Abstract
Ovarian cancer is the most lethal gynecologic malignancy. Cisplatin is a very effective cancer chemotherapy drug, but cisplatin resistance is a crucial problem of therapy failure. Overexpression of PVT1 has been demonstrated in ovarian cancer. The mRNA level of PVT1 in ovarian cancer tissues of cisplatin-resistant patients and cisplatin-sensitive patients, cisplatin-resistant cells SKOV-3/DDP and A2780/DDP, cisplatin-sensitive cells SKOV-3 and A2780 were determined by qRT-PCR. The influence of the knockdown or overexpression of PVT1 on cisplatin resistance was measured by measuring the cytotoxicity of cisplatin and the apoptotic rate of ovarian cancer cells was detected by CCK-8 assay and flow cytometry, respectively. The mRNA levels and protein expression of TGF-β1, Smad4, p-Smad4 and Caspase-3 in apoptotic pathways were determined. The mRNA level of PVT1 was significantly higher in ovarian cancer tissues of cisplatin-resistant patients and cisplatin-resistant cells. SKOV-3/DDP and A2780/DDP cell viability and the percentage of apoptotic cells after transfection with PVT-1 siRNA and treated with cisplatin was markedly lower and higher than the control, respectively. Moreover, the overexpression of PVT1 exhibited the anti-apoptotic property in SKOV-3 and A2780 cells after transfection with LV-PVT1-GFP and treated with cisplatin. The mRNA levels and protein expression of TGF-β1, p-Smad4 and Caspase-3 were much higher in cisplatin-resistant cells transfected with siPVT1. Overexpression of LncRNA PVT1 in ovarian cancer promotes cisplatin resistance by regulating apoptotic pathways.
Keywords: Ovarian cancer, PVT1, cisplatin, resistance, apoptosis
Introduction
Ovarian cancer is the most lethal gynecologic malignancy. It accounts for approximately 3% of all female cancers and is the fifth most common cause of cancer death in women (accounting for 6% of cancer deaths) h. Efforts at early detection and new therapeutic approaches to reduce mortality have been largely unsuccessful because the origin and pathogenesis of epithelial ovarian cancer are poorly understood [1]. Most ovarian cancer patients are diagnosed at an advanced stage and prospects for significant improvement in survival reside in early diagnosis [2]. Early-stage malignancy is frequently asymptomatic and difficult to detect and thus, by the time of diagnosis, most women have advanced disease. Most of these patients, although initially responsive, eventually develop and succumb to drug-resistant metastases [3]. Ovarian cancer is often associated with drug resistance but the cellular pathways contributing to this effect [4]. Standard treatment for ovarian cancer involves chemotherapy with a platinum agent (cisplatin or carboplatin) and a taxane (paclitaxel), but a successful long-term treatment is prevented by the development of drug resistance [5]. One of the major obstacles in the treatment of ovarian cancer is the development of multidrug resistance. In ovarian cancer, the taxane of choice has historically been casplatin; however, there is now substantial evidence that casplatin also may be the preferred taxane in this disease [6]. The cytotoxic effect of casplatin is primarily due to its ability to promote tubulin assembly and inhibit microtubule depolymerization [7]. In vitro studies have shown that casplatin has a broad spectrum of activity against a variety of tumor types, including breast cancer, ovarian cancer, non-small cell lung cancer, head and neck cancer, colorectal cancer, and melanoma. In vivo experiments in animal models and clinical trials have also shown that casplatin is more potent than paclitaxel in the treatment of advanced breast cancer and other solid tumors [8].
PVT1 is a candidate oncogene located adjacent to the MYC locus on chromosomal region 8q24. It has been shown to act as a noncoding RNA with many alternatively spliced isoforms [9]. The PVT1 locus has recently been found to contain a cluster of at least six microRNAs (miRNAs) (such as miR-1204, -1205, -1206, -1207-3p, -1207-5p, and -1208) that span the PVT1 region, adding further complexity to the locus. Both PVT1 copy number gains (CNGs) and PVT1 overexpression have been implicated in the pathophysiology of many tumors, including breast and ovarian cancers and acute myeloid leukemia [10]. Overexpression of PVT1 has been demonstrated in breast cancer, ovarian cancer, pediatric malignant. Screening for PVT1 expression could potentially improve early diagnosis, therapy, and drug resistance [11]. Zhang er al reported the overexpression of long non-coding RNA PVT1 in gastric cancer cells promotes the development of multidrug resistance [12]. However, the roles that PVT1 play in docetaxel drug resistance remain unclear.
