Abstract
Bioassay-guided fractionation of two marine cyanobacterial extracts using the H-460 human lung cancer cell line and the OVC-5 human ovarian cancer cell line led to the isolation of three related α-methoxy-β, β’-dimethyl-γ-pyrones each containing a modified alkyl chain, one of which was identified as the previously reported kalkipyrone and designated kalkipyrone A. The second compound was an analog designated kalkipyrone B. The third was identified as the recently reported yoshipyrone A, also isolated from a marine cyanobacterium. Kalkipyrone A and B were obtained from a field-collection of the cyanobacterium Leptolyngbya sp. from Fagasa Bay, American Samoa, while yoshipyrone A was isolated from a field-collection of cyanobacteria (cf. Schizothrix sp.) from Panama. One-dimensional and two-dimensional NMR experiments were used to determine the overall structures and relative configurations of the kalkipyrones, and the absolute configuration of kalkipyrone B was determined by 1H NMR analysis of diastereomeric Mosher’s esters. Kalkipyrone A showed good cytotoxicity to H-460 human lung cancer cells (EC50 = 0.9 µM), w M), while kalkipyrone B and yoshipyrone A were less active (EC50 = 9.0 µM and > 10 µM, respectively). Both kalkipyrone A and B showed moderate toxicity to Saccharomyces cerevisiae ABC16-Monster strain (IC50 = 14.6 and 13.4 µM, respectively), whereas yoshipyrone A was of low toxicity to this yeast strain (IC50 = 63.8 µM).
Keywords: cyanobacteria, polyketide, kalkipyrone, yoshipyrone, Leptolyngbya, Moorea, Schizothrix
1. Introduction
Cyanobacteria are prolific producers of secondary metabolites, namely compounds which are not used for growth, structure or reproduction. These structurally diverse substances have displayed intriguing bioactivities (Nunnery et al., 2010; Tan, 2007) and their continued discovery remains an important pipeline in the development of therapeutic agents (Simmons et al., 2008; Tan, 2005). Many of these compounds arise from either polyketide synthases (PKS), nonribosomal peptide synthetases (NRPS), or hybrids of these two pathways. Unique arrangements of modifying enzymatic machinery in these modular pathways gives rise to a varied array of natural products. Moreover, enzymatic reactions at the terminal end of these pathways create rings of different sizes, (Jones et al., 2010) side-chains of varying length, or terminal unsaturations (Chang et al., 2004; Edwards et al., 2004). The diversity of resulting functionalities can provide target selectivity as well as favorable pharmacokinetic properties to polyketide molecules (Driggers et al. 2008).
Kalkipyrone, a polyketide natural product featuring a α-pyrone, was originally isolated from a mixed assemblage of Moorea producens (formerly Lyngbya majuscula) and Tolypothrix sp. collected from the splash zone at Playa Kalki, Curaçao (Graber and Gerwick, 1998). This compound showed toxicity to brine shrimp (1 µg/mL) and goldfish (2 µg/mL) and was structurally related to the actinopyrones, which were obtained from a Streptomyces sp. (Yano et al., 1986a; Yano et al., 1986b). The actinopyrones have displayed a diverse suite of bioactivities including anti-microbial, cytotoxicity, vasodilating properties, and EGF signaling inhibitory properties (Schleissner et al., 2011; Yano et. al, 1986). Aureothin, isolated from S. spectabilis, features a α-pyrone with a tetrahydrofuran-containing aryl polyene side chain and has displayed significant anti-fungal and anti-proliferative activity (Werneberg et al., 2010). The neofusapyrones and dactylfungins are pyrone-containing polyketides isolated from fungi with antifungal activity (Honma et al., 2010; Xaio et al., 1993). The structures of the yoshipyrones, kalkipyrone analogs from a field-collected Leptolyngbya sp., have recently been characterized and yoshipyrone A was shown to inhibit adipogenic differentiation in 3T3-L1 cells (Inuzuka et al., 2014).
In this work, reported are the re-isolation of kalkipyrone A (1) and yoshipyrone A (3), and the structure characterization of a new analog, kalkipyrone B (2), all of which are polyketides featuring a pyrone head group and side-chains with varying levels of unsaturation. Kalkipyrone A (1) and B (2) were isolated from a cyanobacterium of the Leptolyngbya lineage collected from American Samoa, whereas yoshipyrone A (3) was isolated from a field-collection of the cyanobacterium cf. Schizothrix sp. from Panama. The structures of these compounds were determined using 1D and 2D NMR techniques, in addition to subsequent spectroscopic and spectrometric techniques. The absolute configuration of kalkipyrone B (2) was determined by a modified Mosher’s ester method (Hoye et al., 2007). These kalkipyrones were evaluated for cytotoxicity against the H-460 human lung cancer cell line and the Saccharomyces cerevisiae ABC16-Monster strain. Kalkipyrone A (1) was the most potent to H-460 cells (EC50 = 0.9 µM), while kalkipyrone B (2) was less active (EC50 = 9.9 µM), and yoshipyrone A (3) showed little cytotoxicity to this cell line. Kalkipyrone A (1) and B (2) also showed moderate cytotoxicity to S. cerevisiae ABC16-Monster whereas yoshipyrone A (3) was essentially inactive against this strain.
