Abstract
Objective
To evaluate the association between use of antiplatelet (AP) or anticoagulant (AC) drugs and retinal/subretinal hemorrhage in participants with neovascular age-related macular degeneration (AMD) in the Comparison of AMD Treatments Trials (CATT).
Design
Cohort study within CATT.
Methods
1185 CATT participants with untreated active neovascular AMD were interviewed for use of AP/AC drugs. Trained readers evaluated photographs for the presence and size of retinal/subretinal hemorrhage associated with the neovascular lesion at baseline and years 1 and 2. Associations between use of AP/AC drugs and hemorrhage were evaluated among all participants and by baseline hypertension status using Fisher exact test and multivariate logistic regression models.
Main Outcome Measures
Odds ratio for association with AP/AC use.
Results
Among 1165 participants with gradable photographs, 724 (62.1%) had retinal/subretinal hemorrhage at baseline, 84.4% of hemorrhages were ≤ 1 DA, 8.1% were 1 to 2 DA, and 7.5% were >2 DA. 608 (52.2%) participants used AP/AC drugs at baseline, including 514 (44.1%) AP only, 77 (6.6%) AC only, and 17 (1.5%) both AP and AC. Participants with retinal/subretinal hemorrhage at baseline were comparable to those without retinal/subretinal hemorrhage except that they were older (80 vs. 78 years, p<0.0001) and had lower diastolic blood pressure (74.9 vs. 76.2 mmHg, p=0.03). Retinal/subretinal hemorrhage was present in 64.5% of AP/AC users and in 59.6% of non-users (p=0.09), the adjusted odds ratio (OR) was 1.18 (95% CI: 0.91–1.51, p=0.21). Neither presence nor size of baseline retinal/subretinal hemorrhage was associated with the type, dose or duration of AP/AC use. Forty-four (4.08%) of 1078 participants had retinal/subretinal hemorrhage detected on 1-year or 2-year photographs; these hemorrhages were not associated with AP/AC use at baseline (p=0.28) or during follow-up (p=0.64). Among participants with hypertension (N=807), AP/AC use was associated with higher rate of retinal/subretinal hemorrhage at baseline (66.8% vs. 56.4%, adjusted OR=1.48, p=0.01), but not size of retinal/subretinal hemorrhage (p=0.41).
Conclusions
The majority of retinal/subretinal hemorrhages in eyes enrolled in CATT were <1 DA. Among all CATT participants, AP/AC use was not significantly associated with hemorrhage, but was significantly associated with hemorrhage in participants with hypertension.
INTRODUCTION
The number of people aged 65 years and older in the United States is increasing, expected to reach 79.7 million by 2040.1 This population shift carries with it an increased burden of age-related diseases such as cardiovascular disease (CVD) and age-related macular degeneration (AMD).2 Both antiplatelet (AP) drugs such as aspirin and anticoagulant (AC) drugs such as warfarin and clopidogrel are commonly used to treat and manage cardiovascular diseases.3 Use of these AP/AC drugs is associated with increased risk of bleeding, including intracerebral and gastrointestinal hemorrhaging.3–5 However, the effect of AP/AC drugs on ocular hemorrhage is less clear. A few studies have investigated the association of AP/AC use with ocular hemorrhage among AMD patients,6–14 but the results from these studies have been conflicting and inconclusive. Because AP/AC drugs are frequently used in older people and ocular hemorrhage is generally associated with poor vision outcome in noevascular AMD,15–17 a better understanding of the association of AP/AC use with ocular hemorrhage is important.
In this study, we sought to evaluate the association between AP/AC use and retinal/subretinal hemorrhage in the participants of the Comparisons of AMD Treatments Trials (CATT). In the CATT study, a large number of retinal/subretinal hemorrhage cases (n=608) were identified at baseline from the standard grading of color fundus photographs and detailed information on the use of AP/AC drugs was collected; providing an opportunity to study this association.
METHODS
Details on the study design and methods have been reported in our previous publications18–19 and on ClinicalTrials.gov (NCT00593450). Only the major features related to this paper are described here.
Study Participants
The institutional review board associated with each center approved the study protocol (https://www.med.upenn.edu/cpob/studies/documents/CATTManualofProceduresJan2011.pdf) and written consent was obtained from each participant. Participants from 43 clinical centers in the United States were randomized to one of the four treatment groups: (1) ranibizumab monthly; (2) bevacizumab monthly; (3) ranibizumab as needed (pro re nata, PRN); and (4) bevacizumab PRN. At 1 year, participants initially assigned to monthly treatment retained their drug assignment but were reassigned randomly to either monthly or as needed treatment. Participants initially assigned to PRN treatment retained both their drug and regimen for year 2.
The study enrollment criteria included age of 50 or older, untreated active choroidal neovascularization (CNV) due to AMD in the study eye (one eye per participant), and visual acuity between 20/25 and 20/320 on electronic visual acuity testing. The presence of active CNV, as seen on fluorescein angiography, and fluid, as seen on time-domain optical coherence tomography, located either within or below the retina or below the retinal pigment epithelium (RPE) were required to establish the presence of active CNV. The study eye was not allowed to have current vitreous hemorrhage, or diabetic retinopathy that might require medical or surgical intervention during 2-year CATT follow-up.
At enrollment, participants provided information on demographic characteristics and a medical history, including a history of cardiovascular diseases and hypertension. At baseline and follow-up visits every 4 weeks, the participants were interviewed by the study coordinator about the use of AP/AC drugs, including the name of the drug, administration dose, frequency, and the dates of use. At baseline, 1 and 2 years of follow-up, each participant had stereoscopic color photographs and fluorescein angiograms of the macula obtained using standard protocols. These images were submitted to the Fundus Photograph Reading Center for grading.
