Abstract
Sphingoid base derivatives have attracted increasing attention as promising chemotherapeutic candidates against lifestyle diseases such as diabetes and cancer. Natural sphingoid bases can be a potential resource instead of those derived by time-consuming total organic synthesis. In particular, glucosylceramides (GlcCers) in food plants are enriched sources of sphingoid bases, differing from those of animals. Several chemical methodologies to transform GlcCers to sphingoid bases have already investigated; however, these conventional methods using acid or alkaline hydrolysis are not efficient due to poor reaction yield, producing complex by-products and resulting in separation problems. In this study, an extremely efficient and practical chemoenzymatic transformation method has been developed using microwave-enhanced butanolysis of GlcCers and a large amount of readily available almond β-glucosidase for its deglycosylation reaction of lysoGlcCers. The method is superior to conventional acid/base hydrolysis methods in its rapidity and its reaction cleanness (no isomerization, no rearrangement) with excellent overall yield.
Keywords: sphingolipids, chemoenzymatic synthesis, ceramide glucoside, enzymology, mass spectrometry
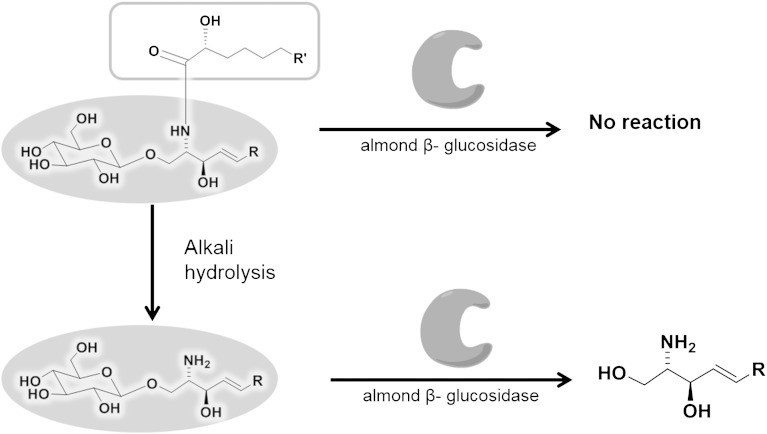
Glucosylceramides (GlcCers) are found as one of the major lipid components in plants, animals, human epidermis, and fungi (1, 2). GlcCers are composed of a sphingoid base whose amino group at the C-2 positon is N-acylated with a fatty acid and a hydroxyl group at the C-1 position glycosylated with a glucose. LysoGlcCers are defined as GlcCers that lack fatty acids and are found rarely with relatively small amounts compared to GlcCers in plasma membranes. Loss of glucose from LysoGlcCer leads to sphingoid bases, which are basic and characteristic structural units of GlcCers with more than 60 structural variations in nature (3).
Although sphingoid bases possess the basic structures of the sphingolipid family, little research has been done on them due to their low abundance in nature. It has been reported that the sphingoid bases have a wide range of biological activity, including anticoagulant, mycostatic and bacteriostatic properties as well as changes in circulation dynamics (4). Recently, considerable attention has been devoted toward their properties against human colon cancer, intestinal cancer, breast cancer, and various other pathogens (5–7). In order to investigate detailed biological activities, an adequate supply of diverse sphingoid bases is essential. Natural sphingoid bases can be used as resources instead of those produced by time-consuming total organic synthesis. In particular, GlcCers in food plants are enriched sources of sphingoid bases, differing from those of animals. The conventional transformation of GlcCers to sphingoid bases is accomplished by an acid or alkali hydrolysis reaction with heating. However, these methods have practical disadvantages, such as purification problems and low efficiency.
In the case of the acid-catalyzed hydrolysis reaction, isomerization and degradation are frequently observed. This is caused by an allylic alcohol group of GlcCers, which readily reacts with solvents such as methanol leading to the introduction of a methoxy group at the C3 position, which also reacts as an ester via N-acyl migration and more allylic rearrangements (3, 8). These complex reactions cause a separation problem as well as a low yield. In the case of base-catalyzed hydrolysis, the β-glycosidic bond is much more resistant than the N-acylated bond against alkali hydrolysis and thus, the reaction does not produce sphingoid bases but lysoGlcCers as a major product (9, 10). Besides acid- or base-catalyzed hydrolysis, an oxidative-reductive hydrolysis method is also used to prepare ceramides from GlcCers and to further to synthesize sphingosine by alkali hydrolysis (11); however, this method is relatively laborious and N-acyl linkages of the GlcCer must be protected during hydrolysis.
