Abstract
Purpose
To evaluate and compare the diagnostic accuracy of the global and sector analyses for detection of early visual field damage using the retinal nerve fiber layer (RNFL) reference databases of the Spectralis and Cirrus spectral-domain optical coherence tomography (SD-OCT) devices.
Methods
Healthy subjects and glaucoma suspects from the Diagnostic Innovations in Glaucoma Study (DIGS) and African Descent and Glaucoma Evaluation Study (ADAGES) with at least 2 years of follow-up were included. Global and sectoral RNFL measures were classified as within normal limits, borderline and outside normal limits based on the device reference databases. The sensitivity of outside normal limits classification was estimated in glaucoma suspect eyes that developed repeatable visual field (VF) damage.
Results
353 glaucoma suspect eyes and 279 healthy eyes were included. 34 (9.6%) of glaucoma suspect eyes developed VF damage. In glaucoma suspect eyes, Spectralis and Cirrus outside normal limits classification was present in 47 eyes (13.3%) and 24 eyes (6.8%), respectively. The sensitivity of the global RNFL outside normal limits classification among eyes that developed VF damage was 23.5% for Cirrus and 32.4% for Spectralis. The specificity of within normal limits global classification in healthy eyes was 100% for Cirrus and 99.6% for Spectralis. There was moderate to substantial agreement between Cirrus and Spectralis classification as outside normal limits.
Conclusions
The Spectralis and Cirrus reference databases have a high specificity for identifying healthy eyes, and good agreement for detection of eyes with early glaucoma damage.
INTRODUCTION
Glaucoma is a progressive optic neuropathy characterized by loss of retinal ganglion cells (RGCs) and associated morphological changes to the optic nerve and retinal nerve fiber layer (RNFL). [1] For many patients, structural changes in the neuroretinal rim and RNFL precede the detection of visual field (VF) deficits in early glaucoma, [2] [3] emphasizing that structural assessment of the optic nerve is an essential component of timely glaucoma diagnosis and management. [4–8]
The last two decades have seen a proliferation of imaging instruments that provide objective and quantitative measures of retinal tissue that cannot be assessed with standard fundus photography. Most recently, spectral domain optical coherence tomography (SD-OCT) has allowed clinicians to obtain unprecedented high-resolution images of the optic nerve head (ONH) and RNFL and has become the standard of care for management of many ophthalmic conditions. [9, 10] SD-OCT instruments often use proprietary reference databases composed of measurements of healthy eyes to set limits of normality for optic disc, RNFL and ganglion cell measurements. Classification as within normal limits (WNL), borderline (BL) and outside normal limits (ONL) provide clinicians with a reference for making clinical decisions. [11]
In the United States, the Food and Drug Administration regulates the commercialization of the reference databases, but there are no standards or guidelines for the types or numbers of subjects that should be included or how the data should be analyzed or presented in the reference databases. [12] In addition, there is sparse literature evaluating the accuracy of the reference database algorithms for detection of glaucomatous structural damage, with reports of false-positive RNFL results in healthy eyes. [13, 14] Moreover, to our knowledge, there are no published reports comparing the agreement between different SD-OCT instruments when their specific databases are used to classify glaucoma and healthy eyes.
The purpose of this study is to evaluate and compare the diagnostic accuracy of Spectralis (Heidelberg Engineering, Heidelberg Germany) and Cirrus (Carl Zeiss Meditec, Dublin, CA) SD-OCT global and sector RNFL classification for detection of early glaucomatous changes.
METHODS
Description of Study Population
This was an observational cross-sectional study. Healthy participants and glaucoma suspects without repeatable visual field damage at baseline with at least two years of follow-up were included from two prospective longitudinal studies designed to evaluate optic nerve structure and visual function in glaucoma; the African Descent and Glaucoma Evaluation Study (ADAGES) and the Diagnostic Innovations in Glaucoma Study (DIGS). The three-site ADAGES collaboration includes the Hamilton Glaucoma Center at the Department of Ophthalmology, University of California, San Diego (UCSD) (Data Coordinating Center), New York Eye and Ear Infirmary, and the Department of Ophthalmology, University of Alabama at Birmingham (UAB). DIGS includes only patients recruited at UCSD and used protocols identical to that of ADAGES. Methodological details have been described previously. [15] Institutional Review Board (IRB)/Ethics Committee approval was obtained. Written informed consent was obtained from all participants. The institutional review boards and human subjects committees of all three sites (University of California, San Diego, CA, New York Eye and Ear Infirmary, New York, NY;, and University of Alabama at Birmingham, Birmingham, AL) approved all methods used to conduct this study. All methods adhered to the tenets of the Declaration of Helsinki for research involving human subjects and to the Health Insurance Portability and Accountability Act. DIGS and ADAGES were registered at http://cilincaltrials.gov (NCT00221897 and NCT00221923, respectively) on September 14, 2005.
