Significance
Whether or not SRY (sex-determining region Y)-box 9+ (Sox9+) pancreatic ductal cells can give rise to insulin-producing β cells in adult mice remains controversial. Using lineage-tracing techniques, we demonstrate that pancreatic Sox9+ ductal cells can be induced to differentiate into β cells under conditions of medium hyperglycemia. Long-term administration of low-dose, but not short-term administration of high-dose, gastrin and epidermal growth factors can augment this differentiation, resulting in formation of new β cells with reversal of diabetes. This study provides previously unidentified insight into β-cell regeneration from ductal cells in diabetic adult individuals.
Keywords: Sox9+ ductal cells, β-cell differentiation, hyperglycemia, gastrin, epidermal growth factor
Abstract
We previously reported that long-term administration of a low dose of gastrin and epidermal growth factor (GE) augments β-cell neogenesis in late-stage diabetic autoimmune mice after eliminating insulitis by induction of mixed chimerism. However, the source of β-cell neogenesis is still unknown. SRY (sex-determining region Y)-box 9+ (Sox9+) ductal cells in the adult pancreas are clonogenic and can give rise to insulin-producing β cells in an in vitro culture. Whether Sox9+ ductal cells in the adult pancreas can give rise to β cells in vivo remains controversial. Here, using lineage-tracing with genetic labeling of Insulin- or Sox9-expressing cells, we show that hyperglycemia (>300 mg/dL) is required for inducing Sox9+ ductal cell differentiation into insulin-producing β cells, and medium hyperglycemia (300–450 mg/dL) in combination with long-term administration of low-dose GE synergistically augments differentiation and is associated with normalization of blood glucose in nonautoimmune diabetic C57BL/6 mice. Short-term administration of high-dose GE cannot augment differentiation, although it can augment preexisting β-cell replication. These results indicate that medium hyperglycemia combined with long-term administration of low-dose GE represents one way to induce Sox9+ ductal cell differentiation into β cells in adult mice.
Autoimmune type 1 diabetes (T1D) results from autoimmune attack on insulin-secreting β cells and subsequent insulin deficiency (1). Cure of T1D requires both reversal of autoimmunity and resupply of insulin-secreting β cells by islet transplantation or augmentation of endogenous β-cell regeneration (2). Because of the lack of donors for islet transplantation and because islet grafts only last for ∼3 y (3), augmentation of endogenous β-cell regeneration would be the more favorable approach. We previously reported that combination therapy of induction of mixed chimerism and administration of gastrin and epidermal growth factor (GE) not only reversed autoimmunity, but also augmented β-cell neogenesis and replication and subsequently cured late-stage T1D in autoimmune nonobese diabetic (NOD) mice (4). However, the origin of neogenesis remains unknown.
It has been proposed that β-cell neogenesis in adult mice can derive from pancreatic ductal cells (5, 6), cells in the islet (7, 8), transdifferentiation from glucagon-producing α cells (9, 10), or from acinar cells (11–13). Although it has been consistently reported that pancreatic ductal epithelial cells give rise to insulin-producing β cells during embryonic development (14–17), whether the pancreatic ductal progenitors can give rise to insulin-producing β cells in neonates and adult mice remains controversial. Using a Cre-based lineage tracing with a human carbonic anhydrase II (CAII) promoter, Inada et al. reported that pancreatic ductal cells were able to give rise to insulin-producing β cells in neonates and in pancreatic duct ligation (PDL)-treated adult mice (5). Using a cyclization recombinase (Cre)-based lineage tracing model with a neurogenin 3 (Ngn3) promoter, Xu et al. also found that cells in the pancreatic ductal lining could give rise to β cells in PDL-treated adult mice (6). Conversely, using a lineage-tracing model with a hepatocyte nuclear factor 1-β (Hnf1β) promoter, Solar et al. found that pancreatic ductal cells did not give rise to β cells in neonates, PDL-treated adult mice, or Alloxan-induced diabetic adult mice treated for 1 wk with GE (14). Using a lineage-tracing model with a SRY (sex-determining region Y)-box 9 (Sox9) promoter, Kopp et al. also showed that Sox9+ ductal cells did not give rise to β cells postnatally after β-cell ablation or after PDL (17, 18). Similarly, Furuyama et al. reported that Sox9+ pancreatic ductal cells were not able to give rise to β cells in PDL-treated adult mice or streptozotocin (STZ)-induced diabetic mice (16). These reports suggest that PDL injury with normal glycemia, hyperglycemia alone, or hyperglycemia plus short-term (1 wk) administration of GE is not able to augment pancreatic Sox9+ ductal cell differentiation into insulin-producing β cells in adult mice. Actually, how hyperglycemia regulates progenitor differentiation into β cells remains largely unknown, although it has been reported that hyperglycemia is toxic to β cells (19, 20).
Several reports have shown that a subpopulation of cells in the pancreatic ducts of adult mice is clonogenic and can give rise to insulin-producing β cells in various in vitro culture systems (21, 22). One recent study, which used in vitro semisolid medium culture, showed that Sox9+CD133+ pancreatic ductal cells from adult mice are able to give rise to three cell lineages, including insulin-producing β cells, ductal epithelial cells, and acinar cells (23, 24). These results indicate that Sox9+CD133+ pancreatic ductal cells from adult mice have the potential to differentiate into insulin-producing β cells in culture, if induced by specific conditions.
Because treatment with GE was reported to induce β-cell neogenesis from adult pancreatic ductal cells in vitro (25), and because we observed neogenesis in mixed chimeric late-stage diabetic NOD mice after long-term (8 wk) administration of GE (4), in the present studies, we tested whether long-term administration of GE was able to induce Sox9+ pancreatic ductal cell differentiation into insulin-producing β cells in diabetic C57BL/6 mice, using a Cre-based lineage-tracing construct driven by Sox9 regulatory sequences. The same strain of mice was used as in the studies of Kopp et al. (17), but a founder with higher recombination efficiency was used in the present studies. We found that islets consisting of β cells differentiated from Sox9+ ductal cells in adult mice with hyperglycemia (>300 mg/dL) and medium hyperglycemia (300–450 mg/dL) in combination with long-term administration of GE synergistically augments the differentiation of Sox9+ ductal cells into β cells with reversal of hyperglycemia. These results indicate that Sox9+ pancreatic ductal cells in adult mice can differentiate into insulin-producing β cells under specific conditions, which include medium hyperglycemia and long-term administration of low-dose GE, as used in previous reports (26).
Results
Long-Term Administration of Low-Dose GE Augments Differentiation of Pancreatic Sox9+ Ductal Cells into β Cells with Reversal of Hyperglycemia.
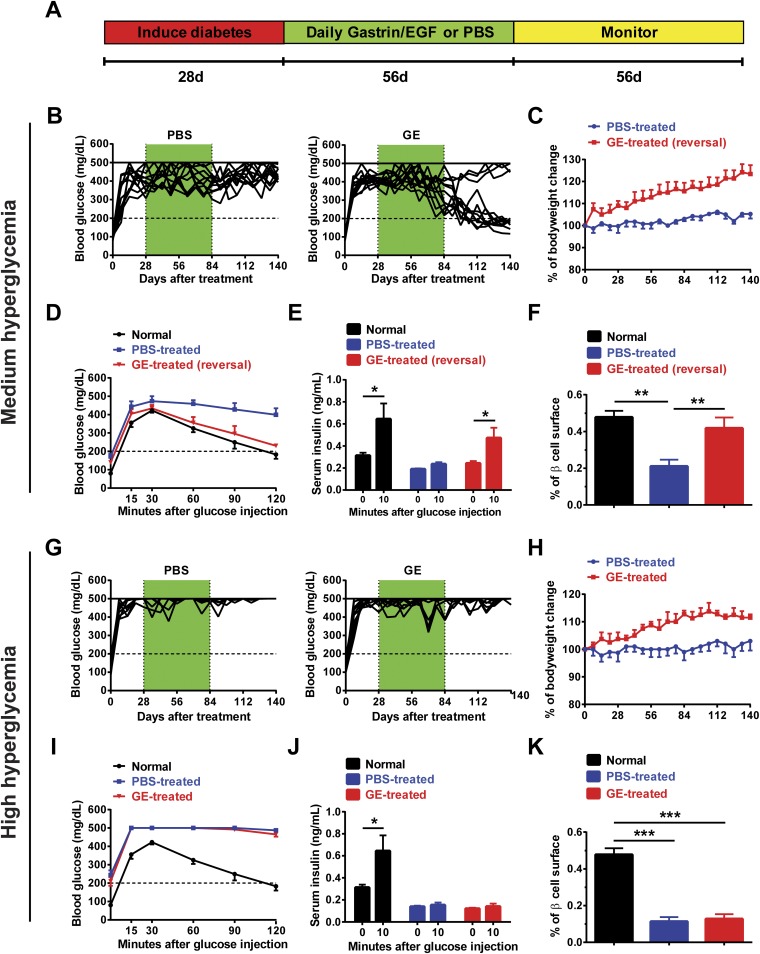
We tested whether Sox9+ pancreatic ductal cells could give rise to insulin-producing β cells in adult diabetic mice after GE treatment, using nonautoimmune Sox9CreERT2R26mT/mG C57BL/6 mice. First, we established a β-cell neogenesis mouse model in nonautoimmune diabetic C57BL/6 mice. The 8-wk-old adult female C57BL/6 mice were induced to develop diabetes by one i.v. injection of Alloxan (70 mg per kg of body weight) as described (14, 27). Mice developed variable levels of hyperglycemia, including mild (<300 mg/dL), medium (300–450 mg/dL), and high (>450 mg/dL) hyperglycemia (Table S1). Because some diabetic mice with mild hyperglycemia recovered spontaneously, only diabetic mice with medium or high hyperglycemia were used in this study. As illustrated in Fig. S1A, diabetic mice with medium and high hyperglycemia were given a daily injection of low-dose GE (gastrin, 3 µg per kg of body weight; EGF, 1 µg per kg of body weight), as described (4, 26) or control PBS for 8 wk, starting at 4 wk after induction of diabetes. The treated mice were monitored for blood glucose for another 8 wk before ending the experiments.