Evaluation of retinal/subretinal hemorrhage
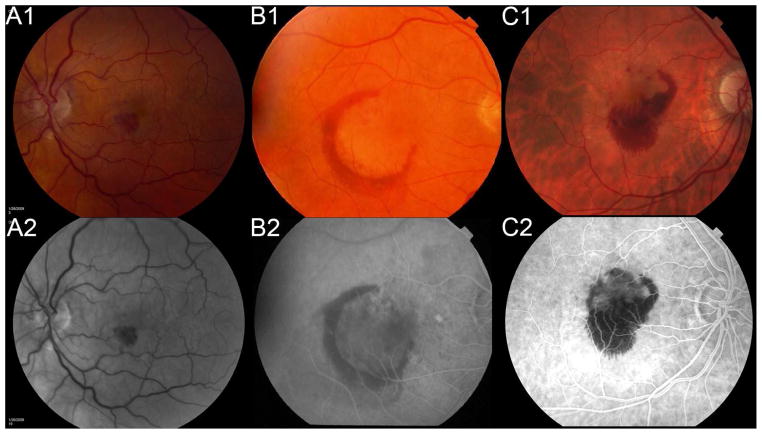
The details on the image grading for color fundus photographs and fluorescein angiogram images have been reported.20 As part of the grading of the CNV lesion characteristics, two certified graders in the CATT Fundus Reading Center, masked to the participants AP/AC use status, independently graded the baseline and follow-up photographic images on the presence and size of retinal/subretinal hemorrhage (≤1 DA, >1 to ≤2 DA, >2 DA) in the study eye (Figure 1), regardless of hemorrhage location (retinal, subretinal or sub-RPE). Discrepancies between two graders were adjudicated by graders. In cases where persisting discrepancies existed, the images were adjudicated by the Photograph Reading Center principal investigator. We previously reported the reproducibility from grading of a random sample of 84 image sets,20 the grading for presence and size of retinal/subretinal hemorrhage (none, ≤1 DA, >1 to ≤2 DA, >2 DA) had good grade-regrade agreement (percent of agreement 80%, weighted kappa 0.72 (95% CI: 0.59 – 0.86)), and inter-grader agreement (percent of agreement = 85%, weighted kappa 0.74 (95% CI: 0.63 – 0.83)).
Figure 1.
The retinal hemorrhage in color fundus photograph (top row) and fluorescein angiogram (bottom row) for size of ≤ 1DA (A), 1 – 2 DA (B), and > 2 DA (C).
Statistical Analysis/Power Consideration
Baseline hypertension was defined as systolic blood pressure at least 160 mmHg or diastolic blood pressure at least 95 mmHg or reported history of past or ongoing hypertension. The comparison of characteristics between participants with vs. without retinal/subretinal hemorrhage at baseline was performed using the independent group t-test for means, and the Fisher exact test for proportions. The association between use of AP/AC drugs and retinal/subretinal hemorrhage was assessed among all CATT participants and among subgroups based on hypertension status using the Fisher exact test, the odds ratio (OR), and its 95% confidence intervals (95% CI) from univariate and multivariate logistic regression models. In the multivariate logistic regression models, we adjusted for the same baseline covariates as a similar study 6 (age, gender, smoking status, diabetes, medical history of cardiovascular diseases and CNV in the fellow eye), so that the results can be compared between the two studies. The association between use of AP/AC drugs and the size of retinal/subretinal hemorrhage at baseline was evaluated using the chi-square test and Cochran-Armitage trend test. The association of AP/AC use with presence and size of retinal/subretinal hemorrhage was evaluated for any AP/AC use, for each specific type of AP/AC drugs, the dose and duration of AP/AC use. All data analyses were performed using SAS (v9.4, SAS Institute Inc., Cary, NC).
For this secondary data analysis, power calculation suggests that the CATT study provided at least 80% power to detect an odds ratio of 1.4 or higher risk of retinal/subretinal hemorrhage at baseline associated with AP/AC use and at least 78% power to detect an odds ratio of 2.0 or higher risk of retinal/subretinal hemorrhage during Years 1 or 2.
RESULTS
Characteristics of AP/AC use
Among 1185 CATT participants, 1165 participants with gradable fundus photographs for determination of retinal/subretinal hemorrhage were included in this study. Among these 1165 participants, 608 (52.2%) used one or more AP/AC drugs at baseline, including 514 (44.1%) with AP drugs only, 77 (6.6%) with AC drugs only, and 17 (1.5%) with both AP and AC drugs. Among 608 participants who used AP/AC drugs, the majority (87.2%) took only one AP/AC drug. Among AP/AC drugs used, the most frequently used drugs were aspirin (69.4%), warfarin (14.7%) or clopidogrel (11.4%). The median duration of AP/AC use at enrollment was 6.6 years (inter-quartile: 3.2 to 11.0 years); duration of use was <5 years for 231 (38.0%) participants, 5 to 10 years for 177 (29.1%), and over 10 years for 200 (32.9%) participants.
Retinal/subretinal hemorrhage at baseline
Among 1165 CATT participants with gradable photographs, 724 (62.1%) had retinal/subretinal hemorrhage at baseline. The majority (611 participants, 84.4%) of the retinal/subretinal hemorrhages were ≤1DA, while 59 (8.1%) were 1 to 2 DA and 54 (7.5%) were >2 DA.
