Abstract
Background
Because ambient air pollution exposure occurs as mixtures, consideration of joint effects of multiple pollutants may advance our understanding of air pollution health effects.
Methods
We assessed the joint effect of air pollutants in selected combinations (representative of oxidant gases, secondary, traffic, power plant, and criteria pollutants; constructed using combinations of criteria pollutants and fine particulate matter (PM2.5) components) on pediatric asthma emergency department (ED) visits in Atlanta during 1998–2004. Joint effects were assessed using multi-pollutant Poisson generalized linear models controlling for time trends, meteorology and daily non-asthma upper respiratory ED visit counts. Rate ratios (RR) were calculated for the combined effect of an interquartile-range increment in each pollutant’s concentration.
Results
Increases in all of the selected pollutant combinations were associated with increases in warm-season pediatric asthma ED visits [e.g., joint effect rate ratio=1.13 (95% confidence interval 1.06–1.21) for criteria pollutants (including ozone, carbon monoxide, nitrogen dioxide, sulfur dioxide, and PM2.5)]. Cold-season joint effects from models without non-linear effects were generally weaker than warm-season effects. Joint effect estimates from multi-pollutant models were often smaller than estimates calculated based on single-pollutant model results, due to control for confounding. Compared with models without interactions, joint effect estimates from models including first-order pollutant interactions were largely similar. There was evidence of non-linear cold-season effects.
Conclusions
Our analyses illustrate how consideration of joint effects can add to our understanding of health effects of multi-pollutant exposures, and also illustrate some of the complexities involved in calculating and interpreting joint effects of multiple pollutants.
Background
Although many air pollution health effects studies focus on individual pollutants, actual air pollution exposures are to multi-pollutant mixtures. Recognition of the importance of air pollution mixtures has led to increased interest in assessment of their health effects.1–3 Various approaches to assessing the health effects of air pollution mixtures have been employed.1,4 For example, some studies have assessed health effects using markers of exposure to specific mixtures, such as measures of traffic volume or proximity to roadways.5 Other studies have assessed health effects of mixtures characterized through source apportionment metrics,6 air pollution indices or sums of pollutant concentrations.7–12 In this study we illustrate and discuss an approach to assessing air pollution mixture health effects that extends traditional single-pollutant epidemiologic models of measured pollutant concentrations by using models that include several pollutants to estimate the combined effect of multiple pollutants (i.e. joint effects).
Considering the joint effects of pollutants, rather than their individual effects, can advance our understanding of air pollutant mixture health effects. For example, considering the joint effects of groups of pollutants from particular sources may increase our understanding of important sources contributing to health effects. In addition, consideration of joint effects can address issues of confounding between pollutants and may avoid some of the difficulties involved in attempts to isolate individual effects of several correlated pollutants.13,14
To date, joint effects have infrequently been considered in air pollution health effects studies,15–21 with few studies assessing the precision of the joint effect estimates.17–21 Moreover, discussion of the potential complexities involved with this approach has been limited.
In this study, we assess the joint effect of pollutants in several combinations of criteria gases, particulate matter less than 2.5 μm in diameter (PM2.5), and PM2.5 components, using data from a time-series study of air pollution and pediatric asthma emergency department (ED) visits in the Atlanta metropolitan area for which model performance has been previously described.22 We selected specific combinations representing pollutants with shared properties (oxidant gases and secondary pollutants) or sources (traffic and coal-fired power plant pollutants), or common air pollutants with U.S. National Ambient Air Quality Standards (NAAQS). We also discuss issues that must be considered when applying this analytic approach.
Methods
We used the data and modeling strategy applied by Strickland et al.,22 building upon previous results by considering joint effects of pollutant combinations. Methods for the original study are described in detail elsewhere.22 Briefly, daily concentrations of ambient 1-hour maximum carbon monoxide (CO), nitrogen dioxide (NO2), and sulfur dioxide (SO2); 8-hour maximum ozone (O3); and 24-hour average PM2.5 and the PM2.5 components elemental carbon (EC), sulfate (SO42−), nitrate (NO3−) and ammonium (NH4+) for the period August 1, 1998 through December 31, 2004 were obtained from several ambient monitoring networks in Atlanta.23,24 Daily pollutant measurements across monitors were combined using population weighting.25 Individual-level data on ED visits during this time period were obtained from metropolitan Atlanta hospitals. Using International Classification of Diseases, 9th Revision (ICD-9), pediatric asthma ED visits were defined as visits with a code for asthma (493.0–493.9) or wheeze (786.09 before October 1, 1998; 786.07 after October 1, 1998) that did not have a code for an external injury or poisoning (E800-E999) among children aged 5 to 17 years. ED visits for acute upper respiratory infections (codes 460.0–466.0) among children in the same age group who did not also have a code for asthma or wheeze were also identified.
We examined the joint effects of combinations of commonly measured pollutants, selected to represent oxidant gases, secondary pollutants, pollutants from traffic and coal-fired power plant sources, and criteria pollutants (Table 1). The “oxidant gases” combination included the major gaseous oxidant air pollutants (O3, NO2 and SO2). The “secondary” pollutant combination included O3 and secondary PM2.5 (for which the concentration was calculated as the sum of the concentrations of the major inorganic PM2.5 components SO42−, NO3−, and NH4+). The pollutants in the “traffic” combination were those most closely associated with traffic pollution in Atlanta (CO, NO2 and EC).10 Organic carbon (OC) measurements were not used in these analyses because OC originates from both primary (e.g. traffic) and secondary sources, and speciated organic data needed to apportion OC to sources were available for only a limited time period. The pollutants in the “power plant” combination (SO2 and SO42−) were selected to be relatively (although not completely) specific for pollution from coal-fired power plants.26,27 The “criteria pollutants” combination included five of the pollutants for which NAAQS are set (including O3, CO, NO2, SO2 and PM2.5; excluding lead and particulate matter less than 10 μm in diameter). The “criteria pollutants” combination was included because of its potential regulatory significance.
