Abstract
X-chromosome-linked inhibitor of apoptosis protein (XIAP) has an important regulatory role in programmed cell death by inhibiting the caspase cascade. Activation of XIAP-dependent signaling culminates into regulation of multiple cellular processes including apoptosis, innate immunity, epithelial-to-mesenchymal transition, cell migration, invasion, metastasis and differentiation. Although XIAP localizes to the cytosolic compartment, XIAP-mediated cellular signaling encompasses mitochondrial and post-mitochondrial levels. Recent findings demonstrate that XIAP also localizes to mitochondria and regulates mitochondria functions. XIAP acts upstream of mitochondrial cytochrome c release and modulates caspase-dependent apoptosis. The new function of XIAP has potential to enhance mitochondrial membrane permeabilization and other cellular functions controlling cytochrome c release. These findings could exploit the overexpression of XIAP in human tumors for therapeutic benefits.
Keywords: Mitochondria, apoptosis, cancer therapy, X-linked inhibitor of apoptosis protein, mitochondria permeabilization, Bax
Introduction
The inhibitor of apoptosis proteins (IAPs) are potentially involved in multiple cellular signaling pathways including cell death, immunity, inflammation, cell cycle and cell migration [1,2]. Members of the IAP family have the unique ability to regulate cell death and survival induced by numerous stimuli [1,3,4]. IAPs were first identified in the baculoviral genome in 1993 by genetic screening, and were found to be inhibitors of apoptosis, a form of programmed cell death [5,6]. X-chromosome-linked IAP (XIAP) is a key member of the IAP protein family and a central regulator of apoptotic cell death [6]. XIAP is ubiquitously expressed in all human adult and fetal tissues except in peripheral blood leukocytes [7]. XIAP inhibits active caspase-9, −3 and −7, thus functioning as an endogenous inhibitor of caspase-dependent apoptotic cell death [6,8–11].
Alterations in IAPs including XIAP overexpression in numerous types of human cancer associate with chemoresistance, progression of disease and poor prognosis [12–14]. Additionally, XIAP also provides apoptosis resistance to post-mitotic cardiomyocytes and sympathetic neurons [15,16]. One of the features of mitochondrial apoptosis is the mitochondrial outer membrane permeabilization (MOMP) leading to the release of cytochrome c into the cytosol which triggers the activation of caspases [17–19]. The findings from the past few years suggest that XIAP also regulates mitochondrial membrane permeabilization, which provides avenues to enhance apoptosis and develop new agents for cancer therapy [20–23]. In this review, we briefly discuss the impact of established and new functions of XIAP in regulating apoptotic cell death in cancer via modulation of mitochondrial permeabilization.
Types and functions of IAPs
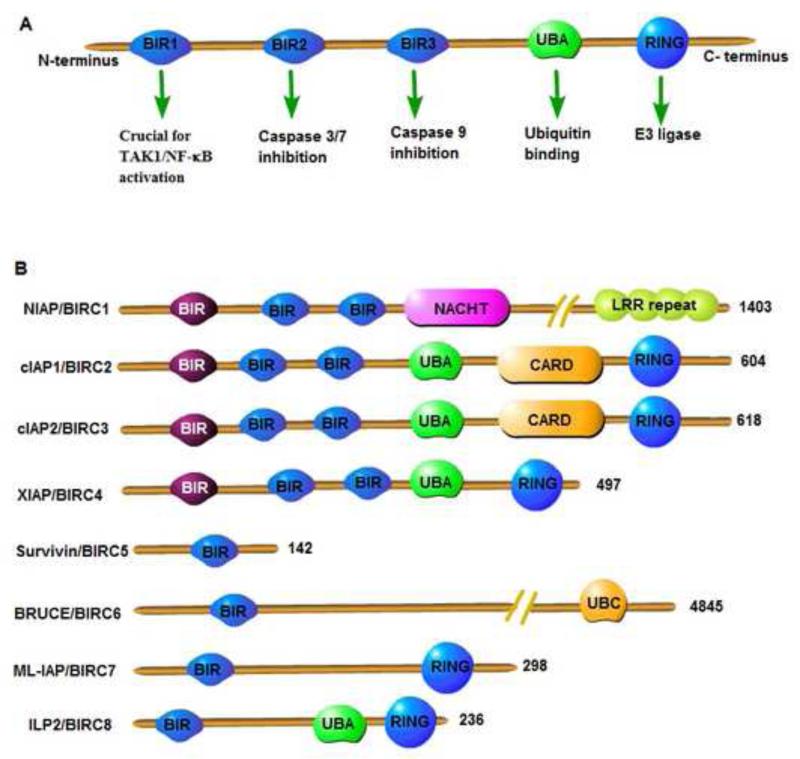
IAPs were first identified in a genetic screen designed to evaluate suppressors of apoptosis and were called baculovirus IAP repeat (BIR)-containing proteins (BIRCs) [5,24]. Currently, the mammalian IAP family has eight members characterized by the presence of 1–3 BIR domains (Figure 1). Neuronal AIP[s1]/BIRC1 contains three BIR domains, a NACHT domain and a leucine-rich repeat (LRR), which can modulate innate immune signaling [25]. Cellular IAP (c-IAP)1/[s2]BIRC2 and c-IAP2[s3]/BIRC3 have three BIR domains, one ubiquitin-associated domain (UBA), one caspase-recruitment domain (CARD) and a really interesting new gene (RING) domain. The RING domain imparts the E3 ubiquitin ligase activity to IAPs and is important for mono- or poly-ubiquitination of substrate proteins and other IAPs. The UBA domain binds to mono- or poly-ubiquitin chains to promote protein complex assembly during cellular signaling. XIAP/BIRC4 is the most studied member of the IAP family and has three BIR domains, one UBA and one RING domain. Additional functions of XIAP are described in detail in the next section. Survivin/BIRC5 is the smallest protein of the group and has only one BIR domain. Survivin has been shown to play a part in chromosome segregation and spindle checkpoint control during cell division [26,27]. BRUCE[s4]/Apollon/BIRC6 contains one BIR domain and lacks the UBA and RING domains, but interestingly has one ubiquitin conjugation (UBC) domain. The UBC domain of BRUCE can catalyze the conjugation of ubiquitin to the substrates such as second mitochondria-derived activator of caspase (Smac) [28]. BRUCE is required for placental development and cellular differentiation [29]. Melanoma IAP (ML-IAP)/[s5]Livin/BIRC7 contains one BIR domain and one RING domain and it is highly expressed in melanomas. ML-IAP has been shown to be involved in cell migration of melanoma cells but dispensable for mouse development [30,31]. Lastly, IAP-like protein 2/ILP[s6]2/BIRC8 is the final member of the group and has one BIR domain, one UBA domain and one RING domain. It is a tissue-specific homolog of XIAP and could be important in inhibiting caspase-9-mediated apoptosis with the help from an as yet unidentified protein [32,33].
Figure 1.
[s10]Types and domains of mammalian inhibitor of apoptosis proteins (IAPs). (a) Functional domains of IAPs. (b) Types of mammalian IAPs. Abbreviations: BIR, baculoviral IAP repeat domain; CARD, caspase recruitment domain; LRR, leucine-rich repeats; NACHT/NOD, nucleotide-binding and oligomerization domain; RING, really interesting new gene domain; UBA, ubiquitin-associated domain; UBC, ubiquitin conjugating domain.
Regulation of IAP function occurs at multiple levels and IAPs can be auto-ubiquitinated or cross-ubiquitinated by their RING domain [34,35]. Endogenous inhibitors of IAPs such as Smac/DIABLO and Omi/HtrA2 [s7]also regulate the levels and function of IAPs by competitively binding the BIR2 and BIR3 domains of XIAP. As a result the binding of caspases to XIAP and subsequent caspase sequestration by XIAP will be prevented [36]. Gene deletion of most IAP proteins has been carried out and single gene knockout studies in mice show that c-IAP1−/−, c-IAP2−/− and XIAP−/− mice mostly develop normally [34,37,38]. This indicates that there exists a redundancy in function between various IAPs. Studies carried out by Vaux and colleagues elegantly demonstrated that mice lacking c-IAP2 and XIAP were viable and fertile whereas mice lacking the combination of c-IAP1 and c-IAP2 or c-IAP1 and XIAP died at 10 days post-conception [39]. Thus, c-IAP1 seems to be more important in regulating various functions of IAPs.