In this study, we aimed to explore the expression of PVT1 in patients with ovarian cancer being resistant to cisplatin and the normal, and the related mechanism.
Material and methods
Cell culture
Tumor tissues were obtained from 30 patients with ovarian cancer sensitive to cisplatin and 30 patients with ovarian cancer resistant to cisplatin. Cisplatin resistance cell lines SKOV-3/DDP and A2780/DDP, parental strains A2780 and A2780/DDP were obtained from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. The cells were routinely cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin in a humidified cell incubator with an atmosphere of 5% CO2 at 37°C. Cells growing at an exponential rate were used for the experiments.
RNA isolation and quantitative RT-PCR
To quantitatively determine mRNA expression of PVT1 in tumor tissues, cell lines SKOV-3/DDP, A2780/DDP, parental strains A2780 and A2780/DDP, qRT-PCR was used. The cells were seeded in a 6-well plate at concentration of 1 × 105 cells per well and incubated in DMEM.
For RNA isolation, total RNA was extracted and isolated from tissue samples or cell lines using either the mirVana miRNA isolation kit (Ambion, Austin, TX) or the TRIzol method. Trizol of 1 mL was added and the solution was mixed homogeneously for 10 min. The mixture was then transferred into eppendorf tubes (EP, 1.5 mL) with 200 μL chloroform. After 15 min shake, the EP tubes were centrifuged at 4°C for 15 min (12000 × g). The supernate was transferred into other EP tubes and mixed with isopyknic isopropanol for 15 s. The centrifugation (4°C, 10 min, 12000 × g) was performed again and the supernate was discarded. The precipitate was washed by 75% ethonal twice and dissolved into 30 μL diethypyrocarbonate (DEPC) after drying to obtain RNA stock solution. After isolation, the concentration of RNA was assessed using a NanoDrop 1000 spectrophotometer (NanoDrop Technologies, Wilmington, Delaware, USA) and the RAN solution was stored at -80°C for further use.
For qRT-PCR, genes were amplified by specific oligonucleotide primer, and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an endogenous control. The detection and quantification contained the following steps: first, reverse transcription was performed at 55°C for 30 min, initial activation for 15 min at 95°C, next 40 cycles of denaturation were conducted at 94°C for 15 s, then annealing for 30 s at 55°C, extension for 30 s at 72°C. The expression level was normalized using U6 small nuclear RNA by the 2-ΔCt method. The ΔCt values were normalized to GAPDH level.
Western blotting
Cultured cells were lysed in RIPA buffer consisting of 1% sodium dodecyl sulfate (SDS), 0.1 mM phenylmethylsulfonyl fluoride (PMSF) and complete protease inhibitors (Roche, Mannheim, Germany). The lysates were centrifuged at 13,000 × g for 15 min, and the supernatants were frozen at -80°C for use. BCA Protein Assay Kit (Pierce Chemical Co., LTD. Rockford, IL, USA) was used to measure the protein concentration of the lysates. Protein samples of 20 μg prepared as indicated above were resolved by SDS-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Millipore Corp., Bedford, MA, USA). After blocking in 5% non-fat milk in Tris-buffered saline for 1 h at room temperature, membranes were incubated in mouse anti-human PTEN monoclonal antibody (Santa Cruz Biotech, Santa Cruz, CA, USA), and followed by horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotech, Santa Cruz, CA, USA). Blots were scanned on the Fluor-S MAX MultiImager (Bio-Rad, Hercules, USA), and signal intensities were determined by Quantity One image software (Bio-Rad, Hercules, USA).
