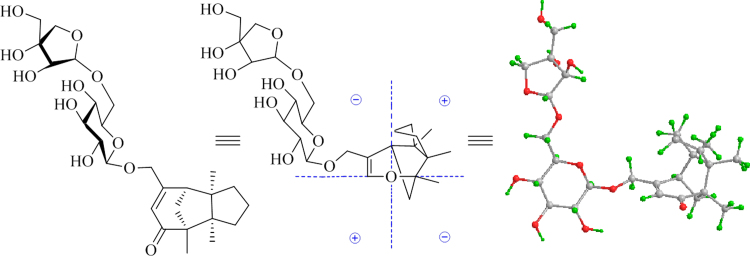
Abstract
Three new sesquiterpene glycosides, named codonopsesquilosides A−C (1−3), were isolated from an aqueous extract of the dried roots of Codonopsis pilosula. Their structures including absolute configurations were determined by spectroscopic and chemical methods. These glycosides are categorized as C15 carotenoid (1), gymnomitrane (2), and eudesmane (3) types of sesquiterpenoids, respectively. Compound 1 is the first diglycoside of C15 carotenoids to be reported. Compound 2 represents the second reported example of gymnomitrane-type sesquiterpenoids from higher plants. The absolute configurations were supported by comparison of the experimental circular dichroism (CD) spectra with the calculated electronic CD (ECD) spectra of 1−3, their aglycones, and model compounds based on quantum-mechanical time-dependent density functional theory. The influences of the glycosyls on the calculated ECD spectra of the glycosidic sesquiterpenoids, as well as some nomenclature and descriptive problems with gymnomitrane-type sesquiterpenoids are discussed.
Keywords: Codonopsis pilosula, Campanulaceae, Sesquiterpene glycoside, Codonopsesquilosides A−C, C15 carotenoid, Gymnomitrane, Eudesmane
Graphical abstract
Three new sesquiterpene glycoside codonopsesquilosides A−C (1−3) were isolated from an aqueous extract of the Codonopsis pilosula roots. Their structures including absolute configurations were determined by spectroscopic and chemical methods. Compound 1 is the first diglycoside of C15 carotenoids, and 2 represents the second example of gymnomitrane-type sesquiterpenoids from higher plants. The influences of the glycosyls on the calculated ECD spectra of the glycosidic sesquiterpenoids are discussed. Some suggestions to solve problems with gymnomitrane-type sesquiterpenoids nomenclature and descriptions are proposed.
1. Introduction
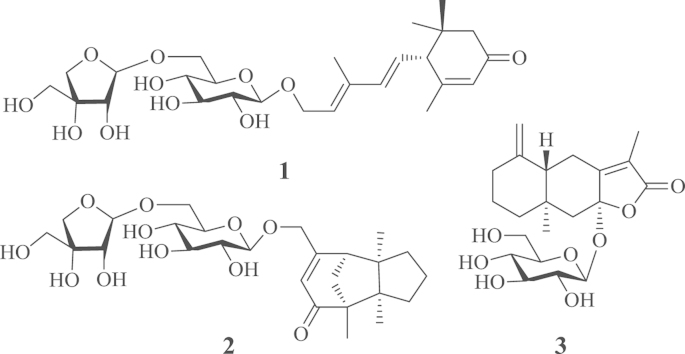
The dried roots of Codonopsis pilosula (Franch.) Nannf. (Campanulaceae), known as “dang shen” in Chinese, are one of the most common drugs in traditional Chinese medicine and normally are used as a substitute for the much more costly Panax ginseng as tonic agents exhibiting similar therapeutic effects1. Although some chemical constituents and pharmacological activities have been reported, previous work was mainly carried out on ethanol or methanol extracts of this natural product2, which is not consistent with practical utilization of herbal medicines including various formulations containing “dang shen” by decocting with water. As part of a program to systematically study the chemical diversity of traditional Chinese medicines and their biological effects, focusing on water-soluble and minor constituents3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, an aqueous decoction of “dang shen” was investigated. Previously, we reported twelve new constituents from the decoction2, 15, including an unsaturated ω-hydroxy fatty acid, 4 acetylenes, and 7 C14-polyacetylene glucosides, along with preliminary bioassays. Continuation of our investigation on the same extract led to characterization of three new minor sesquiterpene glycosides named codonopsesquilosides A−C (1−3) (Fig. 1). Herein, reported are details of the isolation and structural elucidation of the new isolates.
Figure 1.
The structures of compounds 1−3.
2. Results and discussion
Compound 1, a white amorphous powder with [α]20D +26.3 (c 0.04, MeOH), showed IR absorption bands for hydroxyl (3395 cm−1), double bond (3010 cm−1), and conjugated carbonyl (1645 cm−1) functional groups. The molecular formula of 1, C26H40O11, was indicated by HR-ESI-MS at m/z 551.2461 [M+Na]+ (Calcd. for C26H40O11Na, 551.2463) and NMR spectroscopic data (Table 1). The 1H NMR spectrum of 1 in DMSO-d6 showed signals attributable to a trans-disubstituted double bond at δH 6.23 (d, J=15.6 Hz, H-8) and 5.61 (dd, J=15.6 and 9.6 Hz, H-7); two trisubstituted double bonds at δH 5.80 (s, H-4) and 5.60 (d, J=7.2 and 6.6 Hz, H-10); an oxygen-bearing methylene at δH 4.32 (dd, J=12.6 and 6.6, H-11a) and 4.19 (dd, J=12.6 and 7.2 Hz, H-11b); an aliphatic methine at δH 2.67 (dd, J=9.6, H-6); an isolated aliphatic methylene at δH 2.41 (d, J=16.8 Hz, H-2a) and 1.97 (d, J=16.8 Hz, H-2b); and four tertiary methyl groups at δH 1.84 (s, H3-13), 1.73 (s, H3-12), 0.95 (s, H3-14), and 0.88 (s, H3-15). Additionally, the spectrum showed characteristic signals for two glycosyl units (Table 1) with anomeric protons at δH 4.13 (d, J=7.8 Hz, H-1′) and 4.86 (d, J=3.0 Hz, H-1″), respectively. The 13C NMR and distortionless enhanced polarization transfer (DEPT) spectra of 1 showed 26 carbon resonances (Table 1) corresponding the above units, a carbonyl (δC 197.9, C-3), and a quaternary carbon (δC 35.9, C-1). These spectroscopic data indicates that 1 is a monocyclic sesquiterpene diglycoside for which the structure was further elucidated by 2D NMR data analysis.
Table 1.