All subjects had open angles on gonioscopy. Subjects were excluded if they presented at study entry with a best-corrected visual acuity less than 20/40, spherical refraction greater than 5.0 diopters and/or cylinder correction greater than 3.0 diopters, or any other ocular or systemic disease that could affect the optic nerve or the visual field.
All subjects underwent an annual comprehensive ophthalmologic examination including review of medical history, best-corrected visual acuity, slit-lamp biomicroscopy, dilated funduscopic examination and stereoscopic optic disc photography. Semi-annual examination included intraocular pressure (IOP), SD-OCT imaging and visual field testing.
Healthy subjects were recruited from the general population through advertisements, from family members of patients, and from primary eye care clinics at the three study centers. Healthy eyes had an IOP of 21 mmHg or less with no history of elevated IOP, healthy-appearing optic disc and no visual field abnormalities (pattern standard deviation (PSD) within 95% confidence limits and a glaucoma hemifield test (GHT) result within normal limits). Glaucoma suspect eyes were defined as eyes with ocular hypertension (OHT, IOP > 21 mmHg in the presence of a healthy-appearing optic disc and normal visual field), a history of elevated IOP, or an optic disc appearance suspicious of glaucoma (neuroretinal rim thinning or RNFL defects on masked simultaneous stereophotograph assessment) in the presence of a normal visual field at the time of SD-OCT imaging. [15]
An abnormal visual field result was defined as having a pattern standard deviation outside the 95% confidence limits or a glaucoma hemifield test (GHT) result outside the reference range on the Swedish interactive threshold algorithm (SITA Standard 24-2) of the Humphrey visual field analyzer (Carl Zeiss Meditec, Inc., Dublin, California, USA). An optic disc suspicious of glaucomatous optic neuropathy (GON) was defined as suspicion of neuroretinal rim thinning or RNFL defects on masked simultaneous stereophotograph assessment by two experienced graders. Adjudication or consensus grading resolved disagreements in photograph assessment.
Standard Automated Perimetry
All patients underwent visual field testing using the SITA-standard 24-2 strategy within 30 days of SD-OCT imaging. All visual fields were evaluated by the UCSD Visual Field Assessment Center (VisFACT). [16] Visual fields with more than 33% fixation losses or false-negative errors, or more than 15% false-positive errors were excluded. The only exception was the inclusion of visual fields with false-negative errors of more than 33% when the field showed advanced disease (visual field MD worse than −12 dB). Visual fields exhibiting a learning effect (i.e. initial tests showing consistent improvement on visual field indices) were also excluded. Visual fields were further reviewed for the following artifacts: lid and rim artifacts, fatigue effects, inappropriate fixationm evidence that the visual field results were due to a disease other than glaucoma (such as homonymous hemianopia) and inattention.
Optical Coherence Tomography
Each subject was required to have a good-quality Cirrus SD-OCT and a Spectralis SD-OCT acquired on the same day. The most recent good quality images (as determined by the Imaging Data Analysis and Evaluation (IDEA) Reading Center) were included in the analysis for non-progressing eyes. Images obtained within 6 months of the date progression was detected were included for progressing eyes that developed VF damage.
The Cirrus SD-OCT uses a superluminescent diode laser with a center wavelength of 840 nm and an acquisition rate of 27,000 A-scans per second. [17] The protocol used for RNFL thickness evaluation was the optic disc cube. This protocol is based on a tridimensional scan of a 6 × 6-mm2 area centered on the optic disc where information from a 1024 (depth) × 200 × 200-point parallelepiped is collected. Image acquisition time is approximately 2 seconds. Parapapillary RNFL thickness is measured along a 3.46-mm-diameter circular automatically placed around the optic disc; there are approximately 362 A-scans sampled to obtain these RNFL parapapillary measurements. Based on IDEA Center review, well-centered scans with signal strength ≥7 and the absence of movement artifact were included.