Cell viability
To analyze SKOV3/DDP and A2780/DDP cell viability, Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan) was used in accordance with the manufacturer’s instructions. 100 mL suspension of SKOV3 and A2780 were plated in the 96-well plate supplemented with DMEM added 10% FBS with different concentration (0, 1, 2.5, 5, 10, 20, 40, 80, 150 μM) of cisplatin (Sigma-Aldrich, St. Louis, Missouri, USA). After culture for 24 h at 37°C with 5% CO2, CCK-8 (10 mL) was added into each well. After incubation, the optical density (OD) values were measured by enzyme-labeled instrument (Varian, Palo Alto, CA, USA) at 450 nm. The cell viability was then calculated as follows:
Cell Viability % = (ODt-ODb)/(ODc-ODb) × 100%
Where ODt, ODb and ODc was the OD value of BX647187 siRNA-MG63 group, scramble siRNA- MG63 group and MG63 group, respectively.
Cell apoptosis analysis
At interference with siPVT1 and cisplatin, ovarian cancer cells SKOV3/DDP and A2780/DDP were collected by trypsinisation and washed with phosphate-buffered saline (PBS). In apoptosis analysis, an Annexin-V-FITC Apoptosis Detection Kit I (BD Pharmingen) was used according to the manufacturer’s instructions. After washing with PBS, cells were resuspended in 1 × binding buffer at a concentration of 1 × 106 cells/mL. Then, 5 mL of FITC Annexin-V and 5 μL PI were added to 100 μL of the cell suspension and the samples were incubated for 15 min in the dark. After incubation, 400 μL 1 × binding buffer was added. Apoptosis was analyzed by flow cytometry (FACSCalibur, Becton-Dickinson) using the Cell-Quest software (Becton-Dickinson). The cells undergoing apoptosis were Annexin-V-FITC-positive and PI-negative.
Transfection
RNA interference (RNAi) is an evolutionarily conserved surveillance mechanism that responds to double-stranded RNA by sequence-specific silencing of homologous genes. To investigate the role of PVT1 in cell proliferation and apoptosis, we used a RNAi-based strategy to specifically silence PVT1 expression and PVT1-overexpression lentiviral vector (LV-PVT1-GFP) (Shanghai Cancer Institute, China) to make the overexpression of PVT1. China. siRNA specific for PVT1 (sense 50-GCUUGGAGGCUGAGGAGUUTT-30 and antisense 50-AACUCCUCAGCC UCCAAGCTT-30) were synthesized (Ribobio, Guangzhou, China). PVT1 siRNA and LV-PVT1-GFP transfection (50 nmol/L) was performed in cultured gastric cancer cells. Then, qRT-PCR was used to detect relative mRNA level. Cells were then treated with cisplatin. Colorimetric water-soluble tetrazolium salt (CCK-8) assay using a Cell Counting Kit-8 (Dojindo) was used for cell viability detection and Annexin-V-FITC Apoptosis Detection Kit I (BD Pharmingen) was used for apoptosis analysis.
Data analysis
Mean ± standard deviation (SD) was applied to express the statistics from three separate experiments. The differences between groups were analyzed using SPSS 10.0 software and considered statistically significant at P value less than 0.05.
Results
Relationship between PVT1 and cisplatin resistance
To explore the relationship between PVT1 and cisplatin resistance, we examined the mRNA levels of PVT1 in the ovarian cancer tissues of cisplatin-sensitive patients and cisplatin-resistant patients. As a result, mRNA level of PVT1 increased significantly in the cancer tissues of cisplatin-resistant patients comparing to cisplatin-sensitive patients (Figure 1A). To further study the differential expression of PVT1, we determined the PVT1 expression in cell lines SKOV-3/DDP, A2780/DDP, A2780 and A2780/DDP. Of which, SKOV-3/DDP and A2780/DDP were drug resistant cell lines, while A2780 and A2780/DDP were drug sensitive cell lines. The results were shown in Figure 1B. As shown, overexpression of PVT1 were observed in cell lines SKOV-3/DDP and A2780/DDP comparing with A2780 and A2780/DDP, which indicated PVT1 may be related with the development of cisplatin resistance in ovarian cancer.
