Abstract
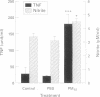
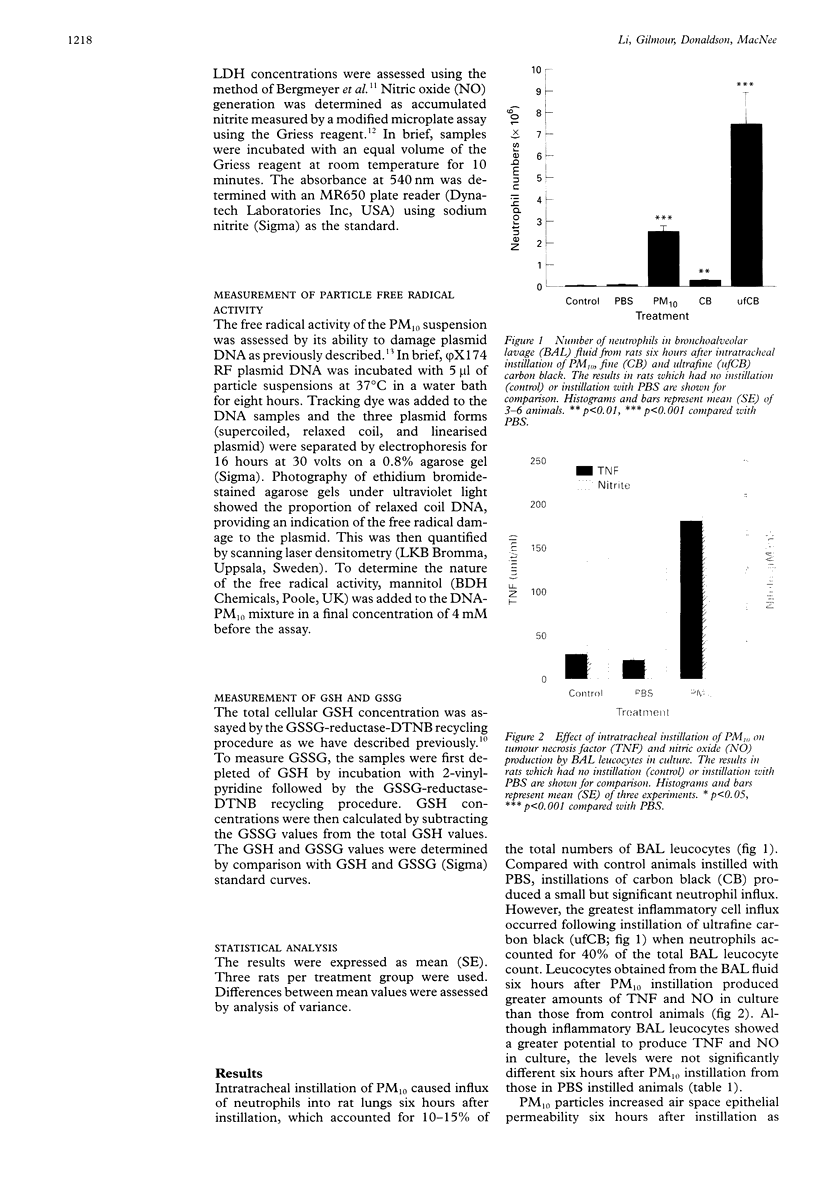
BACKGROUND: Epidemiological evidence has implicated fine particulate air pollution, particularly particles less than 10 microns in diameter (PM10), in the development of exacerbations of asthma and chronic obstructive pulmonary disease (COPD) although the mechanism is unknown. The hypothesis that PM10 particles induce oxidant stress, causing inflammation and injury to airway epithelium, was tested. METHODS: The effects of intratracheal instillation of PM10 was assessed in rat lungs (three per group). Inflammatory cell influx was measured by bronchoalveolar lavage (BAL) and air space epithelial permeability was assessed as the total protein in BAL fluid in vivo. The oxidant properties of PM10 particles were determined by their ability to cause damage to plasmid DNA and by changes in reduced (GSH) and oxidised (GSSG) glutathione. The effects of PM10 particles were compared in some experiments with those of fine (CB) and ultrafine (ufCB) carbon black particles. RESULTS: Six hours after intratracheal instillation of PM10 there was an influx of neutrophils (up to 15% of total cells in BAL fluid) into the alveolar space, increased epithelial permeability, the mean (SE) total protein in the BAL fluid increasing from 0.39 (0.01) to 0.62 (0.01) mg/ml, and increased lactate dehydrogenase (LDH) concentrations in the BAL fluid. An even greater inflammatory response was seen following intratracheal instillation of ufCB but not following CB instillation. PM10 particles had free radical activity in vivo, as shown by a decrease in GSH levels in the BAL fluid from 0.36 (0.05) to 0.25 (0.01) nmol/ml following instillation. The free radical activity of PM10 was confirmed in vitro by its ability to deplete supercoiled plasmid DNA, an effect which could be reversed by mannitol, a specific hydroxyl radical scavenger. BAL fluid leucocytes from rats treated with PM10 produced greater amounts of nitric oxide (NO), measured as nitrite (control 3.07 (0.33), treated 4.45 (0.23) microM/1 x 10(6) cells), and tumour necrosis factor alpha (control 21.0 (3.1), treated 179.2 (29.4) units/l x 10(6) cells) in culture than those obtained from control animals. Since the PM10 preparation was contaminated with small amounts of filter fibres due to the extraction process, the effects of instillation of filter fibres alone was assessed. These studies showed that filter fibres did not account for the proinflammatory and injurious effects of the PM10 suspension. CONCLUSIONS: These findings provide evidence that PM10 has free radical activity and causes lung inflammation and epithelial injury. These data support the proposed hypothesis for the mechanism by which particulate air pollution causes adverse effects in patients with airways diseases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corradin S. B., Fasel N., Buchmüller-Rouiller Y., Ransijn A., Smith J., Mauël J. Induction of macrophage nitric oxide production by interferon-gamma and tumor necrosis factor-alpha is enhanced by interleukin-10. Eur J Immunol. 