Table 1.
Pollutant combinations for calculation of joint effects
| Combination Name | O3 | CO | NO2 | SO2 | PM2.5 | EC | SO42− | Secondary PM2.5a |
|---|---|---|---|---|---|---|---|---|
| Oxidant Gases | X | X | X | |||||
| Secondary | X | X | ||||||
| Traffic | X | X | X | |||||
| Power Plant | X | X | ||||||
| Criteria Pollutants | X | X | X | X | X |
Definition of Abbreviations: O3=ozone, CO=carbon monoxide, NO2=nitrogen dioxide, SO2=sulfur dioxide, PM2.5=particulate matter less than 2.5 μm in diameter, EC=elemental carbon component of PM2.5, SO42−=sulfate component of PM2.5.
Secondary PM2.5 was calculated as the sum of the concentrations of selected PM2.5 components including sulfate, nitrate, and ammonium.
We examined joint effects of pollutant combinations on pediatric asthma ED visits using Poisson generalized linear models that accounted for overdispersion. Because previous analyses showed different warm and cold-season effects,22 analyses were season-specific. We used the same general model form as Strickland et al.22 The dependent variable was the hospital-specific daily pediatric asthma ED visit count. For each pollutant in a given combination, the primary models included a linear term for the three-day moving average of pollutant concentrations [the average of the pollutant concentration on a given day (lag 0) and the previous two days (lags 1 and 2)]. Models also included a linear term for the logarithm of the daily non-asthma pediatric ED visit count for acute upper respiratory infections; cubic polynomials for day-of-season, the moving average of dew point (lags 0–2) and the moving average of minimum temperature (lags 1 and 2); indicator variables for year, month, day of week or holiday (with holidays having a separate indicator), hospital and same-day (lag 0) maximum temperature (for each degree Celsius); and interaction terms between month and year, month and lag 0 maximum temperature, and month and day of week. We estimated joint effects for an interquartile-range (IQR) increment in the three-day moving average of each pollutant concentration as the exponentiated sum (across the pollutants in the combination) of the product of each pollutant’s model coefficient and that pollutant’s IQR. Standard errors for joint effects were calculated using the variance-covariance matrices for the individual-pollutant coefficients (for a mathematical representation of models and joint effect calculations, see Supplemental Material, Model Details).
Initial models included only the pollutants in the specified combination, without interaction terms. The results of these models were compared with the results of models that also included linear terms for all first-order multiplicative interactions between the pollutants in the combination (e.g., for the oxidant gases combination, models included terms for O3, NO2, SO2, and the product terms O3*NO2, O3*SO2 and NO2*SO2). In sensitivity analyses, we considered models with linear, quadratic and cubic terms for each pollutant, to assess the adequacy of using linear pollutant terms. In interaction models and models with quadratic and cubic terms, joint effects were calculated for a concentration change equal in magnitude to each pollutant’s IQR, starting from each pollutant’s 15th, 25th, or 35th percentile levels. We also considered models that controlled for other potentially confounding pollutants.
Concurvity (the analogue of collinearity in non-linear models) was calculated for each pollutant as the correlation between observed pollutant concentrations and predicted concentrations from linear models including all other model variables (i.e., other pollutant terms, time variables and meteorological variables).28 We also calculated concurvity for each pollutant in relation to only the other pollutant terms in the models (without time and meteorological variables).
Results
The distributions of the individual pollutants during the 1998–2004 warm and cold seasons are characterized in Table 2, and correlations between them are shown in Table 3. Warm-season, correlations ranged from moderately high to high (Spearman correlation coefficient (r) 0.62–0.98) among PM2.5, secondary PM2.5, and the PM2.5 component SO42−. There were moderately high correlations among the traffic-related pollutants CO, NO2, and EC (r= 0.57–0.75) in both seasons; and between PM2.5 and O3 (r=0.66) in the warm season. Cold-season correlations were generally lower than warm-season correlations, except for correlations between PM2.5 and CO and PM2.5 and EC, which were slightly higher in the cold season. For all of the pollutant combinations, all pollutants were simultaneously observed in their highest or lowest quartiles on some days in both seasons, so effect estimates for IQR increments in each pollutant represent pollutant changes that actually occur (eTable 1). The mean daily number of pediatric asthma ED visits was 26.2 (standard deviation 14.8) in the warm season and 32.5 (standard deviation 14.1) in the cold season.
Table 2.