Figure 1.
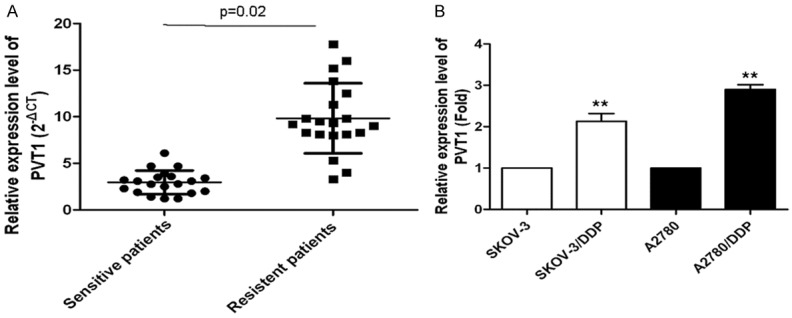
Relationship between PVT1 expression and cisplatin-resistance. A. The mRNA levels of PVT1 in ovarian cancer tissues of cisplatin-sensitive patients and cisplatin-resistant patients; B. The mRNA levels of PVT1 in the cisplatin-resistant SKOV-3/DDP and A2780/DDP cells, and cisplatin-sensitive SKOV-3 and A2780 cells. All values were mean ± SD, **P<0.01, compared with the control, the difference had statistical significance.
PVT1 knockdown reverses the cisplatin resistance in cisplatin-resistant cell lines
Based on the results above, we know PVT1 may be related with the development of cisplatin resistance in ovarian cancer, so we further determined the effect of PVT1 knockdown on cisplatin-induced cytotoxicity and apoptosis in SKOV-3/DDP and A2780/DDP cells. The PVT1 expression in SKOV-3/DDP and A2780/DDP cells after transfection with PVT1 small RNA comparing with the control was shown in Figure 2A. As shown, PVT1 expression in cells after transfection with siPVT1 decreased significantly comparing to the control. Then cells transfected with siPVT1 and the control were treated with 0, 1, 2.5, 5, 10, 20, 40, 80 and 150 μM cisplatin for 24 h, SKOV-3/DDP and A2780/DDP cell viability were determined by CCK-8 assay. As shown in Figure 2B and 2C, cell viability after transfection with siPVT1 decreased markedly with the increase of cisplatin concentration comparing to the control. Furthermore, we determined the percent of apoptotic tumor cells in cells by flow cytometry and the results were shown in Figure 2D. As shown, the percent of apoptotic tumor cells after transfection with siPVT1 was significantly higher than the control. All those indicated knockdown of PVT1 reverses the cisplatin resistance in cisplatin-resistant cell lines.
Figure 2.
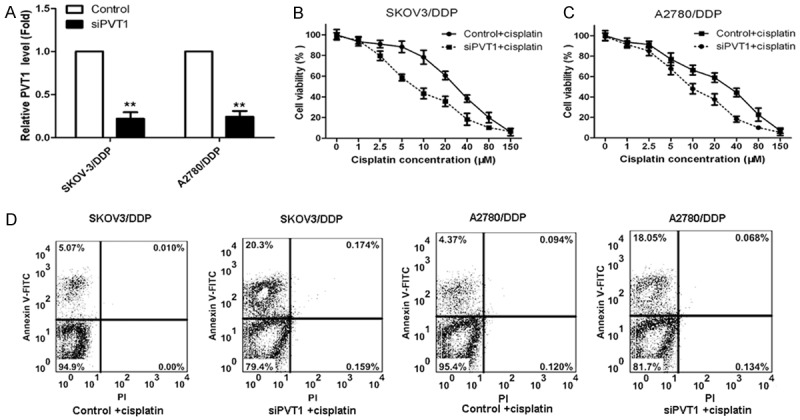
PVT1 knockdown reverses the cisplatin resistance in cisplatin-resistant cell lines. A. PVT1 expression in cisplatin-resistant SKOV-3/DDP and A2780/DDP cells transfected with siPVT1 and the control; B. The influence of PVT1 knockdown on cell viability of SKOV-3/DDP cells transfected with siPVT1 and treated with cisplatin; C. The influence of PVT1 knockdown on cell viability of A2780/DDP cells transfected with siPVT1 and treated with cisplatin; D. The effect of PVT1 knockdown on the percent of apoptotic tumor cells in cells by flow cytometry. All values were mean ± SD, **P<0.01, compared with the control, the difference had statistical significance.
