Abstract
The ability of cancer cells to suppress apoptosis is critical for carcinogenesis. The BCL-2 family proteins comprise the sentinel network that regulates the mitochondrial or intrinsic apoptotic response. Recent advances in our understanding of apoptotic signaling pathways have enabled methods to identify cancers that are “primed” to undergo apoptosis, and have revealed potential biomarkers that may predict which cancers will undergo apoptosis in response to specific therapies. Complementary efforts have focused on developing novel drugs that directly target anti-apoptotic BCL-2 family proteins. In this review, we summarize the current knowledge of the role of BCL-2 family members in cancer development and response to therapy, focusing on targeted therapeutics, recent progress in the development of apoptotic biomarkers, and therapeutic strategies designed to overcome deficiencies in apoptosis.
Introduction
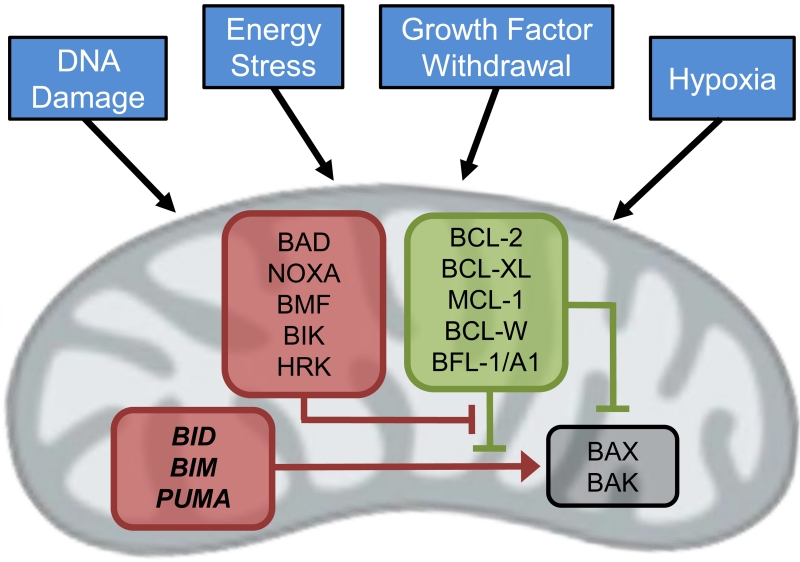
In 2002, Sydney Brenner, Robert Horvitz and John Sulston were awarded the Nobel Prize in Physiology or Medicine largely for their contributions to the understanding of the highly regulated form of cell death known as apoptosis. Based on their work and that of many others, it is now well appreciated that apoptosis is a highly conserved mechanism critical for normal development and tissue homeostasis, with roughly 50-70 million cells undergoing apoptosis daily in an adult human (1). As there are significant pathological consequences of unrestrained apoptosis, it is perhaps not surprising that apoptosis is governed by a complex network of molecular sentinels-- the BCL-2 family of proteins. Diverse inputs such as DNA damage, energy stress, loss of growth factor signaling and hypoxia can trigger apoptosis by activation of these proteins (Figure 1). In cancer, suppression of apoptotic signaling contributes significantly to carcinogenesis and tumor progression (2). Over the past two decades, many studies have elucidated the mechanisms by which this occurs in cancers, and these insights have laid the groundwork for therapies that directly target the apoptotic machinery.
Figure 1. The intrinsic apoptotic pathway is regulated by BCL-2 family proteins at the level of the mitochondria.
Multiple cellular stressors modulate the expression levels of pro- and anti-apoptotic BCL-2 family proteins (red and green respectively), leading to the activation of BAX and/or BAK and mitochondrial depolarization.
The BCL-2 protein family
Thirty years ago, several groups reported a novel translocation between chromosomes 14 and 18 t(14;18) resulting in fusion of the immunoglobin heavy chain and BCL2 loci in acute B cell leukemia and follicular lymphoma cells, leading to overexpression of BCL-2 (3-7). It was subsequently shown that BCL-2 enhanced the survival of these cells by inhibiting apoptosis (8-11). Additional genes with varying degrees of homology to BCL2 have since been identified that code for both anti-apoptotic and pro-apoptotic proteins (12). The anti-apoptotic BCL-2 family proteins, which include BCL-2, BCL-XL, BCL-W, MCL-1 and BFL-1/A1, share structural homology in the BCL-2 homology (BH) 1, 2, 3, and 4 domains. These anti-apoptotic proteins directly interact with pro-apoptotic BH3-only proteins BIM, PUMA, BAD, BID, BIK, BMF, HRK and NOXA, which share homology solely in the BH3 domain. Apoptotic stimuli lead to upregulation of BH3-only proteins and/or down-regulation of anti-apoptotic BCL-2 family proteins. This change in the balance of pro- versus anti-apoptotic BCL-2 family proteins leads to activation of the multi-domain (BH1, 2, 3) effector proteins BAK and BAX, which assemble into multimeric pores in the mitochondrial membrane and facilitate mitochondrial outer membrane permeabilization (MOMP) and cytochrome c release into the cytosol (13).
Recent studies have clarified how the BCL-2 family proteins interact to prevent or induce apoptosis (Figure 1) (14). “Activator” BH3-only proteins (BID, BIM, PUMA) directly interact with “effector” BAX and/or BAK proteins, inducing conformational changes that lead to the assembly of BAX/BAK multimeric pores in the mitochondrial membraine (15-21). Recent data suggests that activators may possess functional differences, with BIM preferentially activating BAX, and BID preferentially activating BAK (22). Anti-apoptotic BCL-2 family members (BLC-2, BCL-XL, MCL-1, BCL-W, BFL-1/A1) inhibit apoptosis by sequestering the activators from engaging BAX and BAK (23-25). “Sensitizer” BH3 proteins (e.g. BAD, NOXA, etc.) induce apoptosis by binding to anti-apoptotic proteins, thereby displacing activators that are then free to activate BAX and BAK (25, 26). Additionally, anti-apoptotic BCL-2 family proteins may bind activated BAX and BAK in some settings, thus promoting cell survival by both directly inhibiting BAX and BAK (27-29) as well as sequestering BH3-only proteins.
The complex network of interactions between pro- and anti-apoptotic BCL-2 family proteins tightly regulates the mitochondrial apoptotic response, allowing for a swift response to specific stimuli, while preventing unwanted cell death during normal cellular functioning. The binding affinities of the various pro- and anti-apoptotic BCL-2 family protein interactions have been characterized in solution using BH3 peptides and truncated proteins (23), however this may not reflect the nature of the interactions between proteins that occur at the mitochondrial membrane (25, 30). More recent work has focused on visualizing interactions between BCL-2 family members in intact living cells, and has revealed complex spatio-temporal dynamics that govern activation of BAX and BAK (29, 31). Additionally, there are marked differences in expression profiles of the BCL-2 family proteins in different tissue and cell types (32). This complexity poses distinct challenges in elucidating the exact roles of individual BCL-2 family proteins in regulating apoptosis in different cancer types, but also suggests that there could be a high degree of specificity for therapeutic modalities that directly target these proteins.
BCL-2 family proteins and cancer
Overexpression of anti-apoptotic BCL-2 family proteins is observed in many cancers, and can result from chromosomal translocations, gene amplification, increased gene transcription, and/or altered post-translational processing. As mentioned above, increased expression of BCL-2 resulting from the t(14;18) translocation occurs in follicular lymphoma (3-5) and diffuse large B cell lymphoma (33). While this translocation is rarely seen in solid tumors, BCL-2 protein overexpression is observed in some breast and prostate cancers (34-36), and other mechanisms of BCL-2 overexpression have been identified such as transcriptional activation by NF-<kappa>B signaling (37) or promoter hypo-methylation (38). MCL1 and BCL2L1 (BCL-XL) are frequently amplified or overexpressed in numerous tumor types (39-42), and increased MCL1 transcription can result from amplification of the transcription factor DEK (43) or constitutive activation of STAT3 (44). Post-translational mechanisms that negatively regulate protein degradation pathways may also contribute to elevated expression of anti-apoptotic BCL-2 family proteins. For instance, MCL-1 protein overexpression can result from enhanced protein stability due to genetic inactivation of the ubiquitin ligase complex protein FBW7 (39, 45, 46).
Overexpression of anti-apoptotic BCL-2 family proteins facilitates tumorigenesis and tumor progression (for more comprehensive reviews see (47, 48)). Transgenic mice overexpressing BCL-2 or MCL-1 develop B-cell lymphomas (11, 49), but the long latency period and low tumor incidence (in the case of BCL-2) suggests a permissive rather than causative role. Supporting this notion, numerous studies using transgenic mouse models have demonstrated that BCL-2, BCL-XL and MCL-1 can accelerate the development of MYC-driven lymphoma and leukemia (9, 50-54). Similarly, BCL-2 has also been shown cooperate with MYC and accelerate tumorigenesis in a mouse breast cancer model (50, 51). Once a tumor is established, anti-apoptotic BCL-2 family proteins also facilitate tumor cell maintenance and survival. For example, loss of BCL-2 in a transgenic mouse leukemia model driven by BCL-2 and c-MYC led to leukemic cell death and prolonged survival (55). MCL-1 has been demonstrated to play a particularly critical role in the survival of multiple myeloma cells, and ablation of MCL-1 expression alone stimulates apoptosis and leads to decreased cell survival (56, 57). As discussed in detail below, this provides rationale for therapeutic targeting of specific anti-apoptotic BCL-2 family proteins in cancer.