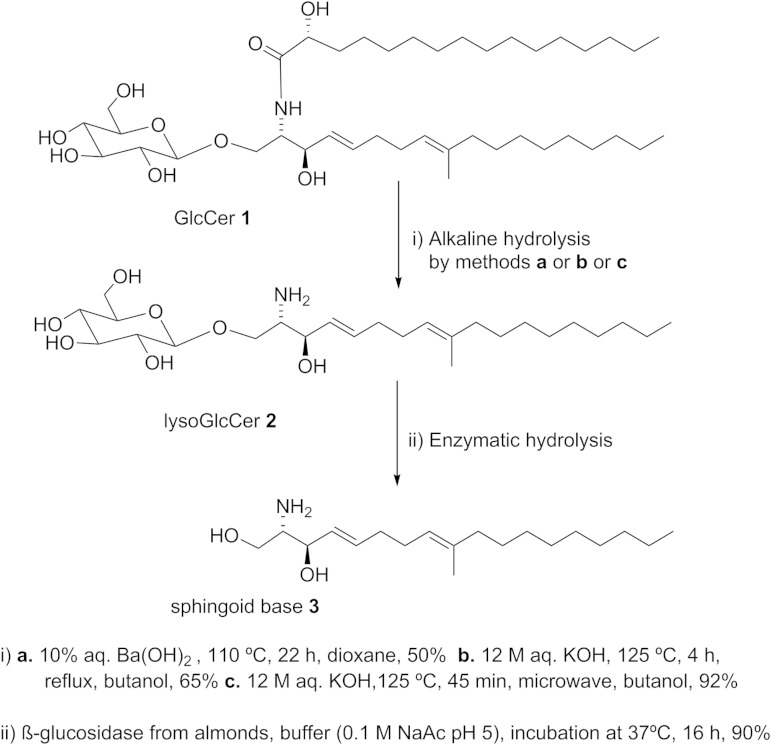
In this study, a novel chemoenzymatic method (Fig. 1)was developed to prepare sphingoid bases from various kinds of GlcCers with high efficiency. The first step is performed by an alkali-catalyzed hydrolysis reaction accelerated by microwave irradiation leading to selective and efficient cleavage of the amide bond to obtain lysoGlcCers. The second step is accomplished by an enzymatic hydrolysis of the β-glycosidic bond in GlcCers by almond β-glucosidase for industrial use. The removal of the acyl chain increases the hydrophilic character to increase accessibility toward the enzymatic active center of β-glucosidase. In fact, hydrolysis of original GlcCer from the golden oyster mushroom (Pleurotus citrinopileatus) by almond β-glucosidase did not work even after adding Triton X-100. Our strategy to increase accessibility toward the enzyme by removing the hydrophobic fatty acid chain by microwave-assisted efficient basic hydrolysis is quite unique and practical. Several sphingoid bases were successfully prepared in high yield by employing the designed method from a wide variety of GlcCers. This novel method provides a new way to prepare various kinds of sphingoid bases from GlcCers of dietary natural resources such as rice, wheat, and soy, which are suitable for practical use.
Fig. 1.
Chemoenzymatic methodology for the preparation of sphingoid bases.
MATERIALS AND METHODS
Materials
Standard GlcCer mixtures from the golden oyster mushroom (tamogitake in Japanese), rice, wheat, and soy were purchased from Funakoshi (Tokyo, Japan). Cerebrosides from porcine brain (C18 galactosylceramide, purity >99%) were purchased from Avanti Polar Lipids (Alabaster, AL). β-Glucosidase from almonds (amygdalase from almonds), p-nitrophenyl (pNP) glucoside, and pNP galactoside were obtained from Tokyo Chemical Industry (Tokyo, Japan). Defatted sweet-almond flour was purchased at Asahi Food Industries (Hyogo, Japan). Ammonia solution (28% in water), n-butanol (>98%), TritonTM X-100, and ninhydrin spray (0.5% in n-butanol) were purchased from Wako Pure Chemical Industries (Tokyo, Japan), Potassium hydroxide and barium hydroxide were purchased from Nacalai Tesque (Tokyo, Japan). All the other solvents used were of reagent grade and were purchased from Kanto Chemical (Tokyo, Japan). NMR solvents, C5D5N, CDCl3, and CD3OD were purchased from Cambridge Isotope Laboratories (Tewksbury, MA). A methanol extract of dried fruiting bodies of golden oyster mushrooms was kindly supplied by Nissei Bio (Sapporo, Japan).
1H-NMR, 13C, 1H-1H correlation spectroscopy (COSY), heteronuclear multiple quantum coherence (HMQC), and heteronuclear multiple bond coherence (HMBC) were recorded on a Varian Inova instrument (500 MHz) at 25°C in pyridine-d5, CDCl3, and CD3OD. Chemical shifts (δ) are reported in parts per million and coupling constant values (J) are reported in Hertz (Hz). ESI mass spectra were obtained by a JEOL JMS-T100LP spectrometer. Optical rotation measurements were carried out by JASCO-P-1020 polarimeter. Analytical TLC was performed on 0.2 mm silica gel plates (Merck 60 F-254). Silica gel column chromatography was carried out by using silica gel (Kanto Silica Gel 60, spherical, 40–50 μm) with air flashing. Microwave reaction was performed by using Biotage® Initiator+ (maximum temperature 300°C and pressure 30 bar) with Biotage® microwave reaction kits (2 ml). Shaking incubator (EYELA FMC-100) was used to carry out an enzymatic reaction.
