Abstract
The extracellular matrix (ECM) is important in creating cellular environments in tissues. Recent studies have demonstrated that ECM components are localized in anterior pituitary cells and affect cell activity. Thus, clarifying the mechanism responsible for ECM maintenance would improve understanding of gland function. Tissue inhibitors of metalloproteinases (TIMPs) are endogenous inhibitors of matrix metalloproteinases and participate in ECM degradation. In this study, we investigated whether cells expressing TIMPs are present in rat anterior pituitary gland. Reverse transcription polymerase chain reaction was used to analyze expression of the TIMP family (TIMP1-4), and cells producing TIMPs in the gland were identified by using in situ hybridization. Expression of TIMP1, TIMP2, and TIMP3 mRNAs was detected, and the TIMP-expressing cells were located in the gland. The TIMP-expressing cells were also investigated by means of double-staining with in situ hybridization and immunohistochemical techniques. Double-staining revealed that TIMP1 mRNA was expressed in folliculostellate cells. TIMP2 mRNA was detected in folliculostellate cells, prolactin cells, and thyroid-stimulating hormone cells. TIMP3 mRNA was identified in endothelial cells, pericytes, novel desmin-immunopositive perivascular cells, and folliculostellate cells. These findings indicate that TIMP1-, TIMP2-, and TIMP3-expressing cells are present in rat anterior pituitary gland and that they are involved in maintaining ECM components.
Keywords: extracellular matrix, tissue inhibitor of metalloproteinases, anterior pituitary, in situ hybridization, immunohistochemistry
I. Introduction
Extracellular matrix (ECM) components provide mechanical support for tissues and are important in cell growth, proliferation, differentiation, and migration. In the creation of the cellular environment, turnover of ECM components is highly regulated by (1) the production of ECM components, (2) the cleavage of ECM components by enzymes, and (3) the inhibition of degradation. Matrix metalloproteases (MMPs) are a major group of enzymes involved in ECM degradation (for a review, see [3]). Tissue inhibitors of metalloproteases (TIMPs) are secretory proteins that function as endogenous inhibitors of MMPs. Four such molecules have been identified in vertebrates: TIMP1, TIMP2, TIMP3, and TIMP4 [8, 25, 31, 34]. The balance between MMPs and TIMPs is critical for ECM deposition and remodeling in tissues.
The anterior pituitary gland is composed of hormone-secreting cells, folliculostellate cells which do not produce classical anterior pituitary hormones, and capillary endothelial cells and pericytes. These cells are surrounded by various ECMs such as laminin, collagen, and fibronectin [9, 18, 20, 33]. Anterior pituitary cells are responsive to ECMs by adhesion. Horacek et al. showed that adult rat anterior pituitary cells express integrin, a receptor of ECM, and adhere to ECMs [10]. ECM components and signal transduction help control anterior pituitary hormone production and cell proliferation (for a review, see [24]). We previously reported that particular cells actively respond to ECM in the anterior pituitary gland, which suggests that the ECM and cell-ECM interaction are important in regulating gland function [11, 12].
Recently, our group identified various types of ECM-producing cells for type I and III collagens [6], laminin chains [28], and small leucine-rich proteoglycans [13] in rat anterior pituitary gland. Our study of the catabolic enzyme for ECM components in the gland revealed that folliculostellate cells express MMP9 [14]. These studies helped to show that the mechanism of ECM remodeling in the gland involved production and degradation of ECM components. However, information on inhibition of degradation is essential for a complete understanding of this mechanism. Although TIMP1, TIMP2, and TIMP3 were previously detected in normal human pituitary by Western blotting [1], there are no data on expression of TIMPs in rat anterior pituitary gland. Therefore, using in situ hybridization, we investigated the possibility that TIMPs and TIMP-expressing cells are present in the rat anterior pituitary gland.
II. Materials and Methods
Animals
Four male Wistar rats (age 8–10 weeks) were purchased from Japan SLC (Shizuoka, Japan), maintained on a 12-hr light/dark cycle, and given conventional food and water ad libitum. Room temperature was maintained at approximately 22°C. All animal experiments were performed after receiving approval from the Institutional Animal Experiment Committee of Jichi Medical University and were conducted in accordance with the Institutional Regulations for Animal Experiments and Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the Jurisdiction of the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Analysis by reverse transcription polymerase chain reaction
Reverse transcription polymerase chain reaction (RT-PCR) was performed as described previously [11]. Total RNA was extracted by using an RNeasy mini-kit and an RNase-free DNase set (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. cDNA was synthesized by a Superscript III reverse transcription kit with oligo-(dT)20 primer (Life Technologies, Carlsbad, CA, USA). Sequences of the gene-specific primers are described in Table 1. Samples were subjected to 2 min at 94°C, 30 cycles of 30 s at 94°C, 30 s at 58°C, 30 s at 72°C, and then an additional 7 min at 72°C in a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA). Amplified products were analyzed on 1.5% agarose gels and visualized by ethidium bromide staining. Negative controls were subjected to RT-PCR without reverse transcriptase, cDNA, or Blend Taq DNA polymerase (Toyobo, Osaka, Japan) and showed no reaction bands.