Conversely, decreased expression of pro-apoptotic BH3-only proteins facilitates tumor formation and progression (58). Suppression of BH3-only protein expression permits the survival of malignant clones, and similar to the role of anti-apoptotic BCL-2 proteins in tumorigenesis, animal models reveal a largely permissive effect of loss of BH3-only protein expression. BIM (59), BID (60), PUMA (61, 62) and NOXA (61) deficient mice exhibit apoptotic defects but do not spontaneously develop cancers. BAD deficient mice develop diffuse large B cell lymphomas late in life, which can be accelerated by sub-lethal doses of radiation, supporting a role for BAD in facilitating the survival of tumorigenic lymphocyte clones (63). Similarly, genetic disruption of one Bcl2l11 (Bim) allele resulting in haploinsufficiency accelerates the formation of B-cell leukemias in Eμ-Myc transgenic mice (64). Bcl2l11 loss has also been shown to cooperate with cyclin D1 overexpression in the development of mantle cell lymphoma in mice (65), mimicking human mantle cell lymphomas that exhibit cyclin D1 overexpression (due to a t(11;14) translocation) and, in some cases, homozygous deletions of BCL2L11 (66).
Apoptotic stimuli such as DNA damage activate the tumor suppressor p53, leading to apoptosis via upregulation of pro-apoptotic genes including PUMA, NOXA, BID and BAX (61, 67-70). TP53 is the most frequently altered gene across all cancers, and loss of TP53 accelerates and potentiates tumorigenesis in multiple murine cancer models (71). PUMA (p53 upregulated mediator of apoptosis) is the primary mediator of p53-induced apoptosis in response to DNA damage (67, 68), and the observation that TP53 mutations typically occur as late events in tumorigenesis (72) raises the possibility that loss of p53-induced expression of BH3-only proteins such as PUMA may contribute to disease progression. In one study, decreased PUMA expression was observed in melanoma compared to dysplastic nevi, and metastatic compared to primary lesions (73). Although alterations in TP53 were not examined in this study, another study reported that BRAF mutant melanomas have impaired expression of p53 target genes compared with nevi (74), suggesting a link between loss of p53 signaling, down-regulation of the PUMA and melanoma disease progression.
Under homeostatic conditions, the expression of pro-apoptotic BH3-only proteins is regulated by growth promoting signaling pathways. Hyperactivation of these same pathways by oncogenic kinases can lead to diminished expression or function of BH3-only proteins by suppressing transcription or by post-translational modifications that decrease BH3-only protein stability or lead to sequestration away from the mitochondria. Phosphorylation of BIM by ERK leads to RSK1/2-sensitive, βTRCP-mediated proteasomal degradation (75, 76), suggesting that hyperactivation of MAP kinase signaling may allow cancer cells to suppress BIM proteins levels and evade apoptosis. Indeed, we speculate that this may be one of the key downstream effectors of activation of ERK signaling in cancers (77, 78). Similarly, BAD can be phosphorylated by both AKT and MAPK, thereby promoting binding to 14-3-3 proteins and sequestration (79-82). In addition to regulation by p53, PUMA expression can be modulated by growth factor stimulation via PI3K and FOXO3A (83). Thus, as discussed further below, suppression of BH3-only protein activity by activation of the MEK/ERK and PI3K/AKT signaling pathways may play a central role in the survival of cancers driven by constitutively activated oncogenic kinases such as EGFR (84-88), BRAF (89), KRAS (90), and BCR-ABL (91).
BCL-2 family proteins and response to targeted therapies
Though cancers typically harbor numerous genetic alterations, certain genetic events may lead to activation of oncogenic signaling pathways that are required for cancer cell survival--so called “oncogene addition.” The discovery that the ABL kinase inhibitor imatinib could inhibit the survival of chronic myelogenous leukemia (CML) cells harboring the BCR-ABL translocation ushered in the era of targeted therapies (92, 93). In 2004, non-small cell lung cancers (NSCLCs) harboring activating mutations in EGFR were demonstrated to have exquisite sensitivity to the EGFR inhibitors gefitinib and erlotinib (94-96), and EGFR inhibitors have now supplanted chemotherapy as first-line therapy for EGFR mutant NSCLC (97-100). Subsequently, dramatic clinical responses of BRAF mutant melanoma (101) and EML4-ALK NSCLC (102-104) to BRAF and ALK inhibitors, respectively, have been observed. With recent advances in genomics, additional oncogenic driver mutations in different cancer types have been identified, and a myriad of novel therapies targeting many different signaling pathways are currently being evaluated in clinical trials.
Over the years, it has become clear that the induction of apoptosis is a critical component of effective targeted therapies. The majority of targeted therapies currently approved or in clinical trials are inhibitors of kinase signaling cascades, and thus lead to perturbation of BCL-2 family proteins to effect apoptosis. Since many oncogenic drivers activate common downstream signaling pathways such as MEK/ERK and PI3K/AKT/FOXO3A, therapies targeting different oncogenic kinases often lead to similar changes in BCL-2 family proteins. Targeted therapies that lead to inhibition of MEK/ERK signaling almost invariably increase BIM protein levels, while those that cause downstream inhibition of mTORC1 typically induce PUMA expression. Importantly, multiple BCL-2 family proteins may be affected simultaneously, which contributes to response to therapy (Figure 2). For example, induction of both PUMA and BIM have been shown to be important in the response of mouse models of EGFR mutant and HER2 positive breast cancer to EGFR and HER2 inhibitors, respectively (105). In KRAS mutant NSCLC, combined MEK and PI3K inhibitors lead to upregulation of PUMA and BIM, both of which are necessary for the induction of an apoptotic response (90). In BRAF mutant melanoma, BIM, PUMA and BMF contribute to apoptosis induced by BRAF and/or MEK inhibitor treatment (89, 106, 107). Both BIM and BAD have been implicated in the apoptotic response of CML to imatinib (91). Conversely, downregulation of anti-apoptotic BCL-2 proteins may also play a role in response to targeted therapies, often in concert with upregulation of pro-apoptotic proteins. For instance, in EGFR mutant NSCLC treated with EGFR inhibitors, the suppression of PI3K/mTORC1 signaling leads to a reduction in MCL-1 expression which acts in concert with BIM induction to trigger an apoptotic response and induce tumor regression in vivo (108, 109).
Figure 2. Targeted therapies inhibit oncogenic kinase signaling cascades and modulate BCL-2 family proteins to induce apoptosis.
Examples of commonly occuring cancers driven by specific oncogenic driver mutations that result in constitutively activated downstream kinase signaing pathways and suppression of the mitochondrial apoptotic pathway. By inhibiting these pathways, targeted therapies lead to upregulation of pro-apoptotic BH3-only proteins and/or downregulation of pro-survival BCL-2 family proteins, ultimately inducing apoptosis. (See references: EGFR (84, 87, 88, 105, 108), BRAF (89, 106, 181), KRAS (90), CML (76, 91, 113))
While these studies have demonstrated that targeted therapies may impact multiple BCL-2 family proteins in a complex manner, BIM has repeatedly emerged as a critical mediator of targeted therapy-induced apoptosis in multiple cancer types, perhaps because many of the current kinase inhibitor targeted therapy paradigms involve modulation of the MEK/ERK and PI3K/FOXO3A signaling axes. Indeed, BIM expression may serve as a potential biomarker useful for predicting response to targeted therapies (110). The first clear evidence that oncogenic signaling led to BIM suppression was provided by studies of BCR-ABL signaling in CML. BCR-ABL-induced ERK signaling leads to suppression of BIM protein levels via phosphorylation and subsequent proteasomal degradation, and treatment of BCR-ABL positive cells with imatinib increases BIM protein levels and induces apoptosis (111, 112). Importantly, siRNA targeting of BIM protects these cells from imatinib-induced cell death. Additionally, BIM is transcriptionally upregulated following inhibition of BCR-ABL by imatinib via activation of FOXO3A (113). Thus, multiple pathways regulated by BCR-ABL converge on BIM, making it a key effector of apoptosis induced by ABL kinase inhibitors.