1H and 13C NMR spectral data (δ) for compounds 1−3.a
| No. |
1 |
2 |
3b |
|||
| δH | δC | δH | δC | δH | δC | |
| 1a | 35.9 | 61.4 | 1.51 m | 41.8 | ||
| 1b | 1.30 ddd (10.5, 6.0, 4.5) | |||||
| 2a | 2.41 d (16.8) | 47.3 | 56.3 | 1.61 m | 23.2 | |
| 2b | 1.97 d (16.8) | |||||
| 3a | 197.9 | 1.38 dd (12.0, 6.0) | 38.8 | 2.32 ddd (13.2, 3.0, 1.8) | 36.7 | |
| 3b | 1.12 m | 1.98 ddd (13.2, 7.2, 6.0) | ||||
| 4a | 5.80 s | 124.8 | 1.48 m | 27.6 | 149.9 | |
| 4b | 1.37 m | |||||
| 5a | 162.0 | 1.64 ddd (12.0, 6.0, 3.6) | 40.0 | 1.91 dd (12.6, 1.2) | 52.0 | |
| 5b | 1.34 dd (12.0, 6.0) | |||||
| 6a | 2.67 d (9.6) | 55.2 | 57.9 | 2.76 ddd (13.2, 12.6, 1.2) | 26.3 | |
| 6b | 2.66 dd (13.2, 3.0) | |||||
| 7 | 5.61 dd (15.6, 9.6) | 126.5 | 2.19 d (4.8) | 50.3 | 162.3 | |
| 8 | 6.23 d (15.6) | 137.2 | 170.6 | 105.3 | ||
| 9a | 135.9 | 6.08 d (0.6) | 123.8 | 2.27 d (13.2) | 51.7 | |
| 9b | 1.49 d (13.2) | |||||
| 10 | 5.60 dd (7.2, 6.6) | 127.3 | 208.3 | 37.4 | ||
| 11a | 4.32 dd (12.6, 6.6) | 64.4 | 2.03 dd (12.0, 4.8) | 47.9 | 123.1 | |
| 11b | 4.19 dd (12.6, 7.2) | 1.82 d (12.0) | ||||
| 12 | 1.73 s | 12.6 | 0.98 s | 17.9 | 171.9 | |
| 13 | 1.84 s | 23.0 | 0.96 s | 23.0 | 1.78 d (0.6) | 8.2 |
| 14 | 0.95 s | 27.4 | 1.15 s | 28.3 | 1.00 s | 17.3 |
| 15a | 0.88 s | 26.8 | 4.42 dd (17.4, 1.8) | 72.2 | 4.81 d (1.2) | 107.0 |
| 15b | 4.33 dd (17.4, 1.8) | 4.63 d (1.2) | ||||
| 1ʹ | 4.13 d (7.8) | 101.8 | 4.26 d (7.8) | 104.5 | 4.15 d (7.8) | 97.3 |
| 2ʹ | 2.95 t (7.8) | 73.3 | 3.29 t (7.8) | 78.0 | 3.23 ddd (9.0, 7.8, 3.0) | 74.3 |
| 3ʹ | 3.23 dd (9.0, 7.8) | 76.6 | 3.19 t (7.8) | 75.0 | 3.36 t (9.0) | 77.9 |
| 4ʹ | 2.97 t (9.0) | 70.3 | 3.24 m | 71.5 | 3.30 ddd (9.6, 6.0, 3.0) | 71.7 |
| 5ʹ | 3.11 t (9.0) | 75.6 | 3.34 m | 78.0 | 3.12 m | 78.2 |
| 6ʹa | 3.83 d (11.4) | 67.7 | 3.90 dd (11.4, 1.8) | 68.5 | 3.80 ddd (11.4, 4.8, 3.0) | 62.8 |
| 6ʹb | 3.40 dd (11.4, 7.2) | 3.55 dd (11.4, 6.0) | 3.58 ddd (11.4, 6.6, 6.0) | |||
| 1ʺ | 4.86 d (3.0) | 109.3 | 4.93 d (2.4) | 111.0 | ||
| 2ʺ | 3.74 (1.8) | 75.9 | 3.84 d (2.4) | 78.0 | ||
| 3ʺ | 78.8 | 80.5 | ||||
| 4ʺa | 3.84 d (9.0) | 73.2 | 3.89 d (9.6) | 75.0 | ||
| 4ʺb | 3.57 d (9.0) | 3.69 d (9.6) | ||||
| 5ʺ | 3.31 m | 63.1 | 3.52 s | 65.6 | ||
NMR data (δ) were measured at 600 MHz for 1H NMR and 150 MHz for 13C NMR in DMSO-d6 for 1, in MeOH-d4 for 2, and in acetone-d6 for 3, respectively. Proton coupling constants (J) in Hz are given in parentheses. The assignments were based on 1H–1H COSY, HSQC, and HMBC experiments.
1H NMR data for hydroxyl protons of 3: δH 4.23 (1H, brs, OH-4ʹ), 4.21 (1H, d, J=3.0 Hz, OH-2ʹ), 4.12 (1H, d, J=3.0 Hz, OH-3ʹ), 3.40 (1H, t, J=6.6 Hz, OH-6ʹ).
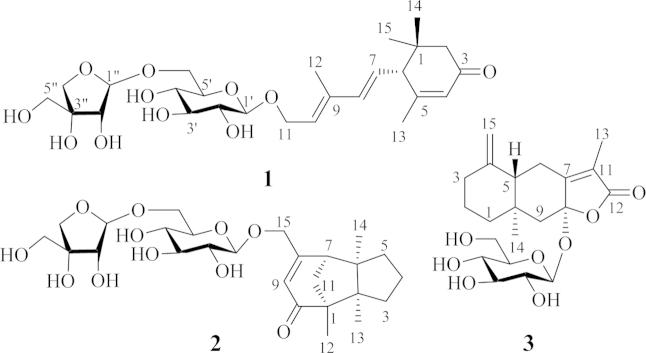
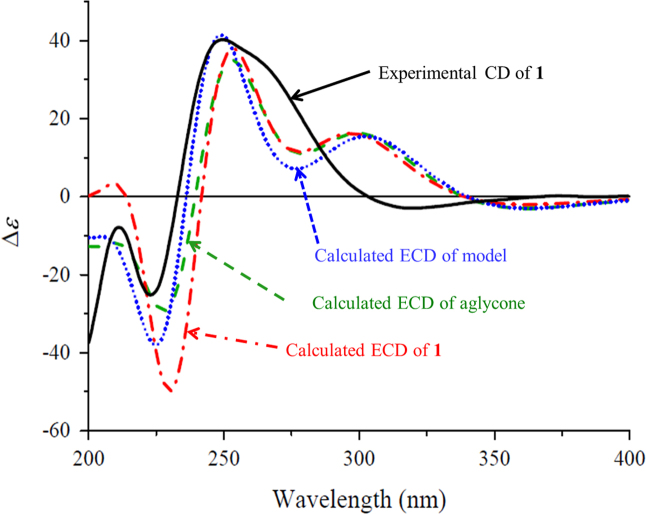
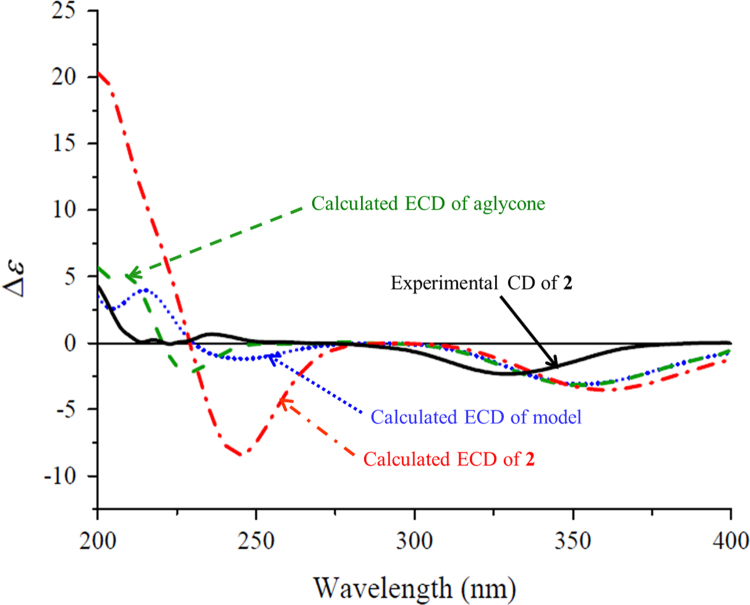
The proton and proton-bearing carbon signals in the NMR spectra were assigned by cross-peaks in the 1H–1H COSY and HSQC spectra. The heteronuclear multiple bond correlation (HMBC) correlations from H2-2 to C-1, C-3, C-6, C-14, and C-15; from H-4 to C-3, C-6, and C-13; from H-6 to C-1, C-2, C-4, C-5, C-13, C-14 and C-15; from H3-13 to C-4, C-5, and C-6; and from both H3-14 and H3-15 to C-1, C-2, and C-6 (Fig. 2); together with their chemical shifts, revealed the presence of a 6-substituted 1,1,5-trimethylhex-4-en-3-one moiety in 1. The 1H–1H COSY correlations of H-6/H-7/H-8 and H-10/H-11 and the HMBC correlations from H-6 to C-7 and C-8; from H-8 to C-6, C-9, C-10, and C-12; from H-10 to C-8 and C-12; from H2-11 to C-9 and C-10; and from H3-12 to C-8, C-9, and C-10 demonstrated that there was an 11-oxygen substituted 9-methylpenta-7,9-dien-7-yl side chain at C-6 of the hex-4-en-3-one moiety. In addition, the 1H–1H COSY correlations of H-1′/H-2′/H-3′/H-4′/H-5′/H2-6′ and their vicinal coupling constants, combined with the HMBC correlations from H2-11 to C-1′ and from H-1′ to C-11, indicated the occurrence of a β-glucopyranosyloxy at C-11. The 1H–1H COSY cross-peaks H-1″/H-2″ and the HMBC correlations from H-1″ to C-4″ and C-6′, from H2-6′ to C-1″, from both H2-4″ and H2-5″ to C-3″, in combination with the quaternary nature of C-3″, suggested that there was a β-apiofuranosyloxy at C-6′ of the β-glucopyranosyl. This was supported by NMR data comparison of the diglycosyl in 1 with those previously reported for structurally related compounds16, 17 and further confirmed by enzymatic hydrolysis of 1 with snailase. From the hydrolysate of 1, a sugar mixture of glucose and apiose was isolated by column chromatography over silica gel (CH3CN–H2O, 8:1, v/v) and identified by thin-layer liquid chromatography (TLC) comparison with authentic sugar samples. The sugar mixture and authentic d- and l-glucose and d- and l-apiose were separately allowed to react with l-cycteine methyl ester and arylisothiocyanate18. Subsequent HPLC analysis indicated that two sugar derivatives from the mixture had retention time (tR) identical to those of d-glucose and d-apiose derivatives. This verified that both glycosyl units in 1 possessed the d-configuration. Similarity of the NMR data and the circular dichroism (CD) spectra between 1 and the reported acetylated β-d-glucopyranoside19 indicated that these two compounds have the same aglycone moiety including geometric and absolute configurations. The configuration was further supported by comparison of the experimental CD spectrum of 1 with the calculated electronic CD (ECD) spectra of 1, its aglycone, and a model compound with substitution of the diglycosyl unit by a methyl group (Fig. 3) predicted from the quantum-mechanical, time-dependent density functional theory (TDDFT) calculations20. Therefore, the structure of compound 1 was determined and named codonopsesquiloside A. This compound can also be named (R)-dehydroxyabscisic alcohol β-d-apiofuranosyl-(1″→6′)-β-d-glucopyranoside since the aglycone differs from (S)-abscisic alcohol only in the absence of hydroxyl group at the chiral center21.
