Abstract
It is important to realize that guidelines cannot always account for individual variation among patients. They are not intended to supplant physician judgment with respect to particular patients or special clinical situations. IDSA considers adherence to these guidelines to be voluntary, with the ultimate determination regarding their application to be made by the physician in the light of each patient's individual circumstances.
Keywords: candidemia, invasive candidiasis, fungal diagnostics, azoles, echinocandins
EXECUTIVE SUMMARY
Background
Invasive infection due to Candida species is largely a condition associated with medical progress, and is widely recognized as a major cause of morbidity and mortality in the healthcare environment. There are at least 15 distinct Candida species that cause human disease, but >90% of invasive disease is caused by the 5 most common pathogens, C. albicans, C. glabrata, C. tropicalis, C. parapsilosis, and C. krusei. Each of these organisms has unique virulence potential, antifungal susceptibility, and epidemiology, but taken as a whole, significant infections due to these organisms are generally referred to as invasive candidiasis. Mucosal Candida infections—especially those involving the oropharynx, esophagus, and vagina—are not considered to be classically invasive disease, but they are included in these guidelines. Since the last iteration of these guidelines in 2009 [1], there have been new data pertaining to diagnosis, prevention, and treatment for proven or suspected invasive candidiasis, leading to significant modifications in our treatment recommendations.
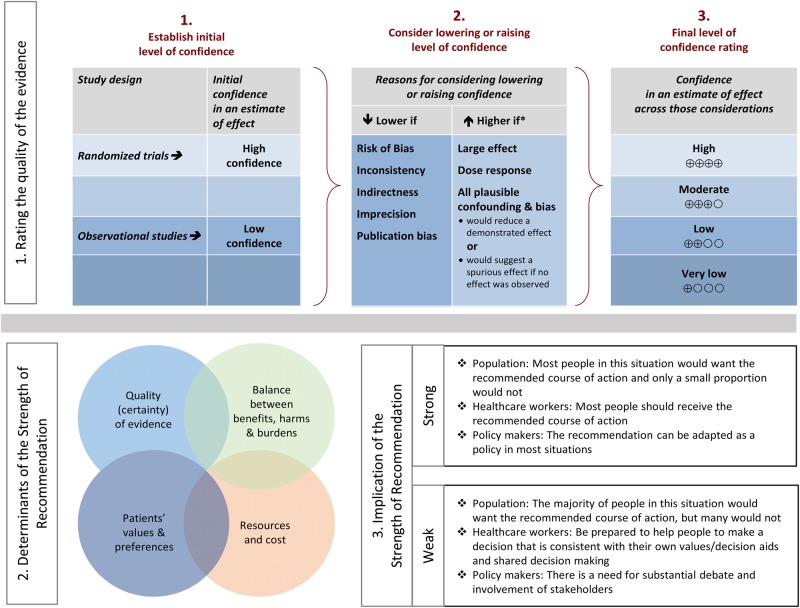
Summarized below are the 2016 revised recommendations for the management of candidiasis. Due to the guideline's relevance to pediatrics, the guideline has been reviewed and endorsed by the American Academy of Pediatrics (AAP) and the Pediatric Infectious Diseases Society (PIDS). The Mycoses Study Group (MSG) has also endorsed these guidelines. The panel followed a guideline development process that has been adopted by the Infectious Diseases Society of America (IDSA), which includes a systematic method of grading both the quality of evidence (very low, low, moderate, and high) and the strength of the recommendation (weak or strong) [2] (Figure 1). [3] The guidelines are not intended to replace clinical judgment in the management of individual patients. A detailed description of the methods, background, and evidence summaries that support each recommendation can be found in the full text of the guideline.
Figure 1.
Approach and implications to rating the quality of evidence and strength of recommendations using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (unrestricted use of the figure granted by the US GRADE Network) [3].
I. What Is the Treatment for Candidemia in Nonneutropenic Patients?
Recommendations
1. An echinocandin (caspofungin: loading dose 70 mg, then 50 mg daily; micafungin: 100 mg daily; anidulafungin: loading dose 200 mg, then 100 mg daily) is recommended as initial therapy (strong recommendation; high-quality evidence).
2. Fluconazole, intravenous or oral, 800-mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily is an acceptable alternative to an echinocandin as initial therapy in selected patients, including those who are not critically ill and who are considered unlikely to have a fluconazole-resistant Candida species (strong recommendation; high-quality evidence).
3. Testing for azole susceptibility is recommended for all bloodstream and other clinically relevant Candida isolates. Testing for echinocandin susceptibility should be considered in patients who have had prior treatment with an echinocandin and among those who have infection with C. glabrata or C. parapsilosis (strong recommendation; low-quality evidence).
4. Transition from an echinocandin to fluconazole (usually within 5–7 days) is recommended for patients who are clinically stable, have isolates that are susceptible to fluconazole (eg, C. albicans), and have negative repeat blood cultures following initiation of antifungal therapy (strong recommendation; moderate-quality evidence).
5. For infection due to C. glabrata, transition to higher-dose fluconazole 800 mg (12 mg/kg) daily or voriconazole 200–300 (3–4 mg/kg) twice daily should only be considered among patients with fluconazole-susceptible or voriconazole-susceptible isolates (strong recommendation; low-quality evidence).
6. Lipid formulation amphotericin B (AmB) (3–5 mg/kg daily) is a reasonable alternative if there is intolerance, limited availability, or resistance to other antifungal agents (strong recommendation; high-quality evidence).
7. Transition from AmB to fluconazole is recommended after 5–7 days among patients who have isolates that are susceptible to fluconazole, who are clinically stable, and in whom repeat cultures on antifungal therapy are negative (strong recommendation; high-quality evidence).
8. Among patients with suspected azole- and echinocandin-resistant Candida infections, lipid formulation AmB (3–5 mg/kg daily) is recommended (strong recommendation; low-quality evidence).
9. Voriconazole 400 mg (6 mg/kg) twice daily for 2 doses, then 200 mg (3 mg/kg) twice daily is effective for candidemia, but offers little advantage over fluconazole as initial therapy (strong recommendation; moderate-quality evidence). Voriconazole is recommended as step-down oral therapy for selected cases of candidemia due to C. krusei (strong recommendation; low-quality evidence).
10. All nonneutropenic patients with candidemia should have a dilated ophthalmological examination, preferably performed by an ophthalmologist, within the first week after diagnosis (strong recommendation; low-quality evidence).
11. Follow-up blood cultures should be performed every day or every other day to establish the time point at which candidemia has been cleared (strong recommendation; low-quality evidence).
12. Recommended duration of therapy for candidemia without obvious metastatic complications is for 2 weeks after documented clearance of Candida species from the bloodstream and resolution of symptoms attributable to candidemia (strong recommendation; moderate-quality evidence).
II. Should Central Venous Catheters Be Removed in Nonneutropenic Patients With Candidemia?
Recommendation
13. Central venous catheters (CVCs) should be removed as early as possible in the course of candidemia when the source is presumed to be the CVC and the catheter can be removed safely; this decision should be individualized for each patient (strong recommendation; moderate-quality evidence).
III. What Is the Treatment for Candidemia in Neutropenic Patients?
Recommendations
14. An echinocandin (caspofungin: loading dose 70 mg, then 50 mg daily; micafungin: 100 mg daily; anidulafungin: loading dose 200 mg, then 100 mg daily) is recommended as initial therapy (strong recommendation; moderate-quality evidence).
15. Lipid formulation AmB, 3–5 mg/kg daily, is an effective but less attractive alternative because of the potential for toxicity (strong recommendation; moderate-quality evidence).
16. Fluconazole, 800-mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily, is an alternative for patients who are not critically ill and have had no prior azole exposure (weak recommendation; low-quality evidence).
17. Fluconazole, 400 mg (6 mg/kg) daily, can be used for step-down therapy during persistent neutropenia in clinically stable patients who have susceptible isolates and documented bloodstream clearance (weak recommendation; low-quality evidence).
18. Voriconazole, 400 mg (6 mg/kg) twice daily for 2 doses, then 200–300 mg (3–4 mg/kg) twice daily, can be used in situations in which additional mold coverage is desired (weak recommendation; low-quality evidence). Voriconazole can also be used as step-down therapy during neutropenia in clinically stable patients who have had documented bloodstream clearance and isolates that are susceptible to voriconazole (weak recommendation; low-quality evidence).
19. For infections due to C. krusei, an echinocandin, lipid formulation AmB, or voriconazole is recommended (strong recommendation; low-quality evidence).
20. Recommended minimum duration of therapy for candidemia without metastatic complications is 2 weeks after documented clearance of Candida from the bloodstream, provided neutropenia and symptoms attributable to candidemia have resolved (strong recommendation; low-quality evidence).
21. Ophthalmological findings of choroidal and vitreal infection are minimal until recovery from neutropenia; therefore, dilated funduscopic examinations should be performed within the first week after recovery from neutropenia (strong recommendation; low-quality evidence).
22. In the neutropenic patient, sources of candidiasis other than a CVC (eg, gastrointestinal tract) predominate. Catheter removal should be considered on an individual basis (strong recommendation; low-quality evidence).
23. Granulocyte colony-stimulating factor (G-CSF)–mobilized granulocyte transfusions can be considered in cases of persistent candidemia with anticipated protracted neutropenia (weak recommendation; low-quality evidence).
IV. What Is the Treatment for Chronic Disseminated (Hepatosplenic) Candidiasis?
Recommendations
24. Initial therapy with lipid formulation AmB, 3–5 mg/kg daily OR an echinocandin (micafungin: 100 mg daily; caspofungin: 70-mg loading dose, then 50 mg daily; or anidulafungin: 200-mg loading dose, then 100 mg daily), for several weeks is recommended, followed by oral fluconazole, 400 mg (6 mg/kg) daily, for patients who are unlikely to have a fluconazole-resistant isolate (strong recommendation; low-quality evidence).
25. Therapy should continue until lesions resolve on repeat imaging, which is usually several months. Premature discontinuation of antifungal therapy can lead to relapse (strong recommendation; low-quality evidence).
26. If chemotherapy or hematopoietic cell transplantation is required, it should not be delayed because of the presence of chronic disseminated candidiasis, and antifungal therapy should be continued throughout the period of high risk to prevent relapse (strong recommendation; low-quality evidence).
27. For patients who have debilitating persistent fevers, short-term (1–2 weeks) treatment with nonsteroidal anti-inflammatory drugs or corticosteroids can be considered (weak recommendation; low-quality evidence).
V. What Is the Role of Empiric Treatment for Suspected Invasive Candidiasis in Nonneutropenic Patients in the Intensive Care Unit?
Recommendations
28. Empiric antifungal therapy should be considered in critically ill patients with risk factors for invasive candidiasis and no other known cause of fever and should be based on clinical assessment of risk factors, surrogate markers for invasive candidiasis, and/or culture data from nonsterile sites (strong recommendation; moderate-quality evidence). Empiric antifungal therapy should be started as soon as possible in patients who have the above risk factors and who have clinical signs of septic shock (strong recommendation; moderate-quality evidence).
29. Preferred empiric therapy for suspected candidiasis in nonneutropenic patients in the intensive care unit (ICU) is an echinocandin (caspofungin: loading dose of 70 mg, then 50 mg daily; micafungin: 100 mg daily; anidulafungin: loading dose of 200 mg, then 100 mg daily) (strong recommendation; moderate-quality evidence).
30. Fluconazole, 800-mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily, is an acceptable alternative for patients who have had no recent azole exposure and are not colonized with azole-resistant Candida species (strong recommendation; moderate-quality evidence).
31. Lipid formulation AmB, 3–5 mg/kg daily, is an alternative if there is intolerance to other antifungal agents (strong recommendation; low-quality evidence).
32. Recommended duration of empiric therapy for suspected invasive candidiasis in those patients who improve is 2 weeks, the same as for treatment of documented candidemia (weak recommendation; low-quality evidence).
33. For patients who have no clinical response to empiric antifungal therapy at 4–5 days and who do not have subsequent evidence of invasive candidiasis after the start of empiric therapy or have a negative non-culture-based diagnostic assay with a high negative predictive value, consideration should be given to stopping antifungal therapy (strong recommendation; low-quality evidence).
VI. Should Prophylaxis Be Used to Prevent Invasive Candidiasis in the Intensive Care Unit Setting?
Recommendations
34. Fluconazole, 800-mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily, could be used in high-risk patients in adult ICUs with a high rate (>5%) of invasive candidiasis (weak recommendation; moderate-quality evidence).
35. An alternative is to give an echinocandin (caspofungin: 70-mg loading dose, then 50 mg daily; anidulafungin: 200-mg loading dose and then 100 mg daily; or micafungin: 100 mg daily) (weak recommendation; low-quality evidence).
36. Daily bathing of ICU patients with chlorhexidine, which has been shown to decrease the incidence of bloodstream infections including candidemia, could be considered (weak recommendation; moderate-quality evidence).
VII. What Is the Treatment for Neonatal Candidiasis, Including Central Nervous System Infection?
What Is the Treatment for Invasive Candidiasis and Candidemia?
Recommendations
37. AmB deoxycholate, 1 mg/kg daily, is recommended for neonates with disseminated candidiasis (strong recommendation; moderate-quality evidence).
38. Fluconazole, 12 mg/kg intravenous or oral daily, is a reasonable alternative in patients who have not been on fluconazole prophylaxis (strong recommendation; moderate-quality evidence).
39. Lipid formulation AmB, 3–5 mg/kg daily, is an alternative, but should be used with caution, particularly in the presence of urinary tract involvement (weak recommendation; low-quality evidence).
40. Echinocandins should be used with caution and generally limited to salvage therapy or to situations in which resistance or toxicity preclude the use of AmB deoxycholate or fluconazole (weak recommendation; low-quality evidence).
41. A lumbar puncture and a dilated retinal examination are recommended in neonates with cultures positive for Candida species from blood and/or urine (strong recommendation; low-quality evidence).
42. Computed tomographic or ultrasound imaging of the genitourinary tract, liver, and spleen should be performed if blood cultures are persistently positive for Candida species (strong recommendation; low-quality evidence).
43. CVC removal is strongly recommended (strong recommendation; moderate-quality evidence).
44. The recommended duration of therapy for candidemia without obvious metastatic complications is for 2 weeks after documented clearance of Candida species from the bloodstream and resolution of signs attributable to candidemia (strong recommendation; low-quality evidence).
What Is the Treatment for Central Nervous System Infections in Neonates?
Recommendations
45. For initial treatment, AmB deoxycholate, 1 mg/kg intravenous daily, is recommended (strong recommendation; low-quality evidence).
46. An alternative regimen is liposomal AmB, 5 mg/kg daily (strong recommendation; low-quality evidence).
47. The addition of flucytosine, 25 mg/kg 4 times daily, may be considered as salvage therapy in patients who have not had a clinical response to initial AmB therapy, but adverse effects are frequent (weak recommendation; low-quality evidence).
48. For step-down treatment after the patient has responded to initial treatment, fluconazole, 12 mg/kg daily, is recommended for isolates that are susceptible to fluconazole (strong recommendation; low-quality evidence).
49. Therapy should continue until all signs, symptoms, and cerebrospinal fluid (CSF) and radiological abnormalities, if present, have resolved (strong recommendation; low-quality evidence).
50. Infected central nervous system (CNS) devices, including ventriculostomy drains and shunts, should be removed if at all possible (strong recommendation; low-quality evidence).
What Are the Recommendations for Prophylaxis in the Neonatal Intensive Care Unit Setting?
Recommendations
51. In nurseries with high rates (>10%) of invasive candidiasis, intravenous or oral fluconazole prophylaxis, 3–6 mg/kg twice weekly for 6 weeks, in neonates with birth weights <1000 g is recommended (strong recommendation; high-quality evidence).
52. Oral nystatin, 100 000 units 3 times daily for 6 weeks, is an alternative to fluconazole in neonates with birth weights <1500 g in situations in which availability or resistance preclude the use of fluconazole (weak recommendation; moderate-quality evidence).
53. Oral bovine lactoferrin (100 mg/day) may be effective in neonates <1500 g but is not currently available in US hospitals (weak recommendation; moderate-quality evidence).
VIII. What Is the Treatment for Intra-abdominal Candidiasis?
Recommendations
54. Empiric antifungal therapy should be considered for patients with clinical evidence of intra-abdominal infection and significant risk factors for candidiasis, including recent abdominal surgery, anastomotic leaks, or necrotizing pancreatitis (strong recommendation; moderate-quality evidence).
55. Treatment of intra-abdominal candidiasis should include source control, with appropriate drainage and/or debridement (strong recommendation; moderate-quality evidence).
56. The choice of antifungal therapy is the same as for the treatment of candidemia or empiric therapy for nonneutropenic patients in the ICU (See sections I and V) (strong recommendation; moderate-quality evidence).
57. The duration of therapy should be determined by adequacy of source control and clinical response (strong recommendation; low-quality evidence).
IX. Does the Isolation of Candida Species From the Respiratory Tract Require Antifungal Therapy?
Recommendation
58. Growth of Candida from respiratory secretions usually indicates colonization and rarely requires treatment with antifungal therapy (strong recommendation; moderate-quality evidence).
X. What Is the Treatment for Candida Intravascular Infections, Including Endocarditis and Infections of Implantable Cardiac Devices?
What Is the Treatment for Candida Endocarditis?
Recommendations
59. For native valve endocarditis, lipid formulation AmB, 3–5 mg/kg daily, with or without flucytosine, 25 mg/kg 4 times daily, OR high-dose echinocandin (caspofungin 150 mg daily, micafungin 150 mg daily, or anidulafungin 200 mg daily) is recommended for initial therapy (strong recommendation; low-quality evidence).
60. Step-down therapy to fluconazole, 400–800 mg (6–12 mg/kg) daily, is recommended for patients who have susceptible Candida isolates, have demonstrated clinical stability, and have cleared Candida from the bloodstream (strong recommendation; low-quality evidence).
61. Oral voriconazole, 200–300 mg (3–4 mg/kg) twice daily, or posaconazole tablets, 300 mg daily, can be used as step-down therapy for isolates that are susceptible to those agents but not susceptible to fluconazole (weak recommendation; very low-quality evidence).
62. Valve replacement is recommended; treatment should continue for at least 6 weeks after surgery and for a longer duration in patients with perivalvular abscesses and other complications (strong recommendation; low-quality evidence).
63. For patients who cannot undergo valve replacement, long-term suppression with fluconazole, 400–800 mg (6–12 mg/kg) daily, if the isolate is susceptible, is recommended (strong recommendation; low-quality evidence).
64. For prosthetic valve endocarditis, the same antifungal regimens suggested for native valve endocarditis are recommended (strong recommendation; low-quality evidence). Chronic suppressive antifungal therapy with fluconazole, 400–800 mg (6–12 mg/kg) daily, is recommended to prevent recurrence (strong recommendation; low-quality evidence).
What Is the Treatment for Candida Infection of Implantable Cardiac Devices?
Recommendations
65. For pacemaker and implantable cardiac defibrillator infections, the entire device should be removed (strong recommendation; moderate-quality evidence).
66. Antifungal therapy is the same as that recommended for native valve endocarditis (strong recommendation; low-quality evidence).
67. For infections limited to generator pockets, 4 weeks of antifungal therapy after removal of the device is recommended (strong recommendation; low-quality evidence).
68. For infections involving the wires, at least 6 weeks of antifungal therapy after wire removal is recommended (strong recommendation; low-quality evidence).
69. For ventricular assist devices that cannot be removed, the antifungal regimen is the same as that recommended for native valve endocarditis (strong recommendation; low-quality evidence). Chronic suppressive therapy with fluconazole if the isolate is susceptible, for as long as the device remains in place is recommended (strong recommendation; low-quality evidence).
What Is the Treatment for Candida Suppurative Thrombophlebitis?
Recommendations
70. Catheter removal and incision and drainage or resection of the vein, if feasible, is recommended (strong recommendation; low-quality evidence).
71. Lipid formulation AmB, 3–5 mg/kg daily, OR fluconazole, 400–800 mg (6–12 mg/kg) daily, OR an echinocandin (caspofungin 150 mg daily, micafungin 150 mg daily, or anidulafungin 200 mg daily) for at least 2 weeks after candidemia (if present) has cleared is recommended (strong recommendation; low-quality evidence).
72. Step-down therapy to fluconazole, 400–800 mg (6–12 mg/kg) daily, should be considered for patients who have initially responded to AmB or an echinocandin, are clinically stable, and have a fluconazole-susceptible isolate (strong recommendation; low-quality evidence).
73. Resolution of the thrombus can be used as evidence to discontinue antifungal therapy if clinical and culture data are supportive (strong recommendation; low-quality evidence).
XI. What Is the Treatment for Candida Osteoarticular Infections?
What Is the Treatment for Candida Osteomyelitis?
Recommendations
74. Fluconazole, 400 mg (6 mg/kg) daily, for 6–12 months OR an echinocandin (caspofungin 50–70 mg daily, micafungin 100 mg daily, or anidulafungin 100 mg daily) for at least 2 weeks followed by fluconazole, 400 mg (6 mg/kg) daily, for 6–12 months is recommended (strong recommendation; low-quality evidence).
75. Lipid formulation AmB, 3–5 mg/kg daily, for at least 2 weeks followed by fluconazole, 400 mg (6 mg/kg) daily, for 6–12 months is a less attractive alternative (weak recommendation; low-quality evidence).
76. Surgical debridement is recommended in selected cases (strong recommendation; low-quality evidence).
What Is the Treatment for Candida Septic Arthritis?
77. Fluconazole, 400 mg (6 mg/kg) daily, for 6 weeks OR an echinocandin (caspofungin 50–70 mg daily, micafungin 100 mg daily, or anidulafungin 100 mg daily) for 2 weeks followed by fluconazole, 400 mg (6 mg/kg) daily, for at least 4 weeks is recommended (strong recommendation; low-quality evidence).
78. Lipid formulation AmB, 3–5 mg/kg daily, for 2 weeks, followed by fluconazole, 400 mg (6 mg/kg) daily, for at least 4 weeks is a less attractive alternative (weak recommendation; low-quality evidence).
79. Surgical drainage is indicated in all cases of septic arthritis (strong recommendation; moderate-quality evidence).
80. For septic arthritis involving a prosthetic device, device removal is recommended (strong recommendation; moderate-quality evidence).
81. If the prosthetic device cannot be removed, chronic suppression with fluconazole, 400 mg (6 mg/kg) daily, if the isolate is susceptible, is recommended (strong recommendation; low-quality evidence).
XII. What Is the Treatment for Candida Endophthalmitis?
What Is the General Approach to Candida Endophthalmitis?
Recommendations
82. All patients with candidemia should have a dilated retinal examination, preferably performed by an ophthalmologist, within the first week of therapy in nonneutropenic patients to establish if endophthalmitis is present (strong recommendation; low-quality evidence). For neutropenic patients, it is recommended to delay the examination until neutrophil recovery (strong recommendation; low-quality evidence).
83. The extent of ocular infection (chorioretinitis with or without macular involvement and with or without vitritis) should be determined by an ophthalmologist (strong recommendation; low-quality evidence).
84. Decisions regarding antifungal treatment and surgical intervention should be made jointly by an ophthalmologist and an infectious diseases physician (strong recommendation; low-quality evidence).
What Is the Treatment for Candida Chorioretinitis Without Vitritis?
Recommendations
85. For fluconazole-/voriconazole-susceptible isolates, fluconazole, loading dose, 800 mg (12 mg/kg), then 400–800 mg (6–12 mg/kg) daily OR voriconazole, loading dose 400 mg (6 mg/kg) intravenous twice daily for 2 doses, then 300 mg (4 mg/kg) intravenous or oral twice daily is recommended (strong recommendation; low-quality evidence).
86. For fluconazole-/voriconazole-resistant isolates, liposomal AmB, 3–5 mg/kg intravenous daily, with or without oral flucytosine, 25 mg/kg 4 times daily is recommended (strong recommendation; low-quality evidence).
87. With macular involvement, antifungal agents as noted above PLUS intravitreal injection of either AmB deoxycholate, 5–10 µg/0.1 mL sterile water, or voriconazole, 100 µg/0.1 mL sterile water or normal saline, to ensure a prompt high level of antifungal activity is recommended (strong recommendation; low-quality evidence).
88. The duration of treatment should be at least 4–6 weeks, with the final duration depending on resolution of the lesions as determined by repeated ophthalmological examinations (strong recommendation; low-quality evidence).
What Is the Treatment for Candida Chorioretinitis With Vitritis?
Recommendations
89. Antifungal therapy as detailed above for chorioretinitis without vitritis, PLUS intravitreal injection of either amphotericin B deoxycholate, 5–10 µg/0.1 mL sterile water, or voriconazole, 100 µg/0.1 mL sterile water or normal saline is recommended (strong recommendation; low-quality evidence).
90. Vitrectomy should be considered to decrease the burden of organisms and to allow the removal of fungal abscesses that are inaccessible to systemic antifungal agents (strong recommendation; low-quality evidence).
91. The duration of treatment should be at least 4–6 weeks, with the final duration dependent on resolution of the lesions as determined by repeated ophthalmological examinations (strong recommendation; low-quality evidence).
XIII. What Is the Treatment for Central Nervous System Candidiasis?
Recommendations
92. For initial treatment, liposomal AmB, 5 mg/kg daily, with or without oral flucytosine, 25 mg/kg 4 times daily is recommended (strong recommendation; low-quality evidence).
93. For step-down therapy after the patient has responded to initial treatment, fluconazole, 400–800 mg (6–12 mg/kg) daily, is recommended (strong recommendation; low-quality evidence).
94. Therapy should continue until all signs and symptoms and CSF and radiological abnormalities have resolved (strong recommendation; low-quality evidence).
95. Infected CNS devices, including ventriculostomy drains, shunts, stimulators, prosthetic reconstructive devices, and biopolymer wafers that deliver chemotherapy should be removed if possible (strong recommendation; low-quality evidence).
96. For patients in whom a ventricular device cannot be removed, AmB deoxycholate could be administered through the device into the ventricle at a dosage ranging from 0.01 mg to 0.5 mg in 2 mL 5% dextrose in water (weak recommendation; low-quality evidence).
XIV. What Is the Treatment for Urinary Tract Infections Due to Candida Species?
What Is the Treatment for Asymptomatic Candiduria?
Recommendations
97. Elimination of predisposing factors, such as indwelling bladder catheters, is recommended whenever feasible (strong recommendation; low-quality evidence).
98. Treatment with antifungal agents is NOT recommended unless the patient belongs to a group at high risk for dissemination; high-risk patients include neutropenic patients, very low-birth-weight infants (<1500 g), and patients who will undergo urologic manipulation (strong recommendation; low-quality evidence).
99. Neutropenic patients and very low–birth-weight infants should be treated as recommended for candidemia (see sections III and VII) (strong recommendation; low-quality evidence).
100. Patients undergoing urologic procedures should be treated with oral fluconazole, 400 mg (6 mg/kg) daily, OR AmB deoxycholate, 0.3–0.6 mg/kg daily, for several days before and after the procedure (strong recommendation; low-quality evidence).
What Is the Treatment for Symptomatic Candida Cystitis?
Recommendations
101. For fluconazole-susceptible organisms, oral fluconazole, 200 mg (3 mg/kg) daily for 2 weeks is recommended (strong recommendation; moderate-quality evidence).
102. For fluconazole-resistant C. glabrata, AmB deoxycholate, 0.3–0.6 mg/kg daily for 1–7 days OR oral flucytosine, 25 mg/kg 4 times daily for 7–10 days is recommended (strong recommendation; low-quality evidence).
103. For C. krusei, AmB deoxycholate, 0.3–0.6 mg/kg daily, for 1–7 days is recommended (strong recommendation; low-quality evidence).
104. Removal of an indwelling bladder catheter, if feasible, is strongly recommended (strong recommendation; low-quality evidence).
105. AmB deoxycholate bladder irrigation, 50 mg/L sterile water daily for 5 days, may be useful for treatment of cystitis due to fluconazole-resistant species, such as C. glabrata and C. krusei (weak recommendation; low-quality evidence).
What Is the Treatment for Symptomatic Ascending Candida Pyelonephritis?
Recommendations
106. For fluconazole-susceptible organisms, oral fluconazole, 200–400 mg (3–6 mg/kg) daily for 2 weeks is recommended (strong recommendation; low-quality evidence).
107. For fluconazole-resistant C. glabrata, AmB deoxycholate, 0.3–0.6 mg/kg daily for 1–7 days with or without oral flucytosine, 25 mg/kg 4 times daily, is recommended (strong recommendation; low-quality evidence).
108. For fluconazole-resistant C. glabrata, monotherapy with oral flucytosine, 25 mg/kg 4 times daily for 2 weeks, could be considered (weak recommendation; low-quality evidence).
109. For C. krusei, AmB deoxycholate, 0.3–0.6 mg/kg daily, for 1–7 days is recommended (strong recommendation; low-quality evidence).
110. Elimination of urinary tract obstruction is strongly recommended (strong recommendation; low-quality evidence).
111. For patients who have nephrostomy tubes or stents in place, consider removal or replacement, if feasible (weak recommendation; low-quality evidence).
What Is the Treatment for Candida Urinary Tract Infection Associated With Fungus Balls?
Recommendations
112. Surgical intervention is strongly recommended in adults (strong recommendation; low-quality evidence).
113. Antifungal treatment as noted above for cystitis or pyelonephritis is recommended (strong recommendation; low-quality evidence).
114. Irrigation through nephrostomy tubes, if present, with AmB deoxycholate, 25–50 mg in 200–500 mL sterile water, is recommended (strong recommendation; low-quality evidence).
XV. What Is the Treatment for Vulvovaginal Candidiasis?
Recommendations
115. For the treatment of uncomplicated Candida vulvovaginitis, topical antifungal agents, with no one agent superior to another, are recommended (strong recommendation; high-quality evidence).
116. Alternatively, for the treatment of uncomplicated Candida vulvovaginitis, a single 150-mg oral dose of fluconazole is recommended (strong recommendation; high-quality evidence).
117. For severe acute Candida vulvovaginitis, fluconazole, 150 mg, given every 72 hours for a total of 2 or 3 doses, is recommended (strong recommendation; high-quality evidence).
118. For C. glabrata vulvovaginitis that is unresponsive to oral azoles, topical intravaginal boric acid, administered in a gelatin capsule, 600 mg daily, for 14 days is an alternative (strong recommendation; low-quality evidence).
119. Another alternative agent for C. glabrata infection is nystatin intravaginal suppositories, 100 000 units daily for 14 days (strong recommendation; low-quality evidence).
120. A third option for C. glabrata infection is topical 17% flucytosine cream alone or in combination with 3% AmB cream administered daily for 14 days (weak recommendation; low-quality evidence).
121. For recurring vulvovaginal candidiasis, 10–14 days of induction therapy with a topical agent or oral fluconazole, followed by fluconazole, 150 mg weekly for 6 months, is recommended (strong recommendation; high-quality evidence).
XVI. What Is the Treatment for Oropharyngeal Candidiasis?
Recommendations
122. For mild disease, clotrimazole troches, 10 mg 5 times daily, OR miconazole mucoadhesive buccal 50-mg tablet applied to the mucosal surface over the canine fossa once daily for 7–14 days are recommended (strong recommendation; high-quality evidence).
123. Alternatives for mild disease include nystatin suspension (100 000 U/mL) 4–6 mL 4 times daily, OR 1–2 nystatin pastilles (200 000 U each) 4 times daily, for 7–14 days (strong recommendation; moderate-quality evidence).
124. For moderate to severe disease, oral fluconazole, 100–200 mg daily, for 7–14 days is recommended (strong recommendation; high-quality evidence).
125. For fluconazole-refractory disease, itraconazole solution, 200 mg once daily OR posaconazole suspension, 400 mg twice daily for 3 days then 400 mg daily, for up to 28 days are recommended (strong recommendation; moderate-quality evidence).
126. Alternatives for fluconazole-refractory disease include voriconazole, 200 mg twice daily, OR AmB deoxycholate oral suspension, 100 mg/mL 4 times daily (strong recommendation; moderate-quality evidence).
127. Intravenous echinocandin (caspofungin: 70-mg loading dose, then 50 mg daily; micafungin: 100 mg daily; or anidulafungin: 200-mg loading dose, then 100 mg daily) OR intravenous AmB deoxycholate, 0.3 mg/kg daily, are other alternatives for refractory disease (weak recommendation; moderate-quality evidence).
128. Chronic suppressive therapy is usually unnecessary. If required for patients who have recurrent infection, fluconazole, 100 mg 3 times weekly, is recommended (strong recommendation; high-quality evidence).
129. For HIV-infected patients, antiretroviral therapy is strongly recommended to reduce the incidence of recurrent infections (strong recommendation; high-quality evidence).
130. For denture-related candidiasis, disinfection of the denture, in addition to antifungal therapy is recommended (strong recommendation; moderate-quality evidence).
XVII. What Is the Treatment for Esophageal Candidiasis?
Recommendations
131. Systemic antifungal therapy is always required. A diagnostic trial of antifungal therapy is appropriate before performing an endoscopic examination (strong recommendation; high-quality evidence).
132. Oral fluconazole, 200–400 mg (3–6 mg/kg) daily, for 14–21 days is recommended (strong recommendation; high-quality evidence).
133. For patients who cannot tolerate oral therapy, intravenous fluconazole, 400 mg (6 mg/kg) daily, OR an echinocandin (micafungin, 150 mg daily, caspofungin, 70-mg loading dose, then 50 mg daily, or anidulafungin, 200 mg daily) is recommended (strong recommendation; high-quality evidence).
134. A less preferred alternative for those who cannot tolerate oral therapy is AmB deoxycholate, 0.3–0.7 mg/kg daily (strong recommendation; moderate-quality evidence).
135. Consider de-escalating to oral therapy with fluconazole 200–400 mg (3–6 mg/kg) daily once the patient is able to tolerate oral intake (strong recommendation; moderate-quality evidence).
136. For fluconazole-refractory disease, itraconazole solution, 200 mg daily, OR voriconazole, 200 mg (3 mg/kg) twice daily either intravenous or oral, for 14–21 days is recommended (strong recommendation; high-quality evidence).
137. Alternatives for fluconazole-refractory disease include an echinocandin (micafungin: 150 mg daily; caspofungin: 70-mg loading dose, then 50 mg daily; or anidulafungin: 200 mg daily) for 14–21 days, OR AmB deoxycholate, 0.3–0.7 mg/kg daily, for 21 days (strong recommendation; high-quality evidence).
138. Posaconazole suspension, 400 mg twice daily, or extended-release tablets, 300 mg once daily, could be considered for fluconazole-refractory disease (weak recommendation; low-quality evidence).
139. For patients who have recurrent esophagitis, chronic suppressive therapy with fluconazole, 100–200 mg 3 times weekly, is recommended (strong recommendation; high-quality evidence).
140. For HIV-infected patients, antiretroviral therapy is strongly recommended to reduce the incidence of recurrent infections (strong recommendation; high-quality evidence).
INTRODUCTION
In the first section, the panel summarizes background information relevant to the topic. In the second section, the panel poses questions regarding the management of candidiasis, evaluates applicable clinical trial and observational data, and makes recommendations using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework [2]. The following 17 questions were answered:
I. What is the treatment for candidemia in nonneutropenic patients?
II. Should central venous catheters be removed in nonneutropenic patients with candidemia?
III. What is the treatment for candidemia in neutropenic patients?
IV. What is the treatment for chronic disseminated (hepatosplenic) candidiasis?
V. What is the role of empiric treatment for suspected invasive candidiasis in nonneutropenic patients in the intensive care unit?
VI. Should prophylaxis be used to prevent invasive candidiasis in the intensive care unit setting?
VII. What is the treatment for neonatal candidiasis, including central nervous system infection?
VIII. What is the treatment for intra-abdominal candidiasis?
IX. Does the isolation of Candida species from the respiratory tract require antifungal therapy?
X. What is the treatment for Candida intravascular infections, including endocarditis and infections of implantable cardiac devices?
XI. What is the treatment for Candida osteoarticular infections?
XII. What is the treatment for Candida endophthalmitis?
XIII. What is the treatment for central nervous system candidiasis?
XIV. What is the treatment for urinary tract infections due to Candida species?
XV. What is the treatment for vulvovaginal candidiasis?
XVI. What is the treatment for oropharyngeal candidiasis?
XVII. What is the treatment for esophageal candidiasis?
Infections due to Candida species are major causes of morbidity and mortality in humans, causing a diverse spectrum of clinical disease ranging from superficial and mucosal infections to invasive disease associated with candidemia and metastatic organ involvement. As an entity, candidemia is one of the most common healthcare-associated bloodstream infections in US hospitals, typically ranking as the third or fourth most common cause of healthcare–associated bloodstream infection. A recent multicenter point-prevalence survey identified Candida species as the most commonly isolated healthcare-associated bloodstream pathogen [4]. Among patients with candidemia and other forms of invasive candidiasis, non-albicans Candida species constitute approximately 50% of all relevant isolates, representing a steady trend in many regions throughout the world for more than a decade [5–12].
Among the many clinical manifestations of candidiasis, candidemia and invasive candidiasis have been given the most attention in clinical trials. Candidemia is associated with up to 47% attributable mortality [5–13], and this is even higher among persons with septic shock [14]. Several authors have demonstrated that mortality is closely linked to both timing of therapy and/or source control [14–19]. That is, earlier intervention with appropriate antifungal therapy and removal of a contaminated central venous catheter (CVC) or drainage of infected material is generally associated with better overall outcomes [14–19]. CVCs are commonly linked with candidemia, but catheters are not always the source, especially among neutropenic patients in whom the gastrointestinal tract is a common source. Most experts agree that thoughtful patient-specific management of CVCs is critical in the overall management of the infection [19].
The continued reliance on blood cultures, which are notoriously insensitive as markers of disease, remains a significant obstacle to early intervention for this condition. The development of reliable nonculture assays is critical to providing the opportunity for earlier intervention and more targeted antifungal therapy among large numbers of patients in whom traditional blood cultures are insensitive or provide untimely results [20].
Species distribution is also a significant challenge for all forms of candidiasis, and there is considerable geographic, center-to-center, and even unit-to-unit variability in the prevalence of pathogenic Candida species [8–12]. Indeed, candidiasis is not one but rather several diseases, with each Candida species presenting its own unique characteristics with respect to tissue tropism, propensity to cause invasive disease, virulence, and antifungal susceptibility. A working knowledge of the local epidemiology and rates of antifungal resistance is critical in making informed therapeutic decisions while awaiting culture and susceptibility data.
Despite the overall robust nature of the randomized controlled trials examining treatment of candidemia and other forms of invasive candidiasis [21–34], no single trial has demonstrated clear superiority of one therapeutic agent over another. Careful analysis of these clinical data sometimes leads to conflicting conclusions. For instance, the use of amphotericin B (AmB) plus fluconazole is as least as effective as higher-dose (800 mg daily) fluconazole given alone for patients with candidemia [22], but there is little role for this combination in current practice, especially as echinocandins are such a safe and effective alternative. Similarly, voriconazole is as effective as the strategy of sequential AmB and fluconazole for candidemia, but few would choose voriconazole in this setting as there is little advantage and potentially greater toxicity associated with using this agent compared to other therapies [23].