2. Results and discussion
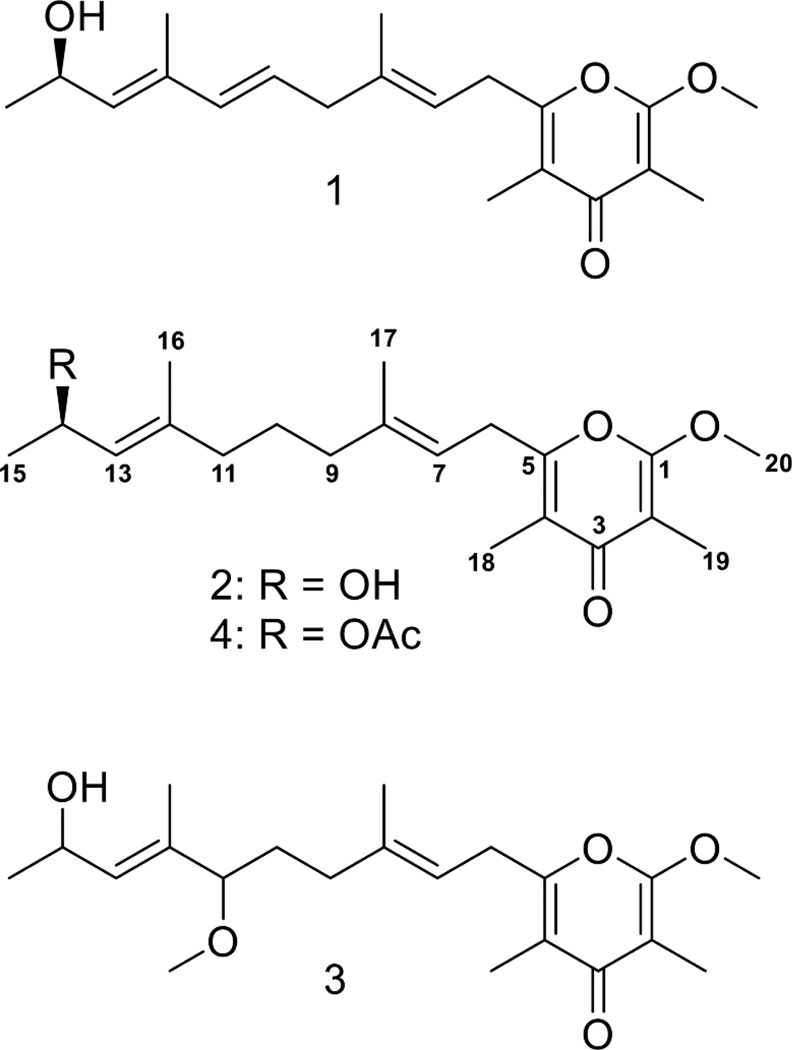
Bioassay-guided fractionation using the H-460 human lung cancer cell line led to the isolation of one minor and one major metabolite from a collection of cyanobacteria biomass from Fagasa Bay, American Samoa (Supplemental Fig. 1). HRESIMS of the minor metabolite (1) gave an [M + Na]+ at m/z 355.1883 suggesting a molecular formula of C20H28O4 and a requirement of seven degrees of unsaturation. Examination of two-dimensional NMR data, the IR spectrum, and UV absorbance (see Supplementary data) led to the identification of this minor metabolite as kalkipyrone (Graber and Gerwick, 1998). Here, this originally described ‘kalkipyrone’ is designated as ‘kalkipyrone A’ (1) (Fig. 1).
Fig. 1.
Chemical structures of compounds 1–4
HRESIMS of the major compound 2 gave an [M + Na]+ at m/z 357.2035, suggesting a molecular formula of C20H30O4 and a requirement for six degrees of unsaturation. Examination of the 1H NMR spectrum of 2 (Supplemental Fig. 2) suggested a compound related to kalkipyrone A (1) with a less complex olefinic proton region. One doublet and four singlet 3H peaks (δ 1.00–2.00) and one deshielded 3H singlet (δ 3.93) suggested a total of six methyl groups, one of which was an O-methyl. Three high-field methylene resonances (δ 1.52, 1.93 and 2.00) and one deshielded methylene (δ 3.29) doublet were also observed. The remaining proton resonances included a deshielded methine proton (δ 4.58) and two olefinic proton resonances (δ 5.20 and 5.21).
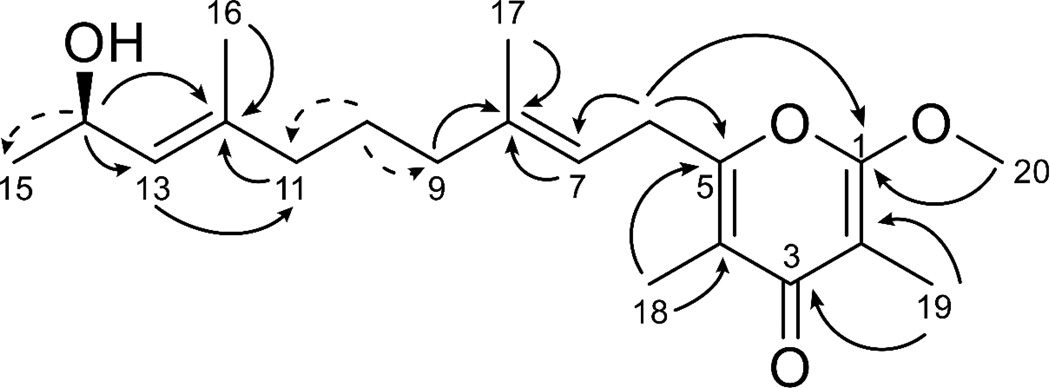
HSQC, HMBC, COSY and TOCSY correlations (Fig. 2, Table 1, Table S1 and Supplemental Figs. 4–8) were used to determine the structure of 2. The oxymethine at H-14 showed HMBC correlations to C-12 and C-13 and COSY correlations to H-13 and H-15 placing it adjacent to a methyl group and an olefin. The olefin proton H-13 showed HMBC correlations to the methylene C-11 and methyl C-16. In turn, the H-16 methyl protons showed HMBC correlations to the quaternary carbon at C-12 (δ 137.4), confirming the presence of a methyl substituted olefin. Bidirectional HMBC correlations, COSY correlations, 1H NMR splitting patterns and δ 1H and 13C values established the presence of three methylene groups between C-12 and C-8. HMBC correlations from H-7 and H3-17 to C-8 supported a second methyl substituted olefin. While these two olefin proton signals were overlapped (δ 5.20, 5.21) in the CDCl3 1H NMR spectrum, a second 1H NMR in DMSO (Supplemental Fig. 3) showed H-7 as a triplet (δ 5.25) and H-13 to be a doublet (δ 5.07), as expected for 2. The deshielded H2-6 methylene protons showed HMBC correlations to both C-7 and the quaternary carbon C-5, demonstrating a side-chain nearly identical to 1 with the exception of a fully saturated bond between C-10 and C-11 for 2. The 13C chemical shift of C-5 (δ 157.2) was in agreement with an olefinic carbon attached to an electron-withdrawing group; this was further supported by observation of an HMBC correlation between H2-6 and C-1 in a Long Range HMBC experiment (LR HMBC; 1H-13C J = 4 Hz), suggesting a pyrone moiety in 2 as in 1. This was confirmed by HMBC correlations between the H3-18 methyl protons and C-3, C-4 and C-5. Complementing this, the H3-19 methyl protons showed HMBC correlations to C-1, C-2 and C-3. An HMBC correlation between the H3-20 methoxy protons and C-1 located this functionality and completed the planar structure of kalkipyrone B (2).