Table S1.
Alloxan treatment induces diabetes with different levels of blood glucose in C57BL/6 mice
| Blood glucose | BG < 300 mg/dL | 300 ≤ BG < 450 mg/dL | BG ≥ 450 mg/dL |
| Glucose level | Mild | Medium | High |
| Mouse no. | 38 | 101 | 83 |
| Percentage, % | 17 | 45 | 38 |
C57BL/6 mice (n = 222) were induced to rendered diabetic by i.v. injecting one dose of Alloxan (70 mg per kg of body weight) at day 0. Blood glucose (BG) concentrations in mice were measured twice a week for 4 wk. By day 28, 17% of the mice had mild hyperglycemia (<300 mg/dL), 45% had medium hyperglycemia (300–450 mg/dL), and 38% had high hyperglycemia (≥450 mg/dL).
Fig. S1.
Long-term administration of low-dose GE reverses diabetes in C57BL/6 mice with medium hyperglycemia. Adult female C57BL/6 mice were induced to develop diabetes by i.p. injection of one dose of Alloxan (70 mg/kg). At 28 d after injection of Alloxan, diabetic mice with medium (300–450 mg/dL) and high (≥450 mg/dL) hyperglycemia were selected for experiments and were treated with gastrin (3 µg/kg) plus EGF (1 μg/kg) (GE) for 56 d. Thereafter, the mice were monitored for another 56 d. The mice were monitored for body weight and blood glucose twice a week for up to 140 d. Before ending the experiments, mice were measured with an IPGTT and for insulin secretion. After ending the experiments, pancreases were harvested and stained for Insulin to measure β-cell surface. (A) Experimental scheme. (B–F) Diabetic mice with medium hyperglycemia were treated with GE and then monitored for blood glucose for 16 wk. (B) Blood glucose levels of diabetic C57BL/6 mice treated with GE or PBS control. Mice with “reversal of diabetes” had lasting stable blood glucose levels that was close to 200 mg/dL. There are 12 mice in each group combined from three experiments. (C) The percentage of body weight change (mean ± SEM, n = 12). (D) IPGTT blood glucose (n = 6). (E) Serum insulin levels before and 10 min after glucose injection during IPGTT (mean ± SEM, n = 6). (F) The percentage of β-cell surface (mean ± SEM, n = 4). (G–K) Diabetic mice with high hyperglycemia were treated with GE and monitored as described above. (G) Blood glucose levels of diabetic C57BL/6 mice treated with GE or PBS control. There are 12 mice in each group combined from three experiments. (H) The percentage of body weight change (mean ± SEM, n = 12). (I) IPGTT blood glucose (n = 6). (J) Serum insulin levels before and 10 min after glucose injection during IPGTT (mean ± SEM, n = 6). (K) The percentage of β-cell surface (mean ± SEM, n = 4). *P < 0.05; **P < 0.01; ***P < 0.001.
GE treatment gradually led to reversal of hyperglycemia in 75% (9/12) of diabetic mice with medium hyperglycemia, whereas no reversion was seen (12/12) in PBS-treated mice (P < 0.01; Fig. S1B). Compared with PBS-treated diabetic mice, GE-treated mice with reversal of diabetes showed marked improvement in body weight growth (P < 0.01; Fig. S1C), rapid blood glucose recovery during i.p. glucose tolerance tests (IPGTT) (P < 0.01; Fig. S1D), marked increase of serum insulin secretion during IPGTT (P < 0.05; Fig. S1E), and increase of β-cell surface, which reached levels similar to normal control mice (P < 0.01; Fig. S1F). In contrast, GE treatment was not able to reverse diabetes in mice with high hyperglycemia (Fig. S1G). Although GE treatment was able to improve body weight growth (P < 0.05; Fig. S1H), it was not able to augment blood glucose recovery or increase insulin production during fasting IPGTT or increase β-cell surface (Fig. S1 I–K). These results indicate that GE treatment can augment β-cell regeneration only in mice with medium hyperglycemia.
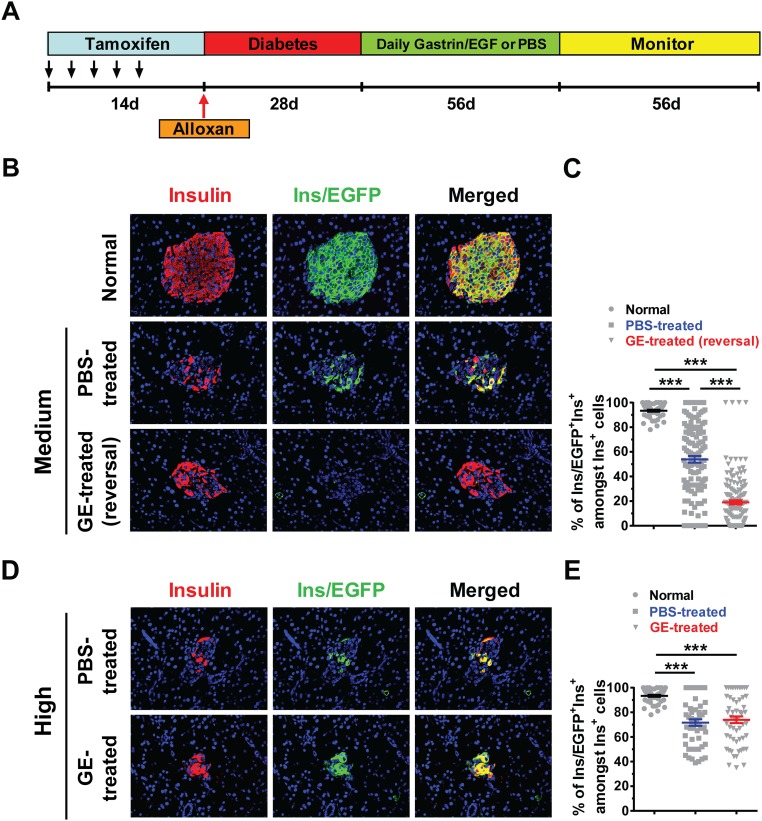
Next, we used lineage tracing to find out whether there is neogenesis of β cells in the GE-treated mice with reversal of diabetes. Accordingly, C57BL/6 mice with Ins1CreERT R26mT/mG transgene were treated with Tamoxifen (TM) to label the preexisting insulin-producing β cells. Thereafter, the mice were induced to develop diabetes with Alloxan and then treated with GE and monitored for blood glucose as described in the diagram in Fig. S2A. In nondiabetic mice, TM treatment labeled >90% of the preexisting β cells in the islets with enhanced green fluorescent protein (EGFP) (Fig. S2 B, Top, and C), but only ∼50% of the Insulin+ islet remnants in the pancreas of PBS-treated diabetic mice with medium hyperglycemia were labeled with EGFP—that is, EGFP+Insulin+ (P < 0.001; Fig. S2 B, Middle, and C). The EGFP+Insulin+ islets and clusters in the GE-treated mice with reversal of diabetes were further reduced to ∼20% (Fig. S2 B, Bottom, and C), which is markedly different from PBS-treated mice (P < 0.001; Fig. S2 B and C). In addition, the Insulin+ clusters in the diabetic mice with high hyperglycemia after GE or PBS treatment were both ∼70% EGFP+, which was still significantly lower than that of control mice with normal glycemia (P < 0.001; Fig. S2 D and E); even GE treatment did not change the percentage compared with PBS treatment (Fig. S2E). These results indicate that hyperglycemia can induce β-cell neogenesis and that medium hyperglycemia combined with administration of low-dose GE can markedly augment the process.
