Abstract
The goal of this critical review is to comprehensively assess the evidence for the molecular, physiologic, and phenotypic skeletal muscle responses to resistance exercise (RE) combined with the nutritional intervention of protein and/or amino acid (AA) ingestion in young adults. We gathered the literature regarding the translational response in human skeletal muscle to acute exposure to RE and protein/AA supplements and the literature describing the phenotypic skeletal muscle adaptation to RE and nutritional interventions. Supplementation of protein/AAs with RE exhibited clear protein dose–dependent effects on translational regulation (protein synthesis) through mammalian target of rapamycin complex 1 (mTORC1) signaling, which was most apparent through increases in p70 ribosomal protein S6 kinase 1 (S6K1) phosphorylation, compared with postexercise recovery in the fasted or carbohydrate-fed state. These acute findings were critically tested via long-term exposure to RE training (RET) and protein/AA supplementation, and it was determined that a diminishing protein/AA supplement effect occurs over a prolonged exposure stimulus after exercise training. Furthermore, we found that protein/AA supplements, combined with RET, produced a positive, albeit minor, effect on the promotion of lean mass growth (when assessed in >20 participants/treatment); a negligible effect on muscle mass; and a negligible to no additional effect on strength. A potential concern we discovered was that the majority of the exercise training studies were underpowered in their ability to discern effects of protein/AA supplementation. Regardless, even when using optimal methodology and large sample sizes, it is clear that the effect size for protein/AA supplementation is low and likely limited to a subset of individuals because the individual variability is high. With regard to nutritional intakes, total protein intake per day, rather than protein timing or quality, appears to be more of a factor on this effect during long-term exercise interventions. There were no differences in strength or mass/muscle mass on RET outcomes between protein types when a leucine threshold (>2 g/dose) was reached. Future research with larger sample sizes and more homogeneity in design is necessary to understand the underlying adaptations and to better evaluate the individual variability in the muscle-adaptive response to protein/AA supplementation during RET.
Keywords: leucine, exercise training, protein synthesis, skeletal muscle, mTORC1
Introduction and Regulation of Protein Metabolism
Human skeletal muscle protein metabolism is an intriguing and relevant area of investigation. The dynamic nature of this integrated system of physiology is challenged by the demands and consequences of human performance, nutrition, aging, inactivity, and disease. Protein turnover is simply defined as the constant cellular processes of protein synthesis [using amino acids (AAs)4 to make peptides and proteins] and protein breakdown (degrading proteins or peptides into AAs) controlling the quantity and quality of protein in a biological system.
An inequality between muscle protein synthesis (MPS) and muscle protein breakdown (MPB) can lead to muscle protein accrual/hypertrophy (e.g., exercise training and nutrition) or muscle loss/atrophy (e.g., sarcopenia, inactivity, malnutrition, and muscle wasting). Considering that muscle contains approximately half of the body’s protein, muscle loss is a concerning issue. The maintenance of muscle quality and mass is necessary for muscle to fulfill its adaptive roles in physical movement, energy metabolism, immunity, and temperature regulation. In addition, as the largest protein source available (∼15–20% protein/tissue weight), the muscle serves as a reservoir for water, minerals, vitamins, and AAs, all of which are essential during periods of stress. Most research studies examined mixed-muscle protein turnover, which is the turnover of all the proteins in muscle mixed together. However, the contractile proteins (i.e., myofibrillar proteins), including myosin and actin among other proteins, make up a larger proportion of the total protein content of muscle compared with the noncontractile sarcoplasmic and mitochondrial proteins, which serve more direct roles in the regulation of muscle energy metabolism. Although these noncontractile proteins are less abundant than the myofibrillar proteins, they have a faster turnover rate (1).
Resistance exercise (RE), when repeated over a period of time, can stimulate a range of health benefits, such as improving body composition and neuroendocrine and cardiovascular function and increasing muscle size and strength (2–4). A prodigious amount of investigation has been directed toward understanding these adaptations and determining if an enhancement effect occurs with protein and/or AA supplementation. Because of the vast body of knowledge and the conflicting conclusions derived from sections of the literature, we undertook a critical examination of the diverse body of evidence characterizing the physiologic and phenotypic response of human muscle growth and to ascertain if an enhancement from protein/AAs is present both acutely and chronically when these nutrients are given in close proximity to exercise. The evidence was collected by ≥6 y of hand-searching the author lists of research articles and systematic reviews on the topic.
The population studied examined healthy young men and women aged <40 y and who engaged in any degree of physical activity (i.e., untrained, recreationally active, and trained individuals).
The intervention was RE with or without protein and/or AA nutrition.
The comparisons included RE type, training and duration, protein and/or AA nutritional interventions, study size, methods, nutritional timing, and period of measurement.
The outcomes included MPS rate, including type of muscle protein being synthesized, MPS, fractional synthesis rate (FSR), skeletal muscle mammalian target of rapamycin complex 1 (mTORC1) signaling, lean mass, muscle mass, and strength.
Type of study designs included randomized clinical placebo-controlled trials, observational studies, and crossover counterbalanced designs. The acute studies were characterized by the examination of these responses in the hours or days after ≥1 bouts of RE. We also collected the literature describing the chronic (>4 wk of exercise training) phenotypic skeletal muscle adaptation (muscle size and strength) in young individuals to RE with protein/AA nutritional intervention. The studies required an arm with a protein supplement and a placebo arm and/or other comparison with nutritional supplementation.
We searched MEDLINE (including in-process and other nonindexed citations), Biomedical Reference Collection: Basic, E-Journals, ERIC, Health Source–Nursing/Academic Edition, CINAHL, AMED, Web of Science SPORTDiscus, and reference lists of articles (in 2010, 2011, 2012, 2013, 2014, and 2015). This search was programmed into PubMed’s weekly update, which was e-mailed to one of the authors (PTR). The most fruitful method of discovering studies was 1) hand-searching for articles published by researchers who are well known in the area of muscle protein metabolism/exercise training and 2) using the reference lists of all retrieved articles to identify potentially missing sources. Several studies were not retrieved via standard methods.
As such, we assessed all of the literature, to our knowledge, with regard to muscle protein turnover and the related cell signaling response (young adult human skeletal muscle) to RE and protein/AA. The compiled evidence was extensive and was summarized in an effort to provide current and future researchers and nutritional practitioners with a unified resource informing the acute and chronic effect of protein/AAs in the adaptation to exercise. To understand any effect from RE and protein/AA feeding, a detailed understanding of RE in the fasted state is needed. The evidence with regard to the physiologic response to RE in the fasted state is enormous, and because our focus was to comprehensively ascertain the effect of feeding we provide evidence to adequately characterize the fasted state response and apologize in advance to any researchers whose valuable contributions we were unable to include. For further information on the topic, readers are encouraged to read the following publications (5–11). Supplemental References and Supplemental Glossary are provided with the Online Supporting Material.
Acute Physiologic Adaptation to RE with and without Protein and/or AA Feeding
Over the past 30 y, a dedicated effort has been made to study how an acute bout of RE can influence muscle protein metabolism during the early stages (hours to days) of postexercise recovery. This early phase of adaptation in muscle protein metabolism involves a complex interaction of signal transduction, gene transcription, translation, and protein degradation, among many other changes (11). Our main focus in the following sections is to comprehensively examine the evidence characterizing the physiologic (protein metabolism) and molecular response (cell signaling) of human muscle protein anabolism and whether an enhancement from protein/AAs is present when these nutrients are given in close proximity to exercise.
Human muscle protein turnover after RE in the fasted and protein- and/or AA-fed state.
To assess the acute muscle protein anabolism (growth) response researchers have used AAs as tracers (stable and isotopically labeled) alongside muscle biopsies to measure MPS and MPB in humans in vivo. Several assumptions and many different methodologic approaches explain some of the inherent variability with the in vivo assessment of human MPS (12). Thus, direct comparisons of qualitative values across laboratories should be interpreted with caution. Nonetheless, some general trends can be gleaned from the percentage changes occurring in each investigation. For mixed-MPS, increases from a resting value of ∼0.05–0.07% to ∼0.07–0.12%/h are common after acute RE (Tables 1 and 2). It is rare to see a value for MPS >0.15%/h after RE, but these values are highly dependent on several methodologic choices, including, but not limited to, the time between biopsies and the chosen tracer precursor. For myofibrillar MPS, it is common to find a maximal stimulation of ∼0.07–0.09%/h after RE compared with a resting value of ∼0.02–0.05%/h. However, the duration and magnitude of post-RE MPS are highly dependent on the exercise intensity and volume (75, 76, 106, 107). These “maximal" values appear to stay elevated for only ∼1–3 h before starting to decline, depending on the exercise intensity, precursor, and muscle fraction studied and the type and timing of the protein/AA feeding.
TABLE 1.
Summary of human skeletal protein turnover responses after RE in the fasted state1
| MPS, %/h |
||||||||||
| First author, year (ref) | Subjects: status, n, age2 | Tracer | Exercise3 | Protein fraction | FSR Bx time PEx | Group | Rest4 | Ex | PEx4 | Net bal |
| MacDougall, 1995 (13) | ST, 6 M, 23 ± 2 y | 2-[13C] Leu | 3 bicep Ex: 4 × failure; 80%1 RM | Mixed | 31–41 h infusion | Ex vs. Cntl | 0.041 | — | 0.047 | — |
| Biolo, 1995 (14) | UT, 5 M+W, 24 ± 5 y | [13C6] Phe | LP, Sq, KC, KE: 4–5 × 8–10; 75% 1 RM | Mixed | During 3 h PEx | Ex vs. Cntl | 0.045 | — | 0.11 | ↑ |
| Tipton, 1996 (15) | TR, 7 W, 20 ± 1 y | [13H6] Phe | WB RE: 3 × 6–10; 65–80% 1 RM | Mixed | 1.5–6.5 h | RE | ∼0.04 | — | 0.05 | — |
| Swim trained | Swim = 4600 m | Swim | — | — | 0.065 | — | ||||
| Both | Swim + RE | — | — | 0.085 | — | |||||
| Phillips, 1997 (16) | Rec, UT, 4 M, 4 W, 23 ± 1 y | [2H5] Phe | KE: 8 × 8; 80% 1 RM; Con/Ecc | Mixed | Day 1: rest | Rest | ∼0.06 | — | — | — |
| Day 2: 0–3 h | 0–3 h | — | — | ∼0.13 | ↑ | |||||
| Day 3: 21–24 h | 21–24 h | — | — | ∼0.09 | ↑ | |||||
| Day 4: 45–48 h | 45–48 h | — | — | ∼0.08 | ↑ | |||||
| Biolo, 1999 (17) | UT, 5 M, 29 ± 5 y | [13C6]Phe | LP: 5 × 10; 12 RM; Sq, KC, KE: 4 × 8; 10 RM | Mixed | 1–4 h | Rest | 0.048 | — | 0.095 | ↔↑ |
| 4–7 h, Ex: 1–4 h | Insulin INF | — | — | 0.075 | ↑ | |||||
| Fowles, 2000 (18) | Rec, 8 M, 26 ± 4 y | 1-[13C]Leu | Isometric stretch = to max passive stretch of 40% MVCs ∼27 min to fatigue | Mixed | 10–22 h | Isometric | 0.049 | — | 0.074 | — |
| Stretch | 0.067 | — | 0.086 | — | ||||||
| Trappe, 2002 (19) | Rec, 8 M, 25 ± 3 y | [2H5]Phe | Si-KE: 10–14 × 10; Ecc of 120% Con | Mixed | 24 h | PLA | ∼0.08 | — | ∼0.14 | — |
| ACET | 0.08 | — | 0.085 | — | ||||||
| IBU | 0.085 | — | 0.105 | — | ||||||
| Pitkanen, 2003 (20) | Rec, 6 M, 26 ± 5 y | [2H5]Phe | 4–5 leg Ex: 1–3 × 1–10; 10 RM; or control non-Ex leg | 3-Pool | 1, ∼3h PEx | Exercise | — | — | ↔, ↑ | ↔ |
| Durham 2004 (21) | Rec,5 M, 2 W, 27 ± 3 y | [2H5]Phe | LP: 8 × 10; 70% 1 RM; KE: 8 × 8; ∼80% 1 RM | 3-Pool | Pre and PEx | Exercise | ↔ | ↔ | — | ↔ |
| Trappe, 2004 (22) | Rec,8 M, 27 ± 4 y | [2H5]Phe | Si-calf Ex: 4 × 15; 70% 1 RM | Mixed | 0–3 h | Cntl | 0.051 | — | 0.069 | — |
| Sheffield-Moore, 2005 (23) | Rec, 6 M, 22 ± 2 y | [2H5]Phe | KE: 6 × 8; 80% 1 RM | Mixed | 0–10 min, 0–1, 0–3 h | Young | 0.072 | — | 0.072, 0.091, 0.102 | ↔ |
| Dreyer, 2006 (24) | UT, 7 M, 4 W, 27 ± 2 y | [2H5]Phe | KE: 10 × 10; 70% 1 RM; some subjects 60–65% | Mixed | Rest, during Ex, 0–1 and1–2 h PEx | — | ∼0.063 | 0.045 | 0.085, 0.095 | ↔ |
| Carrithers, 2007 (25) | Rec, 6 M, 6 W, 26 ± 2 y | [2H5] Phe | Si-RE + AE 90 min: 60% 1 RM | Myo | 0–4 h | AE + RE | — | — | 0.01 | — |
| KE,LP: 4 × 10; 80% 1 RM | RE | — | — | 0.092 | — | |||||
| Fujita, 2007 (26) | UT, 6 M, 32 ± 2 y | [13C6]Phe | BFR KE: 1 × 30, 3 × 15; 20% 1 RM | Mixed | 0–3 h | RE only | ∼0.055 | — | ∼0.06 | — |
| RE + BFR | ?0.06 | — | ?0.085 | — | ||||||
| Drummond, 2009 (27) | UT, 8 M, 29 ± 2 y | [2H5]Phe | KE: 11 × 10; 70% 1 RM | Mixed | 0–2 h | Cntl | 0.06 | — | 0.095 | — |
| Rap | 0.061 | — | 0.058 | — | ||||||
| Fujita, 2009 (28) | 13 M, 9 W, 26 ± 3 y | [2H5]Phe | KE: 10 × 10; 70% 1 RM | Mixed | Rest, during Ex, 0–1, 1–2, 0–2 h PEx | Fast | 0.06 | 0.047 | 0.08, 0.09, 0.073 | ↔ |
| Mayhew, 2009 (29) | UT, 8, ∼28 y | [2H5]Phe | Sq, LP and KE: 3 × 10–12 RM | Mixed | 24–27 h | Young | 0.055 | — | 0.11 | — |
| Moore, 2009 (30) | Rec, 6 M, 29 ± 2 y | [13C]Leu | LP, KE, KC: 4 × 8–10 | Mixed | 1–4 h postingestion | 0 | — | — | 0.053 | — |
| Burd, 2010 (31) | Rec, 16 M, 23 ± 1 y | [2H5]Phe | Si-KE, Ecc | Mixed | 24–27 h | COX-2 | 0.056 | — | 0.108 | — |
| PLA | 0.074 | — | 0.091 | — | ||||||
| Kumar, 2009 (32) | Rec, 25 M, 24 ± 6 y | 1-[13C]Leu | Si-KE: 3 × 27; 20% 1 RM | Myo | 0–4 h | Young 20 | 0.039 | — | 0.06 | — |
| Si-KE: 3 × 14, 40% 1 RM | Young 40 | — | — | 0.068 | — | |||||
| Si-KE: 3 × 9, 60% 1 RM | Young 60 | — | — | 0.095 | — | |||||
| Si-KE: 3 × 8; 75% 1 RM | Young 75 | — | — | 0.105 | — | |||||
| Si-KE: 6 × 3; 90% 1 RM | Young 90 | — | — | 0.094 | — | |||||
| Rec, 25 M, 24 ± 6 y | Averaged 60–90% 1 RM | (0–1, 1–2, 2–4 h) | Young | 0.04 | — | 0.058, 0.108, 0.055 | — | |||
| Dreyer, 2010 (33) | Rec, 9 M, 27 ± 2 y | [2H5]Phe | KE: 10 × 10; 70% 1 RM | Mixed | Rest, 0–2 h | M | 0.057 | — | 0.085 | — |
| Rec, 8 W, 26 ± 3 y | W | 0.06 | — | 0.091 | — | |||||
| Doessing, 2010 (34) | Sed,10 M, 30 ± 2 y | 1-[13C]Pro, [15N]Pro | Si-KE: 10 × 10; 70% 1 RM | Myo/Col | 24 h PEx | Cntl | 0.047 | — | 0.05/0.03 | — |
| rhGH | 0.049 | — | 0.051/0.06 | — | ||||||
| Holm, 2010 (35) | UT, 20 M, 25 ± 1 y | [13C]Leu | Si-KE: LL 17% 1 RM | Myo | Early (0.5–3 h), late (3–5 h) | LL | 0.08 | — | 0.115, 0.095 | — |
| Si-KE: LL 17% 1 RM | Col | LL | — | — | 0.14, 0.188 | — | ||||
| Si-KE: HL 70% 1 RM | Myo | HL | 0.08 | — | 0.086, 0.14 | — | ||||
| Si-KE: HL 70% 1 RM | Col | HL | — | — | 0.163, 0.15 | — | ||||
| Etheridge, 2011 (36) | Rec, 7 M, 21 ± 1 y | 2-[13C]Leu | Si-KE: 6 × 8; 70% 1 RM | Myo | 0–3.5 h | Normoxia | 0.033 | — | 0.104 | — |
| Hypoxia | 0.043 | — | 0.06 | — | ||||||
| Dideriksen, 2011 (37) | Rec, 15 M, 9 W, ∼68 y | [13C]Leu | Si-KE, LP: 5 × 8; ∼80% 1 RM | Myo/Col | 30–390 min post-RE | Water imed PEx | — | — | 0.07 | — |
| Fry, 2011 (38) | Rec, 8 M, 8 W, 27 ± 2 y | [13C6]Phe | KE: 10 × 10; 70% 1 RM | Mixed | Rest, 0–3, 3–6, 24–27 h | Young | 0.051 | — | 0.065, 0.078, 0.079 | — |
| Kumar, 2012 (39) | Rec, 12 M, 24 ± 6 y | [13C]Leu | Si-KE: 3 × 14; 40% 1 RM | Myo | Rest, 0–1, 1–2, 2–4 h | Y 40 3 set | — | 0.042 | ↔ | — |
| Si-KE: 6 × 14; 40% 1 RM | Y 40 6 set | — | ↔ | — | ||||||
| Si-KE: 3 × 8; 75% 1 RM | Y 75 3 set | — | 0.07, 0.12, 0.05 | — | ||||||
| Si-KE: 6 × 8; 75% 1 RM | Y 75 6 set | 0.04 | 0.04, 0.08, 0.05 | — | ||||||
| Camera, 2012 (40) | TR, 8 M, 23 ± 3 y | [13C6]Phe | LP: 2 × 5; 55% 1 RM; 8 × 5; 80% 1 RM | Myo | 1–4 h PEx | Normal glycogen | — | — | 0.045 | — |
| TR, 8 M, 23 ± 4 y | Glycogen depleted | — | — | 0.049 | — | |||||
| Gundermann, 2012 (41) | Rec, 6 M, 24 ± 2 y | [13C6]Phe | BFR KE: 1 × 30, 3 × 15; 20% 1 RM | Mixed | Rest, 1–3 h | BFR | 0.056 | — | 0.078 | — |
| SNP | 0.057 | — | 0.045 | — | ||||||
| Res, 2012 (42) | Rec, 8 M, 23 ± 1 y | [2H5]Phe | LP, KE: 8 × 8: ∼70% 1 RM | Mixed | 2330–0700 h (8 h) | Sleep | — | — | 0.048 | — |
| Gundermann, 2014 (43) | Rec, 8 M, ∼25 y | [13C6]Phe | BFR KE: 1 × 30, 3 × 15; 20% 1 RM | Mixed | Rest, 0–3, 5–6, and 22–24 h, MPB (rest, 6, 24 h) | BFR | ∼0.048 | — | ∼0.07, 0.05, 0.08 | ↑ at 24 h |
| Rec, 8 M, ∼25 y | BFR + Rap | ∼0.055 | — | ∼0.057, 0.05, 0.07 | ↔ | |||||
| Witard, 2014 (44) | ST, 12 M, 22 ± 3 y | [13C6]Phe | Si-LP, Si-KE: 8 × 10; 80% 1 RM | Myo | 0–4 h PEx | 0 | 0.032 | — | 0.052 | — |
| Effect of RET | ||||||||||
| Yarasheski, 1993 (45) | 2 M, 4 W, 24 ± 1 y | [13C]Leu | WB, PRT: 3–4 × 4–8 per Ex; 75–90% 1 RM | Mixed | 0–4 h | Y | 0.049 | — | 0.075 | — |
| Yarasheski, 1993 (46) | ST, 7 M, 23 ± 2 y | 1-[13C] and 1,2-[13C2]Leu | WB, PRT 5–10 Ex: 75–90% 1 RM, 3–6 d/wk | Mixed | 6 h, 2–8 h | Initial | — | — | 0.034 | — |
| GH | — | — | 0.034 | — | ||||||
| Welle, 1995 (47) | 5 M, 4 W, 22–31 y | [13C]Leu | 3 × 8: 80% 3 RM; Ex on day 1, day 3 as inpatients, and Myo MPS on day 4 | Myo | 0–6 h | Y | 0.061 | — | 0.062 | ↑ with 3-MH |
| Hasten, 2000 (48) | 4 M, 3 W, 23–32 y | [13C]Leu | WB,PRT 9 Ex: 2–3 × 8–12; 60–90% 1 RM | Mixed/MHC | 12–13 h | Y | 0.048, 0.038 | — | 0.10, 0.072 | — |
| Phillips, 1999 (49) | 3 M, 3 W, 25 ± 3 y | 2H5, 15N Phe | Si-KE: 8 × 10; 120% 1 RM; Ex and rest leg | Mixed | 0–4 h | UT | 0.045 | — | 0.067 | < Neg |
| ST, 3 M/3 W, 26 ± 3 y | TR | 0.073 | ↔ | 0.082 | < Neg | |||||
| Kim, 2005 (50) | Sed, 8 M, 25 ± 2 y | [13C6]Phe | LP, KE: 4 × 10; 80% 1 RM; 8-wk PRT training | Mixed/Myo | 0–4 h | UT | 0.041, 0.027 | — | 0.093, 0.039 | — |
| TR | 0.061, 0.030 | — | 0.075, 0.043 | — | ||||||
ACET, acetaminophen group; AE, aerobic exercise; BFR, blood flow restriction; Bx, biopsy; Cntl, control; Col, collagen fraction; Con, concentric contractions; COX, cyclooxygenase; Ecc, eccentric contractions; Ex, exercise; Failure, exercise to failure; FSR, fractional synthesis rate; GH, growth hormone; HL, high load; IBU, ibuprofen group; imed, immediate; INF, infusion, KE, knee extension; LL, low load; LP, leg press; M, men; max, maximum; MHC, myosin heavy chain fraction; MPB, muscle protein breakdown; MPS, muscle protein synthesis; MVC, maximal voluntary contraction; Myo, myofibrillar protein fraction; Neg, negative; Net bal, net balance; PEx, postexercise; PLA, placebo; PRT, progressive resistance training; Rap, rapamycin; RE, resistance exercise; Rec, recreationally active; ref, reference; RET, resistance exercise training; rhGH, recombinant human growth hormone; RM, repetition maximum; Sed, sedentary; Si, single leg; SNP, sodium nitroprusside; Sq, squats; ST, strength-trained; TR, trained; UT, untrained; W, women; WB, whole body; Y, young; 3-MH, 3-methylhistidine; ↔, no change; ↔↑, trend to increase; ↑, increase from basal values.