Based on the above information it is clear that domains of IAPs including XIAP are key regulatory elements of various functions of IAPs. Therefore, the absence of only one IAP protein such as c-IAP2 might not have significant deleterious effect on physiological function including embryonic development owing to the fact that c-IAP1 and/or XIAP with similar domain structure could compensate for c-IAP2 function.
Structure and function of XIAP
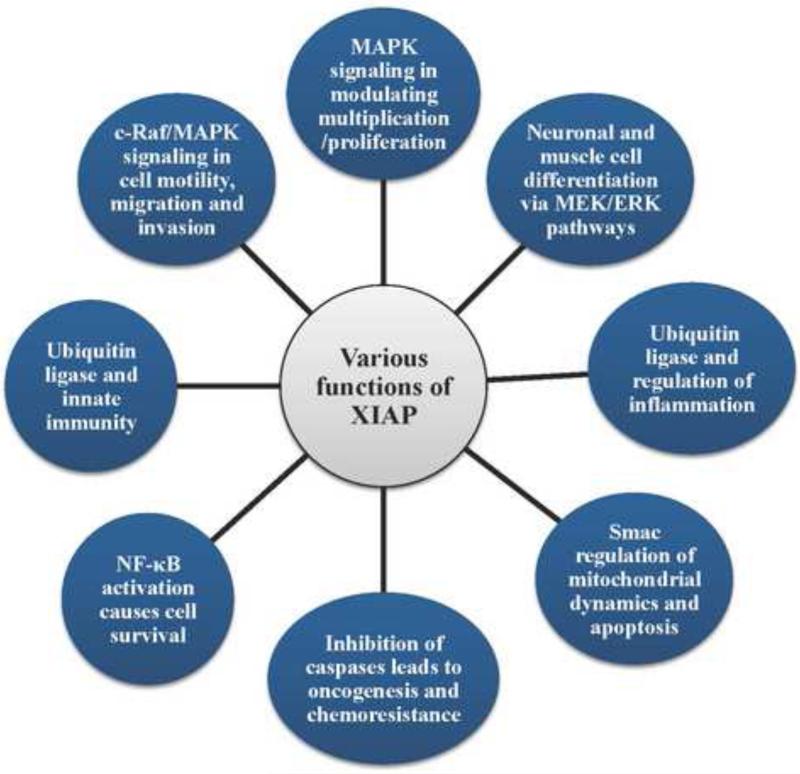
XIAP is a 497 amino acid E3 ubiquitin protein ligase encoded by the Xq25 region of the X chromosome. Genetic evidence shows that BIR domains are essential for the caspase inhibitory function of XIAP, because these domains directly bind to active caspase-9, −3 and −7; and thus protect cells from apoptosis induction (Figure 2) [8,40,41]. XIAP also performs prosurvival function by interacting and facilitating the ubiquitination of caspases such as caspase-9 and caspase-3, as well as its inhibitor, Smac [42–44]. XIAP, like other members of the IAP family proteins such as c-IAP1 and c-IAP2, is expressed in many types of cells [10,11]. XIAP has unique roles in cellular signaling pathways including innate immunity and inflammation, mitosis, c-Jun N-terminal kinase (JNK) activation and nuclear factor (NF)-κB activation [1,45–48]. XIAP also possesses other nonclassical functions including cell motility and migration and human muscle cell differentiation as depicted in Figure 3 [49–53]. These diverse functions of XIAP as well as c-IAPs are attributed to the basic structure of XIAP with multiple domains.
Figure 2.
Apoptotic pathways in mammalian cells and key regulators of apoptosis. Abbreviations: Apaf-1, apoptotic protease activating factor 1; FADD, Fas-associated death domain; Smac, second mitochondria-derived activator of caspase; XIAP, X-chromosome-linked inhibitor of apoptosis protein.
Figure 3.
Various classical functions of X-chromosome-linked inhibitor of apoptosis protein (XIAP).
NF-κB is a protein complex that controls transcription of DNA in eukaryotes. NF-κB is found in almost all animal cells and is involved in cellular responses to different stimuli such as cytokines, free radicals and bacterial or viral antigens [54]. NF-κB activation has been linked to many aspects of the tumorigenesis process. XIAP involvement in NF-κB activation is reported to occur via a novel method where BIR1 domain directly interacts with transforming growth factor (TGF)-β-activated kinase (TAK)1-binding protein (TAB)1 to form the BIR1/TAB1 complex, which induces TAK1 and NF-κB activation [55]. In addition to TAB1 binding, XIAP also interacts with the receptor for bone morphogenetic protein (BMP), thus regulating early developmental processes in mammals [56]. Dimerization of BIR1 domain is sufficient to induce NF-κB activation, which is, however, disrupted by Smac binding to XIAP leading to inhibition of the XIAP–TAB1 interaction [55]. Because NF-κB upon activation mediates various pathways including the cellular processes discussed above, BIR1 domain could be linked to mitochondrial dynamics and apoptosis. Additionally, association of XIAP with mitochondrial apoptosis-related protein in the TGF-β signaling pathway (ARTS) is facilitated by BIR1 domain of XIAP. ARTS-binding with XIAP via BIR1 domain is also associated with recruitment of seven in absentia homolog (Siah)-1 causing ubiquitination and degradation of XIAP [57]. It is also interesting to note that XIAP has been linked to the regulation of stem-cell-dependent skin regeneration [58]. Thus, targeting XIAP by Smac or Smac-like mimetics of BIR1 dimerization will affect multiple cellular functions regulated by NF-κB and mitochondrial pathways.
In addition to NF-κB regulation, IAP proteins play an important part in signaling pathways, such as JNK, mitogen-activated protein kinase (MAPK), TGF-β, Myc and phosphoinositide-3-kinase (PI3K)/Akt pathways [52,59–62]. Fado et al. reported that XIAP negatively regulates neuronal differentiation and these effects of XIAP are mediated by the mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinases (ERK) pathway. It is interesting to note that XIAP binds to cRaf and Trk receptors causing negative regulation of neuronal differentiation [63]. XIAP binding to c-Raf associates with ubiquitination of c-Raf through heat shock protein (Hsp)-90-mediated signaling, which is independent of E3-ligase activity. Thus, XIAP as well as c-IAPs has an important role in regulating turnover of c-Raf modulating the MAPK signaling, which regulates diverse cellular functions including cell growth, proliferation, migration, differentiation and survival. Further studies demonstrate that XIAP also directly interacts with mitogen-activated protein kinase kinase kinase (MEKK)2/3 and competes with PB1 domain-mediated binding to MEK5 [mitogen-activated protein (MAP)/extracellular-signal-regulated kinase (ERK) kinase 5] [52,64]. The above discussion clearly indicates that XIAP not only functions as an inhibitor of apoptosis but also plays an important part in diverse cellular signaling and functions (Figure 3). It is also important to note that the endogenous level of XIAP in cancer cells is regulated by factors such as heat shock transcription factor (HSF)1 and XIAP-associated factor (XAF)1 [65]. XAF1 binds XIAP and, thus, blocks XIAP-mediated inhibition of active caspases [65]. XAF1 is under the negative transcriptional control of HSF1 [66,67]. Therefore, it is possible that, under environmental stress, HSF1 downregulates the expression of XAF1, thus making XIAP available to bind active caspases and inhibiting apoptosis. The diverse cellular functions of XIAP can be assigned to its domains. The most important domains for caspase inhibition are: (i) BIR2/BIR3 domain inhibiting active caspases; and (ii) RING domain with E3-ligase activity causing ubiquitination of XIAP-bound caspases. As discussed in the previous section, c-IAPs and XIAP possess redundant functions as a result of the presence of a similar domain structure. In contrast to the IAPs redundant function, XIAP and c-IAPs differ in some key aspects. For example, XIAP inhibits initiator caspase-9 and executioner caspase-3 and −7 via direct physical interaction, whereas c-IAP1 and c-IAP2 bind caspase-3 and −7 but the inhibition of caspase activity is not efficient, instead these two proteins favor proteasomal degradation of caspases.
Mitochondrial apoptosis and death-receptor signaling
Neoplastic transformation to cancer cells is characterized by reprogrammed energy metabolism and evasion of cell death among other crucial hallmarks [68]. At the root of these changes are the mitochondria. The mitochondrion, a cellular organelle, is ultra-structurally composed of the outer mitochondrial membrane (OMM), inner mitochondrial membrane (IMM), inter-membrane space (IMS) and the mitochondrial matrix. Mitochondria function as the powerhouses of the cell by generating energy (ATP) via oxidative phosphorylation (OXPHOS) [19,69]. Byproducts of OXPHOS are reactive oxygen species (ROS), which act as signaling molecules in cells [70]. Mitochondria also act as a signaling center for the cell death mechanism by playing an essential part in programmed cell death or apoptosis [18,19]. The crucial mediators of apoptosis are cysteine proteases called caspases that cleave crucial protein substrates after the aspartate residues and lead to the disassembly of cellular components and ultimately cell death. Caspase-dependent apoptosis can be divided into two main pathways: one receiving and responding to stress signals from outside the cell (extrinsic pathway) and the other activated by stress signals from within the cells (intrinsic pathway) [18,19,71,72]. Activation of either of these pathways leads to the initiation of the caspase cascade and execution of apoptosis (Figure 2).
