Abstract
The methionine sulfoxide reductase (Msr) family of enzymes has been shown to protect cells against oxidative damage. The two major Msr enzymes, MsrA and MsrB, can repair oxidative damage to proteins due to reactive oxygen species, by reducing the methionine sulfoxide in proteins back to methionine. A role of MsrA in animal aging was first demonstrated in D. melanogaster where transgenic flies over-expressing recombinant bovine MsrA had a markedly extended life span. Subsequently, MsrA was also shown to be involved in the life span extension in C. elegans. These results supported other studies that indicated up-regulation, or activation, of the normal cellular protective mechanisms that cells use to defend against oxidative damage could be an approach to treat age related diseases and slow the aging process. In this study we have identified, for the first time, compounds structurally related to the natural products fusaricidins that markedly activate recombinant bovine and human MsrA and human MsrB.
Keywords: Methionine sulfoxide reductases, fusaricidin analogues, Msr activation
Introduction
The free radical or oxidative damage theory of aging in animals was first proposed by Harman who postulated that aging in all animals was due to the accumulation of free radicals resulting in damage to tissues [1]. It is now well established that increased levels of reactive oxygen species (ROS), produced primarily from cellular respiration, are a significant factor in causing tissue damage [2]. However, it is also known that low levels of ROS play an important protective role in cells by initiating mechanisms, such as ischemic preconditioning, that protect cells against oxidative damage [3–5]. Thus, the level of ROS must be balanced carefully, and cells must protect against increased levels of ROS, that occur during aging. It is known that cells have developed mechanisms to specifically guard against excess formation of ROS, and aging appears to be due to an imbalance between the level of ROS production and the ability of the cellular protective mechanisms to prevent oxidative damage. Included in the protective mechanisms are antioxidants such as glutathione, ROS destroying enzymes including superoxide dismutase (SOD) and catalase, and systems to repair oxidative damage, including DNA repair enzymes [6] and the methionine sulfoxide reductase system (Msr) that repairs damage to proteins due to oxidation of methionine (Met) residues in proteins to methionine sulfoxide (Met-(o)([7]. Our interest has been in the Msr system which was discovered in our laboratory more than 30 years ago [8]. Methionine in proteins is easily oxidized by ROS to methionine sulfoxide (Met(o)), which is present in the protein as two epimers, Met-S-(o) and Met-R-(o). The two major enzymes in the Msr family are MsrA and MsrB which reduce the S and R epimers of Met-(o), respectively, back to Met [7]. Several important enzymes are known to be inactivated by Met oxidation, and their function restored by the Msr system. Those studied in detail, include alpha-1-proteinase inhibitor and calmodulin [9, 10]. However, the repair function of the Msr system may not be its only role in protecting cell against oxidative damage. Levine and coworkers [11] were the first to propose that exposed Met residues in proteins could act as catalytic antioxidants through the action of the Msr system and scavenge ROS. The physiological importance of the Msr system, especially MsrA, became most apparent when it was shown that transgenic flies that over-expressed bovine MsrA (bMsrA) had a markedly extended life span, especially when the bMsrA was specifically expressed in their neuronal cells [12]. More recent studies showed that MsrA is also involved in the life extension seen in C. elegans mutants in which the daf 2 gene was knocked out [13] or in life span extension seen with caloric restriction [14]. It seemed clear that in lower animals increased levels of MsrA activity could extend life span. These results supported other studies showing that flies that over-expressed SOD and mice that were engineered to increase catalase activity in their mitochondria also had extended life spans [15, 16]. These previous results suggested that over-expression or activation of known mechanisms that cells use to protect against oxidative damage, such as the Msr system, could serve as a therapeutic approach for the treatment of age related diseases and for the extension of life span of humans. In this study we report, for the first time, the identification of a class of small cyclic peptides that markedly activate recombinant bovine and human MsrA and human MsrB.