Values for age are means ± SDs or SEMs (see corresponding reference).
Exercise column denotes the exercise (sets × repetitions), at exercise intensity as a percentage of 1 RM unless otherwise stated.
Multiple values in the PEx column indicate the various postexercise MPS assessments for the reference when they are reported. These time periods are described in the column “FSR Bx time PEx” for the respective reference.
TABLE 2.
Summary of human skeletal muscle protein turnover responses after RE in the fed state1
| MPS (fasted), %/h |
MPS (fed), %/h |
||||||||||||
| First author, year (ref) | Subjects: status, n, age2 | Tracer | Exercise3 | Protein fraction | FSR Bx time PEx | Nutrition/group | Leu, g | Nutrition type | Basal | PEx4 | Basal | PEx4 | Net bal |
| Biolo, 1997 (51) | UT, 6 M, 29 ± 5 y | [13C6]Phe | None | Mixed | During 3 h INF | AA INF | >5 | 3 h Travasol (Baxter Healthcare Corporation) | 0.064 | — | 0.1 | 0.144 | — |
| LP, Sq, LC, KE: 4–5 × 8–10; 75% 1 RM | Mixed | >5 | 3h Travasol (Baxter Healthcare Corporation) (1–4 h PEx) | — | — | — | — | — | |||||
| Tipton, 1999 (52)5 | UT, 3 M, 3 W, 22 ± 2 y | [2H5]Phe | LP, Sq, LC, KE: 4–5 × 8–10; 75% 1 RM | N/A, AV-Bal | 45 min after Nutr (∼4–5 h) | 40 g mixed AAs | 4.4 | 1 L Total, given as 100 mL every 18–20 min | No | — | — | 93 | 10 |
| 40 g EAAs | 8.3 | (∼30 min PEx to ∼4 h PEx) | No | — | — | 80 | 30 | ||||||
| PLA | 0 | No | 53 | — | — | — | |||||||
| Rasmussen, (53)5 2000 | Rec, 3 M, 3 W, 34 ± 3 y | [2H5]Phe | LP, KE: 8–10 × 8; 80% 1 RM | N/A, AV-Bal | 1, 3 h | 1 h | 1.2 × 2 | (6 g EAAs, 35 g sucrose) or PLA 1 h or 3 h | No | — | — | 175 | 90 |
| 3 h | 1.2 × 2 | No | — | — | 160 | 75 | |||||||
| Borsheim, 2004 (54)5 | Rec, 5 M, 3 W, 29 y | [2H5]Phe | KE: 10 × 8; 80%1 RM | N/A, AV-Bal | −2, +4 h | EAAs+PRO+CHO | ∼2 | 77 g Malto, 18 WPC, 5 g AAs, 1 h PEx | No | — | — | ↑ at 1h | ↑ NB |
| CHO | 0 | 100 g Malto, 1 h PEx | No | — | — | ↔ | ↔ | ||||||
| Borsheim, 2002 (55)5 | Rec, 3 M, 3 F, 23 ± 2 y | [2H5]Phe | LP, KE: 8–10 × 8; 80% 1 RM | N/A, AV-Bal | Pre, 1, 2, and 3 h | EAAs, given 2× | 1.2 | EAAs | No | — | — | 310 | 225 |
| Louis, 2003 (56) | UT, 7 M, 21 ± 1 y | 1-[13C]Leu | Si-LC, Si-KE: 20 × 10; 75% MVC | Myo | 0–3 h | CHO+PRO | ? | 3 h orally fed CHO (0.3 g/kg), PRO (∼0.08 g/kg milk powder) | — | — | 0.057 | 0.164 | — |
| Cr+CHO+PRO | ? | ±7 g CrM every 20 min | — | — | 0.062 | 0.119 | — | ||||||
| Sarc | CHO+PRO | ? | — | — | 0.068 | 0.218 | — | ||||||
| Cr+CHO+PRO | ? | — | — | 0.07 | 0.22 | — | |||||||
| Miller, 2003 (57) | 6 M, 4 F, ? y | [2H5]Phe | LP, KE: 8–10 × 8; 80% 1 RM | N/A, AV-Bal | 1, 2, and 3 h | CHO | — | 35 g, 1 and 2 h PEx | — | — | — | ↔↕ | — |
| CHO+AAs | 0.54 | 35 + 6 g AAs, 1 and 2 h PEx | — | — | — | ↔↕ | — | ||||||
| AAs | 0.54 | 6 g AAs, 1 and 2 h PEx | — | — | — | ↔↕ | — | ||||||
| Tipton, 2004 (58), 2007 (59), 2009 (60) | UT, 9 M+W, 28 ± 2 y | None | KE: 10 × 8; 80% 1 RM | N/A | −1, 1, 2, 5 h | WP PEx | 2.3 | 20 g WP 1 h PEx, 300 mL | No | — | — | — | ↑ at 1 h |
| UT, 7 M+W, 24 ± 3 y | Casein | 1.7 | 20 g Casein, 1 h PEx, 300 mL | No | — | — | — | ↑ at 1 h | |||||
| UT, 7 M+W, 23 ± 1 y | PLA | 0 | Water, 1 h PEx, 300 mL | No | — | — | — | ↔, Neg | |||||
| UT, 8 M+W, 26 ± 3 y | WP Pre Ex | ∼1.5–2 | 20 g WP imed pre-Ex, 300 mL | No | — | — | — | ↑ at 1 h PEx | |||||
| UT, 5 M,3 F, 30 ± 3 y | PLA | 0 | Water, 1 h PEx, 300 mL | No | — | — | — | ↔, Neg | |||||
| UT, 6 M, 1 W, 25 ± 2 y | WP+Leu | 3.4 + 2 | 16.6 + 3.4g Leu, 1 h PEx, 300 mL | No | — | — | — | ↑ | |||||
| Moore, 2005 (61) | Rec, 8 M, 22 ± 1 y | 1,2-[13C]Leu | Ecc KE: 6 × 10; maximal | Myo | 1–4.5, 1–8.5 h | Ecc | >5 | Myoplex every 30 min, 0.1 g ⋅ kg−1 ⋅ h−1 | — | — | 0.07 | 0.11, 0.105 | — |
| Con | At rest: ∼36 g PRO, 320 kcal | — | — | — | 0.09, 0.115 | — | |||||||
| Col | Ecc | Exercise: ∼67 g PRO, 590 kcal | — | — | 0.015 | 0.06, 0.059 | — | ||||||
| Con | — | — | — | 0.06, 0.058 | — | ||||||||
| Koopman, 2005 (62) | UT, 8 M, 22 ± 1 y | [13C6]Phe | LP, KE: 8 × 8; 80% 1 RM | Mixed | 0–6 h P-In | CHO | 0 | 25g Malto and 25 g glucose | No | — | NM | 0.061 | — |
| CHO+PRO | 9 | 33 g WPH, 25 g Malto, 25 g glucose | No | — | — | 0.082 | — | ||||||
| CHO+PRO+Leu | 54 | 33 g WPH, 16.6 g Leu, 25 g Malto, and 25 g glucose | No | — | — | 0.095 | — | ||||||
| Cuthbertson, 2006 (63) | UT, 8 M, 25 ± 5 y | [13C] Leu | 12 min of steps | Myo | Rest, 0–3, 0–6, 0–24 h | Ecc | >5 | 2h prior to each Bx 45g EAAs + 135g CHO (sucrose) | — | — | 0.042 | 0.051, 0.133, 0.132 | — |
| [13C] Val | Myo | Con | to meet participants 24h energy needs | — | — | 0.048 | 0.048, 0.118, 0.139 | — | |||||
| Sarc | Ecc | — | — | 0.061 | 0.06, 0.146, 0.125 | — | |||||||
| Sarc | Con | — | — | — | 0.066, 0.14, 0.117 | — | |||||||
| Col | Ecc | — | — | 0.016 | 0.048, 0.051 | — | |||||||
| Col | Con | — | — | — | 0.032, 0.058 | — | |||||||
| Elliot 2006 (64) | UT, 3 M, 5 W, 26 ± 2 y | None | KE: 10 × 8; 80% 1 RM | N/A | −1, 1, 2, 5 h | FF milk | <1 | FF-milk (237 g), 8 g PRO, 12 g CHO, 0.6 g fat | — | — | — | — | ↑ at 1, 2 h |
| UT, 6 M, 2 W, 28 ± 3 y | Whole milk | <1 | Whole-milk (237g) 8g PRO, 11g CHO 8g Fat | — | — | — | — | ||||||
| UT, 7 M, 1 W, 24 ± 1 y | Isocaloric FF milk | ∼1–1.2 | Isocaloric FFM (393 g), 14.5 g PRO, 20 g CHO, 1g Fat | — | — | — | — | ||||||
| Koopman, 2006 (65) | UT, 8 M, 20 ± 1 y | [13C6], [2H2] Tyr | LP, KE: 6 × 10; 40–75% 1 RM | Mixed | 0–6 h P-In | CHO | 0 | 92 g Malto and 92 g glucose, 60 g WPH, ∼10 g Leu | No | — | — | 0.06 | — |
| UT, 8 M, 20 ± 1 y | CHO+PRO+LEU | ∼18 | ∼70 g WP | No | — | — | 0.082 | ↑ | |||||
| Tang, 2007 (66) | TR, 8 M, 21 ± 1 y | [2H5]Phe | Si-KE, Si-LP, 4 × 8–10; 80% 1 RM | Mixed | 1–3 h | WP+CHO | ∼1 | 10 g WPI + 21 g fructose, 227 mL, imed PEx | — | — | 0.061 | ∼0.12 | — |
| CHO | 0 | 10 g Malto + 21 g fructose, 227 mL, imed PEx | — | — | 0.049 | ∼0.08 | — | ||||||
| Wilkinson, 2007 (67) | TR, 8 M, 22 ± 1 y | 1-[13C]Leu, [2H5]Phe | LP, LC, Si-KE: 4 × 10; 80% 1 RM | Mixed | 0–3 h | Milk | ∼1.5 | Soy with Malto, 500 mL, 18.2 g PRO, 1.5 g fat, 23 g CHO | No | — | — | 0.100 | — |
| Soy milk | ∼1.8–2 | Milk with lactose 500 mL, 18.2 g PRO, 1.5 g fat, 23 g CHO | No | — | — | 0.070 | — | ||||||
| Beelen 2008 (68) | UT, 10 M, 20 ± 1 y | [13C6]Phe | 2 h RE with 4 × 5 min cycle; 65% Wmax | Mixed | 0–2 h | CHO | >5 | Specific dosing given at intervals, mix of CHO | — | — | — | During Ex, 0.06 | — |
| [2H2]Tyr | CHO+PRO | Specific dosing given at intervals, mix of CHO+CPH PRO | — | — | — | During Ex, 0.088 | WB ↑ | ||||||
| Beelen, 2008 (69) | UT, 20 M, 20 ± 1 y | [13C6]Phe | 2 h RE with 4 × 5 min cycle; 65% Wmax | Mixed | 0–2 h | 2 h PLA | None | Water only | — | — | — | — | — |
| [2H2]Tyr | 9 h PLA | — | 0.057 | — | — | — | |||||||
| 0–9 h | 2 h CP | >5 | Specific dosing given at intervals, mix of CHO+PRO | — | — | — | During Ex, 0.083 | — | |||||
| 9 h CP | Specific dosing given at intervals, mix of CHO+PRO | — | — | — | 0.056 | — | |||||||
| Dreyer, 2008 (70) | UT, 8 M, 27 ± 2 y | [2H5]Phe | KE: 10 × 10; 70% 1 RM | Mixed | 1–2 h PEx, 0–1 h P-In | Leu-EAAs+CHO | 7 | 20 g EAAs +35 g CHO | 0.062 | — | — | 0.165 | — |
| UT, 8 M, 30 ± 2 y | 1–2 h PEx | None | 0 | None | — | 0.09 | — | — | — | ||||
| Drummond, 2008 (71) | UT, 7 M, 30 ± 2 y | [2H5]Phe | KE: 8 × 10; 70% 1 RM | Mixed | 1–3, 3–6, 1–5 h | Young EAA | 7 | 20g EAA, 1h PEx | 0.04 | — | — | 0.11, 0.1, 0.11 | — |
| Fujita, 2009 (28) | UT, 7 M, 4 F, 27 ± 2 y | [2H5]Phe | KE: 10 × 10; 70% 1 RM | Mixed | Rest, Ex, 0–1, 1–2, 0–2 h PEx | Fast | Fasted | 0.06 | 0.08, 0.09, 0.073 | — | — | — | |
| UT, 6 M, 5 F, 25 ± 1 y | EAAs+CHO | 7 | ∼20 g EAAs,∼25 g sucrose | — | — | 0.12 | 0.12, 0.089, 0.098 | — | |||||
| Moore, 2009 (72)6 | Rec, 7 M, 26 ± 3 y | [13C6]Phe | LP, KE: 5 × 8–10 | Myo (Sarc) | 1–3, 3–5 h P-In | 25 g WP Ex | 3 | Bolus imed PEx | 0.025,(0.052) | — | — | 0.066, 0.07 (0.084, 0.077) | — |
| None–nonexercise leg | Myo (Sarc) | 25 g WP | — | — | 0.051, 0.049 (0.086, 0.074) | — | |||||||
| Moore, 2009 (30) | TR, 6 M, 29 ± 2 y | [13C]Leu | LP, KE, LC: 4 × 8–10 | Mixed | 1–4 h P-In | 0 | 0 | Bolus imed PEx | Only PEx | 0.053 | — | — | — |
| 5 g | 0.4 | — | — | 0.075 | — | ||||||||
| 10 g | 0.8 | — | — | 0.08 | — | ||||||||
| 20 g | 1.6 | — | — | 0.11 | — | ||||||||
| 40 g | 3.2 | — | — | 0.115 | — | ||||||||
| Tang, 2009 (73) | ST, 6 M, 23 ± 4 y | [13C6]Phe | Si-KE, Si-LP: 4 × 10–12 RM | Mixed | 3 h, single Bx | WP | 2.3 | WPH 21.4 g, bolus imed PEx | No | — | 0.091 | 0.15 | — |
| ST, 6 M, 23 ± 4 y | Casein | 1.8 | Micellular casein 21.4 g, bolus imed PEx | No | — | 0.047 | 0.072 | — | |||||
| ST, 6 M, 23 ± 4 y | Soy | 1.8 | Soy isolate 21.4 g, bolus imed PEx | No | — | 0.078 | 0.125 | — | |||||
| West, 2009 (74) | Rec, 8 M, 20 ± 1 y | [13C6]Phe | LH: bicep: 4 × 10; ∼95% 10 RM | Mixed | 4 h, single Bx | LH | ∼2.5–3 | 25 g WP PRO post-arm Ex | 0.06 | — | — | 0.08 | — |
| HH: above + LP: 5 × 10; ∼90% 10 RM; KE, KC: 3 × 12 | HH | 0.06 | — | — | 0.081 | — | |||||||
| Myo | LH | 0.04 | — | — | 0.071 | — | |||||||
| HH | 0.04 | — | — | 0.064 | — | ||||||||
| Burd, 2010 (75) | ST, 8 M, 24 ± 5 y | [13C6]Phe | Si-KE: 1 or 3 sets; 70% 1 RM to fatigue; rest leg control | Myo | Rest, 5 h fed, 24 h fast and 29 h fed | 1 set | ∼2–2.5 | 20 g WP | 0.03 | — | — | 0.065, 0.035 | — |
| 3 set | — | — | — | 0.078, 0.06 | — | ||||||||
| Burd, 2010 (76) | TR, 15 M, 21 ± 1 y | [13C6]Phe | KE: 4×, 90% 1 RM | Mixed | 4, 24 h | 90 Fail | ? | Ensure ∼15% of caloric needs | 0.048 | — | 0.049 | 0.16, 0.08 | — |
| KE: 4×, 30% 1 RM (30 WM to 90%) | 30 Fail | Breakfast, 2 h before arrival | 0.048 | — | — | 0.14, 0.095 | — | ||||||
| KE: 4×, 30% 1 RM | 30 WM | 0.047 | — | — | 0.085, 0.075 | — | |||||||
| KE: 4×, 90% 1 RM | Myo | 90 Fail | 0.047 | — | 0.025 | 0.08, 0.055 | — | ||||||
| KE: 4×, 30% 1 RM (30 WM to 90%) | 30 Fail | 0.046 | — | — | 0.095, 0.08 | — | |||||||
| KE: 4×, 30% 1 RM | 30 WM | 0.046 | — | — | 0.06, 0.05 | — | |||||||
| KE: 4×, 90% 1 RM | Sarc | 90 Fail | 0.025 | — | 0.05 | 0.085, 0.05 | — | ||||||
| KE: 4×, 30% 1 RM (30 WM to 90%) | 30 Fail | 0.025 | — | — | 0.075, 0.075 | — | |||||||
| KE: 4×, 30% 1 RM | 30 WM | 0.025 | — | — | 0.06, 0.06 | — | |||||||
| Holm, 2010 (35) | UT, 20 M, 25 ± 1 y | [13C]Leu | Si-KE: LL 17% 1 RM | Myo | Early (0.5–3 h), late (3–5 h) | LL | ? | Fed multinutrient supplement every 30 min or water | 0.08 | 0.115, 0.095 | 0.18 | 0.139,0.17 | — |
| Si-KE: HL 70% 1 RM | HL | — | 0.086, 0.14 | — | 0.15, 0.21 | — | |||||||
| Col | LL | 0.08 | 0.14, 0.188 | 0.06 | 0.1, 0.124 | — | |||||||
| HL | — | 0.163, 0.15 | — | 0.123, 0.126 | — | ||||||||
| Mikkelsen, 2010 (77) | TR, 8 M, 23 ± 1 y | 1-2[13C2]Leu | 200 Maximal Ecc KE each leg | Myo/Col | 24–28 h | PLA | ∼2 | 18–23 g PRO and 26–34 g CHO PEx | — | — | — | 0.11/0.06 | — |
| NSAIDs | — | — | — | 0.14/0.11 | — | ||||||||
| Pennings, 2011 (78) | Rec, 12 M, 21 ± 1 y | [13C6], [2H2]Tyr | Cycling: LP, KE: 6 × 10 | Mixed | 0–6 h P-In | Casein | 1.7 | 20 g Bolus of 250 mL | — | — | 0.061 | 0.072 | — |
| Symons, 2011 (79) | Rec, 3 M, 4 W, 29 ± 3 y | [13C6]Phe | KE: 6 × 8; ∼80% 1 RM | Mixed | Meal to 3 h PEx | Beef | ∼6 | 340 g Beef patty ingestion 1 h pre-Ex | 0.073 | — | — | 0.156 | — |
| West, 2011 (80) | Rec, 8 M, 22 ± 1 y | [13C6]Phe | KE: 8 × 8–10; 10 RM | Myo | 1, 3, 5 h | Bolus | 3.5 | Bolus 25 g WP imed PEx | 0.02 | — | — | 0.041, 0.06 | — |
| Pulse | 3.5 | Pulse: 10 × 2.5 g, every 20 min | — | — | — | 0.03, 0.045 | — | ||||||
| Reitelseder, 2011 (81) | Rec, 9 M, 28 ± 2 y | 1-[13C]Leu | Si-KE: 10 × 8; 80% 1 RM | Myo | 1–3.5, 3.5–6, 1–6 h | Casein | 1.53 | Casein, 17.5 g, bolus imed pre-Ex | ∼0.056 | — | — | 0.098, 0.105, 0.10 | — |
| Myo | WP | 2.06 | WPI, 17.5 g, bolus imed pre-Ex | — | — | — | 0.123, 0.098, 0.10 | — | |||||
| Rec, 8 M, 26 ± 2 y | Myo | PLA | N/A | N/A | — | 0.072, 0.075, 0.073 | — | — | — | ||||
| Burd, 2012 (82) | TR, 8 M, 24 ± 1 y | [13C6]Phe | Slow (6-s Con/Ecc) 1 leg | Myo, Mito, Sarc | 0–6, 24–30 h | Slow | ∼2–2.5 | 20 g WP | 0.021 | — | — | 0.024, 0.053 | — |
| Cntl (1-s Con/Ecc) other leg | Cntl | — | — | — | 0.026, 0.03 | — | |||||||
| Burke, 2012 (83) | TR, 12 M, 27 ± 1 y | [13C6]Phe | KE: 8 × 8–10; 10 RM | Mixed | 0–5 h | Bolus 25 g + 5 Leu | 8 | Before Ex (1 × 25-g dose) | — | — | — | 0.085 | — |
| Pulse 25 g + 5 Leu | 8 | Before Ex (15 × 2 g every 15 min) | — | — | — | 0.095 | — | ||||||
| PLA | — | — | — | — | 0.037 | — | |||||||
| Camera, 2012 (40) | TR, 8 M, 23 ± 3 y | [13C6]Phe | LP: 10 × 5; 55–80% 1 RM | Myo | 0–4 h | Normal glycogen | ∼2–2.5 | 20 g WP + 40 g maltodextrin | — | 0.045 | — | 0.07 | — |
| TR, 8 M, 23 ± 4 y | Glycogen-depleted leg | — | 0.049 | — | 0.068 | — | |||||||
| Churchward-Venne, 2012 (84) | Rec, 8 M, 22 ± 1 y | [13C6]Phe | Si-KE, Si-LP: 4 × 10; ∼95% 10 RM; none–nonexercise leg | Myo | 1–3, 3–5 h PEx | WP | 3 | 25 g WPI, bolus imed PEx | ∼0.03 | — | 0.061, ∼0.05 | 0.064, 0.088 | — |
| Rec, 8 M, 22 ± 1 y | Low WP+Leu | 3 | 6.25 g WPI, bolus imed PEx | — | — | 0.068, ∼0.049 | 0.068, 0.048 | — | |||||
| Rec, 8 M, 23 ± 1 y | Low WP+EAAs, no Leu | 0.75 | 6.25 g WPI, bolus imed PEx | — | — | 0.063, ∼0.050 | 0.069, 0.050 | — | |||||
| Gasier, 2012 (85) | Rec, 12 M, 22 y | D2O | 5× Fail; 85%1 RM | Mixed/Myo | 24 h (16 h PEx) | One leg Con, 1 leg Ex | ? | Normal day | — | — | 0.76/0.94 | 0.69/0.75 | — |
| Res, 2012 (42) | Rec, 8 M, 23 ± 1 y | [2H5]Phe | LP, KE: 8 × 8; ∼70% 1 RM | Mixed | 8 h sleep | PRO (40 g casein) | 3–4 | Bolus 450 mL, 2.5 h PEx | — | 0.048 | — | — | — |
| Rec, 7 M, 22 ± 1 y | PLA (water) | — | — | — | 0.059 | — | |||||||
| West, 2012 (86) | Rec, 8 M, 20 ± 1 y | [13C6]Phe | KE: 8 × 8–10; 10 RM | Myo | 1, 3, 5, 24–26 h | M: 25 g WP | 3.5 | 25 g WP, 12.8 g EAAs, 3.5 g Leu | 0.021 | — | — | 0.057, 0.071, 0.06 | — |
| Rec, 8 W, 22 ± 2 y | W: 25 g WP | 3.5 | 0.020 | — | — | 0.054, 0.068, 0.06 | — | ||||||
| Bechshoeft, 2013 (87) | Sed,10 M, 23 ± 5 y | 1-[13C]Leu | Si-KE:10 × 36; 16% 1 RM (10 sets of 3 min) | Myo | 30–630, 30–180, 180,330, 330–480, 480–630 | Ex or IM feeding | 7.1 | 65 g Oral PRO, initial WP then casein | — | — | 0.059, 0.052, 0.055, 0.041 | 0.064, 0.053, 0.057, 0.062 | — |
| Reidy, 2013 (88) | Rec, 8 M, 1 F, 25 ± 1 y | [13C6]Phe | KE: 8 × 10; 70% 1 RM | Mixed | 1, 3, 5 h | WP (17.5 g PRO) | 1.90 | Bolus of 300 mL 1 h PEx | 0.056 | — | — | 0.078, 0.074, 0.077 | — |
| Rec, 9 M, 1 F, 23 ± 1 y | Blend (19 g PRO) | 1.80 | 0.055 | — | — | 0.088, 0.087, 0.087 | — | ||||||
| Wilkinson, 2014 (89) | Rec, 8 M, 22 ± 4 y | D2O | Si-KE: 4 × 8; 80% 1 RM RET | Myo, Sarc, Col | 0–2, 2–4, 4–8 d | Exercise training leg (5 sessions + 20 g WP) | ∼2 | 20 g WPI, Muscletech | — | — | — | 0.082, 0.082, 0.075 | — |
| None | Cntl leg | 0.06, 0.06, 0.056 | — | — | — | — | |||||||
| Areta, 2014 (90) | ST, 8 M, 25 ± 2 y | [13C6]Phe | KE: 2,4 × 10; 80% 1 RM | Myo | 0, 1, 4, 6, 12 h | Bolus 40 g, 2 servings in 12 h | 3–4 | 2 Boluses of 500 mL | ∼0.03 | — | — | 0.055 | — |
| ST, 7 M, 25 ± 1 y | Med bolus 20 g, 4× servings in 12 h | ∼2 | 4 Boluses of 250 mL | — | — | — | 0.079 | — | |||||
