Abstract
Background Selenium (Se) is an essential mineral element for animals and humans, which they acquire largely from plants. The Se concentration in edible plants is determined by the Se phytoavailability in soils. Selenium is not an essential element for plants, but excessive Se can be toxic. Thus, soil Se phytoavailability determines the ecology of plants. Most plants cannot grow on seleniferous soils. Most plants that grow on seleniferous soils accumulate <100 mg Se kg–1 dry matter and cannot tolerate greater tissue Se concentrations. However, some plant species have evolved tolerance to Se, and commonly accumulate tissue Se concentrations >100 mg Se kg–1 dry matter. These plants are considered to be Se accumulators. Some species can even accumulate Se concentrations of 1000–15 000 mg Se kg–1 dry matter and are called Se hyperaccumulators.
Scope This article provides an overview of Se uptake, translocation and metabolism in plants and highlights the possible genetic basis of differences in these between and within plant species. The review focuses initially on adaptations allowing plants to tolerate large Se concentrations in their tissues and the evolutionary origin of species that hyperaccumulate Se. It then describes the variation in tissue Se concentrations between and within angiosperm species and identifies genes encoding enzymes limiting the rates of incorporation of Se into organic compounds and chromosomal loci that might enable the development of crops with greater Se concentrations in their edible portions. Finally, it discusses transgenic approaches enabling plants to tolerate greater Se concentrations in the rhizosphere and in their tissues.
Conclusions The trait of Se hyperaccumulation has evolved several times in separate angiosperm clades. The ability to tolerate large tissue Se concentrations is primarily related to the ability to divert Se away from the accumulation of selenocysteine and selenomethionine, which might be incorporated into non-functional proteins, through the synthesis of less toxic Se metabilites. There is potential to breed or select crops with greater Se concentrations in their edible tissues, which might be used to increase dietary Se intakes of animals and humans.
Keywords: Arabidopsis, Astragalus, ecology, evolution, genetic variation, hyperaccumulation, metabolism, quantitative trait locus (QTL), selenium, Stanleya, sulphur
INTRODUCTION: SELENIUM IN SOILS, PLANTS AND ANIMALS
Selenium (Se) is an essential mineral element for both human and animal nutrition (White and Brown, 2010). In humans, Se deficiency is associated with hypothyroidism, cardiovascular disease, a weakened immune system, male infertility, cognitive decline and increased incidence of various cancers (Fairweather-Tait et al., 2011; Rayman, 2012; Fordyce, 2013). The Institute of Medicine (USA) has proposed a recommended dietary allowance of 55 μg Se d–1 for adult humans (Institute of Medicine, 2000). Unfortunately, it is estimated that the diets of as many as 1 billion people might lack sufficient Se for their well-being (Combs, 2001; Fairweather-Tait et al., 2011; Joy et al., 2014; Stoffaneller and Morse, 2015). Since much of the Se in human diets is derived, either directly or indirectly, from edible plants, the lack of Se in human diets is generally attributed to crop production on soils with low Se content or Se phytoavailability (Broadley et al., 2006; White and Broadley, 2009; Chilimba et al., 2011; Fairweather-Tait et al., 2011; Rayman, 2012; Fordyce, 2013; Joy et al., 2015).
Excessive dietary Se intakes can also be harmful to humans and animals (Fairweather-Tait et al., 2011; Rayman, 2012; Fordyce, 2013). The symptoms of mild selenosis in humans include dermatitis, cracking of nails, hair loss and garlicky breath (due to exhalation of dimethylselenide), while severe selenosis can cause acute respiratory distress, myocardial infarction and renal failure. The Institute of Medicine (USA) has suggested a tolerable upper intake of 400 μg Se d–1 for adults (Institute of Medicine, 2000). The symptoms of selenosis in animals, which occur when they consume feed with >1–5 mg Se kg–1 dry matter (DM), include garlicky breath, hair loss, hoof deformation (in cattle), abnormal posture, lack of vitality, slow growth, anorexia, diarrhoea, reduced reproductive performance, fetal deformities and respiratory failure (Dhillon and Dhillon, 2003; Fordyce, 2013). Plants growing on seleniferous soils have tissue Se concentrations sufficient to cause selenosis in animals (Rosenfeld and Beath, 1964; Brown and Shrift, 1982; Dhillon and Dhillon, 2003; Fordyce, 2013).
Selenium concentrations in plants are directly related to Se phytoavailability in the soil, as witnessed by the larger Se concentrations in (1) plants growing in natural soils with greater Se phytoavailability (Rosenfeld and Beath, 1964; Brown and Shrift, 1982; Ihnat, 1989); (2) plants growing in soils anthropogenically contaminated with Se (Fang and Wu, 2004; Wu, 2004); (3) produce grown on agricultural soils with greater Se phytoavailability (Ihnat, 1989; Broadley et al., 2006; Williams et al., 2009; Lee et al., 2011; Garrett et al., 2013; Joy et al., 2015); and (4) produce to which soil or foliar Se fertilizers have been applied (Broadley et al., 2006; White and Broadley, 2009; Chilimba et al., 2012; Fordyce, 2013; Alfthan et al., 2015). Indeed, the application of inorganic Se fertilizers has been particularly effective in increasing Se concentrations in edible crops, increasing the Se content of diets and improving the Se status, and health, of both animals and humans (White and Broadley, 2009; Alfthan et al., 2015).
The concentration and chemical forms of Se in natural soils are determined primarily by geology (Dhillon and Dhillon, 2003; Broadley et al., 2006; White et al., 2007b; Fordyce, 2013; Pilbeam et al., 2015). Selenium concentrations in most soils lie in the range 0·01–2·0 mg Se kg–1, but soils associated with particular geological features can reach concentrations of 1200 mg Se kg–1 (Dhillon and Dhillon, 2003; Fordyce, 2013; Pilbeam et al., 2015). The Se concentrations in the latter soils are toxic to many plants and they support a unique flora (Rosenfield and Beath, 1964; Brown and Shrift, 1982). Seleniferous soils are widespread in the Great Plains of the USA, Canada, South America, Australia, India, China and Russia (Dhillon and Dhillon, 2003; Fordyce, 2013; Pilbeam et al., 2015).
Selenate (SeO42–) is the main water-soluble form of Se in oxic soils (pH + pe > 15), which include most cultivated soils, whereas selenite (SeO32–) predominates in anaerobic soils with a neutral to acidic pH (pH + pe = 7·5–15), such as paddy soils (Mikkelsen et al., 1989; White et al., 2007b; Fordyce, 2013; Pilbeam et al., 2015). Selenide (Se2–) species are stable only under low redox conditions (pH + pe < 7·5) and are rarely present in cultivated soils. Selenate is relatively mobile in the soil solution, but selenite is strongly absorbed by iron and aluminium oxides/hydroxides and, to a lesser extent, by clays and organic matter (Fordyce, 2013; Pilbeam et al., 2015). Thus, the addition of selenate to soils facilitates immediate Se accumulation by plants, while selenite provides a longer lasting Se fertilizer (Broadley et al., 2006; Fordyce, 2013; Pilbeam et al., 2015).
Selenium is not considered to be an essential element for flowering plants (angiosperms), although it is considered to be a beneficial element since it can stimulate growth, confer tolerance to environmental factors inducing oxidative stress, and provide resistance to pathogens and herbivory (Quinn et al., 2007; Pilon-Smits et al., 2009; White and Brown, 2010; El Mehdawi and Pilon-Smits, 2012; Feng et al., 2013). Angiosperm species have been divided into three ecological types according to their ability to accumulate Se in their tissues (Rosenfeld and Beath, 1964; Brown and Shrift, 1982; White et al., 2007a). These types are designated non-accumulator, Se-indicator and Se-accumulator species. Most angiosperm species are non-accumulator species. These species cannot tolerate tissue Se concentrations >10–100 μg Se g–1 DM and cannot colonize seleniferous soils (Rosenfeld and Beath, 1964; White et al., 2004; Dhillon and Dhillon, 2009; Fordyce, 2013). In contrast, Se-indicator species are able to tolerate tissue Se concentrations approaching 1 mg Se g–1 DM and colonize both non-seleniferous and seleniferous soils (Rosenfeld and Beath, 1964; Moreno Rodriguez et al., 2005). Tissue Se concentration in Se-indicator plants is directly related to Se phytoavailability in the soil and, therefore ‘indicates’ soil Se phytoavailability (cf. Baker, 1981). The distribution of Se-accumulator species is generally restricted to seleniferous soils, where their leaf Se concentrations can exceed 1 mg Se g–1 DM (Table 1; Rosenfeld and Beath, 1964; Brown and Shrift, 1982). These species include several members of the Asteraceae, Brassicaceae and Fabaceae, which accommodate large Se concentrations in leaf trichomes and epidermal cells (Freeman et al., 2006, 2010; El Mehdawi and Pilon-Smits, 2012). Several members of the Lecythidaceae family [e.g. Brazil nut (Bertholletia excelsa Humb. and Bonpl.), paradise nut (Lecythis zabucajo Aubl.), coco de mono (Lecythis ollaria Loefl.) and monkeypot nut (Lecythis minor Jacq., syn. Lecythis elliptica Kunth.)] are also renowned for accumulating large Se concentrations in their fruit and seed (Chang et al., 1995; Hammel et al., 1996; Dernovics et al., 2007). Selenium concentrations can reach 512 μg g–1 f. wt, which is equivalent to about 530 μg g–1 DM, in Brazil nuts (Chang et al., 1995), 5–12 mg g–1 DM in seeds of coco de mono (Hammel et al., 1996; Ferri et al., 2004) and 4–6 mg g–1 in monkeypot nuts (Dernovics et al., 2007; Németh et al., 2013). It is thought that the ability to accumulate Se arose by convergent evolution of appropriate Se transport and biochemical pathways in disparate angiosperm clades during geological periods when seleniferous soils were more widespread than they are today (Brown and Shrift, 1982; White et al., 2007a; Cappa and Pilon-Smits, 2014). Species are defined as ‘Se-hyperaccumulators’ if their leaves contain >1 mg Se g–1 DM when sampled from the natural environment (Reeves and Baker, 2000; Terry et al., 2000), although there is debate as to whether this threshold should be lowered to 100 μg Se g–1 DM (Reeves and Baker, 2000; van der Ent et al., 2013). Thus, species that hyperaccumulate Se are an extreme sub-set of Se-accumulator species.
Table 1.
Angiosperm species credited with the appellation of Se-(hyper)accumulator which is formally defined as a species for which plants with shoot Se concentrations >1000 mg Se kg–1 dry matter have been sampled from a natural environment
| Species | Authority | Synonyms | Location | Se concentration (mg Se kg–1 DM) | Reference |
|---|---|---|---|---|---|
| Asteraceae (Asterales) | |||||
| Dieteria canescens | (Pursh) Nutt. | Machaeranthera ramosa | Midwest USA | 1600 | Beath et al. (1939a) |
| Grindelia squarrosa | (Pursh) Dunal | Lower Brule Reservation, SD, USA | 930 | Lakin and Byers (1941) | |
| Gutierrezia microcephala | (DC.) A.Gray | Thompson, UT, USA | 1287 | Beath (1943) | |
| Oonopsis foliosa | Greene | Haplopappus fremontii var. fremontii | Lascar, CO, USA | 3630 | Beath et al. (1939b) |
| Oonopsis wardii | (A.Gray) Greene | O. condensata, Haplopappus fremontii var. wardii | Albany County, WY, USA | 9120 | Byers (1935) |
| Symphyotrichum ascendens | (Lindl.) G.L.Nesom | Soda Springs, ID, USA | 4455 | Pfister et al. (2013) | |
| Symphyotrichum ericoides | (L.) G.L.Nesom | Aster ericoides | Pine Ridge, Fort Collins, CO, USA | 1378 | El Mehdawi et al. (2015) |
| Symphyotrichum lateriflorum | (L.) Á.Löve & D.Löve | Aster multiflorus | SD, USA | 1800 | Moxton et al. (1939) |
| Xylorhiza glabriuscula | Nutt. | X. villosa, Machaeranthera glabriuscula, Aster parryi | Huerfano County, CO, USA | 1750 | Byers et al. (1938) |
| Xylorhiza parryi | Greene | Machaeranthera parryi | Albany County, WY, USA | 5390 | Byers (1935) |
| Xylorhiza venusta | (M.E.Jones) A.Heller | Machaeranthera venusta | Midwest USA | 3486 | Rosenfeld and Beath (1964) |
| Fabaceae (Fabales) | |||||
| Acacia cana | Maiden | NW Queensland, Australia | 1121 | McCray and Hurwood (1963) | |
| Astragalus albulus | Wooton & Standl. | La Ventana, NM, USA | 530 | Beath et al. (1941), listed by Rosenfeld and Beath (1964) | |
| Astragalus asclepiadoides | M.E.Jones | Listed by Brown and Shrift (1982) | |||
| Astragalus beathii | C.L.Porter | Cameron, AZ, USA | 3135 | Beath et al. (1940) | |
| Astragalus beckwithii var. purpureus | M.E.Jones | A. artemisarium | Clark County, NE, USA | 970 | Lakin and Byers (1941) |
| Astragalus bisulcatus | (Hook.) A.Gray | A. bisulcatus var. bisulcatus, A. diholcus, A. scobinatulus | Pine Ridge, Fort Collins, CO, USA | 13 685 | Sura-de Jong et al. (2015) |
| Astragalus bisulcatus var. haydenianus | (A.Gray) Barneby | A. haydenianus | Cuba, NM, USA | 2377 | Beath et al. (1941) |
| Astragalus bisulcatus var. nevadensis | (M.E.Jones) Barneby | Listed by Brown and Shrift (1982) | |||
| Astragalus canadensis | L. | A. carolianus | Las Vegas, NE, USA | 1110 | Byers et al. (1938) |
| Astragalus crotalariae | A.Gray | A. limatus | Truckhaven, CA, USA | 2175 | Beath et al. (1941) |
| Astragalus eastwoodiae | M.E.Jones | A. preusii var. eastwoodiae | Utah, USA | 1664 | Beath (1943) |
| Astragalus flavus | Torr. & A.Gray | A. flavus var. flavus, A. convertiflorus var. flaviflorus, A. flaviflorus | Aztec, NM, USA | 1361 | Beath et al. (1941) |
| Astragalus flavus var. argillosus | (M.E.Jones) Barneby | A. argillosus | Greenriver, UT, USA | 631 | Beath et al. (1941), listed by Brown and Shrift (1982) |
| Astragalus flavus var. candicans | A.Gray | A. convertiflorus | Thompson, UT, USA | 1322 | Beath (1943) |
| Astragalus grayi | S.Watson | Carbon County, WY, USA | 4450 | Byers (1935) | |
| Astragalus linifolius | (Osterh.) Osterh. | Listed by Brown and Shrift (1982) | |||
| Astragalus mokiacensis | A.Gray | Listed by Brown and Shrift (1982) | |||
| Astragalus moencoppensis | M.E.Jones | Listed by Brown and Shrift (1982) | |||
| Astragalus nelsonianus | Barneby | A. pectinatus var. platyphyllus | Listed by Brown and Shrift (1982) | ||
| Astragalus oocalycis | M.E.Jones | Listed by Brown and Shrift (1982) | |||
| Astragalus osterhoutii | M.E.Jones | Kremmling, CO, USA | 2678 | Beath et al. (1940) | |
| Astragalus pattersonii | A.Gray | Thompson, UT, USA | 8512 | Beath (1943) | |
| Astragalus pectinatus | (Hook.) G.Don | Teton County, MT, USA | 5170 | Williams et al. (1940) | |
| Astragalus praelongus | E.Sheld. | A. pattersoni var. praelongus, A. recedens | Leupp, AZ, USA | 4835 | Beath et al. (1941) |
| Astragalus praelongus var. ellisiae | (Rydb.) B.L.Turner | A. ellisiae | Valmont, NM, USA. | 656 | Beath et al. (1941), listed by Brown and Shrift (1982) |
| Astragalus praelongus var. longchopus | Listed by Brown and Shrift (1982) | ||||
| Astragalus preussii | A.Gray | A. preussii var. preusii, A. preussii var. latus | Thompson, UT, USA | 4188 | Beath (1943) |
| Astragalus preussii var. laxiflorus | A.Gray | Listed by Brown and Shrift (1982) | |||
| Astragalus racemosus | Pursh. | A. racemosus var. racemosus, A. racemosus var. treleasei | WY, USA | 14 920 | Knight and Beath (1937) |
| Astragalus racemosus var. longisetus | M.E.Jones | Listed by Brown and Shrift (1982) | |||
| Astragalus rafaelensis | M.E.Jones | Jensen, TX, USAA | 716 | Beath et al. (1941), listed by Brown and Shrift (1982) | |
| Astragalus sabulosus | M.E.Jones | Thompson, UT, USA | 2210 | Beath et al. (1941) | |
| Astragalus saurinus | Barneby | Listed by Brown and Shrift (1982) | |||
| Astragalus toanus | M.E.Jones | ID, USA | 990 | Lakin and Byers (1948) | |
| Astragalus urceolatus | (Greene ex Rydb.) Greene ex Ch. Porter | Listed by Beath et al. (1940) | |||
| Astragalus woodruffi | M.E.Jones | Listed by Brown and Shrift (1982) | |||
| Neptunia amplexicaulis | Domin | Richmond, Queensland, Australia | 4334 | Knott and McCray (1959) | |
| Brassicaceae (Brassicales) | |||||
| Cardamine hupingshanensis | K.M.Liu, L.B.Chen, H.F.Bai & L.H.Liu | Yutangba, Enshi, China | 1965 | Yuan et al. (2013) | |
| Stanleya bipinnata | Greene | S. pinnata var. gibberosa, S. pinnata var. bipinnata | Laramie, WY, USA | 2490 | Beath et al. (1940) |
| Stanleya pinnata | (Pursh) Britton | S. pinnata var. pinnata | Pine Ridge, Fort Collins, CO, USA | >4000 | Galeas et al. (2007) |
| Stanleya pinnata var. integrifolia | (E. James) Rollins | S. integrifolia | Vernal, UT, USA | 977 | Beath et al. (1941) |
| Amaranthaceae (Caryophyllales) | |||||
| Atriplex confertifolia | (Torr. & Frém.) S.Watson | Thompson, UT, USA | 1734 | Beath (1943) | |
| Atriplex nuttallii | S.Watson | WY, USA | 930 | Beath et al. (1937) | |
| Rubiaceae (Gentianales) | |||||
| Coelospermum decipiens | Baill. | Morinda reticulata | Cape York Peninsula, Queensland, Australia | 1141 | Knott and McCray (1959) |
| Orobanchaceae (Lamiales) | |||||
| Castilleja angustifolia var. dubia | A.Nelson | C. chromosa | Lysite, WY, USA | 3460 | Beath et al. (1941) |
For each species the largest tissue Se concentration known to the author, and location of the plant that was analysed, are listed.
