Abstract
Metals are required for proper brain development and play an important role in a number of neurobiological functions. The Divalent Metal Transporter 1 (DMT1) is a major metal transporter involved in the absorption and metabolism of several essential metals like iron and manganese. However, non-essential divalent metals are also transported through this transporter. Therefore, altered expression of DMT1 can modify the absorption of toxic metals and metal-induced toxicity. An accumulating body of evidence has suggested that increased metal stores in the brain are associated with elevated oxidative stress mediated by the ability of metals to catalyze redox reactions, resulting in abnormal neurobehavioral function and the progression of neurodegenerative diseases. Metal overload has also been implicated in impaired emotional behavior, although the underlying mechanisms are not well understood with limited information. The current review focuses on psychiatric dysfunction associated with imbalanced metabolism of metals that are transported by DMT1. The investigations with respect to the toxic effects of metal overload on behavior and their underlying mechanisms of toxicity could provide several new therapeutic targets to treat metal-associated affective disorders.
Keywords: Cadmium, Divalent Metal Transporter 1, Emotion, Iron, Lead, Manganese
INTRODUCTION
Transition metals are essential for maintaining proper body functions through a number of biological processes. In particular, the brain requires several important transition metals including iron (Fe), cobalt (Co), manganese (Mn), copper (Cu) and zinc (Zn) for optimal physiological function, and their transport to the brain is regulated by unique transport systems. In the central nervous system (CNS), these metals function as catalysts for biochemical reactions, regulators of gene expression, second messengers in signaling pathways and cofactors for many vital enzymes. For example, several enzymes involved in antioxidant functions use metals as cofactors; superoxide dismutase requires Mn in the mitochondria and Zn/Cu in the cytosol [1,2]. Also, both Zn and Fe are cofactors for serine/threonine phosphatases and kinases like human calcineurin [3]. Imbalanced metal homeostasis either by deficiency or by overload of metals is associated with organ dysfunction that leads to various disorders. For example, anemia due to nutritional iron deficiency or gene mutations results in impaired production of iron-requiring proteins (e.g. hemoglobin), inhibition of cell growth and ultimately cell death. In contrast, iron overload causes tissue damage due to elevated oxidative stress. Because of the consequences of metal imbalance, the homeostasis of metals in healthy organisms is tightly regulated at the levels of uptake, metabolism and excretion of metals.
Non-essential metals can exert toxic effects even at low levels. Inorganic salts of several metals, such as aluminum, lead and alkyl derivatives of lead, mercury and tin have toxic effects on behavior and brain function. The toxicity of metals occurs by the disruption of metal homeostasis and is manifested by DNA damage, alterations of gene expression and damage by oxidative stress [4–7]. The role of metal toxicity in neurological disorders by redox mechanisms has been well-documented [8]. Metal overload is also associated with altered expression of nuclear factors like NF-κB, activator protein-1 (AP-1), nuclear factor of activated T-cells (NFAT) and the tumor suppressor protein p53 [9–11]. Alteration of these nuclear factors result in dysregulation of cell proliferation, apoptosis and cell cycle progression, which ultimately leads to cancer, likely by distinct mechanisms occurring in metal deficiency [12].
The absorption of metals from dietary sources occurs by a number of metal transporters to provide specific needs for individual nutrient metals. In particular, the Divalent Metal Transporter 1 (DMT1) is a major iron transporter essential for iron absorption from diet [13–15]. DMT1 also plays an important role in the uptake of several other divalent metals, including cadmium (Cd) and lead (Pb) which are significant toxicants in our environment. Since the expression levels of DMT1 are regulated by several factors, such as body iron status, gene polymorphism and inflammation [16,17], changes in these factors could also alter the transport and neurotoxicity of metals. For example, iron deficiency up-regulates intestinal DMT1 levels [18] and increases the absorption of Mn and Cd [19,20]. Environmental and physiological factors alter the expression of DMT1, which in turn alters systemic and brain metal status [21,22]. While the role of metal overload in cognitive function in the context of neurodegenerative diseases has been extensively reviewed [23–25], the current review discusses the behavior dysfunctions, with a focus on affective disorders, caused by overload of four metals that are transported by DMT1 [26–28], as well as potential mechanisms of toxicities induced by these metals. The metals selected in this review are two essential nutrient metals (Fe and Mn) as well as two non-essential metals (Pb and Cd) that are prominently found in the environment. The investigations with respect to the toxic effects of metal overload on behavior and their underlying mechanisms of toxicity could provide several new therapeutic targets to treat metal-associated affective disorders.
DIVALENT METAL TRANSPORTER 1 (DMT1): DISTRIBUTION AND FUNCTION
Intestine and erythroid cells
DMT1, coded by the Slc11a2 gene, is a transmembrane protein widely expressed almost throughout the body, although the magnitude of its expression levels varies in different tissues. In the duodenum, DMT1 is primarily involved in the dietary absorption of iron as well as other essential divalent cations, including Mn2+ and Zn2+ [14,15]. The transport of copper through DMT1 has been called into question; DMT1 may transport Cu+ [29], whereas Illing et al. [30] claimed that DMT1 does not transport copper. DMT1 also plays an important role in the uptake of iron into erythroid cells and promotes heme synthesis. Studies have demonstrated that ferrous iron uptake into reticulocytes (immature red blood cells) is DMT1 dependent [31,32]. Briefly, transferrin-bound iron in the circulation is endocytosed by interaction with membrane-associated transferrin receptor 1 (TfR1). In the acidic pH of the endosome, the complex is dissociated and releases iron, while DMT1 expressed in endosomes and lysosomes [33,34] exports iron to the cytosol. The essential role of DMT1 in iron transport was confirmed by two rodent models that carry mutations in DMT1: the mk/mk mice and Belgrade (b) rats. Both mk mice and b rats have a missense mutation (G185R) of DMT1 in transmembrane-4, which causes the expression of non-functional DMT1 [35,36]. As a result, these animals display hypochromic, microcytic anemia [35,37]. DMT1 is also expressed in the outer membrane of the mitochondria [38], suggesting its involvement in mitochondrial iron acquisition, potentially coupled with mitoferrin which is localized to the inner membrane [39]. The exact mechanisms about how these two proteins collaborate for the transport of iron into the mitochondria remains to be elucidated.
Liver and kidney
Other tissues also express DMT1. Mixed results exist with respect to DMT1 expression in the liver. Trinder et al. [40] determined that DMT1 is evenly distributed in the liver and its expression is up-regulated in iron loading and downregulated in iron deficiency states after 8 weeks of diet. However, Nam et al. [41] demonstrated that liver DMT1 levels are increased in iron deficiency and decreased during iron loading after 3 weeks of diet. It is possible that prolonged exposure to iron could have caused a compensatory up-regulation of DMT1 to import and store excess iron in the liver. Further studies to determine the relationship between the activity of liver DMT1 and duration of iron overload/deficiency will help to address this question. In the kidney, DMT1 is believed to participate in the reuptake of divalent cations. Although kidney DMT1 levels are reduced in both mk mice and b rats [42,43], Ferguson et al. [43] found that DMT1 mutation does not alter urinary excretion of iron and suggested that lack of DMT1 is compensated by an unidentified alternate mechanism. A study by Veuthey et al. [44] demonstrated that b rats exhibit higher expression of lipocalin-2 (LCN-2), a protein that sequester iron. It can be postulated that this up-regulation may aid in regulating iron excretion in DMT1 mutations. Further investigation is required to accurately determine and identify these mechanisms.
Respiratory tracts
Human lungs are a site of abundant DMT1 expression [45,46]. In rat lungs, DMT1 protein is predominantly found in normal airway and alveolar epithelium, especially in type II cells [45]. Several investigations have identified that both external stimuli and internal changes can regulate DMT1 activity in the lung. For example, proinflammatory cytokines, such as TNF-α and interferon-γ, as well as inorganic fiber like asbestos, promote DMT1 up-regulation in bronchial epithelial cells [47,48]. Interestingly, DMT1 deficiency is associated with elevated pulmonary inflammation [49]. Increasing evidence has suggested that pulmonary DMT1 functions to provide a protective barrier from toxic effects resulting from inhaled metals [49–51]. DMT1 is also found in the olfactory epithelium [52], where the transporter could mediate the nasal uptake of inhaled divalent metals. In support, up-regulation of DMT1 in response to iron deficiency is associated with increased uptake of intranasal Mn into the brain [52,53]. These results suggest that altered expression of DMT1 in the airborne routes could modify the neurotoxicity of environmental metals.
Brain
In the brain, DMT1 expression is highest in the neurons of the striatum, cerebellum and the vascular and ependymal cells lining the third ventricle [54]. The transport of metals through the blood-brain barrier (BBB) is the first step in regulating the entries of these metals into the CNS, as well as in maintaining their levels at proper concentrations [55]. Transferrin receptor-mediated endocytosis is the primary mechanism by which iron is transported across the BBB based on the accumulating body of evidence from functional and genetic studies [56,57]. Although DMT1 is expressed in brain vascular endothelial cells, its role in iron transport through the BBB is unclear [58]. After uptake through the BBB, iron is transported into various neuronal and non-neuronal cells by DMT1-dependent pathways. Neuronal cells exhibit high expression of DMT1 [59], presumably transporting iron into neurons. DMT1 is also expressed in astrocyte cells located near the BBB [60], likely buffering the neurons from iron overload. Siddappa et al. [61] found that iron deficiency during gestation results in regional up-regulation of DMT1 in the brain. Belgrade rats carrying DMT1 mutation exhibit decreased brain iron levels in various regions, suggesting direct involvement of DMT1 in brain iron transport [62]. The role of DMT1 in brain metal transport is discussed in detail in the following sections for individual metals.
Regulation of DMT1 expression and metal transport
The homeostasis of essential metals is disrupted by changes in DMT1 activity. Conversely, altered status of metals can modify DMT1 expression. DMT1 is up-regulated by Mn exposure, iron deficiency or hypoxic conditions [63–66] and down-regulated in dietary iron overload conditions [67]. These changes can increase the uptake of other metals that are transported by DMT1. For example, it has been recognized that Pb absorption is enhanced in iron deficiency anemia [68,69]. Similarly, Mn uptake is up-regulated in iron deficiency [52]. Taken together, these observations indicate that altered expression of DMT1 could modulate the systemic and brain toxicity of several divalent metals. In this following section, we discuss the neurobehavioral effects of four metals that are transported by DMT1 and potential mechanisms of neurotoxicity as well as the impact of DMT1 on metal toxicity.
IRON
Iron and iron deficiency
Iron is the most abundant transition metal in the body. The metal is essential for various body functions, including ATP production, DNA synthesis and oxygen transport. In the brain, iron is the second most abundant metal, after zinc. Brain iron is required for myelination [70] and serves as a cofactor for indispensable enzymes like tyrosine hydroxylase and tryptophan hydroxylase, which are involved in neurotransmitter synthesis and metabolism [71,72]. Dietary iron deficiency is widespread worldwide in both developing and developed countries. In the United States, an estimated 9–16% adolescent and adult females and 7% of toddlers suffer from iron deficiency [73]. The development of iron-deficiency anemia is of importance, especially in the prenatal and early postnatal life because of increased iron utilization during these periods [74,75]. While more comprehensive information about the role of brain iron in emotional and cognitive behavior is available in recent reviews [76–78], here we have captured several important points for iron overload in the context of mood-associated behaviors along with recent updates.
Iron overload and behavioral dysfunction
Iron overload is best represented by hereditary hemochromatosis (HH), an iron overload disorder most prominently found in North American populations. HH is characterized by significantly higher body iron stores, resulting in liver cirrhosis, cardiomyopathy, diabetes, arthritis, hyperlipidemia and premature death [79–81]. HH is primarily caused by mutations in the HFE (High Fe) gene, which is responsible for regulating body iron status. H63D and C282Y are the two most common mutations associated with HH [82]. While most individuals with HH do not display increased iron levels in the brain, several case studies have demonstrated iron accumulation in the brain in HH patients [83], likely due to pathological events secondary to HH. In addition, other types of brain iron overload have been identified; these include Friedrich ataxia, Kufor-Rakeb disease, Pantothenate kinase 2-associated neurodegeneration, collectively known as ‘neurodegeneration with brain iron accumulation’ (NBIA) [84]. Fewer studies have been conducted with a focus on iron overload than deficiency in the context of neurobehavioral function. Rats exposed to iron overload diet demonstrated decreased activity, startle reflexes, habituation and conditioned avoidance responses [85]. In addition, these iron-loaded rats displayed enhanced prepulse modulation of startle, indicating impaired sensorimotor functioning [85]. Studies have also determined that traumatic brain injury (TBI) results in brain iron overload [86]. Excess brain iron found in TBI is associated with increased free radicals and decreased antioxidant enzymes. The behavioral effects of TBI have been extensively studied and reviewed [87–89]. Oxidative stress in TBI is associated with neuronal dysfunction and reduced learning capacity [90], which is improved by the iron chelator deferoxamine [91,92].
Mouse models of brain iron loading provide a useful tool to study emotional response and the behavioral consequences of iron-associated neurotoxicity. Mice that carry H67D mutation in the mouse Hfe gene, homologous to the H63D HFE mutation in humans, display elevated brain iron levels [93]. These mice have shown increased repetitive behavior, a symptom associated with psychiatric disorders [94], indicating that either HFE mutation or iron loading causes behavioral dysfunction. Mouse models of HFE deficiency (Hfe-knockout mice) show systemic iron overload and recapitulate HH in humans [95–97], but Hfe-deficient mice do not show brain iron loading and do not exhibit abnormal behavioral phenotypes [98,99]. These data indicate that behavior disorders caused by iron overload are more dependent on brain iron rather than systemic iron status. Since most HH patients do not display brain iron loading, hemochromatosis may not directly predispose to behavioral disorders. Alternatively, these studies suggest that brain iron loading could be caused by gain of mutant function rather than loss of HFE function (Hfe-knockout). Likewise, it would be of interest if brain iron loading during development is necessary for abnormal behavior and also if therapeutic intervention (i.e. iron chelation) can reverse impaired behavior. The consequence of the C282Y mutation, another prevalent HFE mutation, should also be tested in the future.
The behavioral influences of iron overload appear to be dose-dependent; Maaroufi et al. [100] demonstrated that 3.0 mg/kg administration of iron sulfate, but not 1.5 mg/kg, produced significant changes in the performance of adult Wistar rats during the elevated plus maze test. Furthermore, there have been incidences of schizophrenic spectrum disorders in patients with thalassemia [101], a secondary (transfusion) iron overload disorder resulting from abnormal hemoglobin production [102]. Bock et al. [103] found an increase in iron-related proteins transferrin and ceruloplasmin, an enzyme that catalyzes the oxidation of ferrous iron, in the blood of psychiatric patients. A study conducted by Blasco et al. [104] demonstrated that brain iron levels correlate with cognitive dysfunction in obese women. This study suggests that brain iron may be potentially used as a biomarker for cognitive dysfunction associated with obesity. These animal and clinical studies have demonstrated the involvement of iron overload in impairment of neurobehavioral functioning. Freret et al. [105] discovered that chronic treatment with the iron chelator, deferoxamine, improves impaired behavior after ischemia. Deferoxamine has also improved symptoms of Alzheimer’s disease in both human patients and animal models. McLachlan et al. [106] demonstrated that chronic deferoxamine administration significantly reduces the progression of dementia in Alzheimer’s disease. Fine et al. [107] demonstrated that intranasal deferoxamine treatment results in ameliorating the loss of spatial memory in mouse models of Alzheimer’s disease and restored inflammatory and protein oxidation markers to normal. These chelator studies further strengthen the possible involvement of metal overload in inducing neurobehavioral disorders. Thus, normalizing brain iron levels should be explored as a probable therapy for restoring dysfunctional behavior. Further investigation is required to determine if deferoxamine and other chelators may be successfully implemented as therapies for brain iron loading disorders.