Fig. S2.
Long-term administration of low-dose GE augments both β-cell neogenesis and replication in diabetic mice with medium, but not high, hyperglycemia. The 8-wk-old female Ins1CreERTR26mT/mG mice were first given i.p. injection of TM every 2 d over a 2-wk period to induce EGFP expression and label the preexisting β cells. Thereafter, mice were induced to develop diabetes with Alloxan, and mice with medium or high hyperglycemia were treated with GE or PBS control. At the end of the experiments, the pancreases were harvested and stained for Insulin and EGFP, and merged staining was also shown. Nuclei were labeled by DAPI. The percentage of EGFP+Ins+ cells among total Insulin+ cells was calculated. Normal nondiabetic mice were used as a control for checking EGFP labeling efficiency. (A) Experimental scheme. (B) The islets in mice with normal or medium hyperglycemia after PBS or GE treatment were stained for Insulin (red), EGFP (green), or merged colors. (C) The percentage of Ins/EGFP+Ins+ cells among Insulin+ cells in islets of mice in C is shown (mean ± SEM, n = 4). (D) Representative staining pattern of islets in mice with high hyperglycemia after PBS or GE treatment. (E) The percentage of Ins/EGFP+Ins+ cells among Insulin+ cells in the islets of mice in D is shown (mean ± SEM, n = 4). ***P < 0.001. (Original magnification: B, D, 400×.)
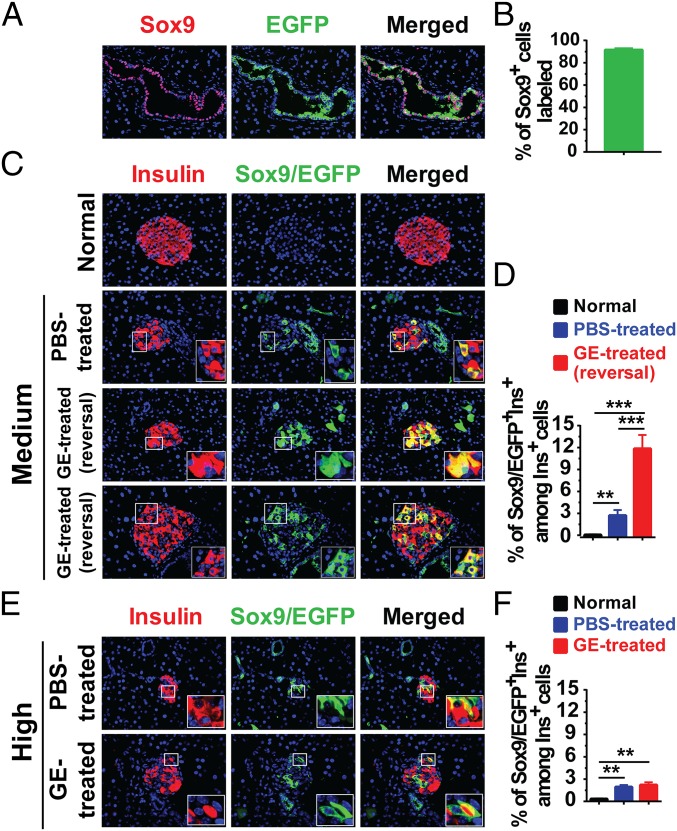
Next, we used Sox9CreERT2R26mT/mG mice to determine whether newly generated β cells in the GE-treated diabetic mice originate from Sox9+ cells in the pancreatic ducts. The mice were induced to develop diabetes and then treated with GE and monitored for blood glucose as described above (Fig. S2A). TM treatment was able to label >90% of pancreatic Sox9-expression ductal epithelial cells with EGFP in Sox9CreERT2R26mT/mG C57BL/6 mice (Fig. 1 A and B). However, most of islets in the normal control did not have any Sox9/EGFP+Insulin+ cells (Fig. 1C, first row). The percentage of Sox9/EGFP+Insulin+ cells among total Insulin+ β cells in normal control mice was <0.4% (Fig. 1D). There were scattered Sox9/EGFP+ cells among Insulin+ islet remnants or Insulin+ cell clusters in medium diabetic mice treated with PBS (Fig. 1C, second row). The percentage of Sox9/EGFP+Insulin+ cells among total Insulin+ cells was ∼3%, and it was a significant increase compared with normal glycemia control mice (P < 0.01; Fig. 1D).
Fig. 1.
Long-term administration of low-dose GE augments Sox9+ ductal cell differentiation into β cells in diabetic mice with medium hyperglycemia. The 8-wk-old female Sox9CreERT2 R26mT/mG mice were first injected with TM to label the Sox9/EGFP+ cells, and then the mice were induced to develop diabetes and treated with PBS or GE and monitored as described in Fig. S2A. At the end of experiments, pancreases were stained for Insulin and EGFP, and merged staining was also shown. (A) Representative pattern of EGFP labeling Sox9+ pancreatic ductal cells in the normal glycemia control mice. (B) Quantification of Sox9+EGFP+ cells relative to the total number of Sox9+ cells in the duct in A is shown (mean ± SEM, n = 4). (C) Representative staining pattern of islets in mice with normal or medium hyperglycemia after PBS or GE treatment. (D) The percentage of Sox9/EGFP+Ins+ cells among total Insulin+ cells in islets of mice in C is shown (mean ± SEM, n = 4). (E) Representative staining pattern of islets in mice with high hyperglycemia after PBS or GE treatment. (F) The percentage of Sox9/EGFP+Ins+ cells among total Insulin+ cells in islets of mice in E is shown (mean ± SEM, n = 4). **P < 0.01; ***P < 0.001. (Original magnification: A, C, E, 400×.)
In contrast, there were two types of islets or clusters in the GE-treated mice with reversal of diabetes (Fig. 1C, bottom two rows): One type is all Sox9/EGFP+Insulin+ cells; the other is a mixture of Sox9/EGFP+Insulin+ and Sox9/EGFP−Insulin+ cells. The percentage of Sox9/EGFP+ cells among total Insulin+ cells increased by more than fourfold in the GE-treated mice compared with PBS-treated control mice (P < 0.001; Fig. 1 C and D). In addition, GE treatment did not significantly increase the percentage of Sox9/EGFP+ cells among total Insulin+ cells in diabetic mice with high hyperglycemia when compared with PBS-treated control mice (Fig. 1 E and F). Collectively, these results indicate that hyperglycemia can induce differentiation of Sox9/EGFP+ pancreatic ductal cells into insulin-producing cells; GE treatment can augment this differentiation in diabetic mice with medium hyperglycemia, but not in the mice with high hyperglycemia.