The echinocandins have emerged as preferred agents for most episodes of candidemia and invasive candidiasis, with the exception of central nervous system (CNS), eye, and urinary tract infections due to these organisms. This preference is based on a strong safety profile, convenience, early fungicidal activity, a trend toward better outcomes based on data from individual studies and combined analyses of candidemia studies [19, 25], and the emergence of azole-resistant Candida species. The recent emergence of multidrug-resistant Candida species further complicates the selection of antifungal therapy for the immediate future [10, 12, 35–38] as there are no good prospective data to guide therapy.
There is an abundance of clinical data generated from large randomized clinical trials for candidemia, Candida esophagitis, oropharyngeal candidiasis, and prophylaxis studies in special populations, such as patients in intensive care units (ICUs), neonates, and selected transplant recipients, and these studies have led to important insights into optimal therapeutic approaches in these vulnerable populations. For those with less common manifestations of disease, such as osteomyelitis, endophthalmitis, and infective endocarditis, treatment recommendations are largely based on extrapolation from randomized studies of patients with other forms of disease, small retrospective series, and anecdotal reports. Thus, there is a critical need to assess these data in an ongoing manner to provide timely recommendations pertaining to the management of patients with these less common forms of candidiasis.
METHODS
Panel Composition
The most recent version of the Infectious Diseases Society of America (IDSA) guideline on the management of patients with candidiasis was published in 2009 [1]. For this update, the IDSA Standards and Practice Guidelines Committee (SPGC) convened a multidisciplinary panel of 12 experts in the management of patients with candidiasis. The panel consisted of 12 members of IDSA, and included 11 adult infectious diseases physicians and 1 pediatric infectious diseases physician. All panel members were selected on the basis of their expertise in clinical and/or laboratory mycology with a focus on candidiasis.
Literature Review and Analysis
Panel members were each assigned to review the recent literature for at least 1 topic, evaluate the evidence, determine the strength of recommendations, and develop written evidence in support of these recommendations. PubMed, which includes Medline (1946 to present), was searched to identify relevant studies for the Candida guideline PICO (population/patient, intervention/indicator, comparator/control, outcome) questions. Search strategies were developed and built by 2 independent health sciences librarians from the Health Sciences Library System, University of Pittsburgh. For each PICO question, the librarians developed the search strategies using PubMed's command language and appropriate search fields. Medical Subject Headings (MeSH) terms and keywords were used for the main search concepts of each PICO question. Articles in all languages and all publication years were included. Initial searches were created and confirmed with input from the guideline committee chairs and group leaders from August to November 2013. The searches were finalized and delivered between late November 2013 and January 2014. After the literature searches were performed, authors continued to review the literature and added relevant articles as needed.
Process Overview
The panel met face-to-face twice and conducted a series of conference calls over a 2-year period. The panel reviewed and discussed all recommendations, their strength, and the quality of evidence. Discrepancies were discussed and resolved, and all final recommendations represent a consensus opinion of the entire panel. For the final version of these guidelines, the panel as a group reviewed all individual sections.
Evidence Review: The GRADE Method
GRADE is a systematic approach to guideline development that has been described in detail elsewhere [2, 39]. The IDSA adopted GRADE in 2008. In the GRADE system, the guideline panel assigns each recommendation with separate ratings for the underlying quality of evidence supporting the recommendation and for the strength with which the recommendation is made (Figure 1). Data from randomized controlled trials begin as “high” quality, and data from observational studies begin as “low” quality. However, the panel may judge that specific features of the data warrant decreasing or increasing the quality of evidence rating, and GRADE provides guidance on how such factors should be weighed [39]. The strength assigned to a recommendation chiefly reflects the panel's confidence that the benefits of following the recommendation are likely to outweigh potential harms. While the quality of evidence is an important factor in choosing recommendation strength, it is not prescriptive.
Guidelines and Conflicts of Interest
The expert panel complied with the IDSA policy on conflicts of interest, which requires disclosure of any financial or other interest that may be construed as constituting an actual, potential, or apparent conflict. Panel members were provided IDSA's conflicts of interest disclosure statement and were asked to identify ties to companies developing products that may be affected by promulgation of the guideline. Information was requested regarding employment, consultancies, stock ownership, honoraria, research funding, expert testimony, and membership on company advisory committees. Decisions were made on a case-by-case basis as to whether an individual's role should be limited as a result of a conflict. Potential conflicts of interests are listed in the Acknowledgments section.
Consensus Development Based on Evidence
The panel obtained feedback from 3 external peer reviewers. The guidelines were reviewed and endorsed by the MSG, the American Academy of Pediatrics (AAP) and the Pediatric Infectious Diseases Society (PIDS). The guideline was reviewed and approved by the IDSA SPGC and the IDSA Board of Directors prior to dissemination.
Revision Dates
At annual intervals, the panel chairs will be asked for their input on the need to update the guideline based on an examination of the current literature. The IDSA SPGC will consider this input and determine the necessity and timing of an update. If warranted, the entire panel or a subset thereof will be convened to discuss potential changes.
BACKGROUND
Antifungal Agents
Pharmacologic Considerations for Therapy for Candidiasis
Systemic antifungal agents shown to be effective for the treatment of invasive candidiasis comprise 4 major categories: the polyenes (amphotericin B [AmB] deoxycholate, liposomal AmB, AmB lipid complex [ABLC], and amphotericin B colloidal dispersion [ABCD, not available in the United States]), the triazoles (fluconazole, itraconazole, voriconazole, and posaconazole), the echinocandins (caspofungin, anidulafungin, and micafungin), and flucytosine. Data from a recently completed clinical trial comparing isavuconazole to an echinocandin for treatment of invasive candidiasis are unavailable at this time. Clinicians should become familiar with strategies to optimize efficacy through an understanding of relevant pharmacokinetic properties.
Amphotericin B
Most experience with AmB is with the deoxycholate preparation. Three lipid formulations of AmB have been developed and approved for use in humans: ABLC, ABCD, and liposomal AmB. These agents possess the same spectrum of activity as AmB deoxycholate, but daily dosing regimens and toxicity profiles differ for each agent. The 3 lipid formulation AmB agents have different pharmacological properties and rates of treatment-related adverse events and should not be interchanged without careful consideration. In this document, a reference to AmB, without a specific dose or other discussion of form, should be taken to be a reference to the general use of any of the AmB preparations. For most forms of invasive candidiasis, the typical intravenous dosage for AmB deoxycholate is 0.5–0.7 mg/kg daily, but dosages as high as 1 mg/kg daily should be considered for invasive Candida infections caused by less susceptible species, such as C. glabrata and C. krusei. The typical dosage for lipid formulation AmB is 3–5 mg/kg daily when used for invasive candidiasis. Nephrotoxicity is the most common serious adverse effect associated with AmB deoxycholate therapy, resulting in acute kidney injury in up to 50% of recipients and an electrolyte-wasting tubular acidosis in a majority of patients [40, 41]. Lipid formulations of AmB are more expensive than AmB deoxycholate, but all have considerably less nephrotoxicity [42, 43]. Most observers agree that lipid formulations, with the exception of ABCD, have fewer infusion-related reactions than AmB deoxycholate. The impact of the pharmacokinetics and differences in toxicity of lipid formulations of AmB have not been formally examined in clinical trials. We are not aware of any forms of candidiasis for which lipid formulations of AmB are superior to AmB deoxycholate in terms of clinical efficacy. In addition, we are not aware of any situation in which lipid formulations should not be used, with the exception of urinary tract infections, because of reduced renal excretion of these formulations. Animal model studies suggest a pharmacokinetic and therapeutic advantage for liposomal AmB in the CNS [44]. Data demonstrating that AmB deoxycholate–induced nephrotoxicity is associated with a 6.6-fold increase in mortality have led many clinicians to use lipid formulations of AmB in proven or suspected candidiasis, especially among patients in a high-risk environment, such as an ICU [45].
Triazoles
Fluconazole, itraconazole, voriconazole, posaconazole, and a new expanded-spectrum triazole, isavuconazole, demonstrate similar activity against most Candida species [46–51]. Each of the azoles has less activity against C. glabrata and C. krusei than against other Candida species. All of the azole antifungals inhibit cytochrome P450 enzymes to some degree [52]. Thus, clinicians must carefully consider the influence on a patient's drug regimen when adding or removing an azole. In large clinical trials, fluconazole demonstrated efficacy comparable to that of AmB deoxycholate for the treatment of candidemia [21, 22] and is also considered to be standard therapy for oropharyngeal, esophageal, and vaginal candidiasis, as well as urinary tract infections [53, 54]. Fluconazole is readily absorbed, with oral bioavailability resulting in concentrations equal to approximately 90% of those achieved by intravenous administration [55]. Absorption is not affected by food consumption, gastric pH, or disease state. Among the triazoles, fluconazole has the greatest penetration into the cerebrospinal fluid (CSF) and vitreous, achieving concentrations of >70% of those in serum [56–59]. For this reason, it is often used in the treatment of CNS and intraocular Candida infections. Fluconazole achieves urine concentrations that are 10–20 times the concentrations in serum and, thus, is the preferred treatment option for symptomatic cystitis [59]. For patients with invasive candidiasis, fluconazole should be administered with an average loading dose of 800 mg (12 mg/kg), followed by an average daily dose of 400 mg (6 mg/kg). The higher-dose level (800 mg daily, 12 mg/kg) is often recommended for therapy of susceptible C. glabrata infections, but this has not been validated in clinical trials. Fluconazole elimination is almost entirely renal; thus, a dose reduction is needed in patients with creatinine clearance <50 mL/minute.
Itraconazole is only available in oral formulations. It has not been well studied for invasive candidiasis, and is generally reserved for patients with mucosal candidiasis, especially those who have experienced treatment failure with fluconazole [60]. Gastrointestinal absorption is variable among patients and is greater for the oral solution compared with the capsule formulation. Histamine receptor antagonists and proton pump inhibitors result in decreased absorption of the capsule formulation, whereas acidic beverages enhance absorption [61]. Administration of the capsule formulation with food increases absorption, but the oral solution is better absorbed on an empty stomach [62]. Oral formulations are dosed in adults at 200 mg 3 times daily for 3 days, then 200 mg once or twice daily thereafter.
Voriconazole has demonstrated effectiveness for both mucosal and invasive candidiasis [23, 63]. Its clinical use has been primarily for step-down oral therapy in patients with infection due to C. krusei and fluconazole-resistant, voriconazole-susceptible C. glabrata. CSF and vitreous concentrations are >50% of serum concentration, and voriconazole has been shown to be efficacious in case series for these infection sites [64–66]. Voriconazole does not accumulate in active form in the urine and thus should not be used for urinary candidiasis. The oral bioavailability of voriconazole is excellent and is not affected by gastric pH, but it decreases when the drug is administered with food [67, 68]. In adults, the recommended oral dosing regimen for candidiasis includes a loading dose of 400 mg (6 mg/kg) twice daily for 2 doses, followed by 200–300 mg (3–4 mg/kg) twice daily.
Intravenous voriconazole is complexed to a cyclodextrin molecule; after 2 loading doses of 6 mg/kg every 12 hours, a maintenance dosage of 3–4 mg/kg every 12 hours is recommended. Because of the potential for cyclodextrin accumulation and possible nephrotoxicity among patients with significant renal dysfunction, intravenous voriconazole is not currently recommended for patients with a creatinine clearance <50 mL/minute. However, retrospective examination of intravenous voriconazole use in patients with varying degrees of renal function below this cutoff value has not identified toxic effects, mitigating some of these concerns [69, 70]. Oral voriconazole does not require dosage adjustment for renal insufficiency, but it is the only triazole that requires dosage reduction for patients with mild to moderate hepatic impairment [71].
Common polymorphisms in the gene encoding the primary metabolic enzyme for voriconazole result in wide variability of serum levels [72]. Drug–drug interactions are common with voriconazole and should be considered when initiating and discontinuing treatment with this compound [52]. Voriconazole has not been studied systematically in fluconazole-resistant Candida species, and with the exception of C. krusei, use is currently discouraged. Each of the triazoles can be associated with uncommon side effects. However, several effects are unique to voriconazole or more commonly associated with higher voriconazole concentrations, including hepatic injury, visual side effects, photosensitivity, periostitis, and CNS side effects [73–75].
Posaconazole does not have an indication for primary candidiasis therapy. It demonstrates in vitro activity against Candida species that is similar to that of voriconazole, but clinical data are inadequate to make an evidence-based recommendation for treatment of candidiasis other than oropharyngeal candidiasis [76]. Posaconazole is currently available as an extended-release tablet, an oral suspension, and an intravenous solution. The tablet formulation, given as 300 mg twice daily for 2 doses, then 300 mg daily produces predictable serum concentrations and excellent drug exposure and requires only once-daily dosing [77, 78]. The oral suspension has unpredictable bioavailability [79–81]. Intravenous posaconazole is given as 300 mg twice daily for 2 doses, then 300 mg daily.
Isavuconazole is a recently approved expanded-spectrum triazole antifungal with excellent in vitro activity against Candida species. Preliminary analysis of the recently completed large international double-blind trial comparing isavuconazole to an echinocandin for invasive candidiasis suggests that isavuconazole did not meet criteria for noninferiority (personal communication, Astellas US).
Echinocandins
Caspofungin, anidulafungin, and micafungin are available only as parenteral preparations [82–84]. The minimum inhibitory concentrations (MICs) of the echinocandins are low for most Candida species, including C. glabrata and C. krusei [48–50]. However, recent case series have described treatment failure associated with resistant strains of C. glabrata [85, 86]. Candida parapsilosis demonstrates innately higher MICs to the echinocandins than do most other Candida species, which raises the concern that C. parapsilosis may be less responsive to the echinocandins.
Each of these agents has been studied for the treatment of esophageal candidiasis [24, 87, 88] and invasive candidiasis [25–34], and each has demonstrated efficacy in these situations. Recent pooled analyses of almost exclusively nonneutropenic patients included in randomized invasive candidiasis treatment trials suggest a survival advantage associated with initial echinocandin therapy [19].
All echinocandins have minimal adverse effects. The pharmacologic properties in adults are also very similar, and each is administered once daily intravenously [82–84]. Echinocandins achieve therapeutic concentrations in all infection sites with the exception of the eye, CNS, and urine [59]. The major route of elimination is nonenzymatic degradation. None of the echinocandins require dosage adjustment for renal insufficiency or dialysis. Both caspofungin and micafungin undergo minimal hepatic metabolism, but neither drug is a major substrate for cytochrome P450. Caspofungin is the only echinocandin for which dosage reduction is recommended for patients with moderate to severe hepatic dysfunction. The usual intravenous dosing regimens for invasive candidiasis are as follows: caspofungin: loading dose 70 mg, then 50 mg daily; anidulafungin: loading dose 200 mg, then 100 mg daily; and micafungin: 100 mg daily (no loading dose needed).
Flucytosine
Flucytosine demonstrates broad antifungal activity against most Candida species, with the exception of C. krusei. The compound is available in the United States only as an oral formulation. The drug has a short half-life (2.4–4.8 hours) and is ordinarily administered at a dosage of 25 mg/kg 4 times daily for patients with normal renal function. Flucytosine demonstrates excellent absorption after oral administration (80%–90%), and most of the drug is excreted unchanged (microbiologically active) in the urine [89, 90]; dose adjustment is necessary for patients with renal dysfunction [91, 92].The compound exhibits high penetration into the CNS and eye. Concentration-dependent toxicity results in bone marrow suppression and hepatitis.
Flucytosine is usually given in combination with another antifungal agent due to a high rate of emergence of resistance during monotherapy [93]. The most common use of flucytosine in the setting of Candida infection is in combination with AmB for patients with more refractory infections, such as Candida endocarditis, meningitis, or endophthalmitis. Occasionally, it is used for the treatment of symptomatic urinary tract candidiasis due to fluconazole-resistant C. glabrata [94].
Pediatric Dosing
There is considerable variation in the pharmacokinetics of antifungal agents between adult and pediatric patients, and the data on dosing in pediatric patients are limited. The pharmacological properties of antifungal agents in children and infants have been reviewed in detail [95]. The optimal dose of AmB deoxycholate in neonates has not been clearly defined; a dosage of 1 mg/kg is generally used [96–98]. The safety, efficacy, area under the curve, and maximal concentration of ABLC 2–5 mg/kg day are similar in adults and children [99]. The pharmacokinetics of liposomal AmB in neonates and children suggest that both volume and clearance are affected by weight [100].
Flucytosine clearance is directly proportional to glomerular filtration rate, and infants with a very low birth weight may accumulate high plasma concentrations because of poor renal function due to immaturity [101]. Thus, the use of flucytosine without careful monitoring of serum drug levels is discouraged in this group of patients.
Fluconazole pharmacokinetics vary with age, and the drug is rapidly cleared in children. Thus, a daily fluconazole dose of 12 mg/kg is necessary for neonates and children [102–105]. Voriconazole pharmacokinetics are also highly variable in children [106–108]. To attain plasma exposures comparable to those in adults receiving 4 mg/kg every 12 hours, a loading dose of intravenous voriconazole of 9 mg/kg twice daily, followed by 8 mg/kg twice daily is recommended in children. The recommended oral dose is 9 mg/kg twice daily (maximum dose 350 mg) [95, 107]. There are no data on voriconazole dosing in children <2 years old, and there are no pediatric studies examining the pharmacokinetics of the intravenous formulation, the oral suspension, or the extended-release tablets of posaconazole.
Caspofungin and micafungin are approved by the US Food and Drug Administration (FDA) for use in children. Caspofungin dosing is based on body surface area rather than weight. Dosing in children is a loading dose of 70 mg/m2, followed by 50 mg/m2/day. Preliminary studies suggest an optimal dose of caspofungin in neonates of 25 mg/m2/day. The current recommendation for micafungin for invasive candidiasis is 2 mg/kg/day, with the option to increase to 4 mg/kg/day in children <40 kg. The optimal dose of micafungin in neonates is unknown, but likely to be 10 mg/kg/day or greater [109]. Anidulafungin should be dosed at 1.5 mg/kg/day for neonates and children [110–112].
Considerations During Pregnancy
AmB is the treatment of choice for invasive candidiasis in pregnant women [113]. Fluconazole, itraconazole, posaconazole, and isavuconazole should be avoided in pregnant women, especially those in the first trimester, because of the possibility of birth defects associated with their use. Voriconazole is contraindicated during pregnancy because of fetal abnormalities observed in animals. There are few data concerning the echinocandins; thus, their use is cautioned during pregnancy. Flucytosine is contraindicated during pregnancy because of fetal abnormalities observed in animals.
Therapeutic Drug Monitoring
Therapeutic drug monitoring (TDM) for itraconazole, voriconazole, posaconazole, and flucytosine has been shown to be useful for optimizing efficacy and limiting toxicity in patients receiving therapy for a variety of invasive fungal infections, including mucosal and invasive candidiasis [114]. The basis for TDM is widely variable concentrations among patients and a strong relationship between concentration and efficacy and/or toxicity.
For itraconazole, when measured by high-pressure liquid chromatography (HPLC), both itraconazole and its bioactive hydroxy-itraconazole metabolite are reported, the sum of which should be considered in assessing drug levels. Treatment success has been associated with concentrations ≥1 mg/L and toxicity with concentrations >5 mg/L. Bioassay levels are 3- to 7-fold higher than those measured by HPLC. Because of nonlinear pharmacokinetics in adults and genetic differences in metabolism, there is both intrapatient and interpatient variability in serum voriconazole concentrations [115–118]. TDM should be considered for patients receiving voriconazole, because drug toxicity has been observed at higher serum concentrations and reduced clinical response has been observed at lower concentrations [117, 118]. The therapeutic trough concentration window for voriconazole is 1–5.5 mg/L. Few data are available to support a specific concentration to optimize posaconazole efficacy. Flucytosine monitoring is predominantly used to prevent concentration-associated toxicity. Peak concentrations <100 mg/L are recommended to avoid the predictable liver and bone marrow effects [119].
Antifungal Susceptibility Testing
Intensive efforts to develop standardized, reproducible, and relevant susceptibility testing methods for fungi have resulted in the development of the Clinical and Laboratory Standards Institute (CLSI) M27-A3 and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) methodologies for susceptibility testing of yeasts [120]. Interpretive breakpoints for susceptibility take into account the MIC, as well as pharmacokinetic/pharmacodynamic data and animal model data. They are reported for each species. Breakpoints have been established for most, but not all, drugs for the 5 most common Candida species [47, 50, 121, 122] (Table 1).
Table 1.
Clinical Breakpoints for Antifungal Agents Against Common Candida Species
| Candida Organism | Clinical Breakpoint, µg/mLa |
||||
|---|---|---|---|---|---|
| Antifungal Agent | S | SDD | I | R | |
| C. albicans | Fluconazole | ≤2 | 4 | ≥8 | |
| Itraconazole | ≤0.12 | 0.25–0.5 | ≥1 | ||
| Voriconazole | ≤0.12 | 0.25–0.5 | ≥1 | ||
| Posaconazole | |||||
| Anidulafungin | ≤0.25 | 0.5 | ≥1 | ||
| Caspofungin | ≤0.25 | 0.5 | ≥1 | ||
| Micafungin | ≤0.25 | 0.5 | ≥1 | ||
| C. glabrata | Fluconazole | 32 | ≥64 | ||
| Itraconazole | |||||
| Voriconazole | |||||
| Posaconazole | |||||
| Anidulafungin | ≤0.12 | 0.25 | ≥0.5 | ||
| Caspofungin | ≤0.12 | 0.25 | ≥0.5 | ||
| Micafungin | ≤0.06 | 0.12 | ≥0.25 | ||
| C. parapsilosis | Fluconazole | ≤2 | 4 | ≥8 | |
| Itraconazole | |||||
| Voriconazole | ≤0.12 | 0.25–0.5 | ≥1 | ||
| Posaconazole | |||||
| Anidulafungin | ≤2 | 4 | ≥8 | ||
| Caspofungin | ≤2 | 4 | ≥8 | ||
| Micafungin | ≤2 | 4 | ≥8 | ||
| C. tropicalis | Fluconazole | ≤2 | 4 | ≥8 | |
| Itraconazole | |||||
| Voriconazole | ≤0.12 | 0.25–0.5 | ≥1 | ||
| Posaconazole | |||||
| Anidulafungin | ≤0.25 | 0.5 | ≥1 | ||
| Caspofungin | ≤0.25 | 0.5 | ≥1 | ||
| Micafungin | ≤0.25 | 0.5 | ≥1 | ||
| C. krusei | Fluconazole | ||||
| Itraconazole | |||||
| Voriconazole | ≤0.5 | 1 | ≥2 | ||
| Posaconazole | |||||
| Anidulafungin | ≤0.25 | 0.5 | ≥1 | ||
| Caspofungin | ≤0.25 | 0.5 | ≥1 | ||
| Micafungin | ≤0.25 | 0.5 | ≥1 | ||
Where no values are entered, there are insufficient data to establish clinical breakpoints.
Abbreviations: I, intermediate; MIC, minimum inhibitory concentration; R, resistant; S, susceptible; SDD, susceptible dose-dependent.
a Clinical breakpoints adopted by the Clinical and Laboratory Standards Institute.
In many instances, clinical breakpoints have decreased from those used previously. For example, the prior Candida clinical breakpoint for susceptibility to fluconazole was ≤8 mg/L. With the new interpretation, the susceptible value has been reduced to ≤2 mg/L for C. albicans. For C. glabrata, there is no breakpoint established for susceptibility to fluconazole, itraconazole, posaconazole, or voriconazole (Table 1).
When there is no clinical breakpoint established, the epidemiologic cutoff value (ECV) based on an examination of the distribution of MICs within a species can be used. The ECV is defined as the MIC value that excludes non–wild type strains, notably isolates that are likely to contain a resistant mutant [50, 123]. The addition of the ECV method is particularly useful for detecting emergence of resistance in a Candida species at an institution.
The susceptibility of Candida to the currently available antifungal agents is generally predictable if the species of the infecting isolate is known. Currently, antifungal resistance in C. albicans is uncommon. However, individual isolates may not necessarily follow this general pattern [124]. Recent surveillance studies suggest that triazole resistance among C. glabrata isolates has increased to a degree that is it difficult to rely upon these agents for therapy in the absence of susceptibility testing [12, 125, 126]. A similar trend has begun to emerge for a smaller proportion of C. glabrata isolates and the echinocandins [35, 85, 125]. The value of susceptibility testing for other Candida species is less clear, although resistance among C. tropicalis and C. parapsilosis has been reported from tertiary care institutions that have extensive use of antifungal agents [127, 128]. Because of these trends, susceptibility testing is increasingly used to guide the management of candidemia and invasive candidiasis.
Diagnosis of Candidiasis
Cultures of blood or other samples collected under sterile conditions have long been considered diagnostic gold standards for invasive candidiasis. Nonculture diagnostic tests, such as antigen, antibody, or β-D-glucan detection assays, and polymerase chain reaction (PCR) are now entering clinical practice as adjuncts to cultures. If used and interpreted judiciously, these tests can identify more patients with invasive candidiasis and better direct antifungal therapy. To fully realize the benefits of combining culture and nonculture tests, however, clinicians must carefully consider the types of invasive candidiasis, understand the strengths and limitations of each assay, and interpret test results in the context of the clinical setting.
Use of Cultures in the Diagnosis of Invasive Candidiasis
Invasive candidiasis encompasses 3 entities: candidemia in the absence of deep-seated candidiasis, candidemia associated with deep-seated candidiasis, and deep-seated candidiasis in the absence of candidemia [20]. The distribution of these entities is likely to differ among centers; on balance, data suggest that the groups are approximately equal in size [129].
The overall sensitivity of blood cultures for diagnosing invasive candidiasis is roughly 50% [20]. The limit of detection of blood cultures is ≤1 colony-forming unit/mL [130, 131]. The limit of detection for cultures is at or below that of PCR [132–135]. As such, blood cultures should be positive during the vast majority of active Candida bloodstream infections. They may be negative in cases of extremely low-level candidemia, intermittent candidemia, deep-seated candidiasis that persists after sterilization of the bloodstream, or deep-seated candidiasis resulting from direct inoculation of Candida in the absence of candidemia. Blood cultures are limited by slow turnaround times (median time to positivity of 2–3 days, ranging from 1 to ≥7 days), and the fact that they may become positive relatively late in the disease course [130, 136]. Cultures of tissues or fluid recovered from infected sites during deep-seated candidiasis also exhibit poor sensitivity (often <50%) and slow turnaround times, and require invasive sampling procedures that may be dangerous or contraindicated due to underlying medical conditions [137].
Antigen and Antibody Detection
Candida antigen and anti-Candida antibody detection has gained greater acceptance in Europe than the United States. In general, antigen detection is limited by rapid clearance from the bloodstream [138]. Concerns have been expressed about the reliability of antibody detection in immunosuppressed hosts, but assays have performed well in patients with neutropenia and cell-mediated immune defects (including hematopoietic cell and solid organ transplant recipients) [138, 139]. Serum immunoglobulin G (IgG) responses against specific antigens have typically performed better than immunoglobulin M (IgM) responses, suggesting that many patients mount amnestic responses or have ongoing, subclinical tissue invasion [139]. The best-studied test is a combined mannan/antimannan antibody assay, which is currently approved for use in Europe, but not the United States (Platelia Candida Ag and Ab; Bio-Rad). In a meta-analysis of 14 studies, the sensitivity/specificity for the diagnosis of invasive candidiasis of mannan and antimannan IgG individually were 58%/93% and 59%/83%, respectively [140]. Values for the combined assay were 83% and 86%, with best performances for C. albicans, C. glabrata, and C. tropicalis infections. In one study of candidemia, at least one test was positive before blood culture in 73% of patients [141]. In a study of hepatosplenic candidiasis, at least one test was positive before radiographic changes in 86% of patients [142]. This assay is not used widely in the United States, and its role in the diagnosis and management of invasive candidiasis is unclear.
β-D-Glucan detection
β-D-glucan is a cell wall constituent of Candida species, Aspergillus species, Pneumocystis jiroveci, and several other fungi. A serum β-D-glucan assay (Fungitell; Associates of Cape Cod, East Falmouth, Massachusetts) has been approved by the FDA as an adjunct to cultures for the diagnosis of invasive fungal infections. True-positive results are not specific for invasive candidiasis, but rather suggest the possibility of an invasive fungal infection. For this reason, among patient populations that are also at risk for invasive mold infections, such as hematopoietic cell transplant recipients, β-D-glucan offers a theoretical advantage over more narrow assays for candidiasis. β-D-glucan detection can identify cases of invasive candidiasis days to weeks prior to positive blood cultures, and shorten the time to initiation of antifungal therapy [143]. Prophylactic or empiric antifungal treatment is likely to impact test performance. On the one hand, antifungal agents may reduce diagnostic sensitivity [144–146], but decreasing β-D-glucan levels may also correlate with responses to antifungal therapy [147].
In meta-analyses of β-D-glucan studies, the pooled sensitivity and specificity for diagnosing invasive candidiasis were 75%–80% and 80%, respectively [144–146]. A number of issues complicate the interpretation of these data, including uncertainties about the best cutoff value for a positive result, number of positive tests required to establish a diagnosis, and optimal timing and frequency of testing among at-risk patients. There is marked heterogeneity among studies in how they address these issues, as well as in patient and control populations, range and type of fungal pathogens targeted, invasive candidiasis disease entities, distributions of Candida species, prior antifungal use, specific β-D-glucan assays employed, and other aspects of study design and statistical interpretation.
The major concern about β-D-glucan detection is the potential for poor specificity and false positivity, which may be particularly problematic in the patient populations for which nonculture diagnostics would be most helpful. For example, false-positive results are rare in healthy controls, but decidedly more common among patients in an ICU [148]. Causes of false positivity include other systemic infections, such as gram-positive and gram-negative bacteremia, certain antibiotics, such as intravenous amoxicillin-clavulanate (not available in the United States), hemodialysis, fungal colonization, receipt of albumin or immunoglobulin, use of surgical gauze or other material containing glucan, and mucositis or other disruptions of gastrointestinal mucosa [149–154]. The specificity of β-D-glucan can be improved by requiring consecutive positive results rather than a single result, but false positivity remains a significant limitation if the above-listed factors are common in the population tested. As an extreme example, the per-patient sensitivity/specificity and positive and negative predictive values of routine surveillance β-D-glucan testing in a recent study of lung transplant recipients were 64%/9% and 14%/50%, respectively [155]. Moreover, 90% of patients had at least one positive β-D-glucan result. Therefore, the test will be most useful if targeted to subgroups of patients whose clinical course or risk factors are particularly suggestive of invasive candidiasis or other fungal infection.
The role of β-D-glucan testing of samples other than serum in the diagnosis of invasive candidiasis is not established. Studies of β-D-glucan testing of CSF reported sensitivity and specificity of 100% and 95%–98%, respectively, for the diagnosis of non-Candida fungal CNS infections [156, 157]. β-D-glucan detection was highly sensitive and specific in a rabbit model of hematogenous C. albicans meningoencephalitis [158]. Limited data suggest that positive predictive values of β-D-glucan in bronchoalveolar lavage fluid are poor for diagnosing fungal pneumonia [159]. There are case reports for testing of samples collected from other sites of invasive Candida infection [160].
Limited data exist pertaining to the usefulness of β-D-glucan testing in children [161]. The optimal threshold for positivity of β-D-glucan testing in children is not known. In studies of uninfected immunocompetent individuals, mean β-D-glucan levels are slightly higher in children than adults [162]. Currently, it is not recommended to use β-D-glucan testing to guide pediatric clinical decision making.
Polymerase Chain Reaction
Candida PCR shares many of the potential benefits and shortcomings of β-D-glucan detection. Compared to cultures, PCR assays of various blood fractions have been shown to shorten the time to diagnosis of invasive candidiasis and initiation of antifungal therapy [134, 135]. The pooled sensitivity and specificity of PCR for suspected invasive candidiasis in a recent meta-analysis were 95% and 92%, respectively [134]. In probable invasive candidiasis, sensitivity of PCR and blood cultures was 85% and 38%, respectively. The impact of antifungal agents on diagnostic sensitivity was unclear. Data among patients colonized with Candida were surprisingly limited, but there was a trend toward lower specificity.
A major limitation of PCR studies is the lack of standardized methodologies and multicenter validation of assay performance. A multicenter US study assessing the performance of a self-contained instrument that amplifies and detects Candida DNA by PCR and T2 magnetic resonance (T2 Biosystems, Lexington, Massachusetts), respectively, has been completed [163]. This assay is FDA approved, but its role in the early diagnosis and management of candidemia remains unclear until more data are available. PCR has potential advantages over β-D-glucan or antigen-antibody assays, including the capacity for species identification, detection of molecular markers for drug resistance, and multiplex formatting. In Europe, a whole-blood, multiplex real-time PCR assay (SeptiFast, Roche) that detects 19 bacteria and 6 fungi (C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, C. krusei, and Aspergillus fumigatus) has been investigated in several studies of sepsis and neutropenic fever. Among patients with candidemia in one study, the sensitivity of the test was 94%; the only negative result was observed with C. famata candidemia [164]. The role of PCR in testing samples other than blood is not established.
Nonculture Diagnostic Testing for Blood Culture–Negative Invasive Candidiasis
The overwhelming majority of studies have examined nonculture diagnostics in the setting of candidemia. More limited data on deep-seated candidiasis demonstrate how these tests may identify cases that are currently missed by blood cultures. In a single-center study of prospectively enrolled patients, the sensitivities/specificities of the Fungitell β-D-glucan assay and a real-time quantitative PCR assay (ViraCor-IBT, Lee's Summit, Missouri) for invasive candidiasis were 56%/73% and 80%/70%, respectively [132]. More importantly, the sensitivities of contemporaneously collected blood cultures, β-D-glucan assay, and PCR samples among patients with deep-seated candidiasis (mostly intra-abdominal candidiasis) were 21%, 67%, and 88%, respectively. The combination of either a positive blood culture or positive β-D-glucan assay had sensitivity for invasive candidiasis of 79%; a positive blood culture or positive PCR sample was 98% sensitive. A second study investigated the serum β-D-glucan assay, Candida score (a predictive score for invasive candidiasis based on clinical parameters and burden of Candida colonization), and Candida colonization indices (predictive scores based on burden of colonization) among prospectively enrolled patients who were in surgical ICUs at 2 hospitals and who were at particularly high risk for intra-abdominal candidiasis [143]. The sensitivity/specificity of 2 consecutive positive β-D-glucan results was 65%/78%. In contrast, the sensitivity of blood cultures was only 7%. In addition to identifying cases missed by blood cultures, the β-D-glucan assay was positive a median of 5 and 6 days prior to positive intra-abdominal cultures and institution of antifungal therapy, respectively. The sensitivities of Candida scores and colonization indices were comparable to β-D-glucan, but specificities were poorer (≤43%).
The interpretation of specificity in these studies was complicated by the fact that negative controls were also at risk for invasive candidiasis. Therefore, it is unclear if positive test results for controls were false positives (as defined in the studies) or true positives that were missed due to the poor sensitivity of intra-abdominal and blood cultures. Indeed, this is a central challenge in assessing new diagnostics for invasive candidiasis: How can test performance be accurately measured when the gold standard is inadequate?
I. What Is the Treatment for Candidemia in Nonneutropenic Patients?
Recommendations
1. An echinocandin (caspofungin: loading dose 70 mg, then 50 mg daily; micafungin: 100 mg daily; anidulafungin: loading dose 200 mg, then 100 mg daily) is recommended as initial therapy (strong recommendation; high-quality evidence).
2. Fluconazole, intravenous or oral, 800-mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily is an acceptable alternative to an echinocandin as initial therapy in selected patients, including those who are not critically ill and who are considered unlikely to have a fluconazole-resistant Candida species (strong recommendation; high-quality evidence).
3. Testing for azole susceptibility is recommended for all bloodstream and other clinically relevant Candida isolates. Testing for echinocandin susceptibility should be considered in patients who have had prior treatment with an echinocandin and among those who have infection with C. glabrata or C. parapsilosis (strong recommendation; low-quality evidence).
4. Transition from an echinocandin to fluconazole (usually within 5–7 days) is recommended for patients who are clinically stable, have isolates that are susceptible to fluconazole (eg, C. albicans), and have negative repeat blood cultures following initiation of antifungal therapy (strong recommendation; moderate-quality evidence).
5. For infection due to C. glabrata, transition to higher-dose fluconazole 800 mg (12 mg/kg) daily or voriconazole 200–300 (3–4 mg/kg) twice daily should only be considered among patients with fluconazole-susceptible or voriconazole-susceptible isolates (strong recommendation; low-quality evidence).
6. Lipid formulation AmB (3–5 mg/kg daily) is a reasonable alternative if there is intolerance, limited availability, or resistance to other antifungal agents (strong recommendation; high-quality evidence).
7. Transition from AmB to fluconazole is recommended after 5–7 days among patients who have isolates that are susceptible to fluconazole, who are clinically stable, and in whom repeat cultures on antifungal therapy are negative (strong recommendation; high-quality evidence).
8. Among patients with suspected azole- and echinocandin-resistant Candida infections, lipid formulation AmB (3–5 mg/kg daily) is recommended (strong recommendation; low-quality evidence).
9. Voriconazole 400 mg (6 mg/kg) twice daily for 2 doses, then 200 mg (3 mg/kg) twice daily is effective for candidemia, but offers little advantage over fluconazole as initial therapy (strong recommendation; moderate-quality evidence). Voriconazole is recommended as step-down oral therapy for selected cases of candidemia due to C. krusei (strong recommendation; low-quality evidence).
10. All nonneutropenic patients with candidemia should have a dilated ophthalmological examination, preferably performed by an ophthalmologist, within the first week after diagnosis (strong recommendation; low-quality evidence).
11. Follow-up blood cultures should be performed every day or every other day to establish the time point at which candidemia has been cleared (strong recommendation; low-quality evidence).
12. Recommended duration of therapy for candidemia without obvious metastatic complications is for 2 weeks after documented clearance of Candida species from the bloodstream and resolution of symptoms attributable to candidemia (strong recommendation; moderate-quality evidence).
Evidence Summary
Candidemia has emerged as one of the most common causes of healthcare-associated bloodstream infections, and in many US hospitals, candidemia represents the third or fourth most common hospital-acquired bloodstream isolate. In most clinical settings, C. albicans is the most commonly isolated species, but the non-albicans Candida species together represent approximately 50% of the bloodstream isolates, and this has been a growing trend in many hospitals throughout the world for more than a decade [8–12].
There are significant challenges in treating candidemia and invasive candidiasis. First, the infection is associated with high mortality. Earlier therapy is associated with better overall outcomes [14–18], but there remain significant limitations to early diagnosis. The development of rapid diagnostic assays has been slow; thus, clinicians continue to rely on cultures to establish a diagnosis [20]. Second, there is considerable geographic, center-to-center, and even unit-to-unit variability of species causing candidemia [12]; each Candida species presents its own unique challenges with respect to virulence, pathogenicity, and antifungal susceptibility. Third, despite the overall robust nature of the randomized controlled trials examining treatment of candidemia and other forms of invasive candidiasis, no single trial has demonstrated the clear superiority of one therapeutic agent over another [19, 21–34]. Fourth, the recent emergence of multidrug-resistant Candida species will complicate the selection of antifungal therapy in the immediate future [10, 12, 35–38].