The comparisons of characteristics between participants with versus without retinal/subretinal hemorrhage at baseline are reported in Table 1. Participants with retinal/subretinal hemorrhage at baseline were comparable to those without retinal/subretinal hemorrhage except that they were older (mean 80 vs. 78 years, p<0.0001) and had lower mean diastolic blood pressure (74.9 vs. 76.2 mmHg, p=0.03). They had a similar reported history of cardiovascular diseases (p=0.57), hypertension (p=0.75), diabetes (p=0.81), osteoarthritis (p=0.43), and rheumatoid arthritis (p=0.61) (Table 1).
Table 1.
Comparison of baseline characteristics between participants with vs. without retinal/subretinal hemorrhage in the study eye at baseline (N=1165)
| Baseline characteristics | Absence of retinal/subretinal hemorrhage at baseline (N=441) | Presence of retinal/subretinal hemorrhage at baseline (N=724) | P-value |
|---|---|---|---|
| Age (years) | <0.0001 | ||
| <70 | 56 (12.7%) | 79 (10.9%) | |
| 70–79 | 190 (43.1%) | 211 (29.1%) | |
| 80–89 | 177 (40.1%) | 371 (51.2%) | |
| ≥90 | 18 (4.1%) | 63 (8.7%) | |
| Mean (SD) | 78.0 (7.2) | 80.0 (7.6) | <0.0001 |
| Female (%) | 278 (63.0%) | 443 (61.2%) | 0.53 |
| Former or current cigarette smoker (%) | 264 (59.9%) | 402 (55.5%) | 0.16 |
| Taking AREDS supplement (%) | 287 (65.1%) | 448 (61.9%) | 0.29 |
| Presence of diabetes (%) | 76 (17.2%) | 129 (17.8%) | 0.81 |
| Presence of hypertension (%) | 303 (68.7%) | 504 (69.6%) | 0.75 |
| Systolic BP (mmHg): mean (SD) | 136 (18.4) | 134 (17.4) | 0.08 |
| Diastolic BP (mmHg): mean (SD) | 76 (10.0) | 75 (9.9) | 0.03 |
| History of cardiovascular diseases | 100 (22.7%) | 176 (24.3%) | 0.57 |
| Myocardial infarction | 51 (11.6%) | 87 (12.0%) | 0.85 |
| Congestive heart failure | 24 (5.4%) | 49 (6.8%) | 0.39 |
| Stroke | 23 (5.2%) | 46 (6.4%) | 0.45 |
| Transient ischemic attack | 23 (5.2%) | 44 (6.1%) | 0.60 |
| Osteoarthritis | 202 (45.8%) | 349 (48.2%) | 0.43 |
| Rheumatoid arthritis | 24 (5.44%) | 45 (6.22%) | 0.61 |
| CNV in the fellow eye | 129 (29.3%) | 216 (29.8%) | 0.84 |
SD=Standard deviation, BP=blood pressure, CNV=Choroid neovascularization.
Association between AP/AC use and retinal/subretinal hemorrhage at baseline
Table 2 shows the overall association between use of AP/AC drugs and retinal/subretinal hemorrhage at baseline. Among 608 participants who took AP/AC drugs at baseline, 64.5% had retinal/subretinal hemorrhage, as compared to 59.6% in 507 participants who did not take AP/AC drugs (p=0.09, Fisher exact test). The unadjusted odds ratio for this association is 1.23 (95% CI: 0.97–1.56, p=0.09). Adjustment by the baseline characteristics (age, gender, smoking status, medical history of CVD and CNV in the fellow eye) did not change the association (adjusted OR 1.18, 95% CI: 0.91 – 1.51, p=0.21). The association with retinal/subretinal hemorrhage was also not significant for use of AP drugs (p=0.22), use of AC drugs (p=0.18), or the combination of AP and AC drugs (p=0.24) (Table 2). When analyzed for use of each of the three most common AP/AC drugs (aspirin, warfarin, and clopidogrel), baseline retinal/subretinal hemorrhage was present in 64.5% of aspirin users, in 68.1% of warfarin users, and in 68.8% of clopidogrel users; these were not statistically different from the percentage of retinal/subretinal hemorrhage (59.6%) in the participants without use of any AP/AC drugs (all p≥0.10, Table 2).
Table 2.