The Cirrus SD-OCT algorithm provides RNFL thickness measurement of the global, superior, nasal, inferior, temporal and twelve clock-hour sectors along the measurement circle. [2] The patient’s RNFL scan results are compared with reference data and are presented as color-coded maps. The Cirrus age-adjusted reference database has been described previously. [18] In short, it is composed of 284 healthy Caucasian (43%), Asian (24%), African (18%), Hispanic (12%) and Indian (1%) subjects between the ages of 19 and 84. Subjects with IOP > 21 mm Hg, refractive error outside the range of −12 to 8 diopters, and/or evidence of visual field damage, and/or RNFL defect or disc hemorrhage were not included in the reference database. [18] White, green, yellow and red are color codes which depict RNFL thicknesses that are within the 95% of the reference database measurements (WNL), thinner than the reference database range at the 5% level (BL) and thinner than the reference database range at the 1% level (outside normal limits) respectively. Sector outside normal limits was defined as at least 1 of 12 Cirrus clock hours classified as outside normal limits.
Spectralis OCT uses a dual-beam SD-OCT, a confocal laser-scanning ophthalmoscope (CSLO) with a wavelength of 870 nm and an infrared reference image to obtain images of ocular microstructures. [17] The instrument has an acquisition rate of 40,000 A-scans per second. Spectralis OCT incorporates a real-time eye tracking system that couples CSLO and SD-OCT scanners to adjust for eye movements and to ensure that the same location of the retina is scanned over time. The protocol used was the high resolution RNFL circle scan, which consists of 1536 A-scan points from a 3.45-mm circle centered on the optic disc. All patients had their corneal curvature inputted into the machine before the examination. These A-scans are averaged into 768 data points. Image acquisition time is approximately 3 seconds. The scan time varies due to the Spectralis eye tracking system which enables the capture of multiple B-scans in the exact same location and due to the automatic real time (ART) mean function which combines these B-scans to reduce noise and improve the signal to noise ratios of the images. For these reasons, the scan time ranges between 1–5 seconds depending on eye movement and image quality. Based on IDEA Center review, well-centered scans with a signal strength ≥15 dB and the absence of movement artifact were included.
The Spectralis SD-OCT age-adjusted reference database is composed of 201 healthy Caucasian subjects between the ages of 18 and 78 years. Subjects with diabetes, IOP > 21 mm Hg, evidence of visual field damage, refractive error worse than -7 diopters, and/or a family history of glaucoma were not included in the reference database (personal communication; A. Tafreshi, Heidelberg Engineering). The algorithms provide RNFL thickness measurements of the global, temporal superior, nasal superior, temporal, nasal, temporal inferior and nasal inferior sectors. Green, yellow and red are color codes, which depict RNFL thicknesses that are within the 95% of the reference database measurements (WNL), thinner than the reference database range at the 5% level (BL) and thinner than the reference database range at the 1% level (outside normal limits) respectively. Sector outside normal limits was defined as ≥ 1 of 7 Spectralis sectors classified as outside normal limits.
Statistical Analysis
The specificity of within normal limits (WNL) classification was estimated in healthy eyes and the sensitivity of outside normal limits (ONL) classification was estimated at the time glaucoma suspect eyes developed repeatable visual field damage (VFD) and/or abnormal photos. Kappa agreement was estimated between Cirrus and Spectralis images with interpretation as suggested by Landis and Koch. [19] McNemar’s (Stuart-Maxwell) tests were used to assess the difference in proportions. We acquire images on both eyes, and given that the most efficient use of our data is to include both eyes in the analysis and that glaucoma often presents first in one eye we included both eyes in the analysis.
