Abstract
Background
Bisphenol A (BPA) is a widely used industrial chemical and suspected endocrine disruptor to which humans are ubiquitously exposed. Liver metabolizes and facilitates BPA excretion through glucuronidation and sulfonation. The sulfotransferase enzymes contributing to BPA sulfonation (detected in human and rodents) is poorly understood.
Objectives
To determine the impact of metabolic and liver disease on BPA sulfonation in human and mouse livers.
Methods
The capacity for BPA sulfonation was determined in human liver samples that were categorized into different stages of metabolic and liver disease (including obesity, diabetes, steatosis, and cirrhosis) and in livers from ob/ob mice.
Results
In human liver tissues, BPA sulfonation was substantially lower in livers from subjects with steatosis (23%), diabetes cirrhosis (16%), and cirrhosis (18%), relative to healthy individuals with non-fatty livers (100%). In livers of obese mice (ob/ob), BPA sulfonation was lower (23%) than in livers from lean wild-type controls (100%). In addition to BPA sulfonation activity, Sult1a1 protein expression decreased by 97% in obese mouse livers.
Conclusion
Taken together these findings establish a profoundly reduced capacity of BPA elimination via sulfonation in obese or diabetic individuals and in those with fatty or cirrhotic livers versus individuals with healthy livers.
Keywords: Bisphenol A, phase-II, sulfotransferase, obesity, diabetes, steatosis, cirrhosis
Introduction
Bisphenol A is an industrial chemical and suspected endocrine disruptor with a widespread exposure in humans. Urinary BPA (total, indicating free BPA plus BPA-conjugates) has been detected at a mean of 2.6 μg/L in ~96% of samples from 2011–2012 NHANES study conducted by the Center of Disease Control (CDC) (http://wwwn.cdc.gov/nchs/nhanes/2011-2012/EPH_G.htm), as well as fetus, adult blood and placenta (Volkel et al., 2002). According to the most recent U.S. Food and Drug Administration (FDA) update, the average dietary exposure of BPA from food is estimated to be 0.2–0.4 microgram per kilogram body weight per day (μg/kg bw/day) for infants and 0.1–0.2 μg/kg bw/day for children and adults (FDA, 2010). Although human exposure to BPA is widespread, studies report a wide range of effective concentrations for specific pathways of BPA mediated endocrine disruption such as estrogenicity, aromatase and androgen receptor inhibition (Judson et al., 2010; Reif et al., 2010).
BPA is predominantly metabolized in the liver to corresponding glucuronide and sulfate conjugates (Pritchett et al., 2002; Hanioka et al., 2008). In both humans and rodents, BPA-glucuronide is the major metabolite detected in blood and urine, whereas sulfated conjugates (mono- and di-sulfates) are minor metabolites (Nishiyama et al., 2002; Volkel et al., 2002; Teeguarden et al., 2015; Thayer et al., 2015). Glucuronide and sulfate conjugated BPA metabolites are eliminated from the body into the urine via glomerular filtration. In addition, in rodents biliary excretion of BPA-glucuronide into the feces is substantial. In vitro ATPase activity assays have demonstrated that BPA-glucuronide has a high affinity for rodent Mrp2 and human MRP3 (ABCC3, basolateral) but is a non-substrate for human MRP2 (ABCC2, apical) transporters (Mazur et al., 2012). In rats, conjugated and unconjugated BPA is primarily (~66%) disposed through biliary excretion and detected in feces 6 hrs after oral or i.v administration (Kurebayashi et al., 2003) potentially due to high BPA-G affinity to Mrp2. In rats administered BPA, ~81% of administered dose was detected (measured as total BPA- conjugated and unconjugated) in feces, ~16% in urine while ~0.1% accumulated in tissue. However, urinary excretion is the major route of BPA elimination from the body in humans, which have higher affinity of BPA-G to basolateral MRP3 and relatively low affinity to apical MRP2 (Mazur et al., 2012). Conjugated BPA (glucuronide/sulfate) may be de-conjugated in the intestinal tract by glucuronidases/sulfatases and undergo enterohepatic recirculation that has been reported in rodents, but not humans (Ginsberg and Rice, 2009).
BPA-sulfate metabolites are detected in human serum and urine at a geometric mean of 0.124 ng/mL and 0.104 ng/mL, respectively (Liao and Kannan, 2012) with females having lower glucuronidated and higher sulfated BPA conjugates relative to males (Kim et al., 2003; Kurebayashi et al., 2003; Ye et al., 2005). BPA sulfonation is potentially SULT1A1-mediated, as determined using in vitro enzymatic methods (Nishiyama et al., 2002). However, the majority of studies describing BPA sulfonation utilize recombinant enzyme systems to determine BPA sulfonation by SULTs, and further studies are needed to determine and confirm BPA sulfonation in human liver.
Rodent studies and human epidemiological studies have revealed a significant correlation between BPA exposure and endocrine disruption, reproductive and developmental defects in rodents, as well as with metabolic disorders such as hypertension, diabetes and obesity (Christiansen et al., 2014; Khalil et al., 2014; Alonso-Magdalena et al., 2015). Extrapolation of observed BPA effects in rodents to humans is controversial, although building evidence suggests refinement of risk assessment towards more vulnerable populations such as fetuses, infants (Myers et al., 2009; Valentino et al., 2015) and potentially disease states with compensated liver function. Two studies have demonstrated ability of BPA to promote lipid accumulation in hepatocytes (Huc et al., 2012; Wang et al., 2013); the effect of this morphological and phenotypic change on BPA metabolism needs to be explored.
Non Alcoholic Fatty Liver Disease (NAFLD) is the accumulation of lipids exceeding 5% by weight of hepatocytes. NAFLD has also been referred to as “hepatic manifestation of insulin resistance” ranging from steatosis (fatty liver) to non-alcoholic steatohepatitis (fatty liver with liver cell damage and inflammation) to progressive hepatic fibrosis, cirrhosis and hepatocellular carcinoma (McCullough, 2011). In the United States, prevalence of NAFLD alone or in combination with increased liver enzymes in serum, as diagnosed by various techniques, was between 5–33% among adults (Lazo and Clark, 2008). Studies have shown that expression of several drug metabolism enzymes and transporters is altered in humans and rodent models of nonalcoholic fatty liver disease (steatosis) and obesity (Merrell and Cherrington, 2011). In addition, our recent studies showed that SULT1A1 expression and activity with a probe substrate was reduced in steatosis, diabetic cirrhosis, and alcoholic cirrhosis (Hardwick et al., 2013; Yalcin et al., 2013). This may result in modified metabolism and disposition of BPA, and potentially modified toxicity and adverse effects.
While sulfotransferase enzymes are an important class of Phase-II detoxification enzymes known to metabolize endogenous and xenobiotic compounds (James and Ambadapadi, 2013), sulfotransferase expression and activity for BPA has not been characterized in human livers under diseased conditions. Although secondary to glucuronidation, repression of SULT1A1 and SULT1A3 protein and activity in diseased human livers (Yalcin et al., 2013) points towards a potentially decreased ability for BPA biotransformation in the diseased liver. The purpose of this study herein was to characterize BPA sulfonation and SULT1A1 expression in human livers from individuals diagnosed with metabolic or liver disease, as well as in the ob/ob mouse under normal and fasted conditions to model metabolic-induced fatty liver disease. Herein, we describe decreased BPA sulfonation in diseased livers from both humans and mice.