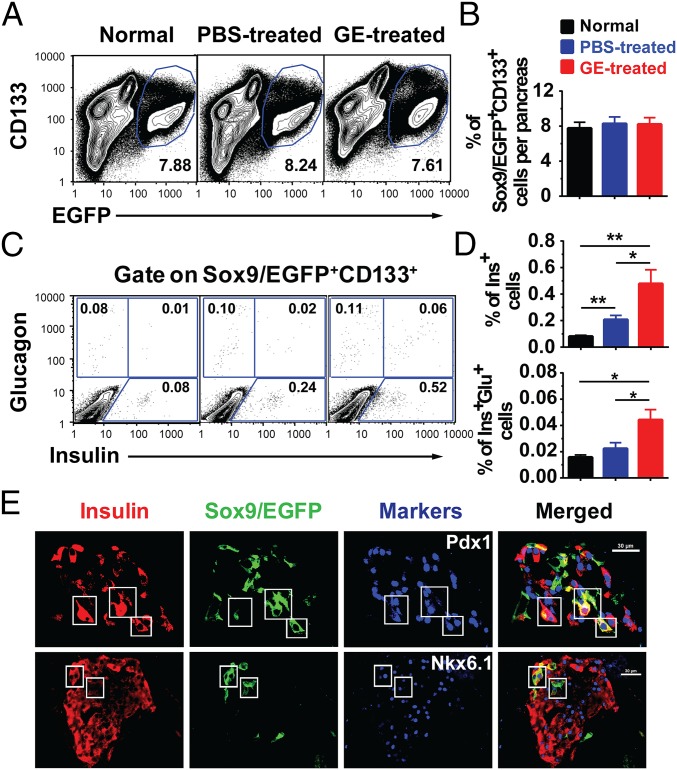
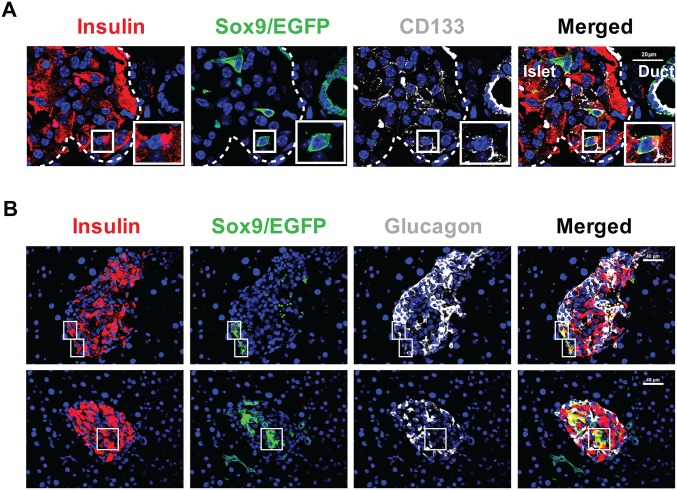
A previous report showed that Sox9–GFP+CD133+, but not Sox9–GFP+CD133−, cells contain cells that gave rise to insulin-producing colonies in an in vitro culture (23); therefore, we tested whether GE treatment increased the percentage of Sox9/EGFP+CD133+ ductal cells using flow cytometry analysis. We found that GE treatment did not expand the Sox9/EGFP+CD133+ cell population, because the percentage of Sox9/EGFP+CD133+ cells among total pancreatic cells was similar to control mice (Fig. 2 A and B). However, GE treatment significantly increased the percentage of Insulin+ cells among Sox9/EGFP+CD133+ cells, which appears to have lower levels of insulin expression (insulinlo) compared with PBS-treated or normal controls (Fig. 2 C and D). The Sox9/EGFP+CD133+Ins+ cells were also identified by histoimmunofluorescent staining (Fig. S3A). These results suggest that Sox9/EGFP+CD133+Ins+ cells may be newly generated β cells or Insulin+ progenitors as described (28).
Fig. 2.
Long-term administration of low-dose GE increases Insulinlo cells among Sox9/EGFP+CD133+ cells, although it does not increase the percentage of Sox9/EGFP+CD133+ in diabetic mice with medium hyperglycemia. After treated by TM and Alloxan as described in Fig. S2A, the pancreatic tissues from normal and diabetic Sox9CreERT2 R26mT/mG mice with medium hyperglycemia after 56-d treatment with PBS or GE were dissociated into single cells. Sox9/EGFP+CD133+, Insulin+, and Insulin+Glucagon+ cell populations were analyzed by flow cytometry. (A and B) The percentage of Sox9/EGFP+CD133+ among total pancreatic mononuclear cells is shown (mean ± SEM, n = 4). (C and D) After gating on Sox9/EGFP+CD133+ cell population, the percentage of Insulin+ and Insulin+Glucagon+ cells is shown. (E) Pancreatic tissues were stained for Insulin, EGFP, and Pdx1 or NKX6.1. One representative staining pattern of islets in mice is shown from 4 replicate experiments. Triple-positive cells are shown in the box. (Scale bars: 30 µm.) *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. S3.
Long-term administration of low-dose GE induces the presence of Sox9/EGFP+CD133+Ins+ or Sox9/EGFP+Ins+Glu+ triple-positive cells in the islets of diabetic mice with medium hyperglycemia. After treated by TM and Alloxan as described in Fig. S2A, the pancreatic tissues from diabetic Sox9CreERT2R26mT/mG mice with medium hyperglycemia after 56-d treatment with GE were stained for Insulin, EGFP, and CD133 or Glucagon. (A) Cell costained with Sox9/EGFP+CD133+Ins+ in islet is showed in the box. (Scale bar: 20 µm.) (B) Cells costained with Sox9/EGFP+Ins+Glu+ in immature islet are showed in the box in Upper. Cells costained with Sox9/EGFP+Ins+Glu− in mature islet are showed in the box in Lower. (Scale bars: 40 µm.)
β cells express pancreatic and duodenal homeobox 1 (Pdx1) and NK6 homeobox 1 (Nkx6.1) (12). Consistently, the Sox9/EGFP+Ins+ cells in the GE-treated recipients also expressed Pdx1 and Nkx6.1 (Fig. 2E). In addition, although the percentage of Insulin+Glucagon+ cells among Sox9/EGFP+CD133+ cells was very low, there was a significant difference between GE- and PBS-treated recipients (P < 0.05; Fig. 2 C and D). Sox9/EGFP+Ins+Glu+ cells were also clearly observed in the islets of GE-treated mice as judged with histoimmunofluorescent staining (Fig. S3B). Collectively, these results suggest that GE treatment can augment differentiation of Sox9+CD133+ cells into insulin-producing β cells.
Medium Hyperglycemia Is Required for Effective Augmentation of Sox9+ Ductal Cells Differentiation into Insulin-Producing β Cells During GE Treatment.
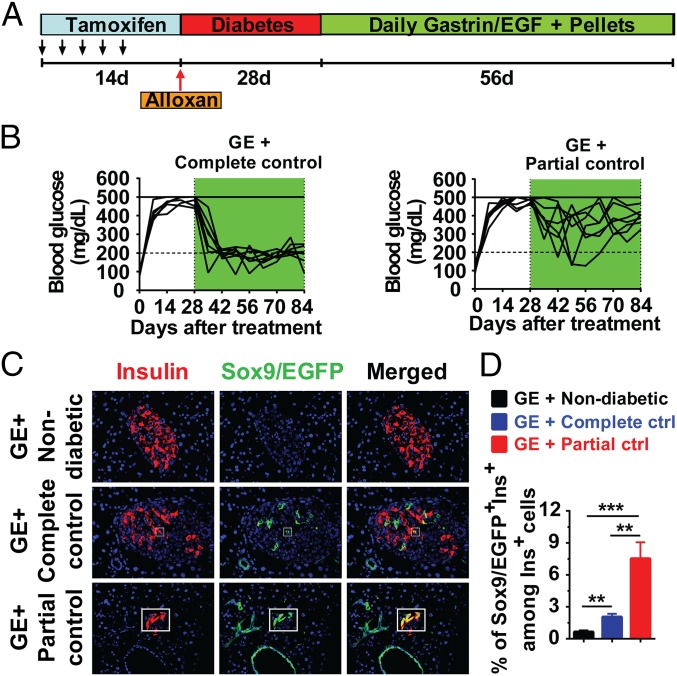
Because significant β-cell neogenesis from Sox9+ ductal cells was observed in GE-treated diabetic mice with medium, but not high, hyperglycemia (Fig. 1), we tested whether medium hyperglycemia is required for augmenting β-cell neogenesis from Sox9+ cells during GE treatment. Accordingly, we compared the effect of GE treatment in nondiabetic normal control and diabetic mice with high hyperglycemia under partial or complete normalization of hyperglycemia by implanting insulin pellets (Fig. 3A). GE treatment hardly induced Sox9/EGFP+Ins+ cells in the islets of nondiabetic normal control mice (Fig. 3 C and D). Compared with complete control of blood glucose, partial normalization of blood glucose markedly increased β cells differentiated from Sox9/EGFP+ cells in diabetic recipients after GE treatment (P < 0.01; Fig. 3 B–D). These results indicate that medium hyperglycemia is required for effective augmentation of Sox9+ ductal cells differentiation into insulin-producing β cells during GE treatment.
Fig. 3.
Medium hyperglycemia is required for inducing Sox9+ ductal cells differentiation into β cells. After treated by TM and Alloxan as described in Fig. S2A, Sox9CreERT2 R26mT/mG mice with high hyperglycemia were chosen for long-term administration of low-dose GE. During GE treatment, insulin pellets were implanted to completely control hyperglycemia to normal level (<200 mg/dL) or partially control to medium level (200–450 mg/dL). At the end of GE treatment, pancreatic tissues were stained for Insulin and EGFP, and merged staining was also shown. (A) Experimental scheme. (B) Blood glucose levels from GE + complete control and GE + partial control groups (n = 6). (C) Representative staining pattern of islets in mice from GE + nondiabetic, GE + complete control, and GE + partial control groups. (Original magnification: 400×.) (D) Quantification of Sox9/EGFP+Ins+ relative to the total number of Insulin+ cells in islets of mice in C is shown (mean ± SEM, n = 4). **P < 0.01; ***P < 0.001.
