Abstract
In contrast to our understanding of testicular differentiation, ovarian differentiation is less well understood in vertebrates. In mammals, R-spondin1 (Rspo1), an activator of Wnt/β-catenin signaling pathway, is located upstream of the female sex determination pathway. However, the functions of Rspo1 in ovarian differentiation remain unclear in non-mammalian species. In order to elucidate the detailed functions of Rspo/Wnt signaling pathway in fish sex determination/differentiation, the ectopic expression of the Rspo1 gene was performed in XY medaka (Oryzias latipes). The results obtained demonstrated that the gain of Rspo1 function induced femininity in XY fish. The overexpression of Rspo1 enhanced Wnt4b and β-catenin transcription, and completely suppressed the expression of male-biased genes (Dmy, Gsdf, Sox9a2 and Dmrt1) as well as testicular differentiation. Gonadal reprograming of Rspo1-over-expressed-XY (Rspo1-OV-XY) fish, induced the production of female-biased genes (Cyp19a1a and Foxl2), estradiol-17β production and further female type secondary sexuality. Moreover, Rspo1-OV-XY females were fertile and produced successive generations. Promoter analyses showed that Rspo1 transcription was directly regulated by DM domain genes (Dmy, the sex-determining gene, and Dmrt1) and remained unresponsive to Foxl2. Taken together, our results strongly suggest that Rspo1 is sufficient to activate ovarian development and plays a decisive role in the ovarian differentiation in medaka.
Sexual differentiation represents a tug of war between male-dominated and female-activated genes in mammals1,2. In XY gonads, transiently activated SRY/Sry gene expression in the supporting cell lineage has been shown to initiate the male differentiation pathway by up-regulating Sox9 and Fgf9 expression3,4,5,6. On the other hand, although several candidate genes are known to be important in female sex differentiation, the master regulator/regulatory pathway has not yet been identified for XX embryos. Previous studies revealed that a Foxl2-leading pathway and Rspo1-activating signalling pathway act independently and complementary to each other, in order to promote ovarian development1,7,8,9,10,11,12,13.
In mammalian species, RSPO1/Rspo1 is involved in ovarian determination and differentiation, by synergizing with specific Wnt ligands to stabilize intracellular β-catenin1,14. Murine Rspo1, specifically up regulated in the XX gonad at E11.5, is necessary for gonadal somatic and germ cell differentiation10,15. In XX mouse gonads, the loss of Rspo1 induces ectopic steroidogenic precursors, endothelial cell migration, formation of a coelomic vessel, Sox9 expression in supporting cells lineages, and formation of ovotestis16,17. RSPO1 is specifically expressed in the foetal ovary between 6 and 9 weeks after conception18, and a single nucleotide insertion in the RSPO1 gene results in sex reversal and testicular development in XX humans19,20. A previous study revealed that the duplication of chromosome 1p, containing Rspo1 and Wnt4 loci, resulted in male-to-female sex reversal in humans18. The stabilization of β-catenin, the downstream gene of Rspo1, in the XY gonad successfully induces sex reversal, suggesting that elevations in Wnt4 and Rspo1 or their downstream signals are sufficient to override testicular development9. Therefore, the Rspo1/Wnt/β-catenin pathway might be instrumental in repressing the Sry-activated male pathway, which, in turn, activates ovarian determination and differentiation in XY mice. Recent findings demonstrated that Rspo1 displays a conserved, female-specific increase in expression in non-mammalian vertebrates, which highlights its conserved role in ovarian development21. Avian Rspo1 is predominantly expressed in the ovaries, and was found to be down regulated in hormone-induced sex reversed ZW embryos21,22. In medaka, up-regulated levels of Rspo1 were noticed in the gonadal primordium during the sex determination period23. Rspo1 expression was detected in the somatic cells of zebrafish from 30 days after fertilization (daf) to 150 daf, as well as in germ cells between 30 and 60 daf in the ovary24. These findings emphasize the importance of Rspo1 in non-mammalian vertebrate sex differentiation. However, the function of Rspo1 has not yet been corroborated in teleostean species.
Medaka (Oryzias latipes) is a much appreciated model for sex determination and differentiation studies25,26,27,28,29,30, owing to its XX-XY heterogamety, thorough embryology, Dmy/Dmrt1b-based genetic sex determination, and distinct sex-specific secondary sex characteristics. We have previously demonstrated that over-expression of Dmy in XX medaka favours male specific genes expression and induces female to male sex reversal29, while XY Dmrt1 (the downstream gene of Dmy) mutant medaka shows female specific genes expressions and further ovarian development30. This suggests that, both Dmy and Dmrt1 are crucial to maintain the balance between the male- and female-dominated gene transcriptions, which eventually determines the gonadal sexuality in medaka29,30. Recently, we also recorded a female-biased and oestrogen-responsive Rspo1 transcription during the sex determination period in medaka23. Previous in vivo ChIP analysis demonstrated that Dmy/Dmrt1b are adjoined to the Rspo1 gene promoter thereby suggesting the interaction between Dmy/Dmrt1b and Rspo1 in medaka31. Therefore, in order to assess the importance of Rspo1 in the induction of femininity, in the present study, Rspo1 was overexpressed in XY fish and sexual development was chronologically investigated. The relationship between the master sex-determining gene Dmy and Rspo1 was also examined to substantiate the sex differentiation mechanism in medaka. Our results reveal that Rspo1 is sufficient to activate female sex determination/differentiation in medaka. To the best of our knowledge, this is the first study to functionally characterize Rspo1 in a non-mammalian vertebrate.