Materials and Methods
Chemicals
[35S]PAPS (1.5–2.54 Ci/mmol) and scintillation fluid (Ultima Flo-M) were purchased from PerkinElmer Life and Analytical Sciences. p-Nitrophenol, and BPA were purchased from Sigma Aldrich. Sult1a1 antibody was obtained from Santa Cruz Biotechnology (TX, USA) and Gapdh antibody was obtained from Cell Signaling Technologies (MA, USA).
Animal treatment and fasting
Adult male C57BL/6 (WT, n=6–8/group) and Lep−/− (B6.V-Lepob/J, ob/ob, n=6–8/group) mice (Jackson Laboratories, Bar Harbor, ME, USA) were fed Harlan TekladLM-485 Mouse/Rat sterilizable diet or food-withheld for 24 hrs. Mice were housed in a temperature-, light-, and humidity-controlled environment in cages with corncob bedding. All the animal experiments were carried at University of Rhode Island, Fogarty facility with IACUC approval. Livers were collected, snap frozen in liquid nitrogen, and stored in −80°C until further analysis.
Cytosol isolation from human and mouse liver tissue
Human liver tissues were purchased from Liver Tissue Cell Distribution System (LTCDS), University of Minnesota, Minneapolis, MN. Details of the human liver donors are described in Yalcin et al. 2013. Liver samples were stored frozen at −80°C until the cytosolic fractions were prepared. Method for isolating cytosolic fractions has been reported previously (Yalcin et al., 2013). Briefly, liver tissue was homogenized by sonication in buffer containing sucrose/Tris/EDTA buffer supplemented with 0.01 M EDTA and 0.5 mM BHT, and cytosolic fractions were used for sulfonation assays.
BPA Sulfonation Activity
Activity assays for each liver tissue were performed in duplicate, and the average of duplicate data was analyzed. Human liver cytosols were incubated with radiolabeled sulfonyl donor [35S]-3′-phosphoadenosine-5′-phosphosulfate (35S-PAPS, 4 μM) and 4 μM BPA in 20 mM potassium phosphate (pH 7.0). Reaction mixture was incubated for 30 min at 37°C, stopped by heating in boiling water for 30 sec, and centrifuged at 14,000*g for 1 min to pellet the protein. To separate reaction components, supernatant was injected onto Phenomenex Synergi Polar-RP column (50 x 2.00 mm, 4 micron). A linear gradient of 15–80% acetonitrile and 20 mM potassium phosphate (pH 2.7) in 8 min was used as mobile phase at 1 mL/min flow to separate excess 35S-PAPS from 35S-BPA. 35S-labeled peaks were quantified on a flow scintillation analyzer (Packard Bioscience, 500 TR series) with Perkin-Elmer Ultima Flo-M scintillation cocktail. 35S-PAPS was eluted at 0.5 min, 35S-BPA-disulfate at 4.2 min, and 35S-BPA-monosulfate at 4.8 min.
Probe Sulfonation Activity Assays
Sulfonation assays for probe substrates was performed as described in (Yalcin et al. 2013). Briefly, cytosolic fraction of liver tissue was incubated with radiolabeled sulfonyl donor 35S-PAPS (4 μM) and a prototype substrate. Sulfated products of p-nitrophenol (pNP, 4 μM), estradiol (20 nM), and Dehydroepiandrosterone (DHEA, 10 μM) were separated on Synergi Polar-RP column (Phenomenex, Torrance, CA) and dopamine-sulfate on a Hypersil Duet C18/SAX column (Thermo Fisher Scientific). Radiolabeling was detected and quantified on a flow scintillation analyzer (500 TR series; Packard Bioscience, Meriden, CT) with PerkinElmer Ultima Flo-M scintillation cocktail.
Western Blotting
Cytosols (50 μg) were electrophoretically separated by SDS-PAGE and transferred onto a polyvinylidene difluoride membrane. After blocking the membrane with non-fat dry milk, blot was incubated with SULT1A1 antibody (Santa Cruz Biotechnology, TX, USA) and subsequently with corresponding HRP-labeled secondary antibody. Blots were visualized using Pierce ECL-Plus Western blot detection reagent (Thermo Fisher Scientific, Rockford, IL, USA) and quantified using ImageQuant software (Bio-Rad, Hercules, CA).
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 6 software. The correlation plots in human and mouse models were obtained by Pearson correlation and multiple regression analysis, respectively. The sample-sets were tested for normal distribution. For sample-sets without normality distribution, Logn transformation was performed to ensure all data set normality. A two-way ANOVA was performed followed by Duncan’s multiple range post hoc test. Probability (p) values less than 0.05 were considered significant.
Results
BPA sulfonation in non-fatty and diseased human livers
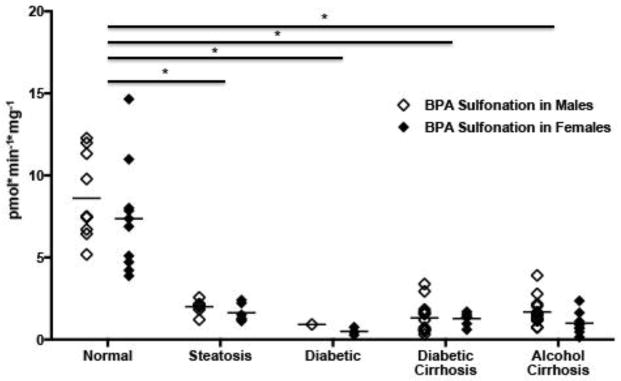
BPA sulfonation was quantified in 81 human liver samples from individuals diagnosed with steatosis (n=13), diabetes (n=4), diabetic cirrhosis (n=22), alcoholic cirrhosis (n=22), and non-fatty (n=20) livers (Supplemental Table 1). BPA sulfonation in non-fatty normal liver tissue cytosolic fraction averaged 7.99 pmol/min/mg. Hepatic BPA sulfonation was significantly lowered in steatosis (23% activity remaining), diabetes (8%), diabetes cirrhosis (16%), and alcoholic cirrhosis (18%) relative to non-fatty livers from healthy subjects (Figure 1). There was no statistical difference observed in BPA sulfonation between the genders.
Figure 1. Bisphenol A (BPA) sulfonation is decreased in cytosolic fractions isolated from diseased human livers.
Each data point represents a single tissue (average of determinations) categorized by gender and disease type. (n=20 for nonfatty, n=13 for steatosis, n=4 for diabetes, n=21 diabetic cirrhosis, and n=21 for alcohol cirrhosis). Females are displayed as black diamonds (◆), males as white diamonds (□). BPA sulfonation was determined by incubating 4 μM bisphenol A with human liver cytosols for 30 min in the presence of the 35S-labeled cofactor 3′-Phosphoadenosine-5′-phosphosulfate (PAPS). The reaction rate was calculated as product formation (pmol)/reaction time (min) / amount of enzyme used (mg). Asterisks (*) represent statistically significant differences between non-fatty and diseased human livers.