Preparation of crude enzyme from almonds
Two grams of defatted sweet-almond flour was shaken with 15 ml of 0.1 M sodium acetate buffer (pH 5) for 15 min., kept for 2 h at 4°C, and filtered by using a Buchner funnel to give the clear stock enzyme solution. This was used for experiments as a crude enzyme preparation.
Extraction and GlcCer purification
Dried methanol extract of the golden oyster mushroom (100 g) was extracted between chloroform-water (3:1, v/v). The chloroform layer obtained was washed with chloroform-methanol-water (3:48:47, v/v, 250 ml × 3) and the combined chloroform extract was concentrated under vacuum to afford yellow oil. The yellow oil was dissolved in hexane-isopropanol (7:3, v/v, 150 ml) and kept at room temperature for 24 h. The mixture was dried under vacuum, redissolved in chloroform, and purified by silica gel chromatography (400 g, 90 cm i.d × 16 cm) with gradient elution [from chloroform to chloroform-methanol (95:5, v/v) to chloroform-methanol (90:10, v/v)] to obtain GlcCer 1.
Alkaline hydrolysis: preparation of lysoGlcCer 2
GlcCer 1 was deacylated by the well-known classical alkali hydrolysis methods (12, 13) as shown by methods a and b in Fig. 2, although method c was the most efficient method established by employing a microwave. Obtained products were detected on the TLC plate by spraying with 5% H2SO4-methanol or ninhydrin and purified by silica gel chromatography eluting with ethyl acetate-methanol-water (4:1:1, v/v).
Fig. 2.
Preparation of 4E,8E-9-methyl-sphingadienine.
Method a: hydrolysis using Ba(OH)2/dioxane.
GlcCer 1 (200 mg) was dissolved in 10 ml dioxane in a round bottom flask at room temperature and the solution was heated to 110°C by oil bath. Subsequently, 10 ml of warm 10% aqueous (aq.) Ba(OH)2 was added and the reaction mixture was refluxed for 22 h at 110°C. After the reaction mixture was cooled to room temperature, 20 ml of 2% aq. ammonium sulfate was added to precipitate barium ions, followed by the addition of 20 ml of diethyl ether. The samples were shaken, vortexed, and centrifuged to separate the phases. The upper ethereal layer was concentrated under vacuum and purified by silica gel chromatography by using chloroform-methanol-water (70:27:3, v/v). By using this method, sphingoid base 3 was also produced as a minor component in qualitative yield.
Method b: butanolysis.
GlcCer 1 (100 mg) was dissolved in 4 ml of butanol and 4 ml of KOH (12.5 M, aq.) was added. The reaction mixture was refluxed at 125°C for 4 h and then cooled to room temperature. Chloroform-methanol-water (2:2:1.3, v/v) mixture solvent (5.3 ml) was added, followed by vortex-mixing and centrifugation. The chloroform phase was collected and the aqueous phase was extracted twice with 2 ml chloroform. Combined chloroform extract was concentrated under vacuum and purified by silica gel column chromatography by using chloroform-methanol-water (70:27:3, v/v).
Method c: microwave-assisted butanolysis.
GlcCer 1 (50 mg) was suspended in 1 ml of butanol in a 2 ml screw capped microwave vial. One milliliter KOH (12 M, aq.) was added and the reaction mixture was stirred under the microwave with high absorption at 125°C for 60 min. After the microwave treatment, lysoGlcCer 2 (1-β-glucosyl-4E,8E-9-methyl-sphingadienine) was extracted from the reaction mixture and purified by the same procedure as described in method b.
Enzymatic hydrolysis: preparation of sphingoid base 3
Three milliliters of β-glucosidase solution from almond [2 mg/ml in 0.1 M sodium acetate buffer (pH 5)] were added to 6 mg lysoGlcCer 2 and the reaction mixture was vortexed and incubated at 37°C for 16 h. After the starting material completely disappeared, 5.3 ml chloroform-methanol-water (2:2:1.3, v/v) was added. The mixture was vortexed and centrifuged. The lower chloroform phase was collected after repeated extraction of aqueous phases with chloroform. The combined chloroform extract was concentrated under vacuum and subsequently purified by silica gel flash column chromatography by using chloroform-methanol-aq. NH3 (40:10:1, v/v).
RESULTS
Characterization of GlcCer from golden oyster mushroom
GlcCer 1 was isolated from golden oyster mushroom by the method previously reported in (14). The residue of chloroform extract was 64 g yellow oil, which, upon purification, yielded 2.5 g GlcCer 1 as white amorphous powder, which had the same Rf value of 0.25 (chloroform-methanol-water = 65:16:2, v/v) as the authentic standard. The detailed analysis of GlcCer 1 by mass spectra and NMR (1H, 13C, 1H-1H COSY, HMBC, and HMQC) spectra, and in comparison with the reported spectra, helped us confirm it as N-(2-hydroxy-hexadecanoyl)-1-β-glucosyl-4E,8E-9-methyl-sphingadienine (supplementary Figs. 1–4).