Results
The Rspo1 transgene and GFP expression
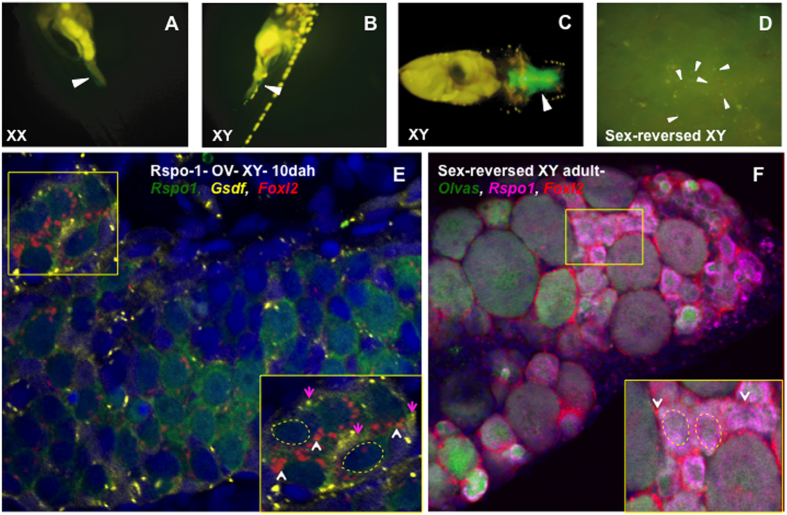
In order to determine the importance of Rspo1 in ovarian development, we constructed pIRES-hrGFP1a-Rspo1 plasmid by fusing Rspo1 ORF with hrGFP1a, in order to drive the hCMV promoter dependent Rspo1-GFP fused protein expression in XY medaka (Fig. S1A). Subsequently, the linearized pIRES-hrGFP1a-Rspo1 plasmid was injected into XY medaka embryos at the one/two-cell stage and individually grew them on 24-well plates (1 embryo/ well) at 26 °C. The microinjected embryos were periodically screened for GFP expression, and the GFP-positive embryos were grown until adulthood. Genomic PCR was performed using a transgene-specific primer pair to confirm the genome integration of the Rspo1 transgene (Fig. S1B). Live fluorescent microscopy depicted strong GFP signals in cells surrounding the germ cells in the XY sex-reversed ovary (Fig. 1A,D). Strong GFP expression was detected in the gonads and brains of 5 days after hatching (dah) embryos in the F1 generation (Fig. 1B,C). To further check the tissue specificity of Rspo1 transgene, we analysed the expression level of Rspo1 in brain, gonad and the remaining body (RB) in Rspo1-OV-XY (Rspo1- overexpressed XY) fish by real-time PCR. The results showed that Rspo1 gene expression was significantly higher in gonad and brain than the other tissues (Fig. S1C). In gonads, the GFP expression was scattered in germ cells and germ cell surrounding cells (Fig. 1E,F). Furthermore, multicolour-fluorescence in situ hybridization (FISH) was performed using different stages of Rspo1-OV-XY fish gonads to identify the cell type in which GFP expressions were prevalent in transgenic gonads. Abundant expression of Rspo1, Foxl2 (female-biased genes) and Gsdf (male dominated) gene was observed in Rspo1-OV-XY fish at 10 dah (Fig. 1E). Meanwhile, co-localization between Rspo1 and Gsdf, Foxl2 and Gsdf were also found in germ cell surrounding somatic cells (Fig. 1E inset). Similarly, co-localization of Rspo1 and Vasa was detected in the cytoplasm of young oocytes (Fig. 1F). Meanwhile, partial overlap of Rspo1 and Foxl2 gene expression in the germ cell surrounding cells was also observed (Fig. 1F, inset).
Figure 1. Localization of Rspo1 transgene in XY medaka embryos and adults.
Rspo1-GFP expression (arrowheads) in gonads of Rspo1 overexpressed XX (A) and XY (B) at 5 dah. Strong Rspo1-GFP expression is also seen in the brain (C) at 5 dah. In adults, GFP fluorescence (marked with white arrow head) is found in various gonadal cells (D). Photographs were taken from F1 generation embryos and F0 adults. Multicolor- fluorescent in situ hybridization FISH shows that Rspo1 (green) expresses in germ cells and germ cell surrounding cells of 10 dah Rspo1-OV-XY fish in which either Foxl2 (red) or Gsdf (yellow) expressions are evident (E). The representative Foxl2-Gsdf and Rspo1-Gsdf expressing somatic cells are respectively marked with white and pink arrowheads in the inset. The yellow dotted line represents candidate germ cells. Similar analysis shows that, Rspo1 (magenta) mRNAs are congregated in the young oocytes, early germ cells and early germ cell surrounding cells at 60 dah (F). The representative Foxl2-Rspo1 expressing early germ cell surrounding cells are marked with white arrowhead in the inset.
Ectopic Rspo1 expression induced ovarian development in XY fish
We observed the changes in secondary sexual characteristics between normal XX, XY, and Rspo1-OV-XY fish under a stereomicroscope. Normal XX female medaka generally possesses short, tapering, smooth anal and dorsal fins (Fig. 2A), while XY males are characterized by long, serrated and forked fins (Fig. 2B). Additionally, the leucophores, a Y-chromosome-derived trait, which doesn’t change with phenotypic sex reversal, were used for determining the genetic sex of medaka. In contrast to normal female and male medaka, the Rspo1-OV-XY female possessed leucophores and female secondary sex characteristics (short anal and dorsal fins, genital papilla, egg spawning) (Fig. 2C).
Figure 2. Fin structures and fertility of Rspo1-OV-XY medaka.
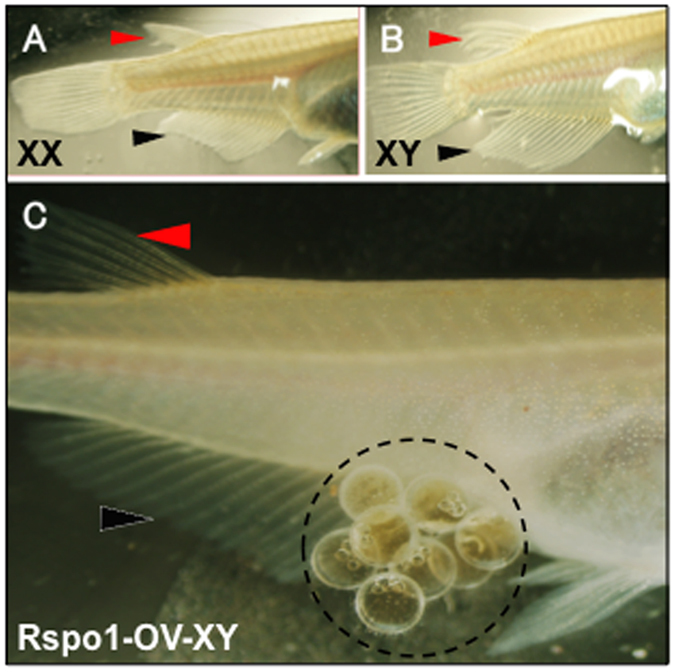
XX females (A) of QurtE medaka were devoid of leucophores, and possessed fused dorsal fin and tapered anal fin, while XY males (B) were characterized by abundant leucophores, forked dorsal fin and fan like anal fin. Rspo1-OV-XY fish (C), despite abundant leucophores, possessed fused dorsal and tapered anal fin, and had the ability to produce viable eggs. Red and black arrowhead indicates the dorsal and anal fin, respectively.
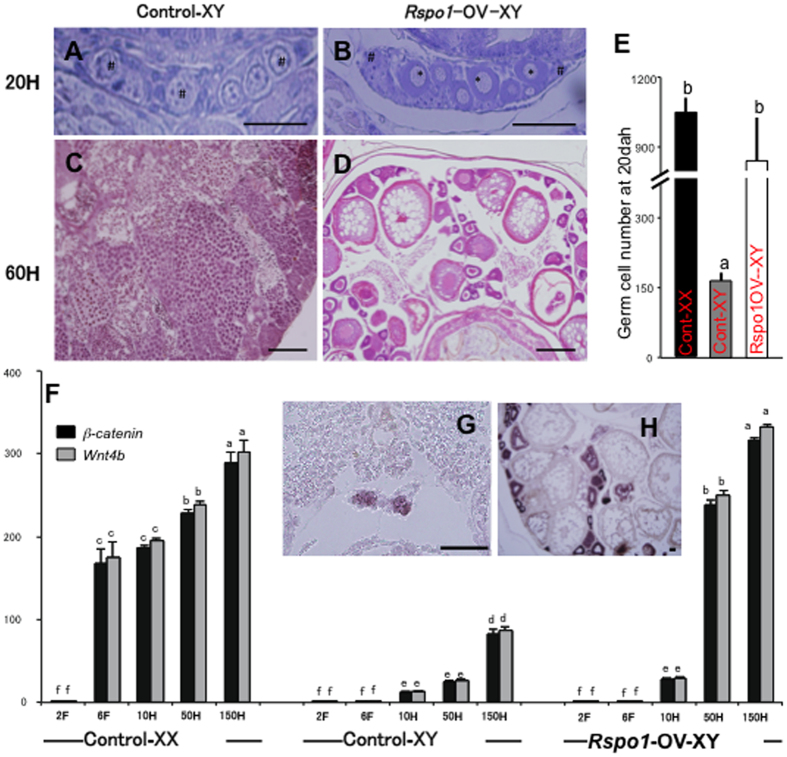
To further confirm the morphological changes in the gonads, HE (hematoxylin & eosin) staining of Rspo1-OV-XY and control-XY fish was performed at 20 dah and adult stages. The germ cell numbers were also counted at 20 dah to further validate the morphological observations. In medaka, slowly dividing mitotic germ cells and meiotic block define male gonadal sexuality at 20 dah. In our present study, similar characteristics were also observed in the control XY gonad (Fig. 3A,E). However, the overexpression of Rspo1 in XY fish accelerated primordial germ cell (PGC) proliferation and preponed the meiotic initiation, before 20 dah (Fig. 3B,E). In contrast to the normal XY testis (Fig. 3C), Rspo1 transgene subsequently activated the female developmental pathway and induced the differentiation of well-organized ovaries, including oocytes at various stages of development, as well as normal ovarian cavity in XY fish (Fig. 3D). Despite the XY genetic background, Rspo1 overexpression resulted in complete sex reversal and production of fertile female XY fish.