RESULTS
The study included 632 eyes of 353 subjects (279 healthy eyes (149 subjects) and 353 glaucoma suspect eyes (204 subjects)) (Table 1). One hundred ninety-nine of the 353 glaucoma suspect eyes (56.4%) had glaucomatous optic neuropathy (GON) without visual field abnormalities, and 154 (43.6%) had ocular hypertension (OHT). Of the 353 glaucoma suspect eyes, 34 (9.6%) developed evidence of progressive glaucoma (the development of glaucomatous visual field defects) during follow-up. The mean age of 204 glaucoma suspects and 149 healthy participants was 65.6 ± 12 years and 54.4 ± 14.8 years, respectively. Fifty percent of the healthy eyes were of African descent, while 31% of the glaucoma suspects and 41% of progressors were of African descent. The average MD of the most recent VF for healthy, glaucoma suspect and progressing eyes was −0.042 ± 1.3 dB, −0.12 ± 1.6 dB and −1.97 ± 2.2 dB, respectively.
Table 1.
Demographic and Ocular Characteristics of the Healthy and Glaucoma Suspect Participants.
| Characteristic | Healthy | Glaucoma Suspects (GON or OHT) |
|
|---|---|---|---|
| Did not Develop Visual Field Damage |
Developed Visual Field Damage |
||
| Number of subjects (eyes) | 149 (279) | 173 (319) | 32 (34) |
| Mean age (95% CI), years | 54.4 (14.8) | 65.5 (63.6, 67.3) | 66.7 (62.7, 70.8) |
| Race % African descent | 50% | 29% | 42% |
| Mean MD (95% CI), dB | −0.042 (1.312) | 0.08 (−0.07, 0.23) | −1.97 (−2.7, −1.2) |
| Mean Cirrus global retinal nerve fiber layer thickness (um)* | 93.85 (92.7, 95.0) | 84.9 (83.6, 86.1) | 74.9 (70.9, 79.0) |
| Mean Spectralis global retinal nerve fiber layer thickness (um)* | 98.60 (97.4, 99.8) | 87.92 (86.6, 89.2) | 80.25 (74.21, 86.3) |
| % Eyes Cirrus global retinal nerve fiber layer thickness outside normal limits | 0% | 5% | 23.5% |
| % Eyes Spectralis global retinal nerve fiber layer thickness outside normal limits | 0.4% | 11.3% | 32.4% |
From most recent visit
The specificity of global RNFL thickness classification as within normal limits (WNL) or borderline (BL) among healthy eyes was excellent for both Cirrus (279/279 (100%)) and Spectralis (278/279 (99.6%)). The specificity of having all sectors and global RNFL thickness within normal limits or borderline was also high for both Cirrus and Spectralis (94.3% and 96.4%, respectively). The sensitivity for identifying progressing eyes at the visit when progression was first detected with at least one RNFL sector outside normal limits (ONL) was 50% for Cirrus and 52.9% for Spectralis (Table 2). The sensitivity of global RNFL classification of ONL in progressors at the time when progressive visual field or optic disc changes were first detected was lower for both Cirrus and for Spectralis, 23.5% and 32.4%, respectively. Few Of the 154 OHT eyes, few (14 (9%)) developed VF damage. Of the 140 OHT eyes that did not develop VFD, 16 (11.4%) had an outside normal limits sector classification on Cirrus and 15 (10.7%) on Spectralis at their last follow-up visit.
Table 2.
Specificity and Sensitivity of Cirrus and Spectralis RNFL Global and Sector Analysis
| Specificity (n=279 healthy eyes) |
Specificity (n=140 OHT eyes that did not develop VF damage) |
Specificity (n=154 GON eyes that did not develop VF damage) |
Sensitivity (n=34 Developed visual field damage) |
|||||
|---|---|---|---|---|---|---|---|---|
|
No RNFL sectors Outside Normal Limits |
No Global sectors Outside Normal Limits |
No RNFL sectors Outside Normal Limits |
Global RNFL Not Outside Normal Limits |
No RNFL sectors Outside Normal Limits |
Global RNFL Not Outside Normal Limits |
≥1 RNFL sector Outside Normal Limits |
Global RNFL Outside Normal Limits |
|
| Cirrus | 263 (94.3%) | 279 (100%) | 118 (84.3%) | 137 (97.9%) | 148 (82.7%) | 166 (92.7%) | 17 (50.0%) | 8 (23.5%) |
| Spectralis | 269 (96.4%) | 278 (99.6%) | 125 (89.3%) | 133 (95.0%) | 138 (77.1%) | 150 (83.8%) | 18 (52.9%) | 11 (32.4%) |
RNFL: retinal nerve fiber layer
Agreement between instruments
There was moderate agreement between instruments for classification as a 2×2 comparison of outside normal limits (ONL) versus not ONL (exact agreement = 91.8%, kappa=0.55, Table 3) and for classification as a 3×3 comparison of outside normal limits, borderline, and within normal limits (exact agreement =82.7%, weighted kappa=0.64, Table 4). For 2×2 overall exact analysis, Spectralis identified a significantly larger proportion of eyes as outside normal limits based on global RNFL 47/353 (13.3%) compared to Cirrus 24/353 (6.8%), (p<0.0001). However, most of the disagreement between instruments was between borderline and outside normal limits or borderline and within normal limits for specific global or sector classifications as illustrated in Figures 1 and 2.