Species binomials, authorities and synonyms were consistent with The Plant List (http://www.theplantlist.org/) in July 2015.
SELENIUM UPTAKE, TRANSLOCATION AND METABOLISM IN PLANTS
Plant roots can take up Se as selenate (SeO42–), selenite (SeO32–; HSeO3–; H2SeO3) or organoselenium compounds, such as selenocysteine (SeCys) and selenomethionine (SeMet), but are unable to take up colloidal elemental Se or metal selenides (White and Broadley, 2009). Selenate uptake by root cells from the rhizosphere is catalysed by high-affinity sulphate transporters (HASTs) homologous to the arabidopsis (Arabidopsis thaliana [L.] Heynh.) AtSULTR1;1 and AtSULTR1;2 transporters (Terry et al., 2000; White et al., 2004, 2007b; Sors et al., 2005b; Shinmachi et al., 2010; Gigolashvili and Kopriva, 2014). In arabidopsis, AtSULTR1;1 contributes little to selenate uptake in S-replete plants, but its relative contribution is increased greatly when plants have insufficient S for growth (El Kassis et al., 2007; White et al., 2007b). Phosphate transporters, such as rice OsPT2, catalyse the uptake of HSeO3– (Zhang et al., 2014), and homologues of the rice aquaporin channel OsNIP2;1 catalyse the uptake of H2SeO3 (Zhao et al., 2010; Pommerrenig et al., 2015). Transporters that catalyse the uptake and movement of cysteine and methionine within the plant might transport SeCys and SeMet (Tegeder, 2012).
The arabidopsis genome contains at least 12 genes encoding sulphate transporters, which are divided into four distinct groups that encode proteins with contrasting physiological functions (Gigolashvili and Kopriva, 2014). An equivalent number of genes encoding sulphate transporters are likely to be present in the genomes of other angiosperms, including species that hyperaccumulate Se (Buchner et al., 2004, 2010; Shinmachi et al., 2010; Cabannes et al., 2011; Takahashi et al., 2012; Gigolashvili and Kopriva, 2014). The expression of genes encoding SULTR1;1 and SULTR1;2 generally increases in roots of non-accumulator and Se-indicator species when their growth is restricted by S supply (El Kassis et al., 2007; Rouached et al., 2008; Shinmachi et al., 2010; Schiavon et al., 2015), or when tissue Se concentrations rise (Takahashi et al., 2000; Van Hoewyk et al., 2005; Zhang et al., 2006a; Rouached et al., 2008; Hsu et al., 2011; Inostroza-Blancheteau et al., 2013). Roots of Se-hyperaccumulator species have constitutively high expression of these genes, which might account for their large selenate uptake capacity (Freeman et al., 2010; Cabannes et al., 2011; Schiavon et al., 2015). The increased expression of genes encoding HASTs, particularly SULTR1;1, results in greater uptake capacity for both sulphate and selenate, and accounts for the greater tissue Se concentrations in S-starved plants compared with S-replete plants (Terry et al., 2000; White et al., 2004, 2007b; Hsu et al., 2011). Sulphur-replete arabidopsis mutants lacking SULTR1;2, but not those lacking other sulphate transporters, take up less selenate and exibit greater tolerance to Se in the rhizosphere than wild-type plants (Shibagaki et al., 2002; El Kassis et al., 2007; Barberon et al., 2008). Similarly, the expression of OsPT2 increases in roots of plants lacking sufficient phosphorus and results in a greater capacity for selenite uptake (Zhang et al., 2014), and rice mutants lacking OsPT2 take up significantly less selenite than wild-type plants (Zhang et al., 2014).
To account for the characteristically greater Se/S quotient in shoots of Se-hyperaccumulator plants than in shoots of other plants growing under the same conditions (Rosenfeld and Beath, 1964; Bell et al., 1992; Feist and Parker, 2001; Galeas et al., 2007; White et al., 2007b; Freeman et al., 2010; Cappa et al., 2014; Harris et al., 2014; DeTar et al., 2015; Schiavon et al., 2015), it has been proposed that the complement of HASTs present in the plasma membranes of root cells differs in its selenate/sulphate selectivity between Se-hyperaccumulator and non-accumulator plants (White et al., 2004, 2007a). Specifically, it is hypothesized that the dominant HASTs in the plasma membrane of roots of Se-hyperaccumulator plants are selective for selenate, whereas those in other angiosperms are selective for sulphate. Interestingly, Cabannes et al. (2011) reported that the amino acid sequence of the SULTR1 transporters cloned from all the Astragalus species they studied (the Se-hyperaccumulator species A. bisulcatus [Hook.] A. Gray, A. crotalariae A. Gray and A. racemosus Pursh., and the non-Se-hyperaccumulator species A. glycyphyllos L. and A. drummondii Hook.) differed from that of other angiosperms. In particular, they identified an alanine residue in the SULTR1 cloned from the Astragalus species that corresponded to a conserved glycine residue in all other transporters of the eukaryotic sulphate permease (SulP) family in a position that might determine the selectivity of this transporter. Harris et al. (2014) observed that increasing sulphate concentration in the rhizosphere reduced leaf molybdenum (Mo) concentration in the Se-hyperaccumulator species Stanleya pinnata (Pursh) Britton but not in the Se-indicator plant Indian mustard [Brassica juncea (L.) Czern.] which, they suggested, might reflect different specificities of the complement of selenate/sulphate/molybdate transporters in Se-hyperaccumulator species and those of other angiosperms. Conversely, increasing the molybdate concentration in the rhizosphere had no effect on shoot S concentration in the Se-hyperaccumulator species Astragalus bisulcatus and A. racemosus, but reduced shoot S concentration in congeneric non-hyperaccumulator species (DeTar et al., 2015).
Selenite is rapidly converted to organoselenium compounds in the root, whereas selenate is delivered immediately to the xylem (White et al., 2004; Ximénez-Embún et al., 2004; Li et al., 2008). Sulphate transporters homologous to arabidopsis AtSULTR2;1, AtSULTR2;2 and AtSULTR3;5 have been implicated in the long-distance transport of selenate in the xylem (Takahashi et al., 2000; Gigolashvili and Kopriva, 2014). Selenium is also transported, to a very limited extent, as SeMet and selenomethionine Se-oxide (SeOMet) in the xylem (Li et al., 2008). In arabidopsis, the low-affinity sulphate transporters AtSULTR2;1 and AtSULTR2;2 are thought to catalyse selenate uptake into cells within the stele, whereas AtSULTR3;5 appears to modulate the activity of AtSULTR2;1, but does not catalyse transport itself (Kataoka et al., 2004a). The expression of AtSULTR2;1, AtSULTR2;2 and their homologues in other plants is induced both by S starvation and by increasing Se availability (Takahashi et al., 2000; Buchner et al., 2004, 2010; Van Hoewyk et al., 2005; Gigolashvili and Kopriva, 2014). Interestingly, the expression of SULTR2 genes in roots of S-replete plants of Se-hyperaccumulating Astragalus species is greater than in S-replete plants of non-Se-hyperaccumulator Astragalus species and S-starved plants of other non-Se-hyperaccumulator species (Cabannes et al., 2011). This might account for the constitutively large Se fluxes from the root to the shoot in Astragalus species that hyperaccumulate Se. In addition, the amino acid sequences of SULTR2 and SULTR3;4 from the Se-hyperaccumulator species A. racemosus and A. bisulcatus differ from those of the congeneric non-Se-hyperaccumulator species A. drummondii (Cabannes et al., 2011). Stanleya pinnata also exhibits a high constitutive expression of SpSULTR2;1 (Schiavon et al., 2015).
Selenate is assimilated into organoselenium compounds in plastids (White et al., 2007b; Pilon-Smits and LeDuc, 2009; Pilon-Smits, 2012). The sulphate transporter AtSULTR3;1 is localized in the chloroplast membrane (Cao et al., 2013) and might catalyse selenate transport into plastids. Selenate is first activated by adenosine triphosphate sulphurylase (ATPS) to form adenosine 5'-phosphoselenate (APSe), which is then reduced to selenite by adenosine 5'-phosphosulphate reductase (APR) using reduced glutathione (GSH) as the electron donor. There are four genes encoding ATPS and three genes encoding APR in the arabidopsis genome, and equivalent numbers in the genomes of other plant species (Schiavon et al., 2015). In non-accumulator and Se-indicator species, the expression of genes encoding ATPS (APS) decreases as S supply is reduced, whereas in Se-hyperaccumulator species, such as S. pinnata, they appear to be constitutively expressed (Freeman et al., 2010; Schiavon et al., 2015). Intriguingly, the expression of APS and several SULTR genes appears to be co-regulated through the expression of micro RNA (miRNA), such as miRNA395 (Paul et al., 2015). The conversion of selenate to selenite appears to be the rate-limiting step in the assimilation of Se into organic compounds (Pilon-Smits et al., 2009). Overexpressing genes encoding ATPS or APR in transgenic plants leads to the accumulation of organic Se in their leaves (Pilon-Smits et al., 1999b; Van Huysen et al., 2004; Bañuelos et al., 2005b; Sors et al., 2005a). Selenite is reduced to selenide enzymatically by sulphite reductase (Pilon-Smits, 2012) or non-enzymatically by reduced glutathione (Terry et al., 2000).
The synthesis of SeCys from serine and selenide is catalysed by cysteine synthase, an enzyme complex containing both serine acetyl transferase (SAT) and O-acetylserine (thiol) lyase (OAS-TL) subunits (Birringer et al., 2002; Sors et al., 2005b; White et al., 2007b; Ogra and Anan, 2012; Pilon-Smits, 2012). Many genes encoding enzymes in the primary S/Se assimilation pathway are upregulated when plant Se supply is increased, and often exhibit constitutively high expression in Se-hyperaccumulator species (Van Hoewyk et al., 2005, 2008b; Freeman et al., 2010). Selenomethionine is synthesized from SeCys and O-phosphohomoserine (OPHS) through the sequential actions of cystathionine γ-synthase (CγS), which produces selenocystathionine (SeCysta), cystathionine β-lyase (CBL), which produces selenohomocysteine (SeHCys), and methionine synthase (MTR). Selenocysteine is the most abundant form of Se in unselenized garlic (Allium sativum L.; Cai et al., 1995), and SeMet is often the most abundant form of Se in edible seeds and cereal grains (Smrkolj et al., 2005, 2006, 2007; Broadley et al., 2006; Kápolna et al., 2007; Rayman et al., 2008; Thavarajah et al., 2008; Zhu et al., 2009; Seppänen et al., 2010; Hart et al., 2011; Fairweather-Tait et al., 2011; Shao et al., 2014), in seeds of Lecythidaceae (Vonderheide et al., 2002; Dumont et al., 2006; Ferri et al., 2004; Németh et al., 2013; da Silva et al., 2013) and in potato (Solanum tuberosum L.) tubers (Gionfriddo et al., 2012). Selenocystathionine appears to be the most abundant form of Se in the non-Se-hyperaccumulator species Stanleya albescens M.E. Jones, and is also present at high concentrations in tissues of several Se-hyperaccumulator species (Birringer et al., 2002; Ferri et al., 2004; Freeman et al., 2006, 2010; Németh et al., 2013). It is also the main Se compound in cladodes and fruit of selenized prickly pear (Opuntia ficus-indica [L.] Mill.; Bañuelos et al., 2011). Interestingly, most of the Se in roots and shoots of the Se-hyperaccumulator species Cardamine hupingshanensis KM Liu et al. is found as selenocystine (SeCys2; Yuan et al., 2013), which is also abundant in fruits of Lecythidaceae (Dumont et al., 2006; da Silva et al., 2013), and Se biofortification of some plants, such as Japanese pungent radish (Raphanus sativus L.), results in the formation of selenohomolanthionine from SeHCys (Ogra et al., 2007). Selenized brassicas, such as broccoli, cauliflower (Brassica oleracea L.) and black mustard (Brassica nigra [L.] K.Koch), can also contain large concentrations of seleno-glucosinolates and their Se-aglycons (Matich et al., 2012, 2015; Ouerdane et al., 2013), and selenosugars, possibly of cell wall origin, have also been reported in appreciable concentrations in selenized plants (Aureli et al., 2012).
Selenium toxicity has been attributed to the non-specific replacement of cysteine and methionine in proteins by SeCys and SeMet (Brown and Shrift, 1982; Van Hoewyk, 2013). The magnitude of this appears to be related to the tissue Se/S quotient, rather than the Se content alone (White et al., 2004; El Kassis et al., 2007). In particular, the replacement of cysteine with SeCys prevents the formation of disulphide bridges, which are essential for protein structure and function, and the replacement of cysteine with SeCys in the active site of enzymes impairs catalytic activity (Brown and Shrift, 1982; Van Hoewyk, 2013). Thus, the conversion of SeCys and SeMet to non-toxic or volatile Se metabolites can increase plant Se tolerance (Sors et al., 2005b; White et al., 2007b; Pilon-Smits and LeDuc, 2009; Van Hoewyk, 2013). Selenocysteine methyltransferase (SMT) catalyses the methylation of SeCys to Se-methylselenocysteine (SeMSeCys), and S-adenosyl-methionine:methionine methyl transferase (MMT) catalyses the methylation of SeMet to Se-methylselenomethionine (SeMSeMet; Sors et al., 2005b; White et al., 2007b; Pilon-Smits and LeDuc, 2009; Van Hoewyk, 2013). Genes encoding functional SMT are not thought to exist in plants with little Se tolerance, such as arabidopsis (Lyi et al., 2005; Van Hoewyk, 2013; Zhao et al., 2015), and there is only a single gene encoding MMT in the arabidopsis genome (Tagmount et al., 2002). The expression of BoSMT increases upon exposure of broccoli to selenate and correlates with the accumulation of SeMSeCys (Lyi et al., 2005), while differences among Astragalus and Stanleya species in their ability to accumulate Se appear to be directly correlated with SMT activity (Sors et al., 2005a, 2009; Freeman et al., 2010). The AbSMT gene appears to be expressed constitutively in Astragalus bisulcatus (Pickering et al., 2003). SeMSeCys is the most abundant form of Se in roots and shoots of Se-hyperaccumulator species, such as A. bisulcatus and Stanleya pinnata (Birringer et al., 2002; Pickering et al., 2003; Sors et al., 2005a; Freeman et al., 2006, 2010; Lindblom et al., 2013; Alford et al., 2014), in allium (chive, garlic, leek, onion) and brassica (broccoli, Brussels sprouts, cabbage, cauliflower, Chinese cabbage, kale) crops fertilized with either selenate or selenite (Birringer et al., 2002; Sugihara et al., 2004; Rayman et al., 2008; Zhu et al., 2009; Fairweather-Tait et al., 2011; Kápolna et al., 2012; Ávila et al., 2014; Thosaikham et al., 2014), and in leaves of other vegetable crops fertilized with selenite (Sugihara et al., 2004; Mazej et al., 2008). It is also present in large concentrations in tubers of selenized potato (Gionfriddo et al., 2012) and seeds of selenized legumes (Smrkolj et al., 2007; Shao et al., 2014). Selenocysteine can also be converted to alanine and elemental Se by a SeCyslyase (cpNifS) located in the chloroplast (van Hoewyk et al., 2008a; Pilon-Smits and Leduc, 2009). Although elemental Se is not commonly observed in leaves, significant amouts of elemental Se have been found in stems, nodules and roots of Se-hyperaccumulator plants grown in the presence of appropriate endosymbiotic bacteria and fungi (Valdez Barillas et al., 2012; Lindblom et al., 2013; Sura-de Jong et al., 2015). It is also noteworthy that plant genomes contain genes encoding putative Se-binding proteins (SBPs) that might contribute to Se tolerance in plant tissues (Agalou et al., 2005; Dutilleul et al., 2008). In the arabidopsis genome, there are three genes encoding SBPs. The expression of AtSBP1, and its homologues in other plants, is upregulated in response to S starvation (Hugouvieux et al., 2009; Byrne et al., 2010).