The association between use of antiplatelet or anticoagulant drugs and retinal/subretinal hemorrhage at baseline
| Unadjusted Analysis | Adjusted Analysis* | |||||
|---|---|---|---|---|---|---|
| Use of anti-coagulant or anti-platelet drugs at baseline | n | Retinal/subretinal hemorrhage at baseline n (%) | Odds ratio (95% CI) | p-value | Odds ratio (95% CI)* | P-value |
| Any use of antiplatelet or anticoagulant drugs | 0.09 | 0.21 | ||||
| No | 557 | 332 (59.6%) | 1.00 | 1.00 | ||
| Yes | 608 | 392 (64.5%) | 1.23 (0.97 – 1.56) | 1.18 (0.91 – 1.51) | ||
| Use of antiplatelet drugs | 0.18 | 0.33 | ||||
| No | 634 | 383 (60.4%) | 1.00 | 1.00 | ||
| Yes | 531 | 341 (64.2%) | 1.18 (0.93 – 1.49) | 1.13 (0.88 – 1.45) | ||
| Use of anticoagulant drugs | 0.22 | 0.33 | ||||
| No | 1071 | 660 (61.6%) | 1.00 | 1.00 | ||
| Yes | 94 | 64 (68.1%) | 1.33 (0.85 – 2.09) | 1.26 (0.79 – 2.00) | ||
| Combination on the use of antiplatelet or anticoagulant drugs | 0.25 | 0.43 | ||||
| None | 557 | 332 (59.6%) | 1.00 | 1.00 | ||
| Antiplatelet drugs only | 514 | 328 (63.8%) | 1.20 (0.93 – 1.53) | 0.16 | 1.15 (0.89 – 1.49) | 0.29 |
| Anticoagulant drugs only | 77 | 51 (62.2%) | 1.33 (0.81 – 2.20) | 0.27 | 1.24 (0.74 – 2.09) | 0.41 |
| Both antiplatelet drugs and anticoagulant drugs | 17 | 13 (76.5%) | 2.20 (0.71 – 6.83) | 0.17 | 2.16 (0.68 – 6.85) | 0.19 |
| Type of antiplatelet or anticoagulant drug at baseline | ||||||
| None | 557 | 332 (59.6%) | 1.00 | 1.00 | ||
| Aspirin | 476 | 307 (64.5%) | 1.23 (0.96 – 1.59) | 0.11 | 1.20 (0.92 – 1.57) | 0.18 |
| Clopidogrel bisulfate | 80 | 55 (68.8%) | 1.49 (0.90 – 2.46) | 0.12 | 1.66 (0.92 – 3.03) | 0.10 |
| Warfarin sodium | 94 | 64 (68.1%) | 1.45 (0.91 – 2.30) | 0.12 | 1.46 (0.87 – 2.43) | 0.15 |
OR = odds ratio, CI = confidence interval.
Adjusted by age (as continuous), gender, smoking status, diabetes, medical history of cardiovascular diseases, and CNV in fellow eye.
The dose of AP/AC drugs was not significantly associated with retinal/subretinal hemorrhage for both aspirin (p=0.38) and warfarin (p=0.25) at baseline (Table 3). The duration of AP/AC use was also not associated with retinal/subretinal hemorrhage (p=0.23). These associations remained not significant after adjustment by baseline covariates (all p≥0.10, Table 3).
Table 3.
The association between dose and duration of antiplatelet or anticoagulant use and retinal/subretinal hemorrhage at baseline
| Unadjusted Analysis | Adjusted Analysis* | |||||
|---|---|---|---|---|---|---|
| Use of antiplatelet or anticoagulant drugs at baseline | n | Retinal/subretinal hemorrhage at baseline n (%) | Odds ratio (95% CI) | p-value | Odds ratio (95% CI)* | P-value |
| Aspirin | 0.38 | 0.55 | ||||
| None | 689 | 417 (60.5%) | 1.00 | 1.00 | ||
| 1–81 mg | 386 | 250 (64.8%) | 1.20 (0.93 – 1.55) | 0.17 | 1.16 (0.89 – 1.51) | 0.28 |
| ≥82 mg | 90 | 57 (63.3%) | 1.13 (0.72 – 1.78) | 0.61 | 1.10 (0.69 – 1.75) | 0.69 |
| Warfarin sodium | 0.25 | 0.30 | ||||
| None | 1071 | 660 (61.6%) | 1.00 | 1.00 | ||
| 1–4 mg | 48 | 30 (62.5%) | 1.04 (0.57 – 1.89) | 0.90 | 0.97 (0.52 – 1.78) | 0.91 |
| ≥5 mg | 46 | 34 (73.9%) | 1.76 (0.90 – 3.45) | 0.097 | 1.70 (0.86 – 2.25) | 0.13 |
| Duration of or antiplatelet anticoagulants use | 0.23 | 0.37 | ||||
| Never use | 557 | 332 (59.6%) | 1.00 | 1.00 | ||
| <5 years | 231 | 148 (64.1%) | 1.21 (0.88 – 1.66) | 0.24 | 1.17 (0.84 – 1.62) | 0.36 |
| ≥5, <10 years | 177 | 120 (67.8%) | 1.43 (0.99 – 2.04) | 0.06 | 1.36 (0.94 – 1.97) | 0.10 |
| ≥10 years | 200 | 124 (62.0%) | 1.11 (0.79 – 1.54) | 0.55 | 1.03 (0.72 – 1.46) | 0.88 |
Adjusted by age (as continuous), gender, smoking status, diabetes, medical history of cardiovascular diseases, and CNV in fellow eye.
The associations of AP/AC use with the size of retinal/subretinal hemorrhage (≤1 DA, 1–2 DA, >2 DA) are reported in Table 4. The use of any AP/AC drugs, specific type of AP/AC drug, its dose and duration of AP/AC use were all not significantly associated with size of retinal/subretinal hemorrhage (all p>0.20, Table 4).
Table 4.