| ST, 8 M, 25 ± 2 y | Pulse 10 g, 8 servings in 12 h | <1 | 8 Boluses of 125 mL | — | — | — | 0.057 | — | |||||
| Areta, 2014 (91) | TR, 8 M, 8 F, 27 ± 4 y | [13C6]Phe | KE: 8 × 8; 50–80% 1 RM | Myo | Rest, 0–4 h | Energy balance | — | 45 kcal ⋅ kg−1 ⋅ FFM−1 | 0.026 | — | — | — | — |
| Energy deficit–PLA | — | 30 kcal ⋅ kg−1 ⋅ FFM−1 – water | 0.019 | 0.024 | — | — | — | ||||||
| Energy deficit–15 g | ∼1.5 | 15 g WP – 500-mL bolus | — | — | — | 0.030 | — | ||||||
| Energy deficit– 30 g | ∼3 | 30 g WP – 500-mL bolus | — | — | — | 0.038 | — | ||||||
| Camera, 2015 (92) | TR, 8 M, 8 F, 19 ± 1 y | [13C6]Phe | KE: 8 × 5; 80% 1 RM and 30 min, 63% PPO | Myo | Rest, 0–4 h | PRO or PLA | ∼2.5–3 | 25 g WP or flavored water | 0.030 | 0.052 | — | 0.072 | — |
| Churchward-Venne, 2014 (93) | Rec, 8 M, 21 ± 1 y | [13C6]Phe | Si-KE: 8 × 10–12; ∼80% 1 RM; none–nonexercise leg | Myo | 0–1.5, 1.5–4.5 h PEx | 25 g WP | 3 | Bolus imed PEx | — | — | ∼0.05, 0.063 | ∼0.052, 0.065 | — |
| Rec, 8 M, 20 ± 1 y | 6.15 g WP+Gly+Ala | 0.75 | Bolus imed PEx | — | — | 0.063, 0.050 | 0.069, 0.050 | — | |||||
| Rec, 8 M, 21 ± 1 y | 6.15 g WP+low Leu+Gly+Ala | 3 | Bolus imed PEx | — | — | 0.052, 0.042 | 0.062, 0.038 | — | |||||
| Rec, 8 M, 20 ± 1 y | 6.15 g WP+high Leu+Gly+Ala | 5 | Bolus imed PEx | — | — | ∼0.057, 0.059 | ∼0.054, 0.063 | — | |||||
| Rec, 8 M, 21 ± 1 y | 6.15 g WP+BCAAs +Gly+Ala | 5 | Bolus imed PEx | — | — | 0.048, 0.052 | 0.057, 0.048 | — | |||||
| Mitchell, 2014 (94) | Rec, 23 M, 24 ± 1 y | [13C6]Phe | LP, KE, LC, CP: 4 × 8 | Myo | Rest, 1–3, 3–6, 1–6 | 30 g milk PRO | ∼3 | Milk PRO imed PEx and/or with breakfast | ∼0.033 | — | — | ∼0.06, 0.05 | — |
| Parr, 2014 (95) | TR, 8 M, 21 ± 5 y | [13C6]Phe | KE: 8 × 5; 80% 1 RM and 30 min, 63% PPO and 10 × 30 s, 110% PPO cycling | Myo | 2–8 h PEx | PRO (25 g WP 2×) | 1.4 | Bolus 500 mL imed and 4 h PEx | 0.025 | — | — | 0.052 | — |
| ALC-PRO,25 g WP 2× | 2.8 | — | — | — | 0.039 | — | |||||||
| Reitelseder, 2014 (96) | Rec, 8 M, 24 ± 2 y | 1-[13C]Leu | Si-KE: 10 × 8; 80% 1 RM | 1–3.5, 3.5–6 h | Casein | 1.53 | Casein, 17.5 g, bolus imed pre-Ex | — | — | — | ↔ | ↑90 min | |
| Rec, 6 M, 26 ± 3 y | WP | 2.06 | WPI, 17.5 g, bolus imed pre-Ex | — | — | — | ↑↔ | ||||||
| Rec, 7 M, 24 ± 2 y | PLA | N/A | N/A | — | ↔ | — | — | ↔ | |||||
| Rahbek, 2014 (97) | Rec, 24 M, 24 ± 1 y | [13C6]Phe | KE Con: 6 × 10; Max | Myo | 1–3 h, 3–5 h PEx | WP+CHO | ∼1.5–1.9 | Bolus 500 mL, ∼18 g PRO + ∼18 g CHO | No | — | — | 0.106, 0.106 | — |
| KE Ecc: 6 × 10; Max | — | — | 0.106, 0.09 | — | |||||||||
| KE Con: 6 × 10; Max | CHO | — | Bolus 500 mL, ∼36 g CHO | — | — | 0.08, 0.10 | — | ||||||
| KE Ecc: 6 × 10; Max | — | — | 0.095, 0.09 | — | |||||||||
| Reidy, 2014 (98) | Rec, 7 M, 1 W, 24 ± 1 y | [13C6]Phe | KE: 8 × 10; 70% 1 RM | Myo | 3, 5 h | WP; 17.3 g PRO | 1.90 | Bolus 300 mL, 1 h PEx | 0.041 | — | — | 0.093 | ↑ at 1 h |
| Rec, 7 M, 1 W, 22 ± 1 y | Blend; 20 g PRO | 1.90 | 0.035 | — | — | 0.081 | ↑ at 1 and 2 h | ||||||
| Witard, 2014 (44) | ST, 12 M, 22 ± 3 y | [13C6]Phe | Si-LP, Si-KE: 8 × 10; 80% 1 RM; 3 h after breakfast | Myo | 0–4 h PEx | 0 | — | None | 0.032 | 0.052 | — | — | — |
| ST, 12 M, 20 ± 1 y | 10 g WP | 0.67 | Bolus imed PEx | — | — | 0.04 | 0.059 | — | |||||
| ST, 12 M, 22 ± 3 y | 20 g WP | 1.34 | — | — | 0.05 | 0.069 | — | ||||||
| ST, 12 M, 20 ± 1 y | 40 g WP | 2.68 | — | — | 0.049 | 0.071 | — | ||||||
| Witard, 2014 (99) | Rec, 5 M, 3 F, 30 ± 3 y | [13C6]Phe | KE: 8 × 10; 80% 1 RM | None | 1, 2, 3, 7 h | EAAs+CHO/PLA | 2.7 | 50 g Sucrose + 15 g EAAs 1 h PEx | — | — | — | 0.11,0. 0.086 | — |
| CHO/EAAs | 50 g sucrose 1 h PEx + 15 g EAAs 2 h | — | — | — | 0.109, 0.089 | ↑ at 1 h | |||||||
| Effect of RET | — | — | — | — | — | ||||||||
| Yarasheski, 1992 (100) | 9 M; 27 ± 1 y | 1-[13C]Leu | WB Ex: 4 × 4–8; 75–90% 1 RM | Mixed | 6 h | PLA+Ex | ? | 1/12 daily intake/30 min | — | — | 0.048 | 0.066 | — |
| 7 M; 27 ± 1 y | GH | — | — | 0.048 | 0.07 | — | |||||||
| Phillips, 2002 (101) | UT,11 M, 8 W, 24 ± 3 y | 2H5, 15N Phe | 8-wk WB, split-routine RET (1 h/d, 6 d/wk): acute Si-LP, Si-KE: 2–6 × 10; 80% pre-TR 1 RM | Mixed | 6–7 h | UT | ? | Fed IM 3847 ± 1029 kJ during infusion | — | — | ∼0.065 | ∼0.083 | ↔, ↑ PEx |
| TR | — | — | ∼0.082 | ∼0.1 | ↔ | ||||||||
| Tang, 2008 (102) | Rec, 10 M, 21 ± 2 y | 2H5 Phe, α-KIC | Si-KE: 6 × 10; 80% 1 RM; 8 wk RET | Mixed | 3 h rest, 4 h PEx, 3 h at 28 h | UT | ? | Specific intermittent dosing of Boost (Nestle) | — | — | 0.045 | 0.090, 0.074 | ↑ at 4 and 24 h |
| [13C6]Phe | TR | — | — | 0.048 | 0.123, 0.062 | ↑ at 4 h, not 24 h | |||||||
| Wilkinson, 2008 (103) | UT, 10 M, 21 ± 1 y | D3-α-KIC | KE: 5 × 10: 80% 1 RM; 10 wk RET | Myo/Mito | 4 h | UT | ? | Fed IM 1.1 g PRO/kg | — | — | 0.054, 0.080 | 0.12, 0.15 | — |
| TR | — | — | 0.08, 0.075 | 0.12, 0.052 | — | ||||||||
45 min 75%  O2max; 10 wk cycling O2max; 10 wk cycling |
UT | — | — | 0.051, 0.074 | 0.051, 0.18 | — | |||||||
| TR | — | — | 0.054, 0.072 | 0.075, 0.15 | — | ||||||||
| Brook, 2015 (104) | Rec, 10 M, 24 ± 1 y | D2O | Si-KE: 6 × 8; 75% 1 RM; 6 wk RET | Myo | 0–3, 3–6, 0–6 wk | TR | — | Assumed to be normal diet | 0.056 | 0–3 wk (0.067), 3–6 wk (0.054) | |||
| UT leg | UT | 0.056 | |||||||||||
| Lambert, 2015 (105) | 16 M, 40 ± 4 y; 9W, 38 ± 4 y | D2O | 11 wk training; acute: WB, 4 × 12; 50–60% 1 RM | Myo (UT/TR) | 0–24 h | RET | ? | 5 IM meals of Boost (Nestle): total ∼8037 kJ | — | — | — | 0.37, 0.43 | — |
| RET-Land TM | — | — | — | 0.44, 0.42 | — | ||||||||
| RET-Aquatic TM | — | — | — | 0.61, 0.52 | — | ||||||||
AA, amino acid; ALC, alcohol; AV-Bal, arterial-venous balance; Bx, biopsy; CHO, carbohydrate; Cntl, control; Col, collagen fraction; Con, concentric contractions; CP, carbohydrate and protein; D2O, deuterium; EAA, essential amino acid; Ecc, eccentric contractions; Ex, exercise; FF, fat-free; FFM, fat-free mass; FSR, fractional synthesis rate; GH, growth hormone; HH, high hormone; HL, high load; IM, intermittent; imed, immediate; INF, infusion, KE, knee extension; KIC, α-ketoisocaproate; LC, leg curls; LL, low load; LP, leg press; M, men; Malto, maltodextrin; Max, maximum; Mito, mitochondrial fraction; MPS, muscle protein synthesis; Myo, myofibrillar protein fraction; MVC, maximal voluntary contractions; N/A, not applicable; NB, net balance; Neg, negative; Net bal, net balance; NM, not mentioned; NSAID, nonsteroidal anti-inflammatory drug; Nutr, nutrition; PEx, postexercise; P-In, postingestion; PLA, placebo; PPO, peak power output; PRO, protein; RE, resistance exercise; Rec, recreationally active; ref, reference; RET, resistance exercise training; RM, repetition maximum; Sarc, sarcoplasmic protein fraction; Sed, sedentary; Si, single leg; Sq, squats; ST, strength-trained; TM, treadmill; TR, trained; UT, Untrained;  O2max, maximal oxygen uptake; W, women; WB, whole body; WP, whey protein; WPC, whey protein concentrate; WPH, whey protein hydrosolate; WPI, whey protein isolate; WM, work-matched; Wmax, watt max; ↔, no change; ↔↑, trend to increase; ↑, increase from basal values; ?, unknown.
O2max, maximal oxygen uptake; W, women; WB, whole body; WP, whey protein; WPC, whey protein concentrate; WPH, whey protein hydrosolate; WPI, whey protein isolate; WM, work-matched; Wmax, watt max; ↔, no change; ↔↑, trend to increase; ↑, increase from basal values; ?, unknown.
Values for age are means ± SDs or SEMs (see corresponding reference).
Exercise column denotes the exercise (sets × repetitions), at exercise intensity as a percentage of 1 RM unless otherwise stated.
Multiple values in the PEx column indicate the various postexercise MPS assessments for the reference when they are reported. These time periods are described in the column “FSR Bx time PEx” for the respective reference.
The majority of values for MPS are reported as %/hour, but 2- and 3-pool arterial-venous method studies (52–55) presented data as nmol ⋅ min−1 ⋅ leg volume or mass−1, not as %/h.
Values in parentheses represent the sarcoplasmic protein fraction.
Energetic, metabolic, and mechanical stresses during and after RE play dynamic roles in the control of protein turnover. It seems intuitive that during RE the primary goal of skeletal muscle metabolism is to maintain energy for contraction, thus prompting a reduction (108) or no change (21) in basal values in the energy costly process of MPS in human skeletal muscle. As expected, during high-intensity RE, muscle blood flow is increased and, secondary to that, muscle perfusion, shunting, and AA flux are increased as well (21). Reports indicate that during the immediate (0–1 h) period after RE, the metabolic milieu switches from catabolic to anabolic as shown by the release of AMP-activated protein kinase (AMPK) inhibition of translation initiation and MPS (21, 24, 28, 33, 38). During this time, blood flow and lactate concentrations normalize as the muscle becomes more sensitive to nutrients, presumably due to the increased AA flux (14, 98, 109), mTORC1 signaling, most noticeably through p70 ribosomal protein S6 kinase 1 (S6K1) phosphorylation (24, 33, 109), and increased insulin sensitivity. After RE, both MPS and MPB are increased compared with rest; yet, net balance is less negative (14, 16, 43). In the fed state, the FSR increases to a greater extent, fractional breakdown rate is thought to slightly decrease, presumably due to insulin- and/or AA-mediated effects, and net protein balance (between MPS and MPB) becomes positive (51, 101, 110). Because this outcome (fractional breakdown rate or MPB) changes less than FSR (16) in response to RE, interventions have targeted FSR. Gender differences in post-RE protein metabolism do not seem to be present. In the fasted (33, 38) and whey protein–fed (86) conditions after RE, MPS and mTORC1 signaling did not differ between men and women. The majority of the literature examining protein metabolism with RE and protein/AAs studied the intermediate postexercise recovery (1–6 h) period, mainly due to a landmark study (111) and methodologic/logistical difficulties. Less was known concerning the MPS response in the later period (6–24 h) (111), and it has been shown that a single bout of RE improves the MPS response in the morning 24 h postexercise (13, 16, 38, 111–114). Regardless of when MPS is elevated after exercise, we still have very little understanding of the translational relevance of these changes in protein turnover in relation to the chronic changes after exercise training.
To provide a comprehensive view of the effect of protein/AAs on postexercise MPS, we examined all of the literature and estimated the percentage change in MPS in studies with protein/AA feeding during the various post-RE time frames. The following comparisons of estimated mean responses, if present, are highlighted in this review: 1) fasted postexercise MPS compared with basal resting values (Ex-Fast vs. Rest), 2) protein/AA-fed resting values compared with basal resting values (Fed vs. Rest), 3) protein/AA-fed postexercise MPS compared with basal resting values (Ex-Fed vs. Rest), 4) protein/AA-fed postexercise MPS compared with fed resting values (Ex-Fed vs. Fed), 5) fasted postexercise MPS compared with fed resting values (Ex-Fast vs. Fed), and the main focus of this review, 6) protein/AA-fed postexercise MPS compared with fasted or carbohydrate placebo postexercise values (Ex-Fed vs. Ex-PLA/CHO; Figure 1). These comparisons were examined over various postexercise periods of varying duration (Tables 1 and 2).
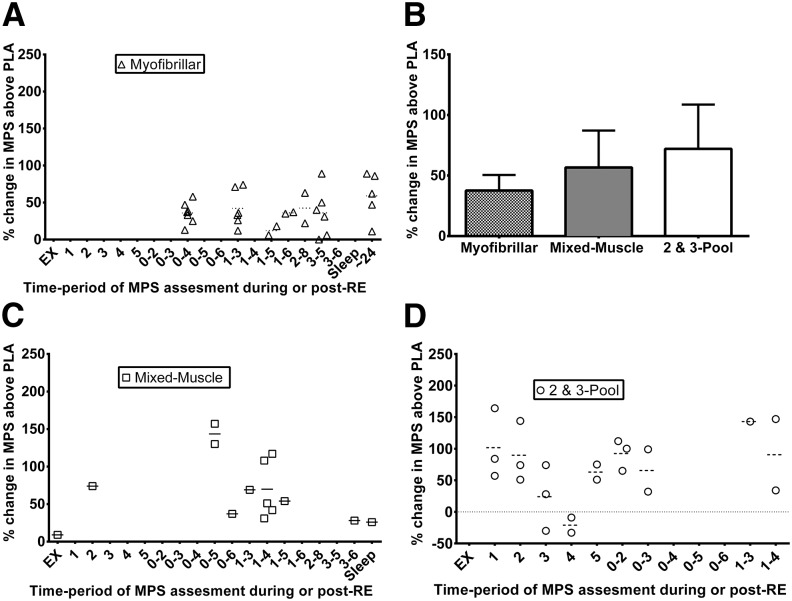
FIGURE 1.
Effect of protein/AA supplementation on postexercise MPS in young adults. Percentage changes from fasted to protein/AA supplemented states on MPS via the direct precursor product method (either the myofibrillar or mixed-muscle protein fractions) and arterial and venous balance methods (2- and 3-pool models) plotted from individual studies according to the time period (h) of assessment post-RE. Studies with an ∼900% response during exercise (115) and ∼600% responses at 1–2 and 3–4 h (53) were removed from panel D to shorten the y axis. Each data point represents a mean response value from a treatment arm in a clinical trial: n = 30 for myofibrillar (A), n = 16 for mixed muscle (C), and n = 21 for 2- and 3-pool models (D) treatment arms. The horizontal (dashed or dotted) line in each column represents the mean response for all treatments in that time period. Panel B shows the mean (95% CI) pooled treatment responses over all time periods. AA, amino acid; EX, exercise; MPS, muscle protein synthesis; PLA, placebo; RE, resistance exercise.
RE alone exerts an obvious increase in postexercise mixed-MPS (Tables 1 and 2). Although the magnitude of the increase may vary between investigations, it appears that postexercise mixed-MPS increases ∼65% from resting basal values. Myofibrillar MPS increases to a similar extent (∼40%), and the muscle collagen fraction is most sensitive with a ∼89% increase. The 2- and 3-pool stable-isotopic mathematical models appear to be less responsive in this condition, with increases of only ∼29% from basal resting values, and only when all of the leg muscles are exercised (14, 20). The average fasted-state post-RE increase in MPS for all studies and methods across all time periods suggests a postexercise increase of 56% from resting values. Although the magnitude and duration of MPS response is highly dependent on exercise intensity/volume (35, 39), it appears that a fatiguing bout of RE, studied in the fasted state, results in multiphasic postexercise MPS responses. A sluggish increase in MPS peaks somewhere at 2–3 h postexercise (∼60–70%), declining at ∼4 h, slightly increasing in the ensuing hours, decreasing during sleep, and then rebounding the following morning. The prevailing theory is that the provision of exogenous AAs during the post-RE periods can further increase and/or prolong MPS depending on the particular conditions examined, which we will highlight below.
The maximal MPS response after nutrition alone (no exercise) is rather transient in that it is only captured in the first few hours postingestion, when MPS typically doubles (∼0.10%/h) (116–119). Indeed, examination of the literature suggests increases during the first 1 or 2 h postingestion with Fed vs. Rest MPS of ∼130%, 117%, 50%, and 81% for myofibrillar MPS, sarcoplasmic MPS, mixed-MPS, and 2- or 3-pool models (arterial and venous limb balance methods), respectively.
The majority of research has been confined to the Ex-Fed vs. Rest comparison, which elicits the highest rates of postexercise MPS as evidenced by changes of ∼138%, 54%, 100%, 78%, and 170% for myofibrillar MPS, sarcoplasmic MPS, mitochondrial MPS, mixed-MPS, and 2- or 3-pool models (arterial and venous limb balance methods), respectively. The average increase in MPS for all studies and methods across all time periods suggests a postexercise increase of 129% from resting values.
