shaken for 10 min and the optical absorbance (A) value at 490 nm was detected with Bio-Rad ELISA instrument. The obtained data was statistically analyzed with Student’s t test.
RESULTS
Extract and identification of mAb HAb18
After dialyzed, the abdominal dropsy of IgG mAb HAb18 was purified successfully with chromatography column SP-40HR (Figure 1). Immunocytochemical staining showed that the positive signal was brown, and located mainly within the cytoplasm and/or on the cell membranes. Most of the HHCC cells were positive. There was no detectable positive signal in negative control.
Figure 1.
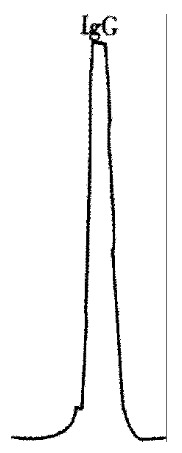
Chromatography for the purification of mAb HAb18.
Purification of HAb18 F(ab’)2-SEA conjugate
There were two peaks in the process of purification and elution of the prepared HAb18 F(ab’)2-SEA conjugate (Figure 2). SDS-PAGE assay demonstrated that the relative molecular mass of the first peak was about Mr. 130 and it was HAb18 F(ab’)2-SEA conjugate. The second peak was the complex of Fab whose relative molecular mass was about 45 and a little F(ab’)2 whose relative molecular mass was about 100 (Figure 3).
Figure 2.
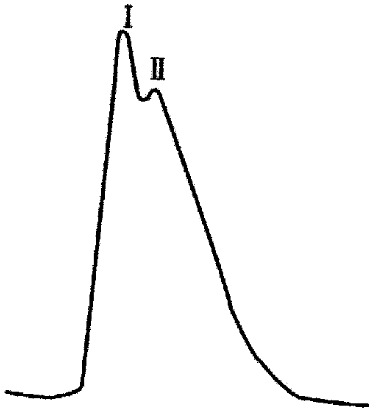
Chromatography for the purification of HAb18 F(ab’)2 SEA conjugate.
Figure 3.
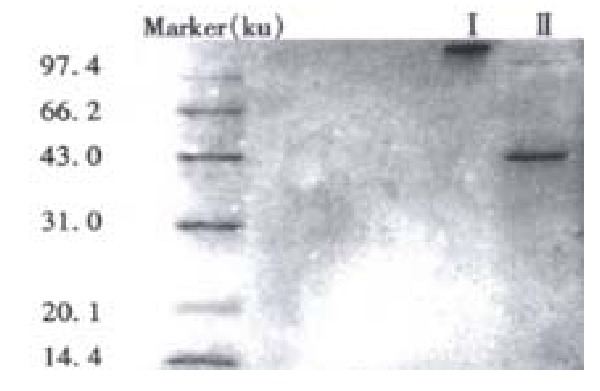
SDS-PAGE assay of the relative molecular mass of purified HAb18 F(ab’)2-SEA conjugate.
Identification of antibody activity of HAb18 F(ab’) 2-SEA conjugate
The result of immunocytochemical staining was similar in HAb18 F(ab’)2-SEA conjugate and HAb18 F(ab’)2, i.e., the cytoplasm and/or cell membranes of most HHCC cells were positively stained, and no detectable positive signal was found in negative control (Figure 4).
Figure 4.