Potential mechanisms of behavioral dysfunction in iron overload
Since excess iron can generate reactive oxygen species (ROS) and increase oxidative stress, most of iron overload studies have been focused on the negative effects on behavioral organization. The effect of iron-induced oxidative stress has been extensively studied in the area of neurodegenerative diseases. Xian-Hui et al. [108] found that brain iron overload associated with DMT1 mutations, in conjunction with aging, may be responsible for the neurodegeneration associated with Alzheimer’s disease. Honda et al. [109] have reviewed the alteration of iron metabolism in Alzheimer’s disease with an emphasis on oxidative stress. Hydroxyl radicals resulting from elevated free iron levels are known to cause lysosomal rupture and leakage of cytosolic contents which promote cell damage [110].
In addition to inducing oxidative stress, iron also influences behavior by altering neurotransmitter turnover. The emotional dysfunction caused by iron overload is in part attributed to impaired dopamine metabolism. Chang et al. [94] found that iron overload caused by Hfe mutation results in decreased dopamine transporters and increased dopamine D2 receptors. In addition, animals fed iron-loading diet decrease brain serotonin and dopamine concentrations [111]. The behavioral deficits by iron overload may be caused by a combination of increased oxidative stress and alterations of the dopaminergic signaling pathway. Less is known about the metabolism of other neurotransmitters like GABA and glutamate under iron overload. Impaired GABA/glutamate homeostasis is implicated in schizophrenia and other psychotic disorders [112–115]. Notably, glutamic acid decarboxylase 67 (GAD67) is decreased in schizophrenic subjects, whereas both GAD65 and GAD67 are reduced in the prefrontal cortex of schizoaffective disorder [114], in which cognitive function is often preserved.
Several lines of evidence suggest that GABAergic pathways are directly affected by iron and also by iron-catalyzed ROS [116–119]. Injections of iron into the brain impairs the release GABA and decreases GABA levels [120,121]. Likewise, increased lipid peroxidation alters GABA uptake [122,123]. These changes can contribute to the neurochemical pathogenesis of mental disorders. Quantitative characterizations of the behavioral and neurochemical phenotypes using animal models of dietary iron overload and/or genetic mutations should provide important information about the role of iron in the development of psychiatric disorders.
Hippocampal DMT1 knockout mice have been generated to study the effect of iron deficiency in the brain without systemic anemia. Carlson et al. [124] postulated the involvement of DMT1 in the hippocampus with spatial memory training. Pisansky et al. [125] found that hippocampus DMT1 knockout mice display alterations in sensorimotor gating, as evidenced by impaired prepulse inhibition and long term potentiation. These studies indicate that altered DMT1 expression in the brain could influence the development of affective disorders. Further investigation is required to determine if DMT1 expression in the brain could be used as a marker for behavioral disorders.
MANGANESE
Manganese physiology and deficiency
Like iron, manganese (Mn) is also an essential trace metal for various biochemical functions. It is required for bone formation and metabolism of carbohydrates and lipids. Studies from animal experiments have demonstrated that dietary Mn deficiency results in various physiological alterations including skeletal abnormalities and alterations in lipid metabolism [126,127]. Since Mn is a cofactor for Mn-dependent (mitochondrial) superoxide dismutase (MnSOD), an essential antioxidant enzyme, Mn deficiency results in decreased activity of MnSOD, thereby increasing mitochondrial lipid peroxidation [128]. Mn is also required for neuronal and glial cell functions. For example, Mn serves as a cofactor for enzymes in neurotransmitter pathways, including glutamine [129,130].
Mn deficiency is not common in humans because a sufficient amount of the metal is provided from the diet. However, Mn deficiency is found in several neurological problems, including bipolar disorder and Hungtington’s disease, as well as epilepsy [131–134]. For example, patients with bipolar affective disorder display a significant decrease in plasma Mn by 18–38% compared with healthy control subjects [131]. Also, Mn levels in the brain cortex are significantly reduced in subjects with Huntington’s disease [135]. Epileptic patients exhibit a decrease in blood Mn [133]. Likewise, deficiencies in MnSOD or Mn-requiring arginase result in a number of neurodegenerative and psychiatric disorders [131,136–139]. These findings suggest that brain Mn transport should be tightly regulated for proper function. Since the role of several metal transporters (e.g. DMT1, ferroportin and SLC30A10) in the uptake and clearance of Mn has been discussed elsewhere [140–144], the current review will focus on the influence of Mn overload on behavioral problems and potential neurotoxic mechanisms.
Manganese exposure and behavioral dysfunction
Manganese absorption occurs through ingestion, dermal absorption and inhalation. A significant association exists between Mn exposure and neurobehavioral and cognitive deficits. Mn neurotoxicity resulting in a Parkinson-like disorder, such as apathy, anorexia, pain in muscles and joints, bradykinesia and rigidity, has been well-documented in people drinking contaminated water, workers employed in mining and Mn ore processing and agricultural workers exposed to Mn-containing pesticides [145–155]. Mn intoxication has also been observed in children during long-term parenteral nutrition and in patients with chronic liver diseases. While psychiatric and overt behavioral problems are observed in children with high Mn exposure, epidemiological studies have also suggested that neurological dysfunction results from chronic, low-level Mn exposure [156–159]. While intestinal bioavailability of Mn is limited due to hepatic first-pass elimination [160], inhaled Mn absorption is much greater than intestinal absorption, indicating that inhalation is the most toxic route of exposure for Mn neurotoxicity [161].
In children, elevated Mn levels have been found in developmental disorders. Increased Mn levels in the hair have been extensively reported in hyperactive children with learning disabilities [162]. Also, in a study by Benko et al. [163] children with ADHD show significantly higher serum Mn concentrations. Hong et al. [164] demonstrated a correlation between blood Mn levels and behavioral problems like anxiety, social behavior and aggression in ADHD. A cross sectional study by Khan et al. [165] demonstrated a dose-response relationship between blood Mn levels and externalizing behavior problems like disruptive behavior and conduct problems. Neurobehavioral toxicities associated with Mn overload include behavioral disinhibition, impulsive errors and attention problems [166]. In addition, impaired psychomotor skills and altered catecholamine metabolism in children has also been linked to prenatal Mn exposure [157]. The behavioral toxicity of Mn could be a consequence of mixed effects on these brain components by dose- and time-dependent manners. Follow up studies must be conducted to determine the effect of Mn exposure on alterations of gene functions involved in neurological processes. The identification of these targets has therapeutic implications to treat Mn-induced neurological disorders.
Mechanisms of Mn-associated neurotoxicity
Mn exposure has been linked to alterations in neurotransmitter pathways like dopamine [167–169] and consequently the development of psychiatric disorders. Kern et al. [170] have demonstrated that prenatal Mn exposure induces behavioral disinhibition and disrupts learning by altering the levels of dopamine receptors and transporters. Tran et al. [171] showed a relationship between striatal dopamine and Mn exposure along with behavioral deficits in rats. Impaired metabolism of GABA and glutamine is also associated with affective disorders caused by Mn exposure. Erikson et al. [172] found a negative correlation between Mn levels in the caudate putamen and GABA concentrations. Other studies, however, have reported increased GABA concentrations and elevated GABA-to-glutamate ratio following Mn overload diet [173]. The effects of Mn exposure on cholinergic systems have received increasing attention and have been reviewed [174]. It has been postulated that Mn toxicity in astrocytes occurs due to the alteration of glutamate transporters. For example, Mn exposure down-regulates the expression and function of glutamate transporters, thereby reducing glutamate uptake [175]. Impaired expression of glutamate/glutamine transporters results in increased extracellular glutamate levels, which promotes excitatory neurotoxicity. The exact mechanism by which Mn-induced neurotransmitter dysfunction causes affective disorders must be characterized by studying the downstream effects of altered neurotransmitter signaling.
An increasing body of evidence suggests that Mn neurotoxicity also results from impaired homeostasis of non-neuronal cells in the brain. Non-neuronal cell injury by Mn exposure results from activation of microglia and astrocytes [176,177]. Mn concentration in astrocytes is much higher than that in neurons, and within the astrocytes, its concentration is highest in the mitochondria [178,179], resulting in mitochondrial dysfunction [180]. Mn exposure causes oxidative stress by inducing nitric oxide synthases (NOS) in neuronal cells [181], which lead to an increased release of inflammatory mediators, such as prostaglandins, cytokines and nitric oxide [182,183]. Moreno et al. [184] found that subchronic Mn exposure causes increased activation of microglia and astrocytes in various regions of the brain. They also found that juveniles were more prone to microglial activation, whereas astrogliosis was more prominent in adults. These results suggest that Mn induced behavioral neurotoxicity occurs by multiple mechanisms that depend on the age and duration of exposure. To accurately characterize the mechanisms responsible for Mn-induced neurotoxicity, prenatal and postnatal exposure studies with different doses and duration should be conducted. It has been postulated that Mn neurotoxicity indirectly results from the production of glial-derived inflammatory products. Dodd et al. [185] demonstrated that Mn exposure alone has a minimal effect on the induction of inducible heme oxygenase (HO-1), an enzyme involved in regulation of oxidative stress, but Mn potentiates the lipopolysaccharide (LPS)-induced expression of HO-1. Loss of HFE function has been associated with protective effects against Mn-induced neurotoxicity [186]. The exact relationship between Mn-induced behavior and neurotransmitter dysfunction and the underlying molecular mechanisms remains to be elucidated. These studies will provide novel molecular targets to treat neurobehavioral dysfunctions induced by heavy metal exposure.
Potential role of DMT1 in Mn neurobehavioral toxicity
The transport of Mn and iron is intimately related; some iron transporters mediate Mn uptake and clearance, including DMT1, transferrin receptor 1 (TfR1) and ferroportin. The importance of DMT1 in Mn transport has been noted in Mn deficiency in Belgrade rats [187]. Erikson et al. [188] demonstrated that Mn uptake into astrocytes occurred via DMT1 and was dependent on iron status. Although the affinity of intestinal DMT1 for Mn is relatively high, the transport of Mn through DMT1 to the brain has been called into question. Crossgrove et al. [189] determined that Mn transport to the brain occurred by mechanisms independent of DMT1. Aschner et al. [190] found that Mn transport across the BBB occurs through transferrin-TfR1 interaction. Mn exposure influences iron concentrations in various brain regions, as well as expression of iron-related proteins, such as ferritin and transferrin [191]. Although DMT1 mediates the absorption of Mn, its exact role in brain Mn transport is still unclear. Further investigation is required to accurately determine the involvement of DMT1 in transporting Mn to the brain. These studies will help elucidate the role of DMT1 in Mn-induced neurotoxicity.
LEAD
Lead exposure and toxicity
Lead (Pb) is an environmental toxicant with no known biological function. Pb is often used in industrial settings worldwide and can be found in many aspects of daily lives such as drinking water, food packaging products, dietary supplements, and even ceramics [192–194]. There are reports of increased blood levels of Pb that can be traced back to Pb glazes used for ceramics produced in Southern Europe [193]. Pb has also been used in various commercial products, such as gasoline and paint, in order to enhance the economic and functional benefits of such products throughout the world. Leaded gasoline and paint were banned due to increased observations of Pb toxicity. However, old houses that are still painted with leaded paint, and Pb that has been emitted to the environment as a result of use of leaded gasoline still remain potential sources of Pb toxicity [192–194]. Past mining areas, as exemplified by the Tar Creek in Ottawa County, Oklahoma, USA, also serve as significant sources of Pb and other toxic metals [195]. Various biological damages including hypertension and sterility in adults and delayed puberty in children have been caused by Pb toxicity [192,193]. Pb exposure in females poses a more serious problem due to the fact that Pb can be stored in bones for decades and leaks out to the fetus via placental transport during pregnancy [194].
Effect of lead on behavioral function
Behavioral effects of Pb have been extensively studied in both animal models and humans [196]. For example, monkeys receiving dietary Pb acetate demonstrated impaired learning and social function [197]. A recent link has been found between Pb toxicity and development of Alzheimer’s disease (AD) with an association between Pb-induced apoptosis and its accumulation in amyloid plaques that are known to promote AD [198,199]. Another proposed link is that Pb exposure decreases DNA methyltransferase and amyloid precursor protein (APP) promoter methylation which in turn increases APP mRNA and amyloid β protein levels [200–202]. Although Pb has been implicated in neurodegenerative diseases, less is known regarding the relationship between time and duration of Pb exposure and the initiation and accumulation of amyloid plaques. Thus, Pb-induced dementia is a field that warrants further investigation, especially in the context of epigenetic regulation of Pb neurotoxicity.
A number of studies have been conducted to evaluate the effects of Pb in humans and many are targeting children due to the fact that Pb toxicity influences affective behavior that is mainly observed in children from an early age. Also, children are more susceptible to heavy metal toxicity due to 1) incompletely developed BBB and CNS, 2) increased absorption of Pb from the gut of children and 3) increased exposure caused by hand-to-mouth activities [203]. Children with increased blood Pb levels were shown to exhibit increased anxiety, social problems and inattention [204]. Analysis of the behavior of 301 school children with elevated bone Pb levels indicates aggression and restlessness in these children [205]. The behavioral effects of Pb are also manifested as hyperactivity and irritability, and these symptoms are not well characterized until children attend school [206]. Mendelsohn and colleagues [207] found that children with Pb levels of 25 µg/dl had significantly lower scores in the Behavior Rating Scale of the Bayley’s Scales of Infant Development. Moreover, Sciarillo et al. [206] found that children who were exposed to Pb had significantly worse results in the Total Behavioral Problem Score in the Child Behavior Checklist. This study found that the Pb exposed children displayed behavioral problems (e.g. aggression and destructive behavior) and reported somatic and sleep problems [206]. In mice, Kasten-Jolly et al. [208] explored the effect of gestational and lactation Pb exposure and found that early Pb exposure resulted in significant alterations in exploratory behavior and spatial memory and was associated with emergence of aggressive behavior. Nation et al. [209] demonstrated that Pb-associated behavior problems lead to other secondary problems. For example, Pb exposure increases consumption of free choice and force choice alcohol, possibly due to a tranquilizing effect of alcohol on Pb-induced irritability.
Mechanisms of Pb-induced neurotoxicity
The mechanism of Pb absorption to the brain, and therefore its neurotoxic mechanisms, is not completely understood. Neurobehavioral toxicity by Pb exposure results by multiple mechanisms that are dependent on age and onset and duration of exposure, as well as brain structure.
Neurological damage induced by Pb exposure can be attributed to the alteration of the overall structure of the brain. For example, acute Pb poisoning leads to brain damage by causing swelling, ventricular compression, herniation and interference in collagen synthesis and vascular permeability [210]. Occupational exposure to high concentrations of Pb was associated with enhanced pinocytic activity and increased openings of interendothelial cells, which caused leakage of microvessels [211]. Pb exposure results in the distortion of the BBB structure [212] and the disruption of myelin morphology [213] in rodents. Moreover, Hu et al. [214] found that Pb administration during lactation alters synaptogenesis. There are damages caused at more microscopic levels as well. Pb is a divalent metal cation which binds to the sulfhydryl groups on proteins with strong affinity and results in the structural distortion of enzymes and other functional proteins [215]. Also, Pb exposure alters gene expression, such as an upregulation of proinflammatory genes in the brain, which can lead to problems in the pathways required for the formation of various brain regions [208]. Pb exerts neurotoxic effects by altering the status of various redox parameters. Mateo et al. [216] demonstrated Pb exposure increases lipid peroxidation and glutathione levels and decreases the activity of anti-oxidant enzymes like glutathione peroxidase. These Pb-induced morphological and functional damages in the brain are subsequently manifested as behavioral alterations.