Fig. 2.
Selected HMBC (black arrows) and COSY (dashed arrows) correlations for kalkipyrone B (2).
Table 1.
| position | αC, type | αH, (J in Hz) | COSY | HMBC | NOESYc |
|---|---|---|---|---|---|
| 1 | 162.1, qC | ||||
| 2 | 99.3, qC | ||||
| 3 | 181.0, qC | ||||
| 4 | 118.0, qC | ||||
| 5 | 157.2, qC | ||||
| 6 | 29.9, CH2 | 3.29 d (7.3) | 7, 17, 18 | 5, 7, 8 | 17 |
| 7 | 116.8, CH | 5.21 ovlp | 6, 17, 18 | 6, 9, 17 | 9 |
| 8 | 138.8, qC | ||||
| 9 | 39.2, CH2 | 2.00 t (7.7) | 10 | 7, 8, 10, 11, 17 | |
| 10 | 25.9, CH2 | 1.52 m | 9, 11 | 8, 9 | |
| 11 | 38.9, CH2 | 1.93 t (7.7) | 10 | 9, 10, 12, 13, 16 | |
| 12 | 137.4, qC | ||||
| 13 | 129.0, CH | 5.20 ovlp | 14, 16 | 11, 16 | 11, 15 |
| 14 | 64.7, CH | 4.58 m | 13, 15 | 12, 13 | 15, 16 |
| 15 | 23.5, CH3 | 1.23 d (6.3) | 14 | 13, 14 | |
| 16 | 16.4, CH3 | 1.67 s | 11, 12, 13 | ||
| 17 | 16.2, CH3 | 1.72 s | 6 | 7, 8, 9 | |
| 18 | 10.1, CH3 | 1.96 s | 6 | 3, 4, 5 | |
| 19 | 6.8, CH3 | 1.84 s | 1, 2, 3 | ||
| 20 | 55.4, CH3 | 3.93 s | 1 |
Data obtained on a Varian 500 MHz instrument in CDCl3.
ovlp = overlapped signals.
data taken in DMSO.
The geometries of the two olefinic bonds in 2 were determined using NOESY correlations (Supplemental Fig. 9). NOE correlations from H-13 to H2-11, in addition to correlations between H-14 and the H3-16, supported assignment of the position 12–13 double bond in the E configuration. Similarly, NOE correlations between H2-6 and H3-17 and between H-7 and H2-9 supported assignment of the position 7, 8 olefin as having an E configuration, the same as in kalkipyrone A (1).
The absolute configuration of 2 was determined using a modified Mosher’s esterification protocol (Hoye et al., 2007), in which two equal portions of 2 were acylated with R-(−)- and S-(+)-α-methoxy-α-(trifluoromethyl)phenylacetyl chloride (MTPA-Cl). This yielded the C-14 R-ester from (S)-MTPA-Cl and the C-14 S-ester from (R)-MTPA-Cl. The 1H NMR spectra of the diastereomeric MTPA esters were examined (Supplemental Figs. 10 and 11) and 1H resonances assigned for each ester so as to calculate α (δS-δR) values. A positive value for H-15 and negative values for H-14, H-13, H-12, and H-16 supported a 14R configuration, again the same as in kalkipyrone A (1) (Fig. 1).
A cyanobacterial collection from Panama was extracted and fractionated by VLC and a relatively polar fraction (‘H’ eluting with 75% EtOAc in MEOH) showed potent and selective toxicity to the OVC-5 human ovarian cancer cell line. Semi-preparative HPLC of this active fraction yielded 3 which gave by HRESIMS an [M + Na]+ at m/z 387.2146, suggesting a molecular formula of C21H32O5 with six degrees of inherent unsaturation. Comparison of the molecular formulas of 1, 2 and 3, along with the presence of a new deshielded 3H singlet (δ 3.15) in the 1H NMR spectrum of 3 (cf. Supplemental Figs. 1 A and 1 C), suggested the presence of a second O-methyl group within a kalkipyrone framework. Examination of the 2D NMR data confirmed the position of the O-methyl group as attached to C-11. The remaining correlations surrounding the O-methyl position were identical to 1 and 2 and confirmed the structure of 3 as that of yoshipyrone A (Fig. 1) (Inuzuka et al., 2014).