Figure 2.
The 1H–1H COSY (thick line) and key HMBC (arrows, from 1H NMR to 13C NMR) correlations of compounds 1−3.
Figure 3.
The experimental CD spectrum of 1 (black) and the calculated ECD spectra of 1 (dashed red) and its aglycone (dash-dotted blue) and the model compound (dotted green) in MeOH.
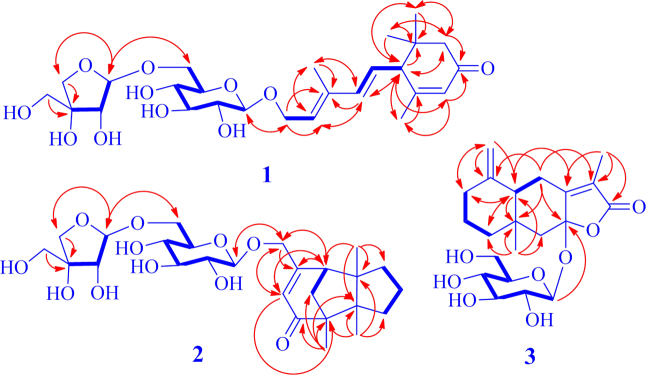
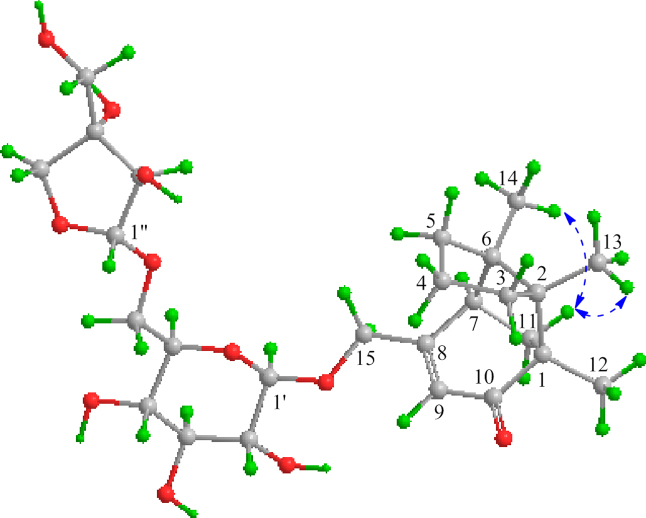
Compound 2, a white amorphous powder with [α]20D −46.7 (c 0.04, MeOH), has the molecular formula C26H40O11 as indicated by HR-ESI-MS at m/z 529.2637 [M+H]+ (Calcd. for C26H41O11, 529.2643) and 551.2462 [M+Na]+ (Calcd. for C26H40O11Na, 551.2463). The IR spectrum of 2 showed the presence of hydroxyl (3395 cm−1) and conjugated carbonyl (1747 and 1647 cm−1) groups. The NMR spectra of 2 (Table 1) displayed the resonances attributable to three tertiary methyls, five methylenes (an oxygen-bearing), one methine, a trisubstituted double bond, and four quaternary carbons including a carbonyl carbon (δC 208.3, C-10), as well as the signals ascribable to the same diglycosyl moiety as that of 1. These spectroscopic data indicates that 2 is a tricyclic sesquiterpene β-d-apiofuranosyl-(1″→6′)-β-d-glucopyranoside, with an aglycone moiety different from that of 1. In the 1H–1H COSY spectrum of 2, the cross-peaks between H2-4 with both H2-3 and H2-5 and between H-7 and H2-11, together with their chemical shifts and coupling patterns, demonstrated that there were two vicinal coupling aliphatic units in the aglycone moiety. In the HMBC spectrum of 2, correlations from H3-13 to C-1, C-2, C-3, and C-6 and from H3-14 to C-2, C-5, C-6, and C-7 (Fig. 2) established connections between the quaternary C-2 with C-1, C-3, C-6, and C-13 and between the quaternary C-6 with C-2, C-5, C-7, and C-14. These connections formed a 2,6-disubstituted 2,6-dimethyl five-membered ring. The HMBC correlations from H3-12 to C-1, C-2, C-10, and C-11 revealed linkages between the quaternary C-1 with C-10, C-11, and C-12, and confirmed the connection between the two quaternary carbons C-1 and C-2. The linkages built up a 1-methyl five-membered ring fused with the former. In addition, the HMBC correlations from H-7 to C-8, C-9, and C-15; from H-9 to C-1, C-7, and C-15; from H2-15 to C-8 and C-9; together with the chemical shifts of these proton and carbon resonances, demonstrated that both the oxymethylene (C-15) and the methine (C-7) were connected to one end (C-8) of the trisubstituted double bond and that the quaternary C-1 was linked via the carbonyl (C-10) to the other end (C-9). This constructed an 8-oxymethylene cyclohexene ring fused with the latter five-membered ring to give the tricyclic bridged structure of the aglycone in 2. The presence of β-apiofuranosyl-(1″→6′)-β-glucopyranosyl was confirmed by the 1H–1H COSY and HMBC correlations similar to those of 1. Meanwhile, the HMBC correlations from H2-15 to C-1′ and from H-1′ to C-15 verified that the diglycosyl is located at C-15 of the aglycone. In the NOE difference spectrum of 2, irradiation of H-11 enhanced H3-13 and H3-14 (Fig. 4), indicating that the bridge methylene (CH2-11) and the two methyl groups were oriented in the same direction. Using the same protocol as described for 1, the d-configuration was assigned for the two glycosyl units in 2. The CD spectrum of 2 displayed a negative Cotton effect at 330 nm (Δε −2.32) for the n→π⁎ transition of cyclohexanone chromophore. Based on the octant rule for cyclohexenones22, 23, the Cotton effect suggested that 2 possesses 1S,7R configuration (Fig. 5), which was further supported by comparison of the experimental CD spectrum of 2 with the calculated ECD spectra of 2 and the aglycone methyl ether (model compound) (Fig. 6). Thus, the structure of compound 2 was determined and named codonopsesquiloside B, which is the first glycoside of gymnomitrane-sesquiterpenoids. According to nomenclature of the parent skeleton of gymnomitrol24, this compound is systematically named (−)-(1S,2R,6R,7R)-1,2,6-trimethyl-8-hydroxy methyltricyclic[5.3.1.02,6]-undec-8-en-10-one β-d-apiofuranosyl-(1″→6′)-β-d-glucopyranoside.
Figure 4.
The NOE enhancements induced by irradiation of H-11a (dashed arrows) for compound 2.
Figure 5.
The octant projection and relationship between chirality and sign of the n→π* transition Cotton effect of compound 2.
Figure 6.
The experimental CD spectrum of 2 (black) and the calculated ECD spectra of 2 (dashed red) and its aglycone (dash-dotted blue) and the model compound (dotted green) in MeOH.
