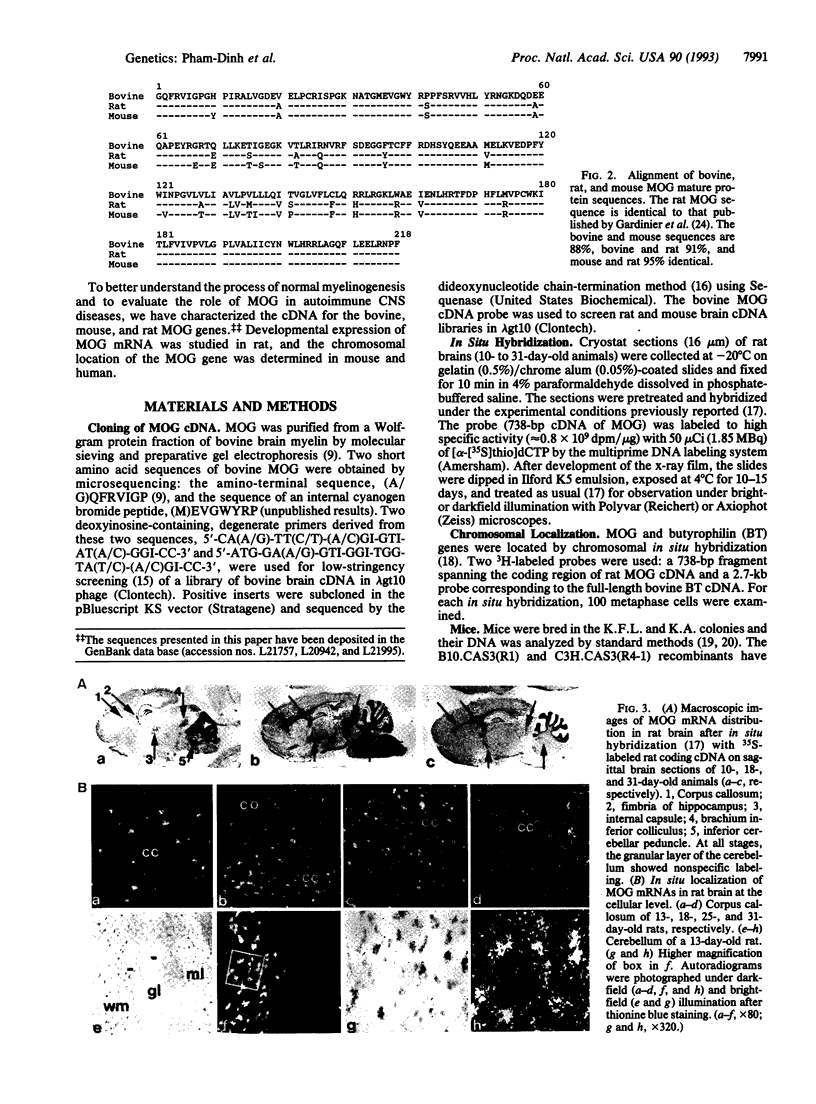
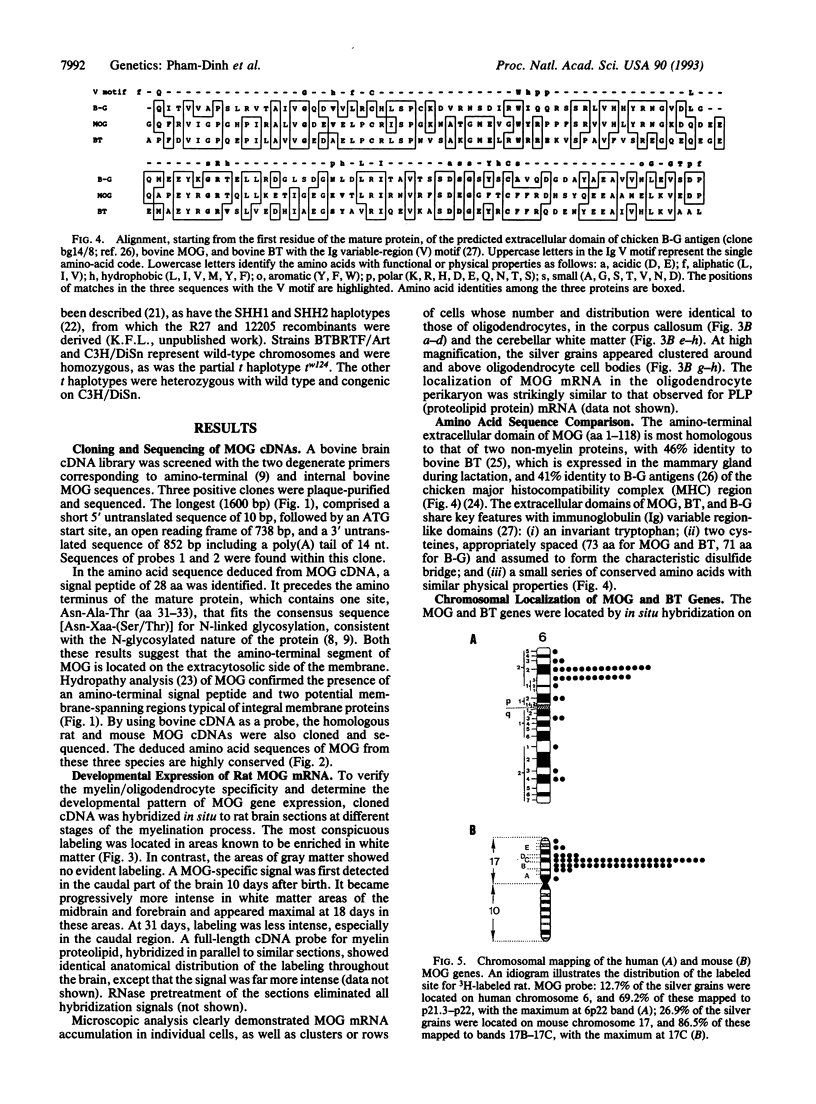
Abstract
Myelin/oligodendrocyte glycoprotein (MOG) is found on the surface of myelinating oligodendrocytes and external lamellae of myelin sheaths in the central nervous system, and it is a target antigen in experimental autoimmune encephalomyelitis and multiple sclerosis. We have isolated bovine, mouse, and rat MOG cDNA clones and shown that the developmental pattern of MOG expression in the rat central nervous system coincides with the late stages of myelination. The amino-terminal, extracellular domain of MOG has characteristics of an immunoglobulin variable domain and is 46% and 41% identical with the amino terminus of bovine butyrophilin (expressed in the lactating mammary gland) and B-G antigens of the chicken major histocompatibility complex (MHC), respectively; these proteins thus form a subset of the immunoglobulin superfamily. The homology between MOG and B-G extends beyond their structure and genetic mapping to their ability to induce strong antibody responses and has implications for the role of MOG in pathological, autoimmune conditions. We colocalized the MOG and BT genes to the human MHC on chromosome 6p21.3-p22. The mouse MOG gene was mapped to the homologous band C of chromosome 17, within the M region of the mouse MHC.