Subsequently, other groups have reported that BIM is essential for induction of apoptosis in multiple cancer types in response to various targeted therapies. In EGFR mutant NSCLC, EGFR inhibitor results in downregulation of PI3K/AKT and MEK/ERK signaling (114), and loss of MEK/ERK signaling leads to accumulation of BIM. Depletion of BIM by RNAi abrogates the apoptotic response to EGFR inhibition (84, 85, 87, 88). The central role of BIM in promoting apoptosis in response to targeted therapies has also been demonstrated in other targeted therapy paradigms including HER2 amplified breast cancers (115), ALK-positive NSCLCs (116), BRAF mutant melanomas (106), BRAF mutant colorectal cancers (117), and PIK3CA mutant breast cancers (115). These studies provide strong experimental evidence that loss of apoptotic signaling—specifically, reduced BIM expression—significantly hinders the response to targeted therapies that either directly or indirectly inhibiting MEK/ERK and/or PI3K/AKT signaling pathways.
Assessment of BCL-2 family proteins as biomarkers of response to anti-cancer therapies
Given the central role of BCL-2 family proteins in mediating the apoptotic response to anti-cancer therapies, there has been interest in determining whether they may have potential to serve as biomarkers predicting treatment response. Letai and colleagues recently developed an experimental method termed “BH3 profiling” that quantifies the intrinsic propensity of a cell to undergo apoptosis, or apoptotic “priming” (23, 118). Conceptually, priming can be understood as the proximity of a tumor cell to the apoptotic threshold, and is a function of the collective expression of pro- versus anti-apoptotic BCL-2 family proteins. BH3 profiling indirectly assesses this balance of BCL-2 family proteins by perturbing cells with exogenous BH3 peptides that mimic the pro-apoptotic activity of promiscuous BH3-only proteins such as BIM, BMF and PUMA (23, 118). In this assay, cells are challenged with low concentrations of BH3 peptides and the degree of MOMP is measured using a fluorescent dye that is sensitive to mitochondrial membrane potential. In cells with a low degree of priming, the relative excess of anti-apoptotic BCL-2 family proteins will bind the exogenously added BH3 peptides without displacement of bound endogenous BH3 activator proteins, and no MOMP will be observed. In contrast, in cells with greater expression of endogenous activator BH3 proteins (or lower relative expression of anti-apoptotic BCL-2 family proteins), binding of BH3 peptides to anti-apoptotic BCL-2 family proteins will liberate the activators to bind BAX and BAK with subsequent MOMP. Thus the experimentally observed MOMP can be interpreted to be a function of the relative balance of endogenous pro-apoptotic BH3 activator proteins sequestered by anti-apoptotic BCL-2 family proteins.
BH3 profiling has been successfully used to predict chemotherapeutic sensitivity of lymphoma cell lines (118) as well as the clinical response of a diverse set of cancers including acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML), multiple myeloma and ovarian cancer (119, 120). Chemosensitive cancer cells have significantly higher apoptotic priming than traditionally chemoresistant cancer sub-types or normal cells, suggesting a possible explanation for the therapeutic window for chemotherapeutic agents. In addition to conventional chemotherapy, BH3 profiling also appears to be effective for identifying highly primed cancers that are more likely to respond to BH3 mimetics (24, 121, 122) as these agents act by directly binding to anti-apoptotic BCL-2 proteins and liberating BH3 proteins (123, 124). Whether baseline global BH3 profiling or assessment of specific BCL-2 family proteins will be useful in predicting response of oncogene-addicted cancers to targeted therapies such as kinase inhibitors remains an open question (90). For example, BRAF mutant melanoma and EGFR mutant NSCLC are relatively chemoresistant, yet they are exquisitely sensitive to BRAF and EGFR inhibitors, respectively, that induce apoptosis by altering expression of specific BCL-2 family proteins. Performing BH3 profiling on these cancers following drug treatment, a technique recently described as Dynamic BH3 Profiling, may more effectively predict induction of apoptosis by targeted kinase inhibitor therapy (125).
It is notable that recent work has suggested that pre-treatment BIM expression levels may indicate the likelihood of response to an array of targeted therapies. Indeed, BIM protein expression levels predict the apoptotic response of EGFR mutant, BRAF mutant and HER2 amplified cell lines to the appropriate targeted therapies (115). Furthermore, we previously observed that pre-treatment BIM mRNA expression levels in EGFR mutant NSCLC specimens correlated with both the magnitude and the duration of response to EGFR inhibitor therapy, suggesting that low BIM expression may be a biomarker of poor response despite the presence of an activating EGFR mutation. This concept has been supported by analysis of BIM mRNA levels in patients enrolled in the EURTAC trial of erlotinib for EGFR mutant NSCLC, which revealed that high BIM expression was associated with an overall response rate (ORR) of 87.5% and progression free survival (PFS) of 12.9 months in the erlotinib treatment group, while those patients with low or moderate BIM expression had an ORR of 34.6% and PFS of 7.2 months. Importantly, elevated BIM expression levels also correlated with improved overall survival (126). Interestingly, a germline polymorphism in intron 2 of BIM that results in aberrant RNA splicing and decreased levels of BIM transcripts containing the BH3-domain was associated with decreased responsiveness of EGFR mutant NSCLC to EGFR inhibitor therapy (127-130). This same polymorphism has also been associated with decreased duration of remission induced by imatinib in CML (131), and a separate polymorphism in the BIM BH3 domain has been identified that is associated with decreased BIM mRNA expression and prolonged time to major molecular response after initiation of imatinib treatment (132). Altogether, these data suggest that BIM expression levels may have prognostic value in predicting response to kinase inhibitors in oncogene addicted cancers.
Therapeutic targeting of BCL-2 family proteins
If a minimal apoptotic response to a given targeted therapy translates into a poor clinical response, it follows that drugs that specifically target apoptotic regulators may be useful to enhance the apoptotic response and improve clinical outcomes. As discussed above, the apoptotic response of a cell is governed by the relative balance of pro- and anti-apoptotic BCL-2 proteins. Therefore, direct inhibition of anti-apoptotic BCL-2 family members may be useful in cancers with marked overexpression of these proteins, or in combination with other therapies whose efficacy is limited by the expression of anti-apoptotic BCL-2 proteins. As a class, agents that inhibit anti-apoptotic BCL-2 family proteins act by binding within the BH3 binding groove of anti-apoptotic BCL-2 proteins and disrupting the interaction with BH3 proteins, are thus termed “BH3 mimetics.” Currently, there are inhibitors of BCL-2 family proteins under development including pan-BCL-2 inhibitors, as well as selective inhibitors of BCL-2/BCL-XL, BCL-2 only, or MCL-1. However, achieving a high degree of selectivity for induction of apoptosis via inhibition of BCL-2 family proteins has proven to proven challenging, with many putative BH3 mimetics leading to cell death in a BAX/BAK independent manner (133).
The most clinically advanced BCL-2 family inhibitors target either BCL-2 and BCL-XL (BCL-2/BCL-XL inhibitors) or BCL-2 only. ABT-737 and its clinical analogue ABT-263 (navitoclax) are small molecule BAD BH3 mimetics that bind the hydrophobic BH3 binding groove of BCL-2, BCL-XL and BCL-W and prevent binding of pro-apoptotic family members such as BIM, BID and BAD (123, 134). Initial studies suggested single-agent efficacy in cancer models characterized by BCL-2 overexpression such as B cell malignancies and small cell lung cancer (SCLC). Recent clinical trials of navitoclax have demonstrated activity in CLL (135), however the efficacy of single-agent BCL-2/BCL-XL inhibitors in SCLC has been underwhelming (136). Use of navitoclax is currently limited by its major dose-limiting toxicity of thrombocytopenia, an on-target consequence of BCL-XL inhibition in platelets (137). In contrast, ABT-199 (venetoclax/GDC-0199), which selectively inhibits BCL-2 but not BCL-XL and thus does not cause thrombocytopenia, may be useful for malignancies in which BCL-2 plays a more central role than BCL-XL, such as in CLL and AML (122). Indeed, a phase I study of ABT-199 for relapsed/refractory CLL showed an overall objective response rate of 79%, with equivalent response rates in del(17p) and chemo-refractory patients (124, 138).
Given the importance of BCL-2 family proteins in regulating the response to kinase pathway inhibition, there has been interest in combining BCL-2/BCL-XL inhibitors with kinase inhibitors. ABT-737/navitoclax has been shown to enhance the efficacy of EGFR inhibitors against EGFR mutant NSCLC cells (84, 87) and MEK or BRAF inhibitors for BRAF mutant melanoma (89, 139, 140). Whether the additional combination benefit of targeting BCL-2/BCL-XL outweighs the potential increase in toxicity for EGFR or BRAF mutant cancers, which generally respond well to tyrosine kinase inhibitor (TKI) alone, remains to be determined. The triple combination of dabrafenib (BRAF), trametinib (MEK) and navitoclax is currently being tested in a phase I/II trial for advanced BRAF mutant melanoma, which will provide a direct assessment of the benefit of adding navitoclax to the current standard of care BRAF/MEK inhibitor combination (clinicaltrials.gov NCT01989585).