The association between use of antiplatelet or anticoagulant drugs and size of retinal/subretinal hemorrhage
| n | Size of retinal/subretinal hemorrhage at baseline | P-value (trend P-value) | ||||
|---|---|---|---|---|---|---|
| Use of antiplatelet or anticoagulant or drugs at baseline | No hemorrhage (n=441) | = 1 DA (n=611) | >1, = 2 DA (n=59) | > 2 DA (n=54) | ||
| Use of any antiplatelet or anticoagulant drugs | 0.34 (0.31) | |||||
| No | 557 | 225 (40.4%) | 277 (49.7%) | 29 (5.2%) | 26 (4.7%) | |
| Yes | 608 | 216 (35.5%) | 334 (54.9%) | 30 (4.9%) | 28 (4.6%) | |
| Use of antiplatelet drugs | 0.58 (0.39) | |||||
| No | 634 | 251 (39.6%) | 322 (50.8%) | 31 (4.9%) | 30 (4.7%) | |
| Yes | 531 | 190 (35.8%) | 289 (54.4%) | 28 (5.3%) | 24 (4.5%) | |
| Use of anticoagulant drugs | 0.59 (0.38) | |||||
| No | 1071 | 411 (38.4%) | 556 (51.9%) | 55 (5.1%) | 49 (4.6%) | |
| Yes | 94 | 30 (31.9%) | 55 (58.5%) | 4 (4.3%) | 5 (5.3%) | |
| Combination on the use of antiplatelet or anticoagulant drugs | 0.64 (0.21) | |||||
| None | 557 | 225 (40.4%) | 277 (49.7%) | 29 (5.2%) | 26 (4.7%) | |
| Antiplatelet drugs only | 514 | 186 (36.2%) | 279 (54.3%) | 26 (5.1%) | 23 (4.5%) | |
| Anticoagulant drugs only | 77 | 26 (33.8%) | 45 (58.4%) | 2 (2.6%) | 4 (5.2%) | |
| Both | 17 | 4 (23.5%) | 10 (58.8%) | 2 (11.8%) | 1 (5.9%) | |
| Aspirin | 0.52 (0.29) | |||||
| None | 689 | 272 (39.5%) | 350 (50.8%) | 32 (4.6%) | 35 (5.1%) | |
| 1–81 mg | 386 | 136 (35.2%) | 213 (55.2%) | 20 (5.2%) | 17 (4.4%) | |
| ≥82 mg | 90 | 33 (36.7%) | 48 (53.3%) | 7 (7.8%) | 2 (2.2%) | |
| Warfarin sodium | 0.60 (0.28) | |||||
| None | 1071 | 411 (38.4%) | 556 (51.9%) | 55 (5.1%) | 49 (4.6%) | |
| 1–4 mg | 48 | 18 (37.5%) | 25 (52.1%) | 3 (6.3%) | 2 (4.2%) | |
| ≥5 mg | 46 | 12 (26.1%) | 30 (65.2%) | 1 (2.2%) | 3 (6.5%) | |
| Duration of antiplatelet or anticoagulant use | 0.49 (0.37) | |||||
| Never use | 557 | 225 (40.4%) | 277 (49.7%) | 29 (5.2%) | 26 (4.7%) | |
| <5 years | 231 | 83 (35.9%) | 125 (54.1%) | 12 (5.2%) | 11 (4.8%) | |
| ≥5, <10 years | 177 | 57 (32.2%) | 107 (60.5%) | 5 (2.8%) | 8 (4.5%) | |
| ≥10 years | 200 | 76 (38.0%) | 102 (51.0%) | 13 (6.5%) | 9 (4.5%) | |
DA = Disc area.
Association between AP/AC use and retinal/subretinal hemorrhage at Years 1 or 2
Retinal/subretinal hemorrhage was detected during follow-up in 44 (4.08%) of the 1078 participants who had photographs available from the 1-year or 2-year visit. The proportion with retinal/subretinal hemorrhage did not differ by AP/AC use at baseline (3.4% among AP/AC users, 4.8% among non-users, p=0.28) or AP/AC use during follow-up (3.8% among AP/AC users, and 4.4% among non-users, p=0.64).
Among 425 participants without retinal/subretinal hemorrhage at baseline, 12 (3.8%) had retinal/subretinal hemorrhage detected at either the 1-year or 2-year visits. The proportion developing retinal/subretinal hemorrhages at year 1 or 2 did not differ by AP/AC use at baseline (1.4% among AP/AC users, 4.3% among non-users, p=0.14) or AP/AC use during follow-up (2.7% among AP/AC users, and 3.1% among non-users, p=1.00).
Association of AP/AC use with retinal/subretinal hemorrhage by the baseline hypertension status
There was a significant interaction between AP/AC use and hypertension for their association with retinal/subretinal hemorrhage at baseline (p=0.003). Among participants with hypertension at baseline (N=807), the AP/AC use at baseline was significantly associated with higher proportion of retinal/subretinal hemorrhage (66.8% for AP/AC users vs. 56.4% for non-users, adjusted OR=1.48, p=0.01, Table 5). Similar association with retinal/subretinal hemorrhage was found for AP use (adjusted OR=1.43, p=0.02) but not for AC use (68.4% for AC users vs. 56.4% for non-AC users, adjusted OR=1.24, p=0.42). In particular, higher proportion of retinal/subretinal hemorrhage was observed in both aspirin users (adjusted OR=1.5, p=0.01) and clopidogrel users (adjusted OR=2.4, p=0.01) than non-users. However, AP/AC use was not associated with the size of retinal/subretinal hemorrhage at baseline (p=0.41). Among participants without hypertension at baseline (N=358), AP/AC use was not associated with retinal/subretinal hemorrhage (56.5% for AP/AC users vs. 64.6% for non-users, adjusted OR=0.73, p=0.19). AP/AC use at baseline was not associated with higher rate of retinal/subretinal hemorrhage at years 1 or 2 for both participants with hypertension (2.8% among AP/AC users vs. 5.5% among non-users, p=0.08) and participants without hypertension (5.3% among AP/AC users vs. 3.9% among non-users, p=0.59). AP/AC use at follow-up was also not associated with higher rate of retinal/subretinal hemorrhage at years 1 or 2 for both participants with hypertension (3.5% among AP/AC users, vs. 4.5% among non-users, p=0.56) and participants without hypertension (4.7% among AP/AC users vs. 4.3% among non-users, p=1.00).