In the extrinsic pathway, the binding of the extracellular ligands [FasL or tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)] to their transmembrane receptors (Fas or TRAIL receptor) leads to the formation of death-inducing signaling complex (DISC). DISC activates caspase 8, which then activates downstream caspases to initiate apoptotic cell death [19,73]. In the intrinsic pathway stress signals including DNA damage and loss of survival factors lead to the permeabilization of the OMM, which leads to loss of membrane potential and release of apoptogenic proteins such as cytochrome c from the IMS. The released cytochrome c along with caspase-9 and Apaf-1 forms the apoptosome complex, which then activates downstream caspases to initiate apoptotic cell death [19,74]. The activation of caspases does not always induce apoptosis because of the abundant expression of IAPs including XIAP, which are known to bind active caspase-9, −3 and −7 leading to suppression of the caspase cascade, and thus apoptosis [75]. This suggests that XIAP inhibits the caspase cascade initiated by mitochondrial and death-receptor signaling. Inhibition of RING domain stabilizes XIAP causing TNF sensitivity as a result of increased retention of DISC-mediated active caspase-3, which ultimately overcomes the caspase-inhibitory function of XIAP [76]. Interestingly, active caspase-3 also cleaves XIAP and potentiates the caspase cascade in a positive regulatory feedback loop [77].
Antiapoptotic function of XIAP is inhibited by the release of proapoptotic Smac from mitochondria [44,78,79] as well as binding of XIAP with cellular stress response 1, a tumor suppressor protein [80]. Thus, the release of Smac along with cytochrome c will facilitate the caspase cascade, and XIAP might not significantly influence the outcome of mitochondria-mediated apoptosis. Indeed, XIAP downregulation via genetic and/or pharmacological approaches does not modulate mitochondrial apoptosis but sensitizes tumor cells to TNF exposure [36]. Together, higher expression and prosurvival function of IAPs including XIAP in cancer cells could be attenuated by increased release of proapoptotic proteins including cytochrome c and Smac. Therefore, MOMP regulation in cancer cells remains an important target for the development of anticancer agents for efficient apoptosis induction.
Known regulators of mitochondrial membrane permeabilization
The Bcl-2 family proteins regulate the permeability of the mitochondrial membrane, and thus have key roles in the regulation of apoptosis [18,81]. Extrinsic and intrinsic apoptotic pathways are initiated in response to a variety of stress signals and complex interplay of Bcl-2 family proteins at the mitochondrial outer membrane to initiate Bak and Bax activation, oligomerization and outer mitochondrial membrane damage [18,19]. The proapoptotic BH3-only proteins such as Bim and Bid activate Bax and Bak causing their oligomerization and channel formation [82]. Mitochondrial membrane permeabilization leads to the release of apoptogenic proteins such as cytochrome c, apoptosis-inducing factor (AIF), Smac, HtrA2 and endonuclease G [18,36], which trigger apoptotic cell death [17].
Bax primarily localizes in the cytosol in an inactive state, whereas prosurvival protein Bcl-xL is present in soluble and membrane-bound forms [83]. Additionally, many apoptosis-related proteins have dual functions regulating apoptosis and survival signaling depending on the overall cellular physiology. This dichotomy in their functions has important implications in cellular physiology and could influence efficient execution of the cell death process in response to distinct stimuli. For instance, Bcl-2 family members Bid and Bad in addition to their proapoptotic functions possess cell survival roles in response to DNA damage and altered glucose metabolism, respectively [84,85]. Furthermore, proapoptotic Bax and Bak in the absence of apoptotic stimuli regulate mitochondrial dynamics [86]. These findings suggest that the majority of proteins involved in apoptotic pathways harbor nonapoptotic functions, which is further supported by the fact that IAPs including XIAP have been shown to be involved in apoptotic, survival and nonapoptotic processes [1,47,49–53,87].
XIAP regulation of mitochondrial membrane permeabilization
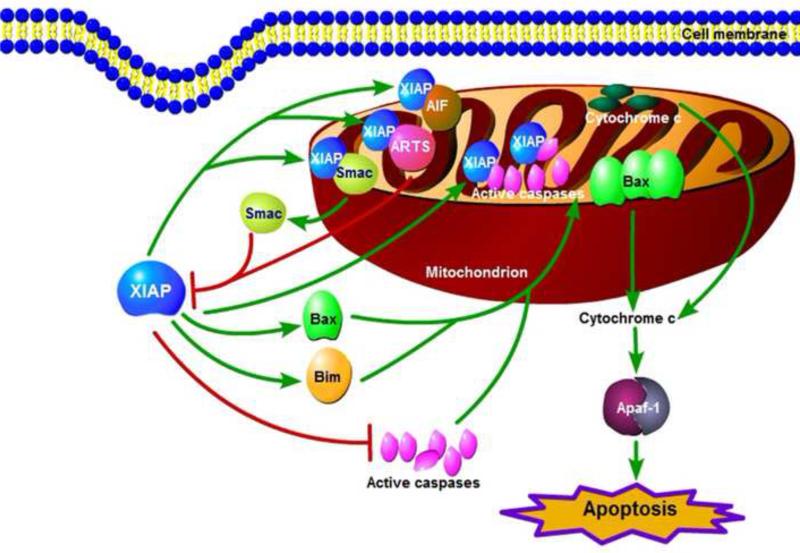
Under physiological conditions, XIAP normally resides in the cytosol and suppresses the caspase cascade favoring cell survival. Surprisingly, evidence supports that abrogation of extracellular matrix-dependent survival signals leads to XIAP translocation to mitochondria prior to mitochondrial membrane permeabilization and caspase activation [20]. The endogenous mitochondrial XIAP then forms a novel ~400 kDa protein complex, which precedes cytochrome c release and apoptosis [20]. Mechanistic analysis reveals that exogenously expressed XIAP associates with mitochondria and induces release of apoptogenic proteins such as cytochrome c and Smac in a Bax/Bak-dependent manner (Figure 4). This proapoptotic function of XIAP could be suppressed by antiapoptotic Bcl-2 family protein Bcl-xL [20]. This unexpected proapoptotic function of XIAP was further supported by our findings on XIAP-mediated cytochrome c release in response to resveratrol in cancer cells [21]. XIAP seems to activate Bax in the cytosolic compartment and could help translocate Bax to the mitochondrion [21], at the mitochondrion Bax undergoes oligomerization and channel formation causing cytochrome c release (Figure 4). Proapoptotic factors like Bim and t-Bid concurrently translocate to mitochondria similar to XIAP during resveratrol-induced apoptosis, suggesting a possibility of crosstalk between proapoptotic BH3-only proteins and XIAP to induce Bax oligomerization on the mitochondrial membrane [21]. Indeed, recent findings provide evidence that Bim overexpression induces XIAP translocation to mitochondria [22]. These findings suggest that XIAP interaction with proapoptotic proteins Bax, Bid and Bim could represent early events during apoptosis [20–23]. Whether XIAP translocation and its role in mitochondria permeabilization during apoptosis are cell-type- or stress-specific are factors that need further evaluation. However, if XIAP has the ability to interact with Bim or Bid either directly or indirectly and activate Bax/Bak this new function of MOMP regulation by XIAP and possibly of other IAPs could be a novel mechanism to regulate apoptotic cell death in cancer.
Figure 4.
X-chromosome-linked inhibitor of apoptosis protein (XIAP) regulation of mitochondrial membrane permeabilization and its impact on the regulation of other mitochondrial residence proteins.