Other proposed mechanisms of toxicity involve the alteration of various neurochemical pathways. Bielarczyk et al. [217] found that developmental Pb exposure results in a decrease in cholineacetyltransferase. Also, administration of Pb to rodents alters dopaminergic and glutamatergic systems and thereby impairs learning [218]. Finkelstein et al. [219] observed an increase in tyrosine hydroxylase in the hippocampus, which resembles the surgical disruption of septohippocampal pathways. These results suggest that the cognitive and memory dysfunctions following Pb exposure may be due to denervation of the cholinergic hippocampal pathway. Further investigation of the mechanisms involved in Pb induced neurological damage can provide new therapies to prevent neurobehavioral problems, especially in Pb poisoning.
Role of metal transport in lead toxicity
Pb is well-absorbed through ingestion, inhalation and skin penetration and can be transported to the CNS via similar pathway as essential metals. Transporters, such as DMT1, allow the entry of essential metals into the CNS and are responsible for the systemic absorption of non-essential heavy metals, such as Pb and mercury (Hg) [26,220,221]. Pb, along with other divalent metals, can be transported by DMT1 [222,223]. Iron deficiency in both animals and humans results in increased blood Pb levels [224,225]. Moreover, Wang et al. [212,226] demonstrated that iron supplementation reduces both blood and brain Pb levels in the rats exposed to Pb and therefore provides a protective role against Pb-induced brain toxicity. However, the involvement of brain DMT1 in the absorption and neurotoxicity of Pb remains unclear, and Pb has been postulated to gain entry into the CNS by other mechanisms. Mounting evidence has indicated that the transport of Pb into the brain is mediated by the same mechanisms as Ca. Pb uptake by brain capillary endothelial cells has been associated with Ca-ATPase, whereas Pb entry into astroglia and neurons is mediated by voltage-sensitive Ca channels [227–229]. Once inside the cells, Pb is accumulated in the mitochondria, the same compartment involving the storage of Ca2+, and thereby disrupts intracellular Ca2+ transport and metabolism and alters Ca signaling pathways like Protein Kinase C [230]. Thus, Ca status appears to play a role in the severity of Pb transport and thereby its toxicity. In addition, Cheong et al. [231] demonstrated that there exists an unidentified mechanism in the brain which has higher affinity for Pb transport as compared with DMT1. These studies also found that, at the neutral pH in the brain, Pb transport is not influenced by iron status and that upregulation of DMT1 does not increase cellular Pb content [231]. From these results, it may be postulated that though DMT1 in the brain could be involved in cellular transport of essential metals to neuronal and non-neuronal cells and exerts neurotoxicity. Since Pb shares similar pathways with other essential metals for transport and interferes with their physiological function, supplementation of essential metals including zinc and iron are often used for the treatment for Pb toxicity [232]. However, such essential metals can also lead to related toxicity when overdosed. Thus, caution must be exercised in using metal supplements as therapies for protecting against Pb toxicity.
CADMIUM
Cadmium exposure
Cadmium (Cd) is a non-essential transition metal. Exposure to Cd is mainly occupational and occurs in metal industries, production of batteries, electroplating processes. Tobacco consumption is a significant source of Cd exposure since Cd levels are high in cigarettes. Occupational Cd exposure has been associated with cancers in various organs (e.g. lungs, prostate, kidney) and therefore Cd was classified as a human carcinogen [233,234]. The most commonly affected organ is kidney; Cd exposure leads to chronic renal failure [235]. Cd also affects hormones that regulate the human reproductive system, thereby causing infertility and premature birth [236]. Cd toxicity has also been linked to hypertension in rats [237], signifying a possible relationship between Cd exposure and cardiovascular diseases.
Behavioral disorders by cadmium
Cd exposure has also been linked to behavioral deficits. Lehotzky et al. [238] demonstrated that prenatal exposure to different doses of Cd alters of motor coordination, avoidance tasks and sociability. Cd exposure in children has been linked to developmental disorders, especially autism spectrum disorder (ASD). Studies have found elevated levels of Cd in the hair and blood of patients with ASD [239–241]. Cd exposure has also been linked to mental retardation and impaired learning and cognitive functions in children. There exists an inverse correlation between levels of Cd in children’s hair with Intelligence Quotient (IQ), learning difficulties and dyslexia [242–244]. In addition to these behavioral deficits, Cd exposure has also been implicated in drug seeking behavior and has been shown to exacerbate drug-induced symptoms and has been linked to an increase in the intake of ethanol and cocaine [245–247]. Thus, increased Cd burden may enhance the vulnerability to the negative effects of psychoactive drugs. Animal studies involving Cd exposure also exhibit behavioral alterations. Rats exposed to Cd had decreased memory, as well as altered anxiety and fear responses [248]. These behavioral deficits correlated with levels of oxidative stress markers in the hippocampus of these rats [248].
Mechanisms of Cd neurotoxicity
Oxidative stress is one of the proposed mechanisms of Cd toxicity. Lopez et al. [249] showed that Cd exposure resulted in ROS formation in cortical neurons. Tobwala et al. [250] also demonstrated that exposure to Pb and Cd in brain microvascular endothelial cells altered cell viability, glutathione levels and catalase enzyme activity by the production of ROS. In addition to oxidative stress, Cd also results in neurological damage as demonstrated by Wong et al. [251] who found that Cd exposure in newborn rats resulted in brain damage, manifested as lesions and cystic cavities in brain regions, and behavioral dysfunctions, which were not observed in adult rats. Thus the age appears to play a significant role in the extent of Cd toxicity. Also, similar to Pb, Cd has increased the affinity for sulfhydryl groups thereby causing structural distortion of enzymes and other functional proteins [252]. At a cellular level, Cd exerts neurotoxic effects by inducing cell apoptosis, altering gene expression and cell signaling [253–255]. Gonçalves et al. [248] demonstrated that N-acetyl cysteine (NAC) reverses cognitive decline induced by Cd. This suggests antioxidant therapies may be utilized to reverse Cd-induced behavioral dysfunctions.
Some neurochemical alterations have also been observed as a result of Cd exposure. Gupta et al. [256] demonstrated a difference in catecholamine metabolism following Cd administration between growing and adult rats; while growing rats showed a decrease in 5-hydroxytryptamine and 5-hydroxyindole acetic acid in most brain regions, adult rats displayed an increase in these catecholamine metabolites. Another study that measured norepinephrine, 5-hydroxytryptamine, and dopamine demonstrated that mean concentrations of these monoamines increased as a result of acute 24-hr exposure to Cd in anterior, mediobasal, and posterior hypothalamus of rats [257]. These observations suggest that the neurobehavioral problems could result from the neurochemical alterations induced by Cd exposure. Studies on temporal and spatial changes in neurotransmitters and regulation of their transporters and receptors are required to provide insights into the mechanisms by which Cd exposure causes behavior dysfunction.
Effect of cadmium transport on neurotoxicity
The absorption of Cd is similar to other divalent metals and occurs from the intestine primarily by DMT1 and is influenced by the status of other metals in the body [258,259]. Park et al. [20] demonstrated that intestinal absorption of Cd through DMT1 increases during iron deficiency. The metabolism of Cd in impaired DMT1 is relatively unknown. In addition to DMT1, some other transporters have been examined in the context of Cd absorption. In adults, intestinal Cd absorption can be mediated by ferroportin, a transmembrane protein involved in cellular iron transport [260]. Neonatal Cd absorption has been linked to multidrug resistance associated protein 1 (MRP1), a transporter involved in the efflux of a number of chemicals and drugs from enterocytes [261]. Absorbed Cd does not generally reach the adult brain due to the presence of the BBB, but this structure is under-developed in children, who may be more susceptible to the neurotoxic effects of Cd. The exact mechanisms that govern Cd transport must be studied in order to determine the susceptibility of underdeveloped BBB to Cd neurotoxicity. Nasal mucosa and olfactory pathways involving olfactory neurons provide an alternate pathway that can bypass the BBB for the absorption of Cd to the brain [262–264]. Accurately characterizing the pathways by which Cd gains entry into the CNS will provide further insights into the mechanisms involved in Cd-induced neurotoxicity.
POLY-METAL EXPOSURE
The expression and activity of the components of metal metabolism is influenced by systemic metal status. This, in turn, affects the absorption and elimination of other metals in the body. Thompson et al. [52] found that the expression of DMT1 is increased in iron-deficient anemia, which enhances olfactory absorption of manganese. Gu et al. [265] found that co-administration of Pb and Cd increases DMT1 expression, thereby altering the transport of toxic metals into the CNS. Combined iron deficiency and Pb exposure potentiates cognitive dysfunctions and neuronal development, as compared with iron deficiency or Pb exposure alone [266]. Concurrent exposure to Pb and Mn leads to increased accumulation of Pb in the brain and alters various behavioral patterns like anxiety and hyperactivity [267,268]. An epidemiological study conducted by Wright et al. [158] demonstrated that Mn and arsenic alters memory function in children and that a significant interaction effect exists between the two metals. Time and duration of exposure are important contributors to poly-metal toxicity. Chandra et al. [267] showed that poly-metal exposure during prenatal and early postnatal life alters brain function, as compared with the exposure during lactation alone. Since poly-metal exposure represents real-life metal exposure and often exhibits synergistic toxicity, more quantitative understanding is necessary to provide therapeutic benefits for the development of metal-induced behavioral disorders and resultant psychiatric alterations following concurrent exposure to multiple metals.
FACTORS INFLUENCING METAL-INDUCED BEHAVIORAL TOXICITIES
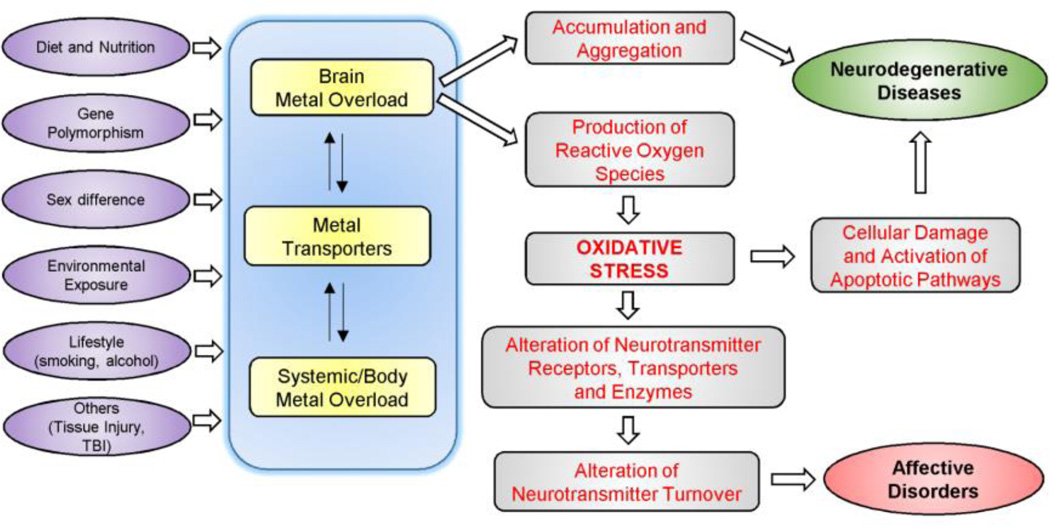
Figure 1 depicts the potential mechanisms of brain metal overload in the development of mental disorders and neurodegenerative diseases, and here we discuss several factors that can influence emotional and affective behavior due to metal overload.
Figure 1. Potential mechanisms of brain metal overload in the development of affective disorders and neurodegenerative diseases.
Various factors alter components of metal metabolism, thereby influencing metal status in the body and brain. Brain metal overload causes neurodegeneration and neurotransmitter dysfunction by inducing oxidative stress. Reactive oxygen species (ROS) impair various components of neurotransmitter pathways, which ultimately manifests as psychiatric/affective disorders.
Metal and nutritional status
Since transition metals possess redox potential, they are selectively involved in a variety of physiological processes, such as gene expression, signaling pathways and electron transport. Thus, it is not surprising that the body has developed complex physiological processes to limit the absorption, distribution and excretion of these metals. The regulation of body metal status is achieved by altering the expression of DMT1 and other metal transporters. For example, iron absorption from the intestine is increased in iron deficiency and decreased upon iron overload [269,270]. Body iron status also influences the pharmacokinetics of other metals in the body; evidence suggests the competitive absorption of Mn and iron from the intestine [271]. Following intravenous injection of 54Mn, rats fed iron overload diet cleared blood Mn more rapidly compared with normal rats [272]. Since both uptake and clearance of metals are affected by the concentrations of other metals in the body, altered body metal status could influence the susceptibility to metal toxicity and potentially to mental disorders.
Gene polymorphism and lifestyle
Other factors, such as gene polymorphisms and different lifestyle, also influence the expression of metal transporters, which in turn alter metal uptake kinetics and toxicity in the body. For example, intestinal Mn absorption is increased in a mouse model of hemochromatosis [273] and this is correlated with elevated activity of iron transporters (ferroportin and DMT1) in hereditary hemochromatosis [274,275]. However, the steady-state concentrations of blood Mn in hemochromatosis are decreased [276], suggesting that Mn clearance is greater than absorption in elevated body iron stores. In addition to these factors, body metal status is also influenced by lifestyle. Smoking is a significant source of cadmium and nickel, while alcohol consumption has been associated with altered iron metabolism [277,278]. These alterations are associated with neurobehavioral dysfunctions. Sassine et al. [279] found that concurrent exposure to Mn, followed by disorders associated with alcohol consumption, were risk factors for psychological distress. Alcohol consumption influences body iron status by regulating the components involved in iron metabolism. Ethanol alters the expression of transcription factors by inducing hypoxia and the expression of hepcidin, a hormone that down-regulates iron transporters, like DMT1 and ferroportin [280–282]. Better understanding of altered metabolism of metals due to gene polymorphism and different lifestyle will help to determine if metal disorders are important risk factors for psychiatric and neurodegenerative diseases.
Sex
Sex differences exist in metal disorders like anemia, with females being more susceptible to these alterations due to a higher prevalence of anemia [283]. Sex differences also exist in behavior and susceptibility to behavior disorders. It is interesting to note that women have been shown to be more predisposed to depression and associated behaviors like apathy and fatigue [284]. Ikeda et al. [285] demonstrated that decreased estrogen levels in ovariectomized mice are associated with decreased levels of hepcidin, the master regulator of iron transport, which ultimately elevates body iron status. The regulation of hepcidin by estrogen is mediated by G-protein coupled receptor 30 (GPR30) signaling and down-regulation of bone-morphologic protein-6 (BMP-6), establishing a molecular relationship between estrogen and hepcidin. On the contrary, Yang et al. [286] showed that estrogen inhibits induction of hepcidin via an estrogen-response element. Notably, testosterone supplementation suppresses the production of hepcidin [287,288]. These studies suggest that body iron status is regulated by additional factors like gender and gonadal hormones. Sex differences also exist between behavior disorders induced by other metals. Menezes-Filho et al. [289] demonstrated that females are more susceptible to attention problems caused by Mn exposure. Anderson et al. [290] also demonstrated sex differences in spatial memory after Pb administration. Studying the correlation between metal metabolism and sex differences in the context of behavioral disorders may help in determining if these molecular mechanisms influence the susceptibility to behavior disorders. The influence of gonadal hormones on metal metabolism provides an important avenue to novel therapeutics to explore molecular targets for behavior disorders that have been linked to hormonal imbalances, e.g. depression.
