Abstract
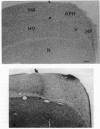
Females of the brood-parasitic brown-headed cowbird (Molothrus ater) search for host nests in which to lay their eggs. Females normally return to lay a single egg from one to several days after first locating a potential host nest and lay up to 40 eggs in a breeding season. Male brown-headed cowbirds do not assist females in locating nests. We predicted that the spatial abilities required to locate and return accurately to host nests may have produced a sex difference in the size of the hippocampal complex in cowbirds, in favor of females. The size of the hippocampal complex, relative to size of the telencephalon, was found to be greater in female than in male cowbirds. No sex difference was found in two closely related nonparasitic icterines, the red-winged blackbird (Agelaius phoeniceus) and the common grackle (Quiscalus quiscula). Other differences among these species in parental care, migration, foraging, and diet are unlikely to have produced the sex difference attributed to search for host nests by female cowbirds. This is one of few indications, in any species, of greater specialization for spatial ability in females and confirms that use of space, rather than sex, breeding system, or foraging behavior per se, can influence the relative size of the hippocampus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Buylla A., Ling C. Y., Nottebohm F. High vocal center growth and its relation to neurogenesis, neuronal replacement and song acquisition in juvenile canaries. J Neurobiol. 1992 Jun;23(4):396–406. doi: 10.1002/neu.480230406. [DOI] [PubMed] [Google Scholar]
- Armstrong E., Clarke M. R., Hill E. M. Relative size of the anterior thalamic nuclei differentiates anthropoids by social system. Brain Behav Evol. 1987;30(5-6):263–271. doi: 10.1159/000118650. [DOI] [PubMed] [Google Scholar]
- Benowitz L. I., Karten H. J. The tractus infundibuli and other afferents to the parahippocampal region of the pigeon. Brain Res. 1976 Jan 30;102(1):174–180. doi: 10.1016/0006-8993(76)90584-9. [DOI] [PubMed] [Google Scholar]
- Bingman V. P., Ioalè P., Casini G., Bagnoli P. Impaired retention of preoperatively acquired spatial reference memory in homing pigeons following hippocampal ablation. Behav Brain Res. 1987 May;24(2):147–156. doi: 10.1016/0166-4328(87)90252-x. [DOI] [PubMed] [Google Scholar]
- Casini G., Bingman V. P., Bagnoli P. Connections of the pigeon dorsomedial forebrain studied with WGA-HRP and 3H-proline. J Comp Neurol. 1986 Mar 22;245(4):454–470. doi: 10.1002/cne.902450403. [DOI] [PubMed] [Google Scholar]
- Crusio W. E., Genthner-Grimm G., Schwegler H. A quantitative-genetic analysis of hippocampal variation in the mouse. J Neurogenet. 1986 Jul;3(4):203–214. doi: 10.3109/01677068609106850. [DOI] [PubMed] [Google Scholar]
- Dark J., Dark K. A., Zucker I. Long day lengths increase brain weight and DNA content in the meadow vole, Microtus pennsylvanicus. Brain Res. 1987 Apr 21;409(2):302–307. doi: 10.1016/0006-8993(87)90715-3. [DOI] [PubMed] [Google Scholar]
- Erichsen J. T., Bingman V. P., Krebs J. R. The distribution of neuropeptides in the dorsomedial telencephalon of the pigeon (Columba livia): a basis for regional subdivisions. J Comp Neurol. 1991 Dec 15;314(3):478–492. doi: 10.1002/cne.903140306. [DOI] [PubMed] [Google Scholar]
- Forger N. G., Fishman R. B., Breedlove S. M. Differential effects of testosterone metabolites upon the size of sexually dimorphic motoneurons in adulthood. Horm Behav. 1992 Jun;26(2):204–213. doi: 10.1016/0018-506x(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., Black J. E., Wallace C. S. Experience and brain development. Child Dev. 1987 Jun;58(3):539–559. [PubMed] [Google Scholar]