Distribution of HAb18 F(ab’)2 SEA conjugate in human hepatoma HHCC cells. ABC, × 400
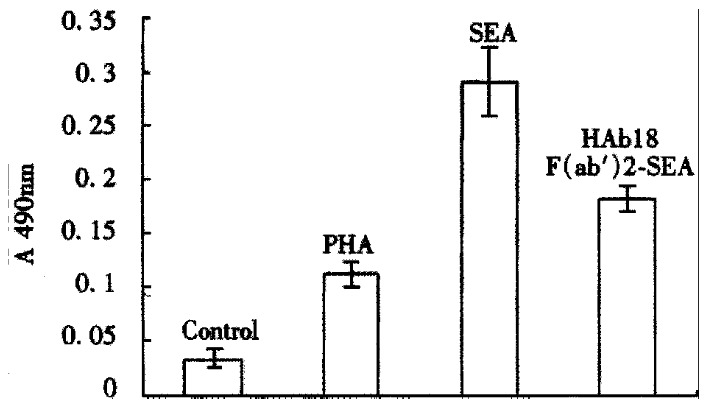
Experimental observation on HAb18 F(ab’)2-SEA conjugate activating PBMC
The result of MTT assay showed that the A value at 490 nm of HAb18 F(ab’)2-SEA conjugate was 0.182 ± 0.012, those of PHA and SEA were respectively 0.112 ± 0.012 and 0.291 ± 0.032, that of negative control was 0.033 ± 0.009. The data of HAb18 F(ab’)2 SEA conjugate, PHA and SEA were all significantly higher than that of negative control (P < 0.05, Figure 5).
Figure 5.
The MTT assay result of HAb18 F(ab’)2-SEA conjugate stimulating PBMC to proliferate.
DISCUSSION
HCC is a common malignant tumor, and there has been no effective treatment up to date[23,24]. Besides the 3 conventional therapeutics, i.e., surgical operation, chemotherapy and radiotherapy, targeting diagnosis and therapy of HCC with anti- HCC mAb have been studied extensively, giving a hopeful prospect to HCC treatment[25-36]. Targeting therapy is a common means of tumor immunotherapy, and is called “biological missile”[37-47]. The “warheads” of” “biological missiles” are usually radioactive nuclides, chemotherapeutants or toxins. Because of the radioactive pollution, “warheads” falling off and other side effects to normal tissue in vivo, the conjugate of mAb and radioactive nuclides, chemotherapeutants or toxins have limited effects and can not be used widely. SAg is a new “warhead” of “biological missiles”. It can kill tumor cells through activating esoteric T lymphocytes, and make the activated T lymphocytes release much useful cytokines. The anti-tumor effect of mAb-SAg conjugate is significantly greater than that of bispecial mAb-mediated cytokines or mononuclear-microphage[8]. Dohlsten et al[7-9] have respectively prepared the chemical conjugates of SEA and anti-colon carcinoma mAb C215, C242, and the fusion proteins of SEA and the Fab fragment of mAb C215, C242 are prepared by gene engineering method. The cytotoxic experiment indicates that these conjugates or fusion proteins do not lose the ability of activating T lymphocytes. RT-PCR assay shows that SEA-activated monocytes express the mRNAs of many cytokines such as IL-1α, Il-1β, IL-2, IL-6, TNF-α, TNF-β and TNF-γ. Ihle et al[10] have prepared the fusion protein of SEA and staphylococcal protein A (SPA) which has a special affinity to IgG, and the conjugates of SEA and anti-CD7 mAb (IgG2a), anti-CD38 mAb (IgG1) respectively, and the cytotoxic experiment to MHCII negative acute T lymphocytic leukemia cells was performed. The results are excellent. The targeting treatment to severe combined immune deficiency (SCID) mice bearing intraperitoneally growing colon carcinoma colo205 cells using fusion protein SEA-C242 Fab shows that human T lymphocytes must be planted into SCID mice before treatment, indicating that SEA-C242 Fab needs to activate T lymphocytes to develop its anti-tumor effect[11]. Litton et al[12] have observed the reaction of tumors and their spherical tissues to fusion protein SEA-C242 with immunohistochemical staining and computer-assisted image analysis, and the results demonstrate that the T lymphocytes and monocytes concentrate in all the tumor tissues just several hours after the SEA-C242 Fab was injected. The main cytokines produced are TNF-α, IL-2, IL-4, IL-5, IL-10, IL-12, IFN-γ, GMSF and TGF-β. The authors consider that apoptosis in tumor cells happens because of the production of cytokines, T lymphocytes infiltration and CD95 (Fas) receptor expression. That is followed by obvious reduction of tumor mass, which is seen within 24 h after the SEA-C242 Fab infusion. In the previous studies of SAg treatment for tumors mAb-SAg conjugates or their fusion proteins were used. mAb-targeted SAg was mainly used in the studies of anti-tumors including colon carcinoma, melanoma, lymphoma, neuroblastoma, etc. There have been some clinical reports about treating tumors with mAb-SEA conjugate[48,49]. Compared with complete mAb, the main advantages of mAb F(ab’)2 fragment are: ① Its volume is smaller, and easy to penetrate through tumor tissues; ② the human anti-mouse antibodies reaction is milder, and suitable for repeated treatments; ③ It does not conjugate with the Fc receptors on non-tumor cells, and can increase T/ NT ratio in vivo. Therefore, in the present study, we used the F(ab’)2 fragment of mAb HAb18, but not intact mAb, and prepared the conjugate of the F (ab’)2 fragment of anti-HCC mAb HAb18 and SEA protein so as to use it in the experimental study of SAg anti-HCC in the future.