FUTURE DIRECTIONS
Metals are worthy of evaluation for their effects on the nervous system and subsequent behavioral changes. Many metals in excess have been implicated in the development of neurodegenerative diseases (e.g. Alzheimer’s disease) by catalyzing redox reactions that promote oxidative stress [6]. Since body metal status is dependent on the components of its metabolism, the dysfunctions caused by alteration of components involved in metal metabolism must be studied. He et al. [291] have proposed a possible link between single nucleotide polymorphisms in DMT1 and the occurrence of Parkinson’s disease. This indicates altered metal transport could be closely related to the development of neurodegenerative disorders. The progression of these neurodegenerative diseases is linked to age, and it has been demonstrated that there is an age-dependent increase in brain metal concentrations [292,293]. The effects of time and duration of metal exposure will help to determine the long term effects, including neurodegeneration and behavior disorders, induced by metal overload. Conversely, further studies that integrate the mechanisms associated with developmental metal overload and behavior dysfunctions, including therapeutic interventions through the mom, will provide possible new therapies to prevent/treat metal-induced behavior disorders in children. Another important factor to be considered is the route of exposure to these metals. These factors are of critical importance, especially in the context of occupational and lifestyle exposure. Studies must be conducted to determine the effects of particulate and soluble metal exposure. Examining these toxicities at the cellular and molecular level will provide insight into the mechanisms by which overload is associated with emotional dysfunction.
Drugs that alter the molecular mechanisms associated with metal overload can provide new therapeutic targets in the treatment of metal induced behavior disorders. Chelator treatments for metal-induced neurological disorders are a promising therapeutic strategy. Many chelators (e.g. deferoxamine) have been shown to improve cognitive function in rat pups after hemorrhage or traumatic brain injury [91,92,294]. Most chelators, unfortunately, have been shown to interfere with the homeostasis of essential nutrient metals. For example, meso-2,3-dimercaptosuccininic acid (DMSA) administration has been associated with minor changes in copper metabolism [295]. Therefore, it is important to analyze the chemical interactions between different classes of chelators and their metal targets to develop chelators that are highly specific and have minimum effects on other metals in the body. Recent advances in chelator research have provided several drugs that specifically chelate fewer metals. These include the iron chelators deferasirox and Bp44mT that have been developed with higher affinity for iron as compared to zinc or copper [296,297]. The chelation therapy with improved binding specificity and capacity holds great promise for ameliorating psychiatric and neurological disorders, as well as metal-induced neurotoxicity. Another potential therapy for correcting metal-induced oxidative stress is antioxidant supplementation. Indeed, antioxidant therapies have already been shown to alleviate oxidative stress occurring in neurodegenerative disorders and improve behavior symptoms in psychiatric and impulsive disorders [298–300]. Studies have found that antioxidants and chelators, both individually and in combination, reduce the deleterious neurological damage caused by metal overload [301,302]. More mechanistic and quantitative investigations on the association of metal overload with emotional behavior may help in developing new therapeutic targets for psychiatric disorders. The use of drugs that can modulate the levels of neurotoxic metals and resultant oxidative stress (e.g. chelators and antioxidants) provides molecular and pharmacological basis for therapeutic and nutritional intervention for behavior dysfunctions.
ACKNOWLEDGEMENTS
This work was supported by the NIH R00 ES017781 (J.K.).
Abbreviations
- BBB
blood-brain barrier
- Cd
cadmium
- CNS
central nervous system
- Cu
copper
- DMT1
divalent metal transporter 1
- Fe
iron
- Mn
manganese
- NBIA
neurodegeneration with brain iron accumulation
- Pb
lead
- Se
selenium
- ROS
reactive oxidative species
- SOD
superoxide dismutase
- Zn
zinc
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting financial interests.
REFERENCES
- 1.Candas D, Li JJ. MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxid Redox Signal. 2014;20:1599–1617. doi: 10.1089/ars.2013.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crapo JD, Oury T, Rabouille C, Slot JW, Chang LY. Copper,zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci U S A. 1992;89:10405–10409. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu L, Golbeck J, Yao J, Rusnak F. Spectroscopic and enzymatic characterization of the active site dinuclear metal center of calcineurin: implications for a mechanistic role. Biochemistry. 1997;36:10727–10734. doi: 10.1021/bi970519g. [DOI] [PubMed] [Google Scholar]
- 4.Glei M, Latunde-Dada GO, Klinder A, Becker TW, Hermann U, et al. Iron-overload induces oxidative DNA damage in the human colon carcinoma cell line HT29 clone 19A. Mutat Res. 2002;519:151–161. doi: 10.1016/s1383-5718(02)00135-3. [DOI] [PubMed] [Google Scholar]
- 5.Roling JA, Bain LJ, Gardea-Torresdey J, Bader J, Baldwin WS. Hexavalent chromium reduces larval growth and alters gene expression in mummichog (Fundulus heteroclitus) Environ Toxicol Chem. 2006;25:2725–2733. doi: 10.1897/05-659r.1. [DOI] [PubMed] [Google Scholar]
- 6.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 7.Gadhia SR, Calabro AR, Barile FA. Trace metals alter DNA repair and histone modification pathways concurrently in mouse embryonic stem cells. Toxicol Lett. 2012;212:169–179. doi: 10.1016/j.toxlet.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Bush AI. Metals and neuroscience. Curr Opin Chem Biol. 2000;4:184–191. doi: 10.1016/s1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 9.Fry RC, Navasumrit P, Valiathan C, Svensson JP, Hogan BJ, et al. Activation of inflammation/NF-kappaB signaling in infants born to arsenic-exposed mothers. PLoS Genet. 2007;3:e207. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombo M, Hamelin C, Kouassi E, Fournier M, Bernier J. Differential effects of mercury, lead, and cadmium on IL-2 production by Jurkat T cells. Clin Immunol. 2004;111:311–322. doi: 10.1016/j.clim.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Urani C, Melchioretto P, Fabbri M, Bowe G, Maserati E, et al. Cadmium Impairs p53 Activity in HepG2 Cells. ISRN Toxicology. 2014;2014:976428. doi: 10.1155/2014/976428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 13.Gunshin H, Fujiwara Y, Custodio AO, DiRenzo C, Robine S, et al. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. Journal of Clinical Investigation. 2005;115:1258–1266. doi: 10.1172/JCI24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knopfel M, Zhao L, Garrick MD. Transport of divalent transition-metal ions is lost in small-intestinal tissue of b/b Belgrade rats. Biochemistry. 2005;44:3454–3465. doi: 10.1021/bi048768+. [DOI] [PubMed] [Google Scholar]
- 15.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 16.Urrutia P, Aguirre P, Esparza A, Tapia V, Mena NP, et al. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem. 2013;126:541–549. doi: 10.1111/jnc.12244. [DOI] [PubMed] [Google Scholar]
- 17.Canonne-Hergaux F, Levy JE, Fleming MD, Montross LK, Andrews NC, et al. Expression of the DMT1 (NRAMP2/DCT1) iron transporter in mice with genetic iron overload disorders. Blood. 2001;97:1138–1140. doi: 10.1182/blood.v97.4.1138. [DOI] [PubMed] [Google Scholar]
- 18.Zoller H, Koch RO, Theurl I, Obrist P, Pietrangelo A, et al. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology. 2001;120:1412–1419. doi: 10.1053/gast.2001.24033. [DOI] [PubMed] [Google Scholar]
- 19.Flanagan PR, Haist J, Valberg LS. Comparative Effects of Iron Deficiency Induced by Bleeding and a Low-Iron Diet on the Intestinal Absorptive Interactions of Iron, Cobalt, Manganese, Zinc, Lead and Cadmium. The Journal of Nutrition. 1980;110:1754–1763. doi: 10.1093/jn/110.9.1754. [DOI] [PubMed] [Google Scholar]
- 20.Park JD, Cherrington NJ, Klaassen CD. Intestinal Absorption of Cadmium Is Associated with Divalent Metal Transporter 1 in Rats. Toxicological Sciences. 2002;68:288–294. doi: 10.1093/toxsci/68.2.288. [DOI] [PubMed] [Google Scholar]
- 21.Wysokinski D, Zaras M, Dorecka M, Waszczyk M, Szaflik J, et al. An association between environmental factors and the IVS4+44C>A polymorphism of the DMT1 gene in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2012;250:1057–1065. doi: 10.1007/s00417-012-1966-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burdo JR, Simpson IA, Menzies S, Beard J, Connor JR. Regulation of the profile of iron-management proteins in brain microvasculature. J Cereb Blood Flow Metab. 2004;24:67–74. doi: 10.1097/01.WCB.0000095800.98378.03. [DOI] [PubMed] [Google Scholar]
- 23.Salvador GA, Uranga RM, Giusto NM. Iron and mechanisms of neurotoxicity. Int J Alzheimers Dis. 2010;2011:720658. doi: 10.4061/2011/720658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- 25.Mason LH, Harp JP, Han DY. Pb Neurotoxicity: Neuropsychological Effects of Lead Toxicity. BioMed Research International. 2014;2014:8. doi: 10.1155/2014/840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garrick MD, Dolan KG, Horbinski C, Ghio AJ, Higgins D, et al. DMT1: a mammalian transporter for multiple metals. Biometals. 2003;16:41–54. doi: 10.1023/a:1020702213099. [DOI] [PubMed] [Google Scholar]
- 27.Bressler JP, Olivi L, Cheong JH, Kim Y, Bannona D. Divalent metal transporter 1 in lead and cadmium transport. Ann N Y Acad Sci. 2004;1012:142–152. doi: 10.1196/annals.1306.011. [DOI] [PubMed] [Google Scholar]
- 28.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy Metals Toxicity and the Environment. EXS. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arredondo M, Munoz P, Mura CV, Nunez MT. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am J Physiol Cell Physiol. 2003;284:C1525–C1530. doi: 10.1152/ajpcell.00480.2002. [DOI] [PubMed] [Google Scholar]
- 30.Illing AC, Shawki A, Cunningham CL, Mackenzie B. Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J Biol Chem. 2012;287:30485–30496. doi: 10.1074/jbc.M112.364208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang A-S, Canonne-Hergaux F, Gruenheid S, Gros P, Ponka P. Use of Nramp2-transfected Chinese hamster ovary cells and reticulocytes from mk/mk mice to study iron transport mechanisms. Experimental hematology. 2008;36:1227–1235. doi: 10.1016/j.exphem.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canonne-Hergaux F, Zhang AS, Ponka P, Gros P. Characterization of the iron transporter DMT1 (NRAMP2/DCT1) in red blood cells of normal and anemic mk/mk mice. Blood. 2001;98:3823–3830. doi: 10.1182/blood.v98.13.3823. [DOI] [PubMed] [Google Scholar]
- 33.Farcich EA, Morgan EH. Uptake of transferrin-bound and nontransferrin-bound iron by reticulocytes from the Belgrade laboratory rat: comparison with Wistar rat transferrin and reticulocytes. Am J Hematol. 1992;39:9–14. doi: 10.1002/ajh.2830390104. [DOI] [PubMed] [Google Scholar]
- 34.Tabuchi M, Yoshimori T, Yamaguchi K, Yoshida T, Kishi F. Human NRAMP2/DMT1, Which Mediates Iron Transport across Endosomal Membranes, Is Localized to Late Endosomes and Lysosomes in HEp-2 Cells. Journal of Biological Chemistry. 2000;275:22220–22228. doi: 10.1074/jbc.M001478200. [DOI] [PubMed] [Google Scholar]
- 35.Fleming MD, Trenor CC, 3rd, Su MA, Foernzler D, Beier DR, et al. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 36.Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, et al. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci U S A. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson K, Molina RM, Brain JD, Wessling-Resnick M. Belgrade Rats Display Liver Iron Loading. The Journal of nutrition. 2006;136:3010–3014. doi: 10.1093/jn/136.12.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolff NA, Ghio AJ, Garrick LM, Garrick MD, Zhao L, et al. Evidence for mitochondrial localization of divalent metal transporter 1 (DMT1) FASEB J. 2014 doi: 10.1096/fj.13-240564. [DOI] [PubMed] [Google Scholar]
- 39.Shaw GC, Cope JJ, Li L, Corson K, Hersey C, et al. Mitoferrin is essential for erythroid iron assimilation. Nature. 2006;440:96–100. doi: 10.1038/nature04512. [DOI] [PubMed] [Google Scholar]
- 40.Trinder D, Oates PS, Thomas C, Sadleir J, Morgan EH. Localisation of divalent metal transporter 1 (DMT1) to the microvillus membrane of rat duodenal enterocytes in iron deficiency, but to hepatocytes in iron overload. Gut. 2000;46:270–276. doi: 10.1136/gut.46.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nam H, Wang C-Y, Zhang L, Zhang W, Hojyo S, et al. ZIP14 and DMT1 in the liver, pancreas, and heart are differentially regulated by iron deficiency and overload: implications for tissue iron uptake in iron-related disorders. Haematologica. 2013;98:1049–1057. doi: 10.3324/haematol.2012.072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canonne-Hergaux F, Gros P. Expression of the iron transporter DMT1 in kidney from normal and anemic mk mice. Kidney Int. 2002;62:147–156. doi: 10.1046/j.1523-1755.2002.00405.x. [DOI] [PubMed] [Google Scholar]
- 43.Ferguson CJ, Wareing M, Delannoy M, Fenton R, McLarnon SJ, et al. Iron handling and gene expression of the divalent metal transporter, DMT1, in the kidney of the anemic Belgrade (b) rat. Kidney Int. 2003;64:1755–1764. doi: 10.1046/j.1523-1755.2003.00274.x. [DOI] [PubMed] [Google Scholar]
- 44.Veuthey T, Hoffmann D, Vaidya VS, Wessling-Resnick M. Impaired renal function and development in Belgrade rats. American Journal of Physiology - Renal Physiology. 2014;306:F333–F343. doi: 10.1152/ajprenal.00285.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brain JD, Heilig E, Donaghey TC, Knutson MD, Wessling-Resnick M, et al. Effects of iron status on transpulmonary transport and tissue distribution of Mn and Fe. Am J Respir Cell Mol Biol. 2006;34:330–337. doi: 10.1165/rcmb.2005-0101OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heilig EA, Thompson KJ, Molina RM, Ivanov AR, Brain JD, et al. Manganese and iron transport across pulmonary epithelium. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1247–L1259. doi: 10.1152/ajplung.00450.2005. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Garrick MD, Yang F, Dailey LA, Piantadosi CA, et al. TNF, IFN-gamma, and endotoxin increase expression of DMT1 in bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L24–L33. doi: 10.1152/ajplung.00428.2003. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Wu Y, Stonehuerner JG, Dailey LA, Richards JD, et al. Oxidant generation promotes iron sequestration in BEAS-2B cells exposed to asbestos. Am J Respir Cell Mol Biol. 2006;34:286–292. doi: 10.1165/rcmb.2004-0275OC. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Molina RM, Donaghey TC, Buckett PD, Brain JD, et al. Influence of DMT1 and iron status on inflammatory responses in the lung. Am J Physiol Lung Cell Mol Physiol. 2011;300:L659–L665. doi: 10.1152/ajplung.00343.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghio AJ, Piantadosi CA, Wang X, Dailey LA, Stonehuerner JD, et al. Divalent metal transporter-1 decreases metal-related injury in the lung. Am J Physiol Lung Cell Mol Physiol. 2005;289:L460–L467. doi: 10.1152/ajplung.00154.2005. [DOI] [PubMed] [Google Scholar]