Sulfotransferase isoform activity in liver cytosolic fractions from WT and ob/ob mice
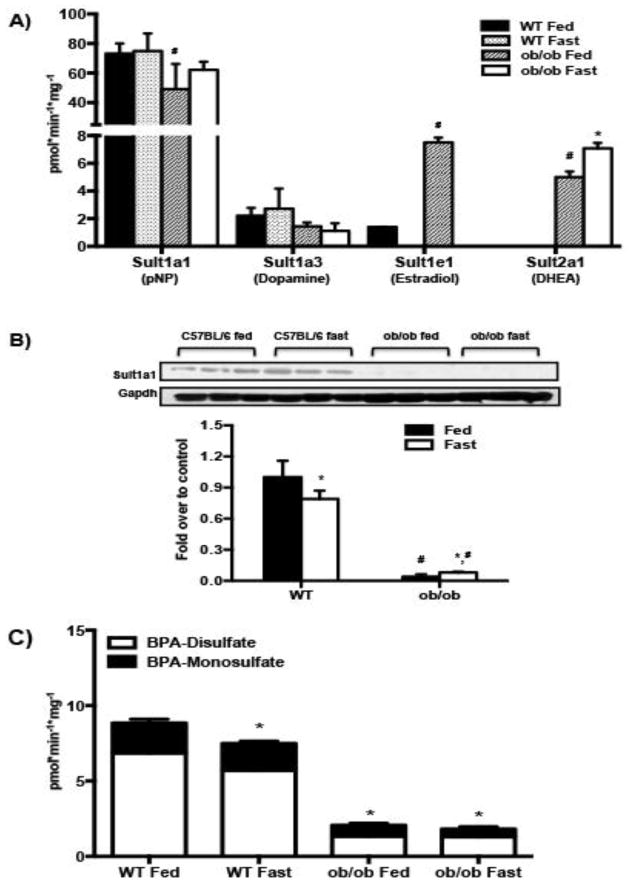
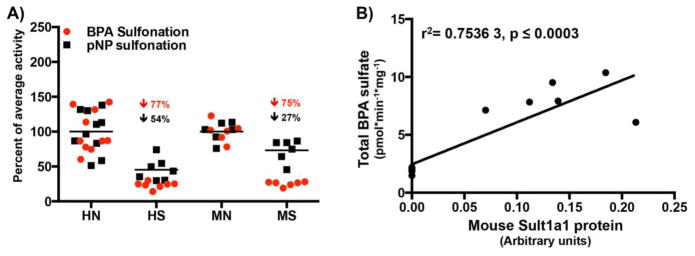
The effect of obesity and fasting on the activity of four major mouse liver cytosolic sulfotransferases was determined by probe substrate sulfonation (as described in material and methods) in C57BL/6 and ob/ob mice, fed or food-withheld for 24 hours (n=3–4/group). The rationale to evaluate Sult activity in both fed and fasted mice was because fasting induces hepatic steatosis, which is exacerbated in obese mice. Our group has demonstrated that hepatic triglycerides are elevated in ob/ob mice after a 24 hour fast (Xu et al., 2012). Fasting did not alter pNP-sulfonation (Sult1a1) or dopamine-sulfonation (Sult1a3) activity in WT mice, and DHEA-sulfonation (Sult2a1) activity remained undetectable in WT mouse livers under both fed (ad libitum) and fasted conditions (Fig. 2A). Under fed conditions, ob/ob mouse livers demonstrated somewhat lower pNP-sulfonation activity (Sult1a1) (78%), but higher estradiol-sulfonation (Sult1e1) (5 fold) and DHEA-sulfonation (Sult2a1) (4 fold) with a similar dopamine-sulfonation (Sult1a3) activity (Fig. 2A) as compared to their WT counterparts. As with the WT mice, fasting did not alter Sult1a1 or Sult1a3 activity in ob/ob mice, but increased DHEA-sulfonation (Sult2a1) activity in ob/ob mouse livers (1.5 fold over ob/ob fed livers) (Fig. 2A). Although Sult1a1 protein expression was undetectable in ob/ob mouse livers (Fig. 2B), we were able to measure pNP sulfonation (Fig. 2A) potentially due to contribution of other mouse Sult isoforms in pNP sulfonation. According to Tabrett and Coughtrie (2003), sulfonation of pNP by purified recombinant human SULT1B1 was significant at concentrations of 4-nitrophenol less than 10 μM, while pNP sulfonation by recombinant SULT1E1, SULT1A3, and SULT2A1 occurred only at higher substrate concentrations (~100 μM). The substrate selectivity of mouse sulfotransferases has not been studied due to lack of recombinant preparations of the mouse Sult isoforms.
Figure 2. Obesity decreases BPA and pNP sulfonation in mice.
(A) Sulfotransferase probe substrate activities in WT and ob/ob mouse livers-C57BL/6 and ob/ob mice (n=8/group) were either fed ad libitum or food withheld for 24 hours. Sult1a1, Sult1a3, Sult1e1 and Sult2a1 activity was determined using probe substrates pNP, dopamine, estradiol and DHEA respectively in liver cytosols as described in methods. (B) Sult1a1 protein expression in WT and ob/ob mouse livers- Representative western blot for Sult1a1 protein expression was determined in liver cytosols from WT and ob/ob mice fed ad libitum or food withheld for 24 hours with gapdh as the loading control. Blots were quantified using ImageJ software. (C) BPA sulfonation in C57BL/6 and ob/ob mouse liver cytosols under fed ad libitum and fasted conditions. BPA sulfonation (BPA-monosulfate, BPA-disulfate) was determined in WT and ob/ob, fed or fasted mouse livers by incubating 4 μM bisphenol A with mouse liver cytosols for 30 min in the presence of the 35S-labeled cofactor PAPS. Reaction components separated with HPLC and 35S labeled compounds were detected with a radiochemical detector. The reaction rate was calculated as product formation (pmol) / reaction time (min) / amount of enzyme used (mg). p<0.05 was considered statistically significant. “*” reflects significant difference between the fed and fasted groups whereas “#” reflects a significant difference between the genotypes.
Effect of obesity and fasting on Sult1a1 protein expression in livers of C57BL/6 and ob/ob mice
Sult1a1 protein expression was barely detectable in ob/ob mice compared to wild-type C57BL/6 mice, regardless of feeding status (p ≤ 0.05) (Figure 2B). The observed decrease in Sult1a1 protein expression in obese mice is a likely explanation for decreased BPA-sulfonation activity in livers of these mice.
BPA sulfonation in WT and ob/ob mouse livers
The effect of obesity and fasting on BPA sulfonation was determined using WT and ob/ob adult male mice. BPA sulfonation in mouse liver cytosols formed two products: monosulfate of BPA (BPS-S, 23%) and bis-sulfate of BPA (BPA-2S, 77%), with BPA-2S:BPA-S ratio being 3.35. Relative to WT mice, total BPA sulfonation was significantly diminished in ob/ob mouse livers accompanied with a decreased BPA-2S:BPA-S to 1.85. Fasting significantly decreased BPA sulfonation in WT livers to about 75% of fed control, without changing the BPA-2S:BPA-S. Combined with these results, there is a potential for decreased renal excretion of BPA in obesity as modeled by the ob/ob mice and 24-hr fasting. Although upon fasting, total BPA sulfonation remained unchanged in ob/ob livers, it increased BPA-2S: BPA-S, to 2.57 as compared 1.85 in the ob/ob fed controls (Fig. 2C).