In the previous reports, the F(ab’)2 fragment of mAb is usually prepared by pepsic digestion method, and the papainic digestion method is mostly used to prepare the Fab fragment of mAb. The productive rate of preparing IgG fragments with papainic digestion is higher than those of other traditional methods and it is helpful in preserving the antibody activity. When an mAb is digested with papain, the F(ab’)2 fragment can be obtained at pH 5.5, and the Fab fragment can be obtained at pH 8.0[50]. The HAb18 F(ab’)2 prepared in this experiment contained a small amount of Fab, which can not be removed because of the limited conditions of purification. The second peak produced during purification of HAb18 F(ab’)2-SEA conjugate was the complex of HAb18 F(ab’)2 and Fab, but the amount of F(ab’)2 was significantly less than that of Fab. These results indicated that most F(ab’)2 had conjugated with SEA protein, whereas Fab seemed not prone to conjugate.
During the preparation of protein conjugate by SPDP method, the reagents with moderate reacting activity should be used, and the reacting conditions should be mild, such as at room temperature, in neutral pH value and water bath, so as to avoid denaturalization of proteins and loss of biological activity. SPDP is a commonly-used protein conjugating reagent. It can introduce sulfhydryl into protein molecules, and then make the protein molecules conjugate with other protein molecules with sulfhydryl exchange or sulfhydryl addition reactions. There are few side effects of this conjugating reaction, but SPDP also has some shortcomings, such as being prone to inactivate because of deliquescence, and there are many other influencing factors during the operating process. The results in present study suggested that the protein conjugate of mAb HAb18 F(ab’)2 and SEA can be prepared by SPDP method successfully, while the activity of proteins can be kept well.
Because the F(ab’)2 fragment of mAb HAb18 used in this study did not contain Fc fragment, mouse anti-human intact IgG antibodies can not react with it effectively, so the antibody activity of HAb18 F (ab’)2-SEA conjugate can not be identified by normal immunocytochemical method. In our experiment, the activity of mAb F(ab’)2 fragment in HAb18 F(ab’)2-SEA conjugate can be identified effectively by indirect immunocytochemical ABC method, in which rabbit anti-mouse IgG F(ab’)2 mAb was used as the second antibody.
SAg has a strong mitogenetic effect on PBMC because it can activate T lymphocytes effectively[51]. The A value of HAb18 F(ab’)2 SEA was significantly higher than that of negative control in this study (P < 0.05), which indicated that the prepared HAb18 F(ab’)2 SEA conjugate had a significant effect on stimulating the proliferation of PBMC and can be used in the experimental study of HCC targeting therapy with mAb-SAg conjugate.