Figure 3. Effects of Rspo1 overexpression on ovarian morphology and Wnt4b/β-catenin signaling.
(A,C) Testis of control XY fish at 20 (A) and 60 (C) dah. (B,D) Ovaries of Rspo1-OV-XY fish at 20 (B) and 60 (D) dah. The mitotic and meiotic germ cells are marked with “*” and “#” respectively. (E) The graphical representation of total germ cells count at 20 dah in different fish groups. Excessive germ cell proliferation was observed in both control-XX (n = 10) and Rspo1-OV-XY (n = 10) gonads at 20 dah. Significant differences (marked by different letter) were detected between control-XY (n = 10) and Rspo1-OV-XY fish. (F) Wnt4b and β-catenin expression in control XX, control XY, and Rspo1-OV-XY fish at different stages of development. Marked increase in the expression of Wnt4b and β-catenin in Rspo1-OV-XY fish were detected at 50 and 150 dah. Data are shown as mean ± S.E., and expressed as relative abundance corrected for Ef1α. Different letters above the bars indicate that these groups differ significantly from each other at p < 0.01. (G,H) Rspo1-OV-XY fish shows β-catenin expression in small germ cell or oocytes and their surrounding cells at 6 (G) and 150 dah (H). Note: Scale bar-50 μm.
Rspo1 affected Wnt4/β-catenin signalling
Rspo1 has been identified as an activator of Wnt4/β-catenin signalling. In order to examine the involvement of both of these genes in Rspo1-associated sex reversal, we measured their mRNA concentrations by real-time PCR. Our results demonstrated a female-biased expression of both of these genes throughout ontogeny. Rspo1-OV-XY fish showed significant induction of Wnt4/β-catenin after 10 dah, which eventually became non-significant compared to the control XX counterpart (Fig. 3F). In situ hybridization (ISH) analysis also confirmed the significant up-regulation of β-catenin in the germ cell cytoplasm of Rspo1-OV-XY fish at both 6 dah and adult stage (Fig. 3G,H).
Fertility and ratio of sex reversal
To further assess the degree of Rspo1-associated ovarian maintenance, the gonadal morphology, papillary process, and secondary sexual characteristics of Rspo1-OV-XY fish were critically analysed at 90 (data not shown) and 150 dah (Fig. 2C). Furthermore, randomly selected Rspo1-OV-XY female fish were used to assess the mating and fertilization ability at 150 dah. Each of the transgenic fish was separately paired with non-transgenic XY fish for 3 days. Observations made within a specific time frame (30 min per pair) revealed that the sex reversed adult fish spawned daily, similar to XX female fish, and further mated with XY male fish to produce the F1 generation. Among the 38 tested Rspo1 transgenic XY fish, nine (24%) displayed usual female-like spawning behaviour, i.e. dancing, coiling, and mating, while another 12 XY female fish (32%) did not participate in mating (SI Table 1). Subsequent gonadal histology failed to recognize any difference between these two groups. Additionally, the remaining 17 injected XY fish (44%) showed the same gonadal morphology, papillary process, secondary sexual characteristics and behaviours as normal XY fish, which might attribute to the non-functioning of Rspo1 transgene.
Rspo1 favoured gonadal femininity
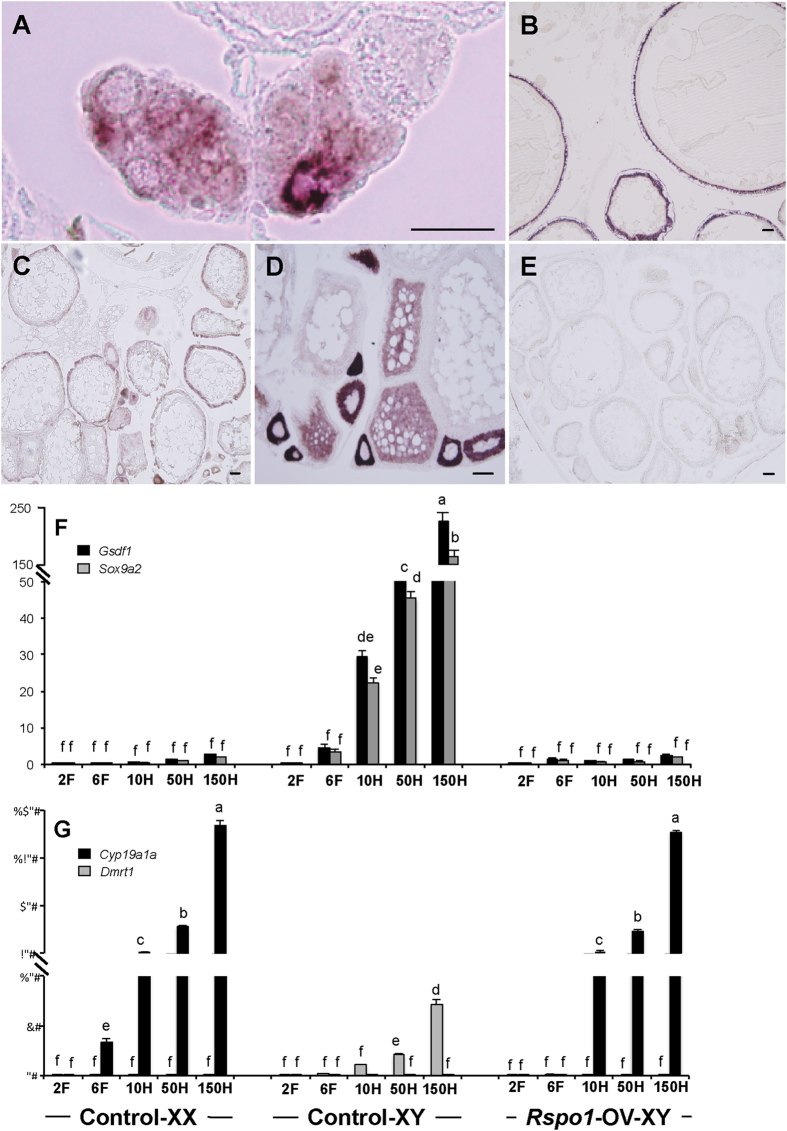
To further clarify the molecular mechanism underlying Rspo1-induced sex reversal, real-time PCR and ISH were performed using male and female-dominated genes. Both these analyses showed that the representative female-specific genes (Foxl2, Figlα, Cyp19a1a) were markedly up regulated (Fig. 4B–D,G), while the expression of Gsdf, Dmrt1 and Sox9a2 (male-dominated genes) was completely repressed in the Rspo1-OV-XY gonads (Fig. 4E–G). Moreover, the expression of Olvas from 10 dah was significantly higher in Rspo1-OV-XY fish than its control XY counterparts (data not shown). Furthermore, the transcription of Scp3 was detected as early as 15 dah in Rspo1-OV-XY fish (Fig. 4A), but absent in control XY fish.