Short-Term Administration of High-Dose GE Does Not Augment β-Cell Neogenesis from Sox9+ Ductal Cells in Mice with Medium Hyperglycemia.
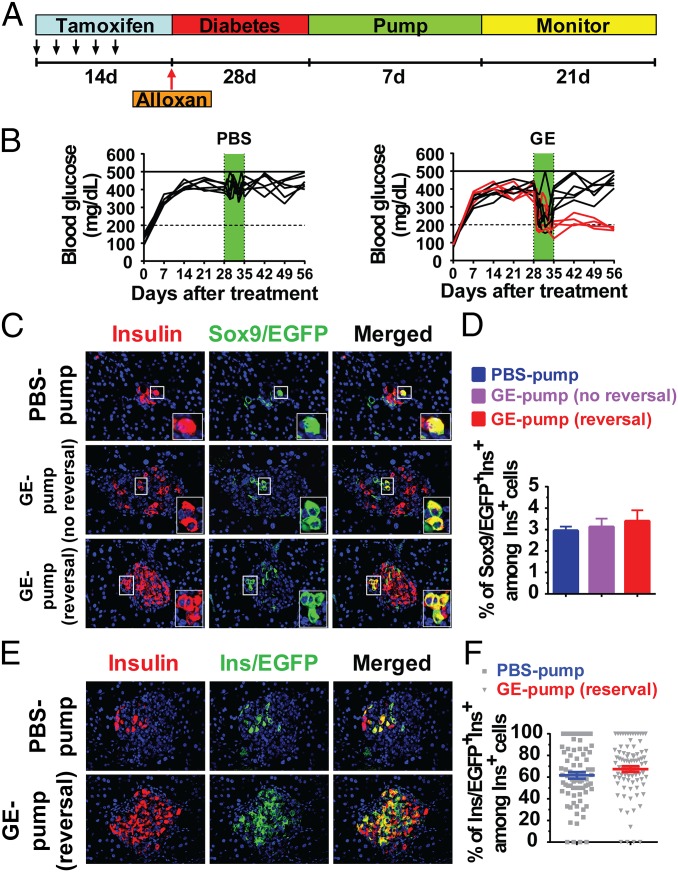
Short-term (1 wk) administration of high-dose GE has been reported to reverse Alloxan-induced diabetes in adult mice, although it was not able to induce Hnf1β+ ductal cell differentiation into β cells (14). Thus, we revisited this issue in diabetic Sox9CreERT2R26mT/mG mice. Similar to the previous reports (14, 27) and in the diagram in Fig. 4A, GE was administered by implanting a pump containing gastrin (release rate: 3 µg per kg of body weight per hour) and EGF (release rate: 10 µg per kg of body weight per hour) for release over 7 d, and then the blood glucose was monitored for 3 wk. We found that 40% (4/10) of GE-treated mice with medium hyperglycemia showed reversal of hyperglycemia, but none in PBS-treated control group (P < 0.01; Fig. 4B). Although there was a small percentage (∼3%) of Sox9/EGFP+Ins+ cells, GE treatment did not significantly increase the percentage between GE-treated mice with or without reversal (Fig. 4 C and D).
Fig. 4.
Short-term administration of high-dose GE does not augment β-cell neogenesis from Sox9+ ductal cells in diabetic mice with medium hyperglycemia. After treated by TM and Alloxan as described in Fig. S2A, Sox9CreERT2R26mT/mG mice with medium hyperglycemia were chosen for short-term administration of high-dose GE. Alzet osmotic mimipumps were i.p. implanted for 7 d, followed by 21 d of monitoring. The pumps contained gastrin (release rate: 3 μg per kg of body weight per hour) and EGF (release rate: 10 μg per kg of body weight per hour) and released their content for ∼7 d. At the ending of high-dose GE treatment, pancreatic tissues were stained for Insulin and EGFP, and merged staining was also shown. (A) Experimental scheme. (B) Blood glucose levels from PBS pump (n = 6) and GE pump (n = 10) groups. (C) Representative staining pattern of islets in mice from PBS and GE pump groups. (D) Quantification of Sox9/EGFP+Ins+ cells relative to the total number of Insulin+ cells in islets of mice in C is shown (mean ± SEM, n = 4). (E) To determine short-term administration of high-dose GE-induced β-cell replication or not, Ins1CreERTR26mT/mG mice with medium hyperglycemia were treated with a PBS or GE pump as described in A. Representative staining pattern of islets in mice from PBS and GE pump groups is shown. (F) Quantification of Ins/EGFP+Ins+ cells relative to the total number of Insulin+ cells in the islets of mice in E is shown (mean ± SEM, n = 4). (Original magnification: C, E, 400×.)
Furthermore, we found that, using Ins1CreERTR26mT/mG mice, the reversal of diabetes after short-term administration of high-dose GE was associated with replication of preexisting β cells, because the percentage of Ins/EGFP+ preexisting β cells among Insulin+ cells of islets in the short-term GE-treated mice was similar to that in the PBS-treated mice (Fig. 4 E and F). Collectively, although short-term administration of GE can augment preexisting β-cell replication, it cannot augment Sox9+ ductal cell differentiation into insulin-producing β cells, and long-term administration of GE is required for augmenting the Sox9+ ductal cell differentiation.
Long-Term Administration of Low-Dose GE Augments Differentiation of Sox9+ Cells in the Pancreatic Ducts into Insulin-Producing β Cells.
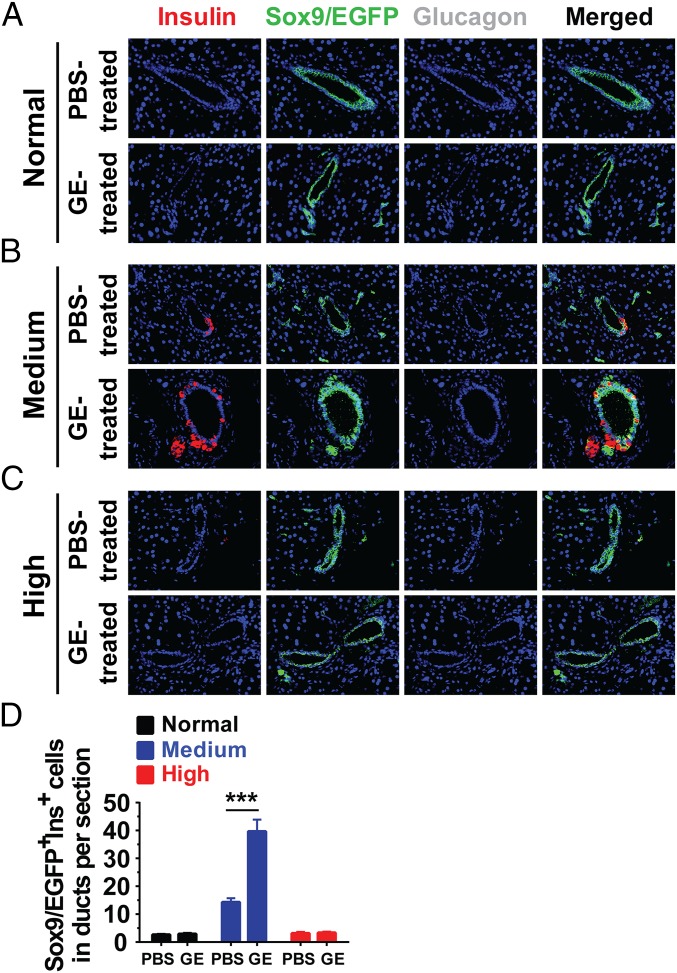
We observed that there were few Sox9/EGFP+Ins+ cells among pancreatic ductal epithelial cells in the normal nondiabetic adult mice, as judged by anti-Insulin immunofluorescent staining; there were fewer than three cells per section, and GE treatment did not show any significant increase (Fig. 5 A and D). However, compared with normal nondiabetic adult mice, medium hyperglycemia alone significantly increased the Sox9/EGFP+Ins+ cells among ductal epithelial cells, which reached ∼15 cells per section (P < 0.01), although high hyperglycemia alone did not increase the number, compared with nondiabetic normal control (Fig. 5 B–D). GE treatment significantly increased Sox9/EGFP+Ins+ cells in medium hyperglycemic mice, which reached ∼40 cells per section (P < 0.001), but GE treatment did not increase the number at all in high hyperglycemic mice (Fig. 5 B–D). Taken together, some Sox9+ cells in the pancreatic ducts could become Insulin+ cells before budding off to form islets, and medium hyperglycemia and GE treatment increase the frequencies of Sox9+Ins+ cells among ductal epithelial cells and augment their differentiation into insulin-producing islet β cells.