To determine the effect of protein ingestion on enhancing the MPS response, a comparison with exercise in the fasting or carbohydrate-fed, postexercise condition is clearly required (Ex-Fed vs. Ex-PLA/CHO) (Figure 1). This comparison has been made (14, 24, 28, 30, 35, 40, 42, 44, 51, 52, 54, 55, 57, 58, 62, 66, 70, 81, 83, 91, 92, 95–97, 114, 120), albeit in restricted conditions due to the logistic difficulty of procuring additional participants or muscle biopsy samples. Interestingly, only 1 study examined the effect of protein/AA feeding on collagen post-RE MPS. The authors found no effect, and even a slight (nonstatistical) decrease in collagen MPS was evident in young adults (35). With myofibrillar MPS a consistent postexercise additive effect (∼45%) of protein/AAs on MPS has been shown. This effect was shown regardless of glycogen depletion (40), energy deficit (91), during sleep (42), after a recent meal (44), or with inclusion of concurrent aerobic exercise (AE) with RE (92), suggesting that this effect is rather robust during the acute-response period. Indeed, this clear protein/AA effect on MPS causally observed in close proximity to exercise has generated recommendations of an optimal postexercise time frame to ingest protein/AA supplements (i.e., the anabolic window).
As a challenge to this recommendation, examination of the various time periods ≥2 h (when myofibrillar/mixed-MPS was assessed) does not seem to indicate an optimal time to maximize the effect for ingestion of protein/AAs. Indeed, anabolic sensitivity to protein/AAs after RE has been shown to be similar at 1 and 3 h post-RE (53) and exists as far as 24 h postexercise in the myofibrillar protein fraction (114). These data highlight the ability of exercise to sensitize the muscle to AAs during postexercise recovery. However, given the multiphasic response of MPS in the fasted state, some have suggested that the additive effect of protein/AAs should be tested at various postexercise time points to determine the most effective synergism/interaction of protein/AA feeding and MPS. A recent investigation examined the repeated timing and dosing of protein/AAs for optimizing the post-RE MPS (90). They suggested that repeated periods of AA flux from postexercise ingestion of 20 g protein every 3 h was more effective than 40 g every 6 h or 10 g every 1.5 h at maximizing myofibrillar MPS throughout a 12-h period (90). However, the optimal timing and dosing of protein supplements around the typical meal pattern are unknown, and these findings should be interpreted with caution, because exercising and prolonged postexercise recovery in a fasted state is not typical practice. From the available literature, it seems that protein dose (30, 44, 91, 121–123) rather than exercise intensity (35) mediates this synergistic effect of protein/AAs (Figure 1). When a maximal dose of protein is given, for the exercise protein/AA fed compared with exercise placebo/carbohydrate comparison, young adults showed a (∼31–89%) change in myofibrillar MPS (44, 91). With mixed-MPS a consistent additive effect (∼23–157%) of protein/AAs on MPS has been shown, illustrating a similar pattern to the myofibrillar fraction, except that, at a maximal dose, young adults can reach an average 89% increase in MPS (30) during a short time frame. Interestingly, only 1 recent study did not show an additive effect of protein after RE (97). A potential explanation is that the participants were accustomed to the exercise bout via an exercise habituation period preceding the metabolism study. Most investigations examining this comparison have used untrained or recreationally active participants (Tables 1 and 2). This theory could be questioned with the observation that resistance-trained participants have also shown this protein/AA effect (30, 40, 44, 91, 92, 95). Yet, even resistance-trained participants do not habitually train higher volumes of knee extension exercise as conducted during these metabolic studies, and one could deduce that these “trained” participants are still experiencing a novel stimulus. Future examination of this comparison should determine if the additive/prolonging effect of protein/AAs may be more beneficial on post-RE MPS during a novel compared with a habituated stimulus. When 2- or 3-pool models (arterial and venous limb balance methods) were used, a slightly higher effect was seen (∼113% change) with this comparison, yet this effect was much more transient, similar to any change in MPS with this methodology examining nutrition alone, lasting only 1–2 h postingestion (14, 51–55, 57, 111, 115, 124).
Several studies have shown an additive effect of protein/AA feeding with protein/AAs (40, 70, 92, 125), during an early postexercise time frame (0–4 h postexercise). However, with regard to the Ex-Fed vs. Fed comparison, others have shown that after exercise, the effect of a maximal dose of protein/AAs on maximizing MPS was similar to that of protein/AAs only in some studies (72, 84) but not others (66, 73, 102); yet, the Ex-Fed vs. Fed response was ∼52% on average. For a maximal protein/AA dose, this effect was nonexistent within the first 3 h postexercise (72, 84, 93) and when protein/AAs were coingested with carbohydrate and fat (93). With a maximal dose, this effect was most evident at 3–5 h (72, 84) (intriguingly, around the time of the next meal) and at 24 h (75, 102, 111, 114, 126) after high-intensity RE. In addition, low-intensity RE may potentiate this effect out to 8–10 h postexercise (87). Yet, this effect of exercise in the fed state was attenuated with RE training (RET) (101–103), presumably due to elevated basal MPS (49, 50, 101, 102) and a more efficient AA utilization within the muscle (127, 128). At the same absolute intensity after RET, a decrease in post-RE MPS was observed and at the same relative intensity the magnitude was increased (102) or unchanged (103), but the time course of the MPS response was attenuated (102). Together, these findings indicate that exercise clearly prolongs the nutrient-induced increase in MPS.
A number of investigators have highlighted the transient effect of protein/AAs on MPS, whereas extracellular AAs are maintained, a phenomenon termed the “muscle full” effect (116). We believe that this effect is largely dependent on the sensitivity of the muscle to nutrients and is most often regulated by physical activity (exercise) or lack thereof (35, 72). We propose that in exercise-stimulated muscle, this “full effect” is attenuated and it is more likely for prolonged aminoacidemia to have an effect on extending MPS. It is interesting to speculate that the attenuation of this “full effect” is partly a consequence of muscle perfusion/swelling that transiently increases myofiber size after RE. Furthermore, the “full effect” first postulated by Atherton et al. (116) is most likely to occur when the muscle AA pools are rapidly filled from the large increase in blood AA concentration that follows a large bolus of a quickly digested protein, such as whey. Thus, blending protein sources with different digestion rates may confer a potential benefit by having just enough AA pool expansion to signal additional MPS while delaying this “filling” and subsequent effect by not overfilling the pool. This effect may be especially relevant in exercise-sensitized muscle because AA flux is increased. Yet, there are several alternative thoughts for the proposed muscle full effect. Some research has suggested, at least in rats, that a means to inhibit prolonged MPS is at the step of translation elongation via a reduction in energy status (129), but this has not yet been shown in human skeletal muscle. Another potential explanation of the muscle full effect is that MPS declines before mTORC1 signaling due to a rate-limiting delay in another step in mRNA translation not regulated by mTORC1. However, this warrants further investigation. These findings have begun to characterize the adaptation in protein metabolism after exercise training; yet, there is much that is not known with regard to the balance of protein synthesis and protein breakdown as adaptation to repeated exercise stimulus occurs.
Protein dose.
Because of the implications of finding an effect, a great deal of attention has been placed on interventions to enhance the acute response of MPS in the early recovery period post-RE. In 2009, Moore et al. (30) used 6 participants to describe a dose effect of post-RE MPS with egg protein ingestion. They discovered that MPS was maximized with 20 g egg protein (30), which corresponds to ∼8–9 g essential amino acids (EAAs) and ∼1.8 g leucine. Follow-up research with whey protein showed similar findings in participants who were provided breakfast (44) in energy deficit (91) or with beef ingestion (123). These and other studies helped shape the general consensus that 20–30 g (containing ∼8–15 g EAAs) is likely to maximize the postexercise MPS response, at least in young men. Yet, certain individuals with a larger lean mass or body mass may benefit from a larger postexercise protein dose (91). These generalized recommendations may not always apply, because there are several modifiers to AA sensitivity in skeletal muscle. Previous physical activity may lower the protein/AA dose, whereas catabolic conditions of energy deficit (91) or various health concerns (inflammation, sickness, aging) may necessitate a higher dose (121–123, 130) to maximize MPS. Although 20–30 g protein seems to be the dose needed to maximize post-RE MPS, given the above points, this maximal dose seems to be conditional on the subject population in question and possibly the type or mix of protein/AAs ingested.
Protein type/source.
In addition to factors intrinsic to the individual, the type or source of protein/AAs ingested has been thought to modulate the postexercise MPS response. Potential differences could be due to the overall protein quality (i.e., AA composition) of the protein source and the extracellular AA appearance reflected by its digestion and absorption rate (i.e., fast, intermediate, or slow). It is clear that crystalline AAs have a potent effect on postexercise MPS (28, 51–53, 55, 70, 71). In addition, intact protein ingestion in the form of soy, casein, whey, egg, or beef increases postexercise MPS (30, 37, 58, 67, 68, 73, 79, 81, 83, 121, 122, 131). Because of several methodologic differences between investigators, there is some disagreement about whether different protein sources produce superior effects on MPS.
An examination of the literature suggests that the intrinsic properties of the ingested protein type/source reflect the physiologic MPS response (Table 2), at least in the immediate hours after ingestion. A fast, rapidly digested source causes a rapid and maximal increase in MPS (58, 73, 80, 122, 132, 133), whereas a slowly digested source is more likely to cause a delayed, more prolonged response (37, 81), at least in the exercised condition. Because of a higher BCAA content (134), and rapid increases in blood AA concentrations, whey protein is often considered superior to other isolated protein sources (80, 121, 122, 135, 136).
However, our scientific interpretations of these findings are shaped by the limits of our observations. Most of the studies examining various protein types/sources use a window of 3–4 h postexercise (Table 2). A study extending the postexercise window to 1–6 h comparing postexercise ingestion of whey with casein found no difference in the MPS but tended to show differences in early and late periods (81). We have shown a similar pattern with a protein blend of multiple AA-release profiles (88). This evidence suggests that the limits in our observation may be skewing our interpretation. It seems clear that when examining the evidence from many acute studies (Table 2), there is no difference in protein source on the magnitude or duration of the MPS response when examined over a longer postexercise incorporation window (past 4 h postexercise). This hypothesis has been tested and is supported by credible evidence in chronic exercise and supplement studies discussed in later sections.
One reason for discrepancies between effects of protein supplement type on the postexercise MPS response is that matching protein by total protein content results in an imbalance of total leucine content across the protein interventions. In studies with this imbalance there are some differences in acute post-RE MPS between protein supplement types (134, 137). It is clear that leucine stimulates MPS (138–144). It seems that the potent stimulatory effect of the higher leucine content of a supplement will affect the MPS response and mTORC1 signaling more than a minor change (1–5 g) in total protein, although the exact titration is unknown. In addition, the difference in total protein ingested is mostly composed of nonessential AAs, which do not further stimulate muscle protein anabolism (52, 145). Although energy status may be important in some cases, such as overall energy deficit (91), but not others (40) a 12- to 40-kcal difference in total energy provided from the few extra grams of protein in the supplement is extremely unlikely to influence the MPS response. We have shown that adding 120 kcal in the form of carbohydrate does not further stimulate muscle protein anabolism when providing sufficient EAAs (146).
It appears that the digestion/absorption rate and AA composition of a protein are 2 factors that should be considered together because they may not act independently. Protein appears to be most effective when given as a bolus (with an adequate amount of leucine) in close proximity to exercise (80) to maximize the feeding effect, because a pulse ingestion (35, 80, 83, 87) poorly mimics the blood AA release from a bolus of more slowly digesting protein (37, 81, 88). Further support to the stimulatory effect of leucine is demonstrated by evidence showing that added free leucine to a whey pulse is just as effective as a whey bolus (83) when given before exercise. Thus, protein/AA ingestion in close proximity (hours or perhaps 1–2 d) to exercise may lower the leucine threshold by exercise-induced facilitation of AA flux. An examination of the literature (Table 2) suggests that a greater leucine stimulus may be needed in the rested compared with exercised condition to prolong and/or enhance the MPS response. We estimate that a protein/AA source containing ∼1.8–2.0 g leucine would be sufficient to activate a postexercise “leucine trigger” due to the exercise-induced AA flux and/or improved muscle sensitivity to AAs. Leucine plays a key role in the postexercise MPS response, at least when total protein intake is lower. Two recent studies have elegantly shown that the leucine content in a supplement is a primary stimulator of MPS, especially when the total protein or content of other AAs is low (84, 93). However, if the protein/AA dose contains sufficient leucine it seems clear that it does not matter what protein source is used provided there is a sufficient quantity of a high-quality source that is digestible and contains all the EAAs. This hypothesis has been tested and proven by chronic exercise and supplement studies discussed in later sections.
A factor overlooked in most studies that considered the impact of protein/AA nutrition on postexercise MPS responses is gut physiology and adaptation. Splanchnic uptake extracts ∼50% or more of the AAs released during digestion during the first-pass splanchnic extraction (147–150). Because of the higher turnover of proteins in those tissues, the splanchnic region is a primary site of AA flux and supply (of certain AAs) to other tissues under various conditions (151–153). This response likely is dependent on frequency/size of the ingested bolus, health and age of the population in question, and the AA composition of the ingested protein source (153–155). We know very little about the interplay of this process with regard to modulating postexercise MPS, especially with regard to chronic exposure to the stimulus. It is possible that the gut or other nonskeletal muscle tissue protein metabolism may play a role in the improved efficiency of protein metabolism that occurs during RET and provide a partial explanation for the negligible effect of protein supplementation after RET.
Most studies that investigated the acute response of postexercise MPS report data in the format of means and then direct generalized conclusions toward the population. Unfortunately, we have very little published information on individual variability in an acute MPS response to nutrition. Our own experiences show that there is significant variability in the magnitude and duration of postexercise MPS between individuals (PT Reidy, unpublished data, 2014). In addition, a few recent publications (92, 94) have shown diverse individual responses. It is possible that a portion of the population (suggested to be ∼25%) may not respond to protein/AA supplements (92, 156). Future research should elucidate more precise estimates of the prevalence and mechanisms underpinning this phenomenon. This may also provide an explanation for the negligible effect of protein supplementation after RET.
Association with acute molecular events and the physiologic response to RE.
Animal, cell, and other basic science models have clearly delineated a necessary role for mTORC1 and other signaling pathways in controlling MPS. Indeed, the large body of evidence in human skeletal muscle also supports the concept that the early protein turnover response to RE is driven largely through translational and post-translational control (112, 157) (Figure 2, Supplemental Tables 1–7, and Supplemental References).
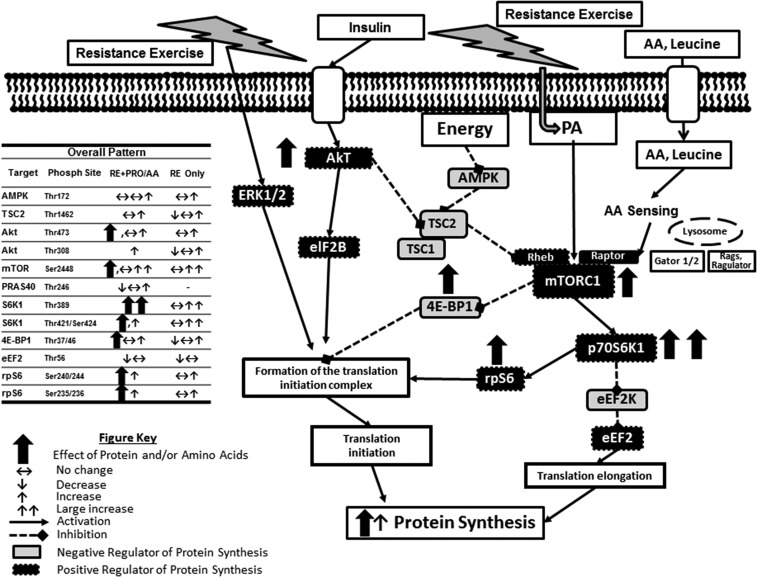
FIGURE 2.
Representative schematic for the effect of postexercise PRO/AA supplementation on the overall mTORC1 signaling and MPS response in human skeletal muscle. AA, amino acid; Akt, protein kinase B; AMPK, AMP-activated protein kinase; eEF2, eukaryotic elongation factor 2; eEF2K, eukaryotic elongation factor 2 kinase; eIF2B, eukaryotic initiation factor 2B; ERK1/2, extracellular-related kinase 1/2; Gator, GTPase-activating protein activity toward Rags; MPS, muscle protein synthesis; mTOR, mammalian target of rapamycin; mTORC1, mammalian target of rapamycin complex 1; PA, phosphatidic acid; Phosph, phosphorylation; PRAS40, proline-rich Akt substrate 40; PRO, protein; Rags, recombination activating genes; Raptor, regulatory-associated protein of mTOR; RE, resistance exercise; Rheb, Ras homolog enriched in brain; rpS6, ribosomal protein S6; S6K1/p70S6K1, p70 ribosomal protein S6 kinase 1; TSC1, tuberous sclerosis complex 1; TSC2, tuberous sclerosis complex 2; 4E-BP1, eukaryotic initiation factor 4E binding protein 1.
The increased translation of mRNA after RE is controlled via the mTORC1 signaling pathway. This signaling pathway is a master growth regulator of translation initiation and elongation, among other processes. Although there are multiple and concerning sources of variance with using the Western blotting method in human studies, the compiled evidence still shows that this control point is upregulated with RE and is enhanced with AA provision, through altered activity of several of its effectors, most prominently S6K1 (Supplemental Tables 1–7). Our laboratory has been able to use the drug rapamycin as a means to gain insight on the cause and effect of RE and EAAs on MPS in human skeletal muscle. Even with a minimal dose of the drug, we were able to block the contraction (27, 43) and EAA (119)-induced stimulation of mTORC1 signaling and MPS in human muscle, indicating that an increase in mTORC1 signaling is necessary to increase MPS as a result of these anabolic stimuli. In review of the literature there is a clear pattern that the increases in postexercise MPS are mechanistically supported by the literature describing an additive effect of protein/AA ingestion on post-RE intracellular signaling through mTORC1 in human skeletal muscle (Figures 1 and 2, Tables 1 and 2, Supplemental Tables 1–7). We presented this review of signaling response to 1) look for patterns in the protein/AA response but also to 2) demonstrate the variability in methods and response patterns by investigators.
After a period of RET some studies showed an attenuation in the acute mTORC1 signaling response (103, 104, 158), as a reflection of the attenuated MPS response (104, 159), but others did not (160, 161). Whether these acute effects add up over the long term to influence muscle mass and consequently strength is the crucial question that needs to be addressed.
As discussed above, although a number of reports showed concomitant activity, several reports did not show concomitant increases in mTORC1 signaling and MPS. Some researchers have voiced frustration regarding “discordance" between mTORC1 signaling and MPS (94, 162). Although much of this inconsistency could be explained by differences in analytical methods, antibody batch effects, or timing of the assessment relative to the assessment of MPS, it is not surprising to see an imperfect concordance between time course and mTORC1 signaling. This should be expected given the multiple and concerning sources of variance with using the Western blotting method in human studies. In particular, the lack of standardized reporting and use of unverified antibodies are especially concerning (163). It would seem presumptuous to assume that a few static 1-s “snapshots" of mTORC1 signaling would be representative of the MPS response over a several-hour (2–6 h) postexercise recovery period. Nonetheless, even with a signal encompassing <0.001% of the MPS time period, several investigations have reported correlations between mTORC1 signaling and MPS after AE (164, 165) and RE in the fasted (32, 38, 75) and fed (76) conditions. Given the lack of consistent protocols (Supplemental Tables 1–7; the normalization marker is diverse or not reported), antibodies, studies, participants, and testing protocols and the fact that one measure of signaling at a specified time point represents a very small percentage of the response time, it is extraordinary that signaling results ever line up with phenotype.
However, we have also shown that rapamycin administration does not influence resting postabsorptive protein synthesis, indicating that other mechanisms besides mTORC1 signaling can be involved. Collectively, our rapamycin data suggest that increases in mTORC1 activity are akin to an “anabolic switch" to turn on MPS in response to a stimulus. It seems very likely that up to a certain point this “switch" may serve as an on/off or on/low/high function in a permissive, but necessary, role to increase MPS rather than as a sensitive “dimmer" switch fine-tuning the MPS response. Indeed, in support of this concept, we showed that additional activation of mTORC1 signaling by adding leucine to a maximal dose of EAAs does not further enhance MPS (118). However, if the overall total protein dose is not optimal or indigestible, slight modifications (i.e., addition of leucine or specific AA combinations) to amplify mTORC1 signaling, and thus MPS, may be successful if conditions are appropriate (84, 93). The evidence from explorations into human skeletal muscle signal transduction shows that an increase in mTORC1 activity and translation initiation occurs after exercise corresponding with increases in MPS; however, although exercise prolongs the MPS response, this effect wanes, which suggests that other factors (energy, available substrate, substrate composition, substrate flux, cell swelling, and changes in AA sensing) may be involved and take precedence over mTORC1 signaling after the initial stimulus fades. Future research needs to delineate the relevance, if any, of these acute signaling effects in relation to the adaptation of long-term outcomes of muscle mass and consequently strength. These signaling markers are clearly not reliable as primary outcomes, but are rather supplemental tools to explore other outcomes.
Chronic Phenotypic Adaptation to RE with and without Protein and/or AA Feeding
Molecular and metabolic investigations have shown the effectiveness of protein or AA supplementation after an acute RE session in the enhancement of MPS and signal transduction (see above). There is a clear benefit of RET to increase muscle size and strength in young adults (166, 167). However, there is lack of clarity with regard to whether chronic protein supplementation during RET further enhances these outcomes compared with RET without protein supplementation.
There have been many systematic reviews, meta-analyses, and even more opinion papers with regard to the effects of protein supplementation on exercise adaptations of muscle mass, body composition, strength, power, and exercise performance (134, 135, 168–176). Given the heterogeneity of long-term exercise-training studies, these reviews have been commendable undertakings, necessary to provide evidence-based application. Nonetheless, further expansion and assessment of the literature on this topic are still needed, because no clear consensus has been found regarding the effects of protein supplementation to augment exercise adaptations. The results and authors’ conclusions from several meta-analyses are summarized in Tables 3 and 4. An effect of protein supplementation was seen with whole-body DXA lean mass and strength when the outcomes were examined independently and unadjusted for any other variables (see footnote) (169, 171). However, after adjusting the analysis to include both young and old participants (and several predictors), the effects of protein/AAs on lean mass and strength were negated (171).
TABLE 3.