Figure 4. Rspo1 overexpression modulates the expression of male and female biased genes.
(A) Specific expression of meiotic marker (Scp3) were recorded in germ cells of Rspo1-OV-XY fish at 15 dah, suggesting the induction of female biased gonadal development in these XY fish. Strong follicular expression of Cyp19a1 (B) and Foxl2 (C) oocyte specific expression of Fig1α (D) and simultaneous reduction of Gsdf (E) in ovarian somatic cells were observed in adult Rspo1-OV-XY fish, demonstrating the female-biased gonadal development in XY fish (scale bar-50 μm). (F) Gsdf and Sox9a2 expression in control XX, control XY, and Rspo1-OV-XY fish at different stages of development. Marked decrease in the expression of Gsdf and Sox9a2 in Rspo1-OV-XY fish was observed at 50 and 150 dah. (G) Cyp19a1a and Dmrt1 expression in control XX, control XY, and Rspo1-OV-XY fish at different stages of development. Marked increase in the expression of Cyp19a1a and decrease in Dmrt1 expression in Rspo1-OV-XY fish was noticed at 10-150 dah. Data are shown as mean ± S.E., and expressed as relative abundance corrected for Ef1α. Different letters (a, b, c, etc.) above the bars indicate that these groups differ significantly from each other at p < 0.01.
Involvement of Rspo1 in the modulation of steroid production
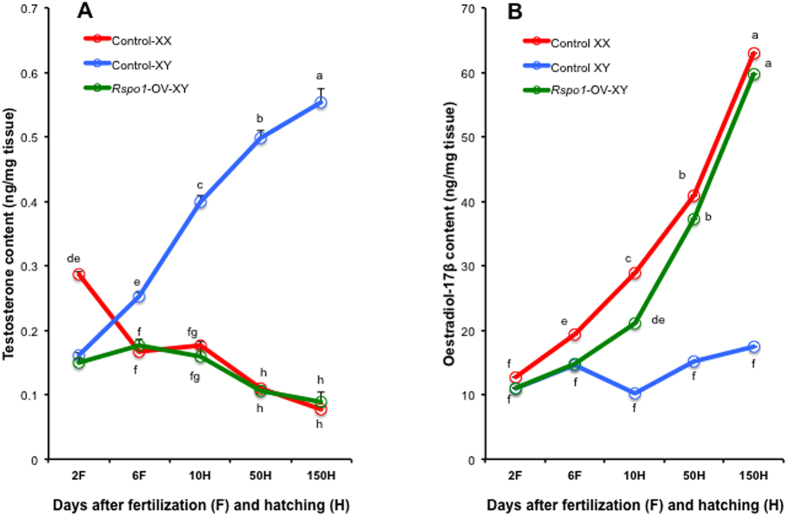
Sex-steroid concentrations play an important role in gonadal sexuality. It was previously reported that Cyp19b (brain type aromatase) starts expressing from 1.5 daf in medaka32. Moreover, Sebillot et al.33, found that the androgen responsive spigging-1 transgene transcription starts very early in medaka (during 2-3 daf), which further suggests some possible zygotic steroid production during early stages. Similarly, in zebrafish, AR (androgen receptor) expression starts from 24 hpf (corresponds to 2 daf medaka embryos)34, which further strengthens the idea that some amount of steroids are being produced in fish embryos during early stages . Previously, we found that Rspo1 transcription can be affected by steroid treatment23 and our present data suggests that Rspo1 over expression induces Foxl2 and Cyp19a expression (major genes in estrogen synthesis). So it will be critical to analyze whether Rspo1 overexpression can alter the estrogen and testosterone concentration in the body. To do so, we measured the estradiol-17β (E2) and testosterone concentrations in control XX, XY, and Rspo1-OV-XY F2 fish at different stages. No significant difference was observed in E2 production between control XX and Rspo1-OV-XY fish during the various stages of ontogeny (Fig. 5). Unlike control females, levels of testosterone were significantly lower in Rspo1-OV-XY at S-21 group samples (Fig. 5). None of the Rspo1-OV-XY samples showed an identical steroid profile to the control XY (Fig. 5). This prompted us to speculate whether Rspo1 overexpression changed the characteristics of Sertoli/Leydig cells to those of granulosa/theca cells. We investigated Cyp19a1a (thecal cell marker) and Dmrt1 (Sertoli cell marker) transcription in Rspo1-OV-XY fish at different stages (Fig. 4G), and consistent with the results for steroid production, we observed increased Cyp19a1a and decreased Dmrt1 expressions in Rspo1-OV-XY gonads at all stages.
Figure 5. Effects of Rspo1 overexpression on sex steroid production.
Testosterone (A) and oestradiol-17β (B) levels in whole embryos and gonads in control XX, control XY, and Rspo1-OV-XY fish, at different stages of development. Markedly reduced testosterone and simultaneously increased estradiol-17β levels in Rspo1-OV-XY fish, demonstrate the female-biased steroid production in Rspo1-OV-XY fish. Data are shown as mean ± S.E. Different letters (a, b, c, etc.) above the bars indicate that these groups differ significantly from each other at p < 0.01.
Antagonism between Rspo1 and DM domain genes
Dmy is the first male sex-determining gene in non-mammalian vertebrates. Furthermore, Dmrt1, supposed to be the downstream gene of Dmy, located upstream of the male cascade, is essential for testicular differentiation. In contrast, Foxl2 has been identified as a female-favouring gene in vertebrates while embryonic medaka Rspo1 expression is initiated much earlier than Foxl223. To further investigate the transcriptional regulation of Rspo1 gene by Foxl2, Dmy and Dmrt1, a dual luciferase promoter analysis was carried out. Our results revealed that both Dmy and Dmrt1 significantly (p < 0.01) repressed the Rspo1 promoter activity (Fig. 6a and S2). However, no such suppression was recorded when Dmy was substituted with mutant Dmy (mutation in DM domain). In contrast to our expectations, Foxl2 failed to induce Rspo1 transcription or reinstate Dmy inhibitory effects on the Rspo1 promoter (Fig. 6a). To further confirm the Rspo1 associated Dmy repression, we measured the expression profiles of Dmy by real-time PCR at different developmental stages. In the different stages of normal XY gonad, the expression of Dmy was very high, however, its expression was significantly repressed from 10 dah and became barely detectable by 50 and 150 dah (Fig. 6b).
Figure 6. Effects of DM domain genes on Rspo1 promoter activity.