Fig. 5.
Long-term administration of low-dose GE increases Sox9/EGFP+Insulin+ cells on the wall of pancreatic ducts. Sox9CreERT2R26mT/mG mice were induced to label pancreatic ductal epithelial cells with TM and then induced to diabetes with Alloxan. Normal nondiabetic mice and diabetic mice with medium or high hyperglycemia were treated with control PBS or GE for 8 wk, followed by 8 wk of monitoring as described in Fig. S2A. At the end of the experiments, pancreatic tissues were stained for Insulin, EGFP, and Glucagon, and merged staining was also shown. (A–C) Representative staining pattern of pancreatic ducts were shown from mice with normal glycemia, medium hyperglycemia, and high hyperglycemia after long-term administration of PBS or low-dose GE. (Original magnification: 400×.) (D) Number of Sox9/EGFP+Ins+ cells on the wall of pancreatic ducts per tissue section in A–C is shown (mean ± SEM, n = 4). ***P < 0.001.
Discussion
Using lineage tracing, we have demonstrated that long-term administration of low-dose GE is able to augment differentiation of pancreatic Sox9+ ductal cells into insulin-producing β cells in nonautoimmune diabetic mice with medium hyperglycemia. We have also shown that, although hyperglycemia is required for initiating the differentiation of Sox9+ ductal cells into insulin-producing β cells, medium hyperglycemia combined with long-term, but not short-term, administration of GE is required for an effective differentiation and reversal of diabetes.
Previous reports showed that, during the embryonic development period, the pancreatic Sox9+ ductal cells differentiated into exocrine acinar cells and endocrine cells, including insulin-producing β cells (14–17); however, whether or not ductal cells can differentiate into β cells in neonates or in adult mice after pancreas injury remains controversial (5, 14, 16, 17), and the cause of controversy remains unclear (29). We find that combination of medium hyperglycemia and long-term administration of GE is able to induce obvious differentiation of Sox9+ ductal cells into insulin-producing β cells, as indicated by the coexistence of Sox9+Ins+Glu+ and Sox9+Ins+Glu− cells in the islets (Fig. S3B). This condition appears to be strict, because a combination of high hyperglycemia and long-term administration of GE did not work. This result may be due to the glucose toxicity to newly generated β cells, as previously reported (19, 20). The combination of medium hyperglycemia and short-term administration of GE did not effectively augment Sox9+ ductal cell differentiation either, although the treatment effectively augmented the replication of preexisting β cells. This result may be due to the fact that the maturation of β cells derived from Sox9+ ductal cells is slower than replication of mature β cells, as indicated by previous publications (13). This observation may provide an explanation for previous publications showing that Sox9+ ductal cells and other pancreatic ductal cells did not differentiate into β cells in Alloxan-induced diabetic mice after 1 wk of treatment with GE (14); this finding also provides an explanation for the lack of Sox9+ ductal-derived β cells in STZ-induced diabetic mice with high hyperglycemia (∼500 mg/dL) and in the absence of administration of growth factors (16, 18). Therefore, we agree with a recent review by Lysy et al. that neogenesis from ducts is influenced by the type and extent of pancreatic injury as well as dependent on the affected cell types (30, 31).
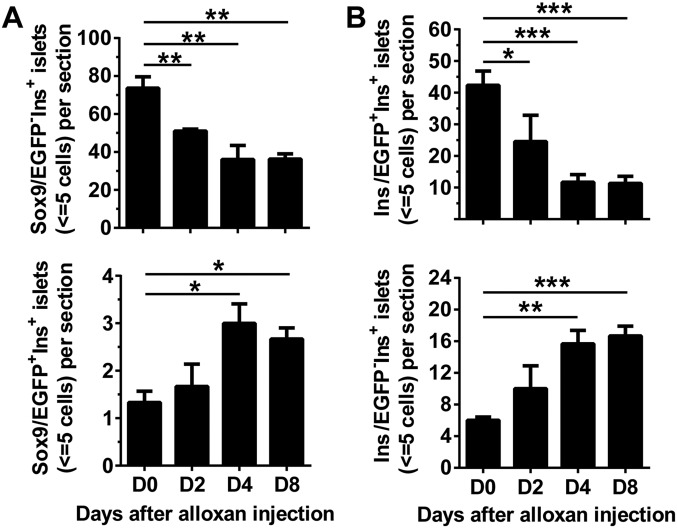
Hybrid Sox9+ periportal hepatocytes were recently reported to have high regenerative capacity (32). Sox9+Ins+ preexisting β cells may also be a type of hybrid cells that can have high regenerative capacity and contribute to β-cell regeneration in the GE-treated diabetic mice. However, the contribution may be moderate, based on our preliminary studies (Fig. S4).
Fig. S4.
Kinetic analysis of small islets (≤5 cells) in Sox9CreERT2R26mT/mG and Ins1CreERTR26mT/mG mice early after induction of diabetes. TM-treated Sox9CreERT2R26mT/mG and Ins1CreERTR26mT/mG mice were given injection of Alloxan as described above (Fig. S2A). The mice with hyperglycemia (blood glucose > 400 mg/dL) on days 2, 4, and 8 after Alloxan injection were used for kinetic analysis of small islets (≤5 cells). Mice before injection (day 0) were used as control. The pancreatic tissues were stained for Insulin and EGFP. The numbers of small islets (≤5 cells) per section was calculated. (A) Sox9/EGFP−Ins+ and Sox9/EGFP+Ins+ small islets per section in Sox9CreERT2R26mT/mG mice (mean ± SEM, n = 4). (B) Ins/EGFP+Ins+ and Ins/EGFP−Ins+ small islets in Ins1CreERTR26mT/mG mice (mean ± SEM, n = 4). *P < 0.05; **P < 0.01; ***P < 0.001.
Hyperglycemia induces mature β cells to replicate (33). Chronic hyperglycemia is also toxic to β cells and causes their dysfunction and dedifferentiation (19, 20). However, the impact of hyperglycemia on β-cell neogenesis remains unclear. We observed that hyperglycemia alone was able to increase the numbers of small islets from neogenesis, indicating that hyperglycemia may induce differentiation of Sox9+ ductal cells or expansion of Sox9+Ins+ ductal epithelial cells. However, pancreatic epithelial cells do not usually express Glut2/Glucokinase machinery for glucose recognition (34), so whether or not the pancreatic ductal epithelial cells can use Glut 10 needs to be tested in the future studies. Medium hyperglycemia, but not high hyperglycemia, in combination with long-term administration of GE was able to effectively augment the differentiation of Sox9+ ductal epithelial cells into β cells. EGF, in combination with other factors, has been reported to activate the PI3K signaling pathway, which augments differentiation of nonendocrine pancreatic cells into insulin-producing cells in vitro (35). However, the pathways involved in the synergistic effect of GE with hyperglycemia remain unclear and need to be addressed in the future studies.
We observed that there were hardly any Sox9+Ins+ cells among ductal epithelial cells in normal control mice. Medium hyperglycemia induced the presence of Sox9/EGFP+Ins+ cells among ductal epithelial cells, and GE treatment increased the frequencies of the Sox9/EGFP+Ins+ cells. A recent study reported that Sox9+CD133+ cells contained β-cell progenitors (23), and multipotent progenitors in the pancreas of mice and human could be Insulin+ (28). Whether or not these Sox9+Ins+ ductal cells are progenitors or “hybrid” cells mentioned above remains unclear, and it is under investigation.