XIAP regulation of NF-κB activation via the BIR1/TAB1 complex [55] can lead to expression of various NF-κB target genes including c-IAP1 and c-IAP2 [88]. As mentioned above, c-IAPs shares similar domains to XIAP, except for the presence of a CARD domain in c-IAP1 and c-IAP2. Thus, increased expression of c-IAPs can manifest and/or regulate mitochondrial membrane permeabilization by interacting with Bax/Bak. Together, combined action of c-IAPs and XIAP could lead to synergistic enhancement of MOMP-dependent apoptosis in cancer cells. In cancer cells that are Bax- and/or Bak-deficient or possess defective Bax/Bak-mediated MOMP, pharmacological inhibition of XIAP or XIAP-silencing will overcome TRAIL resistance in cancer owing to retention of active caspases generated by DISC. Also, stabilization of XIAP or inhibition of the XIAP/proteasome pathway will enhance MOMP in Bax/Bak-sufficient cells causing restoration of mitochondrial apoptosis.
Mechanisms of XIAP translocation to mitochondria
How XIAP translocates to and activates Bax on mitochondria is not clearly defined; however, based on published findings [20–23], multiple possibilities exist. First, XIAP associates with Bax in the cytosol and translocates to mitochondria via a Bim- or t-Bid-dependent manner [21]. However, it is unclear whether Bim or Bid directly interact with the XIAP/Bax complex in the cytosol or mitochondria. It is also interesting to note that reduced expression of XIAP was observed upon exposure to anticancer agents such as mithramycin A and Smac mimetic BV6 to cancer cells that do not express Bax [89]. Further studies using Bax-expressing cells are required to define the correlation of Bax and XIAP expression, which could provide insights on the role of Bax in XIAP translocation to mitochondria. Indeed, recent findings demonstrate the requirement of Bax or Bak, and XIAP translocation is inhibited by prosurvival Bcl-2 proteins such as Bcl-2 and Bcl-xL [23]. Second, Bim could have a direct role in XIAP translocation to mitochondria [22] because, in the absence of Bim, XIAP translocation to mitochondria was inhibited. Third, it is possible that XIAP gains access to the mitochondrial membrane upon mitochondrial depolarization as a result of either exogenous or endogenous stress [22].
In addition to the above-mentioned scenarios including involvement of Bim and Bax in facilitating XIAP translocation to mitochondria, it is also possible that active caspase−3 and −9 could help translocate XIAP to mitochondria. Active caspase-9 and −3 are known to translocate to mitochondria and regulate mitochondria membrane structure and function [90,91]. Active executioner caspases such as caspase-3 and −7 depolarize mitochondria to enhance apoptotic cell death [90,92], whereas active caspase-9 induces cytochrome c release leading to mitochondrial uncoupling and ROS production [90]. Thus, early activation of caspase-3 and caspase-9 could promote apoptosis by depolarizing mitochondrial membrane and ROS production, respectively. Based on well-known prosurvival function of XIAP, it is reasonable to presume that low levels of active caspase-9 and −3 are inhibited by XIAP binding in the cytosol causing inhibition of the caspase cascade. By contrast, XIAP binding with active caspase-9 and −3 forms XIAP/caspase complex, which can amplify the caspase cascade such that XIAP/caspase complex translocates to mitochondria and permeabilizes the mitochondrial membrane. Thus XIAP/caspase complex can function as a unique mechanism to permeabilize the mitochondrial membrane to induce or amplify mitochondrial apoptosis.
Which domain of XIAP has a role in the recruitment of XIAP to mitochondria? Above-mentioned scenarios suggest that XIAP can gain access to mitochondria during early mitochondrial depolarization or through association with Bax, Bak and Bim or through association with active caspases. XIAP interacts with active caspases either via BIR2 or BIR3 domain whereas XIAP interacts with mitochondrial ARTS protein via BIR1 domain [40,41,57]. These findings suggest that all three BIR domains of XIAP can play a part in XIAP translocation to mitochondria. However, molecular analysis suggests that RING domain has a crucial role in XIAP targeting to mitochondria [20,22]. Based on this information, we suggest that XIAP can translocate to mitochondria directly via RING domain association with mitochondria and indirectly via association with XIAP-interacting proteins including active caspases, ARTS or Smac. c-IAP1 and c-IAP2 contain all three BIR domains as well as RING domains, thus it is possible that c-IAPs could also localize to mitochondria and possess functions similar to XIAP. Although BIR2 and BIR3 domains can influence XIAP translocation to mitochondria via interacting with active caspase-3 and −9, direct translocation of XIAP might be mediated by its RING domain. This notion is supported by the fact that caspase-binding mutant XIAP (D148A) can translocate to mitochondria and degrade Smac, whereas RING-less XIAP or RING-less survivin or mutant XIAP (F495L) deficient in ubiquitin transfer activity did not translocate to mitochondria leading to the accumulation of XIAP in the cytosolic compartment [20,22]. It is also important that XIAP-mediated Bax/Bak-dependent MOMP does not require E3-ligase activity, whereas the E3-ligase-activity-dependent mechanism seems to promote XIAP translocation to mitochondria.
Impact of XIAP translocation to mitochondria
Once translocated to the mitochondria, XIAP performs multiple functions. For example, XIAP can activate Bax even in the absence of BH3-only protein signaling, causing Bax/Bak oligomerization and permeabilization of mitochondrial membrane leading to induction of apoptosis [22]. As stated above, this function of XIAP does not require the E3-ligase activity. By contrast, XIAP translocation to mitochondria is associated with mitochondrial ubiquitination in an E3-ligase-dependent manner, suggesting that intramitochondrial XIAP degrades Smac in a manner that requires XIAP binding with Smac (Figure 4) [22,23]. RING-domain-mediated translocation of XIAP can also regulate function or levels of other mitochondria proteins including ARTS, HtrA2 and AIF that are known to interact with XIAP, which can ultimately regulate apoptotic cell death in cancer. Although the underlying mechanism on the role of XIAP in regulating MOMP and consequent apoptosis is not yet defined, further research on the function of mitochondrial XIAP could provide new avenues for cancer therapy.
Our previous findings suggest that apoptosis induced by many stimuli involves an early mitochondrial activation, which might be responsible for the subsequent disruption of mitochondrial respiratory chain (MRC) functions, loss of membrane potential, cytochrome c release and, ultimately, cell death [93]. Early mitochondrial activation and biogenesis was further supported by our recent findings that mitochondria accumulate higher levels of proteins and DNA during DNA-damage-induced apoptosis in cancer cells [94]. The mitochondrial activation leads to translocation and accumulation of multiple proapoptotic and antiapoptotic proteins at the mitochondria including proapoptotic BH3-only protein Bim and XIAP [21,93]. Similar to XIAP overexpression, Bim was constitutively overexpressed in multiple prostate and breast cancer cells as well as in primary tumor cells [95]. These findings suggest that constitutively overexpressed Bim and XIAP could coordinate to perform either proapoptotic or prosurvival functions in epithelial cancer cells. It is also interesting to note that XIAP-mediated cytochrome c release and subsequent apoptosis induction happens in a p53-independent manner [21]. Because cancer cells are known to harbor p53 mutations or loss of p53 function, further characterization of this new role of XIAP will allow development of novel strategies to enhance apoptotic cell death in cancer including in p53-deficient cancers.
Functions of XIAP at mitochondria other than induction of MOMP
The release of cytochrome c induces caspase activation in the cytosol leading to the generation of active caspase-9 and −3, both of which could be inactivated by their binding with XIAP in the cytosol [11,40]. Because caspase-9 can also be activated by redox stress in the mitochondrial compartment [96], there is a possibility that active caspase-9 could be inhibited by the mitochondria translocated and/or resident XIAP. Thus, active caspases generated in the cytosol and mitochondria are inhibited by XIAP. By contrast, XIAP translocates to mitochondria, and this mitochondrial XIAP plays an important part in permeabilization of the mitochondrial membrane to enhance cytochrome-c-mediated apoptosis [20–22]. Mitochondria harbor other proapoptotic proteins such as Smac and AIF [79,97], thus it can also be postulated that mitochondrial XIAP render prosurvival function at mitochondria by binding with Smac and AIF. Indeed, during apoptosis XIAP translocates to mitochondria and binds with Smac [98] and thus prevents the amplification of cytochrome c release and caspase activation. Smac degradation in the mitochondria compartment was further supported by recent findings that XIAP translocates to mitochondria and degrades Smac in a lysosomal- and proteasomal-activity-dependent manner [22,23].
Because BIR2 domain of XIAP binds with AIF [99], the presence of AIF in the mitochondrial compartment suggests that mitochondrial XIAP could bind AIF and inhibit caspase-independent and AIF-dependent apoptosis. Thus, XIAP harbors prosurvival functions including inhibition of caspases, Smac and AIF functions as discussed above. The new XIAP function of promoting mitochondrial permeabilization could be exploited for development of therapeutic agents for human diseases such as cancer. Indeed, increased expression of XIAP does not always provide resistance to anticancer therapeutics but can also be associated with favorable clinical outcome [100–102].