1H-NMR (500 MHz, C5D5N): δ 8.35–8.37 (1H, d, J = 8.4 Hz), δ 6.0–6.04 (1H, dd, J = 5.7, 15.5 Hz), 5.93–5.96 (1H, m), 5.27 (1H, br.s), 4.92–4.93 (1H, d, J = 7.7 Hz), 4.77–4.80 (1H, m), 4.70–4.75 (1H, m), 4.57–4.62 (1H, m), 4.50–4.55 (1H, dd, J = 5.6, 11.7 Hz), 4.35–4.40 (1H, dd, J = 5.6, 11.8 Hz), 4.20–4.28 (3H, m), 4.02–4.07 (1H, m), 3.91–3.95 (1H, br.s), 2.15–2.24 (5H, m), 2.0–2.05 (3H, m), 1.72–1.73 (2H, m), 1.63 (3H, s), 1.38–1.43 (4H, m), 1.27–1.32 (36H, m), 0.86–0.90 (6H, t, J = 6.4 Hz). ESI-MS m/z: 750.5490 [M+Na]+.
Hydrolysis GlcCer by classical methods
Two classical methods, method a and method b, were used for the preparation of lysoGlcCer starting from GlcCer. In method a, 10% aq. Ba(OH)2 was used for hydrolysis of GlcCer 1 resulting in lysoGlcCer 2 (1-β-glucosyl-4E,8E-9-methyl-sphingadienine) with 50% yield; in this method, sphingoid base 3 was also produced as a minor component with a qualitative yield (12). Method b used aq. KOH, hydrolysis of GlcCer 1 resulting in lysoGlcCer 2 with 65% yield. However, these methods have major limitations in the purification process and require a large amount of samples. Hence, these methods are practically invalid for small-scale preparations.
Microwave-enhanced butanolysis of GlcCer
Preparation of 4E,8E-9-methyl-sphingadienine from GlcCer 1 was performed by the two-step chemoenzymatic reaction (Fig. 2), as discussed below. Base-catalyzed hydrolysis of GlcCers by classical methods (a and b) was limited due to poor yield. In terms of the barium hydroxide hydrolysis, removal of barium ion is quite challenging, which often causes purification problems by chelating with the product, and the yield of the desired product is low. The use of KOH water solution under reflux conditions did not push the reaction toward completion and by-products increased when the reaction was conducted for a long time under the same condition (9). To solve these problems, the microwave method was used instead of the classical reflux method to enhance the rate of reaction in a short period of time. GlcCer 1 was subjected to microwave-assisted butanolysis under a different set of experimental conditions (Table 1). It was found that the high concentration of aq. KOH and high temperature favor the hydrolysis reaction to yield lysoGlcCer 2 with 92% yield. Obtained product was characterized by NMR and ESI-MS, respectively (supplementary Fig. 5).
TABLE 1.
Optimization of microwave-assisted alkali hydrolysis of GlcCer 1
| Entry | aq. KOHa (M) | Temperature (°C) | Time (min) | Yield (%) |
| 1 | 1 | 125 | 180 | 66 |
| 2 | 3 | 125 | 120 | 60 |
| 3 | 6 | 125 | 90 | 85 |
| 4 | 6 | 110 | 150 | 72 |
| 5 | 12 | 125 | 60 | 88 |
| 6 | 12 | 125 | 45 | 92 |
In all the experiments, 5 mg of GlcCer 1 was used.
Different concentrations of KOH were made by dissolving in distilled water and in each experiment, 1 ml aq. KOH and 1 ml n-butanol were used.
1H-NMR (500 MHz, C5D5N): δ 5.90–6.00 (2H, m), 5.24–5.27 (1H, t, J = 5.6, 12.2 Hz), 4.98–5.00 (1H, d, J = 7.8 Hz), 4.53–4.57 (2H, m), 4.38–4.46 (2H, m), 4.24–4.31 (3H, m), 4.08–4.11 (1H, m), 3.93–3.99 (1H, m), 4.25–4.28 (1H, dd, J = 4.3, 10.3 Hz), 4.13–4.16 (2H, dd, J = 6.8, 10.6 Hz), 3.51–3.55 (1H, m), 2.11–2.18 (4H, m), 1.99–2.02 (2H, t, J = 7.5, 15.1 Hz), 1.60 (3H, s), 1.37–1.45 (2H, m), 1.18–1.32 (12H, m), 0.85–0.88 (3H, t, J = 7.1, 13.7Hz). ESI-MS m/z: 474.343 [M+H]+.