- 51.Ghio AJ, Turi JL, Madden MC, Dailey LA, Richards JD, et al. Lung injury after ozone exposure is iron dependent. Am J Physiol Lung Cell Mol Physiol. 2007;292:L134–L143. doi: 10.1152/ajplung.00534.2005. [DOI] [PubMed] [Google Scholar]
- 52.Thompson K, Molina RM, Donaghey T, Schwob JE, Brain JD, et al. Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB J. 2007;21:223–230. doi: 10.1096/fj.06-6710com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim J, Li Y, Buckett PD, Bohlke M, Thompson KJ, et al. Iron-responsive olfactory uptake of manganese improves motor function deficits associated with iron deficiency. PLoS One. 2012;7:e33533. doi: 10.1371/journal.pone.0033533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, et al. Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res. 2001;66:1198–1207. doi: 10.1002/jnr.1256. [DOI] [PubMed] [Google Scholar]
- 55.Zheng W, Aschner M, Ghersi-Egea J-F. Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicology and Applied Pharmacology. 2003;192:1–11. doi: 10.1016/s0041-008x(03)00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bradbury MW. Transport of iron in the blood-brain-cerebrospinal fluid system. J Neurochem. 1997;69:443–454. doi: 10.1046/j.1471-4159.1997.69020443.x. [DOI] [PubMed] [Google Scholar]
- 57.Ke Y, Qian ZM. Brain iron metabolism: neurobiology and neurochemistry. Prog Neurobiol. 2007;83:149–173. doi: 10.1016/j.pneurobio.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 58.Siddappa AJ, Rao RB, Wobken JD, Leibold EA, Connor JR, et al. Developmental changes in the expression of iron regulatory proteins and iron transport proteins in the perinatal rat brain. J Neurosci Res. 2002;68:761–775. doi: 10.1002/jnr.10246. [DOI] [PubMed] [Google Scholar]
- 59.Pelizzoni I, Zacchetti D, Smith CP, Grohovaz F, Codazzi F. Expression of divalent metal transporter 1 in primary hippocampal neurons: reconsidering its role in non-transferrin-bound iron influx. J Neurochem. 2012;120:269–278. doi: 10.1111/j.1471-4159.2011.07578.x. [DOI] [PubMed] [Google Scholar]
- 60.Pelizzoni I, Zacchetti D, Campanella A, Grohovaz F, Codazzi F. Iron uptake in quiescent and inflammation-activated astrocytes: A potentially neuroprotective control of iron burden. Biochimica et Biophysica Acta. 2013;1832:1326–1333. doi: 10.1016/j.bbadis.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siddappa AJ, Rao RB, Wobken JD, Casperson K, Leibold EA, et al. Iron deficiency alters iron regulatory protein and iron transport protein expression in the perinatal rat brain. Pediatr Res. 2003;53:800–807. doi: 10.1203/01.PDR.0000058922.67035.D5. [DOI] [PubMed] [Google Scholar]
- 62.Burdo JR, Martin J, Menzies SL, Dolan KG, Romano MA, et al. Cellular distribution of iron in the brain of the Belgrade rat. Neuroscience. 1999;93:1189–1196. doi: 10.1016/s0306-4522(99)00207-9. [DOI] [PubMed] [Google Scholar]
- 63.Jiang L, Garrick MD, Garrick LM, Zhao L, Collins JF. Divalent metal transporter 1 (Dmt1) Mediates Copper Transport in the Duodenum of Iron- Deficient Rats and When Overexpressed in Iron-Deprived HEK-293 Cells. The Journal of Nutrition. 2013;143:1927–1933. doi: 10.3945/jn.113.181867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qian ZM, Wu XM, Fan M, Yang L, Du F, et al. Divalent metal transporter 1 is a hypoxia-inducible gene. J Cell Physiol. 2011;226:1596–1603. doi: 10.1002/jcp.22485. [DOI] [PubMed] [Google Scholar]
- 65.Wang X, Li GJ, Zheng W. Upregulation of DMT1 expression in choroidal epithelia of the blood-CSF barrier following manganese exposure in vitro. Brain Res. 2006;1097:1–10. doi: 10.1016/j.brainres.2006.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Molina RM, Phattanarudee S, Kim J, Thompson K, Wessling-Resnick M, et al. Ingestion of Mn and Pb by rats during and after pregnancy alters iron metabolism and behavior in offspring. Neurotoxicology. 2011;32:413–422. doi: 10.1016/j.neuro.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nam H, Wang CY, Zhang L, Zhang W, Hojyo S, et al. ZIP14 and DMT1 in the liver, pancreas, and heart are differentially regulated by iron deficiency and overload: implications for tissue iron uptake in iron-related disorders. Haematologica. 2013;98:1049–1057. doi: 10.3324/haematol.2012.072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muwakkit S, Nuwayhid I, Nabulsi M, al Hajj R, Khoury R, et al. Iron deficiency in young Lebanese children: association with elevated blood lead levels. J Pediatr Hematol Oncol. 2008;30:382–386. doi: 10.1097/MPH.0b013e318165b283. [DOI] [PubMed] [Google Scholar]
- 69.Landrigan PJ, Baker EL, Jr, Feldman RG, Cox DH, Eden KV, et al. Increased lead absorption with anemia and slowed nerve conduction in children near a lead smelter. The Journal of Pediatrics. 1976;89:904–910. doi: 10.1016/s0022-3476(76)80594-x. [DOI] [PubMed] [Google Scholar]
- 70.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 71.Martinez A, Knappskog PM, Haavik J. A structural approach into human tryptophan hydroxylase and its implications for the regulation of serotonin biosynthesis. Curr Med Chem. 2001;8:1077–1091. doi: 10.2174/0929867013372616. [DOI] [PubMed] [Google Scholar]
- 72.Nagatsu T. Tyrosine hydroxylase: human isoforms, structure and regulation in physiology and pathology. Essays Biochem. 1995;30:15–35. [PubMed] [Google Scholar]
- 73.Iron deficiency--United States, 1999–2000. MMWR Morb Mortal Wkly Rep. 2002;51:897–899. [PubMed] [Google Scholar]
- 74.Bothwell TH, Pribilla WF, Mebust W, Finch CA. Iron metabolism in the pregnant rabbit; iron transport across the placenta. Am J Physiol. 1958;193:615–622. doi: 10.1152/ajplegacy.1958.193.3.615. [DOI] [PubMed] [Google Scholar]
- 75.Siimes MA, Addiego JE, Jr, Dallman PR. Ferritin in serum: diagnosis of iron deficiency and iron overload in infants and children. Blood. 1974;43:581–590. [PubMed] [Google Scholar]
- 76.Lozoff B. Early Iron Deficiency Has Brain and Behavior Effects Consistent with Dopaminergic Dysfunction. The Journal of Nutrition. 2011;141:740S–746S. doi: 10.3945/jn.110.131169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim J, Wessling-Resnick M. Iron and mechanisms of emotional behavior. J Nutr Biochem. 2014;25:1101–1107. doi: 10.1016/j.jnutbio.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- 79.Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Physician. 2013;87:183–190. [PubMed] [Google Scholar]
- 80.Pietrangelo A. Hereditary hemochromatosis--a new look at an old disease. N Engl J Med. 2004;350:2383–2397. doi: 10.1056/NEJMra031573. [DOI] [PubMed] [Google Scholar]
- 81.Kim J, Jia X, Buckett PD, Liu S, Lee CH, et al. Iron loading impairs lipoprotein lipase activity and promotes hypertriglyceridemia. FASEB J. 2013;27:1657–1663. doi: 10.1096/fj.12-224386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steinberg KK, Cogswell ME, Chang JC, Caudill SP, McQuillan GM, et al. Prevalence of C282Y and H63D mutations in the hemochromatosis (HFE) gene in the United States. Jama. 2001;285:2216–2222. doi: 10.1001/jama.285.17.2216. [DOI] [PubMed] [Google Scholar]
- 83.Nielsen JE, Jensen LN, Krabbe K. Hereditary haemochromatosis: a case of iron accumulation in the basal ganglia associated with a parkinsonian syndrome. Journal of neurology, neurosurgery, and psychiatry. 1995;59:318–321. doi: 10.1136/jnnp.59.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schipper HM. Neurodegeneration with brain iron accumulation — Clinical syndromes and neuroimaging. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2012;1822:350–360. doi: 10.1016/j.bbadis.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 85.Sobotka TJ, Whittaker P, Sobotka JM, Brodie RE, Wander DY, et al. Neurobehavioral dysfunctions associated with dietary iron overload. Physiology & Behavior. 1996;59:213–219. doi: 10.1016/0031-9384(95)02030-6. [DOI] [PubMed] [Google Scholar]
- 86.Raz E, Jensen JH, Ge Y, Babb JS, Miles L, et al. Brain Iron Quantification in Mild Traumatic Brain Injury: A Magnetic Field Correlation Study. AJNR American journal of neuroradiology. 2011;32 doi: 10.3174/ajnr.A2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Milman A, Rosenberg A, Weizman R, Pick CG. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J Neurotrauma. 2005;22:1003–1010. doi: 10.1089/neu.2005.22.1003. [DOI] [PubMed] [Google Scholar]
- 88.Karver CL, Wade SL, Cassedy A, Taylor HG, Stancin T, et al. Age at injury and long-term behavior problems after traumatic brain injury in young children. Rehabil Psychol. 2012;57:256–265. doi: 10.1037/a0029522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schwarzbold M, Diaz A, Martins ET, Rufino A, Amante LN, et al. Psychiatric disorders and traumatic brain injury. Neuropsychiatric Disease and Treatment. 2008;4:797–816. doi: 10.2147/ndt.s2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodriguez-Rodriguez A, Egea-Guerrero JJ, Murillo-Cabezas F, Carrillo-Vico A. Oxidative stress in traumatic brain injury. Curr Med Chem. 2014;21:1201–1211. doi: 10.2174/0929867321666131217153310. [DOI] [PubMed] [Google Scholar]
- 91.Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, et al. Long-term effects of experimental intracerebral hemorrhage: the role of iron. J Neurosurg. 2006;104:305–312. doi: 10.3171/jns.2006.104.2.305. [DOI] [PubMed] [Google Scholar]
- 92.Zhang L, Hu R, Li M, Li F, Meng H, et al. Deferoxamine attenuates ironinduced long-term neurotoxicity in rats with traumatic brain injury. Neurol Sci. 2013;34:639–645. doi: 10.1007/s10072-012-1090-1. [DOI] [PubMed] [Google Scholar]
- 93.Nandar W, Neely EB, Unger E, Connor JR. A mutation in the HFE gene is associated with altered brain iron profiles and increased oxidative stress in mice. Biochim Biophys Acta. 2013;1832:729–741. doi: 10.1016/j.bbadis.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 94.Chang J, Kueon C, Kim J. Influence of Lead on Repetitive Behavior and Dopamine Metabolism in a Mouse Model of Iron Overload. Toxicological Research. 2014;30:267–276. doi: 10.5487/TR.2014.30.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou XY, Tomatsu S, Fleming RE, Parkkila S, Waheed A, et al. HFE gene knockout produces mouse model of hereditary hemochromatosis. Proceedings of the National Academy of Sciences. 1998;95:2492–2497. doi: 10.1073/pnas.95.5.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Levy JE, Montross LK, Andrews NC. Genes that modify the hemochromatosis phenotype in mice. J Clin Invest. 2000;105:1209–1216. doi: 10.1172/JCI9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Levy JE, Montross LK, Cohen DE, Fleming MD, Andrews NC. The C282Y mutation causing hereditary hemochromatosis does not produce a null allele. Blood. 1999;94:9–11. [PubMed] [Google Scholar]
- 98.Johnstone DM, Graham RM, Trinder D, Riveros C, Olynyk JK, et al. Changes in brain transcripts related to Alzheimer's disease in a model of HFE hemochromatosis are not consistent with increased Alzheimer's disease risk. J Alzheimers Dis. 2012;30:791–803. doi: 10.3233/JAD-2012-112183. [DOI] [PubMed] [Google Scholar]
- 99.Alsulimani HA, Ye Q, Kim J. Effect of Hfe deficiency on memory capacity and motor coordination after manganese exposure by drinking water in mice. Toxicol Res. 2015 doi: 10.5487/TR.2015.31.4.347. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maaroufi K, Ammari M, Jeljeli M, Roy V, Sakly M, et al. Impairment of emotional behavior and spatial learning in adult Wistar rats by ferrous sulfate. Physiology & Behavior. 2009;96:343–349. doi: 10.1016/j.physbeh.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 101.Borras L, Huguelet P. Schizophrenia and beta-thalassemia: a genetic link? Psychiatry Res. 2008;158:260–261. doi: 10.1016/j.psychres.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 102.Kushner JP, Porter JP, Olivieri NF. Secondary iron overload. Hematology Am Soc Hematol Educ Program. 2001:47, 61. doi: 10.1182/asheducation-2001.1.47. [DOI] [PubMed] [Google Scholar]
- 103.Bock E, Weeke B, Rafaelsen OJ. Serum proteins in acutely psychotic patients. J Psychiatr Res. 1971;9:1–9. doi: 10.1016/0022-3956(71)90003-3. [DOI] [PubMed] [Google Scholar]
- 104.Blasco G, Puig J, Daunis IEJ, Molina X, Xifra G, et al. Brain iron overload, insulin resistance, and cognitive performance in obese subjects: a preliminary MRI case-control study. Diabetes Care. 2014;37:3076–3083. doi: 10.2337/dc14-0664. [DOI] [PubMed] [Google Scholar]
- 105.Freret T, Valable S, Chazalviel L, Saulnier R, Mackenzie ET, et al. Delayed administration of deferoxamine reduces brain damage and promotes functional recovery after transient focal cerebral ischemia in the rat. Eur J Neurosci. 2006;23:1757–1765. doi: 10.1111/j.1460-9568.2006.04699.x. [DOI] [PubMed] [Google Scholar]
- 106.Crapper McLachlan DR, Dalton AJ, Kruck TP, Bell MY, Smith WL, et al. Intramuscular desferrioxamine in patients with Alzheimer's disease. Lancet. 1991;337:1304–1308. doi: 10.1016/0140-6736(91)92978-b. [DOI] [PubMed] [Google Scholar]
- 107.Fine JM, Baillargeon AM, Renner DB, Hoerster NS, Tokarev J, et al. Intranasal deferoxamine improves performance in radial arm water maze, stabilizes HIF-1alpha, and phosphorylates GSK3beta in P301L tau transgenic mice. Exp Brain Res. 2012;219:381–390. doi: 10.1007/s00221-012-3101-0. [DOI] [PubMed] [Google Scholar]
- 108.Xian-hui D, Wei-juan G, Tie-mei S, Hong-lin X, Jiang-tao B, et al. Age-related changes of brain iron load changes in the frontal cortex in APPswe/PS1DeltaE9 transgenic mouse model of Alzheimer's disease. J Trace Elem Med Biol. 2015;30:118–123. doi: 10.1016/j.jtemb.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 109.Honda K, Casadesus G, Petersen RB, Perry G, Smith MA. Oxidative stress and redox-active iron in Alzheimer's disease. Ann N Y Acad Sci. 2004;1012:179–182. doi: 10.1196/annals.1306.015. [DOI] [PubMed] [Google Scholar]
- 110.Brunk UT, Svensson I. Oxidative stress, growth factor starvation and Fas activation may all cause apoptosis through lysosomal leak. Redox Rep. 1999;4:3–11. doi: 10.1179/135100099101534675. [DOI] [PubMed] [Google Scholar]