Both SeMSeCys and SeMSeMet can be conjugated with glutamate to form γ-glutamyl-SeMSeCys (γ-Glu-SeMSeCys) or γ-glutamyl-SeMSeMet (γ-Glu-SeMSeMet), or converted to dimethyldiselenide (DMSe) or dimethylselenide (DMDSe) and volatilized (Sors et al., 2005b; White et al., 2007b; Pilon-Smits and LeDuc, 2009; Ogra and Anan, 2012; Van Hoewyk, 2013). SeMSeMet can also be converted to dimethylselenonium propionate and thence to DMSe (Grant et al., 2004). Many Se-hyperaccumulator species, such as A. bisulcatus (Freeman et al., 2006; Alford et al., 2014), and allium crops (garlic, leek, onion) grown on Se-rich soils accumulate significant concentrations of γ-glutamyl-SeMeSeCys (Sugihara et al., 2004; Ogra et al., 2005; Broadley et al., 2006; White et al., 2007b; Rayman et al., 2008; Fairweather-Tait et al., 2011; Kápolna et al., 2012). In A. bisulcatus, the formation of γ-glutamyl-SeMeSeCys appears to be promoted by rhizobial symbiosis, which has been attributed to a greater supply of glutamate in nodulated plants (Alford et al., 2014). The Se compound γ-glutamyl-Secystathionine has also been reported in some Se-hyperaccumulator plants (e.g. monkeypot nuts; Dernovics et al., 2007). In general, Se is volatilized as DMSe in non-hyperaccumulator species and as DMDSe in Se-hyperaccumulator species (Pilon-Smits and LeDuc, 2009). There is considerable variation among angiosperms in their ability to volatilize Se (Terry et al., 1992; Pilon-Smits et al., 1999a; de Souza et al., 2000), and the production of these volatiles appears to be determined by the conversion of SeCys to SeMet, and transgenic plants overexpressing CγS volatilize more Se than untransformed plants (Pilon-Smits and LeDuc, 2009).
Selenium concentrations tend to be greatest in the younger leaves of plants and generally increase to a maximum during seedling growth, then decline before, or upon, flowering, when Se is translocated from leaves to reproductive organs (Rosenfeld and Beath, 1964; Turakainen et al., 2004; Galeas et al., 2007; White et al., 2007b; Cappa et al., 2014; Harris et al., 2014). This is consistent with transcriptional analyses suggesting that Se/S assimilation occurs predominantly in younger leaves and especially the first leaves a plant produces (White et al., 2007b). Selenium is readily redistributed in the phloem as both selenate and the organoselenium compounds SeMet and SeMSeCys (Carey et al., 2012). In arabidopsis, the HAST AtSULTR1;3 is thought to catalyse selenate uptake into the phloem and the expression of AtSULTR1;3 is increased in S-deficient plants (Yoshimoto et al., 2003).
Most plant cells can accumulate selenate in their vacuoles. When non-accumulator plants are fertilized with selenate, much of this is translocated to the shoot and sequestered in the vacuoles of cells within the vasculature and leaf meophyll (Ximénez-Embún et al., 2004; Mazej et al., 2008). Sulphate transporters homologous to AtSULTR4;1 and AtSULTR4;2 are present in the tonoplast of plant cells and are thought to catalyse the efflux of selenate from the vacuole (Kataoka et al., 2004b; Gigolashvili and Kopriva, 2014). The expression of AtSULTR4;1 and AtSULTR4;2 increases both upon S starvation and when plants are exposed to Se (Van Hoewyk et al., 2005; Gigolashvili and Kopriva, 2014). The expression of both SULTR4;1 and SULTR4;2 is greater in shoots of the Se-hyperaccumulator species Stanleya pinnata than in the congeneric Se-indicator species S. albescens when grown in the presence of selenite (Freeman et al., 2010). Increased expression of TaSULTR4;1 has been linked to greater grain Se concentrations in S-starved wheat than in S-replete wheat (Shinmachi et al., 2010). The expression of a number of genes encoding ABC transporters is increased in roots and leaves of perennial ryegrass (Lolium perenne L.) upon exposure to Se, and it has been suggested that some of these might be involved in the transport of Se compounds within the plant (Byrne et al., 2010), although there is presently no direct evidence to support this hypothesis.
THE EVOLUTION OF SELENIUM HYPERACCUMULATION
There can be considerable variation in shoot Se concentration among angiosperm species growing in the same environment (Rosenfeld and Beath, 1964; Brown and Shrift, 1982; Ihnat, 1989; White et al., 2004, 2007a; Bitterli et al., 2010). However, little of this variation can be attributed to systematic differences between angiosperm orders, and it is thought to reflect species-specific adaptations (White et al., 2004; Watanabe et al., 2007). In general, Se concentration in leaf tissues declines in the order Se-accumulator > Se-indicator > non-accumulator species. Differences in Se accumulation between species are most pronounced within genera containing Se-accumulator or Se-indicator plants, such as Astragalus and Stanleya (White et al., 2004).
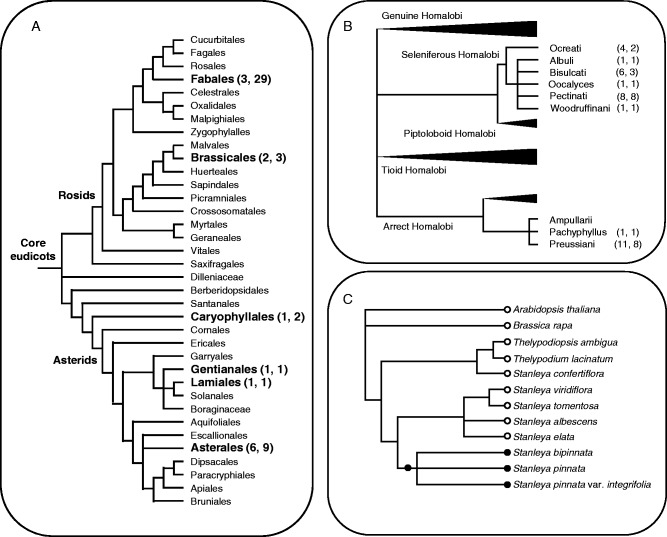
When grown in the same environment, Se concentrations in leaves of Se-hyperaccumulating species are significantly greater than those of other angiosperms (Rosenfeld and Beath, 1964; Brown and Shrift, 1982; White et al., 2007b), suggesting that these species might have distinct physiological adaptations enabling this trait. Since Se-hyperaccumulating species occur in several unrelated families (Table 1; Fig. 1A), it is thought that the traits of Se tolerance and accumulation arose by convergent evolution of appropriate biochemical pathways in several angiosperm clades (Brown and Shrift, 1982; White et al., 2004; Cappa and Pilon-Smits, 2014). The ability to accumulate Se appears to have evolved independently in the core eudicot families Amaranthaceae (Caryophyllales), Asteraceae (Asterales), Brassicaceae (Brassicales), Fabaceae (Fabales), Orobanchaceae (Lamiales) and Rubiaceae (Gentianales). The Fabaceae contains the greatest number of species known to hyperaccumulate Se. The ability to hyperaccumulate Se appears to have evolved several times within the Asteraceae, Brassicaceae and Fabaceae (Table 1). Indeed, it even appears to have evolved several times among North American Astragalus (Fabaceae): in the Homaloboid Phalanx within the seleniferous Homalobi, for which it can be used as a taxonomic character (Barneby, 1964), and the Preussiani (Fig. 1B), and also within the Piptoloboid and Ceridothrix Phalanxes. The evolution of Se hyperaccumulation in Stanleya (Brassicaceae) has also been studied in some detail (Fig. 1C; Cappa et al., 2014, 2015). Cappa et al. (2015) have observed that Se hyperaccumulation is restricted to the S. bipinnata/pinnata clade and is likely to have evolved once and then been lost in various ecotypes, such as those described as S. pinnata var. inyoensis and S. pinnata var. texana. Cappa et al. (2014) reported that S. pinnata ecotypes differed markedly in their ability to hyperaccumulate Se and observed that the trait was restricted to populations on the east side of the continental divide. They suggested that Se hyperaccumulation could have evolved in eastern USA and either (a) the Rocky Mountains formed a geographical barrier for gene flow to the west; (b) a reproductive barrier prevented gene flow because of ploidy differences among populations in the east and west; or (c) there is a greater cost to Se hyperaccumulation in the west. Evidence suggests that the ability to tolerate large tissue Se concentrations evolved earlier than the trait of Se hyperaccumulation in Stanleya and might have been a necessary predisposition enabling Se hyperaccumulation (Cappa et al., 2015).
Fig. 1.
(A) Distribution of proposed Se-hyperaccumulating species among angiosperm orders. Phylogenetic relationships between the angiosperm orders are reproduced from the Angiosperm Phylogeny Group (2009). The number of Se-hyperaccumulating genera and Se-hyperaccumulating species in each order are given in parentheses based on data presented in Table 1. (B) Distribution of proposed Se-hyperaccumulating taxa among sections of the Homaloboid astragali of North America. Taxonomic relationships are derived from Barneby (1964). The number of Se-hyperaccumulating taxa and Se-hyperaccumulating species in each section are given in parentheses based on data presented in Table 1. (C) Distribution of proposed Se-hyperaccumulating taxa among Brassicaceae indicating a single origin of Se hyperaccumulation (filled circles) in the Stanleya pinnata/bipinnata clade (Cappa et al., 2015).
Several hypotheses have been proposed for the evolution of Se hyperaccumulation in plants. First, there is a clear evolutionary advantage in being one of a few stress-tolerant plant species able to colonize seleniferous soils (Brown and Shrift, 1982). This character might have occurred through the evolution of mechanisms for Se exclusion by roots, tissue Se tolerance or Se volatilization. However, although Se exclusion by roots allows non-accumulator plants to survive greater rhizosphere Se concentrations, it does not confer the ability to colonize seleniferous soils (Rosenfeld and Beath, 1964; Brown and Shrift, 1982). In contrast, the ability to accumulate Se in non-toxic forms, and to remove Se by volatilization, are characteristics shared by many Se-indicator and Se-accumulator plants that colonize seleniferous soils, and there is considerable variation in the expression of these between and among plant species (Terry et al., 2000; Bañuelos et al., 2005a; White et al. 2007b; Pilon-Smits and LeDuc, 2009). Since the colonization of seleniferous soils by angiosperm species appears to require the ability to tolerate Se in their tissues, it is unsurprising that biochemical pathways that restrict the incorporation of selenoamino acids into proteins through the production of non-toxic Se metabolites appear to have evolved before those of Se hyperaccumation (Cappa et al., 2015). The accumulation of Se in plant tissues protects them against pathogens and herbivores (Quinn et al., 2007; Pilon-Smits et al., 2009; El Mehdawi and Pilon-Smits, 2012), and it has been proposed that this might be the primary ecological driver for the evolution of Se hyperaccumulation (Quinn et al., 2007; El Mehdawi and Pilon-Smits, 2012). It is also possible that the deposition of leaf litter with large Se concentrations around Se-hyperaccumulator plants could prevent competition by species with less tolerance of Se in the rhizosphere (El Mehdawi et al., 2011).
In addition to their exceptional ability to accumulate Se, Se-hyperaccumulator species have several other characteristics that appear to distinguish them from Se-indicator and non-accumulator species. When compared with other angiosperms, Se-hyperaccumulator species (1) constitutively express genes encoding sulphate transporters (Cabannes et al., 2011; Schiavon et al. 2015); (2) have significantly greater leaf Se/S quotients (Bell et al., 1992; Feist and Parker, 2001; Galeas et al., 2007; White et al., 2007a; Freeman et al., 2010; Cappa et al., 2014; Harris et al., 2014; DeTar et al., 2015); (3) exhibit reduced Mo accumulation with increasing rhizosphere sulphate or selenate concentrations (Harris et al., 2014); (4) restrict the incorporation of selenoamino acids into proteins through greater expression of appropriate genes (see ‘Selenium Uptake, Translocation and Metabolism in Plants’); and (5) accumulate Se in leaf trichomes and epidermal cells (Freeman et al., 2006, 2010; El Mehdawi and Pilon-Smits, 2012). These traits have been proposed as additional diagnostic characteristics for species that hyperaccumulate Se (White et al., 2007a; El Mehdawi and Pilon-Smits, 2012; Harris et al., 2014).
VARIATION IN SELENIUM ACCUMULATION WITHIN PLANT SPECIES
In addition to the considerable variation between species in their ability to accumulate Se in their tissues, there is often significant variation among genotypes of a particular species in this character. It has been observed, for example, that ecotypes of the Se-hyperaccumulator species Stanleya pinnata (Feist and Parker, 2001; Cappa et al., 2014) and Symphyotrichum ericoides (L.) G.L.Nesom (El Mehdawi et al., 2015) differ significantly in their leaf Se concentrations when grown in the same environment. Ecotypes collected from seleniferous soils generally have greater leaf Se concentrations and leaf Se/S quotients than ecotypes collected from soils with less Se in common garden experiments (Feist and Parker, 2001; Cappa et al., 2014; El Mehdawi et al., 2015). Significant genetic variation in shoot Se concentration has also been reported among tall fescue (Festuca arundinacea Schreb.) genotypes (McQuinn et al., 1991; Wu, 1998), and it would appear that there is a negative correlation between shoot Se concentration and shoot yield in this plant species (Wu, 1998).
Arabidopsis accessions differ both in their tolerance of Se in the rhizosphere and in their shoot Se concentration when grown in the same environment (Zhang et al., 2006a, b, 2007; Tamaoki et al., 2008; Chao et al., 2014). However, there appears to be no correlation between tolerance of Se in the rhizosphere and relative shoot Se concentration among arabidopsis accessions (Zhang et al., 2007). Analyses of crosses between arabidopsis accessions suggest that a single major gene controls selenite tolerance in this species, but that at least three chromosomal quantitative trait loci (QTLs) control selenate tolerance (Zhang et al., 2006a, b, 2007). Selenite tolerance in arabidopsis has been correlated with concentrations of non-protein thiols (e.g. cysteine, glutathione, phytochelatins) in roots, and tolerance to both selenate and selenite has been correlated with shoot SeCys and SeCys2 concentrations (Zhang et al., 2006a). In addition, it has been noted that the shoot S concentration of a selenite-tolerant accession of arabidopsis (Col-0) was greater than that of a selenite-sensitive accession (Ws-2) when they were exposed to selenite (Tamaoki et al., 2008). This has been attributed to greater expression of genes encoding SULTR2;2, SURTR3;1 and SULTR3;5, together with several genes involved in S assimilation, in the selenite-tolerant accession than in the selenite-sensitive accession, which is consistent with the hypothesis that upregulation of the S transport and assimilation pathways is one mechanism to increase selenite tolerance (Tamaoki et al., 2008). Chao et al. (2014) failed to identify any QTLs affecting leaf Se concentration in arabidopsis when they applied genome-wide association mapping techniques to a diverse set of 349 accessions, despite most of the variation in leaf Se concentration being accounted for by genotype (heritability 0·68) in their experiments. However, AtAPR2 was inferred to influence leaf Se accumulation in a population of arabidopsis derived from an accession with a large leaf Se concentration (Hodonín) and the Col-0 accession using extreme array mapping (Chao et al., 2014). The influence of AtAPR2 on leaf Se accumulation was further confirmed by phenotyping mutants lacking AtAPR2 and accessions with contrasting AtAPR2 activities (Chao et al., 2014). A single amino acid substitution apparently led to the loss of function of AtAPR2 and Se accumulation in leaves in the Hodonín accession (Chao et al., 2014).