Full text
PDF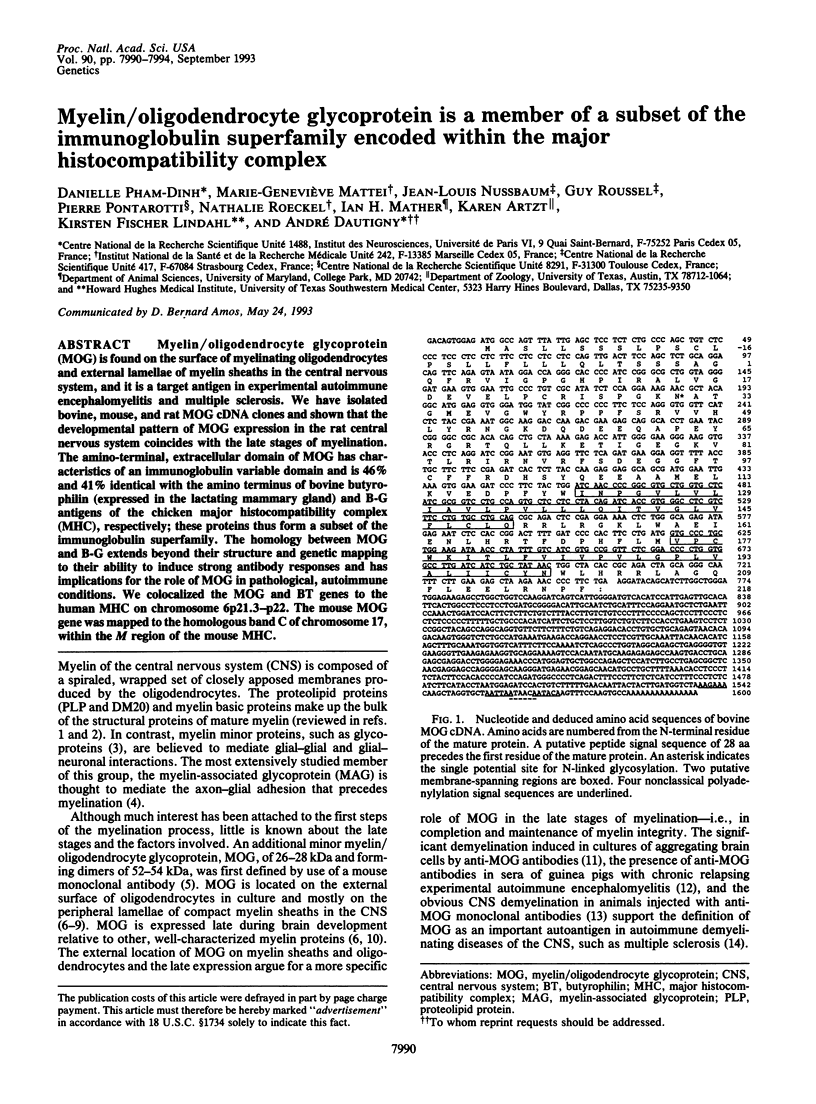


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiguet P., Gardinier M. V., Zanetta J. P., Matthieu J. M. Purification and partial structural and functional characterization of mouse myelin/oligodendrocyte glycoprotein. J Neurochem. 1992 May;58(5):1676–1682. doi: 10.1111/j.1471-4159.1992.tb10040.x. [DOI] [PubMed] [Google Scholar]
- Brunner C., Lassmann H., Waehneldt T. V., Matthieu J. M., Linington C. Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2',3'-cyclic nucleotide 3'-phosphodiesterase in the CNS of adult rats. J Neurochem. 1989 Jan;52(1):296–304. doi: 10.1111/j.1471-4159.1989.tb10930.x. [DOI] [PubMed] [Google Scholar]
- D'Urso D., Brophy P. J., Staugaitis S. M., Gillespie C. S., Frey A. B., Stempak J. G., Colman D. R. Protein zero of peripheral nerve myelin: biosynthesis, membrane insertion, and evidence for homotypic interaction. Neuron. 1990 Mar;4(3):449–460. doi: 10.1016/0896-6273(90)90057-m. [DOI] [PubMed] [Google Scholar]
- Filbin M. T., Walsh F. S., Trapp B. D., Pizzey J. A., Tennekoon G. I. Role of myelin P0 protein as a homophilic adhesion molecule. Nature. 1990 Apr 26;344(6269):871–872. doi: 10.1038/344871a0. [DOI] [PubMed] [Google Scholar]
- Fischer Lindahl K., Hermel E., Loveland B. E., Wang C. R. Maternally transmitted antigen of mice: a model transplantation antigen. Annu Rev Immunol. 1991;9:351–372. doi: 10.1146/annurev.iy.09.040191.002031. [DOI] [PubMed] [Google Scholar]
- Gardinier M. V., Amiguet P., Linington C., Matthieu J. M. Myelin/oligodendrocyte glycoprotein is a unique member of the immunoglobulin superfamily. J Neurosci Res. 1992 Sep;33(1):177–187. doi: 10.1002/jnr.490330123. [DOI] [PubMed] [Google Scholar]
- Howard C. A., Gummere G. R., Lyon M. F., Bennett D., Artzt K. Genetic and molecular analysis of the proximal region of the mouse t-complex using new molecular probes and partial t-haplotypes. Genetics. 1990 Dec;126(4):1103–1114. doi: 10.1093/genetics/126.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller T., Hood L. Diversity of the immunoglobulin gene superfamily. Adv Immunol. 1989;44:1–63. doi: 10.1016/s0065-2776(08)60639-2. [DOI] [PubMed] [Google Scholar]
- Jack L. J., Mather I. H. Cloning and analysis of cDNA encoding bovine butyrophilin, an apical glycoprotein expressed in mammary tissue and secreted in association with the milk-fat globule membrane during lactation. J Biol Chem. 1990 Aug 25;265(24):14481–14486. [PubMed] [Google Scholar]
- Kerlero de Rosbo N., Honegger P., Lassmann H., Matthieu J. M. Demyelination induced in aggregating brain cell cultures by a monoclonal antibody against myelin/oligodendrocyte glycoprotein. J Neurochem. 1990 Aug;55(2):583–587. doi: 10.1111/j.1471-4159.1990.tb04173.x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- LeVine S. M., Wong D., Macklin W. B. Developmental expression of proteolipid protein and DM20 mRNAs and proteins in the rat brain. Dev Neurosci. 1990;12(4-5):235–250. doi: 10.1159/000111853. [DOI] [PubMed] [Google Scholar]