Meta-analyses of chronic effect of RET with PRO/AA supplementation on muscle size and strength1
| Change |
PRO intake |
||||||||||
| First author, year (ref) | Subjects | Groups | PRO/other | Muscle mass/CSA | RET | Size, CSA, μm2 | FFM/LM, kg | Body fat, kg | Strength, 1 RM | g ⋅ kg−1 ⋅ d −1 | g/d |
| Nissen, 2003 (177); reported effect size | n = 64: 52 young and 12 old | PRO (n = 34), PLA (n = 3) | 0.8–1.37 extra grams PRO ⋅ kg−1 ⋅ d−1 | Varied | Varied, <3 wk | — | 0.07 (–0.03, 0.17)3 | — | −0.01 (–0.15, 0.13)3 | — | — |
| Cermak, 2013 (169) | n = 444 young adults; PLA (n = 51–188) | PRO all (n = 67–264) | More PRO (42 ± 30 g (range: 6–106 g) on training days | DXA, some fCSA | Varied, <6 wk | T1: 241 (131, 350); T2: 477 (333, 620)2 | 0.81 (0.53, 1.1)2 | −0.11 (–0.5, 0.29) | 14.4 (5.2, 23.6)2 | — | Median:40; mean: 47 |
| PRO TR (n = 7–47) | — | 0.98 (0.45, 1.5)2 | — | — | Median:84; mean: 72 | ||||||
| PRO UT (n = 5–85) | — | 0.75 (0.42, 1.1)2 | — | — | Median:38; mean: 32 | ||||||
| Miller, 2014 (176) | n = 626 young and old; RET subgroup, n = 258 | Whey, diet replacement | 35–88 g/d | DXA | No-Ex + Ex, <4 wk | — | −0.66 (−2.91, 1.59)3 | −0.60 (–4.08, 2.88)3 | — | 0.23–1.2 (range) | 35–88 (range) |
| Whey, supplement | — | 0.28 (−2.79, 3.35) | −0.21 (−2.16, 1.75) | — | |||||||
| Whey vs. other sources | — | 0.37 (−1.47, 2.21) | 0.14 (−2.05, 1.76) | — | |||||||
| Whey+RET | Varied Ex | — | 2.24 (0.66, 3.81) | — | — | ||||||
| Schoenfeld, 2013 (171); reported effect size | Strength: n = 484 young and old; LM: n = 525 young and old | Overall effect size | All studies pooled | DXA, fCSA | Varied, <6 wk | — | 0.47 (0.31, 0.63)3 | — | 1.39 (0.88,1.90)3 | — | — |
| PRO: basic model | (treatment or control) as a predictor | — | 0.24 (0.04, 0.44)2,3 | — | 0.38 (–0.34, 1.10)3 | — | — | ||||
| PRO: all covariates | Group, PRO matched, training status, blinding, gender, age, body mass, training duration | — | 0.16 (–0.07, 0.38)3 | — | 0.28 (–0.52, 1.07)3 | — | — | ||||
| PRO-reduced model | PRO intake, study duration, and blinding | — | 0.14 (–0.07, 0.35)3 | — | 0.39 (–0.34, 1.11)3 | — | — | ||||
| FFM or CSA | 0.14 (–0.17, 0.46)3 | 0.08 (–0.07, 0.24)3 | — | — | — | — | |||||
| Total PRO intake only model | PRO intake | — | 0.14 (–0.07, 0.35)3 | — | — | — | — | ||||
Values are means (95% CIs) and are effect sizes unless stated otherwise. AA, amino acid; CSA, cross-sectional area; Ex, exercise; fCSA, myofiber cross-sectional area; FFM, fat-free mass; PLA, placebo; PRO, protein; ref, reference; RET, resistance exercise training; RM, repetition maximum; T1, myosin heavy chain I myofibers; T2, myosin heavy chain II myofibers; TR, trained; UT, untrained.
Effect of PRO/AAs.
Standardized effect size.
TABLE 4.
Authors' conclusions from meta-analyses of chronic effects of RET with protein/AA nutrition1
| First author, year (ref) | Conclusions |
| Cermak, 2013 (169) | “Protein supplementation increases muscle mass and strength gains during prolonged resistance-type exercise training.” |
| Miller, 2014 (176) | “The current body of literature supports the use of WP, either as a supplement combined with resistance exercise or as part of a weight loss or weight maintenance diet, to improve body composition parameters.” |
| Schoenfeld, 2013 (171) | “Current evidence does not appear to support the claim that immediate (≤1 h) consumption of protein pre- and/or post-workout significantly enhances strength- or hypertrophic-related adaptations to resistance exercise.” |
AA, amino acid; ref, reference; RET, resistance exercise training; WP, whey protein.
Although a number of studies have shown no effect of added protein/AA supplementation (169, 178–191), other studies with a high-quality protein supplement during RET occasionally showed improved lean mass and, more infrequently, strength compared with no protein supplementation (182, 192–198). The reasons for the confusion in the literature have been suggested to stem from differences in study design, choice and measurement of outcomes, target populations, exercise protocols and timing, and the type and amount of the protein supplement or placebo given. It is likely that other unknown variables are involved as well. However, this area of research is very active, and in the past year alone several new investigations have added to the evidence base. Therefore, to provide a comprehensive critique, we tabulated all of the available literature on younger adults (Supplemental Tables 8–13) that examined the role of protein/AA supplementation (intake, dose, timing, and type) on RET improvements in muscle size, lean mass, and strength.
The pooled results from one meta-analysis showed gains in fat-free mass (FFM), type I and II muscle fiber cross-sectional area (CSA), and 1-repetition-maximum leg-press strength with protein supplementation compared with no protein supplementation after prolonged (>6 wk) RET (169) (Tables 3 and 4). The young adults gained approximately an additional 1 kg FFM, with additional increases of 20% in leg-press strength and an additional 212- and 291-μm2 expansion in myosin heavy-chain fraction (MHC) I and II myofiber CSA in the selected studies, respectively. Interestingly, the younger participants who had previous RET experience had a greater benefit on FFM gains than did untrained participants. The authors suggested that this finding reflected an improved sensitivity of nutritional support to help overcome a plateau or slowing in adaptation to RE (199). This is an interesting hypothesis, although unproven, considering most of the acute investigations of MPS have suggested that RE-trained individuals have a shortened and reduced sensitivity to postexercise protein/AAs compared with resting conditions (see earlier sections). A more probable explanation is that the RE-trained participants were given much more protein (median: 84 g/d; mean: 74 g/d) than the untrained participants (median: 38 g/d; mean: 32 g/d). However, to our knowledge, no longitudinal study has examined the effects of timed protein dosing on the adaptions to RET.
A recent systematic review has suggested that, as RET progresses beyond 6–8 wk and the intensity/volume is increased, an effect of protein/AAs is more likely to occur (174). The longest-running RET and protein supplement study in young adults to date evaluated participants at 12, 24, and 36 wk of a periodized RET program (186). In contrast to popular belief, the authors showed that lean mass gain clearly reached a plateau at 12 wk with protein and further supplementation throughout a progressively difficult RET program had no additional effect. A similar pattern was shown when using ultrasound to assess muscle thickness at 10.5 and 21 wk of progressive RET and protein supplementation (200). Collectively, these data suggest a slowing or limit of muscle growth (hypertrophic plateau) at ∼6–12 wk of RET, even when using untrained participants. This duration coincides with the time course of muscle hypertrophy (201, 202), indicating that ≥90% of the muscle hypertrophy occurs in the first 2 mo of RET. A more in-depth statistical approach examining the effect of previous training was taken by Schoenfeld et al. (171). They could not show that previous training status was an important predictor of lean mass or strength changes with RET and protein supplementation. Their findings do align with the “protein paradox” hidden in the literature. General physical activity (203, 204), and RET (127, 128, 205) in particular, improves efficiency of protein turnover, so theoretically those who become more trained actually would not need more protein; yet, several still posit that RE-trained participants benefit the most. Even so, some have suggested that because trained individuals display a more transient MPS response, protein timing may be important; however, the 2 studies that investigated this hypothesis yielded equivocal results (206, 207). Although RE-trained individuals are unlikely to benefit from protein/AA supplementation, unless through a placebo effect, it is interesting to speculate that previously well trained individuals who restart an intensive program after a period of detraining may have enhanced sensitizing to protein/AA nutrition. Protein/AA supplementation may provide a benefit for elite strength athletes who train at abnormally high intensities and volumes (4–6 h/d), but this protein/AA effect would apply to a very small proportion of the population (208) who consume protein/AA supplements.
One meta-analysis set out to examine if protein timing in close proximity to an exercise bout was an important factor that mediated these exercise adaptations (171). In modeling without covariates, the meta-analysis showed a modest effect of protein supplements on muscle hypertrophy but, as previously mentioned, no effect on strength. When including other variables, such as total protein intake, the effect of protein supplements was negated (171), and they discovered that total protein intake was the best predictor of improvements in muscle mass in their model. Importantly, none of their statistical models showed a protein/AA effect on strength. This finding is contrary to the commonly preached message that protein supplements should be ingested within close proximity of RE, within the so-called anabolic window (168, 209–211) described earlier. Unfortunately, these less-than-convincing reports have followed on the coattails of carbohydrate supplementation for endurance exercise performance, where an anabolic window truly exists. In fact, only a small handful of investigations suggested a benefit from protein timing (206), whereas a host of both acute and chronic investigations clearly indicated that timing may be an inconsequential argument (170, 171), because exercise sensitizes the muscle to protein/AAs up to 24 h postexercise (114, 212, 213). These data suggest that, in the absence of postexercise protein/AA supplementation, the AUC for protein turnover during the day is likely to be similar regardless of when protein is taken for those who are exercising, especially as exercise training progresses. We have high hopes that an emerging methodology (89) will be used to test this hypothesis. The finding of a greater effect from total protein intake, and not protein timing, in relation to an exercise bout during RET should not come as a surprise. As further support behind the role of total protein intake, another review focused on the role of supplemented protein intake during RET (170). The authors discovered that “successful” protein supplementation studies had a significant change (∼66% increase) in protein intake (from baseline habitual intake) and a significantly higher (∼60%) protein intake in the protein-supplemented group compared with the control group (170). This may be an important factor, possibly more relevant than exercise-related supplement timing.
Protein intake and distribution.
A surprising finding of the Cermak et al. (169) meta-analysis was that protein supplementation with RET provided an effect, even though the young participants’ protein intake (1.2 g ⋅ kg−1 ⋅ d−1) was typically well above the 0.8 g ⋅ kg−1 ⋅ d−1 RDA before commencing the intervention (169). This amount of protein per day is within the ranges of the current recommendation of protein intakes for a strength athlete, which is 1.2–1.8 g ⋅ kg−1 ⋅ d−1 (214, 215) and would be well over the per-meal recommendation of 0.25–3 g ⋅ kg−1 ⋅ d−1 (215, 216). Indeed, consuming minimal protein (0.5 g ⋅ kg−1 ⋅ d−1) has been shown to attenuate RET outcomes in some young adults (217). Some have reasoned that higher amounts of protein intake, not supplementation, in proximity to exercise are more likely to affect the responses to RET (170, 171). Yet, evidence suggests that if a certain amount of protein intake is met, any further changes in protein intake have less bearing on the adaptation (169, 181, 209, 218, 219) (Tables 3 and 4, Supplemental Tables 8–13). This fits with a paradigm that distribution, amount, and spread of protein throughout the day may have greater efficiency and relevance on the protein metabolic response (90, 206, 210, 220), particularly with the slowly absorbed intact proteins that humans typically ingest during a complete meal containing all macronutrients. Yet, because the distribution of protein with (221) or without (222) exercise seems to have no effect on lean mass in young adults consuming >1.7 g protein ⋅ kg−1 ⋅ d−1, future research should examine the role of protein supplement distribution during RET under lower protein intakes (<1.7 g protein ⋅ kg−1 ⋅ d−1) or in situations of energy deficit (223). Although a recent recommendation (216, 223) suggests that maintenance of lean mass during energy deficit necessitates protein intakes of 2.3–3.8 g protein ⋅ kg−1 FFM ⋅ d−1), future research should confirm this recommendation. There is no evidence that lean mass gain can occur during energy deficit, but the evidence suggests that maintenance of existent lean mass can occur with higher protein intakes. In addition, because lean mass can increase by provision of additional energy alone (183, 224, 225), the need for supplemental protein/AAs in situations of high energy intake (>1700–2000 kcal/d) is unnecessary during RET (183, 226). A sufficient/overabundant energy intake common to the Western diet may be another potential reason why additional protein/AAs given during RET yield minimal effects on RET outcomes. Energy appears to play a permissive role for lean mass gain, yet the exact energy intake range in which the effect of protein/AAs diminishes to enhance outcomes during RET is unknown. Moreover, a meta-analysis has indicated that total protein intake, with the current Western diet, is more relevant than protein dose or distribution on affecting muscle hypertrophy during RET (171). However, research has yet to determine the role of protein intake, timing, and distribution under variations in total energy/protein intakes.
Protein type.
The authors of several reviews have suggested that future investigations expand the literature on protein supplementation during RET by investigating protein timing and type (169, 209). With regard to protein type, whey protein in its various forms has been the most frequently studied in its ability to augment muscle mass during RET. The amount of evidence comprising whey protein as a supplement prompted another meta-analysis that examined the changes in body composition with supplementation of specifically whey protein (176). The authors concluded that whey protein demonstrates significant increases in lean body mass (∼2.2 kg) when taken throughout RET. Furthermore, they found no effect with regard to whey protein form (isolate compared with concentrate) or when whey was compared with other protein sources (176). It should be noted that these analyses were conducted in only a handful of studies and, as such, are susceptible to greater bias from outlying studies. Thus, further examination of protein type is warranted.
To examine chronic supplementation during RET by protein type we compiled a section of Supplemental Tables 11 and 12 to only include studies (186, 190, 191, 194–196, 227–231) that directly compared ≥2 different protein sources/types/forms on lean mass and strength. Only 2 of these studies actually showed an improved strength outcome when comparing protein forms (195, 229), in this case whey compared with casein, but these studies provided conflicting results, leaving one to conclude that no particular protein source type or form investigated to date provides a greater enhancement of strength over another high-quality source. In addition, 4 studies compared whey or milk with soy protein (186, 194, 196, 230) and 2 studies showed that milk/whey was superior to soy (186, 196) for enhancing lean mass gains, whereas 2 others did not (194, 230). It should be noted that in the studies in which the dairy proteins were more beneficial, a lower protein dose (∼20 g or less) was given, such that the leucine content for soy was likely less than optimal (<1.8 g). However, in the studies (194, 230) in which equivalence in the anabolic response was found between the protein sources, a higher protein dose was given (>28 g). As such, the leucine dose likely “triggered” a maximal response in both treatments (232). This finding is further supported by Joy et al. (227) and Babault et al. (190), who found that protein quality “disparities” between whey and rice protein or whey and pea protein can be overcome by providing a higher protein dose during RET. Indeed, another study comparing whey with a mix of whey, casein, and BCAAs found similar results (231). These data suggest that protein type is likely irrelevant if a high-quality protein is ingested at a dose that stimulates the leucine threshold for that protein.
Critique of the Specificity and Relevance of the Effect of Protein Supplementation
In evaluation of the literature on RET and protein/AA supplementation, the majority of the evidence shows identical increases in whole-body lean mass and especially strength in placebo- and protein-treated groups (Table 5, Supplemental Tables 8–13). However, a few studies showed greater increases in whole-body lean mass with protein than with placebo-alone groups. Several studies showed a trend for a difference in the change between groups, and some showed significant increases with outcomes in the protein group but not in the placebo group. Yet, several of those studies that showed greater increases in outcomes with both protein and placebo treatments displayed very similar absolute values after training. There are several hypotheses/questions for these overall equivalence findings, as we highlight below. Mainly, what is the statistical and meaningful effect of protein supplements during RET, who benefits, what is the functional/physiologic effect, and where is the effect occurring?
TABLE 5.
Summary of the acute and chronic effects of protein/AA supplementation with resistance exercise1
| Results and recommendations | |
| Acute (1 or a few exercise bouts with supplementation) | |
| mTORC1 signaling2 | ↑Akt, mTORC1, rpS6; ↑↑S6K1 |
| Muscle protein synthesis2 | ↑Mixed-muscle, myofibrillar, AV-balance methods |
| Optimal protein dose/serving | ∼20–30 g and >2 g leucine/dose |
| Optimal protein type | Any high-quality protein with >2 g leucine/dose |
| Chronic (exercise training with supplementation) | |
| Whole-body lean mass2 | ↑,↔ (some, inconsistent) |
| Regional lean mass | ↔, Rarely examined |
| Muscle CSA/thickness | ↔, Minimal to no effect |
| Strength | ↔, Minimal to no effect |
| Optimal daily protein intake | ≥0.8–1.0 g ⋅ kg−1 ⋅ d−1 |
| Optimal protein dose/serving | Same as acute, ∼0.25 g ⋅ kg−1 ⋅ d−1 |
| Optimal protein type | Same as acute, but may not be needed |
AA, amino acid; Akt, protein kinase B; AV-balance, arterial-venous balance; mTORC1, mammalian target of rapamycin complex 1; rpS6, ribosomal protein S6; S6K1, p70 ribosomal protein S6 kinase 1; ↔, no change; ↔↑, trend to have an effect; ↑, clear effect, ↑↑, very clear effect.
Effect of protein/AA supplementation.
A slight effect of protein supplementation during RET on whole-body lean gain mass was shown in a few individual studies and when a subset of the literature was pooled in an unadjusted meta-analysis. Whole-body lean mass is typically assessed via DXA and is the main outcome of interest in most studies. Very few studies actually give any information regarding standardization of the DXA scanning protocol. Our own pilot findings and those published from others (233–235) suggest that several variables need to be addressed to obtain precise measurements of body composition, yet these are variables rarely addressed in this field. It is also unfortunate that most studies only reported whole-body lean mass to make conclusions regarding muscle mass and very few (4 of 33) included readily available data on regional lean mass. Trunk or whole-body lean mass includes viscera and vital organs that may change size in response to increased AA supply (236). Future studies are encouraged to report regional (arm/leg/appendicular) lean mass values/changes as a better reflection of muscle mass changes, especially if direct assessments of muscle mass (CSA and muscle thickness) cannot be made. This is of prime importance if conclusions regarding muscle mass continue to be made from studies with DXA as the primary outcome.
Compared with whole-body lean mass changes, the protein effect on strength is even more elusive and is only detected, albeit occasionally, when whole-body RET, not training of isolated limbs, is conducted. This observation may also partly explain why very few studies reported an enhancement in strength or function with protein/AA supplementation during RET. In fact, some of those studies that did show an effect of protein on “estimates of muscle mass” (i.e., DXA lean mass) did not find any suggestion for an enhancement in strength. Taken together, there are several relevant questions regarding these findings:
Do these increases in lean mass constitute limb muscle increases or rather lean trunk/viscera tissue gain or water retention?
Could this be a result of transient expansion in the free AA pool and not muscle protein?
Is this transient expansion of the sarcoplasmic proteins and not myofibrillar protein?
Is the strength testing or muscle mass measure imprecise?
Is the strength testing applied not specific to the area where muscle mass accrual has occurred?
Regardless of these postulations, the end result is a lower force-to-mass ratio compared with the placebo group, which should be a concern to several athletic populations in whom the highest force-to-mass ratio is essential for optimal performance. If anything, this situation of extra “nonfunctional” whole-body lean mass should be further explored to determine the location and specific composition of this accrual.
One meta-analysis showed that protein supplementation during RET enhances muscle CSA and strength concomitantly (169), yet there is little evidence for a coupling of these events with protein supplementation during RET or even RET alone. Indeed, skeletal muscle CSA (via MRI, DXA lean mass, and fiber CSA) has been shown to correlate with strength (237, 238) when assessed as absolute values. But, to our knowledge, changes in CSA have not yet been found to correlate with changes in strength over the course of RET (237). Of the ∼70 studies we examined, there were very few reports of this relation and only 1 laboratory to our knowledge, showed a robust association with changes in muscle strength and fiber CSA during protein supplementation and RET with all treatment groups combined (206, 239, 240). Both muscle size and strength increase with RET, and a common idea persists that increases in lean/muscle mass are coupled to changes in strength, yet the scientific evidence to support such a claim is limited. Indeed, a study found that different cohorts, on the basis of body builds, can show a divergent FFM change while exhibiting identical increases in strength (241). These data together would suggest that the increase in strength may be more independent of muscle mass increases than commonly thought, as previously suggested (8).
One could make the argument that most of the studies that did not see an effect of protein were not appropriately statistically powered for their outcome (e.g., lean mass) of interest. Regrettably, most studies did not report how they determined sample size or were rather ambiguous regarding the method used. Future studies are advised to describe the rationale for sample size selection and to be clearer in reporting variability and sensitivity in outcome assessments and means of the change values of their outcomes, such that effect size/sample size estimations can be calculated for future research. Yet, we attempted to estimate means and SDs and calculate the sample sizes that would be needed to determine an effect of protein/AA supplementation during RET (Supplemental Table 14). We extracted the mean changes and SDs of the changes for the protein and placebo treatments in selected studies and used Statmate 2.0 (GraphPad Software) to calculate the sample size by comparing 2 means with an unpaired t test. The α was set at 0.05 and power at 80%. The meta-analysis of Cermak et al. (169) found an effect of 0.81 kg from protein/AAs compared with placebo during RET in all 444 young participants and an SD of ∼3.1. Using the change divided by the SD we calculated a minimal effect size of 0.26. As Supplemental Table 14 highlights, it would take ∼110 participants at 80% power to have enough participants to detect a significant difference in lean mass between 2 groups. We showed that it would take more untrained instead of trained participants to find a statistical effect on the basis of their data. Data from our own laboratory closely match with the overall estimation from the Cermak et al. (169) meta-analysis: we gave 40 participants protein/AAs during RET and a maltodextrin placebo to 18 participants and found an effect of protein at 0.69 kg with an SD of ∼3.0. This calculates to a minimal effect size of 0.23, and it would take ∼150 participants at 80% power to have enough participants to detect a significant difference in lean mass.
Supplemental Table 14 also highlights the effect sizes and comparisons between several other studies. The effect sizes for lean mass and/or FFM in Cermak et al. (169) are similar to those reported by Schoenfeld et al. (171) (difference = 0.24 ± 0.10; 95% CI: 0.04, 0.44; P = 0.02). It should be noted that, according to Cohen’s method, these are very weak effect sizes. Given that the average total sample size for most studies is 20–30 (which includes both placebo and treatment), it is not unsurprising that differences between protein and placebo are rarely found. However, one could make the argument that the need for these estimated study sample sizes of ∼100 with the use of data extracted from the meta-analysis is the result of a methodologic issue. It could be debated that the studies that used a less-than-optimal training duration or protein/AA supplement (type/timing/dosing) could be diluting the protein/AA effect shown in meta-analyses. In view of this concern, we also provided the sample size estimations from a collection of “high response studies” as “optimal methodologies” to show the sample size estimations based on the maximal responses reported. These optimal method studies typically were whole-body training protocols that maximized exercise intensity and dosed the protein above the leucine threshold (>2 g/serving), although it should be noted that these strategies were not always fruitful (188, 190, 211, 231, 242). On the basis of this subset of “optimal method” studies, these findings suggest that ∼20–30 participants per treatment are needed to capture differences in the changes in whole-body lean mass between placebo and protein/AAs. For whole-body lean mass, 2 of the larger studies in young adults showed that ∼20 participants are needed per group to discern differences in the change between protein and placebo (186, 196), and we have unpublished data (PT Reidy, unpublished data, 2015) that suggest similar findings. When looking at regional changes in lean mass, only 2 other studies and some of our unpublished data suggest that sample sizes of 42–1400 are theoretically needed to detect region-specific lean mass differences (Supplemental Table 14).