Footnotes
Supported by the National 863 Project of China, No.102-09-01-02 and National Natural Science Foundation of China, No.39770827
Edited by Ma JY
References
- 1.Tötterman TH, Gidlöf C, Ragnarsson L, Högbom E, Lindeberg M, von der Lehr N, Einarsson A, Soegaard M, Kristensson K, Kalland T, et al. Targeted superantigens for immunotherapy of haematopoietic tumours. Vox Sang. 1998;74 Suppl 2:483–487. doi: 10.1111/j.1423-0410.1998.tb05461.x. [DOI] [PubMed] [Google Scholar]
- 2.Litton MJ, Dohlsten M, Rosendahl A, Ohlsson L, Søgaard M, Andersson J, Andersson U. The distinct role of CD4+ and CD8+ T-cells during the anti-tumour effects of targeted superantigens. Br J Cancer. 1999;81:359–366. doi: 10.1038/sj.bjc.6690701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papageorgiou AC, Acharya KR. Microbial superantigens: from structure to function. Trends Microbiol. 2000;8:369–375. doi: 10.1016/s0966-842x(00)01793-5. [DOI] [PubMed] [Google Scholar]
- 4.Pulaski BA, Terman DS, Khan S, Muller E, Ostrand-Rosenberg S. Cooperativity of Staphylococcal aureus enterotoxin B superantigen, major histocompatibility complex class II, and CD80 for immunotherapy of advanced spontaneous metastases in a clinically relevant postoperative mouse breast cancer model. Cancer Res. 2000;60:2710–2715. [PubMed] [Google Scholar]
- 5.Fraser J, Arcus V, Kong P, Baker E, Proft T. Superantigens - powerful modifiers of the immune system. Mol Med Today. 2000;6:125–132. doi: 10.1016/s1357-4310(99)01657-3. [DOI] [PubMed] [Google Scholar]
- 6.Yin T, Tong SQ, Xie YC, Lu DY. Cyclosporin A protects Balb/c mice from liver damage induced by superan tigen SEB and D-GalN. World J Gastroenterol. 1999;5:209–212. doi: 10.3748/wjg.v5.i3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dohlsten M, Hedlund G, Akerblom E, Lando PA, Kalland T. Monoclonal antibody-targeted superantigens: a different class of anti-tumor agents. Proc Natl Acad Sci USA. 1991;88:9287–9291. doi: 10.1073/pnas.88.20.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dohlsten M, Abrahmsén L, Björk P, Lando PA, Hedlund G, Forsberg G, Brodin T, Gascoigne NR, Förberg C, Lind P. Monoclonal antibody-superantigen fusion proteins: tumor-specific agents for T-cell-based tumor therapy. Proc Natl Acad Sci USA. 1994;91:8945–8949. doi: 10.1073/pnas.91.19.8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dohlsten M, Sundstedt A, Björklund M, Hedlund G, Kalland T. Superantigen-induced cytokines suppress growth of human colon-carcinoma cells. Int J Cancer. 1993;54:482–488. doi: 10.1002/ijc.2910540321. [DOI] [PubMed] [Google Scholar]
- 10.Ihle J, Holzer U, Krull F, Dohlsten M, Kalland T, Niethammer D, Dannecker GE. Antibody-targeted superantigens induce lysis of major histocompatibility complex class II-negative T-cell leukemia lines. Cancer Res. 1995;55:623–628. [PubMed] [Google Scholar]
- 11.Lando PA, Dohlsten M, Ohlsson L, Kalland T. Tumor-reactive superantigens suppress tumor growth in humanized SCID mice. Int J Cancer. 1995;62:466–471. doi: 10.1002/ijc.2910620418. [DOI] [PubMed] [Google Scholar]
- 12.Litton MJ, Dohlsten M, Lando PA, Kalland T, Ohlsson L, Andersson J, Andersson U. Antibody-targeted superantigen therapy induces tumor-infiltrating lymphocytes, excessive cytokine production, and apoptosis in human colon carcinoma. Eur J Immunol. 1996;26:1–9. doi: 10.1002/eji.1830260102. [DOI] [PubMed] [Google Scholar]
