Abstract
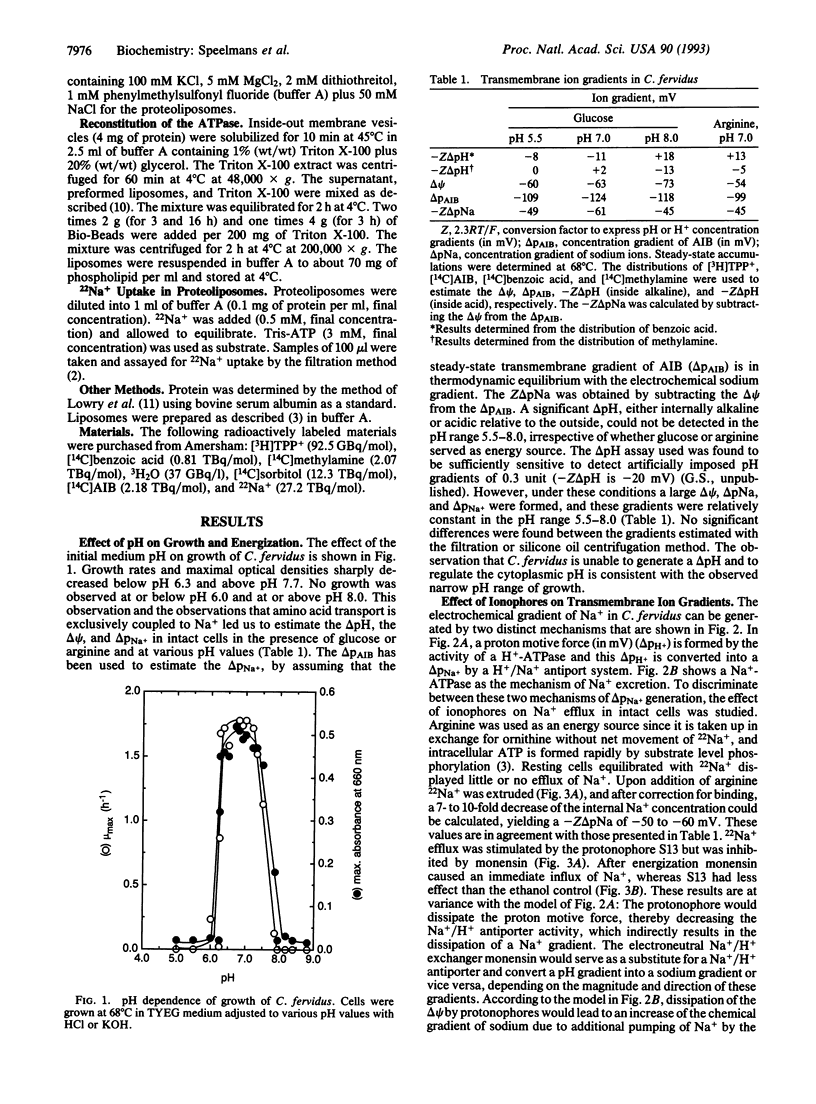
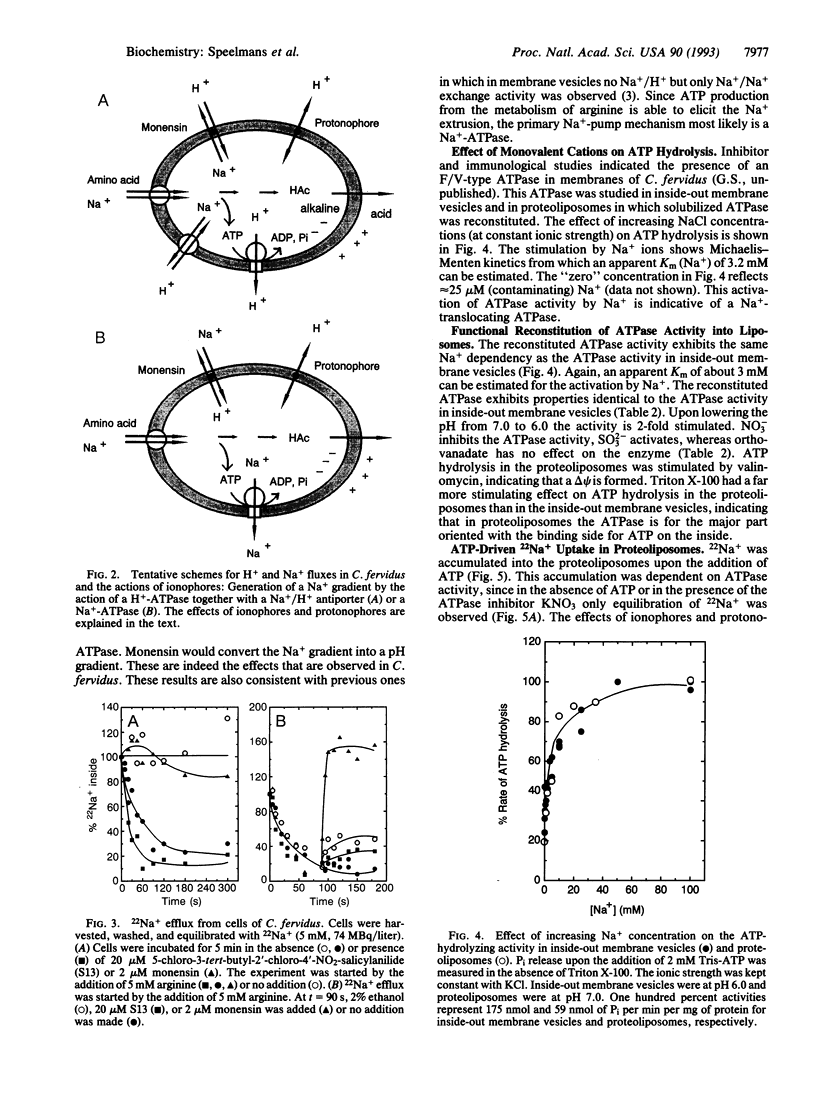
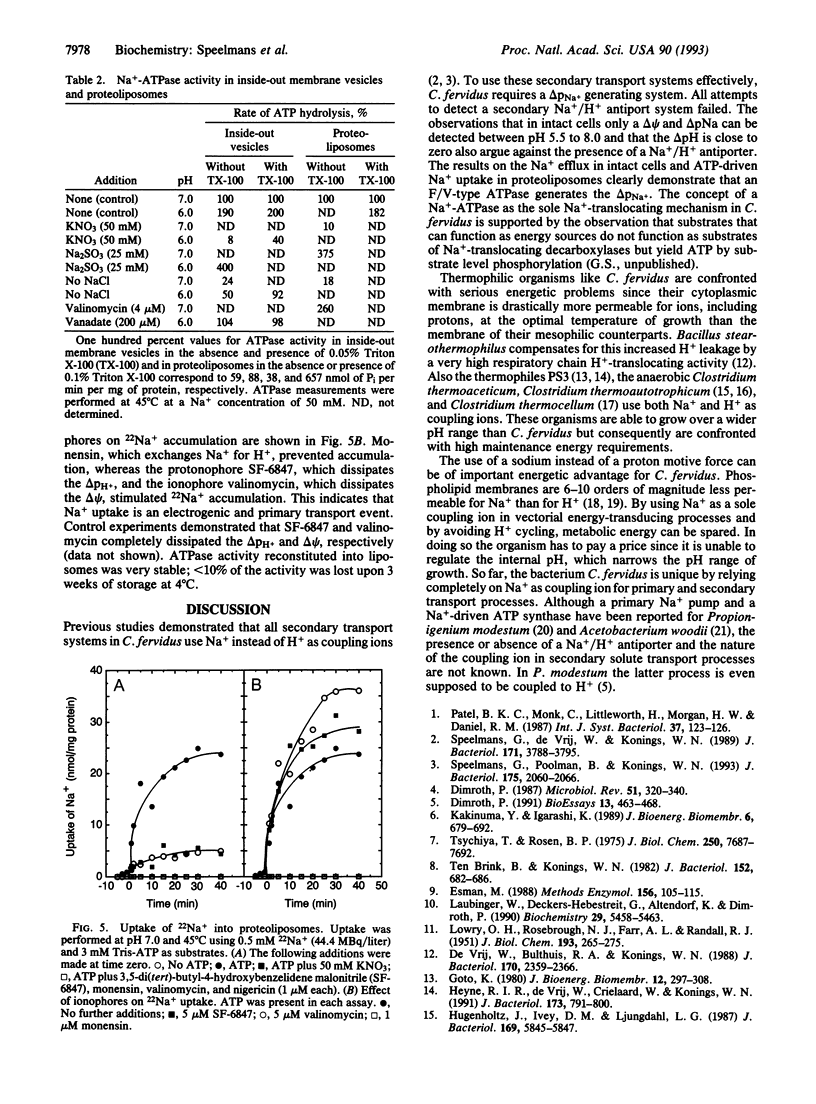
The thermophilic, peptidolytic, anaerobic bacterium Clostridium fervidus is unable to generate a pH gradient in the range of 5.5-8.0, which limits growth of the organism to a narrow pH range (6.3-7.7). A significant membrane potential (delta psi approximately -60 mV) and chemical gradient of Na+ (-Z delta pNa approximately -60 mV) are formed in the presence of metabolizable substrates. Energy-dependent Na+ efflux is inhibited by the Na+/H+ ionophore monensin but is stimulated by uncouplers, suggesting that the Na+ gradient is formed by a primary pumping mechanism rather than by secondary Na+/H+ antiport. This primary sodium pump was found to be an ATPase that has been characterized in inside-out membrane vesicles and in proteoliposomes in which solubilized ATPase was reconstituted. The enzyme is stimulated by Na+, resistant to vanadate, and sensitive to nitrate, which is indicative of an F/V-type Na(+)-ATPase. In the proteoliposomes Na+ accumulation depends on the presence of ATP, is inhibited by the ATPase inhibitor nitrate, and is completely prevented by the ionophore monensin but is stimulated by protonophores and valinomycin. These and previous observations, which indicated that secondary amino acid transport uses solely Na+ as coupling ion, demonstrate that energy transduction at the membrane in C. fervidus is exclusively dependent on a Na+ cycle.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Vrij W., Bulthuis R. A., Konings W. N. Comparative study of energy-transducing properties of cytoplasmic membranes from mesophilic and thermophilic Bacillus species. J Bacteriol. 1988 May;170(5):2359–2366. doi: 10.1128/jb.170.5.2359-2366.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimroth P. Na(+)-coupled alternative to H(+)-coupled primary transport systems in bacteria. Bioessays. 1991 Sep;13(9):463–468. doi: 10.1002/bies.950130906. [DOI] [PubMed] [Google Scholar]