Individual pollutant distributions, Atlanta, August 1998-December 2004
| Pollutant | Season | Units | N | Mean | Standard Deviation | 25th percentilea | 50th percentile | 75th percentilea |
|---|---|---|---|---|---|---|---|---|
| Ozone (8-h maximum) | WARM (May–October) |
ppb | 1196 | 53.9 | 19.2 | 39.8 | 53.3 | 67.7 |
| Carbon monoxide (1-h maximum) | ppm | 1186 | 0.7 | 0.4 | 0.5 | 0.7 | 0.9 | |
| Nitrogen dioxide (1-h maximum) | ppb | 1196 | 22.3 | 10.2 | 14.8 | 20.5 | 28.7 | |
| Sulfur dioxide (1-h maximum) | ppb | 1196 | 8.3 | 7.4 | 2.9 | 6.0 | 11.4 | |
| PM2.5 (24-h average) | μg/m³ | 1196 | 18.4 | 7.6 | 12.8 | 17.0 | 23.1 | |
| PM2.5 elemental carbon (24-h average) | μg/m³ | 1190 | 0.8 | 0.6 | 0.4 | 0.7 | 1.1 | |
| PM2.5 sulfate (24-h average) | μg/m³ | 1179 | 5.9 | 3.5 | 3.4 | 5.1 | 7.8 | |
| Secondary PM2.5b (24-h average) | μg/m³ | 1178 | 8.6 | 4.7 | 5.1 | 7.5 | 10.9 | |
|
| ||||||||
| Ozone (8-h maximum) | COLD (November–April) |
ppb | 1148 | 33.6 | 13.9 | 23.4 | 32.6 | 42.7 |
| Carbon monoxide (1-h maximum) | ppm | 1137 | 0.9 | 0.5 | 0.5 | 0.7 | 1.1 | |
| Nitrogen dioxide (1-h maximum) | ppb | 1149 | 25.6 | 9.8 | 18.4 | 24.9 | 31.7 | |
| Sulfur dioxide (1-h maximum) | ppb | 1149 | 10.8 | 9.0 | 4.1 | 8.2 | 14.6 | |
| PM2.5 (24-h average) | μg/m³ | 1142 | 14.3 | 6.5 | 9.8 | 13.0 | 17.4 | |
| PM2.5 elemental carbon (24-h average) | μg/m³ | 1144 | 0.9 | 0.6 | 0.4 | 0.7 | 1.2 | |
| PM2.5 sulfate (24-h average) | μg/m³ | 1133 | 3.2 | 1.8 | 1.9 | 2.8 | 3.9 | |
| Secondary PM2.5b (24-h average) | μg/m³ | 1133 | 5.6 | 2.7 | 3.7 | 5.1 | 6.9 | |
|
| ||||||||
Definition of Abbreviations: PM2.5=particulate matter less than 2.5 μm in diameter
For comparability with previous analyses,21 the IQRs used in calculation of joint effects were slightly different from those reflected here, due to use of the year-round IQR during 1993–2004 for O3, NO2, CO and SO2, and during August 1, 1998-December 31, 2004 for PM2.5 and PM2.5 components.
Secondary PM2.5 was calculated as the sum of the concentrations of selected PM2.5 components including sulfate, nitrate, and ammonium.
Table 3.
Spearman correlations between pollutants, Atlanta, August 1998-December 2004
| Season | Ozone (8-h maximum) | Carbon monoxide (1-h maximum) | Nitrogen dioxide (1-h maximum) | Sulfur dioxide (1-h maximum) | PM2.5 (24-h average) | PM2.5 elemental carbon (24-h average) | PM2.5 sulfate (24-h average) | |
|---|---|---|---|---|---|---|---|---|
| Ozone (8-h maximum) | WARM (May–October) |
1.00 | ||||||
| Carbon monoxide (1-h maximum) | 0.33 | 1.00 | ||||||
| Nitrogen dioxide (1-h maximum) | 0.54 | 0.75 | 1.00 | |||||
| Sulfur dioxide (1-h maximum) | 0.27 | 0.32 | 0.44 | 1.00 | ||||
| PM2.5 (24-h average) | 0.66 | 0.46 | 0.52 | 0.28 | 1.00 | |||
| PM2.5 elemental carbon (24-h average) | 0.39 | 0.73 | 0.68 | 0.31 | 0.53 | 1.00 | ||
| PM2.5 sulfate (24-h average) | 0.55 | 0.22 | 0.27 | 0.24 | 0.82 | 0.28 | 1.00 | |
| Secondary PM2.5a (24-h average) | 0.55 | 0.27 | 0.31 | 0.24 | 0.86 | 0.33 | 0.98 | |
|
| ||||||||
| Ozone (8-h maximum) | COLD (November–April) |
1.00 | ||||||
| Carbon monoxide (1-h maximum) | 0.12 | 1.00 | ||||||
| Nitrogen dioxide (1-h maximum) | 0.30 | 0.74 | 1.00 | |||||
| Sulfur dioxide (1-h maximum) | 0.05 | 0.22 | 0.41 | 1.00 | ||||
| PM2.5 (24-h average) | 0.17 | 0.57 | 0.49 | 0.07 | 1.00 | |||
| PM2.5 elemental carbon (24-h average) | 0.10 | 0.72 | 0.57 | 0.18 | 0.64 | 1.00 | ||
| PM2.5 sulfate (24-h average) | 0.20 | 0.14 | 0.08 | 0.02 | 0.62 | 0.19 | 1.00 | |
| Secondary PM2.5a (24-h average) | 0.07 | 0.16 | 0.12 | 0.08 | 0.67 | 0.24 | 0.92 | |
|
| ||||||||
Definition of Abbreviations: PM2.5=particulate matter less than 2.5 μm in diameter
Secondary PM2.5 was calculated as the sum of the concentrations of selected PM2.5 components including sulfate, nitrate, and ammonium.