- 13.Gidlöf C, Dohlsten M, Kalland T, Tötterman TH. Antibodies are capable of directing superantigen-mediated T cell killing of chronic B lymphocytic leukemia cells. Leukemia. 1995;9:1534–1542. [PubMed] [Google Scholar]
- 14.Tordsson JM, Ohlsson LG, Abrahmsén LB, Karlström PJ, Lando PA, Brodin TN. Phage-selected primate antibodies fused to superantigens for immunotherapy of malignant melanoma. Cancer Immunol Immunother. 2000;48:691–702. doi: 10.1007/s002620050018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litton MJ, Dohlsten M, Hansson J, Rosendahl A, Ohlsson L, Kalland T, Andersson J, Andersson U. Tumor therapy with an antibody-targeted superantigen generates a dichotomy between local and systemic immune responses. Am J Pathol. 1997;150:1607–1618. [PMC free article] [PubMed] [Google Scholar]
- 16.Rosendahl A, Kristensson K, Hansson J, Riesbeck K, Kalland T, Dohlsten M. Perforin and IFN-gamma are involved in the antitumor effects of antibody-targeted superantigens. J Immunol. 1998;160:5309–5313. [PubMed] [Google Scholar]
- 17.Gidlöf C, Carlson B, Dohlsten M, Tötterman TH. Antibody-directed superantigen-mediated T-cell killing of myeloid leukaemic cell line cells. Eur J Haematol. 1998;60:233–239. doi: 10.1111/j.1600-0609.1998.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosendahl A, Kristensson K, Hansson J, Ohlsson L, Kalland T, Dohlsten M. Repeated treatment with antibody-targeted superantigens strongly inhibits tumor growth. Int J Cancer. 1998;76:274–283. doi: 10.1002/(sici)1097-0215(19980413)76:2<274::aid-ijc16>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 19.Søgaard M, Ohlsson L, Kristensson K, Rosendahl A, Sjoberg A, Forsberg G, Kalland T, Dohlsten M. Treatment with tumor-reactive Fab-IL-2 and Fab-staphylococcal enterotoxin A fusion proteins leads to sustained T cell activation, and long-term survival of mice with established tumors. Int J Oncol. 1999;15:873–882. doi: 10.3892/ijo.15.5.873. [DOI] [PubMed] [Google Scholar]
- 20.Newton DW, Dohlsten M, Lando PA, Kalland T, Olsson C, Kotb M. MHC class II-independent, Vbeta-specific activation of T cells by superantigen mutants fused to anti-tumor Fab fragments: implications for use in treatment of human colon carcinoma. Int J Mol Med. 1998;1:157–162. doi: 10.3892/ijmm.1.1.157. [DOI] [PubMed] [Google Scholar]
- 21.Wahlsten JL, Mills CD, Ramakrishnan S. Antitumor response elicited by a superantigen-transmembrane sequence fusion protein anchored onto tumor cells. J Immunol. 1998;161:6761–6767. [PubMed] [Google Scholar]
- 22.Sui Y, He F, Chen Z. [Radioimmunoimaging of hepatocellular carcinoma with 131I labeled antihepatoma monoclonal antibody Fab fragment] Zhonghua Yixue Zazhi. 1998;78:537–539. [PubMed] [Google Scholar]
- 23.Tang ZY. Advances in clinical research of hepatocellular carcinoma in China. Huaren Xiaohua Zazhi. 1998;6:1013–1016. [Google Scholar]
- 24.Badvie S. Hepatocellular carcinoma. Postgrad Med J. 2000;76:4–11. doi: 10.1136/pmj.76.891.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu SX, Fang GY. Clinical use of hepatic carcinoma associated membrane protein antigen (HAg18-1) for detection of primary hepatocellular carcinoma. China Natl J New Gastroenterol. 1996;2:165–166. [Google Scholar]
