Abstract
Background: Rates of overweight and obesity are on the rise globally, and excess adipose tissue may contribute to elevations in inflammation during pregnancy, leading to pregnancy complications and adverse birth outcomes.
Objective: The purpose of this study was to evaluate adiposity and inflammation in young women as predictors of inflammation in the third trimester of pregnancy in a community-based sample of healthy women.
Methods: Female participants (24–30 y) in a prospective observational cohort study (Cebu Longitudinal Health and Nutrition Survey) were contacted between 2009 and 2014 to identify new pregnancies. A total of 309 women provided data from 409 pregnancies. An in-home interview was scheduled for the third trimester to collect pregnancy information, anthropometric measurements, and a blood sample. Circulating C-reactive protein (CRP) was measured with a high-sensitivity immunoassay. Data collected from assessments in 2005 and 2009 were used to assess body mass index (BMI) and CRP in young adulthood, before pregnancy. Robust regression models were implemented to evaluate BMI and CRP in young adulthood as predictors of pregnancy CRP.
Results: Pre-pregnancy BMI was a stronger predictor of third-trimester circulating CRP than BMI in the third trimester. No association was found between pregnancy weight gain and CRP. Pre-pregnancy CRP was a significant predictor of CRP in pregnancy, independent of BMI.
Conclusions: Levels of overweight/obesity and inflammation in young adulthood, before pregnancy, are important predictors of inflammation in the third trimester of pregnancy. These results may have implications for addressing the growing concern about the contribution of obesity to adverse birth outcomes, and they suggest that factors that influence the regulation of inflammation, before pregnancy and independent of adiposity, may be important in shaping the inflammatory response to pregnancy.
Keywords: inflammation, obesity, C-reactive protein, pregnancy, cohort studies, Philippines
Introduction
Mild increases in inflammation are a normative part of healthy pregnancy, but higher levels of inflammation during pregnancy are associated with adverse birth outcomes (1, 2). For example, circulating concentrations of C-reactive protein (CRP) are elevated slightly among healthy pregnant women (3, 4), but higher-than-normal concentrations in the third trimester predict lower birth weight of offspring (5), preterm delivery (6), chronic placental villitis (7), and preeclampsia (8). Increased inflammation during pregnancy is also associated with gestational diabetes (9) and risk of neurobehavioral disorders in offspring (10, 11). Pregnancy complications and adverse birth outcomes increase offspring risk of cardiovascular and metabolic diseases in adulthood, underscoring the importance of the gestational environment to trajectories of health for the next generation (12, 13).
Adipose tissue is an important site of proinflammatory cytokine production, and higher concentrations of CRP, outside of pregnancy, are consistently associated with measures of overweight and obesity (14, 15) and with elevated risk of cardiovascular disease (16) and type 2 diabetes (17). Obesity in pregnancy also increases risk of gestational diabetes (18) and preeclampsia and preterm delivery (19, 20). Chronic or dysregulated inflammation, driven in part by excess adipose tissue, may therefore be an important contributor to pregnancy complications and adverse birth outcomes.
With rates of obesity on the rise, in the United States and globally (21), several studies have documented positive associations between overweight/obesity and inflammation during pregnancy (22). However, most prior studies have recruited women after they become pregnant, with few studies that included measures of adiposity and inflammation before pregnancy. Considering these factors in adulthood, before pregnancy, may be important for 2 reasons: 1) individual differences in levels of adiposity and inflammation are apparent in adulthood and may be important determinants of how women respond to the inflammatory stress of pregnancy; 2) identifying women who are at increased risk of dysregulated inflammation during pregnancy, before they become pregnant, may provide options for intervention that reduce risk of pregnancy complications.