- Dimroth P. Sodium ion transport decarboxylases and other aspects of sodium ion cycling in bacteria. Microbiol Rev. 1987 Sep;51(3):320–340. doi: 10.1128/mr.51.3.320-340.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmann M. ATPase and phosphatase activity of Na+,K+-ATPase: molar and specific activity, protein determination. Methods Enzymol. 1988;156:105–115. doi: 10.1016/0076-6879(88)56013-5. [DOI] [PubMed] [Google Scholar]
- Goto K., Hirata H., Kagawa Y. A stable Na+/H+ antiporter of thermophilic bacterium PS3. J Bioenerg Biomembr. 1980 Aug;12(3-4):297–308. doi: 10.1007/BF00744690. [DOI] [PubMed] [Google Scholar]
- Heise R., Müller V., Gottschalk G. Presence of a sodium-translocating ATPase in membrane vesicles of the homoacetogenic bacterium Acetobacterium woodii. Eur J Biochem. 1992 Jun 1;206(2):553–557. doi: 10.1111/j.1432-1033.1992.tb16959.x. [DOI] [PubMed] [Google Scholar]
- Heyne R. I., de Vrij W., Crielaard W., Konings W. N. Sodium ion-dependent amino acid transport in membrane vesicles of Bacillus stearothermophilus. J Bacteriol. 1991 Jan;173(2):791–800. doi: 10.1128/jb.173.2.791-800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz J., Ivey D. M., Ljungdahl L. G. Carbon monoxide-driven electron transport in Clostridium thermoautotrophicum membranes. J Bacteriol. 1987 Dec;169(12):5845–5847. doi: 10.1128/jb.169.12.5845-5847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma Y., Igarashi K. Sodium-translocating adenosine triphosphatase in Streptococcus faecalis. J Bioenerg Biomembr. 1989 Dec;21(6):679–692. doi: 10.1007/BF00762686. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laubinger W., Deckers-Hebestreit G., Altendorf K., Dimroth P. A hybrid adenosinetriphosphatase composed of F1 of Escherichia coli and F0 of Propionigenium modestum is a functional sodium ion pump. Biochemistry. 1990 Jun 12;29(23):5458–5463. doi: 10.1021/bi00475a008. [DOI] [PubMed] [Google Scholar]