Overexpression of PVT1 inhibits apoptosis in cisplatin-sensitive cell lines
To study the influence of PVT1 overexpression on cisplatin resistance, cisplatin-sensitive SKOV-3 and A2780 cells were transfected with LV-PVT1-GFP. The PVT1 expression in SKOV-3 and A2780 cells transfected with LV-PVT1-GFP and the control were determined, and results were shown in Figure 3A. As shown, PVT1 expression was enhanced markedly in SKOV-3 and A2780 cells after transfection with LV-PVT1-GFP comparing to the control. Then cells transfected with LV-PVT1-GFP and the control were treated with 0, 1, 2.5, 5, 10, 20, 40, 80 and 150 μM cisplatin for 24 h. The cell viability was determined by CCK-8 assay and percent of apoptotic tumor cells in cells was determined by flow cytometry. Cells transfected with LV-PVT1-GFP had much higher cell viability in the same cisplatin concentration than that in the control and decreased with the increase of cisplatin concentration (Figure 3B and 3C). The result of flow cytometry revealed that SKOV-3 and A2780 cells transfected with LV-PVT1-GFP showed a markedly decrease apoptosis index compared to the control (Figure 3D). Those results indicated overexpression of PVT1 inhibits cisplatin-induced apoptosis in SKOV-3 and A2780 cells.
Figure 3.
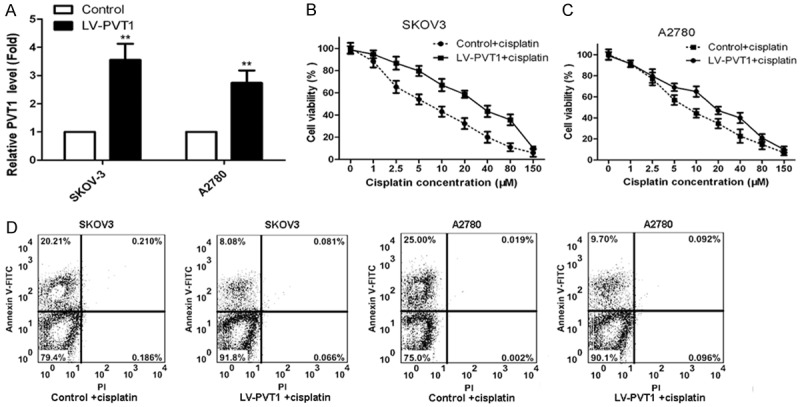
Overexpression of PVT1 inhibits apoptosis in cisplatin-sensitive cell lines. A. PVT1 expression in cisplatin-sensitive SKOV-3 and A2780 cells transfected with LV-PVT1-GFP and the control; B. The influence of PVT1 overexpression on cell viability of SKOV-3 cells transfected with LV-PVT1-GFP and treated with cisplatin; C. The influence of PVT1 overexpression on cell viability of A2780 cells transfected with LV-PVT1-GFP and treated with cisplatin; D. The effect of PVT1 overexpression on the percent of apoptotic tumor cells in cells by flow cytometry. All values were mean ± SD, **P<0.01, compared with the control, the difference had statistical significance.
The mechanism of anticancer activity of PVT1 knockdown
To explore the molecular mechanisms of anticancer activity of PVT1 knockdown, mRNA levels and gene expression on SKOV-3/DDP and A2780/DDP cells transfected with PVT1 siRNA and in the control were determined by qRT-PCR and western bolt assay. Results showed mRNA levels of TGF-β1, p-Smad4 and Caspase-3 in SKOV-3/DDP and A2780/DDP cells after transfection with siPVT1 increased significantly comparing to the control (Figure 4A), whlie mRNA level of Smad 4 had no difference between siPVT1 group and the control. Western bolt analysis also showed protein expression of TGF-β1, p-Smad4 and Caspase-3 in siPVT1 group increased markedly comparing to the control, and protein expression of Smad 4 kept almost the same in the two groups (Figure 4B).