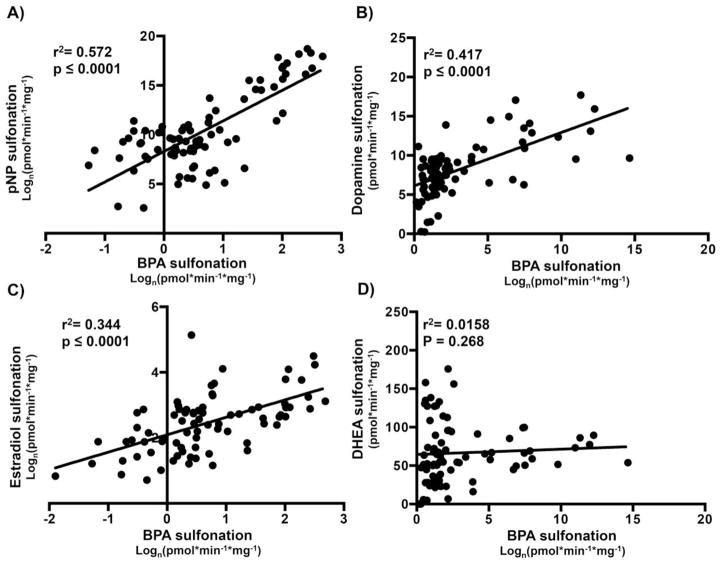
Correlation of BPA sulfonation versus probe substrate sulfonation in human livers
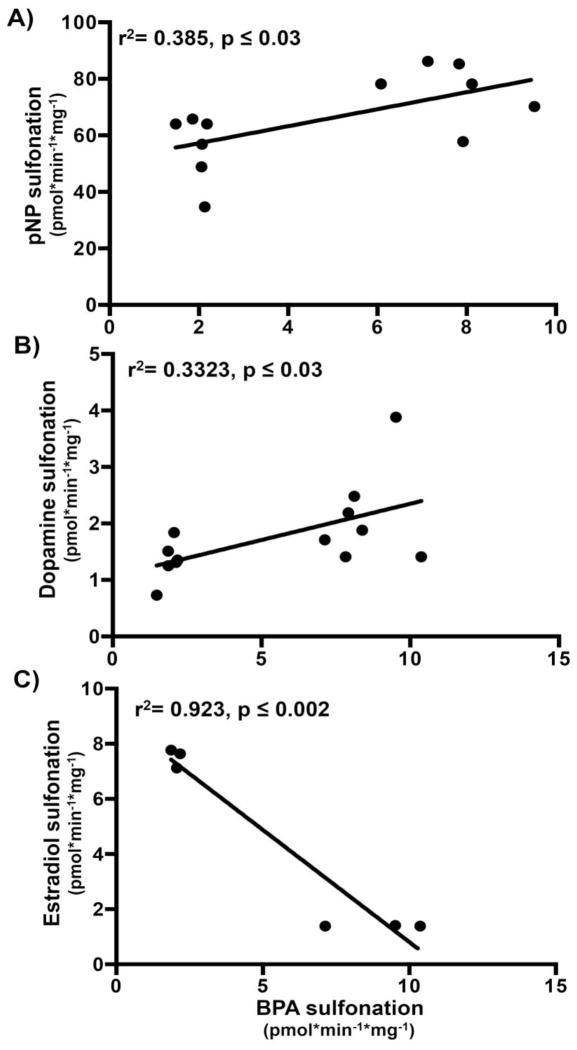
In vitro, BPA is sulfonated by multiple human sulfotransferase isoforms; SULT1A1, SULT2A1, and SULT1E1 with SULT1A1 possessing the highest reaction rate (Nishiyama et al., 2002). A linear correlation between BPA versus pNP sulfonation was detected in normal and diseased human liver cytosols (Fig. 3A). At 4 μM (Tabrett and Coughtrie, 2003), pNP sulfonation is known to be catalyzed mainly by SULT1A1, thus linear correlation indicates that SULT1A1 is potentially responsible for BPA sulfonation in these human liver cytosol samples. In contrast, BPA sulfonation did not yield significant linear correlation with DHEA- (Fig. 3D), while significantly correlated with dopamine- (Fig. 3B), as well as estradiol-sulfonation (Fig. 3C). However, correlation of BPA sulfonation with pNP sulfonation (SULT1A1 activity) remained the highest correlation of the three indicating SULT1A1 as the primary SULT isoform responsible for BPA sulfonation.
Figure 3.
Human BPA sulfonation correlates significantly with (A) SULT1A1 probe activity, (B) SULT1A3 probe activity, and (C) SULT1E1 probe activity, but not (D) SULT2A1 probe activity. The correlation between probe substrate and BPA sulfonation in human livers were obtained by Pearson correlation using GrapPad Prism. p< 0.05 were considered significant.
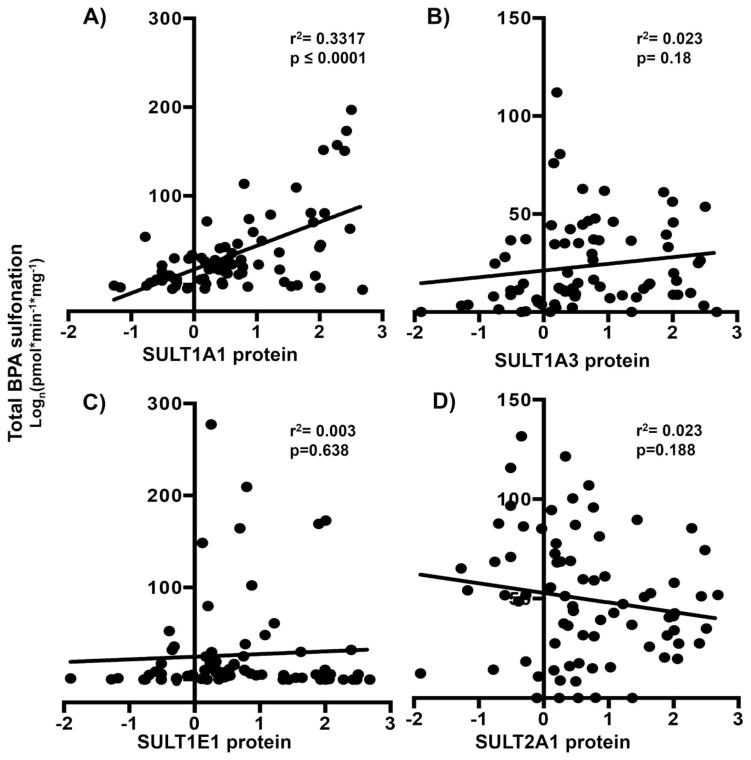
Correlation of BPA sulfonation with SULT isoform protein expression in human livers
It has been shown that sulfotransferase isoforms can compensate for the absence of other isoforms with respect to substrate sulfonation. Hence, it was also important to determine whether protein expression of SULT isoforms in human livers correlates with extent of BPA sulfonation. As demonstrated in Fig. 4, in human livers, BPA sulfonation significantly correlated with SULT1A1 protein (Fig. 4A) expression only. Correlation analysis of BPA sulfonation versus SULT1A3 (Fig. 4B), 1E1 (Fig. 4C) and 2A1 (Fig. 4D) protein expression remained non-significant. These observations further indicate that SULT1A1 primarily sulfonates BPA in human liver.
Figure 4. Human BPA sulfonation correlates significantly only with (A) SULT1A1 protein expression and not (B) 1A3 or (C) 1E1 or (D) SULT2A1 protein expression.