Hydrolysis of lysoGlcCer by β-glucosidase
The idea behind the enzymatic reaction is that the decrease of hydrophobicity will enhance the solubility of lysoGlcCer and thus accelerate the reaction. Also, it was thought that lysoGlcCer molecules could fit into the active site of the enzyme more rigidly than GlcCer. Enzymatic preparation of sphingoid base from lysoGlcCer was demonstrated here for the first time. The lysoGlcCer 2 obtained from alkali hydrolysis was subjected to enzymatic hydrolysis using almond β-glucosidase to give sphingoid base 3 (4E,8E-9-methyl-sphingadienine) as a white amorphous powder in 90% yield, and it was characterized by NMR and ESI-MS (supplementary Figs. 6–8).
1H NMR (500 MHz, C5D5N): δ 5.99–6.01 (2H, m), 5.26–5.29 (1H, t, J = 6.2, 12.7 Hz), 4.57–4.59 (1H, t, J = 5.0, 10.1 Hz), 4.25–4.28 (1H, dd, J = 4.3, 10.3 Hz), 4.13–4.16 (2H, dd, J = 6.8, 10.6 Hz), 3.38–3.42 (1H, q, J = 6.2, 10.9 Hz), 2.14–2.22 (4H, m), 1.99–2.02 (2H, t, J = 7.8, 15.2 Hz), 1.61 (3H, s), 1.39–1.44 (2H, m), 1.20–1.30 (12H, m), 0.85–0.88 (3H, t, J = 7.2, 14.0Hz). ESI-MS 312.2895 [M+H]+. [α]D20 + 4.2°, c = 1, CHCl3.
The positions of double bonds in sphingoid base 3 at C-4 and C-8 were unambiguously assigned from 1H-1H COSY by the observed correlations between H-3 and H-4, H-4 and H-5, H-5 and H-6, H-6 and H-7, and H-7 and H-8. Sphingoid base 3 was acetylated and measured the 1H-NMR to obtain the coupling constant between H4 and H5. The large vicinal coupling constant (J = 15.6 Hz) between H-4 and H-5 was observed, which confirms the geometry around its C-4/C-5 double bond is trans (E). The 13C NMR chemical shift of the C-19 methyl group (δc 14.6) in turn supports C-8 is trans, as demonstrated by comparison of the chemical shifts of the C-3 methyl groups in the E (δc 15.4) and Z (δc 22.7) isomers of 3-methyl-3-hexene (14). The presence of a C-19 allylic methyl group in sphingoid base is confirmed by the HMBC spectrum, in which a correlation between H-8 and C-19 was observed and also in 1H NMR indicates a singlet at δH 1.61.
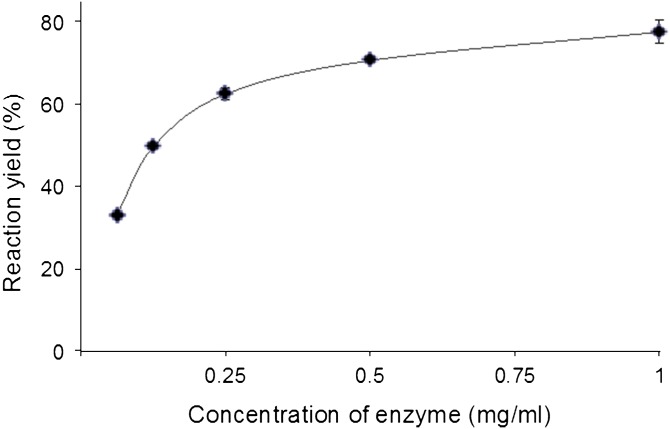
Effect of pH and enzyme concentration on enzymatic hydrolysis
The best pH value for β-glucosidase hydrolysis of lysoGlcCer to sphingoid base was found to be 5.0 (with 0.1 M sodium acetate buffer). As the pH increased, enzyme activity was substantially decreased, leading to the decrease of reaction yield. The reaction was performed by keeping temperature and the concentration of enzyme and substrate constant. Optimal activity of almond β-glucosidase with respect to pH showed identical results, as previously reported (15). The influence of enzyme concentration on the rate of hydrolysis, which is directly proportional to the reaction yield, was studied. At low concentration of the enzyme, hydrolytic rate was limited. As the concentration of enzyme increased, reaction yield increased and reached its maximum level (Fig. 3). When the ratio of enzyme and substrate concentration is 1:1, the hydrolytic activity was found to be maximum, which leads to the formation of sphingoid bases.
Fig. 3.
Influence of enzyme concentration on hydrolysis. The effect of enzyme concentration on hydrolysis of lysoGlcCer. Incubation mixtures, having 1 mg of lysoGlcCer and various concentrations of enzyme in 0.5 ml of buffer at pH 5 were used and the mixtures were incubated for 16 h at 37°C.