- Lebar R., Boutry J. M., Vincent C., Robineaux R., Voisin G. A. Studies on autoimmune encephalomyelitis in the guinea pig. II. An in vitro investigation on the nature, properties, and specificity of the serum-demyelinating factor. J Immunol. 1976 May;116(5):1439–1446. [PubMed] [Google Scholar]
- Lemke G. Unwrapping the genes of myelin. Neuron. 1988 Sep;1(7):535–543. doi: 10.1016/0896-6273(88)90103-1. [DOI] [PubMed] [Google Scholar]
- Lindahl K. F. Genetic variants of histocompatibility antigens from wild mice. Curr Top Microbiol Immunol. 1986;127:272–278. doi: 10.1007/978-3-642-71304-0_31. [DOI] [PubMed] [Google Scholar]
- Linington C., Bradl M., Lassmann H., Brunner C., Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol. 1988 Mar;130(3):443–454. [PMC free article] [PubMed] [Google Scholar]
- Linnington C., Webb M., Woodhams P. L. A novel myelin-associated glycoprotein defined by a mouse monoclonal antibody. J Neuroimmunol. 1984 Sep-Oct;6(6):387–396. doi: 10.1016/0165-5728(84)90064-x. [DOI] [PubMed] [Google Scholar]
- Mattei M. G., Philip N., Passage E., Moisan J. P., Mandel J. L., Mattei J. F. DNA probe localization at 18p113 band by in situ hybridization and identification of a small supernumerary chromosome. Hum Genet. 1985;69(3):268–271. doi: 10.1007/BF00293038. [DOI] [PubMed] [Google Scholar]
- Matthieu J. M., Amiguet P. Myelin/oligodendrocyte glycoprotein expression during development in normal and myelin-deficient mice. Dev Neurosci. 1990;12(4-5):293–302. doi: 10.1159/000111858. [DOI] [PubMed] [Google Scholar]
- Miller M. M., Goto R., Young S., Chirivella J., Hawke D., Miyada C. G. Immunoglobulin variable-region-like domains of diverse sequence within the major histocompatibility complex of the chicken. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4377–4381. doi: 10.1073/pnas.88.10.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadon N. L., Duncan I. D., Hudson L. D. A point mutation in the proteolipid protein gene of the 'shaking pup' interrupts oligodendrocyte development. Development. 1990 Oct;110(2):529–537. doi: 10.1242/dev.110.2.529. [DOI] [PubMed] [Google Scholar]
- Owens G. C., Boyd C. J., Bunge R. P., Salzer J. L. Expression of recombinant myelin-associated glycoprotein in primary Schwann cells promotes the initial investment of axons by myelinating Schwann cells. J Cell Biol. 1990 Sep;111(3):1171–1182. doi: 10.1083/jcb.111.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. I., Roa B. B., Welcher A. A., Schoener-Scott R., Trask B. J., Pentao L., Snipes G. J., Garcia C. A., Francke U., Shooter E. M. The gene for the peripheral myelin protein PMP-22 is a candidate for Charcot-Marie-Tooth disease type 1A. Nat Genet. 1992 Jun;1(3):159–165. doi: 10.1038/ng0692-159. [DOI] [PubMed] [Google Scholar]