Materials and Methods
The general procedures for the synthesis of fusaricidin analogues combinatorial library, including individual peptides used in this study have been described elsewhere [17, 18]. 4-N,N-dimethylamino-azobenzene-4-sulfonyl chloride, (DABS) and DTT were purchased from Sigma-Aldrich. DABS-R-Met-(o) and DABS-S-Met-(o) were prepared as described previously [19]. Human alpha-1-proteinase inhibitor (alpha-1-PI) and human neutrophil elastase were purchased from Peptide Premiere Peptide Solutions and Athens Research & Technologies, respectively. Succinyl-(Ala)3-p-nitroanilide was purchased from Sigma-Aldrich. Clones containing the E. coli TrxA and TrxB, E. coli and bovine MsrA, human MsrB2 and MsrB3 were overexpressed in E. coli, and the respective proteins were purified as described previously [20–23].
For the MsrA and MsrB assays a synthetic substrate, either DABS-Met-S-(o) or DABS-Met-R-(o) was used as described previously [22, 23]. Incubations were performed for 30 minutes. Oxidation of alpha-1-PI and the ability to reduce the chemically oxidized alpha-1-PI with MsrA and determine its effect on elastase activity have been described previously [9, 24, 25].
The preparation of a calf liver 35–60% ammonium sulfate fraction (liver AS) was as follows: Calf liver was homogenized in 3 volumes of 50 mM Tris pH 7.4. The homogenate was first centrifuged for 20 mins at 10,000 x g and the supernatant centrifuged for 2 hrs at 100,000 x g (S-100). The S-100 was subjected to ammonium sulfate precipitation and the proteins precipitating between 35 and 60% saturation were dissolved in 50mm Tris-Cl pH 7.4 and dialyzed against this buffer.
Statistical Analysis
The results reported represent the mean of three replicates in each experiment ± SE.
Results
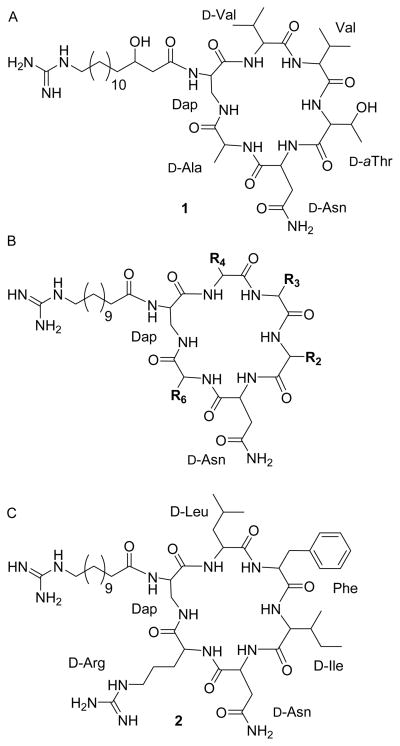
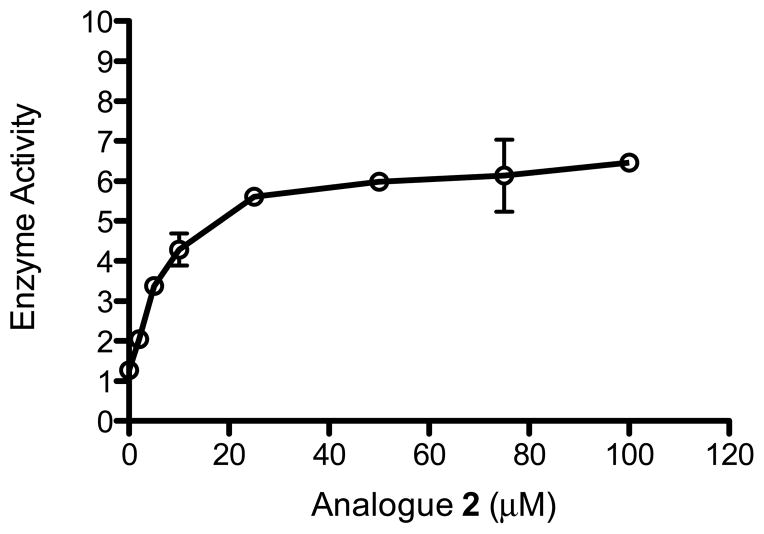
As part of our studies aimed at increasing the activity of MsrA in cells we have used several approaches including viral vectors [26] and screening of more than 380,000 small molecules searching for activators of MsrA. Although the initial screening results, using an assay designed for high throughput screening of activators of MsrA were negative [27], recently it was observed that analogues of naturally occurring fusaricidin A, (Fig. 1A) could significantly stimulate the activity of both recombinant MsrA and MsrB. Fusaricidin A, is a bacterial metabolite with antibiotic activity [28]. Its structure contains a hexadepsipeptide with a 15-guanidino-3-hydroxypentadecanoic tail (Fig. 1A). The analogues that we have tested have a basic scaffold that is similar to fusaricidin A, as shown in Fig. 1B. The analogues differ from fusaricidin A in the amino acid composition of the cyclic hexapeptide and the fatty acid side chain, which is shorter and not hydroxylated. Fig. 1C shows the structure of cyclic lipohexapeptide 2 (analogue 2), the primary compound used in the present studies. This analogue was obtained from screening of a positional scanning library composed of 130,321 hexapeptides. Table 1 shows the effect of amino acid substitutions in the cyclic hexapeptide moiety on the activation of recombinant bovine MsrA (bMsrA). It can be seen that an arginine or lysine in