Table 1. .
Sequences of the specific primers for the studied genes
| Genes | Primer sequence (5'→3') | Product size | Genbank accession number |
|---|---|---|---|
| Timp1 | Forward: TAGAGACACGCTAGAGCAGATACC | 497 bp | NM_053819 |
| Reverse: GCTGAACAGGGAAACACTG | |||
| Timp2 | Forward: AAGGACCTGACAAGGACATC | 542 bp | NM_021989 |
| Reverse: ACCCACTTGGATGATTGATG | |||
| Timp3 | Forward: GGCAACTTTGAGGAAAAGAG | 570 bp | NM_012886 |
| Reverse: CGAAATTGGAGAGCATGTC | |||
| Timp4 | Forward: GATTCTCAGTGATGGAAAAGTC | 736 bp | NM_001109393 |
| Reverse: CAGCCCACTCAGGATATAGTC | |||
| Gapdh | Forward: CCATCACCATCTTCCAGGAG | 457 bp | NM_017008 |
| Reverse: TTCAGCTCTGGGATGACCTT |
In situ hybridization and immunohistochemistry
Rats were perfused through the left ventricle with 4% paraformaldehyde in 0.05 M phosphate buffer (pH 7.4) for 5 min under deep sodium pentobarbital anesthesia. Pituitary glands were then excised and immersed in the same fixative for 24 hr at 4°C, after which the tissues were immersed for at least 2 days in phosphate buffer containing 30% sucrose at 4°C. In situ hybridization was performed with digoxigenin (DIG)-labeled cRNA probes, as described in our previous report [5]. PCR was used to amplify DNA fragments from rat anterior pituitary cDNA. Primer sequences are shown in Table 1. Amplified cDNA fragments were ligated into the pGEM-T vector (Promega, Madison, WI, USA) and cloned. Gene-specific antisense or sense DIG-labeled cRNA probes were made using the Roche DIG RNA labeling kit (Roche Diagnostics, Penzberg, Germany). A cryostat was used to obtain frozen sections (8 μm), which were then mounted on glass slides. DIG-labeled cRNA probe hybridization was performed at 55°C for 16 hr. Each type of mRNA was visualized with an alkaline-phosphatase-conjugated anti-DIG antibody by using 4-nitroblue tetrazolium chloride (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP; Roche Diagnostics). Control experiments were performed, and no specific signal was detected in sections processed with the DIG-labeled sense RNA probe.
For double-staining, after TIMP mRNAs had been detected by in situ hybridization, the section was immunostained as described in our previous report [4]. Sections were then incubated overnight at room temperature in phosphate-buffered saline (PBS) with primary antibodies. Primary antibodies used in the experiments were rabbit anti-porcine adrenocorticotrophic hormone 1–39 (ACTH; Dr. F. Nakamura, Hokkaido University, Hokkaido, Japan; dilution, 1:3,200), anti-rat growth hormone (GH; Dr. K. Wakabayashi, Gunma University, Gunma, Japan; dilution, 1:12,800), anti-rat prolactin (National Institutes of Health (NIH), Bethesda, MD, USA; dilution, 1:12,800), anti-rat thyroid-stimulating hormone (TSH) β subunit (NIH; dilution, 1:3,200), anti-ovine luteinizing hormone (LH) β subunit (Dr. K. Wakabayashi; dilution, 1:12,800), and anti-S100B protein (DAKO, Glostrup, Denmark; dilution, 1:500), as reported previously [4], and desmin (Abcam, Tokyo, Japan; dilution, 1:200), as reported previously [16]. Biotinylated isolectin B4 (1:25; Vector Laboratories, Burlingame, CA, USA) was used for lectin histochemistry. Absence of an observable nonspecific reaction was confirmed by using normal rabbit serum. The ABC method (Vector Laboratories) was performed with 3,3'-diaminobenzidine (Dojindo Laboratories, Kumamoto, Japan) as the substrate.
III. Results
Expression of TIMP family genes in rat anterior pituitary gland
RT-PCR was used to examine expression of TIMP family genes in rat anterior pituitary gland. TIMP1, TIMP2, and TIMP3 mRNAs were detected (Fig. 1a). Next, localization of TIMP1-, TIMP2-, and TIMP3-expressing cells was examined by in situ hybridization with a DIG-labeled antisense cRNA probe. Figure 1b shows hematoxylin-eosin staining of a rat pituitary section. TIMP1-expressing cells were located in the intermediate and anterior lobes (Fig. 1c). TIMP2 and TIMP3 mRNAs were expressed in the posterior, intermediate, and anterior lobes (Fig. 1e, g). No specific signal was detected in adjacent sections processed with the DIG-labeled sense cRNA probes for TIMP1, TIMP2, and TIMP3 (Fig. 1d, f, h).