The selection of any particular agent for the treatment of candidemia should take into account a history of recent azole or echinocandin exposure, a history of intolerance to an antifungal agent, the dominant Candida species and current susceptibility data in a particular clinical unit, severity of illness, relevant comorbidities, and evidence of involvement of the CNS, cardiac valves, and/or visceral organs. The risk of mortality among patients with candidemia ranges from 10% to 47% [6–8, 13], but the actual disease-associated mortality is more likely 10%–20%, with the risk of death being related to increasing age, higher Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, infecting Candida species, immunosuppressive agents, preexisting renal dysfunction, venous catheter retention, and antifungal selection [8, 19, 165–167]. Early initiation of effective antifungal therapy and source control is critical in the successful treatment of candidemia, as demonstrated by data suggesting significantly higher mortality rates among patients with candidemia in whom antifungal therapy was delayed or considered inadequate, and/or in whom source control was not promptly attained [14, 16–18, 168].
The echinocandins demonstrate significant fungicidal activity against most Candida species, and each of these agents has demonstrated success in approximately 70%–75% of patients in randomized, comparative clinical trials [24–28, 31, 32]. Despite the need for intravenous administration, their superb efficacy, favorable safety profile, limited drug interactions, and concerns about fluconazole resistance have led many experts to favor the echinocandins as initial therapy for most adult patients with candidemia. Few studies comparing different echinocandins have been performed [28, 169], but most experts agree that these agents are sufficiently similar to be considered interchangeable.
Only one study comparing an echinocandin to fluconazole has been performed, and the results from this study suggest a strong trend toward more favorable outcomes with anidulafungin compared with fluconazole as primary therapy for candidemia [27]. In a subanalysis of patients with C. albicans infections, there was a significant improvement in global response among those receiving anidulafungin [31]. In another subanalysis of critically ill patients from this trial, those receiving anidulafungin had significantly better responses at end of therapy compared with fluconazole-treated patients [170]. A combined analysis of 7 of the largest randomized clinical trials comparing treatment for candidemia and invasive candidiasis and involving almost 2000 patients found that initial therapy with an echinocandin was a significant predictor of survival [19]. This same analysis identified higher APACHE II score, older age, and infection with C. tropicalis to be associated with worse outcomes and higher mortality [19].
It has become common practice for clinicians treating patients with candidemia to initiate an echinocandin, then change to an oral azole (typically fluconazole) once the patient has become clinically stable [1]. A recent open-label noncomparative trial assessed outcomes of patients who were treated with anidulafungin for at least 5 days followed by step-down therapy to oral fluconazole or voriconazole (if the infecting organism was susceptible) when they were clinically stable and blood cultures had become negative [34]. There was no difference noted in outcomes among patients who continued on anidulafungin throughout the treatment course compared with those who were changed to an oral azole. Smaller pilot studies from Latin America and Asia demonstrated similar findings [33, 171]. Thus, on the basis of these data and other clinical trials [22, 23, 25, 26, 28, 33, 34, 171], the Expert Panel favors step-down therapy to fluconazole or voriconazole for patients who have improved clinically following initial therapy with an echinocandin, have documented clearance of Candida from the bloodstream, and who are infected with an organism that is susceptible to fluconazole (eg, C. albicans, C. parapsilosis, and C. tropicalis) or voriconazole (eg, C. krusei). This transition usually occurs within 5–7 days, but this time is variable and ultimately dependent on patient response and clinician preference.
In many parts of the world, based on success rates reported from well-designed clinical trials, fluconazole remains standard therapy for patients with candidemia [21–23, 27]. However, in light of recent data on the efficacy of echinocandins and increasing resistance to fluconazole, the Expert Panel believes that fluconazole should be considered first-line therapy only in patients who are hemodynamically stable, who have had no previous exposure to azoles, and who do not belong in a group at high risk for C. glabrata infection, including those who are elderly, have underlying malignancy, or are diabetic.
In previous iterations of these guidelines, the Expert Panel favored fluconazole over an echinocandin for treatment of candidemia due to C. parapsilosis based on reports of decreased in vitro activity of echinocandins against this species and of echinocandin resistance among some isolates [11, 12, 172–175]. In spite of these laboratory observations, there have been no clinical studies that have demonstrated superiority of fluconazole over the echinocandins for the treatment of C. parapsilosis infections. Moreover, recent observational data from Spain among almost 200 patients with candidemia due to C. parapsilosis suggested no difference in outcome among patients who received initial treatment with an echinocandin compared with those who received other regimens [176]. Any recommendation supporting fluconazole over an echinocandin is generally based on theoretical concerns rather than on observed therapeutic failure of the echinocandins in these patients.
Voriconazole was shown to be as effective for candidemia and invasive candidiasis as the comparator regimen of sequential therapy with AmB for 4–7 days followed by fluconazole [23]. Voriconazole possesses activity against most Candida species, including C. krusei [177, 178], but the need for more frequent administration, less predictable pharmacokinetics, more drug interactions, and poor tolerance to the drug make it less attractive for initial therapy. Parenteral voriconazole appears to be safe when administered to those with baseline renal dysfunction, despite concerns based on possible nephrotoxicity of its vehicle (sulfobutylether β-cyclodextrin) [70]. Voriconazole does not provide predictable activity against fluconazole-resistant C. glabrata [47, 177–179]. It does, however, fill an important niche for patients who have fluconazole-resistant isolates of C. krusei, C. guilliermondii, or C. glabrata that are voriconazole susceptible and who are ready for transition from an echinocandin or AmB to oral therapy.
There is little role for oral itraconazole for the treatment of candidemia, given the similar antifungal spectrum, ease of administration, superior pharmacokinetics, and better tolerability of fluconazole. Posaconazole has excellent in vitro activity against most Candida species. The extended-release tablet and the intravenous formulation could prove useful in the future, but currently there is no role for posaconazole in the treatment of candidemia. The broad-spectrum azole isavuconazole demonstrates similar in vitro activity against Candida species, as do voriconazole and posaconazole, and could prove useful in the future [180].
AmB has broad activity against all Candida species with the exception of C. lusitaniae, which is frequently resistant. Lipid formulations of AmB are preferred to AmB deoxycholate and should be considered when there is a history of intolerance to echinocandins and/or azoles, the infection is refractory to other therapy, the organism is resistant to other agents, or there is a suspicion of infection due to non-Candida yeasts, such as Cryptococcus neoformans or Histoplasma capsulatum. Liposomal AmB, 3 mg/kg daily, has been shown to be as effective as micafungin for treatment of candidemia [26].
The emergence of echinocandin-resistant and echinocandin-/azole-resistant Candida isolates, especially C. glabrata, clearly has been documented, and this finding appears to be associated with worse clinical outcomes [10, 12, 35–37, 181, 182]. Fluconazole resistance is a frequent finding among echinocandin-resistant isolates [9, 10], further complicating therapeutic choices. There are currently no prospective data to inform a decision, but the Expert Panel favors lipid formulation AmB for treatment of patients with candidemia due to proven or suspected fluconazole and echinocandin-resistant (multidrug resistant) strains until more data become available.
Recent data suggest that as many as 16% of patients with candidemia have some manifestation of ocular involvement, and some of these patients will develop severe, sight-threatening endophthalmitis [70]. Thus, for all patients with candidemia, the Expert Panel strongly advises a dilated funduscopic examination, preferably performed by an ophthalmologist, within the first week after initiation of specific antifungal therapy. Some groups have suggested that it is possible to stratify patients according to risk in an effort to avoid performing ophthalmologic examinations on all candidemic patients [183]. This approach is possibly more cost-effective than examining all patients with candidemia, but the potential benefit of early identification of endophthalmitis and prevention of visual loss far outweighs the expense of performing a dilated funduscopic examination.
Follow-up blood cultures every day or every other day until demonstration of clearance of Candida from the bloodstream are helpful to establish the appropriate duration of antifungal therapy. If there are no metastatic complications of candidemia, the duration of therapy with systemic antifungal agents should be 14 days following documented clearance of Candida species from the bloodstream and resolution of signs and symptoms attributable to infection. This recommendation is based on the results of several prospective, randomized trials in which this rule has been universally and successfully applied, and it is generally associated with few complications and relapses [21–23, 26–28, 30, 32–34].
II. Should Central Venous Catheters Be Removed in Nonneutropenic Patients With Candidemia?
Recommendation
13. CVCs should be removed as early as possible in the course of candidemia when the source is presumed to be the CVC and the catheter can be removed safely; this decision should be individualized for each patient (strong recommendation; moderate-quality evidence).
Evidence Summary
Central venous catheters and other intravascular devices are important risk factors in the development and persistence of candidemia in nonneutropenic patients [5, 7–9, 184]. A CVC is present in at least 70% of nonneutropenic patients with candidemia at the time that the diagnostic blood culture is obtained [5, 7–9, 170, 184–187]. The relationship of candidemia to CVCs has been assumed on the basis of observation, clinical experience, and an understanding of the role of biofilm in the genesis of bloodstream infections [188, 189]. That candidemia in nonneutropenic patients is commonly due to contaminated CVCs is undeniable, but there remains controversy as to how best to distinguish a catheter-associated candidemia from one that is related to another source, such as the gastrointestinal tract.
There have been no prospective clinical studies designed to examine CVC management as a primary measurement related to outcome. Moreover, several retrospective analyses have led to very different conclusions regarding the necessity and timing of CVC removal in the candidemic patient [19, 190–193]. Thus, the controversy continues, with some groups arguing for a strictly individualized approach to each patient [190] and others for an approach that removes CVCs in all nonneutropenic candidemic patients in whom it is safe and feasible to do so [19]. No prospective study has demonstrated a survival benefit to early CVC removal in patients who have candidemia, but most studies have demonstrated a shorter duration of candidemia and/or a trend toward improved outcomes [14, 21–23, 27, 28, 168, 192–200]. The recent combined analysis of 7 candidemia trials observed a survival benefit among those who underwent CVC removal at some time during treatment for candidemia [19]. The survival benefit applied to patients across all levels of severity of illness as determined by APACHE II scores.
The Expert Panel members strongly believe that CVCs should be removed if this can be performed safely when candidemia is documented in the nonneutropenic patient. It is intuitive that each patient with candidemia must be managed individually with respect to CVC removal or retention, but on balance, the bulk of data supports an approach that leads to early removal among nonneutropenic patients in whom the catheter is a likely source of infection.
Among neutropenic patients, the role of the gastrointestinal tract as a source for disseminated candidiasis is evident from autopsy studies, but in an individual patient, it is difficult to determine the relative contributions of the gastrointestinal tract vs the CVC as the primary source of candidemia [195, 201]. An exception is made for candidemia due to C. parapsilosis, which is very frequently associated with CVCs [188, 189, 200, 202]. A recent retrospective analysis that included mostly nonneutropenic patients underscored the influence of early CVC removal, specifically among patients with C. parapsilosis bloodstream infection, on clinical outcome [176].
III. What Is the Treatment for Candidemia in Neutropenic Patients?
Recommendations
14. An echinocandin (caspofungin: loading dose 70 mg, then 50 mg daily; micafungin: 100 mg daily; anidulafungin: loading dose 200 mg, then 100 mg daily) is recommended as initial therapy (strong recommendation; moderate-quality evidence).
15. Lipid formulation AmB, 3–5 mg/kg daily, is an effective but less attractive alternative because of the potential for toxicity (strong recommendation; moderate-quality evidence).
16. Fluconazole, 800-mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily, is an alternative for patients who are not critically ill and have had no prior azole exposure (weak recommendation; low-quality evidence).
17. Fluconazole, 400 mg (6 mg/kg) daily, can be used for step-down therapy during persistent neutropenia in clinically stable patients who have susceptible isolates and documented bloodstream clearance (weak recommendation; low-quality evidence).
18. Voriconazole, 400 mg (6 mg/kg) twice daily for 2 doses, then 200–300 mg (3–4 mg/kg) twice daily, can be used in situations in which additional mold coverage is desired (weak recommendation; low-quality evidence). Voriconazole can also be used as step-down therapy during neutropenia in clinically stable patients who have had documented bloodstream clearance and isolates that are susceptible to voriconazole (weak recommendation; low-quality evidence).
19. For infections due to C. krusei, an echinocandin, lipid formulation AmB, or voriconazole is recommended (strong recommendation; low-quality evidence).
20. Recommended minimum duration of therapy for candidemia without metastatic complications is 2 weeks after documented clearance of Candida from the bloodstream, provided neutropenia and symptoms attributable to candidemia have resolved (strong recommendation; low-quality evidence).
21. Ophthalmological findings of choroidal and vitreal infection are minimal until recovery from neutropenia; therefore, dilated funduscopic examinations should be performed within the first week after recovery from neutropenia (strong recommendation; low-quality evidence).
22. In the neutropenic patient, sources of candidiasis other than a CVC (eg, gastrointestinal tract) predominate. Catheter removal should be considered on an individual basis (strong recommendation; low-quality evidence).
23. Granulocyte colony-stimulating factor (G-CSF)–mobilized granulocyte transfusions can be considered in cases of persistent candidemia with anticipated protracted neutropenia (weak recommendation; low-quality evidence).
Evidence Summary
Candidemia that develops in neutropenic patients is a life-threatening infection associated with acute disseminated candidiasis, a sepsis-like syndrome, multiorgan failure, and death. Outcomes are particularly poor in people with protracted neutropenia, such as that which develops after induction therapy for hematologic malignancies [190, 203, 204]. Candidemia associated with C. tropicalis is associated with particularly poor outcomes in neutropenic hosts. Chronic disseminated candidiasis (hepatosplenic candidiasis) can ensue as a complication of candidemia in neutropenic patients, especially when patients with gastrointestinal tract mucositis do not receive antifungal prophylaxis. There are no adequately powered randomized controlled trials of treatment of candidemia in neutropenic patients. The data are largely derived from single-arm studies, small subsets of randomized controlled studies that have enrolled mostly nonneutropenic patients, and pooled outcomes from randomized trials [205, 206].
Historically, candidemia in neutropenic patients was treated with an AmB formulation. The availability of voriconazole and the echinocandins has led to greater use of these agents, but without compelling clinical data. The extensive use of fluconazole for prophylaxis to prevent invasive candidiasis in neutropenic patients and the lack of meaningful prospective data has led to a diminished therapeutic role for this agent among these patients, except for use as maintenance, or step-down therapy after organism species and susceptibilities are obtained in clinically stable patients [207].
The numbers of neutropenic patients included in candidemia treatment studies are small. In these trials, 50% of caspofungin recipients vs 40% of AmB deoxycholate recipients [25], 68% of micafungin recipients vs 61% of liposomal AmB recipients [26], and 69% of micafungin recipients vs 64% of caspofungin recipients [28] with neutropenia at onset of therapy were successfully treated. The randomized controlled trial of anidulafungin vs fluconazole enrolled too few neutropenic patients with candidemia to generate meaningful data regarding efficacy [27]. In 2 retrospective studies, successful outcomes for primary treatment of neutropenic patients were reported in 64% of those receiving AmB deoxycholate, 64% of those receiving fluconazole, and 68% of those receiving caspofungin [29, 208].
Additional insights can be gleaned from data derived from studies of empiric antifungal therapy involving febrile patients with neutropenia who had candidemia at baseline. In these studies, baseline candidemia was cleared in 73% of those treated with AmB deoxycholate vs 82% of those treated with liposomal AmB [209] and in 67% of those treated with caspofungin vs 50% of those treated with liposomal AmB [210]. Data from a large randomized trial also suggest that voriconazole is a reasonable choice for febrile patients with neutropenia and suspected invasive candidiasis for whom additional mold coverage is desired [211].
A systematic review was conducted to analyze available data generated in treatment trials and empiric therapy trials that enrolled neutropenic patients [205]. This included 17 trials that randomized 342 neutropenic patients with documented invasive candidiasis. Pooling of results favored use of nonpolyenes to AmB-containing comparators. Another pooled analysis that summarized results of treating with micafungin or comparators (liposomal AmB or caspofungin) for candidemia in the setting of malignancy-associated neutropenia from 2 randomized trials demonstrated success rates ranging from 53% to 85%, but no significant differences among treatment groups [206].
On the basis of these limited data, the success rates of antifungal therapy for candidemia in patients with neutropenia do not appear to be substantially different from those reported in the large randomized trials of nonneutropenic patients. However, conclusions may be limited by significant enrollment bias of selected patients. Although these data do not suggest less favorable outcomes associated with fluconazole and voriconazole, many experts prefer lipid formulation AmB or an echinocandin, which are fungicidal, as first-line agents. Similar to the approach in nonneutropenic patients, the recommended duration of therapy for candidemia in neutropenic patients is for 14 days after resolution of attributable signs and symptoms and clearance of the bloodstream of Candida species, provided that there has been recovery from neutropenia. When neutropenia is protracted, an antifungal drug should be continued until engraftment. This recommendation is based on limited data from prospective randomized trials and has been associated with few complications and relapses [209, 210].
The management of intravascular catheters in neutropenic patients with candidemia is less straightforward than in their nonneutropenic counterparts. Distinguishing gut-associated from vascular catheter–associated candidemia can be difficult in these patients [201]. The data for catheter removal are less compelling, and catheter removal often creates significant intravenous access problems. An analysis of 842 patients enrolled in 2 phase 3 treatment trials failed to demonstrate significant clinical benefits of catheter removal in multivariable analyses that adjusted for other measures of prognostic significance [190]. The Expert Panel suggests that catheter removal should be considered on an individual basis, taking into account feasibility and risk of removal.
An extremely important factor influencing the outcome of candidemia in neutropenic patients is the recovery of neutrophils during therapy. In multiple cohort studies of patients with cancer who had candidemia, and pooled analyses of randomized trials, persistent neutropenia was associated with a greater chance of treatment failure [190, 203, 204, 212]. This has led to improvement of strategies to harvest granulocytes from donors (including community volunteers), using G-CSF mobilization, which has been shown to be safe and feasible [213]. Analysis of subsets of people within phase 1/2 granulocyte infusion studies, retrospective observations, and small cohort studies suggest that G-CSF–mobilized granulocyte transfusions may be of benefit in patients with persistent candidemia and prolonged neutropenia [213–215]. In a randomized controlled trial, granulocyte infusions were associated with few toxicities, but small numbers of patients in infection subgroups limited conclusions of efficacy [216]. The panel recommends consideration of granulocyte infusions in select situations, when such technology is feasible.
IV. What Is the Treatment for Chronic Disseminated (Hepatosplenic) Candidiasis?
Recommendations
24. Initial therapy with lipid formulation AmB, 3–5 mg/kg daily OR an echinocandin (micafungin: 100 mg daily; caspofungin: 70-mg loading dose, then 50 mg daily; or anidulafungin: 200-mg loading dose, then 100 mg daily), for several weeks is recommended, followed by oral fluconazole, 400 mg (6 mg/kg) daily, for patients who are unlikely to have a fluconazole-resistant isolate (strong recommendation; low-quality evidence).
25. Therapy should continue until lesions resolve on repeat imaging, which is usually several months. Premature discontinuation of antifungal therapy can lead to relapse (strong recommendation; low-quality evidence).
26. If chemotherapy or hematopoietic cell transplantation is required, it should not be delayed because of the presence of chronic disseminated candidiasis, and antifungal therapy should be continued throughout the period of high risk to prevent relapse (strong recommendation; low-quality evidence).
27. For patients who have debilitating persistent fevers, short term (1–2 weeks) treatment with nonsteroidal anti-inflammatory drugs or corticosteroids can be considered (weak recommendation; low-quality evidence).
Evidence Summary
Chronic disseminated candidiasis is an uncommon syndrome seen almost entirely in patients who have hematologic malignancies and who have just recovered from neutropenia [217–219]. Candida albicans is the species most commonly isolated, but C. tropicalis, C. glabrata, C. krusei, and other Candida species also have been implicated. Fever, right upper quadrant discomfort, nausea, and elevation of liver enzymes occur following return of neutrophils and persist for months unless treatment is initiated. Contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography-CT (PET-CT), and sometimes ultrasound have all been shown to be useful for diagnosis and for follow-up [217, 218, 220, 221]. Biopsy of lesions may reveal budding yeasts and hyphae, but organisms may not be seen on biopsy specimens and often do not grow in culture, leading some to suggest that chronic disseminated candidiasis represents an immune reconstitution syndrome [219].
Approaches to the treatment of chronic disseminated candidiasis are based on anecdotal case reports and open-label series. Early experience with AmB was discouraging; as many as one-third of patients died within 3 months with active infection, and the overall mortality was 74% [222]. With the use of newer antifungal agents, mortality has decreased to 21% overall and is highly linked to relapse of leukemia [223]. Lipid formulations of AmB have proved more efficacious, perhaps related to better tissue concentrations [217, 218, 224, 225]. Fluconazole alone or following AmB induction has been shown to be effective [226, 227]. Increasingly, patients are receiving fluconazole prophylaxis, and thus have an increased risk of developing infection with a fluconazole-resistant organism. In this population, a broader-spectrum azole or an echinocandin is more appropriate therapy. Only a few reports note experience with voriconazole or posaconazole for this condition, but echinocandins are increasingly used to treat this infection [219, 223, 228–231].
Antifungal therapy should be given until all lesions have resolved radiographically in order to prevent relapse. MRI or PET-CT appear to be the most sensitive follow-up modalities, but are expensive [220, 221]; standard contrast-enhanced CT is less expensive and is adequate for follow-up. Additional chemotherapy and hematopoietic cell transplant should be pursued when clinically appropriate and not delayed because of candidiasis. However, antifungal therapy must be continued during the period of immunosuppression to prevent relapse of infection [219, 223, 228–231].
There is evidence that this syndrome could possibly be a form of immune reconstitution and that corticosteroids or anti-inflammatory agents might have a role in selected patients. Several investigators have reported rapid defervescence and improvement in liver enzyme tests when corticosteroids have been given in conjunction with antifungal agents [219, 223, 232, 233]. The dosage of corticosteroids has generally been 0.5–1 mg/kg daily of oral prednisone. The duration of steroid treatment, although highly variable, in most cases has been several weeks, given as a tapering dose [232, 233]. However, the role of corticosteroids in this disease is still not clear.
V. What Is the Role of Empiric Treatment for Suspected Invasive Candidiasis in Nonneutropenic Patients in the Intensive Care Unit?
Recommendations
28. Empiric antifungal therapy should be considered in critically ill patients with risk factors for invasive candidiasis and no other known cause of fever and should be based on clinical assessment of risk factors, surrogate markers for invasive candidiasis, and/or culture data from nonsterile sites (strong recommendation; moderate-quality evidence). Empiric antifungal therapy should be started as soon as possible in patients who have the above risk factors and who have clinical signs of septic shock (strong recommendation; moderate-quality evidence).
29. Preferred empiric therapy for suspected candidiasis in nonneutropenic patients in the ICU is an echinocandin (caspofungin: loading dose of 70 mg, then 50 mg daily; micafungin: 100 mg daily; anidulafungin: loading dose of 200 mg, then 100 mg daily) (strong recommendation; moderate-quality evidence).
30. Fluconazole, 800-mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily, is an acceptable alternative for patients who have had no recent azole exposure and are not colonized with azole-resistant Candida species (strong recommendation; moderate-quality evidence).
31. Lipid formulation AmB, 3–5 mg/kg daily, is an alternative if there is intolerance to other antifungal agents (strong recommendation; low-quality evidence).
32. Recommended duration of empiric therapy for suspected invasive candidiasis in those patients who improve is 2 weeks, the same as for treatment of documented candidemia (weak recommendation; low-quality evidence).
33. For patients who have no clinical response to empiric antifungal therapy at 4–5 days and who do not have subsequent evidence of invasive candidiasis after the start of empiric therapy or have a negative non-culture-based diagnostic assay with a high negative predictive value, consideration should be given to stopping antifungal therapy (strong recommendation; low-quality evidence).
Evidence Summary
Candida species are an increasing cause of invasive infection in nonneutropenic patients in the ICU; half to two-thirds of all episodes of candidemia occur in an ICU [5, 14, 167, 170, 234]. Candida bloodstream infections are associated with increased ICU and hospital stay [129, 235]. Most estimates of attributable mortality rates for invasive candidiasis in this setting are 30%–40% [167, 170]. In those patients who have septic shock due to Candida species and who do not have adequate source control or antifungal therapy begun within 24 hours, the mortality approaches 100% [14]. Prompt initiation of appropriate antifungal therapy has been associated with as much as a 50% reduction in mortality [14, 17, 18, 236]. Prompt and appropriate antifungal therapy is often delayed because of the relative insensitivity of blood cultures, the time needed for blood cultures to yield growth, the possibility of negative blood cultures with invasive abdominal candidiasis, and the lack of specific clinical signs and symptoms. Strategies for initiating empiric antifungal therapy include an evaluation of risk factors and use of surrogate markers.
Optimal utilization of risk factors and colonization status to derive clinical scoring systems and the interpretation of non-culture-based diagnostic tests to identify patients with invasive candidiasis to initiate early empiric antifungal therapy have been the subjects of many investigations. Retrospective and single-center studies have yielded conflicting results, depending on unique patient populations. Well-designed prospective clinical trials in this area have been difficult to perform, and many unanswered questions remain.
Risk factors for development of invasive candidiasis include Candida colonization, severity of illness, exposure to broad-spectrum antibiotics, recent major surgery, particularly abdominal surgery, necrotizing pancreatitis, dialysis, parenteral nutrition, corticosteroids, and the use of CVCs [237, 238]. Empiric therapy based solely on colonization with Candida species appears inadequate [16, 239]. Prospective studies evaluating the extent of Candida colonization with scores or indices have not been shown to change management, and they are labor intensive and expensive [234].
Several studies have looked at prediction models to identify patients at highest risk. These studies are characterized by high specificity, but low sensitivity, thus missing many patients with candidiasis [240–242]. A subset of postsurgical patients, particularly those with recurrent gastrointestinal perforation, anastomotic leaks, or acute necrotizing pancreatitis may be at uniquely high risk for candidiasis [238, 240, 243, 244]. The most important combination of factors in an individual patient has not been established.
Surrogate markers that have been evaluated in the ICU setting include β-D-glucan, mannan-antimannan antibodies, and PCR testing. β-D-glucan appears to be more sensitive than Candida colonization scores or indices, but appears to have low positive predictive value [245–248]. False-positive results are a problem, as noted in the Background section. The optimal timing and number of samples is unknown. In a recent prophylaxis trial of high-risk ICU patients, β-D-glucan testing performed twice weekly identified 87% of patients with proven candidiasis [249]. Small studies basing preemptive therapy on β-D-glucan testing suggest that the high negative predictive value of this test could be useful in excluding invasive candidiasis in the ICU setting [151, 248, 250–252].
Combined mannan-antimannan testing has variable sensitivity and specificity [142, 253]. Real-time PCR appears to have similar sensitivity to β-D-glucan for the diagnosis of candidemia, but may be more sensitive for the diagnosis of other forms of invasive candidiasis [132]. Tests using magnetic biosensor technology for the rapid detection of Candida species from whole-blood samples (T2 Biosystems) are also promising [163]. Recommendations for the clinical use of these tests are challenging without robust data in the at-risk ICU population.
Limited clinical studies have evaluated the efficacy of empiric strategies. Retrospective studies indicate potential for higher survival when empiric antifungal therapy is given to high-risk patients [254]. Prospective clinical trials of empiric antifungal therapy in the ICU are difficult to conduct and have yielded conflicting results. Selected older studies, including those in specific patient populations, such as those with prior gastrointestinal surgery or bowel perforation, demonstrated potential benefit [255, 256]. In a randomized clinical trial of ICU patients at risk for invasive candidiasis and with unexplained fever, empiric fluconazole (800 mg daily for 14 days) was not associated with better outcomes when compared with placebo [257]. A recent study comparing caspofungin to placebo among ICU patients with signs of infection, Candida colonization, and clinical risk factors for invasive candidiasis was stopped prematurely due to poor patient accrual, confirming the difficulty in conducting these trials [249].
Widespread use of antifungal agents must be balanced against the cost, the risk of toxicity, and the emergence of resistance. None of the existing clinical trials have been adequately powered to assess the risk of the emergence of azole or echinocandin resistance. Empiric antifungal therapy should be considered in critically ill patients with risk factors for invasive candidiasis and no other known cause of fever. Preference should be given to an echinocandin in hemodynamically unstable patients, those previously exposed to an azole, and in those colonized with azole-resistant Candida species. Fluconazole may be considered in hemodynamically stable patients who are colonized with azole-susceptible Candida species or who have no prior exposure to azoles. There are no data guiding the appropriate duration of empiric antifungal therapy among patients who have a clinical response to therapy, but it is logical that it should not differ from the treatment of documented candidemia. Conversely, therapy can be stopped after several days in the absence of clinical response if cultures and surrogate markers are negative.
VI. Should Prophylaxis Be Used to Prevent Invasive Candidiasis in the ICU Setting?
Recommendations
34. Fluconazole, 800-mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily, could be used in high-risk patients in adult ICUs with a high rate (>5%) of invasive candidiasis (weak recommendation; moderate-quality evidence).
35. An alternative is to give an echinocandin (caspofungin: 70-mg loading dose, then 50 mg daily; anidulafungin: 200-mg loading dose and then 100 mg daily; or micafungin: 100 mg daily) (weak recommendation; low-quality evidence).
36. Daily bathing of ICU patients with chlorhexidine, which has been shown to decrease the incidence of bloodstream infections including candidemia, could be considered (weak recommendation; moderate-quality evidence).
Evidence Summary
Time to appropriate therapy in candidemia appears to have a significant impact on the outcome of patients with this infection [14, 17, 18]. However, insensitivity and significant delays using culture techniques, as well as limitations of rapid diagnostic tests, remain for this common cause of bloodstream infection among patients in the ICU [258, 259]. A safe and effective prophylactic strategy to prevent candidemia among high-risk patients could be of great benefit [260]. The approach to prophylaxis has been either broad, in which all patients within the ICU setting are treated [261, 262], or selective, in which only specific high-risk groups of patients are targeted for prophylaxis [249, 263, 264].
For ICUs that show very high rates of invasive candidiasis, in excess of the expected rates of <5% of patients, antifungal prophylaxis may be warranted in selected patients who are at highest risk [260]. Two randomized, placebo-controlled trials have shown a reduction in the incidence of invasive candidiasis in single units or single hospitals when fluconazole prophylaxis was used broadly in the ICU; one study targeted all patients in a surgical ICU [262] and, in the other, all patients receiving mechanical ventilation [261]. In both studies, Candida urinary tract infections, as well as invasive candidiasis and candidemia, were included as endpoints.
In a blinded placebo-controlled trial that enrolled a small number of patients, fluconazole prophylaxis was shown to decrease Candida intra-abdominal infections in high-risk patients in the surgical ICU [263]. A noncomparative, open-label trial using caspofungin prophylaxis in a small number of similar high-risk surgical patients also showed benefit [264]. A recent multicenter placebo-controlled, blinded clinical trial of caspofungin prophylaxis targeting only those ICU patients who met specific criteria for high risk for invasive candidiasis showed a trend toward reduction of invasive candidiasis, but was limited by the sample size [249].
Several meta-analyses have assessed the issue of fluconazole prophylaxis in ICU patients [265–268]. Not surprisingly, there were methodological differences among the studies, and there was variation among the study populations. All 4 meta-analyses showed that fluconazole prophylaxis was associated with a reduction in invasive candidiasis, but only 2 showed a reduction in candidemia [267, 268]. Importantly, only one analysis showed a reduction in mortality from invasive candidiasis [268]. None of the meta-analyses assessed the issues of adverse effects of antifungal agents, the emergence of resistance to fluconazole, or major ecological shifts in Candida species, topics of great importance in the ICU setting. A Cochrane analysis confirmed the importance of focusing prophylactic efforts on high-risk patients, noting that the number needed to treat to prevent one case of invasive candidiasis in the ICU setting varied from 9 in high-risk patients to 188 in low-risk patients [269].
Few data exist on risk factors for candidemia in pediatric intensive care unit (PICU) patients. A population-based, case-control study conducted in a large tertiary care pediatric center found an incidence of candidemia of 3.5 per 1000 PICU admissions [270]. The presence of a CVC, a diagnosis of malignancy, and receipt of either vancomycin or an antianaerobic antimicrobial agent for >3 days were independently associated with the development of candidemia. Children who had ≥3 of these risk factors in different combinations had a predicted probability of developing candidemia of between 10% and 46%.
Data are accruing on the use of skin decolonization with antiseptic agents in the ICU to decrease bloodstream infections, including those caused by Candida species [271–274]. Several multicenter randomized clinical trials have shown that daily bathing of ICU patients with chlorhexidine decreases the incidence of both catheter-associated and non-catheter-associated hospital-acquired bloodstream infections [271–273]. These studies were aimed primarily at evaluating the impact on multidrug-resistant bacterial infections and provide few data on Candida infections. However, at least one of these trials found a significant reduction in catheter associated Candida bloodstream infections [272]. A meta-analysis on the effects of daily chlorhexidine bathing included 10 studies performed in an ICU setting, only one of which was a randomized controlled trial. The conclusion was that chlorhexidine bathing reduced the incidence of bloodstream infections, including catheter-associated bacterial infections [274]. Although not proven to prevent candidemia, there is little risk to the use of chlorhexidine in ICU patients, and this practice may prove beneficial.
VII. What Is the Treatment for Neonatal Candidiasis, Including Central Nervous System Infection?
What Is the Treatment for Neonatal Invasive Candidiasis and Candidemia?
Recommendations
37. AmB deoxycholate, 1 mg/kg daily, is recommended for neonates with disseminated candidiasis (strong recommendation; moderate-quality evidence).
38. Fluconazole, 12 mg/kg intravenous or oral daily, is a reasonable alternative in patients who have not been on fluconazole prophylaxis (strong recommendation; moderate-quality evidence).
39. Lipid formulation AmB, 3–5 mg/kg daily, is an alternative, but should be used with caution, particularly in the presence of urinary tract involvement (weak recommendation; low-quality evidence).
40. Echinocandins should be used with caution and generally limited to salvage therapy or to situations in which resistance or toxicity preclude the use of AmB deoxycholate or fluconazole (weak recommendation; low-quality evidence).
41. A lumbar puncture and a dilated retinal examination are recommended in neonates with cultures positive for Candida species from blood and/or urine (strong recommendation; low-quality evidence).
42. CT or ultrasound imaging of the genitourinary tract, liver, and spleen should be performed if blood cultures are persistently positive for Candida species (strong recommendation; low-quality evidence).
43. CVC removal is strongly recommended (strong recommendation; moderate-quality evidence).
44. The recommended duration of therapy for candidemia without obvious metastatic complications is for 2 weeks after documented clearance of Candida species from the bloodstream and resolution of signs attributable to candidemia (strong recommendation; low-quality evidence).
Evidence Summary
Neonatal candidiasis occurs predominately in the neonatal intensive care unit (NICU). Candida species are the third most common pathogen associated with bloodstream infection in NICUs in the United States [275]. However, the incidence of neonatal candidiasis has decreased dramatically over the past decade [276–278]. Neonatal candidiasis is associated with significant risk of death, neurodevelopmental impairment in extremely low-birth-weight infants who weigh ≤1000 g, and increased healthcare costs [279–284]. The primary risk factor for neonatal candidiasis is prematurity with those neonates who have an extremely low birth weight at greatest risk. These infants are at high risk to have CNS involvement as a complication of candidemia [285, 286]. Candida albicans and C. parapsilosis account for 80%–90% of neonatal invasive candidiasis [278, 287].
Neonatal candidiasis differs from invasive disease in older patients in that neonates are more likely to present with nonspecific or subtle signs and symptoms of infection. Candida species invade virtually all tissues, including the retina, brain, heart, lung, liver, spleen, and joints [288]. Endocarditis is an uncommon complication of candidiasis in neonates. Although meningitis is seen frequently in association with candidemia, approximately half of neonates with Candida meningitis do not have a positive blood culture [285]. CNS disease in the neonate typically manifests as meningoencephalitis and should be assumed to be present in the neonate who has candidemia and signs and symptoms suggesting meningoencephalitis, as CSF findings of Candida infection may be unreliable. Neurodevelopmental impairment is common in survivors; therefore, careful follow-up of neurodevelopmental parameters is important [279, 281, 282, 284].
Recent studies have highlighted the significance of candiduria in the absence of candidemia in this population [281]. Extremely low-birth-weight infants with candiduria are at a substantial risk of death or neurodevelopmental impairment. Candiduria in this population should prompt an evaluation (blood cultures, lumbar puncture, and abdominal ultrasound) for disseminated Candida infection and warrants treatment.
The recommendation to treat neonatal candidiasis with AmB deoxycholate or fluconazole is based on small, single-center trials and 2 multicenter cohort studies [279, 289–291]. In contrast to adults and older children, AmB deoxycholate is well tolerated in neonates and does not seem to be associated with a high risk for nephrotoxicity. A recent comparative effectiveness study found that neonates treated with AmB lipid formulations had higher mortality than infants treated with AmB deoxycholate or fluconazole [291]. The difference in outcomes seen with lipid AmB formulations may be related to inadequate penetration of these drugs into the kidneys, inadequate dosing for premature neonates, or unknown confounders. Based on the current evidence, fluconazole and AmB deoxycholate are acceptable choices for therapy, and lipid formulations of AmB should be used with caution. There are few data on the use of echinocandins in neonates. There are concerns with echinocandins because concentrations in the CNS and in the urinary tract are low.
Dosing of antifungal agents is substantially different for neonates than it is for older children and adults. Limited pharmacokinetic data exist regarding dosing of AmB deoxycholate in neonates, and the pharmacokinetics appear to be highly variable in this population [96, 97, 101]. The recommended dose of 1 mg/kg daily results in higher estimates of clearance in infants compared with older children and may partially explain why the drug is better tolerated in neonates [98]. The duration of therapy is based primarily on adult and pediatric data, and there are no data to guide duration specifically in neonates.
Population pharmacokinetic studies have provided dosing information for fluconazole in the neonatal population [105, 292]. Based on these studies, fluconazole, 12/mg/kg daily, can be used to treat neonatal candidiasis. More recent data suggest that a loading dose of fluconazole of 25 mg/kg achieves the therapeutic target more rapidly than traditional dosing [292]. However, further studies of this dosing scheme are required before it can be recommended. Failure to promptly remove or replace CVCs in infants with candidemia places the infant at increased risk of prolonged infection, mortality, and long-term irreversible neurodevelopmental impairment [198, 279]. Removal and replacement of the catheter at an anatomically distinct site should be performed unless contraindicated.
What Is the Treatment for Central Nervous System Infections in Neonates?
Recommendations
45. For initial treatment, AmB deoxycholate, 1 mg/kg intravenous daily, is recommended (strong recommendation; low-quality evidence).