position R6, present in analogues numbered 2,3,4, 8 and 9 listed in Table 1, is able to stimulate MsrA activity between 3.8–6 fold. Other structure activity studies showed that the fatty acid tail is required for activation, and the guanidine group is also required for maximal activation (see Fig. S1 and Table S1) The concentration of analogue 2 and other derivatives used the experiments was 25 μM, but as can be seen in Fig. 2 as little as 10 μM analogue 2 results in >2 fold activation of bMsrA. All of these preliminary experiments were done with full length recombinant bMsrA. However, a distinct shortened form of MsrA, that initiates at the Met residue at position 23 of human MsrA (hMsrA,), is present in the cytoplasm, and this shortened form can be myristoylated [29]. The results with other recombinant MsrA and MsrB enzymes are seen in Table 2. The recombinant short forms of hMsrA, with or without myristoylation, are also activated by analogue 2, but to a lesser extent (3–4 fold) than the full length bMsrA. However, unexpectedly E. coli MsrA is not activated. This latter result is surprising since there is a high degree of homology between the E. coli and mammalian MsrA (see Discussion]. As also shown in Table 2, analogue 2 activates full length recombinant human MsrB2 and MsrB3 and E. coli MsrB, although the activation (2–3 fold) is less than that observed with bMsrA. In addition, when thioredoxin (Trx) was used as the reducing system in place of DTT, there was markedly less activation of bMsrA and hMsrA, generally only between 1.2–1.4 fold (Table S2). The reason for this lower fold activation using the Trx reducing system has not been explained.
Fig. 1.
Structure of fusaricidin A and activators of the Msr enzymes. A- fusaricidin A; B-basic scaffold of activators; C- analogue 2.
Table 1.
Bovine MsrA activation by fusaricidin analogues having various amino acid substitutions in the basic scaffold structure (see Fig. 1). Enzyme specific activity is defined as nmol DABS Met formed/μg protein/30 minutes. The specific activity of the control bMsrA was 6.5±1.19. Because of solubility issues all of the analogues used in this Table were initially dissolved in dimethylformamide (DMF), resulting in final concentrations in the reactions of 0.25% DMF and 25 uM analogue. Using analogue 2, which is soluble in water it was shown that DMF had no effect on the reaction. Fold activity is defined as the increase in enzyme activity compared to the control activity (no activator). For clarity, using the control activity as 1, a fold activity value of 1.2 (e.g., analogue 5 in Table1) represents a 20% increase in activity, whereas a fold activity value of 6.2 (e.g., analogue 2 in Table 1) represents an enzyme activity 6.2 times the control.
| Analogue | R2 | R3 | R4 | R6 | Fold Activity |
|---|---|---|---|---|---|
| 2 | D-Ile | L-Phe | D-Leu | D-Arg | 6.2±0.11 |
| 3 | D-Phe | L-Leu | D-Leu | D-Arg | 5.2±0.51 |
| 4 | D-Leu | L-Leu | D-Phe | D-Arg | 3.8±0.30 |
| 5 | D-Trp | L-Asp | D-Tyr | D-Tyr | 1.2±0.19 |
| 6 | D-Tyr | L-Asp | D-Phe | D-Tyr | 1.2±0.08 |
| 7 | D-Tyr | L-Asp | D-Leu | D-Tyr | 1.6±0.28 |
| 8 | D-Ile | L-Leu | D-Leu | D-Arg | 3.8±0.65 |
| 9 | D-Phe | L-Leu | D-Leu | D-Lys | 4.2±0.43 |
Fig. 2.