(A) Assessment of Rspo1 binding potential to different male and female candidate genes. Expression plasmids of Dmy, mutant Dmy, Dmrt1, and Foxl2 were co-transfected with medaka Rspo1 (4.5 kb) promoter constructs, and relative luciferase activity (RLU) was measured after 48 hours. The RLUs of each expression plasmid (s) were plotted on Y-axis to prepare the graph. (B) Real-time analysis of Dmy expression in Rspo1-OV-XY fish. Marked decrease in Dmy expression was observed from 10 dah. Data are shown as mean ± S.E. of three independent experiments and different letters (a, b, c, etc.) above the bars indicate that these groups differ significantly from each other at p < 0.01.
Discussion
In the present study, we demonstrated that the overexpression of Rspo1 in XY fish induced female sex differentiation, steroid production, and sex reversal. This study showed for the first time that teleostean Rspo1 might be sufficient to activate the canonical Wnt4/β-catenin signalling pathway and estrogen production, which, in turn, favoured femininity. We also found that DM domain genes were sufficient to antagonize Rspo1 transcription, and vice versa, which eventually influenced the gonadal sexuality.
Since the last decade, Rspo1 has been repeatedly proposed as one of the prime femininity regulator in the embryonic gonad1,15,16,17,19,20,21. In our previous study, based on an expressional analysis, we hypothesized that Rspo1 may be critical for female sex determination/differentiation in medaka23. Consistent with our previous work, in the present study, we found that the overexpression of Rspo1 induced ovarian differentiation and sex reversal in XY fish. Contrastingly, the overexpression of Rspo1 alone in XY gonads did not result in sex-reversal in humans and mice8,11,18,35. Such anomalies may be associated with the improper activation of downstream genes/pathways. In this regard, Wnt4, an immediate downstream gene of Rspo1, was dose-dependently upregulated in XY sex reversed humans via the activation of Dax1 (antagonistic factor for SRY)18. In the present study, the Rspo1-OV-XY gonad also demonstrated an early surge in Wnt4b in the gonad, which may have escalated the chances of a gonadal sex change. It is highly likely that the overexpression of Rspo1 induced gonadal Wnt4b transcription and further enhanced the production of β-catenin from 10 dah, reaching a similar abundance of normal XX fish by 50 dah. The stabilization of mouse β-catenin in the XY gonad has also been shown to induce sex reversal. In the present study, we observed an increase in germ cell-specific expression of β-catenin in Rspo1-OV-XY fish at both 6 dah and adult stages. Such tissue-specific induction could be explained using the gonad and brain-specific GFP localization data and supportive realtime profiles of Rspo1 in Rspo1-OV-XY fish. Moreover, in our previous study, we showed that Rspo1 was most abundant in the brain and gonad, the major sex-regulating organs. The considerably late, but consistent up-regulation of β-catenin in germ cells suggests that the elevation in Wnt4b and Rspo1 or their downstream signals are sufficient to override testicular development. Hence, similar to other vertebrates, medaka Rspo1/Wnt4/β-catenin canonical signalling might play a critical role in maintaining the female sexuality.
The molecular perspective of femininity can be easily defined by the increased Foxl2 and Fig1a expression in granulosa and germ cells, respectively. On the other hand, the abundant expression of Dmrt1 and Sox9 are indicative of gonadal masculinity. The strong expression of Fig1a in germ cells and Foxl2 in follicular cells revealed that the overexpression of Rspo1 triggers folliculogenesis and activates the female developmental pathway. Previous studies demonstrated that mouse Foxl2 and Rspo1 regulates distinct female sex-determining pathways and redundantly antagonizes the action of the testis determinants of SRY/Sry10,13,36. In the present study, Foxl2 and Cyp19a1a (encoding the key enzyme for oestrogen production) were both up regulated in Rspo1-OV-XY fish. Moreover, similar to previous findings in mammalian species10,13,36, our promoter analysis data also suggested that Rspo1 transcription was independent of Foxl2. Although both Gsdf-Rspo1 and Gsdf-Foxl2 co-expressing cells were prevalent in 10 dah gonads, Foxl2-Rspo1 cells were very rare. However, at later stages (60 dah), Foxl2 and Rspo1 were restrictively co-localized in early germ cell surrounding cells. Therefore, most probably, the gonadal Foxl2 surge was one of the after-effects of Rspo1 overexpression, which helps in maintaining the femininity. We previously reported that Dmrt1 and Foxl2 play antagonistic roles, and the silencing of either of them, influences the gonadal sexuality in opposite directions by affecting Cyp19a1a transcription and oestrogen production37. In the present study, we also observed a significant increase in E2 production from 10 dah onwards. In medaka, Foxl2 and Cyp19a1a were found to be expressed from 0 dah and 3 dah, respectively38,39. The time frame suggests that the overexpression of Rspo1 counteracted the male gonadal development, which, in turn, induced Foxl2 and Cyp19a1a transcription and further testosterone-to-oestrogen conversion.
Dmy and their downstream genes are known to activate the male developmental program in medaka26,27,30,40. The disruption of Dmrt1 expression in Sertoli cells elevates Foxl2 transcription and promotes Sertoli to granulosa cell trans-differentiation30, which further develops as XY female. In the present study, apart from elevated Foxl2, we also observed a marked reduction of DM domain genes (Dmrt1 and Dmy) in Rspo1-OV-XY fish. Moreover, the dual luciferase promoter analysis revealed that Dmy and Dmrt1 both suppressed the transcription of Rspo1. A ChIP assay also previously demonstrated that, Dmy/Dmrt1b effectively pulled down Rspo131, which led us to hypothesize that, in normal XY males, Dmy/Dmrt1b suppresses Rspo1-activated female responsive genes and favours the male pathway. Moreover, recent studies revealed that the over-expression of Dmy induces testis formation in genetic XX medaka fish29 by suppressing female pathway genes and oestrogen production. In contrast, the overexpression of Rspo1 in XY fish shifted the balance to the female pathway by overriding Dmy actions. Taking these findings into account, we speculated that the sex determination of medaka might be controlled by antagonistic actions between the female pathway (Rspo1/β-catenin signalling pathway) and Dmy-activated male pathway. A previous study showed that oestrogen treatment effectively up-regulated the expression of Rspo1, suppressed the Gsdf transcription, and induced meiosis in XY medaka23,41. Therefore, the reduction of Gsdf in Rspo1-OV-XY medaka might be attributed to excessive oestrogen production in the body. Transcriptional up-regulation of Foxl2 in Gsdf expressing cells further strengthens such possibilities of enhanced oestrogen production. Unlike mammals16,17, our result suggests that Rspo1 possesses the potential to activate female-dominated gene transcription in both somatic and germ cells23 by antagonizing the actions of male pathway genes (Dmy, Gsdf, etc.). Thus, this unique expression profile is presumably an important factor for successful sex reversal.