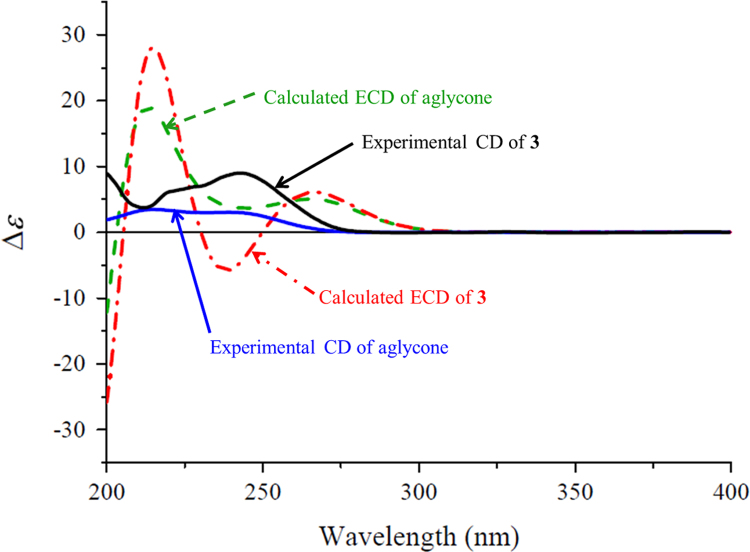
Compound 3 was obtained as a white amorphous powder with [α]20D +94.2 (c 0.15, MeOH). Its molecular formula C21H30O8 was determined by HR-ESI-MS at m/z 433.1827 [M+Na]+ (Calcd. for C21H30O8Na, 433.1833) combined with the NMR data (Table 1). The NMR spectra of 3 resembled those of atractylenolide III that was previously reported from the same plant C. pilosula25, 26 and also obtained in this study, except for the presence of signals due to an additional β-glucopyranosyl unit. This suggests that 3 is the β-glucopyranoside of atractylenolide III, which was proved by experiments of 2D NMR and enzymatic hydrolysis. Especially, in the HMBC spectrum of 3, the correlation from H-1′ to C-8 (Fig. 2) confirmed that the β-glucopyranosyloxy was located at C-8. In addition, from the hydrolysate of 3 with snailase, d-glucose and atractylenolide III were isolated and identified by comparison of their NMR spectroscopic and specific rotation data with those of the authentic samples (see in Experimental section and Supporting information). The absolute configuration of atractylenolide III was supported by comparison of the experimental CD and calculated ECD spectra (Fig. 7). Therefore, the compound 3 was determined and named codonopsesquiloside C. This compound can also be named atractylenolide III β-d-glucopyranoside based on the known aglycone.
Figure 7.
The experimental CD (black and red) and calculated ECD spectra (dashed red and green) of 3 and its aglycone in MeOH.
Comparison of the experimental CD spectra with the ECD spectra predicted from TDDFT calculations has become a recent approach increasingly applied for the determination of absolute configurations of natural products20, and some flexible units including the glycosyls were occasionally replaced27. Since we previously noted that the calculated ECD spectra of several bisindole glucosides were significantly disturbed by the chiral glucopyranosyloxy group on the indole chromophores14, to further investigate the influence of the glycosyl moieties on the ECD calculations of natural products with different chromophores and support the absolute configuration assignments, the ECD spectra of 1−3 and their aglycones, as well as the model compounds with substitution of the diglycosyl unit in 1 and 2 by a methyl group, were calculated. As shown in Fig 3, the ECD spectra of 1, its aglycone, and the model compound displayed Cotton effects with different intensities at similar wavelengths, indicating that the Cotton effect intensities, especially those at the shorter wavelengths, were disturbed by the substituents at C-11. As compared to those in the experimental CD spectra of 1 and the reported analogues19, 28, the similar split Cotton effects between 210 and 270 nm, arising from coupling between the π→π⁎ transitions of the cyclohexanone and conjugated dienol chromophores, were observed in the calculated ECD spectra, whereas the Cotton effect around 300 nm for the n→π⁎ transition of cyclohexanone chromophore has not only different intensities but also opposite signs. Because the exciton chirality method was proved to be applicable for determination of the absolute configurations of the related analogues28, the configuration of 1 was supported by similarity of the split Cotton effects between the experimental CD spectrum of 1 and the calculated ECD spectra. In addition, because the Cotton effects around 300 nm had the opposite signs in the experimental CD and calculated ECD spectra, the empirical octant rule for the measured n→π⁎ transition Cotton effect of cyclohexenones22, 23 is invalid for 1 and the reported analogues19, 28.
The ECD spectra of 2, its aglycone, and the model compound (Fig. 6) exhibited almost overlapping Cotton effects for the n→π⁎ transition of cyclohexanone chromophore, while the intensities and wavelengths for the π→π⁎ transition Cotton effects were affected by the substituents at C-15. In the experimental spectrum of 2 and the calculated ECD spectra, the sign and intensity of the n→π⁎ transition Cotton effects were consistent with each other though the wavelengths of the calculated Cotton effects were red-shifted around 20 nm. This argues that the empirical octant rule for the experimental n→π⁎ transition of cyclohexenones22, 23 is valid for 2 and its analogues (Fig. 5). In addition, the calculated ECD spectra of 3 and its aglycone showed completely different curves (Fig. 7), revealing the significant influence on the Cotton effects by the glucopyranosyl group. In this case, application of the empirical CD rules for the π→π⁎ and n→π⁎ transitions of α,β-unsaturated γ-lactones29 failed to predict the configurations of 3 and the aglycone. Because the calculated ECD and measured CD curves were completely different, direct comparison of the calculated and experimental spectra of 3 also failed to assign the configuration. However, similarity of curve shapes between the calculated and experimental spectra of the aglycone provided a support for the configuration assignment.
Together with our previous work14, the ECD calculations carried out in this study indicate that the glycosyl moieties have a variety of influences on the intensities, wavelengths, and signs of the Cotton effects from both the π→π⁎ and n→π⁎ transitions of the different chromophores. In the ECD calculations, replacement of the glycosyl units may result in an ambiguous assignment of the absolute configurations14. The direct comparison of the calculated and experimental spectra of glycosides may also lead to an inconclusive result27, such as 3. To unambiguously assign the absolute configurations of the glycosides, ECD spectral calculations of both the glycoside and aglycone should be carried out and compared with the corresponding experimental CD spectra. Moreover, ECD calculations may validate whether the empirical CD rules22, 23, 29 are valuable to predict the configurations. The empirical CD rules for the π→π⁎ and/or n→π⁎ transitions of the specific chromophores may be applicable only when the measured and calculated Cotton effects are consistent with each other.
A literature survey demonstrates that compound 1 is the first diglycoside of C15 carotenoid sesquiterpenoids mainly consisting of abscisic acid (ABA) derivatives19. Among the C15 carotenoids, ABA is a widely investigated phytohormone with multiple functions regulating numerous physiological processes in plant growth and development30 and ingested by humans on a daily basis to mediate many beneficial health effects31. ABA is also endogenously produced and released from monocytes, granulocytes, microglia, and insulin-secreting cells with local actions and enhances the ability of pancreatic β-cells to secrete insulin32, 33, 34. Pharmacological studies demonstrated that ABA is involved in various immune and inflammatory responses and has potential in animal models to treat diabetes, atherosclerosis, and inflammatory bowel disease31. Recently, it was found that ABA is a blocker of the bitter taste G protein–coupled receptor T2R435. Therefore, characterization of compound 1 adds a new form to the ABA derivatives with diverse biological functions for future in-depth investigation.