Effect of analogue 2 concentration on bMsrA activity. 0.3 μg of bMsrA was used and incubations were for 30 minutes. Enzyme activity is definied as nmoles DABS Met formed/30minutes.
Table 2.
Activity of different purified recombinant Msr enzymes in the presence of analogue 2. h-human; b-bovine; e-E. coli. The specific activity for each Msr species is as follows: hMsrA, 3.4±0.17; hMsrA myristoylated, 2.9±0.22; eMsrA, 5.3±0.79; bMsrA, 4.2±0.62; E. coli MsrB, 0.6±.08; hMsrB2, 2.9±0.21; hMsrB3, 1.4±0.09. Fold activity and specific activity are defined in Table 1. The concentration of analogue 2 used was 25 μM.
| Msr tested | Fold Activity |
|---|---|
| MsrA | |
| hMsrA | 3.3±0.23 |
| hMsrA (myristoylated) | 3.7±0.30 |
| eMsrA | 1.0**±0.08 |
| bMsrA (full length) | 6.2±0.63 |
| MsrB | |
| eMsrB | 3.1±0.85 |
| hMsrB3 | 1.9±0.12 |
| hMsrB2 | 2.3±0.31 |
Fold Activity of 1 represents no activation.
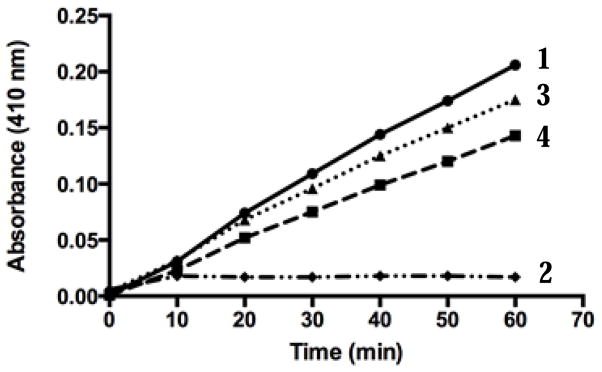
Our routine assay for Msr activity uses a synthetic substrate, either DABS-Met-S-(o) or DABS-Met-R-(o), to measure the activity of MsrA and MsrB, respectively [22, 23]. It was important to demonstrate that activation could also be observed when a natural protein substrate was used. Alpha-1-proteinase inhibitor (alpha-1-PI), an inhibitor of elastase, loses its ability to inhibit elastase upon oxidation of specific Met residues at positions 351 or 358 [30]. We have shown previously that oxidized alpha-1-PI is a substrate for MsrA and reduction with MsrA restores its inhibitory activity on elastase [9]. Oxidized alpha-1-PI was incubated with MsrA in the presence or absence of analogue 2 and its ability to inhibit elastase activity measured as shown in Fig. 3. A limiting amount of MsrA was used in order to see any effect of the activator. Curve 1 in Fig. 3 shows the control elastase activity with or without oxidized alpha-1-PI. When native alpha-1-PI (not oxidized) is present there is complete inhibition of elastase activity (curve 2). Curve 3 shows the results using oxidized alpha-1-PI after incubating it with limiting levels of MsrA (see Methods). This partly reduced alpha-1-PI inhibits elastase activity by 18%. When the oxidized alpha-1-PI is incubated with MsrA in the presence of activator, the inhibition of elastase activity goes up to 40% (curve 4). It should be noted that the most inhibition expected would be 50%, since the chemically oxidized Met residues in alpha-1-PI are an equal mixture of the R and S epimers, and MsrA only reduces the S epimer.
Fig. 3.