Figure 4.
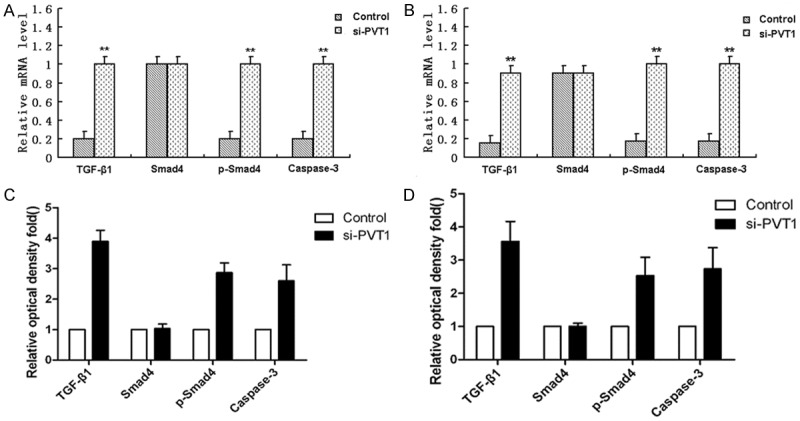
Study on the molecular mechanism about how PVT1 knockdown induce apoptosis. A. mRNA levels of TGF-β1, Smad 4, p-Smad4 and Caspase-3 in SKOV-3/DDP cells after transfection with siPVT1 and in the control group; B. mRNA levels of TGF-β1, Smad 4, p-Smad4 and Caspase-3 in A2780/DDP cells after transfection with siPVT1 and in the control group; C. Protein expression of TGF-β1, Smad 4, p-Smad4 and Caspase-3 in SKOV-3/DDP cells after transfection with siPVT1 and in the control group; D. Protein expression of TGF-β1, Smad 4, p-Smad4 and Caspase-3 in A2780/DDP cells after transfection with siPVT1 and in the control group. All values were mean ± SD, **P<0.01, compared with the control, the difference had statistical significance.
Discussion
Ovarian cancer is the most common gynecologic malignancy and the sixth most common cancer in women worldwide, with highly aggressive natural history causing almost 125,000 deaths yearly [13]. Chemotherapy has been regarded as standard therapy for the majority of women with advanced epithelial ovarian cancer for several decades. Cisplatin is a very effective cancer drug and has had a major clinical impact, particularly for patients with testicular or ovarian cancers [14]. It is generally accepted to be the most active cytotoxic agent for the treatment of ovarian cancer. However, cancer cells often develop multiple mechanisms to cause cisplatin resistance. The reasons why cisplatin resistance occur remains still unclear. Long non-coding RNAs (lncRNAs), a group of non-coding RNAs longer than 200 nucleotides in length, constitute another novel class of non-coding RNAs and regulate gene expression, thus having the possibility to modulate disease progression. Recently, some studies are just beginning to understand the biology of lncRNA in ovarian cancer [15]. PVT1 gene, originally identified as a transcriptional unit from a human homologous sequence to Pvt1, is a lncRNA (1.9 kb) that encodes a number of alternative transcripts and a host gene for several miRNAs [16]. When amplified and overexpressed, PVT1 can increase cell proliferation and inhibit apoptosis, indicating that it is an anti-apoptotic molecule [17] Although several previous reports have identified the functional roles of PVT1 implicated in the pathogenesis of the human diseases, the role that this gene may play in the development of cisplatin resistance in ovarian cancer is few. In this study, we found lncRNA PVT1 is related with cisplatin resistance in ovarian cancer.