There also appears to be sufficient genetic variation to breed for crops that can accumulate more Se in their edible tissues (White and Broadley, 2009). Genetic variation in grain Se concentration has been reported for a number of cereals (Table 1). Although several studies have suggested little genetic variation in grain Se concentration among bread wheat (Triticum aestivum L.) genotypes (Table 2; Lyons et al., 2005a; Zhao et al., 2009; Lee et al., 2011; Nelson et al., 2011), other studies have reported significant genetic variation in this trait (Garvin et al., 2006; Murphy et al., 2008; Rodríguez et al., 2011; Pu et al., 2014). It is evident that the expression of this trait in bread wheat is strongly dependent upon weather conditions, crop husbandry and Se fertilization (Lyons et al., 2005a; Garvin et al., 2006; Zhao et al., 2009; Lee et al., 2011; Nelson et al., 2011). In addition, there appears to be a negative relationship between grain Se concentration and grain yield among genotypes of bread wheat (Zhao et al., 2007; Fan et al., 2008; Murphy et al., 2008), although this is not always observed (Lyons et al., 2005a; Zhao et al., 2009). No genetic variation has been observed to date in the distribution of Se within wheat grain (Lyons et al., 2005b). Significant genetic variation in grain Se concentration has been observed in other cereals including durum wheat (Triticum turgidum L.; Rodríguez et al., 2011), barley (Hordeum vulgare L.; Ilbas et al., 2012; Mangan et al., 2015), wild barley (Hordeum spontaneum K.Koch; Yan et al., 2011), oat (Avena sativa L.; Eurola et al., 2004) and rice (Orzya sativa L.; Zhang et al., 2006c; Norton et al., 2010, 2012).
Table 2.
Examples of the variation in selenium concentrations in edible tissues among genotypes of common crops grown under the same conditions
| Crop | Plant species | Tissue | Trial | Details | Selenium (mg Se kg–1 DM) | Genotypes | Reference |
|---|---|---|---|---|---|---|---|
| Wheat | Triticum aestivum L. | Grain | Field trial, Sonora, Mexico | 0·045 (0·010–0·130) | n = 100 | Lyons et al. (2005a) | |
| Wheat | Triticum aestivum L. | Grain | Field trial, Sonora, Mexico | 0·076 (37-120) | n = 40 | Lyons et al. (2005a) | |
| Wheat | Triticum aestivum L. | Grain | Field trial, Manhattan, KS, USA | 0·045 (0·039–0·055) ns | n = 14 | Garvin et al. (2006) | |
| Wheat | Triticum aestivum L. | Grain | Field trial, Hutchinson, KS, USA | 0·36 (0·28–0·48)*** | n = 14 | Garvin et al. (2006) | |
| Wheat | Triticum aestivum L. | Grain | Field trial, Pullman, WA, USA | 0·015 (0·002–0·030)*** | n = 63 | Murphy et al. (2008) | |
| Wheat | Triticum aestivum L. | Grain | Field trial, Martonvásár, Hungary | 0·099 (0·033–0·238) | n = 150 | Zhao et al. (2009) | |
| Wheat | Triticum aestivum L. | Grain | Six field trials, Europe | 0·070 (0·032–0·091) ns | n = 26 | Zhao et al. (2009) | |
| Spring wheat | Triticum aestivum L. | Grain | Two field trials, SD, USA | 0·832 (0·730–0·940) ns | n = 10 | Lee et al. (2011) | |
| Winter wheat | Triticum aestivum L. | Grain | Two field trials, SD, USA | 0·418 (0·370–0·460) ns | n = 8 | Lee et al. (2011) | |
| Wheat | Triticum aestivum L. | Grain | Three field trials, Edmonton, Canada | Conventional agronomy | 0·023 (0·020–0·028) ns | n = 5 | Nelson et al. (2011) |
| Wheat | Triticum aestivum L. | Grain | Three field trials, Edmonton, Canada | Organic cultivation | 0·131 (0·115–0·151) ns | n = 5 | Nelson et al. (2011) |
| Wheat | Triticum aestivum L. | Grain | Field trial, Canary Islands | 0·072 (0·032–0·130)* | n = 11 | Rodríguez et al. (2011) | |
| Wheat | Triticum aestivum L. | Grain | Hydroponics | 10 μM Na2SeO4 | 0·232 (0·190–0·300) | n = 20 | Souza et al. (2014) |
| Durum wheat | Triticum turgidum L. | Grain | Field Trial, Martonvásár, Hungary | 0·081 (0·039–0·146) | n = 10 | Zhao et al. (2009) | |
| Durum wheat | Triticum turgidum L. | Grain | Field trial, Canary Islands | 0·079 (0·031–0·154)* | n = 8 | Rodríguez et al. (2011) | |
| Einkorn wheat | Triticum monococcum L. | Grain | Field Trial, Martonvásár, Hungary | 0·279 (0·179–0·440) | n = 5 | Zhao et al. (2009) | |
| Emmer wheat | Triticum dicoccon (Schrank) Schübl. | Grain | Field Trial, S. Italy | 0·028 (0·018–0·035) | n = 10 | Piergiovanni et al. (1997) | |
| Emmer wheat | Triticum dicoccon (Schrank) Schübl. | Grain | Field Trial, Martonvásár, Hungary | 0·229 (0·151–0·326) | n = 5 | Zhao et al. (2009) | |
| Spelt wheat | Triticum spelta L. | Grain | Field Trial, S. Italy | 0·039 (0·019–0·058) | n = 10 | Piergiovanni et al. (1997) | |
| Spelt wheat | Triticum spelta L. | Grain | Field trial, Martonvásár, Hungary | 0·209 (0·125–0·244) | n = 5 | Zhao et al. (2009) | |
| Barley | Hordeum vulgare L. | Grain | Field trials on loess soil, China | 0·045 (0·000–0·144) | n = 10 | Yan et al. (2011) | |
| Barley | Hordeum vulgare L. | Grain | Field trial, Moldova | No Se fertilizer | 0·111 (0·084–0·142) ns | n = 3 | Ilbas et al. (2012) |
| Barley | Hordeum vulgare L. | Grain | Field trial, Moldova | 12·5 g ha–1 Na2SeO3 | 0·218 (0·154–0·252)* | n = 3 | Ilbas et al. (2012) |
| Wild barley | Hordeum spontaneum K.Koch | Grain | Field trial on loess soil, China | 0·045 (0·000–0·387) | n = 92 | Yan et al. (2011) | |
| Oat | Avena sativa L. | Grain | Field trials, 8–10 sites × 3 years, Finland | Se fertilizer | 0·110 (0·110–0·140)*** | n = 6 | Eurola et al. (2004) |
| Oat | Avena sativa L. | Grain | Six field trials, ND, USA | 0·380 (0·310 - 0·412) ns | n = 18 | Doehlert et al. (2013) | |
| Rice | Oryza sativa L. | Brown grain | Field trials, Wuxi City, China | 0·057 (0·029–0·103)* | n = 151 | Zhang et al. (2006c) | |
| Common bean | Phaseolus vulgaris L. | Seed | Twelve field trials, Saskachewan, Canada | 0·430 (0·381–0·500) ns | n = 9 | Ray et al. (2014) | |
| Common bean | Phaseolus vulgaris L. | Seed | Glasshouse soil | No Se fertilizer | 0·052 (0·046–0·081) ns | n = 4 | Smrkolj et al. (2007) |
| Common bean | Phaseolus vulgaris L. | Seed | Glasshouse soil | Foliar Se fertilizer | 2·081 (1·892–2·379) ns | n = 4 | Smrkolj et al. (2007) |
| Field pea | Pisum sativum L. | Seed | Twelve field trials, Saskachewan, Canada | 0·457 (0·373–0·519) ns | n = 17 | Thavarajah et al. (2010) | |
| Chickpea | Cicer arietinum L. | Seed | Field trials, ND, USA | 0·333 (0·153–0·563)* | n = 10 | Thavarajah and Thavarajah (2012) | |
| Chickpea | Cicer arietinum L. | Seed | Ten field trials, Saskachewan, Canada | 0·732 (0·629–0·846)** | n = 8 | Ray et al. (2014) | |
| Lentil | Lens culinaris Medik. | Seed | Sixteen field trials, Saskachewan, Canada | 0·523 (0·425–0·672) | n = 19 | Thavarajah et al. (2008) | |
| Lentil | Lens culinaris Medik. | Seed | Field trial, Tel Hadya, Syria (2008) | 0·020 (0·016–0·024)* | n = 10 | Thavarajah et al. (2011) | |
| Lentil | Lens culinaris Medik. | Seed | Field trial, Tel Hadya, Syria (2009) | 0·025 (0·016–0·044)* | n = 17 | Thavarajah et al. (2011) | |
| Lentil | Lens culinaris Medik. | Seed | Field trial, Surkhet, Nepal | 0·439 (0·277–0·577) ns | n = 17 | Thavarajah et al. (2011) | |
| Lentil | Lens culinaris Medik. | Seed | Field trial, Nawalpur, Nepal | 0·064 (0·036–0·117)* | n = 17 | Thavarajah et al. (2011) | |
| Lentil | Lens culinaris Medik. | Seed | Field trial, Annacuur, Morocco | 0·015 (0·008–0·023)* | n = 20 | Thavarajah et al. (2011) | |
| Lentil | Lens culinaris Medik. | Seed | Field trial, Sidi ElAidi, Morocco | 0·042 (0·025–0·063)* | n = 16 | Thavarajah et al. (2011) | |
| Lentil | Lens culinaris Medik. | Seed | Field trial, Pullman, WA, USA | 0. 026 (0·09–0·028) ns | n = 72 | Thavarajah et al. (2011) | |
| Lentil | Lens culinaris Medik. | Seed | Field trial, Horsham, Australia | 0·255 (0·172–0·287) ns | n = 17 | Thavarajah et al. (2011) | |
| Lentil | Lens culinaris Medik. | Seed | Field trial, Melton, Australia | 0·041 (0·027–0·063)* | n = 17 | Thavarajah et al. (2011) | |
| Lentil | Lens culinaris Medik. | Seed | Field trial, Diyarbakir, Turkey (2008) | 0·049 (0·035–0·065)* | n = 11 | Thavarajah et al. (2011) | |
| Lentil | Lens culinaris Medik. | Seed | Field trial, Sanliufa, Turkey (2009) | 0·045 (0·030–0·067)* | n = 10 | Thavarajah et al. (2011) | |
| Lentil | Lens culinaris Medik. | Seed | Four field trials, Saskachewan, Canada | 1·180 (0·970–1·637)** | n = 18 | Ray et al. (2014) | |
| Mung bean | Vigna radiata (L.) R.Wilczek | Seed | Field, ICRISAT, India (2012) | 0·502 (0·210–0·910)** | n = 20 | Nair et al. (2015) | |
| Soybean | Glycine max (L.) Merr. | Seed | Field | 0·111 mg Se kg–1 soil; Se fertilizer | 6·923 (6·355–7·491)** | n = 2 | Yang et al. (2003) |
| Indianmustard | Brassica juncea (L.) Czern. | Leaves | Hydroponics | 2 mg L–1 Na2SeO4 | 707 (501–1092)* | n = 9 | Bañuelos et al. (1997) |
| Indian mustard | Brassica juncea (L.) Czern. | Leaves | Field trial | Se-laden soil | 543 (407–769)* | n = 9 | Bañuelos et al. (1997) |
| Rapid-cycling Brassica | Brassica oleracea L. | Leaves | Hydroponics | E1: 2 mg L–1 Na2SeO4 | 604 (120–988) | n = 219 | Kopsell and Randle (2001) |
| Rapid-cycling Brassica | Brassica oleracea L. | Leaves | Hydroponics | E2: 2 mg L–1 Na2SeO4 | 341 (152–531)** | n = 190 | Kopsell and Randle (2001) |
| Broccoli | Brassica oleracea L. (Italica Group) | Floret | Greenhouse soil | Non-saline irrigation | 0·5 (0·4–0·7) ns | n = 4 | Bañuelos et al. (2003) |
| Broccoli | Brassica oleracea L. (Italica Group) | Leaves | Greenhouse soil | Non-saline irrigation, no Se | 0·3 (0·2–0·5) ns | n = 4 | Bañuelos et al. (2003) |
| Broccoli | Brassica oleracea L. (Italica Group) | Floret | Greenhouse soil | Non-saline irrigation, 250 μg Se L–1 | 48 (43-51)* | n = 4 | Bañuelos et al. (2003) |
| Broccoli | Brassica oleracea L. (Italica Group) | Leaves | Greenhouse soil | Non-saline irrigation, 250 μg Se L–1 | 29 (26-31)* | n = 4 | Bañuelos et al. (2003) |
| Broccoli (hybrid) | Brassica oleracea L. (Italica Group) | Floret | Three field trials, SC, USA | 0·068 (0·053–0·085)** | n = 20 | Farnham et al. (2007) | |
| Broccoli (inbreds) | Brassica oleracea L. (Italica Group) | Floret | Three field trials, SC, USA | 0·063 (0·049–0·080) ns | n = 15 | Farnham et al. (2007) | |
| Broccoli | Brassica oleracea L. (Italica Group) | Leaves | Hydroponics | 20 μM Na2SeO4 | 1100 (801–1798) | n = 38 | Ramos et al. (2011b) |
| Onion | Allium cepa L. | Bulb | Hydroponics | 2 mg L–1 Na2SeO4 | 0·085 (0·060–0·113)*** | n = 16 | Kopsell and Randle (1997) |
| Lettuce | Lactuca sativa L. | Leaves | Hydroponics | 15 μM Na2SeO4 | 5·28 (2·76–7·20) | n = 30 | Ramos et al. (2011a) |
| Lettuce | Lactuca sativa L. | Leaves | Hydroponics | 15 μM Na2SeO3 | 2·87 (1·67–5·33) | n = 30 | Ramos et al. (2011a) |
| Chicory | Cichorium intybus L. | Leaves | Aeroponics | 7 mg Se L–1 (as Na2SeO4) | 391 (167–480)* | n = 4 | Mazej et al. (2008) |
| Tomato | Solanum lycopersicum L. | Fruit | Five glasshouses, Almería, Spain | 0·188 (0·015–0·363)* | n = 8 | Guil-Guerrero and Rebolloso-Fuentes (2009) | |
| Pepper | Capsicum annuum L. | Fruit | Five glasshouses, Almería, Spain | 0·110 (0·047–0·200)* | n = 10 | Guil-Guerrero et al. (2006) | |
| Potato | Solanum tuberosum L. | Tubers | Field, CO, USA | 1·551 (0·014–5·816)* | n = 8 | Perla et al. (2012) |
Data show the mean and, in parentheses, the minimum and maximum Se concentrations for n genotypes
Significant differences are indicated as *P < 0·05, **P < 0·01 and ***P < 0·001.
Chromosomal loci (QTLs) influencing grain Se concentration have been identified in wheat (Yang et al., 2013; Pu et al., 2014). Pu et al. (2014) identified four QTLs affecting grain Se concentration on chromosomes 3D at 218 cM, 4A at 91 cM, 5B at 169 cM and 7D at 215 cM in a cross between a synthetic wheat (SHW-L1) and Chuanmei 32, and a single QTL on chromosome 4D at 100 cM in a cross between Chuanmai 42 and Chuannong 16. Yang et al. (2013) identified four QTLs affecting grain Se concentration in a genetic mapping population derived from a cross between wild emmer wheat (Triticum dicoccoides [Körn. ex Asch. and Graebn.] Schweinf.) and a tetraploid durum wheat. These occurred on chromosomes 5B, 6A and 6B. Chromosomal loci affecting Se concentrations of leaves and grain of paddy rice have also been reported in a genetc mapping population derived from an indica (Bala) and a japonica (Azucena) variety (Norton et al., 2010, 2012). Several QTLs were found to affect grain Se concentration in this population, although the magnitude of their effects differed between environments. Chromosomal loci affecting grain Se concentration in rice were located on chromosome 1 (27·4 and 246·4 cM), chromosome 3 (80·6 cM), chromosome 6 (12·0, 20·3 and 103·5 cM), chromosome 7 (149·8 cM), chromosome 8 (16·9 cM), chromosome 9 (61·5 cM), chromosome 10 (66·1 cM) and chromosome 11 (105·9 cM). Two of these QTLs (on C3 and C7) also influenced leaf Se concentration, suggesting that Se accumulation in leaves, and its subsequent remobilization to developing grain, could be important in determining grain Se concentrations (Norton et al., 2010). None of the causal genes underpinning QTLs affecting Se accumulation in gain of wheat or rice is currently known.