All the reported gymnomitrane-type sesquiterpenes were isolated from lower polar essential oils or extracts of liverworts with chemotaxonomic importance24, 36, 37, 38, 39, 40, 41, except for three derivatives from fungal cultures42, 43 and fruiting bodies44 and only one from a higher plant45. Compound 2 represents the first glycosidic gymnomitrane sesquiterpene. However, there are several unresolved issues with this type of sesquiterpenes in the literature. The first problem is the configuration of bridge-head atoms. To date, all the references indicate that the skeletal configuration of gymnomitrane sesquiterpenes from the liverworts is conservative. (+)-Isogymnomitrene and (−)-gymnomitrene, (+)-α- and (−)-β-barbatenes, and (+)-α- and (−)-β-pompenes are three pairs of synonymous compounds, of which the structures were independently determined. The skeletal configuration of gymnomitrane sesquiterpenes was assigned on the basis of the CD data of gymnomitrol-derived analogues24, 36, 37. X-ray crystallographic analysis of (+)-α-pompene- and (−)-gymnomitrene-derived p-bromobenzoates39, 41 not only verified the absolute configuration assignment, but also confirmed that (+)-α- and (−)-β-pompenes are identical to (+)-isogymnomitrene and (−)-gymnomitrene, respectively, as well as to (+)-α- and (−)-β-barbatenes. Unfortunately, the orientation of the bridge-unit (methylene, oxymethine, or ketone) in the structural drawings was ignored in most of the references40, 41, 43, 45, 46, 48, 49, 51, 52, 54, 55, which causes incorrect illustration of the configurations at the bridge-head atoms. Even in the literature reporting X-ray diffraction analysis41, the orientation of the bridge-methylene and bridge-head H and Me are not properly illustrated in the structural drawing of the reported analogues, wherein the orientation of bridge-methylene is inconsistent with that in a perspective view of the crystal molecular structure. Thus, the absolute configuration of bridge-head atoms, as determined by X-ray crystallographic analysis, should clearly and correctly be illustrated in the structure drawing of gymnomitrane sesquiterpenes.
Secondly, many numbering patterns of the skeletal atoms exist in the literature, e.g. (1) starting at the quaternary bridge-head atom then the neighboring quaternary angular atom and the others: without numbering of the substituent groups (methyl/hydroxymethylene/exomethylene)24 and with the substituent groups numbered 13, 14, 15, and 1246, 47, 48, (2) starting at the bridge-head methine then the neighboring quaternary angular atom and the others: with the substituent groups numbered 14, 13, 12, and 1542, 44; and (3) starting at the bridge-methylene followed by the bridge-head methine then the sp2 hybrid carbon and the others: with the substituting groups correspondingly numbered 13, 14, 15, and 1241 and 14, 13, 12, and 1543, 49, 50, 51, 52, 53, 54, 55. These descriptions have resulted in severe nomenclature confusions of the reported compounds based on the parent structure of gymnomitrane. Therefore, according to the systematic nomenclature of gymnomitrol24, the numbering and structure illustrated by 2 (Fig 1) is recommended, wherein the substituent group at the lower numbered skeletal atom is given the lower number and the bridge-unit has the same orientation as that of the angular methyl groups.
Additionally, among the three gymnomitrane sesquiterpenes from the fungi42, 43, 44, X-ray crystallographic analysis of antrodin F43 demonstrated that this compound has the same skeletal configuration as that from the liverworts. For the only gymnomitrane analogue reported from a higher plant, (−)-α-barbatenal45, because its LiAlH4 reduction product [α]20D −87 (c 0.08) showed an opposite Cotton effect at λ201 nm to that of the (+)-α-barbatene37, the configuration of (−)-α-barbatenal was determined to be reversed to the latter. Identity of the reported NMR data of (−)-α-barbatenal-reduction product45 and gymnomitr-3-en-12-ol {3-gymnomitren-15-ol from the liverwort, [α]20D+27 (c 0.17)}50 confirmed that these two compounds had the same planar structure. The [α]20D values for the two compounds were opposite, there was a large gap between the amplitudes. This prompted us to calculate the ECD spectrum of (−)-α-barbatenal-reduction product (see in Supporting information). For the reported configuration45, the calculated ECD spectrum exhibited the Cotton effect at 201 (Δε −1.60) nm, which is consistent with the reported data and supported the configuration assignment of (−)-α-barbatenal. This, together with the structure determination of 2, demonstrates that the skeletal configuration of gymnomitranes from higher plants is indeed reversed to that from the liverworts and fungi.
3. Conclusions
Three new metabolite codonopsesquilosides A−C (1−3), belonging to C15 carotenoid-, gymnomitrane-, and eudesmane-types of sesquiterpenoids, respectively, were isolated from the aqueous extract of C. pilosula roots. Compounds 1 and 2 represent unique diglycosides of the C15 carotenoids and gymnomitrane-sesquiterpenes. The ECD calculations of 1−3, their aglycones, and the model compounds showed that the glycosyl moieties have a variety of influences on the intensities, wavelengths, and signs of the Cotton effects from both the n→π⁎ and n→π⁎ transitions of different chromophores. To unambiguously assign the absolute configurations, the ECD spectra of the glycoside and aglycone should be calculated and compared with the corresponding experimental CD spectra. All the reported gymnomitrane derivatives from liverworts and fungi have the same skeletal configuration, whereas those from the higher plant have a reversed configuration. To avoid confusion in the nomenclature and illustrating configurations, the numbering of skeletal atoms and the orientation of bridge-unit as shown for 2 (Fig 1) are recommended for gymnomitrane-sesquiterpenoids. Although the biological activity of 1−3 was not assayed in this study due to limited amounts of these samples, the results provide guidance for future studies of the synthesis, chemical transformation, structural modification, biosynthesis, and biological evaluation of these diverse sesquiterpenoids, as well as their potential contribution to the traditional uses of the C. pilosula.
4. Experimental
4.1. General experimental procedures
Optical rotations were measured on P-2000 polarimeter (JASCO, Tokyo, Japan). UV spectra were measured on a V-650 spectrometer (JASCO, Tokyo, Japan). IR spectra were recorded on a Nicolet 5700 FT–IR microscope instrument (FT–IR microscope transmission) (Thermo Electron Corporation, Madison, USA). NMR spectra were obtained at 500 MHz or 600 MHz for 1H NMR, and 125 MHz or 150 MHz for 13C NMR, respectively, on Inova 500 or SYS 600 (Varian Associates Inc., Palo Alto, USA) or Bruker 600 NMR spectrometers (Bruker Corp., Switzerland) in MeOH-d4 with solvent peak used as references. ESI-MS and HR-ESI-MS data were measured using an AccuToFCS JMS-T100CS spectrometer (Agilent Technologies, Ltd., Santa Clara, USA). Column chromatography (CC) was performed with HPD-110 (Cangzhou Bon Absorber Technology Co. Ltd., Cangzhou, China), MCI gel CHP 20P (Mitsubishi Chemical Inc., Tokyo, Japan), silica gel (200–300 mesh, Qingdao Marine Chemical Inc., Qingdao, China), Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden), or Toyopearl HW-40F (Tosoh Corporation, Tokyo, Japan). HPLC separation was performed on an instrument consisting of an Agilent ChemStation for LC system, an Agilent1200 pump, and an Agilent 1100 singel-wavelength absorbance detector (Agilent Technologies, Ltd.) with a YMC-Pack Ph column (250 mm×10 mm, i.d.) packed with phenyl-silica gels (5 μm) (YMC Co. Ltd., Kyoto, Japan) or a Grace column (250 mm×10 mm, i.d.) packed with C18 reversed phase silica gel (5 μm) (W.R. Grace & Co., Maryland, USA). TLC was carried out with precoated silica gel GF254 glass plates (Qingdao Marine Chemical Inc., China). Spots were visualized under UV light or by spraying with 7% H2SO4 in 95% EtOH followed by heating. Unless otherwise noted, all chemicals were obtained from commercially available sources and were used without further purification.
4.2. Plant material
The roots of C. pilosula were collected in October 2012 from a culture field in Weiyuan, Gansu Province, China. Plant identity was verified by Mr. Lin Ma (Institute of Materia Medica, Beijing, China). A voucher specimen (No. ID-S-2503) was deposited at the herbarium of the Department of Medicinal Plants, Institute of Materia Medica, Beijing, China.