46. An alternative regimen is liposomal AmB, 5 mg/kg daily (strong recommendation; low-quality evidence).
47. The addition of flucytosine, 25 mg/kg 4 times daily, may be considered as salvage therapy in patients who have not had a clinical response to initial AmB therapy, but adverse effects are frequent (weak recommendation; low-quality evidence).
48. For step-down treatment after the patient has responded to initial treatment, fluconazole, 12 mg/kg daily, is recommended for isolates that are susceptible to fluconazole (strong recommendation; low-quality evidence).
49. Therapy should continue until all signs, symptoms, and CSF and radiological abnormalities, if present, have resolved (strong recommendation; low-quality evidence).
50. Infected CNS devices, including ventriculostomy drains and shunts, should be removed if at all possible (strong recommendation; low-quality evidence).
Evidence Summary
There are limited data to guide therapy for CNS Candida infections in the neonate. All AmB preparations, including the lipid formulations, penetrate the CNS and have fungicidal activity in the CNS [44]. AmB deoxycholate and liposomal AmB were found to have greater antifungal efficacy when studied in a rabbit model of Candida meningoencephalitis compared with the other formulations [44]. The clinician must weigh the benefits and drawbacks of using liposomal AmB with its good CSF penetration but poor urine levels vs using AmB deoxycholate with less good CSF levels but better urine levels.
The benefit of adding flucytosine for neonates with CNS candidiasis is uncertain. In the largest prospective study evaluating treatment outcomes of CNS candidiasis in neonates, the median time to clear CSF was longer for those who received flucytosine plus AmB deoxycholate (17.5 days; 6 infants), compared with those who received only AmB deoxycholate (6 days; 18 infants) [279]. In addition, flucytosine is poorly tolerated, and gastrointestinal side effects may hinder oral feeding in neonates. In general, flucytosine is used only in neonates who have not responded to AmB alone.
Data supporting the use of echinocandins in neonates are emerging; however, several key issues require further clarification. The optimal dose of echinocandins in neonates remains uncertain [109, 284, 293–297]. Furthermore, there are concerns regarding the penetration of echinocandins into the CSF. Echinocandins appear to penetrate brain tissue, but not the CSF, and achieve concentrations in brain shown to be effective in animal models when dosages higher than those recommended for humans have been used [298, 299]. Limited clinical data suggest that the echinocandins may be effective for the treatment of CNS infections in neonates, but are not adequate to recommend their use at this time [293].
What Are the Recommendations for Prophylaxis in the Neonatal Intensive Care Unit Setting?
Recommendations
51. In nurseries with high rates (>10%) of invasive candidiasis, intravenous or oral fluconazole prophylaxis, 3–6 mg/kg twice weekly for 6 weeks, in neonates with birth weights <1000 g is recommended (strong recommendation; high-quality evidence).
52. Oral nystatin, 100 000 units 3 times daily for 6 weeks, is an alternative to fluconazole in neonates with birth weights <1500 g in situations in which availability or resistance preclude the use of fluconazole (weak recommendation; moderate-quality evidence).
53. Oral bovine lactoferrin (100 mg/day) may be effective in neonates <1500 g but is not currently available in US hospitals (weak recommendation; moderate-quality evidence).
Evidence Summary
Numerous studies examining fluconazole prophylaxis for the prevention of invasive candidiasis in neonates have consistently demonstrated efficacy and possibly reduced mortality [300–310]. Fluconazole, 3 mg/kg or 6 mg/kg twice weekly, significantly reduced rates of invasive candidiasis in premature neonates weighing <1000 g in nurseries with a very high incidence of Candida infections [300, 302]. A 2007 Cochrane review of clinical trials of fluconazole prophylaxis demonstrated efficacy, with a typical relative risk of 0.23 and number needed to treat of 9. The number needed to treat varied substantially depending on the incidence of invasive candidiasis in a particular ICU. The majority of studies have demonstrated the safety of fluconazole prophylaxis and lack of emergence of resistance.
Enteral or orally administered nystatin has been shown to be effective in reducing invasive candidiasis in preterm infants [303, 311–313]. In one study, nystatin prophylaxis was also associated with a reduction in all-cause mortality [313]. However, there remains a paucity of data on nystatin prophylaxis in infants <750 grams (the group at highest risk), and nystatin may not always be able to be administered when there is an ileus, gastrointestinal disease, feeding intolerance, or hemodynamic instability. These clinical situations are very common in low-gestational-age premature infants and limit the broad applicability of nystatin prophylaxis as a preventive strategy.
Lactoferrin is a mammalian milk glycoprotein involved in innate immunity. In a randomized trial of bovine lactoferrin in infants <1500 g, the incidence of late-onset sepsis was significantly lower in the lactoferrin group than in the placebo group [314]. A secondary analysis of the clinical trial showed that lactoferrin also reduced the incidence of invasive fungal infections compared with placebo [314]. Further confirmation of the efficacy and safety of oral bovine lactoferrin for the prevention of invasive candidiasis is needed, especially in infants <750 g, because there were only a few neonates in this category in this trial.
VIII. What Is the Treatment for Intra-abdominal Candidiasis?
Recommendations
54. Empiric antifungal therapy should be considered for patients with clinical evidence of intra-abdominal infection and significant risk factors for candidiasis, including recent abdominal surgery, anastomotic leaks, or necrotizing pancreatitis (strong recommendation; moderate-quality evidence).
55. Treatment of intra-abdominal candidiasis should include source control, with appropriate drainage and/or debridement (strong recommendation; moderate-quality evidence).
56. The choice of antifungal therapy is the same as for the treatment of candidemia or empiric therapy for nonneutropenic patients in the ICU (See sections I and V) (strong recommendation; moderate-quality evidence).
57. The duration of therapy should be determined by adequacy of source control and clinical response (strong recommendation; low-quality evidence).
Evidence Summary
Intra-abdominal candidiasis in patients who have had recent abdominal surgery or intra-abdominal events refers to a heterogeneous group of infections that includes peritonitis, abdominal abscess, and purulent or necrotic infection at sites of gastrointestinal perforation or anastomotic leak. Up to 40% of patients with secondary or tertiary peritonitis, as defined by a multinational consensus panel, may develop intra-abdominal candidiasis with a high mortality rate [243, 244, 315, 316]. A subset of postsurgical patients, particularly those with recurrent gastroduodenal perforation, anastomotic leaks, or acute necrotizing pancreatitis, are at uniquely high risk for invasive candidiasis [243, 244, 263, 316–320]. In other settings, such as perforated appendicitis, invasive candidiasis appears to be a rare complication [316, 319]. Infections are often polymicrobial, with yeast noted in as high as 20% of all cases and 40% in patients with a recent gastroduodenal perforation [319, 320].
Diagnosis is hampered by the lack of specific clinical signs and symptoms. Blood cultures are often negative [321]. A laboratory report of yeast isolated from an abdominal specimen must be evaluated to distinguish between contamination, colonization, and invasive infection. Swabs of superficial wounds and specimens taken from intra-abdominal catheters that have been in place for >24 hours do not provide useful information and should not be performed. In contrast, the presence of yeast obtained from normally sterile intra-abdominal specimens (operative room specimens, and/or drains that have been placed within 24 hours) in patients with clinical evidence for infection should be considered indicative of intra-abdominal candidiasis.
The role of surrogate markers and Candida risk scores in this setting has not been established. There are limited data on the utility of using β-D-glucan in postsurgical patients with suspected intra-abdominal candidiasis. In one study, β-D-glucan had a 72% positive predictive value and an 80% negative predictive value for distinguishing colonization from intra-abdominal invasive candidiasis and performed better than Candida colonization scores or indices [143].
Clinical evidence for the use of antifungal therapy for patients with suspected intra-abdominal invasive candidiasis is limited. Most studies are small, uncontrolled, single-center, or performed in specific populations. Patients who have Candida species isolated from normally sterile abdominal cultures or drains placed within 24 hours and who have clinical evidence of infection should be treated for intra-abdominal candidiasis. Patients who have had gastroduodenal perforations, anastomotic leaks, necrotizing pancreatitis, or other intra-abdominal events without the isolation of Candida species and who are doing poorly despite treatment for bacterial infections may benefit from empiric antifungal therapy. Several meta-analyses of antifungal prophylaxis in high-risk surgical ICU patients have yielded conflicting results [265–268].
Source control with adequate drainage and/or debridement is an important part of therapy of intra-abdominal candidiasis [14]. The choice of antifungal agent should be guided by the Candida species isolated and knowledge of the local epidemiology, including antifungal susceptibility patterns. Duration of antifungal therapy should be guided by clinical response and the adequacy of source control.
IX. Does the Isolation of Candida Species From the Respiratory Tract Require Antifungal Therapy?
Recommendation
58. Growth of Candida from respiratory secretions usually indicates colonization and rarely requires treatment with antifungal therapy (strong recommendation; moderate-quality evidence).
Evidence Summary
The isolation of Candida species from the respiratory tract is commonly encountered among patients who are in the ICU and are intubated or have a chronic tracheostomy. This almost always reflects colonization of the airways and not infection. Candida pneumonia and lung abscess are very uncommon [322, 323]. Only rarely after aspiration of oropharyngeal material has primary Candida pneumonia or abscess been documented [324, 325]. Pneumonia due to Candida species is generally limited to severely immunocompromised patients who develop infection following hematogenous spread to the lungs. CT scan of the thorax usually shows multiple pulmonary nodules. Isolation of Candida species from respiratory samples in a patient who is severely immunosuppressed should trigger a search for evidence of invasive candidiasis.
Although the diagnosis of Candida pneumonia is supported by isolation of the organism from a bronchoalveolar lavage (BAL) specimen, a firm diagnosis requires histopathological evidence of invasive disease. Multiple prospective and retrospective autopsy studies consistently demonstrate the poor predictive value of the growth of Candida from respiratory secretions, including BAL fluid [326–328]. In one prospective study, none of 77 patients who died in an ICU and who had clinical and radiologic evidence of pneumonia and a positive culture for Candida species from BAL or sputum demonstrated evidence of Candida pneumonia at autopsy [328]. Because of the rarity of Candida pneumonia, the extremely common finding of Candida in respiratory secretions, and the lack of specificity of this finding [329–331], a decision to initiate antifungal therapy should not be made on the basis of respiratory tract culture results alone.
Recent observations suggest that colonization of the airway with Candida species is associated with the development of bacterial colonization and pneumonia [332–336]. Candida airway colonization was also associated with worse clinical outcomes and higher mortality in these studies. However, it is not clear if Candida airway colonization has a causal relationship to poorer outcomes or is simply a marker of disease severity.
X. What Is the Treatment for Candida Intravascular Infections, Including Endocarditis and Infections of Implantable Cardiac Devices?
What Is the Treatment for Candida Endocarditis?
Recommendations
59. For native valve endocarditis, lipid formulation AmB, 3–5 mg/kg daily, with or without flucytosine, 25 mg/kg 4 times daily, OR high-dose echinocandin (caspofungin 150 mg daily, micafungin 150 mg daily, or anidulafungin 200 mg daily) is recommended for initial therapy (strong recommendation; low-quality evidence).
60. Step-down therapy to fluconazole, 400–800 mg (6–12 mg/kg) daily, is recommended for patients who have fluconazole-susceptible Candida isolates, have demonstrated clinical stability, and have cleared Candida from the bloodstream (strong recommendation; low-quality evidence).
61. Oral voriconazole, 200–300 mg (3–4 mg/kg) twice daily, or posaconazole tablets, 300 mg daily, can be used as step-down therapy for isolates that are susceptible to those agents but not susceptible to fluconazole (weak recommendation; very low-quality evidence).
62. Valve replacement is recommended; treatment should continue for at least 6 weeks after surgery and for a longer duration in patients with perivalvular abscesses and other complications (strong recommendation; low-quality evidence).
63. For patients who cannot undergo valve replacement, long-term suppression with fluconazole, 400–800 mg (6–12 mg/kg) daily, if the isolate is susceptible, is recommended (strong recommendation; low-quality evidence).
64. For prosthetic valve endocarditis, the same antifungal regimens suggested for native valve endocarditis are recommended (strong recommendation; low-quality evidence). Chronic suppressive antifungal therapy with fluconazole, 400–800 mg (6–12 mg/kg) daily, is recommended to prevent recurrence (strong recommendation; low-quality evidence).
Evidence Summary
The incidence of Candida endocarditis has increased concurrent with the general increase in Candida infections [337]. Endocarditis should be suspected when blood cultures are persistently positive, when a patient with candidemia has persistent fever despite appropriate treatment, or when a new heart murmur, heart failure, or embolic phenomena occur in the setting of candidemia [338]. Most cases occur following cardiac valvular surgery, but other risk factors include injection drug use, cancer chemotherapy, prolonged presence of CVCs, and prior bacterial endocarditis. The signs, symptoms, and complications are generally similar to those of bacterial endocarditis, except for the frequent occurrence of large emboli to major vessels. Cases are fairly evenly divided between C. albicans and non-albicans Candida species [339].
Medical therapy of Candida endocarditis has occasionally been curative [340–348], but the optimum therapy for both native and prosthetic valve endocarditis in adults is a combination of valve replacement and a long course of antifungal therapy based on case reports, case series, cohort studies, a meta-analysis, and clinical experience [339, 349]. Valve repair and vegetectomy are alternatives to valve replacement. Most of the cases reported in the literature have been treated with AmB deoxycholate, with or without flucytosine [339, 342, 349–355]. Fluconazole monotherapy is associated with an unacceptably high rate of relapse and mortality [354]. However, fluconazole is useful for step-down therapy.
AmB deoxycholate and azoles have decreased activity when compared with echinocandins against biofilms formed by Candida in vitro, and they penetrate poorly into vegetations. Echinocandins and lipid formulations of AmB demonstrate more potent activity against Candida biofilms [356]. A prospective, open-label clinical trial, cohort studies, and several case reports show a role for the echinocandins in the treatment of endocarditis [228, 346, 348, 357–365]. Higher dosages of the echinocandins are thought to be necessary to treat endocarditis [228, 365]. Caspofungin has been used as monotherapy and in combination with AmB, azoles, or flucytosine in single case reports, but data are limited for the other echinocandins [346, 360, 361, 363, 365, 366].
Lifelong suppressive therapy with fluconazole has been used successfully after a course of primary therapy in patients for whom cardiac surgery is contraindicated; it has also been advocated to prevent late recurrence of Candida prosthetic valve endocarditis [360, 367, 368]. Because Candida endocarditis has a propensity to relapse months to years later, follow-up should be maintained for several years after treatment [350, 351].
What Is the Treatment for Candida Infection of Implantable Cardiac Devices?
Recommendations
65. For pacemaker and implantable cardiac defibrillator infections, the entire device should be removed (strong recommendation; moderate-quality evidence).
66. Antifungal therapy is the same as that recommended for native valve endocarditis (strong recommendation; low-quality evidence).
67. For infections limited to generator pockets, 4 weeks of antifungal therapy after removal of the device is recommended (strong recommendation; low-quality evidence).
68. For infections involving the wires, at least 6 weeks of antifungal therapy after wire removal is recommended (strong recommendation; low-quality evidence).
69. For ventricular assist devices that cannot be removed, the antifungal regimen is the same as that recommended for native valve endocarditis (strong recommendation; low-quality evidence). Chronic suppressive therapy with fluconazole, if the isolate is susceptible, for as long as the device remains in place is recommended (strong recommendation; low-quality evidence).
Evidence Summary
There are a few case reports and a single retrospective review of Candida infections of pacemakers and cardiac defibrillators [369–374]. The entire device should be removed and antifungal therapy given for 4–6 weeks depending on whether the infection involves the wires in addition to the generator pocket [369, 371–374]. Medical therapy alone has failed [370].
There are isolated case reports and a few case series on Candida infections of ventricular assist devices [375–378]. The Expert Panel believes that suppressive azole therapy after a full course of initial antifungal therapy is warranted. Many of these devices cannot be removed and suppression will be lifelong. The role of antifungal prophylaxis to prevent infection in all patients receiving an assist device remains controversial [378].
What Is the Treatment for Candida Suppurative Thrombophlebitis?
Recommendations
70. Catheter removal and incision and drainage or resection of the vein, if feasible, is recommended (strong recommendation; low-quality evidence).
71. Lipid formulation AmB, 3–5 mg/kg daily, OR fluconazole, 400–800 mg (6–12 mg/kg) daily, OR an echinocandin (caspofungin 150 mg daily, micafungin 150 mg daily, or anidulafungin 200 mg daily) for at least 2 weeks after candidemia (if present) has cleared is recommended (strong recommendation; low-quality evidence).
72. Step-down therapy to fluconazole, 400–800 mg (6–12 mg/kg) daily, should be considered for patients who have initially responded to AmB or an echinocandin, are clinically stable, and have a fluconazole-susceptible isolate (strong recommendation; low-quality evidence).
73. Resolution of the thrombus can be used as evidence to discontinue antifungal therapy if clinical and culture data are supportive (strong recommendation; low-quality evidence).
Evidence Summary
Most experience treating suppurative thrombophlebitis has been with AmB deoxycholate. Fluconazole and caspofungin have also been successful in some cases [379–381], but other agents used for primary treatment of candidemia, including echinocandins and voriconazole, should be effective [382]. Higher-than-usual doses of echinocandins should be used, similar to therapy for endocarditis.
Surgical excision of the vein plays an important role in the treatment of peripheral-vein Candida thrombophlebitis. When a central vein is involved, surgery is usually not an option. In some cases, systemic anticoagulation or thrombolytic therapy has been used as adjunctive therapy, but there are insufficient data to recommend their use. Thrombolytic therapy, in conjunction with antifungal therapy, has been used successfully in the management of an infected thrombus attached to a CVC in a patient with persistent candidemia [381].
XI. What Is the Treatment for Candida Osteoarticular Infections?
What Is the Treatment for Candida Osteomyelitis?
Recommendations
74. Fluconazole, 400 mg (6 mg/kg) daily, for 6–12 months OR an echinocandin (caspofungin 50–70 mg daily, micafungin 100 mg daily, or anidulafungin 100 mg daily) for at least 2 weeks followed by fluconazole, 400 mg (6 mg/kg) daily, for 6–12 months is recommended (strong recommendation; low-quality evidence).
75. Lipid formulation AmB, 3–5 mg/kg daily, for at least 2 weeks followed by fluconazole, 400 mg (6 mg/kg) daily, for 6–12 months is a less attractive alternative (weak recommendation; low-quality evidence).
76. Surgical debridement is recommended in selected cases (strong recommendation; low-quality evidence).
Evidence Summary
Most patients with osteomyelitis present with a subacute to chronic course [383, 384]. The most common mechanism of infection is hematogenous dissemination, but direct inoculation and contiguous spread of infection also occur. Involvement of 2 or more bones is common, and therefore, when a single focus of infection is identified, there should be a search for other sites of involvement. The axial skeleton, especially the spine, is the most common site of involvement in adults; in children, the long bones are more commonly involved [228, 384–388]. Neither the clinical picture nor the findings on radiographic imaging are specific for Candida infection. Candida albicans remains the dominant pathogen. However, 2 retrospective reviews of a large number of cases found that non-albicans Candida were an increasingly frequent cause of Candida osteomyelitis and mixed infections with bacteria, especially Staphylococcus aureus, were not uncommon, underscoring the need for biopsy and culture [384, 389].
Treatment recommendations are based on case reports and case series. Historically, AmB deoxycholate has been the most commonly used agent [388]. Recent literature favors the use of fluconazole or an echinocandin over AmB [228, 384–386]. Fluconazole has been used successfully as initial therapy for patients who have susceptible isolates, but treatment failures have also been reported [390–393]. There are case reports of the successful treatment of osteomyelitis with itraconazole, voriconazole, posaconazole, and caspofungin [228, 229, 394–396].
Cure rates appear to be significantly higher when an antifungal agent is administered for at least 6 months [384, 385]. The addition of AmB deoxycholate or fluconazole to bone cement has been suggested to be of value as adjunctive therapy in complicated cases and appears to be safe, but this practice is controversial [397, 398].
Surgical debridement is frequently performed in conjunction with antifungal therapy. Some reports have found surgical therapy important for Candida vertebral osteomyelitis [387], but others have not found that to be the case [388]. Surgery is indicated in patients who have neurological deficits, spinal instability, large abscesses, or persistent or worsening symptoms during therapy [384].
On the basis of a small number of cases, Candida mediastinitis and sternal osteomyelitis in patients who have undergone sternotomy can be treated successfully with surgical debridement followed by either AmB or fluconazole [391, 399]. Irrigation of the mediastinal space with AmB is not recommended, because it can cause irritation. Antifungal therapy of several months' duration, similar to that needed for osteomyelitis at other sites, is appropriate.
What Is the Treatment for Candida Septic Arthritis?
77. Fluconazole, 400 mg (6 mg/kg) daily, for 6 weeks OR an echinocandin (caspofungin 50–70 mg daily, micafungin 100 mg daily, or anidulafungin 100 mg daily) for 2 weeks followed by fluconazole, 400 mg (6 mg/kg) daily, for at least 4 weeks is recommended (strong recommendation; low-quality evidence).
78. Lipid formulation AmB, 3–5 mg/kg daily, for 2 weeks, followed by fluconazole, 400 mg (6 mg/kg) daily, for at least 4 weeks is a less attractive alternative (weak recommendation; low-quality evidence).
79. Surgical drainage is indicated in all cases of septic arthritis (strong recommendation; moderate-quality evidence).
80. For septic arthritis involving a prosthetic device, device removal is recommended (strong recommendation; moderate-quality evidence).
81. If the prosthetic device cannot be removed, chronic suppression with fluconazole, 400 mg (6 mg/kg) daily, if the isolate is susceptible, is recommended (strong recommendation; low-quality evidence).
Evidence Summary
Adequate drainage is critical to successful therapy of Candida arthritis. In particular, Candida arthritis of the hip requires open surgical drainage. Case reports have documented cures with AmB, fluconazole, and caspofungin therapy in combination with adequate drainage [400–402]. Administration of either AmB or fluconazole produces substantial synovial fluid levels, so that intra-articular injection of antifungal agents is not necessary.
Candida prosthetic joint infection generally requires resection arthroplasty, although success with medical therapy alone has been described rarely [403, 404]. The combination of removal and reimplantation of the prosthesis in 2 stages separated by 3–6 months and a prolonged period of antifungal therapy for at least 12 weeks after the resection arthroplasty and at least 6 weeks after prosthesis implantation is suggested on the basis of limited data [405–407]. The efficacy of antifungal-loaded cement spacers is controversial [408]. If the prosthetic device cannot be removed, chronic suppression with an antifungal agent, usually fluconazole, is necessary.
XII. What Is the Treatment for Candida Endophthalmitis?
What Is the General Approach to Candida Endophthalmitis?
Recommendations
82. All patients with candidemia should have a dilated retinal examination, preferably performed by an ophthalmologist, within the first week of therapy in nonneutropenic patients to establish if endophthalmitis is present (strong recommendation; low-quality evidence). For neutropenic patients, it is recommended to delay the examination until neutrophil recovery (strong recommendation; low-quality evidence).
83. The extent of ocular infection (chorioretinitis with or without macular involvement and with or without vitritis) should be determined by an ophthalmologist (strong recommendation; low-quality evidence).
84. Decisions regarding antifungal treatment and surgical intervention should be made jointly by an ophthalmologist and an infectious diseases physician (strong recommendation; low-quality evidence).
Evidence Summary
Endophthalmitis refers to infections within the eye, usually involving the posterior chamber and sometimes also the anterior chamber. Candida endophthalmitis can be exogenous, initially affecting the anterior chamber and occurring following trauma or a surgical procedure. More often, Candida species cause endogenous infection in which the organism reaches the posterior chamber of the eye via hematogenous spread. Endogenous infections can be manifested as isolated chorioretinitis or as chorioretinitis with extension into the vitreous, leading to vitritis [409–412]. Candida albicans is the species most commonly responsible for endogenous endophthalmitis, but all Candida species that cause candidemia have been reported to cause this complication [411–414]. Outcomes in terms of visual acuity depend on the extent of visual loss at the time of presentation and macular involvement [415].
Several basic principles are important in the approach to treatment of Candida infections of the eye. It should first be determined whether infection involves the anterior and/or posterior segment of the eye and whether the macula or vitreous are involved [70, 416–418]. Achieving adequate concentrations of the appropriate antifungal agent in the area of the eye that is infected is crucial to success [419, 420]. Infections involving the chorioretinal layer are more easily treated because this area of the posterior chamber is highly vascular; many systemic antifungal agents likely reach adequate concentrations within the choroid and the retina [420–422]. The antifungal susceptibilities of the infecting species are important. Species that are susceptible to fluconazole or voriconazole are more easily treated because these agents achieve adequate concentrations in the posterior segment of the eye, including the vitreous [419, 420, 422]. Treatment must be systemic to treat candidemia and other organ involvement, if present, in addition to the ocular infection.
Sight-threatening lesions near the macula or invasion into the vitreous usually necessitate intravitreal injection of antifungal agents, usually AmB deoxycholate or voriconazole, with or without vitrectomy, in addition to systemic antifungal agents [412, 419, 422–425]. The ophthalmologist plays a key role in following the course of endogenous Candida endophthalmitis, deciding when and if to perform intravitreal injections and vitrectomy.
The approach to the patient who has candidemia has evolved over time, and standard practice now includes consultation with an ophthalmologist to do a dilated retinal examination. The basis for the recommendation to perform an ophthalmological evaluation is not a result of randomized controlled trials showing the benefits of such an assessment, but rather clinical judgment that the result of missing and not appropriately treating Candida endophthalmitis could be of great consequence to the patient. The issue of whether an ophthalmological examination of all candidemic patients is cost-effective has been raised [183, 426]. The members of the Expert Panel believe that the risk of missing Candida endophthalmitis outweighs the cost of obtaining an ophthalmological examination. We are concerned about the greater risk of loss of visual acuity in patients who are examined only after manifesting ocular symptoms [415], and note that other centers report higher rates of endophthalmitis than reports from the centers cited by those who question the routine use of ocular examination [417, 418, 421].
What Is the Treatment for Candida Chorioretinitis Without Vitritis?
Recommendations
85. For fluconazole-/voriconazole-susceptible isolates, fluconazole, loading dose, 800 mg (12 mg/kg), then 400–800 mg (6–12 mg/kg) daily OR voriconazole, loading dose 400 mg (6 mg/kg) intravenous twice daily for 2 doses, then 300 mg (4 mg/kg) intravenous or oral twice daily is recommended (strong recommendation; low-quality evidence).
86. For fluconazole-/voriconazole-resistant isolates, liposomal AmB, 3–5 mg/kg intravenous daily, with or without oral flucytosine, 25 mg/kg 4 times daily is recommended (strong recommendation; low-quality evidence).
87. With macular involvement, antifungal agents as noted above PLUS intravitreal injection of either AmB deoxycholate, 5–10 µg/0.1 mL sterile water, or voriconazole, 100 µg/0.1 mL sterile water or normal saline, to ensure a prompt high level of antifungal activity is recommended (strong recommendation; low-quality evidence).
88. The duration of treatment should be at least 4–6 weeks, with the final duration depending on resolution of the lesions as determined by repeated ophthalmological examinations (strong recommendation; low-quality evidence).
Evidence Summary
The greatest clinical experience for treatment of Candida endophthalmitis has been with intravenous AmB deoxycholate, only because it has been available for the longest time. However, this agent does not achieve adequate concentrations in the posterior chamber [419, 420, 427, 428]. In animal experiments in inflamed eyes, liposomal AmB achieved higher concentrations in the eye than either AmB deoxycholate or AmB lipid complex [427]. A few patients have been treated successfully with lipid formulations of AmB, but concentrations in the vitreous in humans have not been reported [429].
Flucytosine provides adjunctive synergistic activity when used with AmB; it should not be used as monotherapy because of development of resistance and reports of decreased efficacy in animal models [428]. It attains excellent levels in the ocular compartments, including the vitreous [412, 430]. Toxicity is common, and flucytosine serum levels must be monitored weekly to prevent dose-related toxicity.
Fluconazole is frequently used for the treatment of Candida endophthalmitis. In experimental animals, this agent achieves excellent concentrations throughout the eye, including the vitreous [428]. In humans, concentrations in the vitreous are approximately 70% of those in the serum [57]. Clinical and microbiological response rates in animals with experimental infection are somewhat conflicting, with most reports showing efficacy of fluconazole, but some noting better efficacy with AmB than fluconazole [428, 431, 432]. Early reports in humans noted the efficacy of fluconazole, but some patients had received intravitreal injection of antifungal agents, as well as systemic fluconazole [433, 434]. Despite the fact that no large published series has defined the efficacy of fluconazole therapy, this agent is routinely used for the treatment of Candida endophthalmitis [410, 411, 415, 421].
Voriconazole has played an increasing role in the treatment of endophthalmitis [419]. Concentrations in the vitreous in humans are approximately 40% of serum concentrations; the drug is relatively safe, and, like fluconazole, can be given by the oral or intravenous route [435–438]. It is more active than fluconazole against C. glabrata, although resistance is increasing and may preclude its use for some patients; it is uniformly active against C. krusei. Efficacy of voriconazole in treating Candida endophthalmitis has been documented, but not compared with fluconazole [429, 436, 438]. Serum and (presumably) intraocular concentrations of voriconazole are quite variable, and serum trough levels should be routinely monitored to achieve concentrations between 2 µg/mL and 5 µg/mL to enhance efficacy and avoid toxicity [118].
There are few data regarding the use of posaconazole for Candida endophthalmitis. Intraocular penetration is poor, this agent has been used in very few patients, and it is not approved for the treatment of candidemia [419].
Echinocandins are first-line agents for the treatment of candidemia. Whether they can effectively treat chorioretinitis without vitreal involvement cannot be answered with the data available. Penetration of all echinocandins into the different chambers of the eye is poor, and is especially poor in the vitreous [412, 419, 420]. When levels have been achieved in experimental animal models and in one study in humans with micafungin, the dosages needed have been higher than those currently licensed for use [112, 439–443]. Only a few case reports of the use of an echinocandin as monotherapy have been published, and the results are contradictory [444, 445]. With the availability of other agents that achieve adequate concentrations in the vitreous, there is little reason to recommend the use of echinocandins for Candida endophthalmitis.
Because involvement of the macula is sight-threatening and concentrations of antifungal agents in the posterior chamber do not immediately reach therapeutic levels, many ophthalmologists perform an intravitreal injection of either AmB deoxycholate or voriconazole to quickly achieve high antifungal activity in the posterior chamber. AmB is the agent that has been used most often for intravitreal injection [422, 423]. A dosage of 5–10 µg given in 0.1 mL sterile water is generally safe [419]. Intravitreal injection of lipid formulations of AmB has been compared with AmB deoxycholate in rabbits; all formulations showed toxicity at higher doses, but at 10 µg, the least toxic was liposomal AmB [446], confirming a prior study using a noncommercial liposomal formulation [447].
Voriconazole is increasingly used for intravitreal injection for both Candida and mold endophthalmitis [438, 448]. It has been shown to be safe in animal eyes at concentrations <250 µg/mL [449]. The usual dose given to humans is 100 µg in 0.1 mL sterile water or normal saline (achieving a final concentration of 25 µg/mL) [419, 438]. In vitrectomized eyes, the half-life of both AmB and voriconazole is shortened, and repeated injections may be required [450, 451].
What Is the Treatment for Candida Chorioretinitis With Vitritis?
Recommendations
89. Antifungal therapy as detailed above for chorioretinitis without vitritis, PLUS intravitreal injection of either amphotericin B deoxycholate, 5–10 µg/0.1 mL sterile water, or voriconazole, 100 µg/0.1 mL sterile water or normal saline, is recommended (strong recommendation; low-quality evidence).
90. Vitrectomy should be considered to decrease the burden of organisms and to allow the removal of fungal abscesses that are inaccessible to systemic antifungal agents (strong recommendation; low-quality evidence).
91. The duration of treatment should be at least 4–6 weeks, with the final duration dependent on resolution of the lesions as determined by repeated ophthalmological examinations (strong recommendation; low-quality evidence).
Evidence Summary
Candida endophthalmitis that has extended into the vitreous results in worse visual outcomes than chorioretinitis without vitritis [415]. This may be related to the inability of many antifungal agents to achieve adequate concentrations in the vitreous body. Poor outcomes could also be due to an increased burden of organisms in the posterior chamber or the existence of an abscess that cannot be visualized through the vitreal haziness. Additionally, in cases of endophthalmitis in which fungemia is not documented and the organism is unknown, vitrectomy provides material for culture that is superior to needle aspiration and allows the proper antifungal agent to be used [422, 424].
The treatment when vitritis is documented is similar to that recommended for chorioretinitis without vitreal involvement, with the added recommendations to (1) inject either AmB deoxycholate or voriconazole into the vitreous to achieve high drug concentrations in the posterior chamber and to (2) consider performing a pars plana vitrectomy. Several small series have noted success in patients in whom early pars plana vitrectomy was accomplished [415, 423, 424, 452]. Removal of the vitreous is usually accompanied by intravitreal injection of antifungal agents, and as noted above, the half-life of injected antifungal agents is shortened with vitrectomy [450, 451]. The risk of retinal detachment, a severe late complication of endophthalmitis with vitreal involvement, is decreased with early vitrectomy [412, 415]. To have the best outcomes, Candida endophthalmitis with vitritis must be managed with close cooperation between ophthalmologists and infectious diseases specialists.
XIII. What Is the Treatment for Central Nervous System Candidiasis?
Recommendations
92. For initial treatment, liposomal AmB, 5 mg/kg daily, with or without oral flucytosine, 25 mg/kg 4 times daily, is recommended (strong recommendation; low-quality evidence).
93. For step-down therapy after the patient has responded to initial treatment, fluconazole, 400–800 mg (6–12 mg/kg) daily, is recommended (strong recommendation; low-quality evidence).
94. Therapy should continue until all signs and symptoms and CSF and radiological abnormalities have resolved (strong recommendation; low-quality evidence).
95. Infected CNS devices, including ventriculostomy drains, shunts, stimulators, prosthetic reconstructive devices, and biopolymer wafers that deliver chemotherapy, should be removed if possible (strong recommendation; low-quality evidence).
96. For patients in whom a ventricular device cannot be removed, AmB deoxycholate could be administered through the device into the ventricle at a dosage ranging from 0.01 mg to 0.5 mg in 2 mL 5% dextrose in water (weak recommendation; low-quality evidence).
Evidence Summary
CNS Candida infections can occur as a manifestation of disseminated candidiasis, as a complication of a neurosurgical procedure, especially when an intracranial device is inserted, or rarely as an isolated chronic infection [453–462]. Meningitis is the most common presentation, but multiple small abscesses throughout the brain parenchyma, large solitary brain abscesses, and epidural abscesses have been reported [462]. Low-birth-weight neonates are at high risk to have CNS infection as a complication of candidemia; neonatal CNS candidiasis is dealt with in the section on neonatal Candida infections. Most infections are due to C. albicans, with few reports of C. glabrata and other species causing infection [453–457, 459, 461, 462]. Treatment is based on the antifungal susceptibilities of the infecting species and the ability of the antifungal agent to achieve appropriate concentrations in the CSF and brain.
No randomized controlled trials have been performed to evaluate the most appropriate treatment for these uncommon infections. Single cases and small series are reported. Most experience has accrued with the use of AmB deoxycholate, with or without flucytosine [453–455, 457, 459, 460, 462]. Liposomal AmB (AmBisome) has been found to attain higher levels in the brain than amphotericin B lipid complex (ABLC) or AmB deoxycholate in a rabbit model of Candida meningoencephalitis [44].
The combination of AmB and flucytosine is recommended because of the in vitro synergism noted with the combination and the excellent CSF concentrations achieved by flucytosine. However, flucytosine's toxic effects on bone marrow and liver must be carefully monitored, preferably with frequent serum flucytosine levels. The optimal length of therapy with AmB alone or in combination with flucytosine has not been studied. Several weeks of therapy are suggested before transitioning to oral azole therapy.
Fluconazole achieves excellent levels in CSF and brain tissue and has proved useful as step-down therapy [453, 454, 459]. Fluconazole also has been used as monotherapy; both success and failure have been noted, and for this reason it is not recommended as first-line therapy [453, 454, 463–465]. Fluconazole combined with flucytosine has been reported to cure Candida meningitis in a few patients [459], and this is a possible regimen for step-down therapy. There are no reports of the use of voriconazole or posaconazole for CNS candidiasis. Voriconazole achieves excellent levels in CSF, and should be considered for the rare case of C. glabrata that is not voriconazole resistant or C. krusei meningitis after initial treatment with AmB with or without flucytosine. Posaconazole does not reach adequate concentrations in the CSF, and this agent is not recommended.
Echinocandins have been used infrequently for CNS candidiasis. There are case reports noting success [466], but CNS breakthrough infections in patients receiving an echinocandin for candidemia have been reported [467]. There are experimental animal data noting that anidulafungin and micafungin can successfully treat C. albicans meningitis, but the doses required in humans are much higher than currently recommended for candidemia [296, 299]. At present, echinocandins are not recommended for CNS candidiasis.
Infected CNS devices should be removed to eradicate Candida. Most experience has been with external ventricular drains and ventriculoperitoneal shunts that have become infected with Candida species [460, 463]. In recent years, infected devices include deep brain stimulators and Gliadel biopolymer wafers that have been placed into the site of a brain tumor to deliver chemotherapy locally. Although difficult to remove, experience has shown that these devices must be taken out for cure of the infection [456, 468, 469].
Intraventricular administration of antifungal agents is not usually necessary for treatment of CNS Candida infections. In patients in whom the removal of a ventricular shunt or external ventriculostomy drain is too risky because of significantly elevated intracranial pressure, or among patients who have not responded to systemic antifungal therapy, intraventricular AmB deoxycholate has proved useful [453, 454, 460, 463, 469]. The dose of intraventricular AmB deoxycholate is not standardized, and recommendations vary from 0.01 mg to 1 mg in 2 mL of 5% dextrose in water daily [455, 463, 466, 469]. Toxicity—mainly headache, nausea, and vomiting—is a limiting factor when administering AmB by this route [454, 463].
XIV. What Is the Treatment for Urinary Tract Infections Due to Candida Species?
What Is the Treatment for Asymptomatic Candiduria?
Recommendations
97. Elimination of predisposing factors, such as indwelling bladder catheters, is recommended whenever feasible (strong recommendation; low-quality evidence).
98. Treatment with antifungal agents is NOT recommended unless the patient belongs to a group at high risk for dissemination; high-risk patients include neutropenic patients, very low-birth-weight infants (<1500 g), and patients who will undergo urologic manipulation (strong recommendation; low-quality evidence).
99. Neutropenic patients and very low-birth-weight infants should be treated as recommended for candidemia (see sections III and VII) (strong recommendation; low-quality evidence).
100. Patients undergoing urologic procedures should be treated with oral fluconazole, 400 mg (6 mg/kg) daily, OR AmB deoxycholate, 0.3–0.6 mg/kg daily, for several days before and after the procedure (strong recommendation; low-quality evidence).