Table 3.
Moderate Agreement between Cirrus and Spectralis Global RNFL Outside Normal Limits (ONL) Versus Not Outside Normal Limits (H, WNL and BL) Classification of Glaucoma Suspects. kappa=0.55
| Spectralis | Total | ||
|---|---|---|---|
| WNL,BL | ONL | ||
|
Cirrus H,WNL,BL ONL |
303 | 26 | 329 (93.2%) |
| 3 | 21 | 24 (6.8%) | |
| Total | 306 (86.7%) | 47 (13.3%) | 353 |
Table 4.
Substantial Agreement between Cirrus and Spectralis Global RNFL Outside Normal Limits (ONL), Borderline (BL) and Within Normal Limits (H and WNL) Classification in Glaucoma Suspects. Weighted kappa=0.64
| Spectralis | Total | |||
|---|---|---|---|---|
| WNL | BL | ONL | ||
|
Cirrus H,WNL BL ONL |
248 | 22 | 8 | 278 (78.8%) |
| 10 | 23 | 18 | 51 (14.4%) | |
| 1 | 2 | 21 | 24 (6.8%) | |
| Total | 259 (73.4%) | 47 (13.3%) | 47 (13.3%) | 353 |
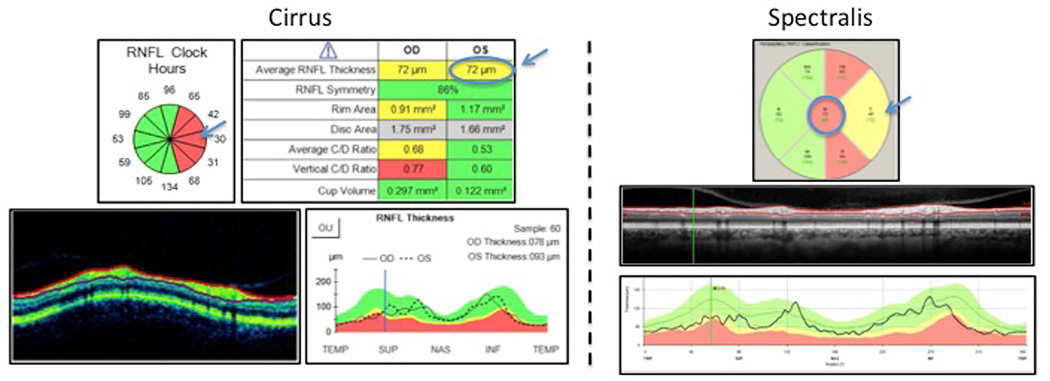
Figure 1.
Case 1. Global RNFL Classification Disagreement between Cirrus (borderline) and Spectralis (outside normal limits) in Glaucoma Suspects
Left: Cirrus scan showing global retinal nerve fiber layer classification as yellow, borderline (top right arrow), while most temporal clock hours are red, outside normal limits (left arrow). Right: Spectralis showing global retinal nerve fiber layer classification as red, outside normal limits (top arrow), while most temporal superior and temporal inferior sectors are red, outside normal limits (left arrow). Although global classification differs, both instruments identify temporal sectors (Spectralis) and clock hours (Cirrus) as outside normal limits.