Effect of analogue 2 on reduction of oxidized alpha-1-PI with bMsrA. bMsrA was used to reduce oxidized alpha-1-PI in the presence or absence of analogue 2, and the inhibitory effect of alpha-1-PI on elastase activity measured (9,23,24). 1- elastase activity alone or with oxidized alpha-1-PI, 2- elastase activity with fully reduced alpha-1-PI, 3- elastase activity with oxidized alpha-1-PI partially reduced with MsrA, 4- elastase activity with alpha-1-PI partially reduced with MsrA in the presence of analogue 2. See text for details.
The studies described above used recombinant forms of bovine and human MsrA and MsrB2, and E. coli MsrB which contain a hexa-His tag, full length E. coli MsrA which was expressed as a GST-fusion protein (GST was removed), and hMsrB3 which has no tag. In order to demonstrate that the native enzyme found in tissues is also activated, studies were initiated using calf liver as the source of both endogenous MsrA and MsrB. Unexpectedly, crude liver extracts markedly inhibited the activation of recombinant bMsrA by analogue 2. The results of a typical experiment using a liver ammonium sulfate fraction (liver AS, see Methods) are shown in Table 3. As seen in lines 1 and 2 the activator has no effect on the liver AS MsrA activity. Lines 3 and 4 show that the recombinant bMsrA can be activated about 6 fold by analogue 2. The combination of liver AS plus recombinant bMsrA is slightly higher (2.9, line 5) than the sum of the individual two activities (lines 1 and 3 = 2.3) indicating a minor activation of the recombinant bMsrA by components in the liver AS fraction. However, line 6 shows that adding the liver AS to the bMsrA and activator yields a small activation of the bMsrA (2.9 vs. 3.6). One would have expected a calculated value of 8.7, i.e., the sum of the MsrA activity in lines 2 and 4. The MsrB in the liver AS fraction was also not significantly activated by analogue 2 (data not shown). It is clear that the liver AS fraction contains a component(s) that markedly inhibits the activation of the recombinant Msr enzymes by analogue 2, which very likely explains why the Msr enzymes present in the liver are not activated. Experiments are in progress to purify the endogenous MsrA and MsrB in the liver AS fraction, and to isolate and identify the inhibitor(s) that are present in the extract.
Table 3.
Effect of a calf liver ammonium sulfate fraction (liver AS) on recombinant bMsrA activity in the presence or absence of analogue 2. Details of the enzyme assays are described elsewhere [22, 23]. 70 μg of liver AS fraction (see Methods) and 0.3 μg of bMsrA were used. The numbers in parenthesis are the expected calculated values. See text for details.
| Fraction | nmoles DABS-Met/30 minutes |
|---|---|
| 1. Liver | 1.0 ±0.09 |
| 2. Liver+ analogue 2 | 1.0±0.03 |
| 3. bMsrA | 1.3 ±0.19 |
| 4. bMsrA+ analogue 2 | 7.7 ±0.30 |
| 5. Liver+bMsrA | 2.9 ±0.31 (2.3) |
| 6. Liver+bMsrA+ analogue 2 | 3.6 ±0.31 (8.7) |
Discussion
The goal of our research has been to up-regulate the normal mechanisms that cells use to protect against oxidative damage. The two mechanisms that have been of interest to us are a protective pharmacological preconditioning response [31, 32]and the Msr protein repair system. The present studies describe for the first time activators of recombinant MsrA and MsrB that are derivatives of the naturally occurring antibiotic fusaricidin A [28]. The major compound we have used in the present study, analogue 2, was derived from a screening of a positional library of > 130,000 cyclic hexapeptides. It should be stressed that the most active derivatives have an arginine or lysine in position 6 of the hexapeptide sequence. Stimulation of recombinant bMsrA activity by 5–6 fold can be obtained and recombinant hMsrA species can also be stimulated, however to a lesser extent. In contrast, E. coli MsrA is not stimulated by these activators. Since the homology between the E. coli and mammalian MsrA species is very high, this is a surprising result, that could eventually give us a clue as to the possible regions on the Msr that are involved in the activation. It is of interest that the most active fusaricidin analogues contain, in addition to a basic amino acid in position 6 of the hexapeptide, a guanidine group at the end of the lipid side chain. If the guanidine group is replaced with an amino group or removed the fold activation is decreased by 25–35% (Table S1). It appears that two strong positively charged groups are required for maximal activation by the fusaricidin derivatives. One possibility is that the lack of specific acidic residues in the E. coli enzyme could explain the inability to activate this enzyme. One distinct difference in the sequences of the mammalian and E. coli enzymes is the presence of Glu residues in the hMsrA (and bMsrA) corresponding to positions 42, 59, 126, 132,185 and 197 in the human sequence, that are missing in E. coli MsrA. Studies are in progress to determine whether any of these residues might be involved in the interaction of the activator with the mammalian MsrA species. Although E. coli MsrA was not activated by analogue 2, E. coli MsrB, and human MsrB2 and B3 were activated 2–3 fold. The MsrB proteins have very different amino acid sequences than MsrA, although the structure of the active site is a “mirror” image to that seen in MsrA [33].