Daily increases in all of the selected pollutant combinations were associated with increases in daily warm-season pediatric asthma ED visit counts (Figure 1, eTable 2). Warm-season rate ratios (RR) for the joint effect of an IQR change in the three-day moving average of all pollutants in the selected combinations, in models without interactions, were 1.10 (95% confidence interval (CI)=1.04–1.16) for oxidant gases, 1.09 (95% CI=1.04–1.14) for secondary pollutants, 1.11 (95% CI=1.06–1.16) for traffic pollutants, 1.06 (95% CI=1.02–1.10) for power plant pollutants, and 1.13 (95% CI 1.06–1.21) for criteria pollutants. Warm-season joint effects were stronger than any of the single-pollutant model effects for the pollutants in each combination (eTable 3). If warm-season joint effects had been calculated using pollutant coefficients from single-pollutant models, joint effect RRs would have been overestimated (calculated RRs: oxidant gases 1.22, secondary 1.12, traffic 1.25, power plant 1.07, criteria pollutants 1.41), because single pollutant models do not control for potential confounding effects of co-varying pollutants. Cold-season joint effects varied across pollutant combinations. Overall, these were not statistically significant with the primary models, but were highest for the secondary combination (RR per IQR change=1.06, 95% CI=0.99–1.14) and the oxidant gases combination (RR per IQR change =1.05, 95% CI=0.99–1.13) (Figure 1, eTable 2). Comparison of cold-season joint effects calculated based on single-pollutant model coefficients with those from the multipollutant models is complicated by some cold-season single pollutant models indicating non-significant protective effects (eTable 3). More detailed results from the single-pollutant and primary joint effect models are shown in eTables 4 and 5 (pollutant coefficients and p-values) and eTables 6,7 (variance-covariance matrix for estimates from primary multi-pollutant models).
Figure 1. Rate ratios and 95% confidence intervals for single pollutant effects per interquartile-range increment in each pollutant from single pollutant models and for corresponding joint effects from multi-pollutant models.
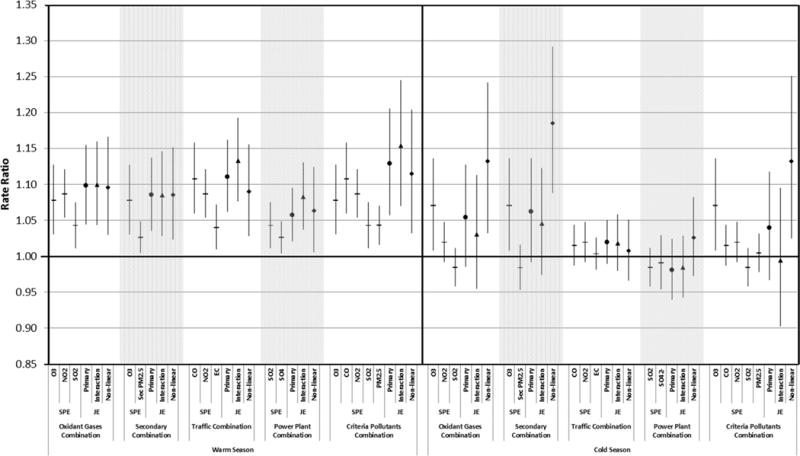
For each combination of pollutants, single-pollutant rate ratios (shown with horizontal line markers) are followed by joint effect rate ratios from the primary multi-pollutant models, which had linear pollutant terms and no interactions (“Primary”, circle markers), multi-pollutant models with linear pollutant terms and first order interactions between pollutants, with IQR increments evaluated starting at the 25th percentile for each pollutant (“Interaction”, triangle markers), and multi-pollutant models with linear, quadratic and cubic pollutant terms and no interactions, with IQR increments evaluated starting at the 25th percentile for each pollutant (“Non-linear”, diamond markers). For comparability with previous analyses,22 the analysis used the year-round IQR during 1993–2004 for O3, NO2, CO and SO2, and during August 1, 1998-December 31, 2004 for PM2.5 and PM2.5 components. The IQRs used in the analysis were: O3 29.18 ppb, CO 0.66 ppm, NO2 12.87 ppb, SO2 10.51 ppb, PM2.5 9.18 μg/m3, EC 0.69 μg/m3, SO42− 3.45 μg/m3, Secondary PM2.5 4.52 μg/m3. PM2.5: particulate matter <2.5 μm in diameter, O3: ozone, NO2: nitrogen dioxide, SO2: sulfur dioxide, Sec PM: Secondary PM2.5, CO: carbon monoxide, EC: elemental carbon component of PM2.5, SO4: Sulfate component of PM2.5. Secondary PM2.5 was calculated as the sum of the concentrations of selected PM2.5 components including nitrate (NO3−), ammonium (NH4+), and sulfate (SO42−).
Compared with joint effect estimates from models without interactions, joint effect estimates from models that included first order interactions between pollutants, with evaluation of the joint effect for a change from the 25th to the 75th percentile level for each pollutant, were generally not substantially different (Figure 1, eTable 2). Sensitivity analyses evaluating joint effects at other points in the pollutant distributions yielded similar conclusions relative to no-interaction models (eFigures 1 and 2). Based on consideration of individual interaction terms or consideration of likelihood ratio tests for the interaction terms together, warm-season models including first-order pollutant interactions generally did not show strong statistical evidence of important interactions (eTables 2 and 4), but cold-season models for the oxidant gases and criteria pollutants combinations did (likelihood ratio test p-values for interaction terms together <0.01, eTables 2 and 5).