The aim of this study is to investigate the predictors of CRP in the third trimester of pregnancy among a cohort of women in the Philippines. The analysis uses data from a large, ongoing cohort study with several rounds of data collection before the pregnancy, to address the following specific objectives: 1) evaluate whether BMI (in kg/m2) during pregnancy or BMI before pregnancy is a stronger predictor of CRP in the third trimester and 2) investigate whether CRP concentration before pregnancy predicts CRP in the third trimester, independently of BMI. The Philippines is a lower-middle income nation undergoing substantial economic, dietary, and lifestyle changes, and it exemplifies current trends toward rising rates of overweight and obesity globally and associated increases in cardiovascular and metabolic diseases (23, 24).
Methods
Participants and data collection.
The Cebu Longitudinal Health and Nutrition Survey is an ongoing birth cohort study that began in 1983 with the recruitment of a community-based sample of 3327 pregnant women (25). Offspring were followed through multiple rounds of data collection, including surveys in young adulthood when participants were 20–22 (2005) and 24–26 (2009) y old. Cebu City is the second-largest urban area in the Philippines, and the CLNHS is notable for the diverse social, economic, and physical environments of study participants, who inhabit agrarian communities, dense urban settlements, and middle-class neighborhoods. Attrition in the Cebu Longitudinal Health and Nutrition Survey is primarily because of factors related to out-migration, and rates of refusal during initial recruitment were low (<4%) (25).
Between 2009 and 2014, when participants were 24–30 y old, women were contacted at 6-mo intervals to identify new pregnancies. An in-home interview was scheduled for the third trimester. Over the 5-y period of pregnancy tracking the individual rate of pregnancy was 1.32 pregnancies per woman with a total of 82.7 pregnancies/y in the sample. Rate of refusal was 1.2% of participants in the cohort, primarily because of loss of contact, presumably resulting from out-migration. All data were collected under conditions of informed consent with institutional review board approval from Northwestern University.
Anthropometric measures were collected with the same standardized procedures at each survey (26). Women provided information on household demographic characteristics and socioeconomic status, pregnancy complications, and timing of last menstrual period. The mean gestational timing of data collection was 29.6 wk from last menstrual period, with 90% of interviews conducted between 26.0 and 35.4 wk.
Blood samples were collected as finger stick capillary whole blood, dried on filter paper [dried blood spots (DBS)], for the analysis of CRP. Each participant’s finger was cleaned with alcohol, and a sterile, disposable microlancet was used to deliver a controlled, uniform puncture. Whole blood was placed directly on standardized filter paper commonly used for neonatal screening (Whatman 903; GE Healthcare). This relatively noninvasive blood collection protocol minimizes pain and inconvenience to the participants, and it facilitates the collection of blood samples despite the constraints of field-based research settings (27). After collection, DBS cards were allowed to dry at room temperature for ∼4 h and were stored in gas-impermeable bags at −20°C before shipment. Samples were express shipped to the United States where they were stored at −30°C before analysis.
Data from the 2005 and 2009 surveys were used to assess BMI and CRP in young adulthood, before pregnancy. In 2005 and 2009, height and weight were measured with the same standardized procedures. In 2005, venous blood samples were collected and measured for high-sensitivity CRP, as described previously (14). BMI data from 2009 were used to define pre-pregnancy BMI, unless women were pregnant at the time of the survey, in which case BMI values from 2005 were used (n = 62).
Complete anthropometric, sociodemographic, and CRP data were available for 409 pregnancies from 309 women. Over the 5-y period of pregnancy tracking, 89 women were pregnant twice, and 11 women were pregnant 3 times.
Measurement of circulating CRP.
Samples were analyzed for CRP in the Laboratory for Human Biology Research at Northwestern University with the use of a modified high-sensitivity enzyme immunoassay protocol previously developed for use with DBS (28). Prior validation of assay performance indicates that the DBS CRP method produces results that are comparable with gold standard plasma-based clinical methods, with a lower limit of detection of 0.03 mg/L (28). To minimize between-assay variation, all samples were analyzed by the same technician with the use of a single lot of capture antibody, detection antibody, and calibration material. Between-assay CVs for low, mid, and high control samples included with all runs were 11.8%, 9.6%, and 8.1%, respectively.