In our research, we found PVT1 was overexpressed in tumor tissues of cisplatin-resistant patients comparing to cisplatin-sensitive patients. It was also overexpressed in cisplatin-resistant cells SKOV-3/DDP and A2780/DDP compared with cisplatin-sensitive SKOV-3 and A2780 cells. It indicated PVT1 may be related with the development of cisplatin resistance in ovarian cancer. The effect of PVT1 knockdown on cisplatin-induced cytotoxicity and apoptosis in SKOV-3/DDP and A2780/DDP cells were than determined. Results of CCK-8 assay and flow cytometry showed PVT1 knockdown significantly lowered cell viability and increased the percentage of apoptotic tumor cells in SKOV-3/DDP and A2780/DDP cells transfected with siPVT1 and treated with cisplatin. It manifested PVT1 knockdown can reverses the cisplatin resistance in cisplatin-resistant cell lines. Cisplatin-sensitive SKOV-3 and A2780 cells were transfected with LV-PVT1-GFP to upregulate PVT1. Then cells were treated with different concentration of cisplatin. The cell viability was determined by CCK-8 assay and percent of apoptotic tumor cells in cells was determined by flow cytometry. Results showed overexpression of PVT1 significantly enhanced cell viability and decreased the percentage of apoptotic tumor cells in SKOV-3 and A2780 cells transfected with LV-PVT1-GFP and treated with cisplatin. Moreover, study by Duarte et al. reported cell apoptosis play a key role in the development of drug resistance [18]. Therefore, we obtained lncRNA PVT1 is closely associated with cisplatin resistance in ovarian cancer cells.
To explore the relationship between PVT1 expression and cell apoptosis in molecular level, we determined the mRNA levels and protein expression of TGF-β1, Smad4, p-Smad4 and Caspase-3 in cell apoptotic pathways by qRT-PCR and western blot. Results showed mRNA levels and protein expression of TGF-β1, p-Smad4 and Caspase-3 increased significantly in tumor cells transfected siPVT1. TGF-β1 is a major pluripotential cytokine with a pronounced immunosuppressive effect [19]. This cytokine has multiple effects that may exacerbate fibrosis. It is a strong extracellular matrix inducer and is chemotactic for fibroblasts and polymorphonuclear neutrophils. In addition, it can induce apoptosis in gastric carcinoma cells, primary hepatocytes, hepatoma cells and human lung epithelial cell lines [20]. Sánchez et al. reported the exposure of cells to TGF-β1 can trigger a variety of cellular responses including the inhibition of cell growth, migration, differentiation and apoptosis [21]. Caspase-3 is an effector of apoptosis in experimental models of Parkinson’s disease (PD) [22]. It is a crucial component of the apoptotic machinery in many cell types [23]. Therefore, we concluded PVT1 knockdown the expression of TGF-β1, p-Smad4 and Caspase-3, which were related with cell apoptosis.
In conclusion, PVT1 expression is closely related with cosplatin resistance in ovarian cancer by regulating cell apoptotic pathways.
Disclosure of conflict of interest
None.
References
- 1.Kurman RJ, Shih IeM. The Origin and pathogenesis of epithelial ovarian cancer-a proposed unifying theory. Am J Surg Pathol. 2010;34:433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Balch C, Huang TH, Brown R, Nephew KP. The epigenetics of ovarian cancer drug resistance and resensitization. Am J Obstet Gynecol. 2004;191:1552–72. doi: 10.1016/j.ajog.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Eliopoulos AG, Kerr DJ, Herod J, Hodgkins L, Krajewski S, Reed JC, Young LS. The control of apoptosis and drug resistance in ovarian cancer: influence of p53 and Bcl-2. Oncogene. 1995;11:1217–1228. [PubMed] [Google Scholar]
- 5.Sorrentino A, Liu CG, Addario A, Peschle C, Scambia G, Ferlini C. Role of microRNAs in drug-resistant ovarian cancer cells. Gynecol Oncol. 2008;111:478–86. doi: 10.1016/j.ygyno.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Vasey PA. Role of docetaxel in the treatment of newly diagnosed advanced ovarian cancer. J. Clin. Oncol. 2003;21:136s–144s. doi: 10.1200/JCO.2003.02.051. [DOI] [PubMed] [Google Scholar]