- Poduslo S. E. Proteins and glycoproteins in plasma membranes and in the membrane lamellae produced by purified oligodendroglia in culture. Biochim Biophys Acta. 1983 Feb 9;728(1):59–65. doi: 10.1016/0005-2736(83)90436-4. [DOI] [PubMed] [Google Scholar]
- Poltorak M., Sadoul R., Keilhauer G., Landa C., Fahrig T., Schachner M. Myelin-associated glycoprotein, a member of the L2/HNK-1 family of neural cell adhesion molecules, is involved in neuron-oligodendrocyte and oligodendrocyte-oligodendrocyte interaction. J Cell Biol. 1987 Oct;105(4):1893–1899. doi: 10.1083/jcb.105.4.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S., Bucan M., Brorson K., Kiefer M. C., Hunt S. W., 3rd, Lehrach H., Lindahl K. F. Genetic and molecular mapping of the Hmt region of mouse. EMBO J. 1989 Dec 1;8(12):3749–3757. doi: 10.1002/j.1460-2075.1989.tb08551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel G., Felix J. M., Dautigny A., Pham-Dinh D., Hindelang C., Jollès P., Nussbaum J. L. In situ localization of NF-H neurofilament subunit mRNAs in rat brain. Dev Neurosci. 1991;13(2):98–103. doi: 10.1159/000112146. [DOI] [PubMed] [Google Scholar]
- Salomonsen J., Dunon D., Skjødt K., Thorpe D., Vainio O., Kaufman J. Chicken major histocompatibility complex-encoded B-G antigens are found on many cell types that are important for the immune system. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1359–1363. doi: 10.1073/pnas.88.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomonsen J., Eriksson H., Skjødt K., Lundgreen T., Simonsen M., Kaufman J. The "adjuvant effect" of the polymorphic B-G antigens of the chicken major histocompatibility complex analyzed using purified molecules incorporated in liposomes. Eur J Immunol. 1991 Mar;21(3):649–658. doi: 10.1002/eji.1830210317. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliess F., Stoffel W. Evolution of the myelin integral membrane proteins of the central nervous system. Biol Chem Hoppe Seyler. 1991 Sep;372(9):865–874. doi: 10.1515/bchm3.1991.372.2.865. [DOI] [PubMed] [Google Scholar]
- Scolding N. J., Frith S., Linington C., Morgan B. P., Campbell A. K., Compston D. A. Myelin-oligodendrocyte glycoprotein (MOG) is a surface marker of oligodendrocyte maturation. J Neuroimmunol. 1989 May;22(3):169–176. doi: 10.1016/0165-5728(89)90014-3. [DOI] [PubMed] [Google Scholar]
- Sun J., Link H., Olsson T., Xiao B. G., Andersson G., Ekre H. P., Linington C., Diener P. T and B cell responses to myelin-oligodendrocyte glycoprotein in multiple sclerosis. J Immunol. 1991 Mar 1;146(5):1490–1495. [PubMed] [Google Scholar]
- Wang C. R., Lindahl K. F. Organization and structure of the H-2M4-M8 class I genes in the mouse major histocompatibility complex. Immunogenetics. 1993;38(4):258–271. doi: 10.1007/BF00188802. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]