Fig. 1. .
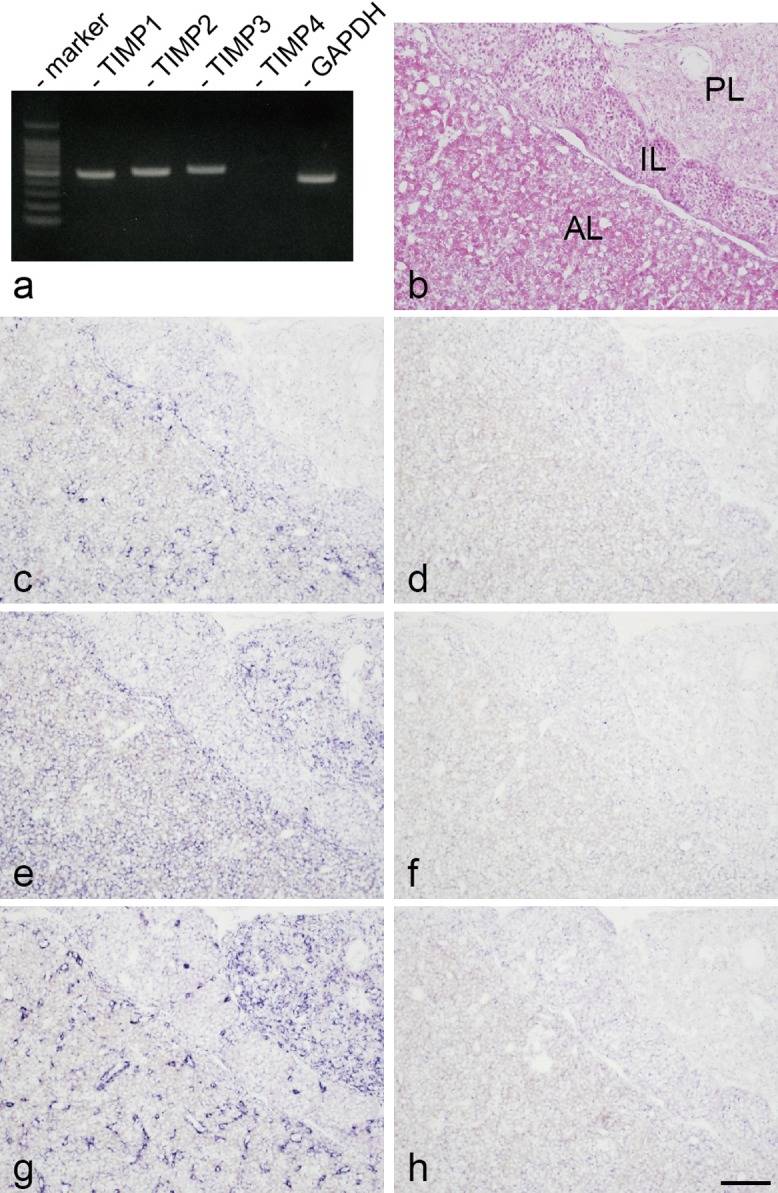
Expression of tissue inhibitor of metalloproteinase (TIMP) family genes in rat anterior pituitary gland. a: Expression of TIMP family (TIMP1-4) mRNAs in rat anterior pituitary gland, as determined by reverse transcription polymerase chain reaction. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal control. b: Hematoxylin and eosin staining of a cryosection of rat anterior pituitary gland (AL: anterior lobe, IL: intermediate lobe, PL: posterior lobe). c, d: In situ hybridization for the TIMP1 antisense probe (c) and sense probe (d). e, f: In situ hybridization for TIMP2 antisense probe (e) and sense probe (f). g, h: In situ hybridization for TIMP3 antisense probe (g) and sense probe (h). Images b–h are consecutive sections. In situ hybridization with NBT/BCIP (blue). Bar=100 μm (b–h).