Evidence Summary
The presence of candiduria is the usual trigger for a physician to consider whether a patient has a urinary tract infection due to Candida species. The patients at most risk for candiduria are those who are elderly, female, diabetic, have indwelling urinary devices, are taking antibiotics, and have had prior surgical procedures [470–475]. In the asymptomatic patient, candiduria almost always represents colonization, and elimination of underlying risk factors, such as indwelling catheters, is often adequate to eradicate candiduria [471].
Multiple studies have noted that candiduria does not commonly lead to candidemia [471, 472, 476–480]. Several of these studies have shown that candiduria is a marker for greater mortality, but death is not related to Candida infection and treatment for Candida infection does not change mortality rates [476, 480, 481]. A prospective study in renal transplant recipients found that although mortality was higher in patients who had candiduria, treatment did not improve outcomes, suggesting again that candiduria is a marker for severity of underlying illness [482].
Several conditions require an aggressive approach to candiduria in asymptomatic patients. These include neonates with very low birth weight, who are at risk for invasive candidiasis that often involves the urinary tract [281, 483]. Many physicians who care for neutropenic patients treat those who have fever and candiduria because the candiduria may indicate invasive candidiasis. However, a recent study from a cancer hospital of a small number of patients, 25% of whom were neutropenic, found that these patients did not develop candidemia or other complications of candiduria [484]. Several reports have documented a high rate of candidemia when patients undergo urinary tract instrumentation [485, 486], which has led to recommendations to treat with antifungal agents periprocedure.
What Is the Treatment for Symptomatic Candida Cystitis?
Recommendations
101. For fluconazole-susceptible organisms, oral fluconazole, 200 mg (3 mg/kg) daily for 2 weeks is recommended (strong recommendation; moderate-quality evidence).
102. For fluconazole-resistant C. glabrata, AmB deoxycholate, 0.3–0.6 mg/kg daily for 1–7 days OR oral flucytosine, 25 mg/kg 4 times daily for 7–10 days is recommended (strong recommendation; low-quality evidence).
103. For C. krusei, AmB deoxycholate, 0.3–0.6 mg/kg daily, for 1–7 days is recommended (strong recommendation; low-quality evidence).
104. Removal of an indwelling bladder catheter, if feasible, is strongly recommended (strong recommendation; low-quality evidence).
105. AmB deoxycholate bladder irrigation, 50 mg/L sterile water daily for 5 days, may be useful for treatment of cystitis due to fluconazole-resistant species, such as C. glabrata and C. krusei (weak recommendation; low-quality evidence).
What Is the Treatment for Symptomatic Ascending Candida Pyelonephritis?
Recommendations
106. For fluconazole-susceptible organisms, oral fluconazole, 200–400 mg (3–6 mg/kg) daily for 2 weeks, is recommended (strong recommendation; low-quality evidence).
107. For fluconazole-resistant C. glabrata, AmB deoxycholate, 0.3–0.6 mg/kg daily for 1–7 days, with or without oral flucytosine, 25 mg/kg 4 times daily, is recommended (strong recommendation; low-quality evidence).
108. For fluconazole-resistant C. glabrata, monotherapy with oral flucytosine, 25 mg/kg 4 times daily for 2 weeks, could be considered (weak recommendation; low-quality evidence).
109. For C. krusei, AmB deoxycholate, 0.3–0.6 mg/kg daily, for 1–7 days is recommended (strong recommendation; low-quality evidence).
110. Elimination of urinary tract obstruction is strongly recommended (strong recommendation; low-quality evidence).
111. For patients who have nephrostomy tubes or stents in place, consider removal or replacement, if feasible (weak recommendation; low-quality evidence).
Evidence Summary
Candida UTI can develop by 2 different routes [487]. Most symptomatic UTIs evolve as an ascending infection beginning in the lower urinary tract, similar to the pathogenesis of bacterial UTI. Patients with ascending infection can have symptoms of cystitis or pyelonephritis. The other route of infection occurs as a consequence of hematogenous spread to the kidneys in a patient who has candidemia. These patients usually have no urinary tract symptoms or signs, and are treated for candidemia.
Diagnostic tests on urine often are not helpful in differentiating colonization from infection or in pinpointing the involved site within the urinary tract [488, 489]. For example, pyuria in a patient with an indwelling bladder catheter cannot differentiate Candida infection from colonization. Similarly, the colony count in the urine, especially when a catheter is present, cannot be used to define infection [488, 489]. Imaging of the urinary tract by ultrasound or CT scanning is helpful in defining structural abnormalities, hydronephrosis, abscesses, emphysematous pyelonephritis, and fungus ball formation [490–492]. Aggregation of mycelia and yeasts (fungus balls) in bladder or kidney leads to obstruction and precludes successful treatment of infection with antifungal agents alone [94]. Rarely, Candida species cause localized infections in prostate, epididymis, or testicles [491, 493–495].
Several basic principles are important in the approach to treatment of Candida UTI. The ability of the antifungal agent to achieve adequate concentrations in the urine is as important as the antifungal susceptibilities of the infecting species [94]. Candida albicans, the most common cause of fungal UTI, is relatively easy to treat because it is susceptible to fluconazole, which achieves high concentrations in the urine. In contrast, UTIs due to fluconazole-resistant C. glabrata and C. krusei can be extremely difficult to treat.
Fluconazole is the drug of choice for treating Candida UTI. It was shown to be effective in eradicating candiduria in the only randomized, double-blind, placebo-controlled trial that has been conducted in patients with candiduria [496]. It is important to note that the patients in this trial were asymptomatic or had minimal symptoms of cystitis. Fluconazole is available as an oral formulation, is excreted into the urine in its active form, and easily achieves urine levels exceeding the MIC for most Candida isolates [94].
Flucytosine demonstrates good activity against many Candida species, with the exception of C. krusei, and is excreted as active drug in the urine [94]. Treatment with flucytosine is limited by its toxicity and the development of resistance when it is used as a single agent.
AmB deoxycholate is active against most Candida species (although some C. krusei isolates are resistant) and achieves concentrations in the urine that exceed the MICs for most isolates, and even low doses have been shown to be effective in treating Candida UTI [497]. The major drawbacks are the need for intravenous administration and toxicity. The lipid formulations of AmB appear to not achieve urine concentrations that are adequate to treat UTI and should not be used [498].
All other antifungal drugs, including the other azole agents and echinocandins, have minimal excretion of active drug into the urine and generally are ineffective in treating Candida UTI [94]. However, there are several reports of patients in whom echinocandins were used, primarily because of UTI due to fluconazole-resistant organisms, and both success and failure were reported [499–502]. Infection localized to the kidney, as occurs with hematogenous spread, probably can be treated with echinocandins because tissue concentrations are adequate even though these agents do not achieve adequate urine concentrations [499].
Irrigation of the bladder with AmB deoxycholate resolves candiduria in 80%–90% of patients, as shown in several open-label trials, but in those studies, recurrent candiduria within several weeks was very common [503–505]. This approach is useful only for bladder infections and generally is discouraged, especially in patients who would not require an indwelling catheter for any other reason [94, 506, 507]. Cystitis due to C. glabrata or C. krusei can sometimes be treated with amphotericin B bladder irrigation and endoscopic removal of any obstructing lesions [94].
What Is the Treatment for Candida Urinary Tract Infection Associated With Fungus Balls?
Recommendations
112. Surgical intervention is strongly recommended in adults (strong recommendation; low-quality evidence).
113. Antifungal treatment as noted above for cystitis or pyelonephritis is recommended (strong recommendation; low-quality evidence).
114. Irrigation through nephrostomy tubes, if present, with AmB deoxycholate, 25–50 mg in 200–500 mL sterile water, is recommended (strong recommendation; low-quality evidence).
Evidence Summary
Fungus balls are an uncommon complication of Candida UTI except in neonates, in whom fungus ball formation in the collecting system commonly occurs as a manifestation of disseminated candidiasis [483]. In adults, surgical or endoscopic removal of the obstructing mycelial mass is central to successful treatment [94, 508, 509]. In neonates, some series documented resolution of fungus balls with antifungal treatment alone [510], but others found that endoscopic removal was necessary [511, 512]. There are anecdotal reports of a variety of techniques used to remove fungus balls from the renal pelvis; these include endoscopic removal via a percutaneous nephrostomy tube, infusion of streptokinase locally, and irrigation with antifungal agents through a nephrostomy tube [511–513]. Fungus balls in the bladder and lower ureter usually can be removed endoscopically [509].
XV. What Is the Treatment for Vulvovaginal Candidiasis?
Recommendations
115. For the treatment of uncomplicated Candida vulvovaginitis, topical antifungal agents, with no one agent superior to another, are recommended (strong recommendation; high-quality evidence).
116. Alternatively, for the treatment of uncomplicated Candida vulvovaginitis, a single 150-mg oral dose of fluconazole is recommended (strong recommendation; high-quality evidence).
117. For severe acute Candida vulvovaginitis, fluconazole 150 mg, given every 72 hours for a total of 2 or 3 doses, is recommended (strong recommendation; high-quality evidence).
118. For C. glabrata vulvovaginitis that is unresponsive to oral azoles, topical intravaginal boric acid, administered in a gelatin capsule, 600 mg daily, for 14 days is an alternative (strong recommendation; low-quality evidence).
119. Another alternative agent for C. glabrata infection is nystatin intravaginal suppositories, 100 000 units daily for 14 days (strong recommendation; low-quality evidence).
120. A third option for C. glabrata infection is topical 17% flucytosine cream alone or in combination with 3% AmB cream administered daily for 14 days (weak recommendation; low-quality evidence).
121. For recurring vulvovaginal candidiasis, 10–14 days of induction therapy with a topical agent or oral fluconazole, followed by fluconazole, 150 mg weekly for 6 months, is recommended (strong recommendation; high-quality evidence).
Evidence Summary
Vulvovaginal candidiasis can be classified as either uncomplicated, which is present in about 90% of cases, or complicated, which accounts for only about 10% of cases, on the basis of clinical presentation, microbiological findings, host factors, and response to therapy [514]. Complicated vulvovaginal candidiasis is defined as severe or recurrent disease, infection due to non-albicans species, and/or infection in an abnormal host. Candida albicans is the usual pathogen, but other Candida species can also cause this infection.
A diagnosis of vulvovaginal candidiasis can usually be made clinically when a woman presents with symptoms of pruritus, irritation, vaginal soreness, external dysuria, and dyspareunia, often accompanied by a change in vaginal discharge. Signs include vulvar edema, erythema, excoriation, fissures, and a white, thick, curdlike vaginal discharge. Unfortunately, these symptoms and signs are nonspecific and can be the result of a variety of infectious and noninfectious etiologies. Before proceeding with empiric antifungal therapy, the diagnosis should be confirmed by a wet-mount preparation with use of saline and 10% potassium hydroxide to demonstrate the presence of yeast or hyphae and a normal pH (4.0–4.5). For those with negative findings, vaginal cultures for Candida should be obtained.
A variety of topical and systemic oral agents are available for treatment of vulvovaginal candidiasis. No evidence exists to show the superiority of any one topical regimen [515, 516], and oral and topical antifungal formulations have been shown to achieve entirely equivalent results [517]. Uncomplicated infection can be effectively treated with either single-dose fluconazole or short-course fluconazole for 3 days, both of which achieve >90% response [517, 518]. Treatment of vulvovaginal candidiasis should not differ on the basis of human immunodeficiency virus (HIV) infection status; identical response rates are anticipated for HIV-positive and HIV-negative women.
Complicated vulvovaginal candidiasis requires that therapy be administered intravaginally with topical agents for 5–7 days or orally with fluconazole 150 mg every 72 hours for 3 doses [54, 514]. Most Candida species, with the exception of C. krusei and C. glabrata, respond to oral fluconazole. Candida krusei responds to all topical antifungal agents. However, treatment of C. glabrata vulvovaginal candidiasis is problematic [514, 516]. The most important decision to make is whether the presence of C. glabrata in vaginal cultures reflects colonization in a patient who has another disease, or whether it indicates true infection requiring treatment. Azole therapy, including voriconazole, is frequently unsuccessful [519]. A variety of local regimens have sometimes proved effective. These include boric acid contained in gelatin capsules and nystatin intravaginal suppositories [520]. Topical 17% flucytosine cream can be used alone or in combination with 3% AmB cream in recalcitrant cases [520, 521]. These topical formulations, as well as boric acid gelatin capsules, must be compounded by a pharmacist for specific patient use. Azole-resistant C. albicans infections are extremely rare. However, recent evidence has emerged documenting fluconazole and azole class resistance in women following prolonged azole exposure [522].
Recurrent vulvovaginal candidiasis, defined as ≥4 episodes of symptomatic infection within one year, is usually caused by azole-susceptible C. albicans [514, 523]. Contributing factors, such as diabetes, are rarely found. Treatment should begin with induction therapy with a topical agent or oral fluconazole for 10–14 days, followed by a maintenance azole regimen for at least 6 months [523–525]. The most convenient and well-tolerated regimen is 150 mg fluconazole once weekly. This regimen achieves control of symptoms in >90% of patients [523]. After cessation of maintenance therapy, a 40%–50% recurrence rate can be anticipated. If fluconazole therapy is not feasible, topical clotrimazole cream, 200 mg twice weekly, clotrimazole vaginal suppository 500 mg once weekly, or other intermittent oral or topical antifungal treatment is recommended [526, 527].
XVI. What Is the Treatment for Oropharyngeal Candidiasis?
Recommendations
122. For mild disease, clotrimazole troches, 10 mg 5 times daily, OR miconazole mucoadhesive buccal 50 mg tablet applied to the mucosal surface over the canine fossa once daily for 7–14 days, are recommended (strong recommendation; high-quality evidence).
123. Alternatives for mild disease include nystatin suspension (100 000 U/mL) 4–6 mL 4 times daily, OR 1–2 nystatin pastilles (200 000 U each) 4 times daily, for 7–14 days (strong recommendation; moderate-quality evidence).
124. For moderate to severe disease, oral fluconazole, 100–200 mg daily, for 7–14 days is recommended (strong recommendation; high-quality evidence).
125. For fluconazole-refractory disease, itraconazole solution, 200 mg once daily OR posaconazole suspension, 400 mg twice daily for 3 days then 400 mg daily, for up to 28 days, are recommended (strong recommendation; moderate-quality evidence).
126. Alternatives for fluconazole-refractory disease include voriconazole, 200 mg twice daily, OR AmB deoxycholate oral suspension, 100 mg/mL 4 times daily (strong recommendation; moderate-quality evidence).
127. Intravenous echinocandin (caspofungin: 70-mg loading dose, then 50 mg daily; micafungin: 100 mg daily; or anidulafungin: 200-mg loading dose, then 100 mg daily) OR intravenous AmB deoxycholate, 0.3 mg/kg daily, are other alternatives for refractory disease (weak recommendation; moderate-quality evidence).
128. Chronic suppressive therapy is usually unnecessary. If required for patients who have recurrent infection, fluconazole, 100 mg 3 times weekly, is recommended (strong recommendation; high-quality evidence).
129. For HIV-infected patients, antiretroviral therapy is strongly recommended to reduce the incidence of recurrent infections (strong recommendation; high-quality evidence).
130. For denture-related candidiasis, disinfection of the denture, in addition to antifungal therapy, is recommended (strong recommendation; moderate-quality evidence).
Evidence Summary
Oropharyngeal and esophageal candidiasis occur in association with HIV infection, diabetes, leukemia and other malignancies, steroid use, radiation therapy, antimicrobial therapy, and denture use [528, 529], and their occurrence is recognized as an indicator of immune dysfunction. In HIV-infected patients, oropharyngeal candidiasis is most often observed in patients with CD4 counts <200 cells/µL [528–530]. The advent of effective antiretroviral therapy has led to a dramatic decline in the prevalence of oropharyngeal candidiasis and a marked diminution in cases of refractory disease [531].
Fluconazole or multiazole resistance is predominantly the consequence of previous repeated and long-term exposure to fluconazole or other azoles [530–533]. Especially in patients with advanced immunosuppression and low CD4 counts, C. albicans resistance has been described, as has gradual emergence of non-albicans Candida species, particularly C. glabrata, as a cause of refractory mucosal candidiasis [532, 533].
Most cases of oropharyngeal candidiasis are caused by C. albicans, either alone or in mixed infections. Symptomatic infections caused by C. glabrata, C. dubliniensis, and C. krusei alone have been described [532–534]. Multiple randomized prospective studies of oropharyngeal candidiasis have been performed involving patients with AIDS and patients with cancer. Most patients will respond initially to topical therapy [532, 535, 536]. In HIV-infected patients, symptomatic relapses occur sooner and more frequently with topical therapy than with fluconazole [535]. In a multicenter randomized study among HIV-infected individuals, 50-mg mucoadhesive buccal tablets of miconazole applied once daily to the mucosal surface over the canine fossa were as effective as 10-mg clotrimazole troches used 5 times daily [537].
Fluconazole tablets and itraconazole solution are superior to ketoconazole and itraconazole capsules [538–540]. Local effects of oral solutions may be as important as the systemic effects. Posaconazole suspension is also as efficacious as fluconazole in patients with AIDS [541]. Posaconazole, 100-mg delayed release tablets, given as 300 mg daily as a single dose, are FDA approved for the prophylaxis of fungal infections in high-risk patients. The tablets provide a stable bioavailability (approximately 55%), once-daily dosing, and the convenience of less stringent food requirements for absorption. This formulation has not been fully evaluated for mucosal candidiasis, but, with further study, could replace the oral suspension for this purpose.
Recurrent infections typically occur in patients who have persistent immunosuppression, especially those who have AIDS and low CD4 cell counts (<50 cells/µL) [530–533]. Long-term suppressive therapy with fluconazole has been shown to be effective in the prevention of oropharyngeal candidiasis [53, 542, 543]. In a large multicenter study of HIV-infected patients, long-term suppressive therapy with fluconazole was compared with the episodic use of fluconazole in response to symptomatic disease. Continuous suppressive therapy reduced the relapse rate more effectively than did intermittent therapy, but was associated with increased in vitro resistance. The frequency of refractory disease was the same for both groups [53]. Oral AmB deoxycholate, nystatin solution, and itraconazole capsules are less effective than fluconazole in preventing oropharyngeal candidiasis [544, 545].
Fluconazole-refractory infections should be treated initially with itraconazole solution; between 64% and 80% of patients will respond to this therapy [546, 547]. Posaconazole suspension is efficacious in approximately 75% of patients with refractory oropharyngeal or esophageal candidiasis [548], and voriconazole also is efficacious for fluconazole-refractory infections [549]. Intravenous caspofungin, micafungin, and anidulafungin have been shown to be effective alternatives to azole agents for refractory candidiasis [24, 87, 88, 550]. Oral or intravenous AmB deoxycholate is also effective in some patients; however, a pharmacist must compound the oral formulation [551]. Immunomodulation with adjunctive granulocyte-macrophage colony-stimulating factor or interferon-γ have been occasionally used in the management of refractory oral and esophageal candidiasis [552, 553].
Decreasing rates of oral carriage of Candida species and a reduced frequency of symptomatic oropharyngeal candidiasis are seen among HIV-infected patients on effective antiretroviral therapy [554]. Thus, antiretroviral therapy should be used whenever possible for HIV-infected patients with oropharyngeal or esophageal candidiasis.
Chronic mucocutaneous candidiasis is a rare condition that is characterized by chronic, persistent onychomycosis and/or mucocutaneous lesions due to Candida species. Some patients have a thymoma or autoimmune polyendocrinopathy syndrome type 1 [555]. Fluconazole should be used as initial therapy for candidiasis in these patients. Response to antifungal therapy may be delayed when there is extensive skin or nail involvement. Because of the intrinsic immunodeficiency, most patients require chronic suppressive antifungal therapy and frequently develop azole-refractory infections [556]. Patients with fluconazole-refractory Candida infections should be treated the same as patients with AIDS who develop azole refractory infections [528].
XVII. What Is the Treatment for Esophageal Candidiasis?
Recommendations
131. Systemic antifungal therapy is always required. A diagnostic trial of antifungal therapy is appropriate before performing an endoscopic examination (strong recommendation; high-quality evidence).
132. Oral fluconazole, 200–400 mg (3–6 mg/kg) daily, for 14–21 days is recommended (strong recommendation; high-quality evidence).
133. For patients who cannot tolerate oral therapy, intravenous fluconazole, 400 mg (6 mg/kg) daily, OR an echinocandin (micafungin: 150 mg daily; caspofungin: 70-mg loading dose, then 50 mg daily; or anidulafungin: 200 mg daily) is recommended (strong recommendation; high-quality evidence).
134. A less preferred alternative for those who cannot tolerate oral therapy is AmB deoxycholate, 0.3–0.7 mg/kg daily (strong recommendation; moderate-quality evidence).
135. Consider de-escalating to oral therapy with fluconazole 200–400 mg (3–6 mg/kg) daily once the patient is able to tolerate oral intake (strong recommendation; moderate-quality evidence).
136. For fluconazole-refractory disease, itraconazole solution, 200 mg daily, OR voriconazole, 200 mg (3 mg/kg) twice daily either intravenous or oral, for 14–21 days is recommended (strong recommendation; high-quality evidence).
137. Alternatives for fluconazole-refractory disease include an echinocandin (micafungin: 150 mg daily; caspofungin: 70-mg loading dose, then 50 mg daily; or anidulafungin: 200 mg daily) for 14–21 days, OR AmB deoxycholate, 0.3–0.7 mg/kg daily, for 21 days (strong recommendation; high-quality evidence).
138. Posaconazole suspension, 400 mg twice daily, or extended-release tablets, 300 mg once daily, could be considered for fluconazole-refractory disease (weak recommendation; low-quality evidence).
139. For patients who have recurrent esophagitis, chronic suppressive therapy with fluconazole, 100–200 mg 3 times weekly, is recommended (strong recommendation; high-quality evidence).
140. For HIV-infected patients, antiretroviral therapy is strongly recommended to reduce the incidence of recurrent infections (strong recommendation; high-quality evidence).
Evidence Summary
Esophageal candidiasis typically occurs at lower CD4 counts than oropharyngeal disease [528–530]. The advent of effective antiretroviral therapy has led to a dramatic decline in the prevalence of esophageal candidiasis and a marked diminution in cases of refractory disease [531]. Most cases of esophageal candidiasis are caused by C. albicans. However, symptomatic infections caused by C. glabrata, C. dubliniensis, and C. krusei have been described [534].
The presence of oropharyngeal candidiasis and dysphagia or odynophagia in an immunocompromised host is frequently predictive of esophageal candidiasis, although esophageal candidiasis can present as odynophagia without concomitant oropharyngeal candidiasis. A therapeutic trial with fluconazole for patients with presumed esophageal candidiasis is a cost-effective alternative to endoscopic examination. In general, most patients with esophageal candidiasis will have improvement or resolution of their symptoms within 7 days after the initiation of antifungal therapy [557].
Fluconazole is superior to ketoconazole, itraconazole capsules, and flucytosine, and is comparable to itraconazole solution for the treatment of esophageal candidiasis [558, 559]; up to 80% of patients with fluconazole-refractory infections will respond to itraconazole solution [547]. Voriconazole is as efficacious as fluconazole and has shown success in the treatment of fluconazole-refractory mucosal candidiasis [63, 549].
The echinocandins are as effective as fluconazole but are associated with higher relapse rates than those observed with fluconazole [24, 87, 88, 550]. Thus, higher doses of echinocandins are recommended for use for esophageal disease than are used for candidemia to decrease relapses. Higher doses have been studied for micafungin [560]. Fluconazole-refractory disease responds to caspofungin, and it is likely that micafungin and anidulafungin are as effective as caspofungin. In patients with advanced AIDS, recurrent infections are common, and long-term suppressive therapy with fluconazole is effective in decreasing the recurrence rates [53]. The use of effective antiretroviral therapy has dramatically decreased the incidence of esophageal candidiasis in HIV-infected patients.
Notes
Acknowledgments. The Expert Panel expresses its gratitude for thoughtful reviews of an earlier version by Anna Thorner and Pranatharthi Chandrasekar; and David van Duin as liaison of the IDSA Standards and Practice Guidelines Committee (SPGC). The panel also greatly appreciates the work of Charles B. Wessels and Michele Klein Fedyshin of the Health Sciences Library System of the University of Pittsburgh for the development and execution of the systematic literature searches for this guideline.
Financial support. Support for this guideline was provided by the Infectious Diseases Society of America.
Potential conflicts of interest. The following list is a reflection of what has been reported to IDSA. To provide thorough transparency, IDSA requires full disclosure of all relationships, regardless of relevancy to the guideline topic. Evaluation of such relationships as potential conflicts of interest (COI) is determined by a review process that includes assessment by the SPGC Chair, the SPGC liaison to the development panel, and the Board of Directors liaison to the SPGC and, if necessary, the COI Task Force of the Board. This assessment of disclosed relationships for possible COI will be based on the relative weight of the financial relationship (ie, monetary amount) and the relevance of the relationship (ie, the degree to which an association might reasonably be interpreted by an independent observer as related to the topic or recommendation of consideration). The reader of these guidelines should be mindful of this when the list of disclosures is reviewed. For activities outside of the submitted work, P. G. P. served as a consultant to Merck, Astellas (past), Gilead, T2 Biosystems, Scynexis, Viamet, IMMY Diagnostics, and Pfizer (past) and has received research grants from T2 Biosystems, Gilead, Merck, Astellas, Scynexis, and IMMY. For activities outside of the submitted work, C. A. K. has received research grants from VA Cooperative Studies, Merck, the Centers for Disease Control and Prevention (CDC) and The National Institute on Aging (all past), and has received royalties from UpToDate. For activities outside of the submitted work, D. A. has served a consultant to Merck, Astellas, Pfizer, Seachaid, Mayne, Roche, Theravance, Viamet, and Scynexis and has received research grants from Merck, Pfizer, MSG, Actellion, Theravance, Scynexis, and Astellas. For activities outside of the submitted work, C. J. C. has consulted for Merck, and received research grants from Pfizer, Merck, Astellas, CSL Behring, and T2 Diagnostics. For activities outside of the submitted work, K. A. M. has received research grants from Pfizer, Astellas, Merck, and the National Institutes of Health (NIH) and served as a consultant for Astellas, Chimerix, Cidara, Genentech, Merck, Revolution Medicines, and Theravance. She has a licensed patent to MycoMed Technologies. For activities outside of the submitted work, L. O.-Z. has served as a consultant to Viracor (past), Novadigm (past), Pfizer (past), Astellas, Cidara, Scynexis, and Merck and has received research grants from Merck (past), Astellas, Pfizer (past), Immunetics, Associates of Cape Cod (past), and T2 Biosystems, and has been on the speakers' bureau for Merck and Pfizer. For activities outside of the submitted work, A. C. R. has received research grants from Merck and T2 Biosystems, and royalties from UpToDate. For activities outside of the submitted work, J. A. V. has served as a consultant for Astellas, Forest, served on promotional speakers' bureau for Astellas, Pfizer, Forest, and Astra Zeneca, and has received research grants from Astellas, Pfizer, Merck, MSG, T2 Biosystems, and NIH/National Institute of Dental and Craniofacial Research. For activities outside of the submitted work, T. J. W. has served as a consultant for Astellas, Drais (past), Novartis, Pfizer, Methylgene, SigmaTau, Merck, ContraFect Trius, and has received research grants from SOS Kids Foundation, Sharpe Family Foundation, Astellas, Cubist, Theravance, Medicines Company, Actavis, Pfizer, Merck, Novartis, ContraFect, and The Schueler Foundation. For activities outside of the submitted work, T. E. Z. has served as a consultant for Astellas, Pfizer, Merck, and Cubist (all past) and has received research grants from Merck (past), Cubist (past), Agency for Health Research and Quality, CDC, NIH, and the Thrasher Foundation. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Pappas PG, Kauffman CA, Andes D et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:503–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guyatt GH, Oxman AD, Vist GE et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US GRADE Network. Approach and implications to rating the quality of evidence and strength of recommendations using the GRADE methodology. Available at: http://www.gradeworkinggroup.org/ Accessed 10 November 2015.