Table 5.
The association between use of antiplatelet or anticoagulant drugs and retinal/subretinal hemorrhage at baseline among those with hypertension and those without hypertension
| With hypertension (N=807) | Without hypertension (N=358) | |||||||
|---|---|---|---|---|---|---|---|---|
| Use of anti-coagulant or anti-platelet drugs at baseline | n | Retinal/subretinal hemorrhage at baseline n (%) | Adjusted odds ratio (95% CI)* | P-value | n | Retinal/subretinal hemorrhage at baseline n (%) | Adjusted odds ratio (95% CI)* | P-value |
| Any use of antiplatelet or anticoagulant drugs | P=0.003 | 0.01 | P=0.13 | 0.19 | ||||
| No | 337 | 190 (56.4%) | 1.00 | 220 | 142 (64.6%) | 1.00 | ||
| Yes | 470 | 314 (66.8%) | 1.48 (1.09 – 2.01) | 138 | 78 (56.5%) | 0.73 (0.45 – 1.18) | ||
| Use of antiplatelet drugs | P=0.007 | 0.02 | P=0.07 | 0.11 | ||||
| No | 398 | 230 (57.8%) | 1.00 | 236 | 153 (64.8%) | 1.00 | ||
| Yes | 409 | 274 (67.0%) | 1.43 (1.06 – 1.92) | 122 | 67 (54.9%) | 0.68 (0.42 – 1.10) | ||
| Use of anticoagulant drugs | P=0.26 | 0.42 | P=0.64 | 0.56 | ||||
| No | 731 | 452 (61.8%) | 1.00 | 340 | 208 (61.2%) | 1.00 | ||
| Yes | 76 | 52 (68.4%) | 1.24 (0.74 – 2.08) | 18 | 12 (66.7%) | 1.37 (0.48 – 3.95) | ||
| Type of antiplatelet or anticoagulant drug at baseline | ||||||||
| None | 337 | 190 (56.4%) | 1.00 | 220 | 142 (64.6%) | 1.00 | ||
| Aspirin | 368 | 245 (66.6%) | 1.50 (1.09 – 2.07) | 0.01 | 108 | 62 (57.4%) | 0.74 (0.44 – 1.23) | 0.23 |
| Clopidogrel bisulfate | 70 | 52 (74.3%) | 2.38 (1.22 – 4.65) | 0.01 | 10 | 3 (30.0%) | 0.27 (0.05 – 1.41) | 0.12 |
| Warfarin sodium | 76 | 52 (68.4%) | 1.67 (0.94 – 2.97) | 0.08 | 18 | 12 (66.7%) | 1.05 (0.34 – 3.26) | 0.93 |
| Dose of Aspirin | P=0.08 | P=0.58 | ||||||
| None | 439 | 259 (59.0%) | 1.00 | 250 | 158 (63.2%) | 1.00 | ||
| 1–81 mg | 297 | 199 (67.0%) | 1.37 (1.00 – 1.87) | 0.049 | 89 | 51 (57.3%) | 0.79 (0.47 – 1.34) | 0.38 |
| ≥82 mg | 71 | 46 (64.8%) | 1.25 (0.73 – 2.12) | 0.42 | 19 | 11 (57.9%) | 0.79 (0.30 – 2.10) | 0.64 |
OR = odds ratio, CI = confidence interval.
Adjusted by age (as continuous), gender, smoking status, diabetes, medical history of cardiovascular diseases, and CNV in fellow eye.
DISCUSSION
Within the CATT, more than half of participants had retinal/subretinal hemorrhage at baseline and majority (84%) of hemorrhages were <1 DA. There was no overall association between use of AP/AC drugs and presence or size of retinal/subretinal hemorrhage. This lack of association was consistent whether we considered AP drugs only, AC drugs only, each specific AP or AC drug, or all AP/AC drugs together. There was no strong evidence of association between AP/AC use and retinal/subretinal hemorrhage even among patients who took higher doses or had longer durations of use. However, among the participants with hypertension, AP/AC use was associated with a higher proportion of retinal/subretinal hemorrhage at baseline, but was not associated with the size of hemorrhage or new/recurrent retinal/subretinal hemorrhage at years 1 or 2.