4.3. Extraction and isolation
The dried and powered roots of C. pilosula (50 kg) were decocted with H2O (150 L, 3×0.5 h). The decoction was evaporated under reduced pressure to yield a brown residue (26 kg). The residue was dissolved in H2O (100 L), loaded on a macroporous adsorbent resin (HPD-110, 20 L) column (200 cm×20 cm), and eluted successively with H2O (100 L), 50% EtOH (120 L), and 95% EtOH (80 L) to yield three corresponding fractions A−C. Fraction B (270 g) was chromatographed over MCI gel CHP 20 P (5.5 L), successively eluting with H2O (20 L), 30% EtOH (30 L), 50% EtOH (30 L), and 95% EtOH (8 L), to give B1−B4. Fraction B3 (22 g) was subjected to flash chromatography over reverse phase (RP) silica gel, eluting with a gradient of increasing MeOH (40−100%) in H2O, to yield subfractions B3-1−B3-5. B3-1 (10 g) was fractionated by CC over silica gel, eluting with a gradient of increasing MeOH (0−100%) in CHCl3, to yield B3-1-1−B3-1-10, of which B3-1-9 (1.1 g) was further separated by CC over HW-40 F using MeOH as the mobile phase to give B3-1-9-1−B3-1-9-5. Purification of B3-1-9-1-2 (80 mg) by RP-HPLC (C18 column, 1.5 mL/min, UV 220 nm) with CH3OH-H2O (58:42, v/v) as the mobile phase yielded 1 (1.6 mg, tR=34 min). Fractionation of B3-2 (5.3 g) by CC over silica gel, eluting with a gradient of increasing MeOH (0−100%) in CHCl3, yielded B3-2-1−B3-2-6, of which B3-2-1 (1.0 g) was further fractionated by CC over Sephadex LH-20 (CHCl3-MeOH, 1:1, v/v) to give B3-2-1-1−B3-2-1-8. Purification of B3-2-1-3 (100 mg) by RP-HPLC (Ph column, 1.5 mL/min, UV 220 nm) with CH3OH-H2O (60:40, v/v) as the mobile phase yielded 2 (3.0 mg, tR=37 min). B3-4 (2.5 g) was separated by CC over silica gel, eluting with a gradient of increasing MeOH (0−100%) in CHCl3, to afford B3-4-1−B3-4-10. Subsequent isolation of B3-4-4 (150 mg) by RP-HPLC (C18 column, 1.5 mL/min, UV 280 nm) with CH3OH-H2O (66:34, v/v) as the mobile phase obtained 3 (8.0 mg, tR=28 min).
4.3.1. Codonopsesquiloside A (1)
White amorphous powder; [α]20D +26.3 (c 0.04, MeOH); UV (MeOH) λmax (logε) 237 (5.69), 344 (4.74) nm; CD (MeOH) 223 (Δε −25.15), 250 (Δε +40.24), 320 (Δε −2.88) nm; IR νmax 3395, 3189, 3010, 2921, 2849, 1646, 1468, 1419, 1373, 1324, 1301, 1246, 1215, 1118, 1053, 1018, 817, 721, 646 cm−1; 1H NMR (DMSO-d6, 600 MHz) data, see Table 1; 13C NMR (DMSO-d6, 150 MHz) data, see Table 1; ESI-MS m/z 551 [M+Na]+, 567 [M+K]+, 563 [M+Cl]−; HR-ESI-MS m/z 551.2461 [M+Na]+ (Calcd. for C26H40O11Na, 551.2463).
4.3.2. Codonopsesquiloside B (2)
White amorphous powder; [α]20D −46.7 (c 0.04, MeOH); UV (MeOH) λmax(logε) 203 (4.70), 239 (4.53) nm; CD (MeOH) 236 (Δε +0.66), 330 (Δε −2.32) nm; IR νmax 3395, 3187, 3010, 2922, 2850, 1747, 1467, 1468, 1420, 1373, 1324, 1300, 1243, 1163, 1118, 1051, 914, 817, 722, 648, 548 cm−1; 1H NMR (CD3OD, 600 MHz) data, see Table 1; 13C NMR (CD3OD, 150 MHz) data, see Table 1; ESI-MS m/z 551 [M+Na]+, 567 [M+K]+, 527 [M − H]−, 563 [M+Cl]−; HR-ESI-MS m/z 529.2637 [M+H]+ (Calcd. for C26H41O11, 529.2643), 551.2462 [M+Na]+ (Calcd. for C26H40O11Na, 551.2463).
4.3.3. Codonopsesquiloside C (3)
White amorphous powder; [α]20D +94.2 (c 0.15, MeOH); UV (MeOH) λmax (logε) 203 (4.67), 222 (4.21) nm; CD (MeOH) 243 (Δε +9.01) nm; IR νmax 3395, 3082, 2930, 2847, 1756, 1688, 1649, 1441, 1411, 1388, 1337, 1322, 1288, 1264, 1251, 1234, 1183, 1160, 1078, 1050, 997, 950, 891, 869, 846, 768, 732, 690, 621, 572, 556 cm−1; 1H NMR (acetone-d6, 600 MHz) data, see Table 1; 13C NMR (acetone-d6, 150 MHz) data, see Table 1; ESI-MS m/z 433 [M+Na]+, 449 [M+K]+, 445 [M+Cl]−; HR-ESI-MS m/z 433.1827 [M+Na]+ (Calcd. for C21H30O8Na, 433.1833).
4.4. Enzymatic hydrolysis of 1−3 and identification of sugars in the hydrolysates
Compounds 1−3 (1.6−3.0 mg) were separately hydrolyzed in H2O (3 mL) with snailase at 37 °C for 24 h, after which the reaction mixture was extracted with EtOAc (3×3 mL). The EtOAc phase was concentrated under reduced pressure, and purified by RP-HPLC using the C18 column (MeOH–H2O, 75:25, v/v, 220 nm, 1.5 mL/min) for the hydrolysate of 2 and the Ph column (MeOH–H2O, 73:27, v/v, 220 nm, 1.5 mL/min) for the hydrolysate of 3 to obtain the corresponding aglycones with following physical-chemical properties. The aglycone of 2 (0.60 mg, tR=20 min): [α]20D +20.1 (c 0.04, MeOH); 1H NMR (600 MHz, acetone-d6) δ 5.98 (s, H-9), 4.30 (m, H-15), 2.17 (d, J=4.2 Hz, H-1), 2.07 (m, H-11a), 1.80 (d, J=11.4 Hz, H-11b), 1.70 (ddd, J=12.6, 6.0, 3.0 Hz, H-3a), 1.52 (m, H-4a), 1.45 (m, H-3b, 4b, 5a), 1.20 (s, MeO-12), 1.15 (m, H-5b), 1.01 (s, MeO-14), 1.00 (s, MeO-13); the aglycone of 3 (1.1 mg, tR=23 min): [α]20D +90.5 (c 0.08, CHCl3); 1H NMR (600 MHz, acetone-d6) δ 5.97 (s, OH-8), 4.85 (s, H-15a), 4.64 (s, H-15b), 2.67 (1H, dd, J=13.2, 3.0 Hz, H-6α), 2.42 (1H, t, J=13.2 Hz, H-6β), 2.35 (1H, brd, J=13.2 Hz, H-3β), 2.23 (1H, d, J=13.2 Hz, H-9α), 2.01 (1H, dt, J=12.6, 6.0 Hz, H-3α), 1.95 (1H, brd, J=12.6 Hz, H-5), 1.75 (3H, s, H3-13), 1.63 (2H, m, H-2), 1.54 (1H, brd, J=13.2 Hz, H-1α), 1.50 (1H, d, J=13.8 Hz, H-9β), 1.33 (1H, dt, J=13.8, 4.8 Hz, H-1β), 1.05 (3H, s, H3-14). The aqueous phase was dried using a stream of N2, followed by CC over silica gel eluting with CH3CN–H2O (8:1, v/v), to yield mixtures of glucose and apiose from the hydrolysates 1 and 2 and glucose from 3. The sugar mixtures from the hydrolysates 1 and 2 exhibited two spots on TLC (CH3CN-H2O, 6:1, v/v) with the retention factors (Rf) identical to those of authentic d-/l-glucose (Rf≈0.33) and d-/l-apiose (Rf≈0.27). The sugar mixtures from hydrolysis of 1 and 2, as well as the authentic d- and l-glucose and d- and l-apiose (0.4 mg, each), were individually reacted with l-cycteine methyl ester (1.0 mg) in pyridine (1.0 mL) at 60 °C for 60 min, then arylisothiocyanate (10 μL) was added and reacted at 60 °C for another 60 min18. The reaction mixtures were separately analyzed by HPLC [Grace C18 (5 μm, 250 mm×4.6 mm), CH3CN–H2O containing 0.1% phosphoric acid, 25:75, v/v, 0.8 mL/min] and monitored by a UV detector (250 nm) at room temperature. The tR for the derivatives of authentic d-glucose, l-glucose, d-apiose, and l-apiose were measured to be 15.30 min, 14.15 min, 24.79 min, and 13.24 min, respectively. For the derivatives of the sugar mixture from the hydrolysate of 1, HPLC analysis displayed two peaks with the tR values of 15.43 min and 24.55 min, which were consistent with those of the derivatives of d-glucose and d-apiose, respectively, and two similar peaks with the tR values of 15.37 min and 24.44 min were observed in the reaction mixture from the hydrolysate of 2 (see in Supporting information). The glucose (1.2 mg) from the hydrolysate of 3 gave retention factor (Rf≈0.33; CH3CN–H2O, 6:1, v/v) on TLC, [α]20D +42.3 (c 0.12, H2O)], and 1H NMR spectral data (D2O) consistent with those of an authentic d-glucose (see in Supporting information).