Proliferative mitosis and meiotic initiation are considered to be the first phenotypic sign of female sex differentiation25,42. The overexpression of Rspo1 in XY fish accelerated the mitotic burst and meiotic initiation of germ cells at 15 dah. A previous study demonstrated that the expression of Dmy activates the male developmental pathway by suppressing the proliferation of PGC and meiotic initiation in XY male medaka28,29. In our experimental fish, meiotic germ cell division was observed at 0 dah in XX ovary, while meiotic germ cells could not be detected until 40 dah in XY testis. The excessive germ cell proliferation and expression of Scp3 (meiotic marker) in Rspo1-OV-XY fish, at 15 dah, implied that the Rspo1 transgene preponed the timing of meiosis in XY fish. Unlike normal XX medaka juveniles, no sign of proliferative mitosis or meiosis was observed in Rspo1-OV-XY fish until 5 dah. We hypothesized that the delayed meiosis in Rspo1-OV-XY fish might be due to the postponed up-regulation of female-biased genes (Wnt4, β-catenin and cyp19a1a) and oestrogen production around 10 dah. Available reports suggest that, in embryonic medaka gonads, Dmy and Gsdf are able to repress germ cell proliferation and meiotic initiation28,29. Similarly we also observed significant down-regulation of male-biased genes at 10 dah Rspo1-OV-XY fish. This, together with the suppressed Dmy/Gsdf/Sox9a2 pathway, indicates that Rspo1 favours gonadal trans-differentiation, by antagonizing the functions of downstream genes in the male pathway. several researchers have demonstrated that androgens are the key regulator of male secondary sex development43,44. In the present study, the overexpression of Rspo1 changed the secondary sex characteristics and induced the development of female-like dorsal and anal fins. The sex-reversed XY female fish possessed both leucophore and female-specific secondary sex features (short anal and dorsal fins, round genital papilla, egg-spawning ability). This strongly suggested that the overexpression of Rspo1 resulted in a decrease in the production of androgens, as revealed by the reduced transcription of Cyp11b (key enzyme for 11-KT biosynthesis) in XY fish. In accordance, testosterone concentrations were markedly reduced in Rspo1-OV-XY fish. This reduction might be associated with depleted number of testosterone-producing cells or increased oestrogen production38.
Taken together, our results indicate that Rspo1 plays a critical role in ovarian differentiation by antagonizing the male axis and activating female-specific genes in the medaka gonad. In females, although Rspo1-activated signalling and Foxl2-responsive E2 production pathways were mutually exclusive, they acted synergistically to promote the female pathway. Therefore, considering the fact that Rspo1 is one of the first female biased gene expressed in medaka23, we postulate that Rspo1 is a foremost factor that shifts the balance towards femininity. In medaka, the balance between Dmy and Rspo1 transcription determines the fate of the gonad. The activation of Dmy induces male differentiation, while Rspo1 favours femininity. Our results further demonstrate that oestrogen-producing and Rspo1-activated-signaling pathways, although independent, mutually ensure the female sex determination/differentiation in fish. In summary, our finding suggests that ectopic Rspo1 expression in XY medaka sufficiently induces ovarian differentiation by suppressing the master male sex determining gene (Dmy) and other male biased genes, elevating ovarian specific gene expression, and altering the steroid production. To our knowledge, the present work is the first report in vertebrates highlighting that Rspo1 alone can completely overturn the genetic male cascade to induce female development. However, further studies are essential to clarify the Rspo1 associated Dmy suppression mechanism in medaka.
Methods
Fish strain and husbandry
The QurtE strain of medaka was used in this study. This strain expresses a male-specific leucophore that facilitates the easy sexing of the fish. Genetic sexing was performed using previously reported protocols26. All fish were maintained at 26 ± 2 °C under a 14-h light and 10-h dark cycle. Eggs were collected within 30 min of fertilization and incubated in distilled water (milli-Q) containing an antifungal solution (Methylene blue, 0.0001%) at 26 ± 2 °C. Brooders and juveniles were fed fresh artemia, while larvae were given artificial food. All in vivo experiments and fish maintenance were conducted following protocols and procedures approved by the Institutional Animal Care and Use committee at Ehime University, Japan, and Southwest University, China.
Plasmid construction
The total RNA of adult ovaries was extracted using the RNeasy mini kit (Qiagen, USA) and cDNA was subsequently synthesized using the Omniscript kit (Qiagen). The ORF of Rspo1 was amplified from the adult ovaries, using two adapter-tagged gene-specific primers (SI Table 2), digested with Sac II and BamH I (New England Biolabs, UK), purified and cloned into similarly digested pIRES-hrGFP-1a vector (Stratagene, USA).
Genomic DNA was isolated from the caudal fin of adult fish using the DNeasy Blood and Tissue kit (Qiagen). The medaka Rspo1 promoter region (−4449bp) was PCR amplified (SI Table 2), cut with restriction enzymes (Mlu I and Xho I), and then directionally inserted into the pGL3-basic vector (Promega, USA). The transcription factors of Foxl2, Dmy and Dmrt1, were sub-cloned into pcDNA3.1 (Invitrogen, USA) from the original clones using gene-specific ORF primers. The inserts and directions of all the plasmids were confirmed by subsequent sequencing. The constructs with correct inserts were purified using the QIAfilter Plasmid Midi kit (Qiagen) and used for subsequent microinjections and luciferase assay.
FISH. The 10 and 60 dah Rspo1-OV-XY medaka gonads were fixed overnight in 4% paraformaldehyde (Nacalai tesque, Kyoto, Japan) at 4 °C. After fixation, the gonads were embedded in paraffin and sectioned at 30 μm, and subjected to FISH. To further confirm the cellular localization of GFP signals in the gonad of Rspo1-OV-XY fish, expression of Gsdf (Sertoli cell), Rspo1 and Folx2 (follicular cell) were investigated at 10 dah, meanwhile, olvas (germ cell), Rspo1 and Foxl2 expression were also examined at 60 dah. FISH was performed as described previously23. Briefly, probes were labeled with fluorescein isothiocyanate (FITC), Digoxigenin (DIG) and Biotin (Roche, Germany), and Alexa fluro-488-anti-FITC, Alexa fluro-546-anti-DIG, and Alexa fluro-591-anti-biotin antibodies were used for the detection. Nuclear staining was carried out using Hoechst dye according to the manufacturer’s instruction (Thermo Scientific, USA). Signals were observed and photographed by a confocal microscope (Zeiss 710, Carl-Zeiss Germany).
Microinjection and detection of transgenes
Microinjection was performed according to the previously reported protocol26. Briefly, fertilized eggs were collected within 15 min of spawning, cleaned off the attaching filaments and injected with linearized Rspo1-GFP plasmid DNA (50 ng/μl in Yamamoto’s solution). Embryonic Rspo1-GFP expression was traced under the microscope at different developmental stages. Genomic DNA was isolated from tail clippings and genetic sex was confirmed by PCR using gene-specific primers (SI Table 2). GFP-expressing genetic XY individuals were sacrificed at 20, 60, and 150 dah to analyse the effects of Rspo1 overexpression by real-time PCR, H&E (Haematoxylin and Eosin) staining, and ISH. The remaining GFP-positive XY fish were further reared until adult stage to assess the sex reversal status, secondary sexuality, breeding ability, and fertilization potential. Furthermore, sex-reversed XY-females were crossed with normal males to determine fertility and produce F1 and F2 generation progeny. Genomic DNA was extracted from the tail of XX/XY control and XY transgene fish of the F0 and F1 generations using the DNeasy Blood and Tissue kit (Qiagen), according to the manufacturer’s protocol. The transgene integration status was examined using a gene-specific forward primer and vector-based reverse primer (SI Table 2). Integration PCR was carried out as follows: 5 min at 94 °C, followed by 40 cycles of 15 sec at 96 °C, 1 min at 68 °C, and a final step of 7 min at 72 °C. The amplified fragment was sub-cloned into pGEM-T easy vector and confirmed by sequencing.