Before data analysis, DBS results were converted to plasma equivalent values with the use of a conversion formula that was based on 69 matched DBS and plasma samples (29). DBS samples were analyzed with the same procedures, lot number of reagents, and technician as applied to the study DBS samples. Plasma samples were analyzed for high-sensitivity CRP in a high-throughput clinical laboratory on the Beckman Coulter Synchron DXC platform. The correlation between DBS and plasma concentrations was high (Pearson r = 0.98), and the resulting Deming regression conversion formula was as follows: plasma (mg/L) = 1.64 × DBS (mg/L).
Data analysis.
Third-trimester CRP was the dependent variable in all analyses, and CRP values were log-transformed (base 10) to normalize the distribution. Pearson correlation coefficients were used to investigate the strength of bivariate associations, and paired t test was used to examine the difference in logCRP in young adulthood and logCRP in pregnancy. A series of multivariate least-squares regression models were implemented to determine the extent to which BMI and CRP before pregnancy and BMI in pregnancy predicted CRP in pregnancy. To facilitate interpretation, median CRP values are presented in the text and in Figure 1, and untransformed values are presented in Figure 2, based on predictions from the final regression model. Descriptive statistics in the text are means ± SDs, except for CRP, which is presented as median (IQR). Regression coefficients are β (95% CI).
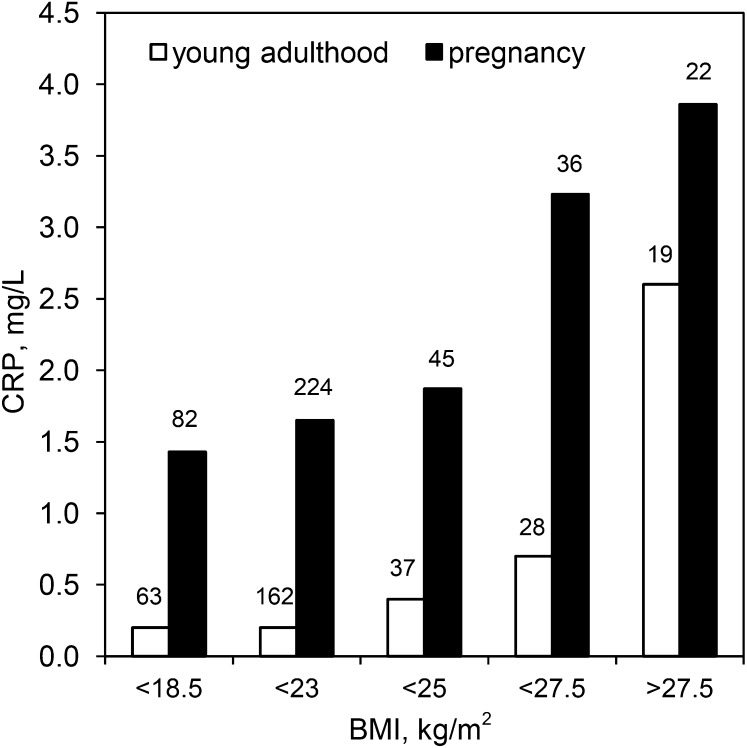
FIGURE 1.
Median CRP concentration during young adulthood and pregnancy in relation to pre-pregnancy BMI in young women in the Philippines. The number of women in each BMI category is presented above the open bars; the number of pregnancies in each BMI category is presented above the closed bars. There were 409 pregnancies in 309 women overall. CRP, C-reactive protein.