4.5. ECD calculation
For details, see Supporting information. Briefly, conformational analysis of 1−3, their aglycones, and the model compounds, were performed by using the MMFF94 molecular mechanics force field via the MOE software package56, respectively. The lowest-energy conformers having relative energies within 2 kcal/mol were optimized at the B3LYP/6-31+G(d) level in MeOH. The energies, oscillator strengths, and rotational strengths of the first 30 electronic excitations were calculated using the TDDFT methodology at the B3LYP/6-311++G (2d, 2p) level. ECD spectra of the conformers were simulated using the Gaussian function with a half-band width of 0.28 eV, and the final ECD spectrum of each compound was simulated according to Boltzmann weighting of each conformer. All quantum computations were performed using Gaussian 09 program package57, on an IBM cluster machine located at the High Performance Computing Center of Peking Union Medical College.
Acknowledgments
Financial support from the National Natural Sciences Foundation of China (NNSFC; Grant Nos. 30825044 and 20932007), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, Grant No. IRT1007), and the National Science and Technology Project of China (Nos. 2012ZX09301002-002 and 2011ZX09307-002-01) is acknowledged.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at 10.1016/j.apsb.2015.09.007.
Appendix A. Supplementary material
Supplementary material
References
- 1.Jiangsu New Medical College. Dictionary of traditional Chinese medicine. vol. 1. Shanghai: Shanghai Science and Technology Publishing House; 1986, p. 1837–9.
- 2.Jiang Y.P., Liu Y.F., Guo Q.L., Jiang Z.B., Xu C.B., Zhu C.G. Acetylenes and fatty acids from Codonopsis pilosula. Acta Pharm Sin B. 2015;5:215–222. doi: 10.1016/j.apsb.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen M.H., Lin S., Li L., Zhu C.G., Wang X.L., Wang Y.N. Enantiomers of an indole alkaloid containing unusual dihydrothiopyran and 1,2,4-thiadiazole rings from the root of Isatis indigotica. Org Lett. 2012;14:5668–5671. doi: 10.1021/ol302660t. [DOI] [PubMed] [Google Scholar]
- 4.Zhao F., Wang S.J., Lin S., Zhu C.G., Yue Z.G., Yu Y. Natural and unnatural anthraquinones isolated from the ethanol extract of the roots of Knoxia valerianoides. Acta Pharm Sin B. 2012;2:260–266. [Google Scholar]
- 5.Yu Y., Zhu C.G., Wang S.J., Song W.X., Yang Y.C., Shi J.G. Homosecoiridoid alkaloids with amino acid units from the flower buds of Lonicera japonica. J Nat Prod. 2013;76:2226–2233. doi: 10.1021/np4005773. [DOI] [PubMed] [Google Scholar]
- 6.Wang F., Jiang Y.P., Wang X.L., Wang S.J., Bu P.B., Lin S. Aromatic glycosides from the flower buds of Lonicera japonica. J Asian Nat Prod Res. 2013;15:492–501. doi: 10.1080/10286020.2013.785531. [DOI] [PubMed] [Google Scholar]
- 7.Tian Y., Guo Q.L., Xu W.D., Zhu C.G., Yang Y.C., Shi J.G. A minor diterpenoid with a new 6/5/7/3 fused-ring skeleton from Euphorbia micractina. Org Lett. 2014;16:3950–3953. doi: 10.1021/ol501760h. [DOI] [PubMed] [Google Scholar]
- 8.Xu W.D., Tian Y., Guo Q.L., Yang Y.C., Shi J.G. Secoeuphoractin, a minor diterpenoid with a new skeleton from Euphorbia micractina. Chin Chem Lett. 2014;25:1531–1534. doi: 10.1021/ol501760h. [DOI] [PubMed] [Google Scholar]
- 9.Song W.X., Yang Y.C., Shi J.G. Two new β-hydroxy amino acid-coupled secoiridoids from the flower buds of Lonicera japonica: isolation, structure elucidation, semisynthesis, and biological activities. Chin Chem Lett. 2014;25:1215–1219. [Google Scholar]
- 10.Yu Y., Jiang Z.B., Song W.X., Yang Y.C., Li Y.H., Jiang J.D. Glucosylated caffeoylquinic acid derivatives from the flower buds of Lonicera japonica. Acta Pharm Sin B. 2015;5:210–214. doi: 10.1016/j.apsb.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Q.L., Wang Y.N., Zhu C.G., Chen M.H., Jiang Z.B., Chen N.H. 4-Hydroxybenzyl-substituted glutathione derivatives from Gastrodia elata. J Asian Nat Prod Res. 2015;17:439–454. doi: 10.1080/10286020.2015.1040000. [DOI] [PubMed] [Google Scholar]
- 12.Guo Q.L., Wang Y.N., Lin S., Zhu C.G., Chen M.H., Jiang Z.B. 4-Hydroxybenzyl-substituted amino acid derivatives from Gastrodia elata. Acta Pharm Sin B. 2015;5:350–357. doi: 10.1016/j.apsb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y.F., Chen M.H., Wang X.L., Zhu C.G., Lin S., Xu C.B. Antiviral enantiomers of a bisindole alkaloid with a new carbon skeleton from the roots of Isatis indigotica. Chin Chem Lett. 2015;26:931–936. [Google Scholar]
- 14.Liu Y.F., Chen M.H., Guo Q.L., Lin S., Xu C.B., Jiang Y.P. Antiviral glycosidic bisindole alkaloids from the roots of Isatis indigotica. J Asian Nat Prod Res. 2015;17:689–704. doi: 10.1080/10286020.2015.1055729. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y.P., Liu Y.F., Guo Q.L., Jiang Z.B., Xu C.B., Zhu C.G. C14-polyacetylene glucosides from Codonopsis pilosula. J Asian Nat Prod Res. 2015;17:601–614. doi: 10.1080/10286020.2015.1041932. [DOI] [PubMed] [Google Scholar]
- 16.Takayanagi T., Ishikawa T., Kitajima J. Sesquiterpene lactone glucosides and alkyl glycosides from the fruit of cumin. Phytochemistry. 2003;63:479–484. doi: 10.1016/s0031-9422(03)00103-1. [DOI] [PubMed] [Google Scholar]
- 17.Tamaki A., Ide T., Otsuka H. Phenolic glycosides from the leaves of Alangium platanifolium var. platanifolium. J Nat Prod. 2000;63:1417–1419. doi: 10.1021/np000119l. [DOI] [PubMed] [Google Scholar]
- 18.Takana T., Nakashima T., Ueda T., Tomi K., Kouno I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem Pharm Bull. 2007;55:899–901. doi: 10.1248/cpb.55.899. [DOI] [PubMed] [Google Scholar]
- 19.Lutz A., Winterhalter P. Isolation of additional carotenoid metabolites from quince fruit (Cydonia oblonga Mill.) J Agric Food Chem. 1992;40:1116–1120. [Google Scholar]
- 20.Li X.C., Ferreira D., Ding Y.Q. Determination of absolute configuration of natural products: theoretical calculation of electronic circular dichroism as a tool. Curr Org Chem. 2010;14:1678–1697. doi: 10.2174/138527210792927717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutz A., Winterhalter P. Abscisic alcohol glucoside in quince. Phytochemistry. 1993;32:57–60. [Google Scholar]
- 22.Snatzke G. Circulardichroismus-VIII, modifizierung der octantenregel für α, β-ungesättigt ketone: theorie. Tetrahedron. 1965;21:413–419. [Google Scholar]
- 23.Snatzke G. Circulardichroismus-IX, modifizierung der octantenregel für α, β-ungesättigt ketone: transoide enone. Tetrahedron. 1965;21:421–438. [Google Scholar]
- 24.Connolly J.D., Harding A.E., Thornton I.M.S. Gymnomitrol and related sesquiterpenoids from the liverwort Gymnomitrion obtusum (Lindb) pears (Hepaticae). A novel tricyclic skeleton. J Chem Soc Perkin Trans I 1974. 1974:2487–2493. [Google Scholar]