Correlation analysis of total BPA sulfate in human livers (control and diseased) with (A) SULT1A1, (B) 1A3, (C) 1E1 and (D) 2A1 protein levels. The correlation analyses in human livers were obtained by Pearson correlation using GraphPad Prism 6. p<0.05 was considered statistically significant.
Human and mouse BPA sulfonation by liver cytosolic sulfotransferases is similar
One of the aims of the present study is to determine whether BPA sulfonation changed similarly in normal and steatotic, human and mouse models. Hence, BPA sulfonation in male human normal (n=10) and steatotic (n=10) livers and male mouse WT (n=6) and ob/ob (n=6) livers was compared. As depicted in Fig. 1, 2A, 2C and 5A, BPA and pNP sulfonation are significantly downregulated in human steatotic samples as well as in ob/ob mouse livers. These observations indicate that presence of increased fat in livers can change sulfonation of BPA in both human and rodents. Total BPA sulfonation in ob/ob mouse livers significantly correlated with Sult1a1 protein expression (Fig. 5B) indicating Sult1a1 as the primary sulfotransferase isoform sulfonating BPA in mouse livers.
Figure 5. Human and mouse BPA sulfonation rates are comparable.
(A) pNP and BPA sulfation in normal and fatty livers in human and mouse (males only). Relative sulfonation of BPA and pNP (Sult1a1/SULT1A1 probe substrate) in male human normal (n=10) and steatotic (n=7) and mouse normal (n=6; 3 fed/3 fasted) and steatotic (n=6;3 fed/3 fasted) liver cytosols. (B) Correlation analysis of total BPA sulfonation versus Sult1a1 protein in WT and obese fed and fasted mouse liver cytosols (n=3/group). The correlation analyses in mouse livers were obtained by Pearson correlation using GrapPad Prism 6. p<0.05 was considered statistically significant.
Correlation of BPA versus pNP sulfonation in WT and ob/ob mouse livers
A linear correlation was detected between total BPA (4 M) versus pNP (4 μM) sulfonation (Fig. 6A) and dopamine (Fig. 6B) sulfonation in control and ob/ob mouse liver cytosols. In contrast to human livers, although significant, mouse estradiol versus BPA sulfonation yielded an inverse correlation (Fig. 6C). Thus, Sult1a1 and/or Sult1a3, and not Sult1e1, contribute to total BPA sulfonation in mouse liver.
Figure 6.
BPA sulfonation in mouse liver highly correlates with Sult1a1 and Sult1a3 activity. Correlation of BPA versus (A) p-nitrophenol, (B) dopamine, (C) estradiol sulfonation in WT and ob/ob mice. Correlations were obtained by Pearson correlation using GraphPad Prism 6. P< 0.05 were considered significant
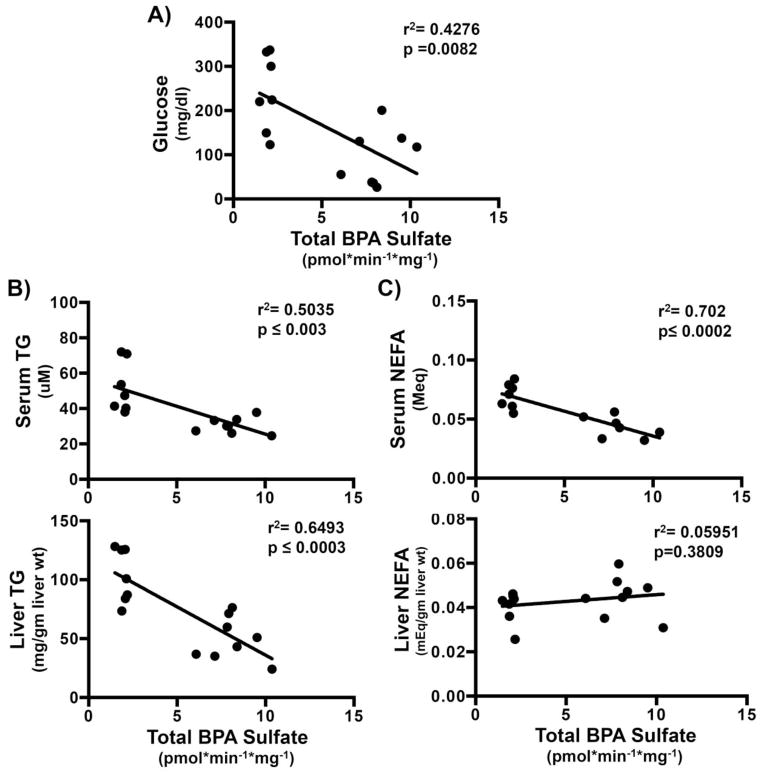
Mouse BPA sulfonation decreases with increasing liver triglycerides, and serum lipid and glucose content
Increased serum triglycerides (TG), non-esterified free fatty acids (NEFA) and glucose along with increased hepatic TG and NEFA are characteristic of obesity/steatosis/metabolic syndrome. Our previous work with the fasted ob/ob mouse model provides a spectrum of serum glucose and lipid concentrations, as well as elevated liver triglyceride content from the wild-type fed mouse to the fasted obese mouse (Xu et al., 2012). Thus, we characterized livers from this sample set to better understand whether serum markers associated with fatty liver disease correspond to BPA sulfonation. Overall, BPA sulfonation significantly decreased in steatotic human and mouse samples (Fig. 5A). To determine whether biochemical parameters significantly contributed to observed decreases in BPA sulfonation, BPA sulfonation was compared with individual biochemical parameters in WT and ob/ob, fed and fasted mice. Fasting results in an increased hepatic TG and NEFA content which has been previously described (Xu et al., 2012). BPA sulfonation inversely correlated with serum glucose levels (Fig. 7A). As seen in Fig. 7B, BPA sulfonation demonstrated a significant inverse correlation with serum and liver TG. BPA sulfonation inversely correlated with serum but not liver NEFA (Fig. 7C). Above observations indicate that the biochemical changes in lipid and glucose concentrations in steatotic models potentially alter the sulfotransferase activity and hence, total BPA sulfonation in the livers.
Figure 7. Increased triglyceride and glucose concentrations are associated with decreased BPA sulfonation.
Corrrelation of total BPA sulfonation in WT and ob/ob fed and fast (n=3/group) mouse cytosol with (A) serum glucose (mg/dl), (B) serum (uM) and liver triglycerides (mg/g liver wt) (TG) and (C) serum (MEq) and liver non-esterified fatty acids (NEFA) (MEq/gm liver wt). Correlations were obtained by Pearson correlation using GraphPad Prism 6. P< 0.05 were considered significant.