- 111.Elseweidy MM, Abd El-Baky AE. Effect of dietary iron overload in rat brain: oxidative stress, neurotransmitter level and serum metal ion in relation to neurodegenerative disorders. Indian J Exp Biol. 2008;46:855–858. [PubMed] [Google Scholar]
- 112.Coyle JT. The GABA-glutamate connection in schizophrenia: which is the proximate cause? Biochem Pharmacol. 2004;68:1507–1514. doi: 10.1016/j.bcp.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 113.Kimoto S, Bazmi HH, Lewis DA. Lower expression of glutamic acid decarboxylase 67 in the prefrontal cortex in schizophrenia: contribution of altered regulation by Zif268. Am J Psychiatry. 2014;171:969–978. doi: 10.1176/appi.ajp.2014.14010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Glausier JR, Kimoto S, Fish KN, Lewis DA. Lower glutamic acid decarboxylase 65-kDa isoform messenger RNA and protein levels in the prefrontal cortex in schizoaffective disorder but not schizophrenia. Biol Psychiatry. 2015;77:167–176. doi: 10.1016/j.biopsych.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40:190–206. doi: 10.1038/npp.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oliveira CR, Agostinho P, Caseiro P, Duarte CB, Carvalho AP. Reactive oxygen species on GABA release. Ann N Y Acad Sci. 1994;738:130–140. doi: 10.1111/j.1749-6632.1994.tb21798.x. [DOI] [PubMed] [Google Scholar]
- 117.Palmeira CM, Santos MS, Carvalho AP, Oliveira CR. Membrane lipid peroxidation induces changes in gamma-[3H]aminobutyric acid transport and calcium uptake by synaptosomes. Brain Res. 1993;609:117–123. doi: 10.1016/0006-8993(93)90863-i. [DOI] [PubMed] [Google Scholar]
- 118.Agostinho P, Duarte CB, Carvalho AP, Oliveira CR. Effect of oxidative stress on the release of [3H]GABA in cultured chick retina cells. Brain Res. 1994;655:213–221. doi: 10.1016/0006-8993(94)91616-0. [DOI] [PubMed] [Google Scholar]
- 119.Swaiman KF, Machen VL. The effect of iron on mammalian cortical neurons in culture. Neurochem Res. 1985;10:1261–1268. doi: 10.1007/BF00964844. [DOI] [PubMed] [Google Scholar]
- 120.Zhang ZH, Zuo QH, Wu XR. Effects of lipid peroxidation on GABA uptake and release in iron-induced seizures. Chin Med J (Engl) 1989;102:24–27. [PubMed] [Google Scholar]
- 121.Shiota A, Hiramatsu M, Mori A. Amino acid neurotransmitters in iron-induced epileptic foci of rats. Res Commun Chem Pathol Pharmacol. 1989;66:123–133. [PubMed] [Google Scholar]
- 122.Braughler JM. Lipid peroxidation-induced inhibition of gamma-aminobutyric acid uptake in rat brain synaptosomes: protection by glucocorticoids. J Neurochem. 1985;44:1282–1288. doi: 10.1111/j.1471-4159.1985.tb08755.x. [DOI] [PubMed] [Google Scholar]
- 123.Chan PH, Kerlan R, Fishman RA. Reductions of gamma-aminobutyric acid and glutamate uptake and (Na+ + K+)-ATPase activity in brain slices and synaptosomes by arachidonic acid. J Neurochem. 1983;40:309–316. doi: 10.1111/j.1471-4159.1983.tb11284.x. [DOI] [PubMed] [Google Scholar]
- 124.Carlson ES, Tkac I, Magid R, O'Connor MB, Andrews NC, et al. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr. 2009;139:672–679. doi: 10.3945/jn.108.096354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pisansky MT, Wickham RJ, Su J, Fretham S, Yuan L-L, et al. Iron Deficiency with or without Anemia Impairs Prepulse Inhibition of the Startle Reflex. Hippocampus. 2013;23:952–962. doi: 10.1002/hipo.22151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Strause LG, Hegenauer J, Saltman P, Cone R, Resnick D. Effects of longterm dietary manganese and copper deficiency on rat skeleton. J Nutr. 1986;116:135–141. doi: 10.1093/jn/116.1.135. [DOI] [PubMed] [Google Scholar]
- 127.Taylor PN, Patterson HH, Klimis-Tavantzis DJ. Manganese deficiency alters high-density lipoprotein subclass structure in the sprague-dawley rat. The Journal of Nutritional Biochemistry. 1996;7:392–396. [Google Scholar]
- 128.Zidenberg-Cherr S, Keen CL, Lonnerdal B, Hurley LS. Superoxide dismutase activity and lipid peroxidation in the rat: developmental correlations affected by manganese deficiency. J Nutr. 1983;113:2498–2504. doi: 10.1093/jn/113.12.2498. [DOI] [PubMed] [Google Scholar]
- 129.Erikson KM, Aschner M. Manganese neurotoxicity and glutamate-GABA interaction. Neurochem Int. 2003;43:475–480. doi: 10.1016/s0197-0186(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 130.Wedler FC, Denman RB. Glutamine synthetase: the major Mn(II) enzyme in mammalian brain. Curr Top Cell Regul. 1984;24:153–169. doi: 10.1016/b978-0-12-152824-9.50021-6. [DOI] [PubMed] [Google Scholar]
- 131.Yanik M, Vural H, Tutkun H, Zoroglu SS, Savas HA, et al. The role of the arginine-nitric oxide pathway in the pathogenesis of bipolar affective disorder. Eur Arch Psychiatry Clin Neurosci. 2004;254:43–47. doi: 10.1007/s00406-004-0453-x. [DOI] [PubMed] [Google Scholar]
- 132.Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M. Manganese Is Essential for Neuronal Health. Annu Rev Nutr. 2015;35:71–108. doi: 10.1146/annurev-nutr-071714-034419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gonzalez-Reyes RE, Gutierrez-Alvarez AM, Moreno CB. Manganese and epilepsy: a systematic review of the literature. Brain Res Rev. 2007;53:332–336. doi: 10.1016/j.brainresrev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 134.Hurley LS, Woolley DE, Rosenthal F, Timiras PS. Influence of manganese on susceptibility of rats to convulsions. Am J Physiol. 1963;204:493–496. doi: 10.1152/ajplegacy.1963.204.3.493. [DOI] [PubMed] [Google Scholar]
- 135.Rosas HD, Chen YI, Doros G, Salat DH, Chen NK, et al. Alterations in brain transition metals in Huntington disease: an evolving and intricate story. Arch Neurol. 2012;69:887–893. doi: 10.1001/archneurol.2011.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pagano G, Talamanca AA, Castello G, Cordero MD, d'Ischia M, et al. Oxidative stress and mitochondrial dysfunction across broad-ranging pathologies: toward mitochondria-targeted clinical strategies. Oxid Med Cell Longev. 2014;2014:541230. doi: 10.1155/2014/541230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Park C, Park SK. Molecular links between mitochondrial dysfunctions and schizophrenia. Mol Cells. 2012;33:105–110. doi: 10.1007/s10059-012-2284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Flynn JM, Melov S. SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic Biol Med. 2013;62:4–12. doi: 10.1016/j.freeradbiomed.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vural H, Sirin B, Yilmaz N, Eren I, Delibas N. The role of arginine-nitric oxide pathway in patients with Alzheimer disease. Biol Trace Elem Res. 2009;129:58–64. doi: 10.1007/s12011-008-8291-8. [DOI] [PubMed] [Google Scholar]
- 140.Au C, Benedetto A, Aschner M. Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology. 2008;29:569–576. doi: 10.1016/j.neuro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Madejczyk MS, Ballatori N. The iron transporter ferroportin can also function as a manganese exporter. Biochim Biophys Acta. 2012;1818:651–657. doi: 10.1016/j.bbamem.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Leyva-Illades D, Chen P, Zogzas CE, Hutchens S, Mercado JM. SLC30A10 is a cell surface-localized manganese efflux transporter, and parkinsonism-causing mutations block its intracellular trafficking and efflux activity. 2014;34:14079–14095. doi: 10.1523/JNEUROSCI.2329-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Martinez-Finley EJ, Gavin CE, Aschner M, Gunter TE. Manganese neurotoxicity and the role of reactive oxygen species. Free Radic Biol Med. 2013;62:65–75. doi: 10.1016/j.freeradbiomed.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen P, Chakraborty S, Mukhopadhyay S, Lee E, Paoliello MM, et al. Manganese homeostasis in the nervous system. J Neurochem. 2015;134:601–610. doi: 10.1111/jnc.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cotzias GC. Manganese in health and disease. Physiol Rev. 1958;38:503–532. doi: 10.1152/physrev.1958.38.3.503. [DOI] [PubMed] [Google Scholar]
- 146.Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect. 2007;115:122–127. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bouchard M, Mergler D, Baldwin ME, Panisset M. Manganese cumulative exposure and symptoms: a follow-up study of alloy workers. Neurotoxicology. 2008;29:577–583. doi: 10.1016/j.neuro.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 148.Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, et al. Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27:315–326. doi: 10.1016/j.neuro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 149.Huang CC, Lu CS, Chu NS, Hochberg F, Lilienfeld D, et al. Progression after chronic manganese exposure. Neurology. 1993;43:1479–1483. doi: 10.1212/wnl.43.8.1479. [DOI] [PubMed] [Google Scholar]
- 150.Lucchini R, Apostoli P, Perrone C, Placidi D, Albini E, et al. Long-term exposure to "low levels" of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicology. 1999;20:287–297. [PubMed] [Google Scholar]
- 151.Claus Henn B, Ettinger AS, Schwartz J, Tellez-Rojo MM, Lamadrid-Figueroa H, et al. Early postnatal blood manganese levels and children's neurodevelopment. Epidemiology. 2010;21:433–439. doi: 10.1097/ede.0b013e3181df8e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mergler D, Baldwin M, Belanger S, Larribe F, Beuter A, et al. Manganese neurotoxicity, a continuum of dysfunction: results from a community based study. Neurotoxicology. 1999;20:327–342. [PubMed] [Google Scholar]
- 153.Barbeau A. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias) Neurotoxicology. 1984;5:13–35. [PubMed] [Google Scholar]
- 154.Donaldson J. The physiopathologic significance of manganese in brain: its relation to schizophrenia and neurodegenerative disorders. Neurotoxicology. 1987;8:451–462. [PubMed] [Google Scholar]
- 155.Aschner M, Guilarte TR, Schneider JS, Zheng W. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol. 2007;221:131–147. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ericson JE, Crinella FM, Clarke-Stewart KA, Allhusen VD, Chan T, et al. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicol Teratol. 2007;29:181–187. doi: 10.1016/j.ntt.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 157.Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology. 2003;24:667–674. doi: 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 158.Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology. 2006;27:210–216. doi: 10.1016/j.neuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 159.Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, et al. Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2006;114:124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bertinchamps AJ, Miller ST, Cotzias GC. Interdependence of routes excreting manganese. Am J Physiol. 1966;211:217–224. doi: 10.1152/ajplegacy.1966.211.1.217. [DOI] [PubMed] [Google Scholar]
- 161.Brenneman KA, Wong BA, Buccellato MA, Costa ER, Gross EA, et al. Direct olfactory transport of inhaled manganese ((54)MnCl(2)) to the rat brain: toxicokinetic investigations in a unilateral nasal occlusion model. Toxicol Appl Pharmacol. 2000;169:238–248. doi: 10.1006/taap.2000.9073. [DOI] [PubMed] [Google Scholar]
- 162.Collipp PJ, Chen SY, Maitinsky S. Manganese in infant formulas and learning disability. Ann Nutr Metab. 1983;27:488–494. doi: 10.1159/000176724. [DOI] [PubMed] [Google Scholar]
- 163.Benko CR, Cordeiro ML, Costa MT, Cunha A, Farias AC, et al. Manganese in children with attention-deficit/hyperactivity disorder: relationship with methylphenidate exposure. Journal of Child and Adolescent Psychopharmacology. 2010;20:113+. doi: 10.1089/cap.2009.0073. [DOI] [PubMed] [Google Scholar]
- 164.Hong SB, Kim JW, Choi BS, Hong YC, Park EJ, et al. Blood manganese levels in relation to comorbid behavioral and emotional problems in children with attention-deficit/hyperactivity disorder. Psychiatry Res. 2014;220:418–425. doi: 10.1016/j.psychres.2014.05.049. [DOI] [PubMed] [Google Scholar]
- 165.Khan K, Factor-Litvak P, Wasserman GA, Liu X, Ahmed E, et al. Manganese Exposure from Drinking Water and Children’s Classroom Behavior in Bangladesh. Environmental Health Perspectives. 2011;119:1501–1506. doi: 10.1289/ehp.1003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ericson JE, Crinella FM, Clarke-Stewart KA, Allhusen VD, Chan T, et al. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicology and Teratology. 2007;29:181–187. doi: 10.1016/j.ntt.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 167.Kern CH, Smith DR. Preweaning Mn exposure leads to prolonged astrocyte activation and lasting effects on the dopaminergic system in adult male rats. Synapse. 2011;65:532–544. doi: 10.1002/syn.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim Y, Kim JM, Kim JW, Yoo CI, Lee CR, et al. Dopamine transporter density is decreased in parkinsonian patients with a history of manganese exposure: what does it mean? Mov Disord. 2002;17:568–575. doi: 10.1002/mds.10089. [DOI] [PubMed] [Google Scholar]
- 169.Chen MK, Lee JS, McGlothan JL, Furukawa E, Adams RJ, et al. Acute manganese administration alters dopamine transporter levels in the non-human primate striatum. Neurotoxicology. 2006;27:229–236. doi: 10.1016/j.neuro.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 170.Kern C, Stanwood G, Smith DR. Pre-weaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse (New York, NY) 2010;64:363–378. doi: 10.1002/syn.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tran TT, Chowanadisai W, Lönnerdal B, Le L, Parker M, et al. Effects of Neonatal Dietary Manganese Exposure on Brain Dopamine Levels and Neurocognitive Functions. NeuroToxicology. 2002;23:645–651. doi: 10.1016/s0161-813x(02)00068-2. [DOI] [PubMed] [Google Scholar]
- 172.Erikson KM, Shihabi ZK, Aschner JL, Aschner M. Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol Trace Elem Res. 2002;87:143–156. doi: 10.1385/BTER:87:1-3:143. [DOI] [PubMed] [Google Scholar]
- 173.Garcia SJ, Gellein K, Syversen T, Aschner M. A manganese-enhanced diet alters brain metals and transporters in the developing rat. Toxicol Sci. 2006;92:516–525. doi: 10.1093/toxsci/kfl017. [DOI] [PubMed] [Google Scholar]
- 174.Finkelstein Y, Milatovic D, Aschner M. Modulation of cholinergic systems by manganese. NeuroToxicology. 2007;28:1003–1014. doi: 10.1016/j.neuro.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 175.Erikson K, Aschner M. Manganese causes differential regulation of glutamate transporter (GLAST) taurine transporter and metallothionein in cultured rat astrocytes. Neurotoxicology. 2002;23:595–602. doi: 10.1016/s0161-813x(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 176.Verity MA. Manganese neurotoxicity: a mechanistic hypothesis. Neurotoxicology. 1999;20:489–497. [PubMed] [Google Scholar]
- 177.Henriksson J, Tjalve H. Manganese taken up into the CNS via the olfactory pathway in rats affects astrocytes. Toxicol Sci. 2000;55:392–398. doi: 10.1093/toxsci/55.2.392. [DOI] [PubMed] [Google Scholar]