- 7.Zeng S, Zu Chen Y, Fu L, Johnson KR, Fan W. In vitro evaluation of schedule-dependent interactions between docetaxel and doxorubicin against human breast and ovarian cancer cells. Clin Cancer Res. 2000;6:3766–3773. [PubMed] [Google Scholar]
- 8.Fumoleau P, Chevallier B, Kerbrat P, Krakowski Y, Misset JL, Maugard-Louboutin C, Dieras V, Azli N, Bougon N, Riva A. A multicentre phase II study of the efficacy and safety of docetaxel as first-line treatment of advanced breast cancer: report of the Clinical Screening Group of the EORTC. Ann Oncol. 1996;7:165–171. doi: 10.1093/oxfordjournals.annonc.a010544. [DOI] [PubMed] [Google Scholar]
- 9.Meyer KB, Maia AT, O’Reilly M, Ghoussaini M, Prathalingam R, Porter-Gill P, Ambs S, Prokunina-Olsson L, Carroll J, Ponder B. A functional variant at a prostate cancer predisposition locus at 8q24 is associated with PVT1 expression. PLoS Genet. 2011;7:e1002165. doi: 10.1371/journal.pgen.1002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huppi K, Volfovsky N, Runfola T, Jones TL, Mackiewicz M, Martin SE, Mushinski JF, Stephens R, Caplen NJ. The identification of microRNAs in a genomically unstable region of human chromosome 8q24. Mol Cancer Res. 2008;6:212–221. doi: 10.1158/1541-7786.MCR-07-0105. [DOI] [PubMed] [Google Scholar]
- 11.Ding J, Li D, Gong M, Wang J, Huang X, Wu T, Wang C. Expression and clinical significance of the long non-coding RNA PVT1 in human gastric cancer. Onco Targets Ther. 2014;7:1625. doi: 10.2147/OTT.S68854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang XW, Bu P, Liu L, Zhang XZ, Li J. Overexpression of long non-coding RNA PVT1 in gastric cancer cells promotes the development of multidrug resistance. Biochem Biophys Res Commun. 2015;462:227–32. doi: 10.1016/j.bbrc.2015.04.121. [DOI] [PubMed] [Google Scholar]
- 13.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 14.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 15.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Beck-Engeser GB, Lum AM, Huppi K, Caplen NJ, Wang BB, Wabl M. Pvt1-encoded microRNAs in oncogenesis. Retrovirology. 2008;5:4. doi: 10.1186/1742-4690-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan Y, Kuo WL, Stilwell JL, Takano H, Lapuk AV, Fridlyand J, Mao JH, Yu M, Miller MA, Santos JL. Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Clin Cancer Res. 2007;13:5745–5755. doi: 10.1158/1078-0432.CCR-06-2882. [DOI] [PubMed] [Google Scholar]
- 18.Duarte N, Varga A, Cherepnev G, Radics R, Molnár J, Ferreira MJ. Apoptosis induction and modulation of P-glycoprotein mediated multidrug resistance by new macrocyclic lathyrane-type diterpenoids. Bioorg Med Chem. 2007;15:546–554. doi: 10.1016/j.bmc.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 19.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+ CD25+ regulatory T cells. J Exp Med. 2005;201:1061–7. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanagihara K, Tsumuraya M. Transforming growth factor β1 induces apoptotic cell death in cultured human gastric carcinoma cells. Cancer Res. 1992;52:4042–4045. [PubMed] [Google Scholar]
- 21.Sánchez-Capelo A. Dual role for TGF-β1 in apoptosis. Cytokine Growth Factor Rev. 2005;16:15–34. doi: 10.1016/j.cytogfr.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M. Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc Natl Acad Sci U S A. 2000;97:2875–2880. doi: 10.1073/pnas.040556597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyas L, Brophy VA, Pope A, Rivett AJ, Tavaré JM. Rapid caspase-3 activation during apoptosis revealed using fluorescence-resonance energy transfer. EMBO Rep. 2000;1:266–70. doi: 10.1093/embo-reports/kvd050. [DOI] [PMC free article] [PubMed] [Google Scholar]