Although HtrA2 was shown to bind and inactivate XIAP, binding of XIAP to HtrA2 does not inhibit the proteolytic activity of HtrA2. Furthermore, overexpression of XIAP does not inhibit the HtrA2-induced caspase-independent atypical cell death [36]. These findings demonstrate an interaction between HtrA2 and XIAP. However, any new scaffolding-like role of XIAP for HtrA2 on the mitochondria is currently unknown because we do not know if this interaction is specific to the cytosol or if it also occurs in mitochondria.
Targeting prosurvival and proapoptotic functions of XIAP for cancer therapy
XIAP binds with active caspases leading to the inhibition of the caspase cascade and apoptosis; thus, blocking prosurvival function of XIAP as well as of c-IAPs has been an attractive target for the development of novel therapeutic agents for cancer treatment. In contrast to the prosurvival function of XIAP, the induction of mitochondrial outer membrane permeabilization by XIAP could provide new avenues for designing novel anticancer agents to harness the therapeutic potential of overexpressed XIAP or c-IAPs in various types of cancer. Targeting the new function of XIAP has significance in cancer therapy because most of the anticancer agents developed based on prosurvival function of XIAP have not been translated successfully in the clinic for cancer therapy. Some of the strategies leading to inhibition of the prosurvival function of XIAP and their possible implications are discussed below.
Caspase inhibitory function of XIAP can be abrogated by its downregulation or silencing using antisense oligonucleotides or siRNA approaches. Additionally, development of small molecules that block caspase-inhibiting functions have therapeutic benefit [103]. Antisense oligonucleotides (AS ODNs) targeting XIAP have been developed for different types of cancer such as human non-small-cell lung carcinoma (NSCLC). AS-ODN-mediated downregulation of XIAP in human NSCLC cells inhibits growth and enhances therapeutic efficacy of the cytotoxic agent vinorelbine in a xenograft model [104]. In some studies, treatment with AS-ODN-mediated downregulation of XIAP or survivin alone did not reduce tumor burden but improved tumor control by radiotherapy in a mouse model of lung cancer [105]. AEG35156, a second-generation antisense oligonucleotide to XIAP, has been used for clinical trials in patients diagnosed with multiple types of cancer including lymphoma, melanoma, leukemia, breast, pancreatic and lung cancers [106]. AEG35156 in combination with standard chemotherapy was well tolerated in acute myeloid leukemia (AML) patients and shows effective antileukemic activity in patients who were refractive to single agents [106,107]. Multicenter Phase I and II trials of AEG35156, in combination with idarubicin or cytarabine, were conducted in relapsed/refractory AML patients. This study shows that AEG35156 effectively silences XIAP in circulating blasts and induces apoptosis in AML stem cells [108]. In spite of initial encouraging effects on tumor control by AEG35156, later studies, however, did not observe beneficial effects rendered by this XIAP inhibitor in other types of cancer [109,110]. These findings suggest that silencing XIAP or other IAPs might not provide therapeutic benefits because reduced levels of IAPs including XIAP will not only inhibit prosurvival function but will also limit the exploitation of their proapoptotic function.
Because the BIR2 domain inhibits the terminal caspase-3 and −7 whereas the BIR3 domain of XIAP inhibits caspase-9 [6,8–10], various inhibitors of BIR3 domain of XIAP have been developed. However, these inhibitors also inhibit c-IAP1/2 causing the release of TNF-α, thus limiting the therapeutic benefit of the XIAP BIR3 inhibitors. To overcome this issue, benzodiazepinone 36 was developed, which shows high selectivity to XIAP BIR3 and c-IAP1 BIR2/3 leading to increased efficacy while inhibiting the release of TNF-α in a xenograft pharmacodynamics model [111]. Recently, another study optimized XIAP-BIR2-selective benzazepinone[s8] screening and identified benzoxazepinone 40, a more potent BIR2-selective inhibitor with better in vivo pharmacokinetic properties. Benzoxazepinone 40 enhances mechanistically distinct apoptotic signaling compared to pan-IAP inhibitors [112]. Because Smac binds with XIAP leading to the induction of apoptotic cell death [44], various Smac mimetics have been developed to inhibit prosurvival function of XIAP, which could have significance in cancer therapy [113–118]. In addition, embelin, a non-peptide inhibitor of XIAP BIR3 domain, has also shown promising anticancer effects by blocking NF-κB activation and promoting caspase activation [119] leading to synthesis of embelin derivatives for the development of XIAP-based anticancer agents. These agents targeting prosurvival function of XIAP might not lead to desirable outcomes to cure cancer because of the inhibition of proapoptotic function of XIAP or other IAPs. Although the exact strategy to design XIAP-based anticancer agents is still elusive because of the prosurvival and proapoptotic functions of XIAP, the screening of small molecules that enhance XIAP translocation to mitochondria while inhibiting BIR2 and BIR3 could provide novel avenues to design better and efficacious anticancer agents. XIAP possesses multiple cellular functions, the implications of which have been discussed in more detail in previous reviews [45,88,89,120,121]. Here, we have focused mostly on how MOMP regulatory function of XIAP could be modulated either alone or in combination with caspase inhibitory function of XIAP.
Concluding remarks and future perspectives
In this review we have summarized a potential role of XIAP in mitochondrial membrane permeabilization (Figure 4), which could potentially be a therapeutic target in cancer prevention and treatment. This novel function of XIAP is different from its classical caspase inhibitory function. Proapoptotic factors like Bim and t-Bid concurrently translocate to mitochondria along with XIAP during resveratrol-induced apoptosis [21]. There is a possibility of crosstalk between proapoptotic BH3-only proteins and XIAP to induce Bax oligomerization on the mitochondrial membrane. Indeed, the recent findings demonstrate the involvement of Bim in XIAP translocation to mitochondria [22]. Because apoptosis induced by many stimuli involves an early mitochondrial activation and loss of membrane potential [93,94], concomitant translocation of XIAP and Bax could represent an early event and enhance cytochrome c release, leading to cell death. The novel function of XIAP is consistent with multiple reports suggesting the nonclassical functions of apoptotic proteins including Bim, Bad, Bid, Apaf-1 and Fas [95,122–124]. Because XIAP is highly expressed in multiple types of cancer [125–127] and XIAP activates Bax clustering and oligomerization at the outer mitochondrial membrane, development of agents that can enhance XIAP-mediated Bax-dependent mitochondrial permeabilization would be an efficient approach for cancer prevention and treatment. Because XIAP exerts its proapoptotic function via Bax/Bak, cancer cells that are Bax/Bak-deficient or harbor nonfunctional Bax/Bak can lead to the inhibition or attenuation of proapoptotic function of XIAP. Thus, in Bax/Bak-deficient cancer cells, XIAP will possess only a prosurvival function causing inhibition of the caspase cascade leading to therapy resistance. Because XIAP-mediated MOMP does not rely on p53 function [21], the XIAP-targeted therapeutics will benefit a majority of cancer patients including those with p53 mutations.
Highlights.
XIAP harbors prosurvival, proapoptotic and nonapoptotic functions
Proapoptotic BH3-only protein Bim can regulate XIAP translocation to mitochondria
XIAP induces Bax-dependent permeabilization of the mitochondrial membrane
XIAP translocation to mitochondria associates with mitochondrial ubiquitination
Targeting the mitochondrial permeabilization function of XIAP could provide novel cancer therapeutics
Teaser.
X-chromosome-linked inhibitor of apoptosis protein (XIAP) inhibits caspases and blocks apoptosis. XIAP can also function as a proapoptotic protein by promoting mitochondrial membrane permeabilization, which could be exploited for therapeutic benefits in cancer.
Acknowledgments
This work was supported in part by the National Cancer Institute of the National Institutes of Health under Award Number R01CA160685 and the American Cancer Society Research Scholar Grant RSG-12-214-01 – CCG; as well as the National Cancer Institute Center Support Grant P30 CA016056 to the Roswell Park Cancer Institute. We apologize to those colleagues whose publications could not be cited owing to space constraints[s9].
Biography
Ajay Chaudhary

Ajay Chaudhary received his PhD in biotechnology from University of Allahabad, India. He was a Research Associate at Advance Centre for Treatment, Research and Education in Cancer (ACTREC), Tata Memorial Hospital, and Postdoctoral Fellow at the National Institute of Immunohematology (NIIH), India. Currently, he is a Research Affiliate at the Department of Pharmacology & Therapeutics, Roswell Park Cancer Institute, New York, USA. He has published 22 scientific papers and his research focuses on mitochondrial signaling in various types of cancer.