Three studies 6,9,11 of AP/AC use and retinal hemorrhage in neovascular AMD have had results that were inconsistent, at least in part, with those of CATT. The study most comparable to CATT with respect to AP/AC use and associations with other risk factors was by Kiernan et al.6 They evaluated the association of AP/AC use with ocular (subretinal or vitreous) hemorrhage among 195 patients with neovascular AMD in a tertiary care university setting. They found that the proportion of with ocular hemorrhage was significantly higher among 96 AP/AC users than 99 non-users (63.5% vs. 29.2%, p<0.0001, OR= 4.2, 95% CI: 2.2–8.3).6 This association was consistent for aspirin (OR=3.8, 95% CI: 1.9 – 7.5), clopidogrel (OR=8.8, 95% CI: 2.3 – 33) and warfarin (OR=11, 95% CI: 2.5 – 47). The differences in findings between Kiernan’s study and the CATT are puzzling, because both studies reported similar percentages of patients taking AP/PC drugs (49.2% in Kiernan’s study and 52.2% in CATT) and a similar distribution of type of AP/AC drugs. Furthermore, both studies found older age was significantly associated with risk of retinal/subretinal hemorrhage, while diabetes and hypertension were not associated with retinal/subretinal hemorrhage. However, the overall percentage of patients having hemorrhage (47%) in the Kiernan study was lower than that in CATT (62%) even though the Kiernan study was more inclusive than CATT by having no restriction on visual acuity and including 7 cases of massive vitreoretinal hemorrhage (6 of them were on 1 or more AP/AC therapies).6 Kuhli-Hattenbach et al. studied 71 consecutive neovascular AMD patients with subretinal hemorrhage and found that AP/AC therapy was strongly associated with larger subretinal hemorrhages (mean size of 9.71 DA in AP/AC users vs. 2.99 DA in non-users, p<0.0001).9 Within a subgroup of 38 patients with arterial hypertension, hemorrhage size was larger for AP/AC users. Tilanus et al. compared 50 AMD patients with massive hemorrhage and 50 AMD patients with small hemorrhage (<1 DA) and found that the association with aspirin was not statistically significant (OR=2.1, 95% CI: 0.7–6.6), but the association with AC use was significant (OR=11.6, 95% CI: 3.1–44).11
However, other large studies have not found an association of ocular hemorrhage and AP/AC use. The Macular Photocoagulation Study did not find a significant association between aspirin use and macular hemorrhage among 732 patients with neovascular AMD.14 The Early Treatment Diabetic Retinopathy Study did not find any significant association between aspirin use and the presence, severity and duration of vitreous/preretinal hemorrhage among patients with diabetes retinopathy.21–22 Brown et al also found that anticoagulant or antiplatelet agents were not associated with postoperative vitreous hemorrhage in diabetic vitrectomy.23
The differences in findings across studies could be due to the differences in sample size, selection of study population, the dose and intensity of AP/AC therapy, and method for determining of hemorrhage and type of ocular hemorrhage investigated. CATT included 1165 study participants from 43 clinical centers with active neovascular AMD. The determination for the presence and size of retinal/subretinal hemorrhage was based on reading center assessment of color fundus photographs by graders masked to AP/AC status, and AP/AC use was obtained by standard interview. Graders did not specify the location of hemorrhage so that retinal, subretinal and sub-RPE hemorrhages were included. Importantly, subjects with vitreous hemorrhage or with baseline VA worse than 20/320 were excluded from enrollment into the CATT, while the previous studies considered both subretinal hemorrhage and vitreous hemorrhage determined mainly based on clinical examination. The exclusion of patients with vitreous hemorrhage and patients with VA worse than 20/320 in the CATT study limits the generalizability of our study findings. It may be that AP/AC use causes massive or vitreal hemorrhages but not moderate or small retinal/subretinal hemorrhage; however, in CATT, there was no indication of an increased association with larger hemorrhages (Table 4), nor with lesions that were composed of at least 50% blood.17
Although our study did not find any statistically significant association between use of any AP/AC drugs and retinal/subretinal hemorrhage, the subgroup analysis by hypertension status at baseline suggests increased risk of retinal/subretinal hemorrhage among AP/AC users with hypertension (OR=1.48, p=0.01), but no association with AP/AC use among participants without hypertension (OR=0.73, p=0.19). A few other subgroup analyses by AP/AC drug, dose and duration also suggested a trend of increased risk of retinal/subretinal hemorrhage. For example, the OR for patients with a warfarin daily dose ≥5mg (n=46) compared to patients who did not use warfarin was 1.76 (95% CI: 0.90 – 3.45, p=0.10). Also, when patients who took both AP and AC drugs at baseline (n=17) were compared to those without AP/AC use, the OR was 2.20 (95% CI: 0.71 – 6.83, p=0.17). With such wide confidence intervals, increased risk cannot be ruled out.
Because ocular hemorrhage is associated with worse vision,15–17 patients with AMD may worry about the risk of ocular hemorrhage. Some ophthalmologists might be tempted to tell their patients to avoid the AP/AC drugs that may promote ocular bleeding. AP/AC drugs are often prescribed for potentially life-saving reasons and our data and data from diabetic retinopathy studies do not provide strong evidence to indicate that they should be discontinued because of fear of intra-ocular hemorrhage.21–22 At the same time, these older patients are more likely to take AP/AC drugs because of the beneficial effect of AP/AC drugs for treating or managing cardiovascular diseases. Our findings suggest that AP/AC use is associated with increased risk of retinal/subretinal hemorrhage in patients with hypertension, and no association in patients without hypertension. These findings are clinically important and suggest that non-hypertensive patients in the need of AP/AC drugs should continue taking AP/AC drugs for appropriate medical indications at the recommended dose without fear of increased risk of visual loss from retinal/subretinal hemorrhage, but for the patients with hypertension, the 1.5-folder increased risk of retinal/subretinal hemorrhage should be considered when taking AP/AC drugs.
In summary, among all CATT participants with active neovascular AMD, the use of antiplatelet and anticoagulant drugs was not significantly associated with retinal/subretinal hemorrhage at baseline or during follow-up. However, among participants with hypertension, the AP/AC use was associated with 1.5-folder increased risk of retinal/subretinal hemorrhage. Hypertension status should be considered in prescribing/taking AP/AC for appropriate medical indications.
Supplementary Material
Among the CATT participants with hypertension, the use of antiplatelet and anticoagulant drugs was associated 1.5-folder increased risk of retinal/subretinal hemorrhage, but no association in participants without hypertension.