Comparison of the cytotoxicity for these three related metabolites showed interesting relationships. Kalkipyrone A (1) with an unsaturation between C-10 and C-11 is the most potent cytotoxic compound, and saturation of this position as in 2 reduces the activity 10-fold (Supplemental Fig. 12). The addition of the methoxy subunit to C-11 as in 3 reduces the cytotoxicity even further. It thus appears that the cytotoxicity properties of the kalkipyrones show distinct structure-activity relationships (SAR), and it would thus be interesting to explore the biological properties of synthetic analogs in this structure class. In this regard, the secondary alcohol of kalkipyrone B (2) could serve as a site for chemical modification (Marel et al., 2008; Gopalakrishnan et al., 1997). As such, an acetyl group was synthetically added to kalkipyrone B (4), but the resulting derivative kalkipyrone B acetate (4) showed no cytotoxic effects even at the highest concentrations tested (50 µM). This observation is consistent M). with the activity profile of 1, 2 and 3. The addition of acyl groups to the pyrone side chain (O-methylation in 3 and acetylation in 4) appears to inhibit cytotoxic activity. It will be interesting to observe if these structure-activity relationships extend to other types of biological activity. For example,the nocayrones B, H and L, γ-pyrone containing polyketides from marine Nocardiopsis strains, were recently shown to be Ca2+ channel modulators (Ochoa et al., 2015; Kim et al., 2014).
The ‘ABC16-Monster’ is a genetically engineered strain of Saccharomyces cerevisiae from which sixteen multi-drug resistance pump genes have been deleted (Suzuki et al., 2011). These deletions make it more sensitive to chemical agents than other laboratory yeast strains (Suzuki et al., 2011). As a result, the ABC16-Monster strain is useful for studies exploring a compound’s mechanism of action (MOA). In addition, drug sensitivity of the ABC16-Monster strain parallels that of neoplastic cells, indicating that it is a good surrogate for MOA studies. When tested against S. cerevisiae ABC16-Monster, 1 and 2 showed nearly the same moderate cytotoxicity (IC50: 1 = 14.6 µM; 2 = 13.4 µM). Yoshipyrone A (3) showed little cytotoxicity (IC50 = 63.8 µM) (Supplemental Fig. 13), consistent with previous results (Liang et al., 2014). While 3 was isolated from a fraction which showed potent activity to OVC-5 human ovarian cancer cells, we were not able to isolate enough material for retesting of pure yoshipyrone A against this cell line, and it remains possible that other metabolites in this fraction were responsible for the observed OVC-5 cytotoxicity.
Live specimens of ASG15JUL14-6 were returned to the laboratory from American Samoa. These cultures were predominately comprised of thin reddish filaments (see Supplementary data for photomicrograph of voucher specimen); by microscopy these were identified as Leptolynbya sp. Thicker filaments, provisionally identified as Moorea sp., were a very minor component of this collection. Unfortunately, the Leptolyngbya component of this collection was not successfully adapted to laboratory culture. Nevertheless, based on its relative prevalence in the collection, Leptolyngbya is most certainly the source of the kalkipyrones A (1) and B (2) reported herein.
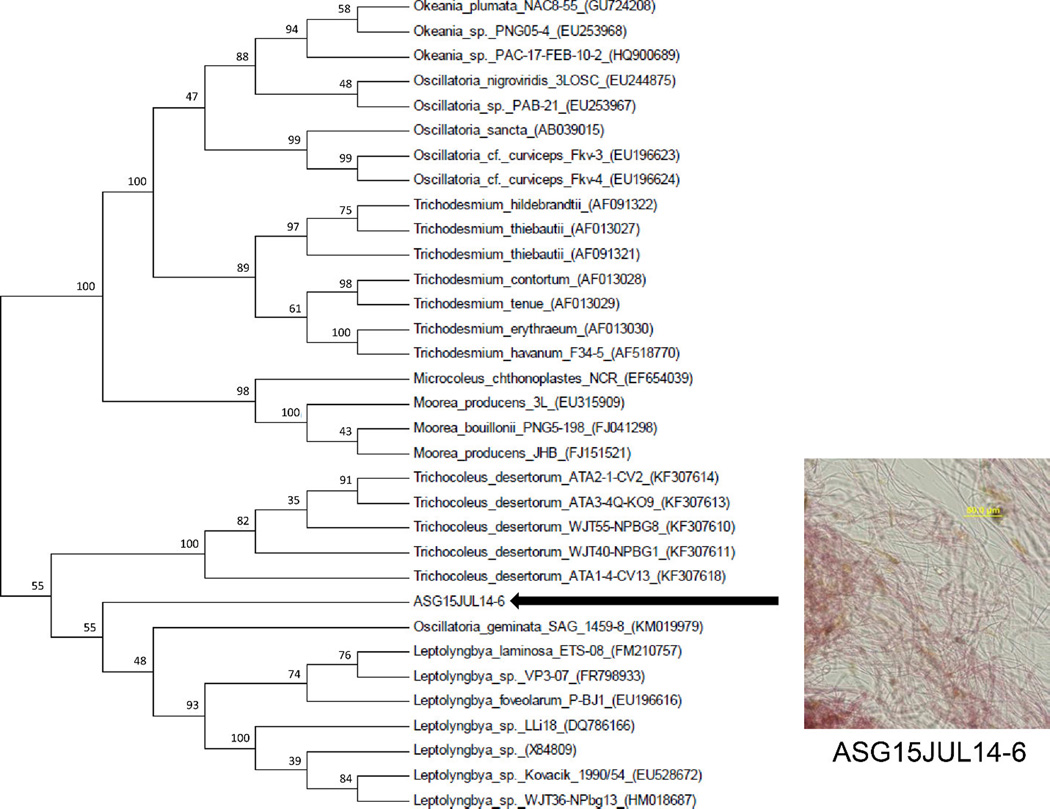
Because many cyanobacterial species have overlapping morphological characteristics, a genetic analysis was applied using a voucher specimen of the field-collected cyanobacteria preserved in RNAlater solution. DNA was isolated from this specimen, the 16S rRNA gene was amplified by PCR and a subcloning procedure was performed. The 16S rRNA sequences of all five clones showed >99% identity to each other and BLAST searching showed highest identity (95%) to sequences from Oscillatoria sp., Leptolyngbya sp. and Trichocoleus sp. Phylogenetic analysis of the consensus 16S rRNA gene sequence of ASG15JUL14-6 (1410 bp) placed it in the Leptolyngbya clade (Figure 3). However, it clustered most closely with Oscillatoria genimata SAG 1459-8 within the Leptolyngbya clade. O. genimata SAG 1459-8 does not cluster with other Oscillatoria strains and this may indicate its reassignment as a Leptolyngbya strain. Summarizing, the morphological and phylogenetic evidence support a taxonomic classification of the kalkipyrone A (1) and B (2) producer from American Samoa as a Leptolyngbya sp.