Steatosis and BPA sulfonation in human and mouse
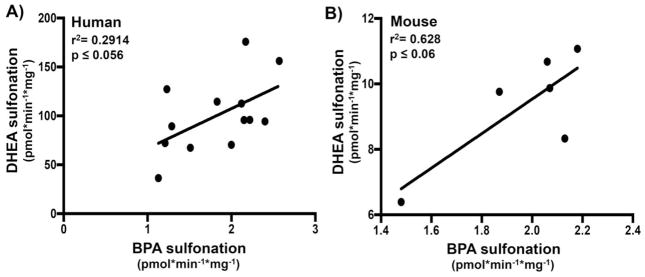
As shown in Fig. 3D, BPA sulfonation did not significantly correlate with DHEA sulfonation/SULT2A1 activity in human livers (normal and diseased). However, a correlation analysis with segregated diseased conditions revealed a near-significant correlation between BPA and DHEA sulfonation in male steatotic and diabetic livers (Fig. 8A and Supp Fig. 1) indicating SULT2A1 could also potentially contribute to BPA sulfonation in steatotic and diabetic livers. It is also interesting to note that BPA versus DHEA sulfonation remained insignificant in other disease conditions of diabetes-cirrhosis, and alcohol cirrhosis (Supp. Fig. 2). DHEA versus BPA sulfonation correlation analysis was performed in ob/ob mouse samples to determine whether animal model of steatosis/hyperglycemia also demonstrated similar trends. It is important to note that in our experiments, DHEA sulfonation was detectable only in ob/ob liver cytosolic fraction, while WT cytosols showed no detectable sulfonation of DHEA. Correlation analysis between DHEA and BPA sulfonation, in ob/ob liver cytosols set demonstrated a near-significant correlation (Fig. 8B).
Figure 8.
BPA sulfonation correlates with dehydroepiandrosterone (DHEA) sulfonation in male (A) human steatotic livers (n=7) and (B) ob/ob fed and fast (n=3/group) mouse livers. Correlations were obtained by Pearson correlation using GraphPad Prism 6. P< 0.05 were considered significant.
Discussion
The present study describes BPA sulfonation in normal and diseased human and mouse liver tissue. Nishiyama et al., 2002, studied participation of human SULTs in BPA metabolism using purified recombinant human sulfotransferases. SULT1A1 demonstrated highest kcat/km (574 min−1mM−1) and lowest km (4.7 μM) values followed by SULT2A1 and 1E1, while SULT1A3 had no effect on BPA sulfonation. In agreement with these data, human liver SULT1A1 (4 μM pNP) activity showed the best correlation towards BPA sulfonation in human liver cytosols (Fig. 3A). SULT1A1 protein decreases progressively from steatotic to cirrhotic liver tissues (Yalcin et al., 2013) similar to the significant decrease in BPA sulfonation in diseased tissues observed herein. These observations along with a significant correlation between pNP and BPA sulfonation suggest primarily SULT1A1 mediated BPA sulfonation in human liver. SULT1A3 activity also significantly correlated with BPA sulfonation in human liver cytosol (Fig. 3B), indicating a potential role for the phenol-preferring cytosolic SULT1A family in BPA sulfonation. Interestingly, SULT1E1 activity (Fig. 3C) also correlated significantly with BPA sulfonation. However, SULT1E1 expression and contribution to sulfonation in liver is lower than the other sulfotransferases (Riches et al., 2009; Yalcin et al., 2013), hence contribution to BPA sulfonation may be insignificant. BPA treatment has also been shown to inhibit SULT1E1 to a greater extent than SULT1A1 in vitro in hepatocytes (Hanet et al., 2008), further diminishing expected SULT1E1 contribution. The specificity of probe substrates for SULT1A1 (Tabrett and Coughtrie, 2003), SULT1A3 (Yasuda et al., 2007), SULT2A1 (Huang et al., 2010), and SULT1E1 (Falany et al., 1995) isoforms were shown using purified recombinant enzymes. In addition, we showed the specificity and selectivity of anti-SULT1A1 and anti-SULT1A3 antibodies using recombinant protein and human cytosolic liver fractions (Yalcin et al., 2013). Anti-SULT2A1 (purchased from Abcam) and anti-SULT1E1 (Aviva Systems Biology) antibodies had previously been tested on human livers.
Compared to human, the contribution of sulfotransferase isoforms to BPA sulfonation in mouse is relatively unknown, with Sult1a1 being suspected as the major contributing isoform to BPA sulfonation. Data obtained in this study after correlating BPA sulfonation with probe substrate sulfonation indicates that in mouse livers, as in humans, Sult1a1 and 1a3 are primarily involved in BPA sulfonation (Fig. 6A and 6B). In steatotic ob/ob mouse, BPA sulfonation was significantly decreased along with Sult1a1 protein expression further suggesting Sult1a1 involvement. However, unlike human, correlation of Sult1e1 activity with BPA sulfonation in mouse liver cytosols demonstrated an inverse correlation (Fig. 6C). Significant induction of Sult1e1/estradiol activity in ob/ob mice could potentially bias the correlation analysis and should be evaluated further. Ob/ob livers show a detectable DHEA sulfonation and a significant correlation between DHEA and BPA sulfonation, potentially due to significantly increased liver Sult2a1 expression relative to WT livers (data not shown) and indicating a Sult2a1 mediated BPA sulfonation in steatosis. We also observed a similar trend in diseased human livers, with SULT2A1 activity significantly correlating with BPA sulfonation only in steatotic and steatotic diabetic livers, indicating a shift in BPA sulfonation in these livers. These observations are in line with higher serum BPA concentration in obese women than lean counterparts, potentially due to changes in BPA metabolism in part mediated by SULTs (Takeuchi et al. 2004).
Increasing fasting duration decreases urinary total BPA concentrations in humans (Christensen et al. 2012). As shown in the present study, decreased Sult1a1 expression along with BPA sulfonation in a murine model could explain the observation (Fig. 2B). However, fasting did not alter Sult1a1 expression or further decrease BPA sulfonation in ob/ob mouse (Fig. 3B and 3C). This unchanged BPA sulfonation upon fasting in ob/ob mouse livers could potentially be explained as follows: Fasting down regulates Sult1a1 activity resulting in potential compensation from Sult2a1. In the absence of one isoform, sulfonation of substrate is compensated by another isoform such as SULT12A1 mediated sulfonation of estradiol, a prototypical SULT1E1 substrate (Riches et al. 2009). It is possible in ob/ob mouse livers, where BPA sulfonation is Sult2a1 mediated; fasting induction of Sult2a1 keeps total BPA sulfonation unchanged (Fig. 2C). Short term fasting increases serum and hepatic TG and NEFA content and in the present study; ob/ob fasted mice have the highest TG and NEFA content (Xu et al., 2012) in contrast to caloric restriction where these parameters decrease (Kulkarni et al., 2013). BPA sulfonation inversely correlated with most of serum and hepatic lipid parameters, as well as, glucose indicating that the metabolic state of the liver is important in determining the extent of BPA sulfonation. To date, there are have been multiple published studies that have associated aspects of metabolic disease with urine BPA concentrations (Rochester, 2013; Lakind et al., 2014). While these do not specifically address risk for liver disease, the studies are evaluating common risk factors associated with NAFLD. For example, a cross-sectional evaluation of urine samples and data from 1455 adults aged 18–74 years from the 2003–2004 National Health and Nutrition Examination Survey 2003–2004 reported higher urinary total BPA concentrations in association with cardiovascular diagnoses, diabetes, and clinically abnormal concentrations of the liver enzymes, i.e. gamma-glutamyltransferase and alkaline phosphatase (Lang et al., 2008). In a separate cross-sectional study analyzing urine from 2747 adults (aged 18–74) using pooled data from the 2003/04 and 2005/06 National Health and Nutrition Examination Surveys, higher BPA exposure was associated with general and central obesity in the general adult population of the United States (Carwile and Michels, 2011). The association between total urinary BPA concentration and generalized obesity or abdominal obesity has also been observed in Chinese populations (Wang et al., 2012), children and adolescents (Trasande et al., 2012; Bhandari et al., 2013; Wang et al., 2013). Recent work also demonstrated an association between urinary BPA content and adverse liver effects in young boys (Khalil et al., 2014). Given that the latter cited studies are cross-sectional in design, there has been concern that reverse causality may be important (Lang et al., 2008). There is recent epidemiological evidence to support this concern. Recent work evaluating the CHAMACOS prenatal and postnatal cohort observed that higher urinary BPA concentrations at 9 years of age were associated with increased adiposity at 9 years, whereas increasing BPA concentrations in mothers during pregnancy were associated with decreased BMI, body fat, and overweight/obesity among their daughters at 9 years of age (Harley et al., 2013). Our work herein supports the notion that reverse causality could occur, which could be due to decreased capacity for BPA biotransformation in association with fatty liver. We acknowledge that additional work addressing BPA glucuronidation is needed. However, these findings using livers from both human and rodent disease models demonstrate decreased BPA biotransformation and strongly argue for additional work to understand the contribution of liver disease to BPA clearance, especially in obesity and conditions of insulin resistance.