BCL-2/BCL-XL inhibitors may also be useful for lowering the apoptotic threshhold in cancers for which a kinase inhibitor alone is insufficient to induce an apoptotic response. We and others have explored the combination of MEK and BCL-2/BCL-XL inhibitors for KRAS mutant cancers, for which single agent MEK inhibition is largely ineffective (141-143). In these cancers, inhibition of MEK leads to stabilization and accumulation of BIM, however, this only leads to apoptosis when BCL-XL is simultaneously neutralized (Figure 3). Given that inhibition of BCL-XL appears to be more important than inhibition of BCL-2 for the anti-cancer effect, it remains to be seen whether the unavoidable thrombocytopenia due to BCL-XL inhibition will limit the clinical efficacy of this combination, which is currently under clinical development (Clinicaltrials.gov NCT02079740). Should toxicity prevent using full doses of navitoclax, intermittent dosing strategies that take full advantage of inducing pronounced apoptosis could be explored.
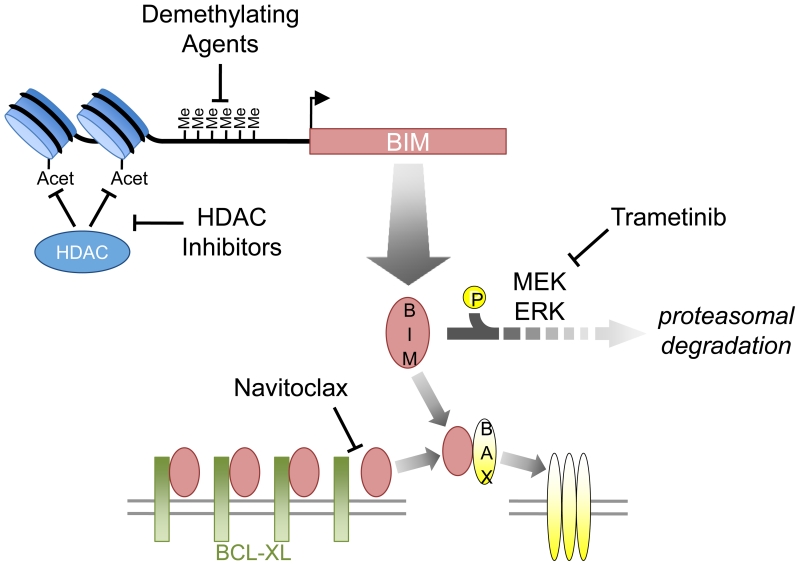
Figure 3. Strategies for enhancing the pro-apoptotic activity of BIM.
Demethylating agents or histone deacetylase (HDAC) inhibitors may overcome epigenetic reppression of BIM transcription, whereas MEK inhibitors (e.g. trametinib) decrease BIM degradation—all leading to increased cellular BIM protein levels and increased activation of BAX. BCL-2/BCL-XL inhibitors (e.g. navitoclax) block the ability for anti-apoptotic BCL-2 family members like BCL-2 and BCL-XL to neutralize BIM.
Beyond BCL-XL and BCL-2, the MCL1 gene is frequently amplified (39) or aberrantly regulated in cancer resulting in high expression levels in a wide range of both solid and hematologic malignancies (32) including lung (41) , breast (144), prostate (145), pancreas (146), leukemia (45, 147, 148). In particular, high MCL-1 expression levels are important for the survival of multiple myeloma cells (56, 57, 149). Additionally, elevated expression of MCL-1 confers resistance to anti-tubulin chemotherapy (46) and BCL-2/BCL-XL inhibitors (150-152). Thus there has been keen interest in developing drugs that selectively bind and inhibit MCL-1 that might be useful as single agent or in combination with chemotherapy or other targeted therapies.
To date, no selective MCL-1 inhibitors have entered clinical trials. The pan-BCL-2 inhibitor obatoclax, which inhibits MCL-1 as well as BCL-2, BCL-XL and BCL-W (153), has been tested in the clinic as single agent and in combination with chemotherapy for a number of cancers including hematologic malignancies, non-small cell lung cancer and small cell lung cancer (154-157). Overall, the clinical activity of obatoclax has been disappointing, and unexpected central nervous system toxicity including disorientation and ataxia has been observed, possibly due to off-target drug activity independent of induction of apoptosis via inhibition of BCL-2 proteins (158). In addition to obatoclax, apogossypol derivatives BI-97C1 (sabutoclax) and BI112D1 that inhibit BH3 peptide binding to BCL-2 ,BCL-xL and MCL-1 have been reported by Pellecchia and colleagues. Sabutoclax induces apoptosis in MCL-1 dependent preclinical cancer models in a BAK/BAX dependent manner (159), as well as mitochondrial fragmentation in an MCL-1 dependent, BAX/BAK independent manner (160), but it has yet to be evaluated in clinical trials.
Additional putative small molecule inhibitors of MCL-1 have been described, however the clinical promise of many of these compounds is diminished by poor selectivity and/or potency (133, 161). Several groups have combined fragment-based screening and structure based design--analogous to the development of ABT-737--to generate MCL-1 inhibitors with improved potency and selectivity (162, 163). Most recently, Abbvie has reported development of series of small molecule MCL-1 inhibitors identified via high-throughput screening and subsequently refined via iterative structure-guided design utilizing drug:MCL-1 co-crystal structures (164). The resulting compounds exhibit sub-nanonmolar binding affinities and high selectivity, and are capable of disrupting MCL-1:BIM complexes in intact cells and inducing apoptosis in MCL-1 dependent cancer cell models (165). These advances raise the exciting possibility that potent and selective MCL-1 inhibitors may soon be available for clinical examination.
In the absence of direct inhibitors of MCL-1, pharmacologic strategies that indirectly suppress MCL-1 activity by diminishing MCL-1 protein expression have been developed. In contrast to the other anti-apoptotic BCL-2 family proteins, the MCL-1 protein has a short half-life (<4 hours), so alterations in transcription, translation and degradation can rapidly impact cellular MCL-1 protein levels. Golub and colleagues utilized a chemical genomic screen to identify several compounds including anthracycline chemotherapeutics (e.g. doxorubicin, daunorubicin, epirubicin) that led to transcriptional repression of MCL1 and subsequent apoptosis (166). Importantly, restoration of physiologic MCL-1 protein levels was capable of rescuing cells from the apoptotic effects of these MCL1 transcriptional repressor compounds, suggesting that MCL-1 suppression may contribute to the clinical activity of anthracyclines. It has also been shown that MCL-1 protein expression can be suppressed by inhibition of mTOR-mediated translation (167), though this effect appears specific to the ATP-competitive TORC inhibitors rather than allosteric TORC1 inhibitors such as rapamycin (108, 168-171). Interestingly, in EGFR mutant NSCLC, EGFR inhibitors lead to inhibition of PI3K-mTOR signaling and down-regulation of MCL-1, which contributes to significantly to the apoptotic response (Figure 2) (108). Exploiting this regulation of MCL-1 protein expression by mTOR, we recently investigated combining mTOR inhibitors (targeting MCL-1) with ABT-263 (targeting BCL-2/XL) and found that this combination was highly effective in pre-clinical models of KRAS and BRAF mutant colorectal cancers as well as SCLCs (170, 172). However, it remains to be determined if this combination will be tolerable in the clinic.
Therapeutic strategies for enhancing BIM activity
As discussed above, oncogene addicted cancers with decreased BIM expression may have a poor response to targeted therapies. While loss of BIM expression may result from genetic mechanisms in some cases, in other cases BIM expression may be suppressed by epigenetic mechanisms such as histone modifications or promoter hypermethylation (173). Thus drugs that target epigenetic regulators might be useful for increasing BIM expression levels and overcoming apoptotic resistance in these cancers (Figure 3).
Aberrant promoter hypermethylation occurs frequently in cancer, and may result in transcriptional repression of tumor suppressor genes (174). The BCL2L11 promoter contains an extensive CpG rich region and hypermethylation of this region is associated with low BIM expression. Notably, BCL2L11 promoter hypermethylation has been correlated with poor prognosis in CML (175) and Burkitt lymphoma (176), which are typically characterized by excellent clinical responses to imatinib and multi-agent chemotherapy, respectively. Demethylating agents (decitabine and azacytadine, currently approved for the treatment of myelodyspastic syndrome) may be useful for reversing BIM suppression due to promoter hypermethylation, possibly by disrupting transcriptional co-repressor complexes (177). Addition of decitabine to imatinib has been shown to restore BIM expression and imatinib-induced apoptosis in CML cells with BCL2L11 promoter hypermethylation (175). Importantly, this study demonstrates the potential of using agents to restore BIM expression in order to sensitize low BIM expressing cancers to targeted therapies.