- 26.Wu YD, Yang KZ, Zhou DN, Gang YQ, Song XQ, Hu XH, Huang BY. Clinical observation of 125 I labeled anti alpha fetoprotein antibody radioimm unotherapy in hepatocellular carcinoma. China Natl J New Gastroenterol. 1997;3:43–46. doi: 10.3748/wjg.v3.i1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Wu MC, Chan H, Zhang BH, Qian GX, Pan WZ, Qiang MY. Anti human AFP variant McAb in radioimmunodetection for primary hepatocellular carcinoma. China Natl J New Gastroenterol. 1997;3:234–235. doi: 10.3748/wjg.v3.i4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong Y, Liu KD, Zhou G, Xue Q, Chen SL, Tang ZY. Tumor radioimmunoimaging of chimeric antibody in nude mice with hepatoma xenograft. World J Gastroenterol. 1998;4:7–9. doi: 10.3748/wjg.v4.i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu K, Wang BC, Chen ZN, Fang P, Liu CG, Wan WX, Liu YF. 99mTc-labeled HAb18 McAb Fab fragment for radioimmunoimaging in nude mice bearing human hepatocellular carcinoma. World J Gastroenterol. 1998;4:117–120. doi: 10.3748/wjg.v4.i2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bian HJ, Chen ZN, Deng JL. Direct technetium-99m labeling of anti-hepatoma monoclonal antibody fragment: a radioimmunoconjugate for hepatocellular carcinoma imaging. World J Gastroenterol. 2000;6:348–352. doi: 10.3748/wjg.v6.i3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu YD, Zhou DN, Gang YQ, Hu XH, Li ZG, Song XQ, He HP, Yang KZ, Huang BY. Double bullet radioimmunotargeting therapy in 31 patients with primary liver cancer. Xin Xiaohuabingxue Zazhi. 1997;5:415–416. doi: 10.3748/wjg.v3.i3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen ZN, Bian HJ, Jiang JL. Kecent progress inanti-hepatoma monoclonal antibody and its application. Huaren Xiaohua Zazhi. 1998;6:461–462. [Google Scholar]
- 33.Wu YD, Song XQ, Zhou DN, Hu XH, Gan YQ, Li ZG, Liao P. Expermental and clinical study on targeting treatment of liver cancer using radionuclide anti-AFP antibody-MMC double bomb. Shijie Huaren Xiaohua Zazhi. 1999;7:387–390. [Google Scholar]
- 34.Kuwata T, Haruta I, Hasegawa K, Yamauchi K, Hayashi N. Antibody dependent cell-mediated cytotoxicity using hepatocellular carcinoma reactive monoclonal antibody. J Gastroenterol Hepatol. 1998;13:137–144. doi: 10.1111/j.1440-1746.1998.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 35.Mohr L, Schauer JI, Boutin RH, Moradpour D, Wands JR. Targeted gene transfer to hepatocellular carcinoma cells in vitro using a novel monoclonal antibody-based gene delivery system. Hepatology. 1999;29:82–89. doi: 10.1002/hep.510290148. [DOI] [PubMed] [Google Scholar]
- 36.Song YQ, Wang GF, Dai XL, Xie H. Enhanced radioimmunotherapeutic efficacy of a monoclonal antibody cocktail against SMMC-7721 human hepatocellular carcinoma. Cell Res. 1998;8:241–247. doi: 10.1038/cr.1998.24. [DOI] [PubMed] [Google Scholar]
- 37.Hu JY, Su JZ, Pi ZM, Zhu JG, Zhou GH, Sun QB. Radioimmunoimaging of colorectal cancer using (99m)Tc labeled monoclonal antibody. World J Gastroenterol. 1998;4:303–306. doi: 10.3748/wjg.v4.i4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan YZ, Wang Q, Xu JY, Zhou WD, Yu YY, Luo W. Measurement of serum carcino associated antigen by anti colon cancer monoclonal antibody in patients with gastric and colon cancer. Xin Xiaohuabingxue Zazhi. 1995;3:3–4. [Google Scholar]
- 39.Lu XP, Li BJ, Chen SL, Lu B, Jiang NY. Anti CEA monoclonal antibody targeting therapy for colorectal carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:329–331. [Google Scholar]