In sensitivity analyses, compared with estimates from models with only linear pollutant terms, joint effect estimates for an IQR change in each pollutant (starting at the 25th percentile) from models including quadratic and cubic pollutant terms were slightly lower for the warm-season traffic and criteria pollutant combinations; and notably higher for several cold-season combinations, particularly the oxidant gases, secondary and oxidant pollutant combinations (Figure 1). Evaluation of joint effects at other points in the pollutant distributions gave similar conclusions (eFigures 1 and 2). Dose-response curves based on the results of the models with and without non-linear terms for the oxidant gases, secondary and criteria pollutants combinations are shown in eFigure 3. Statistical evidence for the importance of the quadratic and cubic terms was observed for the warm-season traffic combination, and the cold-season secondary, criteria pollutants and power plant combinations (p-values for likelihood ratio tests for non-linear terms together <0.03, see eTable 8 for joint effect estimates and eTables 9 and 10 for detailed model results).
Joint effect estimates from models controlling for potentially confounding pollutants that were not included in calculation of joint effects were generally lower than joint effect estimates from models including only the pollutants in the joint effect (except for the traffic combination in both seasons, and the oxidant gases combination in the cold season for which estimates were not substantially different) (see eTable 8 for joint effect estimates and eTables 9, 10 for detailed model results). The difference was most marked for the warm-season secondary combination when controlling for CO, NO2 and SO2 (RR=1.02, 95% CI=0.96–1.08 vs. 1.09, 95% CI=1.04–1.14 in model with only O3 and secondary PM2.5) and for the warm-season power plant combination when controlling for O3, CO, NO2 and EC (RR=1.00, 95% CI 0.96–1.05 vs. 1.06, 95% CI=1.02–1.10 in model with only SO2 and SO42−).
Measures of concurvity between pollutants and all other variables in the joint effect models were high in both seasons, ranging from 0.63 to 0.95 for the various pollutants in the models without interaction terms (eTables 4,5). Concurvity was generally lowest for SO2 and highest for O3. Lower, but still substantial, concurvity values were also seen for the pollutants in single-pollutant models (concurvities of 0.61–0.91), as well as for pollutants in multi-pollutant models in relation to only the other pollutants in the models (warm season 0.26–0.87, cold season 0.05–0.83 in no-interaction models). As expected, concurvities increased when first-order interaction terms were included.
Discussion
We calculated joint effects for several pollutant combinations selected based on common pollutant properties, relevance to air quality standards, or common sources. We observed warm-season joint effects representing increases of 6–13% in daily pediatric asthma ED visit rates for an IQR change in all of the pollutants in the combinations. Warm-season pediatric asthma appears to be impacted by a variety of pollutant combinations, particularly those including O3 and/or NO2. Joint effects for cold-season pediatric asthma showed more heterogeneity across mixtures, with strong effects observed for the oxidant gases, secondary and criteria pollutants combinations when non-linear effects were included in the models. Our results illustrated that calculation of joint effects from multi-pollutant models can avoid overestimation of joint effects that would result from their calculation based on single-pollutant model effect estimates, because single-pollutant model estimates may include some of the effect of correlated pollutants due to mutual confounding, which can be substantial. Our results also illustrate some of the complexities involved in calculating and interpreting joint effect estimates.
To calculate a joint effect, one has to calculate the effect of some specified change in each pollutant in the combination. The degree of change in each pollutant used in the calculation could be determined in several ways. We calculated the joint effect of an IQR change in each pollutant, since that has a basis in actual pollutant distributions. However, the IQR is specific to the location and time period and may vary for a given pollutant depending on the metric used (e.g., central monitor vs. population weighted average). Use of the IQR could also lead to combinations of pollutants with greater variability appearing more harmful (i.e., having a higher effect estimate per IQR change in pollutant concentrations) than combinations of pollutants with less variability, even if those combinations are not inherently more harmful. In addition, contrasts other than an IQR change may be of interest to risk assessors. To allow examination of other contrasts of interest, we have included information in the supplemental material about the individual parameter estimates (eTables 4 and 5) and the corresponding covariance matrix (eTables 6 and 7).
In sensitivity analyses, we explored the impact of several model specification decisions on the joint effect estimates. One decision relates to the approach to potential confounding by pollutants not included in the joint effect of interest. In our sensitivity analyses, models controlling for common, potentially confounding pollutants other than those in the joint effect often showed lower joint effect estimates than models that included only pollutants in the joint effect. Changes in joint effect estimates observed when including additional pollutants in the model could be due to control for confounding, but could also be due to differential measurement error. In a multi-pollutant model, measurement error can cause the effect estimate for a better-measured pollutant to represent some of the effect of a correlated but less-well-measured pollutant, making individual pollutant effect estimates less accurate.29,30 In the presence of differential measurement error, adding other pollutants to the model could decrease the accuracy of the joint effect estimate. Differential measurement error may have impacted our joint effect estimates, with the degree of the impact depending on true pollutant effects, correlations between pollutants, relative degrees of measurement error, and correlations between measurement errors.29,31
Other model specification issues relate to how pollutants are included in the model. Pollutants are often included in models as linear terms, as in our primary analyses. Our sensitivity analyses showed that an assumption of linear pollutant effects (on the log scale) may be inadequate. We observed statistical evidence for the importance of the quadratic and cubic terms in some cases (particularly the secondary, oxidant gases and criteria pollutants combinations in the cold season). Violation of the assumption of a log-linear effect of pollutant concentration across each pollutant’s concentration range could be magnified in a joint effect model because the assumption is made for several pollutants. Decisions also need to be made about which interactions to consider, and how to include those interactions in the model (i.e., linear terms or flexible forms). In some of our models, there was statistical evidence of important interactions. Models that include interaction terms may more accurately represent joint effects than models without interaction terms, but estimates from them may be less precise due to high correlations between model terms.