Characterization of TIMP1-expressing cells
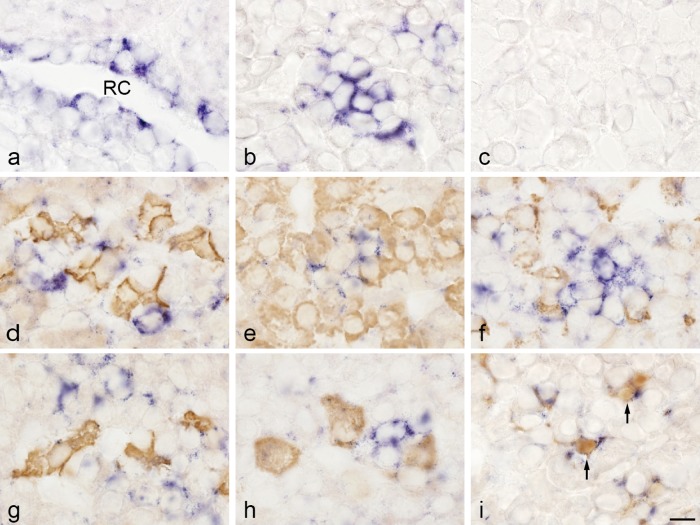
TIMP1 mRNA was detected in cells of the marginal cell layer (in both the anterior and intermediate lobes) (Fig. 2a) and in cells of the anterior lobe (Fig. 2b). A positive signal was observed only in cell cytoplasm. To identify cells that express TIMP1 mRNA, we performed double-staining using in situ hybridization to detect TIMP1 mRNA and immunohistochemistry to detect pituitary hormone and S100 protein. TIMP1 mRNA was detected in folliculostellate cells, which were identified on the basis of their immunoreaction to S100 protein (Fig. 2i). In this experiment, all TIMP1-expressing cells were detected by immunoreaction for S100 protein. TIMP1 mRNA was not expressed in cells that produced ACTH, GH, prolactin, TSH, or LH (Fig. 2d, e, f, g, h).
Fig. 2. .
Double-staining of TIMP1 mRNA detected by in situ hybridization and hormones and S100 protein detected by immunohistochemistry in rat anterior pituitary gland. a, b: In situ hybridization for TIMP1. TIMP1-expressing cells were observed in the marginal layer (a) surrounding Rathke’s cleft (RC) and in the anterior lobe (b). c: Negative control with sense probe. d–i: In situ hybridization of TIMP1 and immunohistochemistry of adrenocorticotropic hormone (ACTH; d), growth hormone (GH; e), prolactin (f), thyroid-stimulating hormone β-subunit (TSHβ; g), luteinizing hormone β-subunit (LHβ; h), and S100 protein (folliculostellate cells; i). In situ hybridization with NBT/BCIP (blue) and immunostaining with 3,3'-diaminobenzidine (brown). TIMP1 mRNA was colocalized with the S100 protein immunoreaction (arrows). Bar=10 μm (a–i).
Characterization of TIMP2-expressing cells
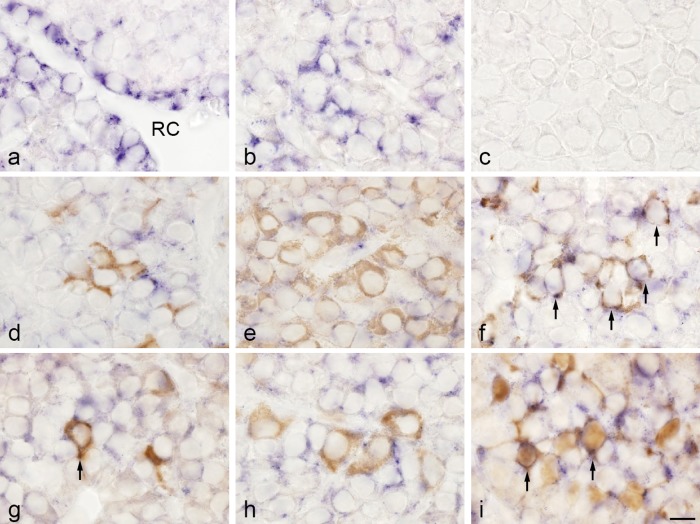
TIMP2 mRNA was detected in the marginal layer cells (Fig. 3a) and in the anterior lobe (Fig. 3b). A positive signal was observed only in cell cytoplasm. To identify cells that express TIMP2 mRNA, we performed double-staining using in situ hybridization to detect TIMP2 mRNA and immunohistochemistry to detect pituitary hormone and S100 protein. TIMP2 mRNA was detected in prolactin cells, TSH cells, and folliculostellate cells, which were identified on the basis of immunoreaction to prolactin, TSHβ, and S100 protein, respectively (Fig. 3f, g, i). TIMP2 mRNA was not expressed in cells that produced ACTH, GH, or LH (Fig. 3d, e, h). TIMP2 mRNA was also expressed in pituicytes, which are glial cells in the posterior lobe (Supplementary Fig. 1).
Fig. 3. .
Double-staining of TIMP2 mRNA detected by in situ hybridization and hormones and S100 protein detected by immunohistochemistry in rat anterior pituitary gland. a, b: In situ hybridization for TIMP2. TIMP2-expressing cells were observed in the marginal layer surrounding Rathke’s cleft (RC) (a) and in the anterior lobe (b). c: Negative control with sense probe. d–i: In situ hybridization of TIMP2 and immunohistochemistry of ACTH (d), GH (e), prolactin (f), TSHβ (g), LHβ (h), and S100 protein (folliculostellate cells; i). In situ hybridization with NBT/BCIP (blue) and immunostaining with 3,3'-diaminobenzidine (brown). TIMP2 mRNA was colocalized with the prolactin, TSHβ, and S100 protein immunoreactions (arrows). Bar=10 μm (a–i).