- 178.Aschner M, Gannon M, Kimelberg HK. Manganese uptake and efflux in cultured rat astrocytes. J Neurochem. 1992;58:730–735. doi: 10.1111/j.1471-4159.1992.tb09778.x. [DOI] [PubMed] [Google Scholar]
- 179.Morello M, Canini A, Mattioli P, Sorge RP, Alimonti A, et al. Sub-cellular localization of manganese in the basal ganglia of normal and manganese-treated rats An electron spectroscopy imaging and electron energy-loss spectroscopy study. Neurotoxicology. 2008;29:60–72. doi: 10.1016/j.neuro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 180.Milatovic D, Yin Z, Gupta RC, Sidoryk M, Albrecht J, et al. Manganese induces oxidative impairment in cultured rat astrocytes. Toxicol Sci. 2007;98:198–205. doi: 10.1093/toxsci/kfm095. [DOI] [PubMed] [Google Scholar]
- 181.Prabhakaran K, Ghosh D, Chapman GD, Gunasekar PG. Molecular mechanism of manganese exposure-induced dopaminergic toxicity. Brain Research Bulletin. 2008;76:361–367. doi: 10.1016/j.brainresbull.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 182.Antonini JM, Sriram K, Benkovic SA, Roberts JR, Stone S, et al. Mild steel welding fume causes manganese accumulation and subtle neuroinflammatory changes but not overt neuronal damage in discrete brain regions of rats after short-term inhalation exposure. Neurotoxicology. 2009;30:915–925. doi: 10.1016/j.neuro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 183.Liu X, Sullivan KA, Madl JE, Legare M, Tjalkens RB. Manganese-induced neurotoxicity: the role of astroglial-derived nitric oxide in striatal interneuron degeneration. Toxicol Sci. 2006;91:521–531. doi: 10.1093/toxsci/kfj150. [DOI] [PubMed] [Google Scholar]
- 184.Moreno JA, Streifel KM, Sullivan KA, Legare ME, Tjalkens RB. Developmental exposure to manganese increases adult susceptibility to inflammatory activation of glia and neuronal protein nitration. Toxicol Sci. 2009;112:405–415. doi: 10.1093/toxsci/kfp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dodd CA, Filipov NM. Manganese Potentiates LPS-Induced Heme- Oxygenase 1 in Microglia but not Dopaminergic Cells: Role in Controlling Microglial Hydrogen Peroxide and Inflammatory Cytokine Output. Neurotoxicology. 2011;32:683–692. doi: 10.1016/j.neuro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ye Q, Kim J. Loss of hfe function reverses impaired recognition memory caused by olfactory manganese exposure in mice. Toxicol Res. 2015;31:17–23. doi: 10.5487/TR.2015.31.1.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chua AC, Morgan EH. Manganese metabolism is impaired in the Belgrade laboratory rat. J Comp Physiol B. 1997;167:361–369. doi: 10.1007/s003600050085. [DOI] [PubMed] [Google Scholar]
- 188.Erikson KM, Aschner M. Increased manganese uptake by primary astrocyte cultures with altered iron status is mediated primarily by divalent metal transporter. Neurotoxicology. 2006;27:125–130. doi: 10.1016/j.neuro.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 189.Crossgrove JS, Yokel RA. Manganese distribution across the blood-brain barrier III. The divalent metal transporter-1 is not the major mechanism mediating brain manganese uptake. Neurotoxicology. 2004;25:451–460. doi: 10.1016/j.neuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 190.Aschner M, Gannon M. Manganese (Mn) transport across the rat blood-brain barrier: Saturable and transferrin-dependent transport mechanisms. Brain Research Bulletin. 1994;33:345–349. doi: 10.1016/0361-9230(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 191.Li GJ, Choi BS, Wang X, Liu J, Waalkes MP, et al. Molecular mechanism of distorted iron regulation in the blood-CSF barrier and regional blood-brain barrier following in vivo subchronic manganese exposure. Neurotoxicology. 2006;27:737–744. doi: 10.1016/j.neuro.2006.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Toscano CD, Guilarte TR. Lead neurotoxicity: from exposure to molecular effects. Brain Res Brain Res Rev. 2005;49:529–554. doi: 10.1016/j.brainresrev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 193.Levin R, Brown MJ, Kashtock ME, Jacobs DE, Whelan EA, et al. Lead exposures in U.S. Children, 2008: implications for prevention. Environ Health Perspect. 2008;116:1285–1293. doi: 10.1289/ehp.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Meyer PA, Brown MJ, Falk H. Global approach to reducing lead exposure and poisoning. Mutat Res. 2008;659:166–175. doi: 10.1016/j.mrrev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 195.Hu H, Shine J, Wright RO. The challenge posed to children's health by mixtures of toxic waste: the Tar Creek superfund site as a case-study. Pediatr Clin North Am. 2007;54:155–175. doi: 10.1016/j.pcl.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schwartz BS, Stewart WF, Bolla KI, Simon PD, Bandeen-Roche K, et al. Past adult lead exposure is associated with longitudinal decline in cognitive function. Neurology. 2000;55:1144–1150. doi: 10.1212/wnl.55.8.1144. [DOI] [PubMed] [Google Scholar]
- 197.Bushnell PJ, Bowman RE. Effects of chronic lead ingestion on social development in infant rhesus monkeys. Neurobehav Toxicol. 1979;1:207–219. [PubMed] [Google Scholar]
- 198.Chen T, Li YY, Zhang JL, Xu B, Lin Y, et al. Protective effect of C(60) - methionine derivate on lead-exposed human SH-SY5Y neuroblastoma cells. J Appl Toxicol. 2011;31:255–261. doi: 10.1002/jat.1588. [DOI] [PubMed] [Google Scholar]
- 199.Tavakoli-Nezhad M, Barron AJ, Pitts DK. Postnatal inorganic lead exposure decreases the number of spontaneously active midbrain dopamine neurons in the rat. Neurotoxicology. 2001;22:259–269. doi: 10.1016/s0161-813x(01)00010-9. [DOI] [PubMed] [Google Scholar]
- 200.Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, et al. Alzheimer's disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci. 2008;28:3–9. doi: 10.1523/JNEUROSCI.4405-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li YY, Chen T, Wan Y, Xu SQ. Lead exposure in pheochromocytoma cells induces persistent changes in amyloid precursor protein gene methylation patterns. Environ Toxicol. 2012;27:495–502. doi: 10.1002/tox.20666. [DOI] [PubMed] [Google Scholar]
- 202.Basha MR, Wei W, Bakheet SA, Benitez N, Siddiqi HK, et al. The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J Neurosci. 2005;25:823–829. doi: 10.1523/JNEUROSCI.4335-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Needleman H. Lead poisoning. Annu Rev Med. 2004;55:209–222. doi: 10.1146/annurev.med.55.091902.103653. [DOI] [PubMed] [Google Scholar]
- 204.Roy A, Bellinger D, Hu H, Schwartz J, Ettinger AS, et al. Lead exposure and behavior among young children in Chennai, India. Environ Health Perspect. 2009;117:1607–1611. doi: 10.1289/ehp.0900625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. Bone lead levels and delinquent behavior. Jama. 1996;275:363–369. [PubMed] [Google Scholar]
- 206.Sciarillo WG, Alexander G, Farrell KP. Lead exposure and child behavior. American Journal of Public Health. 1992;82:1356–1360. doi: 10.2105/ajph.82.10.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mendelsohn AL, Dreyer BP, Fierman AH, Rosen CM, Legano LA, et al. Low-level lead exposure and behavior in early childhood. Pediatrics. 1998;101:E10. doi: 10.1542/peds.101.3.e10. [DOI] [PubMed] [Google Scholar]
- 208.Kasten-Jolly J, Pabello N, Bolivar VJ, Lawrence DA. Developmental lead effects on behavior and brain gene expression in male and female BALB/cAnNTac mice. Neurotoxicology. 2012;33:1005–1020. doi: 10.1016/j.neuro.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nation JR, Baker DM, Taylor B, Clark DE. Dietary lead increases ethanol consumption in the rat. Behav Neurosci. 1986;100:525–530. doi: 10.1037//0735-7044.100.4.525. [DOI] [PubMed] [Google Scholar]
- 210.Struzynska L, Walski M, Gadamski R, Dabrowska-Bouta B, Rafalowska U. Lead-induced abnormalities in blood-brain barrier permeability in experimental chronic toxicity. Mol Chem Neuropathol. 1997;31:207–224. doi: 10.1007/BF02815125. [DOI] [PubMed] [Google Scholar]
- 211.Dingwall-Fordyce I, Lane RE. A Follow-up Study of Lead Workers. British Journal of Industrial Medicine. 1963;20:313–315. doi: 10.1136/oem.20.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang Q, Luo W, Zheng W, Liu Y, Xu H, et al. Iron supplement prevents lead-induced disruption of the blood-brain barrier during rat development. Toxicol Appl Pharmacol. 2007;219:33–41. doi: 10.1016/j.taap.2006.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dabrowska-Bouta B, Sulkowski G, Walski M, Struzynska L, Lenkiewicz A, et al. Acute lead intoxication in vivo affects myelin membrane morphology and CNPase activity. Exp Toxicol Pathol. 2000;52:257–263. doi: 10.1016/S0940-2993(00)80043-3. [DOI] [PubMed] [Google Scholar]
- 214.Hu F, Xu L, Liu Z-H, Ge M-M, Ruan D-Y, et al. Developmental Lead Exposure Alters Synaptogenesis through Inhibiting Canonical Wnt Pathway In Vivo and In Vitro. PLoS ONE. 2014;9:e101894. doi: 10.1371/journal.pone.0101894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hermes-Lima M, Pereira B, Bechara EJ. Are free radicals involved in lead poisoning? Xenobiotica. 1991;21:1085–1090. doi: 10.3109/00498259109039548. [DOI] [PubMed] [Google Scholar]
- 216.Mateo R, Beyer WN, Spann JW, Hoffman DJ, Ramis A. Relationship between oxidative stress, pathology, and behavioral signs of lead poisoning in mallards. J Toxicol Environ Health A. 2003;66:1371–1389. doi: 10.1080/15287390306390. [DOI] [PubMed] [Google Scholar]
- 217.Bielarczyk H, Tomsig JL, Suszkiw JB. Perinatal low-level lead exposure and the septo-hippocampal cholinergic system: selective reduction of muscarinic receptors and cholineacetyltransferase in the rat septum. Brain Res. 1994;643:211–217. doi: 10.1016/0006-8993(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 218.Cory-Slechta DA. Relationships between Pb-induced changes in neurotransmitter system function and behavioral toxicity. Neurotoxicology. 1997;18:673–688. [PubMed] [Google Scholar]
- 219.Finkelstein Y, Markowitz ME, Rosen JF. Low-level lead-induced neurotoxicity in children: an update on central nervous system effects. Brain Res Brain Res Rev. 1998;27:168–176. doi: 10.1016/s0165-0173(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 220.Ballatori N. Transport of toxic metals by molecular mimicry. Environmental Health Perspectives. 2002;110:689–694. doi: 10.1289/ehp.02110s5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Vazquez M, Velez D, Devesa V, Puig S. Participation of divalent cation transporter DMT1 in the uptake of inorganic mercury. Toxicology. 2015;331:119–124. doi: 10.1016/j.tox.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 222.D IB, Portnoy ME, Olivi L, Lees PS, Culotta VC, et al. Uptake of lead and iron by divalent metal transporter 1 in yeast and mammalian cells. Biochem Biophys Res Commun. 2002;295:978–984. doi: 10.1016/s0006-291x(02)00756-8. [DOI] [PubMed] [Google Scholar]
- 223.Garrick MD, Singleton ST, Vargas F, Kuo HC, Zhao L, et al. DMT1: which metals does it transport? Biol Res. 2006;39:79–85. doi: 10.4067/s0716-97602006000100009. [DOI] [PubMed] [Google Scholar]
- 224.Crowe A, Morgan EH. Interactions between tissue uptake of lead and iron in normal and iron-deficient rats during development. Biol Trace Elem Res. 1996;52:249–261. doi: 10.1007/BF02789166. [DOI] [PubMed] [Google Scholar]
- 225.Bradman A, Eskenazi B, Sutton P, Athanasoulis M, Goldman LR. Iron deficiency associated with higher blood lead in children living in contaminated environments. Environ Health Perspect. 2001;109:1079–1084. doi: 10.1289/ehp.011091079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang Q, Luo W, Zhang W, Dai Z, Chen Y, et al. Iron supplementation protects against lead-induced apoptosis through MAPK pathway in weanling rat cortex. Neurotoxicology. 2007;28:850–859. doi: 10.1016/j.neuro.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 227.Kerper LE, Hinkle PM. Lead uptake in brain capillary endothelial cells: activation by calcium store depletion. Toxicol Appl Pharmacol. 1997;146:127–133. doi: 10.1006/taap.1997.8234. [DOI] [PubMed] [Google Scholar]
- 228.Kerper LE, Hinkle PM. Cellular uptake of lead is activated by depletion of intracellular calcium stores. J Biol Chem. 1997;272:8346–8352. doi: 10.1074/jbc.272.13.8346. [DOI] [PubMed] [Google Scholar]
- 229.Legare ME, Barhoumi R, Hebert E, Bratton GR, Burghardt RC, et al. Analysis of Pb2+ entry into cultured astroglia. Toxicol Sci. 1998;46:90–100. doi: 10.1006/toxs.1998.2492. [DOI] [PubMed] [Google Scholar]
- 230.Markovac J, Goldstein GW. Picomolar concentrations of lead stimulate brain protein kinase C. Nature. 1988;334:71–73. doi: 10.1038/334071a0. [DOI] [PubMed] [Google Scholar]
- 231.Cheong JH, Bannon D, Olivi L, Kim Y, Bressler J. Different mechanisms mediate uptake of lead in a rat astroglial cell line. Toxicol Sci. 2004;77:334–340. doi: 10.1093/toxsci/kfh024. [DOI] [PubMed] [Google Scholar]
- 232.Ahamed M, Siddiqui MK. Environmental lead toxicity and nutritional factors. Clin Nutr. 2007;26:400–408. doi: 10.1016/j.clnu.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 233.Waalkes MP. Cadmium carcinogenesis in review. Journal of Inorganic Biochemistry. 2000;79:241–244. doi: 10.1016/s0162-0134(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 234.Pesch B, Haerting J, Ranft U, Klimpel A, Oelschlagel B, et al. Occupational risk factors for renal cell carcinoma: agent-specific results from a case-control study in Germany. MURC Study Group. Multicenter urothelial and renal cancer study. Int J Epidemiol. 2000;29:1014–1024. doi: 10.1093/ije/29.6.1014. [DOI] [PubMed] [Google Scholar]
- 235.Buchet JP, Lauwerys R, Roels H, Bernard A, Bruaux P, et al. Renal effects of cadmium body burden of the general population. Lancet. 1990;336:699–702. doi: 10.1016/0140-6736(90)92201-r. [DOI] [PubMed] [Google Scholar]
- 236.Henson MC, Chedrese PJ. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp Biol Med (Maywood) 2004;229:383–392. doi: 10.1177/153537020422900506. [DOI] [PubMed] [Google Scholar]
- 237.Schroeder HA. Cadmium Hypertension in Rats. Am J Physiol. 1964;207:62–66. doi: 10.1152/ajplegacy.1964.207.1.62. [DOI] [PubMed] [Google Scholar]
- 238.Lehotzky K, Ungvary G, Polinak D, Kiss A. Behavioral deficits due to prenatal exposure to cadmium chloride in CFY rat pups. Neurotoxicol Teratol. 1990;12:169–172. doi: 10.1016/0892-0362(90)90130-5. [DOI] [PubMed] [Google Scholar]
- 239.Blaurock-Busch E, Amin OR, Rabah T. Heavy metals and trace elements in hair and urine of a sample of arab children with autistic spectrum disorder. Maedica (Buchar) 2011;6:247–257. [PMC free article] [PubMed] [Google Scholar]
- 240.Al-Ayadhi LY. Heavy metals and trace elements in hair samples of autistic children in central Saudi Arabia. Neurosciences (Riyadh) 2005;10:213–218. [PubMed] [Google Scholar]