The reduction of oxidized alpha-1-PI by bMsrA is also stimulated by the fusaricidin analogue 2, showing that the reduction of a Met(o) residue in an oxidized protein that is a known substrate of MsrA, can also be stimulated by this activator. An unexpected problem has been the inability to activate the endogenous liver MsrA or MsrB with analogue 2 or other of the activators. One reason is the presence of an inhibitor(s) in the liver extract that prevents the activation of the endogenous Msr enzymes, since the liver extract also inhibits the activation of the bMsrA by analogue 2 (Table 3). Experiments are in progress to purify the MsrA and MsrB from calf liver, as well as isolate and identify any inhibitors of the activation. Preliminary results indicate that a 40 fold purified MsrB1 fraction from the liver extract can be activated >2 fold by analogue 2 (data not shown). We also cannot explain the much lower activation of MsrA when the Trx system is used as the reducing agent in place of DTT. This will be studied in more detail after purification of the endogenous MsrA from the liver extract.
We are particularly interested in studying oxidative damage in the retina, where we have recently shown that sulindac, a substrate for the Msr enzymes, initiates a pharmacological preconditioning response that protects retinal pigmented epithelial (RPE) cells against oxidative damage [32]. MsrA is found in high concentrations in RPE cells [34], and the retina is very sensitive to oxidative damage which can lead to loss of the photoreceptor cells that are involved in diseases including age related macular degeneration, diabetic retinopathy, and the genetic disease, retinitis pigmentosa. Any drug that could activate the Msr enzymes would have therapeutic potential.
Supplementary Material
Research Highlights.
Activation of methionine sulfoxide reductases MsrA and MsrB
Fusaricidine derivatives activate methionine sulfoxide reductases
Activation of MsrA and MsrB, protective enzymes against oxidative damage
Acknowledgments
The research was supported in part through the Florida Drug Discovery Acceleration Program by the State of Florida, Department of Health, NIH grant 1R21AI119288-01 (PC) and the FAU Foundation (HW). The authors thank Dr. Rodney Levine at the NIH for supplying the purified human recombinant myristoylated and non-myristoylated MsrA, Dr. Todd Lowther, Wake Forest University School of Medicine for the hMsrB clone and Dr. J. Fielding Hejtmancik at the NIH for the hMsrB3 clone. We also thank Dr. Shailaja Kesaraju-Allani for her advice and help in preparing the manuscript.