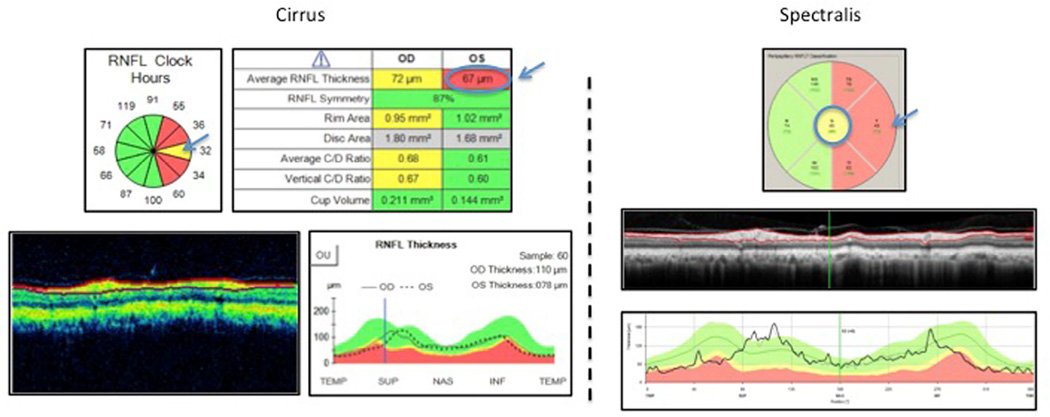
Figure 2.
Case 2. Global RNFL Classification Disagreement between Cirrus (outside normal limits) and Spectralis (borderline) in Glaucoma Suspects
Left: Cirrus scan showing global retinal nerve fiber layer classification as red, outside normal limits (top right arrow), while most temporal clock hours are red, outside normal limits (left arrow). Right: Spectralis showing global retinal nerve fiber layer classification as yellow, borderline (top arrow), while most temporal superior and temporal inferior sectors are red, outside normal limits (left arrow). Although global classification differs, both instruments identify temporal sectors (Spectralis) and clock hours (Cirrus) as outside normal limits.
In Figure 1, Cirrus (left) global RNFL classified as borderline (yellow), whereas Spectralis (right) as outside normal limits (ONL) (red), while in Figure 2 Cirrus (left) global RNFL classified as ONL (red) whereas Spectralis (right), borderline (yellow). However in both cases, there was overall consistency between instruments. The sectors in the temporal region of the optic disc were classified as outside normal limits.
DISCUSSION
The ability to discriminate healthy from glaucomatous optic nerves is limited by the quality of reference databases of imaging platforms. Our results suggest that Spectralis and Cirrus reference databases have excellent specificity (>99%) for correctly identifying healthy eyes and have modest sensitivity for detecting the first signs of progressive glaucoma in glaucoma suspect eyes. We found that while maintaining high specificity (>94%), Spectralis and Cirrus tend to have higher sensitivity using the definition of glaucoma as at least 1 RNFL sector outside normal limits than global RNFL outside normal limits. We also found generally good agreement between Cirrus and Spectralis reference database classification as outside normal limits, with most disagreements between a borderline classification with either an outside normal limits or within normal limits classification. Moreover, when there was a disagreement in the classification of a specific sector, there was often agreement on the classification of other sectors so that overall clinical interpretation would be similar across instruments.
Our results confirm previous results evaluating the diagnostic accuracy of the Spectralis and Cirrus reference databases. Jeoung et al, in a study that included pre-perimetric glaucoma participants with localized RNFL defects and healthy subjects reported the sensitivity and specificity of Spectralis ≥ 1 sector outside normal limits as 48.7% and 100.0%, respectively. [20] These results are very similar to our findings of a sensitivity of 52.9% for detecting early glaucomatous damage, at a high specificity of 96.4% (Table 2). Chang et al, in a study that included patients with more severe glaucoma than the current study reported a higher Cirrus sensitivity of 85% and a similar specificity of 94% for ≥ 1 sector outside normal limits to our results of 50% and 94.3%, respectively. [21] Differences in the severity of glaucoma patients included likely account for the differences in the specificities and sensitivities reported. Our study is unique in that it directly compares the diagnostic accuracy of reference database derived RNFL classifications of two SDOCT instruments from scans acquired on the same day.