Genetic variation in seed Se concentration has been reported among genotypes of several legume species (Table 2), although data from field trials indicate that genetic effects on seed Se concentration are generally small when compared with environmental effects (Thavarajah et al., 2010; Garrett et al., 2013; Ray et al., 2014). When grown at several sites in Saskatchewan (Canada), genetic effects on seed Se concentration of common bean (Phaseolus vulgaris L.) or field pea (Pisum sativum L.) were not significant (P > 0·1), although genotype × environment interactions did affect seed Se concentrations in common bean (Thavarajah et al., 2010; Garrett et al., 2013; Ray et al., 2014). This is consistent with studies performed in the glasshouse on common bean (Smrkolj et al., 2007). Similarly, no single nucleotide polymorphism (SNP) markers could be associated with variation in seed Se concentration among 94 pea genotypes grown in the field in Saskatchewan (Diapari et al., 2015). In contrast, genotypic variation was found to affect seed Se concentrations in both chickpea (Cicer arietinum L.) and lentil (Lens culinaris Medik.) grown in Saskatchewan (Thavarajah et al., 2008; Thavarajah and Thavarajah, 2012; Ray et al., 2014; Rahman et al., 2015). Significant genetic variation in seed Se concentration of lentil has also been observed in field trials conducted in other countries including Morocco, Turkey, Syria, Nepal, Australia and the USA (Thavarajah et al., 2011). Significant genetic variation in seed Se concentration has also been observed among genotypes of mung bean (Vigna radiata [L.] R.Wilczek; Nair et al., 2015) and soybean (Glycine max [L.] Merr.; Yang et al., 2003), and two QTLs have been identified, one on chromosome 8 and another on chromosome 18, that explain about 21 % of the variation in seed Se concentration in a recombinant inbred population of soybean derived from a cross between Williams82 and DSR-173 (Ramamurthy et al., 2014). Interestingly, the QTL on chromosome 8 includes GmSULTR2;1 (Ramamurthy et al., 2014).
Despite large environmental effects, significant genetic effects on Se concentration have been observed for onion bulbs (Allium cepa L.; Kopsell and Randle, 1997), leaves of rapid-cycling Brassica oleracea (Kopsell and Randle, 2001), broccoli florets (B. oleracea L. Italica Group; Bañuelos et al., 2003; Farnham et al., 2007; Ramos et al., 2011b), sprouts of cauliflower (B. oleracea L. Botrytis Group), kale (B. oleracea L. Acephala Group) and Chinese cabbage (Brassica rapa L.; Ávila et al., 2014), shoots of Indian mustard (Bañuelos et al., 1997), leaves of chicory (Cichorium intybus L.; Mazej et al., 2007) and leaves of lettuce (Lactuca sativa L.; Ramos et al., 2011a). In lettuce, the ability of genotypes to accumulate Se supplied as selenate was positively correlated with the expression of LsSULTR1;1, LsAPS1 and LsAPR1 (Ramos et al., 2011a). Significant genetic variation has also been observed in tomato (Solanum lycopersicum L.) fruit (Guil-Guerrero and Rebolloso-Fuentes, 2009), pepper (Capsicum annuum L.) fruit (Guil-Guerrero et al., 2006) and potato tubers (Perla et al., 2012).
TRANSGENIC APPROACHES TO INCREASE SELENIUM ACCUMULATION
Transgenic plants have been generated with greater Se tolerance, Se accumulation or Se volatilization than their non-transgenic counterparts (Table 3; Terry et al., 2000; Pilon-Smits and LeDuc, 2009; Pilon-Smits, 2012). These have been created for a variety of purposes. They have been used to provide fundamental knowledge of the transport proteins involved in the uptake and movement of Se in plants and to gain insight into the biochemical pathways and, in particular, the rate-limiting steps and control of Se metabolism in plants. The manipulation of Se transport and biochemistry can benefit crop production either directly, by allowing the development of crops with greater Se tolerance that can grow on soils with high soil Se concentrations, or indirectly, through the remediation of agricultural land with high soil Se concentrations using plants that can remove more Se from soils either by accumulating more Se in harvested tissues or by volatilizing more Se to the atmosphere. It can also benefit crop quality through Se biofortification of produce, not only by enabling greater Se concentrations to be accumulated in edible produce but also by synthesizing the most beneficial Se compounds for human and animal health.
Table 3.
Phenotypes of transgenic plants overexpressing genes involved in selenium uptake and metabolism
| Overexpressed gene | Enzyme | Plant | Tolerance of Se in the rhizosphere |
Leaf Se concentration |
Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Field soil | Selenite | Selenate | Total | Organic | Volatilization | ||||
| ShST1 | Sulphate transporter | Indian mustard | No effect | Increase, selenate supply | de Souza et al. (2000) | ||||
| AtATPS1 | ATP-sulphurylase | Arabidopsis | Decrease | Decrease | Increase | Sors et al. (2005a) | |||
| AtATPS1 | ATP-sulphurylase | Indian mustard | No effect | Increase | Increase | increase | No effect | Pilon-Smits et al. (1999b); | |
| Van Huysen et al. (2004); | |||||||||
| Bañuelos et al. (2005b) | |||||||||
| AtATPS1 | ATP-sulphurylase | Tobacco | No effect | No effect, selenate supply | McKenzie et al. (2009) | ||||
| PaAPR | Adenosine 5′-phosphosulphate reductase | Arabidopsis | Increase | Decrease | Increase | Sors et al. (2005a) | |||
| AtATPS1 + PaAPR | ATP-sulphurylase + adenosine 5′-phosphosulphate reductase | Arabidopsis | Decrease | Increase | Sors et al. (2005a) | ||||
| Escherichia coli gshII | Glutathione synthetase | Indian mustard | Increase | Increase | Bañuelos et al. (2005b) | ||||
| Escherichia coli gshI | γ-Glutamyl-cysteine synthetase | Indian mustard | No effect | Increase | Bañuelos et al. (2005b) | ||||
| TgSAT (m) | Serine acetyltransferase | Arabidopsis | No effect | Decrease | Increase | Sors et al. (2005a) | |||
| SoCS (cp) | Cysteine synthase | Indian mustard | No effect | No effect | de Souza et al. (2000) | ||||
| AtCGS1 (cp) | Cystathionine-γ-synthase | Indian mustard | No effect | Increase | No effect | Decrease, selenite supply | Increase | Van Huysen et al. (2003, 2004) | |
| No effect, selenate supply | |||||||||
| AbSMT1 | SeCys methyltransferase | Arabidopsis | Increase | Increase | Increase | Increase in SeMSeCys and GluMSeCys | Increase | LeDuc et al. (2004); | |
| Ellis et al. (2004) | |||||||||
| AbSMT | SeCys methyltransferase | Indian mustard | No effect | Increase | Increase | Increase | Increase in SeMSeCys | Increase in laboratory, no effect in field | LeDuc et al. (2004); |
| Bañuelos et al. (2007); | |||||||||
| Kubachka et al. (2007) | |||||||||
| AbSMTA | SeCys methyltransferase | Tobacco | No effect | Increase | Increase in SeMSeCys and GluMSeCys | Increase | Matich et al. (2009); | ||
| McKenzie et al. (2009) | |||||||||
| AtATPS1 + AbSMT | ATP-sulphurylase + SeCys methyltransferase | Indian mustard | Increase | Increase | Increase | Increase in SeMSeCys and GluMSeCys | LeDuc et al. (2006) | ||
| Kubachka et al. (2007) | |||||||||
| BoATPS1 + AbSMTA | ATP-sulphurylase + SeCys methyltransferase | Tobacco | No effect | Increase | Increase in SeMSeCys and GluMSeCys | Increase | Matich et al. (2009); | ||
| McKenzie et al. (2009) | |||||||||
| AtCpNifS | Cysteine lyase | Arabidopsis | No effect | Increase | Increase | Decrease in protein | Van Hoewyk et al. (2005) | ||
| Mus musculus SL (cp) | Selenocysteine lyase | Indian mustard | Increase | Decrease | Decrease | Increase | Decrease in protein | No effect | Garifullina et al. (2003); |
| Bañuelos et al. (2007) | |||||||||
| Mus musculus SL (cyt) | Selenocysteine lyase | Arabidopsis | Increase | Increase | Increase | Decrease in protein | Pilon et al. (2003) | ||
| Mus musculus SL (cp) | Selenocysteine lyase | Arabidopsis | Decrease | Decrease | Increase | Decrease in protein | Pilon et al. (2003) | ||
| AtSBP1 | Selenium-binding protein | Arabidopsis | Increase | Agalou et al. (2005) | |||||
The phenotypes listed are tolerance of Se in the soil under field conditions, tolerance of selenite and selenate in the rhizosphere determined in laboratory experiments, effects on total Se concentration and organic Se compounds in leaves, and Se volatilization.
Much of the research using transgenic plants has been directed towards the remediation of land with high soil Se concentrations (Terry et al., 2000; Pilon-Smits and LeDuc, 2009; Zhu et al., 2009; Pilon-Smits, 2012). This research has focused on (a) increasing plant tolerance of high soil Se concentrations; (b) increasing Se transport to the shoot; (c) increasing Se accumulation in shoot tissues; and (d) increasing Se volatilization. Overexpressing genes encoding transporters for selenate, selenite or selenoamino acids in the plasma membrane of particular cells can increase the capacity for Se uptake and transport within the plant. However, unless this is accompanied by an ability to tolerate greater tissue Se concentrations or volatilize more Se, it is unlikely to allow greater tolerance of Se in the rhizosphere or phytoremediation potential.
In non-accumulator plants, the conversion of selenate to selenite within plastids appears to be the rate-limiting step in the assimilation of Se into organic compounds (Pilon-Smits et al., 2009). Overexpression of AtATPS1, PaAPR or both AtATPS1 and PaAPR in arabidopsis results in greater concentrations of organic Se in leaves, but a decrease in total leaf Se concentration (Table 3; Sors et al., 2005a) and, although overexpression of PaAPR results in greater tolerance of selenate in the rhizosphere in arabidopsis, the overexpression of AtATPS1 does not (Sors et al., 2005a). In contrast, the overexpression of AtATPS1 in Indian mustard, a Se-indicator plant, results in greater concentrations of Se and organic Se in leaves and greater tolerance of selenate in the rhizosphere (Pilon-Smits et al., 1999b; Van Huysen et al., 2004; Bañuelos et al., 2005b). The overexpression of genes involved in glutathione synthesis, such as glutathione synthase and γ-glutamyl-cysteine synthase, also appears to increase Se concentrations in leaves and Se tolerance of Indian mustard grown on seleniferous soils (Bañuelos et al., 2005b), whereas overexpression of cystathione-γ-synthase results in greater tolerance of selenite in the rhizosphere, reduced leaf Se concentrations and greater Se volatilization (Van Huysen et al., 2003, 2004).
The ability to tolerate Se in plant tissues and, thereby, to accumulate greater Se concentrations can be increased by the overexpression of genes encoding SMT, particularly if combined with overexpressing ATPS (Table 3). The overexpression of SMT, with or without the overexpression of ATPS, results in greater tolerance of selenite, and sometimes also selenate, in the rhizosphere, greater total Se, SeMSeCys and γ-glutamyl-SeMSeCys concentrations in leaves, and greater Se volatilization in transgenic plants compared with untransformed controls (Ellis et al., 2004; LeDuc et al., 2004, 2006; Bañuelos et al., 2007; Kubachka et al., 2007; Matich et al., 2009; McKenzie et al., 2009). The overexpression of genes encoding SeCyslyases has had variable effects on the tolerance of transgenic plants to selenate and selenite in the rhizosphere, but has consistently resulted in greater leaf Se concentrations and less Se incorporation into proteins in transgenic plants exposed to selenite or selenite than in untransformed plants (Garifullina et al., 2003; Pilon et al., 2003; Van Hoewyk et al., 2005; Bañuelos et al., 2007). Finally, the overexpression of AtSBP1 has been shown to increase selenite tolerance in transgenic arabidopsis (Agalou et al., 2005).
CONCLUSIONS AND PERSPECTIVES
Selenium is an essential mineral element for the well-being of animals and a beneficial element for plants. However, excess Se can be toxic to both animals and plants. There is considerable interest in understanding how plants acquire and accumulate Se, not only to facilitate appropriate dietary Se intakes for animal and humans, which often requires Se biofortification of edible crops, but also to remediate land contaminated anthropogenically by excess Se and to appreciate the ecology of native plants inhabiting seleniferous soils. Recently, researchers have begun to identify the genetic factors influencing Se acquisition and accumulation by plants. Initially, this work focused on elucidating the genes encoding enzymes involved in Se uptake, metabolism and distribution within the plant. Application of this knowledge has allowed the genetic manipulation of Se metabolism to increase Se accumulation in harvested tissues and Se volatilization to the atmosphere, benefitting both biofortification and phytoremediation strategies. It has also informed our appreciation of the possible mechanisms driving the evolution of species that hyperaccumulate Se in their tissues. Appreciable variation in Se concentrations in analogous tissues has been attributed to genetic factors both between and within plant species. Considerable effort is currently being invested in identifying chromosomal loci (QTLs) underlying these differences, which will enable the selection and breeding of crops with greater ability to acquire and accumulate Se in appropriate chemical forms in their edible tissues. Although our knowledge of the genetics of Se accumulation in plants appears rudimentary at present, it will increase rapidly as the modern toolbox of molecular techniques are applied. It is laudable that this effort will be built on the solid foundations of plant physiology and biochemistry.
ACKNOWLEDGEMENTS
This paper is based on a talk at The Fourth International Conference on Selenium in the Environment and Human Health, Sao Paulo, Brazil. I thank Dr Paula Pongrac for bringing several papers to my attention, Ursula McKean for sourcing obscure literature, and Dr Paula Pongrac and Professor Martin Broadley for reading my original manuscript. The work was supported by the Rural and Environment Science and Analytical ServicesDivision (RESAS) of the Scottish Government through WorkPackage 7.2 (2011–2016) and by a Fellowship funded by The National Council for Scientific and Technological Development (CNPq) of Brazil (Grant #402868/2012-9).