- 4.Magill SS, Edwards JR, Bamberg W et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–17. [DOI] [PubMed] [Google Scholar]
- 6.Gudlaugsson O, Gillespie S, Lee K et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis 2003; 37:1172–7. [DOI] [PubMed] [Google Scholar]
- 7.Pappas PG, Rex JH, Lee J et al. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis 2003; 37:634–43. [DOI] [PubMed] [Google Scholar]
- 8.Pfaller M, Neofytos D, Diekema D et al. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance(R)) registry, 2004–2008. Diagn Microbiol Infect Dis 2012; 74:323–31. [DOI] [PubMed] [Google Scholar]
- 9.Diekema D, Arbefeville S, Boyken L, Kroeger J, Pfaller M. The changing epidemiology of healthcare-associated candidemia over three decades. Diagn Microbiol Infect Dis 2012; 73:45–8. [DOI] [PubMed] [Google Scholar]
- 10.Pfaller MA, Messer SA, Moet GJ, Jones RN, Castanheira M. Candida bloodstream infections: comparison of species distribution and resistance to echinocandin and azole antifungal agents in intensive care unit (ICU) and non-ICU settings in the SENTRY Antimicrobial Surveillance Program (2008–2009). Int J Antimicrob Agents 2011; 38:65–9. [DOI] [PubMed] [Google Scholar]
- 11.Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. Candida bloodstream infections: comparison of species distributions and antifungal resistance patterns in community-onset and nosocomial isolates in the SENTRY Antimicrobial Surveillance Program, 2008–2009. Antimicrob Agents Chemother 2011; 55:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009). J Clin Microbiol 2011; 49:396–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan J, Meltzer MI, Plikaytis BD et al. Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from population-based candidemia surveillance. Infect Control Hosp Epidemiol 2005; 26:540–7. [DOI] [PubMed] [Google Scholar]
- 14.Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis 2012; 54:1739–46. [DOI] [PubMed] [Google Scholar]
- 15.Grim SA, Berger K, Teng C et al. Timing of susceptibility-based antifungal drug administration in patients with Candida bloodstream infection: correlation with outcomes. J Antimicrob Chemother 2012; 67:707–14. [DOI] [PubMed] [Google Scholar]
- 16.Ostrosky-Zeichner L, Kullberg BJ, Bow EJ et al. Early treatment of candidemia in adults: a review. Med Mycol 2011; 49:113–20. [DOI] [PubMed] [Google Scholar]
- 17.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 2005; 49:3640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garey KW, Rege M, Pai MP et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 2006; 43:25–31. [DOI] [PubMed] [Google Scholar]
- 19.Andes DR, Safdar N, Baddley JW et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis 2012; 54:1110–22. [DOI] [PubMed] [Google Scholar]
- 20.Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis 2013; 56:1284–92. [DOI] [PubMed] [Google Scholar]
- 21.Rex JH, Bennett JE, Sugar AM et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med 1994; 331:1325–30. [DOI] [PubMed] [Google Scholar]
- 22.Rex JH, Pappas PG, Karchmer AW et al. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin Infect Dis 2003; 36:1221–8. [DOI] [PubMed] [Google Scholar]
- 23.Kullberg BJ, Sobel JD, Ruhnke M et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet 2005; 366:1435–42. [DOI] [PubMed] [Google Scholar]
- 24.Krause DS, Simjee AE, van Rensburg C et al. A randomized, double-blind trial of anidulafungin versus fluconazole for the treatment of esophageal candidiasis. Clin Infect Dis 2004; 39:770–5. [DOI] [PubMed] [Google Scholar]
- 25.Mora-Duarte J, Betts R, Rotstein C et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 2002; 347:2020–9. [DOI] [PubMed] [Google Scholar]
- 26.Kuse ER, Chetchotisakd P, da Cunha CA et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007; 369:1519–27. [DOI] [PubMed] [Google Scholar]
- 27.Reboli AC, Rotstein C, Pappas PG et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356:2472–82. [DOI] [PubMed] [Google Scholar]
- 28.Pappas PG, Rotstein CM, Betts RF et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis 2007; 45:883–93. [DOI] [PubMed] [Google Scholar]
- 29.Anaissie EJ, Vartivarian SE, Abi-Said D et al. Fluconazole versus amphotericin B in the treatment of hematogenous candidiasis: a matched cohort study. Am J Med 1996; 101:170–6. [DOI] [PubMed] [Google Scholar]
- 30.Ruhnke M, Paiva JA, Meersseman W et al. Anidulafungin for the treatment of candidaemia/invasive candidiasis in selected critically ill patients. Clin Microbiol Infect 2012; 18:680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reboli AC, Shorr AF, Rotstein C et al. Anidulafungin compared with fluconazole for treatment of candidemia and other forms of invasive candidiasis caused by Candida albicans: a multivariate analysis of factors associated with improved outcome. BMC Infect Dis 2011; 11:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Betts RF, Nucci M, Talwar D et al. A Multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin Infect Dis 2009; 48:1676–84. [DOI] [PubMed] [Google Scholar]
- 33.Nucci M, Colombo AL, Petti M et al. An open-label study of anidulafungin for the treatment of candidaemia/invasive candidiasis in Latin America. Mycoses 2014; 57:12–8. [DOI] [PubMed] [Google Scholar]
- 34.Vazquez J, Reboli AC, Pappas PG et al. Evaluation of an early step-down strategy from intravenous anidulafungin to oral azole therapy for the treatment of candidemia and other forms of invasive candidiasis: results from an open-label trial. BMC Infect Dis 2014; 14:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander BD, Johnson MD, Pfeiffer CD et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 2013; 56:1724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis JS 2nd, Wiederhold NP, Wickes BL, Patterson TF, Jorgensen JH. Rapid emergence of echinocandin resistance in Candida glabrata resulting in clinical and microbiologic failure. Antimicrob Agents Chemother 2013; 57:4559–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castanheira M, Woosley LN, Messer SA, Diekema DJ, Jones RN, Pfaller MA. Frequency of fks mutations among Candida glabrata isolates from a 10-year global collection of bloodstream infection isolates. Antimicrob Agents Chemother 2014; 58:577–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lortholary O, Desnos-Ollivier M, Sitbon K, Fontanet A, Bretagne S, Dromer F. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2,441 patients. Antimicrob Agents Chemother 2011; 55:532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011; 64:380–2. [DOI] [PubMed] [Google Scholar]
- 40.Girmenia C, Gentile G, Micozzi A, Martino P. Nephrotoxicity of amphotericin B desoxycholate. Clin Infect Dis 2001; 33:915–6. [DOI] [PubMed] [Google Scholar]
- 41.Wingard JR, Kubilis P, Lee L et al. Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin Infect Dis 1999; 29:1402–7. [DOI] [PubMed] [Google Scholar]
- 42.Safdar A, Ma J, Saliba F et al. Drug-induced nephrotoxicity caused by amphotericin B lipid complex and liposomal amphotericin B: a review and meta-analysis. Medicine (Baltimore) 2010; 89:236–44. [DOI] [PubMed] [Google Scholar]
- 43.Walsh TJ, Hiemenz JW, Seibel NL et al. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin Infect Dis 1998; 26:1383–96. [DOI] [PubMed] [Google Scholar]
- 44.Groll AH, Giri N, Petraitis V et al. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J Infect Dis 2000; 182:274–82. [DOI] [PubMed] [Google Scholar]
- 45.Bates DW, Su L, Yu DT et al. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin Infect Dis 2001; 32:686–93. [DOI] [PubMed] [Google Scholar]
- 46.Pfaller MA, Andes D, Diekema DJ, Espinel-Ingroff A, Sheehan D. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist Updat 2010; 13:180–95. [DOI] [PubMed] [Google Scholar]
- 47.Pfaller MA, Andes D, Arendrup MC et al. Clinical breakpoints for voriconazole and Candida spp. revisited: review of microbiologic, molecular, pharmacodynamic, and clinical data as they pertain to the development of species-specific interpretive criteria. Diagn Microbiol Infect Dis 2011; 70:330–43. [DOI] [PubMed] [Google Scholar]
- 48.Pfaller MA, Boyken L, Hollis RJ et al. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J Clin Microbiol 2008; 46:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfaller MA, Boyken L, Hollis RJ et al. Wild-type MIC distributions and epidemiological cutoff values for the echinocandins and Candida spp. J Clin Microbiol 2010; 48:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfaller MA, Castanheira M, Diekema DJ, Messer SA, Jones RN. Triazole and echinocandin MIC distributions with epidemiological cutoff values for differentiation of wild-type strains from non-wild-type strains of six uncommon species of Candida. J Clin Microbiol 2011; 49:3800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfaller MA, Castanheira M, Messer SA, Moet GJ, Jones RN. Echinocandin and triazole antifungal susceptibility profiles for Candida spp., Cryptococcus neoformans, and Aspergillus fumigatus: application of new CLSI clinical breakpoints and epidemiologic cutoff values to characterize resistance in the SENTRY Antimicrobial Surveillance Program (2009). Diagn Microbiol Infect Dis 2011; 69:45–50. [DOI] [PubMed] [Google Scholar]
- 52.Bruggemann RJ, Alffenaar JW, Blijlevens NM et al. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis 2009; 48:1441–58. [DOI] [PubMed] [Google Scholar]
- 53.Goldman M, Cloud GA, Wade KD et al. A randomized study of the use of fluconazole in continuous versus episodic therapy in patients with advanced HIV infection and a history of oropharyngeal candidiasis: AIDS Clinical Trials Group Study 323/Mycoses Study Group Study 40. Clin Infect Dis 2005; 41:1473–80. [DOI] [PubMed] [Google Scholar]
- 54.Sobel JD, Kapernick PS, Zervos M et al. Treatment of complicated Candida vaginitis: comparison of single and sequential doses of fluconazole. Am J Obstet Gynecol 2001; 185:363–9. [DOI] [PubMed] [Google Scholar]
- 55.Zimmermann T, Yeates RA, Laufen H, Pfaff G, Wildfeuer A. Influence of concomitant food intake on the oral absorption of two triazole antifungal agents, itraconazole and fluconazole. Eur J Clin Pharmacol 1994; 46:147–50. [DOI] [PubMed] [Google Scholar]
- 56.Thaler F, Bernard B, Tod M et al. Fluconazole penetration in cerebral parenchyma in humans at steady state. Antimicrob Agents Chemother 1995; 39:1154–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tod M, Lortholary O, Padoin C, Chaine G. Intravitreous penetration of fluconazole during endophthalmitis. Clin Microbiol Infect 1997; 3:379A. [DOI] [PubMed] [Google Scholar]
- 58.Tucker RM, Williams PL, Arathoon EG et al. Pharmacokinetics of fluconazole in cerebrospinal fluid and serum in human coccidioidal meningitis. Antimicrob Agents Chemother 1988; 32:369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dodds Ashley ES, Lewis R, Lewis JS, Martin C, Andes D. Pharmacology of systemic antifungal agents. Clin Infect Dis 2006; 43:S28–39. [Google Scholar]
- 60.Eichel M, Just-Nubling G, Helm EB, Stille W. Itraconazole suspension in the treatment of HIV-infected patients with fluconazole-resistant oropharyngeal candidiasis and esophagitis [in German]. Mycoses 1996; 39(suppl 1):102–6. [DOI] [PubMed] [Google Scholar]
- 61.Lange D, Pavao JH, Wu J, Klausner M. Effect of a cola beverage on the bioavailability of itraconazole in the presence of H2 blockers. J Clin Pharmacol 1997; 37:535–40. [DOI] [PubMed] [Google Scholar]
- 62.Van Peer A, Woestenborghs R, Heykants J, Gasparini R, Gauwenbergh G. The effects of food and dose on the oral systemic availability of itraconazole in healthy subjects. Eur J Clin Pharmacol 1989; 36:423–6. [DOI] [PubMed] [Google Scholar]
- 63.Ally R, Schurmann D, Kreisel W et al. A randomized, double-blind, double-dummy, multicenter trial of voriconazole and fluconazole in the treatment of esophageal candidiasis in immunocompromised patients. Clin Infect Dis 2001; 33:1447–54. [DOI] [PubMed] [Google Scholar]
- 64.Kethireddy S, Andes D. CNS pharmacokinetics of antifungal agents. Expert Opin Drug Metab Toxicol 2007; 3:573–81. [DOI] [PubMed] [Google Scholar]
- 65.Purkins L, Wood N, Greenhalgh K, Eve MD, Oliver SD, Nichols D. The pharmacokinetics and safety of intravenous voriconazole—a novel wide-spectrum antifungal agent. Br J Clin Pharmacol 2003; 56(suppl 1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwartz S, Ruhnke M, Ribaud P et al. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood 2005; 106:2641–5. [DOI] [PubMed] [Google Scholar]
- 67.Purkins L, Wood N, Kleinermans D, Nichols D. Histamine H2-receptor antagonists have no clinically significant effect on the steady-state pharmacokinetics of voriconazole. Br J Clin Pharmacol 2003; 56(suppl 1):51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Purkins L, Wood N, Kleinermans D, Greenhalgh K, Nichols D. Effect of food on the pharmacokinetics of multiple-dose oral voriconazole. Br J Clin Pharmacol 2003; 56(suppl 1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neofytos D, Lombardi LR, Shields RK et al. Administration of voriconazole in patients with renal dysfunction. Clin Infect Dis 2012; 54:913–21. [DOI] [PubMed] [Google Scholar]
- 70.Oude Lashof AM, Sobel JD, Ruhnke M et al. Safety and tolerability of voriconazole in patients with baseline renal insufficiency and candidemia. Antimicrob Agents Chemother 2012; 56:3133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alffenaar JW, de Vos T, Uges DR, Daenen SM. High voriconazole trough levels in relation to hepatic function: how to adjust the dosage? Br J Clin Pharmacol 2009; 67:262–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ikeda Y, Umemura K, Kondo K, Sekiguchi K, Miyoshi S, Nakashima M. Pharmacokinetics of voriconazole and cytochrome P450 2C19 genetic status. Clin Pharmacol Ther 2004; 75:587–8. [DOI] [PubMed] [Google Scholar]
- 73.Eiden C, Peyriere H, Cociglio M et al. Adverse effects of voriconazole: analysis of the French Pharmacovigilance Database. Ann Pharmacother 2007; 41:755–63. [DOI] [PubMed] [Google Scholar]
- 74.Hoffman HL, Rathbun RC. Review of the safety and efficacy of voriconazole. Expert Opin Investig Drugs 2002; 11:409–29. [DOI] [PubMed] [Google Scholar]
- 75.Malani AN, Kerr LE, Kauffman CA. Voriconazole: how to use this antifungal agent and what to expect. Semin Respir Crit Care Med 2015; 36:786–95. [DOI] [PubMed] [Google Scholar]
- 76.Pfaller MA, Messer SA, Boyken L et al. In vitro activities of voriconazole, posaconazole, and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS global antifungal surveillance program. Diagn Microbiol Infect Dis 2004; 48:201–5. [DOI] [PubMed] [Google Scholar]
- 77.Krishna G, Ma L, Martinho M, O'Mara E. Single-dose phase I study to evaluate the pharmacokinetics of posaconazole in new tablet and capsule formulations relative to oral suspension. Antimicrob Agents Chemother 2012; 56:4196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krishna G, Ma L, Martinho M, Preston RA, O'Mara E. A new solid oral tablet formulation of posaconazole: a randomized clinical trial to investigate rising single- and multiple-dose pharmacokinetics and safety in healthy volunteers. J Antimicrob Chemother 2012; 67:2725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Courtney R, Wexler D, Radwanski E, Lim J, Laughlin M. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br J Clin Pharmacol 2004; 57:218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ezzet F, Wexler D, Courtney R, Krishna G, Lim J, Laughlin M. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin Pharmacokinet 2005; 44:211–20. [DOI] [PubMed] [Google Scholar]
- 81.Alffenaar JW, van Assen S, van der Werf TS, Kosterink JG, Uges DR. Omeprazole significantly reduces posaconazole serum trough level. Clin Infect Dis 2009; 48:839. [DOI] [PubMed] [Google Scholar]
- 82.Chandrasekar PH, Sobel JD. Micafungin: a new echinocandin. Clin Infect Dis 2006; 42:1171–8. [DOI] [PubMed] [Google Scholar]
- 83.Deresinski SC, Stevens DA. Caspofungin. Clin Infect Dis 2003; 36:1445–57. [DOI] [PubMed] [Google Scholar]
- 84.Vazquez JA, Sobel JD. Anidulafungin: a novel echinocandin. Clin Infect Dis 2006; 43:215–22. [DOI] [PubMed] [Google Scholar]
- 85.Dannaoui E, Desnos-Ollivier M, Garcia-Hermoso D et al. Candida spp. with acquired echinocandin resistance, France, 2004–2010. Emerg Infect Dis 2012; 18:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shields RK, Nguyen MH, Press EG, Updike CL, Clancy CJ. Anidulafungin and micafungin MIC breakpoints are superior to that of caspofungin for identifying FKS mutant Candida glabrata strains and echinocandin resistance. Antimicrob Agents Chemother 2013; 57:6361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Wet N, Llanos-Cuentas A, Suleiman J et al. A randomized, double-blind, parallel-group, dose-response study of micafungin compared with fluconazole for the treatment of esophageal candidiasis in HIV-positive patients. Clin Infect Dis 2004; 39:842–9. [DOI] [PubMed] [Google Scholar]
- 88.Villanueva A, Arathoon EG, Gotuzzo E, Berman RS, DiNubile MJ, Sable CA. A randomized double-blind study of caspofungin versus amphotericin for the treatment of candidal esophagitis. Clin Infect Dis 2001; 33:1529–35. [DOI] [PubMed] [Google Scholar]
- 89.Kauffman CA. Candiduria. Clin Infect Dis 2005; 41(suppl 6):S371–6. [DOI] [PubMed] [Google Scholar]
- 90.Malani AN, Kauffman CA. Candida urinary tract infections: treatment options. Expert Rev Anti Infect Ther 2007; 5:277–84. [DOI] [PubMed] [Google Scholar]
- 91.Francis P, Walsh TJ. Evolving role of flucytosine in immunocompromised patients: new insights into safety, pharmacokinetics, and antifungal therapy. Clin Infect Dis 1992; 15:1003–18. [DOI] [PubMed] [Google Scholar]
- 92.Pasqualotto AC, Howard SJ, Moore CB, Denning DW. Flucytosine therapeutic monitoring: 15 years experience from the UK. J Antimicrob Chemother 2007; 59:791–3. [DOI] [PubMed] [Google Scholar]
- 93.Barchiesi F, Arzeni D, Caselli F, Scalise G. Primary resistance to flucytosine among clinical isolates of Candida spp. J Antimicrob Chemother 2000; 45:408–9. [DOI] [PubMed] [Google Scholar]
- 94.Fisher JF, Sobel JD, Kauffman CA, Newman CA. Candida urinary tract infections—treatment. Clin Infect Dis 2011; 52(suppl 6):S457–66. [DOI] [PubMed] [Google Scholar]
- 95.Lestner JM, Smith PB, Cohen-Wolkowiez M, Benjamin DK Jr, Hope WW. Antifungal agents and therapy for infants and children with invasive fungal infections: a pharmacological perspective. Br J Clin Pharmacol 2013; 75:1381–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Starke JR, Mason EO Jr, Kramer WG, Kaplan SL. Pharmacokinetics of amphotericin B in infants and children. J Infect Dis 1987; 155:766–74. [DOI] [PubMed] [Google Scholar]
- 97.Koren G, Lau A, Klein J et al. Pharmacokinetics and adverse effects of amphotericin B in infants and children. J Pediatr 1988; 113:559–63. [DOI] [PubMed] [Google Scholar]
- 98.Benson JM, Nahata MC. Pharmacokinetics of amphotericin B in children. Antimicrob Agents Chemother 1989; 33:1989–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Walsh TJ, Whitcomb P, Piscitelli S et al. Safety, tolerance, and pharmacokinetics of amphotericin B lipid complex in children with hepatosplenic candidiasis. Antimicrob Agents Chemother 1997; 41:1944–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hong Y, Shaw PJ, Nath CE et al. Population pharmacokinetics of liposomal amphotericin B in pediatric patients with malignant diseases. Antimicrob Agents Chemother 2006; 50:935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baley JE, Meyers C, Kliegman RM, Jacobs MR, Blumer JL. Pharmacokinetics, outcome of treatment, and toxic effects of amphotericin B and 5-fluorocytosine in neonates. J Pediatr 1990; 116:791–7. [DOI] [PubMed] [Google Scholar]
- 102.Lee JW, Seibel NL, Amantea M, Whitcomb P, Pizzo PA, Walsh TJ. Safety and pharmacokinetics of fluconazole in children with neoplastic diseases. J Pediatr 1992; 120:987–93. [DOI] [PubMed] [Google Scholar]
- 103.Saxen H, Hoppu K, Pohjavuori M. Pharmacokinetics of fluconazole in very low birth weight infants during the first two weeks of life. Clin Pharmacol Ther 1993; 54:269–77. [DOI] [PubMed] [Google Scholar]
- 104.Seay RE, Larson TA, Toscano JP, Bostrom BC, O'Leary MC, Uden DL. Pharmacokinetics of fluconazole in immune-compromised children with leukemia or other hematologic diseases. Pharmacotherapy 1995; 15:52–8. [PubMed] [Google Scholar]
- 105.Wade KC, Wu D, Kaufman DA et al. Population pharmacokinetics of fluconazole in young infants. Antimicrob Agents Chemother 2008; 52:4043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Walsh TJ, Karlsson MO, Driscoll T et al. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob Agents Chemother 2004; 48:2166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Driscoll TA, Yu LC, Frangoul H et al. Comparison of pharmacokinetics and safety of voriconazole intravenous-to-oral switch in immunocompromised children and healthy adults. Antimicrob Agents Chemother 2011; 55:5770–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Driscoll TA, Frangoul H, Nemecek ER et al. Comparison of pharmacokinetics and safety of voriconazole intravenous-to-oral switch in immunocompromised adolescents and healthy adults. Antimicrob Agents Chemother 2011; 55:5780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hope WW, Smith PB, Arrieta A et al. Population pharmacokinetics of micafungin in neonates and young infants. Antimicrob Agents Chemother 2010; 54:2633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Benjamin DK Jr, Driscoll T, Seibel NL et al. Safety and pharmacokinetics of intravenous anidulafungin in children with neutropenia at high risk for invasive fungal infections. Antimicrob Agents Chemother 2006; 50:632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dowell JA, Knebel W, Ludden T, Stogniew M, Krause D, Henkel T. Population pharmacokinetic analysis of anidulafungin, an echinocandin antifungal. J Clin Pharmacol 2004; 44:590–8. [DOI] [PubMed] [Google Scholar]
- 112.Livermore JL, Felton TW, Abbott J et al. Pharmacokinetics and pharmacodynamics of anidulafungin for experimental Candida endophthalmitis: insights into the utility of echinocandins for treatment of a potentially sight-threatening infection. Antimicrob Agents Chemother 2013; 57:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moudgal VV, Sobel JD. Antifungal drugs in pregnancy: a review. Expert Opin Drug Saf 2003; 2:475–83. [DOI] [PubMed] [Google Scholar]
- 114.Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother 2009; 53:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johnson LB, Kauffman CA. Voriconazole: a new triazole antifungal agent. Clin Infect Dis 2003; 36:630–7. [DOI] [PubMed] [Google Scholar]
- 116.Neely M, Rushing T, Kovacs A, Jelliffe R, Hoffman J. Voriconazole pharmacokinetics and pharmacodynamics in children. Clin Infect Dis 2010; 50:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis 2008; 46:201–11. [DOI] [PubMed] [Google Scholar]
- 118.Smith J, Safdar N, Knasinski V et al. Voriconazole therapeutic drug monitoring. Antimicrob Agents Chemother 2006; 50:1570–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vermes A, Guchelaar HJ, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother 2000; 46:171–9. [DOI] [PubMed] [Google Scholar]
- 120.Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts. 3rd informational supplement 2008. Wayne, PA: CLSI.
- 121.Pfaller MA, Espinel-Ingroff A, Canton E et al. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B, flucytosine, and itraconazole and Candida spp. as determined by CLSI broth microdilution. J Clin Microbiol 2012; 50:2040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pfaller MA, Diekema DJ, Andes D et al. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist Updat 2011; 14:164–76. [DOI] [PubMed] [Google Scholar]
- 123.Pfaller MA, Boyken L, Hollis RJ et al. Wild-type MIC distributions and epidemiological cutoff values for posaconazole and voriconazole and Candida spp. as determined by 24-hour CLSI broth microdilution. J Clin Microbiol 2011; 49:630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pfaller MA, Diekema DJ, Sheehan DJ. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin Microbiol Rev 2006; 19:435–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pfaller MA, Castanheira M, Lockhart SR, Ahlquist AM, Messer SA, Jones RN. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J Clin Microbiol 2012; 50:1199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pfaller MA, Castanheira M, Messer SA, Moet GJ, Jones RN. Variation in Candida spp. distribution and antifungal resistance rates among bloodstream infection isolates by patient age: report from the SENTRY Antimicrobial Surveillance Program (2008–2009). Diagn Microbiol Infect Dis 2010; 68:278–83. [DOI] [PubMed] [Google Scholar]
- 127.Ben-Ami R, Olshtain-Pops K, Krieger M et al. Antibiotic exposure as a risk factor for fluconazole-resistant Candida bloodstream infection. Antimicrob Agents Chemother 2012; 56:2518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oxman DA, Chow JK, Frendl G et al. Candidaemia associated with decreased in vitro fluconazole susceptibility: is Candida speciation predictive of the susceptibility pattern? J Antimicrob Chemother 2010; 65:1460–5. [DOI] [PubMed] [Google Scholar]
- 129.Leroy O, Gangneux JP, Montravers P et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006). Crit Care Med 2009; 37:1612–8. [DOI] [PubMed] [Google Scholar]
- 130.Pfeiffer CD, Samsa GP, Schell WA, Reller LB, Perfect JR, Alexander BD. Quantitation of Candida CFU in initial positive blood cultures. J Clin Microbiol 2011; 49:2879–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Telenti A, Steckelberg JM, Stockman L, Edson RS, Roberts GD. Quantitative blood cultures in candidemia. Mayo Clin Proc 1991; 66:1120–3. [DOI] [PubMed] [Google Scholar]
- 132.Nguyen MH, Wissel MC, Shields RK et al. Performance of Candida real-time polymerase chain reaction, beta-D-glucan assay, and blood cultures in the diagnosis of invasive candidiasis. Clin Infect Dis 2012; 54:1240–8. [DOI] [PubMed] [Google Scholar]
- 133.Schell WA, Benton JL, Smith PB et al. Evaluation of a digital microfluidic real-time PCR platform to detect DNA of Candida albicans in blood. Eur J Clin Microbiol Infect Dis 2012; 31:2237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Avni T, Leibovici L, Paul M. PCR diagnosis of invasive candidiasis: systematic review and meta-analysis. J Clin Microbiol 2011; 49:665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McMullan R, Metwally L, Coyle PV et al. A prospective clinical trial of a real-time polymerase chain reaction assay for the diagnosis of candidemia in nonneutropenic, critically ill adults. Clin Infect Dis 2008; 46:890–6. [DOI] [PubMed] [Google Scholar]
- 136.Ness MJ, Vaughan WP, Woods GL. Candida antigen latex test for detection of invasive candidiasis in immunocompromised patients. J Infect Dis 1989; 159:495–502. [DOI] [PubMed] [Google Scholar]
- 137.Thaler M, Pastakia B, Shawker TH, O'Leary T, Pizzo PA. Hepatic candidiasis in cancer patients: the evolving picture of the syndrome. Ann Intern Med 1988; 108:88–100. [DOI] [PubMed] [Google Scholar]
- 138.Ellepola AN, Morrison CJ. Laboratory diagnosis of invasive candidiasis. J Microbiol 2005; 43(Spec No):65–84. [PubMed] [Google Scholar]
- 139.Clancy CJ, Nguyen ML, Cheng S et al. Immunoglobulin G responses to a panel of Candida albicans antigens as accurate and early markers for the presence of systemic candidiasis. J Clin Microbiol 2008; 46:1647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mikulska M, Calandra T, Sanguinetti M, Poulain D, Viscoli C. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: recommendations from the Third European Conference on Infections in Leukemia. Crit Care 2010; 14:R222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yera H, Sendid B, Francois N, Camus D, Poulain D. Contribution of serological tests and blood culture to the early diagnosis of systemic candidiasis. Eur J Clin Microbiol Infect Dis 2001; 20:864–70. [DOI] [PubMed] [Google Scholar]
- 142.Prella M, Bille J, Pugnale M et al. Early diagnosis of invasive candidiasis with mannan antigenemia and antimannan antibodies. Diagn Microbiol Infect Dis 2005; 51:95–101. [DOI] [PubMed] [Google Scholar]
- 143.Tissot F, Lamoth F, Hauser PM et al. Beta-glucan antigenemia anticipates diagnosis of blood culture-negative intraabdominal candidiasis. Am J Respir Crit Care Med 2013; 188:1100–9. [DOI] [PubMed] [Google Scholar]
- 144.Karageorgopoulos DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas ME. Beta-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis 2011; 52:750–70. [DOI] [PubMed] [Google Scholar]
- 145.Lu Y, Chen YQ, Guo YL, Qin SM, Wu C, Wang K. Diagnosis of invasive fungal disease using serum (1-->3)-beta-D-glucan: a bivariate meta-analysis. Intern Med 2011; 50:2783–91. [DOI] [PubMed] [Google Scholar]
- 146.Onishi A, Sugiyama D, Kogata Y et al. Diagnostic accuracy of serum 1,3-beta-D-glucan for Pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol 2012; 50:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jaijakul S, Vazquez JA, Swanson RN, Ostrosky-Zeichner L. (1,3)-beta-D-glucan as a prognostic marker of treatment response in invasive candidiasis. Clin Infect Dis 2012; 55:521–6. [DOI] [PubMed] [Google Scholar]
- 148.Wheat LJ. Approach to the diagnosis of invasive aspergillosis and candidiasis. Clin Chest Med 2009; 30:367–77, viii. [DOI] [PubMed] [Google Scholar]
- 149.Hachem RY, Kontoyiannis DP, Chemaly RF, Jiang Y, Reitzel R, Raad I. Utility of galactomannan enzyme immunoassay and (1,3) beta-D-glucan in diagnosis of invasive fungal infections: low sensitivity for Aspergillus fumigatus infection in hematologic malignancy patients. J Clin Microbiol 2009; 47:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ellis M, Al-Ramadi B, Finkelman M et al. Assessment of the clinical utility of serial beta-D-glucan concentrations in patients with persistent neutropenic fever. J Med Microbiol 2008; 57(Pt 3):287–95. [DOI] [PubMed] [Google Scholar]
- 151.Pickering JW, Sant HW, Bowles CA, Roberts WL, Woods GL. Evaluation of a (1->3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol 2005; 43:5957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kato A, Takita T, Furuhashi M, Takahashi T, Maruyama Y, Hishida A. Elevation of blood (1-->3)-beta-D-glucan concentrations in hemodialysis patients. Nephron 2001; 89:15–9. [DOI] [PubMed] [Google Scholar]
- 153.Usami M, Ohata A, Horiuchi T, Nagasawa K, Wakabayashi T, Tanaka S. Positive (1-->3)-beta-D-glucan in blood components and release of (1-->3)-beta-D-glucan from depth-type membrane filters for blood processing. Transfusion 2002; 42:1189–95. [DOI] [PubMed] [Google Scholar]
- 154.Mennink-Kersten MA, Warris A, Verweij PE. 1,3-beta-D-glucan in patients receiving intravenous amoxicillin-clavulanic acid. N Engl J Med 2006; 354:2834–5. [DOI] [PubMed] [Google Scholar]
- 155.Alexander BD, Smith PB, Davis RD, Perfect JR, Reller LB. The (1,3){beta}-D-glucan test as an aid to early diagnosis of invasive fungal infections following lung transplantation. J Clin Microbiol 2010; 48:4083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mikulska M, Furfaro E, Del Bono V et al. (1–3)-beta-D-glucan in cerebrospinal fluid is useful for the diagnosis of central nervous system fungal infections. Clin Infect Dis 2013; 56:1511–2. [DOI] [PubMed] [Google Scholar]
- 157.Litvintseva AP, Lindsley MD, Gade L et al. Utility of (1–3)-beta-D-glucan testing for diagnostics and monitoring response to treatment during the multistate outbreak of fungal meningitis and other infections. Clin Infect Dis 2014; 58:622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Petraitiene R, Petraitis V, Hope WW et al. Cerebrospinal fluid and plasma (1-->3)-beta-D-glucan as surrogate markers for detection and monitoring of therapeutic response in experimental hematogenous Candida meningoencephalitis. Antimicrob Agents Chemother 2008; 52:4121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Theel ES, Jespersen DJ, Iqbal S et al. Detection of (1, 3)-beta-D-glucan in bronchoalveolar lavage and serum samples collected from immunocompromised hosts. Mycopathologia 2013; 175:33–41. [DOI] [PubMed] [Google Scholar]
- 160.Jeragh A, Ahmad S, Naseem J, Khan ZU. Candida lusitaniae arthritis in an intravenous drug user. Mycoses 2007; 50:430–2. [DOI] [PubMed] [Google Scholar]
- 161.Mularoni A, Furfaro E, Faraci M et al. High levels of beta-D-glucan in immunocompromised children with proven invasive fungal disease. Clin Vaccine Immunol 2010; 17:882–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Smith PB, Benjamin DK Jr, Alexander BD, Johnson MD, Finkelman MA, Steinbach WJ. Quantification of 1,3-beta-D-glucan levels in children: preliminary data for diagnostic use of the beta-glucan assay in a pediatric setting. Clin Vaccine Immunol 2007; 14:924–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mylonakis E, Clancy CJ, Ostrosky-Zeichner L et al. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clin Infect Dis 2015; 60:892–9. [DOI] [PubMed] [Google Scholar]
- 164.Lucignano B, Ranno S, Liesenfeld O et al. Multiplex PCR allows rapid and accurate diagnosis of bloodstream infections in newborns and children with suspected sepsis. J Clin Microbiol 2011; 49:2252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schuster MG, Meibohm A, Lloyd L, Strom B. Risk factors and outcomes of Candida krusei bloodstream infection: a matched, case-control study. J Infect 2013; 66:278–84. [DOI] [PubMed] [Google Scholar]
- 166.Dupont BF, Lortholary O, Ostrosky-Zeichner L, Stucker F, Yeldandi V. Treatment of candidemia and invasive candidiasis in the intensive care unit: post hoc analysis of a randomized, controlled trial comparing micafungin and liposomal amphotericin B. Crit Care 2009; 13:R159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Horn DL, Neofytos D, Anaissie EJ et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 2009; 48:1695–703. [DOI] [PubMed] [Google Scholar]
- 168.Labelle AJ, Micek ST, Roubinian N, Kollef MH. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med 2008; 36:2967–72. [DOI] [PubMed] [Google Scholar]
- 169.Kohno S, Izumikawa K, Yoshida M et al. A double-blind comparative study of the safety and efficacy of caspofungin versus micafungin in the treatment of candidiasis and aspergillosis. Eur J Clin Microbiol Infect Dis 2013; 32:387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kett DH, Azoulay E, Echeverria PM, Vincent JL. Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit Care Med 2011; 39:665–70. [DOI] [PubMed] [Google Scholar]
- 171.Mootsikapun P, Hsueh PR, Talwar D, Co VM, Rajadhyaksha V, Ong ML. Intravenous anidulafungin followed optionally by oral voriconazole for the treatment of candidemia in Asian patients: results from an open-label phase III trial. BMC Infect Dis 2013; 13:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Walsh TJ. Echinocandins—an advance in the primary treatment of invasive candidiasis. N Engl J Med 2002; 347:2070–2. [DOI] [PubMed] [Google Scholar]
- 173.Bennett JE. Echinocandins for candidemia in adults without neutropenia. N Engl J Med 2006; 355:1154–9. [DOI] [PubMed] [Google Scholar]
- 174.Bassetti M, Merelli M, Righi E et al. Epidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and Spain. J Clin Microbiol 2013; 51:4167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Orasch C, Marchetti O, Garbino J et al. Candida species distribution and antifungal susceptibility testing according to European Committee on Antimicrobial Susceptibility Testing and new vs. old Clinical and Laboratory Standards Institute clinical breakpoints: a 6-year prospective candidaemia survey from the fungal infection network of Switzerland. Clin Microbiol Infect 2014; 20:698–705. [DOI] [PubMed] [Google Scholar]
- 176.Fernandez-Ruiz M, Aguado JM, Almirante B et al. Initial use of echinocandins does not negatively influence outcome in Candida parapsilosis bloodstream infection: a propensity score analysis. Clin Infect Dis 2014; 58:1413–21. [DOI] [PubMed] [Google Scholar]
- 177.Espinel-Ingroff A, Barchiesi F, Cuenca-Estrella M et al. International and multicenter comparison of EUCAST and CLSI M27-A2 broth microdilution methods for testing susceptibilities of Candida spp. to fluconazole, itraconazole, posaconazole, and voriconazole. J Clin Microbiol 2005; 43:3884–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007; 20:133–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Espinel-Ingroff A, Pfaller MA, Bustamante B et al. Multilaboratory study of epidemiological cutoff values for detection of resistance in eight Candida species to fluconazole, posaconazole, and voriconazole. Antimicrob Agents Chemother 2014; 58:2006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pfaller MA, Messer SA, Rhomberg PR, Jones RN, Castanheira M. In vitro activities of isavuconazole and comparator antifungal agents tested against a global collection of opportunistic yeasts and molds. J Clin Microbiol 2013; 51:2608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shields RK, Nguyen MH, Press EG, Updike CL, Clancy CJ. Caspofungin MICs correlate with treatment outcomes among patients with Candida glabrata invasive candidiasis and prior echinocandin exposure. Antimicrob Agents Chemother 2013; 57:3528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Beyda ND, Lewis RE, Garey KW. Echinocandin resistance in Candida species: mechanisms of reduced susceptibility and therapeutic approaches. Ann Pharmacother 2012; 46:1086–96. [DOI] [PubMed] [Google Scholar]
- 183.Vinikoor MJ, Zoghby J, Cohen KL, Tucker JD. Do all candidemic patients need an ophthalmic examination? Int J Infect Dis 2013; 17:e146–8. [DOI] [PubMed] [Google Scholar]
- 184.Wagner M, Bonhoeffer J, Erb TO et al. Prospective study on central venous line associated bloodstream infections. Arch Dis Child 2011; 96:827–31. [DOI] [PubMed] [Google Scholar]
- 185.Chow JK, Golan Y, Ruthazer R et al. Factors associated with candidemia caused by non-albicans Candida species versus Candida albicans in the intensive care unit. Clin Infect Dis 2008; 46:1206–13. [DOI] [PubMed] [Google Scholar]
- 186.Chow JK, Golan Y, Ruthazer R et al. Risk factors for albicans and non-albicans candidemia in the intensive care unit. Crit Care Med 2008; 36:1993–8. [DOI] [PubMed] [Google Scholar]
- 187.Advani S, Reich NG, Sengupta A, Gosey L, Milstone AM. Central line-associated bloodstream infection in hospitalized children with peripherally inserted central venous catheters: extending risk analyses outside the intensive care unit. Clin Infect Dis 2011; 52:1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ruiz LS, Khouri S, Hahn RC et al. Candidemia by species of the Candida parapsilosis complex in children's hospital: prevalence, biofilm production and antifungal susceptibility. Mycopathologia 2013; 175:231–9. [DOI] [PubMed] [Google Scholar]