Currently, each manufacturer’s database differs in size, eligibility criteria, and ethnic makeup. [12] The inclusion and exclusion criteria for subjects included in the Spectralis and Cirrus RNFL reference databases are similar but not identical. Both reference databases exclude subjects with visual field damage, elevated IOP, and high myopia (−7 diopters or more for Spectralisbe −10 diopters for Cirrus). In contrast to the Cirrus, which includes a mix of ethnicities, the Spectralis reference database in this study is comprised of Caucasians only and excludes subjects with a family history of glaucoma. It has been suggested that reference databases should include different ethnicities or have databases for different ethnicities, to take into account racial and ethnic differences in ocular phenotypes such as disc size, RNFL thickness and cup volume. [18, 22] [23] Others suggest that it may be possible to account for much of the racial variation by adjusting for disc size since optic disc size is easily measured, explains a large proportion of the variability in optic nerve head parameters and explains much of the variability by race without the need for separate ethnicity-specific reference databases. [12] [24] In this study, the Spectralis Caucasian only reference database performed as well as the ethnically mixed Cirrus reference database without statistical adjustment for optic disc size in either database. Moreover the specificity in our healthy eyes, which included 50% individuals of African descent, was excellent in both reference databases, suggesting that ethnic specific references databases may not improve detection of RNFL damage. The similar performance of the two instrument reference databases may be due in part to the smaller ethnic and racial differences in RNFL thickness compared to other optic nerve head parameters such as rim area and cup area. However, our study suggests that RNFL thicknesses are less variable across ancestry groups than other structural feature such as optic disc structural parameters.
There are several limitations to this study, which include a relatively small sample size and limited by design to early glaucoma. However, if there were differences in classification between instrument reference databases, the differences would be more pronounced in eyes with early glaucoma, and clinicians often rely on these measurements to assist in the differential diagnosis of glaucoma in glaucoma suspect eyes. [4] We therefore focused our analysis on eyes with the earliest signs of glaucoma.
To increase the utility of SD-OCT in the clinical management of glaucoma, RNFL thickness is compared against a database of subjects who do not have disease in order to provide clinicians with a reference range for comparison to an individual’s measurement. [12] These references databases can describe the likelihood that the RNFL thickness value is within or outside normal limits, but cannot determine the documented outside normal limits RNFL measurement is due to glaucoma. For clinical management decisions, these reference-databases based classifications should be used in conjunction with a thorough clinical examination and visual field testing.
In conclusion, the Spectralis and Cirrus reference databases showed moderate to substantial agreement in the classification of glaucoma suspect eyes, with and without signs of early progressive changes. In addition both reference databases had a high specificity for identifying healthy eyes. Longer follow up is needed to determine whether glaucoma suspect eyes classified as outside normal limits without visual field damage or progressive optic disc change will develop glaucomatous damage in the future.
Acknowledgments
Funding/Support: Supported by National Eye Institute, Eyesight Foundation of Alabama (Birmingham, Alabama, USA), the Edith C. Blum Research Fund of the New York Glaucoma Research Institute (New York City, New York, USA), Japan Eye Bank Association, Overseas Research Grant (Tokyo, Japan), Research to Prevent Blindness, an unrestricted grant (New York City, New York, USA), P30EY022589, EY08208 (LMZ), U10EY14267 (LMZ), EY019869 (LMZ), EY11008 (LMZ), EY021818 (FAM), EY022039 (CB), and EY13959. Alcon Laboratories Inc. (Fort Worth, Texas, USA), Allergan Inc. (Irvine, California, USA), Pfizer Inc. (New York City, New York, USA), Merck Inc. (Whitehouse Station, New Jersey, USA), Santen Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Marvasti AH, et al. The relationship between visual field index and estimated number of retinal ganglion cells in glaucoma. PLoS One. 2013;8(10):e76590. doi: 10.1371/journal.pone.0076590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang YH, et al. Ability of cirrus high-definition spectral-domain optical coherence tomography clock-hour, deviation, and thickness maps in detecting photographic retinal nerve fiber layer abnormalities. Ophthalmology. 2013;120(7):1380–1387. doi: 10.1016/j.ophtha.2012.12.048. [DOI] [PubMed] [Google Scholar]