Abbreviations
- Msr
methionine sulfoxide reductases
- ROS
reactive oxygen species
- Met(o)
methionine sulfoxide
- DABS
4-N,N-dimethylamino-azobenzene-4-sulfonyl chloride
- alpha-1-PI
alpha-1 proteinase inhibitor
- RPE
retinal pigmented epithelial cells
- Myr
myristoylated
- Trx
thioredoxin
- DMF
dimethylformamide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of gerontology. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Stadtman ER. Protein oxidation and aging. Free radical research. 2006;40:1250–1258. doi: 10.1080/10715760600918142. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R, Dawn B, Tang XL, Qiu Y, Ping P, Xuan YT, Jones WK, Takano H, Guo Y, Zhang J. The nitric oxide hypothesis of late preconditioning. Basic research in cardiology. 1998;93:325–338. doi: 10.1007/s003950050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolli R, Dawn B, Xuan YT. Role of the JAK-STAT pathway in protection against myocardial ischemia/reperfusion injury. Trends in cardiovascular medicine. 2003;13:72–79. doi: 10.1016/s1050-1738(02)00230-x. [DOI] [PubMed] [Google Scholar]
- 5.Otani H. Ischemic preconditioning: from molecular mechanisms to therapeutic opportunities. Antioxidants & redox signaling. 2008;10:207–247. doi: 10.1089/ars.2007.1679. [DOI] [PubMed] [Google Scholar]
- 6.Lu AL, Li X, Gu Y, Wright PM, Chang DY. Repair of oxidative DNA damage: mechanisms and functions. Cell biochemistry and biophysics. 2001;35:141–170. doi: 10.1385/CBB:35:2:141. [DOI] [PubMed] [Google Scholar]
- 7.Weissbach H, Resnick L, Brot N. Methionine sulfoxide reductases: history and cellular role in protecting against oxidative damage. Biochimica et biophysica acta. 2005;1703:203–212. doi: 10.1016/j.bbapap.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Brot N, Weissbach L, Werth J, Weissbach H. Enzymatic reduction of protein-bound methionine sulfoxide. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:2155–2158. doi: 10.1073/pnas.78.4.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carp H, Janoff A, Abrams W, Weinbaum G, Drew RT, Weissbach H, Brot N. Human methionine sulfoxide-peptide reductase, an enzyme capable of reactivating oxidized alpha-1-proteinase inhibitor in vitro. The American review of respiratory disease. 1983;127:301–305. doi: 10.1164/arrd.1983.127.3.301. [DOI] [PubMed] [Google Scholar]
- 10.Sun H, Gao J, Ferrington DA, Biesiada H, Williams TD, Squier TC. Repair of oxidized calmodulin by methionine sulfoxide reductase restores ability to activate the plasma membrane Ca-ATPase. Biochemistry. 1999;38:105–112. doi: 10.1021/bi981295k. [DOI] [PubMed] [Google Scholar]
- 11.Levine RL, Mosoni L, Berlett BS, Stadtman ER. Methionine residues as endogenous antioxidants in proteins. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruan H, Tang XD, Chen ML, Joiner ML, Sun G, Brot N, Weissbach H, Heinemann SH, Iverson L, Wu CF, Hoshi T. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2748–2753. doi: 10.1073/pnas.032671199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minniti AN, Cataldo R, Trigo C, Vasquez L, Mujica P, Leighton F, Inestrosa NC, Aldunate R. Methionine sulfoxide reductase A expression is regulated by the DAF-16/FOXO pathway in Caenorhabditis elegans. Aging cell. 2009;8:690–705. doi: 10.1111/j.1474-9726.2009.00521.x. [DOI] [PubMed] [Google Scholar]
- 14.Minnerly ZJJ, Aldunate R, Weissbach H, Jia K. Methionine sulfoxide reductase A mediates dietary restriction-induced lifespan extension in Caenorhabditis elgans. Aging Sci. 2013;1 doi: 10.4172/2329-8847.1000110. [DOI] [Google Scholar]
- 15.Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 16.Schriner SE, Linford NJ. Extension of mouse lifespan by overexpression of catalase. Age (Dordr) 2006;28:209–218. doi: 10.1007/s11357-006-9010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bionda N, Fleeman RM, de la Fuente-Núñez C, Rodriguez MC, Reffuveille F, Shaw LN, Pastar I, Davis SC, Hancock REW, Cudic P. Identification of novel cyclic lipopeptides from a positional scanning combinatorial library with enhanced antibacterial and antibiofilm activities. European journal of medicinal chemistry. 2015 doi: 10.1016/j.ejmech.2015.11.032. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bionda N, Stawikowski M, Stawikowska R, Cudic M, Lopez-Vallejo F, Treitl D, Medina-Franco J, Cudic P. Effects of cyclic lipodepsipeptide structural modulation on stability, antibacterial activity, and human cell toxicity. ChemMedChem. 2012;7:871–882. doi: 10.1002/cmdc.201200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minetti G, Balduini C, Brovelli A. Reduction of DABS-L-methionine-dl-sulfoxide by protein methionine sulfoxide reductase from polymorphonuclear leukocytes: stereospecificity towards the l-sulfoxide. The Italian journal of biochemistry. 1994;43:273–283. [PubMed] [Google Scholar]