Histone modifications such as acetylation may also lead to transcriptional repression of BIM. Histone deacetylase (HDAC) inhibitors, such as vorinostat, have been shown to restore BIM expression in models of anaplastic large cell lymphoma, CLL and pediatric ALL (177-179). The combination of an EGFR inhibitor with vorinostat resulted in increased expression of BH3 domain-containing BIM in EGFR mutant lung cancers that harbor the intronic deletion polymorphism discussed above (180). In this context, HDAC inhibition increased expression of the wild-type BIM protein and re-sensitized to EGFR inhibitor treatment in vivo. Thus, similar to demethylating agents, HDAC inhibitors may be useful in combination with targeted therapies for cancers with low BIM expression. Indeed, a clinical trial investigating the combination of erlotinib and the HDAC inhibitor romidepsin for advanced NSCLC has recently completed enrollment (Clinicaltrials.gov NCT01302808). However, this trial was not restricted to lung cancers with EGFR mutations, and therefore, may not address the concept of restoring BIM levels to increase sensitivity to targeted therapies designed for genetically defined subsets of cancer.
Conclusion
It is now well established that the BCL-2 family of proteins plays an important role in tumorigenesis and tumor maintenance, as well as the response of cancers to both classic chemotherapies and targeted therapies. However, our understanding of the precise mechanisms of apoptotic signaling networks in specific cancer paradigms continues to evolve. Novel methods of interrogating the BCL-2 family proteins to quantify the proximity of a cancer to the apoptotic threshold may be useful for predicting response of specific cancers to chemotherapy and BH3 mimetics. Moreover, BIM levels alone may predict the apoptotic response to TKI treatment for certain oncogene-addicted cancers such as EGFR mutant NSCLC. Therapies that directly target the apoptotic response by inhibiting anti-apoptotic BCL-2 family proteins (e.g. navitoclax, ABT-199) have shown clinical promise for cancers that depend on overexpression of BCL-2 such as CLL, and may be useful in combination with kinase inhibitors for solid tumors. The rational use of these agents has the potential for improving currently available therapies as well as yielding novel therapeutic approaches for a wide range of cancers.
SIGNIFICANCE.
Apoptosis, long known to be important for response to conventional cytotoxic chemotherapy, has more recently been shown to be essential for the efficacy of targeted therapies. Approaches that increase the likelihood of a cancer to undergo apoptosis following therapy may help improve targeted treatment strategies.
Acknowledgments
Grant Support: NIH-NCI R01CA164273 (J. Engelman), NIH-NCI 2RO1CA140594 (J. Engelman), NIH-NCI 2P50CA127003 (J. Engelman), NIH-NCI R01CA137008 (J. Engelman)
Footnotes
Conflict of interest:
J. Engelman: Novartis - research funding, consulting; AstraZeneca - research funding, consulting; Amgen - research funding, consulting; GSK - consulting; Roche - consulting, IP licensed by Ventana (owned by Roche); LOXO- stock ownership, consulting; Gatekeeper Pharmaceuticals - stock ownership (“I have a financial interest in Gatekeeper Pharmaceuticals, a company aimed at developing new therapies for overcoming resistance to erlotinib. Gatekeeper Pharmaceuticals has IP on an EGFR inhibitor. My interests have been reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies.”)
A. Hata: Amgen - consulting
A. Faber: none
References
- 1.Reed JC. Dysregulation of apoptosis in cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17:2941–53. doi: 10.1200/JCO.1999.17.9.2941. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226:1097–9. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 4.Cleary ML, Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985;82:7439–43. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakhshi A, Jensen JP, Goldman P, Wright JJ, McBride OW, Epstein AL, et al. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985;41:899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- 6.Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–3. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 7.Cleary ML, Smith SD, Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986;47:19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- 8.Henderson S, Rowe M, Gregory C, Croom-Carter D, Wang F, Longnecker R, et al. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–15. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 9.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–2. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 10.McDonnell TJ, Deane N, Platt FM, Nunez G, Jaeger U, McKearn JP, et al. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 11.McDonnell TJ, Korsmeyer SJ. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14; 18) Nature. 1991;349:254–6. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- 12.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 14.Villunger A, Labi V, Bouillet P, Adams J, Strasser A. Can the analysis of BH3-only protein knockout mice clarify the issue of ‘direct versus indirect’ activation of Bax and Bak? Cell death and differentiation. 2011;18:1545–6. doi: 10.1038/cdd.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–42. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 16.Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD, et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell. 2013;152:519–31. doi: 10.1016/j.cell.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 17.Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–81. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell. 2010;40:481–92. doi: 10.1016/j.molcel.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–3. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36:487–99. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leshchiner ES, Braun CR, Bird GH, Walensky LD. Direct activation of full-length proapoptotic BAK. Proc Natl Acad Sci U S A. 2013;110:E986–95. doi: 10.1073/pnas.1214313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarosiek KA, Chi X, Bachman JA, Sims JJ, Montero J, Patel L, et al. BID preferentially activates BAK while BIM preferentially activates BAX, affecting chemotherapy response. Mol Cell. 2013;51:751–65. doi: 10.1016/j.molcel.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–65. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. The Journal of clinical investigation. 2007;117:112–21. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–84. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–35. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merino D, Giam M, Hughes PD, Siggs OM, Heger K, O’Reilly LA, et al. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J Cell Biol. 2009;186:355–62. doi: 10.1083/jcb.200905153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C, et al. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104–16. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leber B, Lin J, Andrews DW. Still embedded together binding to membranes regulates Bcl-2 protein interactions. Oncogene. 2010;29:5221–30. doi: 10.1038/onc.2010.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aranovich A, Liu Q, Collins T, Geng F, Dixit S, Leber B, et al. Differences in the mechanisms of proapoptotic BH3 proteins binding to Bcl-XL and Bcl-2 quantified in live MCF-7 cells. Mol Cell. 2012;45:754–63. doi: 10.1016/j.molcel.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 32.Placzek WJ, Wei J, Kitada S, Zhai D, Reed JC, Pellecchia M. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis. 2010;1:e40. doi: 10.1038/cddis.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nunez G, Seto M, Seremetis S, Ferrero D, Grignani F, Korsmeyer SJ, et al. Growth- and tumor-promoting effects of deregulated BCL2 in human B-lymphoblastoid cells. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:4589–93. doi: 10.1073/pnas.86.12.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callagy GM, Pharoah PD, Pinder SE, Hsu FD, Nielsen TO, Ragaz J, et al. Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:2468–75. doi: 10.1158/1078-0432.CCR-05-2719. [DOI] [PubMed] [Google Scholar]
- 35.Bhargava V, Kell DL, van de Rijn M, Warnke RA. Bcl-2 immunoreactivity in breast carcinoma correlates with hormone receptor positivity. The American journal of pathology. 1994;145:535–40. [PMC free article] [PubMed] [Google Scholar]
- 36.Catz SD, Johnson JL. BCL-2 in prostate cancer: a minireview. Apoptosis : an international journal on programmed cell death. 2003;8:29–37. doi: 10.1023/a:1021692801278. [DOI] [PubMed] [Google Scholar]
- 37.Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene. 2001;20:7342–51. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- 38.Hanada M, Delia D, Aiello A, Stadtmauer E, Reed JC. bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood. 1993;82:1820–8. [PubMed] [Google Scholar]
- 39.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho-Vega JH, Rassidakis GZ, Admirand JH, Oyarzo M, Ramalingam P, Paraguya A, et al. MCL-1 expression in B-cell non-Hodgkin’s lymphomas. Human pathology. 2004;35:1095–100. doi: 10.1016/j.humpath.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 41.Song L, Coppola D, Livingston S, Cress D, Haura EB. Mcl-1 regulates survival and sensitivity to diverse apoptotic stimuli in human non-small cell lung cancer cells. Cancer Biol Ther. 2005;4:267–76. doi: 10.4161/cbt.4.3.1496. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Guttikonda S, Roberts L, Uziel T, Semizarov D, Elmore SW, et al. Mcl-1 is critical for survival in a subgroup of non-small-cell lung cancer cell lines. Oncogene. 2011;30:1963–8. doi: 10.1038/onc.2010.559. [DOI] [PubMed] [Google Scholar]