- Laubinger W., Dimroth P. Characterization of the ATP synthase of Propionigenium modestum as a primary sodium pump. Biochemistry. 1988 Sep 20;27(19):7531–7537. doi: 10.1021/bi00419a053. [DOI] [PubMed] [Google Scholar]
- Nichols J. W., Deamer D. W. Net proton-hydroxyl permeability of large unilamellar liposomes measured by an acid-base titration technique. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2038–2042. doi: 10.1073/pnas.77.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speelmans G., Poolman B., Konings W. N. Amino acid transport in the thermophilic anaerobe Clostridium fervidus is driven by an electrochemical sodium gradient. J Bacteriol. 1993 Apr;175(7):2060–2066. doi: 10.1128/jb.175.7.2060-2066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speelmans G., de Vrij W., Konings W. N. Characterization of amino acid transport in membrane vesicles from the thermophilic fermentative bacterium Clostridium fervidus. J Bacteriol. 1989 Jul;171(7):3788–3795. doi: 10.1128/jb.171.7.3788-3795.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano J. S., Schreurs W. J., Kashket E. R. Membrane H Conductance of Clostridium thermoaceticum and Clostridium acetobutylicum: Evidence for Electrogenic Na/H Antiport in Clostridium thermoaceticum. Appl Environ Microbiol. 1987 Apr;53(4):782–786. doi: 10.1128/aem.53.4.782-786.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Rosen B. P. Characterization of an active transport system for calcium in inverted membrane vesicles of Escherichia coli. J Biol Chem. 1975 Oct 10;250(19):7687–7692. [PubMed] [Google Scholar]
- ten Brink B., Konings W. N. Electrochemical proton gradient and lactate concentration gradient in Streptococcus cremoris cells grown in batch culture. J Bacteriol. 1982 Nov;152(2):682–686. doi: 10.1128/jb.152.2.682-686.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