LITERATURE CITED
- Agalou A, Roussis A, Spaink HP. 2005. The Arabidopsis selenium-binding protein confers tolerance to toxic levels of selenium. Functional Plant Biology 32: 881–890. [DOI] [PubMed] [Google Scholar]
- Alford ÉR, Lindblom SD, Pittarello M, et al. 2014. Roles of rhizobial symbionts in selenium hyperaccumulation in Astragalus (Fabaceae). American Journal of Botany 101: 1895–1905. [DOI] [PubMed] [Google Scholar]
- Alfthan G, Eurola M, Ekholm P, et al. 2015. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: from deficiency to optimal selenium status of the population. Journal of Trace Elements in Medicine and Biology 31: 142–147. [DOI] [PubMed] [Google Scholar]
- Angiosperm Phylogeny Group. 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161: 105–121. [Google Scholar]
- Aureli F, Ouerdane L, Bierla K, Szpunar J, Prakash NT, Cubadda F. 2012. Identification of selenosugars and other low-molecular weight selenium metabolites in high-selenium cereal crops. Metallomics 4: 968–978 [DOI] [PubMed] [Google Scholar]
- Ávila FW, Yang Y, Faquin V, et al. 2014. Impact of selenium supply on Se-methylselenocysteine and glucosinolate accumulation in selenium-biofortified Brassica sprouts. Food Chemistry 165: 578–586. [DOI] [PubMed] [Google Scholar]
- Baker AJM. 1981. Accumulators and excluders – strategies in the response of plants to heavy metals. Journal of Plant Nutrition 3: 643–654. [Google Scholar]
- Bañuelos GS, Ajwa HA, Wu L, Guo X, Akohoue S, Zambrzuski S. 1997. Selenium-induced growth reduction in Brassica land races considered for phytoremediation. Ecotoxicology and Environmental Safety 36: 282–287. [DOI] [PubMed] [Google Scholar]
- Bañuelos GS, Pasakdee S, Finley JW. 2003. Growth response and selenium and boron distribution in broccoli varieties irrigated with poor quality water. Journal of Plant Nutrition 26: 2537–2549. [Google Scholar]
- Bañuelos GS, Lin ZQ, Arroyo I, Terry N. 2005a Selenium volatilization in vegetated agricultural drainage sediment from the San Luis Drain, Central California. Chemosphere 60: 1203–1213. [DOI] [PubMed] [Google Scholar]
- Bañuelos G, Terry N, LeDuc DL, Pilon-Smits EAH, Mackey B. 2005b Field trial of transgenic Indian mustard plants shows enhanced phytoremediation of selenium-contaminated sediment. Environmental Science and Technology 39: 1771–1777. [DOI] [PubMed] [Google Scholar]
- Bañuelos G, LeDuc DL, Pilon-Smits EAH, Tagmount A, Terry N. 2007. Transgenic Indian mustard overexpressing selenocysteine lyase or selenocysteine methyltransferase exhibit enhanced potential for selenium phytoremediation under field conditions. Environmental Science and Technology 41: 599–605. [DOI] [PubMed] [Google Scholar]
- Bañuelos GS, Fakra SC, Walse SS, et al. 2011. Selenium accumulation, distribution, and speciation in spineless prickly pear cactus: a drought- and salt-tolerant, selenium-enriched nutraceutical fruit crop for biofortified foods. Plant Physiology 155: 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberon M, Berthomieu P, Clairotte M, Shibagaki N, Davidian J-C, Gosti F. 2008. Unequal functional redundancy between the two Arabidopsis thaliana high-affinity sulphate transporters SULTR1;1 and SULTR1;2 . New Phytologist 180: 608–619 [DOI] [PubMed] [Google Scholar]
- Barneby RC. 1964. Atlas of North American Astragalus. Volumes I and II. New York: The New York Botanical Garden. [Google Scholar]
- Beath OA. 1943. Toxic vegetation growing on the salt wash sandstone member of the Morrison formation. American Journal of Botany 30: 698–706. [Google Scholar]
- Beath OA, Eppson HF, Gilbert CS. 1937. Selenium distribution in and seasonal variation of type vegetation occurring on seleniferous soils. Journal of the American Pharmaceutical Association 26: 394–405. [Google Scholar]
- Beath OA, Gilbert CS, Eppson HF. 1939a The use of indicator plants in locating seleniferous areas in the Western United States. I. General. American Journal of Botany 26: 257–269. [Google Scholar]
- Beath OA, Gilbert CS, Eppson HF. 1939b The use of indicator plants in locating seleniferous areas in the Western United States. II. Correlation studies by states. American Journal of Botany 26: 296–315. [Google Scholar]
- Beath OA, Gilbert CS, Eppson HF. 1940. The use of indicator plants in locating seleniferous areas in western United States. III. Further studies. American Journal of Botany 27: 564–573. [Google Scholar]
- Beath OA, Gilbert CS, Eppson HF. 1941. The use of indicator plants in locating seleniferous areas in western United States. IV. Progress report. American Journal of Botany 28: 887–900. [Google Scholar]
- Bell PF, Parker DR, Page AL. 1992. Contrasting selenate–sulfate interactions in selenium-accumulating and nonaccumulating plant species. Soil Science Society of America Journal 56: 1818–1824. [Google Scholar]
- Birringer M, Pilawa S, Flohé L. 2002. Trends in selenium biochemistry. Natural Product Reports 19: 693–718. [DOI] [PubMed] [Google Scholar]
- Bitterli C, Bañuelos GS, Schulin R. 2010. Use of transfer factors to characterize uptake of selenium by plants. Journal of Geochemical Exploration 107: 206–216. [Google Scholar]
- Broadley MR, White PJ, Bryson RJ, et al. 2006. Biofortification of UK food crops with selenium. Proceedings of the Nutrition Society 65: 169–181. [DOI] [PubMed] [Google Scholar]
- Brown TA, Shrift A. 1982. Selenium: toxicity and tolerance in higher plants. Biological Reviews 57: 59–84 [Google Scholar]
- Buchner P, Stuiver CEE, Westermann S, et al. 2004. Regulation of sulfate uptake and expression of sulfate transporter genes in Brassica oleracea L. as affected by atmospheric H2S and pedospheric sulphate nutrition. Plant Physiology 136: 3396–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner P, Parmar S, Kriegel A, Carpentier M, Hawkesford MJ. 2010. The sulfate transporter family in wheat: tissue-specific gene expression in relation to nutrition. Molecular Plant 3: 374–389. [DOI] [PubMed] [Google Scholar]
- Byers HG. 1935. Selenium occurrence in certain soils in the United States with a discussion of related topics . United States Department of Agriculture Technical Bulletin 482. Washington, DC: US Department of Agriculture. [Google Scholar]
- Byers HB, Miller TJ, Williams KT, Lakin HW. 1938. Selenium occurrence in certain soils in the United States with a discussion of related topics, third report . United States Department of Agriculture Technical Bulletin 601. Washington, DC: US Department of Agriculture. [Google Scholar]
- Byrne SL, Durandeau K, Nagy I, Barth S. 2010. Identification of ABC transporters from Lolium perenne L. that are regulated by toxic levels of selenium. Planta 231: 901–911. [DOI] [PubMed] [Google Scholar]
- Cabannes E, Buchner P, Broadley MR, Hawkesford MJ. 2011. A comparison of sulfate and selenium accumulation in relation to the expression of sulfate transporter genes in Astragalus species. Plant Physiology 157: 2227–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai XJ, Block E, Uden PC, Zhang Z, Quimbly BD, Sullivan JJ. 1995. Allium chemistry: identification of selenoamino acids in ordinary and selenium-enriched garlic, onion, and broccoli using gas chromatography with atomic emission detection. Journal of Agricultural and Food Chemistry 43: 1754–1757. [Google Scholar]
- Cao MJ, Wang Z, Wirtz M, Hell R, Oliver DJ, Xiang CB. 2013. SULTR3;1 is a chloroplast-localized sulphate transporter in Arabidopsis thaliana. The Plant Journal 73: 607–616. [DOI] [PubMed] [Google Scholar]
- Cappa JJ, Pilon-Smits EAH. 2014. Evolutionary aspects of elemental hyperaccumulation. Planta 239: 267–275. [DOI] [PubMed] [Google Scholar]
- Cappa JJ, Cappa PJ, El Mehdawi AF, McAleer JM, Simmons MP, Pilon-Smits EAH. 2014. Characterization of selenium and sulfur accumulation across the genus Stanleya (Brassicaceae): a field survey and common-garden experiment. American Journal of Botany 101: 830–839. [DOI] [PubMed] [Google Scholar]
- Cappa JJ, Yetter C, Fakra S, et al. 2015. Evolution of selenium hyperaccumulation in Stanleya (Brassicaceae) as inferred from phylogeny, physiology and X-ray microprobe analysis. New Phytologist 205: 583–595. [DOI] [PubMed] [Google Scholar]
- Carey A-M, Scheckel KG, Lombi E, et al. 2012. Grain accumulation of selenium species in rice (Oryza sativa L.). Environmental Science and Technology 46: 5557–5564. [DOI] [PubMed] [Google Scholar]
- Chang JC, Gutenmann WH, Reid CM, Lisk DJ. 1995. Selenium content of Brazil nuts from two geographic locations in Brazil . Chemosphere 30: 801–802. [DOI] [PubMed] [Google Scholar]
- Chao D-Y, Baraniecka P, Danku J, et al. 2014. Variation in sulfur and selenium accumulation is controlled by naturally occurring isoforms of the key sulfur assimilation enzyme ADENOSINE 5'-PHOSPHOSULFATE REDUCTASE2 across the Arabidopsis species range. Plant Physiology 166: 1593–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilimba ADC, Young SD, Black CR, et al. 2011. Maize grain and soil surveys reveal suboptimal dietary selenium intake is widespread in Malawi. Scientific Reports 1: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilimba ADC, Young SD, Black CR, Meacham MC, Lammel J, Broadley MR. 2012. Agronomic biofortification of maize with selenium (Se) in Malawi. Field Crops Research 125: 118–128. [Google Scholar]
- Combs GF. 2001. Selenium in global food systems . British Journal of Nutrition 85: 517–547. [DOI] [PubMed] [Google Scholar]
- Dernovics M, García-Barrera T, Bierła K, Preud’homme H, Lobiński R. 2007. Standardless identification of selenocystathionine and its gamma-glutamyl derivatives in monkeypot nuts by 3D liquid chromatography with ICP-MS detection followed by nanoHPLCQ-TOF-MS/MS. Analyst 132: 439–449. [DOI] [PubMed] [Google Scholar]
- DeTar RA, Alford ÉR, Pilon-Smits EAH. 2015. Molybdenum accumulation, tolerance and molybdenum–selenium–sulfur interactions in Astragalus selenium hyperaccumulator and nonaccumulator species. Journal of Plant Physiology 183: 32–40. [DOI] [PubMed] [Google Scholar]
- Dhillon KS, Dhillon SK. 2003. Distribution and management of seleniferous soils. Advances in Agronomy 79: 119–184. [Google Scholar]
- Dhillon KS, Dhillon SK. 2009. Accumulation and distribution of selenium in some vegetable crops grown in selenate-Se treated clay loam soil. Frontiers of Agriculture in China 3: 366–373. [Google Scholar]
- Diapari M, Sindhu A, Warkentin TD, Bett K, Tar’an B. 2015. Population structure and marker-trait association studies of iron, zinc and selenium concentrations in seed of field pea (Pisum sativum L.). Molecular Breeding 35: 30. [Google Scholar]
- Doehlert DC, Simsek S, Thavarajah D, Thavarajah P, Ohm J-B. 2013. Detailed composition analyses of diverse oat genotype kernels grown in different environments in North Dakota. Cereal Chemistry 90: 572–578. [Google Scholar]
- Dumont E, De Pauw L, Vanhaecke F, Cornelis R. 2006. Speciation of Se in Bertholletia excelsa (Brazil nut): a hard nut to crack? Food Chemistry 95: 684–692. [Google Scholar]
- Dutilleul C, Jourdain A, Bourguignon J, Hugouvieux V. 2008. The Arabidopsis putative selenium-binding protein family: expression study and characterization of SBP1 as a potential new player in cadmium detoxification processes. Plant Physiology 147: 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kassis E, Cathala N, Rouached H, et al. 2007. Characterization of a selenate-resistant Arabidopsis mutant. Root growth as a potential target for selenate toxicity. Plant Physiology 143: 1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mehdawi AF, Pilon-Smits EAH. 2012. Ecological aspects of plant selenium hyperaccumulation. Plant Biology 14: 1–10. [DOI] [PubMed] [Google Scholar]
- El Mehdawi AF, Quinn CF, Pilon-Smits EAH. 2011. Effects of selenium hyperaccumulation on plant–plant interactions: evidence for elemental allelopathy? New Phytologist 191: 120–131. [DOI] [PubMed] [Google Scholar]
- El Mehdawi AF, Paschke MW, Pilon-Smits EAH. 2015. Symphyotrichum ericoides populations from seleniferous and nonseleniferous soil display striking variation in selenium accumulation. New Phytologist 206: 231–242. [DOI] [PubMed] [Google Scholar]
- Ellis DR, Sors TG, Brunk DG, et al. 2004. Production of Se-methylselenocysteine in transgenic plants expressing selenocysteine methyltransferase. BMC Plant Biology 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H. 2013. Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant and Soil 362: 319–334. [Google Scholar]
- Eurola M, Hietaniemi V, Kontturi M, et al. 2004. Selenium content of Finnish oats in 1997–1999: effect of cultivars and cultivation techniques. Agricultural and Food Science 13: 46–53. [Google Scholar]
- Fairweather-Tait SJ, Bao Y, Broadley MR, et al. 2011. Selenium in human health and disease. Antioxidants and Redox Signaling 14: 1337–1383. [DOI] [PubMed] [Google Scholar]
- Fan M-S, Zhao F-J, Poulton PR, McGrath SP. 2008. Historical changes in the concentrations of selenium in soil and wheat grain from the Broadbalk experiment over the last 160 years. Science of the Total Environment 389: 532–538. [DOI] [PubMed] [Google Scholar]
- Fang WX, Wu PW. 2004. Elevated selenium and other mineral element concentrations in soil and plant tissues in bone coal sites in Haoping area, Ziyang County, China. Plant and Soil 261: 135–146. [Google Scholar]
- Farnham MW, Hale AJ, Grusak MA, Finley JW. 2007. Genotypic and environmental effects on selenium concentration of broccoli heads grown without supplemental selenium fertilizer. Plant Breeding 126: 195–200. [Google Scholar]
- Feist LJ, Parker DR. 2001. Ecotypic variation in selenium accumulation among populations of Stanleya pinnata. New Phytologist 149: 61–69. [DOI] [PubMed] [Google Scholar]
- Feng R, Wei C, Tud S. 2013. The roles of selenium in protecting plants against abiotic stresses. Environmental and Experimental Botany 87: 58–68. [Google Scholar]
- Ferri T, Coccioli F, De Luca C, Callegari CV, Morabito R. 2004. Distribution and speciation of selenium in Lecythis ollaria plant. Microchemical Journal 78: 195–203. [Google Scholar]
- Fordyce FM. 2013. Selenium deficiency and toxicity in the environment. In: Selinus O, Alloway B, Centeno JA, et al., eds. Essentials of medical geology, revised edn. Dordrecht: Springer, 375–416. [Google Scholar]
- Freeman JL, Zhang L, Marcus MA, Fakra S, McGrath SP, Pilon-Smits EAH. 2006. Spatial imaging, speciation and quantification of Se in the hyperaccumulator plants Astragalus bisulcatus and Stanleya pinnata. Plant Physiology 142: 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JL, Tamaoki M, Stushnoff C, et al. 2010. Molecular mechanisms of selenium tolerance and hyperaccumulation in Stanleya pinnata. Plant Physiology 153: 1630–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeas ML, Zhang LH, Freeman JL, Wegner M, Pilon-Smits EAH. 2007. Seasonal fluctuations of selenium and sulfur accumulation in selenium hyperaccumulators and related nonaccumulators. New Phytologist 173: 517–525. [DOI] [PubMed] [Google Scholar]
- Garifullina GF, Owen JD, Lindblom SD, Tufan H, Pilon M, Pilon-Smits EAH. 2003. Expression of a mouse selenocysteine lyase in Brassica juncea chloroplasts affects selenium tolerance and accumulation. Physiologia Plantarum 118: 538–544 [Google Scholar]
- Garrett RG, Gawalko E, Wang N, Richter A, Warkentin TD. 2013. Macro-relationships between regional-scale field pea (Pisum sativum) selenium chemistry and environmental factors in western Canada. Canadian Journal of Plant Science 93: 1059–1071. [Google Scholar]
- Garvin DF, Welch RM, Finley JW. 2006. Historical shifts in the seed mineral micronutrient concentration of US hard red winter wheat germplasm. Journal of the Science of Food and Agriculture 86: 2213–2220. [Google Scholar]
- Gigolashvili T, Kopriva S. 2014. Transporters in plant sulphur metabolism. Frontiers in Plant Science 5: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gionfriddo E, Naccarato A, Sindona G, Tagarelli A. 2012. A reliable solid phase microextraction-gas chromatography–triple quadrupole mass spectrometry method for the assay of selenomethionine and selenomethylselenocysteine in aqueous extracts: difference between selenized and not-enriched selenium potatoes. Analytica Chimica Acta 747: 58–66. [DOI] [PubMed] [Google Scholar]