Fig. 3.
Phylogenetic analysis of the kalkipyrone producing strain ASG15JUL14-6 (American Samoa collection), which is embedded withing the Leptolynbya sp. clade. The evolutionary history was inferred by using the Maximum Likelihood method based on the General Time Reversible model. The bootstrap consensus tree is inferred from 1000 replicates. Branches corresponding to partitions reproduced in less than 0% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown above the branches. Species are identified by strain number when available and Genbank accession number. Morphological analysis of ASG15JUL14-6 was carried out using an Olympus IX51 epifluorescent microscope equipped with an Olympus U-CMAD3. Scale bar is 50 µM.
The field-collection of cyanobacteria from Panama from which yoshinone (3) was isolated was morphologically identified as a Schizothrix sp. Kalkipyrone A (1) was originally isolated from a mixed collection of Moorea sp. and Tolypothrix sp. from Playa Kalki, Cura•ao, whereas yoshipyrone A (3) was isolated from a sample of Leptolyngbya sp. collected from Ishigaki island, Okinawa, Japan. However, all of these identifications were based on morphological characteristics. To our knowledge, the report herein is the first to incorporate phylogenetic analysis into the identification of a kalkipyrone-producing cyanobacterium, firmly establishing it as a Leptolyngbya sp.
3. Concluding remarks
In summary, one new and two known kalkipyrone-type metabolites were isolated from two field-collections of marine cyanobacteria. Kalkipyrone A (1) and B (2) showed cytotoxicity against human lung cancer cells and yeast cells. The geographic distribution of the kalkipyrones from multiple sites in the Caribbean and North and South Pacific Ocean generates intriguing questions concerning the ecological and physiological role of these molecules in cyanobacteria.
4. Experimental
4.1. General procedures
Optical rotations were measured on a JASCO P-2000 polarimeter, UV spectra on a Beckman Coulter DU-800 spectrophotometer, and IR spectra with a Nicolet IR-100 FT-IR spectrophotometer with a KBr plate. NMR spectra were recorded with residual CHCl3 as the internal standard (δC 77.0, δH 7.26) on a Varian Unity 500 MHz spectrometer. A Bruker 600 MHz spectrometer equipped with a 1.7 mm MicroCryoProbe was also used for some experiments. Semi-preparative HPLC was carried out using a Waters 515 pump system equipped with a Waters 996 PDA. Isolated HPLC peaks were subjected to LC-MS/MS analysis using a ThermoFinnigan LCQ AdvantageMax mass detector equipped with an electrospray ionization (ESI) source. High resolution electrospray ionization mass spectra (HRESIMS) were obtained using an Agilent 1290 Infinity system with an Agilent 6530 Accurate Mass Q-TOF LC/MS.
4.2. Collection and morphological identification of cyanobacteria
Kalkipyrone-producing cyanobacteria were collected from Panama (PAP25JUN12-1) and from Fagasa Bay, American Samoa (ASG15JUL14-6) by hand using SCUBA. Voucher specimens under these collection codes are stored in the Scripps Institution of Oceanography marine cyanobacterial voucher collection, and are available from the corresponding author. Biomass was collected in a 1 L bottle and preserved in seawater:iPrOH (1:1) solution for subsequent extraction. Live specimens were brought back to the laboratory and examined using an Olympus IX51 epifluorescent microscope equipped with an Olympus U-CMAD3 camera for morphological identification.
4.3. Extraction and isolation of kalkipyrones
The cyanobacteria biomass collected from Fagasa Bay, American Samoa (ASG15JUL14-6) was repeatedly extracted using CH2Cl2-MeOH (2:1), affording a 1 g crude extract. The latter was further fractionated over silica gel using vacuum liquid chromatography (VLC) and a solvent system of increasing polarity using a stepped gradient of hexanes, EtOAc and MeOH. Fractions F (EtOAc-hexanes, 5:1), G (EtOAc), and H (EtOAc-MeOH, 4:1) were combined (63.6 mg) and separated into 5 fractions using C18 SPE (1 g) and a stepwise gradient solvent system starting with MeCN-H2O (4:1) and ending with CH2Cl2. Fractions 1 (MeCN-H2O, 4:1) and Fraction 2 (MeCN) were combined (13.7 mg) and subjected to RP semi-preparative HPLC using a Phenomenex 4 µm Hydro column (250 × 10 mm) with a gradient from H2O-MeCN (1:1) to MeCN over 20 minutes yielding kalkipyrone A (1) (1 mg) and kalkipyrone B (2) (2.5 mg). The cyanobacterial biomass from Panama was extracted and fractionated by VLC as described above and Fraction H (EtOAc-MeOH, 4:1) was subjected to RP semi-preparative HPLC yielding yoshipyrone A (3) (0.3 mg).