- 43.Khodadoust MS, Verhaegen M, Kappes F, Riveiro-Falkenbach E, Cigudosa JC, Kim DS, et al. Melanoma proliferation and chemoresistance controlled by the DEK oncogene. Cancer research. 2009;69:6405–13. doi: 10.1158/0008-5472.CAN-09-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. The Journal of clinical investigation. 2001;107:351–62. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS, et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature. 2011;471:104–9. doi: 10.1038/nature09732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471:110–4. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- 47.Kelly PN, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011;18:1414–24. doi: 10.1038/cdd.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 49.Zhou P, Levy NB, Xie H, Qian L, Lee CY, Gascoyne RD, et al. MCL1 transgenic mice exhibit a high incidence of B-cell lymphoma manifested as a spectrum of histologic subtypes. Blood. 2001;97:3902–9. doi: 10.1182/blood.v97.12.3902. [DOI] [PubMed] [Google Scholar]
- 50.Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–3. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 51.Jager R, Herzer U, Schenkel J, Weiher H. Overexpression of Bcl-2 inhibits alveolar cell apoptosis during involution and accelerates c-myc-induced tumorigenesis of the mammary gland in transgenic mice. Oncogene. 1997;15:1787–95. doi: 10.1038/sj.onc.1201353. [DOI] [PubMed] [Google Scholar]
- 52.Linden M, Kirchhof N, Carlson C, Van Ness B. Targeted overexpression of Bcl-XL in B-lymphoid cells results in lymphoproliferative disease and plasma cell malignancies. Blood. 2004;103:2779–86. doi: 10.1182/blood-2003-10-3399. [DOI] [PubMed] [Google Scholar]
- 53.Swanson PJ, Kuslak SL, Fang W, Tze L, Gaffney P, Selby S, et al. Fatal acute lymphoblastic leukemia in mice transgenic for B cell-restricted bcl-xL and c-myc. J Immunol. 2004;172:6684–91. doi: 10.4049/jimmunol.172.11.6684. [DOI] [PubMed] [Google Scholar]
- 54.Xiang Z, Luo H, Payton JE, Cain J, Ley TJ, Opferman JT, et al. Mcl1 haploinsufficiency protects mice from Myc-induced acute myeloid leukemia. The Journal of clinical investigation. 2010;120:2109–18. doi: 10.1172/JCI39964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Letai A, Sorcinelli MD, Beard C, Korsmeyer SJ. Antiapoptotic BCL-2 is required for maintenance of a model leukemia. Cancer Cell. 2004;6:241–9. doi: 10.1016/j.ccr.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 56.Zhang B, Gojo I, Fenton RG. Myeloid cell factor-1 is a critical survival factor for multiple myeloma. Blood. 2002;99:1885–93. doi: 10.1182/blood.v99.6.1885. [DOI] [PubMed] [Google Scholar]
- 57.Derenne S, Monia B, Dean NM, Taylor JK, Rapp MJ, Harousseau JL, et al. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. Blood. 2002;100:194–9. doi: 10.1182/blood.v100.1.194. [DOI] [PubMed] [Google Scholar]
- 58.Elkholi R, Floros KV, Chipuk JE. The Role of BH3-Only Proteins in Tumor Cell Development, Signaling, and Treatment. Genes & cancer. 2011;2:523–37. doi: 10.1177/1947601911417177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–8. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 60.Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–91. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 61.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–8. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 62.Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–8. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 63.Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP, et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100:9324–9. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6164–9. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katz SG, Labelle JL, Meng H, Valeriano RP, Fisher JK, Sun H, et al. Mantle Cell Lymphoma in Cyclin D1 Transgenic Mice with Bim-deficient B-cells. Blood. 2013 doi: 10.1182/blood-2013-04-499079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tagawa H, Karnan S, Suzuki R, Matsuo K, Zhang X, Ota A, et al. Genome-wide array-based CGH for mantle cell lymphoma: identification of homozygous deletions of the proapoptotic gene BIM. Oncogene. 2005;24:1348–58. doi: 10.1038/sj.onc.1208300. [DOI] [PubMed] [Google Scholar]
- 67.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–94. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 68.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–82. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 69.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100:1931–6. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sax JK, Fei P, Murphy ME, Bernhard E, Korsmeyer SJ, El-Deiry WS. BID regulation by p53 contributes to chemosensitivity. Nature cell biology. 2002;4:842–9. doi: 10.1038/ncb866. [DOI] [PubMed] [Google Scholar]
- 71.Donehower LA, Lozano G. 20 years studying p53 functions in genetically engineered mice. Nat Rev Cancer. 2009;9:831–41. doi: 10.1038/nrc2731. [DOI] [PubMed] [Google Scholar]
- 72.Rivlin N, Brosh R, Oren M, Rotter V. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes & cancer. 2011;2:466–74. doi: 10.1177/1947601911408889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karst AM, Dai DL, Martinka M, Li G. PUMA expression is significantly reduced in human cutaneous melanomas. Oncogene. 2005;24:1111–6. doi: 10.1038/sj.onc.1208374. [DOI] [PubMed] [Google Scholar]
- 74.Yu H, McDaid R, Lee J, Possik P, Li L, Kumar SM, et al. The role of BRAF mutation and p53 inactivation during transformation of a subpopulation of primary human melanocytes. The American journal of pathology. 2009;174:2367–77. doi: 10.2353/ajpath.2009.081057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dehan E, Bassermann F, Guardavaccaro D, Vasiliver-Shamis G, Cohen M, Lowes KN, et al. betaTrCP- and Rsk1/2-mediated degradation of BimEL inhibits apoptosis. Molecular cell. 2009;33:109–16. doi: 10.1016/j.molcel.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, et al. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–93. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 77.Wiggins CM, Band H, Cook SJ. c-Cbl is not required for ERK1/2-dependent degradation of BimEL. Cellular signalling. 2007;19:2605–11. doi: 10.1016/j.cellsig.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ewings KE, Hadfield-Moorhouse K, Wiggins CM, Wickenden JA, Balmanno K, Gilley R, et al. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. The EMBO journal. 2007;26:2856–67. doi: 10.1038/sj.emboj.7601723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–9. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 80.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 81.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–28. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 82.She QB, Solit DB, Ye Q, O’Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell. 2005;8:287–97. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–63. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cragg MS, Kuroda J, Puthalakath H, Huang DC, Strasser A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 2007;4:1681–89. doi: 10.1371/journal.pmed.0040316. discussion 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deng J, Shimamura T, Perera S, Carlson NE, Cai D, Shapiro GI, et al. Proapoptotic BH3-only BCL-2 family protein BIM connects death signaling from epidermal growth factor receptor inhibition to the mitochondrion. Cancer Res. 2007;67:11867–75. doi: 10.1158/0008-5472.CAN-07-1961. [DOI] [PubMed] [Google Scholar]
- 86.Costa DB, Nguyen KS, Cho BC, Sequist LV, Jackman DM, Riely GJ, et al. Effects of erlotinib in EGFR mutated non-small cell lung cancers with resistance to gefitinib. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:7060–7. doi: 10.1158/1078-0432.CCR-08-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gong Y, Somwar R, Politi K, Balak M, Chmielecki J, Jiang X, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 2007;4:1655–68. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Costa DB, Halmos B, Kumar A, Schumer ST, Huberman MS, Boggon TJ, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 2007;4:1669–79. doi: 10.1371/journal.pmed.0040315. discussion 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cragg MS, Jansen ES, Cook M, Harris C, Strasser A, Scott CL. Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic. J Clin Invest. 2008;118:3651–9. doi: 10.1172/JCI35437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hata AN, Yeo A, Faber AC, Lifshits E, Chen Z, Cheng KA, et al. Failure to induce apoptosis via BCL-2 family proteins underlies lack of efficacy of combined MEK and PI3K inhibitors for KRAS-mutant lung cancers. Cancer Res. 2014;74:3146–56. doi: 10.1158/0008-5472.CAN-13-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuroda J, Puthalakath H, Cragg MS, Kelly PN, Bouillet P, Huang DC, et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci U S A. 2006;103:14907–12. doi: 10.1073/pnas.0606176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nature medicine. 1996;2:561–6. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 93.Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. The New England journal of medicine. 2001;344:1038–42. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 94.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 95.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–7. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 96.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 97.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. The New England journal of medicine. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 98.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 99.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 100.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 101.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. The New England journal of medicine. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. The New England journal of medicine. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 104.Shaw AT, Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. The New England journal of medicine. 2014;370:1189–97. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bean GR, Ganesan YT, Dong Y, Takeda S, Liu H, Chan PM, et al. PUMA and BIM are required for oncogene inactivation-induced apoptosis. Science signaling. 2013;6:ra20. doi: 10.1126/scisignal.2003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang YF, Jiang CC, Kiejda KA, Gillespie S, Zhang XD, Hersey P. Apoptosis induction in human melanoma cells by inhibition of MEK is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin Cancer Res. 2007;13:4934–42. doi: 10.1158/1078-0432.CCR-07-0665. [DOI] [PubMed] [Google Scholar]