The role of Sox9+ ductal cells in the neogenesis of β cells in mixed chimeric NOD mice remains unclear. Although long-term administration of low-dose GE-augmented β-cell neogenesis in both nonautoimmune C57BL/6 mice and in chimeric late-stage autoimmune NOD mice, the source of neogenesis may still be different. Sox9+ ductal cells gave rise to β cells in nonautoimmune mice with medium hyperglycemia, but they may not give rise to β cells in autoimmune mice. If the Sox9+ “progenitors” or “hybrids” are Insulin+, those Insulin+ cells may also be damaged by antiinsulin antibodies and insulin-reactive T cells. In addition, it was reported that there was no neogenesis, but rather preexisting β cell replication in diabetic Insulin–Cre–DTR mice (33). It was also reported that there was β-cell neogenesis from α cell transdifferentiation, but not from ductal progenitors in Insulin–Cre–DTR mice after severe β-cell depletion and >6 mo incubation time (10). It is unclear yet why ductal epithelial cells did not give rise to β cells in diabetic Insulin–Cre–DTR mice; this outcome may result from diphtheria toxin (DT) elimination of Insulin+ progenitors or hybrids that are required for neogenesis.
In summary, we have demonstrated that pancreatic Sox9+ ductal cells in adult mice can differentiate into insulin-producing β cells and contribute to reversal of diabetes in nonautoimmune mice in the presence of medium hyperglycemia and long-term administration of GE, although Sox9+ ductal cells may only be one of the sources for β-cell neogenesis in the diabetic mice. Our studies also indicate that β-cell neogenesis from Sox9+ ductal cells is a slow process and requires long-term growth factor therapy; additionally, adjustment of hyperglycemia to a medium level is also critical for the optimal therapeutic effect. This information may be useful for improving design of the clinical trial of combination therapy with dipeptidyl peptidase-4 inhibitors and proton-pump inhibitors that would increase Glucagon-like peptide-1 and gastrin concentration in new-onset type 1 diabetic patients, as described in a recent report (36).
Materials and Methods
Wild-type C57BL/6, breeders of Ins1CreERT mice were purchased from The Jackson Laboratory. Breeders of Sox9CreERT2 mice were described (17, 18) and provided by M. Sander’s laboratory at the University of California, San Diego. ROSA26mT/mG breeders were provided by the C.-C.C. laboratory at the City of Hope (COH). All mice were maintained in a pathogen-free room in City of Hope Animal Resources Center. The experimental procedures were approved by COH Institutional Animal Care and Use Committee. TM, human recombinant EGF, and human [Leu15]-gastrin I were purchased from Sigma. Histology and immunofluorescence staining, morphometric analysis and cell counting, IPGTT, pancreas isolation, intracellular staining and flow cytometry, and statistical analysis were described in SI Materials and Methods.
SI Materials and Methods
Mice.
Wild-type C57BL/6, breeders of Ins1CreERT mice were purchased from The Jackson Laboratory. Breeders of Sox9CreERT2 mice were described (17) and provided by M. Sander’s laboratory at the University of California, San Diego. For the present study, we used a founder that induces recombination in cells with high SOX9 expression, and higher recombination efficiency than the founder described (17, 18). ROSA26mT/mG breeders were provided by the C.-C.C. laboratory at the COH. All mice were maintained in a pathogen-free room in the COH Animal Resources Center. The experimental procedures were approved by the COH Institutional Animal Care and Use Committee.
Induction of Diabetes by Alloxan and in Vivo Activation of Reporter Genes by TM.
The 8-wk-old female mice were i.v.-injected with β-cell toxin Alloxan (Sigma) at 70 mg per kg of body weight. Tail vein blood glucose was measured daily or twice a week with Precision Xtra Glucose Meter (Abbott Diabetes Care Inc.) with a maximum reading of 500 mg/dL. TM (Sigma) was prepared at a concentration of 20 mg/mL in corn oil (Sigma). For tracing studies in adult mice, a total of 20 mg of TM was given i.v. in five doses (each 4 mg) over a 2-wk period. Mice rendered diabetic (>300 mg/dL) over a period of 28 d were used in subsequent experiments.
Preparation and Injection of Growth Factors.
For low-dose growth factor injection, human recombinant EGF (Sigma) was dissolved in sterile 10 mmol/L acetic acid solution at a stock concentration of 3 μg/mL. Human [Leu15]-gastrin I (Sigma) was dissolved in PBS to a stock concentration of 3 μg/mL The stocks were stored in aliquots at −80 °C. The stocks were diluted in sterile PBS (pH 7.4) to a working concentration of EGF (1 μg per kg of body weight) and gastrin (3 μg per kg of body weight). PBS vehicle (control) or GE were i.p. administered to the mice daily for 56 d.
For high-dose growth factor treatment, human recombinant EGF was dissolved in sterile 10 mmol/L acetic acid solution at a stock concentration of 1 mg/mL. Human [Leu15]-gastrin I was dissolved in PBS to a stock concentration of 0.5 mg/mL The mixture was injected into miniosmotic pumps (Alzet 1007D) to obtain a flux rate of 3 μg per kg of body weight per hour for gastrin and 10 μg per kg of body weight per hour for EGF. Pumps with growth factors or vehicle composed of 5 mmol/L acetic acid solution were implanted i.p. at day 28 after Alloxan injection. These pumps release their content for ∼7 d.
Histology and Immunofluorescence Staining.
Samples for immunofluorescence analysis were fixed overnight in 10% (wt/vol) formalin or 4% (wt/vol) paraformaldehyde (PFA), embedded in paraffin, and cut to 4- to 5-μm tissue sections. Antigen retrieval in paraffin sections was heat-mediated with Antigen Unmasking Solution, Citric Acid Based (Vector Labs). Primary antibodies include guinea pig anti-insulin (1:4,000; DAKO), mouse anti-glucagon (1:1,000; Sigma), goat anti-GFP conjugated fluorescein isothiocyanate (FITC) (1:100; Abcam), rabbit anti-Sox9 (1:1,000; Millipore), rabbit anti-Pdx1 (1:1,000; Abcam), rabbit anti-Nkx6.1 (1:100; Abcam), and rat anti-CD133 (1:100; eBioscience). Secondary antibodies for indirect fluorescent staining conjugated to aminomethylcoumarin (AMCA), Cy3, or Alexa Fluor 647 were obtained from Jackson ImmunoResearch Labs. The antibodies conjugated to AMCA were used at a concentration of 1:100, and the antibodies conjugated to Cy3 or Alexa Fluor 647 were used at a concentration of 1:1,000. Nuclei were labeled by 4′,6-diamidino-2-phenylindole (DAPI) (1 μg/mL; Sigma). Tissue sections were viewed on an Olympus IX81 Automated Inverted Microscope. Images were captured and processed by using Image-Pro Premier.
Morphometric Analysis and Cell Counting.
For morphometric analyses, the entire adult pancreas was kept flat and totally sectioned horizontally. Thinner sections were taken at 20-µm intervals throughout the organ. Four sections from different levels (∼2% of the pancreas) in one adult mouse were analyzed.
Percentage of EGFP+Sox9+ cells was determined by the number of EGFP+Sox9+ cells divided by the total number of Sox9+ cells. Cells were counted in 10 random fields of view of a tissue section. To quantify the number of lineage-labeled Insulin+ cells, all Insulin+ cells and Ins/EGFP+Ins+ cells on a section were counted, and then the percentage of Ins/EGFP+Ins+ cells among total Insulin+ cells was determined. To quantify the number of lineage-labeled Insulin+ cells arising from Sox9+ cells, all Insulin+ cells and Sox9/EGFP+Ins+ cells on a section were counted, and then the percentage of Sox9/EGFP+Ins+ cells among total Insulin+ cells was determined. To quantify Sox9/EGFP+Ins+ cells among ductal tubes, all Sox9/EGFP+ ductal cells coexpressing Insulin in ducts were counted on a section. For β-cell surface measurements, four sections per mouse were selected for histochemical staining of Insulin. The Insulin+ area of the entire section was captured by using Hamamatsu Nanozoomer 2.0 HT. The percentage of Insulin+ area was determined by Insulin+ area divided by total pancreatic tissue area in a slide, using Image-Pro Premier software. In all of the morphometric analysis and cell counting, four sections from different levels with 20-µm intervals in each mouse were counted. The average count of each mouse was calculated. Mean ± SEM from at least four mice in each group is presented.
IPGTT.
Mice were fasted for 16 h and injected i.p. with glucose (2 g per kg of body weight). Blood glucose concentration was measured from tail vein blood with Glucose Meter. Insulin concentration in plasma from time points at 0 and 10 min after glucose injection was determined with the Mouse Insulin ELISA kit (Mercodia).
Pancreas Isolation, Intracellular Staining, and Flow Cytometry.