- 189.Tumbarello M, Fiori B, Trecarichi EM et al. Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS One 2012; 7:e33705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nucci M, Anaissie E, Betts RF et al. Early removal of central venous catheter in patients with candidemia does not improve outcome: analysis of 842 patients from 2 randomized clinical trials. Clin Infect Dis 2010; 51:295–303. [DOI] [PubMed] [Google Scholar]
- 191.Velasco E, Portugal RD. Factors prompting early central venous catheter removal from cancer patients with candidaemia. Scand J Infect Dis 2011; 43:27–31. [DOI] [PubMed] [Google Scholar]
- 192.Lai YC, Huang LJ, Chen TL et al. Impact of Port-A-Cath device management in cancer patients with candidaemia. J Hosp Infect 2012; 82:281–5. [DOI] [PubMed] [Google Scholar]
- 193.Garnacho-Montero J, Diaz-Martin A, Garcia-Cabrera E, Ruiz Perez de Pipaon M, Hernandez-Caballero C, Lepe-Jimenez JA. Impact on hospital mortality of catheter removal and adequate antifungal therapy in Candida spp. bloodstream infections. J Antimicrob Chemother 2013; 68:206–13. [DOI] [PubMed] [Google Scholar]
- 194.Rex JH, Bennett JE, Sugar AM et al. Intravascular catheter exchange and duration of candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Clin Infect Dis 1995; 21:994–6. [DOI] [PubMed] [Google Scholar]
- 195.Nguyen MH, Peacock JE Jr, Tanner DC et al. Therapeutic approaches in patients with candidemia. Evaluation in a multicenter, prospective, observational study. Arch Intern Med 1995; 155:2429–35. [PubMed] [Google Scholar]
- 196.Luzzati R, Amalfitano G, Lazzarini L et al. Nosocomial candidemia in non-neutropenic patients at an Italian tertiary care hospital. Eur J Clin Microbiol Infect Dis 2000; 19:602–7. [DOI] [PubMed] [Google Scholar]
- 197.Liu CY, Huang LJ, Wang WS et al. Candidemia in cancer patients: impact of early removal of non-tunneled central venous catheters on outcome. J Infect 2009; 58:154–60. [DOI] [PubMed] [Google Scholar]
- 198.Karlowicz MG, Hashimoto LN, Kelly RE Jr, Buescher ES. Should central venous catheters be removed as soon as candidemia is detected in neonates? Pediatrics 2000; 106:E63. [DOI] [PubMed] [Google Scholar]
- 199.Klatte JM, Newland JG, Jackson MA. Incidence, classification, and risk stratification for Candida central line-associated bloodstream infections in pediatric patients at a tertiary care children's hospital, 2000–2010. Infect Control Hosp Epidemiol 2013; 34:1266–71. [DOI] [PubMed] [Google Scholar]
- 200.Devrim I, Yaman Y, Demirag B et al. A single center's experience with Candida parapsilosis related long-term central venous access device infections: the port removal decision and its outcomes. Pediatr Hematol Oncol 2014; 31:435–41. [DOI] [PubMed] [Google Scholar]
- 201.Nucci M, Anaissie E. Revisiting the source of candidemia: skin or gut? Clin Infect Dis 2001; 33:1959–67. [DOI] [PubMed] [Google Scholar]
- 202.Anaissie EJ, Rex JH, Uzun O, Vartivarian S. Predictors of adverse outcome in cancer patients with candidemia. Am J Med 1998; 104:238–45. [DOI] [PubMed] [Google Scholar]
- 203.Nucci M, Silveira MI, Spector N et al. Risk factors for death among cancer patients with fungemia. Clin Infect Dis 1998; 27:107–11. [DOI] [PubMed] [Google Scholar]
- 204.Velasco E, Bigni R. A prospective cohort study evaluating the prognostic impact of clinical characteristics and comorbid conditions of hospitalized adult and pediatric cancer patients with candidemia. Eur J Clin Microbiol Infect Dis 2008; 27:1071–8. [DOI] [PubMed] [Google Scholar]
- 205.Kanji JN, Laverdiere M, Rotstein C, Walsh TJ, Shah PS, Haider S. Treatment of invasive candidiasis in neutropenic patients: systematic review of randomized controlled treatment trials. Leuk Lymphoma 2013; 54:1479–87. [DOI] [PubMed] [Google Scholar]
- 206.Cornely OA, Marty FM, Stucker F, Pappas PG, Ullmann AJ. Efficacy and safety of micafungin for treatment of serious Candida infections in patients with or without malignant disease. Mycoses 2011; 54:e838–47. [DOI] [PubMed] [Google Scholar]
- 207.Ullmann AJ, Akova M, Herbrecht R et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect 2012; 18(suppl 7):53–67. [DOI] [PubMed] [Google Scholar]
- 208.Betts R, Glasmacher A, Maertens J et al. Efficacy of caspofungin against invasive Candida or invasive Aspergillus infections in neutropenic patients. Cancer 2006; 106:466–73. [DOI] [PubMed] [Google Scholar]
- 209.Walsh TJ, Finberg RW, Arndt C et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med 1999; 340:764–71. [DOI] [PubMed] [Google Scholar]
- 210.Walsh TJ, Teppler H, Donowitz GR et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med 2004; 351:1391–402. [DOI] [PubMed] [Google Scholar]
- 211.Walsh TJ, Pappas P, Winston DJ et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med 2002; 346:225–34. [DOI] [PubMed] [Google Scholar]
- 212.Horn DL, Ostrosky-Zeichner L, Morris MI et al. Factors related to survival and treatment success in invasive candidiasis or candidemia: a pooled analysis of two large, prospective, micafungin trials. Eur J Clin Microbiol Infect Dis 2010; 29:223–9. [DOI] [PubMed] [Google Scholar]
- 213.Price TH, Bowden RA, Boeckh M et al. Phase I/II trial of neutrophil transfusions from donors stimulated with G-CSF and dexamethasone for treatment of patients with infections in hematopoietic stem cell transplantation. Blood 2000; 95:3302–9. [PubMed] [Google Scholar]
- 214.Grigull L, Pulver N, Goudeva L et al. G-CSF mobilised granulocyte transfusions in 32 paediatric patients with neutropenic sepsis. Support Care Cancer 2006; 14:910–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Safdar A, Hanna HA, Boktour M et al. Impact of high-dose granulocyte transfusions in patients with cancer with candidemia: retrospective case-control analysis of 491 episodes of Candida species bloodstream infections. Cancer 2004; 101:2859–65. [DOI] [PubMed] [Google Scholar]
- 216.Seidel MG, Peters C, Wacker A et al. Randomized phase III study of granulocyte transfusions in neutropenic patients. Bone Marrow Transplant 2008; 42:679–84. [DOI] [PubMed] [Google Scholar]
- 217.Kontoyiannis DP, Luna MA, Samuels BI, Bodey GP. Hepatosplenic candidiasis. A manifestation of chronic disseminated candidiasis. Infect Dis Clin North Am 2000; 14:721–39. [DOI] [PubMed] [Google Scholar]
- 218.Masood A, Sallah S. Chronic disseminated candidiasis in patients with acute leukemia: emphasis on diagnostic definition and treatment. Leuk Res 2005; 29:493–501. [DOI] [PubMed] [Google Scholar]
- 219.Rammaert B, Desjardins A, Lortholary O. New insights into hepatosplenic candidosis, a manifestation of chronic disseminated candidosis. Mycoses 2012; 55:e74–84. [DOI] [PubMed] [Google Scholar]
- 220.Anttila VJ, Lamminen AE, Bondestam S et al. Magnetic resonance imaging is superior to computed tomography and ultrasonography in imaging infectious liver foci in acute leukaemia. Eur J Haematol 1996; 56:82–7. [DOI] [PubMed] [Google Scholar]
- 221.Hot A, Maunoury C, Poiree S et al. Diagnostic contribution of positron emission tomography with [18F]fluorodeoxyglucose for invasive fungal infections. Clin Microbiol Infect 2011; 17:409–17. [DOI] [PubMed] [Google Scholar]
- 222.Anttila VJ, Elonen E, Nordling S, Sivonen A, Ruutu T, Ruutu P. Hepatosplenic candidiasis in patients with acute leukemia: incidence and prognostic implications. Clin Infect Dis 1997; 24:375–80. [DOI] [PubMed] [Google Scholar]
- 223.De Castro N, Mazoyer E, Porcher R et al. Hepatosplenic candidiasis in the era of new antifungal drugs: a study in Paris 2000–2007. Clin Microbiol Infect 2012; 18:E185–7. [DOI] [PubMed] [Google Scholar]
- 224.Gokhale PC, Barapatre RJ, Advani SH, Kshirsagar NA, Pandya SK. Successful treatment of disseminated candidiasis resistant to amphotericin B by liposomal amphotericin B: a case report. J Cancer Res Clin Oncol 1993; 119:569–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sallah S, Semelka RC, Sallah W, Vainright JR, Philips DL. Amphotericin B lipid complex for the treatment of patients with acute leukemia and hepatosplenic candidiasis. Leuk Res 1999; 23:995–9. [DOI] [PubMed] [Google Scholar]
- 226.Kauffman CA, Bradley SF, Ross SC, Weber DR. Hepatosplenic candidiasis: successful treatment with fluconazole. Am J Med 1991; 91:137–41. [DOI] [PubMed] [Google Scholar]
- 227.Anaissie E, Bodey GP, Kantarjian H et al. Fluconazole therapy for chronic disseminated candidiasis in patients with leukemia and prior amphotericin B therapy. Am J Med 1991; 91:142–50. [DOI] [PubMed] [Google Scholar]
- 228.Cornely OA, Lasso M, Betts R et al. Caspofungin for the treatment of less common forms of invasive candidiasis. J Antimicrob Chemother 2007; 60:363–9. [DOI] [PubMed] [Google Scholar]
- 229.Ostrosky-Zeichner L, Oude Lashof AM, Kullberg BJ, Rex JH. Voriconazole salvage treatment of invasive candidiasis. Eur J Clin Microbiol Infect Dis 2003; 22:651–5. [DOI] [PubMed] [Google Scholar]
- 230.Lehrnbecher T, Attarbaschi A, Duerken M et al. Posaconazole salvage treatment in paediatric patients: a multicentre survey. Eur J Clin Microbiol Infect Dis 2010; 29:1043–5. [DOI] [PubMed] [Google Scholar]
- 231.Poon LM, Chia HY, Tan LK, Liu TC, Koh LP. Successful intensive chemotherapy followed by autologous hematopoietic cell transplantation in a patient with acute myeloid leukemia and hepatosplenic candidiasis: case report and review of literature. Transpl Infect Dis 2009; 11:160–6. [DOI] [PubMed] [Google Scholar]
- 232.Legrand F, Lecuit M, Dupont B et al. Adjuvant corticosteroid therapy for chronic disseminated candidiasis. Clin Infect Dis 2008; 46:696–702. [DOI] [PubMed] [Google Scholar]
- 233.Chaussade H, Bastides F, Lissandre S et al. Usefulness of corticosteroid therapy during chronic disseminated candidiasis: case reports and literature review. J Antimicrob Chemother 2012; 67:1493–5. [DOI] [PubMed] [Google Scholar]
- 234.Eggimann P, Ostrosky-Zeichner L. Early antifungal intervention strategies in ICU patients. Curr Opin Crit Care 2010; 16:465–9. [DOI] [PubMed] [Google Scholar]
- 235.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis 2005; 41:1232–9. [DOI] [PubMed] [Google Scholar]
- 236.Marriott DJ, Playford EG, Chen S et al. Determinants of mortality in non-neutropenic ICU patients with candidaemia. Crit Care 2009; 13:R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pittet D, Monod M, Suter PM, Frenk E, Auckenthaler R. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg 1994; 220:751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Blumberg HM, Jarvis WR, Soucie JM et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey. Clin Infect Dis 2001; 33:177–86. [DOI] [PubMed] [Google Scholar]
- 239.Troughton JA, Browne G, McAuley DF, Walker MJ, Patterson CC, McMullan R. Prior colonisation with Candida species fails to guide empirical therapy for candidaemia in critically ill adults. J Infect 2010; 61:403–9. [DOI] [PubMed] [Google Scholar]
- 240.Leon C, Ruiz-Santana S, Saavedra P et al. A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med 2006; 34:730–7. [DOI] [PubMed] [Google Scholar]
- 241.Michalopoulos AS, Geroulanos S, Mentzelopoulos SD. Determinants of candidemia and candidemia-related death in cardiothoracic ICU patients. Chest 2003; 124:2244–55. [DOI] [PubMed] [Google Scholar]
- 242.Ostrosky-Zeichner L, Pappas PG, Shoham S et al. Improvement of a clinical prediction rule for clinical trials on prophylaxis for invasive candidiasis in the intensive care unit. Mycoses 2011; 54:46–51. [DOI] [PubMed] [Google Scholar]
- 243.Dupont H, Paugam-Burtz C, Muller-Serieys C et al. Predictive factors of mortality due to polymicrobial peritonitis with Candida isolation in peritoneal fluid in critically ill patients. Arch Surg 2002; 137:1341–6; discussion 7. [DOI] [PubMed] [Google Scholar]
- 244.Montravers P, Dupont H, Gauzit R et al. Candida as a risk factor for mortality in peritonitis. Crit Care Med 2006; 34:646–52. [DOI] [PubMed] [Google Scholar]
- 245.Presterl E, Parschalk B, Bauer E, Lassnigg A, Hajdu S, Graninger W. Invasive fungal infections and (1,3)-beta-D-glucan serum concentrations in long-term intensive care patients. Int J Infect Dis 2009; 13:707–12. [DOI] [PubMed] [Google Scholar]
- 246.Mohr JF, Sims C, Paetznick V et al. Prospective survey of (1-->3)-beta-D-glucan and its relationship to invasive candidiasis in the surgical intensive care unit setting. J Clin Microbiol 2011; 49:58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Koo S, Bryar JM, Page JH, Baden LR, Marty FM. Diagnostic performance of the (1-->3)-beta-D-glucan assay for invasive fungal disease. Clin Infect Dis 2009; 49:1650–9. [DOI] [PubMed] [Google Scholar]
- 248.Posteraro B, De Pascale G, Tumbarello M et al. Early diagnosis of candidemia in intensive care unit patients with sepsis: a prospective comparison of (1-->3)-beta-D-glucan assay, Candida score, and colonization index. Crit Care 2011; 15:R249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ostrosky-Zeichner L, Shoham S, Vazquez J et al. MSG-01: a randomized, double-blind, placebo-controlled trial of caspofungin prophylaxis followed by preemptive therapy for invasive candidiasis in high-risk adults in the critical care setting. Clin Infect Dis 2014; 58:1219–26. [DOI] [PubMed] [Google Scholar]
- 250.Digby J, Kalbfleisch J, Glenn A, Larsen A, Browder W, Williams D. Serum glucan levels are not specific for presence of fungal infections in intensive care unit patients. Clin Diagn Lab Immunol 2003; 10:882–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hanson KE, Pfeiffer CD, Lease ED et al. Beta-D-glucan surveillance with preemptive anidulafungin for invasive candidiasis in intensive care unit patients: a randomized pilot study. PLoS One 2012; 7:e42282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Obayashi T, Negishi K, Suzuki T, Funata N. Reappraisal of the serum (1-->3)-beta-D-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis 2008; 46:1864–70. [DOI] [PubMed] [Google Scholar]
- 253.Arendrup MC, Bergmann OJ, Larsson L, Nielsen HV, Jarlov JO, Christensson B. Detection of candidaemia in patients with and without underlying haematological disease. Clin Microbiol Infect 2010; 16:855–62. [DOI] [PubMed] [Google Scholar]
- 254.Parkins MD, Sabuda DM, Elsayed S, Laupland KB. Adequacy of empirical antifungal therapy and effect on outcome among patients with invasive Candida species infections. J Antimicrob Chemother 2007; 60:613–8. [DOI] [PubMed] [Google Scholar]
- 255.Piarroux R, Grenouillet F, Balvay P et al. Assessment of preemptive treatment to prevent severe candidiasis in critically ill surgical patients. Crit Care Med 2004; 32:2443–9. [DOI] [PubMed] [Google Scholar]
- 256.Shan YS, Sy ED, Wang ST, Lee JC, Lin PW. Early presumptive therapy with fluconazole for occult Candida infection after gastrointestinal surgery. World J Surg 2006; 30:119–26. [DOI] [PubMed] [Google Scholar]
- 257.Schuster MG, Edwards JE Jr, Sobel JD et al. Empirical fluconazole versus placebo for intensive care unit patients: a randomized trial. Ann Intern Med 2008; 149:83–90. [DOI] [PubMed] [Google Scholar]
- 258.Ostrosky-Zeichner L. Invasive mycoses: diagnostic challenges. Am J Med 2012; 125(1 suppl):S14–24. [DOI] [PubMed] [Google Scholar]
- 259.Fagan RP, Edwards JR, Park BJ, Fridkin SK, Magill SS. Incidence trends in pathogen-specific central line-associated bloodstream infections in US intensive care units, 1990–2010. Infect Control Hosp Epidemiol 2013; 34:893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Ostrosky-Zeichner L. Prophylaxis or preemptive therapy of invasive candidiasis in the intensive care unit? Crit Care Med 2004; 32:2552–3. [DOI] [PubMed] [Google Scholar]
- 261.Garbino J, Lew DP, Romand JA, Hugonnet S, Auckenthaler R, Pittet D. Prevention of severe Candida infections in nonneutropenic, high-risk, critically ill patients: a randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontamination. Intensive Care Med 2002; 28:1708–17. [DOI] [PubMed] [Google Scholar]
- 262.Pelz RK, Hendrix CW, Swoboda SM et al. Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients. Ann Surg 2001; 233:542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Eggimann P, Francioli P, Bille J et al. Fluconazole prophylaxis prevents intra-abdominal candidiasis in high-risk surgical patients. Crit Care Med 1999; 27:1066–72. [DOI] [PubMed] [Google Scholar]
- 264.Senn L, Eggimann P, Ksontini R et al. Caspofungin for prevention of intra-abdominal candidiasis in high-risk surgical patients. Intensive Care Med 2009; 35:903–8. [DOI] [PubMed] [Google Scholar]
- 265.Playford EG, Webster AC, Sorrell TC, Craig JC. Antifungal agents for preventing fungal infections in non-neutropenic critically ill and surgical patients: systematic review and meta-analysis of randomized clinical trials. J Antimicrob Chemother 2006; 57:628–38. [DOI] [PubMed] [Google Scholar]
- 266.Shorr AF, Chung K, Jackson WL, Waterman PE, Kollef MH. Fluconazole prophylaxis in critically ill surgical patients: a meta-analysis. Crit Care Med 2005; 33:1928–35; quiz 36. [DOI] [PubMed] [Google Scholar]
- 267.Vardakas KZ, Samonis G, Michalopoulos A, Soteriades ES, Falagas ME. Antifungal prophylaxis with azoles in high-risk, surgical intensive care unit patients: a meta-analysis of randomized, placebo-controlled trials. Crit Care Med 2006; 34:1216–24. [DOI] [PubMed] [Google Scholar]
- 268.Cruciani M, de Lalla F, Mengoli C. Prophylaxis of Candida infections in adult trauma and surgical intensive care patients: a systematic review and meta-analysis. Intensive Care Med 2005; 31:1479–87. [DOI] [PubMed] [Google Scholar]
- 269.Playford E, Webster A, Sorrell T. Antifungal agents for preventing fungal infections in non-neutropenic critically ill patients. Cochrane Database Syst Rev 2001; CD004920. [DOI] [PubMed] [Google Scholar]
- 270.Zaoutis TE, Prasad PA, Localio AR et al. Risk factors and predictors for candidemia in pediatric intensive care unit patients: implications for prevention. Clin Infect Dis 2010; 51:e38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Huang SS, Septimus E, Kleinman K et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013; 368:2255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Climo MW, Yokoe DS, Warren DK et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med 2013; 368:533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Montecalvo MA, McKenna D, Yarrish R et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med 2012; 125:505–11. [DOI] [PubMed] [Google Scholar]
- 274.O'Horo JC, Silva GL, Munoz-Price LS, Safdar N. The efficacy of daily bathing with chlorhexidine for reducing healthcare-associated bloodstream infections: a meta-analysis. Infect Control Hosp Epidemiol 2012; 33:257–67. [DOI] [PubMed] [Google Scholar]
- 275.Hocevar SN, Edwards JR, Horan TC, Morrell GC, Iwamoto M, Lessa FC. Device-associated infections among neonatal intensive care unit patients: incidence and associated pathogens reported to the National Healthcare Safety Network, 2006–2008. Infect Control Hosp Epidemiol 2012; 33:1200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Aliaga S, Clark RH, Laughon M et al. Changes in the incidence of candidiasis in neonatal intensive care units. Pediatrics 2014; 133:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Fisher BT, Ross RK, Localio AR, Prasad PA, Zaoutis TE. Decreasing rates of invasive candidiasis in pediatric hospitals across the United States. Clin Infect Dis 2014; 58:74–7. [DOI] [PubMed] [Google Scholar]
- 278.Chitnis AS, Magill SS, Edwards JR, Chiller TM, Fridkin SK, Lessa FC. Trends in Candida central line-associated bloodstream infections among NICUs, 1999–2009. Pediatrics 2012; 130:e46–52. [DOI] [PubMed] [Google Scholar]
- 279.Benjamin DK Jr, Stoll BJ, Fanaroff AA et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 2006; 117:84–92. [DOI] [PubMed] [Google Scholar]
- 280.Zaoutis TE, Heydon K, Localio R, Walsh TJ, Feudtner C. Outcomes attributable to neonatal candidiasis. Clin Infect Dis 2007; 44:1187–93. [DOI] [PubMed] [Google Scholar]
- 281.Wynn JL, Tan S, Gantz MG et al. Outcomes following candiduria in extremely low birth weight infants. Clin Infect Dis 2012; 54:331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Stoll BJ, Hansen NI, Adams-Chapman I et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004; 292:2357–65. [DOI] [PubMed] [Google Scholar]
- 283.Smith PB, Morgan J, Benjamin JD et al. Excess costs of hospital care associated with neonatal candidemia. Pediatr Infect Dis J 2007; 26:197–200. [DOI] [PubMed] [Google Scholar]
- 284.Benjamin DK Jr, Smith PB, Arrieta A et al. Safety and pharmacokinetics of repeat-dose micafungin in young infants. Clin Pharmacol Ther 2010; 87:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Cohen-Wolkowiez M, Smith PB, Mangum B et al. Neonatal Candida meningitis: significance of cerebrospinal fluid parameters and blood cultures. J Perinatol 2007; 27:97–100. [DOI] [PubMed] [Google Scholar]
- 286.Fernandez M, Moylett EH, Noyola DE, Baker CJ. Candidal meningitis in neonates: a 10-year review. Clin Infect Dis 2000; 31:458–63. [DOI] [PubMed] [Google Scholar]
- 287.Steinbach WJ, Roilides E, Berman D et al. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatr Infect Dis J 2012; 31:1252–7. [DOI] [PubMed] [Google Scholar]
- 288.Benjamin DK Jr, Poole C, Steinbach WJ, Rowen JL, Walsh TJ. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics 2003; 112(3 Pt 1):634–40. [DOI] [PubMed] [Google Scholar]
- 289.Driessen M, Ellis JB, Cooper PA et al. Fluconazole vs. amphotericin B for the treatment of neonatal fungal septicemia: a prospective randomized trial. Pediatr Infect Dis J 1996; 15:1107–12. [DOI] [PubMed] [Google Scholar]
- 290.Linder N, Klinger G, Shalit I et al. Treatment of candidaemia in premature infants: comparison of three amphotericin B preparations. J Antimicrob Chemother 2003; 52:663–7. [DOI] [PubMed] [Google Scholar]
- 291.Ascher SB, Smith PB, Watt K et al. Antifungal therapy and outcomes in infants with invasive Candida infections. Pediatr Infect Dis J 2012; 31:439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Piper L, Smith PB, Hornik CP et al. Fluconazole loading dose pharmacokinetics and safety in infants. Pediatr Infect Dis J 2011; 30:375–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Odio CM, Araya R, Pinto LE et al. Caspofungin therapy of neonates with invasive candidiasis. Pediatr Infect Dis J 2004; 23:1093–7. [PubMed] [Google Scholar]
- 294.Saez-Llorens X, Macias M, Maiya P et al. Pharmacokinetics and safety of caspofungin in neonates and infants less than 3 months of age. Antimicrob Agents Chemother 2009; 53:869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Heresi GP, Gerstmann DR, Reed MD et al. The pharmacokinetics and safety of micafungin, a novel echinocandin, in premature infants. Pediatr Infect Dis J 2006; 25:1110–5. [DOI] [PubMed] [Google Scholar]
- 296.Hope WW, Mickiene D, Petraitis V et al. The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: implications for echinocandin therapy in neonates. J Infect Dis 2008; 197:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Smith PB, Walsh TJ, Hope W et al. Pharmacokinetics of an elevated dosage of micafungin in premature neonates. Pediatr Infect Dis J 2009; 28:412–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Groll AH, Mickiene D, Petraitiene R et al. Pharmacokinetic and pharmacodynamic modeling of anidulafungin (LY303366): reappraisal of its efficacy in neutropenic animal models of opportunistic mycoses using optimal plasma sampling. Antimicrob Agents Chemother 2001; 45:2845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Warn PA, Livermore J, Howard S et al. Anidulafungin for neonatal hematogenous Candida meningoencephalitis: identification of candidate regimens for humans using a translational pharmacological approach. Antimicrob Agents Chemother 2012; 56:708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Manzoni P, Stolfi I, Pugni L et al. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. N Engl J Med 2007; 356:2483–95. [DOI] [PubMed] [Google Scholar]
- 301.Manzoni P, Arisio R, Mostert M et al. Prophylactic fluconazole is effective in preventing fungal colonization and fungal systemic infections in preterm neonates: a single-center, 6-year, retrospective cohort study. Pediatrics 2006; 117:e22–32. [DOI] [PubMed] [Google Scholar]
- 302.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med 2001; 345:1660–6. [DOI] [PubMed] [Google Scholar]
- 303.Violaris K, Carbone T, Bateman D, Olawepo O, Doraiswamy B, LaCorte M. Comparison of fluconazole and nystatin oral suspensions for prophylaxis of systemic fungal infection in very low birthweight infants. Am J Perinatol 2010; 27:73–8. [DOI] [PubMed] [Google Scholar]
- 304.Weitkamp JH, Ozdas A, LaFleur B, Potts AL. Fluconazole prophylaxis for prevention of invasive fungal infections in targeted highest risk preterm infants limits drug exposure. J Perinatol 2008; 28:405–11. [DOI] [PubMed] [Google Scholar]
- 305.Uko S, Soghier LM, Vega M et al. Targeted short-term fluconazole prophylaxis among very low birth weight and extremely low birth weight infants. Pediatrics 2006; 117:1243–52. [DOI] [PubMed] [Google Scholar]
- 306.Rolnitsky A, Levy I, Sirota L, Shalit I, Klinger G. Targeted fluconazole prophylaxis for high-risk very low birth weight infants. Eur J Pediatr 2012; 171:1481–7. [DOI] [PubMed] [Google Scholar]
- 307.Kicklighter SD, Springer SC, Cox T, Hulsey TC, Turner RB. Fluconazole for prophylaxis against candidal rectal colonization in the very low birth weight infant. Pediatrics 2001; 107:293–8. [DOI] [PubMed] [Google Scholar]
- 308.Martin A, Pappas A, Lulic-Botica M, Natarajan G. Impact of ‘targeted’ fluconazole prophylaxis for preterm neonates: efficacy of a highly selective approach? J Perinatol 2012; 32:21–6. [DOI] [PubMed] [Google Scholar]
- 309.Healy CM, Campbell JR, Zaccaria E, Baker CJ. Fluconazole prophylaxis in extremely low birth weight neonates reduces invasive candidiasis mortality rates without emergence of fluconazole-resistant Candida species. Pediatrics 2008; 121:703–10. [DOI] [PubMed] [Google Scholar]
- 310.Healy CM, Baker CJ, Zaccaria E, Campbell JR. Impact of fluconazole prophylaxis on incidence and outcome of invasive candidiasis in a neonatal intensive care unit. J Pediatr 2005; 147:166–71. [DOI] [PubMed] [Google Scholar]
- 311.Ozturk MA, Gunes T, Koklu E, Cetin N, Koc N. Oral nystatin prophylaxis to prevent invasive candidiasis in neonatal intensive care unit. Mycoses 2006; 49:484–92. [DOI] [PubMed] [Google Scholar]
- 312.Sims ME, Yoo Y, You H, Salminen C, Walther FJ. Prophylactic oral nystatin and fungal infections in very-low-birthweight infants. Am J Perinatol 1988; 5:33–6. [DOI] [PubMed] [Google Scholar]
- 313.Howell A, Isaacs D, Halliday R. Oral nystatin prophylaxis and neonatal fungal infections. Arch Dis Child Fetal Neonatal Ed 2009; 94:F429–33. [DOI] [PubMed] [Google Scholar]
- 314.Manzoni P, Stolfi I, Messner H et al. Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: a randomized controlled trial. Pediatrics 2012; 129:116–23. [DOI] [PubMed] [Google Scholar]
- 315.Bassetti M, Marchetti M, Chakrabarti A et al. A research agenda on the management of intra-abdominal candidiasis: results from a consensus of multinational experts. Intensive Care Med 2013; 39:2092–106. [DOI] [PubMed] [Google Scholar]
- 316.Sandven P, Qvist H, Skovlund E, Giercksky KE. Significance of Candida recovered from intraoperative specimens in patients with intra-abdominal perforations. Crit Care Med 2002; 30:541–7. [DOI] [PubMed] [Google Scholar]
- 317.Lee BJ, Jeong JH, Wang SG, Lee JC, Goh EK, Kim HW. Effect of botulinum toxin type a on a rat surgical wound model. Clin Exp Otorhinolaryngol 2009; 2:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Vege SS, Gardner TB, Chari ST et al. Outcomes of intra-abdominal fungal vs. bacterial infections in severe acute pancreatitis. Am J Gastroenterol 2009; 104:2065–70. [DOI] [PubMed] [Google Scholar]
- 319.de Ruiter J, Weel J, Manusama E, Kingma WP, van der Voort PH. The epidemiology of intra-abdominal flora in critically ill patients with secondary and tertiary abdominal sepsis. Infection 2009; 37:522–7. [DOI] [PubMed] [Google Scholar]
- 320.Roehrborn A, Thomas L, Potreck O et al. The microbiology of postoperative peritonitis. Clin Infect Dis 2001; 33:1513–9. [DOI] [PubMed] [Google Scholar]
- 321.Montravers P, Lepape A, Dubreuil L et al. Clinical and microbiological profiles of community-acquired and nosocomial intra-abdominal infections: results of the French prospective, observational EBIIA study. J Antimicrob Chemother 2009; 63:785–94. [DOI] [PubMed] [Google Scholar]
- 322.Masur H, Rosen PP, Armstrong D. Pulmonary disease caused by Candida species. Am J Med 1977; 63:914–25. [DOI] [PubMed] [Google Scholar]
- 323.Kontoyiannis DP, Reddy BT, Torres HA et al. Pulmonary candidiasis in patients with cancer: an autopsy study. Clin Infect Dis 2002; 34:400–3. [DOI] [PubMed] [Google Scholar]
- 324.Tamai K, Tachikawa R, Tomii K, Imai Y. Fatal community-acquired primary Candida pneumonia in an alcoholic patient. Intern Med 2012; 51:3159–61. [DOI] [PubMed] [Google Scholar]
- 325.Pasqualotto AC. Candida and the paediatric lung. Paediatr Respir Rev 2009; 10:186–91. [DOI] [PubMed] [Google Scholar]
- 326.Lewis RE, Cahyame-Zuniga L, Leventakos K et al. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: a 20-year autopsy study. Mycoses 2013; 56:638–45. [DOI] [PubMed] [Google Scholar]
- 327.Sharma S, Nadrous HF, Peters SG et al. Pulmonary complications in adult blood and marrow transplant recipients: autopsy findings. Chest 2005; 128:1385–92. [DOI] [PubMed] [Google Scholar]
- 328.Meersseman W, Lagrou K, Spriet I et al. Significance of the isolation of Candida species from airway samples in critically ill patients: a prospective, autopsy study. Intensive Care Med 2009; 35:1526–31. [DOI] [PubMed] [Google Scholar]
- 329.Rello J, Esandi ME, Diaz E, Mariscal D, Gallego M, Valles J. The role of Candida sp isolated from bronchoscopic samples in nonneutropenic patients. Chest 1998; 114:146–9. [DOI] [PubMed] [Google Scholar]
- 330.el-Ebiary M, Torres A, Fabregas N et al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Respir Crit Care Med 1997; 156(2 Pt 1):583–90. [DOI] [PubMed] [Google Scholar]
- 331.Wood GC, Mueller EW, Croce MA, Boucher BA, Fabian TC. Candida sp. isolated from bronchoalveolar lavage: clinical significance in critically ill trauma patients. Intensive Care Med 2006; 32:599–603. [DOI] [PubMed] [Google Scholar]
- 332.Williamson DR, Albert M, Perreault MM et al. The relationship between Candida species cultured from the respiratory tract and systemic inflammation in critically ill patients with ventilator-associated pneumonia. Can J Anaesth 2011; 58:275–84. [DOI] [PubMed] [Google Scholar]
- 333.Delisle MS, Williamson DR, Perreault MM, Albert M, Jiang X, Heyland DK. The clinical significance of Candida colonization of respiratory tract secretions in critically ill patients. J Crit Care 2008; 23:11–7. [DOI] [PubMed] [Google Scholar]
- 334.Roux D, Gaudry S, Khoy-Ear L et al. Airway fungal colonization compromises the immune system allowing bacterial pneumonia to prevail. Crit Care Med 2013; 41:e191–9. [DOI] [PubMed] [Google Scholar]
- 335.Mear JB, Kipnis E, Faure E et al. Candida albicans and Pseudomonas aeruginosa interactions: more than an opportunistic criminal association? Med Mal Infect 2013; 43:146–51. [DOI] [PubMed] [Google Scholar]
- 336.Hamet M, Pavon A, Dalle F et al. Candida spp. airway colonization could promote antibiotic-resistant bacteria selection in patients with suspected ventilator-associated pneumonia. Intensive Care Med 2012; 38:1272–9. [DOI] [PubMed] [Google Scholar]
- 337.Tacke D, Koehler P, Cornely OA. Fungal endocarditis. Curr Opin Infect Dis 2013; 26:501–7. [DOI] [PubMed] [Google Scholar]
- 338.Card L, Lofland D. Candidal endocarditis presenting with bilateral lower limb ischemia. Clin Lab Sci 2012; 25:130–4. [PubMed] [Google Scholar]
- 339.Ellis ME, Al-Abdely H, Sandridge A, Greer W, Ventura W. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Infect Dis 2001; 32:50–62. [DOI] [PubMed] [Google Scholar]
- 340.Venditti M, De Bernardis F, Micozzi A et al. Fluconazole treatment of catheter-related right-sided endocarditis caused by Candida albicans and associated with endophthalmitis and folliculitis. Clin Infect Dis 1992; 14:422–6. [DOI] [PubMed] [Google Scholar]
- 341.Czwerwiec FS, Bilsker MS, Kamerman ML, Bisno AL. Long-term survival after fluconazole therapy of candidal prosthetic valve endocarditis. Am J Med 1993; 94:545–6. [DOI] [PubMed] [Google Scholar]
- 342.Nguyen MH, Nguyen ML, Yu VL, McMahon D, Keys TF, Amidi M. Candida prosthetic valve endocarditis: prospective study of six cases and review of the literature. Clin Infect Dis 1996; 22:262–7. [DOI] [PubMed] [Google Scholar]
- 343.Lejko-Zupanc T, Kozelj M. A case of recurrent Candida parapsilosis prosthetic valve endocarditis: cure by medical treatment alone. J Infect 1997; 35:81–2. [DOI] [PubMed] [Google Scholar]
- 344.Melamed R, Leibovitz E, Abramson O, Levitas A, Zucker N, Gorodisher R. Successful non-surgical treatment of Candida tropicalis endocarditis with liposomal amphotericin-B (AmBisome). Scand J Infect Dis 2000; 32:86–9. [DOI] [PubMed] [Google Scholar]
- 345.Aaron L, Therby A, Viard JP, Lahoulou R, Dupont B. Successful medical treatment of Candida albicans in mechanical prosthetic valve endocarditis. Scand J Infect Dis 2003; 35:351–2. [DOI] [PubMed] [Google Scholar]
- 346.Jimenez-Exposito MJ, Torres G, Baraldes A et al. Native valve endocarditis due to Candida glabrata treated without valvular replacement: a potential role for caspofungin in the induction and maintenance treatment. Clin Infect Dis 2004; 39:e70–3. [DOI] [PubMed] [Google Scholar]
- 347.Westling K, Thalme A, Julander I. Candida albicans tricuspid valve endocarditis in an intravenous drug addict: successful treatment with fluconazole. Scand J Infect Dis 2005; 37:310–1. [DOI] [PubMed] [Google Scholar]
- 348.Rajendram R, Alp NJ, Mitchell AR, Bowler IC, Forfar JC. Candida prosthetic valve endocarditis cured by caspofungin therapy without valve replacement. Clin Infect Dis 2005; 40:e72–4. [DOI] [PubMed] [Google Scholar]
- 349.Steinbach WJ, Perfect JR, Cabell CH et al. A meta-analysis of medical versus surgical therapy for Candida endocarditis. J Infect 2005; 51:230–47. [DOI] [PubMed] [Google Scholar]
- 350.Muehrcke DD, Lytle BW, Cosgrove DM 3rd. Surgical and long-term antifungal therapy for fungal prosthetic valve endocarditis. Ann Thorac Surg 1995; 60:538–43. [DOI] [PubMed] [Google Scholar]
- 351.Mayayo E, Moralejo J, Camps J, Guarro J. Fungal endocarditis in premature infants: case report and review. Clin Infect Dis 1996; 22:366–8. [DOI] [PubMed] [Google Scholar]
- 352.Levy I, Shalit I, Birk E et al. Candida endocarditis in neonates: report of five cases and review of the literature. Mycoses 2006; 49:43–8. [DOI] [PubMed] [Google Scholar]
- 353.Noyola DE, Fernandez M, Moylett EH, Baker CJ. Ophthalmologic, visceral, and cardiac involvement in neonates with candidemia. Clin Infect Dis 2001; 32:1018–23. [DOI] [PubMed] [Google Scholar]
- 354.Smego RA Jr, Ahmad H. The role of fluconazole in the treatment of Candida endocarditis: a meta-analysis. Medicine (Baltimore) 2011; 90:237–49. [DOI] [PubMed] [Google Scholar]
- 355.Rubinstein E, Noriega ER, Simberkoff MS, Rahal JJ Jr. Tissue penetration of amphotericin B in Candida endocarditis. Chest 1974; 66:376–7. [DOI] [PubMed] [Google Scholar]
- 356.Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother 2002; 46:1773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Mrowczynski W, Wojtalik M. Caspofungin for Candida endocarditis. Pediatr Infect Dis J 2004; 23:376. [DOI] [PubMed] [Google Scholar]
- 358.Moudgal V, Little T, Boikov D, Vazquez JA. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob Agents Chemother 2005; 49:767–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Bacak V, Biocina B, Starcevic B, Gertler S, Begovac J. Candida albicans endocarditis treatment with caspofungin in an HIV-infected patient—case report and review of literature. J Infect 2006; 53:e11–4. [DOI] [PubMed] [Google Scholar]
- 360.Lye DC, Hughes A, O'Brien D, Athan E. Candida glabrata prosthetic valve endocarditis treated successfully with fluconazole plus caspofungin without surgery: a case report and literature review. Eur J Clin Microbiol Infect Dis 2005; 24:753–5. [DOI] [PubMed] [Google Scholar]
- 361.Lopez-Ciudad V, Castro-Orjales MJ, Leon C et al. Successful treatment of Candida parapsilosis mural endocarditis with combined caspofungin and voriconazole. BMC Infect Dis 2006; 6:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Baddley JW, Benjamin DK Jr, Patel M et al. Candida infective endocarditis. Eur J Clin Microbiol Infect Dis 2008; 27:519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Falcone M, Barzaghi N, Carosi G et al. Candida infective endocarditis: report of 15 cases from a prospective multicenter study. Medicine (Baltimore) 2009; 88:160–8. [DOI] [PubMed] [Google Scholar]
- 364.Talarmin JP, Boutoille D, Tattevin P et al. Candida endocarditis: role of new antifungal agents. Mycoses 2009; 52:60–6. [DOI] [PubMed] [Google Scholar]
- 365.De Rosa FG, D'Avolio A, Corcione S et al. Anidulafungin for Candida glabrata infective endocarditis. Antimicrob Agents Chemother 2012; 56:4552–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Lefort A, Chartier L, Sendid B et al. Diagnosis, management and outcome of Candida endocarditis. Clin Microbiol Infect 2012; 18:E99–109. [DOI] [PubMed] [Google Scholar]
- 367.Penk A, Pittrow L. Role of fluconazole in the long-term suppressive therapy of fungal infections in patients with artificial implants. Mycoses 1999; 42(suppl 2):91–6. [DOI] [PubMed] [Google Scholar]
- 368.Boland JM, Chung HH, Robberts FJ et al. Fungal prosthetic valve endocarditis: Mayo Clinic experience with a clinicopathological analysis. Mycoses 2011; 54:354–60. [DOI] [PubMed] [Google Scholar]
- 369.Joly V, Belmatoug N, Leperre A et al. Pacemaker endocarditis due to Candida albicans: case report and review. Clin Infect Dis 1997; 25:1359–62. [DOI] [PubMed] [Google Scholar]
- 370.Roger PM, Boissy C, Gari-Toussaint M et al. Medical treatment of a pacemaker endocarditis due to Candida albicans and to Candida glabrata. J Infect 2000; 41:176–8. [DOI] [PubMed] [Google Scholar]
- 371.Tascini C, Bongiorni MG, Tagliaferri E et al. Micafungin for Candida albicans pacemaker-associated endocarditis: a case report and review of the literature. Mycopathologia 2013; 175:129–34. [DOI] [PubMed] [Google Scholar]
- 372.Brown LA, Baddley JW, Sanchez JE, Bachmann LH. Implantable cardioverter-defibrillator endocarditis secondary to Candida albicans. Am J Med Sci 2001; 322:160–2. [DOI] [PubMed] [Google Scholar]
- 373.Hindupur S, Muslin AJ. Septic shock induced from an implantable cardioverter-defibrillator lead-associated Candida albicans vegetation. J Interv Card Electrophysiol 2005; 14:55–9. [DOI] [PubMed] [Google Scholar]
- 374.Halawa A, Henry PD, Sarubbi FA. Candida endocarditis associated with cardiac rhythm management devices: review with current treatment guidelines. Mycoses 2011; 54:e168–74. [DOI] [PubMed] [Google Scholar]
- 375.Bagdasarian NG, Malani AN, Pagani FD, Malani PN. Fungemia associated with left ventricular assist device support. J Card Surg 2009; 24:763–5. [DOI] [PubMed] [Google Scholar]