- 25.Wang Z.T., Xu G.J., Hattori M., Namba T. Constituents of the roots of Codonopsis pilosula. Jap J Pharm. 1988;42:339–342. [Google Scholar]
- 26.Qi H.Y., Wang R., Liu Y., Shi Y.P. Studies on the chemical constituents of codonopsis pilosula. J Chin Med Mater. 2011;34:546–548. [PubMed] [Google Scholar]
- 27.Tatsis E.C., Schaumlöffel A., Warskulat A.C., Massiot G., Schneider B., Bringmann G. Nudicaulins, yellow flower pigments of Papaver nudicaule: revised constitution and assignment of absolute configuration. Org Lett. 2013;15:156–159. doi: 10.1021/ol303211w. [DOI] [PubMed] [Google Scholar]
- 28.Koreeda M., Weiss G., Nakanishi K. Absolute configuration of natural (+)-abscisic acid. J Am Chem Soc. 1973;95:239–240. [Google Scholar]
- 29.Uchida I., Kuriyama K. The π→π⁎ circular dichroism of α,β-unsaturated γ-lactones. Tetrahedron Lett. 1974;15:3761–3764. [Google Scholar]
- 30.Wasilewska A., Vlad F., Sirichandra C., Redko Y., Jammes F., Valon C. An update on abscisic acid signaling in plants and more. Mol Plant. 2008;1:198–217. doi: 10.1093/mp/ssm022. [DOI] [PubMed] [Google Scholar]
- 31.Bassaganya-Riera J., Skoneczka J., Kingston D.G.J., Krishnan A., Misyak S.A. Mechanisms of action and medicinal applications of abscisic acid. Curr Med Chem. 2010;17:467–478. doi: 10.2174/092986710790226110. [DOI] [PubMed] [Google Scholar]
- 32.Bruzzone S., Moreschi I., Usai C., Guida L., Damonte G., Salis A. Abscisic acid is an endogenous cytokine in human granulocytes with cyclic ADP-ribose as second messenger. Proc Natl Acad Sci U S A. 2007;104:5759–5764. doi: 10.1073/pnas.0609379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruzzone S., Bodrato N., Usai C., Guida L., Moreschi I., Nano R. Abscisic acid is an endogenous stimulator of insulin release from human pancreatic islets with cyclic ADP ribose as second messenger. J Biol Chem. 2008;283:32188–32197. doi: 10.1074/jbc.M802603200. [DOI] [PubMed] [Google Scholar]
- 34.Magnone M., Bruzzone S., Guida L., Damonte G., Millo E., Scarfi S. Abscisic acid released by human monocytes activates monocytes and vascular smooth muscle cell responses involved in atherogenesis. J Biol Chem. 2009;284:17808–17818. doi: 10.1074/jbc.M809546200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pydi S.P., Jaggupilli A., Nelson K.M., Abrams S.R., Bhullar R.P., Loewen M.C. Abscisic acid acts as a blocker of the bitter taste G protein-coupled receptor T2R4. Biochemistry. 2015;54:2622–2631. doi: 10.1021/acs.biochem.5b00265. [DOI] [PubMed] [Google Scholar]
- 36.Connolly J.D., Harding A.E. Thornton IMS. Gymnomitrol, a novel tricyclic sesquiterpenoid from gymnomitrion obtusum (Lindb) pears (hepaticae) J Chem Soc Chem Commun 1972. 1972:1320–1321. [Google Scholar]
- 37.Andersen N.H., Costin C.R., Kramer C.M., Ohta Y., Huneck S. Sesquiterpenes of Barbilophozia species. Phytochemistry. 1973;12:2709–2716. [Google Scholar]
- 38.Matsuo A., Maeda T., Nakayama M., Hayashi S. α–Pompene a novel tricyclic sesquiterpene hydrocarbon from the liverwort. Bazzania pompeana. Tetrahedron Lett. 1973;14:4131–4134. [Google Scholar]
- 39.Matsuo A., Nosaki H., Nakayama M., Kushi Y., Hayashi S., Kamijo N. The revised structure for α-pompene and the absolute configurations of (+)-α- and (-)-β-pompene from Bazzania pompeana. Tetrahedron Lett. 1975;16:241–244. [Google Scholar]
- 40.Toyota M., Nagashima F., Asakawa Y. Gymnomitrane-type sesquiterpenoids from the liverwort Plagiochila trabeculata. Phytochemistry. 1988;27:2161–2164. [Google Scholar]
- 41.Matsuo A., Nozaki H., Yano K., Uto S., Nakayama M., Huneck S. Gymnomitrane sesquiterpenoids from the liverwort Marsupella emarginata var. Patens. Phytochemistry. 1990;29:1921–1924. [Google Scholar]
- 42.Rösslein L., Tamm C., Zürcher W., Riesen A., Zehnder M. Sambucinic acid, a new metabolite of Fusarium sambacinum. 45th communication on verrucarins andrordins. Helv Chim Acta. 1988;71:588–595. [Google Scholar]
- 43.Chen Z.M., Chen H.P., Wang F., Li Z.H., Feng T., Liu J.K. New triquinane and gymnomitrane sesquiterpenes from fermentation of the basidiomycete Antrodiella albocinnamomea. Fitoterapia. 2015;102:61–66. doi: 10.1016/j.fitote.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Liu J.Q., Wang C.F., Li Y., Luo H.R., Qiu M.H. Isolation and bioactivity evaluation of terpenoids from the medicinal fungus Ganoderma sinense. Planta Med. 2012;78:368–376. doi: 10.1055/s-0031-1280441. [DOI] [PubMed] [Google Scholar]
- 45.Achenbach H., Benirschke G. Joannesialactone and other compounds from Joannesia princeps. Phytochemistry. 1997;45:149–157. [Google Scholar]
- 46.Nagashima F., Ishimaru A., Asakawa Y. Sesquiterpenoids from the liverwort Marsupella aquatica. Phytochemistry. 1994;37:777–779. [Google Scholar]
- 47.Toyota M., Konoshima M., Asakawa Y. Terpenoid constituents of the liverwort Reboulia hemisphaerica. Phytochemistry. 1999;52:105–112. [Google Scholar]
- 48.Scher J.M., Speakman J.B., Zapp J., Becker H. Bioactivity guided isolation of antifungal compounds from the liverwort Bazzania trilobata (L.) S.F. Gray. Phytochemistry. 2004;65:2583–2588. doi: 10.1016/j.phytochem.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 49.Nagashima F., Momosaki S., Watanabe Y., Takaoka S., Huneck S., Asakawa Y. Sesquiterpenoids from the liverworts Bazzania trilobata and Porella canariensis. Phytochemistry. 1996;42:1361–1366. [Google Scholar]
- 50.Buchanan M.S., Connolly J.D., Kadir A.A., Rycroft D.S. Sesquiterpenoids and diterpenoids from the liverwort Jungermannia truncata. Phytochemistry. 1996;42:1641–1646. [Google Scholar]
- 51.Warmers U., König W.A. Gymnomitrane-type sesquiterpenes of the liverworts Gymnomitrion obtusum and Reboulia hemisphaerica. Phytochemistry. 1999;52:1501–1505. [Google Scholar]
- 52.Warmers U., König W.A. Biosynthesis of the gymnomitrane-type sesquiterpenes in liverworts. Phytochemistry. 2000;53:645–650. doi: 10.1016/s0031-9422(99)00587-7. [DOI] [PubMed] [Google Scholar]
- 53.Wu C.L., Kao T.L. A new gymnomitrane-type sesquiterpenoid from the liverwort Cylindrocolea recurvifolia. J Asian Nat Prod Res. 2002;4:281–285. doi: 10.1080/1028602021000049078. [DOI] [PubMed] [Google Scholar]
- 54.Nagashima F., Kondoh M., Uematsu T., Nishiyama A., Saito S., Sato M. Cytotoxic and apoptosis-inducing ent-kaurane-type diterpenoids from the Japanese liverwort Jungermannia truncata Nees. Chem Pharm Bull. 2002;50:808–813. doi: 10.1248/cpb.50.808. [DOI] [PubMed] [Google Scholar]
- 55.Adio A.M., König W.A. Sesquiterpenoids and norsesquiterpenoids from three liverworts. Tetrahedron: Asymmetry. 2007;18:1693–1700. [Google Scholar]
- 56.Molecular Operating Environment 2008.10. Available from: 〈http://www.chemcomp.com〉.
- 57.Gaussian 09.Available from: 〈http://www.gaussian.com〉.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material