Acknowledgments
Supported by cooperative agreements U10 EY017823, U10 EY017825, U10 EY017826, U10 EY017828 and R21EY023689 from the National Eye Institute, National Institutes of Health, Department of Health and Human Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Administration on Aging, Administration for Community Living. A Profile of Older Americans: 2013. US Department of Health and Human Services; 2013. [Accessed April 21, 2015]. p. 15. Available at http://www.aoa.acl.gov/Aging_Statistics/Profile/2013/docs/2013_Profile.pdf. [Google Scholar]
- 2.Diseases, Conditions, and Injuries Affecting Seniors. U.S. Government; 2015. [Accessed April 21, 2015]. Available at: http://www.usa.gov/Topics/Seniors/Health/Diseases.shtml. [Google Scholar]
- 3.Schulman S. Care of patients receiving long-term anticoagulants therapy. N Engl J Med. 2003;349:675–83. doi: 10.1056/NEJMcp025373. [DOI] [PubMed] [Google Scholar]
- 4.Hylek EM. Complications of oral anticoagulant therapy: bleeding and nonbleeding, rates and risk factors. Semin Vasc Med. 2003;3:271–8. doi: 10.1055/s-2003-44463. [DOI] [PubMed] [Google Scholar]
- 5.Palareti G, Cosmi B. Bleeding with anticoagulation therapy - who is at risk, and how best to identify such patients. Thromb Haemost. 2009;102:268–78. doi: 10.1160/TH08-11-0730. [DOI] [PubMed] [Google Scholar]
- 6.Kiernan DF, Hariprasad SM, Rusu IM, et al. Epidemiology of the association between anticoagulants and intraocular hemorrhage in patients with neovascular age-related macular degeneration. Retina. 2010;30:1573–8. doi: 10.1097/IAE.0b013e3181e2266d. [DOI] [PubMed] [Google Scholar]
- 7.El Baba F, Jarrett WH, Harbin TS, et al. Massive hemorrhage complicating age-related macular degeneration. Clinicopathologic correlation and role of anticoagulants. Ophthalmology. 1986;93:1581–92. doi: 10.1016/s0161-6420(86)33540-1. [DOI] [PubMed] [Google Scholar]
- 8.Knox FA, Johnston PB. Spontaneous suprachoroidal haemorrhage in a patient with age-related macular degeneration on excessive anticoagulation therapy. Eye. 2002;16:669–70. doi: 10.1038/sj.eye.6700109. [DOI] [PubMed] [Google Scholar]
- 9.Kuhli-Hattenbach C, Fischer IB, Schalnus R, Hattenbach L-O. Subretinal hemorrhages associated with age-related macular degeneration in patients receiving anticoagulation or antiplatelet therapy. Am J Ophthalmol. 2010;149:316–21. doi: 10.1016/j.ajo.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Kuhli-Hattenbach C, Miesbach W, Scharrer I, Hattenbach L-O. Massive subretinal hemorrhage and anticoagulants. An unfortunate combination? Ophthalmologe. 2012;109:665–9. doi: 10.1007/s00347-012-2567-2. [DOI] [PubMed] [Google Scholar]
- 11.Tilanus MA, Vaandrager W, Cuypers MH, et al. Relationship between anticoagulant medication and massive intraocular hemorrhage in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2000;238:482–5. doi: 10.1007/pl00007887. [DOI] [PubMed] [Google Scholar]
- 12.Lewis H, Sloan SH, Foos RY. Massive intraocular hemorrhage associated with anticoagulation and age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1988;226:59–64. doi: 10.1007/BF02172720. [DOI] [PubMed] [Google Scholar]
- 13.Olson JM, Scott IU, Kerchner DL, Kunselman AR. Association between systemic anticoagulation and rate of intraocular hemorrhage following intravitreal anti-VEGF therapy for age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retina. 2013;44:455–9. doi: 10.3928/23258160-20130909-06. [DOI] [PubMed] [Google Scholar]
- 14.Klein ML. Macular degeneration: is aspirin a risk for progressive disease? JAMA. 1991;266:2279. doi: 10.1001/jama.266.16.2279b. [DOI] [PubMed] [Google Scholar]
- 15.Bennett SR, Folk JC, Blodi CF, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol. 1990;109:33–7. doi: 10.1016/s0002-9394(14)75575-8. [DOI] [PubMed] [Google Scholar]
- 16.Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina. 1996;16:183–9. doi: 10.1097/00006982-199616030-00001. [DOI] [PubMed] [Google Scholar]
- 17.Altaweel MM, Daniel E, Martin DF, et al. Outcomes of eyes with lesions composed of >50% blood in the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Ophthalmology. 2015;122:391–8. doi: 10.1016/j.ophtha.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The CATT Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: 2-year results. Ophthalmology. 2012;119:1388–98. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grunwald JE, Daniel E, Ying GS, et al. Photographic assessment of baseline fundus morphologic features in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2012;119:1634–41. doi: 10.1016/j.ophtha.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Early Treatment Diabetic Retinopathy Study Research Group. Effects of aspirin treatment on diabetic retinopathy: Early Treatment Diabetic Retinopathy Study Report No 8. Ophthalmology. 1991;98:757–65. [PubMed] [Google Scholar]
- 22.Chew EY, Klein ML, Murphy RP, et al. Effects of aspirin on vitreous/preretinal hemorrhage in patients with diabetes mellitus. Early treatment diabetic retinopathy study report no. 20. Arch Ophthalmol. 1995;113:52–5. doi: 10.1001/archopht.1995.01100010054020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.