Dhyan Chandra

Dr Chandra is an Associate Professor in the Department of Pharmacology and Therapeutics at Roswell Park Cancer Institute, New York, USA. He trained at the University of Texas MD Anderson Cancer Center, USA. He has been a cancer researcher for more than 15 years and his ongoing research focuses on how mitochondria-mediated cell death could be targeted for cancer prevention and therapy. He has published more than 40 peer-reviewed articles in various scientific journals.
Neelu Yadav

Neelu Yadav received her PhD from Graduate School of Biomedical Sciences at the University of Texas MD Anderson Cancer Center, Houston, USA. During her PhD, she studied the role of protein arginine methyltransferases in development and cancer. Currently, she is a HRI Scientist at Roswell Park Cancer Institute, New York, USA. Her research focuses on studying the causes and effects of mitochondria dysfunction in disease, specifically in cancer. She has authored 21 peer-reviewed articles in various international journals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galban S, Duckett CS. XIAP as a ubiquitin ligase in cellular signaling. Cell Death Differ. 2010;17:54–60. doi: 10.1038/cdd.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prakash H, et al. Deficiency of XIAP leads to sensitization for Chlamydophila pneumoniae pulmonary infection and dysregulation of innate immune response in mice. J. Biol. Chem. 2010;285:20291–20302. doi: 10.1074/jbc.M109.096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srinivasula SM, Ashwell JD. IAPs: what's in a name? Mol. Cell. 2008;30:123–135. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez J, Meier P. To fight or die – inhibitor of apoptosis proteins at the crossroad of innate immunity and death. Curr. Opin. Cell Biol. 2010;22:872–881. doi: 10.1016/j.ceb.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 5.Crook NE, et al. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J. Virol. 1993;67:2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deveraux QL, et al. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 7.Liston P, et al. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature. 1996;379:349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- 8.Shiozaki EN, et al. Mechanism of XIAP-mediated inhibition of caspase-9. Mol. Cell. 2003;11:519–527. doi: 10.1016/s1097-2765(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasula SM, et al. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112–116. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- 10.Deveraux QL, et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bratton SB, et al. Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J. 2001;20:998–1009. doi: 10.1093/emboj/20.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakagawa Y, et al. IAP family protein expression correlates with poor outcome of multiple myeloma patients in association with chemotherapy-induced overexpression of multidrug resistance genes. Am. J. Hematol. 2006;81:824–831. doi: 10.1002/ajh.20656. [DOI] [PubMed] [Google Scholar]
- 13.Mizutani Y, et al. Overexpression of XIAP expression in renal cell carcinoma predicts a worse prognosis. Int. J. Oncol. 2007;30:919–925. [PubMed] [Google Scholar]
- 14.Yang XH, et al. XIAP is a predictor of cisplatin-based chemotherapy response and prognosis for patients with advanced head and neck cancer. PLoS One. 2012;7:e31601. doi: 10.1371/journal.pone.0031601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaughn AE, Deshmukh M. Essential postmitochondrial function of p53 uncovered in DNA damage-induced apoptosis in neurons. Cell Death Differ. 2007;14:973–981. doi: 10.1038/sj.cdd.4402084. [DOI] [PubMed] [Google Scholar]
- 16.Potts MB, et al. Reduced Apaf-1 levels in cardiomyocytes engage strict regulation of apoptosis by endogenous XIAP. J. Cell Biol. 2005;171:925–930. doi: 10.1083/jcb.200504082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bratton SB, Salvesen GS. Regulation of the Apaf-1-caspase-9 apoptosome. J. Cell Sci. 2010;123:3209–3214. doi: 10.1242/jcs.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tait SW, Green DR. Mitochondrial regulation of cell death. Cold Spring Harb. Perspect. Biol. 2013;5 doi: 10.1101/cshperspect.a008706. doi: 10.1101/cshperspect.a008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yadav N, Chandra D. Mitochondrial and postmitochondrial survival signaling in cancer. Mitochondrion. 2014;16:18–25. doi: 10.1016/j.mito.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owens TW, et al. Role for X-linked Inhibitor of apoptosis protein upstream of mitochondrial permeabilization. J. Biol. Chem. 2010;285:1081–1088. doi: 10.1074/jbc.M109.072322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gogada R, et al. Resveratrol induces p53-independent, X-linked inhibitor of apoptosis protein (XIAP)-mediated Bax protein oligomerization on mitochondria to initiate cytochrome c release and caspase activation. J. Biol. Chem. 2011;286:28749–28760. doi: 10.1074/jbc.M110.202440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamacher-Brady A, et al. Intramitochondrial recruitment of endolysosomes mediates Smac degradation and constitutes a novel intrinsic apoptosis antagonizing function of XIAP E3 ligase. Cell Death Differ. 2014;21:1862–1876. doi: 10.1038/cdd.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamacher-Brady A, Brady NR. Bax/Bak-dependent, Drp1-independent targeting of XIAP into inner-mitochondrial compartments counteracts Smac-dependent effector caspase activation. J. Biol. Chem. 2015:jbc.M115.643064. doi: 10.1074/jbc.M115.643064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deveraux QL, Reed JC. IAP family proteins--suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 25.Damiano JS, et al. Heterotypic interactions among NACHT domains: implications for regulation of innate immune responses. Biochem. J. 2004;381:213–219. doi: 10.1042/BJ20031506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lens SM, et al. The case for Survivin as mitotic regulator. Curr. Opin. Cell Biol. 2006;18:616–622. doi: 10.1016/j.ceb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Vader G, et al. Survivin mediates targeting of the chromosomal passenger complex to the centromere and midbody. EMBO Rep. 2006;7:85–92. doi: 10.1038/sj.embor.7400562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartke T, et al. Dual role of BRUCE as an antiapoptotic IAP and a chimeric E2/E3 ubiquitin ligase. Mol. Cell. 2004;14:801–811. doi: 10.1016/j.molcel.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Lotz K, et al. BRUCE, a giant E2/E3 ubiquitin ligase and inhibitor of apoptosis protein of the trans-Golgi network, is required for normal placenta development and mouse survival. Mol. Cell Biol. 2004;24:9339–9350. doi: 10.1128/MCB.24.21.9339-9350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oberoi-Khanuja TK, et al. Role of melanoma inhibitor of apoptosis (ML-IAP) protein, a member of the baculoviral IAP repeat (BIR) domain family, in the regulation of C-RAF kinase and cell migration. J. Biol. Chem. 2012;287:28445–28455. doi: 10.1074/jbc.M112.341297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varfolomeev E, et al. Characterization of ML-IAP protein stability and physiological role in vivo. Biochem. J. 2012;447:427–436. doi: 10.1042/BJ20121103. [DOI] [PubMed] [Google Scholar]
- 32.Shin H, et al. The BIR domain of IAP-like protein 2 is conformationally unstable: implications for caspase inhibition. Biochem. J. 2005;385:1–10. doi: 10.1042/BJ20041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richter BW, et al. Molecular cloning of ILP-2, a novel member of the inhibitor of apoptosis protein family. Mol. Cell Biol. 2001;21:4292–4301. doi: 10.1128/MCB.21.13.4292-4301.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conze DB, et al. Posttranscriptional downregulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol. Cell Biol. 2005;25:3348–3356. doi: 10.1128/MCB.25.8.3348-3356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung HH, et al. The RING domain of cIAP1 mediates the degradation of RING-bearing inhibitor of apoptosis proteins by distinct pathways. Mol. Biol. Cell. 2008;19:2729–2740. doi: 10.1091/mbc.E08-01-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sensintaffar J, et al. XIAP is not required for human tumor cell survival in the absence of an exogenous death signal. BMC Cancer. 2010;10:11. doi: 10.1186/1471-2407-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conte D, et al. Inhibitor of apoptosis protein cIAP2 is essential for lipopolysaccharide-induced macrophage survival. Mol. Cell Biol. 2006;26:699–708. doi: 10.1128/MCB.26.2.699-708.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harlin H, et al. Characterization of XIAP-deficient mice. Mol. Cell Biol. 2001;21:3604–3608. doi: 10.1128/MCB.21.10.3604-3608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moulin M, et al. IAPs limit activation of RIP kinases by TNF receptor 1 during development. EMBO J. 2012;31:1679–1691. doi: 10.1038/emboj.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riedl SJ, et al. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 2001;104:791–800. doi: 10.1016/s0092-8674(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 41.Chai J, et al. Structural basis of caspase-7 inhibition by XIAP. Cell. 2001;104:769–780. doi: 10.1016/s0092-8674(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki Y, et al. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8662–8667. doi: 10.1073/pnas.161506698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morizane Y, et al. X-linked inhibitor of apoptosis functions as ubiquitin ligase toward mature caspase-9 and cytosolic Smac/DIABLO. J. Biochem. 2005;137:125–132. doi: 10.1093/jb/mvi029. [DOI] [PubMed] [Google Scholar]