Characterization of TIMP3-expressing cells
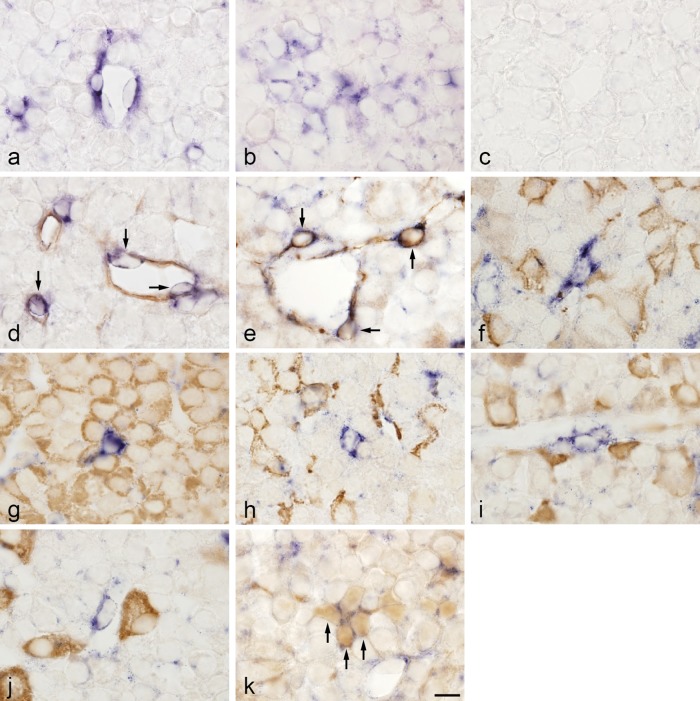
TIMP3 mRNA was detected in cells of capillaries and cells in perivascular spaces (Fig. 4a) and the anterior lobe (Fig. 4b) and was more prominent in perivascular spaces. A positive signal was observed only in cell cytoplasm. To identify cells that express TIMP3 mRNA, we performed double-staining using in situ hybridization to detect TIMP3 mRNA and histochemistry to detect isolectin B4, desmin, pituitary hormone, and S100 protein. TIMP3 mRNA was detected in endothelial cells, desmin-immunopositive cells, and folliculostellate cells, which were identified on the basis of their histochemical reaction to isolectin B4, desmin, and S100 protein, respectively (Fig. 4d, e, k). Recently, our group found two types of desmin-immunopositive cells in rat anterior pituitary gland: pericytes and novel desmin-immunopositive perivascular cells [17]. TIMP3 mRNA was not expressed in cells that produced ACTH, GH, prolactin, TSH, or LH (Fig. 4f–j). TIMP3 mRNA was also detected in cells located around the endocrine cell cluster in the intermediate lobe (Supplementary Fig. 2a). In addition, TIMP3 mRNA was expressed in pituicytes, endothelial cells, and pericytes in the posterior lobe (Supplementary Fig. 2b–e).
Fig. 4. .
Double-staining of TIMP3 mRNA detected by in situ hybridization and isolectin B4, desmin, hormones, and S100 protein detected by immunohistochemistry in rat anterior pituitary gland. a, b: In situ hybridization for TIMP3. TIMP3-expressing cells were observed in blood capillaries, perivascular spaces (a), and the anterior lobe (b). c: Negative control with sense probe. d–k: In situ hybridization of TIMP3 and immunohistochemistry of isolectin B4 (endothelial cells; d), desmin (e), ACTH (f), GH (g), prolactin (h), TSHβ (i), LHβ (j), and S100 protein (folliculostellate cells; k). In situ hybridization with NBT/BCIP (blue) and immunostaining with 3,3'-diaminobenzidine (brown). TIMP3 mRNA was colocalized with the isolectin B4, desmin, and S100 protein immunoreactions (arrows). Bar=10 μm (a–k).
IV. Discussion
This is the first report to describe TIMP expression in rat anterior pituitary gland. Double-staining with in situ hybridization and immunohistochemistry revealed that folliculostellate cells express TIMP1, TIMP2, and TIMP3; that endothelial cells, pericytes, and novel desmin-immunopositive perivascular cells express TIMP3; and that prolactin cells and TSH cells express TIMP2.