On the basis of results in Supplemental Table 14 one could infer that most studies were underpowered, but the case may actually be more complex, and picking which studies to use the mean change to estimate the effect size is difficult. For example, several studies used treatment sizes <10 and found pronounced differences (194, 239, 243) between protein and placebo groups, yet studies with >20/group also showed no effect (190, 191, 242). This finding is puzzling, and “optimal” methodology alone cannot explain this discrepancy but points to the possibility of a potentially interesting selection bias, or other conflict-of-interest concerns, which warrants further investigation. However, similar to large pharmaceutical trials, increasing the sample size is likely to find a statistical effect between treatments but provides little meaning or clinical relevance for the outcome.
It is clear that, for most RET outcomes, the protein/AA effect after RET is moderate to low at best. However, this already small physiologic effect could be overestimated due to the possible influence of a placebo effect. It was briefly mentioned (244) that participants usually know when they are given protein/AA treatments. Unfortunately, the placebo effect of protein/AAs has not been investigated, and the effectiveness of protein/AA masking is rarely discussed. Our own pilot work in untrained participants showed that 83% of those receiving protein and 73% of the participants given maltodextrin placebo correctly guessed their treatment. However, participants could not distinguish between protein/AA supplement types. This placebo effect is likely to be higher in trained individuals, because they have had previous exposure to protein/AA supplements (245–249), believe the supplements enhance performance (246, 249) and muscle size (249), and can likely distinguish protein/AA texture and taste easily. This is another potential reason why some, but not all, studies suggest that the effect of protein/AAs is higher in participants with previous training. To determine the clinically meaningful relevance of a mean protein effect of ∼0.5–1 kg whole-body lean mass gain found in a large cohort or a meta-analysis, there are important questions that should be asked: 1) in which population will the protein effect be found, 2) is there a proportion of the population who are nonresponders to protein, 3) or is there a proportion of the population who is driving the protein effect?
There is no clear pattern, even with “optimal” methodologies and protein type/dosing, that defines the effect of protein/AAs to enhance adaptation to RET. This suggests individual variation or selection bias for responders to exercise/nutrition, and future investigations should seek to examine this further. Although randomly assigned participants undergoing RET will eventually reach the same average absolute lean mass, this pattern infers that protein supplementation may be more effective in those with a lower starting lean mass muscle mass (192, 243, 250) and/or strength (190). Data in support of this contention come from a recent and very large (n = 106) clinical trial from Babault et al. (190). They used a sensitivity analysis to show that protein supplementation was most effective in enhancing biceps muscle thickness (compared with placebo) in a subset of those who had lower starting strength values (190). The concept that “the less a participant has to start the more they have to gain” may apply only to strength values and not lean mass. One study in young adults suggested the opposite effect with body composition: that those with greater FFM at the start of RET show the most change in FFM (241) but similar increases in strength.
Unfortunately, only 2 of ∼50 studies actually showed the individual responses after protein supplementation and RET (186, 196). This is unfortunate because there is marked variability in the responses, and useful information for clinical practice could be gained by understanding the reasons behind the variant responses. We pooled the data from our laboratory and the 2 previously described studies (186, 196) and another study in a milk-protein-only group (94) to generate a diagram of the individual responses in whole-body lean mass after RET with protein or carbohydrate supplementation (Figure 3A, B).
FIGURE 3.
Effect of protein supplementation during resistance exercise training on the individual response for LM or FFM changes. These data were extracted from diverse clinical trials reporting the scatterplot (A) of the individual change after supplementation with 20–30 g protein (n = 95) combining the similar overall average changes in a protein blend (PT Reidy and BB Rasmussen, unpublished data), milk protein (94, 196), and whey protein (186; PT Reidy and BB Rasmussen, unpublished data) compared with isocaloric maltodextrin carbohydrate (n = 57) (186, 196; PT Reidy and BB Rasmussen, unpublished data). The solid lines in panel A represent the means (95% CIs) of each pooled group (PRO and CHO). Hartman et al. (196) used FFM. The data were pooled into a relative frequency histogram (B). CHO, carbohydrate; FFM, fat-free mass; LM, lean mass; PRO, protein.
Although the exercise-training protocols and the protein type given (blend, whey, or milk protein) were diverse, the pattern of magnitude of the change and especially the extreme variability in the change was remarkably similar across studies, so we pooled the data into 2 groups (protein and carbohydrate) (Figure 3A). The variability in the response is remarkable, and it is clear that some individuals do not respond to protein/AAs (Figure 3A, bottom left) and some natural responders do quite well, even with carbohydrate, and are likely to experience minimal benefit from protein/AAs (Figure 3A, top right). These groups represent a third of the sample who experience minimal to no effect of protein/AAs. On the other hand, in the carbohydrate grouping, some potential responders to protein/AAs are mixed with natural nonresponders to RET (Figure 3A, bottom right). Yet, the fourth response grouping (Figure 3A, top left) is a mix of natural responders and individuals who potentially experienced an effect of protein/AAs. We took these data and examined them further in a relative frequency histogram (Figure 3B). The effect of protein/AAs seemed most pronounced in the 1.25- to 3.75-kg response bins. Intriguingly, the lower bins (0–1.25 kg) and upper bins (>3.75 kg) showed very similar patterns in both protein and carbohydrate groupings as a reflection of natural low and high responders, regardless of nutritional supplementation. As has been previously mentioned (156), it is very doubtful that protein/AAs or any form of nutritional supplementation can turn a low responder into a high responder. These pooled data should be interpreted with caution because this was a cross-sectional comparison. In this field, research would greatly benefit from the use of larger sample sizes and possibly the crossover counterbalanced clinical trial as tools to further explore the individual variability in why some individuals respond to protein/AA supplementation and others do not.
As questioned above, where is the additional lean mass distributed (arms, legs, trunk) and what tissue (muscle, visceral organs) does it comprise? In addition, what is the functional/physiologic relevance for increased lean/muscle mass? Most important, if the extra lean/muscle mass accrued with protein supplementation does not affect strength, is there another physiologic benefit or is this simply a nonfunctional cosmetic effect? These are important questions for the nutrition practitioner who works with clients on a client-by-client basis. This could mean examination of the potential for fatigue resistance (191, 251), a site of greater postabsorptive glucose disposal, or the presence of a greater AA reservoir acting as a buffer against acute periods of sickness, injury, or disuse common with aging (252). It is clear that we have yet to define the specific who, what, where, and why of the effect of protein supplementation during RET. Investigators should be encouraged to consider the above points when designing, implementing, and interpreting future research trials.
Relevance of Acute Responses on Chronic Outcomes
The acute physiologic responses to exercise and nutrition have garnered ample attention (discussed above). This is partly due to the mechanistic insight into and physiologic knowledge gained, which is inherently interesting. Recent findings (94, 253) and occasional critique over the years have questioned the relevance of these types of studies in relation to chronic outcomes.
Indeed, the prevailing theory for adaptation is that the recurring summation of molecular and physiologic changes molds the ensuing phenotype (11, 254). The literature supports this concept generally, but we know very little about this adaptive process in specific situations and populations, in particular regarding the interaction between exercise and protein/AA nutrition. An important first step in understanding these changes has been the acute study, which has most frequently explored the immediate hours or occasionally the following day or days after 1 exercise bout. This is a crucial point, because the acute studies summarize their findings with various exercise modalities and/or nutritional interventions with inferences toward chronic outcomes.
Certainly, it is obvious that mTORC1 activity plays a role in the hypertrophic response (29, 162, 255). Future research should seek to examine mechanisms explaining the factors and variability modifying this relation. mTORC1 activation has been linked to MPS on occasions (discussed in above sections). Some investigations (29, 256, 257), but not all (94), have indicated that the acute pretraining postexercise response of S6K1 (a marker of mTORC1 activation) is associated with muscle hypertrophy after RET. It would seem intuitive that a direct estimate of the rate of MPS would be a stronger predictor of muscle mass accrual. However, a recent in-depth investigation (94) and a previous report (29) showed that when using the same cohort of participants to compare the acute FSR response to the change in muscle mass, this relation quantitatively does not exist. This is a puzzling finding for some, because a recent viewpoint article (253) highlighted that there have been several reports in the same laboratory but in different participant cohorts in which acute studies assessing MPS or net balance (35, 67, 74, 120) have reflected chronic outcomes (198, 258–260). However, this is not always evident (76, 97, 261–263). As suggested (253), there exist several reasons for this discordance. They include individual factors such as age, genetic and/or epigenetic factors, transcriptional adaptability, nutritional status, level of physical activity, and/or other environmental influences. In addition, it is possible that variability in the outcomes, changes in protein breakdown, or other factors may be involved. We have little or no information regarding which of these factors is most dominant or how they interact, and future research should seek to elucidate what role these factors play. It seems most evident that acute studies may be useful in presenting the general “hypertrophic” potential of a certain intervention. However, it is clear that there is an inherent variability in an individual’s ability to respond to training, which we are only now beginning to understand.
It is well known that physiologic adaptation to a given stress changes over time. What is clear is that the “law of diminishing returns” exerts a strong precedent on the acute MPS response as an individual becomes more trained (199). Interestingly, this effect has been suggested to occur rather quickly (102, 253). These data could theoretically suggest that the “upper limit” or “set point” of hypertrophy is approaching and that various mechanisms may start to attenuate the anabolic response. An important observation provided support for this hypothesis (102). The authors showed that the cumulative MPS rate over a 3-wk period was strongly correlated to the change in muscle thickness over 6 wk of exercise training and that the increased MPS normalized during weeks 3–6 of RET. This becomes even more complicated with the reflection that this regulation may occur in other time periods (postabsorptive, postprandial), over the course of exercise training (hours, days, and weeks).
Several investigations have sought to determine the effect of later time periods, repeated bouts, exercise habituation, and a few various durations of exercise training. Unfortunately, the majority of the acute MPS studies focused on the immediate postexercise time period. It is very likely that there are other time frames, in addition to the immediate hours postexercise and protein/AA nutrition, in which changes in MPS and MPB are regulated to control hypertrophy. For example, although the acute postexercise response may lessen in trained individuals, it appears that the resting postabsorptive MPS is increased in the trained state MPS (49, 50, 99, 100) (Tables 1 and 2). We know very little regarding the regulation of protein metabolism during those later time frames and the diurnal response of protein turnover during exercise training and how that affects overall phenotypic change (hypertrophy or other outcomes). There likely exists a multifactorial role of protein/AA stimulus on MPS and MPB or even processes of indispensable AA loss or change in protein metabolism in non–muscle tissue during exercise training. Training status alone could be a complicated variable, suggesting differentiated responses on the basis of sessions to years of training. Type of training (aerobic, resistance, concurrent) and when these sessions are applied during a periodized training program are also likely to illicit a variety of responses. Layering these variables together with factors intrinsic to the individual represents the actual complexity of the situation. Because physiology adapts to both exercise and nutritional stimuli, it may also be of benefit to examine how altering or cycling protein/AA form or dose can maintain the sensitivity of AAs during RET. These realities provide a daunting test for investigators.
The compiled evidence from human research models indicates that the transcriptional, post-translational, physiologic, and phenotypic response to exercise and nutrition is highly variable. This fact has provided a layer of ambiguity in our ability to make precise estimates of the effectiveness of protein/AAs during exercise interventions. However, in this critical review we clearly showed that protein/AA ingestion enhances the acute exercise–induced stimulation of both mTORC1 signaling and MPS (i.e., 2 important components of the muscle growth response). Only a small effect of protein/AAs on whole-body lean mass (effect size of 0.2–0.4) was evident in some studies, but minimal to no effect on strength or muscle mass was consistent. During exercise training, the ability of muscle to utilize AAs for MPS improves (i.e., becomes more efficient), which may help to explain the lack of an effect of protein/AA supplementation during RET. Hulmi et al. (200) summarized the disparity for the difference in the protein/AA effect between acute and chronic outcomes: “while the positive effects of the protein or amino acid ingestion on muscle hypertrophy signaling can often be clear when studied acutely after each exercise, especially when the study was performed in a fasting state, the long-term positive effects may not be as robust with normal daily high protein consumption.” Thus, another important variable that may determine the overall effectiveness of protein/AAs during RET is the total daily protein intake. Muscle becomes more sensitive to AA availability for at least 24 h after a bout of exercise. Therefore, participants who consume sufficient daily energy and protein in a balanced diet are not likely to enhance muscle growth and strength with protein/AA supplementation during RET, although slight increases in whole-body lean mass are possible but may not be clinically relevant (Tables 4 and 5, Supplemental Table 14). However, for those who consume a poor diet, with limited energy or protein, the total amount, quality, and distribution pattern of protein intake throughout the day may be useful in enhancing muscle growth and possibly function, if impaired, in response to RET.
Acknowledgments
PTR and BBR formulated the review topic, reviewed the manuscript, wrote the manuscript, and had primary responsibility for the final content; and PTR conducted the literature review, data extraction, and formatting. Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: AA, amino acid; AE, aerobic exercise; AMPK, AMP-activated protein kinase; CSA, cross-sectional area; EAA, essential amino acid; Ex-Fed vs. Ex-PLA/CHO, protein/AA-fed postexercise MPS compared with fasted or carbohydrate placebo postexercise values; Ex-Fed vs. Fed, protein/AA-fed postexercise MPS compared with fed resting values; Ex-Fed vs. Rest, protein/AA-fed postexercise MPS compared with basal resting values; Fed vs. Rest, protein/AA-fed resting values compared with basal resting values; FFM, fat-free mass; FSR, fractional synthesis rate; MHC, myosin heavy-chain fraction; MPB, muscle protein breakdown; MPS, muscle protein synthesis; mTORC1, mammalian target of rapamycin complex 1; RE, resistance exercise; RET, resistance exercise training; S6K1, p70 ribosomal protein S6 kinase 1.
References
- 1.Jaleel A, Short KR, Asmann YW, Klaus KA, Morse DM, Ford GC, Nair KS. In vivo measurement of synthesis rate of individual skeletal muscle mitochondrial proteins. Am J Physiol Endocrinol Metab 2008;295:E1255–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraemer WJ, Deschenes MR, Fleck SJ. Physiological adaptations to resistance exercise: implications for athletic conditioning. Sports Med 1988;6:246–56. [DOI] [PubMed] [Google Scholar]
- 3.Abernethy PJ, Jurimae J, Logan PA, Taylor AW, Thayer RE. Acute and chronic response of skeletal muscle to resistance exercise. Sports Med 1994;17:22–38. [DOI] [PubMed] [Google Scholar]
- 4.Matoba H, Gollnick PD. Response of skeletal muscle to training. Sports Med 1984;1:240–51. [DOI] [PubMed] [Google Scholar]
- 5.Churchward-Venne TA, Burd NA, Phillips SM. Nutritional regulation of muscle protein synthesis with resistance exercise: strategies to enhance anabolism. Nutr Metab (London) 2012;9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murton AJ, Greenhaff PL. Physiological control of muscle mass in humans during resistance exercise, disuse and rehabilitation. Curr Opin Clin Nutr Metab Care 2010;13:249–54. [DOI] [PubMed] [Google Scholar]
- 7.Fry AC. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med 2004;34:663–79. [DOI] [PubMed] [Google Scholar]
- 8.Folland JP, Williams AG. The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med 2007;37:145–68. [DOI] [PubMed] [Google Scholar]
- 9.American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 2009;41:687–708. [DOI] [PubMed] [Google Scholar]
- 10.Toigo M, Boutellier U. New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. Eur J Appl Physiol 2006;97:643–63. [DOI] [PubMed] [Google Scholar]
- 11.Coffey VG, Hawley JA. The molecular bases of training adaptation. Sports Med 2007;37:737–63. [DOI] [PubMed] [Google Scholar]
- 12.Smith GI, Patterson BW, Mittendorfer B. Human muscle protein turnover—why is it so variable? J Appl Physiol 2011;110:480–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacDougall JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interisano SA, Yarasheski KE. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol 1995;20:480–6. [DOI] [PubMed] [Google Scholar]
- 14.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol 1995;268:E514–20. [DOI] [PubMed] [Google Scholar]
- 15.Tipton KD, Ferrando AA, Williams BD, Wolfe RR. Muscle protein metabolism in female swimmers after a combination of resistance and endurance exercise. J Appl Physiol 1996;81:2034–8. [DOI] [PubMed] [Google Scholar]
- 16.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 1997;273:E99–107. [DOI] [PubMed] [Google Scholar]
- 17.Biolo G, Williams BD, Fleming RY, Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes 1999;48:949–57. [DOI] [PubMed] [Google Scholar]
- 18.Fowles JR, MacDougall JD, Tarnopolsky MA, Sale DG, Roy BD, Yarasheski KE. The effects of acute passive stretch on muscle protein synthesis in humans. Can J Appl Physiol 2000;25:165–80. [DOI] [PubMed] [Google Scholar]
- 19.Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Physiol Endocrinol Metab 2002;282:E551–6. [DOI] [PubMed] [Google Scholar]
- 20.Pitkanen HT, Nykanen T, Knuutinen J, Lahti K, Keinanen O, Alen M, Komi PV, Mero AA. Free amino acid pool and muscle protein balance after resistance exercise. Med Sci Sports Exerc 2003;35:784–92. [DOI] [PubMed] [Google Scholar]
- 21.Durham WJ, Miller SL, Yeckel CW, Chinkes DL, Tipton KD, Rasmussen BB, Wolfe RR. Leg glucose and protein metabolism during an acute bout of resistance exercise in humans. J Appl Physiol 2004;97:1379–86. [DOI] [PubMed] [Google Scholar]
- 22.Trappe TA, Raue U, Tesch PA. Human soleus muscle protein synthesis following resistance exercise. Acta Physiol Scand 2004;182:189–96. [DOI] [PubMed] [Google Scholar]
- 23.Sheffield-Moore M, Paddon-Jones D, Sanford AP, Rosenblatt JI, Matlock AG, Cree MG, Wolfe RR. Mixed muscle and hepatic derived plasma protein metabolism is differentially regulated in older and younger men following resistance exercise. Am J Physiol Endocrinol Metab 2005;288:E922–9. [DOI] [PubMed] [Google Scholar]
- 24.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 2006;576:613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrithers JA, Carroll CC, Coker RH, Sullivan DH, Trappe TA. Concurrent exercise and muscle protein synthesis: implications for exercise countermeasures in space. Aviat Space Environ Med 2007;78:457–62. [PubMed] [Google Scholar]
- 26.Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol 2007;103:903–10. [DOI] [PubMed] [Google Scholar]
- 27.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 2009;587:1535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Volpi E, Rasmussen BB. Essential amino acid and carbohydrate ingestion before resistance exercise does not enhance postexercise muscle protein synthesis. J Appl Physiol 2009;106:1730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayhew DL, Kim JS, Cross JM, Ferrando AA, Bamman MM. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol 2009;107:1655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 2009;89:161–8. [DOI] [PubMed] [Google Scholar]
- 31.Burd NA, Dickinson JM, Lemoine JK, Carroll CC, Sullivan BE, Haus JM, Jemiolo B, Trappe SW, Hughes GM, Sanders CE Jr. , et al. . Effect of a cyclooxygenase-2 inhibitor on postexercise muscle protein synthesis in humans. Am J Physiol Endocrinol Metab 2010;298:E354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, et al. . Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 2009;587:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dreyer HC, Fujita S, Glynn EL, Drummond MJ, Volpi E, Rasmussen BB. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol (Oxf) 2010;199:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doessing S, Heinemeier KM, Holm L, Mackey AL, Schjerling P, Rennie M, Smith K, Reitelseder S, Kappelgaard AM, Rasmussen MH, et al. . Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. J Physiol 2010;588:341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holm L, van Hall G, Rose AJ, Miller BF, Doessing S, Richter EA, Kjaer M. Contraction intensity and feeding affect collagen and myofibrillar protein synthesis rates differently in human skeletal muscle. Am J Physiol Endocrinol Metab 2010;298:E257–69. [DOI] [PubMed] [Google Scholar]