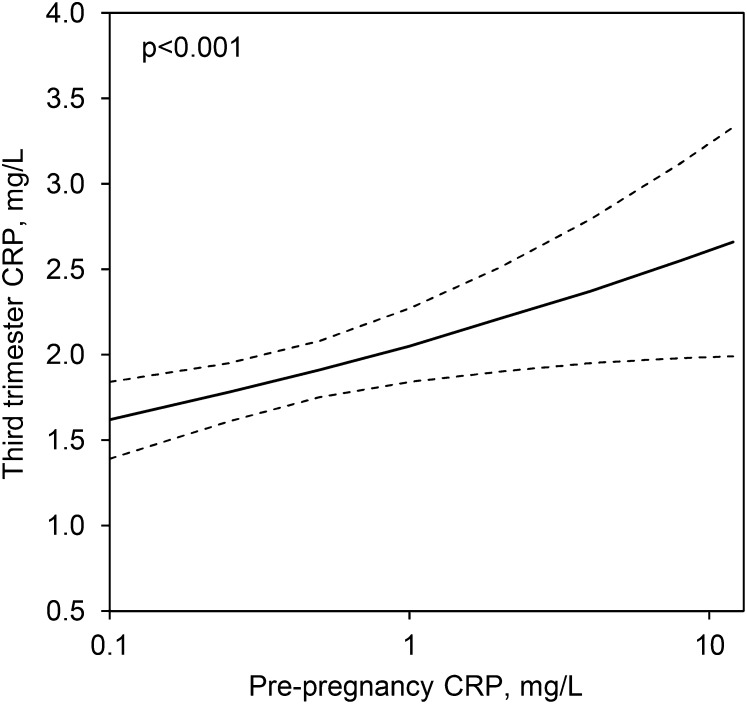
FIGURE 2.
CRP concentrations during pregnancy in young women in the Philippines as predicted by the pre-pregnancy concentration, adjusted for other variables in Model 3, Table 2. The 95% CI is represented by dashed lines. There were 409 pregnancies in 309 women overall. CRP, C-reactive protein.
The sample includes 409 pregnancies, but because 100 women provided data on ≥1 pregnancy, all observations are not independent. Therefore, robust regression procedures were implemented that relax the assumption of independence and produce SEs that account for clustered observations (30). No sample weights or adjustments for missing observations were applied to the analyses. Compared with other women in the cohort, participants in the pregnancy study did not differ for years of education, parity, or height or weight in young adulthood (all P > 0.3). α < 0.05 was set as the criterion for statistical significance. All statistical analyses were conducted with Stata/SE for Windows, version 13.1 (StataCorp).
Preliminary analyses indicated that the timing of data collection in the third trimester was not significantly associated with CRP (β = 0.01; 95% CI: −0.03, 0.05; P = 0.7) and was therefore not considered further. Women who were pregnant in the 2005 survey were not included in this analysis because they did not have a CRP measurement when they were not pregnant.
Several variables were considered as potential confounders/modifiers of associations with pregnancy CRP. Multiple measures of socioeconomic status (education level, home ownership, assets) were assessed as part of the pregnancy interview. Information on pregnancy complications was also collected, including self-reports of gestational diabetes, high blood pressure, urinary tract infection, and placenta previa. Overall, 19.2% of the pregnancies were associated with ≥1 complication. Information on diet during pregnancy was also considered. Women were asked to report on the number of meals and snacks they typically eat in a day and the frequency of fast-food consumption in a month. They were also asked to report on what foods they usually eat for breakfast, lunch, dinner, and snack. This information was summarized by category, to provide measures of the daily frequency of consumption of the following foods: meat (pork, beef, goat, chicken), fish and crustaceans, bread/baked goods, and rice (various preparations).
Results
Mean pre-pregnancy BMI in the sample was 21.4 ± 3.5, mean BMI in the third trimester was 24.5 ± 3.4, and women gained 7.3 ± 5.0 kg from their pre-pregnancy weight (Table 1). With the use of WHO-recommended BMI cutoff values, 15.3% of the women were overweight or obese as young adults, before pregnancy. However, WHO analyses have also acknowledged increased risk of cardiovascular and metabolic diseases in Asian populations, including the Philippines, at BMI < 25 (31). After prior research in this cohort (32), the use of 23 as the BMI cutoff for overweight and 25 as the BMI cutoff for obesity indicated a rate of overweight/obesity of 27.3%. Consistent with prior reports that levels of central body fat are higher among some Asian population at a given BMI value compared with white/European populations (33), mean waist-hip ratio in the sample was 0.88, and 81.4% of the sample was >0.85, the WHO cutoff for increased metabolic risk (34).