- Harvey P. H., Krebs J. R. Comparing brains. Science. 1990 Jul 13;249(4965):140–146. doi: 10.1126/science.2196673. [DOI] [PubMed] [Google Scholar]
- Jacobs L. F., Gaulin S. J., Sherry D. F., Hoffman G. E. Evolution of spatial cognition: sex-specific patterns of spatial behavior predict hippocampal size. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6349–6352. doi: 10.1073/pnas.87.16.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLEN B. II. Embryogenesis of brain nuclei in the chick telencephalon. Ergeb Anat Entwicklungsgesch. 1962;36:62–82. [PubMed] [Google Scholar]
- Krebs J. R., Erichsen J. T., Bingman V. P. The distribution of neurotransmitters and neurotransmitter-related enzymes in the dorsomedial telencephalon of the pigeon (Columba livia). J Comp Neurol. 1991 Dec 15;314(3):467–477. doi: 10.1002/cne.903140305. [DOI] [PubMed] [Google Scholar]
- Krebs J. R., Sherry D. F., Healy S. D., Perry V. H., Vaccarino A. L. Hippocampal specialization of food-storing birds. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1388–1392. doi: 10.1073/pnas.86.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima N., Higashitani F., Teraoka K., Yoshioka R. Sex differences in appetitive learning of mice. Physiol Behav. 1986;37(2):263–268. doi: 10.1016/0031-9384(86)90230-1. [DOI] [PubMed] [Google Scholar]
- Nordeen E. J., Grace A., Burek M. J., Nordeen K. W. Sex-dependent loss of projection neurons involved in avian song learning. J Neurobiol. 1992 Aug;23(6):671–679. doi: 10.1002/neu.480230606. [DOI] [PubMed] [Google Scholar]
- Rehkämper G., Haase E., Frahm H. D. Allometric comparison of brain weight and brain structure volumes in different breeds of the domestic pigeon, Columba livia f.d. (fantails, homing pigeons, strassers). Brain Behav Evol. 1988;31(3):141–149. doi: 10.1159/000116581. [DOI] [PubMed] [Google Scholar]
- Reid S. N., Juraska J. M. Sex differences in the gross size of the rat neocortex. J Comp Neurol. 1992 Jul 15;321(3):442–447. doi: 10.1002/cne.903210310. [DOI] [PubMed] [Google Scholar]
- Roof R. L., Havens M. D. Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Res. 1992 Feb 14;572(1-2):310–313. doi: 10.1016/0006-8993(92)90491-q. [DOI] [PubMed] [Google Scholar]
- Schöpke R., Wolfer D. P., Lipp H. P., Leisinger-Trigona M. C. Swimming navigation and structural variations of the infrapyramidal mossy fibers in the hippocampus of the mouse. Hippocampus. 1991 Jul;1(3):315–328. doi: 10.1002/hipo.450010322. [DOI] [PubMed] [Google Scholar]
- Seress L. Interspecies comparison of the hippocampal formation shows increased emphasis on the regio superior in the Ammon's horn of the human brain. J Hirnforsch. 1988;29(3):335–340. [PubMed] [Google Scholar]
- Sherry D. F., Jacobs L. F., Gaulin S. J. Spatial memory and adaptive specialization of the hippocampus. Trends Neurosci. 1992 Aug;15(8):298–303. doi: 10.1016/0166-2236(92)90080-r. [DOI] [PubMed] [Google Scholar]
- Sherry D. F., Vaccarino A. L., Buckenham K., Herz R. S. The hippocampal complex of food-storing birds. Brain Behav Evol. 1989;34(5):308–317. doi: 10.1159/000116516. [DOI] [PubMed] [Google Scholar]
- Sherry D. F., Vaccarino A. L., Buckenham K., Herz R. S. The hippocampal complex of food-storing birds. Brain Behav Evol. 1989;34(5):308–317. doi: 10.1159/000116516. [DOI] [PubMed] [Google Scholar]
- Turner A. M., Greenough W. T. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Res. 1985 Mar 11;329(1-2):195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- Williams C. L., Barnett A. M., Meck W. H. Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav Neurosci. 1990 Feb;104(1):84–97. doi: 10.1037//0735-7044.104.1.84. [DOI] [PubMed] [Google Scholar]
- Woolley C. S., McEwen B. S. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992 Jul;12(7):2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]