Histology and ISH
At 20 and 60 dah, the trunk portions of control XX, control XY, and Rspo1 transgene XY fish were fixed in Bouin’s fixative, embedded in paraffin, sectioned at 5 μm, and subjected to standard HE staining. 10 individual gonads of 20 dah group samples were serially sectioned (5 μm each), HE stained and total germ cell count was performed using stereo-microscope (400X zoom). The average of 10 individuals was used for graphical representation. Samples (6, 15 and 60 dah), fixed in paraformaldehyde (4%), were embedded in paraffin and sectioned at 5 μm for ISH. ISH was carried out using sense and anti-sense digoxigenin-labelled RNA probes transcribed in vitro with an RNA labelling kit (Roche) from their respective plasmid DNAs containing the ORFs of medaka Foxl2, β-catenin, Figlα, Gsdf, Scp3, and Cyp19a1a. Sections for ISH were deparaffinised, hydrated, and treated with proteinase K (10 μg/ml, Roche), and then hybridized with the sense or anti-sense DIG-labelled RNA probe at 58 °C for 22 h. Hybridization signals were then detected using alkaline phosphatase-conjugated anti-DIG antibody (Roche, Germany) and NBT, as described previously45.
Quantitative gene expression
Changes in gene expression were quantified using the ABI Prism 7000 sequence detection system (Applied Biosystem, USA). One hundred nanograms of total RNA, isolated from embryos or gonads at different developmental stages (2 and 6 daf, 10, 50, and150 dah) were used for cDNA synthesis, using a Quantitect RT PCR kit (Qiagen). First strand cDNAs were diluted to 100 μl for subsequent use. Gene-specific RT-PCR was performed using the SYBR green master mix (Applied Biosystem) and 5 ng of cDNA, according to the manufacturer’s instructions. Real-time PCR primers are listed in SI Table 2. PCR conditions included an initial denaturation at 94 °C (2 min) followed by 40 cycles at 94 °C (30 s) and 60 °C (1 min). Ef1α was used as the internal control. The absolute transcript copy number of each gene was determined with the help of appropriate standard curves and normalized with the Ef1α copy numbers in each sample. The reported values were averaged from sample triplicates of three pooled whole body samples (10 individuals from 2 and 6 daf, and 10 dah, and three gonads from 60 and 150 dah) of XX, XY and Rspo1-OV-XY. The RNAs were used for two independent cDNA synthesis, each group of cDNA were further analysed twice and all the data were used for mean and standard error calculations.
Steroids measurement
One hundred de-yolked embryos (2 and 6 daf)/juveniles (10 and 50 dah)/10 adults (150 dah) were quickly frozen in liquid nitrogen, weighed, homogenized, and used for steroid extraction. The freeze-dried extracts were diluted in appropriate quantities of dilution buffer (20 μl per mg tissue) and used to measure testosterone concentrations with the testosterone high sensitivity ELISA kit (Enzo, Japan), following manufacturer’s instructions. Samples were further diluted (100-fold) to measure oestrogen with the estradiol-17β high sensitivity ELISA kit (Enzo). Steroid concentrations were calculated based on the standard curve prepared using respective steroids, provided by the manufacturer (Enzo). Both tissue and extract duplicates were analysed before final calculations. Preliminary analyses were carried out to standardize the sampling method and assessment protocols.
Luciferase assays and repression of Dmy by Rspo1 transgene
HEK293 cells were grown in DMEM (Sigma) supplemented with 10% foetal bovine serum (JRH Biosciences) and 1Χ penicillin-streptomycin-glutamine (Invitrogen) with 5% CO2 at 37 °C. Confluent HEK293 cells were seeded on 24-well plates (at 5 × 105 cells/well), grown for 24 hours, and transfected using Lipofectamine (Invitrogen) with the following plasmids: 1) 0.5 μg of constructs of the Rspo1 promoter cloned into the pGL3-basic luciferase reporter plasmids; 2) 0.05 μg–0.5 μg of the pcDNA3.1 expression plasmids (Invitrogen) of Dmy and Dmrt1, and 3) pRL-TK (Promega), 100 ng/well. Renilla luciferase was employed as an internal control for transfection efficiency. The transfection solution was made of 100 μl of Opti-MEM I reduced serum medium containing complexed DNA, and 2 μl of Lipofectamine reagent. After 48 h of transfection, the cells were PBS-washed and lysed with 100 μl luciferase lysis buffer. Luciferase activity was measured using the Dual-Luciferase Reporter Assay kit (Promega) and LUMATLB 9507 luminometer (Berthold Technologies GmbH & Co. KG). Relative luciferase activity was calculated by dividing firefly luciferase activity by Renilla luciferase activity. Results are presented as the means ± S.E. of triplicates. Moreover, the expression level of Dmy was compared between the normal XY and Rspo1-OV-XY fish by real-time PCR, according to the methods aforementioned. The copy number of Dmy was measured at 2 and 6 daf, 10, 50 and 150 dah, and data were expressed as the relative mean copy number ± S.E. at each stage.
Statistical analysis
Statistical differences in relative mRNA expression, steroid concentrations, and relative luciferase activity were assessed by one-way ANOVA, followed by Tukey’s test or the Student’s t-test. All statistical analyses were performed using GraphPad prism software. All experimental data are shown as the mean ± S.E. Differences were considered significant at p < 0.01, if not otherwise stated.
Additional Information
How to cite this article: Zhou, L. et al. Rspo1-activated signalling molecules are sufficient to induce ovarian differentiation in XY medaka (Oryzias latipes). Sci. Rep. 6, 19543; doi: 10.1038/srep19543 (2016).
Supplementary Material
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31201986), Science and Technology Agency (SORST Program), the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, Research Foundation of Talent Introduction of Southwest University, China (SWU111003), a grant from Natural Science Foundation Project of CQ CSTC, China (cstc2012jjA80005), and the special fund of Chongqing key laboratory (CSTC). The authors are thankful to National BioResource Project (NBRP) for providing the medaka strains.
Footnotes
Author Contributions L.Z., T.C., and Y.N. conceived the study and all authors participated in its design. L.Z. and T.C. performed the overexpression experiments, germ cell counting, realtime PCR. Q.Z. analysed the data and performed ISH. and genomic PCR. analysis. S.M. performed the FISH. experiments and confocal microscopy. L.Z., T.C., Y.N. and Y.Z. wrote the manuscript. All the authors have read and approved the final manuscript.