- 107.VanBrocklin MW, Verhaegen M, Soengas MS, Holmen SL. Mitogen-activated protein kinase inhibition induces translocation of Bmf to promote apoptosis in melanoma. Cancer research. 2009;69:1985–94. doi: 10.1158/0008-5472.CAN-08-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Faber AC, Li D, Song Y, Liang MC, Yeap BY, Bronson RT, et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci U S A. 2009;106:19503–8. doi: 10.1073/pnas.0905056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Faber AC, Wong KK, Engelman JA. Differences underlying EGFR and HER2 oncogene addiction. Cell Cycle. 2010;9:851–2. doi: 10.4161/cc.9.5.11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Faber AC, Ebi H, Costa C, Engelman JA. Apoptosis In Targeted Therapy Responses: The Role of BIM. Adv Pharmacol. 2012;65:519–42. doi: 10.1016/B978-0-12-397927-8.00016-6. [DOI] [PubMed] [Google Scholar]
- 111.Aichberger KJ, Mayerhofer M, Krauth MT, Vales A, Kondo R, Derdak S, et al. Low-level expression of proapoptotic Bcl-2-interacting mediator in leukemic cells in patients with chronic myeloid leukemia: role of BCR/ABL, characterization of underlying signaling pathways, and reexpression by novel pharmacologic compounds. Cancer research. 2005;65:9436–44. doi: 10.1158/0008-5472.CAN-05-0972. [DOI] [PubMed] [Google Scholar]
- 112.Kuribara R, Honda H, Matsui H, Shinjyo T, Inukai T, Sugita K, et al. Roles of Bim in apoptosis of normal and Bcr-Abl-expressing hematopoietic progenitors. Molecular and cellular biology. 2004;24:6172–83. doi: 10.1128/MCB.24.14.6172-6183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Essafi A, Fernandez de Mattos S, Hassen YA, Soeiro I, Mufti GJ, Thomas NS, et al. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24:2317–29. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- 114.Mukohara T, Engelman JA, Hanna NH, Yeap BY, Kobayashi S, Lindeman N, et al. Differential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutations. Journal of the National Cancer Institute. 2005;97:1185–94. doi: 10.1093/jnci/dji238. [DOI] [PubMed] [Google Scholar]
- 115.Faber AC, Corcoran RB, Ebi H, Sequist LV, Waltman BA, Chung E, et al. BIM expression in treatment naïve cancers predicts responsiveness to kinase inhibitors. Cancer Discov. 2011;1:352–65. doi: 10.1158/2159-8290.CD-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takezawa K, Okamoto I, Nishio K, Janne PA, Nakagawa K. Role of ERK-BIM and STAT3-survivin signaling pathways in ALK inhibitor-induced apoptosis in EML4-ALK-positive lung cancer. Clin Cancer Res. 2011;17:2140–8. doi: 10.1158/1078-0432.CCR-10-2798. [DOI] [PubMed] [Google Scholar]
- 117.Wickenden JA, Jin H, Johnson M, Gillings AS, Newson C, Austin M, et al. Colorectal cancer cells with the BRAF(V600E) mutation are addicted to the ERK1/2 pathway for growth factor-independent survival and repression of BIM. Oncogene. 2008;27:7150–61. doi: 10.1038/onc.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–85. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 119.Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–33. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vo TT, Ryan J, Carrasco R, Neuberg D, Rossi DJ, Stone RM, et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell. 2012;151:344–55. doi: 10.1016/j.cell.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood. 2008;111:2300–9. doi: 10.1182/blood-2007-06-098012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective BCL-2 Inhibition by ABT-199 Causes On Target Cell Death in Acute Myeloid Leukemia. Cancer discovery. 2013 doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 124.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013 doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 125.Montero J, Sarosiek KA, DeAngelo JD, Maertens O, Ryan J, Ercan D, et al. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell. 2015;160:977–89. doi: 10.1016/j.cell.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Costa C, Molina MA, Drozdowskyj A, Gimenez-Capitan A, Bertran-Alamillo J, Karachaliou N, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:2001–10. doi: 10.1158/1078-0432.CCR-13-2233. [DOI] [PubMed] [Google Scholar]
- 127.Ng KP, Hillmer AM, Chuah CT, Juan WC, Ko TK, Teo AS, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med. 2012;18:521–8. doi: 10.1038/nm.2713. [DOI] [PubMed] [Google Scholar]
- 128.Isobe K, Hata Y, Tochigi N, Kaburaki K, Kobayashi H, Makino T, et al. Clinical significance of BIM deletion polymorphism in non-small-cell lung cancer with epidermal growth factor receptor mutation. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2014;9:483–7. doi: 10.1097/JTO.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao M, Zhang Y, Cai W, Li J, Zhou F, Cheng N, et al. The Bim deletion polymorphism clinical profile and its relation with tyrosine kinase inhibitor resistance in Chinese patients with non-small cell lung cancer. Cancer. 2014;120:2299–307. doi: 10.1002/cncr.28725. [DOI] [PubMed] [Google Scholar]
- 130.Lee JH, Lin YL, Hsu WH, Chen HY, Chang YC, Yu CJ, et al. Bcl-2-like protein 11 deletion polymorphism predicts survival in advanced non-small-cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2014;9:1385–92. doi: 10.1097/JTO.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 131.Katagiri S, Umezu T, Ohyashiki JH, Ohyashiki K. The BCL2L11 (BIM) deletion polymorphism is a possible criterion for discontinuation of imatinib in chronic myeloid leukaemia patients. British journal of haematology. 2013;160:269–71. doi: 10.1111/bjh.12111. [DOI] [PubMed] [Google Scholar]
- 132.Augis V, Airiau K, Josselin M, Turcq B, Mahon FX, Belloc F. A Single Nucleotide Polymorphism in cBIM Is Associated with a Slower Achievement of Major Molecular Response in Chronic Myeloid Leukaemia Treated with Imatinib. PloS one. 2013;8:e78582. doi: 10.1371/journal.pone.0078582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Varadarajan S, Vogler M, Butterworth M, Dinsdale D, Walensky LD, Cohen GM. Evaluation and critical assessment of putative MCL-1 inhibitors. Cell Death Differ. 2013;20:1475–84. doi: 10.1038/cdd.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer research. 2008;68:3421–8. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 135.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–96. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18:3163–9. doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–86. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 138.Seymour JF, Davids MS, Pagel JM, Kahl BS, Wierda WG, Puvvada S, et al. ABT-199 (GDC-0199) in relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL): High complete- response rate and durable disease control. J Clin Oncol. 2014;32:5s. [Google Scholar]
- 139.Serasinghe MN, Missert DJ, Asciolla JJ, Podgrabinska S, Wieder SY, Izadmehr S, et al. Anti-apoptotic BCL-2 proteins govern cellular outcome following B-RAF inhibition and can be targeted to reduce resistance. Oncogene. 2014 doi: 10.1038/onc.2014.21. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Frederick DT, Salas Fragomeni RA, Schalck A, Ferreiro-Neira I, Hoff T, Cooper ZA, et al. Clinical profiling of BCL-2 family members in the setting of BRAF inhibition offers a rationale for targeting de novo resistance using BH3 mimetics. PLoS One. 2014;9:e101286. doi: 10.1371/journal.pone.0101286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Corcoran RB, Cheng KA, Hata AN, Faber AC, Ebi H, Coffee EM, et al. Synthetic Lethal Interaction of Combined BCL-XL and MEK Inhibition Promotes Tumor Regressions in KRAS Mutant Cancer Models. Cancer Cell. 2013;23:121–8. doi: 10.1016/j.ccr.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sale MJ, Cook SJ. The BH3 mimetic ABT-263 synergizes with the MEK1/2 inhibitor selumetinib/AZD6244 to promote BIM-dependent tumour cell death and inhibit acquired resistance. The Biochemical journal. 2013;450:285–94. doi: 10.1042/BJ20121212. [DOI] [PubMed] [Google Scholar]
- 143.Tan N, Wong M, Nannini MA, Hong R, Lee LB, Price S, et al. Bcl-2/Bcl-xL inhibition increases the efficacy of MEK inhibition alone and in combination with PI3 kinase inhibition in lung and pancreatic tumor models. Molecular cancer therapeutics. 2013;12:853–64. doi: 10.1158/1535-7163.MCT-12-0949. [DOI] [PubMed] [Google Scholar]