- 376.Shoham S, Shaffer R, Sweet L, Cooke R, Donegan N, Boyce S. Candidemia in patients with ventricular assist devices. Clin Infect Dis 2007; 44:e9–12. [DOI] [PubMed] [Google Scholar]
- 377.Aslam S, Hernandez M, Thornby J, Zeluff B, Darouiche RO. Risk factors and outcomes of fungal ventricular-assist device infections. Clin Infect Dis 2010; 50:664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Cabrera AG, Khan MS, Morales DL et al. Infectious complications and outcomes in children supported with left ventricular assist devices. J Heart Lung Transplant 2013; 32:518–24. [DOI] [PubMed] [Google Scholar]
- 379.Friedland IR. Peripheral thrombophlebitis caused by Candida. Pediatr Infect Dis J 1996; 15:375–7. [DOI] [PubMed] [Google Scholar]
- 380.Benoit D, Decruyenaere J, Vandewoude K et al. Management of candidal thrombophlebitis of the central veins: case report and review. Clin Infect Dis 1998; 26:393–7. [DOI] [PubMed] [Google Scholar]
- 381.Block AA, Thursky KA, Worth LJ, Slavin MA. Thrombolytic therapy for management of complicated catheter-related Candida albicans thrombophlebitis. Intern Med J 2009; 39:61–3. [DOI] [PubMed] [Google Scholar]
- 382.Pan SC, Hsieh SM, Chang SC, Lee HT, Chen YC. Septic Candida krusei thrombophlebitis of inferior vena cava with persistent fungemia successfully treated by new antifungal agents. Med Mycol 2005; 43:731–4. [DOI] [PubMed] [Google Scholar]
- 383.Arias F, Mata-Essayag S, Landaeta ME et al. Candida albicans osteomyelitis: case report and literature review. Int J Infect Dis 2004; 8:307–14. [DOI] [PubMed] [Google Scholar]
- 384.Gamaletsou MN, Kontoyiannis DP, Sipsas NV et al. Candida osteomyelitis: analysis of 207 pediatric and adult cases (1970–2011). Clin Infect Dis 2012; 55:1338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Slenker AK, Keith SW, Horn DL. Two hundred and eleven cases of Candida osteomyelitis: 17 case reports and a review of the literature. Diagn Microbiol Infect Dis 2012; 73:89–93. [DOI] [PubMed] [Google Scholar]
- 386.Neofytos D, Huprikar S, Reboli A et al. Treatment and outcomes of Candida osteomyelitis: review of 53 cases from the PATH Alliance(R) registry. Eur J Clin Microbiol Infect Dis 2014; 33:135–41. [DOI] [PubMed] [Google Scholar]
- 387.Hendrickx L, Van Wijngaerden E, Samson I, Peetermans WE. Candidal vertebral osteomyelitis: report of 6 patients, and a review. Clin Infect Dis 2001; 32:527–33. [DOI] [PubMed] [Google Scholar]
- 388.Miller DJ, Mejicano GC. Vertebral osteomyelitis due to Candida species: case report and literature review. Clin Infect Dis 2001; 33:523–30. [DOI] [PubMed] [Google Scholar]
- 389.Kaldau NC, Brorson S, Jensen PE, Schultz C, Arpi M. Bilateral polymicrobial osteomyelitis with Candida tropicalis and Candida krusei: a case report and an updated literature review. Int J Infect Dis 2012; 16:e16–22. [DOI] [PubMed] [Google Scholar]
- 390.Hennequin C, Bouree P, Hiesse C, Dupont B, Charpentier B. Spondylodiskitis due to Candida albicans: report of two patients who were successfully treated with fluconazole and review of the literature. Clin Infect Dis 1996; 23:176–8. [DOI] [PubMed] [Google Scholar]
- 391.Malani PN, McNeil SA, Bradley SF, Kauffman CA. Candida albicans sternal wound infections: a chronic and recurrent complication of median sternotomy. Clin Infect Dis 2002; 35:1316–20. [DOI] [PubMed] [Google Scholar]
- 392.Sugar AM, Saunders C, Diamond RD. Successful treatment of Candida osteomyelitis with fluconazole. A noncomparative study of two patients. Diagn Microbiol Infect Dis 1990; 13:517–20. [DOI] [PubMed] [Google Scholar]
- 393.Dan M, Priel I. Failure of fluconazole therapy for sternal osteomyelitis due to Candida albicans. Clin Infect Dis 1994; 18:126–7. [DOI] [PubMed] [Google Scholar]
- 394.Petrikkos G, Skiada A, Sabatakou H, Antoniadou A, Dosios T, Giamarellou H. Case report. Successful treatment of two cases of post-surgical sternal osteomyelitis, due to Candida krusei and Candida albicans, respectively, with high doses of triazoles (fluconazole, itraconazole). Mycoses 2001; 44:422–5. [DOI] [PubMed] [Google Scholar]
- 395.Schilling A, Seibold M, Mansmann V, Gleissner B. Successfully treated Candida krusei infection of the lumbar spine with combined caspofungin/posaconazole therapy. Med Mycol 2008; 46:79–83. [DOI] [PubMed] [Google Scholar]
- 396.Legout L, Assal M, Rohner P, Lew D, Bernard L, Hoffmeyer P. Successful treatment of Candida parapsilosis (fluconazole-resistant) osteomyelitis with caspofungin in a HIV patient. Scand J Infect Dis 2006; 38:728–30. [DOI] [PubMed] [Google Scholar]
- 397.Marra F, Robbins GM, Masri BA et al. Amphotericin B-loaded bone cement to treat osteomyelitis caused by Candida albicans. Can J Surg 2001; 44:383–6. [PMC free article] [PubMed] [Google Scholar]
- 398.Sealy PI, Nguyen C, Tucci M, Benghuzzi H, Cleary JD. Delivery of antifungal agents using bioactive and nonbioactive bone cements. Ann Pharmacother 2009; 43:1606–15. [DOI] [PubMed] [Google Scholar]
- 399.Clancy CJ, Nguyen MH, Morris AJ. Candidal mediastinitis: an emerging clinical entity. Clin Infect Dis 1997; 25:608–13. [DOI] [PubMed] [Google Scholar]
- 400.Harris MC, Pereira GR, Myers MD et al. Candidal arthritis in infants previously treated for systemic candidiasis during the newborn period: report of three cases. Pediatr Emerg Care 2000; 16:249–51. [DOI] [PubMed] [Google Scholar]
- 401.Weigl JA. Candida arthritis in a premature infant treated successfully with oral fluconazole for six months. Ann Acad Med Singapore 2000; 29:253–5. [PubMed] [Google Scholar]
- 402.Sim JP, Kho BC, Liu HS, Yung R, Chan JC. Candida tropicalis arthritis of the knee in a patient with acute lymphoblastic leukaemia: successful treatment with caspofungin. Hong Kong Med J 2005; 11:120–3. [PubMed] [Google Scholar]
- 403.Merrer J, Dupont B, Nieszkowska A, De Jonghe B, Outin H. Candida albicans prosthetic arthritis treated with fluconazole alone. J Infect 2001; 42:208–9. [DOI] [PubMed] [Google Scholar]
- 404.Tunkel AR, Thomas CY, Wispelwey B. Candida prosthetic arthritis: report of a case treated with fluconazole and review of the literature. Am J Med 1993; 94:100–3. [DOI] [PubMed] [Google Scholar]
- 405.Dutronc H, Dauchy FA, Cazanave C et al. Candida prosthetic infections: case series and literature review. Scand J Infect Dis 2010; 42:890–5. [DOI] [PubMed] [Google Scholar]
- 406.Anagnostakos K, Kelm J, Schmitt E, Jung J. Fungal periprosthetic hip and knee joint infections clinical experience with a 2-stage treatment protocol. J Arthroplasty 2012; 27:293–8. [DOI] [PubMed] [Google Scholar]
- 407.Ueng SW, Lee CY, Hu CC, Hsieh PH, Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res 2013; 471:3002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Goss B, Lutton C, Weinrauch P, Jabur M, Gillett G, Crawford R. Elution and mechanical properties of antifungal bone cement. J Arthroplasty 2007; 22:902–8. [DOI] [PubMed] [Google Scholar]
- 409.Binder MI, Chua J, Kaiser PK, Procop GW, Isada CM. Endogenous endophthalmitis: an 18-year review of culture-positive cases at a tertiary care center. Medicine (Baltimore) 2003; 82:97–105. [DOI] [PubMed] [Google Scholar]
- 410.Lingappan A, Wykoff CC, Albini TA et al. Endogenous fungal endophthalmitis: causative organisms, management strategies, and visual acuity outcomes. Am J Ophthalmol 2012; 153:162–6. [DOI] [PubMed] [Google Scholar]
- 411.Shah CP, McKey J, Spirn MJ, Maguire J. Ocular candidiasis: a review. Br J Ophthalmol 2008; 92:466–8. [DOI] [PubMed] [Google Scholar]
- 412.Khan FA, Slain D, Khakoo RA. Candida endophthalmitis: focus on current and future antifungal treatment options. Pharmacotherapy 2007; 27:1711–21. [DOI] [PubMed] [Google Scholar]
- 413.Lamaris GA, Esmaeli B, Chamilos G et al. Fungal endophthalmitis in a tertiary care cancer center: a review of 23 cases. Eur J Clin Microbiol Infect Dis 2008; 27:343–7. [DOI] [PubMed] [Google Scholar]
- 414.Fierro JL, Prasad PA, Fisher BT et al. Ocular manifestations of candidemia in children. Pediatr Infect Dis J 2013; 32:84–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Sallam A, Taylor SR, Khan A et al. Factors determining visual outcome in endogenous Candida endophthalmitis. Retina 2012; 32:1129–34. [DOI] [PubMed] [Google Scholar]
- 416.Krishna R, Amuh D, Lowder CY, Gordon SM, Adal KA, Hall G. Should all patients with candidaemia have an ophthalmic examination to rule out ocular candidiasis? Eye (Lond) 2000; 14(Pt 1):30–4. [DOI] [PubMed] [Google Scholar]
- 417.Popovich K, Malani PN, Kauffman CA, Cinti SK. Compliance with Infectious Diseases Society of America guidelines for ophthalmologic evaluation of patients with candidemia. Infect Dis Clin Pract 2007; 15:254–6. [Google Scholar]
- 418.Karmisholt MK, Hjort U, Knudsen LL, Schonheyder HC. Candidaemia and risk of intraocular infection: a Danish hospital-based cohort study. Scand J Infect Dis 2008; 40:241–6. [DOI] [PubMed] [Google Scholar]
- 419.Riddell J, Comer GM, Kauffman CA. Treatment of endogenous fungal endophthalmitis: focus on new antifungal agents. Clin Infect Dis 2011; 52:648–53. [DOI] [PubMed] [Google Scholar]
- 420.Eltoukhy N, Crank C. Antifungal distribution into cerebrospinal fluid, vitreous humor, bone, and other difficult sites. Curr Fungal Infect Rep 2010; 4:111–9. [Google Scholar]
- 421.Blennow O, Tallstedt L, Hedquist B, Gardlund B. Duration of treatment for candidemia and risk for late-onset ocular candidiasis. Infection 2013; 41:129–34. [DOI] [PubMed] [Google Scholar]
- 422.Chhablani J. Fungal endophthalmitis. Expert Rev Anti Infect Ther 2011; 9:1191–201. [DOI] [PubMed] [Google Scholar]
- 423.Essman TF, Flynn HW Jr, Smiddy WE et al. Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg Lasers 1997; 28:185–94. [PubMed] [Google Scholar]
- 424.Zhang YQ, Wang WJ. Treatment outcomes after pars plana vitrectomy for endogenous endophthalmitis. Retina 2005; 25:746–50. [DOI] [PubMed] [Google Scholar]
- 425.Martinez-Vazquez C, Fernandez-Ulloa J, Bordon J et al. Candida albicans endophthalmitis in brown heroin addicts: response to early vitrectomy preceded and followed by antifungal therapy. Clin Infect Dis 1998; 27:1130–3. [DOI] [PubMed] [Google Scholar]
- 426.Dozier CC, Tarantola RM, Jiramongkolchai K, Donahue SP. Fungal eye disease at a tertiary care center: the utility of routine inpatient consultation. Ophthalmology 2011; 118:1671–6. [DOI] [PubMed] [Google Scholar]
- 427.Goldblum D, Rohrer K, Frueh BE, Theurillat R, Thormann W, Zimmerli S. Ocular distribution of intravenously administered lipid formulations of amphotericin B in a rabbit model. Antimicrob Agents Chemother 2002; 46:3719–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Louie A, Liu W, Miller DA et al. Efficacies of high-dose fluconazole plus amphotericin B and high-dose fluconazole plus 5-fluorocytosine versus amphotericin B, fluconazole, and 5-fluorocytosine monotherapies in treatment of experimental endocarditis, endophthalmitis, and pyelonephritis due to Candida albicans. Antimicrob Agents Chemother 1999; 43:2831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Osthoff M, Hilge R, Schulze-Dobold C, Bogner JR. Endogenous endophthalmitis with azole-resistant Candida albicans—case report and review of the literature. Infection 2006; 34:285–8. [DOI] [PubMed] [Google Scholar]
- 430.O'Day DM, Head WS, Robinson RD, Stern WH, Freeman JM. Intraocular penetration of systemically administered antifungal agents. Curr Eye Res 1985; 4:131–4. [DOI] [PubMed] [Google Scholar]
- 431.Filler SG, Crislip MA, Mayer CL, Edwards JE Jr. Comparison of fluconazole and amphotericin B for treatment of disseminated candidiasis and endophthalmitis in rabbits. Antimicrob Agents Chemother 1991; 35:288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Park SS, D'Amico DJ, Paton B, Baker AS. Treatment of exogenous Candida endophthalmitis in rabbits with oral fluconazole. Antimicrob Agents Chemother 1995; 39:958–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Akler ME, Vellend H, McNeely DM, Walmsley SL, Gold WL. Use of fluconazole in the treatment of candidal endophthalmitis. Clin Infect Dis 1995; 20:657–64. [DOI] [PubMed] [Google Scholar]
- 434.Luttrull JK, Wan WL, Kubak BM, Smith MD, Oster HA. Treatment of ocular fungal infections with oral fluconazole. Am J Ophthalmol 1995; 119:477–81. [DOI] [PubMed] [Google Scholar]
- 435.Breit SM, Hariprasad SM, Mieler WF, Shah GK, Mills MD, Grand MG. Management of endogenous fungal endophthalmitis with voriconazole and caspofungin. Am J Ophthalmol 2005; 139:135–40. [DOI] [PubMed] [Google Scholar]
- 436.Varma D, Thaker HR, Moss PJ, Wedgwood K, Innes JR. Use of voriconazole in Candida retinitis. Eye (Lond) 2005; 19:485–7. [DOI] [PubMed] [Google Scholar]
- 437.Hariprasad SM, Mieler WF, Holz ER et al. Determination of vitreous, aqueous, and plasma concentration of orally administered voriconazole in humans. Arch Ophthalmol 2004; 122:42–7. [DOI] [PubMed] [Google Scholar]
- 438.Hariprasad SM, Mieler WF, Lin TK, Sponsel WE, Graybill JR. Voriconazole in the treatment of fungal eye infections: a review of current literature. Br J Ophthalmol 2008; 92:871–8. [DOI] [PubMed] [Google Scholar]
- 439.Suzuki T, Uno T, Chen G, Ohashi Y. Ocular distribution of intravenously administered micafungin in rabbits. J Infect Chemother 2008; 14:204–7. [DOI] [PubMed] [Google Scholar]
- 440.Mochizuki K, Sawada A, Suemori S et al. Intraocular penetration of intravenous micafungin in inflamed human eyes. Antimicrob Agents Chemother 2013; 57:4027–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Groll AH, Mickiene D, Petraitis V et al. Compartmental pharmacokinetics and tissue distribution of the antifungal echinocandin lipopeptide micafungin (FK463) in rabbits. Antimicrob Agents Chemother 2001; 45:3322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Groll AH, Gullick BM, Petraitiene R et al. Compartmental pharmacokinetics of the antifungal echinocandin caspofungin (MK-0991) in rabbits. Antimicrob Agents Chemother 2001; 45:596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Goldblum D, Fausch K, Frueh BE, Theurillat R, Thormann W, Zimmerli S. Ocular penetration of caspofungin in a rabbit uveitis model. Graefes Arch Clin Exp Ophthalmol 2007; 245:825–33. [DOI] [PubMed] [Google Scholar]
- 444.Sarria JC, Bradley JC, Habash R, Mitchell KT, Kimbrough RC, Vidal AM. Candida glabrata endophthalmitis treated successfully with caspofungin. Clin Infect Dis 2005; 40:e46–8. [DOI] [PubMed] [Google Scholar]
- 445.Gauthier GM, Nork TM, Prince R, Andes D. Subtherapeutic ocular penetration of caspofungin and associated treatment failure in Candida albicans endophthalmitis. Clin Infect Dis 2005; 41:e27–8. [DOI] [PubMed] [Google Scholar]
- 446.Cannon JP, Fiscella R, Pattharachayakul S et al. Comparative toxicity and concentrations of intravitreal amphotericin B formulations in a rabbit model. Invest Ophthalmol Vis Sci 2003; 44:2112–7. [DOI] [PubMed] [Google Scholar]
- 447.Tremblay C, Barza M, Szoka F, Lahav M, Baum J. Reduced toxicity of liposome-associated amphotericin B injected intravitreally in rabbits. Invest Ophthalmol Vis Sci 1985; 26:711–8. [PubMed] [Google Scholar]
- 448.Sen P, Gopal L, Sen PR. Intravitreal voriconazole for drug-resistant fungal endophthalmitis: case series. Retina 2006; 26:935–9. [DOI] [PubMed] [Google Scholar]
- 449.Kernt M, Neubauer AS, De Kaspar HM, Kampik A. Intravitreal voriconazole: in vitro safety-profile for fungal endophthalmitis. Retina 2009; 29:362–70. [DOI] [PubMed] [Google Scholar]
- 450.Wingard LB Jr, Zuravleff JJ, Doft BH, Berk L, Rinkoff J. Intraocular distribution of intravitreally administered amphotericin B in normal and vitrectomized eyes. Invest Ophthalmol Vis Sci 1989; 30:2184–9. [PubMed] [Google Scholar]
- 451.Shen YC, Wang MY, Wang CY et al. Clearance of intravitreal voriconazole. Invest Ophthalmol Vis Sci 2007; 48:2238–41. [DOI] [PubMed] [Google Scholar]
- 452.Shen X, Xu G. Vitrectomy for endogenous fungal endophthalmitis. Ocul Immunol Inflamm 2009; 17:148–52. [DOI] [PubMed] [Google Scholar]
- 453.Sanchez-Portocarrero J, Perez-Cecilia E, Corral O, Romero-Vivas J, Picazo JJ. The central nervous system and infection by Candida species. Diagn Microbiol Infect Dis 2000; 37:169–79. [DOI] [PubMed] [Google Scholar]
- 454.Chen TL, Chen HP, Fung CP, Lin MY, Yu KW, Liu CY. Clinical characteristics, treatment and prognostic factors of candidal meningitis in a teaching hospital in Taiwan. Scand J Infect Dis 2004; 36:124–30. [DOI] [PubMed] [Google Scholar]
- 455.Nguyen MH, Yu VL. Meningitis caused by Candida species: an emerging problem in neurosurgical patients. Clin Infect Dis 1995; 21:323–7. [DOI] [PubMed] [Google Scholar]
- 456.O'Brien D, Cotter M, Lim CH, Sattar MT, Smyth E, Fitzpatrick F. Candida parapsilosis meningitis associated with Gliadel (BCNU) wafer implants. Br J Neurosurg 2011; 25:289–91. [DOI] [PubMed] [Google Scholar]
- 457.Casado JL, Quereda C, Oliva J et al. Candidal meningitis in HIV-infected patients: analysis of 14 cases. Clin Infect Dis 1997; 25:673–6. [DOI] [PubMed] [Google Scholar]
- 458.Burgert SJ, Classen DC, Burke JP, Blatter DD. Candidal brain abscess associated with vascular invasion: a devastating complication of vascular catheter-related candidemia. Clin Infect Dis 1995; 21:202–5. [DOI] [PubMed] [Google Scholar]
- 459.Voice RA, Bradley SF, Sangeorzan JA, Kauffman CA. Chronic candidal meningitis: an uncommon manifestation of candidiasis. Clin Infect Dis 1994; 19:60–6. [DOI] [PubMed] [Google Scholar]
- 460.Montero A, Romero J, Vargas JA et al. Candida infection of cerebrospinal fluid shunt devices: report of two cases and review of the literature. Acta Neurochir (Wien) 2000; 142:67–74. [DOI] [PubMed] [Google Scholar]
- 461.McCullers JA, Vargas SL, Flynn PM, Razzouk BI, Shenep JL. Candidal meningitis in children with cancer. Clin Infect Dis 2000; 31:451–7. [DOI] [PubMed] [Google Scholar]
- 462.Fennelly AM, Slenker AK, Murphy LC, Moussouttas M, DeSimone JA. Candida cerebral abscesses: a case report and review of the literature. Med Mycol 2013; 51:779–84. [DOI] [PubMed] [Google Scholar]
- 463.O'Brien D, Stevens NT, Lim CH et al. Candida infection of the central nervous system following neurosurgery: a 12-year review. Acta Neurochir (Wien) 2011; 153:1347–50. [DOI] [PubMed] [Google Scholar]
- 464.Aleixo MJ, Caldeira L, Ferreira ML. Candida albicans meningitis: clinical case. J Infect 2000; 40:191–2. [DOI] [PubMed] [Google Scholar]
- 465.Epelbaum S, Laurent C, Morin G, Berquin P, Piussan C. Failure of fluconazole treatment in Candida meningitis. J Pediatr 1993; 123:168–9. [DOI] [PubMed] [Google Scholar]
- 466.Liu KH, Wu CJ, Chou CH et al. Refractory candidal meningitis in an immunocompromised patient cured by caspofungin. J Clin Microbiol 2004; 42:5950–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Prabhu RM, Orenstein R. Failure of caspofungin to treat brain abscesses secondary to Candida albicans prosthetic valve endocarditis. Clin Infect Dis 2004; 39:1253–4. [DOI] [PubMed] [Google Scholar]
- 468.Pepper J, Zrinzo L, Mirza B, Foltynie T, Limousin P, Hariz M. The risk of hardware infection in deep brain stimulation surgery is greater at impulse generator replacement than at the primary procedure. Stereotact Funct Neurosurg 2013; 91:56–65. [DOI] [PubMed] [Google Scholar]
- 469.Glick JA, Graham RS, Voils SA. Candida meningitis post Gliadel wafer placement successfully treated with intrathecal and intravenous amphotericin B. Ann Pharmacother 2010; 44:215–8. [DOI] [PubMed] [Google Scholar]
- 470.Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev 2010; 23:253–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Kauffman CA, Vazquez JA, Sobel JD et al. Prospective multicenter surveillance study of funguria in hospitalized patients. The National Institute for Allergy and Infectious Diseases (NIAID) Mycoses Study Group. Clin Infect Dis 2000; 30:14–8. [DOI] [PubMed] [Google Scholar]
- 472.Chen SC, Tong ZS, Lee OC et al. Clinician response to Candida organisms in the urine of patients attending hospital. Eur J Clin Microbiol Infect Dis 2008; 27:201–8. [DOI] [PubMed] [Google Scholar]
- 473.Kobayashi CC, de Fernandes OF, Miranda KC, de Sousa ED, Silva Mdo R. Candiduria in hospital patients: a study prospective. Mycopathologia 2004; 158:49–52. [DOI] [PubMed] [Google Scholar]
- 474.Sobel JD, Fisher JF, Kauffman CA, Newman CA. Candida urinary tract infections—epidemiology. Clin Infect Dis 2011; 52(suppl 6):S433–6. [DOI] [PubMed] [Google Scholar]
- 475.Fraisse T, Crouzet J, Lachaud L et al. Candiduria in those over 85 years old: a retrospective study of 73 patients. Intern Med 2011; 50:1935–40. [DOI] [PubMed] [Google Scholar]
- 476.Simpson C, Blitz S, Shafran SD. The effect of current management on morbidity and mortality in hospitalised adults with funguria. J Infect 2004; 49:248–52. [DOI] [PubMed] [Google Scholar]
- 477.Binelli CA, Moretti ML, Assis RS et al. Investigation of the possible association between nosocomial candiduria and candidaemia. Clin Microbiol Infect 2006; 12:538–43. [DOI] [PubMed] [Google Scholar]
- 478.Bougnoux ME, Kac G, Aegerter P, d'Enfert C, Fagon JY. Candidemia and candiduria in critically ill patients admitted to intensive care units in France: incidence, molecular diversity, management and outcome. Intensive Care Med 2008; 34:292–9. [DOI] [PubMed] [Google Scholar]
- 479.Alvarez-Lerma F, Nolla-Salas J, Leon C et al. Candiduria in critically ill patients admitted to intensive care medical units. Intensive Care Med 2003; 29:1069–76. [DOI] [PubMed] [Google Scholar]
- 480.Paul N, Mathai E, Abraham OC, Michael JS, Mathai D. Factors associated with candiduria and related mortality. J Infect 2007; 55:450–5. [DOI] [PubMed] [Google Scholar]
- 481.Viale P. Candida colonization and candiduria in critically ill patients in the intensive care unit. Drugs 2009; 69(suppl 1):51–7. [DOI] [PubMed] [Google Scholar]
- 482.Safdar N, Slattery WR, Knasinski V et al. Predictors and outcomes of candiduria in renal transplant recipients. Clin Infect Dis 2005; 40:1413–21. [DOI] [PubMed] [Google Scholar]
- 483.Robinson JL, Davies HD, Barton M et al. Characteristics and outcome of infants with candiduria in neonatal intensive care—a Paediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study. BMC Infect Dis 2009; 9:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Georgiadou SP, Tarrand J, Sipsas NV, Kontoyiannis DP. Candiduria in haematologic malignancy patients without a urinary catheter: nothing more than a frailty marker? Mycoses 2013; 56:311–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Ang BS, Telenti A, King B, Steckelberg JM, Wilson WR. Candidemia from a urinary tract source: microbiological aspects and clinical significance. Clin Infect Dis 1993; 17:662–6. [DOI] [PubMed] [Google Scholar]
- 486.Beck SM, Finley DS, Deane LA. Fungal urosepsis after ureteroscopy in cirrhotic patients: a word of caution. Urology 2008; 72:291–3. [DOI] [PubMed] [Google Scholar]
- 487.Fisher JF, Kavanagh K, Sobel JD, Kauffman CA, Newman CA. Candida urinary tract infection: pathogenesis. Clin Infect Dis 2011; 52(suppl 6):S437–51. [DOI] [PubMed] [Google Scholar]
- 488.Sobel J. Controversies in the diagnosis of candiduria: what is the critical colony count? Curr Treatment Options Infect Dis 2002; 4:81–3. [Google Scholar]
- 489.Kauffman CA, Fisher JF, Sobel JD, Newman CA. Candida urinary tract infections—diagnosis. Clin Infect Dis 2011; 52(suppl 6):S452–6. [DOI] [PubMed] [Google Scholar]
- 490.Sadegi BJ, Patel BK, Wilbur AC, Khosla A, Shamim E. Primary renal candidiasis: importance of imaging and clinical history in diagnosis and management. J Ultrasound Med 2009; 28:507–14. [DOI] [PubMed] [Google Scholar]
- 491.Erden A, Fitoz S, Karagulle T, Tukel S, Akyar S. Radiological findings in the diagnosis of genitourinary candidiasis. Pediatr Radiol 2000; 30:875–7. [DOI] [PubMed] [Google Scholar]
- 492.Krishnasamy PV, Liby C 3rd. Emphysematous pyelonephritis caused by Candida tropicalis. Am J Med 2010; 123:e7–8. [DOI] [PubMed] [Google Scholar]
- 493.Wise GJ, Shteynshlyuger A. How to diagnose and treat fungal infections in chronic prostatitis. Curr Urol Rep 2006; 7:320–8. [DOI] [PubMed] [Google Scholar]
- 494.Jenkin GA, Choo M, Hosking P, Johnson PD. Candidal epididymo-orchitis: case report and review. Clin Infect Dis 1998; 26:942–5. [DOI] [PubMed] [Google Scholar]
- 495.Wen SC, Juan YS, Wang CJ et al. Emphysematous prostatic abscess: case series study and review. Int J Infect Dis 2012; 16:e344–9. [DOI] [PubMed] [Google Scholar]
- 496.Sobel JD, Kauffman CA, McKinsey D et al. Candiduria: a randomized, double-blind study of treatment with fluconazole and placebo. The National Institute of Allergy and Infectious Diseases (NIAID) Mycoses Study Group. Clin Infect Dis 2000; 30:19–24. [DOI] [PubMed] [Google Scholar]
- 497.Fisher JF, Woeltje K, Espinel-Ingroff A, Stanfield J, DiPiro JT. Efficacy of a single intravenous dose of amphotericin B for Candida urinary tract infections: further favorable experience. Clin Microbiol Infect 2003; 9:1024–7. [DOI] [PubMed] [Google Scholar]
- 498.Agustin J, Lacson S, Raffalli J, Aguero-Rosenfeld ME, Wormser GP. Failure of a lipid amphotericin B preparation to eradicate candiduria: preliminary findings based on three cases. Clin Infect Dis 1999; 29:686–7. [DOI] [PubMed] [Google Scholar]
- 499.Sobel JD, Bradshaw SK, Lipka CJ, Kartsonis NA. Caspofungin in the treatment of symptomatic candiduria. Clin Infect Dis 2007; 44:e46–9. [DOI] [PubMed] [Google Scholar]
- 500.Haruyama N, Masutani K, Tsuruya K et al. Candida glabrata fungemia in a diabetic patient with neurogenic bladder: successful treatment with micafungin. Clin Nephrol 2006; 66:214–7. [DOI] [PubMed] [Google Scholar]
- 501.Malani A. Failure of caspofungin for treatment of Candida glabrata candiduria. Case report and review of the literature. Infect Dis Clin Pract 2010; 18:271–2. [Google Scholar]
- 502.Schelenz S, Ross CN. Limitations of caspofungin in the treatment of obstructive pyonephrosis due to Candida glabrata infection. BMC Infect Dis 2006; 6:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Jacobs LG, Skidmore EA, Cardoso LA, Ziv F. Bladder irrigation with amphotericin B for treatment of fungal urinary tract infections. Clin Infect Dis 1994; 18:313–8. [DOI] [PubMed] [Google Scholar]
- 504.Leu HS, Huang CT. Clearance of funguria with short-course antifungal regimens: a prospective, randomized, controlled study. Clin Infect Dis 1995; 20:1152–7. [DOI] [PubMed] [Google Scholar]
- 505.Jacobs LG, Skidmore EA, Freeman K, Lipschultz D, Fox N. Oral fluconazole compared with bladder irrigation with amphotericin B for treatment of fungal urinary tract infections in elderly patients. Clin Infect Dis 1996; 22:30–5. [DOI] [PubMed] [Google Scholar]
- 506.Drew RH, Arthur RR, Perfect JR. Is it time to abandon the use of amphotericin B bladder irrigation? Clin Infect Dis 2005; 40:1465–70. [DOI] [PubMed] [Google Scholar]
- 507.Tuon FF, Amato VS, Penteado Filho SR. Bladder irrigation with amphotericin B and fungal urinary tract infection—systematic review with meta-analysis. Int J Infect Dis 2009; 13:701–6. [DOI] [PubMed] [Google Scholar]
- 508.Chitale SV, Shaida N, Burtt G, Burgess N. Endoscopic management of renal candidiasis. J Endourol 2004; 18:865–6. [DOI] [PubMed] [Google Scholar]
- 509.Davis NF, Smyth LG, Mulcahy E, Scanlon T, Casserly L, Flood HD. Ureteric obstruction due to fungus-ball in a chronically immunosuppressed patient. Can Urol Assoc J 2013; 7:E355–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Vazquez-Tsuji O, Campos-Rivera T, Ahumada-Mendoza H, Rondan-Zarate A, Martinez-Barbabosa I. Renal ultrasonography and detection of pseudomycelium in urine as means of diagnosis of renal fungus balls in neonates. Mycopathologia 2005; 159:331–7. [DOI] [PubMed] [Google Scholar]
- 511.Shih MC, Leung DA, Roth JA, Hagspiel KD. Percutaneous extraction of bilateral renal mycetomas in premature infant using mechanical thrombectomy device. Urology 2005; 65:1226. [DOI] [PubMed] [Google Scholar]
- 512.Babu R, Hutton KA. Renal fungal balls and pelvi-ureteric junction obstruction in a very low birth weight infant: treatment with streptokinase. Pediatr Surg Int 2004; 20:804–5. [DOI] [PubMed] [Google Scholar]
- 513.Chung BH, Chang SY, Kim SI, Choi HS. Successfully treated renal fungal ball with continuous irrigation of fluconazole. J Urol 2001; 166:1835–6. [PubMed] [Google Scholar]
- 514.Sobel JD, Faro S, Force RW et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol 1998; 178:203–11. [DOI] [PubMed] [Google Scholar]
- 515.Reef SE, Levine WC, McNeil MM et al. Treatment options for vulvovaginal candidiasis, 1993. Clin Infect Dis 1995; 20(suppl 1):S80–90. [DOI] [PubMed] [Google Scholar]
- 516.Sobel JD. Vulvovaginal candidosis. Lancet 2007; 369:1961–71. [DOI] [PubMed] [Google Scholar]
- 517.Watson MC, Grimshaw JM, Bond CM, Mollison J, Ludbrook A. Oral versus intra-vaginal imidazole and triazole anti-fungal agents for the treatment of uncomplicated vulvovaginal candidiasis (thrush): a systematic review. BJOG 2002; 109:85–95. [DOI] [PubMed] [Google Scholar]
- 518.Sobel JD, Brooker D, Stein GE et al. Single oral dose fluconazole compared with conventional clotrimazole topical therapy of Candida vaginitis. Fluconazole Vaginitis Study Group. Am J Obstet Gynecol 1995; 172(4 Pt 1):1263–8. [DOI] [PubMed] [Google Scholar]
- 519.Sood G, Nyirjesy P, Weitz MV, Chatwani A. Terconazole cream for non-Candida albicans fungal vaginitis: results of a retrospective analysis. Infect Dis Obstet Gynecol 2000; 8:240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Sobel JD, Chaim W, Nagappan V, Leaman D. Treatment of vaginitis caused by Candida glabrata: use of topical boric acid and flucytosine. Am J Obstet Gynecol 2003; 189:1297–300. [DOI] [PubMed] [Google Scholar]
- 521.White DJ, Habib AR, Vanthuyne A, Langford S, Symonds M. Combined topical flucytosine and amphotericin B for refractory vaginal Candida glabrata infections. Sex Transm Infect 2001; 77:212–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Marchaim D, Lemanek L, Bheemreddy S, Kaye KS, Sobel JD. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet Gynecol 2012; 120:1407–14. [DOI] [PubMed] [Google Scholar]
- 523.Sobel JD, Wiesenfeld HC, Martens M et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med 2004; 351:876–83. [DOI] [PubMed] [Google Scholar]
- 524.Donders G, Bellen G, Byttebier G et al. Individualized decreasing-dose maintenance fluconazole regimen for recurrent vulvovaginal candidiasis (ReCiDiF trial). Am J Obstet Gynecol 2008; 199:613e1–9. [DOI] [PubMed] [Google Scholar]
- 525.Rosa MI, Silva BR, Pires PS et al. Weekly fluconazole therapy for recurrent vulvovaginal candidiasis: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2013; 167:132–6. [DOI] [PubMed] [Google Scholar]
- 526.Witt A, Kaufmann U, Bitschnau M et al. Monthly itraconazole versus classic homeopathy for the treatment of recurrent vulvovaginal candidiasis: a randomised trial. BJOG 2009; 116:1499–505. [DOI] [PubMed] [Google Scholar]
- 527.Iavazzo C, Gkegkes ID, Zarkada IM, Falagas ME. Boric acid for recurrent vulvovaginal candidiasis: the clinical evidence. J Womens Health (Larchmt) 2011; 20:1245–55. [DOI] [PubMed] [Google Scholar]
- 528.Vazquez JA, Sobel JD. Candidiasis. In: Kauffman CA, Pappas PG, Sobel JD, Dismukes WE, eds. Essentials of Clinical Mycology. 2nd ed New York: Springer, 2011. [Google Scholar]
- 529.Dignani MC, Solomkin JS, Anaissie EJ. Candida. In: Anaissie EJ, McGinnis MR, Pfaller MA, eds. Clinical mycology, 2nd ed. New York: Elsevier, 2009. [Google Scholar]
- 530.Bodhade AS, Ganvir SM, Hazarey VK. Oral manifestations of HIV infection and their correlation with CD4 count. J Oral Sci 2011; 53:203–11. [DOI] [PubMed] [Google Scholar]
- 531.Schwarcz L, Chen MJ, Vittinghoff E, Hsu L, Schwarcz S. Declining incidence of AIDS-defining opportunistic illnesses: results from 16 years of population-based AIDS surveillance. AIDS 2013; 27:597–605. [DOI] [PubMed] [Google Scholar]
- 532.Sangeorzan JA, Bradley SF, He X et al. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am J Med 1994; 97:339–46. [DOI] [PubMed] [Google Scholar]
- 533.Patel PK, Erlandsen JE, Kirkpatrick WR et al. The changing epidemiology of oropharyngeal candidiasis in patients with HIV/AIDS in the era of antiretroviral therapy. AIDS Res Treat 2012; 2012:262471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Bonacini M, Young T, Laine L. The causes of esophageal symptoms in human immunodeficiency virus infection. A prospective study of 110 patients. Arch Intern Med 1991; 151:1567–72. [PubMed] [Google Scholar]
- 535.Pons V, Greenspan D, Debruin M. Therapy for oropharyngeal candidiasis in HIV-infected patients: a randomized, prospective multicenter study of oral fluconazole versus clotrimazole troches. The Multicenter Study Group. J Acquir Immune Defic Syndr 1993; 6:1311–6. [PubMed] [Google Scholar]
- 536.Finlay PM, Richardson MD, Robertson AG. A comparative study of the efficacy of fluconazole and amphotericin B in the treatment of oropharyngeal candidosis in patients undergoing radiotherapy for head and neck tumours. Br J Oral Maxillofac Surg 1996; 34:23–5. [DOI] [PubMed] [Google Scholar]
- 537.Vazquez JA, Patton LL, Epstein JB et al. Randomized, comparative, double-blind, double-dummy, multicenter trial of miconazole buccal tablet and clotrimazole troches for the treatment of oropharyngeal candidiasis: study of miconazole Lauriad(R) efficacy and safety (SMiLES). HIV Clin Trials 2010; 11:186–96. [DOI] [PubMed] [Google Scholar]
- 538.Cartledge JD, Midgely J, Gazzard BG. Itraconazole solution: higher serum drug concentrations and better clinical response rates than the capsule formulation in acquired immunodeficiency syndrome patients with candidosis. J Clin Pathol 1997; 50:477–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Queiroz-Telles F, Silva N, Carvalho MM et al. Evaluation of efficacy and safety of itraconazole oral solution for the treatment of oropharyngeal candidiasis in AIDS patients. Braz J Infect Dis 2001; 5:60–6. [DOI] [PubMed] [Google Scholar]
- 540.Phillips P, De Beule K, Frechette G et al. A double-blind comparison of itraconazole oral solution and fluconazole capsules for the treatment of oropharyngeal candidiasis in patients with AIDS. Clin Infect Dis 1998; 26:1368–73. [DOI] [PubMed] [Google Scholar]
- 541.Vazquez JA, Skiest DJ, Nieto L et al. A multicenter randomized trial evaluating posaconazole versus fluconazole for the treatment of oropharyngeal candidiasis in subjects with HIV/AIDS. Clin Infect Dis 2006; 42:1179–86. [DOI] [PubMed] [Google Scholar]
- 542.Havlir DV, Dube MP, McCutchan JA et al. Prophylaxis with weekly versus daily fluconazole for fungal infections in patients with AIDS. Clin Infect Dis 1998; 27:1369–75. [DOI] [PubMed] [Google Scholar]
- 543.Meunier F, Paesmans M, Autier P. Value of antifungal prophylaxis with antifungal drugs against oropharyngeal candidiasis in cancer patients. Eur J Cancer B Oral Oncol 1994; 30B:196–9. [DOI] [PubMed] [Google Scholar]
- 544.Philpott-Howard JN, Wade JJ, Mufti GJ, Brammer KW, Ehninger G. Randomized comparison of oral fluconazole versus oral polyenes for the prevention of fungal infection in patients at risk of neutropenia. Multicentre Study Group. J Antimicrob Chemother 1993; 31:973–84. [DOI] [PubMed] [Google Scholar]
- 545.Smith D, Midgley J, Gazzard B. A randomised, double-blind study of itraconazole versus placebo in the treatment and prevention of oral or oesophageal candidosis in patients with HIV infection. Int J Clin Pract 1999; 53:349–52. [PubMed] [Google Scholar]
- 546.Phillips P, Zemcov J, Mahmood W, Montaner JS, Craib K, Clarke AM. Itraconazole cyclodextrin solution for fluconazole-refractory oropharyngeal candidiasis in AIDS: correlation of clinical response with in vitro susceptibility. AIDS 1996; 10:1369–76. [DOI] [PubMed] [Google Scholar]
- 547.Saag MS, Fessel WJ, Kaufman CA et al. Treatment of fluconazole-refractory oropharyngeal candidiasis with itraconazole oral solution in HIV-positive patients. AIDS Res Hum Retroviruses 1999; 15:1413–7. [DOI] [PubMed] [Google Scholar]
- 548.Skiest DJ, Vazquez JA, Anstead GM et al. Posaconazole for the treatment of azole-refractory oropharyngeal and esophageal candidiasis in subjects with HIV infection. Clin Infect Dis 2007; 44:607–14. [DOI] [PubMed] [Google Scholar]
- 549.Hegener P, Troke PF, Fatkenheuer G, Diehl V, Ruhnke M. Treatment of fluconazole-resistant candidiasis with voriconazole in patients with AIDS. AIDS 1998; 12:2227–8. [PubMed] [Google Scholar]
- 550.Villanueva A, Gotuzzo E, Arathoon EG et al. A randomized double-blind study of caspofungin versus fluconazole for the treatment of esophageal candidiasis. Am J Med 2002; 113:294–9. [DOI] [PubMed] [Google Scholar]
- 551.Dewsnup DH, Stevens DA. Efficacy of oral amphotericin B in AIDS patients with thrush clinically resistant to fluconazole. J Med Vet Mycol 1994; 32:389–93. [DOI] [PubMed] [Google Scholar]
- 552.Vazquez JA, Hidalgo JA, De Bono S. Use of sargramostim (rh-GM-CSF) as adjunctive treatment of fluconazole-refractory oropharyngeal candidiasis in patients with AIDS: a pilot study. HIV Clin Trials 2000; 1:23–9. [DOI] [PubMed] [Google Scholar]
- 553.Bodasing N, Seaton RA, Shankland GS, Pithie A. Gamma-interferon treatment for resistant oropharyngeal candidiasis in an HIV-positive patient. J Antimicrob Chemother 2002; 50:765–6. [DOI] [PubMed] [Google Scholar]
- 554.Martins MD, Lozano-Chiu M, Rex JH. Declining rates of oropharyngeal candidiasis and carriage of Candida albicans associated with trends toward reduced rates of carriage of fluconazole-resistant C. albicans in human immunodeficiency virus-infected patients. Clin Infect Dis 1998; 27:1291–4. [DOI] [PubMed] [Google Scholar]
- 555.Kirkpatrick CH, Windhorst DB. Mucocutaneous candidiasis and thymoma. Am J Med 1979; 66:939–45. [DOI] [PubMed] [Google Scholar]
- 556.Kamai Y, Maebashi K, Kudoh M et al. Characterization of mechanisms of fluconazole resistance in a Candida albicans isolate from a Japanese patient with chronic mucocutaneous candidiasis. Microbiol Immunol 2004; 48:937–43. [DOI] [PubMed] [Google Scholar]
- 557.Wilcox CM, Alexander LN, Clark WS, Thompson SE 3rd. Fluconazole compared with endoscopy for human immunodeficiency virus-infected patients with esophageal symptoms. Gastroenterology 1996; 110:1803–9. [DOI] [PubMed] [Google Scholar]
- 558.Barbaro G, Barbarini G, Calderon W, Grisorio B, Alcini P, Di Lorenzo G. Fluconazole versus itraconazole for candida esophagitis in acquired immunodeficiency syndrome. Candida esophagitis. Gastroenterology 1996; 111:1169–77. [DOI] [PubMed] [Google Scholar]
- 559.Wilcox CM, Darouiche RO, Laine L, Moskovitz BL, Mallegol I, Wu J. A randomized, double-blind comparison of itraconazole oral solution and fluconazole tablets in the treatment of esophageal candidiasis. J Infect Dis 1997; 176:227–32. [DOI] [PubMed] [Google Scholar]
- 560.de Wet NT, Bester AJ, Viljoen JJ et al. A randomized, double blind, comparative trial of micafungin (FK463) vs. fluconazole for the treatment of oesophageal candidiasis. Aliment Pharmacol Ther 2005; 21:899–907. [DOI] [PubMed] [Google Scholar]