- 40.Lu XP, Li BJ, Chen SL, Lu B, Jiang NY. Effect of chemotherapy or targeting chemotherapy on apoptosis of colorectal carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:332–334. [Google Scholar]
- 41.Schlaeppi JM, Wood JM. Targeting vascular endothelial growth factor (VEGF) for anti-tumor therapy, by anti-VEGF neutralizing monoclonal antibodies or by VEGF receptor tyrosine-kinase inhibitors. Cancer Metastasis Rev. 1999;18:473–481. doi: 10.1023/a:1006358220123. [DOI] [PubMed] [Google Scholar]
- 42.Stein R, Juweid M, Mattes MJ, Goldenberg DM. Carcinoembryonic antigen as a target for radioimmunotherapy of human medullary thyroid carcinoma: antibody processing, targeting, and experimental therapy with 131I and 90Y labeled MAbs. Cancer Biother Radiopharm. 1999;14:37–47. doi: 10.1089/cbr.1999.14.37. [DOI] [PubMed] [Google Scholar]
- 43.Marasco WA. Antibodies for targeted gene therapy: extracellular gene targeting and intracellular expression. Adv Drug Deliv Rev. 1998;31:153–170. doi: 10.1016/s0169-409x(97)00099-9. [DOI] [PubMed] [Google Scholar]
- 44.Tsunoda S, Ohizumi I, Matsui J, Koizumi K, Wakai Y, Makimoto H, Tsutsumi Y, Utoguchi N, Taniguchi K, Saito H, et al. Specific binding of TES-23 antibody to tumour vascular endothelium in mice, rats and human cancer tissue: a novel drug carrier for cancer targeting therapy. Br J Cancer. 1999;81:1155–1161. doi: 10.1038/sj.bjc.6690823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wild MK, Strittmatter W, Matzku S, Schraven B, Meuer SC. Tumor therapy with bispecific antibody: the targeting and triggering steps can be separated employing a CD2-based strategy. J Immunol. 1999;163:2064–2072. [PubMed] [Google Scholar]
- 46.Smith NL, Finley JL, Wennerberg AE, Semer DA, Kearse KP. Immunohistochemically detecting target antigens in patient biopsies for tailoring monoclonal antibody based cancer therapy. Hum Antibodies. 1999;9:61–65. [PubMed] [Google Scholar]
- 47.Cochlovius B, Perschl A, Adema GJ, Zöller M. Human melanoma therapy in the SCID mouse: in vivo targeting and reactivation of melanoma-specific cytotoxic T cells by bi-specific antibody fragments. Int J Cancer. 1999;81:486–493. doi: 10.1002/(sici)1097-0215(19990505)81:3<486::aid-ijc25>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 48.Nielsen SE, Zeuthen J, Lund B, Persson B, Alenfall J, Hansen HH. Phase I study of single, escalating doses of a superantigen-antibody fusion protein (PNU-214565) in patients with advanced colorectal or pancreatic carcinoma. J Immunother. 2000;23:146–153. doi: 10.1097/00002371-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 49.Alpaugh RK, Schultz J, McAleer C, Giantonio BJ, Persson R, Burnite M, Nielsen SE, Vitek L, Persson B, Weiner LM. Superantigen-targeted therapy: phase I escalating repeat dose trial of the fusion protein PNU-214565 in patients with advanced gastrointestinal malignancies. Clin Cancer Res. 1998;4:1903–1914. [PubMed] [Google Scholar]
- 50.Qiu K, Chen ZN, Liu ZG, Wang Q, He FC, Qu P, Mi L, Sui YF, Liu YF. Preparation of anti-hepatoma McAb HAb18 F(ab')2 and Fab fragments by papainic digestions in different conditions. Disi Junyi Daxue Xuebao. 1995;16:414–417. [Google Scholar]
- 51.Hauk PJ, Hamid QA, Chrousos GP, Leung DY. Induction of corticosteroid insensitivity in human PBMCs by microbial superantigens. J Allergy Clin Immunol. 2000;105:782–787. doi: 10.1067/mai.2000.105807. [DOI] [PubMed] [Google Scholar]