- 3.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26(6):688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lisboa R, et al. Comparison of different spectral domain OCT scanning protocols for diagnosing preperimetric glaucoma. Invest Ophthalmol Vis Sci. 2013;54(5):3417–3425. doi: 10.1167/iovs.13-11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson CA, et al. Structure and function evaluation (SAFE): II. Comparison of optic disk and visual field characteristics. Am J Ophthalmol. 2003;135(2):148–154. doi: 10.1016/s0002-9394(02)01930-x. [DOI] [PubMed] [Google Scholar]
- 6.Johnson CA, et al. The relationship between structural and functional alterations in glaucoma: a review. Semin Ophthalmol. 2000;15(4):221–233. doi: 10.3109/08820530009037873. [DOI] [PubMed] [Google Scholar]
- 7.Harwerth RS, et al. Neural losses correlated with visual losses in clinical perimetry. Invest Ophthalmol Vis Sci. 2004;45(9):3152–3160. doi: 10.1167/iovs.04-0227. [DOI] [PubMed] [Google Scholar]
- 8.Medeiros FA, et al. The structure and function relationship in glaucoma: implications for detection of progression and measurement of rates of change. Invest Ophthalmol Vis Sci. 2012;53(11):6939–6946. doi: 10.1167/iovs.12-10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leitgeb R, Hitzenberger C, Fercher A. Performance of fourier domain vs. time domain optical coherence tomography. Opt Express. 2003;11(8):889–894. doi: 10.1364/oe.11.000889. [DOI] [PubMed] [Google Scholar]
- 10.Leung CK, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a variability and diagnostic performance study. Ophthalmology. 2009;116(7):1257–1263. 1263 e1–1263.e2. doi: 10.1016/j.ophtha.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Bendschneider D, et al. Retinal nerve fiber layer thickness in normals measured by spectral domain OCT. J Glaucoma. 2010;19(7):475–482. doi: 10.1097/IJG.0b013e3181c4b0c7. [DOI] [PubMed] [Google Scholar]
- 12.Realini T, et al. Normative Databases for Imaging Instrumentation. J Glaucoma. 2014 doi: 10.1097/IJG.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung CK, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: patterns of retinal nerve fiber layer progression. Ophthalmology. 2012;119(9):1858–1866. doi: 10.1016/j.ophtha.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 14.Kim NR, et al. Spectral-domain optical coherence tomography for detection of localized retinal nerve fiber layer defects in patients with open-angle glaucoma. Arch Ophthalmol. 2010;128(9):1121–1128. doi: 10.1001/archophthalmol.2010.204. [DOI] [PubMed] [Google Scholar]
- 15.Sample PA, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127(9):1136–1145. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Racette L, et al. African Descent and Glaucoma Evaluation Study (ADAGES): III. Ancestry differences in visual function in healthy eyes. Arch Ophthalmol. 2010;128(5):551–559. doi: 10.1001/archophthalmol.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leite MT, et al. Agreement among spectral-domain optical coherence tomography instruments for assessing retinal nerve fiber layer thickness. Am J Ophthalmol. 2011;151(1):85–92. e1. doi: 10.1016/j.ajo.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 18.Knight OJ, et al. Effect of race, age, and axial length on optic nerve head parameters and retinal nerve fiber layer thickness measured by Cirrus HD-OCT. Arch Ophthalmol. 2012;130(3):312–318. doi: 10.1001/archopthalmol.2011.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 20.Jeoung JW, et al. Diagnostic ability of spectral-domain versus time-domain optical coherence tomography in preperimetric glaucoma. J Glaucoma. 2014;23(5):299–306. doi: 10.1097/IJG.0b013e3182741cc4. [DOI] [PubMed] [Google Scholar]
- 21.Chang RT, et al. Sensitivity and specificity of time-domain versus spectral-domain optical coherence tomography in diagnosing early to moderate glaucoma. Ophthalmology. 2009;116(12):2294–2299. doi: 10.1016/j.ophtha.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Zangwill LM, et al. Racial differences in optic disc topography: baseline results from the confocal scanning laser ophthalmoscopy ancillary study to the ocular hypertension treatment study. Arch Ophthalmol. 2004;122(1):22–28. doi: 10.1001/archopht.122.1.22. [DOI] [PubMed] [Google Scholar]
- 23.Rao HL, Babu GJ, Sekhar GC. Comparison of the diagnostic capability of the Heidelberg Retina Tomographs 2 and 3 for glaucoma in the Indian population. Ophthalmology. 2010;117(2):275–281. doi: 10.1016/j.ophtha.2009.06.062. [DOI] [PubMed] [Google Scholar]
- 24.Girkin CA, et al. The effects of race, optic disc area, age, and disease severity on the diagnostic performance of spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(9):6148–6153. doi: 10.1167/iovs.10-6698. [DOI] [PubMed] [Google Scholar]