- 241.Vergani L, Cristina L, Paola R, Luisa AM, Shyti G, et al. Metals, metallothioneins and oxidative stress in blood of autistic children. Research in Autism Spectrum Disorders. 2011;5:286–293. [Google Scholar]
- 242.Thatcher RW, McAlaster R, Lester ML. Evoked potentials related to hair cadmium and lead in children. Ann N Y Acad Sci. 1984;425:384–390. doi: 10.1111/j.1749-6632.1984.tb23560.x. [DOI] [PubMed] [Google Scholar]
- 243.Marlowe M, Errera J, Jacobs J. Increased lead and cadmium burdens among mentally retarded children and children with borderline intelligence. Am J Ment Defic. 1983;87:477–483. [PubMed] [Google Scholar]
- 244.Capel ID, Pinnock MH, Dorrell HM, Williams DC, Grant EC. Comparison of concentrations of some trace, bulk, and toxic metals in the hair of normal and dyslexic children. Clin Chem. 1981;27:879–881. [PubMed] [Google Scholar]
- 245.Miller DK, Nation JR. Chronic cadmium exposure attenuates the conditioned reinforcing properties of morphine and fentanyl. Brain Res. 1997;776:162–169. doi: 10.1016/s0006-8993(97)01013-5. [DOI] [PubMed] [Google Scholar]
- 246.Nation JR, Pugh CK, Von Stultz J, Bratton GR, Clark DE. The effects of cadmium on the self-administration of ethanol and an isocaloric/isohedonic equivalent. Neurotoxicol Teratol. 1989;11:509–514. doi: 10.1016/0892-0362(89)90027-5. [DOI] [PubMed] [Google Scholar]
- 247.Nation JR, Livermore CL, Bratton GR, Schenk S. Chronic cadmium exposure alters cocaine self-administration in adult male rats. Experimental and Clinical Psychopharmacology. 1996;4:264–270. [Google Scholar]
- 248.Gonçalves JF, Fiorenza AM, Spanevello RM, Mazzanti CM, Bochi GV, et al. N-acetylcysteine prevents memory deficits, the decrease in acetylcholinesterase activity and oxidative stress in rats exposed to cadmium. Chemico-Biological Interactions. 2010;186:53–60. doi: 10.1016/j.cbi.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 249.López E, Arce C, Oset-Gasque MJ, Cañadas S, González MP. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radical Biology and Medicine. 2006;40:940–951. doi: 10.1016/j.freeradbiomed.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 250.Tobwala S, Wang H-J, Carey J, Banks W, Ercal N. Effects of Lead and Cadmium on Brain Endothelial Cell Survival, Monolayer Permeability, and Crucial Oxidative Stress Markers in an in Vitro Model of the Blood-Brain Barrier. Toxics. 2014;2:258. [Google Scholar]
- 251.Wong K-L, Klaassen CD. Neurotoxic effects of cadmium in young rats. Toxicology and Applied Pharmacology. 1982;63:330–337. doi: 10.1016/0041-008x(82)90261-7. [DOI] [PubMed] [Google Scholar]
- 252.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 253.Shukla A, Shukla GS, Srimal RC. Cadmium-induced alterations in blood-brain barrier permeability and its possible correlation with decreased microvessel antioxidant potential in rat. Hum Exp Toxicol. 1996;15:400–405. doi: 10.1177/096032719601500507. [DOI] [PubMed] [Google Scholar]
- 254.Ishitobi H, Watanabe C. Effects of low-dose perinatal cadmium exposure on tissue zinc and copper concentrations in neonatal mice and on the reproductive development of female offspring. Toxicology Letters. 2005;159:38–46. doi: 10.1016/j.toxlet.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 255.Yuan Y, Jiang CY, Xu H, Sun Y, Hu FF, et al. Cadmium-induced apoptosis in primary rat cerebral cortical neurons culture is mediated by a calcium signaling pathway. PLoS One. 2013;8:e64330. doi: 10.1371/journal.pone.0064330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Gupta A, Murthy RC, Thakur SR, Dubey MP, Chandra SV. Comparative neurotoxicity of cadmium in growing and adult rats after repeated administration. Biochem Int. 1990;21:97–105. [PubMed] [Google Scholar]
- 257.Lafuente A, Gonzalez-Carracedo A, Romero A, Esquifino AI. Effect of cadmium on 24-h variations in hypothalamic dopamine and serotonin metabolism in adult male rats. Exp Brain Res. 2003;149:200–206. doi: 10.1007/s00221-002-1356-6. [DOI] [PubMed] [Google Scholar]
- 258.Tallkvist J, Bowlus CL, Lonnerdal B. DMT1 gene expression and cadmium absorption in human absorptive enterocytes. Toxicol Lett. 2001;122:171–177. doi: 10.1016/s0378-4274(01)00363-0. [DOI] [PubMed] [Google Scholar]
- 259.Bannon DI, Abounader R, Lees PSJ, Bressler JP. Effect of DMT1 knockdown on iron, cadmium, and lead uptake in Caco-2 cells. 2003:C44–C50. doi: 10.1152/ajpcell.00184.2002. [DOI] [PubMed] [Google Scholar]
- 260.Ryu D-Y, Lee S-J, Park DW, Choi B-S, Klaassen CD, et al. Dietary iron regulates intestinal cadmium absorption through iron transporters in rats. Toxicology Letters. 2004;152:19–25. doi: 10.1016/j.toxlet.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 261.Ohrvik H, Tyden E, Artursson P, Oskarsson A, Tallkvist J. Cadmium Transport in a Model of Neonatal Intestinal Cells Correlates to MRP1 and Not DMT1 or FPN1. ISRN Toxicol. 2013;2013:892364. doi: 10.1155/2013/892364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Bondier JR, Michel G, Propper A, Badot PM. Harmful effects of cadmium on olfactory system in mice. Inhal Toxicol. 2008;20:1169–1177. doi: 10.1080/08958370802207292. [DOI] [PubMed] [Google Scholar]
- 263.Lafuente A, Esquifino AI. Cadmium effects on hypothalamic activity and pituitary hormone secretion in the male. Toxicol Lett. 1999;110:209–218. doi: 10.1016/s0378-4274(99)00159-9. [DOI] [PubMed] [Google Scholar]
- 264.Esquifino AI, Marquez N, Alvarez-Demanuel E, Lafuente A. Effects of chronic alternating cadmium exposure on the episodic secretion of prolactin in male rats. J Trace Elem Med Biol. 1999;12:205–210. doi: 10.1016/S0946-672X(99)80059-5. [DOI] [PubMed] [Google Scholar]
- 265.Gu C, Chen S, Xu X, Zheng L, Li Y, et al. Lead and cadmium synergistically enhance the expression of divalent metal transporter 1 protein in central nervous system of developing rats. Neurochem Res. 2009;34:1150–1156. doi: 10.1007/s11064-008-9891-6. [DOI] [PubMed] [Google Scholar]
- 266.Adhami VM, Husain R, Husain R, Seth PK. Influence of iron deficiency and lead treatment on behavior and cerebellar and hippocampal polyamine levels in neonatal rats. Neurochem Res. 1996;21:915–922. doi: 10.1007/BF02532341. [DOI] [PubMed] [Google Scholar]
- 267.Chandra SV, Murthy RC, Saxena DK, Lal B. Effects of pre- and postnatal combined exposure to Pb and Mn on brain development in rats. Ind Health. 1983;21:273–279. doi: 10.2486/indhealth.21.273. [DOI] [PubMed] [Google Scholar]
- 268.Betharia S, Maher TJ. Neurobehavioral effects of lead and manganese individually and in combination in developmentally exposed rats. NeuroToxicology. 2012;33:1117–1127. doi: 10.1016/j.neuro.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 269.Schumann K, Elsenhans B, Ehtechami C, Forth W. Increased intestinal iron absorption in rats with normal hepatic iron stores. Kinetic aspects of the adaptative response to parenteral iron repletion in dietary iron deficiency. Biochim Biophys Acta. 1990;1033:277–281. doi: 10.1016/0304-4165(90)90133-h. [DOI] [PubMed] [Google Scholar]
- 270.Ruivard M, Laine F, Ganz T, Olbina G, Westerman M, et al. Iron absorption in dysmetabolic iron overload syndrome is decreased and correlates with increased plasma hepcidin. J Hepatol. 2009;50:1219–1225. doi: 10.1016/j.jhep.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 271.Rossander-Hulten L, Brune M, Sandstrom B, Lonnerdal B, Hallberg L. Competitive inhibition of iron absorption by manganese and zinc in humans. Am J Clin Nutr. 1991;54:152–156. doi: 10.1093/ajcn/54.1.152. [DOI] [PubMed] [Google Scholar]
- 272.Thompson K, Molina R, Donaghey T, Brain JD, Wessling-Resnick M. The influence of high iron diet on rat lung manganese absorption. Toxicol Appl Pharmacol. 2006;210:17–23. doi: 10.1016/j.taap.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 273.Kim J, Buckett PD, Wessling-Resnick M. Absorption of manganese and iron in a mouse model of hemochromatosis. PLoS One. 2013;8:e64944. doi: 10.1371/journal.pone.0064944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Montosi G, Donovan A, Totaro A, Garuti C, Pignatti E, et al. Autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. J Clin Invest. 2001;108:619–623. doi: 10.1172/JCI13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Levy JE, Montross LK, Andrews NC. Genes that modify the hemochromatosis phenotype in mice. Journal of Clinical Investigation. 2000;105:1209–1216. doi: 10.1172/JCI9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Claus Henn B, Kim J, Wessling-Resnick M, Téllez-Rojo MM, Jayawardene I, et al. Associations of iron metabolism genes with blood manganese levels: a population-based study with validation data from animal models. Environmental Health. 2011;10:97–97. doi: 10.1186/1476-069X-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Bache CA, Lisk DJ, Doss GJ, Hoffmann D, Adams JD. Cadmium and nickel in mainstream particulates of cigarettes containing tobacco grown on a low-cadmium soil-sludge mixture. J Toxicol Environ Health. 1985;16:547–552. doi: 10.1080/15287398509530762. [DOI] [PubMed] [Google Scholar]
- 278.Whitfield JB, Zhu G, Heath AC, Powell Lw, et al. Effects of alcohol consumption on indices of iron stores and of iron stores on alcohol intake markers. Alcohol Clin Exp Res. 2001;25:1037–1045. [PubMed] [Google Scholar]
- 279.Sassine M-P, Mergler D, Bowler R, Hudnell HK. Manganese accentuates adverse mental health effects associated with alcohol use disorders. Biological Psychiatry. 2002;51:909–921. doi: 10.1016/s0006-3223(01)01350-6. [DOI] [PubMed] [Google Scholar]
- 280.Ohtake T, Saito H, Hosoki Y, Inoue M, Miyoshi S, et al. Hepcidin is down-regulated in alcohol loading. Alcohol Clin Exp Res. 2007;31:S2–S8. doi: 10.1111/j.1530-0277.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- 281.Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, et al. Alcohol Metabolism-mediated Oxidative Stress Down-regulates Hepcidin Transcription and Leads to Increased Duodenal Iron Transporter Expression. Journal of Biological Chemistry. 2006;281:22974–22982. doi: 10.1074/jbc.M602098200. [DOI] [PubMed] [Google Scholar]
- 282.Anderson ER, Taylor M, Xue X, Martin A, Moons DS, et al. The hypoxia-inducible factor-C/EBPalpha axis controls ethanol-mediated hepcidin repression. Mol Cell Biol. 2012;32:4068–4077. doi: 10.1128/MCB.00723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Johnson SR, Winkleby MA, Boyce WT, McLaughlin R, Broadwin R, et al. The association between hemoglobin and behavior problems in a sample of low-income Hispanic preschool children. J Dev Behav Pediatr. 1992;13:209–214. [PubMed] [Google Scholar]
- 284.Rao TSS, Asha MR, Ramesh BN, Rao KSJ. Understanding nutrition, depression and mental illnesses. Indian Journal of Psychiatry. 2008;50:77–82. doi: 10.4103/0019-5545.42391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ikeda Y, Tajima S, Izawa-Ishizawa Y, Kihira Y, Ishizawa K, et al. Estrogen regulates hepcidin expression via GPR30-BMP6-dependent signaling in hepatocytes. PLoS One. 2012;7:e40465. doi: 10.1371/journal.pone.0040465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yang Q, Jian J, Katz S, Abramson SB, Huang X. 17beta-Estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology. 2012;153:3170–3178. doi: 10.1210/en.2011-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Bachman E, Feng R, Travison T, Li M, Olbina G, et al. Testosterone suppresses hepcidin in men: a potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab. 2010;95:4743–4747. doi: 10.1210/jc.2010-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Guo W, Bachman E, Li M, Roy CN, Blusztajn J, et al. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell. 2013;12:280–291. doi: 10.1111/acel.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Menezes-Filho JA, de Carvalho-Vivas CF, Viana GF, Ferreira JR, Nunes LS, et al. Elevated manganese exposure and school-aged children's behavior: a gender-stratified analysis. Neurotoxicology. 2014;45:293–300. doi: 10.1016/j.neuro.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 290.Anderson DW, Pothakos K, Schneider JS. Sex and Rearing Condition Modify the Effects of Perinatal Lead Exposure on Learning and Memory. Neurotoxicology. 2012;33:985–995. doi: 10.1016/j.neuro.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.He Q, Du T, Yu X, Xie A, Song N, et al. DMT1 polymorphism and risk of Parkinson's disease. Neurosci Lett. 2011;501:128–131. doi: 10.1016/j.neulet.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 292.Massie HR, Aiello VR, Iodice AA. Changes with age in copper and superoxide dismutase levels in brains of C57BL/6J mice. Mech Ageing Dev. 1979;10:93–99. doi: 10.1016/0047-6374(79)90073-3. [DOI] [PubMed] [Google Scholar]
- 293.Markesbery WR, Ehmann WD, Alauddin M, Hossain TI. Brain trace element concentrations in aging. Neurobiol Aging. 1984;5:19–28. doi: 10.1016/0197-4580(84)90081-2. [DOI] [PubMed] [Google Scholar]
- 294.Klebe D, Krafft PR, Hoffmann C, Lekic T, Flores JJ, et al. Acute and delayed deferoxamine treatment attenuates long-term sequelae after germinal matrix hemorrhage in neonatal rats. Stroke. 2014;45:2475–2479. doi: 10.1161/STROKEAHA.114.005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Aposhian HV, Maiorino RM, Dart RC, Perry DF. Urinary excretion of meso-2,3-dimercaptosuccinic acid in human subjects. Clin Pharmacol Ther. 1989;45:520–526. doi: 10.1038/clpt.1989.67. [DOI] [PubMed] [Google Scholar]
- 296.Steinhauser S, Heinz U, Bartholomä M, Weyhermüller T, Nick H, et al. Complex Formation of ICL670 and Related Ligands with FeIII and FeII. European Journal of Inorganic Chemistry. 2004;2004:4177–4192. [Google Scholar]
- 297.Yu Y, Rahmanto YS, Richardson DR. Bp44mT: an orally active iron chelator of the thiosemicarbazone class with potent anti-tumour efficacy. British Journal of Pharmacology. 2012;165:148–166. doi: 10.1111/j.1476-5381.2011.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Siedlak SL, Casadesus G, Webber KM, Pappolla MA, Atwood CS, et al. Chronic antioxidant therapy reduces oxidative stress in a mouse model of Alzheimer's disease. Free Radic Res. 2009;43:156–164. doi: 10.1080/10715760802644694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33:2187–2199. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- 300.Grant JE, Odlaug BL, Kim SW. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2009;66:756–763. doi: 10.1001/archgenpsychiatry.2009.60. [DOI] [PubMed] [Google Scholar]
- 301.Lan J, Jiang DH. Desferrioxamine and vitamin E protect against iron and MPTP-induced neurodegeneration in mice. J Neural Transm. 1997;104:469–481. doi: 10.1007/BF01277665. [DOI] [PubMed] [Google Scholar]
- 302.Sripetchwandee J, Pipatpiboon N, Chattipakorn N, Chattipakorn S. Combined Therapy of Iron Chelator and Antioxidant Completely Restores Brain Dysfunction Induced by Iron Toxicity. PLoS ONE. 2014;9:e85115. doi: 10.1371/journal.pone.0085115. [DOI] [PMC free article] [PubMed] [Google Scholar]