Our goal was to evaluate the joint effects approach as a method for examining air pollution mixture effects, but our analyses have limitations. Direct comparison of joint effect magnitudes across our pollutant combinations requires caution. Our combinations were carefully selected, and provided a good framework for examining issues surrounding quantification of joint effects, but they were selected for different reasons and included different numbers of pollutants (which alone can impact joint effect magnitudes). Some pollutants were in multiple combinations, which can contribute to similarities in observed joint effects. In addition, for combinations selected based on common sources, our results should be viewed as estimates of the joint effects of selected pollutants from shared sources and not as the total effects of those sources per se. Finally, results of time-series studies of acute air pollution health effects can be sensitive to model specification decisions other than those related to air pollutants. Our basic model was used in a previous study of acute air pollution effects on pediatric asthma;22 that paper presented several sensitivity analyses (controlling for pollen levels, not controlling for non-asthma upper respiratory infection ED visit counts, and using three alternate methods for time trend control) that indicated that the basic model was robust.22
Some previous studies have used multi-pollutant models to calculate joint effects of pollutant combinations on pediatric respiratory outcomes, using approaches similar to ours. An analysis of pre-randomization-phase data from an asthma management study included estimation of the joint effect of pairs of common air pollutants on pediatric asthma exacerbations.20 In that study, the strongest single-pollutant model effect estimates were observed for CO and NO2. Joint effect estimates from models including CO and NO2 were closer to the strongest single-pollutant model effect estimates than to an effect estimate based on the sum of single-pollutant model effects. A study of associations between PM2.5 and O3 and peak expiratory flow among children in Mexico City found that both pollutants were associated with decreased peak expiratory flow, but the joint effect (per IQR change) of the two pollutants was lower than the sum of their effects from single pollutant models.17
Other studies have considered air pollutant joint effects in relation to other outcomes. Roberts18 and Roberts and Martin19 modeled the total acute effect of PM10, O3, NO2, SO2 and CO on mortality using time-series models that calculated the overall effect of the pollutants (per standard deviation change in each pollutant), estimating the relative contribution of each pollutant to the overall effect through pollutant weights. Burnett, et al16 studied the association between particulate and gaseous air pollutants and cardiorespiratory hospitalizations in Toronto, Canada and calculated the total effect (for a change in each pollutant equal to its mean value) of pollutants selected using a forward selection procedure. In a separate study, Burnett, et al15 examined the association between daily ambient air pollution and mortality in 8 Canadian cities. They presented single-pollutant and joint effects (for a change in each pollutant equal to its mean value) for PM2.5 and PM10-2.5 and for a combination four gases (O3, CO, SO2 and NO2). They reported that the joint effect for the gases calculated in this way was greater than the joint effects for the particles. Jerrett, et al examined the “cumulative risk index” (calculated as for our joint effects) for long-term exposure to O3, PM2.5 and NO2 in relation to cardiovascular mortality.21 They found that the joint effect from a two-pollutant model was larger than the joint effect calculated based on single-pollutant model results. Many other studies have considered multi-pollutant models, but have focused on single pollutant effects, controlling for other pollutants, rather than on joint effects.23,30,32,33
One reason the approach used in our analyses has not been more commonly used is concern that collinearity (also called concurvity in non-linear models) can lead to instability in effect estimates.1 Collinearity can lead to inflation of the estimated variances of effect estimates,34 and the degree of collinearity in time-series models of air pollution health effects can be substantial.28 Given the need to control for confounding by variables, such as temperature, that are highly correlated with pollutant concentrations, some degree of collinearity is inevitable in time-series studies of air pollution health effects. However, collinearity must be considered in relation to power.35 Our study used a long time series (6 years) with fairly high daily event counts, leading to ample power.36 This may allow for a higher degree of collinearity with less adverse impact on model performance than would be the case for a study with a shorter time series or lower average daily counts. Effect estimate variances in our models did not appear severely inflated, providing some reassurance that concurvity was not excessive in relation to our study size. Nevertheless, when considering our results, one should recognize that pollutants vary in the degree to which they demonstrate collinearity with other variables in the model.28 This can lead to better power for detection of effects of some pollutants, or pollutant combinations, than others.36
Recognition of the importance of air pollution mixtures has led to increased interest in air pollution cumulative risk assessments (e.g., a recent risk assessment for PM2.5 and O337). Cumulative risk assessment considers the combined impact of multiple stressors on health outcomes, with consideration of potential interactions between stressors.38,39 Epidemiologic studies like this one can contribute to cumulative risk assessments,40 by providing dose-response functions. To be most useful for risk assessments, epidemiologic studies must present dose-response estimates for the same multi-pollutant contrasts considered in the risk assessment, or pollutant effect estimates from multi-pollutant models with their variance-covariance matrix to allow adaptation of results to other contrasts.14,40 Our consideration of only commonly measured pollutants may facilitate use of our results in risk assessment. We have included information relevant to risk assessment in our supplementary material. However, individual pollutant effect estimates presented in the supplementary material are subject to the issues discussed above relating to confounding and measurement error.
In summary, our analyses illustrate the feasibility of a straightforward approach to modeling joint effects in a study of acute air pollution health effects with a long time series and fairly high average daily outcome counts. Our results shed light on pollutant combinations that may have important acute effects on pediatric asthma ED visits, and provide estimates (potentially useful for risk assessments) of the joint effects of those combinations. They also illustrate some of the complexities involved in calculating and interpreting joint effects of multiple pollutants. If the complexities are appropriately considered, calculation of joint effects is appealing because it transparently and explicitly specifies the contributions of various pollutants to the health effects of mixtures. This approach has promise for application to time-series analyses of joint effects of multiple pollutants in relation to various health endpoints in studies with sufficient power.