Kalkipyrone B (2): yellow oil; [α]25D +9.3 (c 0.2, CHCl3); UV (MEOH) αmax (log α) 249 nm; IR (DCM, neat) αmax 3395, 2928, 2852, 1664, 1581, 1464, 1403, 1375, 1334, 1251, 1162, 1059, 983, 967, 764, 482 cm−1; for 1H NMR (500 MHz, CDCl3) and 13C NMR (500 MHz, CDCl3) spectroscopic data, see Table 1; HRESIMS [M + Na]+ m/z 357.2035 (calcd for C20H30O4, 357.2036).
4.4 Preparation of kalkipyrone B (2) MTPA esters
Compound 2 (0.5 mg) was reconstituted in CH2Cl2 (0.5 mL) in a 2 mL vial to which dry pyridine (5 µL) and (S)-(−)-α-methoxy-α-(trifluoromethyl)phenylacetyl chloride (7 µL) were added. The vial was capped and the reaction was stirred for 15 h. The identical procedure was repeated with an equal amount of 2 and (R)-(+)-α-methoxy-α-(trifluoromethyl)phenylacetyl chloride. The reaction mixtures were quenched with H2O and extracted with CH2Cl2. The preparation was purified over a silica SPE column (50 mg) eluting with EtOAc-hexanes (1:1). R ester: partial 1H NMR (500 MHz, CDCl3) δ 5.85 (1H, m, H-14), 5.21 (1H, m, H-7), 5.19 (1H, m, H-13), 3.92 (3H, s, H-20), 3.30 (2H, d, J = 7.1 Hz, H-6), 2.06 (2H, m, H-9), 1.99 (2H, m, H-11), 1.97 (3H, s, H-19), 1.85 (3H, s, H-18), 1.75 (3H, s, H-17), 1.71 (3H, s, H-16), 1.52 (2H, m, H-10), 1.31 (3H, d, J = 6.3 Hz, H-15). S-ester: partial 1H NMR (500 MHz, CDCl3) δ 5.82 (1H, m, H-14), 5.17 (1H, t, J = 7.1 Hz, H-7), 5.08 (1H, d, J = 9.0 Hz, H-13), 3.92 (3H, s, H-20), 3.30 (2H, d, J = 7.1 Hz, H-6), 1.99 (2H, m, H-9), 1.97 (3H, s, H-19), 1.95 (2H, m, H-11), 1.87 (3H, s, H-18), 1.73 (3H, s, H-17), 1.70 (3H, s, H-16), 1.49 (2H, m, H-10), 1.38 (3H, d, J = 6.4 Hz, H-15).
4.5 Acetylation of kalkipyrone B
A sample of 2 (0.4 mg) was stirred for 24 h in a 1:1 solution of pyridine:Ac2O. The reaction mixture was dried under N2 and partitioned between CH2Cl2 and H2O, following which the CH2Cl2 layer was removed and dried under N2. The residue was reconstituted in MeCN and subjected to RP-HPLC using a Phenomenex 4 µm Hydro c m column (250 × 10 mm) and an isocratic solvent system of MeCN-H2O (9:1). Kalkipyrone B acetate (4) (0.2 mg isolated) eluted at 11 min.
Kalkipyrone B acetate (4): pale yellow oil; 1H NMR (500 MHz, CDCl3) δ 5.59 (1H, m, H-14), 5.21 (1H, t, J = 7.3 Hz, H-7), 5.13 (1H, d, J = 8.7 Hz, H-13), 3.94 (3H, s, H-20), 3.30 (2H, d, J = 7.3 Hz, H-6), 2.02 (3H, s, H-22), 1.99 (2H, m, H-11), 1.96 (3H, s, H-18), 1.94 (2H, m, H-9), 1.85 (3H, s, H-19), 1.72 (3H, s, H-17) 1.69 (3H, s, H-16) 1.52 (2H, m, H-10), 1.25 (3H, d, J = 6.5 Hz, H-15); 13C NMR (600 MHz, CDCl3), δ 180.9 (C, C-3), 170.3 (C, C-21), 162.0 (C, C-1), 157.0 (C, C-5), 139.0 (C, C-12), 138.8. (C, C-8), 125.0 (CH, C-13), 118.0 (C, C-4), 117.4 (CH, C-7), 99.3 (C, C-2), 68.0 (CH, C-14), 55.1 (CH3, C-20), 38.9 (CH2, C-9), 38.8 (CH2, C-11), 29.9 (CH2, C-6), 25.7 (CH2, C-10), 21.3 (CH3, C-22), 20.7 (CH3, C-15), 16.4 (CH3, C-16), 16.2 (CH3, C-17), 9.8 (CH3, C-18), 6.8 (CH3, C-19); HRESIMS [M + Na]+ m/z 399.2147 (calcd for C22H32O5, 399.2142).
4.6. Cytotoxicity assay
Human lung cancer H-460 cells were added to 96-well plates at 3.33 × 104 cells/mL in Roswell Park Memorial Institute (RPMI) 1640 media with fetal bovine serum (FBS) and 1% penicillin/streptomycin. The cells were incubated overnight (37 °C, 5% CO2) in a volume of 180 µL L per well to allow recovery before treatment with test compounds. The test substances were dissolved in DMSO to a stock concentration of 1 mg/mL. Working solutions were made through serial dilution in RPMI 1640 media without FBS, with 20 µL added to each well to produce final compound concentrations of 10, 3, 1, 0.3, 0.1, 0.03, 0.01, 0.003, and 0.001 µg/mL. An equal volume of RPMI 1640 media without FBS was added to wells designated as negative controls for each plate. Plates were incubated for approximately 48 h before MTT staining (Mosmann, 1983). Plates were read at 570 and 630 nm using a ThermoElectron Multiskan Ascent plate reader to determine cell viability (Mosmann, 1983).