A central mechanism in ECM remodeling is the cleavage of ECM components by MMP. TIMPs inhibit MMP activity and are thus important in the maintenance of ECM components. Homeostasis of ECM is controlled by the combination of MMP activation and inhibition by TIMPs (for a review, see [2]). Although all TIMPs inhibit the proteolytic activity of MMPs, they differ somewhat in expression pattern and interaction with MMP zymogens [7, 23]. It has been reported that TIMP1, TIMP2, and TIMP3 are widely expressed. In contrast, TIMP4 was definitely detected in heart, brain, ovary, and skeletal muscle [19, 26]. In the present study, RT-PCR showed expression of TIMP1, TIMP2, and TIMP3 mRNA, but not TIMP4 mRNA, in rat anterior pituitary gland (Fig. 1). To further understanding of the mechanism responsible for maintaining local ECM in the gland, we attempted to identify the cells that express TIMPs. Histological observations using in situ hybridization revealed that TIMP1, TIMP2, and TIMP3 mRNA were distributed in different cell populations in rat anterior pituitary gland (Fig. 1c–h).
Folliculostellate cells express S100 protein and regulate endocrine cells by secreting several humoral factors, such as basic fibroblast growth factor, vascular endothelial growth factor, and interleukin-6 (for a review, see [15]). In this study, we found that folliculostellate cells expressed TIMP1, TIMP2, and TIMP3 in rat anterior pituitary gland and that TIMP1 is expressed specifically in folliculostellate cells (Fig. 2i, Fig. 3i, Fig. 4k). Matsumoto et al. reported that TIMP2 is secreted from TtT/GF, a mouse folliculostellate cell line, as a cell survival factor in the pituitary gland [22]. The present study is the first to show that three types of TIMP are produced in folliculostellate cells of the anterior pituitary gland in vivo. Interestingly, our series of studies demonstrated that folliculostellate cell functions are influenced by the interaction between folliculostellate cell and ECMs [11, 12]. In addition, folliculostellate cells produce MMP9, which is required for interconnection and proliferation of folliculostellate cells on laminin [14]. Our present and previous observations indicate that folliculostellate cells not only secrete growth factors for regulating endocrine cells, but also actively respond to ECMs and modulate the amount of ECMs involved in creating the cellular environment of the gland by producing the required catabolic enzymes and inhibitors. In immunohistochemical studies, Tomita showed that normal human anterior pituitary cells contain MMP2, MMP9, and lesser amounts of TIMP1 and TIMP2 [32]. To further elucidate the mechanism for ECM remodeling in rat anterior pituitary gland, we need to assess the correlations between the enzymes and their inhibitors.
Anterior pituitary cells form lobules surrounded by ECMs, and the blood capillaries run around the lobules in the rat gland [30]. Basement membrane proteins and collagen fibers were observed in the perivascular space of the gland [18, 29]. Our previous studies revealed that the two types of desmin-immunopositive cells (pericytes and novel desmin-immunopositive perivascular cells) in the gland produce type I and III collagens [6, 17]. In addition, the endothelial cells in the gland express the basement membrane component laminin [27, 28]. The present study revealed that the endothelial cells, pericytes, and novel desmin-immunopositive perivascular cells express TIMP3 in rat anterior pituitary gland (Fig. 4d, e). A previous study showed that, unlike other TIMPs, TIMP3 is entrapped in the ECM by binding to sulfated glycosaminoglycans [35]. Our findings suggest that ECM-producing cells, endothelial cells, pericytes, and novel desmin-immunopositive perivascular cells also secrete TIMP3, which remains with the ECM in the perivascular spaces of rat anterior pituitary gland and contributes to the deposition of ECM components in those spaces.
We found that TIMP2 mRNA is expressed in endocrine cells, prolactin cells, and TSH cells (Fig. 3f, g). This suggests that the active state of endocrine cells affects TIMP2 expression and can alter the ECM state in rat anterior pituitary gland. This result is important in clarifying the interaction between hormone-secreting cells and ECM in the gland.
Mander and Morris showed that macrophages are present in rat anterior pituitary gland [21]. We examined expression of TIMPs in macrophages of the gland by double-staining with in situ hybridization for TIMPs and immunohistochemistry for markers of macrophages (data not shown). We found that the number of macrophages was extremely low in the gland. Furthermore, only some macrophages were positive in in situ hybridization for TIMPs in the gland. The low number of macrophages suggests that TIMPs of macrophages are not important in the maintenance of ECM in the gland.
The present results strongly suggest that appropriate utilization of TIMPs and TIMP-expressing cells is crucial for local ECM remodeling in the rat anterior pituitary gland.
V. Declaration of Interest
The authors have no conflict of interest that might prejudice the impartiality of this research.
VI. Acknowledgments
We thank David Kipler, ELS, of Supernatant Communications for assistance in manuscript preparation.
This work was partly supported by JSPS KAKENHI Grants (Numbers 25460275 to MK, 25860147 to TT, 26460281 to KF), by promotional funds from the Keirin Race of the Japan Keirin Association, and by the Jichi Medical University Young Investigator Award from Jichi Medical University School of Medicine.