TABLE 1.
Descriptive characteristics of the study participants1
| Characteristic | Value |
| Age, y | 27.0 ± 1.5 (24.4–30.2) |
| Parity | 2.8 ± 1.6 (1–10) |
| Education, y | 12.0 ± 4.2 (1–23) |
| Waist-hip ratio, before pregnancy | 0.88 ± 0.04 (0.78–0.96) |
| BMI, kg/m2 | |
| Before pregnancy | 21.4 ± 3.5 (14.9–34.8) |
| Third trimester | 24.5 ± 3.4 (16.6–38.2) |
Values are means ± SDs (range), n = 409 pregnancies from 309 women.
Median CRP before pregnancy was 0.3 mg/L (IQR: 0.09, 1.3 mg/L), and it increased significantly in the third trimester to 1.8 mg/L (IQR: 1.0, 3.1 mg/L) (t = −17.5, P < 0.001, for difference between logCRP before and during pregnancy). Pregnancy CRP was positively associated with BMI before pregnancy (Figure 1). Similarly, concentration of CRP in young adulthood was positively associated with BMI in young adulthood, as reported previously in this cohort (14). As expected, pre-pregnancy BMI and BMI in the third trimester of pregnancy were highly correlated (r = 0.80, P < 0.001).
We next examined the predictors of third-trimester CRP in multivariate regression analyses. In preliminary analyses we found that primiparous women had significantly lower CRP during pregnancy (β = −0.11; 95% CI: −0.20, −0.02; P < 0.05). We therefore included this variable and maternal age (β = 0.05; 95% CI: −0.05, 0.14; P = 0.4) in subsequent models.
BMI during pregnancy was significantly and positively associated with CRP (β = 0.028; 95% CI: 0.015, 0.041; P < 0.001). The association between pre-pregnancy BMI and CRP, however, was slightly stronger (Table 2, model 1), and the model R2 was higher (0.10 compared with 0.09), indicating a larger proportion of explained variance in logCRP. When both BMI variables were considered simultaneously, pre-pregnancy BMI was a significant predictor of CRP (β = 0.025; 95% CI: 0.007, 0.043; P < 0.01), whereas BMI in pregnancy was not (β = 0.009; 95% CI: −0.012, 0.030; P = 0.4).
TABLE 2.
Linear regression coefficients that describe the associations between age, parity, BMI before pregnancy, BMI gain in pregnancy, and circulating CRP before pregnancy and log-transformed circulating CRP in the third trimester in young women in the Philippines1
| Characteristic | Model 1 | Model 2 | Model 3 |
| Age, y | 0.078* (−0.010, 0.165) | 0.080* (−0.007, 0.167) | 0.076* (−0.006, 0.158) |
| Primiparity | −0.064 (−0.154, 0.026) | −0.067 (−0.156, 0.022) | −0.048 (−0.133, 0.038) |
| BMI before pregnancy, kg/m2 | 0.032*** (0.021, 0.043) | 0.034*** (0.022, 0.046) | 0.028*** (0.014, 0.043) |
| BMI gain, kg/m2 | 0.009 (−0.012, 0.030) | 0.009 (−0.011, 0.029) | |
| logCRP before pregnancy | 0.104** (0.031, 0.176) | ||
| Model R2 | 0.10 | 0.10 | 0.14 |
Values are β (95% CI); n = 409 pregnancies from 309 women. *P < 0.10, **P < 0.01, and ***P < 0.001.
Because BMI values before and during pregnancy were highly correlated, it is difficult to distinguish their independent associations with CRP. We therefore calculated a variable that represented BMI gain in pregnancy (BMIpregnancy − BMIpre-pregnancy), which is only moderately correlated with the BMI before pregnancy (r = −0.28, P < 0.001) and third-trimester BMI (r = 0.35, P < 0.001). Because height did not change across the pre-pregnancy and pregnancy measurements, BMI gain can also be interpreted as representing weight gain during pregnancy. BMI gain was not a significant predictor of third-trimester CRP, whereas pre-pregnancy BMI remained a strong predictor (Table 2, model 2).