The dissected pancreas was digested and dissociated to single cells with collagenase B (2–4 mg/mL; Roche) and DNase I (2,000 U/mL per pancreas; Roche), refer to Jin et al. (23). For flow-cytometry analysis, the cell suspension was first incubated with anti-mouse CD16/32 (1:25; eBioscience) and Live/Dead (1:1,000; Life Technology) for 5 min on ice to diminish nonspecific binding. APC-conjugated anti-mouse CD133 (1:100; eBioscience) was added, and the cells were incubated on ice for 20 min. After washing twice, cells were immediately fixed and permeabilized (4% PFA, 0.1% saponin/PBS, 30 min) on ice. After fixation, intracytoplasmic staining was performed with rabbit anti-insulin conjugated Alexa Fluor647 (1:50; Bioss), goat anti-GFP conjugated FITC (1:100; Abcam), and mouse monoclonal IgG1 anti-glucagon (Sigma). Simultaneous staining using mouse IgG1 antibodies required Invitrogen’s Zenon prelabeling technology (Pacific Blue). After the final wash in 0.1% saponin/PBS, cells were postfixed in 2% (wt/vol) PFA and immediately analyzed with flow cytometry, a CyAn immunocytometry system (Dako Cytomation), and the data were analyzed with FlowJo software (TreeStar) as described (4).
Statistical Analysis.
All quantitative data are shown as mean ± SEM. All data were analyzed by using GraphPad software (Prism Version 6.0; GraphPad Software). Comparison of kinetic blood glucose change was evaluated with a two-way ANOVA test. Comparison of means among multiple groups was evaluated with a one-way ANOVA test. Comparison of two means was performed with an unpaired two-tailed Student t test.
Acknowledgments
We thank Drs. Maike Sander and Janel L. Kopp (Department of Pediatrics and Cellular and Molecular Medicine, University of California, San Diego) for providing Sox9CreERT2 mice and critical review of the manuscript; Drs. Hsun Teresa Ku and Hung-Ping Shih at the City of Hope (COH) for critical review of the manuscript; Brian Armstrong and his staff at the COH Light Microscopy Core for providing technical assistance; Lucy Brown and her staff at the COH Flow Cytometry Facility; Dr. Richard Ermel and his staff at the COH Animal Resources Center; and Sofia Loera and her staff at the Anatomic Pathology Facility for providing excellent services. This work was supported by the Institutional Fund of The Beckman Research Institute of City of Hope and by private donations from Legacy Heritage Fund Limited and from Arthur and Judith Lubin.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524200113/-/DCSupplemental.
References
- 1.Castaño L, Eisenbarth GS. Type-I diabetes: A chronic autoimmune disease of human, mouse, and rat. Annu Rev Immunol. 1990;8:647–679. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- 2.Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant. 2008;8(10):1990–1997. doi: 10.1111/j.1600-6143.2008.02353.x. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AM, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 4.Wang M, et al. Mixed chimerism and growth factors augment β cell regeneration and reverse late-stage type 1 diabetes. Sci Transl Med. 2012;4(133):133ra59. doi: 10.1126/scitranslmed.3003835. [DOI] [PubMed] [Google Scholar]
- 5.Inada A, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA. 2008;105(50):19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132(2):197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Guz Y, Nasir I, Teitelman G. Regeneration of pancreatic beta cells from intra-islet precursor cells in an experimental model of diabetes. Endocrinology. 2001;142(11):4956–4968. doi: 10.1210/endo.142.11.8501. [DOI] [PubMed] [Google Scholar]
- 8.Zulewski H, et al. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50(3):521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]
- 9.Chung CH, Hao E, Piran R, Keinan E, Levine F. Pancreatic β-cell neogenesis by direct conversion from mature α-cells. Stem Cells. 2010;28(9):1630–1638. doi: 10.1002/stem.482. [DOI] [PubMed] [Google Scholar]
- 10.Thorel F, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464(7292):1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455(7213):627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan FC, et al. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development. 2013;140(4):751–764. doi: 10.1242/dev.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, et al. Long-term persistence and development of induced pancreatic beta cells generated by lineage conversion of acinar cells. Nat Biotechnol. 2014;32(12):1223–1230. doi: 10.1038/nbt.3082. [DOI] [PubMed] [Google Scholar]
- 14.Solar M, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17(6):849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Kopinke D, Murtaugh LC. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev Biol. 2010;10:38. doi: 10.1186/1471-213X-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furuyama K, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43(1):34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 17.Kopp JL, et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138(4):653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopp JL, et al. Progenitor cell domains in the developing and adult pancreas. Cell Cycle. 2011;10(12):1921–1927. doi: 10.4161/cc.10.12.16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weir GC, Aguayo-Mazzucato C, Bonner-Weir S. β-cell dedifferentiation in diabetes is important, but what is it? Islets. 2013;5(5):233–237. doi: 10.4161/isl.27494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weir GC, Bonner-Weir S. Islet β cell mass in diabetes and how it relates to function, birth, and death. Ann N Y Acad Sci. 2013;1281:92–105. doi: 10.1111/nyas.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonner-Weir S, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000;97(14):7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada T, et al. Reprogramming mouse cells with a pancreatic duct phenotype to insulin-producing β-like cells. Endocrinology. 2015;156(6):2029–2038. doi: 10.1210/en.2014-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin L, et al. Colony-forming cells in the adult mouse pancreas are expandable in Matrigel and form endocrine/acinar colonies in laminin hydrogel. Proc Natl Acad Sci USA. 2013;110(10):3907–3912. doi: 10.1073/pnas.1301889110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin L, et al. In vitro multilineage differentiation and self-renewal of single pancreatic colony-forming cells from adult C57BL/6 mice. Stem Cells Dev. 2014;23(8):899–909. doi: 10.1089/scd.2013.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suarez-Pinzon WL, Lakey JR, Brand SJ, Rabinovitch A. Combination therapy with epidermal growth factor and gastrin induces neogenesis of human islet beta-cells from pancreatic duct cells and an increase in functional beta-cell mass. J Clin Endocrinol Metab. 2005;90(6):3401–3409. doi: 10.1210/jc.2004-0761. [DOI] [PubMed] [Google Scholar]
- 26.Suarez-Pinzon WL, Yan Y, Power R, Brand SJ, Rabinovitch A. Combination therapy with epidermal growth factor and gastrin increases beta-cell mass and reverses hyperglycemia in diabetic NOD mice. Diabetes. 2005;54(9):2596–2601. doi: 10.2337/diabetes.54.9.2596. [DOI] [PubMed] [Google Scholar]
- 27.Rooman I, Bouwens L. Combined gastrin and epidermal growth factor treatment induces islet regeneration and restores normoglycaemia in C57Bl6/J mice treated with alloxan. Diabetologia. 2004;47(2):259–265. doi: 10.1007/s00125-003-1287-1. [DOI] [PubMed] [Google Scholar]
- 28.Smukler SR, et al. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell. 2011;8(3):281–293. doi: 10.1016/j.stem.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Houbracken I, Mathijs I, Bouwens L. Lineage tracing of pancreatic stem cells and beta cell regeneration. Methods Mol Biol. 2012;933:303–315. doi: 10.1007/978-1-62703-068-7_20. [DOI] [PubMed] [Google Scholar]
- 30.Lysy PA, Weir GC, Bonner-Weir S. Concise review: Pancreas regeneration: recent advances and perspectives. Stem Cells Transl Med. 2012;1(2):150–159. doi: 10.5966/sctm.2011-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Criscimanna A, et al. Duct cells contribute to regeneration of endocrine and acinar cells following pancreatic damage in adult mice. Gastroenterology. 2011;141(4):1451–1462. doi: 10.1053/j.gastro.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Font-Burgada J, et al. Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell. 2015;162(4):766–779. doi: 10.1016/j.cell.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 34.Pang K, Mukonoweshuro C, Wong GG. Beta cells arise from glucose transporter type 2 (Glut2)-expressing epithelial cells of the developing rat pancreas. Proc Natl Acad Sci USA. 1994;91(20):9559–9563. doi: 10.1073/pnas.91.20.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koblas T, Zacharovova K, Berkova Z, Girman P, Saudek F. An acidic pH and activation of phosphoinositide 3-kinase stimulate differentiation of pancreatic progenitors into insulin-producing cells. Transplant Proc. 2010;42(6):2075–2080. doi: 10.1016/j.transproceed.2010.05.087. [DOI] [PubMed] [Google Scholar]
- 36.Griffin KJ, Thompson PA, Gottschalk M, Kyllo JH, Rabinovitch A. Combination therapy with sitagliptin and lansoprazole in patients with recent-onset type 1 diabetes (REPAIR-T1D): 12-month results of a multicentre, randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2014;2(9):710–718. doi: 10.1016/S2213-8587(14)70115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]