- 44.Wu G, et al. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408:1008–1012. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 45.Estornes Y, Bertrand MJ. IAPs, regulators of innate immunity and inflammation. Semin. Cell Dev. Biol. 2015;39:106–114. doi: 10.1016/j.semcdb.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 46.Damgaard RB, et al. The ubiquitin ligase XIAP recruits LUBAC for NOD2 signaling in inflammation and innate immunity. Mol. Cell. 2012;46:746–758. doi: 10.1016/j.molcel.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Bauler LD, et al. XIAP regulates cytosol-specific innate immunity to Listeria infection. PLoS Pathog. 2008;4:e1000142. doi: 10.1371/journal.ppat.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levkau B, et al. xIAP induces cell-cycle arrest and activates nuclear factor-kappaB: new survival pathways disabled by caspase-mediated cleavage during apoptosis of human endothelial cells. Circ. Res. 2001;88:282–290. doi: 10.1161/01.res.88.3.282. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, et al. E3 ligase activity of XIAP RING domain is required for XIAP-mediated cancer cell migration, but not for its RhoGDI binding activity. PLoS One. 2012;7:e35682. doi: 10.1371/journal.pone.0035682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, et al. X-linked inhibitor of apoptosis protein (XIAP) mediates cancer cell motility via Rho GDP dissociation inhibitor (RhoGDI)-dependent regulation of the cytoskeleton. J. Biol. Chem. 2011;286:15630–15640. doi: 10.1074/jbc.M110.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oberoi-Khanuja TK, et al. IAPs on the move: role of inhibitors of apoptosis proteins in cell migration. Cell Death Dis. 2013;4:e784. doi: 10.1038/cddis.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takeda AN, et al. Ubiquitin-dependent regulation of MEKK2/3-MEK5-ERK5 signaling module by XIAP and cIAP1. EMBO J. 2014;33:1784–1801. doi: 10.15252/embj.201487808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oberoi TK, et al. IAPs regulate the plasticity of cell migration by directly targeting Rac1 for degradation. EMBO J. 2012;31:14–28. doi: 10.1038/emboj.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 55.Lu M, et al. XIAP induces NF-kappaB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol. Cell. 2007;26:689–702. doi: 10.1016/j.molcel.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamaguchi K, et al. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. EMBO J. 1999;18:179–187. doi: 10.1093/emboj/18.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garrison JB, et al. ARTS and Siah collaborate in a pathway for XIAP degradation. Mol. Cell. 2011;41:107–116. doi: 10.1016/j.molcel.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fuchs Y, et al. Sept4/ARTS regulates stem cell apoptosis and skin regeneration. Science. 2013;341:286–289. doi: 10.1126/science.1233029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Y, et al. Tumor necrosis factor receptor 2 signaling induces selective c-IAP1-dependent ASK1 ubiquitination and terminates mitogen-activated protein kinase signaling. J. Biol. Chem. 2007;282:7777–7782. doi: 10.1074/jbc.M609146200. [DOI] [PubMed] [Google Scholar]
- 60.Neil JR, et al. X-linked inhibitor of apoptosis protein and its E3 ligase activity promote transforming growth factor-{beta}-mediated nuclear factor-{kappa}B activation during breast cancer progression. J. Biol. Chem. 2009;284:21209–21217. doi: 10.1074/jbc.M109.018374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Themsche C, et al. XIAP gene expression and function is regulated by autocrine and paracrine TGF-beta signaling. Mol. Cancer. 2010;9:216. doi: 10.1186/1476-4598-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaur S, et al. X-linked inhibitor of apoptosis (XIAP) inhibits c-Jun N-terminal kinase 1 (JNK1) activation by transforming growth factor beta1 (TGF-beta1) through ubiquitin-mediated proteosomal degradation of the TGF-beta1-activated kinase 1 (TAK1). J. Biol. Chem. 2005;280:38599–38608. doi: 10.1074/jbc.M505671200. [DOI] [PubMed] [Google Scholar]
- 63.Fado R, et al. X-linked inhibitor of apoptosis protein negatively regulates neuronal differentiation through interaction with cRAF and Trk. Sci. Rep. 2013;3:2397. doi: 10.1038/srep02397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klein AM, Cobb MH. ERK5 signaling gets XIAPed: a role for ubiquitin in the disassembly of a MAPK cascade. EMBO J. 2014;33:1735–1736. doi: 10.15252/embj.201489205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liston P, et al. Identification of XAF1 as an antagonist of XIAP anti-Caspase activity. Nat. Cell Biol. 2001;3:128–133. doi: 10.1038/35055027. [DOI] [PubMed] [Google Scholar]
- 66.Santagata S, et al. Tight coordination of protein translation and HSF1 activation supports the anabolic malignant state. Science. 2013;341:1238303. doi: 10.1126/science.1238303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J, et al. HSF1 down-regulates XAF1 through transcriptional regulation. J. Biol. Chem. 2006;281:2451–2459. doi: 10.1074/jbc.M505890200. [DOI] [PubMed] [Google Scholar]
- 68.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 69.Acin-Perez R, Enriquez JA. The function of the respiratory supercomplexes: the plasticity model. Biochim. Biophys. Acta. 2014;1837:444–450. doi: 10.1016/j.bbabio.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 70.Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014;2:17. doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiong S, et al. Mitochondria-mediated apoptosis in mammals. Protein Cell. 2014;5:737–749. doi: 10.1007/s13238-014-0089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 73.Zaman S, et al. Targeting the apoptosis pathway in hematologic malignancies. Leuk. Lymphoma. 2014;55:1980–1992. doi: 10.3109/10428194.2013.855307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu CC, Bratton SB. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid. Redox Signal. 2013;19:546–558. doi: 10.1089/ars.2012.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holcik M, et al. XIAP: apoptotic brake and promising therapeutic target. Apoptosis. 2001;6:253–261. doi: 10.1023/a:1011379307472. [DOI] [PubMed] [Google Scholar]
- 76.Gillissen B, et al. Targeted therapy of the XIAP/proteasome pathway overcomes TRAIL-resistance in carcinoma by switching apoptosis signaling to a Bax/Bak-independent 'type I' mode. Cell Death Dis. 2013;4:e643. doi: 10.1038/cddis.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hornle M, et al. Caspase-3 cleaves XIAP in a positive feedback loop to sensitize melanoma cells to TRAIL-induced apoptosis. Oncogene. 2011;30:575–587. doi: 10.1038/onc.2010.434. [DOI] [PubMed] [Google Scholar]
- 78.Chai J, et al. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature. 2000;406:855–862. doi: 10.1038/35022514. [DOI] [PubMed] [Google Scholar]
- 79.Du C, et al. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 80.Zheng ZL, et al. Interaction of CSR1 with XIAP reverses inhibition of caspases and accelerates cell death. Am. J. Pathol. 2012;181:463–471. doi: 10.1016/j.ajpath.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dewson G, Kluck RM. Mechanisms by which Bak and Bax permeabilise mitochondria during apoptosis. J. Cell Sci. 2009;122:2801–2808. doi: 10.1242/jcs.038166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Czabotar PE, et al. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 83.Hsu YT, et al. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Danial NN, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- 85.Kamer I, et al. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell. 2005;122:593–603. doi: 10.1016/j.cell.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 86.Karbowski M, et al. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- 87.Burstein E, et al. A novel role for XIAP in copper homeostasis through regulation of MURR1. EMBO J. 2004;23:244–254. doi: 10.1038/sj.emboj.7600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Latour S, Aguilar C. XIAP deficiency syndrome in humans. Semin. Cell Dev. Biol. 2015;39:115–123. doi: 10.1016/j.semcdb.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 89.Dubrez L, Rajalingam K. IAPs and cell migration. Semin. Cell Dev. Biol. 2015;39:124–131. doi: 10.1016/j.semcdb.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 90.Cepero E, et al. Caspase-9 and effector caspases have sequential and distinct effects on mitochondria. Oncogene. 2005;24:6354–6366. doi: 10.1038/sj.onc.1208793. [DOI] [PubMed] [Google Scholar]