In summary, the data herein report a significant decrease in BPA sulfonation in liver samples from humans diagnosed with steatosis, diabetes, and cirrhosis via decreased sulfotransferase enzyme activity, primarily SULT1A1 expression and activity. Furthermore, BPA sulfonation is directly correlated with SULT2A1/Sult2a1 activity in human and mouse steatotic livers and not cirrhotic livers. These new results are especially important considering that BPA-sulfate conjugates have been detected in human urine, suggesting that BPA sulfonation occurs even at common (and relatively low) BPA exposures.
Conclusion
We report novel findings that liver disease negatively impacts BPA biotransformation. Decreased BPA sulfonation in association with impaired liver function or increased hepatic lipid content and markers of metabolic syndrome may result in increased free BPA or total urinary BPA levels.
Supplementary Material
Highlights.
Present study demonstrates that hepatic SULT 1A1/1A3 are primarily sulfonate BPA in mouse and human.
Hepatic BPA sulfonation is profoundly reduced steatosis, diabetes and cirrhosis.
With BPA-S detectable in urine under low or common exposures, these findings are novel and important.
Acknowledgments
This work was supported by National Institute of Health [R01ES016042 and K22ES013782] to AS, University of Rhode Island Council For Research Proposal Development Program Award to RK, and by the URI Foundation, Metabolism and Enzymology Laboratory funding to RK. Normal human liver and diseased human livers were obtained through the Liver Tissue Cell Distribution System, Minneapolis, Minnesota and Pittsburgh, Pennsylvania, which was funded by NIH Contract #N01-DK-7-0004 / HHSN267200700004C. Rhode Island IDeA Network of Biomedical Research Excellence Award Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 2 P20 GM103430.
Abbreviations
- BPA
Bisphenol A
- pNP
para-Nitrophenol
- PAPS
3′-Phosphoadenosine-5′-phosphosulfate
- SULT
sulfotransferase
- DHEA
dehydroepiandrosterone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso-Magdalena P, Garcia-Arevalo M, Quesada I, Nadal A. Bisphenol-A treatment during pregnancy in mice: a new window of susceptibility for the development of diabetes in mothers later in life. Endocrinology. 2015;156:1659–1670. doi: 10.1210/en.2014-1952. [DOI] [PubMed] [Google Scholar]
- Bhandari R, Xiao J, Shankar A. Urinary bisphenol A and obesity in U.S. children. Am J Epidemiol. 2013;177:1263–1270. doi: 10.1093/aje/kws391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ Res. 2011;111:825–830. doi: 10.1016/j.envres.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen S, Axelstad M, Boberg J, Vinggaard AM, Pedersen GA, Hass U. Low-dose effects of bisphenol A on early sexual development in male and female rats. Reproduction. 2014;147:477–487. doi: 10.1530/REP-13-0377. [DOI] [PubMed] [Google Scholar]
- Falany CN, Krasnykh V, Falany JL. Bacterial expression and characterization of a cDNA for human liver estrogen sulfotransferase. The Journal of steroid biochemistry and molecular biology. 1995;52:529–539. doi: 10.1016/0960-0760(95)00015-r. [DOI] [PubMed] [Google Scholar]
- Ginsberg G, Rice DC. Does rapid metabolism ensure negligible risk from bisphenol A? Environmental health perspectives. 2009;117:1639–1643. doi: 10.1289/ehp.0901010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanet N, Lancon A, Delmas D, Jannin B, Chagnon MC, Cherkaoui-Malki M, Latruffe N, Artur Y, Heydel JM. Effects of endocrine disruptors on genes associated with 17beta-estradiol metabolism and excretion. Steroids. 2008;73:1242–1251. doi: 10.1016/j.steroids.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Hanioka N, Naito T, Narimatsu S. Human UDP-glucuronosyltransferase isoforms involved in bisphenol A glucuronidation. Chemosphere. 2008;74:33–36. doi: 10.1016/j.chemosphere.2008.09.053. [DOI] [PubMed] [Google Scholar]
- Hardwick RN, Ferreira DW, More VR, Lake AD, Lu Z, Manautou JE, Slitt AL, Cherrington NJ. Altered UDP-glucuronosyltransferase and sulfotransferase expression and function during progressive stages of human nonalcoholic fatty liver disease. Drug metabolism and disposition: the biological fate of chemicals. 2013;41:554–561. doi: 10.1124/dmd.112.048439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Aguilar Schall R, Chevrier J, Tyler K, Aguirre H, Bradman A, Holland NT, Lustig RH, Calafat AM, Eskenazi B. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environmental health perspectives. 2013;121:514–520. doi: 10.1289/ehp.1205548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Bathena SP, Tong J, Roth M, Hagenbuch B, Alnouti Y. Kinetic analysis of bile acid sulfation by stably expressed human sulfotransferase 2A1 (SULT2A1) Xenobiotica; the fate of foreign compounds in biological systems. 2010;40:184–194. doi: 10.3109/00498250903514607. [DOI] [PubMed] [Google Scholar]
- Huc L, Lemarie A, Gueraud F, Helies-Toussaint C. Low concentrations of bisphenol A induce lipid accumulation mediated by the production of reactive oxygen species in the mitochondria of HepG2 cells. Toxicology in vitro : an international journal published in association with BIBRA. 2012;26:709–717. doi: 10.1016/j.tiv.2012.03.017. [DOI] [PubMed] [Google Scholar]
- James MO, Ambadapadi S. Interactions of cytosolic sulfotransferases with xenobiotics. Drug metabolism reviews. 2013;45:401–414. doi: 10.3109/03602532.2013.835613. [DOI] [PubMed] [Google Scholar]
- Judson RS, Houck KA, Kavlock RJ, Knudsen TB, Martin MT, Mortensen HM, Reif DM, Rotroff DM, Shah I, Richard AM, Dix DJ. In vitro screening of environmental chemicals for targeted testing prioritization: the ToxCast project. Environmental health perspectives. 2010;118:485–492. doi: 10.1289/ehp.0901392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N, Ebert JR, Wang L, Belcher S, Lee M, Czerwinski SA, Kannan K. Bisphenol A and cardiometabolic risk factors in obese children. The Science of the total environment. 2014;470–471:726–732. doi: 10.1016/j.scitotenv.2013.09.088. [DOI] [PubMed] [Google Scholar]
- Kim YH, Kim CS, Park S, Han SY, Pyo MY, Yang M. Gender differences in the levels of bisphenol A metabolites in urine. Biochemical and biophysical research communications. 2003;312:441–448. doi: 10.1016/j.bbrc.2003.10.135. [DOI] [PubMed] [Google Scholar]
- Kulkarni SR, Xu J, Donepudi AC, Wei W, Slitt AL. Effect of caloric restriction and AMPK activation on hepatic nuclear receptor, biotransformation enzyme, and transporter expression in lean and obese mice. Pharmaceutical research. 2013;30:2232–2247. doi: 10.1007/s11095-013-1140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi H, Betsui H, Ohno Y. Disposition of a low dose of 14C-bisphenol A in male rats and its main biliary excretion as BPA glucuronide. Toxicological sciences : an official journal of the Society of Toxicology. 2003;73:17–25. doi: 10.1093/toxsci/kfg040. [DOI] [PubMed] [Google Scholar]