- 20.Rahman MA, Nelson H, Weissbach H, Brot N. Cloning, sequencing, and expression of the Escherichia coli peptide methionine sulfoxide reductase gene. The Journal of biological chemistry. 1992;267:15549–15551. [PubMed] [Google Scholar]
- 21.Moskovitz J, Weissbach H, Brot N. Cloning the expression of a mammalian gene involved in the reduction of methionine sulfoxide residues in proteins. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2095–2099. doi: 10.1073/pnas.93.5.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagher D, Brunell D, Brot N, Vallee BL, Weissbach H. Selenocompounds can serve as oxidoreductants with the methionine sulfoxide reductase enzymes. The Journal of biological chemistry. 2006;281:31184–31187. doi: 10.1074/jbc.M606962200. [DOI] [PubMed] [Google Scholar]
- 23.Sagher D, Brunell D, Hejtmancik JF, Kantorow M, Brot N, Weissbach H. Thionein can serve as a reducing agent for the methionine sulfoxide reductases. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8656–8661. doi: 10.1073/pnas.0602826103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abrams WR, Weinbaum G, Weissbach L, Weissbach H, Brot N. Enzymatic reduction of oxidized alpha-1-proteinase inhibitor restores biological activity. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:7483–7486. doi: 10.1073/pnas.78.12.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bieth J, Spiess B, Wermuth CG. The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochemical medicine. 1974;11:350–357. doi: 10.1016/0006-2944(74)90134-3. [DOI] [PubMed] [Google Scholar]
- 26.Prentice HM, Moench IA, Rickaway ZT, Dougherty CJ, Webster KA, Weissbach H. MsrA protects cardiac myocytes against hypoxia/reoxygenation induced cell death. Biochemical and biophysical research communications. 2008;366:775–778. doi: 10.1016/j.bbrc.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 27.Brunell D, Weissbach H, Hodder P, Brot N. A high-throughput screening compatible assay for activators and inhibitors of methionine sulfoxide reductase A. Assay and drug development technologies. 2010;8:615–620. doi: 10.1089/adt.2009.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kajimura Y, Kaneda M. Fusaricidin A, a new depsipeptide antibiotic produced by Bacillus polymyxa KT-8. Taxonomy, fermentation, isolation, structure elucidation and biological activity. The Journal of antibiotics. 1996;49:129–135. doi: 10.7164/antibiotics.49.129. [DOI] [PubMed] [Google Scholar]
- 29.Kim G, Cole NB, Lim JC, Zhao H, Levine RL. Dual sites of protein initiation control the localization and myristoylation of methionine sulfoxide reductase A. The Journal of biological chemistry. 2010;285:18085–18094. doi: 10.1074/jbc.M110.119701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taggart C, Cervantes-Laurean D, Kim G, McElvaney NG, Wehr N, Moss J, Levine RL. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. The Journal of biological chemistry. 2000;275:27258–27265. doi: 10.1074/jbc.M004850200. [DOI] [PubMed] [Google Scholar]
- 31.Moench I, Prentice H, Rickaway Z, Weissbach H. Sulindac confers high level ischemic protection to the heart through late preconditioning mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19611–19616. doi: 10.1073/pnas.0911046106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sur A, Kesaraju S, Prentice H, Ayyanathan K, Baronas-Lowell D, Zhu D, Hinton DR, Blanks J, Weissbach H. Pharmacological protection of retinal pigmented epithelial cells by sulindac involves PPAR-alpha. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16754–16759. doi: 10.1073/pnas.1419576111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowther WT, Weissbach H, Etienne F, Brot N, Matthews BW. The mirrored methionine sulfoxide reductases of Neisseria gonorrhoeae pilB. Nature structural biology. 2002;9:348–352. doi: 10.1038/nsb783. [DOI] [PubMed] [Google Scholar]
- 34.Moskovitz J, Jenkins NA, Gilbert DJ, Copeland NG, Jursky F, Weissbach H, Brot N. Chromosomal localization of the mammalian peptide-methionine sulfoxide reductase gene and its differential expression in various tissues. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3205–3208. doi: 10.1073/pnas.93.8.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.