Application for preparation of various sphingoid bases
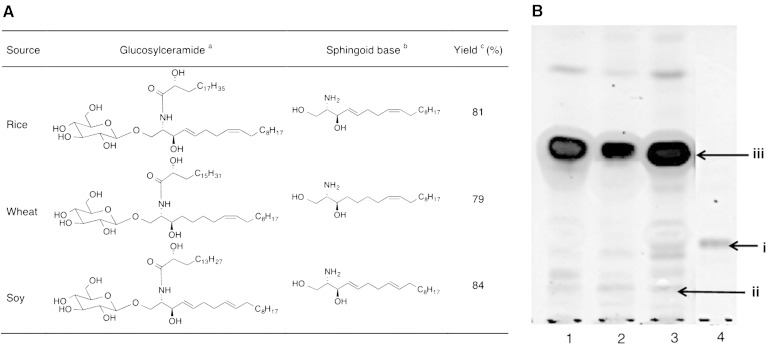
The accuracy and validity of the present chemoenzymatic method, employing microwave-facilitated alkali hydrolysis followed by β-glucosidase treatment, were confirmed with a variety of GlcCers from alimentary natural resources. The various commercially available GlcCer mixtures from rice, wheat, and soy were purchased and hydrolyzed by this method to prepare their respective sphingoid bases (Fig. 4A). All GlcCers hydrolyzed with more than 70% of reaction yield were calculated from their TLC data (Fig. 4B), and the sphingoid bases obtained were characterized by ESI-MS (supplementary Figs. 9–11).
Fig. 4.
Preparation of different kinds of dietary sphingoid bases. A: GlcCers from different sources and their respective sphingoid bases. aThe GlcCer mixture was used for hydrolysis and the structure shown here is a major component of it. bSphingoid bases obtained are also mixtures. cOverall yield was calculated with respect to representative sphingoid bases. B: TLC profiles of hydrolyzed lysoGlcCers. Lane 1, rice lysoGlcCer; lane 2, wheat lysoGlcCer; lane 3, soy lysoGlcCer; lane 4, a standard GlcCer (golden oyster mushroom). The TLC plate was developed with chloroform-methanol-aq. NH3 (40:10:1, v/v), stained by cupric sulfate in phosphoric acid. i, standard GlcCer; ii, lysoGlcCers from rice, wheat, and soy; iii, sphingoid bases.
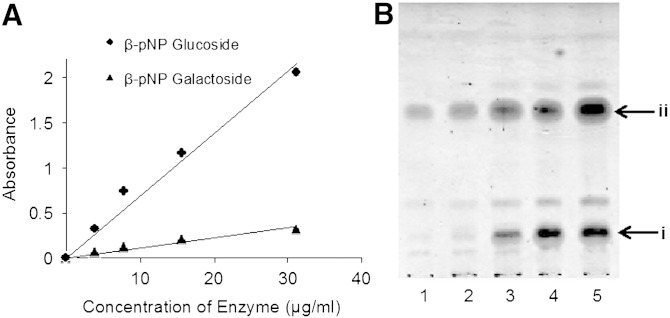
To show the substrate specificity of the enzyme used in our experiments, galactosyl-sphingosine prepared from galactosylceramide from porcine brain was used as a substrate for almond β-glucosidase. Surprisingly, the reaction successfully gives sphingosine. In order to understand the extent of contamination of β-galactosidase in this commercial enzyme product, further β-glucosidase assay was performed using pNP glucoside and pNP galactoside substrates, and it was found that the enzyme used is a mixture with β-galactosidase activity also. Results of this assay showed that β-glucosidase was 6-fold more reactive than β-galactosidase (Fig. 5A) in the commercially available almond β-glucosidase (total amount of protein: 363 μg/ml). This result suggests that the purification of the enzyme is not essential to β-glucosidase hydrolysis of lysoGlcCer. To improve the cost-effectiveness of our method, sodium acetate buffer (pH 5) extract of partially defatted sweet-almond flour was used as an enzyme stock solution, which was prepared as crude enzyme (15). The total amount of protein present in the stock solution, determined by bicinchoninic acid assay, was 260 μg/ml. This stock solution was used to carry out the hydrolysis of lysoGlcCer 2 at various concentrations (Fig. 5B). The β-glucosidase activity of our crude preparation was found to be 10% less than the commercial β-glucosidase, but gave the same result as the commercial product with respect to conversion of lysoGlcCer into sphingoid bases when the reaction was conducted under the same experimental conditions. Currently, to the best of our knowledge, this is the most economical and efficient way to prepare sphingoid bases and, hence, this methodology will have wide industrial applications.
Fig. 5.
A: β-glucosidase assay using standard β-pNP glucoside and β-pNP galactoside substrates, absorbance was recorded using Bio-Rad micro plate reader at 405 nm. B: Hydrolysis of lysoGlcCer by crude enzyme from almonds. Lanes 1–5 are reactions at different concentrations of lysoGlcCer (100, 250, 500, and 1,000 μg/ml). In each reaction, 1 ml of enzyme stock solution was used. i, lysoGlcCer 2; ii, sphingoid base (4E,8E-9Me-d18:2).