4.7. Yeast cytotoxicity assay
S. cerevisiae ABC16-Monster cells were inoculated from single colonies grown on agar plates stored at 4 °C into 2 mL of synthetic complete minimal media in 5 mL snap-cap culture tubes. The tubes were then incubated overnight at 250 RPM in a shaking incubator (Controlled Environment Incubator Shaker, Model G-25, New Brunswick Scientific Co., Inc.). Cultures were removed in mid-log phase (OD600 reading between 0.1 and 0.5) and the cells were then diluted to OD600 0.01. Aliquots of cells (30 µL) were added to a 384 well plate. Kalkipyrone A (1) and B (2) and yoshipyrone A (3) were reconstituted in DMSO (1.5%) at a starting concentration of 150 or 300 µM and fifteen serial dilutions (1:2) were performed for dosing. An initial OD600 reading was performed at time 0 and then the plate was covered with a lid in an incubator at 30 °C for 18 hr. The plates were shaken for 1 min on the ‘high’ setting in a Synergy HT spectrophotometer and then an OD600 measurement was immediately recorded.
IC50 values were determined by subtracting OD600 measurement at time 0 hr from that at 18 hr. Nonlinear regression was performed on the log (inhibitor) vs. response using the variable slope model. Minimum values are constrained to 0.0 in case any compounds have a higher OD value at time 0 hr than at 18 hr. For each determined IC50 value, three experimental replicates of two technical duplicates were performed. Fold changes in IC50 values were compared using a one-way ANOVA, followed by Dunnett’s post-hoc test when comparing multiple treatments to a control treatment.
4.8. DNA extraction, amplification, sequencing and phylogenetic analysis
Wet biomass of field-collected cyanobacterial tissue (ca. 5 g) from American Samoa were stored in RNA-preserving reagent RNAlater solution (Life Technologies), comprising two finger-sized tufts of cyanobacteria. Half of the sample was frozen using liquid N2 and ground via mortar and pestle into a powder. The pulverized frozen biomass was then subjected to the G20 Genomic Tip - Qiagen bacterial DNA isolation protocol following the manufacturer’s instructions (Qiagen). Primers used to amplify 16S rRNA sequences were GGGGAATYTTCCGCAATGGG (forward) and GGCTACCTTGTTACGACTT (reverse) (Nubel et al., 1997; Martinez-Murcia et al., 1995). The PCR reaction contained 2X Taq Master Mix (12.5 µL) (Promega), H2O (10 µL), forward and reverse primers (1 µL) at a final primer concentration of 0.4 µM, and DNA (1 µL) (~50 ng). The PCR cycling conditions were as follows: 95 °C for 1.5 min initial denaturation, followed by 35 cycles of 95 °C denaturation for 30 s, 50.5 °C annealing for 30 s, 72 °C for 2 min, with a final extension time of 4 min. PCR products were cloned via the TOPO-TA cloning kit (Life Technologies) into DH5α E. coli as per manufacturer’s instructions, grown overnight, then transferred into liquid media and grown overnight. Plasmids from five clones were harvested using the QIAprep Spin Miniprep kit (Qiagen), and Sanger sequenced with M13 primers. The 16S rRNA sequence of ASG15JUL14-6 was aligned with other cyanobacterial 16S rRNA genes using MUSCLE. The evolutionary history was inferred by using the Maximum Likelihood method based on the General Time Reversible model. A bootstrap consensus tree was inferred from 1000 replicates. Sequence alignments and phylogenetic trees were constructed in MEGA v. 5.2.
Supplementary Material
Highlights.
Bioassay guided isolation gave three γ-pyrones from a marine cyanobacterium
Kalkipyrone B was determined by NMR and Mosher’s ester analysis
Kalkipyrone A was cytotoxic while kalkipyrone A and B were equipotent antifungals
16S rRNA analysis identified the producing strain to be a Leptolyngbya species
Acknowledgments
We thank E. Glukhov for photomicrographs of the voucher specimen of Leptolyngbya sp. (ASG15Jul14-6). O. Demirkiran is grateful to The Scientific and Technological Research Council of Turkey (TUBITAK) for a fellowship that enabled her to conduct research at the Scripps Institution of Oceanography, UCSD. This work was supported by NIH Grants TW006634 and CA100851 (to WHG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data associated with this article can be found in the online version.