Supplementary Materials
VII. References
- 1.Beaulieu E., Kachra Z., Mousseau N., Delbecchi L., Hardy J. and Béliveau R. (1999) Matrix metalloproteinases and their inhibitors in human pituitary tumors. Neurosurgery 45; 1432–1440; discussion 1440–1441. [DOI] [PubMed] [Google Scholar]
- 2.Brew K., Dinakarpandian D. and Nagase H. (2000) Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim. Biophys. Acta 1477; 267–283. [DOI] [PubMed] [Google Scholar]
- 3.Chakraborti S., Mandal M., Das S., Mandal A. and Chakraborti T. (2003) Regulation of matrix metalloproteinases: an overview. Mol. Cell. Biochem. 253; 269–285. [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara K., Kikuchi M., Takigami S., Kouki T. and Yashiro T. (2007) Expression of retinaldehyde dehydrogenase 1 in the anterior pituitary glands of adult rats. Cell Tissue Res. 329; 321–327. [DOI] [PubMed] [Google Scholar]
- 5.Fujiwara K., Maekawa F., Kikuchi M., Takigami S., Yada T. and Yashiro T. (2007) Expression of retinaldehyde dehydrogenase (RALDH) 2 and RALDH3 but not RALDH1 in the developing anterior pituitary glands of rats. Cell Tissue Res. 328; 129–135. [DOI] [PubMed] [Google Scholar]
- 6.Fujiwara K., Jindatip D., Kikuchi M. and Yashiro T. (2010) In situ hybridization reveals that type I and III collagens are produced by pericytes in the anterior pituitary gland of rats. Cell Tissue Res. 342; 491–495. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg G. I., Strongin A., Collier I. E., Genrich L. T. and Marmer B. L. (1992) Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J. Biol. Chem. 267; 4583–4591. [PubMed] [Google Scholar]
- 8.Greene J., Wang M., Liu Y. E., Raymond L. A., Rosen C. and Shi Y. E. (1996) Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J. Biol. Chem. 271; 30375–30380. [DOI] [PubMed] [Google Scholar]
- 9.Holck S., Albrechtsen R. and Wewer U. M. (1987) Laminin in the anterior pituitary gland of the rat. Laminin in the gonadotrophic cells correlates with their functional state. Lab. Invest. 56; 481–488. [PubMed] [Google Scholar]
- 10.Horacek, M. J., Kawaguchi, T. and Terracio, L. (1994) Adult adenohypophysial cells express beta 1 integrins and prefer laminin during cell-substratum adhesion. In Vitro Cell. Dev. Biol. Anim. 30A; 35–40. [DOI] [PubMed] [Google Scholar]
- 11.Horiguchi K., Kikuchi M., Kusumoto K., Fujiwara K., Kouki T., Kawanishi K. and Yashiro T. (2010) Living-cell imaging of transgenic rat anterior pituitary cells in primary culture reveals novel characteristics of folliculo-stellate cells. J. Endocrinol. 204; 115–123. [DOI] [PubMed] [Google Scholar]
- 12.Horiguchi K., Kouki T., Fujiwara K., Kikuchi M. and Yashiro T. (2011) The extracellular matrix component laminin promotes gap junction formation in the rat anterior pituitary gland. J. Endocrinol. 208; 225–232. [DOI] [PubMed] [Google Scholar]
- 13.Horiguchi K., Syaidah R., Fujiwara K., Tsukada T., Ramadhani D., Jindatip D., Kikuchi M. and Yashiro T. (2013) Expression of small leucine-rich proteoglycans in rat anterior pituitary gland. Cell Tissue Res. 351; 207–212. [DOI] [PubMed] [Google Scholar]
- 14.Ilmiawati C., Horiguchi K., Fujiwara K. and Yashiro T. (2012) Matrix metalloproteinase-9 expression in folliculostellate cells of rat anterior pituitary gland. J. Endocrinol. 212; 363–370. [DOI] [PubMed] [Google Scholar]
- 15.Inoue K., Couch E. F., Takano K. and Ogawa S. (1999) The structure and function of folliculo-stellate cells in the anterior pituitary gland. Arch. Histol. Cytol. 62; 205–218. [DOI] [PubMed] [Google Scholar]
- 16.Jindatip D., Fujiwara K., Kouki T. and Yashiro T. (2012) Transmission and scanning electron microscopy study of the characteristics and morphology of pericytes and novel desmin-immunopositive perivascular cells before and after castration in rat anterior pituitary gland. Anat. Sci. Int. 87; 165–173. [DOI] [PubMed] [Google Scholar]
- 17.Jindatip D., Fujiwara K., Horiguchi K., Tsukada T., Kouki T. and Yashiro T. (2013) Changes in fine structure of pericytes and novel desmin-immunopositive perivascular cells during postnatal development in rat anterior pituitary gland. Anat. Sci. Int. 88; 196–203. [DOI] [PubMed] [Google Scholar]