- Grant TD, Montes-Bayón M, LeDuc D, Fricke MW, Terry N, Caruso JA. 2004. Identification and characterization of Se-methyl selenomethionine in Brassica juncea roots. Journal of Chromatography A 1026: 159–166. [DOI] [PubMed] [Google Scholar]
- Guil-Guerrero JL, Rebolloso-Fuentes MM. 2009. Nutrient composition and antioxidant activity of eight tomato (Lycopersicon esculentum) varieties. Journal of Food Composition and Analysis 22: 123–129. [Google Scholar]
- Guil-Guerrero JL, Martínez-Guirado C, del Mar Rebolloso-Fuentes M, Carrique-Pérez A. 2006. Nutrient composition and antioxidant activity of 10 pepper (Capsicum annuum) varieties. European Food Research and Technology 224: 1–9. [Google Scholar]
- Hammel C, Kyriakopoulos A, Behne D, Gawlik D, Brätter P. 1996. Protein-bound selenium in the seeds of coco de mono (Lecythis ollaria). Journal of Trace Elements in Medicine and Biology 10: 96–102. [DOI] [PubMed] [Google Scholar]
- Harris J, Schneberg KA, Pilon-Smits EAH. 2014. Sulfur–selenium–molybdenum interactions distinguish selenium hyperaccumulator Stanleya pinnata from non-hyperaccumulator Brassica juncea (Brassicaceae). Planta 239: 479–491. [DOI] [PubMed] [Google Scholar]
- Hart DJ, Fairweather-Tait SJ, Broadley MR, et al. 2011. Selenium concentration and speciation in biofortified flour and bread: retention of selenium during grain biofortification, processing and production of Se-enriched food. Food Chemistry 126: 1771–1778. [DOI] [PubMed] [Google Scholar]
- Hsu F-C, Wirtz M, Heppel SC, et al. 2011. Generation of Se-fortified broccoli as functional food: impact of Se fertilization on S metabolism. Plant, Cell and Environment 34: 192–207. [DOI] [PubMed] [Google Scholar]
- Hugouvieux V, Dutilleul C, Jourdain A, Reynaud F, Lopez V, Bourguignon J. 2009. Arabidopsis putative Selenium-Binding Protein1 expression is tightly linked to cellular sulfur demand and can reduce sensitivity to stresses requiring glutathione for tolerance. Plant Physiology 151: 768–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihnat M. 1989. Plants and agricultural materials. In: Ihnat M, ed. Occurrence and distribution of selenium. Boca Raton, FL: CRC Press, 33–105. [Google Scholar]
- Ilbas AI, Yılmaz S, Akbulut M, Bogdevich O. 2012. Uptake and distribution of selenium, nitrogen and sulfur in three barley cultivars subjected to selenium applications. Journal of Plant Nutrition 35: 442–452. [Google Scholar]
- Inostroza-Blancheteau C, Reyes-Díaz M, Alberdi M, et al. 2013. Influence of selenite on selenium uptake, differential antioxidant performance and gene expression of sulfate transporters in wheat genotypes. Plant and Soil 369: 47–59. [Google Scholar]
- Institute of Medicine. 2000. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington DC: National Academies Press. [PubMed] [Google Scholar]
- Joy EJM, Ander EL, Young SD, et al. 2014. Dietary mineral supplies in Africa. Physiologia Plantarum 151: 208–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy EJM, Broadley MR, Young SD, et al. 2015. Soil type influences crop mineral composition in Malawi. Science of the Total Environment 505: 587–595. [DOI] [PubMed] [Google Scholar]
- Kápolna E, Gergely V, Dernovics M, Illés A, Fodor P. 2007. Fate of selenium species in sesame seeds during simulated bakery process. Journal of Food Engineering 79: 494–501. [Google Scholar]
- Kápolna E, Laursen KH, Husted S, Larsen EH. 2012. Bio-fortification and isotopic labelling of Se metabolites in onions and carrots following foliar application of Se and 77Se. Food Chemistry 133: 650–657. [Google Scholar]
- Kataoka T, Hayashi N, Yamaya T, Takahashi H. 2004a Root-to-shoot transport of sulfate in Arabidopsis. Evidence for the role of SULTR3;5 as a component of low-affinity sulfate transport system in the root vasculature. Plant Physiology 136: 4198–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T, Watanabe-Takahashi A, Hayashi N, et al. 2004b Vacuolar sulfate transporters are essential determinants controlling internal distribution of sulfate in Arabidopsis. The Plant Cell 16: 2693–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight SH, Beath OA. 1937. The occurrence of selenium and seleniferous vegetation in Wyoming . University of Wyoming Agricultural Experiment Station Bulletin 221. Laramie: University of Wyoming. [Google Scholar]
- Knott SG, McCray CWR. 1959. Two naturally occurring outbreaks of selenosis in Queensland. Australian Vetinary Journal 35: 161–165 [Google Scholar]
- Kopsell DA, Randle WM. 1997. Short-day onion cultivars differ in bulb selenium and sulphur accumulation which can affect bulb pungency. Euphytica 96: 385–390. [Google Scholar]
- Kopsell DA, Randle WM. 2001. Genetic variances and selection potential for selenium accumulation in a rapid-cycling Brassica oleracea population. Journal of the American Society for Horticultural Science 126: 329–335. [Google Scholar]
- Kubachka KM, Meija J, LeDuc DL, Terry N, Caruso JA. 2007. Selenium volatiles as proxy to the metabolic pathways of selenium in genetically modified Brassica juncea. Environmental Science and Technology 41: 1863–1869. [DOI] [PubMed] [Google Scholar]
- Lakin HW, Byers HG. 1941. Selenium occurrence in certain soils in the United States with a discussion of related topics, sixth report . United States Department of Agriculture Technical Bulletin 530. Washington, DC: US Department of Agriculture. [Google Scholar]
- Lakin HW, Byers HG. 1948. Selenium occurrence in certain soils in the United States with a discussion of related topics, seventh report . United States Department of Aghriculture Technical Bulletin 530. Washington, DC: US Department of Agriculture. [Google Scholar]
- LeDuc DL, Tarun AS, Montes-Bayon M, et al. 2004. Overexpression of selenocysteine methyltransferase in Arabidopsis and Indian mustard increases selenium tolerance and accumulation. Plant Physiology 135: 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDuc DL, AbdelSamie M, Montes-Bayón M, Wu CP, Reisinger SJ, Terry N. 2006. Overexpressing both ATP sulfurylase and selenocysteine methyltransferase enhances selenium phytoremediation traits in Indian mustard. Environmental Pollution 144: 70–76. [DOI] [PubMed] [Google Scholar]
- Lee S, Woodward HJ, Doolittle JJ. 2011. Selenium uptake response among selected wheat (Triticum aestivum) varieties and relationship with soil selenium fractions. Soil Science and Plant Nutrition 57: 823–832. [Google Scholar]
- Li H-F, McGrath SP, Zhao F-J. 2008. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytologist 178: 92–102. [DOI] [PubMed] [Google Scholar]
- Lindblom SD, Valdez-Barillas JR, Fakra SC, Marcus MA, Wangeline AL, Pilon-Smits EAH. 2013. Influence of microbial associations on selenium localization and speciation in roots of Astragalus and Stanleya hyperaccumulators. Environmental and Experimental Botany 88: 33–42. [Google Scholar]
- Lyi SM, Heller LI, Rutzke M, Welch RM, Kochian LV, Li L. 2005. Molecular and biochemical characterization of the selenocysteine Se-methyltransferase gene and Se-methylselenocysteine synthesis in broccoli. Plant Physiology 138: 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons G, Ortiz-Monasterio I, Stangoulis J, Graham R. 2005a Selenium concentration in wheat grain: is there sufficient genotypic variation to use in breeding? Plant and Soil 269: 269–380. [Google Scholar]
- Lyons GH, Genc Y, Stangoulis JCR, Palmer LT, Graham RD. 2005b Selenium distribution in wheat grain, and the effect of postharvest processing on wheat selenium content. Biological Trace Element Research 103: 155–168. [DOI] [PubMed] [Google Scholar]
- Mangan B-u-N, Hui L, Lashari MS, Shah AN, Licao C, Weining S. 2015. Nutritional characteristics and starch properties of Tibetan barley. International Journal of Agricultural Policy and Research 3: 293–299. [Google Scholar]
- Matich AJ, McKenzie MJ, Brummell DA, Rowan DD. 2009. Organoselenides from Nicotiana tabacum genetically modified to accumulate selenium. Phytochemistry 70: 1098–1106. [DOI] [PubMed] [Google Scholar]
- Matich AJ, McKenzie MJ, Lill RE, et al. 2012. Selenoglucosinolates and their metabolites produced in Brassica spp. fertilised with sodium selenate. Phytochemistry 75: 140–152. [DOI] [PubMed] [Google Scholar]
- Matich AJ, McKenzie MJ, Lill RE, McGhie TK, Chen RKY, Rowan DD. 2015. Distribution of selenoglucosinolates and their metabolites in Brassica treated with sodium selenate. Journal of Agricultural and Food Chemistry 63: 1896–1905. [DOI] [PubMed] [Google Scholar]
- Mazej D, Osvald J, Stibilj V. 2008. Selenium species in leaves of chicory, dandelion, lamb’s lettuce and parsley. Food Chemistry 107: 75–83. [Google Scholar]
- McCray CWR, Hurwood IS. 1963. Selenosis in North-Western Queensland associated with a marine Cretaceous formation. Queensland Journal of Agricultural Science 20: 475–498. [Google Scholar]
- McKenzie MJ, Hunter DA, Pathirana R, et al. 2009. Accumulation of an organic anticancer selenium compound in a transgenic Solanaceous species shows wider applicability of the selenocysteine methyltransferase transgene from selenium hyperaccumulators. Transgenic Research 18: 407–424. [DOI] [PubMed] [Google Scholar]
- McQuinn SD, Sleper DA, Mayland HF, Krause GF. 1991. Genetic variation for selenium content in tall fescue. Crop Science 31: 617–620. [Google Scholar]
- Mikkelsen RL, Page AL, Bingham FT. 1989. Factors affecting selenium accumulation by agricultural crops. In: Jacobs LW, Chang AC, Dowdy RH, Severson RC, Sommers LE, Volk VV, eds. Selenium in agriculture and the environment. Soil Science Society of America, Special Publication; 23: 65–94. [Google Scholar]
- Moreno Rodriguez MJ, Cala Rivero V, Jiménez Ballesta R. 2005. Selenium distribution in topsoils and plants of a semi-arid Mediterranean environment. Environmental Geochemistry and Health 27: 513–519. [DOI] [PubMed] [Google Scholar]
- Moxton AL, Olson OE, Searight WV. 1939. Selenium in rocks, soils and plants. South Dakota Agricultural Experimental Station Technical Bulletin 2. Brookings, SD: South Dakota Agricultural Experimental Station. [Google Scholar]
- Murphy KM, Reeves PG, Jones SS. 2008. Relationship between yield and mineral nutrient concentrations in historical and modern spring wheat cultivars. Euphytica 163: 381–390. [Google Scholar]
- Nair RM, Thavarajah P, Giri RR, et al. 2015. Mineral and phenolic concentrations of mungbean [Vigna radiata (L.) R. Wilczek var. radiata] grown in semi-arid tropical India. Journal of Food Composition and Analysis 39: 23–32. [Google Scholar]
- Nelson AG, Quideau SA, Frick B, et al. 2011. The soil microbial community and grain micronutrient concentration of historical and modern hard red spring wheat cultivars grown organically and conventionally in the black soil zone of the Canadian prairies. Sustainability 3: 500–517. [Google Scholar]
- Németh A, Reyes JFG, Kosáry J, Dernovics M. 2013. The relationship of selenium tolerance and speciation in Lecythidaceae species. Metallomics 5: 1663–1673. [DOI] [PubMed] [Google Scholar]
- Norton GJ, Deacon CM, Xiong L, Huang S, Meharg AA, Price AH. 2010. Genetic mapping of the rice ionome in leaves and grain: identification of QTLs for 17 elements including arsenic, cadmium, iron and selenium. Plant and Soil 329: 139–153. [Google Scholar]
- Norton GJ, Duan GL, Lei M, Zhu YG, Meharg AA, Price AH. 2012. Identification of quantitative trait loci for rice grain element composition on an arsenic impacted soil: influence of flowering time on genetic loci. Annals of Applied Biology 161: 46–56. [Google Scholar]
- Ogra Y, Anan Y. 2012. Selenometabolomics explored by speciation. Biological and Pharmaceutical Bulletin 35: 1863–1869. [DOI] [PubMed] [Google Scholar]
- Ogra Y, Ishiwata K, Iwashita Y, Suzuki KT. 2005. Simultaneous speciation of selenium and sulfur species in selenized odorless garlic (Allium sativum L. Shiro) and shallot (Allium ascalonicum) by HPLC-inductively coupled plasma-(octopole reaction system)-mass spectrometry and electrospray ionization-tandem mass spectrometry. Journal of Chromatography A 1093: 118–125. [DOI] [PubMed] [Google Scholar]
- Ogra Y, Kitaguchi T, Ishiwata K, Suzuki N, Iwashita Y, Suzuki KT. 2007. Identification of selenohomolanthionine in selenium-enriched Japanese pungent radish . Journal of Analytical Atomic Spectrometry 22: 1390–1396. [Google Scholar]
- Ouerdane L, Aureli F, Flis P, et al. 2013. Comprehensive speciation of low-molecular weight selenium metabolites in mustard seeds using HPLC–electrospray linear trap/orbitrap tandem mass spectrometry. Metallomics 5: 1294–1304. [DOI] [PubMed] [Google Scholar]
- Paul S, Datta SK, Datta K. 2015. miRNA regulation of nutrient homeostasis in plants. Frontiers in Plant Science 6: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perla V, Holm DG, Jayanty SS. 2012. Selenium and sulfur content and activity of associated enzymes in selected potato germplasm. American Journal of Potato Research 89: 111–120. [Google Scholar]
- Pfister JA, Davis TZ, Hall JO. 2013. Effect of selenium concentration on feed preferences by cattle and sheep . Journal of Animal Science 91: 5970–5980. [DOI] [PubMed] [Google Scholar]
- Pickering IJ, Wright C, Bubner B, et al. 2003. Chemical form and distribution of selenium and sulfur in the selenium hyperaccumulator Astragalus bisulcatus. Plant Physiology 131: 1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piergiovanni AR, Rizzi R, Pannacciulli E, Gatta CD. 1997. Mineral composition in hulled wheat grains: a comparison between emmer (Triticum dicoccon Schrank) and spelt (T. spelta L.) accessions. International Journal of Food Sciences and Nutrition 48: 381–386. [Google Scholar]
- Pilbeam DJ, Greathead HMR, Drihem K. 2015. Selenium. In: Barker AV, Pilbeam DJ, eds. A handbook of plant nutrition, 2nd edn Boca Raton, FL: CRC Press, 165–198. [Google Scholar]
- Pilon M, Owen JD, Garifullina GF, et al. 2003. Enhanced selenium tolerance and accumulation in transgenic Arabidopsis expressing a mouse selenocysteine lyase. Plant Physiology 131: 1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon-Smits EAH. 2012. Plant selenium metabolism – genetic manipulation, phytotechnological applications, and ecological implications. In: Womg MH, ed. Environmental contamination: health risks and ecological restoration. Boca Raton, FL: CRC Press, 293–311. [Google Scholar]
- Pilon-Smits EAH, LeDuc DL. 2009. Phytoremediation of selenium using transgenic plants. Current Opinion in Biotechnology 20: 207–212. [DOI] [PubMed] [Google Scholar]
- Pilon-Smits EAH, de Souza MP, Hong G, et al. 1999a Selenium volatalization and accumulation by twenty aquatic plant species. Journal of Environmental Quality 28: 1011–1018. [Google Scholar]
- Pilon-Smits EAH, Hwang SB, Lytle CM, et al. 1999b Overexpression of ATPsulphurylase in Brassica juncea leads to increased selenite uptake, reduction and tolerance. Plant Physiology 119: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon-Smits EAH, Quinn CF, Tapken W, Malagoli M, Schiavon M. 2009. Physiological functions of beneficial elements. Current Opinion in Plant Biology 12: 267–274. [DOI] [PubMed] [Google Scholar]
- Pommerrenig B, Diehn TA, Bienert GP. 2015. Metalloido-porins: essentiality of Nodulin 26-like intrinsic proteins in metalloid transport. Plant Science 238: 212–227. [DOI] [PubMed] [Google Scholar]
- Pu ZE, Yu M, He QY, et al. 2014. Quantitative trait loci associated with micronutrient concentrations in two recombinant inbred wheat lines. Journal of Integrative Agriculture 13: 2322–2329. [Google Scholar]
- Quinn CF, Galeas ML, Freeman JL, Pilon-Smits EAH. 2007. Selenium: deterrence, toxicity, and adaptation. Integrated Environmental Assessment and Management 3: 460–462. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Erskine W, Materne MA, McMurray LM, Thavarajah P, Thavarajah D, Siddique KHM. 2015. Enhancing selenium concentration in lentil (Lens culinaris subsp. culinaris) through foliar application. Journal of Agricultural Science 153: 656–665. [Google Scholar]