1993 Aug;23(8):2045–2048. doi: 10.1002/eji.1830230851. [DOI] [PubMed] [Google Scholar]
- Dalal N. S., Suryan M. M., Vallyathan V., Green F. H., Jafari B., Wheeler R. Detection of reactive free radicals in fresh coal mine dust and their implication for pulmonary injury. Ann Occup Hyg. 1989;33(1):79–84. doi: 10.1093/annhyg/33.1.79. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Driscoll K. E., Lindenschmidt R. C., Maurer J. K., Higgins J. M., Ridder G. Pulmonary response to silica or titanium dioxide: inflammatory cells, alveolar macrophage-derived cytokines, and histopathology. Am J Respir Cell Mol Biol. 1990 Apr;2(4):381–390. doi: 10.1165/ajrcmb/2.4.381. [DOI] [PubMed] [Google Scholar]
- Faux S. P., Howden P. J., Levy L. S. Iron-dependent formation of 8-hydroxydeoxyguanosine in isolated DNA and mutagenicity in Salmonella typhimurium TA102 induced by crocidolite. Carcinogenesis. 1994 Aug;15(8):1749–1751. doi: 10.1093/carcin/15.8.1749. [DOI] [PubMed] [Google Scholar]
- Gilmour P. S., Beswick P. H., Brown D. M., Donaldson K. Detection of surface free radical activity of respirable industrial fibres using supercoiled phi X174 RF1 plasmid DNA. Carcinogenesis. 1995 Dec;16(12):2973–2979. doi: 10.1093/carcin/16.12.2973. [DOI] [PubMed] [Google Scholar]
- Kennedy T. P., Dodson R., Rao N. V., Ky H., Hopkins C., Baser M., Tolley E., Hoidal J. R. Dusts causing pneumoconiosis generate .OH and produce hemolysis by acting as Fenton catalysts. Arch Biochem Biophys. 1989 Feb 15;269(1):359–364. doi: 10.1016/0003-9861(89)90118-5. [DOI] [PubMed] [Google Scholar]
- Li X. Y., Donaldson K., Brown D., MacNee W. The role of tumor necrosis factor in increased airspace epithelial permeability in acute lung inflammation. Am J Respir Cell Mol Biol. 1995 Aug;13(2):185–195. doi: 10.1165/ajrcmb.13.2.7626286. [DOI] [PubMed] [Google Scholar]
- Li X. Y., Donaldson K., Rahman I., MacNee W. An investigation of the role of glutathione in increased epithelial permeability induced by cigarette smoke in vivo and in vitro. Am J Respir Crit Care Med. 1994 Jun;149(6):1518–1525. doi: 10.1164/ajrccm.149.6.8004308. [DOI] [PubMed] [Google Scholar]
- Lund L. G., Aust A. E. Iron-catalyzed reactions may be responsible for the biochemical and biological effects of asbestos. Biofactors. 1991 Jun;3(2):83–89. [PubMed] [Google Scholar]