Supplementary Material
Acknowledgments
Sources of Financial Support:
This publication was made possible by US Environmental Protection Agency (USEPA), contract #EP-12-H-000093, NIH grant K01ES019877, and USEPA grant R834799. The views expressed in this manuscript are those of the authors and do not necessarily represent the views or policies of the US EPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication. Support for the original emergency department study was provided by grants from NIH (R01ES011294), USEPA (STAR R829213-01), and the Electric Power Research Institute (EP-P27723/C13172).
Footnotes
List of Supplemental Digital Content
Supplemental Materials. Text file that includes supplemental text on model details, supplemental tables, and supplemental figures.
References
- 1.Dominici F, Peng RD, Barr CD, Bell ML. Protecting human health from air pollution: shifting from a single-pollutant to a multipollutant approach. Epidemiology. 2010;21:187–194. doi: 10.1097/EDE.0b013e3181cc86e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vedal S, Kaufman JD. What does multi-pollutant air pollution research mean? Am J Respir Crit Care Med. 2011;183:4–6. doi: 10.1164/rccm.201009-1520ED. [DOI] [PubMed] [Google Scholar]
- 3.Mauderly JL, Burnett RT, Castillejos M, et al. Is the air pollution health research community prepared to support a multipollutant air quality management framework? Inhal Toxicol. 2010;22(suppl 1):1–19. doi: 10.3109/08958371003793846. [DOI] [PubMed] [Google Scholar]
- 4.Billionnet C, Sherrill D, Annesi-Maesano I. Estimating the health effects of exposure to multi-pollutant mixture. Ann Epidemiol. 2012;22:126–141. doi: 10.1016/j.annepidem.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 5.McConnell R, Berhane K, Yao L, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006;114:766–772. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarnat JA, Marmur A, Klein M, et al. Fine particle sources and cardiorespiratory morbidity: an application of chemical mass balance and factor analytical source-apportionment methods. Environ Health Perspect. 2008;116:459–466. doi: 10.1289/ehp.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letz AG, Quinn JM. Relationship of basic military trainee emergency department visits for asthma and San Antonio air quality. Allergy Asthma Proc. 2005;26:463–467. [PubMed] [Google Scholar]
- 8.Stieb DM, Burnett RT, Smith-Doiron M, Brion O, Shin HH, Economou V. A new multipollutant, no-threshold air quality health index based on short-term associations observed in daily time-series analyses. J Air Waste Manag Assoc. 2008;58:435–450. doi: 10.3155/1047-3289.58.3.435. [DOI] [PubMed] [Google Scholar]
- 9.Hong YC, Leem JH, Ha EH, Christiani DC. PM10 exposure, gaseous pollutants, and daily mortality in Inchon, South Korea. Environ Health Perspect. 1999;107:873–878. doi: 10.1289/ehp.99107873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pachon JE, Balachandran S, Hu Y, et al. Development of outcome-based, multipollutant mobile source indicators. J Air Waste Manag Assoc. 2012;62:431–442. doi: 10.1080/10473289.2012.656218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kesten S, Szalai J, Dzyngel B. Air quality and the frequency of emergency room visits for asthma. Ann Allergy Asthma Immunol. 1995;74:269–273. [PubMed] [Google Scholar]
- 12.Roberts S, Martin MA. Using supervised principal components analysis to assess multiple pollutant effects. Environ Health Perspect. 2006;114:1877–1882. doi: 10.1289/ehp.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg MS. On the interpretation of epidemiological studies of ambient air pollution. J Expo Sci Environ Epidemiol. 2007;17(suppl 2):S66–S70. doi: 10.1038/sj.jes.7500629. [DOI] [PubMed] [Google Scholar]
- 14.Levy JI. Is Epidemiology the key to cumulative risk assessment? Risk Anal. 2008;28:1507–1513. doi: 10.1111/j.1539-6924.2008.01121.x. [DOI] [PubMed] [Google Scholar]
- 15.Burnett RT, Brook J, Dann T, et al. Association between particulate- and gas-phase components of urban air pollution and daily mortality in eight Canadian cities. Inhal Toxicol. 2000;12(suppl 4):15–39. doi: 10.1080/08958370050164851. [DOI] [PubMed] [Google Scholar]
- 16.Burnett RT, Smith-Doiron M, Stieb D, Cakmak S, Brook JR. Effects of particulate and gaseous air pollution on cardiorespiratory hospitalizations. Arch Environ Health. 1999;54:130–139. doi: 10.1080/00039899909602248. [DOI] [PubMed] [Google Scholar]
- 17.Gold DR, Damokosh AI, Pope CA, et al. Particulate and ozone pollutant effects on the respiratory function of children in southwest Mexico City. Epidemiology. 1999;10:8–16. [PubMed] [Google Scholar]
- 18.Roberts S. A new model for investigating the mortality effects of multiple air pollutants in air pollution mortality time-series studies. J Toxicol Env Health A. 2006;69:417–435. doi: 10.1080/15287390500246761. [DOI] [PubMed] [Google Scholar]
- 19.Roberts S, Martin MA. Investigating the mixture of air pollutants associated with adverse health outcomes. Atmos Environ. 2006;40:984–991. [Google Scholar]