- 144.Ding Q, He X, Xia W, Hsu JM, Chen CT, Li LY, et al. Myeloid cell leukemia-1 inversely correlates with glycogen synthase kinase-3beta activity and associates with poor prognosis in human breast cancer. Cancer Res. 2007;67:4564–71. doi: 10.1158/0008-5472.CAN-06-1788. [DOI] [PubMed] [Google Scholar]
- 145.Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, et al. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. The American journal of pathology. 1996;148:1567–76. [PMC free article] [PubMed] [Google Scholar]
- 146.Miyamoto Y, Hosotani R, Wada M, Lee JU, Koshiba T, Fujimoto K, et al. Immunohistochemical analysis of Bcl-2, Bax, Bcl-X, and Mcl-1 expression in pancreatic cancers. Oncology. 1999;56:73–82. doi: 10.1159/000011933. [DOI] [PubMed] [Google Scholar]
- 147.Kaufmann SH, Karp JE, Svingen PA, Krajewski S, Burke PJ, Gore SD, et al. Elevated expression of the apoptotic regulator Mcl-1 at the time of leukemic relapse. Blood. 1998;91:991–1000. [PubMed] [Google Scholar]
- 148.Yoshimoto G, Miyamoto T, Jabbarzadeh-Tabrizi S, Iino T, Rocnik JL, Kikushige Y, et al. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 activation. Blood. 2009;114:5034–43. doi: 10.1182/blood-2008-12-196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wuilleme-Toumi S, Robillard N, Gomez P, Moreau P, Le Gouill S, Avet-Loiseau H, et al. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia. 2005;19:1248–52. doi: 10.1038/sj.leu.2403784. [DOI] [PubMed] [Google Scholar]
- 150.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–88. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 151.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin X, Morgan-Lappe S, Huang X, Li L, Zakula DM, Vernetti LA, et al. ‘Seed’ analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene. 2007;26:3972–9. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 153.Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–7. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.O’Brien SM, Claxton DF, Crump M, Faderl S, Kipps T, Keating MJ, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113:299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chiappori A, Williams C, Northfelt DW, Adams JW, Malik S, Edelman MJ, et al. Obatoclax mesylate, a pan-bcl-2 inhibitor, in combination with docetaxel in a phase 1/2 trial in relapsed non-small-cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2014;9:121–5. doi: 10.1097/JTO.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Paik PK, Rudin CM, Pietanza MC, Brown A, Rizvi NA, Takebe N, et al. A phase II study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan in relapsed small cell lung cancer. Lung Cancer. 2011;74:481–5. doi: 10.1016/j.lungcan.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Parikh SA, Kantarjian H, Schimmer A, Walsh W, Asatiani E, El-Shami K, et al. Phase II study of obatoclax mesylate (GX15-070), a small-molecule BCL-2 family antagonist, for patients with myelofibrosis. Clinical lymphoma, myeloma & leukemia. 2010;10:285–9. doi: 10.3816/CLML.2010.n.059. [DOI] [PubMed] [Google Scholar]
- 158.McCoy F, Hurwitz J, McTavish N, Paul I, Barnes C, O’Hagan B, et al. Obatoclax induces Atg7-dependent autophagy independent of beclin-1 and BAX/BAK. Cell Death Dis. 2010;1:e108. doi: 10.1038/cddis.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wei J, Stebbins JL, Kitada S, Dash R, Placzek W, Rega MF, et al. BI-97C1, an optically pure Apogossypol derivative as pan-active inhibitor of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 2010;53:4166–76. doi: 10.1021/jm1001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Varadarajan S, Butterworth M, Wei J, Pellecchia M, Dinsdale D, Cohen GM. Sabutoclax (BI97C1) and BI112D1, putative inhibitors of MCL-1, induce mitochondrial fragmentation either upstream of or independent of apoptosis. Neoplasia. 2013;15:568–78. doi: 10.1593/neo.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Belmar J, Fesik SW. Small molecule Mcl-1 inhibitors for the treatment of cancer. Pharmacol Ther. 2015;145:76–84. doi: 10.1016/j.pharmthera.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Friberg A, Vigil D, Zhao B, Daniels RN, Burke JP, Garcia-Barrantes PM, et al. Discovery of potent myeloid cell leukemia 1 (Mcl-1) inhibitors using fragment-based methods and structure-based design. J Med Chem. 2013;56:15–30. doi: 10.1021/jm301448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Petros AM, Swann SL, Song D, Swinger K, Park C, Zhang H, et al. Fragment-based discovery of potent inhibitors of the anti-apoptotic MCL-1 protein. Bioorg Med Chem Lett. 2014;24:1484–8. doi: 10.1016/j.bmcl.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 164.Bruncko M, Wang L, Sheppard GS, Phillips DC, Tahir SK, Xue J, et al. Structure-Guided Design of a Series of MCL-1 Inhibitors with High Affinity and Selectivity. J Med Chem. 2015;58:2180–94. doi: 10.1021/jm501258m. [DOI] [PubMed] [Google Scholar]
- 165.Leverson JD, Zhang H, Chen J, Tahir SK, Phillips DC, Xue J, et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax) Cell Death Dis. 2015;6:e1590. doi: 10.1038/cddis.2014.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wei G, Twomey D, Lamb J, Schlis K, Agarwal J, Stam RW, et al. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell. 2006;10:331–42. doi: 10.1016/j.ccr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 167.Chen MH, Yang WL, Lin KT, Liu CH, Liu YW, Huang KW, et al. Gene expression-based chemical genomics identifies potential therapeutic drugs in hepatocellular carcinoma. PloS one. 2011;6:e27186. doi: 10.1371/journal.pone.0027186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Muller A, Zang C, Chumduri C, Dorken B, Daniel PT, Scholz CW. Concurrent inhibition of PI3K and mTORC1/mTORC2 overcomes resistance to rapamycin induced apoptosis by down-regulation of Mcl-1 in mantle cell lymphoma. International journal of cancer Journal international du cancer. 2013;133:1813–24. doi: 10.1002/ijc.28206. [DOI] [PubMed] [Google Scholar]
- 169.Hsieh AC, Costa M, Zollo O, Davis C, Feldman ME, Testa JR, et al. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell. 2010;17:249–61. doi: 10.1016/j.ccr.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Faber AC, Coffee EM, Costa C, Dastur A, Ebi H, Hata AN, et al. mTOR inhibition specifically sensitizes colorectal cancers with KRAS or BRAF mutations to BCL-2/BCL-XL inhibition by suppressing MCL-1. Cancer Discov. 2014;4:42–52. doi: 10.1158/2159-8290.CD-13-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Preuss E, Hugle M, Reimann R, Schlecht M, Fulda S. Pan-mammalian target of rapamycin (mTOR) inhibitor AZD8055 primes rhabdomyosarcoma cells for ABT-737-induced apoptosis by down-regulating Mcl-1 protein. The Journal of biological chemistry. 2013;288:35287–96. doi: 10.1074/jbc.M113.495986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Faber AC, Farago AF, Costa C, Dastur A, Gomez-Caraballo M, Robbins R, et al. Assessment of ABT-263 activity across a cancer cell line collection leads to a potent combination therapy for small-cell lung cancer. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1411848112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Harada H, Grant S. Targeting the regulatory machinery of BIM for cancer therapy. Critical reviews in eukaryotic gene expression. 2012;22:117–29. doi: 10.1615/critreveukargeneexpr.v22.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ohtani-Fujita N, Fujita T, Aoike A, Osifchin NE, Robbins PD, Sakai T. CpG methylation inactivates the promoter activity of the human retinoblastoma tumor-suppressor gene. Oncogene. 1993;8:1063–7. [PubMed] [Google Scholar]
- 175.San Jose-Eneriz E, Agirre X, Jimenez-Velasco A, Cordeu L, Martin V, Arqueros V, et al. Epigenetic down-regulation of BIM expression is associated with reduced optimal responses to imatinib treatment in chronic myeloid leukaemia. Eur J Cancer. 2009;45:1877–89. doi: 10.1016/j.ejca.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 176.Richter-Larrea JA, Robles EF, Fresquet V, Beltran E, Rullan AJ, Agirre X, et al. Reversion of epigenetically mediated BIM silencing overcomes chemoresistance in Burkitt lymphoma. Blood. 2010;116:2531–42. doi: 10.1182/blood-2010-02-268003. [DOI] [PubMed] [Google Scholar]
- 177.Piazza R, Magistroni V, Mogavero A, Andreoni F, Ambrogio C, Chiarle R, et al. Epigenetic silencing of the proapoptotic gene BIM in anaplastic large cell lymphoma through an MeCP2/SIN3a deacetylating complex. Neoplasia. 2013;15:511–22. doi: 10.1593/neo.121784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bachmann PS, Piazza RG, Janes ME, Wong NC, Davies C, Mogavero A, et al. Epigenetic silencing of BIM in glucocorticoid poor-responsive pediatric acute lymphoblastic leukemia, and its reversal by histone deacetylase inhibition. Blood. 2010;116:3013–22. doi: 10.1182/blood-2010-05-284968. [DOI] [PubMed] [Google Scholar]
- 179.Inoue S, Riley J, Gant TW, Dyer MJ, Cohen GM. Apoptosis induced by histone deacetylase inhibitors in leukemic cells is mediated by Bim and Noxa. Leukemia. 2007;21:1773–82. doi: 10.1038/sj.leu.2404760. [DOI] [PubMed] [Google Scholar]
- 180.Nakagawa T, Takeuchi S, Yamada T, Ebi H, Sano T, Nanjo S, et al. EGFR-TKI resistance due to BIM polymorphism can be circumvented in combination with HDAC inhibition. Cancer Res. 2013;73:2428–34. doi: 10.1158/0008-5472.CAN-12-3479. [DOI] [PubMed] [Google Scholar]
- 181.Corcoran RB, Rothenberg SM, Hata AN, Faber AC, Piris A, Nazarian RM, et al. TORC1 suppression predicts responsiveness to RAF and MEK inhibition in BRAF-mutant melanoma. Science translational medicine. 2013;5:196ra98. doi: 10.1126/scitranslmed.3005753. [DOI] [PMC free article] [PubMed] [Google Scholar]