- 91.Chandra D, Tang DG. Mitochondrially localized active caspase-9 and caspase-3 result mostly from translocation from the cytosol and partly from caspase-mediated activation in the organelle. Lack of evidence for Apaf-1-mediated procaspase-9 activation in the mitochondria. J. Biol. Chem. 2003;278:17408–17420. doi: 10.1074/jbc.M300750200. [DOI] [PubMed] [Google Scholar]
- 92.Lakhani SA, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chandra D, et al. Early mitochondrial activation and cytochrome c up-regulation during apoptosis. J. Biol. Chem. 2002;277:50842–50854. doi: 10.1074/jbc.M207622200. [DOI] [PubMed] [Google Scholar]
- 94.Yadav N, et al. Transformations of the macromolecular landscape at mitochondria during DNA-damage-induced apoptotic cell death. Cell Death Dis. 2014;5:e1453. doi: 10.1038/cddis.2014.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gogada R, et al. Bim, a proapoptotic protein, up-regulated via transcription factor E2F1-dependent mechanism, functions as a prosurvival molecule in cancer. J. Biol. Chem. 2013;288:368–381. doi: 10.1074/jbc.M112.386102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Katoh I, et al. Dimerization and processing of procaspase-9 by redox stress in mitochondria. J. Biol. Chem. 2004;279:15515–15523. doi: 10.1074/jbc.M311819200. [DOI] [PubMed] [Google Scholar]
- 97.Susin SA, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 98.Flanagan L, et al. XIAP impairs Smac release from the mitochondria during apoptosis. Cell Death Dis. 2010;1:e49. doi: 10.1038/cddis.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilkinson JC, et al. Apoptosis-inducing factor is a target for ubiquitination through interaction with XIAP. Mol. Cell Biol. 2008;28:237–247. doi: 10.1128/MCB.01065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seeger JM, et al. Elevated XIAP expression alone does not confer chemoresistance. Br. J. Cancer. 2010;102:1717–1723. doi: 10.1038/sj.bjc.6605704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hwang C, et al. X-linked inhibitor of apoptosis deficiency in the TRAMP mouse prostate cancer model. Cell Death Differ. 2008;15:831–840. doi: 10.1038/cdd.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seligson DB, et al. Expression of X-linked inhibitor of apoptosis protein is a strong predictor of human prostate cancer recurrence. Clin. Cancer Res. 2007;13:6056–6063. doi: 10.1158/1078-0432.CCR-07-0960. [DOI] [PubMed] [Google Scholar]
- 103.Fulda S. Exploiting inhibitor of apoptosis proteins as therapeutic targets in hematological malignancies. Leukemia. 2012;26:1155–1165. doi: 10.1038/leu.2012.4. [DOI] [PubMed] [Google Scholar]
- 104.Hu Y, et al. Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer cells in vitro and in vivo. Clin. Cancer Res. 2003;9:2826–2836. [PubMed] [Google Scholar]
- 105.Cao C, et al. XIAP and survivin as therapeutic targets for radiation sensitization in preclinical models of lung cancer. Oncogene. 2004;23:7047–7052. doi: 10.1038/sj.onc.1207929. [DOI] [PubMed] [Google Scholar]
- 106.Dean E, et al. Phase I trial of AEG35156 administered as a 7-day and 3-day continuous intravenous infusion in patients with advanced refractory cancer. J. Clin. Oncol. 2009;27:1660–1666. doi: 10.1200/JCO.2008.19.5677. [DOI] [PubMed] [Google Scholar]
- 107.Katragadda L, et al. XIAP antisense therapy with AEG 35156 in acute myeloid leukemia. Expert Opin. Investig. Drugs. 2013;22:663–670. doi: 10.1517/13543784.2013.789498. [DOI] [PubMed] [Google Scholar]
- 108.Carter BZ, et al. XIAP antisense oligonucleotide (AEG35156) achieves target knockdown and induces apoptosis preferentially in CD34+38- cells in a Phase 1/2 study of patients with relapsed/refractory AML. Apoptosis. 2011;16:67–74. doi: 10.1007/s10495-010-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schimmer AD, et al. Addition of AEG35156 XIAP antisense oligonucleotide in reinduction chemotherapy does not improve remission rates in patients with primary refractory acute myeloid leukemia in a randomized Phase II study. Clin. Lymphoma Myeloma Leuk. 2011;11:433–438. doi: 10.1016/j.clml.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 110.Mahadevan D, et al. Phase I trial of AEG35156 an antisense oligonucleotide to XIAP plus gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma. Am. J. Clin. Oncol. 2013;36:239–243. doi: 10.1097/COC.0b013e3182467a13. [DOI] [PubMed] [Google Scholar]
- 111.Kester RF, et al. Optimization of benzodiazepinones as selective inhibitors of the X-linked inhibitor of apoptosis protein (XIAP) second baculovirus IAP repeat (BIR2) domain. J. Med. Chem. 2013;56:7788–7803. doi: 10.1021/jm400732v. [DOI] [PubMed] [Google Scholar]
- 112.Donnell AF, et al. Benzazepinones and benzoxazepinones as antagonists of inhibitor of apoptosis proteins (IAPs) selective for the second baculovirus IAP repeat (BIR2) domain. J. Med. Chem. 2013;56:7772–7787. doi: 10.1021/jm400731m. [DOI] [PubMed] [Google Scholar]
- 113.Petersen SL, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Greer RM, et al. SMAC mimetic (JP1201) sensitizes non-small cell lung cancers to multiple chemotherapy agents in an IAP-dependent but TNF-alpha-independent manner. Cancer Res. 2011;71:7640–7648. doi: 10.1158/0008-5472.CAN-10-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eschenburg G, et al. Smac mimetic LBW242 sensitizes XIAP-overexpressing neuroblastoma cells for TNF-alpha-independent apoptosis. Cancer Res. 2012;72:2645–2656. doi: 10.1158/0008-5472.CAN-11-4072. [DOI] [PubMed] [Google Scholar]
- 116.Edison N, et al. Peptides mimicking the unique ARTS-XIAP binding site promote apoptotic cell death in cultured cancer cells. Clin. Cancer Res. 2012;18:2569–2578. doi: 10.1158/1078-0432.CCR-11-1430. [DOI] [PubMed] [Google Scholar]
- 117.Ramachandiran S, et al. The Smac mimetic RMT5265.2HCL induces apoptosis in EBV and HTLV-I associated lymphoma cells by inhibiting XIAP and promoting the mitochondrial release of cytochrome C and Smac. Leuk. Res. 2012;36:784–790. doi: 10.1016/j.leukres.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peng Y, et al. Bivalent Smac mimetics with a diazabicyclic core as highly potent antagonists of XIAP and cIAP1/2 and novel anticancer agents. J. Med. Chem. 2012;55:106–114. doi: 10.1021/jm201072x. [DOI] [PubMed] [Google Scholar]
- 119.Nikolovska-Coleska Z, et al. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J. Med. Chem. 2004;47:2430–2440. doi: 10.1021/jm030420+. [DOI] [PubMed] [Google Scholar]
- 120.Silke J, Vaux DL. IAP gene deletion and conditional knockout models. Semin. Cell Dev. Biol. 2015;39:97–105. doi: 10.1016/j.semcdb.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 121.Kenneth NS, Duckett CS. IAP proteins: regulators of cell migration and development. Curr. Opin. Cell Biol. 2012;24:871–875. doi: 10.1016/j.ceb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 122.Hardwick JM, Soane L. Multiple functions of BCL-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013;5 doi: 10.1101/cshperspect.a008722. doi: 10.1101/cshperspect.a008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zermati Y, et al. Nonapoptotic role for Apaf-1 in the DNA damage checkpoint. Mol. Cell. 2007;28:624–637. doi: 10.1016/j.molcel.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 124.Chen L, et al. CD95 promotes tumour growth. Nature. 2010;465:492–496. doi: 10.1038/nature09075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gyrd-Hansen M, et al. IAPs contain an evolutionarily conserved ubiquitin-binding domain that regulates NF-kappaB as well as cell survival and oncogenesis. Nat. Cell Biol. 2008;10:1309–1317. doi: 10.1038/ncb1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kashkar H. X-linked inhibitor of apoptosis: a chemoresistance factor or a hollow promise. Clin. Cancer Res. 2010;16:4496–4502. doi: 10.1158/1078-0432.CCR-10-1664. [DOI] [PubMed] [Google Scholar]
- 127.LaCasse EC, et al. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–6275. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]