- Ramamurthy RK, Jedlicka J, Graef GL, Waters BM. 2014. Identification of new QTLs for seed mineral, cysteine, and methionine concentrations in soybean [Glycine max (L.) Merr.]. Molecular Breeding 34: 431–445. [Google Scholar]
- Ramos SJ, Rutzke MA, Hayes RJ, Faquin V, Guilherme LRG, Li L. 2011a Selenium accumulation in lettuce germplasm. Planta 233: 649–660. [DOI] [PubMed] [Google Scholar]
- Ramos SJ, Yuan Y, Faquin V, Guilherme LRG, Li L. 2011b Evaluation of genotypic variation of broccoli (Brassica oleracea var. italic) in response to selenium treatment. Journal of Agricultural and Food Chemistry 59: 3657–3665. [DOI] [PubMed] [Google Scholar]
- Ray H, Bett K, Tar’an B, Vandenberg A, Thavarajah D, Warkentin T. 2014. Mineral micronutrient content of cultivars of field pea, chickpea, common bean, and lentil grown in Saskatchewan, Canada. Crop Science 54: 1698–1708. [Google Scholar]
- Rayman MP. 2012. Selenium and human health. Lancet 379: 1256–1268. [DOI] [PubMed] [Google Scholar]
- Rayman MP, Infante HG, Sargent M. 2008. Food-chain selenium and human health: spotlight on speciation. British Journal of Nutrition 100: 238–253. [DOI] [PubMed] [Google Scholar]
- Reeves RD, Baker AJM. 2000. Metal-accumulating plants. In: Raskin I, Ensley BD, eds. Phytoremediation of toxic metals: using plants to clean up the environment. New York: Wiley, 193–229. [Google Scholar]
- Rodríguez LH, Morales DA, Rodríguez ER, Romero CD. 2011. Minerals and trace elements in a collection of wheat landraces from the Canary Islands. Journal of Food Composition and Analysis 24: 1081–1090 [Google Scholar]
- Rosenfeld I, Beath OA. 1964. Selenium: geobotany, biochemistry, toxicity, and nutrition. New York: Academic Press. [Google Scholar]
- Rouached H, Wirtz M, Alary R, et al. 2008. Differential regulation of the expression of two high-affinity sulfate transporters, SULTR1.1 and SULTR1.2, in Arabidopsis. Plant Physiology 147: 897–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavon M, Pilon M, Malagoli M, Pilon-Smits EAH. 2015. Exploring the importance of sulphate transporters and ATPsulphurylases for selenium hyperaccumulation – comparison of Stanleya pinnata and Brassica juncea (Brassicaceae). Frontiers in Plant Science 6: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppänen MM, Kontturi J, Heras IL, Madrid Y, Cámara C, Hartikainen H. 2010. Agronomic biofortification of Brassica with selenium – enrichment of SeMet and its identification in Brassica seeds and meal. Plant and Soil 337: 273–283. [Google Scholar]
- Shao SX, Mi XB, Ouerdane L, et al. 2014. Quantification of Se-methylselenocysteine and its γ-glutamyl derivative from naturally Se-enriched green bean (Phaseolus vulgaris vulgaris) after HPLC-ESI-TOF-MS and orbitrap MSn-based identification. Food Analytical Methods 7: 1147–1157. [Google Scholar]
- Shibagaki N, Rose A, McDermott JP, et al. 2002. Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots. The Plant Journal 29: 475–486. [DOI] [PubMed] [Google Scholar]
- Shinmachi F, Buchner P, Stroud JL, et al. 2010. Influence of sulfur deficiency on the expression of specific sulfate transporters and the distribution of sulfur, selenium, and molybdenum in wheat. Plant Physiology 153: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva EG, Mataveli LR, Arruda MA. 2013. Speciation analysis of selenium in plankton, Brazil nut and human urine samples by HPLC-ICP-MS. Talanta 110: 53–57. [DOI] [PubMed] [Google Scholar]
- Smrkolj P, Stibilj V, Kreft I, Kapolna E. 2005. Selenium species determination in selenium enriched pumpkin (Cucurbita pepo L.) seeds by HPLC–UV–HG-AFS. Analytical Sciences 21: 1501–1504. [DOI] [PubMed] [Google Scholar]
- Smrkolj P, Stibilj V, Kreft I, Germ M. 2006. Selenium species in buckwheat cultivated with foliar addition of Se(VI) and various levels of UV-B radiation. Food Chemistry 96: 675–681. [Google Scholar]
- Smrkolj P, Osvald M, Osvald J, Stibilj V. 2007. Selenium uptake and species distribution in selenium-enriched bean (Phasolus vulgaris L.) seeds obtained by two different cultivations. European Food Research and Technology 225: 233–237. [Google Scholar]
- Sors TG, Ellis DR, Na GN, et al. 2005a Analysis of sulfur and selenium assimilation in Astragalus plants with varying capacities to accumulate selenium. The Plant Journal 42: 785–797. [DOI] [PubMed] [Google Scholar]
- Sors TG, Ellis DR, Salt DE. 2005b Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynthesis Research 86: 373–389. [DOI] [PubMed] [Google Scholar]
- Sors TG, Martin CP, Salt DE. 2009. Characterization of selenocysteine methyltransferases from Astragalus species with contrasting selenium accumulation capacity. The Plant Journal 59: 110–122. [DOI] [PubMed] [Google Scholar]
- Souza GA, Hart JJ, Carvalho JG, et al. 2014. Genotypic variation of zinc and selenium concentration in grains of Brazilian wheat lines. Plant Science 224: 27–35. [DOI] [PubMed] [Google Scholar]
- de Souza MP, Pilon-Smits EAH, Terry N. 2000. The physiology and biochemistry of selenium volatilization by plants. In: Raskin I, Ensley BD, eds Phytoremediation of toxic metals: using plants to clean-up the environment. New York: John Wiley and Sons, 171–190. [Google Scholar]
- Stoffaneller R, Morse NL. 2015. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 7: 1494–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara S, Kondo M, Chihara Y, Yuji M, Hattori H, Yoshida M. 2004. Preparation of selenium-enriched sprouts and identification of their selenium species by high-performance liquid chromatography-inductively coupled plasma mass spectrometry. Bioscience, Biotechnology, and Biochemistry 68: 193–199. [DOI] [PubMed] [Google Scholar]
- Sura-de Jong M, Reynolds RJB, Richterova K, et al. 2015. Selenium hyperaccumulators harbor a diverse endophytic bacterial community characterized by high selenium resistance and plant growth promoting properties. Frontiers in Plant Science 6: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagmount A, Berken A, Terry N. 2002. An essential role of S-adenosyl-l-methionine:l-methionine S-methyltransferase in selenium volatilization by plants. Methylation of selenomethionine to selenium-methyl-l-selenium-methionine, the precursor of volatile selenium. Plant Physiology 130: 847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Watanabe-Takahashi A, Smith FW, Blake-Kalff M, Hawkesford MJ, Saito K. 2000. The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant Journal 23: 171–182. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Buchner P, Yoshimoto N, Hawkesford MJ, Shiu S-H. 2012. Evolutionary relationships and functional diversity of plant sulfate transporters. Frontiers in Plant Science 2: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki M, Freeman JL, Pilon-Smits EAH. 2008. Cooperative ethylene and jasmonic acid signaling regulates selenite resistance in Arabidopsis. Plant Physiology 146: 1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeder M. 2012. Transporters for amino acids in plant cells: some functions and many unknowns. Current Opinion in Plant Biology 15: 315–321. [DOI] [PubMed] [Google Scholar]
- Terry N, Carlson C, Raab TK, Zayed AM. 1992. Rates of selenium volatilization among crop species. Journal of Environmental Quality 21: 341–344. [Google Scholar]
- Terry N, Zayed AM, de Souza MP, Tarun AS. 2000. Selenium in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology 51: 401–432. [DOI] [PubMed] [Google Scholar]
- Thosaikham W, Jitmanee K, Sittipout R, Maneetong S, Chantiratikul A, Chantiratikul P. 2014. Evaluation of selenium species in selenium-enriched pakchoi (Brassica chinensis Jusl var parachinensis (Bailey) Tsen & Lee) using mixed ion-pair reversed phase HPLC–ICP-MS. Food Chemistry 145: 736–742. [DOI] [PubMed] [Google Scholar]
- Thavarajah D, Thavarajah P. 2012. Evaluation of chickpea (Cicer arietinum L.) micronutrient composition: biofortification opportunities to combat global micronutrient malnutrition. Food Research International 49: 99–104. [Google Scholar]
- Thavarajah D, Ruszkowski J, Vandenberg A. 2008. High potential for selenium biofortification of lentils (Lens culinaris L.). Journal of Agriculture and Food Chemistry 57: 10747–10753. [DOI] [PubMed] [Google Scholar]
- Thavarajah D, Warkentin T, Vandenberg A. 2010. Natural enrichment of selenium in Saskachewan field peas (Pisum sativum L.). Canadian Journal of Plant Science 90: 383–389. [Google Scholar]
- Thavarajah D, Thavarajah P, Sarker A, et al. 2011. A global survey of effects of genotype and environment on selenium concentration in lentils (Lens culinaris L.): implications for nutritional fortification strategies. Food Chemistry 125: 72–76. [Google Scholar]
- Turakainen M, Hartikainen H, Seppänen MM. 2004. Effects of selenium treatments on potato (Solanum tuberosum L.) growth and concentrations of soluble sugars and starch . Journal of Agricultural and Food Chemistry 52: 5378–5382. [DOI] [PubMed] [Google Scholar]
- Valdez Barillas JR, Quinn CF, Freeman JL, et al. 2012. Selenium distribution and speciation in the hyperaccumulator Astragalus bisulcatus and associated ecological partners. Plant Physiology 159: 1834–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoewyk D. 2013. A tale of two toxicities: malformed selenoproteins and oxidative stress both contribute to selenium stress in plants. Annals of Botany 112: 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoewyk D, Garifullina GF, Ackley AR, et al. 2005. Overexpression of AtCpNifS enhances selenium tolerance and accumulation in Arabidopsis. Plant Physiology 139: 1518–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoewyk D, Pilon M, Pilon-Smits EAH. 2008a The functions of NifS-like proteins in plant sulfur and selenium metabolism. Plant Science 174: 117–123. [Google Scholar]
- Van Hoewyk D, Takahashi H, Hess A, Tamaoki M, Pilon-Smits EAH. 2008b Transcriptome and biochemical analyses give insights into selenium-stress responses and selenium tolerance mechanisms in Arabidopsis. Physiologia Plantarum 132: 236–253. [DOI] [PubMed] [Google Scholar]
- Van Huysen T, Abdel-Ghany S, Hale KL, LeDuc D, Terry N, Pilon-Smits EAH. 2003. Overexpression of cystathionine-γ-synthase enhances selenium volatilisation in Brassica juncea. Planta 218: 71–78. [DOI] [PubMed] [Google Scholar]
- Van Huysen T, Terry N, Pilon-Smits EAH. 2004. Exploring the selenium phytoremediation potential of transgenic Brassica juncea overexpressing ATP sulfurylase or cystathionine-γ-synthase. International Journal of Phytoremediation 6: 111–118 [DOI] [PubMed] [Google Scholar]
- Vonderheide AP, Wrobel K, Kannamkumarath SS, et al. 2002. Characterization of selenium species in Brazil nuts by HPLC-ICP-MS and ES-MS. Journal of Agricultural and Food Chemistry 50: 5722–5728. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Broadley MR, Jansen S, et al. 2007. Evolutionary control of leaf element composition in plants. New Phytologist 174: 516–523. [DOI] [PubMed] [Google Scholar]
- White PJ, Broadley MR. 2009. Biofortification of crops with seven mineral elements often lacking in human diets – iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytologist 182: 49–84. [DOI] [PubMed] [Google Scholar]
- White PJ, Brown PH. 2010. Plant nutrition for sustainable development and global health. Annals of Botany 105: 1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Bowen HC, Parmaguru P, et al. 2004. Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. Journal of Experimental Botany 55: 1927–1937. [DOI] [PubMed] [Google Scholar]
- White PJ, Bowen HC, Marshall B, Broadley MR. 2007a Extraordinarily high leaf selenium to sulphur ratios define ‘Se-accumulator’ plants. Annals of Botany 100: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Broadley MR, Bowen HC, Johnson SE. 2007b Selenium and its relationship with sufur. In: Hawkesford MJ, de Kok LJ, eds. Sulfur in plants – an ecological perspective. Dordrecht: Springer, 225–252. [Google Scholar]
- Williams KT, Lakin HW, Byers HG. 1940. Selenium occurrence in certain soils in the United States, with a discussion of related topics: fourth report . United States Department of Agriculture Technical Bulletin 702. Washington, DC: United States Department of Agriculture. [Google Scholar]
- Williams PN, Lombi E, Sun GX, et al. 2009. Selenium characterization in the global rice supply chain. Environmental Science and Technology 43: 6024–6030. [DOI] [PubMed] [Google Scholar]
- Wu L. 2004. Review of 15 years of research on ecotoxicology and remediation of land contaminated by agricultural drainage sediment rich in selenium. Ecotoxicology and Environmental Safety 57: 257–269. [DOI] [PubMed] [Google Scholar]
- Ximénez-Embún P, Alonso I, Madrid-Albarrán Y, Cámara C. 2004. Establishment of selenium uptake and species distribution in lupine, Indian mustard, and sunflower plants. Journal of Agricultural and Food Chemistry 52: 832–838. [DOI] [PubMed] [Google Scholar]
- Yan J, Wang F, Qin H, et al. 2011. Natural variation in grain selenium concentration of wild barley, Hordeum spontaneum, populations from Israel. Biological Trace Element Research 142: 773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Chen L, Hu Q, Pan G. 2003. Effect of the application of selenium on selenium content of soybean and its products. Biological Trace Element Research 93: 249–256. [DOI] [PubMed] [Google Scholar]
- Yang R, Wang R, Xue W, et al. 2013. QTL location and analysis of selenium content in tetraploid wheat grain. Guizhou Agricultural Sciences 10: 1–4. [In Chinese] [Google Scholar]
- Yoshimoto N, Inoue E, Saito K, Yamaya T, Takahashi H. 2003. Phloem-localizing sulfate transporter, Sultr1;3, mediates re-distribution of sulfur from source to sink organs in Arabidopsis . Plant Physiology 131: 1511–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan LX, Zhu YY, Lin ZQ, Banuelos G, Li W, Yin XB. 2013. A novel selenocystine-accumulating plant in selenium-mine drainage area in Enshi, China. PLoS Ine 8: e65615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L-H, Abdel-Ghany SE, Freeman JL, Ackley AR, Schiavon M, Pilon-Smits EAH. 2006a Investigation of selenium tolerance mechanisms in Arabidopsis thaliana. Physiologia Plantarum 128: 212–223. [Google Scholar]
- Zhang L, Byrne PF, Pilon-Smits EAH. 2006b Mapping quantitative trait loci associated with selenate tolerance in Arabidopsis thaliana. New Phytologist 170: 33–42. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shi W, Wang X, Zhou X. 2006c Genotypic differences in selenium accumulation in rice seedlings at early growth stage and analysis of dominant factors influencing selenium content in rice seeds. Journal of Plant Nutrition 29: 1601–1618. [Google Scholar]
- Zhang L, Ackley AR, Pilon-Smits EAH. 2007. Variation in selenium tolerance and accumulation among 19 Arabidopsis thaliana accessions. Journal of Plant Physiology 164: 327–336. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hu B, Li W, et al. 2014. OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytologist 201: 1183–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D-Y, Sun F-L, Zhang B, Zhang Z-Q, Yin LQ. 2015. Systematic comparisons of orthologous selenocysteine methyltransferase and homocysteine methyltransferase genes from seven monocots species. Notulae Scientia Biologicae 7: 210–216. [Google Scholar]
- Zhao F-J, Lopez-Bellido FJ, Gray CW, Whalley WR, Clark LJ, McGrath SP. 2007. Effects of soil compaction and irrigation on the concentrations of selenium and arsenic in wheat grains. Science of the Total Environment 372: 433–439. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Su YH, Dunham SJ, et al. 2009. Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. Journal of Cereal Science 49: 290–295. [Google Scholar]
- Zhao XQ, Mitani N, Yamaji N, Shen RF, Ma JF. 2010. Involvement of silicon influx transporter OsNIP2;1 in selenite uptake in rice. Plant Physiology 153: 1871–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y-G, Pilon-Smits EAH, Zhao F-J, Williams PN, Meharg AA. 2009. Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends in Plant Science 14: 436–442. [DOI] [PubMed] [Google Scholar]