- Lakind JS, Goodman M, Mattison DR. Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: a systematic review of epidemiologic research. Critical reviews in toxicology. 2014;44:121–150. doi: 10.3109/10408444.2013.860075. [DOI] [PubMed] [Google Scholar]
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. Jama. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Seminars in liver disease. 2008;28:339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K. Determination of free and conjugated forms of bisphenol A in human urine and serum by liquid chromatography-tandem mass spectrometry. Environmental science & technology. 2012;46:5003–5009. doi: 10.1021/es300115a. [DOI] [PubMed] [Google Scholar]
- Mazur CS, Marchitti SA, Dimova M, Kenneke JF, Lumen A, Fisher J. Human and rat ABC transporter efflux of bisphenol a and bisphenol a glucuronide: interspecies comparison and implications for pharmacokinetic assessment. Toxicological sciences : an official journal of the Society of Toxicology. 2012;128:317–325. doi: 10.1093/toxsci/kfs167. [DOI] [PubMed] [Google Scholar]
- McCullough AJ. Epidemiology of the metabolic syndrome in the USA. Journal of digestive diseases. 2011;12:333–340. doi: 10.1111/j.1751-2980.2010.00469.x. [DOI] [PubMed] [Google Scholar]
- Merrell MD, Cherrington NJ. Drug metabolism alterations in nonalcoholic fatty liver disease. Drug metabolism reviews. 2011;43:317–334. doi: 10.3109/03602532.2011.577781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JP, Zoeller RT, vom Saal FS. A clash of old and new scientific concepts in toxicity, with important implications for public health. Environmental health perspectives. 2009;117:1652–1655. doi: 10.1289/ehp.0900887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama T, Ogura K, Nakano H, Kaku T, Takahashi E, Ohkubo Y, Sekine K, Hiratsuka A, Kadota S, Watabe T. Sulfation of environmental estrogens by cytosolic human sulfotransferases. Drug metabolism and pharmacokinetics. 2002;17:221–228. doi: 10.2133/dmpk.17.221. [DOI] [PubMed] [Google Scholar]
- Pritchett JJ, Kuester RK, Sipes IG. Metabolism of bisphenol a in primary cultured hepatocytes from mice, rats, and humans. Drug metabolism and disposition: the biological fate of chemicals. 2002;30:1180–1185. doi: 10.1124/dmd.30.11.1180. [DOI] [PubMed] [Google Scholar]
- Reif DM, Martin MT, Tan SW, Houck KA, Judson RS, Richard AM, Knudsen TB, Dix DJ, Kavlock RJ. Endocrine profiling and prioritization of environmental chemicals using ToxCast data. Environmental health perspectives. 2010;118:1714–1720. doi: 10.1289/ehp.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riches Z, Stanley EL, Bloomer JC, Coughtrie MW. Quantitative evaluation of the expression and activity of five major sulfotransferases (SULTs) in human tissues: the SULT “pie”. Drug metabolism and disposition: the biological fate of chemicals. 2009;37:2255–2261. doi: 10.1124/dmd.109.028399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester JR. Bisphenol A and human health: a review of the literature. Reproductive toxicology. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Tabrett CA, Coughtrie MW. Phenol sulfotransferase 1A1 activity in human liver: kinetic properties, interindividual variation and re-evaluation of the suitability of 4-nitrophenol as a probe substrate. Biochemical pharmacology. 2003;66:2089–2097. doi: 10.1016/s0006-2952(03)00582-3. [DOI] [PubMed] [Google Scholar]
- Teeguarden JG, Twaddle NC, Churchwell MI, Yang X, Fisher JW, Seryak LM, Doerge DR. 24-hour human urine and serum profiles of bisphenol A: Evidence against sublingual absorption following ingestion in soup. Toxicology and applied pharmacology. 2015;288:131–142. doi: 10.1016/j.taap.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Thayer KA, Doerge DR, Hunt D, Schurman SH, Twaddle NC, Churchwell MI, Garantziotis S, Kissling GE, Easterling MR, Bucher JR, Birnbaum LS. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environment international. 2015;83:107–115. doi: 10.1016/j.envint.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. Jama. 2012;308:1113–1121. doi: 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- Valentino R, D’Esposito V, Ariemma F, Cimmino I, Beguinot F, Formisano P. Bisphenol A environmental exposure and the detrimental effects on human metabolic health: is it necessary to revise the risk assessment in vulnerable population? Journal of endocrinological investigation. 2015 doi: 10.1007/s40618-015-0336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkel W, Colnot T, Csanady GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chemical research in toxicology. 2002;15:1281–1287. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- Wang J, Sun B, Hou M, Pan X, Li X. The environmental obesogen bisphenol A promotes adipogenesis by increasing the amount of 11beta-hydroxysteroid dehydrogenase type 1 in the adipose tissue of children. International journal of obesity. 2013;37:999–1005. doi: 10.1038/ijo.2012.173. [DOI] [PubMed] [Google Scholar]
- Wang T, Li M, Chen B, Xu M, Xu Y, Huang Y, Lu J, Chen Y, Wang W, Li X, Liu Y, Bi Y, Lai S, Ning G. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. The Journal of clinical endocrinology and metabolism. 2012;97:E223–227. doi: 10.1210/jc.2011-1989. [DOI] [PubMed] [Google Scholar]
- Xu J, Kulkarni SR, Li L, Slitt AL. UDP-glucuronosyltransferase expression in mouse liver is increased in obesity- and fasting-induced steatosis. Drug metabolism and disposition: the biological fate of chemicals. 2012;40:259–266. doi: 10.1124/dmd.111.039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin EB, More V, Neira KL, Lu ZJ, Cherrington NJ, Slitt AL, King RS. Downregulation of sulfotransferase expression and activity in diseased human livers. Drug metabolism and disposition: the biological fate of chemicals. 2013;41:1642–1650. doi: 10.1124/dmd.113.050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S, Liu MY, Suiko M, Sakakibara Y, Liu MC. Hydroxylated serotonin and dopamine as substrates and inhibitors for human cytosolic SULT1A3. Journal of neurochemistry. 2007;103:2679–2689. doi: 10.1111/j.1471-4159.2007.04948.x. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. Quantification of urinary conjugates of bisphenol A, 2,5-dichlorophenol, and 2-hydroxy-4-methoxybenzophenone in humans by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Analytical and bioanalytical chemistry. 2005;383:638–644. doi: 10.1007/s00216-005-0019-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.