- Oberdorster G., Gelein R. M., Ferin J., Weiss B. Association of particulate air pollution and acute mortality: involvement of ultrafine particles? Inhal Toxicol. 1995 Jan-Feb;7(1):111–124. doi: 10.3109/08958379509014275. [DOI] [PubMed] [Google Scholar]
- Ostro B. The association of air pollution and mortality: examining the case for inference. Arch Environ Health. 1993 Sep-Oct;48(5):336–342. doi: 10.1080/00039896.1993.9936722. [DOI] [PubMed] [Google Scholar]
- Pope C. A., 3rd, Bates D. V., Raizenne M. E. Health effects of particulate air pollution: time for reassessment? Environ Health Perspect. 1995 May;103(5):472–480. doi: 10.1289/ehp.95103472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C. A., 3rd, Kanner R. E. Acute effects of PM10 pollution on pulmonary function of smokers with mild to moderate chronic obstructive pulmonary disease. Am Rev Respir Dis. 1993 Jun;147(6 Pt 1):1336–1340. doi: 10.1164/ajrccm/147.6_Pt_1.1336. [DOI] [PubMed] [Google Scholar]
- Punjabi C. J., Laskin J. D., Pendino K. J., Goller N. L., Durham S. K., Laskin D. L. Production of nitric oxide by rat type II pneumocytes: increased expression of inducible nitric oxide synthase following inhalation of a pulmonary irritant. Am J Respir Cell Mol Biol. 1994 Aug;11(2):165–172. doi: 10.1165/ajrcmb.11.2.7519435. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Air pollution and daily mortality: a review and meta analysis. Environ Res. 1994 Jan;64(1):36–52. doi: 10.1006/enrs.1994.1005. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Air pollution and hospital admissions for the elderly in Detroit, Michigan. Am J Respir Crit Care Med. 1994 Sep;150(3):648–655. doi: 10.1164/ajrccm.150.3.8087333. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Slater D., Larson T. V., Pierson W. E., Koenig J. Q. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis. 1993 Apr;147(4):826–831. doi: 10.1164/ajrccm/147.4.826. [DOI] [PubMed] [Google Scholar]
- Seaton A., MacNee W., Donaldson K., Godden D. Particulate air pollution and acute health effects. Lancet. 1995 Jan 21;345(8943):176–178. doi: 10.1016/s0140-6736(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Thiemermann C., Wu C. C., Szabó C., Perretti M., Vane J. R. Role of tumour necrosis factor in the induction of nitric oxide synthase in a rat model of endotoxin shock. Br J Pharmacol. 1993 Sep;110(1):177–182. doi: 10.1111/j.1476-5381.1993.tb13789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallyathan V., Shi X. L., Dalal N. S., Irr W., Castranova V. Generation of free radicals from freshly fractured silica dust. Potential role in acute silica-induced lung injury. Am Rev Respir Dis. 1988 Nov;138(5):1213–1219. doi: 10.1164/ajrccm/138.5.1213. [DOI] [PubMed] [Google Scholar]