DISCUSSION
GlcCers can be acid/base hydrolyzed to generate sphingoid bases; however, their application has been limited as a result of the low yield by the reaction byproducts. In this study, we present a highly efficient chemoenzymatic method to produce sphingoid bases from GlcCers, conveniently. This method is applicable to many GlcCers and is easily scalable, even for large-scale preparation of lysoGlcCers and sphingoid bases.
The chemoenzymatic method described in this work has several distinguishing features, which make it a satisfactory method for production of high-yield sphingoid bases from GlcCers, in contrast to other previously reported methods. As discussed in an earlier section, acid-catalyzed hydrolysis of GlcCers could produce many kinds of byproducts via rearrangements and isomerization reactions (8). An intermediate product of base-catalytic hydrolysis reaction, a lysosphingolipid, could reduce the by-product formation of methanolic HCl; however, the isomerization reactions cannot be completely prevented (16). Another chemical transformation has been examined by using an oxidative-reductive hydrolysis method. Preparation of sphingoid bases by the oxidative-reduction hydrolysis method has two drawbacks. First, the essential three continuous reaction steps are not efficient. Second, the phytosphingosine type of ceramides, which possess a vicinal diol group, tend to be oxidized by an oxidation reagent, sodium periodate, leading to a complex reaction mixture (17).
On the other hand, an enzymatic hydrolysis of GlcCer could be expected to be a mild, selective, and efficient method. In fact, a hydrolysis by β-glucosidase from ox brain has been reported as a sole enzymatic method (18). However, this method requires conversion of ceramides to sphingoid bases by either strong alkali hydrolysis or N-deacylase treatment. We also carried out hydrolysis of GlcCer 1 using β-glucosidase in the presence of Triton X-100. Unfortunately, reactions do not proceed even after a 48 h incubation at 37°C (pH 5). This might be due to the low solubility and high hydrophobicity of GlcCer 1. This limitation makes previously reported methods inefficient for the preparation of sphingoid bases, especially on a large-scale. As shown in other studies (5, 11), a quality yield of sphingoid bases was prepared only under drastic conditions. In contrast, an extremely high yield (83%) was achieved following the simple two-step protocol for the preparation of sphingoid bases from GlcCer 1 in this report.
To the best of our knowledge, no efficient methods, which can practically prepare sphingoid bases from GlcCers, have been reported. In contrast, our two-step protocol has many advantages compared with all the other methods, as shown below. Because deacylation was carried out under microwave-assisted conditions with a yield higher than 90%, all types of acyl chains were hydrolyzed without leaving any GlcCers for short period. Microwave is an emerging technique to accelerate reactions, and this is the first example of its application to a GlcCer hydrolysis reaction. The specific cleavage of the glycosidic bond between glucose and sphingoid base portion in lysoGlcCer with a mild reaction conditions was obtained using β-glucosidase instead of drastic acid hydrolysis. The elimination of the acyl chains as well as the enhanced solubility of lysoGlcCer compared with GlcCer will increase accessibility of the enzymatic active center of the β-glucosidase. Furthermore, this method could be expanded for more complex glycosphingolipids, such as lactosylceramides and gangliosides.
In summary, analysis and functional studies of naturally occurring sphingoid bases are an emerging area of lipid research and are promising drug leads (19). A practical efficient method for the preparation of sphingoid bases starting from naturally abundant GlcCers was provided. In this study, the two-stage cleavage procedure for sphingoid base preparation was performed on a medium and small scale. However, this procedure has the potential to be expanded to large-scale experiments. This method is simple, economical, and reliable, requires a limited amount of starting material, and provides a suitable tool for convenient preparation and the proper analysis of sphingoids without the interference of artifacts. Furthermore, this method may also be applicable for the preparation of sphingoid bases from other glycolipids, which helps in their structural characterization. Application of this method toward construction of a natural sphingoid base chemical library and studies thereof is in progress and it will be published in near future.
Supplementary Material
Acknowledgments
The authors thank Nissei Bio Company, Ltd. (Sapporo, Japan) for providing golden oyster mushroom samples.
Footnotes
Abbreviations:
- aq.
- aqueous
- br.s
- broad singlet
- COSY
- correlation spectroscopy
- d
- doublet
- dd
- double doublet
- GlcCer
- glucosylceramide
- HMBC
- heteronuclear multiple bond coherence
- HMQC
- heteronuclear multiple quantum coherence
- m
- multiplet
- pNP
- p-nitrophenyl
- t
- triplet
This work was partially supported by the grant of “Innovation COE project for Future Medicine and Medicinal Research,” a grant-in-aid for Scientific Research (15H03111) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and a scholarship from Hokkaido University through the International Graduate Program (S.G.B.G.).