References
- Chang C, Sitachitta N, Rossi JV, Roberts MA, Flatt PM, Jia J, Sherman DH, Gerwick WH. Biosynthetic pathway and gene cluster analysis of curacin A, an antitubulin natural product from the tropical marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2004;67:1356–1367. doi: 10.1021/np0499261. [DOI] [PubMed] [Google Scholar]
- Driggers EM, Hale SP, Lee J, Terrett NK. The exploration of macrocycles for the drug discovery-an underexploited structural class. Nat. Rev. Drug Discov. 2008;7:608–624. doi: 10.1038/nrd2590. [DOI] [PubMed] [Google Scholar]
- Edwards DJ, Marquez BL, Nogle LM, McPhail K, Goeger DE, Roberts MA, Gerwick WH. Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbya majuscula. Chem. Biol. 2004;11:817–833. doi: 10.1016/j.chembiol.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan G, Banumathi B, Suresh G. Evaluation of the antifungal activity of natural xanthones from Garcinia mangostana and their synthetic derivatives. J. Nat. Prod. 1997;60:519–524. doi: 10.1021/np970165u. [DOI] [PubMed] [Google Scholar]
- Graber MA, Gerwick WH. Kalkipyrone, a toxic gamma-pyrone from an assemblage of the marine cyanobacteria Lyngbya majuscula and Tolypothrix sp. J. Nat. Prod. 1998;61:677–680. doi: 10.1021/np970539j. [DOI] [PubMed] [Google Scholar]
- Honma M, Kudo S, Takada N, Tanaka K, Miura T, Hashimoto M. Novel neofusapyrones isolated from Verticillium dahlia as potent antifungal substances. Bioorg. Med. Chem. Lett. 2010;20:709–712. doi: 10.1016/j.bmcl.2009.11.063. [DOI] [PubMed] [Google Scholar]
- Hoye T, Jeffrey CS, Shao F. Mosher ester analysis for the determination of absolute configuration of the stereogenic (chiral) carbinol carbons. Nat. Protoc. 2007;2:2451–2458. doi: 10.1038/nprot.2007.354. [DOI] [PubMed] [Google Scholar]
- Inuzuka T, Yamamoto K, Iwasaki A, Ohno O, Suenaga K, Kawazoe Y, Uemura D. An inhibitor of the adipogenic differentiation of 3T3-L1 cells, yoshinone A, and its analogs, isolated from the marine cyanobacterium Leptolyngbya sp. Tetrahedron Lett. 2014;55:6711–6714. [Google Scholar]
- Jones AC, Monroe EA, Eisman EB, Gerwick L, Sherman DH, Gerwick WH. The unique mechanistic transformations involved in the biosynthesis of modular natural products from marine cyanobacteria. Nat. Prod. Rep. 2010;27:1048–1065. doi: 10.1039/c000535e. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ogura H, Akasaka K, Oikawa T, Matsuura N, Imada C, Yasuda H, Igarashi Y. Nocapyrones: α - and α-pyrones from a marine-derived Nocardiopsis sp. Marine Drugs. 2014;12:4110–4125. doi: 10.3390/md12074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marel AK, Lizard G, Izard JC, Latruffe N, Delmas D. Inhibitory effects of trans-resveratrol analog molecules on the proliferation and the cell cycle progression of human colon tumoral cells. Mol. Nutr. Food Res. 2008;52:538–548. doi: 10.1002/mnfr.200700185. [DOI] [PubMed] [Google Scholar]
- Martínez-Murica AJ, Acinas SG, Rodriguez-Valera F. Evaluation of prokaryotic diversity by restrictase digestion of 16S rDNA directly amplified from hypersaline environments. FEMS Microbiol. Ecol. 1995;17:247–255. [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nübel U, Garcia-Pichel F, Muyzer G. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl. Environ. Microbiol. 1997;63:3327–3332. doi: 10.1128/aem.63.8.3327-3332.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnery JK, Mevers E, Gerwick WG. Biologically active secondary metabolites from marine cyanobacteria. Curr. Opin. Biotechnol. 2010;21:787–793. doi: 10.1016/j.copbio.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa JL, Bray WM, Lokey RS, Linington RG. Phenotype-guided natural products discovery using cytological profiling. J. Nat. Prod. 2015 doi: 10.1021/acs.jnatprod.5b00455. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleissner C, Pérez M, Losada A, Rodriguez P, Crespo C, Zúniga P, Fernández R, Reyes F, de la Calle F. Antitumor actinopyrones produced by Streptomyces ablus POR-04-15-053 isolated from a marine sediment. J. Nat. Prod. 2011;74:1590–1596. doi: 10.1021/np200196j. [DOI] [PubMed] [Google Scholar]
- Simmons TL, Coates RC, Clark BR, Engene N, Gonzalez D, Esquenazi E, Dorrestein PC, Gerwick WH. Biosynthetic origin of natural products isolated from marine organism-invertebrate assemblages. Proc. Natl. Acad. Sci. USA. 2008;105:4587–4594. doi: 10.1073/pnas.0709851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, St. Onge RP, Mani R, King OD, Heilbut A, Labunskyy VM, Chen W, Pham L, Zhang LV, Tong AH, Nislow C, Giaever G, Gladyshev VN, Vidal M, Schow P, Lehár J, Roth FP. Knocking out multigene redundancies via cycles of sexual assortment and fluorescence selection. Nat. Methods. 2011;8:159–164. doi: 10.1038/nmeth.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LT. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry. 2007;68:954–979. doi: 10.1016/j.phytochem.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Tan LT. Pharmaceutical agents from filamentous marine cyanobacteria. Drug Discov. Today. 2005;18:863–871. doi: 10.1016/j.drudis.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Werneburg M, Busch B, He J, Richter MEA, Xiang L, Moore BS, Roth M, Dahse H, Hertweck C. Exploiting enzymatic promiscuity to engineer a focused library of highly selective antifungal and antiproliferative aureothin analogues. J. Am. Chem. Soc. 2010;132:10407–10413. doi: 10.1021/ja102751h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xaio J, Kumazawa S, Yoshikawa N, Mikawa T, Sato Y. Dactylfungins, novel antifungal antibiotics produced by Dactylaria parvispora. J. Antibiotics. 1993;46:48–55. doi: 10.7164/antibiotics.46.48. [DOI] [PubMed] [Google Scholar]
- Yano KK, Yokoi K, Sato J, Oono J, Kouda T, Ogawa Y, Nakashima T. Actinopyrones A, B and C, new physiologically active substances. I. Producing organism, fermentation, isolation, and biological properties. J. Antibiotics. 1986a;39:32–37. doi: 10.7164/antibiotics.39.32. [DOI] [PubMed] [Google Scholar]
- Yano KK, Yokoi K, Sato J, Oono J, Kouda T, Ogawa Y, Nakashima T. Actinopyrones A, B and C, new physiologically active substances. II. Physico-chemical properties and chemical structures. J. Antibiotics. 1986b;39:38–43. doi: 10.7164/antibiotics.39.38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.