- 36.Etheridge T, Atherton PJ, Wilkinson D, Selby A, Rankin D, Webborn N, Smith K, Watt PW. Effects of hypoxia on muscle protein synthesis and anabolic signaling at rest and in response to acute resistance exercise. Am J Physiol Endocrinol Metab 2011;301:E697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dideriksen KJ, Reitelseder S, Petersen SG, Hjort M, Helmark IC, Kjaer M, Holm L. Stimulation of muscle protein synthesis by whey and caseinate ingestion after resistance exercise in elderly individuals. Scand J Med Sci Sports 2011;21:e372–83. [DOI] [PubMed] [Google Scholar]
- 38.Fry CS, Drummond M, Glynn E, Dickinson J, Gundermann D, Timmerman K, Walker D, Dhanani S, Volpi E, Rasmussen B. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 2011;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar V, Atherton PJ, Selby A, Rankin D, Williams J, Smith K, Hiscock N, Rennie MJ. Muscle protein synthetic responses to exercise: effects of age, volume, and intensity. J Gerontol A Biol Sci Med Sci 2012;67:1170–7. [DOI] [PubMed] [Google Scholar]
- 40.Camera DM, West DW, Burd NA, Phillips SM, Garnham AP, Hawley JA, Coffey VG. Low muscle glycogen concentration does not suppress the anabolic response to resistance exercise. J Appl Physiol (1985) 2012;113:206–14. [DOI] [PubMed] [Google Scholar]
- 41.Gundermann DM, Fry CS, Dickinson JM, Walker DK, Timmerman KL, Drummond MJ, Volpi E, Rasmussen BB. Reactive hyperemia is not responsible for stimulating muscle protein synthesis following blood flow restriction exercise. J Appl Physiol 2012;112:1520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Res PT, Groen B, Pennings B, Beelen M, Wallis GA, Gijsen AP, Senden JM, VAN Loon LJ. Protein ingestion before sleep improves postexercise overnight recovery. Med Sci Sports Exerc 2012;44:1560–9. [DOI] [PubMed] [Google Scholar]
- 43.Gundermann DM, Walker DK, Reidy PT, Borack MS, Dickinson JM, Volpi E, Rasmussen BB. Activation of mTORC1 signaling and protein synthesis in human muscle following blood flow restriction exercise is inhibited by rapamycin. Am J Physiol Endocrinol Metab 2014;306:E1198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr 2014;99:86–95. [DOI] [PubMed] [Google Scholar]
- 45.Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol 1993;265:E210–4. [DOI] [PubMed] [Google Scholar]
- 46.Yarasheski KE, Zachweija JJ, Angelopoulos TJ, Bier DM. Short-term growth hormone treatment does not increase muscle protein synthesis in experienced weight lifters. J Appl Physiol 1993;74:3073–6. [DOI] [PubMed] [Google Scholar]
- 47.Welle S, Thornton C, Statt M. Myofibrillar protein synthesis in young and old human subjects after three months of resistance training. Am J Physiol 1995;268:E422–7. [DOI] [PubMed] [Google Scholar]
- 48.Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab 2000;278:E620–6. [DOI] [PubMed] [Google Scholar]
- 49.Phillips SM, Tipton KD, Ferrando AA, Wolfe RR. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol 1999;276:E118–24. [DOI] [PubMed] [Google Scholar]
- 50.Kim PL, Staron RS, Phillips SM. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol 2005;568:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol 1997;273:E122–9. [DOI] [PubMed] [Google Scholar]
- 52.Tipton KD, Ferrando AA, Phillips SM, Doyle D Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol 1999;276:E628–34. [DOI] [PubMed] [Google Scholar]
- 53.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol 2000;88:386–92. [DOI] [PubMed] [Google Scholar]
- 54.Børsheim E, Aarsland A, Wolfe RR. Effect of an amino acid, protein, and carbohydrate mixture on net muscle protein balance after resistance exercise. Int J Sport Nutr Exerc Metab 2004;14:255–71. [DOI] [PubMed] [Google Scholar]
- 55.Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab 2002;283:E648–57. [DOI] [PubMed] [Google Scholar]
- 56.Louis M, Poortmans JR, Francaux M, Berre J, Boisseau N, Brassine E, Cuthbertson DJ, Smith K, Babraj JA, Waddell T, et al. . No effect of creatine supplementation on human myofibrillar and sarcoplasmic protein synthesis after resistance exercise. Am J Physiol Endocrinol Metab 2003;285:E1089–94. [DOI] [PubMed] [Google Scholar]
- 57.Miller SL, Tipton KD, Chinkes DL, Wolf SE, Wolfe RR. Independent and combined effects of amino acids and glucose after resistance exercise. Med Sci Sports Exerc 2003;35:449–55. [DOI] [PubMed] [Google Scholar]
- 58.Tipton KD, Elliott TA, Cree MG, Wolf SE, Sanford AP, Wolfe RR. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sports Exerc 2004;36:2073–81. [DOI] [PubMed] [Google Scholar]
- 59.Tipton KD, Elliott TA, Cree MG, Aarsland AA, Sanford AP, Wolfe RR. Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am J Physiol Endocrinol Metab 2007;292:E71–6. [DOI] [PubMed] [Google Scholar]
- 60.Tipton KD, Elliott TA, Ferrando AA, Aarsland AA, Wolfe RR. Stimulation of muscle anabolism by resistance exercise and ingestion of leucine plus protein. Appl Physiol Nutr Metab 2009;34:151–61. [DOI] [PubMed] [Google Scholar]
- 61.Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab 2005;288:E1153–9. [DOI] [PubMed] [Google Scholar]
- 62.Koopman R, Wagenmakers AJ, Manders RJ, Zorenc AH, Senden JM, Gorselink M, Keizer HA, van Loon LJ. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab 2005;288:E645–53. [DOI] [PubMed] [Google Scholar]
- 63.Cuthbertson DJ, Babraj J, Smith K, Wilkes E, Fedele MJ, Esser K, Rennie M. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab 2006;290:E731–8. [DOI] [PubMed] [Google Scholar]
- 64.Elliot TA, Cree MG, Sanford AP, Wolfe RR, Tipton KD. Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Med Sci Sports Exerc 2006;38:667–74. [DOI] [PubMed] [Google Scholar]
- 65.Koopman R, Verdijk L, Manders RJ, Gijsen AP, Gorselink M, Pijpers E, Wagenmakers AJ, van Loon LJ. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am J Clin Nutr 2006;84:623–32. [DOI] [PubMed] [Google Scholar]
- 66.Tang JE, Manolakos JJ, Kujbida GW, Lysecki PJ, Moore DR, Phillips SM. Minimal whey protein with carbohydrate stimulates muscle protein synthesis following resistance exercise in trained young men. Appl Physiol Nutr Metab 2007;32:1132–8. [DOI] [PubMed] [Google Scholar]
- 67.Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr 2007;85:1031–40. [DOI] [PubMed] [Google Scholar]
- 68.Beelen M, Koopman R, Gijsen AP, Vandereyt H, Kies AK, Kuipers H, Saris WH, van Loon LJ. Protein coingestion stimulates muscle protein synthesis during resistance-type exercise. Am J Physiol Endocrinol Metab 2008;295:E70–7. [DOI] [PubMed] [Google Scholar]
- 69.Beelen M, Tieland M, Gijsen AP, Vandereyt H, Kies AK, Kuipers H, Saris WH, Koopman R, van Loon LJ. Coingestion of carbohydrate and protein hydrolysate stimulates muscle protein synthesis during exercise in young men, with no further increase during subsequent overnight recovery. J Nutr 2008;138:2198–204. [DOI] [PubMed] [Google Scholar]
- 70.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 2008;294:E392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol 2008;104:1452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol 2009;587:897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol 2009;107:987–92. [DOI] [PubMed] [Google Scholar]
- 74.West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, De Lisio M, Tang JE, Parise G, Rennie MJ, et al. . Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J Physiol 2009;587:5239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burd NA, Holwerda AM, Selby KC, West DW, Staples AW, Cain NE, Cashaback JG, Potvin JR, Baker SK, Phillips SM. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J Physiol 2010;588:3119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, et al. . Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One 2010;5:e12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mikkelsen UR, Schjerling P, Helmark IC, Reitelseder S, Holm L, Skovgaard D, Langberg H, Kjaer M, Heinemeier KM. Local NSAID infusion does not affect protein synthesis and gene expression in human muscle after eccentric exercise. Scand J Med Sci Sports 2011;21:630–44. [DOI] [PubMed] [Google Scholar]
- 78.Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr 2011;93:322–31. [DOI] [PubMed] [Google Scholar]
- 79.Symons TB, Sheffield-Moore M, Mamerow MM, Wolfe RR, Paddon-Jones D. The anabolic response to resistance exercise and a protein-rich meal is not diminished by age. J Nutr Health Aging 2011;15:376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, Moore DR, Stellingwerff T, Phillips SM. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr 2011;94:795–803. [DOI] [PubMed] [Google Scholar]
- 81.Reitelseder S, Agergaard J, Doessing S, Helmark IC, Lund P, Kristensen NB, Frystyk J, Flyvbjerg A, Schjerling P, van Hall G, et al. . Whey and casein labeled with L-[1-13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. Am J Physiol Endocrinol Metab 2011;300:E231–42. [DOI] [PubMed] [Google Scholar]
- 82.Burd NA, Andrews RJ, West DW, Little JP, Cochran AJ, Hector AJ, Cashaback JG, Gibala MJ, Potvin JR, Baker SK, et al. . Muscle time under tension during resistance exercise stimulates differential muscle protein sub-fractional synthetic responses in men. J Physiol 2012;590:351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burke LM, Hawley JA, Ross ML, Moore DR, Phillips SM, Slater GR, Stellingwerff T, Tipton KD, Garnham AP, Coffey VG. Preexercise aminoacidemia and muscle protein synthesis after resistance exercise. Med Sci Sports Exerc 2012;44:1968–77. [DOI] [PubMed] [Google Scholar]
- 84.Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol 2012;590:2751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gasier HG, Riechman SE, Wiggs MP, Buentello A, Previs SF, Fluckey JD. Cumulative responses of muscle protein synthesis are augmented with chronic resistance exercise training. Acta Physiol (Oxf) 2011;201:381–9. [DOI] [PubMed] [Google Scholar]
- 86.West DW, Burd NA, Churchward-Venne TA, Camera DM, Mitchell CJ, Baker SK, Hawley JA, Coffey VG, Phillips SM. Sex-based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. J Appl Physiol 2012;112:1805–13. [DOI] [PubMed] [Google Scholar]
- 87.Bechshoeft R, Dideriksen KJ, Reitelseder S, Scheike T, Kjaer M, Holm L. The anabolic potential of dietary protein intake on skeletal muscle is prolonged by prior light-load exercise. Clin Nutr 2013;32:236–44. [DOI] [PubMed] [Google Scholar]
- 88.Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, Fry CS, Borack MS, Cope MB, Mukherjea R, et al. . Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. J Nutr 2013;143:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilkinson DJ, Franchi MV, Brook MS, Narici MV, Williams JP, Mitchell WK, Szewczyk NJ, Greenhaff PL, Atherton PJ, Smith K. A validation of the application of D(2)O stable isotope tracer techniques for monitoring day-to-day changes in muscle protein subfraction synthesis in humans. Am J Physiol Endocrinol Metab 2014;306:E571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Areta JL, Burke LM, Ross ML, Camera DM, West DW, Broad EM, Jeacocke NA, Moore DR, Stellingwerff T, Phillips SM, et al. . Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol 2013;591:2319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Areta JL, Burke LM, Camera DM, West DW, Crawshay S, Moore DR, Stellingwerff T, Phillips SM, Hawley JA, Coffey VG. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Am J Physiol Endocrinol Metab 2014;306:E989–97. [DOI] [PubMed] [Google Scholar]
- 92.Camera DM, West DW, Phillips SM, Rerecich T, Stellingwerff T, Hawley JA, Coffey VG. Protein ingestion increases myofibrillar protein synthesis after concurrent exercise. Med Sci Sports Exerc 2015;47:82–91. [DOI] [PubMed] [Google Scholar]
- 93.Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK, et al. . Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr 2014;99:276–86. [DOI] [PubMed] [Google Scholar]
- 94.Mitchell CJ, Churchward-Venne TA, Parise G, Bellamy L, Baker SK, Smith K, Atherton PJ, Phillips SM. Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PLoS One 2014;9:e89431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parr EB, Camera DM, Areta JL, Burke LM, Phillips SM, Hawley JA, Coffey VG. Alcohol ingestion impairs maximal post-exercise rates of myofibrillar protein synthesis following a single bout of concurrent training. PLoS One 2014;9:e88384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reitelseder S, Agergaard J, Doessing S, Helmark IC, Schjerling P, van Hall G, Kjaer M, Holm L. Positive muscle protein net balance and differential regulation of atrogene expression after resistance exercise and milk protein supplementation. Eur J Nutr 2014;53:321–33. [DOI] [PubMed] [Google Scholar]
- 97.Rahbek SK, Farup J, Moller AB, Vendelbo MH, Holm L, Jessen N, Vissing K. Effects of divergent resistance exercise contraction mode and dietary supplementation type on anabolic signalling, muscle protein synthesis and muscle hypertrophy. Amino Acids 2014;46:2377–92. [DOI] [PubMed] [Google Scholar]
- 98.Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, Cope MB, Mukherjea R, Jennings K, Volpi E, et al. . Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J Appl Physiol 2014;116:1353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Witard OC, Cocke TL, Ferrando AA, Wolfe RR, Tipton KD. Increased net muscle protein balance in response to simultaneous and separate ingestion of carbohydrate and essential amino acids following resistance exercise. Appl Physiol Nutr Metab 2014;39:329–39. [DOI] [PubMed] [Google Scholar]
- 100.Yarasheski KE, Campbell JA, Smith K, Rennie MJ, Holloszy JO, Bier DM. Effect of growth hormone and resistance exercise on muscle growth in young men. Am J Physiol 1992;262:E261–7. [DOI] [PubMed] [Google Scholar]
- 101.Phillips SM, Parise G, Roy BD, Tipton KD, Wolfe RR, Tamopolsky MA. Resistance-training-induced adaptations in skeletal muscle protein turnover in the fed state. Can J Physiol Pharmacol 2002;80:1045–53. [DOI] [PubMed] [Google Scholar]
- 102.Tang JE, Perco JG, Moore DR, Wilkinson SB, Phillips SM. Resistance training alters the response of fed state mixed muscle protein synthesis in young men. Am J Physiol Regul Integr Comp Physiol 2008;294:R172–8. [DOI] [PubMed] [Google Scholar]
- 103.Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 2008;586:3701–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Szewczyk NJ, Greenhaff PL, Smith K, Atherton PJ. Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide-derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. FASEB J 2015;29:4485–96. [DOI] [PubMed] [Google Scholar]
- 105.Lambert BS, Shimkus KL, Fluckey JD, Riechman SE, Greene NP, Cardin JM, Crouse SF. Anabolic responses to acute and chronic resistance exercise are enhanced when combined with aquatic treadmill exercise. Am J Physiol Endocrinol Metab 2015;308:E192–200. [DOI] [PubMed] [Google Scholar]
- 106.Kumar V, Atherton P, Smith K, Rennie MJ. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol 2009;106:2026–39. [DOI] [PubMed] [Google Scholar]
- 107.Moore DR, Burd NA. Exercise intensity matters for both young and old muscles. J Physiol 2009;587:511–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rose AJ, Richter EA. Regulatory mechanisms of skeletal muscle protein turnover during exercise. J Appl Physiol 2009;106:1702–11. [DOI] [PubMed] [Google Scholar]
- 109.Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, Gundermann DM, Volpi E, Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol 2011;111:135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Glynn EL, Fry CS, Drummond MJ, Dreyer HC, Dhanani S, Volpi E, Rasmussen BB. Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am J Physiol Regul Integr Comp Physiol 2010;299:R533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tipton KD, Borsheim E, Wolf SE, Sanford AP, Wolfe RR. Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion. Am J Physiol Endocrinol Metab 2003;284:E76–89. [DOI] [PubMed] [Google Scholar]
- 112.Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA, Smith K. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol 1992;73:1383–8. [DOI] [PubMed] [Google Scholar]
- 113.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Volpi E, Rasmussen BB. Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci 2013;68:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Phillips SM. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr 2011;141:568–73. [DOI] [PubMed] [Google Scholar]
- 115.Tipton KD, Rasmussen BB, Miller SL, Wolf SE, Owens-Stovall SK, Petrini BE, Wolfe RR. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol Endocrinol Metab 2001;281:E197–206. [DOI] [PubMed] [Google Scholar]
- 116.Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 2010;92:1080–8. [DOI] [PubMed] [Google Scholar]
- 117.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 2010;298:E1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr 2010;140:1970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr 2011;141:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Holm L, Esmarck B, Suetta C, Matsumoto K, Doi T, Mizuno M, Miller BF, Kjaer M. Postexercise nutrient intake enhances leg protein balance in early postmenopausal women. J Gerontol A Biol Sci Med Sci 2005;60:1212–8. [DOI] [PubMed] [Google Scholar]
- 121.Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr 2012;108:1780–8. [DOI] [PubMed] [Google Scholar]
- 122.Yang Y, Churchward-Venne TA, Burd NA, Breen L, Tarnopolsky MA, Phillips SM. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab (London) 2012;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Robinson MJ, Burd NA, Breen L, Rerecich T, Yang Y, Hector AJ, Baker SK, Phillips SM. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl Physiol Nutr Metab 2013;38:120–5. [DOI] [PubMed] [Google Scholar]
- 124.Børsheim E, Cree MG, Tipton KD, Elliott TA, Aarsland A, Wolfe RR. Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise. J Appl Physiol 2004;96:674–8. [DOI] [PubMed] [Google Scholar]
- 125.Witard OC, Tieland M, Beelen M, Tipton KD, van Loon LJ, Koopman R. Resistance exercise increases postprandial muscle protein synthesis in humans. Med Sci Sports Exerc 2009;41:144–54. [DOI] [PubMed] [Google Scholar]
- 126.Welle S, Thornton CA. High-protein meals do not enhance myofibrillar synthesis after resistance exercise in 62- to 75-yr-old men and women. Am J Physiol 1998;274:E677–83. [DOI] [PubMed] [Google Scholar]
- 127.Moore DR, Del Bel NC, Nizi KI, Hartman JW, Tang JE, Armstrong D, Phillips SM. Resistance training reduces fasted- and fed-state leucine turnover and increases dietary nitrogen retention in previously untrained young men. J Nutr 2007;137:985–91. [DOI] [PubMed] [Google Scholar]
- 128.Hartman JW, Moore DR, Phillips SM. Resistance training reduces whole-body protein turnover and improves net protein retention in untrained young males. Appl Physiol Nutr Metab 2006;31:557–64. [DOI] [PubMed] [Google Scholar]
- 129.Wilson GJ, Moulton CJ, Garlick PJ, Anthony TG, Layman DK. Post-meal responses of elongation factor 2 (eEF2) and adenosine monophosphate-activated protein kinase (AMPK) to leucine and carbohydrate supplements for regulating protein synthesis duration and energy homeostasis in rat skeletal muscle. Nutrients 2012;4:1723–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005;19:422–4. [DOI] [PubMed] [Google Scholar]
- 131.Harber MP, Konopka AR, Jemiolo B, Trappe SW, Trappe TA, Reidy PT. Muscle protein synthesis and gene expression during recovery from aerobic exercise in the fasted and fed states. Am J Physiol Regul Integr Comp Physiol 2010;299:R1254–62. [DOI] [PubMed] [Google Scholar]
- 132.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA 1997;94:14930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, Ballevre O, Beaufrere B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab 2001;280:E340–8. [DOI] [PubMed] [Google Scholar]
- 134.Tang JE, Phillips SM. Maximizing muscle protein anabolism: the role of protein quality. Curr Opin Clin Nutr Metab Care 2009;12:66–71. [DOI] [PubMed] [Google Scholar]
- 135.Hulmi JJ, Lockwood CM, Stout JR. Effect of protein/essential amino acids and resistance training on skeletal muscle hypertrophy: a case for whey protein. Nutr Metab (London) 2010;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pennings B, Groen B, de Lange A, Gijsen AP, Zorenc AH, Senden JM, van Loon LJ. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab 2012;302:E992–9. [DOI] [PubMed] [Google Scholar]
- 137.Burd NA, Yang Y, Moore DR, Tang JE, Tarnopolsky MA, Phillips SM. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br J Nutr 2012;108:958–62. [DOI] [PubMed] [Google Scholar]
- 138.Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol 1998;275:E73–8. [DOI] [PubMed] [Google Scholar]
- 139.Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with L-[1-13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1-13C]valine. Am J Physiol 1992;262:E372–6. [DOI] [PubMed] [Google Scholar]
- 140.Anthony TG, Anthony JC, Yoshizawa F, Kimball SR, Jefferson LS. Oral administration of leucine stimulates ribosomal protein mRNA translation but not global rates of protein synthesis in the liver of rats. J Nutr 2001;131:1171–6. [DOI] [PubMed] [Google Scholar]
- 141.Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr 2001;131(Suppl):856S–60S. [DOI] [PubMed] [Google Scholar]
- 142.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 2000;130:2413–9. [DOI] [PubMed] [Google Scholar]
- 143.Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 2010;38:1533–9. [DOI] [PubMed] [Google Scholar]
- 144.Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, et al. . Effects of leucine and its metabolite beta-hydroxy-beta-methylbutyrate on human skeletal muscle protein metabolism. J Physiol 2013;591:2911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 2003;78:250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Glynn EL, Fry CS, Timmerman KL, Drummond MJ, Volpi E, Rasmussen BB. Addition of carbohydrate or alanine to an essential amino acid mixture does not enhance human skeletal muscle protein anabolism. J Nutr 2013;143:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cayol M, Boirie Y, Rambourdin F, Prugnaud J, Gachon P, Beaufrere B, Obled C. Influence of protein intake on whole body and splanchnic leucine kinetics in humans. Am J Physiol 1997;272:E584–91. [DOI] [PubMed] [Google Scholar]
- 148.Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK, Lemosquet S, Saris WH, Boirie Y, van Loon LJ. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr 2009;90:106–15. [DOI] [PubMed] [Google Scholar]
- 149.Koopman R, Walrand S, Beelen M, Gijsen AP, Kies AK, Boirie Y, Saris WHM, van Loon LJC. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J Nutr 2009;139:1707–13. [DOI] [PubMed] [Google Scholar]
- 150.Engelen MP, Rutten EP, De Castro CL, Wouters EF, Schols AM, Deutz NE. Supplementation of soy protein with branched-chain amino acids alters protein metabolism in healthy elderly and even more in patients with chronic obstructive pulmonary disease. Am J Clin Nutr 2007;85:431–9. [DOI] [PubMed] [Google Scholar]
- 151.Bos C, Metges CC, Gaudichon C, Petzke KJ, Pueyo ME, Morens C, Everwand J, Benamouzig R, Tome D. Postprandial kinetics of dietary amino acids are the main determinant of their metabolism after soy or milk protein ingestion in humans. J Nutr 2003;133:1308–15. [DOI] [PubMed] [Google Scholar]
- 152.Fouillet H, Mariotti F, Gaudichon C, Bos C, Tome D. Peripheral and splanchnic metabolism of dietary nitrogen are differently affected by the protein source in humans as assessed by compartmental modeling. J Nutr 2002;132:125–33. [DOI] [PubMed] [Google Scholar]
- 153.Fouillet H, Bos C, Gaudichon C, Tome D. Approaches to quantifying protein metabolism in response to nutrient ingestion. J Nutr 2002;132(Suppl):3208S–18S. [DOI] [PubMed] [Google Scholar]
- 154.Cynober LA. Plasma amino acid levels with a note on membrane transport: characteristics, regulation, and metabolic significance. Nutrition 2002;18:761–6. [DOI] [PubMed] [Google Scholar]
- 155.Young VR. Amino acids and proteins in relation to the nutrition of elderly people. Age Ageing 1990;19:S10–24. [DOI] [PubMed] [Google Scholar]