We next evaluated whether CRP, measured before pregnancy, was an independent predictor of CRP in the third trimester. When considered in a model with age and primiparity, pre-pregnancy CRP was a strong and significant predictor of CRP in pregnancy (β = 0.136; 95% CI: 0.064, 0.207; P < 0.001). When pre-pregnancy BMI was added to the model, pre-pregnancy CRP remained as an independent predictor of CRP in the third trimester, and the association between pre-pregnancy BMI and pregnancy CRP was attenuated (Table 2, model 3). The model R2 is higher when pre-pregnancy CRP is included (0.14 compared with 0.10), further underscoring the predictive value of pre-pregnancy CRP. Figure 2 shows CRP in pregnancy as a function of CRP before pregnancy, independent of BMI and other covariates in the final model.
Finally, we considered several sets of variables as potential confounders of associations with pregnancy CRP (results not shown). Measures of socioeconomic status (home ownership, maternal education, household assets) did not approach significance as predictors of pregnancy CRP. We also confirmed that the pattern of results did not differ across primiparous compared with multiparous pregnancies by running separate models for each. Similarly, pregnancy complications, and measures of food frequency, did not modify patterns of association between pregnancy CRP and other variables in the final model.
Discussion
Pregnancy is an inflammatory event, and effective regulation of inflammation is an important part of healthy pregnancy. Inflammation is also associated with overweight and obesity, but the extent to which additional adipose tissue, before or during pregnancy, may contribute to excessive inflammation is not known. In this study we take advantage of data from a prospective cohort study to investigate the extent to which BMI and inflammation in young adulthood, before pregnancy, predict the inflammatory response to pregnancy.
Consistent with prior research, across a wide range of populations, we find a positive association between BMI and CRP in pregnancy (35–37). However, our results suggest that BMI in adulthood, before pregnancy, is a stronger predictor of CRP in pregnancy. Cross-sectional studies do not include pre-pregnancy BMI, raising the possibility that prior reports of an association between BMI and CRP in pregnancy reflect the strong correlation between BMI before pregnancy and BMI in pregnancy. Consistent with this idea, a study of healthy pregnant women (9) documents a strong positive association between pre-pregnancy BMI and late second/early third-trimester CRP, with no association between CRP and pregnancy weight gain. Similarly, we find no association between pregnancy weight gain and CRP in our sample.
The clinical implications of our findings are not clear and will require additional research. Elevations in CRP are a normative part of healthy pregnancy (3, 4), but excessive inflammation was associated with adverse birth and developmental outcomes in a wide range of populations globally (5–11). Our results may have important implications for addressing growing concern about the contribution of overweight and obesity to adverse birth outcomes, because it appears that limiting weight gain during pregnancy may not be effective in reducing levels of inflammation.
To the best of our knowledge, this study is the first to report an association between the level of systemic inflammation in adulthood, before pregnancy, and the inflammatory response to pregnancy. We find a strong positive association that is independent of BMI. Although prior research has emphasized the potential for synergy between pregnancy and adiposity for increasing levels of inflammation during gestation (22, 36), our findings suggest that additional factors that contribute to the regulation of inflammation, before pregnancy and independent of adiposity, may be important in shaping the inflammatory response to pregnancy. For example, prior research has identified nutritional and microbial exposures in infancy (38, 39) and psychosocial stressors in childhood (40) as important determinants of chronic inflammation in adulthood. These kinds of exposures, during sensitive periods of immune development, may therefore have lasting effects on the inflammatory response to pregnancy (41).