- 18.Kaidzu S., Noda T., Tane N. and Yashiro T. (2000) Collagen synthesis by rat anterior pituitary cells in vivo and in vitro. Acta Histochem. Cytochem. 33; 81–87. [Google Scholar]
- 19.Leco K. J., Apte S. S., Taniguchi G. T., Hawkes S. P., Khokha R., Schultz G. A. and Edwards D. R. (1997) Murine tissue inhibitor of metalloproteinases-4 (Timp-4): cDNA isolation and expression in adult mouse tissues. FEBS Lett. 401; 213–217. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y. C., Tanaka S., Inoue K. and Kurosumi K. (1989) Localization of fibronectin in the folliculo-stellate cells of the rat anterior pituitary by the double bridge peroxidase-antiperoxidase method. Histochemistry 92; 43–45. [DOI] [PubMed] [Google Scholar]
- 21.Mander T. H. and Morris J. F. (1996) Development of microglia and macrophages in the postnatal rat pituitary. Cell Tissue Res. 286; 347–355. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto H., Ishibashi Y., Ohtaki T., Hasegawa Y., Koyama C. and Inoue K. (1993) Newly established murine pituitary folliculo-stellate-like cell line (TtT/GF) secretes potent pituitary glandular cell survival factors, one of which corresponds to metalloproteinase inhibitor. Biochem. Biophys. Res. Commun. 194; 909–915. [DOI] [PubMed] [Google Scholar]
- 23.Nagase H. (1998) Cell surface activation of progelatinase A (proMMP-2) and cell migration. Cell Res. 8; 179–186. [DOI] [PubMed] [Google Scholar]
- 24.Paez-Pereda M., Kuchenbauer F., Arzt E. and Stalla G. K. (2005) Regulation of pituitary hormones and cell proliferation by components of the extracellular matrix. Braz. J. Med. Biol. Res. 38; 1487–1494. [DOI] [PubMed] [Google Scholar]
- 25.Pavloff N., Staskus P. W., Kishnani N. S. and Hawkes S. P. (1992) A new inhibitor of metalloproteinases from chicken: ChIMP-3. A third member of the TIMP family. J. Biol. Chem. 267; 17321–17326. [PubMed] [Google Scholar]
- 26.Rahkonen O. P., Koskivirta I. M., Oksjoki S. M., Jokinen E. and Vuorio E. I. (2002) Characterization of the murine Timp4 gene, localization within intron 5 of the synapsin 2 gene and tissue distribution of the mRNA. Biochim. Biophys. Acta 1577; 45–52. [DOI] [PubMed] [Google Scholar]
- 27.Ramadhani D., Tsukada T., Fujiwara K., Horiguchi K., Kikuchi M. and Yashiro T. (2012) Laminin isoforms and laminin-producing cells in rat anterior pituitary. Acta Histochem. Cytochem. 45; 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramadhani D., Tsukada T., Fujiwara K., Azuma M., Kikuchi M. and Yashiro T. (2014) Changes in laminin chain expression in pre- and postnatal rat pituitary gland. Acta Histochem. Cytochem. 47; 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirasawa N., Mabuchi Y., Sakuma E., Horiuchi O., Yashiro T., Kikuchi M., Hashimoto Y., Tsuruo Y., Herbert D. C. and Soji T. (2004) Intercellular communication within the rat anterior pituitary gland: X. Immunohistocytochemistry of S-100 and connexin 43 of folliculo-stellate cells in the rat anterior pituitary gland. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 278; 462–473. [DOI] [PubMed] [Google Scholar]
- 30.Soji T. and Herbert D. C. (1989) Intercellular communication between rat anterior pituitary cells. Anat. Rec. 224; 523–533. [DOI] [PubMed] [Google Scholar]
- 31.Stetler-Stevenson W. G., Krutzsch H. C. and Liotta L. A. (1989) Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J. Biol. Chem. 264; 17374–17378. [PubMed] [Google Scholar]
- 32.Tomita T. (1997) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in pituitary adenomas: possible markers of neuroendocrine cells. Endocr. Pathol. 8; 305–313. [DOI] [PubMed] [Google Scholar]
- 33.Tougard C., Louvard D., Picart R. and Tixier-Vidal A. (1985) Immunocytochemical localization of laminin in rat anterior pituitary cells in vivo and in vitro. In Vitro Cell. Dev. Biol. 21; 57–61. [DOI] [PubMed] [Google Scholar]
- 34.Woolley D. E., Roberts D. R. and Evanson J. M. (1975) Inhibition of human collagenase activity by a small molecular weight serum protein. Biochem. Biophys. Res. Commun. 66; 747–754. [DOI] [PubMed] [Google Scholar]
- 35.Yu W. H., Yu S., Meng Q., Brew K. and Woessner J. F. Jr. (2000) TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J. Biol. Chem. 275; 31226–31232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.