- 156.Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F, Helge JW, Timmons JA, Phillips SM. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol 2011;110:309–17. [DOI] [PubMed] [Google Scholar]
- 157.Waterlow JC, Waterlow JC. Protein turnover. Wallingford (United Kingdom); CABI Publishing; 2006. [Google Scholar]
- 158.Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR, Hawley JA. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J 2006;20:190–2. [DOI] [PubMed] [Google Scholar]
- 159.Damas F, Phillips S, Vechin FC, Ugrinowitsch C. A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Med 2015;45:801–7. [DOI] [PubMed] [Google Scholar]
- 160.Farnfield MM, Breen L, Carey KA, Garnham A, Cameron-Smith D. Activation of mTOR signalling in young and old human skeletal muscle in response to combined resistance exercise and whey protein ingestion. Appl Physiol Nutr Metab 2012;37:21–30. [DOI] [PubMed] [Google Scholar]
- 161.Figueiredo VC, Caldow MK, Massie V, Markworth JF, Cameron-Smith D, Blazevich AJ. Ribosome biogenesis adaptation in resistance training-induced human skeletal muscle hypertrophy. Am J Physiol Endocrinol Metab 2015;309:E72–83. [DOI] [PubMed] [Google Scholar]
- 162.Phillips BE, Williams JP, Gustafsson T, Bouchard C, Rankinen T, Knudsen S, Smith K, Timmons JA, Atherton PJ. Molecular networks of human muscle adaptation to exercise and age. PLoS Genet 2013;9:e1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gilda JE, Ghosh R, Cheah JX, West TM, Bodine SC, Gomes AV. Western blotting inaccuracies with unverified antibodies: need for a Western Blotting Minimal Reporting Standard (WBMRS). PLoS One 2015;10:e0135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reidy PT, Konopka AR, Hinkley JM, Undem MK, Harber MP. The effect of feeding during recovery from aerobic exercise on skeletal muscle intracellular signaling. Int J Sport Nutr Exerc Metab 2014;24:70–8. [DOI] [PubMed] [Google Scholar]
- 165.Di Donato DM, West DW, Churchward-Venne TA, Breen L, Baker SK, Phillips SM. Influence of aerobic exercise intensity on myofibrillar and mitochondrial protein synthesis in young men during early and late postexercise recovery. Am J Physiol Endocrinol Metab 2014;306:E1025–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rhea MR, Alvar BA, Burkett LN, Ball SD. A meta-analysis to determine the dose response for strength development. Med Sci Sports Exerc 2003;35:456–64. [DOI] [PubMed] [Google Scholar]
- 167.Peterson MD, Rhea MR, Alvar BA. Maximizing strength development in athletes: a meta-analysis to determine the dose-response relationship. J Strength Cond Res 2004;18:377–82. [DOI] [PubMed] [Google Scholar]
- 168.Stark M, Lukaszuk J, Prawitz A, Salacinski A. Protein timing and its effects on muscular hypertrophy and strength in individuals engaged in weight-training. J Int Soc Sports Nutr 2012;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 2012;96:1454–64. [DOI] [PubMed] [Google Scholar]
- 170.Bosse JD, Dixon BM. Dietary protein to maximize resistance training: a review and examination of protein spread and change theories. J Int Soc Sports Nutr 2012;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schoenfeld BJ, Aragon AA, Krieger JW. The effect of protein timing on muscle strength and hypertrophy: a meta-analysis. J Int Soc Sports Nutr 2013;10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dideriksen K, Reitelseder S, Holm L. Influence of amino acids, dietary protein, and physical activity on muscle mass development in humans. Nutrients 2013;5:852–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hayes A, Cribb PJ. Effect of whey protein isolate on strength, body composition and muscle hypertrophy during resistance training. Curr Opin Clin Nutr Metab Care 2008;11:40–4. [DOI] [PubMed] [Google Scholar]
- 174.Pasiakos SM, McLellan TM, Lieberman HR, . T. he effects of protein supplements on muscle mass, strength, and aerobic and anaerobic power in healthy adults: a systematic review. Sports Med 2015;45:111–31. [DOI] [PubMed] [Google Scholar]
- 175.Pasiakos SM, Lieberman HR, McLellan TM. Effects of protein supplements on muscle damage, soreness and recovery of muscle function and physical performance: a systematic review. Sports Med 2014;44:655–70. [DOI] [PubMed] [Google Scholar]
- 176.Miller PE, Alexander DD, Perez V. Effects of whey protein and resistance exercise on body composition: a meta-analysis of randomized controlled trials. J Am Coll Nutr 2014;33:163–75. [DOI] [PubMed] [Google Scholar]
- 177.Nissen SL, Sharp RL. Effect of dietary supplements on lean mass and strength gains with resistance exercise: a meta-analysis. J Appl Physiol (1985) 2003;94:651–9. [DOI] [PubMed] [Google Scholar]
- 178.Antonio J, Sanders MS, Ehler LA, Uelmen J, Raether JB, Stout JR. Effects of exercise training and amino-acid supplementation on body composition and physical performance in untrained women. Nutrition 2000;16:1043–6. [DOI] [PubMed] [Google Scholar]
- 179.Beck TW, Housh TJ, Johnson GO, Coburn JW, Malek MH, Cramer JT. Effects of a drink containing creatine, amino acids, and protein combined with ten weeks of resistance training on body composition, strength, and anaerobic performance. J Strength Cond Res 2007;21:100–4. [DOI] [PubMed] [Google Scholar]
- 180.Chromiak JA, Smedley B, Carpenter W, Brown R, Koh YS, Lamberth JG, Joe LA, Abadie BR, Altorfer G. Effect of a 10-week strength training program and recovery drink on body composition, muscular strength and endurance, and anaerobic power and capacity. Nutrition 2004;20:420–7. [DOI] [PubMed] [Google Scholar]
- 181.Lemon PW, Tarnopolsky MA, MacDougall JD, Atkinson SA. Protein requirements and muscle mass/strength changes during intensive training in novice bodybuilders. J Appl Physiol 1992;73:767–75. [DOI] [PubMed] [Google Scholar]
- 182.Rankin JW, Goldman LP, Puglisi MJ, Nickols-Richardson SM, Earthman CP, Gwazdauskas FC. Effect of post-exercise supplement consumption on adaptations to resistance training. J Am Coll Nutr 2004;23:322–30. [DOI] [PubMed] [Google Scholar]
- 183.Rozenek R, Ward P, Long S, Garhammer J. Effects of high-calorie supplements on body composition and muscular strength following resistance training. J Sports Med Phys Fitness 2002;42:340–7. [PubMed] [Google Scholar]
- 184.White KM, Bauer SJ, Hartz KK, Baldridge M. Changes in body composition with yogurt consumption during resistance training in women. Int J Sport Nutr Exerc Metab 2009;19:18–33. [DOI] [PubMed] [Google Scholar]
- 185.Candow DG, Chilibeck PD, Burke DG, Davison KS, Smith-Palmer T. Effect of glutamine supplementation combined with resistance training in young adults. Eur J Appl Physiol 2001;86:142–9. [DOI] [PubMed] [Google Scholar]
- 186.Volek JS, Volk BM, Gomez AL, Kunces LJ, Kupchak BR, Freidenreich DJ, Aristizabal JC, Saenz C, Dunn-Lewis C, Ballard KD, et al. . Whey protein supplementation during resistance training augments lean body mass. J Am Coll Nutr 2013;32:122–35. [DOI] [PubMed] [Google Scholar]
- 187.Erskine RM, Fletcher G, Hanson B, Folland JP. Whey protein does not enhance the adaptations to elbow flexor resistance training. Med Sci Sports Exerc 2012;44:1791–800. [DOI] [PubMed] [Google Scholar]
- 188.Weisgarber KD, Candow DG, Vogt ES. Whey protein before and during resistance exercise has no effect on muscle mass and strength in untrained young adults. Int J Sport Nutr Exerc Metab 2012;22:463–9. [DOI] [PubMed] [Google Scholar]
- 189.Mitchell CJ, Oikawa SY, Ogborn DI, Nates NJ, MacNeil LG, Tarnopolsky M, Phillips SM. Daily chocolate milk consumption does not enhance the effect of resistance training in young and old men: a randomized controlled trial. Appl Physiol Nutr Metab 2015;40:199–202. [DOI] [PubMed] [Google Scholar]
- 190.Babault N, Paizis C, Deley G, Guerin-Deremaux L, Saniez MH, Lefranc-Millot C, Allaert FA. Pea proteins oral supplementation promotes muscle thickness gains during resistance training: a double-blind, randomized, placebo-controlled clinical trial vs. whey protein. J Int Soc Sports Nutr 2015;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Babault N, Deley G, Le Ruyet P, Morgan F, Allaert FA. Effects of soluble milk protein or casein supplementation on muscle fatigue following resistance training program: a randomized, double-blind, and placebo-controlled study. J Int Soc Sports Nutr 2014;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Andersen LL, Tufekovic G, Zebis MK, Crameri RM, Verlaan G, Kjaer M, Suetta C, Magnusson P, Aagaard P. The effect of resistance training combined with timed ingestion of protein on muscle fiber size and muscle strength. Metabolism 2005;54:151–6. [DOI] [PubMed] [Google Scholar]
- 193.Burke DG, Chilibeck PD, Davidson KS, Candow DG, Farthing J, Smith-Palmer T. The effect of whey protein supplementation with and without creatine monohydrate combined with resistance training on lean tissue mass and muscle strength. Int J Sport Nutr Exerc Metab 2001;11:349–64. [DOI] [PubMed] [Google Scholar]
- 194.Candow DG, Burke NC, Smith-Palmer T, Burke DG. Effect of whey and soy protein supplementation combined with resistance training in young adults. Int J Sport Nutr Exerc Metab 2006;16:233–44. [DOI] [PubMed] [Google Scholar]
- 195.Cribb PJ, Williams AD, Carey MF, Hayes A. The effect of whey isolate and resistance training on strength, body composition, and plasma glutamine. Int J Sport Nutr Exerc Metab 2006;16:494–509. [DOI] [PubMed] [Google Scholar]
- 196.Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr 2007;86:373–81. [DOI] [PubMed] [Google Scholar]
- 197.Hulmi JJ, Kovanen V, Selanne H, Kraemer WJ, Hakkinen K, Mero AA. Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids 2009;37:297–308. [DOI] [PubMed] [Google Scholar]
- 198.Josse AR, Tang JE, Tarnopolsky MA, Phillips SM. Body composition and strength changes in women with milk and resistance exercise. Med Sci Sports Exerc 2010;42:1122–30. [DOI] [PubMed] [Google Scholar]
- 199.Burd NA, Tang JE, Moore DR, Phillips SM. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol 2009;106:1692–701. [DOI] [PubMed] [Google Scholar]
- 200.Hulmi JJ, Tannerstedt J, Selanne H, Kainulainen H, Kovanen V, Mero AA. Resistance exercise with whey protein ingestion affects mTOR signaling pathway and myostatin in men. J Appl Physiol 2009;106:1720–9. [DOI] [PubMed] [Google Scholar]
- 201.DeFreitas JM, Beck TW, Stock MS, Dillon MA, Kasishke PR, II. An examination of the time course of training-induced skeletal muscle hypertrophy. Eur J Appl Physiol 2011;111:2785–90. [DOI] [PubMed] [Google Scholar]
- 202.Wernbom M, Augustsson J, Thomee R. The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Sports Med 2007;37:225–64. [DOI] [PubMed] [Google Scholar]
- 203.Todd KS, Butterfield GE, Calloway DH. Nitrogen balance in men with adequate and deficient energy intake at three levels of work. J Nutr 1984;114:2107–18. [DOI] [PubMed] [Google Scholar]
- 204.Butterfield GE, Calloway DH. Physical activity improves protein utilization in young men. Br J Nutr 1984;51:171–84. [DOI] [PubMed] [Google Scholar]
- 205.Castaneda C, Gordon PL, Uhlin KL, Levey AS, Kehayias JJ, Dwyer JT, Fielding RA, Roubenoff R, Singh MF. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency: a randomized, controlled trial. Ann Intern Med 2001;135:965–76. [DOI] [PubMed] [Google Scholar]
- 206.Cribb PJ, Hayes A. Effects of supplement timing and resistance exercise on skeletal muscle hypertrophy. Med Sci Sports Exerc 2006;38:1918–25. [DOI] [PubMed] [Google Scholar]
- 207.Hoffman JR, Ratamess NA, Tranchina CP, Rashti SL, Kang J, Faigenbaum AD. Effect of protein-supplement timing on strength, power, and body-composition changes in resistance-trained men. Int J Sport Nutr Exerc Metab 2009;19:172–85. [DOI] [PubMed] [Google Scholar]
- 208.Dragan GI, Vasiliu A, Georgescu E. Researches concerning the effects of Refit on elite weightlifters. J Sports Med Phys Fitness 1985;25:246–50. [PubMed] [Google Scholar]
- 209.Lemon PW, Berardi JM, Noreen EE. The role of protein and amino acid supplements in the athlete’s diet: does type or timing of ingestion matter? Curr Sports Med Rep 2002;1:214–21. [DOI] [PubMed] [Google Scholar]
- 210.Candow DG, Chilibeck PD. Timing of creatine or protein supplementation and resistance training in the elderly. Appl Physiol Nutr Metab 2008;33:184–90. [DOI] [PubMed] [Google Scholar]
- 211.Kerksick C, Harvey T, Stout J, Campbell B, Wilborn C, Kreider R, Kalman D, Ziegenfuss T, Lopez H, Landis J, et al. . International Society of Sports Nutrition position stand: nutrient timing. J Int Soc Sports Nutr 2008;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Timmerman KL, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Jennings K, Rasmussen BB, Volpi E. A moderate acute increase in physical activity enhances nutritive flow and the muscle protein anabolic response to mixed nutrient intake in older adults. Am J Clin Nutr 2012;95:1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, Volpi E. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 2007;56:1615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rodriguez NR, DiMarco NM, Langley S; American Dietetic Association, Dietitians of Canada; American College of Sports Medicine. Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: nutrition and athletic performance. J Am Diet Assoc 2009;109:509–27. [DOI] [PubMed] [Google Scholar]
- 215.Phillips SM. Dietary protein requirements and adaptive advantages in athletes. Br J Nutr 2012;108(Suppl 2):S158–67. [DOI] [PubMed] [Google Scholar]
- 216.Phillips SM. A brief review of higher dietary protein diets in weight loss: a focus on athletes. Sports Med 2014;44(Suppl 2):S149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Torún B, Scrimshaw NS, Young VR. Effect of isometric exercises on body potassium and dietary protein requirements of young men. Am J Clin Nutr 1977;30:1983–93. [DOI] [PubMed] [Google Scholar]
- 218.Thalacker-Mercer AE, Petrella JK, Bamman MM. Does habitual dietary intake influence myofiber hypertrophy in response to resistance training? A cluster analysis. Appl Physiol Nutr Metab 2009;34:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Antonio J, Peacock CA, Ellerbroek A, Fromhoff B, Silver T. The effects of consuming a high protein diet (4.4 g/kg/d) on body composition in resistance-trained individuals. J Int Soc Sports Nutr 2014;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care 2009;12:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.MacKenzie-Shalders KL, King NA, Byrne NM, Slater GJ. Increasing protein distribution has no effect on changes in lean mass during a rugby preseason. Int J Sport Nutr Exerc Metab 2015. In press. [DOI] [PubMed] [Google Scholar]
- 222.Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, Ritz P, Antoine JM, Prugnaud J, Beaufrere B, et al. . Protein feeding pattern does not affect protein retention in young women. J Nutr 2000;130:1700–4. [DOI] [PubMed] [Google Scholar]
- 223.Churchward-Venne TA, Murphy CH, Longland TM, Phillips SM. Role of protein and amino acids in promoting lean mass accretion with resistance exercise and attenuating lean mass loss during energy deficit in humans. Amino Acids 2013;45:231–40. [DOI] [PubMed] [Google Scholar]
- 224.Forbes GB. Body composition as affected by physical activity and nutrition. Fed Proc 1985;44:343–7. [PubMed] [Google Scholar]
- 225.Bray GA, Smith SR, de Jonge L, Xie H, Rood J, Martin CK, Most M, Brock C, Mancuso S, Redman LM. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA 2012;307:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hambre D, Vergara M, Lood Y, Bachrach-Lindstrom M, Lindstrom T, Nystrom FH. A randomized trial of protein supplementation compared with extra fast food on the effects of resistance training to increase metabolism. Scand J Clin Lab Invest 2012;72:471–8. [DOI] [PubMed] [Google Scholar]
- 227.Joy JM, Lowery RP, Wilson JM, Purpura M, De Souza EO, Wilson SM, Kalman DS, Dudeck JE, Jager R. The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Nutr J 2013;12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wilborn CD, Taylor LW, Outlaw J, Williams L, Campbell B, Foster CA, Smith-Ryan A, Urbina S, Hayward S. The effects of pre- and post-exercise whey vs. casein protein consumption on body composition and performance measures in collegiate female athletes. J Sports Sci Med 2013;12:74–9. [PMC free article] [PubMed] [Google Scholar]
- 229.Demling RH, DeSanti L. Effect of a hypocaloric diet, increased protein intake and resistance training on lean mass gains and fat mass loss in overweight police officers. Ann Nutr Metab 2000;44:21–9. [DOI] [PubMed] [Google Scholar]
- 230.Brown EC, DiSilvestro RA, Babaknia A, Devor ST. Soy versus whey protein bars: effects on exercise training impact on lean body mass and antioxidant status. Nutr J 2004;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kerksick CM, Rasmussen C, Lancaster S, Starks M, Smith P, Melton C, Greenwood M, Almada A, Kreider R. Impact of differing protein sources and a creatine containing nutritional formula after 12 weeks of resistance training. Nutrition 2007;23:647–56. [DOI] [PubMed] [Google Scholar]
- 232.Phillips SM. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med 2014;44(Suppl 1):S71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nana A, Slater GJ, Stewart AD, Burke LM. Methodology review: using dual-energy X-ray absorptiometry (DXA) for the assessment of body composition in athletes and active people. Int J Sport Nutr Exerc Metab 2015;25:198–215. [DOI] [PubMed] [Google Scholar]
- 234.Nana A, Slater GJ, Hopkins WG, Halson SL, Martin DT, West NP, Burke LM. Importance of standardized DXA protocol for assessing physique changes in athletes. Int J Sport Nutr Exerc Metab 2014. In press. [DOI] [PubMed] [Google Scholar]
- 235.Nana A, Slater GJ, Hopkins WG, Burke LM. Effects of exercise sessions on DXA measurements of body composition in active people. Med Sci Sports Exerc 2013;45:178–85. [DOI] [PubMed] [Google Scholar]
- 236.Waterlow JC, Garlick PJ, Millward DJ. Protein turnover in mammalian tissues and in the whole body. Amsterdam: Elsevier North-Holland; 1978. [Google Scholar]
- 237.Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB, van Loon LJ. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 2013;48:492–8. [DOI] [PubMed] [Google Scholar]
- 238.Verdijk LB, Snijders T, Beelen M, Savelberg HH, Meijer K, Kuipers H, Van Loon LJ. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J Am Geriatr Soc 2010;58:2069–75. [DOI] [PubMed] [Google Scholar]
- 239.Cribb PJ, Williams AD, Stathis CG, Carey MF, Hayes A. Effects of whey isolate, creatine, and resistance training on muscle hypertrophy. Med Sci Sports Exerc 2007;39:298–307. [DOI] [PubMed] [Google Scholar]
- 240.Cribb PJ, Williams AD, Hayes A. A creatine-protein-carbohydrate supplement enhances responses to resistance training. Med Sci Sports Exerc 2007;39:1960–8. [DOI] [PubMed] [Google Scholar]
- 241.Van Etten LM, Verstappen FT, Westerterp KR. Effect of body build on weight-training-induced adaptations in body composition and muscular strength. Med Sci Sports Exerc 1994;26:515–21. [DOI] [PubMed] [Google Scholar]
- 242.Herda AA, Herda TJ, Costa PB, Ryan ED, Stout JR, Cramer JT. Muscle performance, size, and safety responses after eight weeks of resistance training and protein supplementation: a randomized, double-blinded, placebo-controlled clinical trial. J Strength Cond Res 2013;27:3091–100. [DOI] [PubMed] [Google Scholar]
- 243.Bird SP, Tarpenning KM, Marino FE. Independent and combined effects of liquid carbohydrate/essential amino acid ingestion on hormonal and muscular adaptations following resistance training in untrained men. Eur J Appl Physiol 2006;97:225–38. [DOI] [PubMed] [Google Scholar]
- 244.Nissen SL, Sharp RL. Effect of dietary supplements on lean mass and strength gains with resistance exercise: a meta-analysis. J Appl Physiol 2003;94:651–9. [DOI] [PubMed] [Google Scholar]
- 245.Goston JL, Correia MI. Intake of nutritional supplements among people exercising in gyms and influencing factors. Nutrition 2010;26:604–11. [DOI] [PubMed] [Google Scholar]
- 246.Dascombe BJ, Karunaratna M, Cartoon J, Fergie B, Goodman C. Nutritional supplementation habits and perceptions of elite athletes within a state-based sporting institute. J Sci Med Sport 2010;13:274–80. [DOI] [PubMed] [Google Scholar]
- 247.Morrison LJ, Gizis F, Shorter B. Prevalent use of dietary supplements among people who exercise at a commercial gym. Int J Sport Nutr Exerc Metab 2004;14:481–92. [DOI] [PubMed] [Google Scholar]
- 248.Froiland K, Koszewski W, Hingst J, Kopecky L. Nutritional supplement use among college athletes and their sources of information. Int J Sport Nutr Exerc Metab 2004;14:104–20. [DOI] [PubMed] [Google Scholar]
- 249.El Khoury D, Antoine-Jonville S.. Intake of nutritional supplements among people exercising in gyms in Beirut City. J Nutr Metabol 2012;2012:703490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Snijders T, Res PT, Smeets JS, van Vliet S, van Kranenburg J, Maase K, Kies AK, Verdijk LB, van Loon LJ. Prot ein ingestion before sleep increases muscle mass and strength gains during prolonged resistance-type exercise training in healthy young men. J Nutr 2015;145:1178–84. [DOI] [PubMed] [Google Scholar]
- 251.Gryson C, Ratel S, Rance M, Penando S, Bonhomme C, Le Ruyet P, Duclos M, Boirie Y, Walrand S. Four-month course of soluble milk proteins interacts with exercise to improve muscle strength and delay fatigue in elderly participants. J Am Med Dir Assoc 2014;15:958, e1–9. [DOI] [PubMed] [Google Scholar]
- 252.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 2006;84:475–82. [DOI] [PubMed] [Google Scholar]
- 253.Mitchell CJ, Churchward-Venne TA, Cameron-Smith D, Phillips SM. What is the relationship between the acute muscle protein synthetic response and changes in muscle mass? J Appl Physiol 2015;118:495–7. [DOI] [PubMed] [Google Scholar]
- 254.Booth FW, Tseng BS, Fluck M, Carson JA. Molecular and cellular adaptation of muscle in response to physical training. Acta Physiol Scand 1998;162:343–50. [DOI] [PubMed] [Google Scholar]
- 255.Dennis RA, Przybyla B, Gurley C, Kortebein PM, Simpson P, Sullivan DH, Peterson CA. Aging alters gene expression of growth and remodeling factors in human skeletal muscle both at rest and in response to acute resistance exercise. Physiol Genomics 2008;32:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mitchell CJ, Churchward-Venne TA, Bellamy L, Parise G, Baker SK, Phillips SM. Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PLoS One 2013;8:e78636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E. Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 2008;102:145–52. [DOI] [PubMed] [Google Scholar]
- 258.Holm L, Olesen JL, Matsumoto K, Doi T, Mizuno M, Alsted TJ, Mackey AL, Schwarz P, Kjaer M. Protein-containing nutrient supplementation following strength training enhances the effect on muscle mass, strength, and bone formation in postmenopausal women. J Appl Physiol 2008;105:274–81. [DOI] [PubMed] [Google Scholar]
- 259.Holm L, Reitelseder S, Pedersen TG, Doessing S, Petersen SG, Flyvbjerg A, Andersen JL, Aagaard P, Kjaer M. Changes in muscle size and MHC composition in response to resistance exercise with heavy and light loading intensity. J Appl Physiol 2008;105:1454–61. [DOI] [PubMed] [Google Scholar]
- 260.West DW, Burd NA, Tang JE, Moore DR, Staples AW, Holwerda AM, Baker SK, Phillips SM. Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training-induced muscle hypertrophy nor strength of the elbow flexors. J Appl Physiol 2010;108:60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 2005;82:1065–73. [DOI] [PubMed] [Google Scholar]
- 262.Moore DR, Young M, Phillips SM. Similar increases in muscle size and strength in young men after training with maximal shortening or lengthening contractions when matched for total work. Eur J Appl Physiol 2012;112:1587–92. [DOI] [PubMed] [Google Scholar]
- 263.Mitchell CJ, Churchward-Venne TA, West DW, Burd NA, Breen L, Baker SK, Phillips SM. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol 2012;113:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]