A major strength of our study is the use of a large, prospective, community-based cohort, with measures of adiposity and inflammation collected before pregnancy. A limitation is our use of single CRP measures to assess chronic inflammation, both in adulthood and during pregnancy. Similarly, because we have only 1 pregnancy CRP measure, we cannot determine the level of inflammation earlier in gestation. In addition, the average BMI in our sample is relatively low at 21.4, compared with an average BMI of ∼26 for comparably aged young women in the United States (42). As such, generalizability may be limited, although we note that the patterns of association between BMI, pregnancy, and CRP in our sample are consistent with prior research in the United States and other international settings. In addition, the range of BMI in our sample includes a substantial number of overweight/obese individuals, and most women in our sample had waist-hip ratio above the WHO cutoff for increased metabolic risk, which should enhance generalizability to more obesigenic environments. Additional research in this and other populations may generate novel insights into the factors that contribute to inflammation during pregnancy, with implications for birth outcomes and trajectories of health in the next generation.
Acknowledgments
TWM, JBB, and CWK designed the research, conducted the research, and provided essential materials; FL and LSA conducted the research and provided essential materials; and TWM performed statistical analysis, wrote the paper, and had primary responsibility for final content. All authors read and approved the final manuscript.
References
- 1.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, Petraglia F. Inflammation and Pregnancy. Reprod Sci 2009;16:206–15. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev 2007;65:S194–202. [DOI] [PubMed] [Google Scholar]
- 3.Watts DH, Krohn MA, Wener MH, Eschenbach DA. C-reactive protein in normal pregnancy. Obstet Gynecol 1991;77:176–80. [DOI] [PubMed] [Google Scholar]
- 4.Kuzawa CW, Adair LS, Borja J, McDade TW. C-reactive protein by pregnancy and lactational status among Filipino young adult women. Am J Hum Biol 2013;25:131–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowe LP, Metzger BE, Lowe WL Jr, Dyer AR, McDade TW, McIntyre HD. Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab 2010;95:5427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorokin Y, Romero R, Mele L, Wapner RJ, Iams JD, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Harper M, et al. . Maternal serum interleukin-6, C-reactive protein, and matrix metalloproteinase-9 concentrations as risk factors for preterm birth <32 weeks and adverse neonatal outcomes. Am J Perinatol 2010;27:631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst LM, Grobman WA, Wolfe K, Huang MH, McDade TW, Holl JL, Borders AE. Biological markers of stress in pregnancy: associations with chronic placental inflammation at delivery. Am J Perinatol 2013;30:557–64. [DOI] [PubMed] [Google Scholar]
- 8.Teran E, Escudero C, Calle A. C-reactive protein during normal pregnancy and preeclampsia. Int J Gynaecol Obstet 2005;89:299–300. [DOI] [PubMed] [Google Scholar]
- 9.Retnakaran R, Hanley AJG, Raif N, Connelly PW, Sermer M, Zinman B. C-reactive protein and gestational diabetes: the central role of maternal obesity. J Clin Endocrinol Metab 2003;88:3507–12. [DOI] [PubMed] [Google Scholar]
- 10.Saliba E, Henrot A. Inflammatory mediators and neonatal brain damage. Biol Neonate 2001;79:224–7. [DOI] [PubMed] [Google Scholar]
- 11.Ponzio NM, Servatius R, Beck K, Marzouk A, Kreider T. Cytokine levels during pregnancy influence immunological profiles and neurobehavioral patterns of the offspring. Ann N Y Acad Sci 2007;1107:118–28. [DOI] [PubMed] [Google Scholar]
- 12.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008;359:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuzawa CW, Quinn EA. Developmental origins of adult function and health: evolutionary hypotheses. Annu Rev Anthropol 2009;38:131–47. [Google Scholar]
- 14.McDade TW, Rutherford JN, Adair L, Kuzawa C. Population differences in associations between C-reactive protein concentration and adiposity: comparison of young adults in the Philippines and the United States. Am J Clin Nutr 2009;89:1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford ES, Giles WH, Mokdad AH, Myers GL. Distribution and correlates of C-reactive protein concentrations among adult US women. Clin Chem 2004;50:574–81. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 1998;98:731–3. [DOI] [PubMed] [Google Scholar]