References
- Capel B. R-spondin1 tips the balance in sex determination. Nat. Genet. 38, 1233–1234 (2006). [DOI] [PubMed] [Google Scholar]
- Kim Y. et al. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol . 4, e187 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman P., Munsterberg A., Capel B., Vivian N. & Lovell-badge R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature 348, 450–452 (1990). [DOI] [PubMed] [Google Scholar]
- Gubbay J. et al. A gene-mapping to the sex-determining region of the mouse Y-chromosome is a member of a novel family of embryonically expressed genes. Nature 346, 245–250 (1990). [DOI] [PubMed] [Google Scholar]
- Sinclair A. H. et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346, 240–244 (1990). [DOI] [PubMed] [Google Scholar]
- Lovell-badge R. & Robertson E. XY-female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development 109, 635–646 (1990). [DOI] [PubMed] [Google Scholar]
- Biason-Lauber A. WNT4, RSPO1, and FOXL2 in sex development. Semin. Reprod. Med. 30, 387–395 (2012). [DOI] [PubMed] [Google Scholar]
- Chassot A. A. et al. WNT4 and RSPO1 together are required for cell proliferation in the early mouse gonad. Development 139, 4461–4472 (2012). [DOI] [PubMed] [Google Scholar]
- Maatouk D. M. et al. Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum. Mol. Genet. 17, 2949–2955 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nef S. & Vassalli J. D. Complementary pathways in mammalian female sex determination. J. Biol. 8, 74 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolenghi C. et al. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum. Mol. Genet. 16, 2795–2804 (2007). [DOI] [PubMed] [Google Scholar]
- Schlessinger D. et al. Determination and stability of gonadal sex. J. Androl. 31, 16–25 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocer A. et al. R-spondin1 and Foxl2 act into two distinct cellular types during goat ovarian differentiation. BMC Dev. Biol. 8, 36 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. A. et al. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol. Biol. Cell 19, 2588–2596 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizuka K. et al. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum. Mol. Genet. 17, 1278–1291 (2008). [DOI] [PubMed] [Google Scholar]
- Chassot A. A. et al. Rspo1, an essential gene for ovarian differentiation in mammals. Sex. Dev. 2, 280–280 (2008). [Google Scholar]
- Chassot A. A. et al. RSPO1/beta-catenin signaling pathway regulates oogonia differentiation and entry into meiosis in the mouse fetal ovary. PLoS One 6, e25641 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan B. K. et al. Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am J Hum Genet 68, 1102–1109 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma P. et al. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat. Genet. 38, 1304–1309 (2006). [DOI] [PubMed] [Google Scholar]
- Tomaselli S. et al. Human RSPO1/R-spondin1 is expressed during early ovary development and augments beta-catenin signaling. PLoS One 6, e16366 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A. et al. Cloning and expression of R-Spondin1 in different vertebrates suggests a conserved role in ovarian development. BMC Dev. Biol. 8, 72 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth L. S., Cummins D., Doran T. J., Sinclair A. H. & Smith C. A. Overexpression of aromatase alone is sufficient for ovarian development in genetically male chicken embryos. PLoS One 8, e68362 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L. Y. et al. R-spondins are involved in the ovarian differentiation in a teleost, medaka (Oryzias latipes). BMC Dev. Biol. 12, 36 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. M. et al. Molecular analysis shows differential expression of R-spondin1 in zebrafish (Danio rerio) gonads. Mol. Biol. Rep. 38, 275–282 (2011). [DOI] [PubMed] [Google Scholar]
- Kobayashi T. et al. Two DM domain genes, Dmy and Dmrt1, involved in testicular differentiation and development in the medaka, Oryzias latipes. Dev. Dyn. 231, 518–526 (2004). [DOI] [PubMed] [Google Scholar]
- Matsuda M. et al. Dmy is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 417, 559–563 (2002). [DOI] [PubMed] [Google Scholar]
- Kondo M. et al. Absence of the candidate male sex-determining gene dmrt1b(Y) of medaka from other fish species. Curr. Biol. 13, 416–420 (2003). [DOI] [PubMed] [Google Scholar]
- Paul-Prasanth B. et al. Knock-down of DMY initiates female pathway in the genetic male medaka, Oryzias latipes. Biochem. Biophys. Res. Commun. 351, 815–819 (2006). [DOI] [PubMed] [Google Scholar]
- Matsuda M. et al. Dmy gene induces male development in genetically female (XX) medaka fish. Proc. Natl. Acad. Sci. USA 104, 3865–3870 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama H. et al. Dmrt1 mutation causes a male-to-female sex reversal after the sex determination by Dmy in the medaka. Chromosome Res. 20, 163–176 (2012). [DOI] [PubMed] [Google Scholar]
- Herpin A. et al. Divergent expression regulation of gonad development genes in medaka shows incomplete conservation of the downstream regulatory network of vertebrate sex deetermination. Mol. Biol. Evol. 30, 2328–2346 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo K. et al. Sex differences in aromatase gene expression in the medaka brain. J. Neuroendocrinol. 23, 412–423 (2011). [DOI] [PubMed] [Google Scholar]
- Sébillot A. et al. Rapid fluorescent detection of (anti)androgens with spiggin-gfp medaka. Environ. Sci. Technol. 48, 10919–10928 (2014). [DOI] [PubMed] [Google Scholar]
- Hossain M. S. et al. Zebrafish androgen receptor: Isolation, molecular and biochemical characterization. Biol. Repro . 78, 361–369 (2008) [DOI] [PubMed] [Google Scholar]
- Buscara L. et al. Goat RSPO1 overexpression rescues sex-reversal in Rspo1-knockout XX mice but does not perturb testis differentiation in XY or sex-reversed XX mice. Transgenic Res. 18, 649–654 (2009). [DOI] [PubMed] [Google Scholar]
- Auguste A. et al. Loss of R-spondin1 and Foxl2 amplifies female-to-male sex reversal in XX mice. Sex. Dev. 5, 304–317 (2011). [DOI] [PubMed] [Google Scholar]
- Li M. H. et al. Antagonistic roles of Dmrt1 and Foxl2 in sex differentiation via estrogen production in tilapia as demonstrated by TALENs. Endocrinology 154, 4814–4825 (2013). [DOI] [PubMed] [Google Scholar]
- Nakamoto M., Matsuda M., Wang D. S., Nagahama Y. & Shibata N. Molecular cloning and analysis of gonadal expression of Foxl2 in the medaka, Oryzias latipes. Biochem. Biophys. Res. Commun. 344, 353–361 (2009). [DOI] [PubMed] [Google Scholar]
- Suzuki A., Tanaka M. & Shibata N. Expression of aromatasemRNAand effect of aromatase inhibitor during ovarian development in the medaka, Oryzias latipes. J. Exp. Zool. 301, 266–273 (2004). [DOI] [PubMed] [Google Scholar]
- Nanda I. et al. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc. Natl. Acad. Sci. USA 99, 11778–11783 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y. et al. Expression of gonadal soma derived factor (GSDF) is spatially and temporally correlated with early testicular differentiation in medaka. Gene Expr. Patterns 10, 283–289 (2010). [DOI] [PubMed] [Google Scholar]
- Kurokawa H. et al. Germ cells are essential for sexual dimorphism in the medaka gonad. Proc Natl. Acad. Sci. USA 104, 16958–16963 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Suzuki A., Shibata N., Sakaizumi M. & Hamaguchi S. The novel mutant scl of the medaka fish, Oryzias latipes, shows no secondary sex characters. Zool. Sci. 25, 299–306 (2008). [DOI] [PubMed] [Google Scholar]
- Ogino Y. et al. Bmp7 and Lef1 are the downstream effectors of androgen signaling in androgen-induced sex characteristics development in medaka. Endocrinology 155, 449–462 (2014). [DOI] [PubMed] [Google Scholar]
- Zhou L. Y. et al. A novel type of P450c17 lacking the lyase activity is responsible for C21-steroid biosynthesis in the fish ovary and head kidney. Endocrinology 148, 4282–4291 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.