- 20.Schildcrout JS, Sheppard L, Lumley T, Slaughter JC, Koenig JQ, Shapiro GG. Ambient air pollution and asthma exacerbations in children: an eight-city analysis. Am J Epidemiol. 2006;164:505–517. doi: 10.1093/aje/kwj225. [DOI] [PubMed] [Google Scholar]
- 21.Jerrett M, Burnett RT, Beckerman BS, Turner MC, Krewski D, Thurston G, Martin RV, van Donkelaar A, Hughes E, Shi Y, Gapstur SM, Thun MJ, Pope CA., 3rd Spatial analysis of air pollution and mortality in California. Am J Respir Crit Care Med. 2013;188:593–599. doi: 10.1164/rccm.201303-0609OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strickland MJ, Darrow LA, Klein M, et al. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med. 2010;182:307–316. doi: 10.1164/rccm.200908-1201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peel JL, Tolbert PE, Klein M, et al. Ambient air pollution and respiratory emergency department visits. Epidemiology. 2005;16:164–174. doi: 10.1097/01.ede.0000152905.42113.db. [DOI] [PubMed] [Google Scholar]
- 24.Van Loy M, Bahadori T, Wyzga R, Hartsell B, Edgerton E. The Aerosol Research and Inhalation Epidemiology Study (ARIES): PM2.5 mass and aerosol component concentrations and sampler intercomparisons. J Air Waste Manag Assoc. 2000;50:1446–1458. doi: 10.1080/10473289.2000.10464187. [DOI] [PubMed] [Google Scholar]
- 25.Ivy D, Mulholland JA, Russell AG. Development of ambient air quality population-weighted metrics for use in time-series health studies. J Air Waste Manag Assoc. 2008;58:711–720. doi: 10.3155/1047-3289.58.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wade KS, Mulholland JA, Marmur A, et al. Effects of instrument precision and spatial variability on the assessment of the temporal variation of ambient air pollution in Atlanta, Georgia. J Air Waste Manag Assoc. 2006;56:876–888. doi: 10.1080/10473289.2006.10464499. [DOI] [PubMed] [Google Scholar]
- 27.Marmur A, Unal A, Mulholland JA, Russell AG. Optimization-based source apportionment of PM2.5 incorporating gas-to-particle ratios. Environ Sci Technol. 2005;39:3245–3254. doi: 10.1021/es0490121. [DOI] [PubMed] [Google Scholar]
- 28.Ito K, Thurston GD, Silverman RA. Characterization of PM2.5, gaseous pollutants, and meteorological interactions in the context of time-series health effects models. J Expo Sci Environ Epidemiol. 2007;17:S45–S60. doi: 10.1038/sj.jes.7500627. [DOI] [PubMed] [Google Scholar]
- 29.Zeger SL, Thomas D, Dominici F, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108:419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tolbert PE, Klein M, Peel JL, Sarnat SE, Sarnat JA. Multipollutant modeling issues in a study of ambient air quality and emergency department visits in Atlanta. J Expo Sci Environ Epidemiol. 2007;17:S29–S35. doi: 10.1038/sj.jes.7500625. [DOI] [PubMed] [Google Scholar]
- 31.Carrothers TJ, Evans JS. Assessing the impact of differential measurement error on estimates of fine particle mortality. J Air Waste Manag Assoc. 2000;50:65–74. doi: 10.1080/10473289.2000.10463988. [DOI] [PubMed] [Google Scholar]
- 32.Mortimer K, Neugebauer R, Lurmann F, Alcorn S, Balmes J, Tager I. Air pollution and pulmonary function in asthmatic children: effects of prenatal and lifetime exposures. Epidemiology. 2008;19:550–557. doi: 10.1097/EDE.0b013e31816a9dcb. [DOI] [PubMed] [Google Scholar]
- 33.Levy JI, Diez D, Dou Y, Barr CD, Dominici F. A meta-analysis and multisite time-series analysis of the differential toxicity of major fine particulate matter constituents. Am J Epidemiol. 2012;175:1091–1099. doi: 10.1093/aje/kwr457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lesaffre E, Marx BD. Collinearity in generalized linear-regression. Commun Stat Theory Methods. 1993;22:1933–1952. [Google Scholar]
- 35.Mason CH, Perreault WD. Collinearity, power and interpretation of multiple regression analysis. J Mark Res. 1991;28:268–280. [Google Scholar]
- 36.Winquist A, Klein M, Tolbert P, Sarnat SE. Power estimation using simulations for air pollution time-series studies. Environ Health. 2012;11:68. doi: 10.1186/1476-069X-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fann N, Lamson AD, Anenberg SC, Wesson K, Risley D, Hubbell BJ. Estimating the national public health burden associated with exposure to ambient PM2.5 and ozone. Risk Anal. 2012;32:81–95. doi: 10.1111/j.1539-6924.2011.01630.x. [DOI] [PubMed] [Google Scholar]
- 38.Sexton K. Cumulative risk assessment: an overview of methodological approaches for evaluating combined health effects from exposure to multiple environmental stressors. Int J Environ Res Public Health. 2012;9:370–390. doi: 10.3390/ijerph9020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menzie CA, MacDonell MM, Mumtaz M. A phased approach for assessing combined effects from multiple stressors. Environ Health Perspect. 2007;115:807–816. doi: 10.1289/ehp.9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fann N, Bell ML, Walker K, Hubbell B. Improving the linkages between air pollution epidemiology and quantitative risk assessment. Environ Health Perspect. 2011;119:1671–1675. doi: 10.1289/ehp.1103780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