- 17.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–34. [DOI] [PubMed] [Google Scholar]
- 18.Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, Dietz PM. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007;30:2070–6. [DOI] [PubMed] [Google Scholar]
- 19.Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health 2001;91:436–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siega-Riz AM, Siega-Riz AM, Laraia B. The implications of maternal overweight and obesity on the course of pregnancy and birth outcomes. Matern Child Health J 2006;10:S153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popkin BM, Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes Relat Metab Disord 2004;28:S2–9. [DOI] [PubMed] [Google Scholar]
- 22.Denison FC, Roberts KA, Barr SM, Norman JE. Obesity, pregnancy, inflammation, and vascular function. Reproduction 2010;140:373–85. [DOI] [PubMed] [Google Scholar]
- 23.Adair LS. Dramatic rise in overweight and obesity in adult Filipino women and risk of hypertension. Obes Res 2004;12:1335–41. [DOI] [PubMed] [Google Scholar]
- 24.Tanchoco CC, Cruz AJ, Duante CA, Litonjua AD. Prevalence of metabolic syndrome among Filipino adults aged 20 years and over. Asia Pac J Clin Nutr 2003;12:271–6. [PubMed] [Google Scholar]
- 25.Adair LS, Popkin BM, Akin JS, Guilkey DK, Gultiano S, Borja J, Perez L, Kuzawa CW, McDade T, Hindin MJ. Cohort profile: the Cebu Longitudinal Health and Nutrition Survey. Int J Epidemiol 2011;40:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 27.McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography 2007;44:899–925. [DOI] [PubMed] [Google Scholar]
- 28.McDade TW, Burhop J, Dohnal J. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin Chem 2004;50:652–4. [DOI] [PubMed] [Google Scholar]
- 29.McDade TW. Development and validation of assay protocols for use with dried blood spot samples. Am J Hum Biol 2014;26:1–9. [DOI] [PubMed] [Google Scholar]
- 30.Rogers WH. Regression standard errors in clustered samples. Stata Tech Bull 1993;13:19–23. [Google Scholar]
- 31.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. [DOI] [PubMed] [Google Scholar]
- 32.Dahly DL, Gordon-Larsen P, Emch M, Borja J, Adair LS. The spatial distribution of overweight and obesity among a birth cohort of young adult Filipinos (Cebu Philippines, 2005): an application of the Kulldorff spatial scan statistic. Nutr Diabetes 2013;3:e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Thornton JC, Russell M, Burastero S, Heymsfield S, Pierson RN. Asians have lower-body mass index (BMI) but higher percent body-fat than do whites - comparisons of anthropometric measurements. Am J Clin Nutr 1994;60:23–8. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. Waist circumference and waist-hip ratio: report of a WHO expert consultation. WHO: Geneva; 2011. [Google Scholar]
- 35.Madan JC, Davis JM, Craig WY, Collins M, Allan W, Quinn R, Dammann O. Maternal obesity and markers of inflammation in pregnancy. Cytokine 2009;47:61–4. [DOI] [PubMed] [Google Scholar]
- 36.Christian LM, Porter K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: effects of maternal body mass index. Cytokine 2014;70:134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab 2007;92:969–75. [DOI] [PubMed] [Google Scholar]
- 38.McDade TW, Rutherford J, Adair L, Kuzawa CW. Early origins of inflammation: microbial exposures in infancy predict lower levels of C-reactive protein in adulthood. Proc Biol Sci 2010;277:1129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDade TW, Metzger MW, Chyu L, Duncan GJ, Garfield C, Adam EK. Long-term effects of birth weight and breastfeeding duration on inflammation in early adulthood. Proc Biol Sci 2014;281:20133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA 2007;104:1319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDade TW. Early environments and the ecology of inflammation. Proc Natl Acad Sci USA 2012;109:17281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee H, Lee D, Guo G, Harris KM. Trends in body mass index in adolescence and young adulthood in the United States: 1959–2002. J Adolesc Health 2011;49:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]