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Cahoon E. B., and Lynch D. V.. 1991. Analysis of glucocerebrosides of rye (Secale cereale L. cv Puma) leaf and plasma membrane. Plant Physiol. 95: 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin-Ming G., Wei-Ming Z., She-Qi Z., Xing Z., An-Ling Z., Hui C., Yue-Ying S., and Ming T.. 2004. Sphingolipids from the edible fungus Tuber indicum. Eur. J. Lipid Sci. Technol. 106: 815–821. [Google Scholar]
- 3.Karlsson K. A. 1970. On the chemistry and occurrence of sphingolipid long-chain bases. Chem. Phys. Lipids. 5: 6–43. [DOI] [PubMed] [Google Scholar]
- 4.Karlsson K. A. 1970. Sphingolipid long chain bases. Lipids. 5: 878–891. [DOI] [PubMed] [Google Scholar]
- 5.Sugawara T., Zaima N., Yamamoto A., Sakai S., Noguchi R., and Hirata T.. 2006. Isolation of sphingoid bases of sea cucumber cerebrosides and their cytotoxicity against human colon cancer cells. Biosci. Biotechnol. Biochem. 70: 2906–2912. [DOI] [PubMed] [Google Scholar]
- 6.Struckhoff A. P., Bittman R., Burow M. E., Clejan S., Elliott S., Hammond T., Tang Y., and Beckman B. S.. 2004. Novel ceramide analogs as potential chemotherapeutic agents in breast cancer. J. Pharmacol. Exp. Ther. 309: 523–532. [DOI] [PubMed] [Google Scholar]
- 7.Fyrst H., Oskouian B., Bandhuvula P., Gong Y., Byun H. S., Bittman R., Lee A. R., and Saba J. D.. 2009. Natural sphingadienes inhibit Akt-dependent signaling and prevent intestinal tumorigenesis. Cancer Res. 69: 9457–9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sambasivarao K., and McCluer R. H.. 1963. Thin-layer chromatographic separation of sphingosine and related bases. J. Lipid Res. 4: 106–108. [PubMed] [Google Scholar]
- 9.Morrison W. R., and Hay J. D.. 1970. Polar lipids in bovine milk. II. Long-chain bases, normal and 2-hydroxy fatty acids, and isomeric cis and trans monoenoic fatty acids in the sphingolipids. Biochim. Biophys. Acta. 202: 460–467. [DOI] [PubMed] [Google Scholar]
- 10.Kadowaki H., Bremer E. G., Evans J. E., Jungalwala F. B., and McCluer R. H.. 1983. Acetonitrile-hydrochloric acid hydrolysis of gangliosides for high performance liquid chromatographic analysis of their long chain bases. J. Lipid Res. 24: 1389–1397. [PubMed] [Google Scholar]
- 11.Carter H. E., Rothfus J. A., and Gigg R.. 1961. Biochemistry of the sphingolipids. XII. Conversion of cerebrosides to ceramides and sphingosine; structure of Gaucher cerebroside. J. Lipid Res. 2: 228–234. [Google Scholar]
- 12.Markham J. E., Li J., Cahoon E. B., and Jaworski J. G.. 2006. Separation and identification of major plant sphingolipid classes from leaves. J. Biol. Chem. 281: 22684–22694. [DOI] [PubMed] [Google Scholar]
- 13.Radin N. S. 1974. Preparation of psychosines (1-O-hexosyl sphingosine) from cerebrosides. Lipids. 9: 358–360. [DOI] [PubMed] [Google Scholar]
- 14.Meng T-X., Ishikawa H., Shimizu K., Ohga S., and Kondo R.. 2012. A glucosylceramide with antimicrobial activity from the edible mushroom Pleurotus citrinopileatus. J. Wood Sci. 58: 81–86. [Google Scholar]
- 15.Heyworth R., and Walker P. G.. 1962. Almond-emulsin beta-D-glucosidase and beta-D-galactosidase. Biochem. J. 83: 331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taketomi T., and Kawamura N.. 1970. Preparation of lysohematoside (neuraminyl-galactosyl-glucosylsphingosine) from hematoside of equine erythrocyte and its chemical and hemolytic properties. J. Biochem. (Tokyo). 68: 475–485. [DOI] [PubMed] [Google Scholar]
- 17.Iga S., Iga D. P., Nicolescu A., Florentina D., Gitman S., and Cîmpeanu G.. 2011. Preparation of ceramide and sphingosine by chemical and biochemical methods–an instrument for the evaluation of compatibility between sphinganine and fatty acids. Rom. Biotechnol. Lett. 16: 6611–6617. [Google Scholar]
- 18.Gatt S. 1966. Enzymic hydrolysis of sphingolipids: hydrolysis of ceramide glucoside by an enzyme from ox brain. Biochem. J. 101: 687–691. [PMC free article] [PubMed] [Google Scholar]
- 19.Pruett S. T., Bushnev A., Hagedorn K., Adiga M., Haynes C. A., Sullards M. C., Liotta D. C., and Merrill A. H. Jr. 2008. Biodiversity of sphingoid bases (“sphingosines”) and related amino alcohols. J. Lipid Res. 49: 1621–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.